Abstract
Stony Coral Tissue Loss Disease (SCTLD) has devastated Caribbean coral reefs since 2014, but its potential for global impact remains uncertain. We developed predictive models to assess the worldwide vulnerability of coral reefs to SCTLD under different origin and spread hypotheses. Using random forest regression models incorporating coral taxonomy and zooxanthellae clade associations from 52 taxa, we projected SCTLD susceptibility and mortality patterns globally using six indices: Mean susceptibility per genus per location, Summed susceptibilities across genera per location, Summed susceptibilities across genera per realm, Mean mortality per genus per location, Summed mortalities across genera per location, and Summed mortalities across genera per realm. Models demonstrated strong predictive performance (R² = 0.57 for susceptibility; R² = 0.73 for mortality) and revealed that about 7% of coral genera per location are potentially susceptible to SCTLD. While mean susceptibility and mortality per genus were highest in the Tropical Atlantic, the summed susceptibility and mortality across genera were much higher in the biodiverse Central Indo-Pacific. Natural barriers could limit SCTLD’s spread, including the mid-Atlantic gap and the low diversity of the Tropical Eastern Pacific, supporting the contained disease hypothesis. However, the widespread distribution of susceptible genera across coral reef realms indicates significant vulnerability should SCTLD circumvent these barriers through human-mediated transport, particularly via ballast water or the aquarium trade. If SCTLD is an invasive pathogen originating in the Pacific, as shipping patterns for the aquarium trade suggest, mortality in its native range would likely be lower than our projections. These findings point to targeted intervention strategies, including enhanced monitoring at key locations, assessment of biosecurity needs in high-risk areas, and prioritized conservation efforts in vulnerable high-diversity regions to prevent SCTLD from spreading globally.
1 Introduction
Reef fish diversity could decline by half if corals were removed from the oceans (Strona et al., 2021). Although coral extinction is a hypothetically extreme case, Stony Coral Tissue Loss Disease (SCTLD) has caused substantial declines in Caribbean coral populations since 2014, reducing coral cover by up to 60% in some areas, with particularly high impacts to reefs dominated by susceptible coral families (Walton et al., 2018; Papke et al., 2024). This aggressive disease spreads rapidly (Figure 1) (Alvarez-Filip et al., 2019), killing coral colonies within weeks of infection and reshaping entire reef ecosystems (Alvarez-Filip et al., 2022; Swaminathan et al., 2024). As SCTLD continues to spread through the Caribbean, coral reef scientists and managers face an urgent question: Could this deadly disease become the next global coral pandemic, threatening the biodiversity hotspots of the Indo-Pacific (Rosenau et al., 2021)?
Figure 1
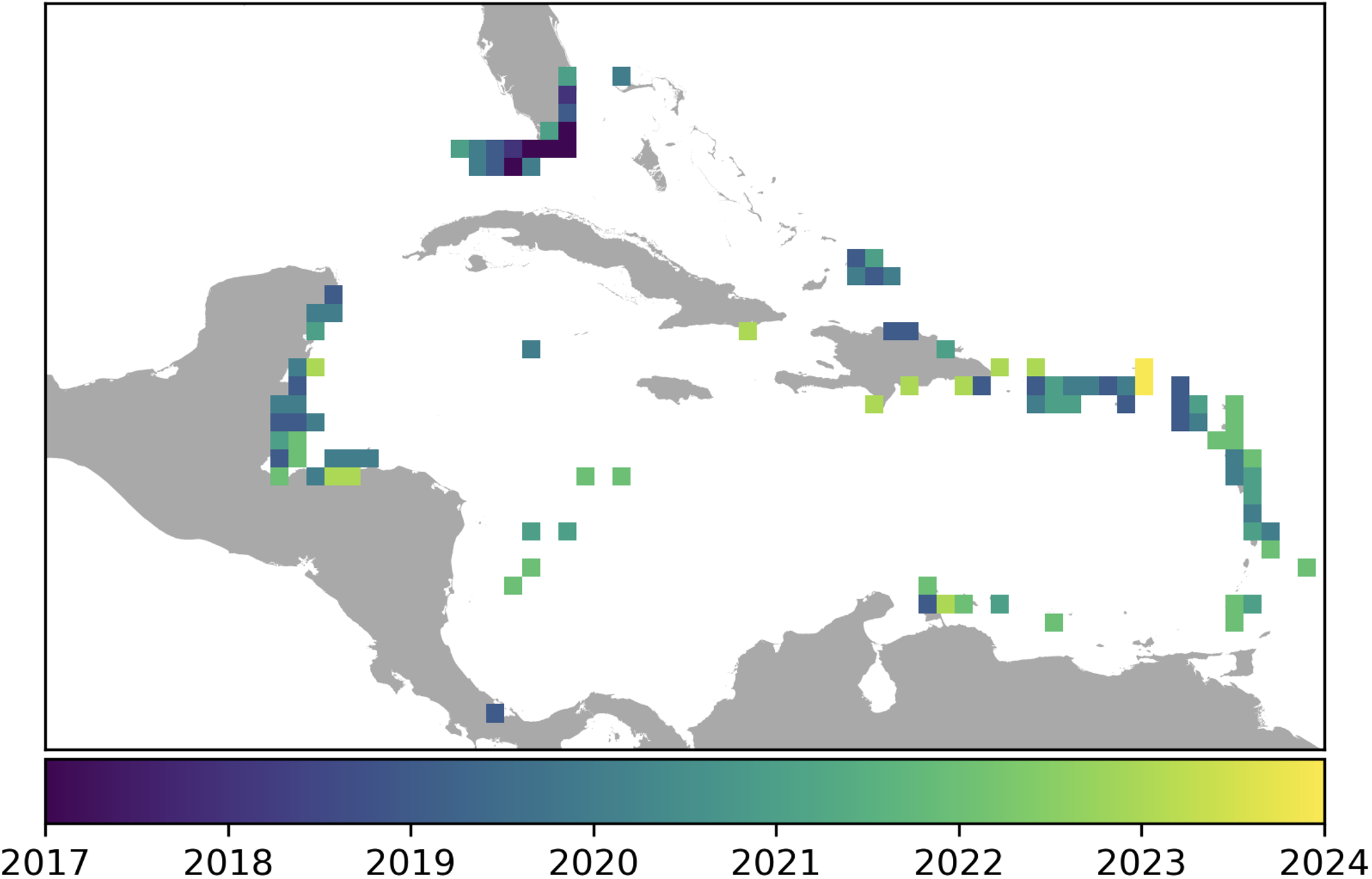
Stony Coral Tissue Loss Disease detections over time through the Caribbean (Kramer et al., 2024) reprojected to the grid approach used in our methods. Each color represents a different year.
Given competing hypotheses about this disease’s origin and potential for spread, we aimed to quantify coral reef vulnerability worldwide based on taxonomic relationships and symbiont associations observed in affected Caribbean species. This approach made it possible to evaluate which global regions face the highest risk if SCTLD were to spread beyond its current range, while identifying potential natural barriers to dispersal and high-risk pathways for transmission. By modeling vulnerability at multiple scales, we hope to inform new monitoring efforts and targeted biosecurity measures.
Multiple potential causal agents of SCTLD are being investigated, including bacteria, which are associated with infection (Rosales et al., 2023), while some evidence points to a virus that targets zooxanthellae—the symbiotic algae essential for coral survival (Work et al., 2021). The disease’s impact varies significantly among coral species, with massive corals and those hosting specific zooxanthellae clades being particularly vulnerable. High-diversity reefs have suffered the most severe impacts from this disease (Costa et al., 2021).
It is widely assumed that coral disease increases with temperature and poor water quality (Lafferty et al., 2004). The initial detection of SCTLD at the Port of Miami is consistent with this hypothesis (Precht et al., 2016), but SCTLD progression is not clearly linked with temperature or nutrients (Carreiro et al., 2024) and its spread to many relatively pristine locations independent of temperature suggests that SCTLD is not a disease of stress (Muller et al., 2020), making temperature extremes and other stressors unsuitable variables for predictive analyses. Thus we excluded the stressful environment hypothesis from our analysis.
The novel disease hypothesis posits that SCTLD is a new pathogen that arose in the Caribbean and is spreading outward (Figure 1). This scenario is plausible because diseases routinely evolve into global pandemics with rapid spread—as HIV, SARS, Avian Influenza, and COVID-19 demonstrate. New marine diseases can emerge in aquaculture settings, where species from different regions are mixed at high density under stressful or novel conditions (Lafferty et al., 2015). It is not clear how SCTLD might have emerged as a novel disease, but, if it has, this hypothesis implies SCTLD could become a global coral pandemic with impacts we can map based on how SCTLD susceptibility and mortality correlate with coral phylogeny and zooxanthellae clades.
The invasive disease hypothesis proposes that SCTLD may be endemic and non-pathogenic elsewhere, and was only recently introduced to the Caribbean where it impacts naïve hosts (Rosenau et al., 2021). History shows that when invasive pathogens encounter naïve hosts with limited evolved tolerance or resistance, the consequences can be notable (Lafferty and Gerber, 2002). In marine systems, disease introductions are best known from aquaculture (Lafferty et al., 2015). An insightful example is the abalone sabellid worm that infects mollusk shells; the worm causes no harm to the South African abalone it coevolved with, but after being introduced by farmers, its disfigurement of California abalone nearly destroyed the industry (Kuris and Culver, 1999). Similarly, an asymptomatic fungal infection carried by Asian salamanders was spread through the pet trade into Europe, where it has been linked to declines in European salamanders (Nguyen et al., 2017). The well-documented escape of pet Pacific lionfish into Florida waters (Semmens et al., 2004) shows how aquarium-related pathways could similarly introduce coral pathogens into new marine ecosystems.
As for the future spread of SCTLD, the contained disease hypothesis suggests that impacts from SCTLD will hopefully remain confined to the Tropical Atlantic. This could occur if SCTLD is an invasive disease that is already prevalent and benign elsewhere. However, this scenario may still leave susceptible areas outside the Tropical Atlantic where SCTLD has yet to arrive. Alternatively, if SCTLD is a novel disease, its dispersal could be limited due to barriers such as currents and continents (Dobbelaere et al., 2022). Thus, the mid-Atlantic and west coast of Africa may hinder eastward spread to the Indian Ocean, while the Isthmus of Panama might constrain westward spread into the Eastern Pacific.
The potential for spread through ballast water (Rosenau et al., 2021) challenges the assumption that natural barriers will limit SCTLD’s global impact. However, while the initial outbreak near the Port of Miami (Precht et al., 2016) suggests a potential ballast-water introduction, limited direct shipping from Pacific coral reefs to Miami challenges this specific explanation. A plausible alternative to ballast water would be the importation of infected Pacific corals to Miami aquarium dealers. This route is worrisome because other tropical ports besides Miami import live organisms for the marine aquarium trade (e.g., Honolulu, Hong Kong, Dubai, Singapore (Green, 2003)). Such human-mediated pathways could circumvent natural boundaries.
Assuming that SCTLD is uncontained leads to the spreading disease hypothesis, which posits that SCTLD will expand beyond the Tropical Atlantic (Muller et al., 2020; Studivan et al., 2022). If SCTLD has a broad endemic range, then impacts might be mostly limited to the Tropical Atlantic. If SCTLD has a narrow endemic range, impacts could be substantial. However, the most impacts would occur if SCTLD is a novel disease that spreads globally. Although the susceptibility of Pacific coral species Pavona clavus and Pocillopora sp. (but not Porites lobata) experimentally exposed to SCTLD experienced rapid tissue loss (Layagala, 2024). The spreading disease hypothesis represents both the most concerning potential outcome for global reef systems and, importantly for our study, the hypothesis most amenable to modeling with available data.
Our analytical approach integrates these source and spread hypotheses to assess the potential global vulnerability to SCTLD. To better understand and prepare for SCTLD’s potential global impact, we used known information about SCTLD’s effects and host range in the Caribbean to map the potential susceptibility of corals to SCTLD (i.e., assuming no barriers to spread). We also mapped the potential impact of SCTLD to corals under the assumption that it is a novel disease with mortality rates similar to that seen in the Caribbean. We assessed SCTLD susceptibility and mortality at the genus, location (degree) and realm (Spalding et al., 2017) scales. The resulting information might also indicate barriers to spread based on pockets of low-susceptibility. These predictive maps are the first global assessment of potential SCTLD vulnerability.
While scientists work to understand SCTLD better, the National Oceanic and Atmospheric Administration (NOAA) has outlined a course of action acknowledging that SCTLD will likely persist on U.S. coral reefs (Skrivanek and Wusinich-Mendez, 2020). The plan aims to reduce spread, identify vulnerable areas, preserve at-risk coral species, and identify restoration opportunities. Such management initiatives could inform global responses if SCTLD were to spread beyond the Caribbean.
Although we present multiple hypotheses about SCTLD’s origin and spread, our primary aim was to model two specific scenarios: (1) the potential distribution of SCTLD if it is an invasive infectious disease, assuming patterns of infection seen in the Caribbean apply elsewhere, and (2) its distribution and impacts if it is a novel disease, assuming patterns of infection and mortality seen in the Caribbean apply elsewhere. These scenarios correspond most directly to testing predictions from the invasive disease and novel disease hypotheses.
2 Materials and methods
We modeled the potential spread of a novel disease statistically. Specifically, we estimated SCTLD’s potential impact on coral populations worldwide based on the assumption that the relationships observed in the Caribbean between coral susceptibility to SCTLD and coral family, as well as the types of algae (zooxanthellae clades) they host (Beavers et al., 2023), can be extrapolated to coral reefs outside the Caribbean. To do so, we compiled SCTLD susceptibility and mortality data for 40 coral species from two key studies (Alvarez-Filip et al., 2019; Estrada-Saldívar et al., 2020).
We based our mortality estimates on published data. Corals are long-lived hosts whose dead skeleton remains fixed in place, giving researchers more confidence in mortality rates than for just about any non-human host. If mortality parameters are biased because researchers focus on heavily impacted sites, our estimates may represent upper limits rather than average values. Although this potential bias wouldn’t change our qualitative conclusions about relative vulnerability across regions, it could affect impact magnitude.
We used machine learning to make predictions using global coral distribution data. Range data were taken from a public IUCN database (https://www.iucnredlist.org/resources/spatial-data-download) and mapped onto reef polygons rasterized at a resolution of 1° × 1° latitude/longitude (Strona et al., 2021), which resulted in the exclusion of small reefs (UNEP-WCMC, 2025). After excluding species absent from the GeoSymbio dataset (Franklin et al., 2012), we aggregated the remaining data at species, genus, and family levels into a comprehensive dataset of 52 taxa (28 species, 15 genera, and 9 families). From this, we developed a multivariate dataset incorporating SCTLD susceptibility, mortality, zooxanthellae clade associations, and taxonomic levels for each taxon. Given the lack of support for the stressful environment hypothesis, we did not consider additional drivers like water quality or temperature when predicting susceptibility and mortality. Using our prepared dataset, we created two random forest regression models with zooxanthellae associations and taxonomic levels as independent variables to predict SCTLD susceptibility and mortality for nearly all coral genera worldwide. We evaluated model accuracy with leave-one-out cross validation. This approach generated 52 partial models for both susceptibility and mortality, each based on 51 observations. We then quantified accuracy as the R2 between the observed and the predicted susceptibility/mortality in the left-out records. All models were stored and used in global projections, providing mean and standard errors for each location. Additionally, for realm-scale bar plots, we bootstrapped the 95% confidence intervals, which underscores their robustness (Supplementary Table S1 and indicated by maps of standard errors in the supplementary).
To visualize the results globally, we developed six indices to represent SCTLD risk and impact:
Mean susceptibility per genus, per location, . Summed susceptibilities across genera, per location, . Summed susceptibilities across genera, per realm, N . Mean mortality per genus, per location, . Summed mortalities across genera, per location, . Summed mortalities across genera, per realm, N . The six indices give a multi-scale understanding of SCTLD risk across different coral genera, locations, and realms. To show their application, we present these indices for a hypothetical community of three coral genera (A, B, and C) with the following values in a realm with 30 locations:
-
30% susceptibility, 50% mortality
-
10% susceptibility, 10% mortality
-
0% susceptibility, 0% mortality
Then:
-
= 0.4/3 = 0.13
-
= 0 + 0.3 + 0.1 = 0.4 (can be > 1)
-
= 30*0.4 = 12
-
= 0.16/3 = 0.053
-
= 0.15 + 0.01 = 0.16 (can be > 1)
-
= 30*0.16 = 4.8
A consequence of being limited to presence-absence coral data is that predictions might overestimate SCTLD’s impact if susceptible species are less abundant. Therefore, we compared our indices calculated from presence-absence data against indices calculated from coral abundance data published by NOAA’s National Coral Reef Monitoring Program (Coral Reef Conservation Program (CRCP), 2016; Towle et al., 2021), assuming that relationships seen in this national level data could be generalized to other coral reef realms (many other realms have higher coral diversity than occur in the NOAA data). These measures were highly correlated and their regression slope generates a coefficient that we multiplied our indices by in order to translate impacts per coral genus to impacts per reef. We compared in-sample predictions with actual data to validate the model, estimate its error, and confirm the model was not overfit.
We used a heat map to represent the indices globally. On this map, mean susceptibility per genus illustrates the expected susceptibility for an average coral genus at each location. The mean mortality per genus map was built off the mean-susceptibility per genus map to illustrate the expected mortality for the average coral genus at a location. The summed susceptibilities across genera map illustrates where SCTLD is likely to thrive due to having many susceptible hosts, whereas the summed mortality across genera map showed locations where SCTLD is likely to have higher absolute (rather than relative) impacts to corals. We addressed potential uncertainties in our predictions through sensitivity analyses and by creating uncertainty maps generated using the leave-one-out procedure above (See Supplementary Figures).
To make broader scale comparisons in per genus and per location metrics, we calculated mean indices for locations within five distinct coral reef realms (Spalding et al., 2007): Western Indo-Pacific (WIP), Central Indo-Pacific (CIP), Eastern Indo-Pacific (EIP)), Tropical Eastern Pacific (TEP), and Tropical Atlantic (TA) as illustrated in Figure 2. These realms vary considerably in their number of locations, so we also compared cumulative susceptibility and impacts among realms after summing across locations.
Figure 2
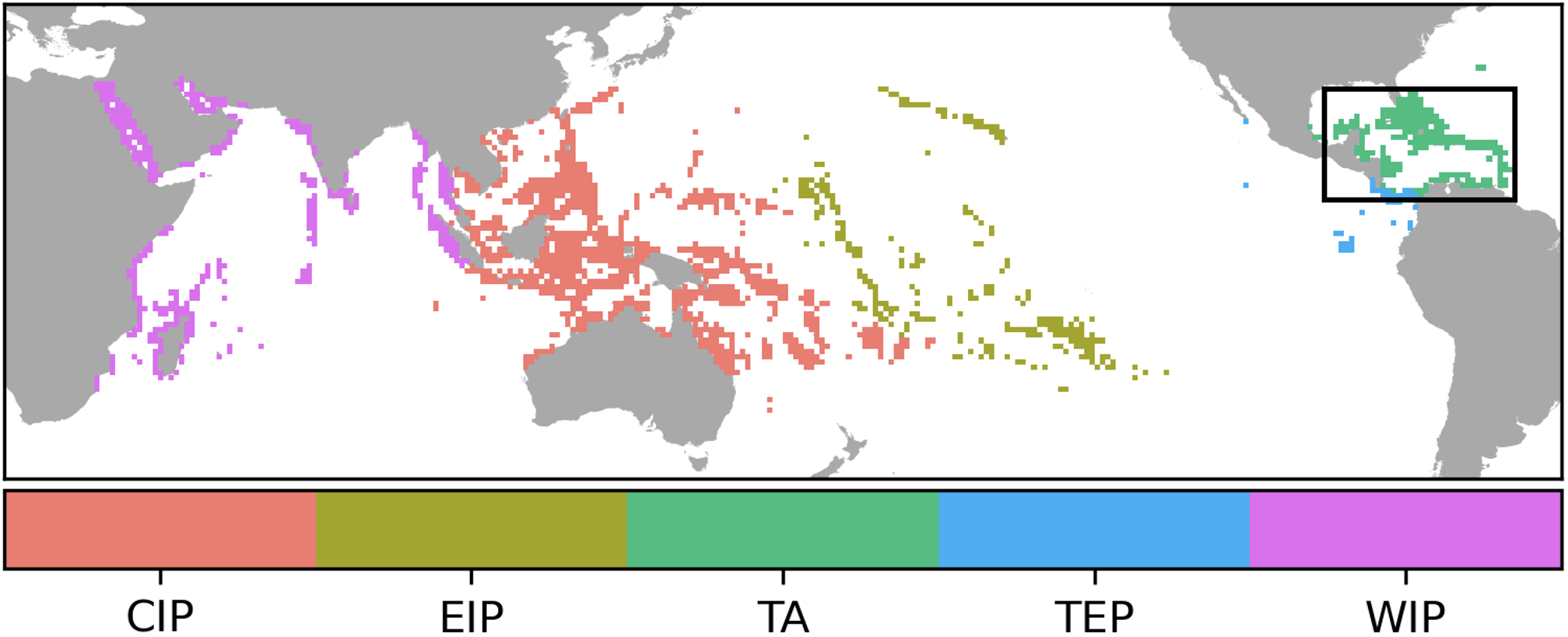
Five coral reef regions used for summarizing and contrasting projected SCTLD susceptibility and mortality: Western Indo-Pacific (WIP), Central Indo-Pacific (CIP), Eastern Indo-Pacific (EIP)), Tropical Eastern Pacific (TEP), Tropical Atlantic (TA). The inset map refers to Figure 1. Each color represents a different realm (UNEP-WCMC, 2025).
Model validation revealed strong predictive performance. The susceptibility model achieved an overall R² of 0.57, with R² values of 0.48, 0.57, and 0.85 for species, genus, and family levels, respectively. The mortality model showed even stronger predictive power, with an overall R² of 0.73, and R² values of 0.69 (species), 0.76 (genus), and 0.9 (family). We found good correlations (R² between 0.47 and 0.79) between the four indices and their weighted counterparts. Notably, the weighted versions were consistently lower than the unweighted indices, likely due to higher susceptibility and mortality in less common coral species. To account for this, we applied correction factors to our unweighted indices, adjusting the map scales accordingly.
3 Results
Consistent with concerns from the spreading disease hypothesis, presumably susceptible genera were widely distributed across all major coral reef realms. Some locations with low coral diversity outside the Caribbean, such as reefs in the parts of India and Vietnam, showed particularly high mean susceptibility per genus (Figure 3A). Otherwise, predicted mean susceptibility per genus at a location was lower than what was observed throughout the Tropical Atlantic (Figures 3A, 4A). When weighted to favor common genera, potentially susceptible SCTLD hosts comprised a mean of 7% of genera per location. These patterns reveal that SCTLD infects only a small percentage of coral genera globally, though that might not fully indicate susceptibility to invasion.
Figure 3
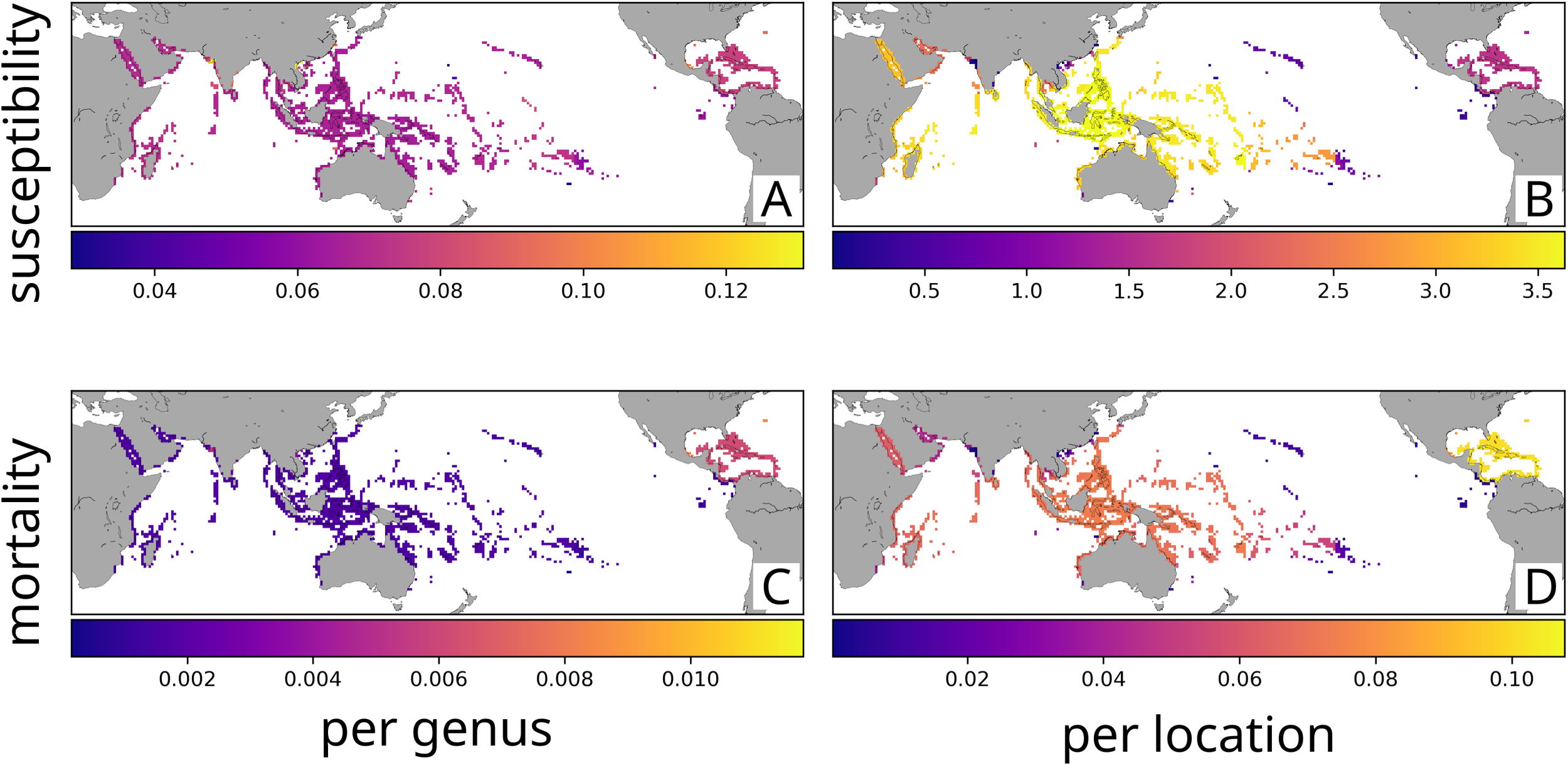
World maps of projected SCTLD susceptibility and impact per location. (A) Mean susceptibility per genus, per location (weighted by relative abundance). (B) Summed susceptibilities across genera, per location (weighted by relative abundance). (C) Mean mortality per genus, per location (weighted by relative abundance). (D) Summed mortalities across genera, per location (weighted by relative abundance).
Figure 4
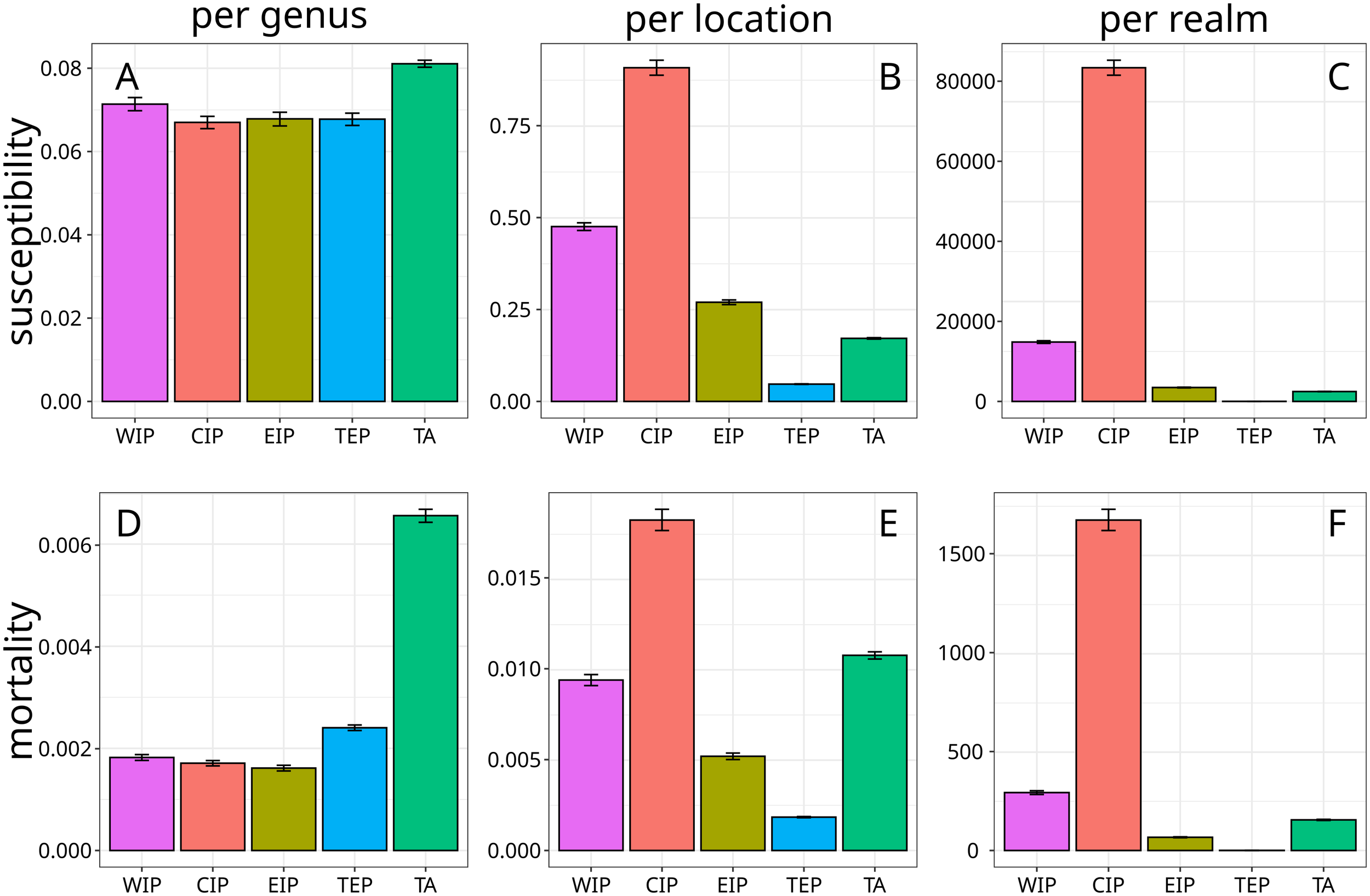
Variation in projected SCTLD susceptibility and impact by realm with 99% bootstrapped confidence intervals. Western Indo-Pacific (WIP), Central Indo-Pacific (CIP), Eastern Indo-Pacific (EIP)), Tropical Eastern Pacific (TEP), Tropical Atlantic (TA). (A) Mean susceptibility per genus, per location. (B) Summed susceptibilities across genera, per location (weighted by relative abundance). (C) Summed susceptibilities across genera, per location (weighted by relative abundance) times realm area. (D) Mean mortality per genus, per location. (E) Summed mortalities across genera, per location (weighted by relative abundance). (F) Summed mortalities across genera, per location (weighted by relative abundance) times realm area. See Supplementary Table S1 for the robustness of these patterns to bootstrapping.
To better predict potential SCTLD invasion, we assessed summed susceptibility across genera per location (Figures 3B, 4B), revealing substantial variation across realms driven by differences in coral diversity. Except for the low diversity Tropical Eastern Pacific, all realms had higher summed susceptibility across genera per location than the Tropical Atlantic (Figure 4B). For instance, summed susceptibility across genera per location was three times higher in the Western Indo-Pacific (Figure 4B). The Central Indo-Pacific’s exceptional coral diversity made it the most vulnerable realm per location in absolute terms, despite having lower mean susceptibility per genus than the Caribbean (Figure 4B). The total coverage of susceptible corals varied dramatically across realms, with the small, low-diversity Tropical Eastern Pacific containing the least susceptible area, while the massive and biodiverse Central Indo-Pacific harbored the greatest extent of potentially vulnerable coral ecosystems by far (Figure 4C).
Several of our findings support the contained disease hypothesis. For example, there were few patches of susceptible corals between the Caribbean and the Indian Ocean (Figure 3B), creating a natural barrier against eastward spread. Furthermore, the low diversity of potentially susceptible corals in the Eastern Tropical Pacific (Figure 3B) would fortify the natural barrier created by the Isthmus of Panama. Finally, the expected moderate susceptibility of Hawaiian corals would limit the vulnerability of introduction through the port of Honolulu.
Under the novel disease hypothesis, our models predicted relatively low mean mortality impacts per coral genus globally (0.24%), though with substantial regional variation. Mean mortality decreased significantly outside the Tropical Atlantic, with the Tropical Eastern Pacific showing only slightly higher mean mortality per genus per location than other Pacific Realms (Figures 3C, 4D). Exceptions included high predicted mean mortality in India and Vietnam that were comparable with the Tropical Atlantic (Figure 3C).
When considering overall reef impact, summed mortalities across genera per location provides a more comprehensive measure of potential disease effects. This metric projected the Central Indo-Pacific would be the most severely impacted realm due to its high diversity, followed by the Tropical Atlantic with its high mean susceptibility and mortality (Figures 3D, 4E). The Western Indo-Pacific and Eastern Indo-Pacific were projected to have intermediate summed mortalities per location, and the Tropical Eastern Pacific had the lowest projected summed mortalities per location due to its low diversity (Figures 3D, 4E). The relative amount of coral mortality across realms was similar to that seen for susceptibility due to the vast differences in the number of locations among realms (Figure 4F).
Although we did not explicitly model the invasive disease hypothesis, the Coral Triangle’s high diversity of potentially susceptible and tolerant hosts (Figure 3B) points to the possibility that SCTLD might have Pacific origins. If so, the actual mortality rates at locations where SCTLD is native could be less than predicted in Figures 3C, D, 4D–F which assume that all realms have similar mortality to the Caribbean.
4 Discussion
SCTLD currently appears contained within the Caribbean, and there are natural barriers to its spread reinforced by pockets of low susceptible coral communities. However, ballast water dispersal over long distances might bypass these natural barriers. Furthermore, the ports of Dubai, Singapore, and Hong Kong import coral for the pet trade and have many susceptible genera on nearby reefs, putting those areas at risk if SCTLD is an invasive disease. Regardless of the vector, the potential for spread to a realm seems most likely to be driven by summed susceptibility, which increases in high-diversity coral communities. Thus, SCTLD could do best in biodiverse realms like the Coral Triangle but have limited spread through the Tropical Eastern Pacific and moderate spread through the Central Indo-Pacific. Additional studies of Pacific coral susceptibility are needed to refine these predictions.
SCTLD research faces several important limitations that affect our predictions. Scientists have studied this coral disease extensively, but the unknown causal agent and unclear host relationships make some results speculative. Our analysis used data from only 52 coral genera, which may miss local variations and differences in disease response outside the Caribbean. We trained our models exclusively on Mexican Caribbean data, so they may not predict disease outcomes accurately in other regions where coral communities and environmental conditions differ. Our analysis also excluded oceanographic factors like temperature and water quality, which might influence SCTLD progression. Random forest models excel at prediction but remain sensitive to data quality and may miss complex ecological processes such as disease transmission routes or environmental feedback loops. Future studies examining Pacific coral susceptibility would strengthen these predictive models and expand their global applicability.
If SCTLD is a novel disease, then its impacts could expand considerably out from the Tropical Atlantic. The loss in absolute coral cover per location would be much lower in the low-diversity Tropical Eastern Pacific and Eastern Indo-Pacific realms, but much higher in the biodiverse Central Indo-Pacific. Furthermore, coupling the large extent of the Indo-Pacific with the relatively high per-location cumulative mortality rates indicates that a novel SCTLD would have the potential to cause coral mortality on a scale several times greater than already experienced in the Caribbean.
If SCTLD was introduced to the Caribbean, aquarium trade shipping patterns might point to a source. In the years before SCTLD was detected, nearly all marine invertebrate shipments to Miami, USA came from locations with many SCTLD susceptible coral genera: Sri Lanka (23%), Indonesia (27%), or the Philippines (49%) (Rhyne et al., 2015). Based on these export hubs, and the relatively high susceptibility around them, the Pacific seems the most likely potential source of SCTLD in the Caribbean. Assuming Pacific corals have coevolved with the agent causing SCTLD, their mortality rates might be too low for noticeable disease signs.
The actual future distribution of SCTLD will depend on which assumptions about spread and origin prove to be correct. However, testing these key assumptions will remain challenging until either SCTLD is observed outside the Caribbean, or exposure experiments can be conducted with corals from different realms.
Once the causative agent for SCTLD is identified, and an effective diagnostic assay is developed for asymptomatic corals, it should be possible to examine whether SCTLD is already endemic elsewhere and whether it has less virulence there. Doing so would help identify a potential pathogen source as well as areas that might be susceptible, but not yet invaded. Should evidence build for the invasive disease hypothesis, it may be possible to identify and minimize introduction vectors for this and potentially other coral diseases.
Managers might combat SCTLD through rescuing colonies of vulnerable species, preserving their genetic material, and implementing restoration efforts, which might focus on reducing other threats like water quality and overfishing (Alvarez-Filip et al., 2022). Treatment options include applying antibiotics or probiotics to individual coral colonies, though the scientific community remains divided on these approaches (Papke et al., 2024). Preventative measures could include biosecurity for tropical marine aquarium imports into ports with nearby coral reefs. Our research helps identify locations where such interventions would yield the greatest conservation benefits. These findings also support existing management frameworks like NOAA’s Strategy for SCTLD Response and Prevention (Skrivanek and Wusinich-Mendez, 2020), which identifies Pacific expansion as a major threat. We pinpointed high-risk entry points and vulnerable biodiversity hotspots that managers can use to develop response strategies. This research provides guidance for long-term conservation planning across realms that extend beyond the Caribbean.
The different outcomes expected under these four key hypotheses show that further research into SCTLD’s causative agent(s) and origin would refine predictions about its future spread and impact. While managing coral diseases has historically proven challenging, our findings provide a roadmap for targeted intervention strategies—including enhanced monitoring at key locations, strengthened biosecurity in high-risk areas, identification of potential disease vector pathways, such as through the aquarium trade, and prioritized conservation efforts in regions with many susceptible species—that together could significantly reduce the risk of SCTLD transforming from a regional problem into a global coral epidemic.
Statements
Data availability statement
Publicly available datasets were analyzed in this study. These datasets can be found here: IUCN Red List Spatial Data Download; Habitats Ocean+; NOAA NCEI Accession 0157633; AGRRA Map of Coral Disease in the Caribbean and Florida; https://github.com/giovannistrona/SCTLD.
Author contributions
KL: Project administration, Funding acquisition, Methodology, Writing – review & editing, Investigation, Conceptualization, Writing – original draft. GS: Methodology, Formal analysis, Writing – original draft, Writing – review & editing, Software, Data curation, Visualization, Conceptualization, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. KL received funding from the U.S. Geological Survey Ecosystems Mission Area.
Acknowledgments
Sara Swaminathan provided suggestions and comments. Any use of trade, firm, or product names in this publication is for descriptive purposes only and does not imply endorsement by the U.S. Government. Anthropic Claude 3.5 Sonnet was used to help proof English and formatting.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1608622/full#supplementary-material
References
1
Alvarez-Filip L. Estrada-Saldívar N. Pérez-Cervantes E. Molina-Hernández A. González-Barrios F. J. (2019). A rapid spread of the stony coral tissue loss disease outbreak in the Mexican Caribbean. PeerJ7, e8069. doi: 10.7717/peerj.8069
2
Alvarez-Filip L. González-Barrios F. J. Pérez-Cervantes E. Molina-Hernández A. Estrada-Saldívar N. (2022). Stony coral tissue loss disease decimated Caribbean coral populations and reshaped reef functionality. Commun. Biol.5, 440. doi: 10.1038/s42003-022-03398-6
3
Beavers K. M. Van Buren E. W. Rossin A. M. Emery M. A. Veglia A. J. Karrick C. E. et al . (2023). Stony coral tissue loss disease induces transcriptional signatures of in situ degradation of dysfunctional Symbiodiniaceae. Nat. Commun.14, 2915. doi: 10.1038/s41467-023-38612-4
4
Carreiro A. M. Eckert R. J. Sturm A. B. Ingalls T. C. Combs I. R. Walker B. K. et al . (2024). Assessment of nutrient amendments on stony coral tissue loss disease in Southeast Florida. Front. Mar. Sci.11, 1384534. doi: 10.3389/fmars.2024.1384534
5
Coral Reef Conservation Program (CRCP) (2016). Documentation for NOAA’s Coral Reef Conservation Program (CRCP) National Coral Reef Monitoring Program (NCRMP) data archived at NCEI (NCEI Accession 0157633). Available online at: https://www.ncei.noaa.gov/archive/accession/0157633 (Accessed April 26, 2024).
6
Costa S.V. Hibberts S.J. Olive D.A. Budd K.A. Long A.E. Meiling S.S. et al . (2021). Diversity and disease: The effects of coral diversity on prevalence and impacts of stony coral tissue loss disease in Saint Thomas, US Virgin Islands. Front. Mar. Sci.8, 682688.
7
Dobbelaere T. Holstein D. M. Muller E. M. Gramer L. J. McEachron L. Williams S. D. et al . (2022). Connecting the dots: transmission of stony coral tissue loss disease from the Marquesas to the Dry Tortugas. Front. Mar. Sci.9, 778938. doi: 10.3389/fmars.2022.778938
8
Estrada-Saldívar N. Molina-Hernández A. Pérez-Cervantes E. Medellín-Maldonado F. González-Barrios F. J. Alvarez-Filip L. (2020). Reef-scale impacts of the stony coral tissue loss disease outbreak. Coral Reefs39, 861–866. doi: 10.1007/s00338-020-01949-z
9
Franklin E. C. Stat M. Pochon X. Putnam H. M. Gates R. D. (2012). GeoSymbio: a hybrid, cloud-based web application of global geospatial bioinformatics and ecoinformatics for Symbiodinium–host symbioses. Mol. Ecol. Resour.12, 369–373. doi: 10.1111/j.1755-0998.2011.03081.x
10
Green E. (2003). International trade in marine aquarium species: using the global marine aquarium database. Mar. Ornamental Species: Collection Cult. Conserv., 31–47. doi: 10.1002/9780470752722.ch3
11
Kramer P. R. Roth L. Lang J. C. (2024). Map of Stony Coral Tissue Loss Disease Outbreak in the Caribbean. Available online at: www.aggra.org (Accessed February 15, 2025).
12
Kuris A. M. Culver C. S. (1999). An introduced sabellid polychaete pest infesting cultured abalones and its potential spread to other California gastropods. Invertebrate Biol.118, 391–403. doi: 10.2307/3227008
13
Lafferty K. D. Gerber L. R. (2002). Good medicine for conservation biology: The intersection of epidemiology and conservation theory. Conserv. Biol.16, 593–604. doi: 10.1046/j.1523-1739.2002.00446.x
14
Lafferty K. D. Harvell C. D. Conrad J. M. Friedman C. S. Kent M. L. Kuris A. M. et al . (2015). Infectious diseases affect marine fisheries and aquaculture economics. Annu. Rev. Mar. Sci.7, 471–496. doi: 10.1146/annurev-marine-010814-015646
15
Lafferty K. D. Porter J. W. Ford S. E. (2004). Are diseases increasing in the ocean? Annu. Rev. Ecol. Evol. Syst.35, 31–54. doi: 10.1146/annurev.ecolsys.35.021103.105704
16
Layagala K. (2024). Transmissibility of Stony Coral Tissue Loss Disease (SCTLD) to Pacific Coral Species (William & Mary). Available online at: https://scholarworks.wm.edu/honorstheses/2245/.
17
Muller E. M. Sartor C. Alcaraz N. I. Van Woesik R. (2020). Spatial epidemiology of the stony-coral-tissue-loss disease in Florida. Front. Mar. Sci.7, 163. doi: 10.3389/fmars.2020.00163
18
Nguyen T. T. Van Nguyen T. Ziegler T. Pasmans F. Martel A. (2017). Trade in wild anurans vectors the urodelan pathogen Batrachochytrium salamandrivorans into Europe. Amphibia-Reptilia38, 554–556. doi: 10.1163/15685381-00003125
19
Papke E. Carreiro A. Dennison C. Deutsch J. M. Isma L. M. Meiling S. S. et al . (2024). Stony coral tissue loss disease: a review of emergence, impacts, etiology, diagnostics, and intervention. Front. Mar. Sci.10, 1321271. doi: 10.3389/fmars.2023.1321271
20
Precht W. F. Gintert B. E. Robbart M. L. Fura R. Van Woesik R. (2016). Unprecedented disease-related coral mortality in Southeastern Florida. Sci. Rep.6, 31374. doi: 10.1038/srep31374
21
Rhyne A. L. Tlusty M. F. Holmberg R. Szczebak J. (2015). Marine aquarium biodiversity and trade flow. Available online at: https://aquariumtradedata.org/members/ (Accessed March 18, 2025).
22
Rosales S. M. Huebner L. K. Evans J. S. Apprill A. Baker A. C. Becker C. C. et al . (2023). A meta-analysis of the stony coral tissue loss disease microbiome finds key bacteria in unaffected and lesion tissue in diseased colonies. ISME Commun.3, 19. doi: 10.1038/s43705-023-00220-0
23
Rosenau N. A. Gignoux-Wolfsohn S. Everett R. A. Miller A. W. Minton M. S. Ruiz G. M. (2021). Considering commercial vessels as potential vectors of stony coral tissue loss disease. Front. Mar. Sci.8, 709764. doi: 10.3389/fmars.2021.709764
24
Semmens B. X. Buhle E. R. Salomon A. K. Pattengill-Semmens C. V. (2004). A hotspot of non-native marine fishes: evidence for the aquarium trade as an invasion pathway. Mar. Ecol. Prog. Ser.266, 239–244. doi: 10.3354/meps266239
25
Skrivanek A. Wusinich-Mendez D. (2020). NOAA Strategy for Stony Coral Tissue Loss Disease Response and Prevention (Washington D.C.: U.S. Department of Commerce, National Oceanic and Atmospheric Administration). Available online at: https://coast.noaa.gov/data/coralreef_noaa_gov/media/docs/NOAA_SCTLD_Strategy_2020.pdf.
26
Spalding M. Burke L. Wood S. A. Ashpole J. Hutchison J. Zu Ermgassen P. (2017). Mapping the global value and distribution of coral reef tourism. Mar. Policy82, 104–113. doi: 10.1016/j.marpol.2017.05.014
27
Spalding M. D. Fox H. E. Allen G. R. Davidson N. Ferdaña Z. A. Finlayson M. et al . (2007). Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. BioScience57, 573–583. doi: 10.1641/B570707
28
Strona G. Lafferty K. D. Fattorini S. Beck P. S. Guilhaumon F. Arrigoni R. et al . (2021). Global tropical reef fish richness could decline by around half if corals are lost. Proc. R. Soc. B.288, 20210274. doi: 10.1098/rspb.2021.0274
29
Studivan M. S. Baptist M. Molina V. Riley S. First M. Soderberg N. et al . (2022). Transmission of stony coral tissue loss disease (SCTLD) in simulated ballast water confirms the potential for ship-born spread. Sci. Rep.12, 19248. doi: 10.1038/s41598-022-21868-z
30
Swaminathan S. D. Lafferty K. D. Knight N. S. Altieri A. H. (2024). Stony coral tissue loss disease indirectly alters reef communities. Sci. Adv.10, eadk6808. doi: 10.1126/sciadv.adk6808
31
Towle E. K. Allen M. E. Barkley H. Besemer N. (2021). National coral reef monitoring plan. Washington D.C.: NOAA Coral Reef Conservation Program (U.S.)
32
UNEP-WCMC (2025). Ocean+ Habitats. Available online at: habitats.oceanplus.org (Accessed May 9, 2025).
33
Walton C. J. Hayes N. K. Gilliam D. S. (2018). Impacts of a regional, multi-year, multi-species coral disease outbreak in Southeast Florida. Front. Mar. Sci.5, 323. doi: 10.3389/fmars.2018.00323
34
Work T. M. Weatherby T. M. Landsberg J. H. Kiryu Y. Cook S. M. Peters E. C. (2021). Viral-like particles are associated with endosymbiont pathology in Florida corals affected by stony coral tissue loss disease. Front. Mar. Sci.8, 750658.
Summary
Keywords
coral, disease, invasive, spread, map
Citation
Lafferty KD and Strona G (2025) Mapping global coral vulnerability to stony coral tissue loss disease: implications for biosecurity and conservation. Front. Mar. Sci. 12:1608622. doi: 10.3389/fmars.2025.1608622
Received
09 April 2025
Accepted
30 June 2025
Published
25 July 2025
Volume
12 - 2025
Edited by
Lorenzo Alvarez-Filip, National Autonomous University of Mexico, Mexico
Reviewed by
Abigail S. Clark, Boy Scouts of America - Florida Sea Base: Brinton Environmental Center, United States
Andrea Rivera-Sosa, Coral Reef Alliance, United States
Updates
Copyright
© 2025 Lafferty and Strona.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kevin D. Lafferty, klafferty@usgs.gov; Giovanni Strona, giovanni.strona@ec.europa.eu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.