- 1Ministry of Education Key Laboratory for Ecology of Tropical Islands, Key Laboratory of Tropical Animal and Plant Ecology of Hainan Province, College of Life Sciences, Hainan Normal University, Haikou, Hainan, China
- 2Department of Environmental Health Sciences, Soonchunhyang University, Asan, Republic of Korea
- 3Department of Marine Science and Convergence Engineering, College of Science and Convergence Technology, Hanyang University, Ansan, Republic of Korea
- 4Maritime Safety and Environmental Research Division, Korea Research Institute of Ships and Ocean Engineering, Daejeon, Republic of Korea
Cyclic and linear siloxanes are widely used in personal care, industrial, and consumer products. To better understand their fate and ecological risks in estuarine environments, this study investigated spatial distribution, seasonal variations, and the bioaccumulation potential of siloxanes in surface water, sediment, and in benthic organisms in an industrialized bay of Korea. Cyclic siloxanes exhibited higher concentrations than linear siloxanes across multiple environmental matrices, indicating their predominance and persistence. Water and sediment had distinct spatial distributions of siloxanes, which were influenced by local sources and hydrodynamic conditions. Industrial activities and power plant effluents were identified as major contributors to siloxane contamination in the coastal environment. Overall, the concentration of siloxanes in surface water was highest in winter. In particular, cyclic siloxanes showed a greater sensitivity to seasonal variation than linear siloxanes, with concentrations fluctuating significantly across sampling periods. The spatial distribution of siloxanes in sediment was strongly associated with organic carbon. Benthic invertebrates exhibited a strong potential for bioaccumulation of D5 and L9, with the highest bioaccumulation factors and biota-sediment accumulation factors among the detected compounds. These findings highlight the need for continued monitoring and management of siloxane contamination in industrialized estuarine environments.
1 Introduction
Siloxanes, including both cyclic (Dn, where n represents the number of silicon atoms) and linear (Ln) forms, are used in a broad range of products, such as cosmetics and personal care products (CPCPs), cooking utensils, pharmaceuticals, and as intermediates in the production of silicone polymers (Horii et al., 2022a; Jessup et al., 2022). The main chain of siloxane molecules is composed of repeated silicon oxygen (Si-O) bonds, where silicon atoms are not only connected to oxygen atoms to form a skeleton, but also typically bonded to organic groups such as methyl groups. This structure endows siloxanes with a range of excellent properties, including high thermal stability (derived from strong Si-O bonds), excellent lubricity, extremely low surface tension, good hydrophobicity, flexibility, physiological inertness, and a wide viscosity range (depending on molecular weight, low molecular weight is in liquid form, high molecular weight can be in rubber or resin form) (Abe and Gunji, 2004; Baney et al., 1995; Colas and Curtis, 2013; Shimizu et al., 2017). Their essential properties, such as high thermal stability, lubricity, and low surface tension, make them commercially useful and thus in strong demand (Horii et al., 2021; Liu et al., 2022). Global silicone production has been estimated to be ~8.5 million metric tons in 2021 (Garside, 2022), with the market expected to grow from USD 16.7 billion in 2021 to USD 23.4 billion by 2026 (IMARC, 2021). The large consumption of siloxanes has led to widespread contamination across multiple environmental matrices, including air, water, soil, sediment, and biological samples, on a global scale (Wang et al., 2017; Lee et al., 2018; Guo et al., 2021; Chen et al., 2022; Horii et al., 2022a; Chen et al., 2024; Kang et al., 2024).
Previous studies have reported ecotoxicological effects at concentrations below 10 μg/L for several cyclic siloxanes, such as octamethylcyclotetrasiloxane (D4) and decamethylcyclopentasiloxane (D5), in several freshwater and marine species (Sousa et al., 1995). More recent studies have confirmed that siloxanes exert toxic effects on the nervous, immune, endocrine, and reproductive systems in both animals and humans (Guo et al., 2021; Xiang et al., 2021). However, earlier ecotoxicological assessments of cyclic siloxanes–including D4, D5, and D6–suggested no significant health risks to aquatic organisms (Redman et al., 2012; Fairbrother and Woodburn, 2016; Powell et al., 2017; Zhang et al., 2018; Cantu and Gobas, 2021). Despite these findings, subsequent research has reported a significant potential for bioaccumulation and biomagnification of siloxanes in a variety of aquatic ecosystems (Jia et al., 2015; Cui et al., 2019; Xue et al., 2019; Guo et al., 2021; Wang et al., 2021; Kim et al., 2022). Additionally, high concentrations of siloxanes, ~1,000 ng/g lipid weight, have been detected in a range of aquatic species, including benthic invertebrates and fish (Borga et al., 2013; Powell et al., 2017, Powell et al., 2018; Cui et al., 2019; Chen et al., 2022).
Regulatory actions on the use of cyclic siloxanes (D4, D5, and D6) in CPCPs have been implemented by the European Commission (ECHA, 2019), the United Kingdom (Brooke et al., 2009a, Brooke et al., 2009b, Brooke et al., 2009c), Canada (Environment Canada, 2008a, Environment Canada, 2008b, Environment Canada, 2008c), the United States (USEPA, 2011), and Japan (METI-Japan, 2018). However, no specific regulations on the use of siloxanes currently exist in Korea. Although the release of polydimethylsiloxane (PDMS) that contains primary siloxanes into the marine environment is legally prohibited in Korea, PDMS is still used as an antifoaming agent in fossil fuel power plants. In 2016, government investigations revealed that several fossil fuel power plants had discharged siloxanes into the marine environment, raising both social and environmental concerns (Chen et al., 2024).
Our previous studies confirmed that industrial activities are major sources of siloxane contamination in coastal environments (Lee et al., 2018; Chen et al., 2023; Kang et al., 2024). Due to their extensive use in CPCPs and a number of industrial applications, siloxanes are continuously released into aquatic systems. Their physicochemical properties, such as hydrophobicity, volatility, and environmental persistence, raise concerns about their potential for long-term accumulation and ecological impact in marine ecosystems (Mojsiewicz-Pienkowska and Krenczkowska, 2018). Given these concerns, a comprehensive understanding of the distribution of siloxanes across different environmental matrices is essential for evaluating their environmental fate and ecological risks. In the present study, the occurrence and bioaccumulation potential of cyclic and linear siloxanes were investigated in multiple environmental matrices present in coastal waters influenced by large-scale industrial complexes and fossil fuel power plants in Korea. The seasonal variability in the concentration of siloxanes in surface water was also examined to gain insight into the environmental dynamics and potential influencing factors in the aquatic environment.
2 Materials and methods
2.1 Sampling
Ulsan Bay and its connected river and stream systems are among the most heavily industrialized coastal zones and host the largest commercial harbors in Korea (Kim et al., 2020). A total of 100 surface water and 25 surface sediment samples were collected from 25 locations that were categorized into streams, rivers, and the bay. Additionally, 80 surface water samples were collected from the outfalls of three power plants between 2017 and 2018 (Figure 1). Surface water sampling was conducted four times–in June, September, and December 2017, and January 2018–while sediment samples were collected in June 2017. Water and sediment were collected using stainless steel baskets and Van Veen grab samplers, respectively. In June 2017, three benthic species, mussels (Mytilus coruscus), conches (Strombus gigas), and sea cucumber (Holothuroidea), were collected near the power plant outfalls. Benthic invertebrates were collected and analyzed due to their sedentary nature and close association with sediments, particularly under strong tidal current conditions. Given the high hydrophobicity of PDMS and its strong affinity for sediment particles, these organisms serve as suitable bioindicators for assessing PDMS bioaccumulation. Their presence near the power plant’s drainage outlet provides an effective means of evaluating PDMS exposure levels in areas directly influenced by point source contamination. The soft tissues of each species were pooled and homogenized for analysis. All samples were stored at –20°C until further processing. In addition, PDMS (n = 5), which was used as an anti-foaming agent in the fossil fuel power plants, was collected for siloxane analysis.
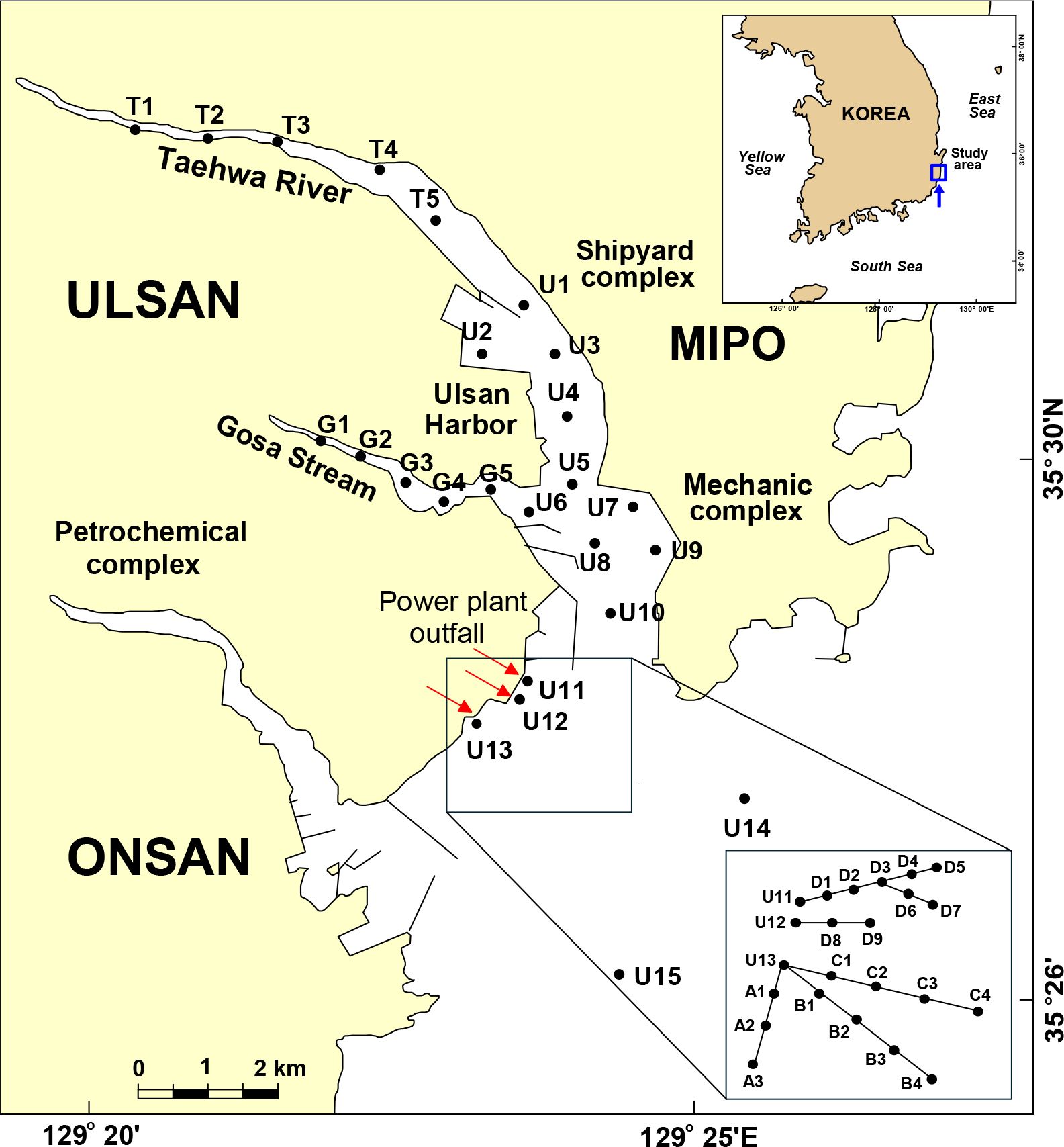
Figure 1. Sampling locations of surface water and sediment were collected from Ulsan Bay and its adjacent regions of Korea.
2.2 Sample pretreatment and instrumental analysis
The experimental standards and reagents used in this study were consistent with those described in previous studies (Lee et al., 2018; Chen et al., 2022). Water samples (~2L) were extracted using liquid-liquid extraction following the addition of mass-labeled internal standards (13C12-D4, D5, and D6; 100ng each; Moravek Biochemicals and Radiochemicals, Brea, CA, USA). Sequential extractions were performed using hexane (ultra-trace residue analysis grade; J.T. Baker, Phillipsburg, NJ, USA), a mixture of hexane and dichloromethane (ultra-trace residue analysis grade; J.T. Baker), and a mixture of hexane and ethyl acetate (HPLC grade; Sigma-Aldrich, St. Louis, MO, USA). All extracts were combined and concentrated to less than 1mL under a nitrogen stream. Freeze-dried sediment (~5g) or biological samples (~0.5g) were placed in polypropylene (PP) tubes and spiked with the same internal standards (100ng each). The samples were extracted by shaking on an orbital shaker at 250rpm for 60min. For sediment, the same solvents and extraction method used for the water samples were applied. Biological samples were extracted three times with hexane. After each extraction, the mixtures were centrifuged at 3000rpm for 5min, and the supernatants were transferred to clean PP tubes. Sulfur in sediment extracts was removed using activated copper pre-washed with hydrochloric acid (Sigma-Aldrich). For biological extracts, 10% of each was used to determine lipid content. A Turbovap evaporator (Classic LV; Vimpelgatan, Uppsala, Sweden) was used to concentrate each extract to 1mL of hexane for instrumental analysis.
A total of four cyclic siloxanes (D4–D7) and 15 linear siloxanes (L3–L17) were analyzed using a gas chromatograph coupled with a tandem mass spectrometer (GC-MS/MS; Agilent 7890/7000C, Wilmington, TX, USA) equipped with a DB-5MS capillary column (30m length, 0.25mm inner diameter, 0.25µm film thickness; J&W Scientific, Palo Alto, CA, USA). Analyses were performed in electron impact ionization mode, and siloxanes were identified and quantified using the multiple reaction monitoring method. Instrumental conditions followed those reported by Lee et al. (2018). Briefly, Oven temperature was programmed to increase from 40°C (2 min) to 220°C (20°C/min) and to 280°C (5°C/min). This latter temperature was held for 10 min followed by 5 min at 300°C. The MS was operated in electron impact ionization mode at 70 eV. D4, D5, and D6 were quantified using the internal standard method, while D7 and linear siloxanes (L3–L17) were quantified using an external standard method.
Water temperature and salinity were measured in real time using a CTD instrument (SBE 19 plus V2, Sea-Bird Electronics, Bellevue, WA, USA). Total organic carbon (TOC) content in sediment samples was analyzed using a CHN analyzer (FLASH 2000 Series, Thermo Scientific, Boston, MA, USA).
2.3 Quality control
To minimize background contamination, the use of CPCPs was strictly avoided during all experimental procedures, following protocols established in previous studies (Lee et al., 2018; Choi et al., 2020). The overall linearity (R2) of the calibration curves for siloxane standards in GC-MS instrument was 0.9991, with a range of 0.9919–0.9998. Limits of quantification (LOQs), calculated based on a signal-to-noise ratio of 10, ranged from 0.07ng/g dry weight (dw) (L6) to 0.97ng/g dw (L17). For water samples, the recoveries of the internal standards 13C12-D4, 13C12-D5, and 13C12-D6 were 62%±28%, 71%±21%, and 76%±22% (mean ± standard deviation), respectively. In sediment samples, the recoveries were 72%±18%, 82%±15%, and 83%±12%, respectively. For biological samples, recoveries of 13C12-D4, 13C12-D5, and 13C12-D6 were 71%±3.4%, 74%±4.2%, and 79%±4.7%, respectively. Matrix-spiked sample test was performed on water, sediment, and biological samples to assess the matrix effect. The overall recoveries of matrix-spike samples were in the ranges of 69%–118% (mean: 88%) for cyclic siloxanes and 65%–107% (mean: 86%) for linear siloxanes. To ensure instrumental stability and accuracy throughout the analysis, test standards were analyzed after every 10 sample injections. Detailed quality control results are provided in Supplementary Table S1.
2.4 Statistical analysis
Concentrations of individual siloxanes below the LOQs were substituted with zero for the calculation of mean and total concentrations. Spearman correlation analysis was conducted to assess the relationships between the concentration of siloxanes and other parameters. One-way ANOVA was performed to identify significant differences in the concentrations of siloxanes across operational groups (e.g., seasonal variability and spatial distribution). All statistical analyses were conducted using SPSS® version 23.0 (Armonk, NY, USA), with a significance level set at p < 0.05.
3 Results and discussion
3.1 Occurrence and concentrations of siloxanes
The concentrations of cyclic and linear siloxanes in water, sediment, and biological samples collected from Ulsan Bay and its adjacent regions of Korea are summarized in Table 1. With the exception of L4, all siloxanes were detected in at least one matrix, indicating widespread contamination in the coastal environment. Specifically, D5–D7 and L7–L15 in water, D4–D7 and L5–L17 in sediment, and D4–D7, L6, and L8–L12 in biological samples were detected in more than 50% of the total samples. In contrast, D4, L3–L6, and L16–L17 in water, L3–L4 in sediment, and L3–L5, L7, and L13–L17 in biological samples were detected less frequently (< 30% of total samples). The frequent detection of specific siloxanes for multiple environmental matrices suggests their environmental persistence, consistent with previous studies (Lee et al., 2018; Lee et al., 2019; Horii et al., 2022a; Chen et al., 2022). Species-specific accumulation patterns of siloxanes were observed, as evidenced by variations in the concentration of siloxanes among target species. This suggests that selective bioaccumulation and species-dependent metabolic processes are crucial in the distribution of siloxanes in biological samples (Gobas et al., 2009; Chen et al., 2022).
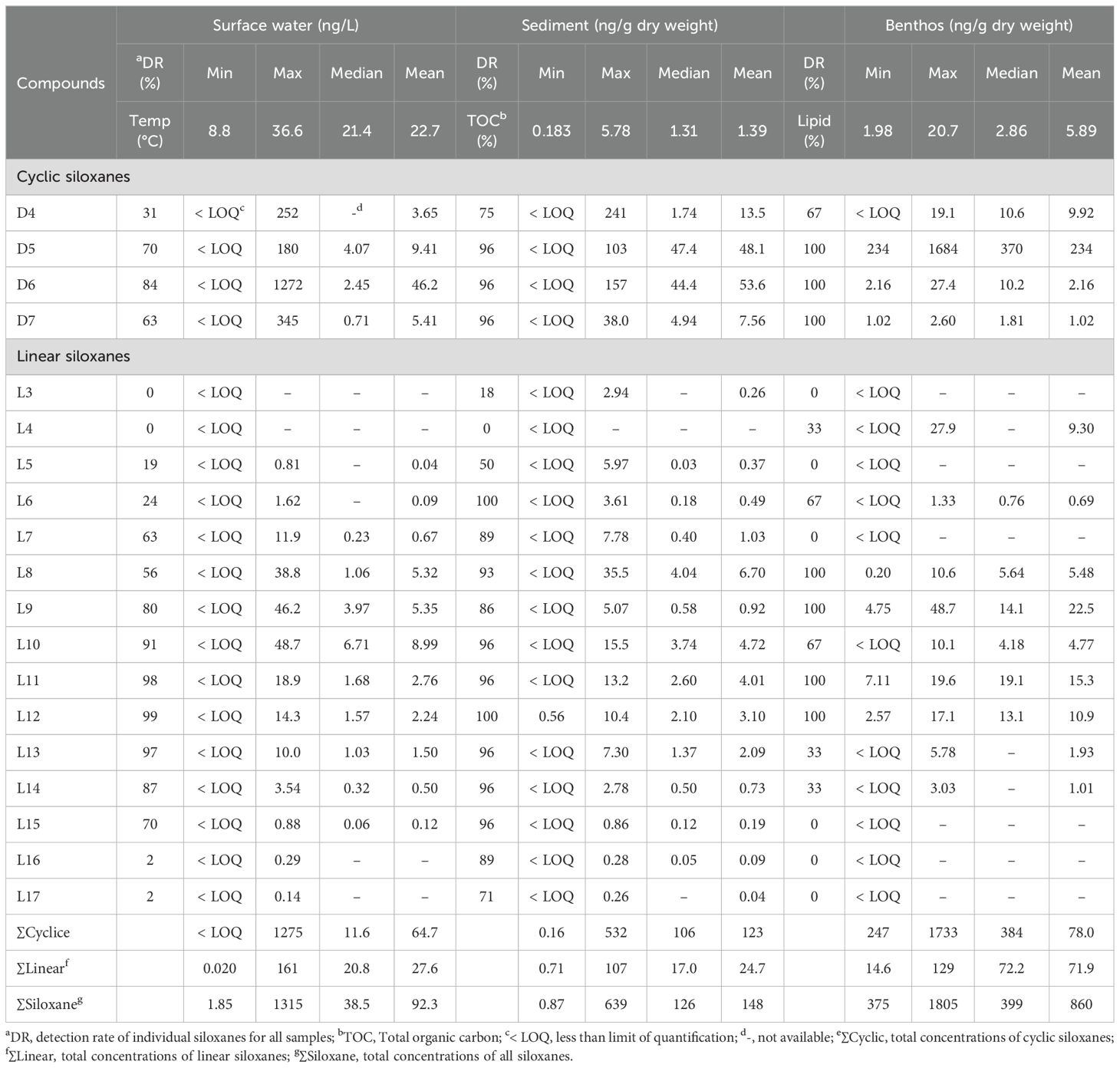
Table 1. Summary of the concentration of siloxanes in surface water, sediment, and benthic invertebrates collected from Ulsan Bay and its adjacent regions of Korea.
Siloxane concentrations varied significantly among sampling locations within three orders of magnitude. The concentration of all types of siloxanes (∑Siloxane) ranged from 1.85 to 1315ng/L (mean: 92.3ng/L) in surface water, 0.87 to 639ng/g dw (mean: 147ng/g dw) in sediment, and 375 to 1805ng/g dw (mean: 860ng/g dw) in biological samples. Significantly higher concentrations of siloxanes in biological samples compared to water and sediment indicated a strong potential for bioaccumulation (Xue et al., 2019; Zhi et al., 2019; Chen et al., 2022; Kim et al., 2020). Specifically, the concentration of total cyclic siloxanes (∑Cyclic) in the biological samples ranged from 247 to 1733ng/g dw (mean: 788ng/g dw), significantly higher (p<0.05) than those in sediment (0.16–532ng/g dw; mean: 123ng/g dw). Similarly, the total concentration of linear siloxanes (∑Linear) in biological samples ranged from 14.6 to 129ng/g dw (mean: 71.9ng/g dw), also significantly higher (p<0.05) than those in sediment (0.71–107ng/g dw; mean: 24.7ng/g dw). In the water samples, ∑Cyclic concentrations ranged from below the LOQ to 1274ng/L (mean: 64.7ng/L), while ∑Linear concentrations ranged from 0.020 to 161ng/L (mean: 27.6ng/L). Across all matrices, ∑Cyclic concentrations were consistently higher than ∑Linear, likely due to the greater use and discharge of cyclic siloxanes (Lee et al., 2019; Wang et al., 2021; Chen et al., 2022).
Among individual siloxanes, the highest concentrations in both water and sediment were observed for D6, with mean values of 46.2ng/L and 53.6ng/g dw, respectively, followed by D5 (mean: 9.41ng/L in water and 48.1ng/g dw in sediment). These results were consistent with previous findings from industrialized coastal regions, where D5 and D6 were the predominant siloxanes detected in sediments (Lee et al., 2018, Lee et al., 2019; Chen et al., 2022). In the biological samples, D5 exhibited the highest mean concentration among cyclic siloxanes (763ng/g dw), followed by D6 (13.3ng/g dw). These results suggested a strong tendency for benthic organisms to selectively bioaccumulate D5 over D6, as was described in previous studies (Jia et al., 2015; Chen et al., 2022). For linear siloxanes, the highest concentration in water was observed for L10 (mean: 8.99ng/L), followed by L9 (5.35ng/L) and L8 (5.32ng/L). In sediment and biological samples, L8 had the highest concentrations (6.70 and 22.5ng/g dw, respectively), followed by L10 (4.72 and 15.3ng/g dw) and L11 (4.01 and 10.9ng/g dw). Notably, L7–L13 exhibited higher concentrations in the industrialized bays of Korea compared to other linear siloxanes, consistent with previous reports (Lee et al., 2018; Lee et al., 2019; Chen et al., 2022).
Spearman correlation analysis was performed on siloxanes detected in water, sediment, and biota samples to further investigate the relationships among these environmental media (Supplementary Table S2). Significant correlations were observed between water and sediment samples collected in different seasons (r = 0.743–0.867, p < 0.01), suggesting the potential for siloxane exchange within the water-sediment system. Additionally, the concentration of siloxanes in biota showed significant correlations with those in water and sediment (r = 0.525–0.704, p < 0.01), indicating that organisms may bioaccumulate siloxanes from both sources. When examining individual siloxanes, only certain linear siloxanes (L10–L14) in surface water displayed significant correlations with one another (r = 0.614–0.847, p < 0.01) (Supplementary Table S3), while correlations among the remaining compounds were weak or absent. This suggests the presence of multiple sources for siloxane contamination in water. In contrast, almost all siloxanes in sediment samples exhibited significant correlations (r = 0.330–0.968, p < 0.05), implying a common source or similar environmental behavior in a sedimentary environment. Furthermore, individual siloxanes were significantly correlated with TOC content in sediments (r = 0.376–0.727, p < 0.05), indicating that TOC may play a crucial role in governing siloxane distribution in sedimentary environments.
3.2 Global comparison of siloxane concentration in water, sediment, and biological samples
The concentration of siloxanes in water, sediment, and biological samples observed in this study was compared with those reported in previous research to better understand the current global status of siloxane contamination. Although the specific types and numbers of siloxanes measured varied across the studies, the primary siloxanes detected were largely comparable. For samples of water and sediment, comparisons were made using data from natural water bodies in rivers, lakes, and coastal environments. For biological samples, benthic invertebrates and small, low-trophic-level fish were considered. Overall, the concentrations of siloxanes in water, sediment, and marine benthos in this study fell within the worldwide ranges reported in previous studies (Supplementary Table S4).
The mean concentration of ΣCyclic siloxanes in water samples (64.7 ng/L) was higher than that reported in some freshwater samples from China (18.3–41.3 ng/L; Hong et al., 2014; Zhi et al., 2018; Guo et al., 2019; Jiang et al., 2022) and the Geum River in Korea (49.6 ng/L; Kim et al., 2022). However, it was lower than concentrations reported in other regions, including China (78.6–291 ng/L; Zhang et al., 2018; Liu et al., 2022; Jiang et al., 2022), several rivers in Korea (88.7–630 ng/L; Wang et al., 2021; Kim et al., 2022), Turkey (96.9 ng/L; Yaman et al., 2020), Japan (221 ng/L; Horii et al., 2017), Spain (203–1531 ng/L; Sanchís et al., 2013), and Vietnam (350 ng/L; Nguyen et al., 2022). The mean concentration of ΣLinear siloxanes in water (27.6 ng/L) was higher than those previously reported in China (<LOQ–9.93 ng/L; Hong et al., 2014; Jiang et al., 2022), but was 4–10 times lower than levels observed in China (180 ng/L; Zhi et al., 2018) and Korea (96.7–304 ng/L; Wang et al., 2021; Kim et al., 2022). The detection of siloxanes, especially linear types, in surface waters remains limited and warrants greater attention.
In sediments, the mean concentration of ΣCyclic siloxanes (123 ng/g dw) was higher than that reported in the Llobregat River, Spain (3.39 ng/g dw; Sanchís et al., 2013), some rivers in Korea (3.39–106 ng/g dw; Wang et al., 2021; Chen et al., 2022; Kim et al., 2022), China (28.5–58.1 ng/g dw; Zhi et al., 2018; He et al., 2021b; Jiang et al., 2022), Canada (50.9 ng/g dw; Pelletier et al., 2022), and Japan (71.9 ng/g dw; Horii et al., 2022b). However, these concentrations were 2–20 times lower than those reported in coastal sediments from Korea (219–245 ng/g dw; Lee et al., 2018, Lee et al., 2019), freshwater sediments in China (170–1035 ng/g dw; Zhang et al., 2018; Zhi et al., 2018; Guo et al., 2019; Zhi et al., 2019; Jiang et al., 2022; Liu et al., 2022), Japan (905 ng/g dw; Horii et al., 2022b), Spain (2070 ng/g dw; Sanchís et al., 2013), and Vietnam (2518 ng/g dw; Nguyen et al., 2022). The mean concentration of ΣLinear siloxanes in sediment (24.7 ng/g dw) was higher than those in coastal sediments from Korea (12.5–22.8 ng/g dw; Wang et al., 2021; Chen et al., 2022; Kim et al., 2022) and rivers in China (12.2 ng/g dw; Jiang et al., 2022). However, it was 3–50 times lower than values reported in sediments from Korean waters (70.1–467 ng/g dw; Lee et al., 2018, Lee et al., 2019; Kim et al., 2022), Chinese rivers (84.0–1558 ng/g dw; Zhi et al., 2018, Zhi et al., 2019; He et al., 2021b; Jiang et al., 2022), and Tokyo Bay, Japan (117–1310 ng/g dw; Horii et al., 2022b).
Studies on siloxanes in biological samples are limited and often report concentrations using different units. Nevertheless, the mean ΣCyclic concentration in marine benthos (788 ng/g dw) observed in our study was within the ranges reported in China (9.96 ng/g dw, 11.9–95 ng/g wet wt; Jia et al., 2015; Cui et al., 2019; Xue et al., 2019; Zhi et al., 2019; He et al., 2021a), Canada (18.2–236.7 ng/g wet wt; Pelletier et al., 2022), Japan (155 ng/g wet wt; Powell et al., 2017), Korea (139–4309 ng/g lw; Chen et al., 2022; Kim et al., 2022), and Norway (719–1042 ng/g lw; Borga et al., 2012, Borga et al., 2013). The mean ΣLinear concentration in marine benthos (71.9 ng/g dw) was also within the range reported in Canada (1.4 ng/g wet wt; Pelletier et al., 2022), China (11.3–21.9 ng/g wet wt; Xue et al., 2019; Zhi et al., 2019), and Korea (272 ng/g dw; Chen et al., 2022).
3.3 Spatial distribution of siloxanes
The spatial distribution of cyclic and linear siloxanes in surface water collected from Ulsan Bay and its adjacent regions of Korea is presented in Figure 2. The mean concentrations of ΣSiloxane in surface water across the different coastal zones were in the following order: Taehwa River (120 ng/L) > Gosa Stream (118 ng/L) > Ulsan Bay (106 ng/L) > plant outfall (73.4 ng/L), with no significant differences observed. The contamination status by ΣSiloxane varied slightly depending on the sampling period. In June, the ΣSiloxane concentrations were in the following order: Gosa Stream (mean: 94.2 ng/L) > Taehwa River (76.7 ng/L) > Ulsan Bay (45.5 ng/L) > plant outfall (36.1 ng/L). In September, the order shifted to: Taehwa River (261 ng/L) > plant outfall (220 ng/L) > Ulsan Bay (164 ng/L) > Gosa Stream (145 ng/L). In December, the order was: Gosa Stream (169 ng/L) > Ulsan Bay (143 ng/L) > Taehwa River (83.0 ng/L) > plant outfall (34.9 ng/L). In January, Gosa Stream (78.9 ng/L) > Taehwa River (60.0 ng/L) > Ulsan Bay (35.2 ng/L) > plant outfall (11.3 ng/L). These results suggested that siloxane contamination in surface water was generally similar across sampling sites when seasonal variability was not considered. Notably, the highest ΣSiloxane concentrations were detected in September at Stations B01 (1315 ng/L) and D04 (1176 ng/L), both located near the power plant outfall. This indicated that power plant discharges may play a crucial role in siloxane contamination in water environments, consistent with previous findings (Jin et al., 2016; Chen et al., 2022; Kim et al., 2022).
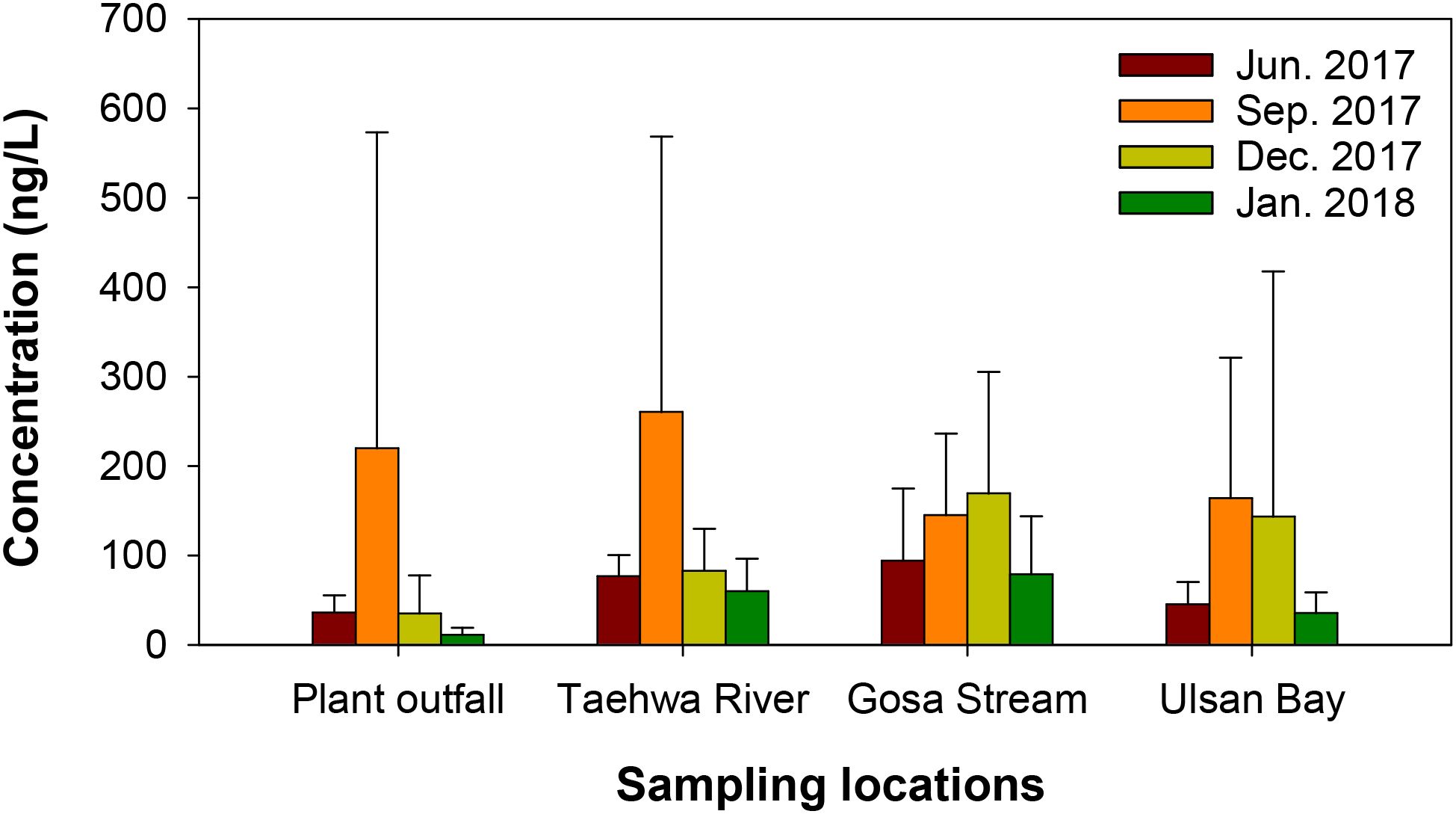
Figure 2. Spatial distribution of cyclic and linear siloxanes in surface water collected from Ulsan Bay and its adjacent regions, sampled in June, September and December 2017 and January 2018. Bar graphs and error bars indicate mean concentrations and standard deviations of siloxanes, respectively.
The concentrations of ΣSiloxane in sediment were in the following order: Gosa Stream (mean: 303 ng/g dw) > plant outfall (134 ng/g dw) > Ulsan Bay (108 ng/g dw) > Taehwa River (103 ng/g dw) (Figure 3). A similar trend was observed for sedimentary TOC content: Gosa Stream (mean: 2.26%) > Ulsan Bay (1.38%) > plant outfall (1.15%) > Taehwa River (0.84%). Unlike the spatial distribution in surface water, the sedimentary concentrations of siloxanes were influenced by both TOC content and source inputs, due to the hydrophobic nature of siloxanes. Previous studies also reported significant correlations between the sedimentary concentrations of siloxanes and TOC content (Zhang et al., 2011; Lee et al., 2014, Lee et al., 2018; Lee et al., 2019). In our study, the ΣCyclic concentrations in the sediment were 4–7 times higher than those of ΣLinear, indicating that cyclic siloxanes are preferentially enriched in the sedimentary environment, in line with previous findings (Lee et al., 2018, Lee et al., 2019; Chen et al., 2022). The highest sedimentary concentrations of ΣSiloxane were observed at Stations G2 (639 ng/g dw) and G3 (393 ng/g dw), both located in Gosa Stream. Previous studies identified Gosa Stream as a major contamination route for the flame retardants, plasticizers, and other contaminants (Kim et al., 2020; Lee et al., 2020, Liu et al., 2023). Our findings suggested that industrial activities contribute more significantly to sedimentary siloxane contamination than power plant discharges.
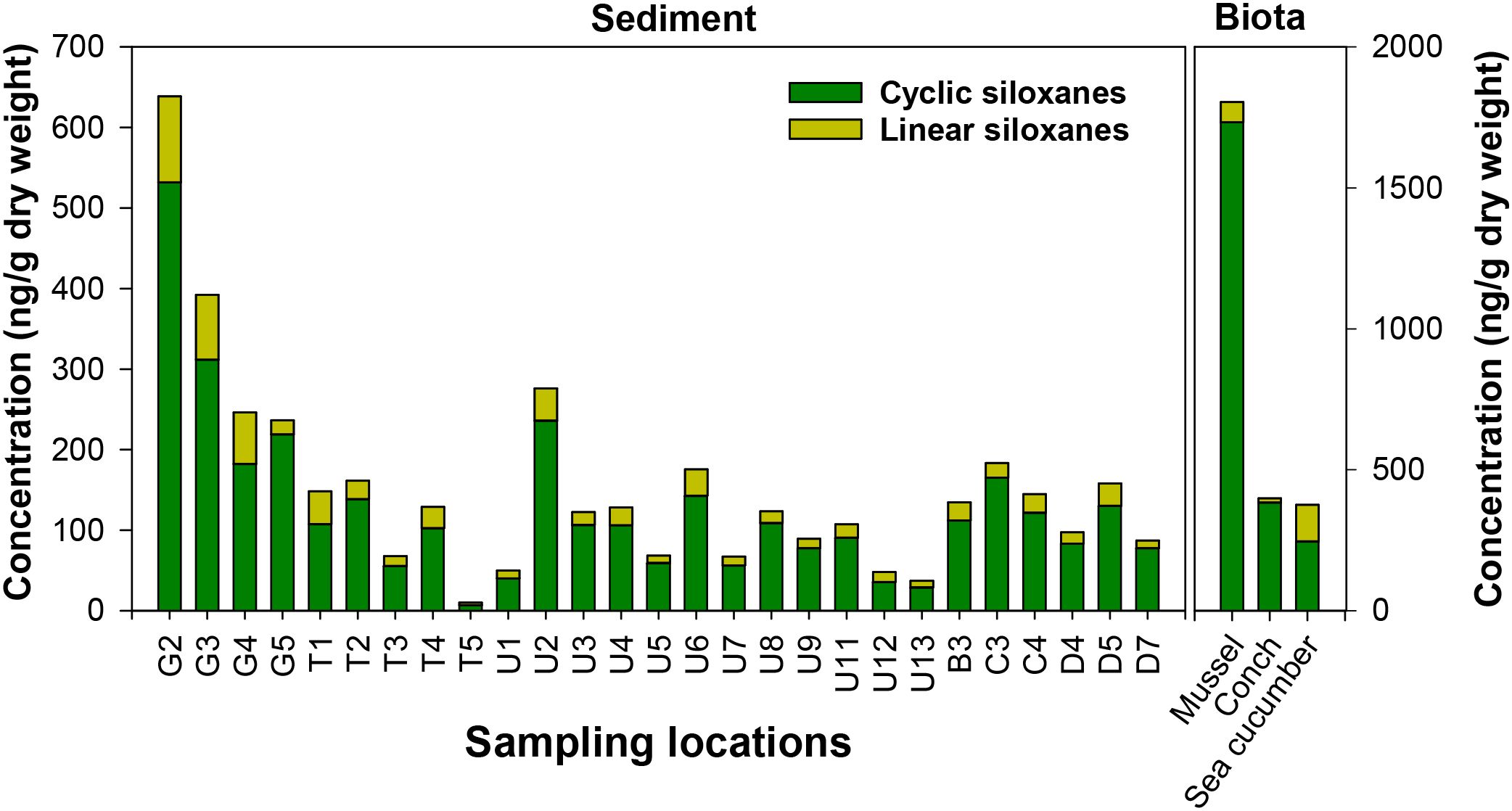
Figure 3. Spatial distribution of cyclic and linear siloxanes in sediment and benthic invertebrates collected from Ulsan Bay and its adjacent regions of Korea.
3.4 Seasonal variability of siloxanes in surface water
The seasonal variability in the concentrations of ΣSiloxane in surface water collected from Ulsan Bay and its adjacent regions of Korea is presented in Figure 4. The ΣCyclic concentrations in surface water collected in September (169 ± 277 ng/L) were significantly or slightly higher than those measured in December (64.0 ± 147 ng/L; p > 0.05), June (10.3 ± 8.18 ng/L; p < 0.05), and January (8.98 ± 17.4 ng/L; p < 0.05). Similarly, the mean surface water temperature in September (32.0 ± 2.78°C) was significantly higher than in June (21.4 ± 2.71°C; p < 0.05), December (19.4 ± 4.31°C; p < 0.05), and January (18.9 ± 4.31°C; p < 0.05). The ΣLinear concentrations in surface water collected in June (38.3 ± 28.7 ng/L) and September (31.6 ± 16.2 ng/L) were significantly higher than those in December (20.0 ± 27.1 ng/L; p < 0.05) and January (19.8 ± 24.4 ng/L; p < 0.05). In September and December, the ΣCyclic concentrations were 6.1 and 2.4 times higher than the corresponding ΣLinear concentrations, respectively. In contrast, in June and January, the ΣCyclic concentrations were lower than those of ΣLinear. These findings suggested that cyclic siloxanes in surface water may be more sensitive to seasonal variability than linear siloxanes. Previous studies reported higher levels of cyclic siloxanes in lake water during colder seasons, which were attributed to their low volatility and increased partitioning from sediment to water at lower temperatures (Krogseth et al., 2017; Guo et al., 2019). However, such patterns were not observed in our study. This discrepancy could be due to the fact that seasonal variability affects not only temperature, but also factors such as salinity, chlorophyll content, suspended solids, surface runoff, and the half-life of organic matter (Wu et al., 2017; Horii et al., 2022a). Further research is needed to better understand the seasonal behavior of siloxanes in aquatic environments.
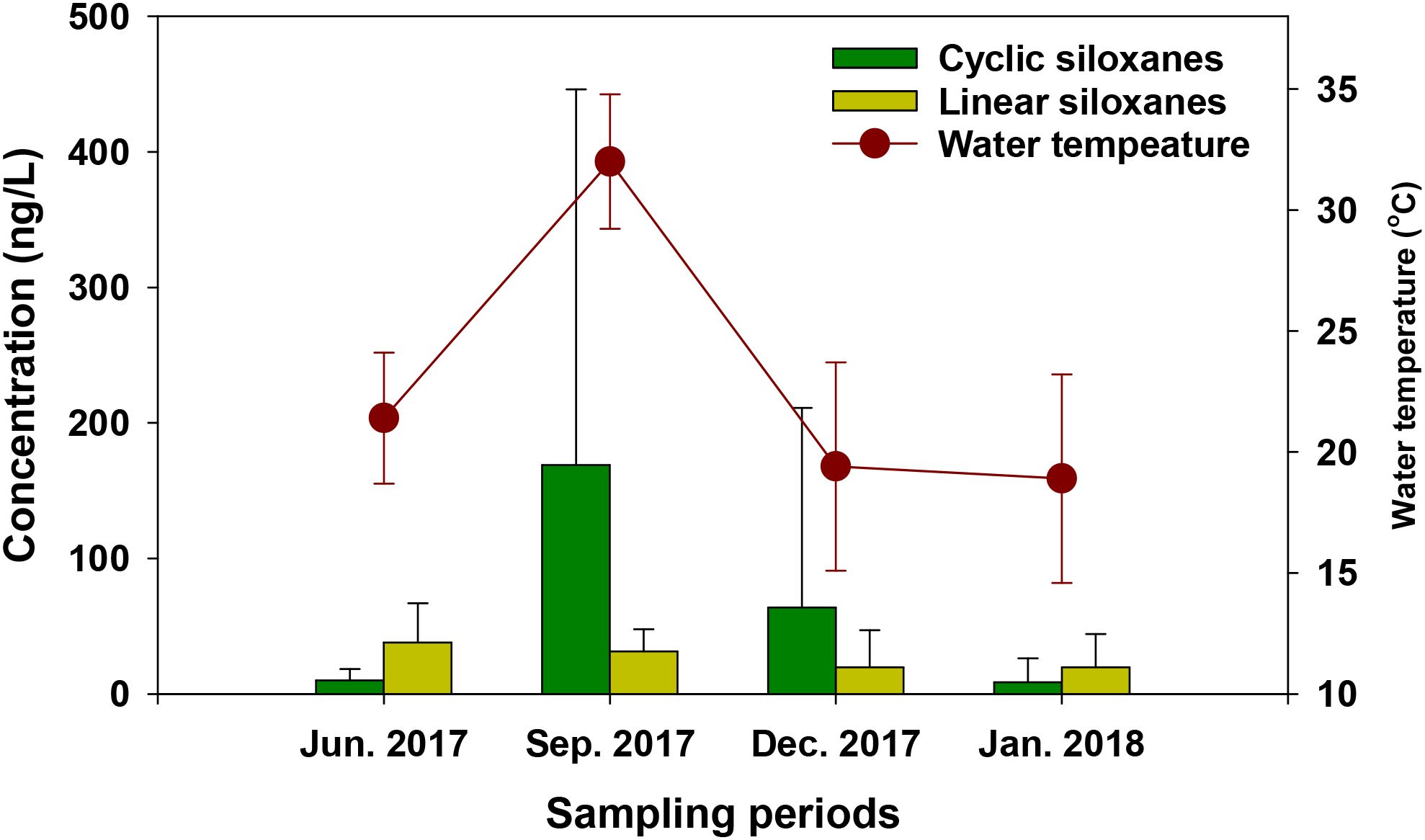
Figure 4. Concentrations of cyclic and linear siloxanes and water temperature (mean ± standard deviation) in surface water samples collected from Ulsan Bay and its adjacent regions of Korea across four sampling periods (June, September and December 2017 and January 2018). Bar graphs and error bars indicate mean concentrations and standard deviations of siloxanes, respectively.
3.5 Compositional profiles and source tracking of siloxanes
The relative contributions of individual siloxanes to the ΣSiloxane concentrations in water, sediment, and benthic invertebrates collected from Ulsan Bay and its adjacent regions are shown in Figure 5. Additionally, the composition profile of siloxanes in defoamer used in power plant effluent is presented (Supplementary Table S5). Overall, the compositional profiles of siloxanes in defoamer, water, and sediment differed notably. In the defoamer, the contributions of ΣCyclic (mean: 55%) and ΣLinear (45%) were similar. In contrast, the relative contributions in surface water varied considerably by sampling period: ΣCyclic accounted for 23% (June), 63% (September and December), and 28% (January), while ΣLinear accounted for 77%, 37%, 37%, and 72%, respectively. In sediment, ΣCyclic contributed 80%, significantly higher than ΣLinear (20%), which aligned with previous findings from industrialized bays in Korea (Lee et al., 2018, Lee et al., 2019). For benthic invertebrates, ΣCyclic dominated in mussels (96%), conches (96%), and sea cucumbers (66%), suggesting selective bioaccumulation of cyclic siloxanes from sedimentary environments. Similar patterns were reported in mollusks from the Bohai Sea (China) and biota from the Geum River (Korea) (Zhi et al., 2019; Kim et al., 2022).
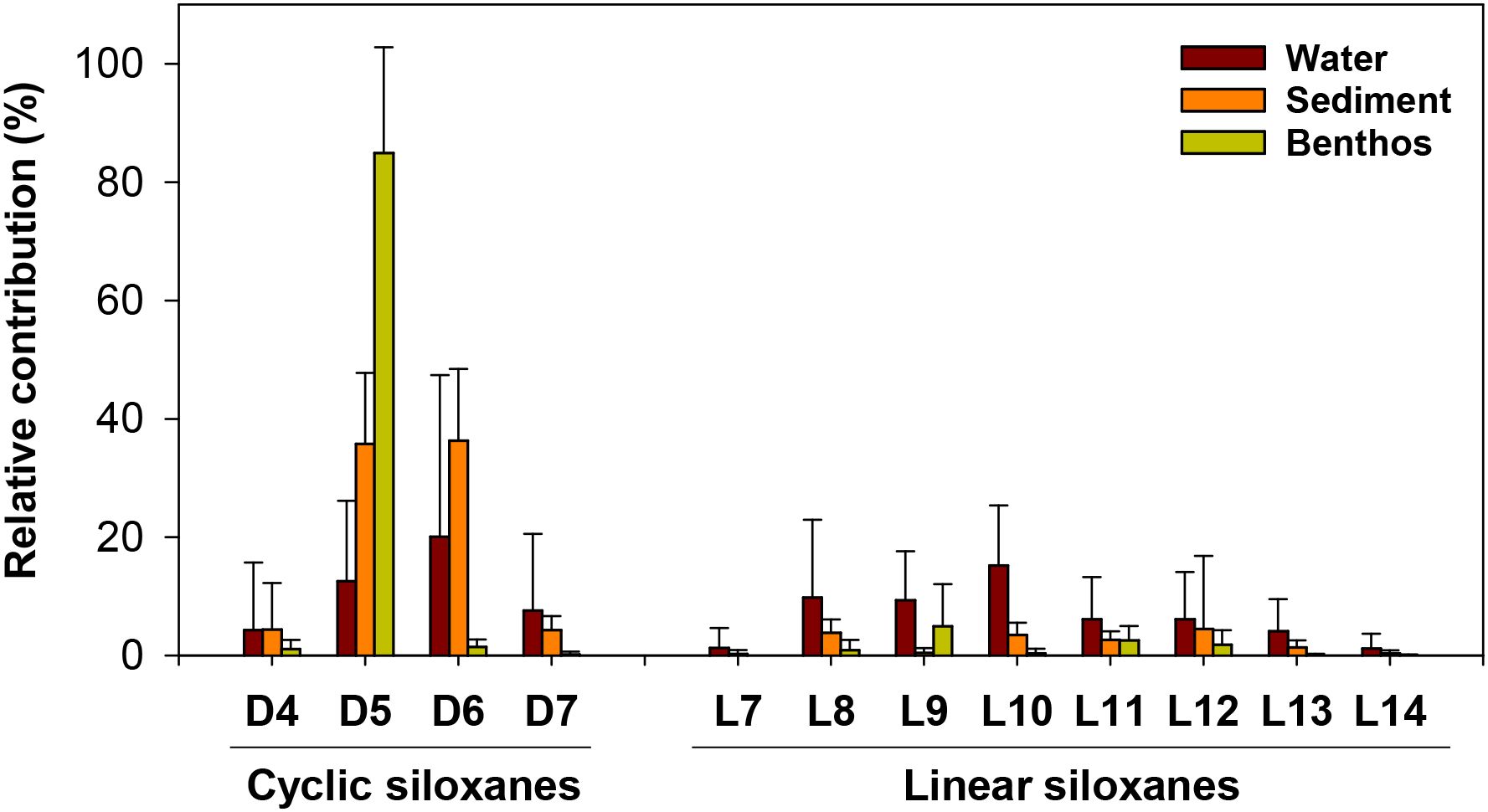
Figure 5. Relative compositional profiles of cyclic and linear siloxanes in surface water, sediment and benthic invertebrates collected from Ulsan Bay and its adjacent regions of Korea.
The predominant siloxanes in surface water also varied by sampling period. In June, L8 (24%) and L10 (23%) were dominant, followed by L9 (18%) and D5 (15%). In September, D6 (46%) was most abundant, followed by L10 (13%) and D5 (12%). December samples showed a higher contribution of D7 (26%), followed by D6 (20%), D5 (15%), L10 (13%), and L9 (12%). In January, L12 (18%) was dominant, with L11 (15%), L13 (12%), L10 (12%), and D4 (11%) also contributing. These seasonal differences suggested that the composition of siloxanes in surface water is significantly influenced by the sampling period. Moreover, compositional profiles of siloxanes differed between the different water bodies (Ulsan Bay, Gosa Stream, Taehwa River, and power plant outfall), likely reflecting varying contamination sources in coastal zones. Previous studies of siloxanes in wastewater treatment plants (WWTPs) in Korea reported distinct composition profiles between domestic and industrial WWTPs, which was linked to differing usage patterns in household and industrial products (Lee et al., 2014).
In sediment samples, D5 and D6 collectively accounted for 72% of the ΣSiloxane concentrations, consistent with earlier findings (Hong et al., 2014; Lee et al., 2014; Lee et al., 2018; Zhi et al., 2019). This is likely reflected the widespread use of D5 and D6 in industrial applications, as well as their association with high TOC content in sediments (Lu et al., 2011; Lee et al., 2018; Zhang et al., 2018). In contrast to surface water and sediment, D5 was predominant in benthic organisms, comprising 93% of ΣSiloxane concentrations in mussels and conches, and 62% in sea cucumbers, indicating a strong potential for selective bioaccumulation of D5. A previous study reported that the weak binding between D5 and the cytochrome leads to higher bioaccumulation in benthic invertebrates (Chen et al., 2024).
3.6 Potential for bioaccumulation of siloxanes
The bioaccumulation factor (BAF) and biota-sediment accumulation factor (BSAF) are commonly used indicators to assess the bioaccumulation potential of chemicals in aquatic organisms (Gu et al., 2017; Wang et al., 2021; Bernardo et al., 2022). In this study, field-based BAF (L/kg) was calculated as the ratio of lipid-normalized concentrations of siloxanes in aquatic organisms (CB, ng/g lipid weight) to the concentration of siloxanes in water (CW, ng/L). According to the Canadian Environmental Protection Act (CEPA, 1999) and the Stockholm Convention on Persistent Organic Pollutants (United States Environmental Production Agency (USEPA), 2014), BAF values exceeding 5,000 L/kg are indicative of significant bioaccumulation potential. BSAF was calculated as the ratio of the lipid-normalized concentration of siloxanes in organisms (CB, ng/g lw) to the TOC-normalized concentration in sediment (CS, ng/g TOC), with values > 1.7 indicating preferential partitioning into biological lipids (Wang et al., 2021; Bernardo et al., 2022). Among the 19 target siloxanes, 11 compounds (D4–D7 and L8–L14) were available for the BAF and BSAF calculation based on the detection rate (Supplementary Table S6).
Mussels exhibited notable bioaccumulation potential for D4–D6 and L8–L14, with field-based BAFs ranging from 1.30 × 104 to 6.12 × 106. They also showed significant BSAFs for D5 (6.45) and L9 (6.13), indicating strong sediment-associated uptake. Conchs showed high BAFs for D4–D6, L9, and L11–L12 (1.27 × 104–1.35 × 106), and also accumulated L9 from sediment (BSAF = 2.06). Sea cucumbers had bioaccumulation potential for D5–D6 and L8–L12 (BAF = 7.28 × 10³–8.50 × 105), and particularly high BSAF for L9 (21.1). Overall, D5 and L9 showed the highest bioaccumulation potential among the measured siloxanes across all three benthic species. Previous studies reported BAFs for D5 ranging from 7.06 × 10³ to 1.33 × 104 (Brooke et al., 2009b; Wang et al., 2021), and BSAF v7alues from 1.5 to 2.1 (Warner et al., 2010; Chen et al., 2022), confirming its notable bioaccumulation potential. However, data on the bioaccumulation behavior of linear siloxanes remain limited, suggesting the need of further studies.
4 Conclusions
This study provides a comprehensive assessment of the occurrence, distribution, seasonal variability, and bioaccumulation of cyclic and linear siloxanes in an industrialized estuarine environment. The predominance of cyclic siloxanes across water, sediment, and biota indicates their persistence and widespread contamination. The study finds distinct spatial distributions of siloxanes, which are driven by local industrial sources and hydrodynamic conditions. Siloxane contamination in water and sediment appears to be jointly influenced by industrial activities and power plant discharges. Seasonal variation significantly affects siloxane levels, particularly for cyclic siloxanes in surface water. The strong bioaccumulation potential of D5 and L9 in benthic invertebrates suggests ecological health risks. These findings highlight the need for regulatory action and continued monitoring of siloxanes in estuarine ecosystems impacted by industrial and energy production activities.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Wild benthic organisms were collected for this study; however, no ethical approval was required or obtained for these research activities.
Author contributions
WC: Data curation, Formal Analysis, Methodology, Writing – original draft. SL: Data curation, Formal Analysis, Investigation, Software, Writing – review & editing. H-KL: Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. ML: Funding acquisition, Investigation, Project administration, Supervision, Writing – review & editing. H-BM: Conceptualization, Funding acquisition, Investigation, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the Korea Institute of Marine Science and Technology Promotion (KIMST) through projects entitled “Development of management technology for persistent organic pollutants (POPs) in the marine environment (RS-2024-00417889)”, “Development of source identification and apportionment methods for toxic substances in marine environments (KIMST-20220534)”, and “Development of technology for impact assessment and management of HNS discharged from marine industrial facilities (KIMST-20210660)” funded by the Ministry of Oceans and Fisheries (MOF), Korea.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer SM declared a shared affiliation with the authors H-KL and H-BM to the handling editor at the time of review.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1621429/full#supplementary-material
References
Abe Y. and Gunji T. (2004). Oligo-and polysiloxanes. Prog. Polym. Sci. 29, 149–182. doi: 10.1016/j.progpolymsci.2003.08.003
Baney R. H., Itoh M., Sakakibara A., and Suzuki T. (1995). Silsesquioxanes. Chem. Rev. 95, 1409–1430. doi: 10.1021/cr00037a012
Bernardo F., Alves A., and Homem V. (2022). A review of bioaccumulation of volatile methylsiloxanes in aquatic ecosystems. Sci. Total Environ. 824, 153821. doi: 10.1016/j.scitotenv.2022.153821
Borga K., Fjeld E., Kierkegaard A., and McLachlan M. S. (2012). Food web accumulation of cyclic siloxanes in Lake Mjosa, Norway. Environ. Sci. Technol. 46, 6347–6354. doi: 10.1021/es300875ddoi.org/10.1021/es300875d
Borga K., Fjeld E., Kierkegaard A., and McLachlan M. S. (2013). Consistency in trophic magnification factors of cyclic methyl siloxanes in pelagic freshwater food webs leading to brown trout. Environ. Sci. Technol. 47, 14394–14402. doi: 10.1021/es404374j
Brooke D. N., Crookes M. J., Gray D., and Robertson S. (2009a). Environmental risk assessment report: decamethylcyclopentasiloxane (Bristol: Environment Agency of England and Wales). Available online at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/290565/scho0309bpqz-e-e.pdf.
Brooke D. N., Crookes M. J., Gray D., and Robertson S. (2009b). Environmental risk assessment report: dodecamethylcyclohexasiloxane (Bristol: Environment Agency of England and Wales). Available online at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/290561/scho0309bpqx-e-e.pdf.
Brooke D. N., Crookes M. J., Gray D., and Robertson S. (2009c). Environmental risk assessment report: octamethylcyclotetrasiloxane (Bristol: Environment Agency of England and Wales). Available online at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/290562/scho0309bpqy-e-e.pdf.
Canadian Environmental Protection Act (CEPA) (1999). “Canada gazette, part III, vol 22,” in Public works and government services(Ottawa, Ontario, Canada: Environment and Climate Change Canada).
Cantu M. A. and Gobas F. A. P. C. (2021). Bioaccumulation of dodecamethylcyclohexasiloxane (D6) in fish. Chemosphere 281, 130948. doi: 10.1016/j.chemosphere.2021.130948
Chen W., Kang Y.-J., Lee H.-K., Lee M. J., and Moon H.-B. (2022). Nationwide monitoring of cyclic and linear siloxanes in sediment and bivalves from Korean coastal waters: Occurrence, geographical distribution, and bioaccumulation potential. Mar. pollut. Bull. 185, 114201. doi: 10.1016/j.marpolbul.2022.114201
Chen W., Kang Y., Lee H.-K., Lim J.-E., Lee M., and Moon H.-B. (2023). Spatial distribution and temporal trends of cyclic and linear siloxanes in sediment from semi-enclosed and industrialized bays of Korea, in 2013 and 2021. Front. Mar. Sci. 10, 1185314. doi: 10.3389/fmars.2023.1185314
Chen W., Lee S., and Moon H.-B. (2024). Cyclic and linear siloxane contamination in sediment and invertebrates around a thermal power plant in Korea: Source impact, distribution, seasonal variation, and potential for bioaccumulation. Chemosphere 349, 140779. doi: 10.1016/j.chemosphere.2023.140779
Choi W., Lee S., Lee H.-K., and Moon H.-B. (2020). Organophosphate flame retardants and plasticizers in sediment and bivalves along the Korean coast: Occurrence, geographical distribution, and a potential for bioaccumulation. Mar. pollut. Bull. 156, 111275. doi: 10.1016/j.marpolbul.2020.111275
Colas A. and Curtis J. (2013). Handbook of polymer applications in medicine and medical devices. Silicones 7, 131–143. doi: 10.1016/B978-0-323-22805-3.00007-4
Cui S., Fu Q., An L., Yu T., Zhang F., Gao S., et al. (2019). Trophic transfer of cyclic methyl siloxanes in the marine food web in the Bohai Sea, China. Ecotox. Environ. Saf. 178, 86–93. doi: 10.1016/j.ecoenv.2019.04.034
ECHA (2019). “REACH Annex XV restriction report,” in Octamehylcyclotetrasiloxane (D4), decamethylcyclopentasiloxane (D5), dodecamethylcyclohexasiloxane (D6). Available online at: https://echa.europa.eu/documents/10162/039f5415-d7a2-b279-d270-0d07e18f6392.
Environment Canada (2008a). Screening assessment for the challenge decamethylcyclopentasiloxane (D5). Available online at: https://www.ec.gc.ca/ese-ees/13CC261E-5FB0-4D33-8000-EA6C6440758A/batch2_541-02-6_en.pdf (Accessed March 29, 2011).
Environment Canada (2008b). Screening assessment for the challenge dodecamethylcyclohexasiloxane (D6). Available online at: https://www.ec.gc.ca/ese-ees/FC0D11E7-DB34-41AA-B1B3-E66EFD8813F1/batch2_540-97-6_en.pdf (Accessed March 29, 2011).
Environment Canada (2008c). Screening assessment for the challenge octamethylcyclotetrasiloxane (D4). Available online at: https://www.ec.gc.ca/ese-ees/2481B508-1760-4878-9B8A-270EEE8B7DA4/batch2_556-67-2_en.pdf (Accessed March 29, 2011).
Fairbrother A. and Woodburn K. (2016). Assessing the aquatic risks of the cyclic volatile methyl siloxane D4. Environ. Sci. Technol. Lett. 3, 359–363. doi: 10.1021/acs.estlett.6b00341
Garside M. (2022). Silicon production worldwide 2010–2021. Available online at: https://www.statista.com/statistics/573585/global-silicon-production/ (Accessed February 2, 2025).
Gobas F. A. P. C., Wolf W., De Burkhard L. P., Verbruggen E., and Plotzke K. (2009). Revisiting bioaccumulation criteria for POPs and PBT assessments. Integr. Environ. Assess. Manage. 5, 624–637. doi: 10.1897/IEAM_2008-089.1
Gu S.-Y., Ekpeghere K. I., Kim H.-Y., Lee I.-S., Kim D.-H., Choo G. J., et al. (2017). Brominated flame retardants in marine environment focused on aquaculture area: occurrence, source and bioaccumulation. Sci. Total Environ. 601, 1182–1191. doi: 10.1016/j.scitotenv.2017.05.209
Guo W. J., Dai Y., Chu X. T., Cui S., Sun Y. P., Li Y. F., et al. (2021). Assessment bioaccumulation factor (BAF) of methyl siloxanes in crucian carp (Carassius auratus) around a siloxane production factory. Ecotox. Environ. Saf. 213, 111983. doi: 10.1016/j.ecoenv.2021.111983
Guo J. Y., Zhou Y., Zhang B. Y., and Zhang J. B. (2019). Distribution and evaluation of the fate of cyclic volatile methyl siloxanes in the largest lake of southwest China. Sci. Total Environ. 657, 87–95. doi: 10.1016/j.scitotenv.2018.11.454
He Y., Shuai S. C., Yang L., and Tang Z. W. (2021b). Occurrence of methylsiloxanes in sediments from a subtropical river-lake system in eastern China and its implication for ecological risks. Ecotox. Environ. Saf. 223, 112627. doi: 10.1016/j.ecoenv.2021.112627
He Y., Su S., Cheng J., Tang Z., Ren S., and Lyu Y. (2021a). Bioaccumulation and trophodynamics of cyclic methylsiloxanes in the food web of a large subtropical lake in China. J. Hazard. Mater. 413, 125354. doi: 10.1016/j.jhazmat.2021.125354
Hong W., Jia H., Liu C., Zhang Z., Sun Y., and Li Y. (2014). Distribution, source, fate and bioaccumulation of methyl siloxanes in marine environment. Environ. pollut. 191, 175–181. doi: 10.1016/j.envpol.2014.04.033
Horii Y., Minomo K., Lam J. C. W., and Yamashita N. (2022a). Spatial distribution and accumulation profiles of volatile methylsiloxanes in Tokyo Bay, Japan: Mass loadings and historical trends. Sci. Total Environ. 806, 150821. doi: 10.1016/j.scitotenv.2021.150821
Horii Y., Minomo K., Ohtsuka N., Mmotegi M., Nojiri K., and Kannan K. (2017). Distribution characteristics of volatile methyl siloxanes in Tokyo Bay watershed in Japan: Analysis of surface water by purge and trap method. Sci. Total Environ. 587, 57–65. doi: 10.1016/j.scitotenv.2017.02.014
Horii Y., Ohtsuka N., Minomo K., Motegi M., Takemine S., Motegi M., et al. (2021). Distribution characteristics of methylsiloxanes in atmospheric environment of Saitama, Japan: Diurnal and seasonal variations and emission source apportionment. Sci. Total Environ. 754, 142399. doi: 10.1016/j.scitotenv.2020.142399
Horii Y., Ohtsuka N., Nishino T., Kroda K., Imaizumi Y., and Sakurai T. (2022b). Spatial distribution, mass balance, and benthic risk assessment of cyclic, linear, and modified methylsiloxanes in sediments from the Tokyo Bay catchment Basin, Japan: Mass balance in extracta ble organosilicon. Sci. Total Environ. 838, 155956. doi: 10.1016/j.scitotenv.2022.155956
IMARC (2021). Silicones market: Global industry trends, share, size, growth, opportunity and forecast 2021–2026. Available online at: https://www.marketsandmarkets.com/Market-Reports/silicone-market-709.html (Accessed May, 2024).
Jessup W. H., Wiegand J., Delbridge-Perry M., MacAvoy S. E., and Connaughton V. P. (2022). Developmental effects of siloxane exposure in zebrafish: A comparison study using laboratory-mixed and environmental water samples. J. Appl. Toxicol. 42, 1986–2004. doi: 10.1002/jat.4369
Jia H. L., Zhang Z. F., Wang C. Q., Hong W. J., Sun Y. Q., and Li Y. F. (2015). Trophic transfer of methyl siloxanes in the marine food web from coastal area of northern China. Environ. Sci. Technol. 49, 2833–2840. doi: 10.1021/es505445e
Jiang Y., Guo J. Y., Zhou Y., Zhang B. Y., and Zhang J. B. (2022). Occurrence and behavior of methylsiloxanes in urban environment in four cities of China. Int. J. Environ. Res. Pub. Health 19, 13869. doi: 10.3390/ijerph192113869
Jin X., Lee H.-K., Badejo A. C., Lee S.-Y., Shen A., Lee S., et al. (2016). Decline in sediment contamination by persistent toxic substances from the outfall of wastewater treatment plant: Effectiveness of legislative actions in Korea. Chemosphere 453, 426–435. doi: 10.1016/j.chemosphere.2016.03.075
Kang Y., Lee S., Chen W., and Moon H.-B. (2024). Factors determining contamination and time trends in cyclic and linear siloxanes in sediments from an industrialized lake in Korea. Ecotox. Environ. Saf. 269, 115817. doi: 10.1016/j.ecoenv.2023.115817
Kim D. Y., Cho H.-E., Won E.-J., Kim H.-J., Lee S. G., An K.-G., et al. (2022). Environmental fate and trophic transfer of synthetic musk compounds and siloxanes in Geum River, Korea: Compound-specific nitrogen isotope analysis of amino acids for accurate trophic position estimation. Environ. Int. 161, 107123. doi: 10.1016/j.envint.2022.107123
Kim S., Kim Y., and Moon H.-B. (2020). Contamination and historical trends of legacy and emerging plasticizers in sediment from highly industrialized bays of Korea. Sci. Total Environ. 765, 142751. doi: 10.1016/j.scitotenv.2020.142751
Krogseth I. S., Whelan M., Christensen G., Breivik K., Evenset A., and Warner N. (2017). Understanding of cyclic volatile methyl siloxane fate in a high latitude lake is constrained by uncertainty in organic carbon–water partitioning. Environ. Sci. Technol. 51, 401–409. doi: 10.1021/acs.est.6b04828
Lee S.-Y., Lee. S., Choi M., Kannan K., and Moon H.-B. (2018). An optimized method for the analysis of cyclic and linear siloxanes and their distribution in surface and core sediments from industrialized bays in Korea. Environ. pollut. 236, 111–118. doi: 10.1016/j.envpol.2018.01.051
Lee H.-K., Lee S., Lim J.-E., and Moon H.-B. (2020). Legacy and novel flame retardants in water and sediment from highly industrialized bays of Korea: Occurrence, source tracking, decadal time trend, and ecological risks. Mar. pollut. Bull. 160, 111639. doi: 10.1016/j.marpolbul.2020.111639
Lee S., Moon H.-B., Song G.-J., Ra K., Lee W.-C., and Kannan K. (2014). A nationwide survey and emission estimates of cyclic and linear siloxanes through sludge from wastewater treatment plants in Korea. Sci. Total Environ. 497–498, 106–112. doi: 10.1016/j.scitotenv.2014.07.083
Lee D., Park M.-K., Lee I.-S., and Choi S.-D. (2019). Contamination characteristics of siloxanes in coastal sediment collected from industrialized bays in South Korea. Ecotox. Environ. Saf. 182, 109457. doi: 10.1016/j.ecoenv.2019.109457
Liu N. N., Zhang J., He X. D., Xu L., and Cai Y. Q. (2023). Occurrence and fate of chlorinated methylsiloxanes in surrounding aqueous systems of Shengli oilfield. China. J. Environ. Sci. 125, 332–339. doi: 10.1016/j.jes.2021.11.033
Liu N. N., Zhao X. S., Xu L., and Cai Y. Q. (2022). Temporal and spatial variation, input fluxes and risk assessment of cyclic methylsiloxanes in rivers-Bohai Sea system. Ecotox. Environ. Saf. 231, 113169. doi: 10.1016/j.ecoenv.2022.113169
Lu Y., Yuan T., Wang W. H., and Kannan K. (2011). Concentrations and assessment of exposure to siloxanes and synthetic musks in personal care products from China. Environ. pollut. 159, 3522–3528. doi: 10.1016/j.envpol.2011.08.015
METI-Japan (2018). The evaluation of chemical substances and regulation of their manufacture act (in Japanese). Available online at: https://www.meti.go.jp/policy/chemical_management/kasinhou/information/bulletin_kan.html (Accessed April 2, 2018).
Mojsiewicz-Pienkowska K. and Krenczkowska D. (2018). Evolution of consciousness of exposure to siloxanes-Review of publications. Chemosphere 191, 204–217. doi: 10.1016/j.chemosphere.2017.10.045
Nguyen H. M. N., Khieu H. T., Le H. Q., Duong T. T., Do T. Q., Minh T. B., et al. (2022). Assessment of distributional characteristics and ecological risks of cyclic volatile methylsiloxanes in sediments from urban rivers in northern Vietnam. Environ. Sci. pollut. Res. 29, 29917–29926. doi: 10.1007/s11356-021-18487-y
Pelletier M., Armellin A., McDaniel T., Martin P., McGoldrick D., Clark M., et al. (2022). Influence of wastewater effluents on the bioaccumulation of volatile methylsiloxanes in the St. Lawrence River. Sci. Total Environ. 806, 151267. doi: 10.1016/j.scitotenv.2021.151267
Powell D. E., Schøyen M., Øxnevad S., Gerhards R., Böhmer T., Koerner M., et al. (2018). Bioaccumulation and trophic transfer of cyclic volatile methylsiloxanes (cVMS) in the aquatic marine food webs of the Oslofjord, Norway. Sci. Total Environ. 622, 127–139. doi: 10.1016/j.scitotenv.2017.11.237
Powell D. E., Suganuma N., Kobayashi K., Nakamura T., Ninomiya K., Matsumura K., et al. (2017). Trophic dilution of cyclic volatile methylsiloxanes (cVMS) in the pelagic marine food web of Tokyo Bay, Japan. Sci. Total Environ. 578, 366–382. doi: 10.1016/j.scitotenv.2016.10.189
Redman A. D., Mihaich E., Woodburn K., Paquin P., Powell D., Mcgrath J. A., et al. (2012). Tissue-based risk assessment of cyclic volatile methyl siloxanes. Environ. Toxicol. Chem. 31, 1911–1919. doi: 10.1002/etc.1900
Sanchís J., Martínez E., Ginebreda A., Farré M., and Barceló D. (2013). Occurrence of linear and cyclic volatile methylsiloxanes in wastewater, surface water and sediments from Catalonia. Sci. Total Environ. 443, 30–38. doi: 10.1016/j.scitotenv.2012.10.047
Shimizu T., Kanamori K., and Nakanishi K. (2017). Silicone-based organic-inorganic hybrid aerogels and xerogels. Chem. Eur. J. 23, 5176–5187. doi: 10.1002/chem.201603680
United Nations Environmental Programme (UNEP) (2011). Final act of the conference of pleniopotentiaries on the Stockholm Convention on persistent organic pollutants (Geneva, Switzerland: Secretariat of the Stockholm Convention). Available online at: http://www.pops.int/documents/meetings/dipcon/25june2001/conf4_finalact/en/FINALACT-English.PDF (Accessed October 19, 2010).
United States Environmental Production Agency (USEPA). (2014). Enforceable Consent Agreement for Environmental Testing for Octamethylcyclotetrasiloxane (D4). Available online at: https://www.epa.gov/sites/production/files/2015-01/documents/signed_siloxanes_eca_4-2-14.pdf (Accessed February 6, 2014).
Wang W. T., Cho H.-S., Kim K., Park K.-H., and Oh J.-E. (2021). Tissue-specific distribution and bioaccumulation of cyclic and linear siloxanes in South Korean crucian carp (Carassius carassius). Environ. Pollut. 288, 117789. doi: 10.1016/j.envpol.2021.117789
Wang D. G., de Solla S. R., Lebeuf M., Bisbicos T., Barrett G. C., and Alaee M. (2017). Determination of linear and cyclic volatile methylsiloxanes in blood of turtles, cormorants, and seals from Canada. Sci. Total Environ. 574, 1254–1260. doi: 10.1016/j.scitotenv.2016.07.133
Warner N. A., Evenset A., Christensen G., Gabrielsen G. W., Borga K., and Leknes H. (2010). Volatile siloxanes in the European Arctic: Assessment of sources and spatial distribution. Environ. Sci. Technol. 44, 7705–7710. doi: 10.1021/es101617k
Wu Y. L., Wang X. H., Li Y. Y., Ma M. L., Luo H., and Hong H. S. (2017). Polybrominated diphenyl ethers, organochlorine pesticides, and polycyclic aromatic hydrocarbons in water from the Jiulong River Estuary, China: levels, distributions, influencing factors, and risk assessment. Environ. Sci. pollut. Res. 24, 89338945. doi: 10.1007/s11356-015-4782-2
Xiang X. L., Liu N. N., Xu L., and Cai Y. Q. (2021). Review of recent findings on occurrence and fates of siloxanes in environmental compartments. Ecotox. Environ. Saf. 224, 112631. doi: 10.1016/j.ecoenv.2021.112631
Xue X. H., Jia H. L., and Xue J. C. (2019). Bioaccumulation of methyl siloxanes in common carp (Cyprinus carpio) and in an estuarine food web in northeastern China. Arch. Environ. Contam. Toxicol. 76, 496–507. doi: 10.1007/s00244-018-0569-z
Yaman B., Erkuzu H., Okan F., and Odabasi M. (2020). Spatial variations of linear and cyclic volatile methyl siloxanes in a river basin and their air-water exchange. Atmos. pollut. Res. 11, 2308–2316. doi: 10.1016/j.apr.2020.06.005
Zhang Z. F., Qi H., Ren N. Q., Li Y. F., Gao D. W., and Kannan K. (2011). Survey of cyclic and linear siloxanes in sediment from the Songhua River and in sewage sludge from wastewater treatment plants, northeastern China. Arch. Environ. Contam. Toxicol. 60, 204–211. doi: 10.1007/s00244-010-9619-x
Zhang Y. X., Shen M. C., Tian Y., and Zeng G. M. (2018). Cyclic volatile methylsiloxanes in sediment, soil, and surface water from Dongting Lake, China. J. Soil Sediment 18, 2063–2071. doi: 10.1007/s11368-018-1948-9
Zhi L. Q., Xu L., He X. D., Zhang C. H., and Cai Y. Q. (2018). Occurrence and profiles of methylsiloxanes and their hydrolysis product in aqueous matrices from the Daqing oilfield in China. Sci. Total Environ. 631, 879–886. doi: 10.1016/j.scitotenv.2018.03.098
Keywords: siloxane, cyclic, linear, seasonal variation, benthos, bioaccumulation
Citation: Chen W, Lee S, Lee H-K, Lee M and Moon H-B (2025) Multi-matrix contamination by cyclic and linear siloxanes in a highly industrialized estuarine environment of Korea: source identification, seasonal variation, and bioaccumulation potential. Front. Mar. Sci. 12:1621429. doi: 10.3389/fmars.2025.1621429
Received: 01 May 2025; Accepted: 30 June 2025;
Published: 21 July 2025.
Edited by:
Zhiyuan Wu, Changsha University of Science and Technology, ChinaReviewed by:
Jiashen Tian, Arizona State University, United StatesMin-Kyu Park, Jeju National University, Republic of Korea
Sori Mok, Hanyang University, Republic of Korea
Yohei Sato, National Institute of Advanced Industrial Science and Technology (AIST), Japan
Copyright © 2025 Chen, Lee, Lee, Lee and Moon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hyo-Bang Moon, aGJtb29uQGhhbnlhbmcuYWMua3I=
 Wenming Chen
Wenming Chen Sunggyu Lee
Sunggyu Lee Hyun-Kyung Lee3
Hyun-Kyung Lee3 Hyo-Bang Moon
Hyo-Bang Moon