- 1Integrative Marine Ecology Department, Stazione Zoologica Anton Dohrn, Naples, Italy
- 2National Biodiversity Future Center (NBFC), Palermo, Italy
- 3Department of Research Infrastructures for Marine Research, Stazione Zoologica di Napoli Anton Dohrn, Naples, Italy
- 4Aix Marseille University, Université de Toulon, CNRS, IRD, MIO, Marseille, France
- 5UMR TELEMMe, MMSH, Aix-Marseille University, CNRS, Aix-en-Provence, France
True jellyfish (Cnidaria, Scyphozoa) often appear in large aggregations along the coasts, where they interfere with human activities (tourism, fisheries, power plants). Therefore, defining their distribution and predicting their outbreaks is crucial for effective coastal management. In this study, we tested the combination of modeling based on the Lagrangian approach and stable isotope (SI) analysis to define the trajectories of the scyphomedusa Pelagia noctiluca in the Gulf of Naples (GoN, western Mediterranean Sea) during 4 outbreaks recorded in March, June, July, and November 2019. SIs were determined in scyphomedusae and their potential planktonic prey collected at the Long Term Research site MareChiara (LTER-MC) during the outbreaks and during the previous three weeks, to account for the turnover rate of medusae. Numerical simulations were performed using a particle tracking model forced by a Regional Ocean Modeling System (ROMS) developed for the GoN. Lagrangian simulations were performed releasing particles 20 days before the outbreaks to align with SI determinations. SI ratios of scyphomedusae indicated offshore foraging, with Lagrangian simulations confirming offshore-to-coastal transport via south Tyrrhenian surface dynamic and southern winds regime. During the outbreak in November, carbon and nitrogen SIs of medusae (-18.7‰ and 1.9‰, respectively) reflected the SIs of plankton typically found in offshore waters. The model corroborated this finding, suggesting a rapid transport of medusae by surface currents driven by intense southerly winds (gusts up to 18.7 m/s). During the other three outbreaks, SI values of medusae (δ13C ranging between -20.1 and -18.5‰, δ15N between 4.6 and 5.9‰) were intermediate between prey found offshore and those in the coastal area. Simulations indicated that surface circulation patterns promoted the permanence of medusae within the coastal area, particularly in summer. Our results suggest that SI ratios of scyphomedusae are intimately dependent on their movements across diverse isoscapes. Therefore, predictive models integrating SI analysis and ocean circulation data could improve early warning systems for jellyfish outbreaks, aiding coastal management.
1 Introduction
“True” jellyfish (Cnidaria, Scyphozoa) often appear in large aggregations of numerous individuals as a result of high reproductive rates (blooms), oceanographic conditions (wind, water currents) (outbreaks) or a combination of the two factors (Fernández-Alías et al., 2024; Lucas and Dawson, 2014). Mass appearances of jellyfish are recorded mainly in coastal areas, where human activities concentrate and appear vulnerable to these phenomena. Jellyfish sting bathers, damage fishing nets and fish caught within them, stop power plant activities, with a not yet accurately determined economic loss (De Donno et al., 2014; Palmieri et al., 2015; Mghili et al., 2022). Additionally, because jellyfish are generally considered potential predators and competitors of fish as they prey upon plankton, including fish eggs and larvae (Purcell and Arai, 2001), they appear to deplete fish stocks (Lynam et al., 2006; Robinson et al., 2014). While jellyfish outbreaks are blamed for having a negative effect on the economy, their ecological role within the food web is still underestimated. Their position at intermediate trophic levels within marine food webs (Hays et al., 2018) likely makes large aggregations of jellyfish a resource for predators at higher trophic levels (sea turtles, mammals, birds, sharks) and benthic organisms, which rely on jelly-falls to survive on the seafloor (Sweetman et al., 2014).
Within the Mediterranean Sea, the scyphomedusa Pelagia noctiluca (Forsskål, 1775) is an example of the multi-faceted role of jellyfish within marine ecosystems. Renowned for its remarkable outbreaks in coastal areas ( (Brotz and Pauly, 2012), this species is commonly called “the mauve stinger” and appears to negatively affect touristic and fishery activities of coastal areas within the Mediterranean Sea (Canepa et al., 2014). In order to evaluate the effects of predation by this species upon fish stocks, the dietary composition of P. noctiluca has been defined using analysis of gut contents alone (Malej, 1989; Rosa et al., 2013; Tilves et al., 2016) or combined with stable isotope (SI) analysis (Milisenda et al., 2018; Tilves et al., 2018) and experimental work under controlled conditions (Tilves et al., 2012). Results indicated that P. noctiluca is an opportunistic predator with remarkable feeding rates upon a variety of planktonic prey. However, fish (Milisenda et al., 2014) and corals (Musco et al., 2018) have been found to prey upon P. noctiluca, while the observation of several fish with mouths full of nematocysts after Pelagia’s outbreaks suggested that fish which did not feed upon jellyfish on a regular basis, could take advantage of the sudden and unexpected availability of prey (Orsi Relinii et al., 2010).
Despite the importance of jellyfish mass appearances within the economy of human activities and the functioning of ecosystems, the mechanisms regulating jellyfish outbreaks are defined only in part (Fernández-Alías et al., 2024; Lucas and Dawson, 2014). The presence of jellyfish is detected easily at the surface because they can attain a large size and attract the general public, which prompted the success of citizen science projects (Boero, 2013; Marambio et al., 2021). However, direct observations at sea did not allow tracking jellyfish in space and time, but they contributed to developing numerical models to define the movements of jellyfish and unravel the physical processes that locally drive the outbreaks. These processes are difficult to assess from either in situ or remote observations, which do not provide information about the circulation patterns. Hydrodynamic circulation models overcome this limitation by reproducing surface current and water column dynamics at high spatio-temporal resolution. In order to simulate the transport and distribution of particles (passive or active) as forced by hydrodynamic processes, the Lagrangian approach is usually applied because it allows to reconstruct, for a fixed geographic region, particles’ history in space and time (van Sebille et al., 2018). Particle tracking models offer high flexibility in setting different scenarios and have been applied successfully in the last 20 years to track real buoys (Rubio et al., 2009), marine debris (Gifuni et al., 2023) as well as planktonic organisms (Cianelli et al., 2009; Ciannelli et al., 2022; Qiu et al., 2010) including jellyfish (Henschke et al., 2018). In the Mediterranean Sea, Lagrangian particle models have been used to simulate the transport of P. noctiluca from the pelagic zone to the coast with consequent beaching (Berline et al., 2013; Edelist et al., 2022) to infer connectivity between different sub-regions (Bergamasco et al., 2022) and to identify suitable wind and current patterns that may favor the accumulation of individuals and thus the development of outbreaks, especially in semi-enclosed areas such as bays (Aouititen et al., 2019; Malačič et al., 2007).
In this study, we combined SI analysis and Lagrangian particle modeling to the distribution of P. noctiluca in the Gulf of Naples (GoN, central-southern Tyrrhenian Sea) during 4 outbreaks recorded in March, June, July and November 2019 in order to test the effectiveness of the coupled approaches and to improve our understanding of the patterns driving jellyfish outbreaks at local scale. Carbon and nitrogen SI ratios of P. noctiluca and potential prey were determined, while particle tracking simulations were forced with current fields generated by a Regional Ocean Modeling System (ROMS) developed for the GoN.
2 Materials and methods
2.1 Study area
The Gulf of Naples (GoN) is a semi-enclosed embayment in the central-southern Tyrrhenian Sea along the Campania Region (southern Italy) (Figure 1). The area has a typically temperate Mediterranean climate with marked seasonality. A complex geomorphology and wind-driven water movements result in a regular alternation between coastal and offshore waters within the gulf (Cianelli et al., 2015). From October to May, intense meteorological events in the area are triggered mainly by westerly and southern air flows, driven over by cyclonic circulations resulting from the oscillation of the polar front. In summer (June-September), however, the area is characterized by almost stable high pressure (Capozzi et al., 2023; Saviano et al., 2023; Saviano et al., 2022). During spring, the strong northwestward Tyrrhenian current, which is the prevailing driving force of the circulation patterns within the GoN during winter, becomes unstable due to the weakening of the cyclonic wind-stress curl. As a result, the local forcing becomes the main driver of the water masses within the basin, with the formation of an anticyclonic circulation within the GoN (de Ruggiero et al., 2020; Gifuni et al., 2022). Within this highly dynamic system, planktonic communities appear to have a stable composition. Phytoplankton form assemblages that occur consistently in time and are formed mainly by typical coastal species of temperate areas (Bosso et al., 2025; Bellardini et al., 2024; Zingone et al., 2023). Similarly, heterotrophic and mixotrophic protists as well as zooplankton communities include mainly coastal species, although the number of offshore species seems to have increased in recent years (Mazzocchi et al., 2023; Del Gaizo et al., 2021).
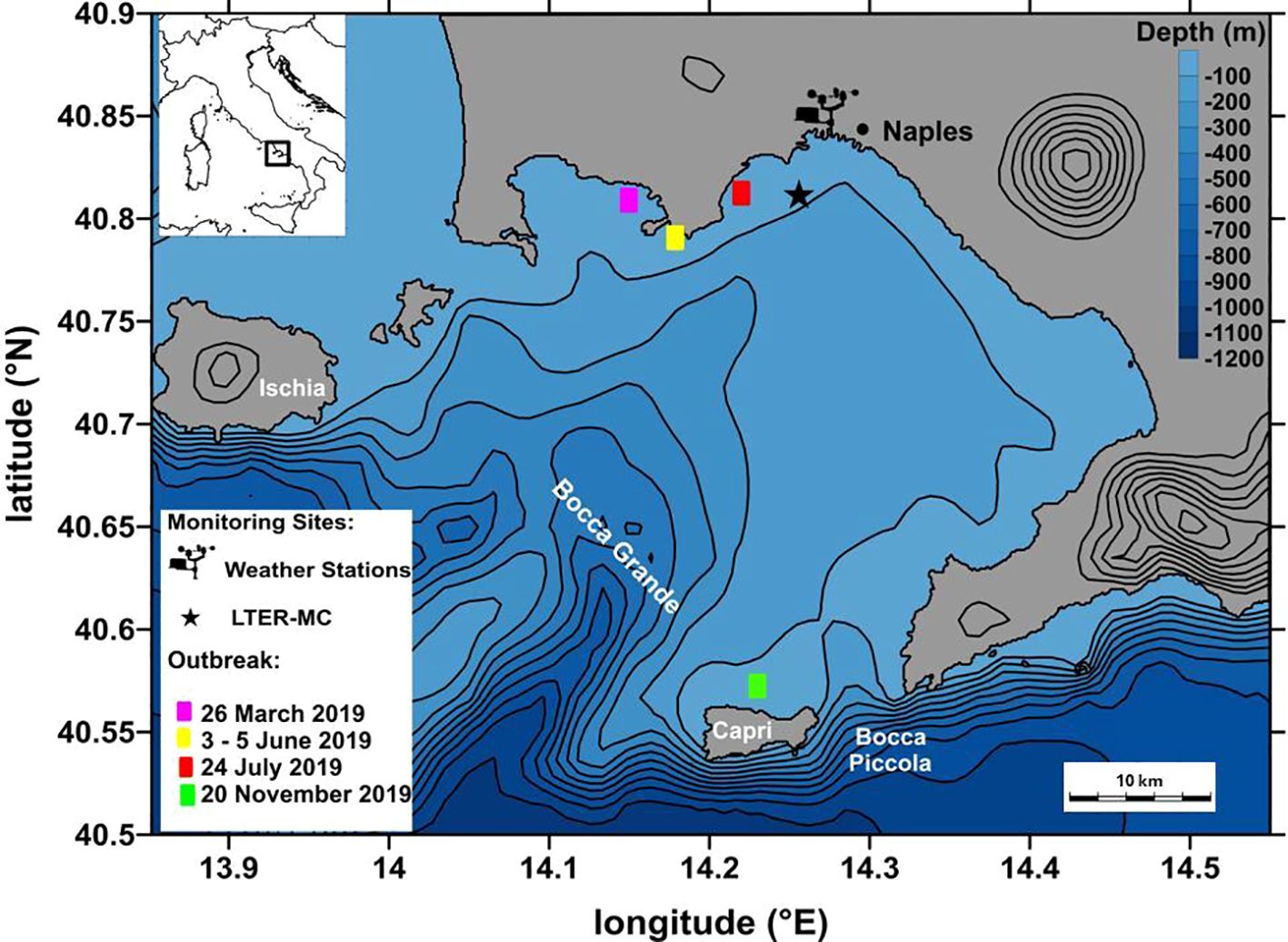
Figure 1. Map of the Gulf of Naples (central-southern Tyrrhenian Sea, western Mediterranean Sea) with the weather stations by the Istituto Superiore per la Ricerca sull’Ambiente (ISPRA) and Fondazione Meteorologica Milano Duomo (FOMD), the Long-Term Ecological Research site MareChiara (LTER-MC) and the sites where the outbreaks of the scyphomedusa Pelagia noctiluca were recorded in 2019.
2.2 Sample collection
Pelagia noctiluca were collected within the GoN at the sea surface using a dip net during the 4 outbreaks recorded in March, June, July and November 2019 (Figure 1). Each specimen was placed in a plastic bag with seawater to allow gut evacuation. Plankton samples, which encompassed all potential prey of P. noctiluca within the GoN, were collected weekly throughout 2019 within the monitoring at the Long-Term Ecological Research site MareChiara (LTER-MC) (Kokoszka et al., 2023) by vertical tows (from about 70 m depth to surface) using 20 and 200 µm plankton nets and transferred into 0.5L plastic jars. To isolate the smallest planktonic fractions, water was sampled by deploying a 5L Niskin bottle at the subsurface (1 m depth) and then transferred into plastic bins. All samples were kept fresh and transported to the laboratory on ice. Because the turnover rate of scyphomedusae is about 20 days (D’Ambra et al., 2014), only the samples collected 3 weeks before each outbreak of P. noctiluca were considered in the data analysis within the present study.
2.3 Sample processing
In the laboratory, each P. noctiluca was sized (bell diameter as the distance between two opposite rhopalia, cm), rinsed with sterilized filtered seawater to remove detritus and/or other planktonic organisms and frozen at – 30°C. Water and plankton samples were processed as described in detail in Merquiol et al. (2023), in order to isolate 4 distinct size fractions: 0-20 µm, which included pico- and nanoplankton; 20-200 µm (microplankton); 200-2,000 µm (mesozooplankton); > 2,000 µm (macrozooplankton). Seawater samples were pre-filtered through a 20 µm mesh nand the fraction <20 µm was concentrated onto pre-combusted Whatmann GF/F 0.7 µm filters to isolate pico- and nanoplankton (<20 µm), Net samples were concentrated onto a 20 and a 200 µm meshes and transferred into vials. Macrozooplankton were found only in March within the 200 µm sample and were sorted at the lowest taxon possible. Individuals were then pooled into two separate groups, Siphonophora and Salpida, to ensure sufficient organic matter to determine SIs. All samples were freeze-dried and the powder was homogenized using a mortar and a pestle.
2.4 Stable isotope determination and analysis
Carbon and nitrogen SI ratios were determined for each scyphomedusa and for each planktonic size fraction and taxonomic group. According to the diverse organic content of each group, 4.0 ± 0.1 mg of scyphomedusae and macrozooplankton, and 2.0 ± 0.1mg of the other groups were packed into tin capsules and sent to the Stable Isotope Facility at the University of California in Davis (USA) for determination of δ13C and δ15N (for details about the instrument and standards see Merquiol et al. (2023). Duplicate aliquots of 26 samples were determined in order to ensure reproducibility, which was ± 0.04‰ for δ13C and ± 0.06‰ for δ15N.
The C:N of P. noctiluca and Siphonophora were lower than 3.5, while ratios were higher in all other groups (Supplementary Table S1). Therefore, the δ13C of pico- and nanoplankton and Salpida was corrected according to Post et al. (2007) due to the lack of a specific equation, while the δ13C of mesozooplankton was normalized following Smyntek et al. (2007).
Bell diameters of scyphomedusae were compared among the 4 outbreaks using an analysis of variance (ANOVA). The relationship between the δ13C and δ15N and the bell diameter of scyphomedusae was defined using Pearson’s correlations.
2.5 Numerical models
Numerical simulations were performed using the Regional Ocean Modeling System (ROMS), a 3-D free-surface hydrostatic primitive-equation and finite-difference model widely used by the oceanographic scientific community for a wide range of applications (Haidvogel et al., 2008). A one-way nesting approach was adopted to obtain high resolution for the Campania coastal area. Firstly, simulations were performed on a grid covering the whole Tyrrhenian Sea with a 2 km resolution and 60 vertical levels, using the reanalysis data of the Mediterranean Forecasting System (https://medforecast.bo.ingv.it/ as initial and boundary conditions with a resolution of ~ 6–7 km and available with a daily frequency (Dobricic et al., 2005). Results of this first simulation were then used as initial and boundary conditions for a finer-grid computation, covering the Campania coast with a 500 m resolution and 50 vertical levels [see Kokoszka et al. (2022)]. Lateral conditions at the open boundaries were the same as those proposed by Marchesiello et al. (2001), with an adaptive algorithm where inward and outward fluxes were treated separately. The turbulence model used for the vertical mixing in the vertical direction was the K-Profile Parameterization (KPP) introduced by Large et al. (1994). In the horizontal field a constant value (equal to 10 m2 s-1) was attributed to the diffusion coefficient for velocity. Surface forcing was calculated from atmospheric values using the bulk parameterization of Fairall et al. (1996) based on the Coupled Ocean-Atmosphere Response Experiment (COARE) algorithm. The river inflows were modelled as point sources, with daily flow rate data obtained from a variety of sources, in particular from the Italian State agencies in charge of monitoring the environment (personal communications). The numerical configuration adopted in this work is an improved version of the GoN high resolution model (GNAM) validated in (Kokoszka et al., 2022). The major improvement regards atmospheric data, such as winds and air temperature, that were previously obtained from ERA-Interim reanalysis data (Dee et al., 2011) at horizontal resolution of about 16 km, while, in the present version, they were derived from dynamically downscaled ERA5 reanalysis data at a 2.2 km resolution. Lagrangian trajectories were simulated using the ROMS floats module, which allows the release and tracking of numerical particles, using a fourth-order Milne predictor and fourth-order Hamming corrector. Floats were all treated as geopotential, i.e. they were kept at the same depth during all simulations. The time step used in all simulations was 60 s, while particle locations were stored every 6 hours. While P. noctiluca may exhibit vertical migrations, our simulations assume passive surface transport due to limited data on vertical behavior in the GoN, thus the approximation of a purely horizontal displacement of the particles was selected for all the numerical simulations, in line with other studies using a Lagrangian approach for P. noctiluca in the Mediterranean Sea (Malačič et al., 2007; Bergamasco et al., 2022).
2.6 Simulation set-up
The following set-up was used for all Lagrangian simulations: 20 particles were released in random positions within each grid cell contained in the study area [lon = (13.6, 14.7), lat = (40.25, 41.05)], for a total of 892,960 particles. The release area was chosen based on initial tests (not shown here) to cover a sufficiently large area around the target zone. Particles were all treated as geopotential; thus, they were kept at the same depth during all the simulations. The time step used in all simulations was 60 s, while particle locations were stored every 6 hours. . In each of the 4 simulation scenarios, all particles were released at the same time, 20 days before the outbreak date and after 6 weeks from the start of the simulation, to allow for an initial spin-up of the velocity field from initial conditions. The choice of starting simulations 20 days before the outbreaks considers the surface dynamics of the study area (Cianelli et al., 2015) and the turnover rate of isotopes in scyphomedusae (D’Ambra et al., 2014). Post-processing of the simulations was made by individuating the trajectories that cross a target area in the 24 hours of the outbreak date (from 0:00 h to 24:00 h). The choice of the size of the target area, centered on the position of jellyfish outbreaks, was made by balancing the need to identify trajectories passing very close to the outbreak’ locations and the resolution of the numerical model, in order to gain a realistic accuracy. After a series of tests for all the 4 outbreaks presented here, a square target area of 1 km size was chosen in all simulations, corresponding to two grid cells at the 500 m resolution of the model.
2.7 Wind data
The accuracy of the model trajectories was tested by a wind analysis which was carried out for 20 days before each outbreak to align with models and SI determinations, using in situ wind data (speed (m/s) and direction (°)).”Wind data were collected by two weather stations. The wind data used for the outbreaks in June, July and November were collected by the weather station operated by ISPRA that is located in the harbor of Naples (Molo del Carmine: lon:14.2699220345E; lat: 40.84149055N; anemometric sensor height: 10 m a.m.s.l.; data freely downloadable at http://www.mareografico.it/; date of access: 19 May 2024) (Figure 1). Due to a lack of data in the ISPRA dataset, we used for the event in March the data retrieved by the weather station located at the University of Naples “Parthenope” (lon: 14.2452361E; lat: 40.8321083N) owned by Fondazione Osservatorio Meteorologico Milano Duomo (FOMD Network), a private and professional network of urban meteorological stations in Italy developed since 2010 (Curci et al., 2017). The weather stations are equipped with ultrasonic anemometer sensors located approximately 3 km away. Hourly averaged data were used in the analysis. Stick plots of the wind data for each outbreak were used to directly indicate the magnitude and direction of the wind during the 20 days before each outbreak (Figure 2).
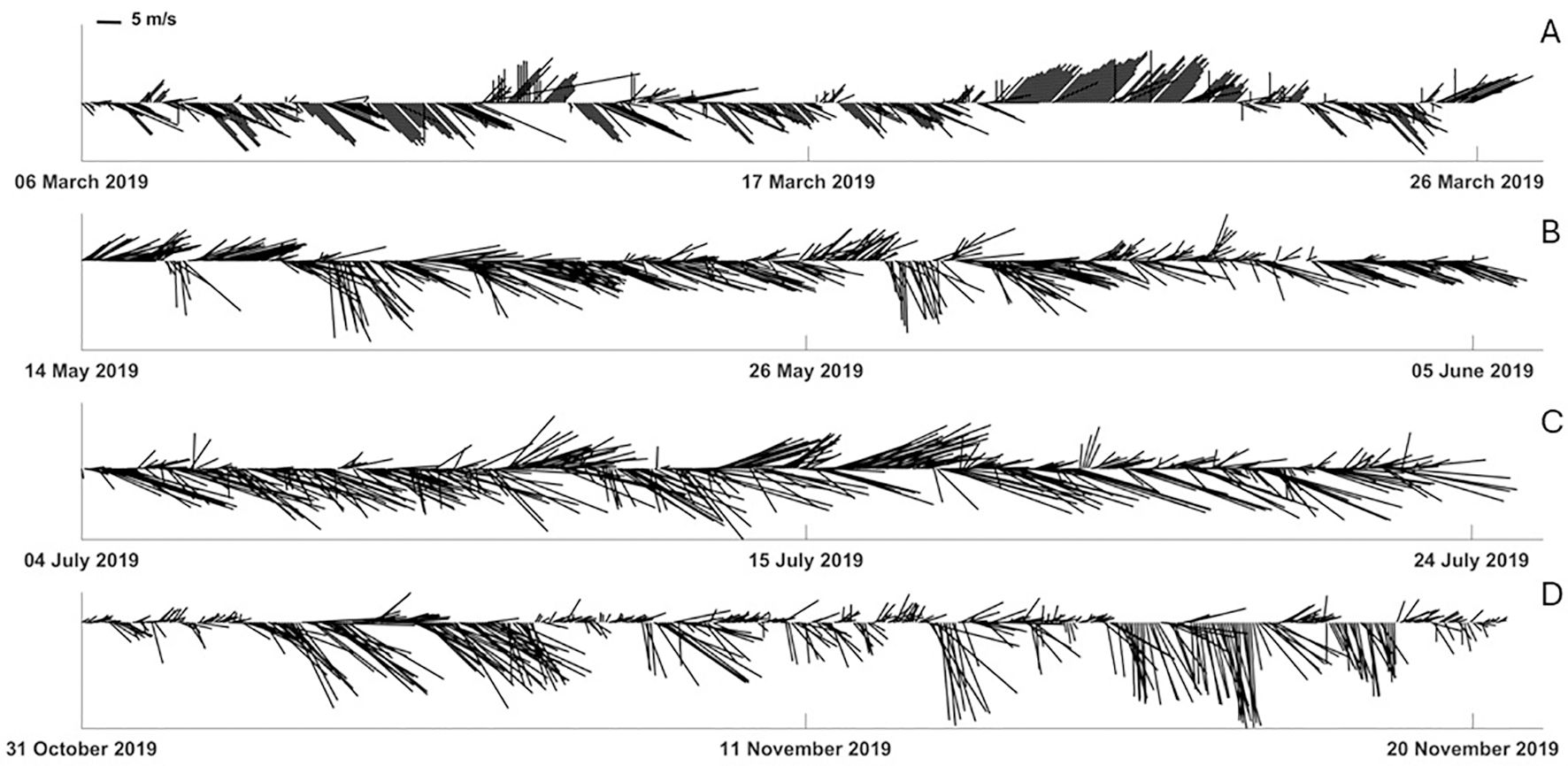
Figure 2. Stick plots of wind data during the 20 days before the outbreaks of Pelagia noctiluca recorded in the Gulf of Naples (central-southern Tyrrhenian sea) during 2019. (A) Outbreak 1 (6–26 March); (B) Outbreak 2 (14 May - 5 June); (C) Outbreak 3 (4–24 July); (D) Outbreak 4 (31 October - 20 November).
3 Results
3.1 Stable isotope ratios of Pelagia noctiluca and plankton
Overall, the mean bell diameter of scyphomedusae collected during the 4 outbreaks in 2019 was 7.6 ± 1.7 cm and varied across collections (Supplementary Table S2). While the δ13C of scyphomedusae was -18.9 ± 0.7 ‰ and it was not correlated with the bell diameter (R2 = -0.069, p = 0.056), the δ15N averaged 4.7 ± 1.6 ‰ and decreased with increasing bell diameter (R2 = -0.856, p < 0.01). This finding is unusual because the correlation is positive as larger medusa can capture larger prey with higher δ15N (Schaub et al., 2023).
When looking at the relative position of P. noctiluca and plankton collected within the GoN, the δ13C and δ15N of scyphomedusae did not reflect the SI composition of any size fractions and/or taxonomic group (Figure 3). During the outbreaks in March, June and July (Figures 3A–C), the SI composition of scyphomedusae fell within the range of SI values of their potential prey. However, if the diet-tissue fractionation factor (DTDF), which accounts for the trophic enrichment between the predator and the prey, is applied, P. noctiluca will remain outside the polygon of the potential prey. This suggests that prey with a very low δ15N are required to close the polygon around scyphomedusae. Conversely, in November (Figure 3D), the δ15N of scyphomedusae was already lower than the δ15N of all planktonic groups. By accounting for the DTDF, the δ15N would be similar to values determined in plankton collected in pelagic areas (Montoya et al., 2002).
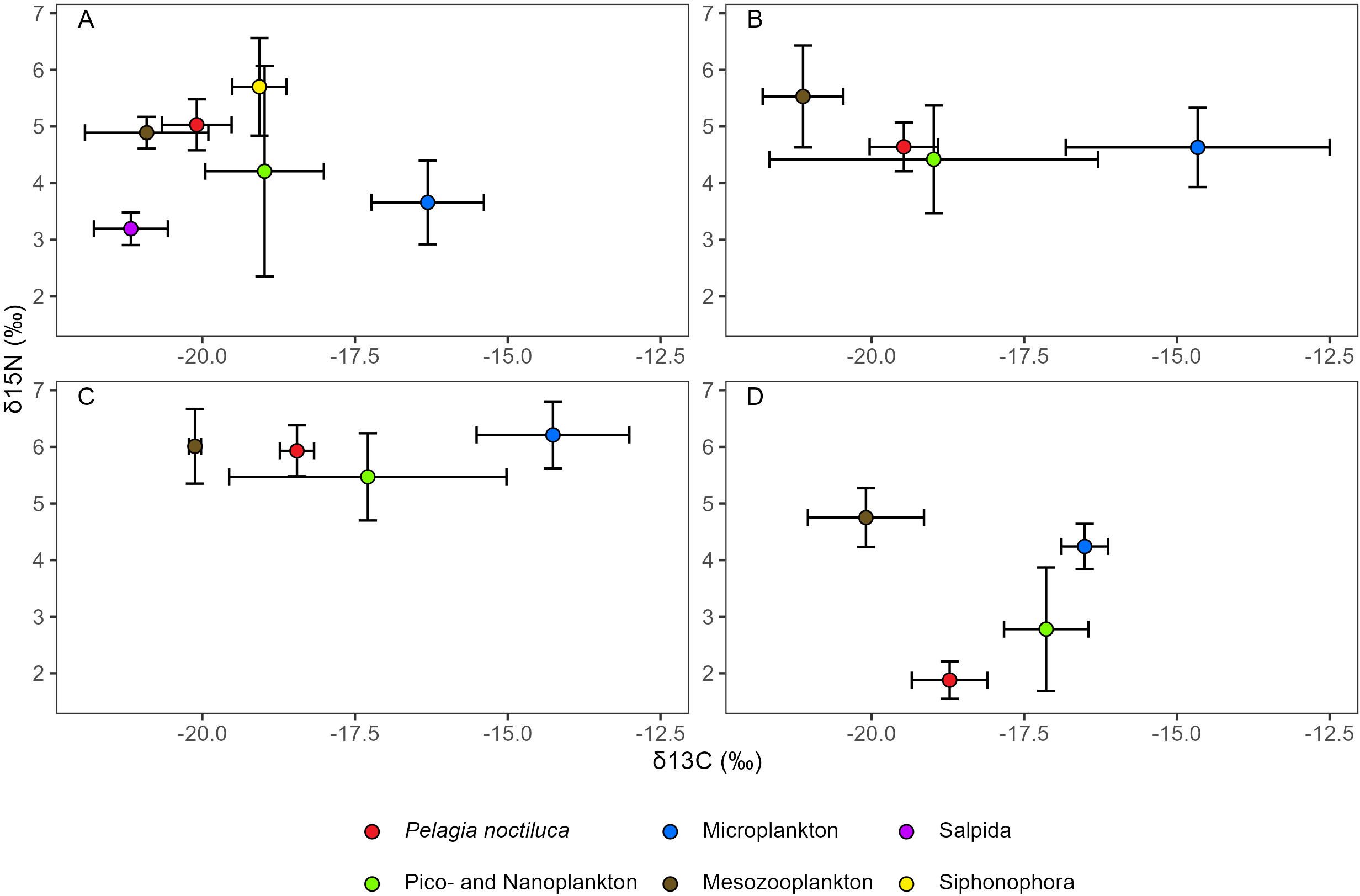
Figure 3. Bulk δ15N and lipid-corrected δ13C (mean ± standard deviation) of the scyphomedusa Pelagia noctiluca and planktonic size fractions (nano- and picoplankton (< 20 µm), microplankton (20-200 µm), mesozooplankton (200-2,000 µm)) and macrozooplankton (> 2,000 µm, divided into the two taxonomic groups of Salpida and Siphonophora) collected weekly for 20 days before the outbreaks of P. noctiluca recorded in: (A) March, (B) June, (C) July and (D) November 2019 within the Gulf of Naples (central-southern Tyrrhenian Sea).
3.2 Lagrangian simulations of Pelagia noctiluca trajectories
The simulations were conducted for each outbreak observed in 2019, and the trajectories of particles were analyzed up to 20 days before the event.
3.2.1 Outbreak 1: 26 March 2019
The simulations of the group of P. noctiluca observed on 26 March (Figure 1) indicated the origin of the trajectories in the GoN area and the presence of a northward current flow (Figure 4A). The days preceding the outbreak were characterized by strong SW winds and re-circulation within the GoN (Figures 4B, C). Subsequently, weak winds favored the permanence of P. noctiluca in the GoN for several days, with aggregation of individuals mainly in the northern part of the GoN (Figures 4C, D).
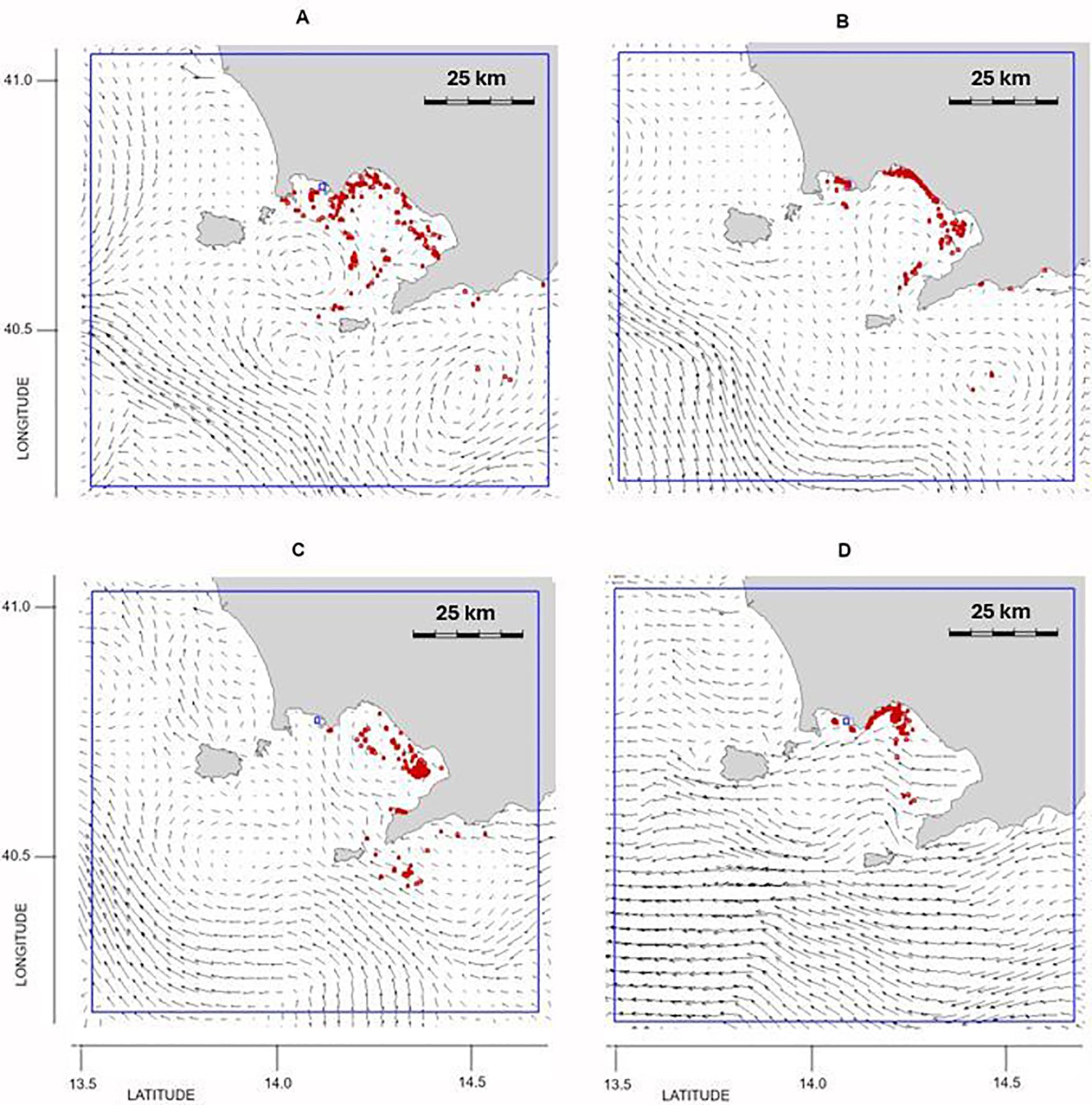
Figure 4. Outbreak 1 (26 March 2019): particle position and current field (A) 20 days, (B) 15 days, (C) 10 days, and (D) 5 days before the outbreak. The blue line indicates the particles’ released area: lon = [13.6, 14.7], lat = [40.25, 41.05].
3.2.2 Outbreak 2: 3–5 June 2019
This was the longest event (Figure 1). The simulations showed a large number of particles coming from offshore, likely due to strong SW-WSW winds and consequent surface currents. In the 20 days preceding the observation, the trajectories originated outside the GoN, mainly from Bocca Grande (Figures 5A, B). The wind regime in the following days favored the development of currents directed toward the inner part of the GoN (Figures 5C, D), resulting in the transport of most released particles along the Naples urban area.
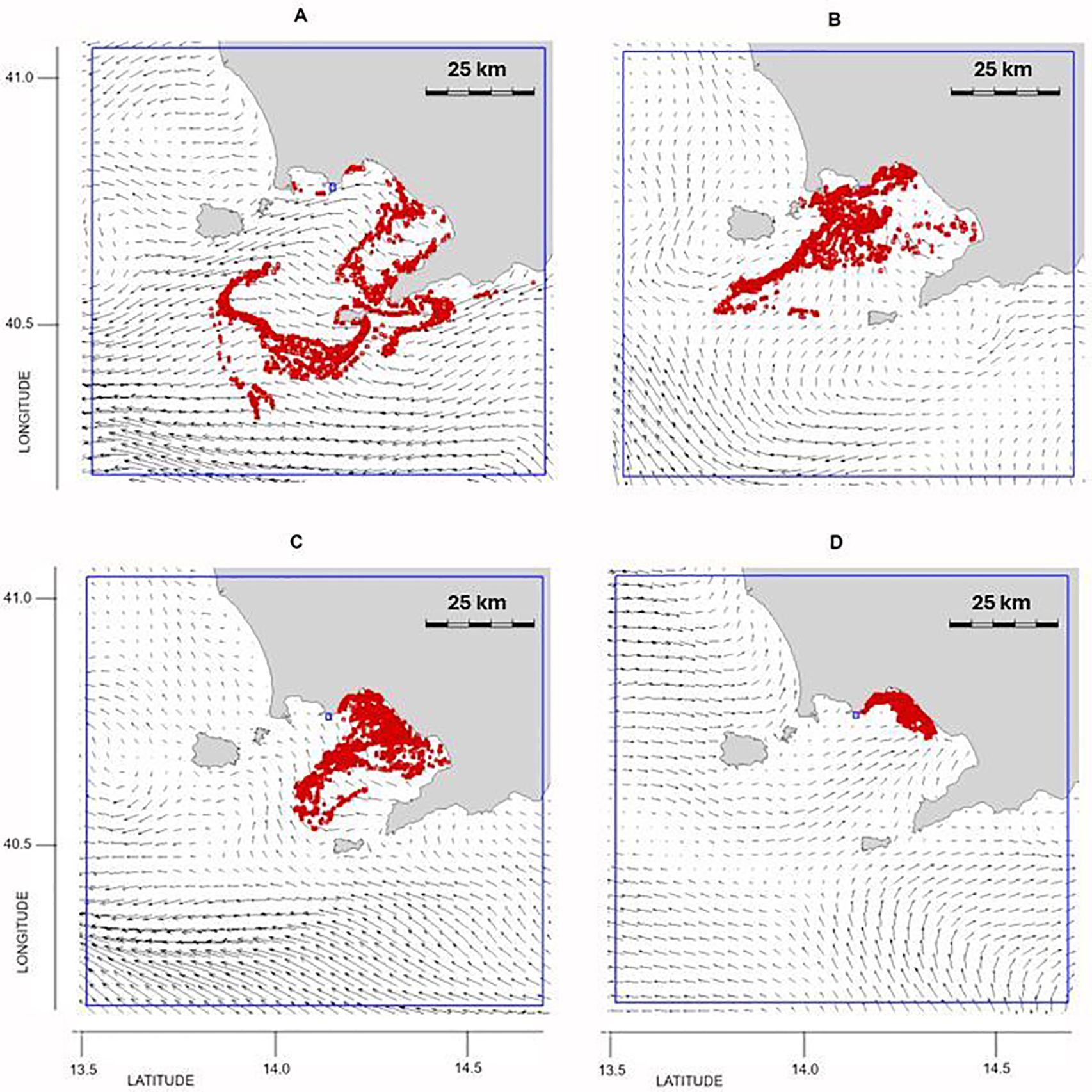
Figure 5. Outbreak 2: (3–5 June 2019): particle position and current field (A) 20 days, (B) 15 days, (C) 10 days and (D) 5 days before the outbreak. The blue line indicates the particles’ released area: lon = [13.6, 14.7], lat = [40.25, 41C05].
3.2.3 Outbreak 3: 24 July 2019
The summer outbreak (Figure 1) reflected the typical conditions of surface dynamics during this season, characterized by weak currents and recirculation in the internal part of the GoN. The particle trajectories in the 20 days preceding the outbreak originated within the GoN (Figures 6A, B), and the weak recirculation favored the stagnation of P. noctiluca near the north coast of the GoN (Figures 6C, D).
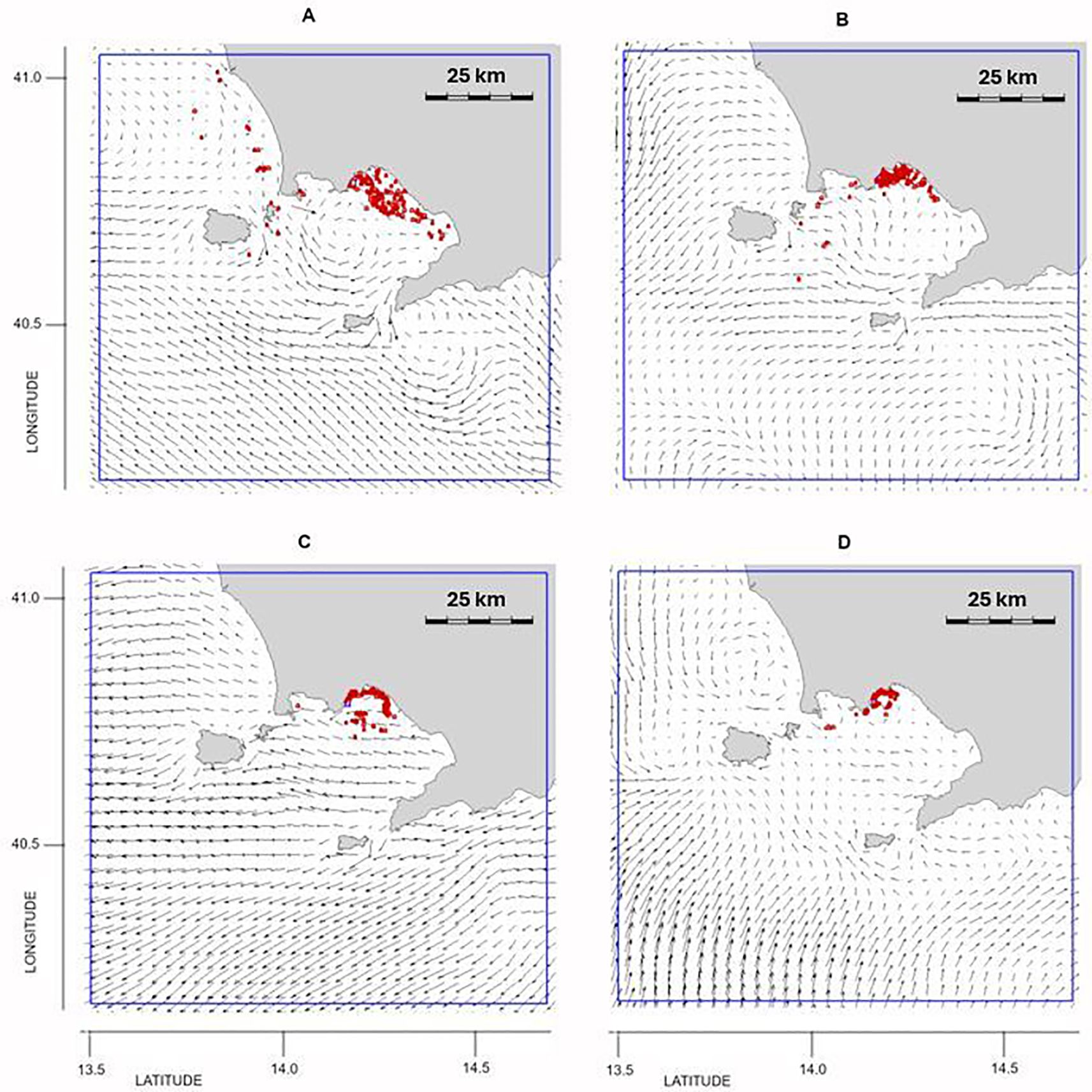
Figure 6. Outbreak 3 (24 July 2019): particle position and current field (A) 20 days, (B) 15 days, (C) 10 days and (D) 5 days before the outbreak. The blue line indicates the particles’ released area: lon = [13.6, 14.7], lat = [40.25, 41.05].
3.2.4 Outbreak 4: 20 November 2019
The autumn outbreak was observed in Capri (Figure 1A). Particle trajectories originated from the southern part of the GoN and from Bocca Piccola, due to the presence of a gyre drawn by the local currents pattern (Figures 7A, B). In the following days, the intensification of southerly winds allowed the establishment of a strong northward current with the presence of particles directed north of Bocca Grande (Figures 7C, D). The individuals of P. noctiluca were partly confined outside the GoN due to the autumnal dynamics of the southern Tyrrhenian Sea and partly reached the inner part of the GoN from Bocca Piccola (Figure 7D).
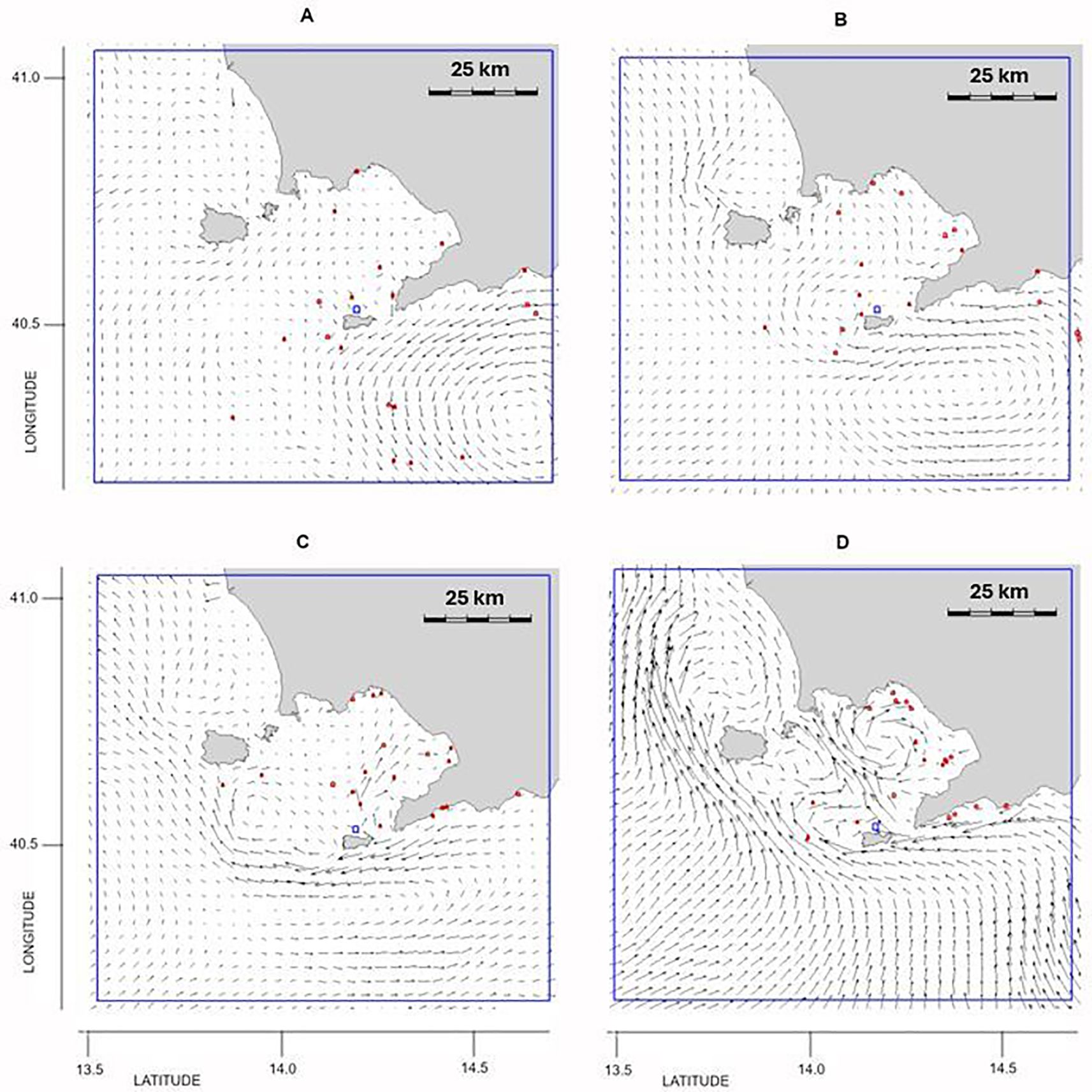
Figure 7. Outbreak 4 (20 November 2019): particle position and current field (A) 20 days, (B) 15 days, (C) 10 days and (D) 5 days before the outbreak. The blue line indicates the particles’ released area: lon = [13.6, 14.7], lat = [40.25, 41.05].
To support the analysis of the trajectories over the 20 days preceding the outbreaks, a probability density function (PDF) was performed for each outbreak to determine the origin of the particles. This analysis (Supplementary Figure S1) corroborates the qualitative analysis of the trajectories indicated by models.
4 Discussion
Determinations of δ13C and δ15N in scyphomedusae have been prompted by the advantages of using this approach alone or in combination with gut content analysis, which allowed to detect specific prey types, such as soft-bodied organisms like salps and siphonophores (Tilves et al., 2018) or highly digestible small plankton (D’Ambra et al., 2018). The application of SI ratios in scyphozoan trophic ecology highlighted a seasonal pattern in different species and ecosystems. Both Aurelia sp. in the northern Gulf of Mexico (USA) (D’Ambra et al., 2018) and P. noctiluca in the Strait of Sicily (southern Italy) (Milisenda et al., 2018) exhibited an increase of δ13C and δ15N in summer. Until now, the application of SI ratios in scyphomedusae appeared to be challenged only by the variability of the diet-tissue discrimination factors (DTDFs), which differed widely across species and studies (D’Ambra et al., 2014; Schaub et al., 2021). However, in the present study, DTDFs are not the key to resolve the mismatch between P. noctiluca and their potential prey. Conversely, our findings suggest that the SI signature of scyphomedusae is intimately related to the physical processes that dictate the transport of individuals in the study area. Therefore, the correct interpretation of SIs cannot foreclose the knowledge of the movements of medusae across isoscapes.
The coupling between current-driven transport and SIs affects planktonic communities within the GoN (Merquiol et al., 2023). P. noctiluca follow the same pattern observed in pico- and nanoplankton collected at LTER-MC across the whole 2019 (Merquiol et al., 2023). Like scyphomedusae, this small planktonic size fraction is passively transported by offshore waters entering the GoN and keeps a distinctly low SI signature (-22 ‰ for δ13C and 2 ‰ for δ15N)until it starts incorporating the SI signature of coastal waters (-16 ‰for δ13C and 7 ‰ for δ15N) (Merquiol et al., 2023). Unlike pico- and nanoplankton, microplankton and mesozooplankton include mainly coastal species, which reflect the SI signature of the inner part of the GoN (Merquiol et al., 2023). In November, the SI ratios of scyphomedusae reflected the SI signature of prey ingested offshore before being rapidly transported along the coastline. During the other 3 outbreaks, surface circulation patterns favored the permanence of jellyfish in the inner part of the GoN where they started to incorporate the SI signature of prey captured in this area. This scenario was observed in co-occurrence with atmospheric high pressure which generally dominates during the summer season (Saviano et al., 2021). The coupling between the jellyfish trajectories and their turnover rates corroborates the intimate relationship between SI composition of medusae and their movements across isoscapes.
The combination of particle tracking and SI approaches shed light on the close interplay between P. noctiluca and local circulation patterns within this highly dynamic coastal system of the Mediterranean Sea. Our simulations are in line with the observations by Lo Bianco (1909), who recorded the sudden appearance of large aggregations of P. noctiluca within the GoN in co-occurrence with strong southern winds, particularly during winter months. A similar pattern was found by Berline et al. (2013) for P. noctiluca in the Ligurian Sea, where the presence of scyphomedusae was regulated by the interplay between the Ligurian Northern Current and wind, with southern winds favoring standings on the most touristic beaches along the French Riviera.
In the present study, we did not find young medusae nor ephyrae during our sampling, which leaves unknown the reproductive area(s) of P. noctiluca in the central Tyrrhenian Sea. P. noctiluca is a holoplanktonic scyphomedusa and likely reproduces in the pelagic environment (Russell, 1970; Sandrini and Avian, 1991). Data collected in the Ligurian Sea and the Strait of Messina suggest that P. noctiluca overwinter at depth and then are lifted to the surface by upwelling currents, where ephyra production occurs to maximize survival (Bergamasco et al., 2022; Benedetti-Cecchi et al., 2015). Migrations of zooplankton along the water column have been rarely described in the Mediterranean Sea (Sabatés et al., 2010) but have not been recorded within the GoN. Most assessments of jellyfish distribution cover surface waters (Doyle et al., 2008; Long et al., 2024; Mghili et al., 2020), while their distribution at different depths and potential vertical migrations remain an hypothesis to be corroborated by data collected in situ The available data do not allow us to infer the permanence of medusae at depth and to define their reproductive area(s) in the central Tyrrhenian Sea, but this knowledge gap may be filled by multi-scale monitoring which integrates horizontal transport coupled with movements along the water column.
5 Conclusions
In the present study, the mismatch between SI ratios of Pelagia noctiluca medusae and their potential planktonic prey collected within the GoN, suggested that medusae were foraging outside the GoN. Lagrangian simulations corroborated this interpretation of SI ratios, indicating that medusae were transported from offshore waters into the GoN by local circulation patterns and intense southerly winds. However, recirculation patterns within the GoN promoted the permanence of medusa along the coast in summer. By integrating the spatial and temporal resolution of observations in situ with particle tracking simulations and SI determinations, we provided a robust interpretation of SI ratios of scyphomedusae and an insight into their trophic ecology in a highly dynamic system. Additionally, we defined the patterns that regulate the distribution of P. noctiluca within the GoN and the oceanographic conditions potentially favoring the formation of outbreaks. This knowledge may be used to promote early alert systems for outbreaks which will result into a better management of coastal areas, for example limiting bathing upon imminent arrival of mass aggregations of jellyfish. Overall, our results suggest that multi-disciplinary and multi-scale monitoring will allow to refine our understanding of the ecology of scyphomedusae, while improving early alert systems for jellyfish mass appearances for a better management of marine coastal areas.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because We targeted scyphomedusae for this study, which are a group of invertebrate organisms not protected by any restriction.
Author contributions
ID’A: Project administration, Data curation, Software, Validation, Methodology, Conceptualization, Visualization, Funding acquisition, Writing – review & editing, Writing – original draft, Investigation, Resources, Formal analysis, Supervision. SS: Validation, Project administration, Methodology, Data curation, Formal analysis, Investigation, Conceptualization, Writing – review & editing, Software, Writing – original draft. MAA: Data curation, Writing – review & editing. VB: Visualization, Writing – review & editing, Data curation, Software. DI: Writing – review & editing. MGM: Writing – review & editing. LM: Investigation, Writing – review & editing, Data curation. DC: Investigation, Conceptualization, Writing – review & editing, Supervision, Resources, Writing – original draft, Validation, Project administration, Methodology.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project was funded under the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.4 -Call for tender No. 3138 of 16 December 2021, rectified by Decree n.3175 of 18 December 2021 of Italian Ministry of University and Research funded by the European Union -NextGenerationEU; Project code CN_00000033, Concession Decree No. 1034 of 17 June 2022 adopted by the Italian Ministry of University and Research, CUP C63C22000520001 -”National Biodiversity Future Center -NBFC”.
Acknowledgments
Louise Merquiol was supported by a PhD fellowship funded by the Stazione Zoologica Anton Dohrn (Open University—Stazione Zoologica Anton Dohrn PhD Program). We thank Marco Cannavacciuolo, Raffaella Casotti, Fabio Conversano, Iole Di Capua, Roberto Gallia, Francesca Margiotta, Augusto Passarelli, Isabella Percopo, Captain Enzo Rando, Maria Saggiomo, Francesco Terlizzi, Ferdinando Tramontano, and Gianluca Zazo for collecting samples at sea, Oliver Lincoln for graphical support and Jonathan Houghton for commenting a draft version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1608726/full#supplementary-material
References
Aouititen M., Bekkali R., Nachite D., Luan X., and Mrhraoui M. (2019). Predicting jellyfish strandings in the moroccan north-west mediterranean coastline. Eur. Sci. J. ESJ 15. doi: 10.19044/esj.2019.v15n2p72
Bellardini D., Russo L., Di Tuccio V., De Luca D., Del Gaizo G., Zampicinini G., et al. (2024). Spatiotemporal changes of pelagic food webs investigated by environmental DNA metabarcoding and connectivity analysis. Philos. Trans. R. Soc. B: Biol. Sci. 379, 20230178. doi: 10.1098/rstb.2023.0178
Benedetti-Cecchi L., Canepa A., Fuentes V., Tamburello L., Purcell J. E., Piraino S., et al. (2015). Deterministic factors overwhelm stochastic environmental fluctuations as drivers of jellyfish outbreaks. PloS One 10, e0141060. doi: 10.1371/journal.pone.0141060
Bergamasco A., Cucco A., Guglielmo L., Minutoli R., Quattrocchi G., Guglielmo R., et al. (2022). Observing and modeling long-term persistence of P. noctiluca in coupled complementary marine systems (Southern Tyrrhenian Sea and Messina Strait). Sci. Rep. 12, 14905. doi: 10.1038/s41598-022-18832-2
Berline L., Zakardjian B., Molcard A., Ourmières Y., and Guihou K. (2013). Modeling jellyfish Pelagia noctiluca transport and stranding in the Ligurian Sea. Mar. pollut. Bull. 70, 90–99. doi: 10.1016/j.marpolbul.2013.02.016
Boero F. (2013). Review of jellyfish blooms in the Mediterranean and Black Sea. Studies and Reviews. General Fisheries Commission for the Mediterranean. Rome: FAO.
Bosso L., Saviano S., Abagnale M., Bellardini D., Bolinesi F., Botte V., et al. (2025). GIS-based integration of marine data for assessment and management of a highly anthropized coastal area. Sci. Rep. 15, 16200. doi: 10.1038/s41598-025-00206-z
Brotz L. and Pauly D. (2012). Jellyfish populations in the Mediterranean Sea. Acta Adriatica 53, 213–232.
Canepa A., Fuentes V., Sabatés A., Piraino S., Boero F., and Gili J.-M. (2014). “Pelagia noctiluca in the Mediterranean sea,” in Jellyfish Blooms. Eds. Pitt K. and Lucas C. (Haidelberg: Springer).
Capozzi V., Annella C., and Budillon G. (2023). Classification of daily heavy precipitation patterns and associated synoptic types in the Campania Region (southern Italy). Atmos. Res. 289, 106781.
Cianelli D., Falco P., Iermano I., Mozzillo P., Uttieri M., Buonocore B., et al. (2015). Inshore/offshore water exchange in the Gulf of Naples. J. Mar. Syst. 145, 37–52. doi: 10.1016/j.jmarsys.2015.01.002
Cianelli D., Sabia L., D’alcalà M. R., and Zambianchi E. (2009). An individual-based analysis of the dynamics of two coexisting phytoplankton species in the mixed layer. Ecol. Model. 220, 2380–2392. doi: 10.1016/j.ecolmodel.2009.06.016
Ciannelli L., Cannavacciuolo A., Konstandinidis P., Mirasole A., Wong-Ala J. A. T. K., Guerra M. T., et al. (2022). Ichthyoplankton assemblages and physical characteristics of two submarine canyons in the south central Tyrrhenian Sea. Fisheries Oceanography 31, 480–496. doi: 10.1111/fog.12596
Curci S., Lavecchia C., Frustaci G., Paolini R., Pilati S., and Paganelli C. (2017). Assessing measurement uncertainty in meteorology in urban environments. Measurement Sci. Technol. 28, 104002. doi: 10.1088/1361-6501/aa7ec1
D’Ambra I., Carmichael R. H., and Graham W. M. (2014). Determination of δ13C and δ15N and trophic fractionation in jellyfish: implications for food web ecology. Mar. Biol. 161, 473–480. doi: 10.1007/s00227-013-2345-y
D’Ambra I., Graham W. M., Carmichael R. H., and Hernandez F. J. Jr (2018). Dietary overlap between jellyfish and forage fish in the northern Gulf of Mexico. Mar. Ecol. Prog. Ser. 587, 31–40. doi: 10.3354/meps12419
De Donno A., Idolo A., Bagordo F., Grassi T., Leomanni A., Serio F., et al. (2014). Impact of stinging jellyfish proliferations along south Italian coasts: human health hazards, treatment and social costs. Int. J. Environ. Res. Public Health 11, 2488–2503. doi: 10.3390/ijerph110302488
Dee D. P., Uppala S. M., Simmons A. J., Berrisford P., Poli P., Kobayashi S., et al. (2011). The ERA-Interim reanalysis: configuration and performance of the data assimilation system. Q. J. R. Meteorological Soc. 137, 553–597. doi: 10.1002/qj.828
Del Gaizo G., Russo L., Abagnale M., Buondonno A., Furia M., Saviano S., et al. (2021). An autumn biodiversity survey on heterotrophic and mixotrophic protists along a coast-to-offshore transect in the Gulf of Naples (Italy). Adv. Oceanography Limnology 12, 1–9. doi: 10.4081/aiol.2021.10018
de Ruggiero P., Esposito G., Napolitano E., Iacono R., Pierini S., and Zambianchi E. (2020). Modelling the marine circulation of the Campania coastal system (Tyrrhenian Sea) for the year 2016: Analysis of the dynamics. J. Mar. Syst. 210, 103388. doi: 10.1016/j.jmarsys.2020.103388
Dobricic S., Pinardi N., Adani M., Bonazzi A., Fratianni C., and Tonani M. (2005). Mediterranean Forecasting System: An improved assimilation scheme for sea-level anomaly and its validation. Q. J. R. Meteorological Soc. 131, 3627–3642. doi: 10.1256/qj.05.100
Doyle T. K., De Haas H., Cotton D., Dorschel B., Cummins V., Houghton J. D. R., et al. (2008). Widespread occurrence of the jellyfish Pelagia noctiluca in Irish coastal and shelf waters. J. Plankton Res. 30, 963–968. doi: 10.1093/plankt/fbn052
Edelist D., Knutsen Ø., Ellingsen I., Majaneva S., Aberle N., Dror H., et al. (2022). Tracking jellyfish swarm origins using a combined oceanographic-genetic-citizen science approach. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.869619
Fairall C. W., Bradley E. F., Rogers D. P., Edson J. B., and Young G. S. (1996). Bulk parameterization of air-sea fluxes for Tropical Ocean-Global Atmosphere Coupled-Ocean Atmosphere Response Experiment. J. Geophysical Research: Oceans 101, 3747–3764. doi: 10.1029/95JC03205
Fernández-Alías A., Marcos C., and Pérez-Ruzafa A. (2024). The unpredictability of scyphozoan jellyfish blooms. Front. Mar. Sci. 11. doi: 10.3389/fmars.2024.1349956
Gifuni L., de Ruggiero P., Cianelli D., Pierini S., and Zambianchi E. (2023). Numerical investigation of the three-dimensional paths of plastic polymers in the Gulf of Naples. Mar. pollut. Bull. 193, 115259. doi: 10.1016/j.marpolbul.2023.115259
Gifuni L., de Ruggiero P., Cianelli D., Zambianchi E., and Pierini S. (2022). Hydrology and dynamics in the gulf of naples during spring of 2016: in situ and model data. J. Mar. Sci. Eng. 10, 1776. doi: 10.3390/jmse10111776
Haidvogel D. B., Arango H., Budgell W. P., Cornuelle B. D., Curchitser E., Di Lorenzo E., et al. (2008). Ocean forecasting in terrain-following coordinates: Formulation and skill assessment of the Regional Ocean Modeling System. J. Comput. Phys. 227, 3595–3624. doi: 10.1016/j.jcp.2007.06.016
Hays G., Doyle T., and Houghton J. (2018). A paradigm shift in the trophic importance of jellyfish. Trends Ecol. Evol. 33, 874–884. doi: 10.1016/j.tree.2018.09.001
Henschke N., Stock C. A., and Sarmiento J. L. (2018). Modeling population dynamics of scyphozoan jellyfish (Aurelia spp.) in the Gulf of Mexico. Mar. Ecol. Prog. Ser. 591, 167–183. doi: 10.3354/meps12255
Kokoszka F., Le Roux B., Iudicone D., Conversano F., and Ribera d’Alcalá M. (2023). Long-term variability of the coastal ocean stratification in the Gulf of Naples: Two decades of monitoring the marine ecosystem at the LTER–MC site, between land and open Mediterranean Sea. Mar. Ecol. 44, e12725. doi: 10.1111/maec.12725
Kokoszka F., Saviano S., Botte V., Iudicone D., Zambianchi E., and Cianelli D. (2022). Gulf of naples advanced model (GNAM): A multiannual comparison with coastal HF radar data and hydrological measurements in a coastal tyrrhenian basin. J. Mar. Sci. Eng. 10, 1044. doi: 10.3390/jmse10081044
Large W. G., Mcwilliams J. C., and Doney S. C. (1994). Oceanic vertical mixing: A review and a model with a nonlocal boundary layer parameterization. Rev. Geophysics 32, 363–403. doi: 10.1029/94RG01872
Lo Bianco S. (1909). Notizie biologiche riguardanti specialmente il periodo di maturità sessuale degli animali del Golfo di Napoli. Mittheilungen aus der Zoologischen Station zu Neapal 19, 513–761.
Long A. P., Bastian T., Haberlin D., Stokes D., Lyashevska O., Brophy D., et al. (2024). Regular widespread aggregations of the oceanic jellyfish Pelagia noctiluca in the northeast Atlantic over 11 years. Estuarine Coast. Shelf Sci. 303, 108805. doi: 10.1016/j.ecss.2024.108805
Lucas C. H. and Dawson M. N. (2014). “What are jellyfishes and thaliaceans and why do they bloom?,” in Jellyfish blooms (Haidelberg: Springer).
Lynam C. P., Gibbons M. J., Axelson B. E., Sparks C. A. J., Coetzee J., Heywood B. J., et al. (2006). Jellyfish overtake fish in a heavily fished ecosystem. Curr. Biol. 16, 492–493. doi: 10.1016/j.cub.2006.06.018
Malačič V., Petelin B., and Malej A. (2007). Advection of the jellyfish Pelagia noctiluca (Scyphozoa) studied by the Lagrangian tracking of water mass in the climatic circulation of the Adriatic Sea. Geophysical Res. Abstracts 9, 02802.
Malej A. (1989). Behaviour and trophic ecology of the jellyfish Pelagia noctiluca (Forsskål 1775). J. Exp. Mar. Biol. Ecol. 126, 259–270. doi: 10.1016/0022-0981(89)90191-3
Marambio M., Canepa A., Lòpez L., Gauci A. A., Gueroun S. K. M., Zampardi S., et al. (2021). Unfolding jellyfish bloom dynamics along the Mediterranean basin by transnational citizen science initiatives. Diversity 13, 274. doi: 10.3390/d13060274
Marchesiello P., Mcwilliams J. C., and Shchepetkin A. (2001). Open boundary conditions for long-term integration of regional oceanic models. Ocean Model. 3, 1–20. doi: 10.1016/S1463-5003(00)00013-5
Mazzocchi M. G., Di Capua I., Kokoszka F., Margiotta F., D’alcalà M. R., Sarno D., et al. (2023). Coastal mesozooplankton respond to decadal environmental changes via community restructuring. Mar. Ecol. 44, e12746. doi: 10.1111/maec.12746
Merquiol L., Mazzocchi M. G., and D’Ambra I. (2023). The planktonic food web in the Gulf of Naples based on the analysis of carbon and nitrogen stable isotope ratios. Mar. Ecol., e12762. doi: 10.1111/maec.12762
Mghili B., Analla M., and Aksissou M. (2020). Temporal dynamics of jellyfish pelagia noctiluca stranded on the mediterranean coast of Morocco. Turkish J. Fisheries Aquat. Sci. 21, 87–94. doi: 10.4194/1303-2712
Mghili B., Analla M., and Aksissou M. (2022). Estimating the economic damage caused by jellyfish to fisheries in Morocco. Afr. J. Mar. Sci. 44, 271–277. doi: 10.2989/1814232X.2022.2105949
Milisenda G., Rosa S., Fuentes V. L., Boero F., Guglielmo L., Purcell J. E., et al. (2014). Jellyfish as Prey: Frequency of Predation and Selective Foraging of Boops boops (Vertebrata, Actinopterygii) on the Mauve Stinger Pelagia noctiluca (Cnidaria, Scyphozoa). PloS One 9, e94600. doi: 10.1371/journal.pone.0094600
Milisenda G., Rossi S., Vizzini S., Fuentes V. L., Purcell J. E., Tilves U., et al. (2018). Seasonal variability of diet and trophic level of the gelatinous predator Pelagia noctiluca (Scyphozoa). Sci. Rep. 8, 12140. doi: 10.1038/s41598-018-30474-x
Montoya J. P., Carpenter E. J., and Capone D. G. (2002). Nitrogen fixation and nitrogen isotope abundances in zooplankton of the oligotrophic North Atlantic. Limnology Oceanography 47, 1612–1628. doi: 10.4319/lo.2002.47.6.1617
Musco L., FernáNdez T. S. V., Caroselli E., Roberts J. M., and Badalamenti F. C. (2018). Protocooperation among small polyps allows the coral Astroydes calycularis to prey on large jellyfish. Bull. Ecol. Soc. Am. 99, 1–6. doi: 10.1002/bes2.1445
Orsi Relinii L., Lantieri L., and Garibaldi F. (2010). Medusivorous fishes of the Mediterranean. A coastal safety system against jellyfish blooms. Biol. Mar. Mediterr. 17, 348–349.
Palmieri M. G., Schaafsma M., Luisetti T., Barausse A., Harwood A., Sen A., et al. (2015). “Jellyfish blooms and their impacts on welfare benefits: recreation in the UK and fisheries in Italy,” in Coastal Zones Ecosystem Services: From Science to Values and Decision Making. Eds. Turner K. R. and Schaafsma M. (Springer International Publishing, Cham).
Post D. M., Layman C. A., Arrington D. A., Takimoto G., Quattrocchi J., and Montaña G. C. (2007). Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analysis. Oecologia 152, 179–189. doi: 10.1007/s00442-006-0630-x
Purcell J. E. and Arai M. N. (2001). Interactions of pelagic cnidarians and ctenophores with fish: a review. Hydrobiologia 451, 27–44. doi: 10.1023/A:1011883905394
Qiu Z. F., Doglioli A. M., Hu Z. Y., Marsaleix P., and Carlotti F. (2010). The influence of hydrodynamic processes on zooplankton transport and distributions in the North Western Mediterranean: Estimates from a Lagrangian model. Ecol. Model. 221, 2816–2827. doi: 10.1016/j.ecolmodel.2010.07.025
Robinson K. L., Ruzicka J. J., Decker M. B., Brodeur R. D., Hernandez F. J., Quiñones J., et al. (2014). Jellyfish, forage fish, and the world’s major fisheries. Oceanography 27, 104–115. doi: 10.5670/oceanog.2014.90
Rosa S., Pansera M., Granata A., and Guglielmo L. (2013). Interannual variability, growth, reproduction and feeding of Pelagia noctiluca (Cnidaria: Scyphozoa) in the Straits of Messina (Central Mediterranean Sea): Linkages with temperature and diet. J. Mar. Syst. 111–112, 97–107. doi: 10.1016/j.jmarsys.2012.10.001
Rubio A., Taillandier V., and Garreau P. (2009). Reconstruction of the Mediterranean northern current variability and associated cross-shelf transport in the Gulf of Lions from satellite-tracked drifters and model outputs. J. Mar. Syst. 78, S63–S78. doi: 10.1016/j.jmarsys.2009.01.011
Russell F. R. S. (1970). The Medusae of the British Isles. II. Pelagic Scyphozoa, with a supplement to the first volume on Hydromedusae (Cambridge: Cambridge University Press).
Sabatés A., Pagès F., Atienza D., Fuentes V., Purcell J., and Gili J.-M. (2010). Planktonic cnidarian distribution and feeding of Pelagia noctiluca in the NW Mediterranean Sea. Hydrobiologia 645, 153–165. doi: 10.1007/s10750-010-0221-z
Sandrini L. R. and Avian M. (1991). Reproduction of Pelagia noctiluca in the central and northern Adriatic Sea. Hydrobiologia 216-217, 197–202. doi: 10.1007/BF00026462
Saviano S., Biancardi A. A., Uttieri M., Zambianchi E., Cusati L. A., Pedroncini A., et al. (2022). Sea storm analysis: evaluation of multiannual wave parameters retrieved from HF radar and wave model. Remote Sens. 14, 1696.
Saviano S., Biancardi A. A., Kokoszka F., Uttieri M., Zambianchi E., Cusati L. A., et al. (2023). HF radar wind direction: multiannual analysis using model and HF network. Remote Sens. 15, 1–20. doi: 10.3390/rs15122991
Saviano S., Esposito G., Di Lemma R., de Ruggiero P., Zambianchi E., Pierini S., et al. (2021). Wind direction data from a coastal HF radar system in the gulf of naples (Central Mediterranean sea). Remote Sens. 13, 1–16. doi: 10.3390/rs13071333
Schaub J., Mclaskey A. K., Forster I., and Hunt B. P. V. (2021). Experimentally derived estimates of turnover and modification for stable isotopes and fatty acids in scyphozoan jellyfish. J. Exp. Mar. Biol. Ecol. 545, 151631. doi: 10.1016/j.jembe.2021.151631
Schaub J., Mclaskey A. K., Forster I., and Hunt B. P. V. (2023). Size-based changes in trophic ecology and nutritional quality of moon jellyfish (Aurelia labiata). Ecosphere 14, e4430. doi: 10.1002/ecs2.4430
Smyntek P. M., Teece M. A., Schulz K. L., and Thackeray S. G. (2007). A standard protocol for stable isotope analysis of zooplankton in aquatic food web researchusing mass balance correction models. Limnology Oceanography 52, 2135–2146. doi: 10.4319/lo.2007.52.5.2135
Sweetman A. K., Smith C. R., Dale T., and Jones D. O. B. (2014). Rapid scavenging of jellyfish carcasses reveals the importance of gelatinous material to deep-sea food webs. Proc. R. Soc. London B: Biol. Sci. 281, 20142210. doi: 10.1098/rspb.2014.2210
Tilves U., Fuentes V. L., Milisenda G., Parrish C. C., Vizzini S., and Sabatés A. (2018). Trophic interactions of the jellyfish Pelagia noctiluca in the NW Mediterranean: evidence from stable isotope signatures and fatty acid composition. Mar. Ecol. Prog. Ser. 591, 101–116. doi: 10.3354/meps12332
Tilves U., Purcell J., Fuentes V., Torrents A., Pascual M., Raya V., et al. (2016). Natural diet and predation impacts of Pelagia noctiluca on fish eggs and larvae in the NW Mediterranean. J. Plankton Res. 38. 1243–1254. doi: 10.1093/plankt/fbw059
Tilves U., Purcell J. E., Marambio M., Canepa A., Olariaga A., and Fuentes V. (2012). Predation by the scyphozoan Pelagia noctiluca on Mnemiopsis leidyi ctenophores in the NW Mediterranean Sea. J. Plankton Res. 35, 218–224. doi: 10.1093/plankt/fbs082
van Sebille E., Griffies S. M., Abernathey R., Adams T. P., Berloff P., Biastoch A., et al. (2018). Lagrangian ocean analysis: Fundamentals and practices. Ocean Model. 121, 49–75. doi: 10.1016/j.ocemod.2017.11.008
Keywords: jellyfish, outbreaks, carbon, nitrogen, Lagrangian trajectories, surface currents, Mediterranean Sea
Citation: D’Ambra I, Saviano S, Ambrosio MA, Botte V, Iudicone D, Mazzocchi MG, Merquiol L and Cianelli D (2025) Tracking Pelagia noctiluca scyphomedusae by combining modeling and stable isotope approaches. Front. Mar. Sci. 12:1608726. doi: 10.3389/fmars.2025.1608726
Received: 09 April 2025; Accepted: 20 June 2025;
Published: 22 July 2025.
Edited by:
Mustapha Aksissou, Abdelmalek Essaadi University, MoroccoReviewed by:
Eva Garcia-Vazquez, Universidad de Oviedo Mieres, SpainBilal Mghili, Abdelmalek Essaadi University, Morocco
Mohamed Keznine, Abdelmalek Essaadi University, Morocco
Copyright © 2025 D’Ambra, Saviano, Ambrosio, Botte, Iudicone, Mazzocchi, Merquiol and Cianelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Isabella D’Ambra, aXNhYmVsbGEuZGFtYnJhQHN6bi5pdA==
†These authors have contributed equally to this work
 Isabella D’Ambra
Isabella D’Ambra Simona Saviano
Simona Saviano Maria Assunta Ambrosio3
Maria Assunta Ambrosio3 Daniela Cianelli
Daniela Cianelli