- 1Discipline of Genetics, School of Life Sciences, University of KwaZulu-Natal, Durban, South Africa
- 2Department of Agriculture and Animal Health, University of South Africa (UNISA), Johannesburg, South Africa
- 3Centre for Plant Metabolomics Research, Department of Biochemistry, Faculty of Science, University of Johannesburg, Johannesburg, South Africa
Introduction: Sea cucumbers are ecologically and economically significant marine invertebrates, yet the metabolic diversity and bioactive potential of noncommercialized, endemic species remains poorly understood.
Methods: This study presents the first intra-species metabolomic analysis of Pseudocnella sykion, a species endemic to the Eastern coast of Southern Africa, using untargeted 1HNMR metabolomics and full-scan UPLC-QTOF-HR-MS.
Results: The analysis revealed a diverse array of metabolites associated with protein synthesis, tissue growth, osmoregulation, and energy utilization, with distinct tissue-specific patterns across the body wall, gonad, and gut/mesentery. The gut/mesentery tissue showed higher levels of amino acids and energy-related compounds. UPLCQTOF-HR-MS tentatively identified several metabolites, including triterpene glycosides and rosmarinic acid, a phenolic compound typically associated with plants. Online resources, including the Dictionary of Marine Natural Products, contained no previously recorded compounds for P. sykion.
Discussion: These findings underscore the untapped potential of P. sykion as a source of novel metabolites and demonstrate the utility of untargeted metabolomics in generating baseline profiles for underexplored marine species. The results offer a foundation for future research into bioactivity, environmental monitoring, and cultivation strategies. While this study provides critical baseline data, challenges in metabolite identification and extraction underscore the need for further targeted analyses. Overall, this research enhances our understanding of the metabolic dynamics of sea cucumbers and advocates for continued exploration of lesser-known species to support conservation, bioprospecting, and sustainable aquaculture. It represents a pioneering effort in metabolomic profiling of Southern African sea cucumber species and lays the groundwork for future investigations into their metabolic pathways and potential bioactivities.
Introduction
Metabolomics, a burgeoning field in “omics” research, delves into the exploration of an organism’s metabolome–an intricate collection of small chemical compounds that represents the endpoint of gene expression, offering a direct physiological snapshot of an organism’s biochemical state (Clish, 2015; Nalbantoglu, 2019; Tebani and Bekri, 2019; Smith, 2020; Nkobole and Prinsloo, 2021; Raletsena et al., 2022).
In light of the quest for natural therapeutics, biologically active metabolites are gaining traction due to their superior efficacy, fewer side effects, and lower toxicity compared to synthetic drugs (Xu et al., 2018; Kellner Filho et al., 2019; Kamyab et al., 2020; Fasakhodi et al., 2021; More et al., 2021; Lemes et al., 2022; More et al., 2022). Consequently, metabolomics has become a pivotal tool with applications spanning the identification of disease-causing metabolites, drug discovery, precision medicine, pharmacology, cosmetics, and aquaculture (Clish, 2015; Xu et al., 2018; Nalbantoglu, 2019; Tebani and Bekri, 2019; Lemes et al., 2022; More et al., 2022).
However, the sensitivity of metabolites and high chemical diversity, shaped by species-specific genetics and environmental responses, can influence on biological properties and efficacy (Xu et al., 2017, 2018; Tebani and Bekri, 2019; David and Rostkowski, 2020; Kamyab et al., 2020; Telahigue et al., 2020; Hossain et al., 2022). This metabolic variability may suggest the presence of novel or understudied metabolite variations within lesser-studied or endemic species, accentuating the need to expand metabolomic research to understand the physiological and metabolic processes of living organisms.
Marine organisms have garnered global recognition as rich sources of pharmaceutically beneficial metabolites, owing to their unique metabolic adaptations to harsh marine environments and constant exposure to pathogens (Castillo, 2006; Bordbar et al., 2011; Bahrami et al., 2014a; Honey-Escandón et al., 2015; Khotimchenko, 2018; Telahigue et al., 2020). Among these, sea cucumbers–soft-bodied, marine echinoderms found marine environments worldwide–play vital ecological roles in sediment aeration, nutrient recycling, maintaining water quality, balancing ocean acidification, and even acting as hosts to various symbiotes (Bordbar et al., 2011; Xu et al., 2016; Ayyar and Mistry, 2020; Iwalaye et al., 2020). They have been consumed for centuries as delicacies, referred to as “Beche-de-Mer”, “Gamat” or “Trepang”, and used as a medicinal ingredient in traditional Asian medicine (Bordbar et al., 2011; Wen et al., 2011; Bahrami et al., 2014b; Purcell, 2014; Wijesinghe et al., 2015; Pangestuti and Arifin, 2018; Biandolino et al., 2019). Metabolomics has become increasingly utilized in sea cucumber research due to growing evidence of their diverse and health-promoting bioactive compounds.
However, despite the significance of sea cucumbers as valuable reservoirs of bioactive metabolites, current research predominantly spotlights a handful of commercially important species. Detrimentally, even with wildlife protection measures, catch restrictions, and legal efforts to curb poaching, the populations of these high-value species continue to decline, placing many at risk of extinction should alternative and sustainable solutions not be implemented (Ayyar and Mistry, 2020). Furthermore, structural and chemical variations among metabolites can significantly modify their bioactivities, and such differences can demonstrate considerable diversity across species, often being species-specific, making some compounds useful as metabolic biomarkers for species identification (Senni et al., 2011; Wu et al., 2015; Carvalhal et al., 2019; Grauso et al., 2019; Kamyab et al., 2020; Kalinin et al., 2021). Nevertheless, the metabolite composition and bioactive potential of most sea cucumber species remains largely unexplored, and the ongoing decline of high-value species highlights the urgent need for expanded investigation into lesser-known and potentially endemic species. This approach is critical for both biodiversity conservation and the development of sustainable bioprospecting solutions.
To date, it is estimated that there are over 1700 extant sea cucumber species (Pangestuti and Arifin, 2018; Gajdosechova et al., 2020; Imbs et al., 2021; Salindeho et al., 2022). Of these, 163 species from 74 genera, can be found in Southern African waters (Thandar, 2015). The Dendrochirotida represents the largest holothurian order in this region, comprising 73 species distributed along the southern coastlines of South Africa, inhabiting both the warm eastern and cold western waters of the Agulhas and Benguela water currents, respectively (Thandar, 2015).
Pseudocnella sykion, or the ‘black sea cucumber’, is a highly endemic species belonging to the family Cucumaridae under the Dendrochirotida, and can be found along the southern and eastern coastlines of South Africa, from the Cape Agulhas up to East London (Thandar, 1987, 2015). P. sykion typically inhabits warm tropical waters is often observed either exposed at the base of rocks or wedged between crevices in inter- and subtidal rocky shores (Thandar, 1987, 2015). At sexual maturity, P. sykion typically measures between 2.5 – 2.9 cm3 but can grow up to six cm, and is typically described as dark, olive-green to black, with a plump, barrel-shaped body (Thandar, 1987; Foster and Hodgson, 1995). The gonads of P. sykion are described as a group of unbranched tubules of equal diameter and are present year-round, with seasonal variation in gonadal tube diameter, oocyte size, and spermatozoa abundance corresponding to different reproductive stages (Thandar, 1987; Foster and Hodgson, 1995). Reproductive events in sea cucumbers, including gonadal growth and spawning, are generally influenced by environmental factors, with decreasing seawater temperatures and day length triggering gonadal growth, whilst warmer temperatures and longer day lengths acting as a spawning signal. Longer periods of gonadal maturity may also indicate favorable environmental conditions and sufficient food supply (Foster and Hodgson, 1995). However, due to an overall lack of scientific research regarding P. sykion, there is little to no information regarding its reproductive biology. As with most sea cucumber species, P. sykion likely follows an annual reproductive cycles involving a long gametogenesis and maturity phase during the winter months (March-September in the Southern hemisphere), followed by spawning in summer (December/January) and brief resting phase (Foster and Hodgson, 1995). Interestingly, some Dendrochirotida species are known to brood their young or exhibit more than one spawning season throughout the year (Martinez et al., 2020, 2024). However, in the absence of species-specific reproductive studies, it remains unclear whether P. sykion follows such reproductive strategies, and further research is needed to confirm this possibility.
Owing to its endemicity and overall lack of research on sea cucumbers, especially those found surrounding developing nations, there is very little known about P. sykion outside of basic morphology. This knowledge gap paves the way for metabolomic research to assess the biological value of lesser-known species, like P. sykion. Such investigations are not only crucial to biodiversity conservation but also offer potential commercial alternatives to high-value species at risk of overexploitation. Moreover, expanded research can open economic pathways for sustainable marine resource use, particularly in developing nations.
To our knowledge, no previous metabolomic investigations have been done to assess the intra-species metabolic profile of P. sykion. This served as the rationale for this study, aimed at carrying out an untargeted metabolomic analysis on the body wall, gonad, and gut/mesentery tissues of P. sykion over summer and winter using Proton Nuclear Magnetic Resonance (1H-NMR) and Ultra Performance Liquid Chromatography Quadruple Time-of-Flight High-Resolution Mass Spectrometry (UPLC-QTOF-HR-MS). The results from this study will provide foundational insights into the metabolite compositions of this unique, highly neglected endemic species, and contribute to the global understanding of sea cucumber biology, with implications for future research, conservation, and cultivation prospects.
Materials and methods
Ethical statement
Lower-order marine invertebrates, such as sea cucumbers, are not currently subject to ethical clearance requirements according to the University of KwaZulu-Natal Animal Research Ethics Committee (AREC) guidelines. Nevertheless, sample collection, transportation, and handling adhered to recommendations the Department of Forestry, Fisheries, and the Environment (DFFE) and the University of KwaZulu-Natal.
Sample collection and storage
Specimens of Pseudocnella sykion were collected by hand-picking during spring tide from the rocky intertidal region at Park Rynie, KwaZulu-Natal, South Africa (30° 19′ 246 S; 30° 44′ E) in August 2021 (Winter) and January 2022 (Summer). The samples were frozen, cleaned, and dissected into their body wall (a), gonads (b), and gut/mesentery tissue (c). Due to the small body size and low tissue mass of P. sykion, approximately five individuals were pooled to obtain sufficient material for each investigated replicate. Subsequently, they were frozen in liquid nitrogen, freeze-dried, and ground into a fine powder which was stored at -80°C until analysis. Notably, during dissection, the coelomic fluid appeared blood-red, suggesting potential factors such as the presence of hemoglobin, stress, or contamination. It is important to note that Park Rynie is frequently subjected to contamination factors, due to sewage outlets and chemical spills, such as the UPL chemical spills that occurred on 12 July 2021 prior to the time of sample collection (article: https://mg.co.za/environment/2021-10-09-sewage-chemical-spills-undermine-environmental-groups-marine-clean-up-efforts/; https://www.news24.com/news24/southafrica/news/upl-disaster-initial-tests-found-high-levels-of-arsenic-from-durbans-chemical-spill-20210909). The impact of external factors, such as chemical spills or sewage contamination, on the nutritional profile or physiological responses of this species warrants further testing.
1H-NMR metabolomic analysis
Tissue-specific analyses were conducted in triplicate across both summer and winter seasons, with three replicates per tissue type (body wall (a), gonad (b), and gut/mesentery (c)), yielding nine samples per season. Aqueous-methanol extraction was conducted using equal volumes (600 µl) of deuterated methanol (CH3OH – d4) and 0.01% 3–(trimethylsilyl)propionic acid, deuterium oxide–potassium dihydrogen phosphate (TSP, D2O – KH2PO4) buffer added to 50 mg of the freeze-dried sample in a 2 ml Eppendorf tube. The solution was sonicated for 15 minutes, followed by centrifugation for 15 minutes at 12–000 rpm (10–625 xg). The supernatant was carefully extracted and placed in a labelled NMR tube and analyzed by performing 32 scans on each sample using a 600 MHz spectrometer.
Spectral data were processed using MestReNova software v14.2.2. Pre-processing involved referencing to the internal TSP standard, normalization, and baseline correction. This was followed by reduction of the spectral intensities into integrated regions (bins) at 0.04 ppm widths between 0.04–10 ppm. Multivariate data analysis was performed using SIMCA software v17.0.1 (Umetrics, Sweden). Prior to analysis, the water (4.6 – 5.0 ppm) and methanol peaks (3.28 – 3.36 ppm) were excluded from the data set. Tissue and seasonal variabilities were assessed out using Principal Component Analysis (PCA) and Orthogonal Partial Least Square Discriminatory Analysis (OPLS-DA). Validation of the OPLS-DA models were carried out using the permutation test with 100 permutations and the Hierarchical Cluster Analysis (HCA). S-plot, VIP Scores plots, and contribution loadings plots were then used to determine the chemical shift regions related to metabolite differentiation between the body tissue extracts and seasons. Compound annotation was conducted by cross-referencing the identified NMR spectral bin values with online databases, such as Chenomx NMR Suite Software (v9.02) and the Human Metabolome Database (HMDB), alongside published literature.
Ultra-performance liquid chromatography quadrupole time-of-flight high resolution mass spectrometry
Full-scan UPLC-QTOF-HR-MS was performed on approximately 40 mg freeze-dried tissue samples, using representatives of each tissue type–body wall, gonad, and gut/mesentery–collected during summer and winter. Metabolite extraction was carried out by mixing the samples with 2 ml cold 70% HPLC-grade methanol, followed by sonication and vortexing to ensure complete metabolite dissolution. Subsequently, the solution was centrifuged, and the supernatant was carefully collected and filtered using Pall acrodisc GHB filters (13 mm; 0.2-micron) and a glass syringe with a Teflon plunger head before UPLC analysis.
The UPLC analysis was performed using the Waters Premier UPLC system (Waters Corporation, Milford, MA, USA). The separation was achieved on a Waters T3 C18 column (measuring at 150 mm x 2.1 mm, 1.8 µm) with a column temperature maintained at 60°C. The mobile phase consisted of two solvents: Solvent A (water containing 10mM formic acid) and Solvent B (Acetonitrile containing 10mM formic acid). The gradient program was set to initiate at 100% solvent A for one minute, followed by a linear gradient to 1% Solvent A at 16 minutes. The total runtime of the UPLC analysis was 20 minutes with a flow rate set at 0.4 mL/min.
The UPLC was followed by the Quadruple Time-of-Flight (QTOF) mass spectrometry analysis conducted using the Waters SYNAPT XS mass spectrometer (Waters Corporation, Milford, MA, USA). The instrument operated in both positive and negative electrospray ionization modes (ESIPos and ESINeg) to obtain Centroid data. The capillary voltage was set to 0.6 kV and the cone voltage was set to 30V. The source temperature was maintained at 120°C, while the desolvation temperature was set to 450°C. Nitrogen (N2) was used as the desolvation gas with a flow rate of 600 L/h. For LockMass correction, leucine enkephalin (100 pg/uL) was sampled every 20 seconds to ensure accurate mass data.
The generated data was processed using MassLynx 4.2 (SCN 1028), with the mass accuracy maintained below 1 mDA. Elemental composition analysis was performed using the Elemental Composition software embedded in MassLynx, using predefined element limits for carbon (C) (1 – 80), hydrogen (H) (1 – 200), oxygen (O) (0 – 35), and sulphur (S) (0 – 2). Only monoisotopic mass data was used and both odd and even electron states were considered with a mass tolerance of 3 mDa. Tentative compound identification for the metabolites was conducted by comparing the mass spectral data with online databases, including ChemSpider, PubChem, The Dictionary of Marine Natural Products, and the NIST 2014 Mass Spectral Library, along with online databases and relevant literature.
Results
The 1H-NMR data underwent comprehensive analysis using SIMCA software v17.0.1, employing both unsupervised Principal Components Analysis (PCA-X) and supervised Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA) to compare the chemical profiles among the different tissue samples of P. sykion collected over summer and winter.
Results from the PCA-X (Figure 1A) indicated notable separation among the body wall, gonad, and gut/mesentery tissues from P. sykion. The body wall and gut/mesentery tissues formed distinct, widespread clusters separating away from a central cluster of gonadal tissue. This suggests shared similarities between the gonadal tissue with both the body wall and gut/mesentery tissues. Notably, one winter sample, “PS_W_3b”, stood out as the most distinct gonadal sample, aligning closer to the gut/mesentery cluster. Conversely, the gut/mesentery tissue sample “PS_S_2c” appeared as the most differentiated in its class, positioned within the gonadal tissue cluster and near the “PS_W_1a” body wall sample. Additionally, a single body wall sample, “PS_W_3a”, exhibited clear separation, falling outside the 95% interval. However, this sample was not identified as an outlier according to the Hotelling’s T2Range Line Plot. The PCA-X model demonstrated a robust predictive capacity and goodness-of fit, as evidenced by an R2X(cum) = 0.975 and Q2(cum) = 0.855.
![Three scatter plots (A, B, C) depict data on tissue types from different body regions labeled as body wall, gonad, and gut/mesentery. Plot A shows body wall (green), gonad (blue), and gut/mesentery (red). Plot B uses similar colors to represent the same tissues. Plot C extends this with additional colors indicating seasonal variations: body wall summer (green), winter (dark green); gonad summer (orange), winter (blue); gut/mesentery summer (red), winter (purple). Each plot features labeled data points within an ovoid confidence ellipse on axes t[1] and t[2].](https://www.frontiersin.org/files/Articles/1609951/fmars-12-1609951-HTML/image_m/fmars-12-1609951-g001.jpg)
Figure 1. A representation of the PCA-X results (A) and OPLS-DA score plots from the 1H-NMR comparing the body tissues (B) from P. sykion over summer and winter (C).
The OPLS-DA score plot (Figure 1B) confirmed the distinct segregation of body wall and gut/mesentery tissues from the central gonadal tissue cluster. The “PS_W_3b” gonadal sample remained segregated from the main gonadal cluster, though now positioned away from the gut/mesentery tissues. Similarly, the body wall sample “PS_W_3a” remained outside the 95% confidence interval, but was not identified as an outlier according to the Hotelling’s T2Range Line Plot and DModX Line Plot. The model exhibited an R2X(cum) of 0.933, a goodness of fit R2Y(cum) = 0.544, and a predictability of Q2(cum) = 0.4, suggesting little significant variability across the three tissues. Permutation validation conducted with 100 permutations showed intercepts at R2 = (0.0; 0.217) and Q2 = (0.0, -0.438) (Supplementary Figure 1). While distinct segregation was observed between the tissue types, overall seasonal comparison (Figure 1C) revealed limited seasonal variability, with slight disparities observed within the body wall and gut/mesentery tissues. Collectively, these results underscore the substantial role of body tissue in metabolic variation, with notable segregation between the body wall and gut/mesentery tissues and considerable intra-tissue variation. Notably, the gonadal tissue extract displayed the greatest consistency in metabolic composition and may share metabolic similarities with both the body wall and gut/mesentery extracts due to their close proximity.
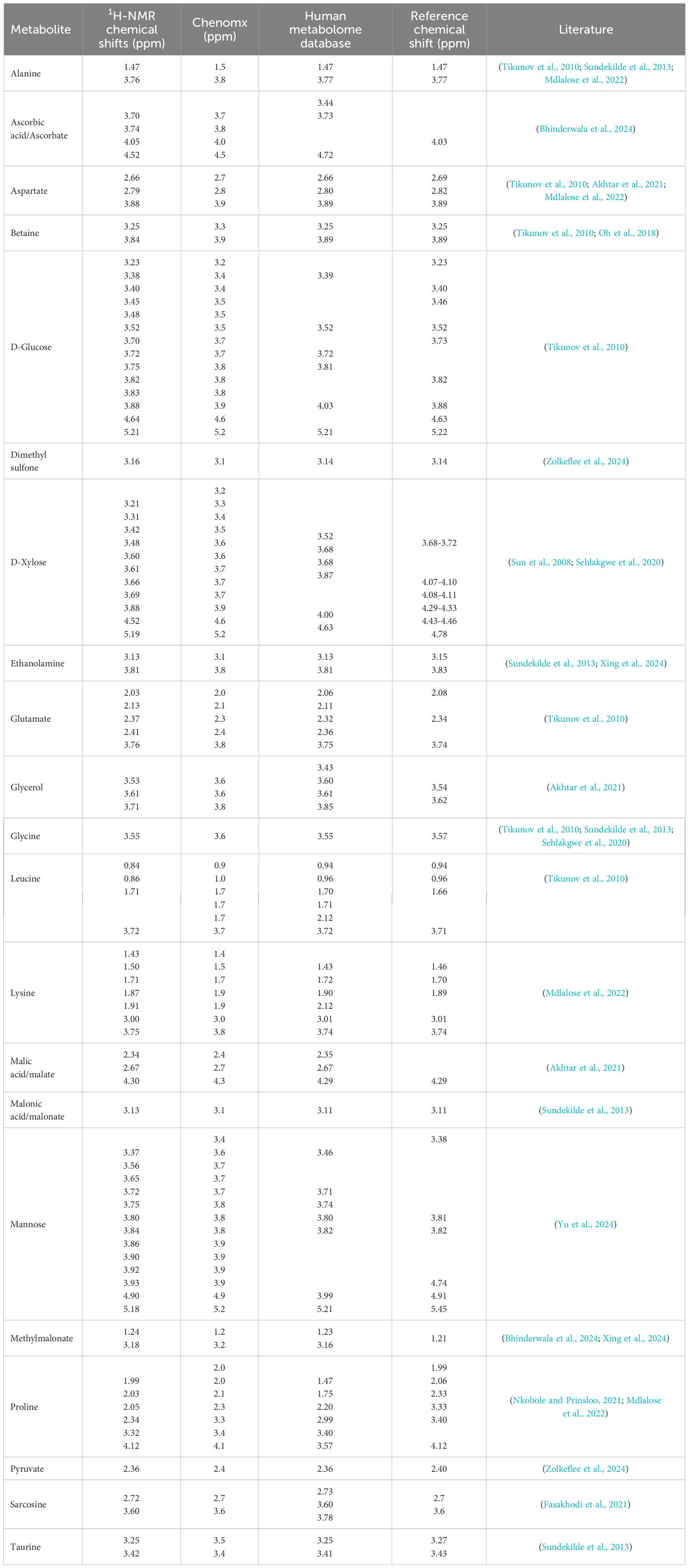
The NMR spectral comparison of body wall, gonad, and gut/mesentery tissue extracts from P. sykion (Figures 2A, B) was conducted to discern the spectral regions contributing to metabolic variations among these tissues. This analysis revealed distinct areas of spectral differentiation alongside notable similarities, particularly between the gonad and gut/mesentery tissues, suggesting a higher degree of metabolic resemblance and potential compared to the body wall.
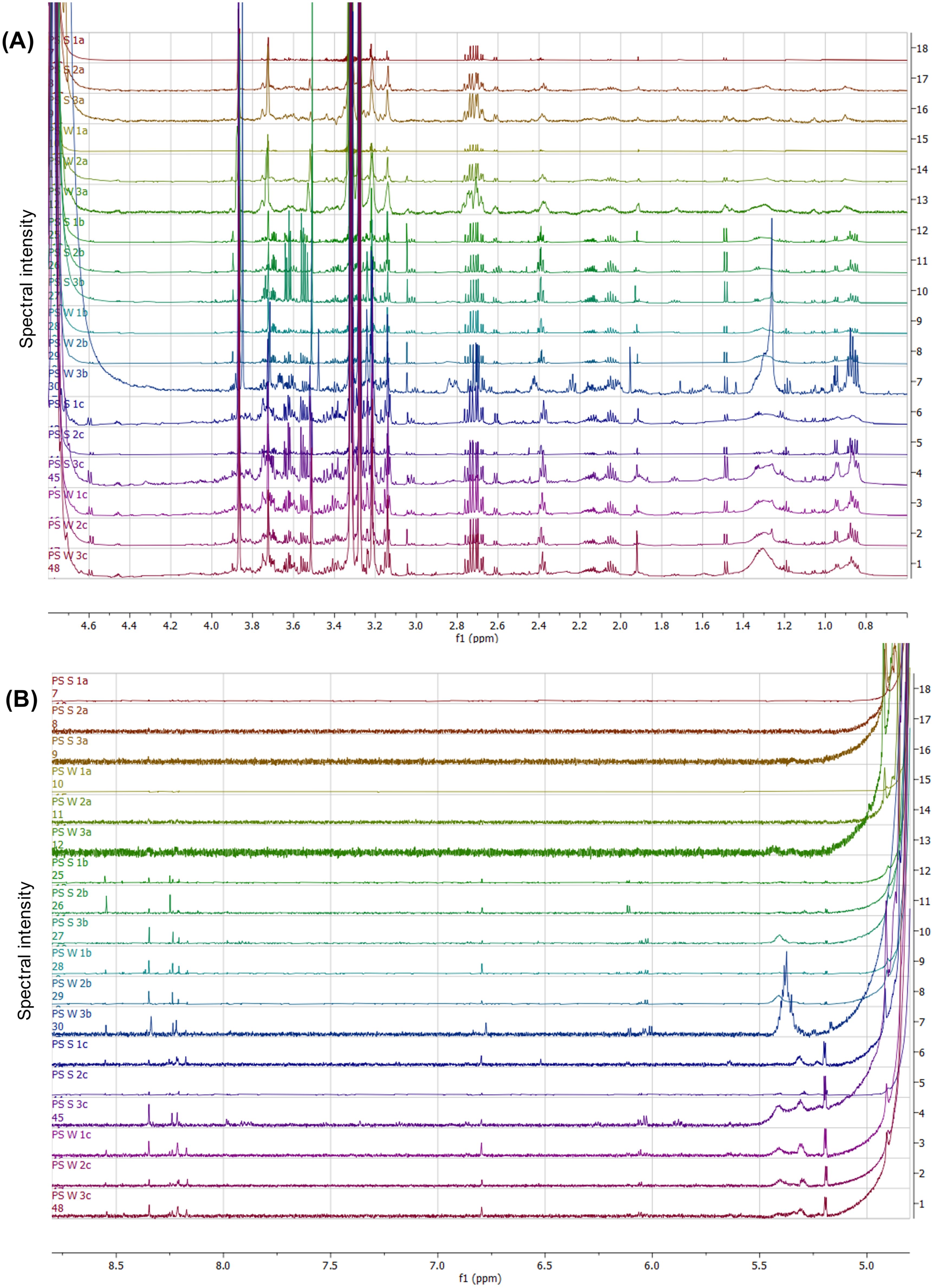
Figure 2. NMR spectral stack showing the differentiated spectral regions within the sugar-aliphatic (A) and aromatic (B) zones from the various body tissues from P. sykion. With the letters “a” representing body wall, “b” = gonad, “c” = gut/mesentery tissues, “S” = summer samples, and “W” = winter samples.
In the sugar-aliphatic region (0.8–1.5 ppm, Figure 2A), the body wall displayed limited spectral peaks, primarily a minor doublet around 1.5 ppm. In contrast, the gonad and gut/mesentery tissues exhibited a more complex spectral pattern within this region, including two partially overlapping triplets around 1.9 ppm, doublets around 1.95, 1.04, and 1.08 ppm, a singlet around 1.1 ppm, a sloping peak around 1.3 ppm, and a doublet around 1.5 ppm. While the gonadal sample “PS_W_3b” demonstrated the highest concentration within this region, the gut/mesentery tissues consistently displayed higher spectral intensity. Between 1.9–2.4 ppm, minimal spectral patterns were observed in the body wall, whereas the gonad and gut/mesentery shared similar spectral peak patterns. Notably, all tissue samples contained a distinct doublet of quartets around 2.7 ppm, preceded by a doublet around 2.6 ppm. The pattern at 2.7 ppm could potentially signify a species- or group-specific metabolic compound or collection of compounds. Between 3.0 – 4.0 ppm, distinct spectral patterns were observed among the three tissues, with the body wall having the lowest abundance and concentration of spectral peaks, the gonadal tissue exhibiting exhibited moderate intensities, and the gut/mesentery tissue displaying the highest abundance and concentrations. Notable spectral patterns included those corresponding to glycerol (3.5–3.8 ppm) and betaine (3.26 and 3.84 ppm), observed primarily in the gonad and gut/mesentery tissues. Additionally, although faintly present in the gonadal tissue, the gut/mesentery tissue displayed a minor peak around 4.5 ppm and a doublet at 4.6 ppm.
Within the aromatic region (Figure 2B), the body wall tissue exhibited only a single peak around 4.8 ppm, also present within the gonad and gut/mesentery tissues. Around 5.2 ppm and 5.4 ppm both the gonad and gut/mesentery tissues displayed a doublet and a sloped peak, more pronounced in the gut/mesentery tissue. Between 6.1–6.2 ppm, varying doublets and a singlet at 6.8 ppm were observed in the gonad and gut/mesentery tissues, with no distinct pattern differentiating the two. Additionally, between 8.2–8.6 ppm, the gonad and gut/mesentery tissues exhibited a range of spectral peaks, absent within the body wall tissue. These findings highlight metabolic differences among these three body tissues and suggest specific spectral patterns associated with distinct metabolic compounds or processes.
Tissue x tissue pairwise comparisons
The tissue-to-tissue pairwise OPLS-DA comparisons shed light on the metabolic variability among the body wall vs gonad (Figure 3A), body wall vs gut/mesentery (Figure 3B), and gonad vs gut/mesentery (Figure 3C) tissues from P. sykion. These analyses complemented the insights gained from the NMR spectral stack (Figure 2) and were used to identify specific spectral regions contributing to the observed metabolic differences. The models exhibited varying levels of robustness and predictability, with the body wall vs gonad showing an R2X(cum) = 0.904, R2Y(cum) = 0.533, and Q2(cum) = -0.291, the body wall vs gut/mesentery comparison with an R2X(cum) = 0.958, R2Y(cum) = 0.782, and Q2(cum) = 0.677, and the gonad vs gut/mesentery comparison with an R2X(cum) = 0.91, R2Y(cum) = 0.591, and Q2(cum) values = 0.517. Permutation validation tests further supported the reliability of these models (Supplementary Figure 2).
![Three scatter plots labeled A, B, and C show data points within confidence ellipses on axes t[1] and to[1]. Each plot has data grouped by color: Plot A compares “Body wall” (green) to “Gonad” (blue). Plot B compares “Body wall” (green) to “Gut/mesentery” (blue). Plot C compares “Gonad” (green) to “Gut/mesentery” (blue). Points are labeled with tags like PS_S_3a and PS_W_2b.](https://www.frontiersin.org/files/Articles/1609951/fmars-12-1609951-HTML/image_m/fmars-12-1609951-g003.jpg)
Figure 3. OPLS-DA score plots from the 1H-NMR spectral data showing the body wall vs gonad (A), body wall vs gut/mesentery (B), and gonad vs gut/mesentery (C) body tissue comparison in P. sykion.
S-plots and VIP scores plots were generated for each significant pairwise tissue comparison to identify the influential spectral regions contributing to segregation between body wall vs gut/mesentery (Figure 4), and gonad vs gut/mesentery (Figure 5) tissue extracts. Additionally, contribution loadings plots were used to visualize the regions of spectral variability (Figures 4C, 5C). Detailed descriptions of the influential 1H-NMR spectral regions associated with P. sykion tissue comparisons are provided in Supplementary Table 1.
![(A) Scatter plot illustrating the relationship between p(corr)[1] and p[1], with larger blue and red circles representing significant values. (B) Bar graph displaying VIP scores, with prominent blue and green bars. (C) Bar graph showing score contributions, highlighting variations with blue, green, orange, and red bars.](https://www.frontiersin.org/files/Articles/1609951/fmars-12-1609951-HTML/image_m/fmars-12-1609951-g004.jpg)
Figure 4. S-plot (A), VIP score plot (B), and contribution loadings plot (C) showing differentiating 1H-NMR spectral regions between the body wall and gut/mesentery tissue from P. sykion. Bars above the X-axis (C) showing regions positively associated with the gut/mesentery tissue.
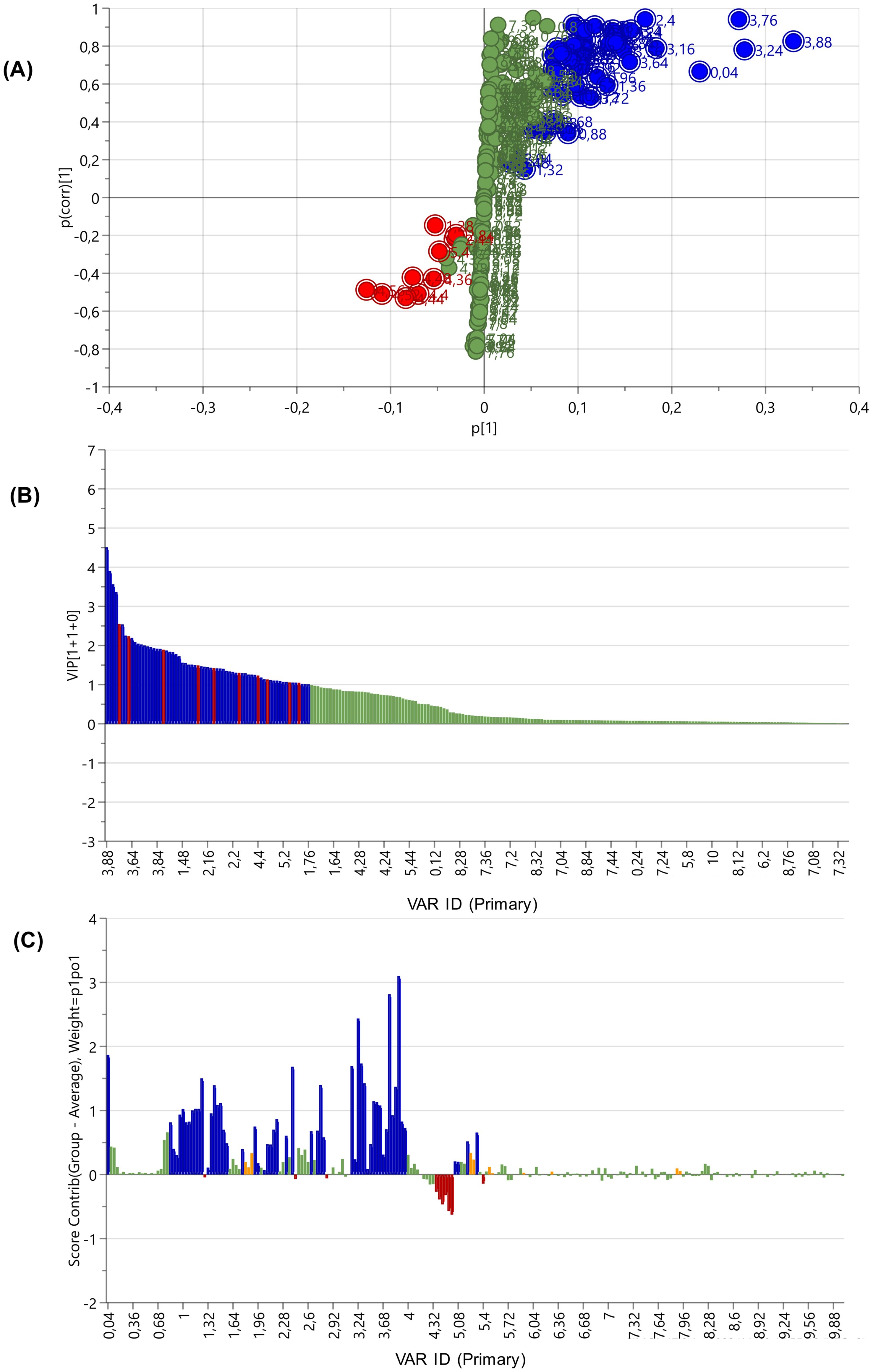
Figure 5. S-plot (A), VIP score plot (B), and contribution loadings plot (C) showing the 1H-NMR spectral regions differentiating the gonad and gut/mesentery tissues from P. sykion. Bars above the X-axis (C) showing regions positively associated with the gut/mesentery tissue.
1H-NMR compound annotation
The annotated binned regions derived from the contribution loadings plots were cross-referenced with online databases, including Chenomx, HMDB, and NP_MRD, as well as published literature. The untargeted metabolomics comparison among the body wall, gonadal, and gut/mesentery tissues of P. sykion revealed distinct differences in metabolite composition across and within the three tissues, as illustrated in Tables 1 and 2, respectively.
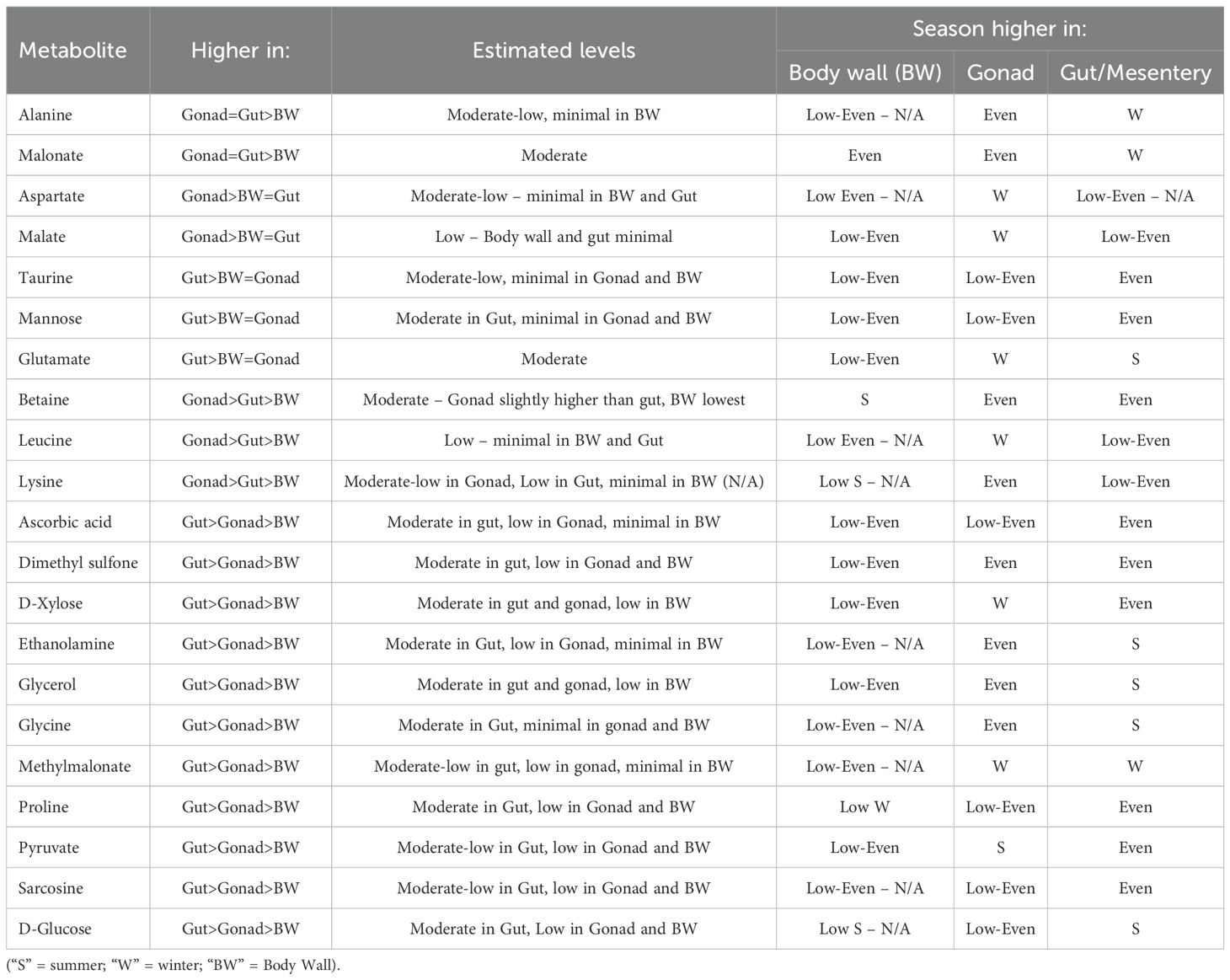
Table 2. 1H-NMR metabolite comparisons between and within the body wall, gonad, and gut/mesentery tissue from P. Sykion.
Notably, the gonadal and gut/mesentery tissues exhibited a higher metabolic potential compared to the body wall. Specifically, the gonad and gut/mesentery tissues displayed moderate levels of malonate and alanine in comparison to the body wall. Moreover, the gonadal tissue demonstrated higher levels of malate, butyrate, aspartate, betaine, lysine, and leucine compared to the body wall or gut/mesentery tissues. In contrast, the gut/mesentery tissue showcased elevated levels of D-xylose, D-glucose, mannose, glycerol, methylmalonate, pyruvate, glycine, proline, taurine, glutamate, ascorbic acid, dimethyl sulfone, ethanolamine, and sarcosine. Overall, the results suggest distinct metabolic profiles and functional roles across the different tissue types in P. sykion.
Seasonal comparisons within body tissues
Body wall
The results of the PCA-X analysis on the body wall tissue extracts of P. sykion across summer and winter (Figure 6) did not reveal significant seasonal variation. The model proved robust, as evidenced by an R2X = 0.957 and Q2(cum) = 0.83.
![Scatter plot depicting summer and winter body wall data points on a t[1] versus t[2] grid. Summer body wall points are green, while winter body wall points are blue. An ellipse encloses most points, indicating the data spread.](https://www.frontiersin.org/files/Articles/1609951/fmars-12-1609951-HTML/image_m/fmars-12-1609951-g006.jpg)
Figure 6. Representation of the PCA-X score plot results from the 1H-NMR spectral data showing the body wall tissue comparison between P. sykion over summer and winter.
Although seasonal variation was minimal, NMR spectral stacks (Figures 7A, B) were generated to assess metabolic composition within the body wall tissues within the sugar-aliphatic (Figure 7A) and the aromatic (Figure 7B) spectral regions. In the aliphatic region spanning 0.8–2.2 ppm (Figure 7A), small undefined spectral peaks were observed, with a notable doublet more pronounced in the summer body wall samples at 1.5 ppm. A multiplet around 2.4 ppm was evident, albeit less distinct in the “PS_S_1a” and “PS_W_1a” samples. Notably, a doublet peak at 2.6 ppm and a distinct multiplet pattern at 2.7 ppm were observed, particularly prominent and in higher concentration within the summer body wall samples. Additionally, an array of peaks between 3.0 and 4.0 ppm was noted, with varying peak intensities across seasons, with “PS_W_3a” showing the highest overall concentration. Furthermore, two singlets between 4.8–5.0 ppm were observed, with no additional peaks evident within the aromatic region. Overall, the body wall tissues of P. sykion exhibited minimal metabolite potential and negligible seasonal variability, as indicated by the NMR spectral analysis.
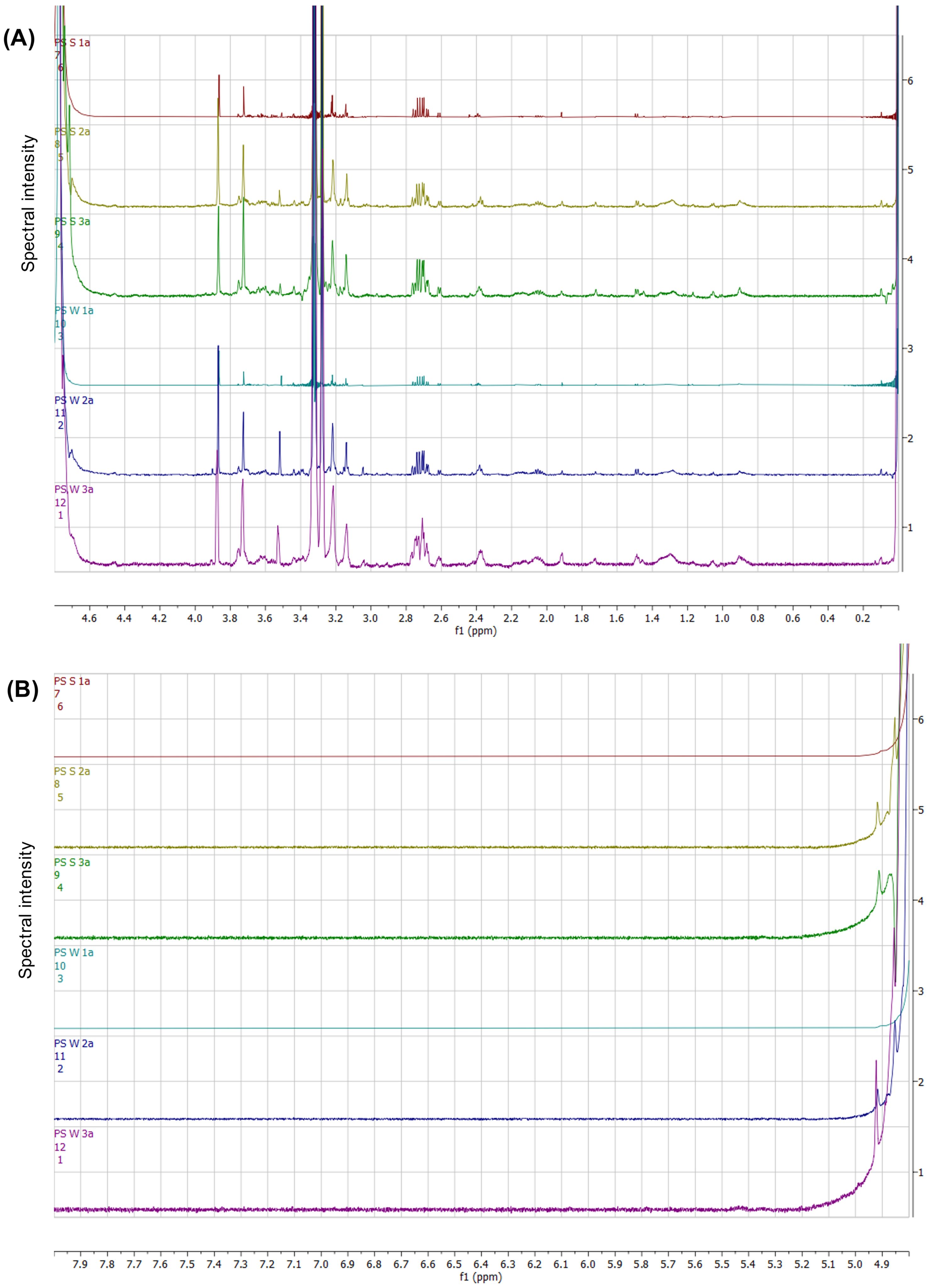
Figure 7. NMR spectral stack highlighting the chemical shift regions within the sugar-aliphatic (A) and aromatic (B) regions from the body wall tissue comparison from P. sykion between summer and winter.
Gonad
The analysis of gonadal tissues from P. sykion revealed subtle seasonal distinctions, as depicted in Figure 8. The PCA-X results (Figure 8A) proved robust, with an R2X(cum) of 0.959 and a Q2(cum) of 0.877. Notably, the winter sample “PS_W_3b” stood out as distinctly different from the rest of the gonadal tissues, while the summer “PS_S_1b” and winter “PS_W_1b” samples appeared closer to each other, suggesting an overlap in metabolic composition between seasons. Further insights were provided by the OPLS-DA (Figure 8B), which emphasized the seasonal separation within the gonadal tissues, with the exception of the “1b” summer and winter samples. Notably, greater variability was observed within the winter samples, particularly due to the pronounced separation of the “W_3b” sample. The model demonstrated a high goodness of fit but with limited predictive power, supported by an R2X(cum) = 0.958, R2Y(cum) = 0.659, and Q2(cum) = 0.297. To validate these findings, permutation plot and hierarchical cluster analysis (HCA) were conducted, with the permutation plot having intercepts at R2 = (0.0; 0.805) and Q2 = (0.0; 0.131) (Supplementary Figures 3A, B).
![Two scatter plots labeled (A) and (B) display data points for “Summer gonad” in green and “Winter gonad” in blue. Both plots feature an ellipse centered around the origin, with axes labeled as t[1] vs t[2] for (A) and t[1] vs to[1] for (B). Data points are labeled and plotted within the ellipse, showing separation between summer and winter data sets.](https://www.frontiersin.org/files/Articles/1609951/fmars-12-1609951-HTML/image_m/fmars-12-1609951-g008.jpg)
Figure 8. PCA-X (A) and OPLS-DA score plot (B) from the 1H-NMR showing the seasonal separation of gonadal tissue samples from P. sykion.
NMR spectral analysis of the gonadal tissues (Figure 9) provided insights into the spectral regions distinguishing between summer and winter gonadal samples. In the sugar-aliphatic (Figure 9A), notable spectral similarities were observed between 0.8–1.5 ppm, with the winter “PS_W_3b” sample displaying significantly higher spectral intensities within this region. Additionally, distinct patterns were noted in the 3.0 to 4.0 ppm region, with the “PS_S_3b” summer sample exhibiting significantly higher spectral intensities, likely corresponding to a glycerol pattern, and with all samples displaying substantial singlets around 3.26 and 3.85 ppm, potentially associated with betaine. In the aromatic region (Figure 9B), the samples exhibited singlets around 4.9 and 5.2 ppm, likely related to sugar monosaccharides. Despite there being a high degree of similarity between the summer and winter samples, minor peak and concentration differences can be observed in the spectral patterns.
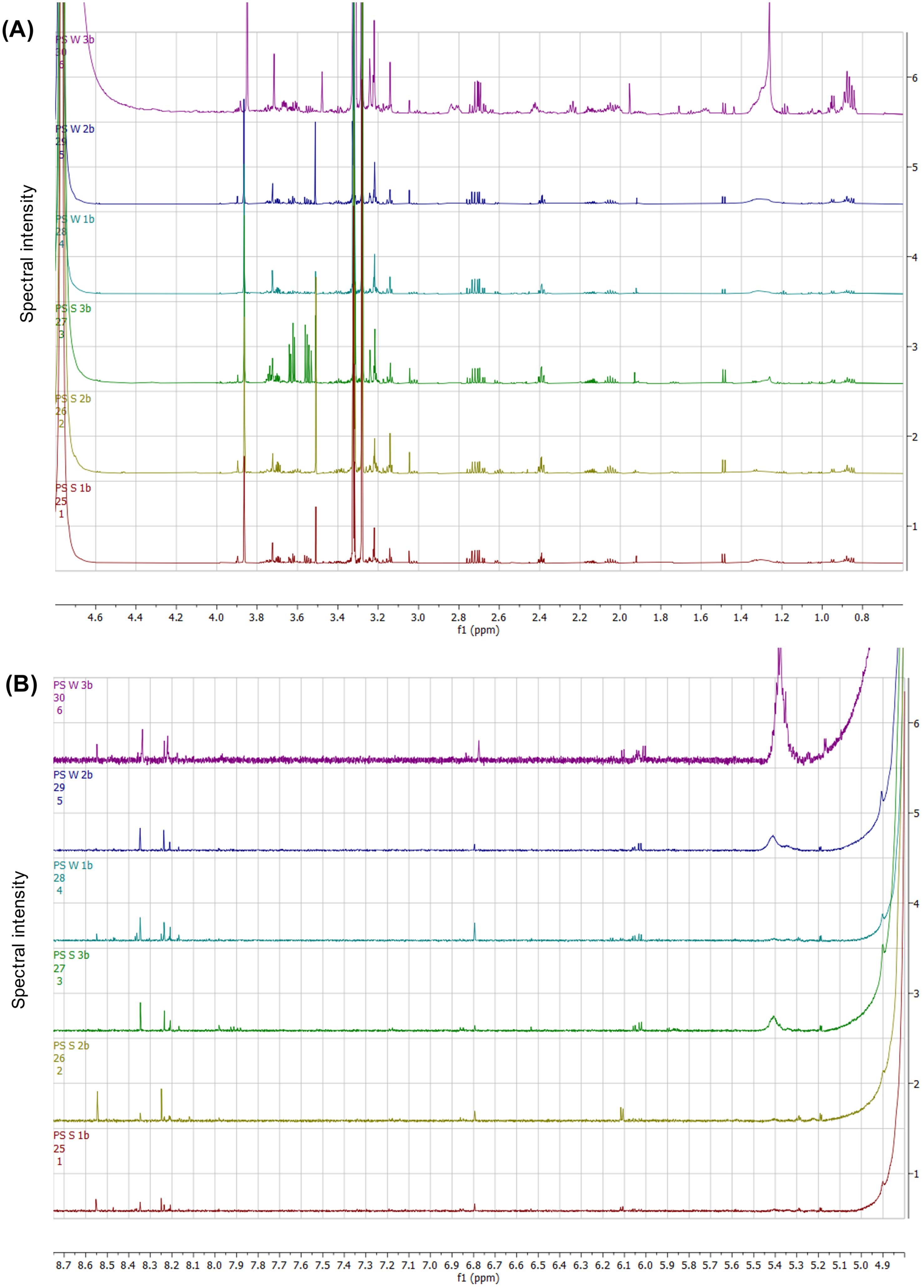
Figure 9. NMR spectral stack identifying the differentiated chemical shift regions within the sugar-aliphatic (A) and aromatic (B) regions from the gonadal tissue comparison from P. sykion between summer and winter.
The S-plot and VIP scores plot (Figures 10A, B) further elucidated the spectral regions responsible for the seasonal separation of the gonadal samples from P. sykion. Influential scores were identified from both ends of the S-plot and in VIP scores ≥ 1.0. The contribution loadings plot (Figure 10C) identified positively associated regions corresponding with the winter samples at 0.04, 0.88, 0.92, 0.96, 1.2, 1.24, 1.28, 1.32, 1.36, 1.6, 2.04, 2.28, 2.44, 2.72, 2.84, 3.2, 3.24, 3.48, 3.68, 3.72, 3.88, 4.32, 4.36, 4.4, 4.44, 4.48, 4.52, 4.56, 5.04, 5.36, and 5.4 ppm, and to a lesser extent, the 0.84, 1, 1.04, 1.64, 1.96, 2, 2.08, 2.12, 2.16, 2.24, 2.36, 2.68, 2.76, 2.8, 2.88, 3.16, 3.44, 3.8, 3.84, 3.92, 4.04, 4.08, 4.12, 4.16, 4.2, 4.24, 4.28, 5.08, and 5.12 ppm regions. Negatively associated regions corresponded with the spectral bins at 2.4, 3.52, 3.56, 3.64, and 3.76 ppm.
![Panel A shows a scatter plot with colored dots representing different variables in clusters along axes p[1] and p(corr)[1]. Panel B displays a bar chart with blue and red bars of varying heights indicating VIP scores for each variable. Panel C presents another bar chart illustrating score contributions across different variables, with blue and red bars depicting positive and negative values.](https://www.frontiersin.org/files/Articles/1609951/fmars-12-1609951-HTML/image_m/fmars-12-1609951-g010.jpg)
Figure 10. S-plot (A), VIP score plot (B), and contribution loadings plot (C) showing 1H-NMR spectral regions contributing to the clustering and separation of the gonadal tissues from P. sykion between summer and winter. Bars above the X-axis (C) show regions positively associated with the winter gonadal tissue.
Analysis of the contribution values, in comparison with online databases Chenomx and HMDB revealed notable fluctuations in metabolite levels. Chemical shift regions correlating with certain metabolites, such as malate, aspartate, butyrate, leucine, glutamate, D-xylose, and methylmalonate, were more abundant in winter, while others like pyruvate, were more abundant in summer.
Gut/mesentery tissue
Similar to the body wall comparison, the results of the PCA-X on the gut/mesentery seasonal tissue from P. sykion (Figure 11) indicated no significant seasonal variation within this tissue. However, there was greater dispersal within the summer tissue samples, whereas the winter samples clustered near the center along with one summer replicate, “PS_S_1c”. Despite the lack of clear seasonal distinction, the model demonstrated a high level of predictability and robustness, with R2X = 0.933 and Q2(cum) = 0.791. This observation could be attributed to the ecological niche of P. sykion, being wedged into rock crevices and consistently exposed to the same types of organic and fecal matter produced by the surrounding organisms, P. sykion likely experiences a relatively stable dietary environment, which could lead to a more consistent metabolic profile within the gut/mesentery tissues across seasons. The minor variations in the summer samples may be related to natural dietary and metabolic fluctuations but do not appear significant enough to create a clear seasonal separation.
![Scatter plot showing data points for summer and winter gut/mesentery samples. Green circles represent summer samples and blue circles indicate winter samples. Data is plotted on axes labeled t[1] and t[2], with an ellipse indicating data variance.](https://www.frontiersin.org/files/Articles/1609951/fmars-12-1609951-HTML/image_m/fmars-12-1609951-g011.jpg)
Figure 11. Representation of the PCA-X score plot results from the 1H-NMR spectral data showing the seasonal comparison between the gut/mesentery tissue from P. sykion. Green representing summer samples, blue representing winter.
The NMR spectral data from the summer and winter gut/mesentery tissues of P. sykion were stacked to compare the spectral patterns within the sugar-aliphatic (Figure 12A) and aromatic (Figure 12B) regions. The results revealed significant spectral similarities between the two seasons, with the slightly higher spectral intensities observed in the summer “S_3c” sample. Several commonalities were identified between the seasonal samples, including the presence of a multiplet at 2.7 ppm and doublet around 2.6 ppm, previously observed in both the body wall and gonadal tissues. Spectral patterns between 3.0 and 4.0 ppm were shared by both summer and winter samples, and appeared to be related to glycerol and betaine. Although these spectral peaks appeared with higher intensities in the summer “S_3c” sample, they remained consistent across the winter samples. Additionally, a low multiplet around 4.45 ppm and a doublet around 4.6 ppm, likely related to sugar-type compounds, were also observed.
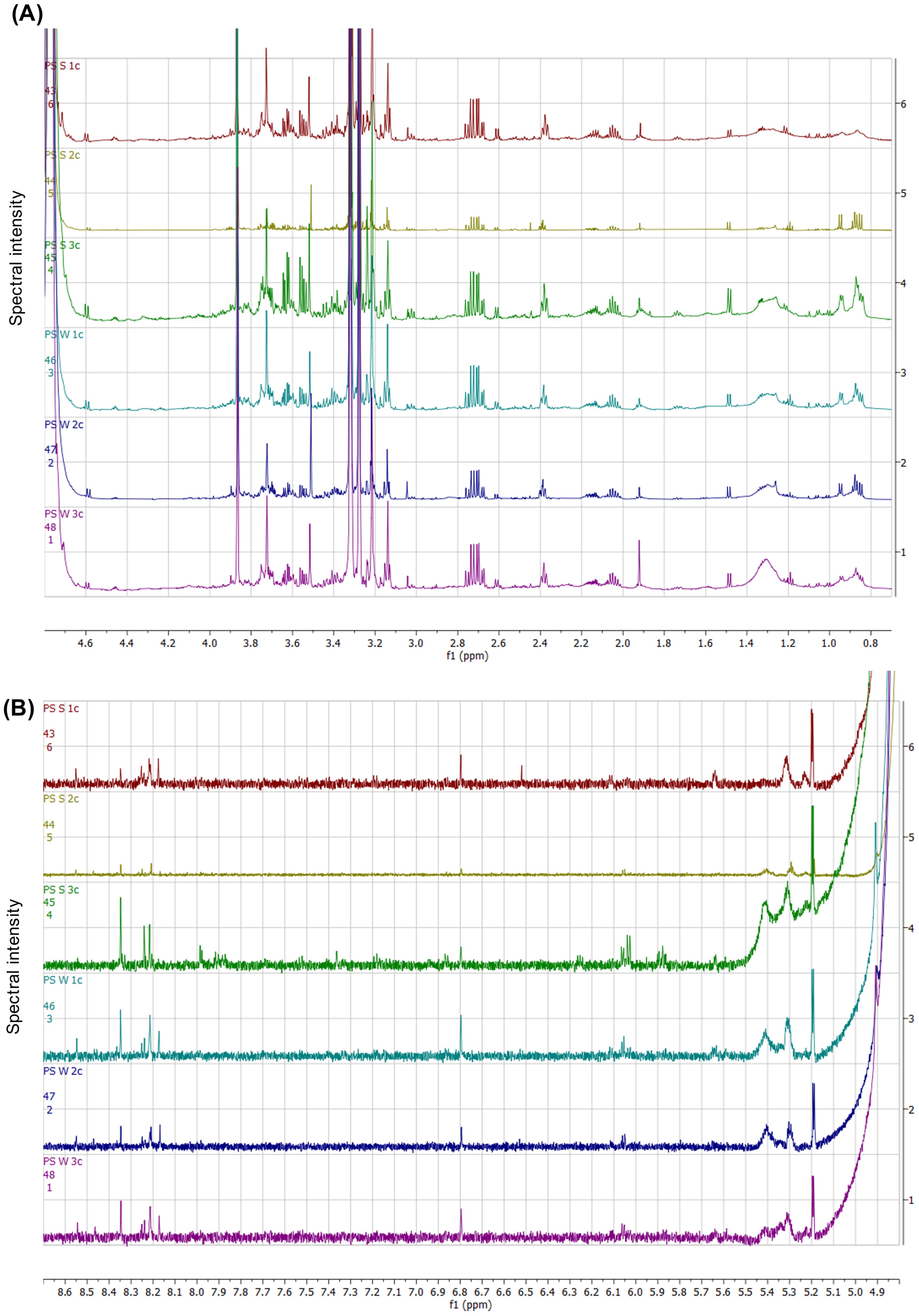
Figure 12. NMR spectral stack comparing the spectral regions within the sugar-aliphatic (A) and aromatic (B) regions from the gut/mesentery tissues from P. sykion between summer and winter.
In the high sugar-aromatic region (Figure 12B), the samples exhibited similar spectral peaks. However, at around 5.4 ppm, the winter samples displayed a spectral peak that was only present in one of the summer samples, “S_3c”. Doublets observed between 6.0–6.15 ppm were of higher intensity within the “S_3c” summer sample but were more consistent in the winter samples. Although a distinct singlet appeared at 6.8 ppm, it had greater intensity within the winter samples. In the 8.1 and 8.6 ppm region, all samples displayed distinctive spectral peaks, but there were no discernible patterns that distinguished them between seasons or samples. Overall, the results correlate with the PCA-X analysis (Figure 11) and suggest a high degree of metabolic similarity between the summer and winter gut/mesentery tissues. This indicates that the metabolic composition of the gut/mesentery tissue remains relatively consistent across seasons, possibly reflecting the stability of the ecological niche and dietary sources of P. sykion.
Despite the lack of overall significant seasonal variability, the results showed slight fluctuations in some metabolites within the gut/mesentery tissue. Alanine, malonate, and methylmalonate appeared with slightly higher peaks in winter, while glutamate, ethanolamine, glycerol, glycine, and D-glucose were higher in summer. These findings suggest that metabolic processes, dietary composition, or food abundance may fluctuate slightly, albeit not necessarily significantly, across seasons. However, further investigations need to be undertaken to assess the extent of metabolic composition based on environmental fluctuations.
Overall, the gonadal and gut/mesentery tissues of P. sykion exhibited greater metabolic potential and metabolite diversity compared to the body wall tissue. The gonadal tissue showed a predilection for amino acids, whereas the gut/mesentery tissue displayed a broader spectrum of metabolites, encompassing sugars, amino acids, and organic acids. Seasonal variations were observed, with winter gonadal and summer gut/mesentery tissues favoring a higher metabolite composition and potential. These findings provide insights into the metabolite dynamics of sea cucumbers in response to seasonal changes, underscoring the importance of considering tissue-specific and environmental influences in metabolomic studies. Seasonal differences in metabolite levels suggest potential adaptations of P. sykion to environmental changes, which could have implications for its ecological role and overall health, especially given the environmental changes such as sewage contamination and chemical spills faced by the study area, Park Rynie. Such environmental conditions may impact the nutritional quality, bioactive potential, and health of the species. Further research into the functional significance of these metabolic changes and compositions is warranted to gain a deeper understanding of the physiological mechanisms underlying these patterns. This could help elucidate the biological potential of P. sykion and assess its suitability for sustainable cultivation and natural drug discovery for human use.
UPLC-QTOF-HR-MS analysis
The metabolic composition of P. sykion was investigated through UPLC-QTOF-HR-MS analysis (Supplementary Figure 4), focusing on three body tissues collected during summer and winter. This analytical technique enables the calculation of empirical formulas via accurate mass measurements of small molecules. However, the precise identification of these compounds is reliant upon access to reference standards, up-to-date mass spectral databases and comprehensive raw data from literature. The lack of holothurian-specific databases posed a significant challenge, as reliance on broader, non-holothurian databases may have affected the accuracy and confidence in compound identification. The Dictionary of Marine Natural Products do not have any compounds listed for P. sykion, highlighting the novelty of metabolomic investigations on this species.
Supplementary Table 2 summarizes the tentative metabolite profiles identified in the tissues of P. sykion, including details on observed and monoisotopic masses, retention times, and calculated empirical formulas. The analysis revealed that while metabolites were present in both summer and winter samples, summer specimens exhibited a greater metabolite diversity. Notably, the gonad and gut/mesentery tissues demonstrated higher metabolic potential compared to the body wall, aligning with the 1H-NMR findings (Figure 2; Table 2).
Among the compounds tentatively identified was rosmarinic acid, a phenolic compound typically associated with plants; although its presence has also been recorded in Holothuria forskali (Telahigue et al., 2020). Additionally, the results suggest that P. sykion has the potential for several triterpene glycosides, including scabrasides A and D, holothurin A1, and A4, 24-dehydroechinoside A, fuscocineroside B, and 17-dehydroxyholothurin A (fuscocineroside C). Several detected compounds could not be assigned specific names or classes due to insufficient reference spectra. These unidentified compounds may be unique to holothurians or specific to this species, potentially indicating novel bioactive molecules. However, it is also possible that some of these compounds, such as rosmarinic acid, may have originated from dietary consumption of organic matter, such as phytoplankton or microalgae. As this is an untargeted analysis with the aim of providing a baseline metabolic profile for P. sykion, there is an inherent degree of uncertainty in metabolite confirmation. The mass spectral data were obtained using broad parameters set within the MS software, while some compound identifications were based on generalized matches with mass spectral databases; thus, these may not accurately reflect the true compound conformations present in the samples. Additionally, uncertainties in metabolite characterization reported in the literature further complicated metabolite annotation. Interestingly, P. sykion exhibits a high potential for metabolite isomers, underscoring the need to expand holothurian research to better understand the metabolic complexity of these organisms. Consequently, further targeted analyses and compound isolation studies employing fragmentation analyses are warranted to comprehensively elucidate the compound structures and their biological properties.
Discussion
Pseudocnella sykion is an endemic sea cucumber species found along the Eastern Coast of Southern Africa and remains relatively unknown due to limited academic attention. This study represents the first untargeted 1H-NMR metabolomics and full-scan UPLC-QTOF-HR-MS analysis on P. sykion, aiming to establish a baseline metabolic profile and determine metabolic variability between three body tissues (body wall, gonad, and gut/mesentery) across seasonal (summer and winter) changes. The results displayed slight seasonal and tissue distinctions, with gonad and gut/mesentery tissue extracts displaying greater metabolic potential compared to the body wall. Seasonal variation was most prominent in the gonadal tissue extract, which exhibited elevated metabolic activity in winter. Although the gut/mesentery tissue extract did not show significant seasonal separation (Figure 11), certain compounds were more abundant in summer, potentially reflecting improved food availability during this period.
Body wall extracts exhibited low metabolite concentrations, which remained relatively stable across seasons. This may suggest limited environmental stress or metabolic variability within this tissue. Notably, malonate and betaine were detected at moderate concentrations, with betaine–an osmoregulatory compound–showing higher levels in summer.
Reproductive mechanisms influenced by seasonal fluctuations and environmental cues likely contribute to tissue-specific metabolic variability in sea cucumbers (Ru et al., 2017; Cheng et al., 2021; Jiang et al., 2021). Many sea cucumber species exhibit species-specific seasonal reproductive cycles, which may account for observed gonadal metabolite differences (Foster and Hodgson, 1995; Dolmatov, 2014; Domínguez-Godino et al., 2015; Madduppa et al., 2017; Jiang et al., 2021). In this study, gonadal tissue extracts of P. sykion displayed a slight increase in several metabolites during winter, including aspartate, leucine, and glutamate, malate, D-xylose, and methylmalonate. Conversely, pyruvate levels were elevated in summer, potentially indicating increased energy mobilization likely linked to reproductive activity, such as spawning. Notably, D-xylose–a major component of triterpene glycosides which are known to have roles in reproduction and oogenesis (Kalinin et al., 2005; Bahrami et al., 2014a; Bahrami et al., 2014b; Kalinin et al., 2015; Bahrami et al., 2018; Puspitasari et al., 2021)–was also higher during winter, suggesting potential reproductive activity or recovery during this season.
Complementary UPLC-QTOF-HR-MS analyses (Supplementary Table 2; Supplementary Figure 4) revealed high levels of tentatively identified compounds in the gonad and gut/mesentery tissue extracts, including scabrasides A and D, holothurin A1, and A4, 24-dehydroechinoside A, fuscocineroside B, and 17-dehydroxyholothurin A (fuscocineroside C). The potential presence of these triterpene glycosides across both seasons and in association with reproductive tissues suggests potential roles in reproductive physiology, supporting the possibility of dual spawning or extended recovery phases, which have been observed within other Dendrochirotid species (Foster and Hodgson, 1995; Hamel and Mercier, 1996; Peters-Didier et al., 2018). However, due to limited biological data on P. sykion, including its reproductive biology, further investigations are warranted to confirm these patterns and clarify the relationship between reproductive mechanisms and metabolic variation. Such insights could inform optimal strategies for sustainable cultivation while enhancing the production of beneficial metabolites. Additionally, the tentative identifications, which were based on literature and compound databases, also face challenges related to compound ambiguity and the high occurrence of isomers among the identified compounds, underscoring the need for further targeted analyses involving, employing compound isolation and fragmentation to accurately determine the complete structures and biological functions of these metabolites within P. sykion.
Triterpene glycosides can serve as species biomarkers, with variations in holostane triterpenoids used to resolve taxonomic discrepancies in Cucumaria species (Kalinin et al., 2015; Xu et al., 2018). P. sykion, initially classified as Cucumaria sykion before taxonomic re-evaluation (Thandar, 1987), may share metabolic similarities with other Cucumaria species. However, due to limited research on the Cucumariidae family, further targeted investigations of triterpene glycosides within P. sykion are warranted using alternative solvents and analytical techniques to validate their structures and explore their nutritional and pharmacological potential. Such studies could also contribute to reassessing or validating the taxonomic classification of this species.
The gut/mesentery tissue exhibited the highest abundance of many identified metabolites (Table 1), including amino acids and compounds associated with energy metabolism and resource acquisition. These include glycine, glutamate, taurine, proline, sarcosine, ethanolamine, ascorbic acid, dimethyl sulfone, glycerol, methylmalonate, pyruvate, mannose, D-xylose, and D-glucose, indicating diverse physiological functions and possible accumulation of dietary compounds. Several of these compounds, such as glycine, taurine, proline, sarcosine, and glycerol can act as osmolytes, assisting marine organisms in regulating cellular osmotic pressure in high salinity marine environments, while also serving as antioxidant, protein stabilizing, and cytoprotective agents (Kinne, 1993; Yancey et al., 2002; Yancey, 2005; McParland et al., 2021). Their presence and higher relative abundance within the gut/mesentery tissue raises questions about endogenous synthesis versus dietary or microbial origin.
The detection of rosmarinic acid from the UPLC-QTOF-HR-MS was particularly interesting. Rosmarinic acid is a complex polyphenolic compound with potent bioactive properties commonly found in terrestrial plant species, especially those from the Boraginaceae family and Rosmarinus officinalis from the Lamiaceae family (Kamatou et al., 2010; Shekarchi et al., 2012; Elufioye and Habtemariam, 2019; Nadeem et al., 2019). Given its wide range of biological activities, including anti-inflammatory, anti-microbial, and anti-carcinogenic properties, the identification of rosmarinic acid, along with triterpene glycosides tentatively identified from the UPLC-QTOF-HR-MS (Supplementary Table 2) highlights the biological potential of P. sykion metabolites (Li et al., 2013; Honey-Escandón et al., 2015; Janakiram et al., 2015; Khotimchenko, 2018; Salindeho et al., 2022; Puspitasari et al., 2023).
However, although rosmarinic acid has been previously identified in H. forskali (Telahigue et al., 2020), its presence in P. sykion is noteworthy given its strong association with plant species, raising questions about compound origin. Sea cucumbers, including P. sykion, are known to feed on detritus, microalgae, phytoplankton, and other organic matter present either in the sediment or suspended in the water column, depending on whether they are deposit or suspension feeders (Foster and Hodgson, 1995; Xu et al., 2016). Being a suspension feeder and often found wedged under rocks along with other marine species, P. sykion may be exposed to a higher abundance of organic plant or fecal matter as a food source, particularly during high tide when water levels are elevated. The strong association of rosmarinic acid with plants, along with the uncertainty of several compounds identified in the UPLC-QTOF-HR-MS (Supplementary Table 2), warrants further investigation.
The dietary composition and food availability can be influenced by various factors, including environmental conditions, seasonal changes, and water quality. Additionally, the energy requirements and feeding rates of sea cucumbers can be affected by reproductive seasons and fluctuating water temperatures. Increased food consumption typically occurs during warmer seasons and spawning periods, while decreased food intake may occur during unfavorable environmental conditions, such as lower temperatures, reduced food availability or periods of hibernation or reproductive dormancy. In addition, water pollution poses a significant threat to the food and water quality of sea cucumber habitats, particularly in shallow coastal regions where sea cucumbers may be more susceptible to contamination from sewage outlets, industrial releases, and chemical spills during extreme weather events (Dupont et al., 2010; Sicuro et al., 2012; Pangestuti and Arifin, 2018; Gajdosechova et al., 2020). Consequently, the metabolic composition within tissues, particularly within the gut tissue, can vary significantly based on seasonal changes, food availability and composition, water quality, and proximity to pollution sources (Sicuro et al., 2012; Pangestuti and Arifin, 2018; Biandolino et al., 2019; Ahmed et al., 2023). For instance, the study site, Park Rynie, is known to have an active sewage outlet and experienced a chemical spill along the coastline shortly before the time of sample collection. This may explain the potential presence of lauryl sulphate, a surfactant commonly used in household practices and wastewater treatment (Jönander et al., 2022). However, these events are often located further offshore and unlikely to have significantly impacted the intertidal sampling site outside of high tide or extreme weather events. Further environmental studies need to be conducted to confirm the presence of this compound in the surrounding environment and the impact of external factors and potential contaminants on the metabolite profile of marine organisms. Due to the endemic nature of P. sykion and the absence of research on this species, comparative references are currently unavailable. Therefore, it is crucial that future research focuses on investigating the structures of the detected compounds and ascertain whether they are endogenously produced by the sea cucumber or exogenously acquired through dietary consumption or microbial activity. This exploration is essential to fully understand the metabolic dynamics and potential bioactive properties of P. sykion.
Metabolites play crucial roles in mediating the organism’s response to environmental stressors, facilitating adaptation to temperature changes and exposure to pollutants (Xu et al., 2017; Pangestuti and Arifin, 2018; Xu et al., 2018; David and Rostkowski, 2020). The findings of this study highlight the metabolic potential exhibited by P. sykion, particularly in compounds crucial for protein synthesis, tissue growth and regeneration, osmoregulation, and energy utilization. While specific research on the metabolites of lesser-known sea cucumbers remain limited, their known roles in other organisms suggest their importance in the metabolic activities and survival of these marine organisms. Overall, the metabolic diversity and complexity of sea cucumbers underscore the need for further research to fully understand the metabolic origins and functions of these compounds. Such investigations are crucial for understanding the ecological roles and nutritional potential of sea cucumbers and to provide essential information to aid in conservation and resource management efforts aimed at preserving these marine organisms and their habitats, and to assist in improving aquaculture integration of alternative species to encourage sustainable resource use.
Additionally, these findings underscore the significance of employing untargeted 1H-NMR and UPLC-QTOF-HR-MS as robust tools for exploring the metabolic diversity and complexity of sea cucumbers. This approach not only allows for the comprehensive exploration of the metabolome, but also serves to monitor an organisms’ health status and environmental conditions (Xu et al., 2016; Sehlakgwe et al., 2020). Moreover, these results shed light on the biological potential of endemic sea cucumber species, highlighting the need to broaden research endeavors aimed at unravelling the metabolic compositions, functional roles, and potential bioactive properties of lesser-studied sea cucumber species. In contrast to targeted metabolomics approaches, untargeted analysis offers an unbiased view of the metabolome, enabling the identification of a broad range of metabolites, including those that may be previously unknown or unexpected (Nalbantoglu, 2019; David and Rostkowski, 2020; Nkobole and Prinsloo, 2021), such as the presence of glycerol and dimethyl sulfone in this study.
However, despite the advantages afforded by untargeted metabolomics, several limitations were encountered in this study that need to be considered for future research endeavors. To enhance the robustness and reproducibility of findings, future research should prioritize increasing the sample size. Additionally, careful consideration should be given to the selection of extraction solvents and analysis methods because the vast variability in chemical structures, polarities, and chemical properties of metabolites may impact the accuracy and comprehensiveness of the metabolic profile obtained (Wang et al., 2010; Nalbantoglu, 2019; David and Rostkowski, 2020; Hossain et al., 2022). Furthermore, the identification of metabolites in this study relied on comparisons with existing literature and databases, which may not be comprehensive or entirely accurate for sea cucumber metabolites. This limitation is especially significant given the uncertainties surrounding compound confirmations in the literature and the high likelihood of isomers–compounds sharing the same empirical formula but differing in structural configurations–within the results. Consequently, certain metabolites remain unidentified due to the absence of reference spectra and the inherent complexity of untargeted NMR spectra, which often display overlapping peaks. The use of targeted analysis techniques can be used to target specific compounds and obtain mass spectral fragmentation data for each compound, allowing the use of software tools to attempt to match mass spectra with proposed structure. Moving forward, future research endeavors should aim to isolate and purify novel/unidentified compounds to conduct targeted investigations to refine the identification metabolites, elucidate their metabolic pathways and functional roles within P. sykion, and assess their potential utility for isolation, commercial exploitation, nutritional supplementation, and pharmaceutical applications. Moreover, endeavors to ascertain the nutritional potential of lesser-studied species could be used to validate the feasibility of sustainable cultivation of this species.
Conclusion
In conclusion, this study underscores the significance of untargeted 1H-NMR and UPLC-QTOF-HR-MS as powerful tools to provide insights into the metabolic diversity and complexity of sea cucumbers. The findings shed light on the metabolic potential of P. sykion and emphasize the need for further research to elucidate the metabolic compositions, functional roles, and potential bioactive properties of under-investigated, endemic sea cucumber species. However, while untargeted metabolomics provides an overview of the metabolome, limitations exist in metabolite identification due to the complexity of metabolic compounds, the efficacy of the selected solvent in metabolite extraction, and the lack of comprehensive holothurian metabolite databases, highlighting areas for improvement in future research. By addressing these limitations and incorporating a wider range of extraction solvents and analysis techniques, we can deepen our understanding of the sea cucumber metabolome and their potential for commercial, nutritional, and pharmaceutical purposes. Ultimately, this research paves the way for further exploration into the metabolic dynamics of sea cucumbers to comprehend their intricate biochemical mechanisms, ecological roles, and physiological adaptations. This information will aid in improving conservation and sustainable cultivation practices by exploring sea cucumbers as valuable marine resources with diverse applications in industry and medicine.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because Lower-order marine invertebrates, such as sea cucumbers, are not currently subject to ethical clearance requirements according to the University of KwaZulu-Natal Animal Research Ethics Committee (AREC) guidelines. However, at the time of study, sea cucumbers were on the University of KwaZulu-Natal marine collection permit.
Author contributions
CU: Data curation, Methodology, Investigation, Conceptualization, Writing – original draft, Formal analysis, Writing – review & editing. GP: Methodology, Data curation, Writing – review & editing, Investigation, Resources, Formal analysis. PS: Formal analysis, Writing – review & editing, Investigation, Data curation, Resources, Methodology. MO: Project administration, Conceptualization, Writing – review & editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to thank the Council for Scientific and Industrial Research (CSIR) in Pretoria for granting access to the 1H-NMR and UPLC-QTOF-HR-MS facilities used in this study. We also extend our gratitude to the reviewers for their time and thoughtful consideration in evaluating this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1609951/full#supplementary-material
References
Ahmed Q., Mohammad Ali Q., Bat L., ÖZtekİN A., Ghory F., Shaikh I., et al. (2023). Gut content analysis in Holothuria leucospilota and Holothuria cinerascens (Echinodermata: Holothuroidea: Holothuriidae) from Karachi coast. J. Materials Environ. Sci. 14, 31–40. Available online at: https://www.jmaterenvironsci.com/Document/vol14/vol14_N1/JMES-2023-14003-Ahmed.pdf (Accessed December 6, 2024).
Akhtar M. T., Samar M., Shami A. A., Mumtaz M. W., Mukhtar H., Tahir A., et al. (2021). 1H-NMR-based metabolomics: an integrated approach for the detection of the adulteration in chicken, chevon, beef and donkey meat. Molecules 26, 4643. doi: 10.3390/molecules26154643
Ayyar K. and Mistry U. (2020). Sea Cucumbers: Countering the Illegal Trade (Mhali Punjab, India: RoundGlass Sustain). Available online at: https://sustain.round.glass/conservation/sea-cucumbers-illegal-trade/.
Bahrami Y., Zhang W., Chataway T., and Franco C. (2014a). Structural elucidation of novel saponins in the sea cucumber holothuria lessoni. Mar. Drugs 12, 4439–4473. doi: 10.3390/md12084439
Bahrami Y., Zhang W., and Franco C. (2014b). Discovery of novel saponins from the viscera of the sea cucumber Holothuria lessoni. Mar. Drugs 12, 2633–2667. doi: 10.3390/md12052633
Bahrami Y., Zhang W., and Franco C. M. M. (2018). Distribution of saponins in the sea cucumber holothuria lessoni; the body wall versus the viscera, and their biological activities. Mar. Drugs 16, 423. doi: 10.3390/md16110423
Bhinderwala F., Roth H. E., Filipi M., Jack S., and Powers R. (2024). Potential metabolite biomarkers of multiple sclerosis from multiple biofluids. ACS Chem. Neurosci. 15, 1110–1124. doi: 10.1021/acschemneuro.3c00678
Biandolino F., Parlapiano I., Denti G., Fanelli G., and Prato E. (2019). Can different body tissues of two sea cucumbers supply a fair amount of omega 3 for health benefit? J. Aquat. Food Product Technology. 28, 821–836. doi: 10.1080/10498850.2019.1652217
Bordbar S., Anwar F., and Saari N. (2011). High-value components and bioactives from sea cucumbers for functional foods–a review. Mar. Drugs 9, 1761–1805. doi: 10.3390/md9101761
Carvalhal F., Cristelo R. R., Resende D. I. S. P., Pinto M. M. M., Sousa E., and Correia-da-Silva M. (2019). Antithrombotics from the sea: polysaccharides and beyond. Mar. Drugs 17, 170. doi: 10.3390/md17030170
Castillo J. A. (2006). Predator defense mechanisms in shallow water sea cucumbers (Holothuroidea). UC Berkeley: UCB Moorea Class: Biol. Geomorphology Trop. Islands. Student Research Papers, Fall 2006. Available online at: https://escholarship.org/uc/item/355702bs
Cheng C., Wu F., Ren C., Jiang X., Zhang X., Li X., et al. (2021). Aquaculture of the tropical sea cucumber, Stichopus monotuberculatus: Induced spawning, detailed records of gonadal and embryonic development, and improvements in larval breeding by digestive enzyme supply in diet. Aquaculture 540, 736690. doi: 10.1016/j.aquaculture.2021.736690
Clish C. B. (2015). Metabolomics: an emerging but powerful tool for precision medicine. Cold Spring Harbor Mol. Case Stud. 1, a000588. doi: 10.1101/mcs.a000588
David A. and Rostkowski P. (2020). Chapter 2 - Analytical techniques in metabolomics. Environ. Metabolomics., 35–64. doi: 10.1016/B978-0-12-818196-6.00002-9
Dolmatov I. Y. (2014). Asexual reproduction in holothurians. Sci. World J. 2014, 527234. doi: 10.1155/2014/527234
Domínguez-Godino J. A., Slater M. J., Hannon C., and González-Wangüermert M. (2015). A new species for sea cucumber ranching and aquaculture: Breeding and rearing of Holothuria arguinensis. Aquaculture. 438, 122–128. doi: 10.1016/j.aquaculture.2015.01.004
Dupont S., Ortega-Martínez O., and Thorndyke M. (2010). Impact of near-future ocean acidification on echinoderms. Ecotoxicology. 19, 449–462. doi: 10.1007/s10646-010-0463-6
Elufioye T. O. and Habtemariam S. (2019). Hepatoprotective effects of rosmarinic acid: Insight into its mechanisms of action. Biomedicine Pharmacotherapy 112, 108600. doi: 10.1016/j.biopha.2019.108600
Fasakhodi M. T., Abed-Elmdoust A., Farhangi M., Hosseini S. V., and Fasakhodi M. T. (2021). 1H NMR spectroscopy for identification of metabolic profile fluctuations in the extract, powder and pellet produced from sea cucumber (Holothuria leucospilota). Aquaculture Res. 52, 1715–1723. doi: 10.1111/are.15027
Foster G. G. and Hodgson A. N. (1995). Annual reproductive cycles of three sympatric species of intertidal holothurians (Echinodermata) from the coast of the Eastern Cape Province of South Africa. Invertebrate Reprod. Dev. 27, 49–59. doi: 10.1080/07924259.1995.9672433
Gajdosechova Z., Palmer C. H., Dave D., Jiao G., Zhao Y., Tan Z., et al. (2020). Arsenic speciation in sea cucumbers: Identification and quantitation of water-extractable species. Environ. Pollution. 266, 115190. doi: 10.1016/j.envpol.2020.115190
Grauso L., Yegdaneh A., Sharifi M., Mangoni A., Zolfaghari B., and Lanzotti V. (2019). Molecular networking-based analysis of cytotoxic saponins from sea cucumber holothuria atra. Mar. Drugs 17, 86. doi: 10.3390/md17020086
Hamel J.-F. and Mercier A. (1996). Evidence of chemical communication during the gametogenesis of holothuroids. Ecology 77, 1600–1616. doi: 10.2307/2265555
Honey-Escandón M., Arreguín-Espinosa R., Solís-Marín F. A., and Samyn Y. (2015). Biological and taxonomic perspective of triterpenoid glycosides of sea cucumbers of the family Holothuriidae (Echinodermata, Holothuroidea). Comp. Biochem. Physiol. Part B: Biochem. Mol. Biol. 180, 16–39. doi: 10.1016/j.cbpb.2014.09.007
Hossain A., Dave D., and Shahidi F. (2022). Antioxidant potential of sea cucumbers and their beneficial effects on human health. Mar. Drugs 20, 521. doi: 10.3390/md20080521
Imbs A. B., Ermolenko E. V., Grigorchuk V. P., Sikorskaya T. V., and Velansky P. V. (2021). Current progress in lipidomics of marine invertebrates. Mar. Drugs 19, 660. doi: 10.3390/md19120660
Iwalaye O. A., Moodley G. K., and Robertson-Andersson D. V. (2020). The possible routes of microplastics uptake in sea cucumber Holothuria cinerascens (Brandt 1835). Environ. Pollution. 264, 114644. doi: 10.1016/j.envpol.2020.114644
Janakiram N. B., Mohammed A., and Rao C. V. (2015). Sea cucumbers metabolites as potent anti-cancer agents. Mar. Drugs 13, 2909–2923. doi: 10.3390/md13052909
Jiang J., Zhao Z., Gao S., Chen Z., Dong Y., He P., et al. (2021). Divergent metabolic responses to sex and reproduction in the sea cucumber Apostichopus japonicus. Comp. Biochem. Physiol. Part D: Genomics Proteomics 39, 100845. doi: 10.1016/j.cbd.2021.100845
Jönander C., Backhaus T., and Dahllöf I. (2022). Single substance and mixture toxicity of dibutyl-phthalate and sodium dodecyl sulphate to marine zooplankton. Ecotoxicology Environ. Saf. 234, 113406. doi: 10.1016/j.ecoenv.2022.113406
Kalinin V. I., Avilov S. A., Silchenko A. S., and Stonik V. A. (2015). Triterpene glycosides of sea cucumbers (Holothuroidea, echinodermata) as taxonomic markers. Natural Product Commun. 10:21–26. doi: 10.1177/1934578X1501000108
Kalinin V. I., Silchenko A. S., Avilov S. A., and Stonik V. A. (2021). Progress in the studies of triterpene glycosides from sea cucumbers (Holothuroidea, echinodermata) between 2017 and 2021. Natural Product Commun. 16, 1934578X2110539. doi: 10.1177/1934578X211053934
Kalinin V. I., Silchenko A. S., Avilov S. A., Stonik V. A., and Smirnov A. V. (2005). Sea cucumbers triterpene glycosides, the recent progress in structural elucidation and chemotaxonomy. Phytochem. Rev. 4, 221–236. doi: 10.1007/s11101-005-1354-y
Kamatou G. P. P., Viljoen A. M., and Steenkamp P. (2010). Antioxidant, antiinflammatory activities and HPLC analysis of South African Salvia species. Food Chem. 119, 684–688. doi: 10.1016/j.foodchem.2009.07.010
Kamyab E., Kellermann M. Y., Kunzmann A., and Schupp P. J. (2020). “Chemical biodiversity and bioactivities of saponins in echinodermata with an emphasis on sea cucumbers (Holothuroidea),” in YOUMARES 9 - The Oceans: Our Research, Our Future Proceedings of the 2018 conference for YOUng MArine RESearcher in Oldenburg (Germany: Springer Nature), 121–157. doi: 10.1007/978-3-030-20389-4_7
Kellner Filho L. C., Picão B. W., Silva M. L. A., Cunha W. R., Pauletti P. M., Dias G. M., et al. (2019). Bioactive aliphatic sulfates from marine invertebrates. Mar. Drugs 17, 527. doi: 10.3390/md17090527
Khotimchenko Y. (2018). Pharmacological potential of sea cucumbers. Int. J. Mol. Sci. 19, 1342. doi: 10.3390/ijms19051342
Kinne R. K. H. (1993). The role of organic osmolytes in osmoregulation: From bacteria to mammals. J. Exp. Zoology 265, 346–355. doi: 10.1002/jez.1402650403
Lemes A. C., Egea M. B., Oliveira Filho J. G., Gautério G. V., Ribeiro B. D., and Coelho M. A. Z. (2022). Biological approaches for extraction of bioactive compounds from agro-industrial by-products: A review. Front. Bioengineering Biotechnol. 9. doi: 10.3389/fbioe.2021.802543
Li Y.-X., Himaya S. W. A., and Kim S.-K. (2013). Triterpenoids of marine origin as anti-cancer agents. Molecules 18, 7886–7909. doi: 10.3390/molecules18077886
Madduppa H., Taurusman A. A., Subhan B., Anggraini N. P., Fadillah R., and Tarman K. (2017). DNA barcoding reveals vulnerable and not evaluated species of sea cucumbers (Holothuroidea and Stichopodidae) from Kepulauan Seribu reefs, Indonesia. Biodiversitas 18, 893–898. doi: 10.13057/biodiv/d180305
Martinez M., Alba-Posse E., Lauretta D., and Penchaszadeh P. (2020). Reproductive features in the sea cucumber Pentactella perrieri (Ekman 1927) (Holothuroidea: Cucumariidae): a brooding hermaphrodite species from the southwestern Atlantic Ocean. Polar Biol. 43, 1383–1389. doi: 10.1007/s00300-020-02715-1
Martinez M. I., Martínez-Salinas A. P., and Moura R. B. (2024). “Chapter 14 - Knowledge of biodiversity and reproduction in sea cucumbers from southern South America to the Antarctic Peninsula,” in The World of Sea Cucumbers. Eds. Mercier A., Hamel J.-F., Suhrbier A. D., and Pearce C. M. (Academic Press, Elsevier Inc.). doi: 10.1016/B978-0-323-95377-1.00006-0
McParland E. L., Alexander H., and Johnson W. M. (2021). The osmolyte ties that bind: genomic insights into synthesis and breakdown of organic osmolytes in marine microbes. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.689306
Mdlalose S. P., Raletsena M., Ntushelo K., Bodede O., and Modise D. M. (2022). 1H-NMR-based metabolomic study of potato cultivars, markies and fianna, exposed to different water regimes. Front. Sustain. Food Syst. 6. doi: 10.3389/fsufs.2022.801504
More G. K., Meddows-Taylor S., and Prinsloo G. (2021). Metabolomic profiling of antioxidant compounds in five vachellia species. Molecules 26, 6214. doi: 10.3390/molecules26206214
More G. K., Vervoort J., Steenkamp P. A., and Prinsloo G. (2022). Metabolomic profile of medicinal plants with anti-RVFV activity. Heliyon 8, e08936. doi: 10.1016/j.heliyon.2022.e08936
Nadeem M., Imran M., Aslam Gondal T., Imran A., Shahbaz M., Muhammad Amir R., et al. (2019). Therapeutic potential of rosmarinic acid: A comprehensive review. Appl. Sci. 9, 3139. doi: 10.3390/app9153139
Nalbantoglu S. (2019). Metabolomics: basic principles and strategies. Mol. Med. doi: 10.5772/intechopen.88563
Nkobole N. and Prinsloo G. (2021). 1H-NMR and LC-MS based metabolomics analysis of wild and cultivated amaranthus spp. Molecules 26, 795. doi: 10.3390/molecules26040795
Oh J., Yoon D. H., Han J. G., Choi H. K., and Sung G. H. (2018). (1)H NMR based metabolite profiling for optimizing the ethanol extraction of Wolfiporia cocos. Saudi J. Biol. Sci. 25, 1128–1134. doi: 10.1016/j.sjbs.2018.04.007
Pangestuti R. and Arifin Z. (2018). Medicinal and health benefit effects of functional sea cucumbers. J. Traditional Complementary Med. 8, 341–351. doi: 10.1016/j.jtcme.2017.06.007
Peters-Didier J., Pardo L. M., Garrido O., and Gallardo C. S. (2018). Reproductive biology of the commercial sea cucumber Athyonidium Chilensis (Holothuroidea: Dendrochirotida) in southern Chile. J. Mar. Biol. Assoc. United Kingdom 98, 311–323. doi: 10.1017/S0025315416001193
Purcell S. W. (2014). Value, market preferences and trade of beche-de-mer from pacific island sea cucumbers. PloS One 9, e95075. doi: 10.1371/journal.pone.0095075
Puspitasari Y. E., De Bruyne T., Foubert K., Aulanni’Am A. A., Pieters L., Hermans N., et al. (2021). Holothuria triterpene glycosides: a comprehensive guide for their structure elucidation and critical appraisal of reported compounds. Phytochem. Rev. 21, 1315–1358. doi: 10.1007/s11101-021-09783-z
Puspitasari Y. E., Tuenter E., Foubert K., Herawati H., Hariati A. M., Aulanni’Am A. A., et al. (2023). Saponin and fatty acid profiling of the sea cucumber holothuria atra, α-glucosidase inhibitory activity and the identification of a novel triterpene glycoside. Nutrients 15, 1033. doi: 10.3390/nu15041033
Raletsena M. V., Mdlalose S., Bodede O. S., Assress H. A., Woldesemayat A. A., and Modise D. M. (2022). 1H-NMR and LC-MS based metabolomics analysis of potato (Solanum tuberosum L.) cultivars irrigated with fly ash treated acid mine drainage. Molecules 27, 1187. doi: 10.3390/molecules27041187
Ru X., Zhang L., Liu S., and Yang H. (2017). Reproduction affects locomotor behaviour and muscle physiology in the sea cucumber. Apostichopus japonicus. Anim. Behaviour. 133, 223–228. doi: 10.1016/j.anbehav.2017.09.024
Salindeho N., Nurkolis F., Gunawan W. B., Handoko M. N., Samtiya M., and Muliadi R. D. (2022). Anticancer and anticholesterol attributes of sea cucumbers: An opinion in terms of functional food applications. Front. Nutr. 9. doi: 10.3389/fnut.2022.986986
Sehlakgwe P. F., Lall N., and Prinsloo G. (2020). 1H-NMR metabolomics and LC-MS analysis to determine seasonal variation in a cosmeceutical plant leucosidea sericea. Front. Pharmacol. 11. doi: 10.3389/fphar.2020.00219
Senni K., Pereira J., Gueniche F., Delbarre-Ladrat C., Sinquin C., Ratiskol J., et al. (2011). Marine polysaccharides: a source of bioactive molecules for cell therapy and tissue engineering. Mar. Drugs 9, 1664–1681. doi: 10.3390/md9091664
Shekarchi M., Hajimehdipoor H., Saeidnia S., Gohari A. R., and Hamedani M. P. (2012). Comparative study of rosmarinic acid content in some plants of Labiatae family. Pharmacogn Mag 8, 37–41. doi: 10.4103/0973-1296.93316
Sicuro B., Piccinno M., Gai F., Abete M. C., Danieli A., Dapra F., et al. (2012). Food quality and safety of Mediterranean Sea cucumbers Holothuria tubulosa and Holothuria polii in southern Adriatic Sea. Asian J. Anim. Veterinary Advances. 7, 851–859. doi: 10.3923/ajava.2012.851.859
Smith Y. (2020). What is Metabolomics? (Manchester, United Kingdom: AZoLifeSciences). Available online at: https://www.azolifesciences.com/article/What-is-Metabolomics.aspx.
Sun G.-Q., Li L., Yi Y.-H., Yuan W.-H., Liu B.-S., Weng Y.-Y., et al. (2008). Two new cytotoxic nonsulfated pentasaccharide holostane (=20-hydroxylanostan-18-oic acid γ-lactone) glycosides from the sea cucumber holothuria grisea. Helv. Chimica Acta 91, 1453–1460. doi: 10.1002/hlca.200890158
Sundekilde U. K., Larsen L. B., and Bertram H. C. (2013). NMR-based milk metabolomics. Metabolites 3, 204–222. doi: 10.3390/metabo3020204
Tebani A. and Bekri S. (2019). Paving the way to precision nutrition through metabolomics. Front. Nutr. 6. doi: 10.3389/fnut.2019.00041
Telahigue K., Ghali R., Nouiri E., Labidi A., and Hajji T. (2020). Antibacterial activities and bioactive compounds of the ethyl acetate extract of the sea cucumber Holothuria forskali from Tunisian coasts. J. Mar. Biol. Assoc. United Kingdom 100, 229–237. doi: 10.1017/S0025315420000016
Thandar A. (1987). The status of some southern African nominal species of Cucumaria (s.e.) referable to a new genus and their ecological isolation. South Afr. J. Zoology. 22, 287–296. doi: 10.1080/02541858.1987.11448059
Thandar A. S. (2015). Biodiversity and distribution of the southern African sea cucumbers (Echinodermata: Holothuroidea). Zootaxa. 4058, 341–361. doi: 10.11646/zootaxa.4058.3.3
Tikunov A. P., Johnson C. B., Lee H., Stoskopf M. K., and Macdonald J. M. (2010). Metabolomic investigations of American oysters using H-NMR spectroscopy. Mar. Drugs 8, 2578–2596. doi: 10.3390/md8102578
Wang J. H., Byun J., and Pennathur S. (2010). Analytical approaches to metabolomics and applications to systems biology. Semin. Nephrol. 30, 500–511. doi: 10.1016/j.semnephrol.2010.07.007
Wen J., Hu C., Zhang L., and Fan S. (2011). Genetic identification of global commercial sea cucumber species on the basis of mitochondrial DNA sequences. Food Control. 22, 72–77. doi: 10.1016/j.foodcont.2010.06.010
Wijesinghe W. A. J. P., Vairappan C. S., and Jeon Y. J. (2015). Exploitation of health promoting potentials of edible sea cucumber (Holothuria edulis): search of new bioactive components as functional ingredients. Int. Proc. Chemical Biol. Environ. Engineering. 86, 36–41. Available online at: http://www.ipcbee.com/vol86/rp008_ICNFS2015-N0013.pdf (Accessed November 13, 2024).
Wu M., Xu L., Zhao L., Xiao C., Gao N., Luo L., et al. (2015). Structural analysis and anticoagulant activities of the novel sulfated fucan possessing a regular well-defined repeating unit from sea cucumber. Mar. Drugs 13, 2063–2084. doi: 10.3390/md13042063
Xing M., Liu F., Lin J., Xu D., Zhong J., Xia F., et al. (2024). Origin tracing and adulteration identification of bird’s nest by high- and low-field NMR combined with pattern recognition. Food Res. Int. 175, 113780. doi: 10.1016/j.foodres.2023.113780
Xu C., Zhang R., and Wen Z. (2018). Bioactive compounds and biological functions of sea cucumbers as potential functional foods. J. Funct. Foods 49, 4973–4984. doi: 10.1016/j.jff.2018.08.009
Xu D., Zhou S., and Yang H. (2017). Carbohydrate and amino acids metabolic response to heat stress in the intestine of the sea cucumber Apostichopus japonicus. Aquaculture Res. 48, 5883–5891. doi: 10.1111/are.13411
Xu Q., Xu Q., Zhang X., Peng Q., and Yang H. (2016). Fatty acid component in sea cucumber Apostichopus japonicus from different tissues and habitats. J. Mar. Biol. Assoc. United Kingdom. 96, 197–204. doi: 10.1017/S002531541500168X
Yancey P. H. (2005). Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J. Exp. Biol. 208, 2819–2830. doi: 10.1242/jeb.01730
Yancey P. H., Blake W. R., and Conley J. (2002). Unusual organic osmolytes in deep-sea animals: adaptations to hydrostatic pressure and other perturbants. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 133, 667–676. doi: 10.1016/S1095-6433(02)00182-4
Yu F., Yang Q., Cui T., Luo L., Zhao M., and Long X. (2024). Preparation and isolation of hydrophilic mannosylerythritol lipids via chemical modification and stepwise extraction. J. Surfactants Detergents 27, 725–736. doi: 10.1002/jsde.12738
Zolkeflee N. K. Z., Wong P. L., Maulidiani M., Ramli N. S., Azlan A., Mediani A., et al. (2024). Revealing metabolic and biochemical variations via 1H NMR metabolomics in streptozotocin-nicotinamide-induced diabetic rats treated with metformin. Biochem. Biophys. Res. Commun. 708, 149778. doi: 10.1016/j.bbrc.2024.149778
Keywords: Pseudocnella sykion, endemic, dendrochirotida, metabolomics, 1H-NMR, UPLC-QTOF-HR-MS
Citation: Upton C, Prinsloo G, Steenkamp PA and Okpeku M (2025) Untargeted metabolomic profiling of Pseudocnella sykion from KwaZulu-Natal, South Africa using 1H-NMR and UPLC-QTOF-HR-MS. Front. Mar. Sci. 12:1609951. doi: 10.3389/fmars.2025.1609951
Received: 11 April 2025; Accepted: 05 August 2025;
Published: 01 September 2025.
Edited by:
Satheesh Sathianeson, King Abdulaziz University, Saudi ArabiaReviewed by:
Khaled Mohammed Geba, Menoufia University, EgyptGuohua Sun, Ludong University, China
Radwa Hassan, National Research Centre, Egypt
Copyright © 2025 Upton, Prinsloo, Steenkamp and Okpeku. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Moses Okpeku, T2twZWt1TUB1a3puLmFjLnph
†ORCID: Moses Okpeku, orcid.org/0000-0002-8337-6294
 Cassandra Upton
Cassandra Upton Gerhard Prinsloo
Gerhard Prinsloo Paul Anton Steenkamp
Paul Anton Steenkamp Moses Okpeku
Moses Okpeku