- 1College of Fisheries and Ocean Sciences, University of Alaska Fairbanks, Fairbanks, AK, United States
- 2Office of Research, University of Alaska Anchorage, Anchorage, AK, United States
Killer whales (Orcinus orca) are cosmopolitan, apex predators that sometimes interact with commercial fisheries. These fishery interactions can affect killer whales, sometimes harmfully, and cause negative socioeconomic consequences for the fishing industry. This review examines global trends in commercial fishery interactions with killer whales by analyzing 69 articles published between 1963 and 2024. These articles noted interactions between killer whales and fisheries in all oceans, but especially at high latitudes. Most documented interactions involved the depredation of longlines. Killer whales have been observed depredating a minimum of 30 species, mainly large fish such as tunas (Thunnus spp.). Bycatch, injuries, fishers’ retaliatory measures, and artificial provisioning impacted killer whales that interacted with fisheries. Various mitigation measures have been tested with mixed success. This review outlines policy options to address interactions between killer whales and fisheries and identifies existing knowledge gaps.
1 Introduction
Conflicts between humans and wildlife occur when the needs and behavior of wildlife intersect negatively with human activities and are often exacerbated by competition for resources and habitat loss/fragmentation (Woodroffe et al., 2005). In terrestrial habitats, these conflicts manifest as damage to crops, livestock injury or death, and threats to human safety and socioeconomic wellbeing (Woodroffe et al., 2005; Torres et al., 2018). In marine systems, human-wildlife conflicts often center on fishing activities, which frequently overlap spatially and temporally with many marine predators, such as sharks and marine mammals (Tixier et al., 2021a). Fatal encounters with fishing gear are the largest direct cause of cetacean mortality globally, accounting for an estimated 650,000 marine mammal deaths annually and representing a significant conservation concern for many populations and species (Read et al., 2006; Read, 2008). Furthermore, competition for fisheries’ target species and damage to fishing gear by marine mammals can strain fishers’ livelihoods and hinder recovery objectives for some endangered and threatened marine mammal species (Baird et al., 2021).
Fishery interactions with marine mammals have been increasingly documented in recent decades and involve all marine mammal families (Jog et al., 2022). These interactions may be divided into two broad categories: indirect and direct interactions. Indirect interactions, also known as biological or ecological interactions, refer to the population-level effects a fishery may exert on a marine mammal population, such as reducing prey availability or altering the composition of prey populations important to marine mammals (DeMaster et al., 2001; Read, 2008; Northridge, 2018). In contrast, direct, or operational, interactions encompass the immediate encounters between marine mammals and fishing operations and gear, such as fishing nets, lines, or vessels, and tend to affect individual animals rather than entire populations (Read, 2008; Northridge, 2018). However, some population-level impacts have been documented resulting from operational interactions (Tixier et al., 2017; Ballance et al., 2021).
Operational interactions between marine mammals and commercial fisheries are multifaceted. These interactions include but are not limited to depredation, where predators directly remove catch from fishing gear, and commensalism, where animals forage around fishing gear on fish or other species that would otherwise not be caught or retained in the fishery (Read, 2008; Northridge, 2018; Tixier et al., 2021a) A major distinction between depredation and commensalism is that depredation ultimately results in greater costs to fishers, such as loss or damage of catch and/or gear, increased expenditures and fishing time to replace depredated catch, and the costs of mitigation measures to avoid depredation (Northridge, 2018; Tixier et al., 2021a). In contrast, commensalism usually results in little to no economic detriment to fisheries because interacting individuals typically do not damage gear or remove target catch from the gear itself (Luque et al., 2006; Perez, 2006; Northridge, 2018). In commensal interactions, marine mammals feed on fishery discards, fish injured but not captured by the fishing gear (e.g., shaken off hooks, extruded through nets, or injured by ropes and cables), or other animals attracted by fishing activity. Foraging on discards is a commonly documented interaction often associated with net fisheries, especially trawl fisheries (Bonizzoni et al., 2022). Operational interactions that begin as commensalism may eventually change into depredation over time (Chilvers et al., 2003; Northridge, 2018; Bonizzoni et al., 2022). Marine mammals interacting with fisheries risk entanglement and injury from the fishing gear, injury from the vessel, and potentially injurious deterrence measures employed by fishers (Matkin et al., 2008; Jog et al., 2022). Reliance on fisheries for food may also alter natural foraging habits and dietary composition (Chilvers et al., 2003; Bonizzoni et al., 2022).
Killer whales (Orcinus orca), apex predators with a cosmopolitan distribution (Forney and Wade, 2006), have a long history of interacting with fisheries and other harvesting activities at sea. Whaling logs from the 1700s documented killer whales scavenging on baleen whales captured by commercial whaling operations, especially the tongue and lips (Whitehead and Reeves, 2005). In southeastern Australia, a small population of killer whales and European colonial whalers cooperatively hunted baleen whales from 1844 through 1928, though local Indigenous knowledge holders report this mutualistic behavior predates colonization (Reeves et al., 2023). More recently, killer whales have been documented interacting with commercial fisheries, particularly with longline gear at high latitudes (Dahlheim, 1988; Purves et al., 2004; Kock et al., 2006). These diverse behaviors are reflective of the species’ broad ecological variation. Killer whales are known to feed on more than 140 species of fish, cephalopods, seals, sea lions, dolphins, porpoises, whales, seabirds, and marine reptiles (Ford, 2018). However, killer whale subspecies, ecotypes, and populations often display dietary specializations. In the North Pacific, resident killer whales (O. o. ater) feed exclusively on fish, mostly salmonids (Van Cise et al., 2024; Filatova et al., 2023; Ford et al., 2016; Ford and Ellis, 2006; Saulitis et al., 2000). In contrast, sympatric Bigg’s killer whales (O. o. rectipinnus) feed only on other marine mammals (Filatova et al., 2023; Ford and Ellis, 2006; Dahlheim and White, 2010; Herman et al., 2005; Saulitis et al., 2000). Some generalist forms do exist, however. Killer whales in the tropics, subtropics, and portions of the sub-Antarctic have a broad dietary niche and are more opportunistic in prey choice, likely due to seasonal fluctuations in prey availability or low regional productivity (Kiszka et al., 2021; Reisinger et al., 2016; Tixier et al., 2019).
Given this species’ ecological diversity, geographic variability, and frequent encounters with fisheries, a global, comprehensive synthesis of commercial fishery interactions with killer whales is needed to understand overarching patterns in fishery interactions and the potential for mitigating harmful impacts. Drawing upon published literature, this review describes killer whale interactions with commercial fisheries around the world and identifies shared patterns and key differences in the types of fishing gear and species targeted, onset and spread of interactions, emerging interactions, and their behavioral and ecological consequences. We aim to provide a comprehensive resource to inform management options and identify knowledge gaps for further research.
2 Methods
The search engines Web of Science and SCOPUS were used to identify peer-reviewed journal articles related to fishery interactions with killer whales. Identical search terms were used for both search engines (see Supplementary Material). Gray literature, such as reports, white papers, and government documents, frequently contains information regarding odontocete depredation not captured in traditional academic papers. Articles from gray literature were sourced through government databases, regional fisheries management organization (RFMO) websites, and other sources. Following the methods used by Jog et al. (2022) and Tixier et al. (2021a), additional materials were collected through a “snowball search” of citations in reference lists of selected papers to locate relevant articles missed by the search engines. Titles, abstracts, and report summaries were screened for relevant content and were discarded if they did not contain information about killer whales and fishery interactions. Additionally, articles were excluded if they did not readily distinguish between killer whales and false killer whales (Pseudorca crassidens).
Articles were examined for the following content: interaction types, gear types, species targeted by fishery or prey species consumed by killer whales, impacts on killer whales, and management strategies. We followed the general framing of operational (or direct) interactions, as defined by Read (2008) and Northridge (2018). Operational interactions were classified into two main categories: depredation and commensalism. Interactions were classified as depredation when texts explicitly reported or described the removal or attempted removal of bait or catch from fishing gear (e.g., lines or nets) by a killer whale. Conversely, interactions were classified as commensalism when texts reported killer whales feeding on spilled catch, discarded catch, or other animals attracted by fishing activity without directly removing catch from fishing gear. Interactions lacking sufficient detail to be confidently assigned to either category were included but categorized as “unspecified.” This framework enabled us to synthesize the literature in a way that captured the full breadth of reported behaviors, allowing for comparisons across fisheries and geographic areas.
To address our objectives, we begin by characterizing key aspects of research on fishery interactions with killer whales, including research trends, the prevalence of interaction types documented in the literature, fishery species targeted, and the general timeline of the emergence of interactions. Next, we contextualize these interactions within the broader history of commercial fisheries development. Third, we discuss the behavioral and ecological patterns across interactions with fisheries and the consequences of these interactions. The final sections assess approaches to management, provide policy insights, and highlight knowledge gaps.
2.1 Limitations
Literature reviews and surveys can miss relevant publications due to insufficient search terms and search algorithms. Further, this review is limited to commercial fishery interactions and relies primarily on materials published in peer-reviewed journals and government repositories indexed in academic databases. Additionally, this review is limited to articles written or translated into English and may miss materials written in other languages.
In addition, killer whale interactions with sport and other small-scale artisanal fisheries (Tixier et al., 2021a) are not systematically assessed here. Scientific surveys and other types of academic research are the standard methods used in most studies on fishery interactions with killer whales. However, local and Indigenous communities may possess present and historical knowledge of fishery interactions that are underrepresented in academic literature. Indigenous knowledge is increasingly recognized as critical for understanding marine sociological ecosystems and can inform fisheries management (Ban et al., 2017; Reid et al., 2021).
3 Results
3.1 Research trends and interaction types
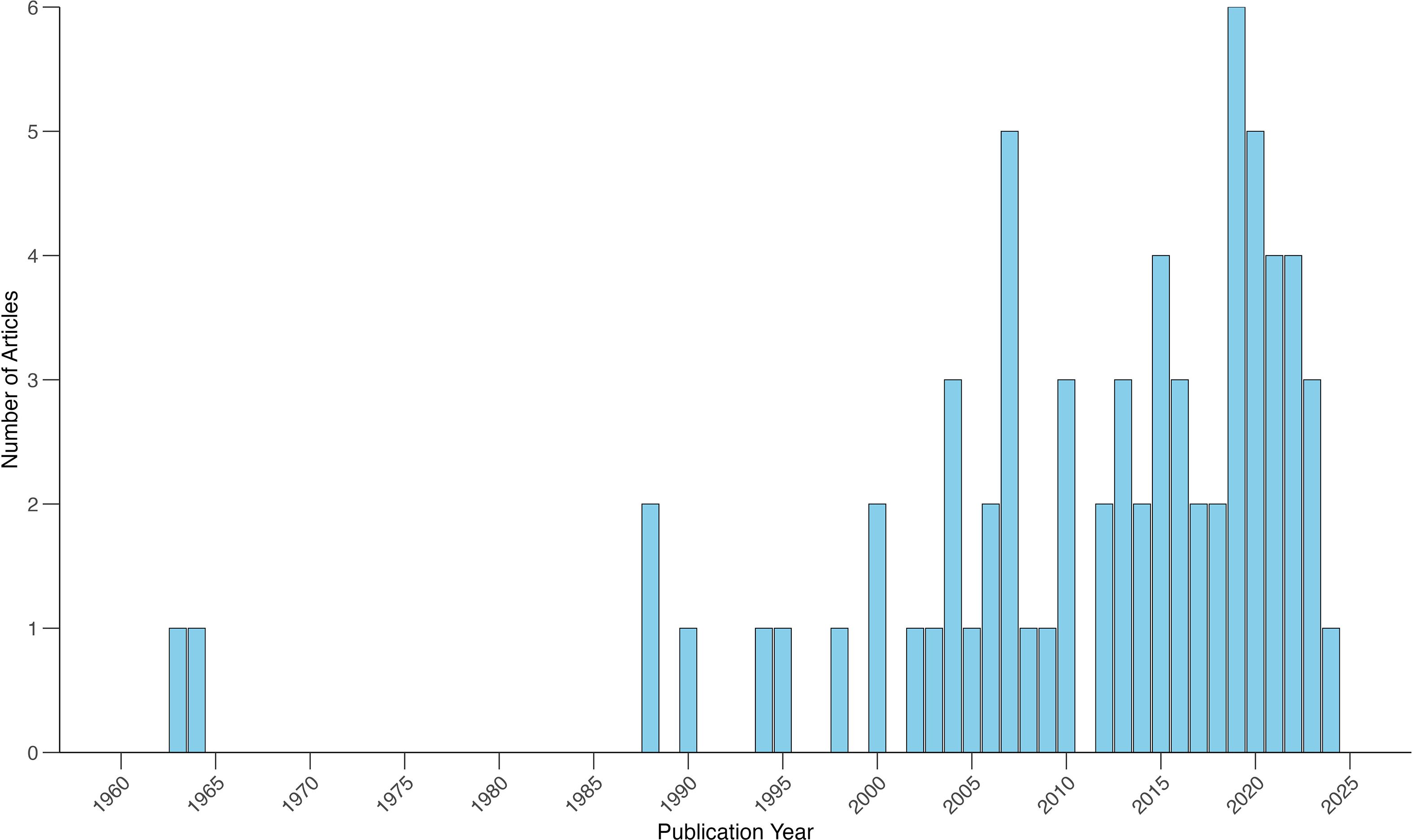
After removing duplicate results, Web of Science and SCOPUS returned a combined total of 61 articles. Of these, 18 were eliminated based on screening criteria for relevancy. An additional 14 peer-reviewed articles were included after identifying them through the “snowball search” method. In total, 69 articles (57 journal articles and 12 gray literature) from 1963–2024 were reviewed, with a notable increase in publications beginning in the mid-2000s (Figure 1). Depredation and commensalism represented the majority of interaction types (Figure 2). Most publications reported cases of killer whale depredation (80%), and 10% of articles documented instances of commensalism. Some articles described multiple interaction types occurring within a single study or region (9%), and a few articles did not provide enough detail to categorize interactions (1%).
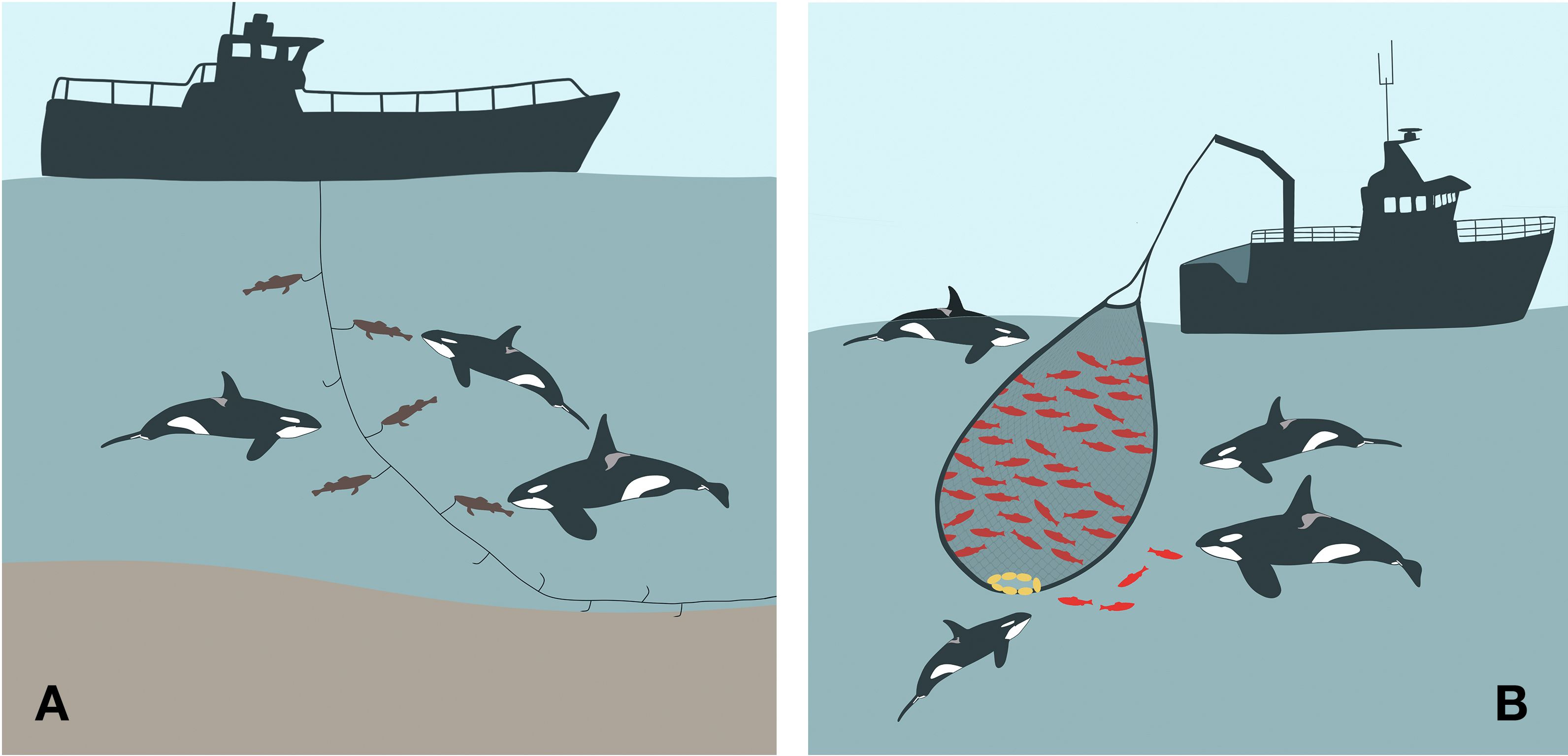
Figure 2. Illustrations of killer whale interactions with fisheries, showing (A) depredation, where whales remove fish from fishing gear and (B) commensalism, where whales feed on discarded fish and lost catch. Not to scale.
Two shared behaviors were documented in both interaction types: potential attraction to vessel sounds indicating gear haulback (the “dinner bell effect”) and following fishing vessels over long distances (Table 1).
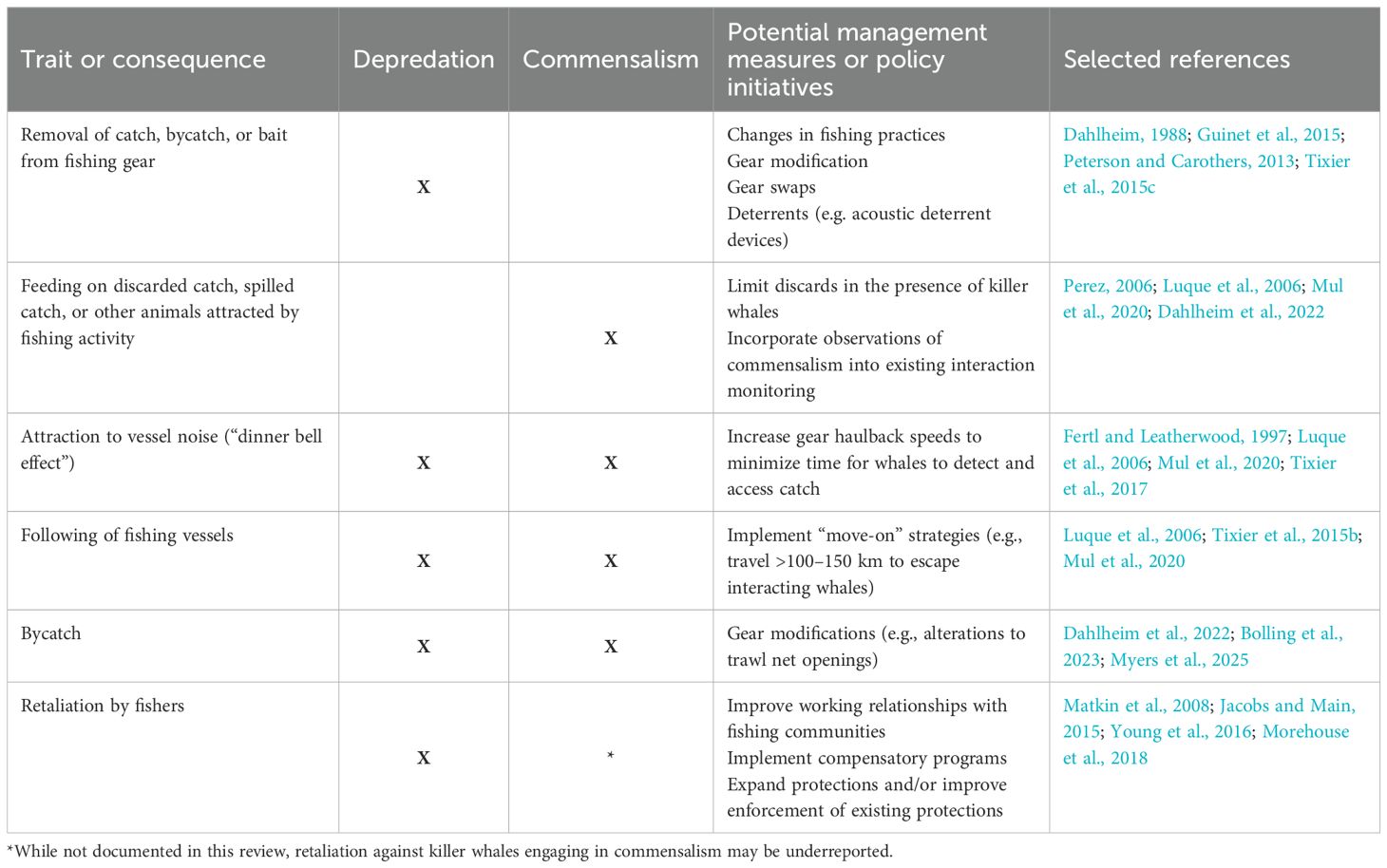
Table 1. Comparison of behavioral traits and consequences of depredation and commensalism in commercial fishery interactions with killer whales, highlighting shared and unique traits and potential management measures to reduce conflict.
3.2 Gear types and species targeted
Fishery interactions with killer whales primarily involved longlines (n=61). Most publications reported killer whale interactions with demersal longlines, followed by pelagic longlines (Figure 3). The skew towards demersal longlines may be the result of the large number of studies (n=17) conducted on killer whale depredation in the Southern Ocean Patagonian toothfish (Dissostichus eleginoides) fishery, which utilizes this gear type (FAO, 2024). Other articles reported interactions involving pelagic and non-pelagic trawl nets, gillnets, purse seines, vertical longlines, unspecified longline types, pots, and troll gear (Figure 3).
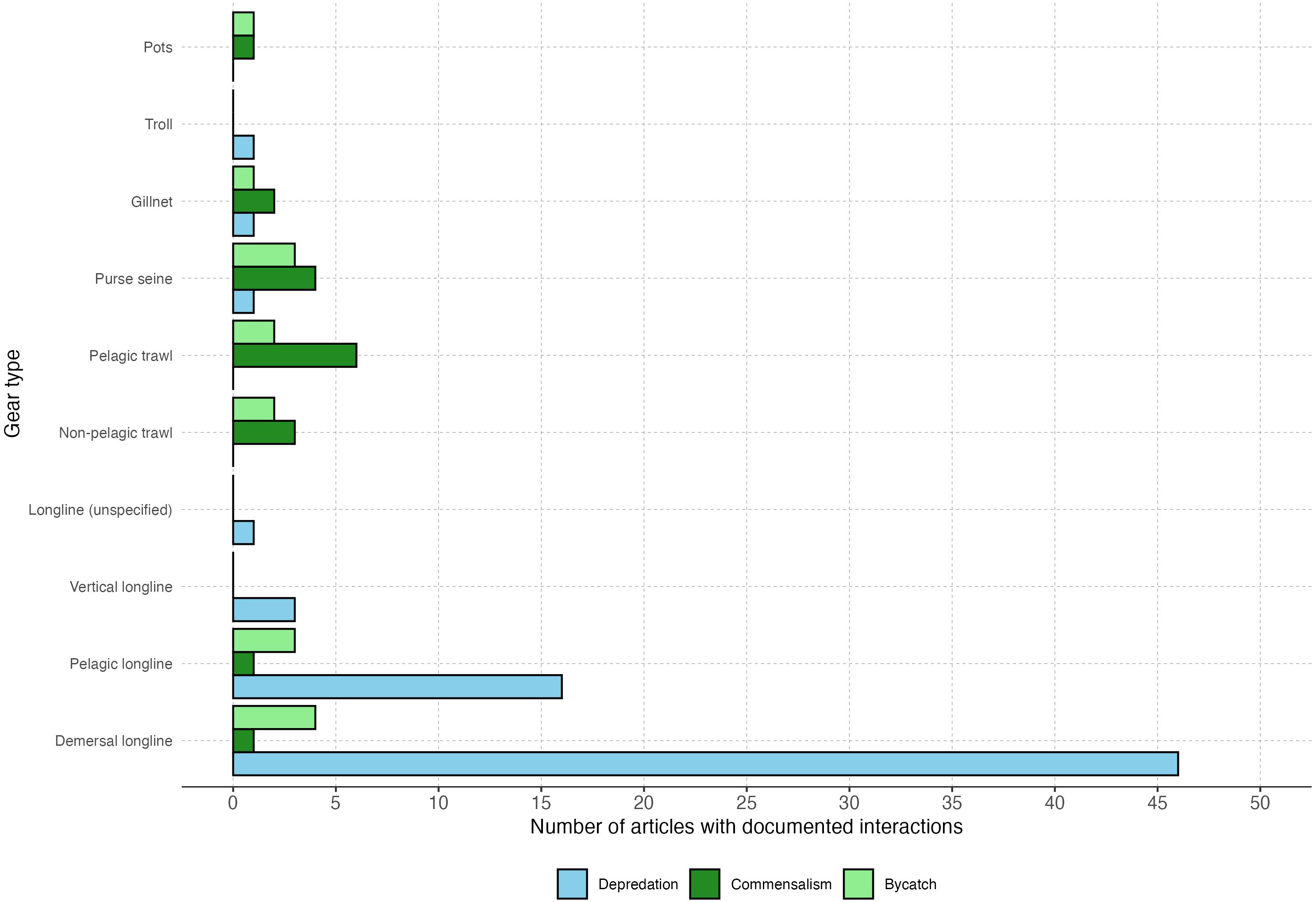
Figure 3. Interactions by gear type. Grouped bar plot showing the number of publications that described interactions, including bycatch and entanglement, across ten different gear types. While 69 publications were reviewed, many contained information regarding multiple interactions and gear types.
Killer whales were observed depredating at least 30 fish species from commercial fisheries between 1952 and 2022 (Supplementary Table 1). Tunas (Thunnus spp.) were the most widely reported genus depredated by killer whales (Supplementary Table 1). In comparison, commensal interactions were documented more frequently with fisheries targeting Atlantic herring (Clupea harengus), mackerel (Scomber scrombus), and groundfish (Table 2). Table 2 and Supplementary Table 1 provide comprehensive accounts of killer whale commensalism and depredation records by area and gear type.
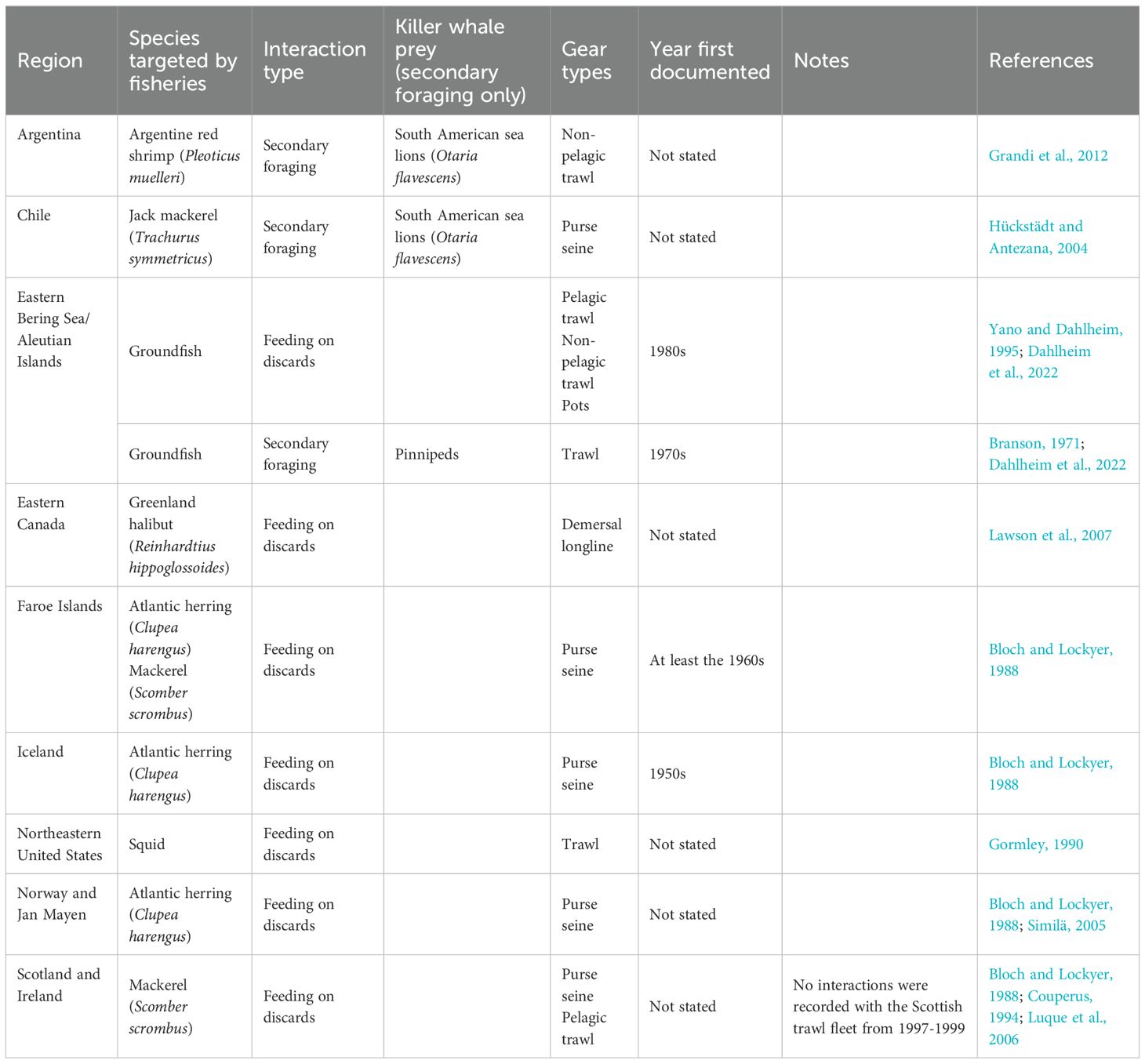
Table 2. Global records of commensal interactions between killer whales and commercial fisheries across locations and gear types from selected references.
3.3 Onset, spread, and types of commercial fishery interactions with killer whales
Documented commercial fishery interactions with killer whales are widespread (Figure 4). The earliest reported interactions we identified were from distant water Japanese tuna longline fisheries in 1952. These interactions entailed killer whales removing and damaging catch in the tropical South Pacific, particularly near Palau (Iwashita et al., 1963). As these fleets expanded into other parts of the South Pacific and Indian Oceans throughout the 1950s and 1960s, reports of killer whale depredation increased, with fishers noting that killer whale depredation typically began within two years of arriving on “virgin” fishing grounds (Iwashita et al., 1963; Sivasubramaniam, 1964). Fishers in the North Atlantic also began documenting killer whales interacting with Atlantic halibut (Hippoglossus hippoglossus), Atlantic herring, and mackerel fisheries around the Faroe Islands, Iceland, and Jan Mayen beginning in the 1950s (Bloch and Lockyer, 1988).
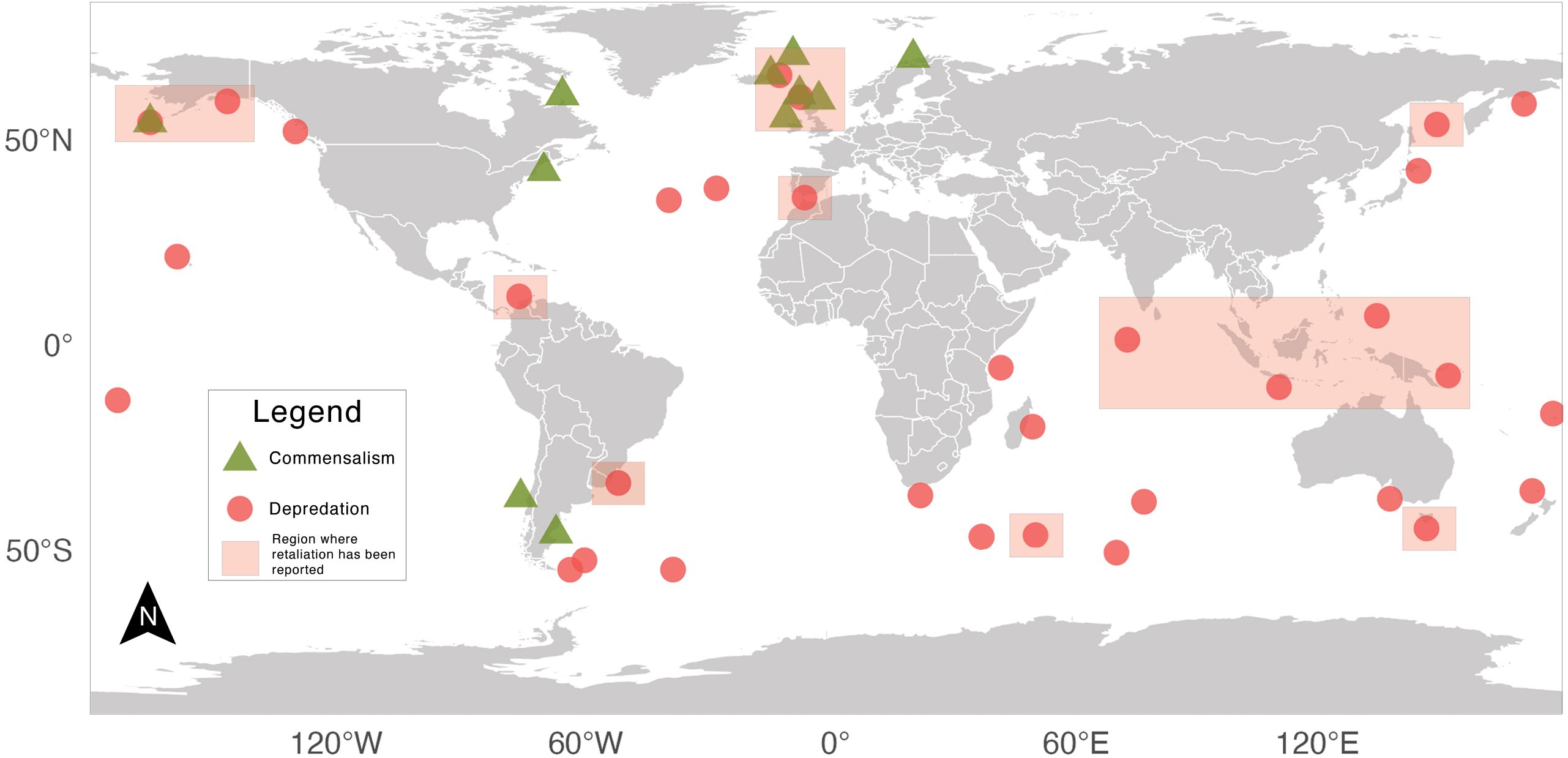
Figure 4. General areas of reported killer whale interactions with commercial fisheries. Red circles denote depredation, while triangles indicate reports of commensalism. Semi-transparent boxes indicate regions where retaliatory actions have been reported. All symbols and shaded areas represent general approximations rather than precise coordinates.
Killer whale depredation was next reported in other areas as commercial fisheries expanded operations. Japanese longliners operating off Alaska in the eastern Bering Sea reported depredation of sablefish (Anoplopoma fimbria) longlines as early as the 1960s (Dahlheim, 1988). Reports of similar interactions in the Gulf of Alaska arose in the 1980s (Dahlheim, 1988). Killer whale depredation of longlines and gillnets was reported from the western Bering Sea and the Sea of Okhotsk in Russia by the mid-1990s (Belonovich et al., 2021, 2019; Kornev et al., 2014).
In the Southern Hemisphere, killer whales were seen depredating blue-eyed trevalla (Hyperoglyphe antarctica) fisheries and tuna and swordfish (Xiphias gladius) longline fisheries operating off southern Australia in the 1970s and 1980s, respectively (Bell et al., 2006; Tixier et al., 2018; Gimonkar et al., 2022). Longliners fishing off New Zealand’s North Island first documented killer whale depredation in 1984 (Visser, 2000). In the tropical southwest Atlantic, including Brazil and Uruguay, killer whales have been observed depredating a variety of highly migratory species such as tuna, marlin, swordfish, and sharks since at least the 1980s (Charles et al., 2020; Rosa and Secchi, 2007; Secchi and Vaske, 1998). Killer whale depredation has also been prevalent in the Southern Ocean Patagonian toothfish fishery, which operates near the Crozet, Marion, Kerguelen, and Falkland Islands, Prince Edward Island, southern Chile, and Argentina (Kock et al., 2006; Nolan et al., 2000; Purves et al., 2004; Tixier et al., 2016; Hucke-Gaete et al., 2004; Roche and Guinet, 2007). Interactions between killer whales and the toothfish fishery were first reported in 1996 when the fishery began (Tixier et al., 2015a) and have continued through the most recent available studies (Auguin et al., 2024; Towers et al., 2019; Tixier et al., 2019).
Commensalism has often been documented with trawling, purse seining, and occasionally pot and longline fisheries (Table 2). Foraging on discards has been documented with groundfish trawlers in the Bering Sea, Aleutian Islands, and the Gulf of Alaska (Dahlheim et al., 2022), pot fisheries in the Bering Sea and Aleutian Islands (Dahlheim et al., 2022), herring and mackerel seiners in Norway, Iceland, and the Faroe Islands (Bloch and Lockyer, 1988; Mul et al., 2020), Greenland halibut (Reinhardtius hippoglossoides) longliners in eastern Canada (Lawson et al., 2007), squid trawlers in Massachusetts (Gormley, 1990), and pelagic mackerel trawlers off Scotland and Ireland (Couperus, 1994; Luque et al., 2006; Mul et al., 2020; Pinfield et al., 2012; Similä, 2005).
In their review of odontocete interactions with trawl fisheries, Bonizzoni et al. (2022) described “secondary foraging,” where a cetacean forages on species that are attracted by fishing activity (Bonizzoni et al., 2022). For example, mammal-eating killer whales have been documented hunting pinnipeds attracted to fisheries to depredate or feed on lost or discarded catch. In Argentina, killer whales have been documented preying on South American sea lions (Otaria flavescens) associating with shrimp trawlers, which discard Argentine hake (Merluccius hubbsi), the sea lions’ preferred prey (Grandi et al., 2012). Chilean purse seiners targeting jack mackerel (Trachurus lathami) (Hückstädt and Antezana, 2004) have also reported killer whales pursuing South American sea lions associated with fishing activity. Similarly, mammal-eating killer whales were known to prey on sea lions following Soviet Union trawlers in the Bering Sea in 1971 (Branson, 1971). Three Bigg’s killer whales that were caught and killed in the Bering Sea/Aleutian Islands pollock trawl fishery may have been pursuing marine mammals, such as Steller sea lions (Eumetopias jubatus), that were attracted to fishing operations (Dahlheim et al., 2022).
3.4 Emerging interactions and areas without documented interactions
While killer whale interactions with fisheries have occurred for decades in some regions, other areas are experiencing relatively new or newly increasing levels of interactions. For example, news articles from northern Japan indicate increasing reports of killer whales damaging and removing flatfish from gill nets over the last ten years (Narayama, 2023). Mitani et al. (2024) recently confirmed these reports through passive acoustic monitoring of demersal gillnets for slime flounder (Microstomus achne). While there are limited reports of killer whales depredating Pacific halibut (Hippoglossus stenolepis) longlines in British Columbia as early as 1990 (Yano and Dahlheim, 1995), anecdotal reports from local fishers indicate interactions may be on the rise and now include salmon (Oncorhynchus spp.) troll fisheries and sablefish longline fisheries in this area (Dracott et al., 2019).
Additionally, some fishing technologies may facilitate emerging interactions with killer whales. While documentation is limited, there are some records of killer whales interacting with fish aggregating devices (FADs), anchored or free-floating objects used by fisheries to attract pelagic fish. Fishers in the Caribbean (Bolaños-Jiménez et al., 2024) and Indonesia (Soede et al., 2019) have reported killer whales foraging on fish around FADs. Fisheries associated with FADs now account for about 70% of global tuna catches (Hallier and Gaertner, 2008), and the extent of documented tuna fishery operational interactions suggests that killer whale-FAD interactions may be underrecognized. Moreover, observed interactions may underrepresent their frequency and impact because FADs are accessed for infrequent, brief intervals.
The ability for novel behaviors to rapidly spread among killer whale social groups (Whitehead et al., 2004) suggests that what begins as a passive foraging tactic may evolve into more direct contact with fishing gear. For instance, in 2023, six killer whales were caught and killed, and one was seriously injured in non-pelagic bottom trawl gear targeting deep-water flatfish in the Bering Sea and Aleutian Islands (NOAA Fisheries, 2023). These events represent a substantial increase over the annual average of 1.1 killer whale mortalities in Alaskan fisheries from 2016–2020 (Young et al., 2024). This relatively high incidental catch rate coincided with anecdotal reports from captains that killer whale presence around non-pelagic trawl vessels started increasing in 2020 (Myers et al., 2025). Acoustic research aboard a non-pelagic trawl vessel during commercial fishing operations indicated that at least some whales were likely foraging around the net, which could include pursuing fish at the mouth of the net or entering the net (Myers et al., 2025). Killer whales have been known to feed on discards from trawlers in this region since at least the 1980s (Dahlheim et al., 2022). Active foraging near the net opening is a recently documented development; however, whether it had previously occurred undetected is unknown, as data to document this type of behavior had not been collected before.
In contrast, other regions with local killer whale populations have not reported fishery interactions. For example, in Greenland, no recent cases of killer whale depredation have been reported (Lennert and Richard, 2017) despite an ongoing fishery for Greenland halibut (Long et al., 2021), a species that attracts killer whales to fisheries operating in other regions (Belonovich et al., 2021; Peterson et al., 2013; Lawson et al., 2007). This may be due to dietary specialization–killer whales around Greenland prey predominantly on other marine mammals, particularly seals and small cetaceans, and fish constitute a minimal part of their diets (Remili et al., 2023). Similarly, in the Ross Sea, killer whales are frequently sighted from vessels engaged in the toothfish fishery, but depredation has not been reported (Kock et al., 2006). We also found no reported cases of killer whale depredation in the Gulf of Mexico pelagic longline fisheries for billfish and tuna, even though pelagic longline fisheries for these species are depredated by killer whales elsewhere. This may be due to the relatively low effort of these fisheries in the Gulf of Mexico and the limited opportunity for whales to depredate (Carretta et al., 2023), the limited proportion of fish in the diet of killer whales found there (Barry et al., 2024), low regional killer whale abundance (Barry et al., 2024), or a combination of these factors. Except for Morocco, there are no published records of killer whale depredation or feeding on discards in western Africa (Tixier et al., 2021a). Given the high level of artisanal and commercial fishing effort in western Africa, the lack of records may be due to limited reporting or investigation.
4 Discussion
This review presents the first comprehensive synthesis of operational fishery interactions with killer whales. We found that interactions with fisheries occur in all oceans across various gear types (Figure 3), with depredation more widely reported than commensalism (Figure 4). However, commensalism may be underreported, as this behavior is not always perceived as damaging to fisheries or included in fishery interaction analyses (Perez, 2006; Luque et al., 2006) and is therefore less likely to garner the same level of attention as depredation. Additionally, while killer whales interacting with fisheries across different regions exhibited a set of common traits, including potential learned associations with vessel sounds indicating haulbacks and extended following of fishing vessels, interaction types carried differing levels of impacts to fisheries and retaliation risks, suggesting the need for tailored management approaches (Table 1).
4.1 Historical context of fisheries interactions
The historical trajectory of fishery interactions with killer whales, from early localized accounts from the tropics to more widespread patterns observed today, reflects both behavioral plasticity and the influence of changing fishing practices within the last century. The onset of reports of killer whale interactions with commercial fisheries in the literature corresponds with the global expansion of modern fishing fleets following World War I and again following World War II (Cushing, 1988; Sahrhage and Lundbeck, 1992). Industrialization allowed for the development of larger, more powerful ships, durable synthetic fibers, more efficient gear capable of catching large quantities of fish, and the ability to freeze catch on board, all of which enabled fishing vessels to successfully fish in distant and more remote stretches of the open ocean for longer periods (Finley, 2016; Pitcher and Lam, 2015).
Increased fishing worldwide provided killer whales with more opportunities to intercept prey species spatially and temporally concentrated by fishing activity. In addition, expanding fishing efforts, including illegal, unreported, and unregulated fishing, may have depleted local resources for some killer whales, prompting depredation to supplement dwindling natural prey availability (Tixier et al., 2021a).
4.2 Behavioral and ecological patterns across fishery interactions
4.2.1 Overlap and variation in killer whale behavior
Across the reviewed literature, we found that two predominant behavioral patterns are often reported in the context of both depredation and commensal interactions: potential attraction to sounds produced by gear haulback and the tendency of interacting whales to follow fishing vessels over long distances (Table 1). Killer whales may use acoustic cues that vessels produce during gear hauling to home in on fishing locations (Dahlheim, 1988; Fertl and Leatherwood, 1997; Visser, 2000; Thode et al., 2007). Some depredating odontocetes, such as sperm whales (Physeter macrocephalus), are known to cue in on propeller cavitation noise produced by fishing vessels during haul-backs (Thode et al., 2007). It is less clear what specific acoustic signals killer whales may use to detect fishing activity or vessels, or how they may vary across fisheries. However, our review suggests that sounds may serve as a common signal for killer whales to identify foraging opportunities associated with fishing activity. Once killer whales have located fishing vessels, they may continue to follow them over long distances, positioning themselves to exploit later feeding opportunities (Cieslak et al., 2021; Tixier et al., 2015c; Towers et al., 2019).
We also found differences between interaction types across gear types and fisheries (Figure 3). Commensal interactions were associated more often with gear aggregating large schools of fish, such as trawl and purse seine fisheries, which often result in the spillover of fish during hauling (Figure 3) (Luque et al., 2006; Mul et al., 2020). Killer whales also forage around fisheries that discard species that may be high-priority prey (Dahlheim et al., 2022). Additionally, some mammal-eating killer whales take advantage of commercial fisheries by pursuing species, such as pinnipeds, attracted to fishing activities. While potentially less common, this type of secondary foraging highlights the potential for fisheries harvest and management to have cascading effects on multiple predators.
In contrast, depredating killer whales were documented more frequently in association with longline fisheries (Figure 3), which target large fish with exposed, baited hooks. Fish immobilized by their capture on longline gear are vulnerable to depredation by killer whales, which may remove whole fish or leave behind just the heads or lips (Secchi and Vaske, 1998; Peterson et al., 2013; Passadore et al., 2015). Tunas, which can exceed 600 kg (NOAA, 2024), are particularly prone to depredation, especially in the tropics (Supplementary Table 1). Esteban et al. (2016b) estimated that a medium-sized pod of killer whales (n=14) would need to hunt at least 21–141 Atlantic bluefin tuna (Thunnus thynnus) per day if the fish ranged in size from 0.8-1.5 m. However, their energetic requirements could be met by as few as eight larger (≥ 2 m) tuna when depredating from local fisheries (Esteban et al., 2016b). The opportunity to intercept large, immobilized, and unprotected fish captured by fishing gear enables killer whales to feed at low energetic cost.
These findings illustrate the shared mechanisms that may facilitate killer whale interactions with fisheries, while also highlighting how interactions can manifest differently across gear types and fisheries.
4.2.2 Alternatives to natural predation and opportunism
There is often uncertainty regarding whether a depredated species constitutes a natural component of a killer whale’s diet or if it is consumed only (or primarily) due to facilitation by fisheries. This is especially true in regions where information on killer whale diets is poor, such as the tropics and subtropics. However, even in relatively well-studied areas, as research techniques have advanced, recent studies have found killer whales naturally forage on species previously only documented to be consumed through depredation, such as sablefish (Myers et al., 2024; Van Cise et al., 2024) and toothfish (Tixier et al., 2019). Sablefish, a commonly depredated species in the Bering Sea, Aleutian Islands, and Gulf of Alaska (Dahlheim, 1988; Peterson et al., 2013), was only recently confirmed as a natural prey species for resident killer whale populations in the North Pacific through fecal sample analysis (Myers et al., 2024; Van Cise et al., 2024). Consumption of sablefish may have been previously undetected through traditional surface prey sampling of prey remains. Similarly, though killer whales in the Crozet Islands have heavily depredated toothfish fisheries since the mid-1990s, it was not until 2019 that Tixier et al. (2019) showed killer whales from this population rely on medium-to-large toothfish as a natural part of their diet through the use of stable isotope mixing models and dietary reconstruction.
Killer whales often depredate selectively, even if multiple species are available in the fishing gear (Kock et al., 2006; Belonovich and Burkanov, 2012; Tixier et al., 2016). In New Zealand, for example, killer whales have been documented removing school sharks (Galeorhinus galeus) and blue-eyed trevalla while ignoring hāpuku (Polyprion oxygeneios) on the same longline (Visser, 2000). Similar selective depredation behavior has been reported from South Georgia (Purves et al., 2004; Gasco et al., 2015), the Crozet Islands (Tixier et al., 2010), the Sea of Okhotsk (Belonovich et al., 2021), the Bering Sea (Peterson et al., 2013), and Iceland (Samarra et al., 2018). Depredating killer whales can be sufficiently selective in their prey choices that the ratio of target catch to bycatch in the presence or absence of killer whales has been used to estimate catch lost to depredation (Gasco et al., 2015). Additionally, depredation of sharks by killer whales is often associated with the targeted removal of the liver and nearby organs through the pelvic girdle (Morrice, 2004; Silva et al., 2011; Passadore et al., 2015; Mucientes and Gonzalez-Pestana, 2020), a behavior that is also known from killer whales that naturally forage on sharks (Engelbrecht et al., 2019; Towner et al., 2022; Reeves et al., 2025). However, some studies have noted that killer whales removed normally neglected bycatch species when catch amounts of target species were low (Passadore et al., 2015; Charles et al., 2020), indicating some flexibility in choice.
Tixier et al. (2019) hypothesize that depredation may be more likely to develop in killer whale groups if the species captured in the fishing gear are already part of their natural diets. Forty-six percent of the fish species that killer whales were documented depredating from fisheries have also been identified as natural prey (Table 3). However, selectiveness may have developed or compounded over time with repeated exposure to prey sources more readily available through operational fishery interactions. For example, in the Gulf of Alaska, Chinook (O. tshawytscha), chum (O. keta), and coho (O. kisutch) salmon dominate resident killer whale diets in the summer, whereas sablefish make up a smaller proportion (Myers et al., 2024; Van Cise et al., 2024). However, sablefish constitute a high proportion of killer whale depredation records in the same region (Peterson and Hanselman, 2017). Similarly, in the Crozet Islands, killer whales may switch from natural predation of toothfish to depredation when fisheries facilitate access to aggregated toothfish (Tixier et al., 2019). When depredating, an individual killer whale can likely satisfy its daily energy requirements from depredated toothfish alone. However, annual contributions of depredated toothfish fulfill less than an estimated 10% of the Crozet Island killer whale population’s energetic requirements (Faure et al., 2021), as the fishery has a short season. Nevertheless, if killer whales are fulfilling a substantial portion of their caloric needs through depredation, even if only seasonally, trophic interactions within a local ecosystem may shift with the release of predation pressure from other prey species. Such potential ecosystem effects likely depend on seasonal fishing effort, targets, and killer whale foraging activities outside of interactions (Faure et al., 2021).
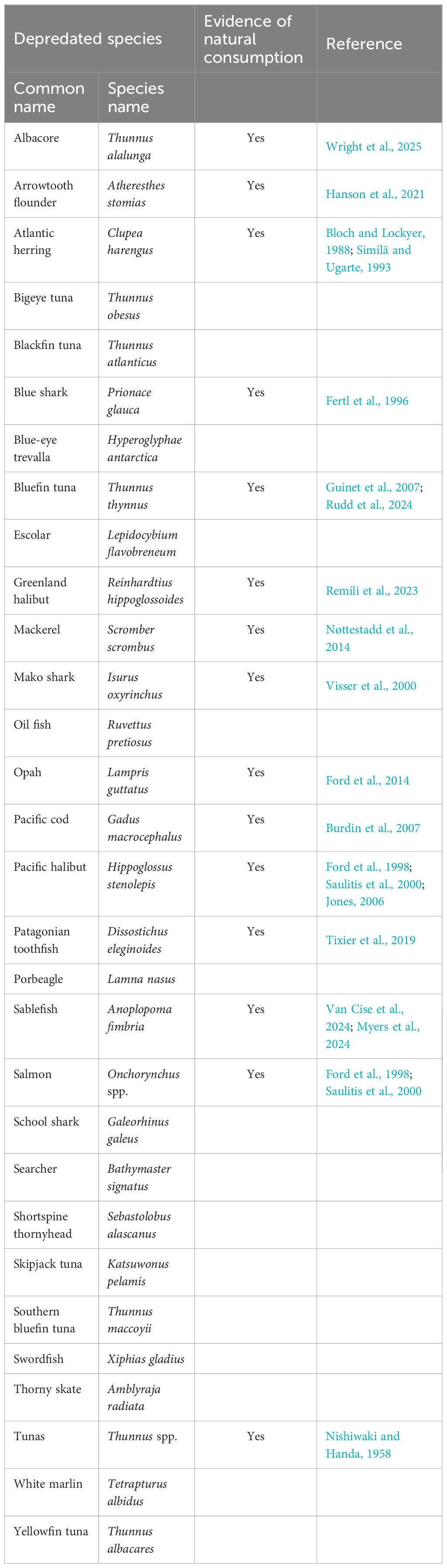
Table 3. Depredated species and evidence of natural consumption by killer whales from selected references.
4.2.3 Accessing prey at depth
Killer whales may modify their diving behavior to facilitate exploitation of fisheries and can also exceed depths previously thought to be a limiting factor in interactions. In the northern and southern hemispheres, killer whales have been documented diving up to 480 m (Schorr et al., 2022) and 767 m (Reisinger et al., 2015), respectively, when not around fishing vessels, though most documented foraging dives for the species are under 200 m (Baird et al., 2005; Miller et al., 2010; Tennessen et al., 2019). Killer whale research efforts have typically been biased towards coastal regions, where study areas are often shallow (e.g., Baird et al., 2005). Consequently, initial efforts to study killer whale depredation of demersal longlines focused primarily on the haulback, the retrieval process when captured fish are brought to the surface (Dahlheim, 1988; Purves et al., 2004; Clark and Agnew, 2010; Werner et al., 2015).
More recent studies have demonstrated that killer whales can access greater depths at which these species occur and where demersal longline gear is deployed (Towers et al., 2019; Richard et al., 2019, 2022). For example, a tagged killer whale depredating toothfish longlines in South Georgia dove up to 1087 m, the deepest documented dive for this species (Towers et al., 2019). Towers et al. (2019) suggested that intra- and interspecific competition during fishery interactions likely drives this extreme diving behavior. In South Georgia, killer whales and sperm whales depredate longlines simultaneously and modify their dive depths and speeds to access toothfish during gear retrieval (Kock et al., 2006; Towers et al., 2019). The benefits of gaining first access to longlines, such as the opportunity to select the largest fish available, may outweigh the energetic and physiological costs of undertaking rapid and deep dives (Towers et al., 2019). Similarly, in the Bering Sea, killer whales foraging around trawl vessels were recorded close to fishing nets at approximately 400 m depth (Myers et al., 2025), a depth that exceeds most other dive records in the North Pacific (Baird et al., 2005; Miller et al., 2010; Tennessen et al., 2019).
Further, recent passive acoustic monitoring in the Crozet Island toothfish fishery revealed the presence of foraging killer whales from at least two different populations around set demersal longlines, suggesting that whales may detect and potentially depredate from demersal longlines before the retrieval process begins (Richard et al., 2022). Some killer whales in South Georgia may also depredate the longlines during the soak period (Towers et al., 2019). In addition, passive acoustic monitoring work from southeastern Australia’s blue-eyed trevalla fishery suggested that killer whales may remove fish from demersal longlines before gear retrieval (Cieslak et al., 2021). Monitoring of demersal longline depredation that relies on confirmation of killer whales at the surface during hauling and counting damaged fish (e.g., Peterson et al., 2014; Passadore et al., 2015) likely underestimates depredation rates and the total amount of fish lost if whales are depredating before gear retrieval.
4.2.4 Artificial provisioning and influence on reproduction
Artificial wildlife provisioning can influence fecundity, offspring quality, and population trajectories of provisioned species (Griffin et al., 2022, 2023). These dynamics are particularly relevant to killer whales exploiting the predictable food sources fisheries provide. For example, in the Crozet Islands, adult female killer whales involved in depredation of the local toothfish fishery demonstrated a 4% increase in the probability of producing a calf the following year compared to non-depredating females (Tixier et al., 2015a). Additionally, Tixier et al. (2015a) found that depredating whales experienced higher survival rates than non-depredating individuals. Further, non-depredating matrilines have declined over the last few decades, possibly due to reduced reproduction and/or changes in natural prey availability (Tixier et al., 2015a, 2017).
While increased access to prey may boost reproductive output and overall fitness, depredation may lead to dependency upon fisheries for food. In the Strait of Gibraltar, a small subpopulation of killer whales is known to hunt Atlantic bluefin tuna through an endurance-exhaustion method, where individuals chase fish for over thirty minutes (Guinet et al., 2007). Increased demand and overfishing of bluefin tuna in the Eastern Atlantic and Mediterranean beginning in the 1960s led to a severe stock decline and reduced natural prey availability for the Gibraltar subpopulation of killer whales, which also depredates tuna from local drop-line fisheries (Esteban et al., 2016b; ICCAT, 2010). Esteban et al. (2016a) found that individuals interacting with the tuna fisheries exhibit higher survival and reduced reproductive intervals than non-interacting individuals (Esteban et al., 2016a). Additionally, a reduction in drop-line tuna harvest was associated with declining killer whale births, suggesting that the population relied on prey availability provided by the fishery (Esteban et al., 2016a). The bluefin tuna stock in this area has largely recovered (Bjørndal, 2023), though how stock recovery has impacted local killer whale depredation rates, survival, or reproduction has not been documented.
Sex biases have been detected in depredating odontocetes, such as Hawaiian false killer whales, where females interact more frequently with pelagic longlines than males (Baird et al., 2015). In 2023, all killer whales bycaught in the Bering Sea and Aleutian Islands fisheries for which sex was confirmed were adult females (NOAA Fisheries, 2023). Sex-associated differences in behavior may also be important in understanding the dynamics of fishery interactions with killer whales, especially as they relate to fecundity and mortality.
4.2.5 Harmful retaliation
In some circumstances, fishers engaged in legal and illegal fishing operations may retaliate against killer whales. Retaliatory measures can include the use of guns, harpoons, explosives, or other projectiles to deter killer whales from accessing catch. Notably, all reports of retaliatory actions in the reviewed literature were associated with cases of depredation, rather than commensalism, though this should be interpreted cautiously, as many events likely go unreported. The loss of catch from fishing gear through depredation is more economically damaging to fisheries than commensal activities and thus may prompt direct action from some fishers, including harmful and injurious measures to repel killer whales from gear. This dynamic mirrors some human-wildlife conflicts in terrestrial environments—depredation of valuable livestock often results in retaliatory killings of large carnivores (Woodroffe et al., 2005; Kissui, 2008).
These sometimes lethal retaliatory tactics have been documented in fisheries in Alaska (Matkin et al., 2008; Fraker, 2013), Australia (Morrice, 2004), the Crozet Islands (Tixier et al., 2017), Colombia (Alvarez-León, 2002) Brazil and Uruguay (Rosa and Secchi, 2007), the Faroe Islands and Jan Mayen (Bloch and Lockyer, 1988), the Sea of Okhotsk (Kornev et al., 2014), the Strait of Gibraltar (De Stephanis et al., 2002), and parts of the equatorial Pacific and Indian Ocean basins (Sivasubramaniam, 1964) (Figure 4.). However, these documented cases likely underestimate the frequency of harmful retaliation due to insufficient reporting in many areas. Fishers have reported that explosives and gunfire may be ineffective in deterring killer whales because the whales returned after initially scattering (Kornev et al., 2014) or learned to stay out of range of projectiles while depredating (Bloch and Lockyer, 1988; Kornev et al., 2014). However, some researchers have noted that killer whales in areas of high depredation have displayed avoidance behaviors around vessels, which may suggest that past negative interactions potentially induced long-lasting behavioral responses (Dahlheim et al., 2022).
One of the most extreme cases of documented retaliation occurred from 1954–1956 in Iceland, when hundreds of killer whales were reportedly killed by the U.S. Navy in response to reports of damage to fishing gear (Jourdain et al., 2019). In Prince William Sound, Alaska, killer whales from the resident AB pod were subject to retaliatory shootings from fishers attempting to deter the whales from depredating sablefish catches (Matkin et al., 2008). Bullet wounds were documented on 13 killer whales from this pod from 1985–1986, and four of these individuals had died by 1987 (Matkin et al., 2008). Similarly, from 1996–2002, killer whales in the Crozet Islands were subject to fatal shootings and explosives intended to reduce their perceived impact on illegal toothfish fisheries; the deterrence efforts caused a 60% population decline (Poncelet et al., 2010; Tixier et al., 2017). Busson et al. (2019) found that these mortalities had a long-term impact on surviving individuals, including weakened social connections and reduced survival rates. Further, Auguin et al. (2024) detected behavioral heterogeneity across depredating killer whale social units in the Crozet Islands, which could suggest that the impacts of lethal retaliation may have been felt unevenly across the population. While illegal fishing in the Crozet Islands has been largely curtailed, there are still concerns that lethal deterrence in illegal, unreported, and unregulated fisheries may persist in neighboring regions as this population decline continued from 2005–2020 (Tixier et al., 2021b).
4.2.6 Entanglement, bycatch, and ship strikes
Killer whales are vulnerable to injury and mortality during fishery interactions. Entanglements and hooking of killer whales have been documented in longline fisheries in Alaska (Bolling et al., 2023; Dahlheim et al., 2022), the Gulf of Mexico (Barry et al., 2024), Australia (Bell et al., 2006), Brazil (Charles et al., 2020), New Zealand (Visser, 2000), the Indian Ocean (Sivasubramaniam, 1964), and the Faroe Islands (Bloch and Lockyer, 1988). Most documented cases of killer whale trawl entanglements have been reported from the Bering Sea, Aleutian Islands, and Gulf of Alaska (Perez, 2006; Bolling et al., 2023), with an additional record from New Zealand (Fertl and Leatherwood, 1997). These reports should be considered conservative estimates, as bycatch is likely underreported in many areas (Donoghue et al., 2003). Curiously, we found no reports of entanglement or incidental catch of killer whales off the Crozet Islands, where interaction rates are among the highest in the world and fisheries observer coverage is 100% (Tixier et al., 2017).
Reports of killer whale bycatch in gillnets are rare. However, a few instances have been reported from California and British Columbia (Carretta et al., 2023), eastern Canada (Lawson et al., 2007), and South Korea (Jin-gu, 2022). Reeves et al. (2013) found that at least 75% of odontocete species are impacted by gillnet bycatch (Reeves et al., 2013), and several species and populations are threatened with extinction due to gillnet entanglement (Read, 2008; Gray and Kennelly, 2018). Several factors may explain the limited number of reported killer whale entanglements in gillnets. Some gillnet fisheries have declined or ceased operations in certain areas of high killer whale abundance, such as off Washington state and California (Carretta et al., 2023). Elsewhere, a lack of spatial or temporal overlap with gillnet fishery operations may explain the low incidence of killer whale bycatch. Furthermore, acoustic deterrent devices are reported to have reduced overall cetacean bycatch in some drift gillnet fisheries (Barlow and Cameron, 2003). However, the recent increase in killer whale gillnet depredation in northern Japan may raise concerns about entanglement risks (Narayama, 2023; Mitani et al., 2024). Additionally, “cryptic” bycatch in gillnets, where an individual becomes entangled in the net but moves away before being observed or documented, may not be accounted for.
Whales depredating or foraging on discards from fisheries are likely to be at higher risk of bycatch because of their proximity to or immediate contact with fishing gear. Whales entangled or entrapped in gear that is soaked or towed for many hours, such as demersal longlines or non-pelagic trawl nets, may be less likely to survive. Only 21% of killer whales found entangled in trawl nets and longlines in Alaskan fisheries from 1991–2023 were released alive (Bolling et al., 2023; NOAA Fisheries, 2023). Most of these released whales had serious injuries and were determined to have a low chance of survival (Bolling et al., 2023).
However, in some bycatch situations, mortality and serious injury rates are low. For example, in northern Norway, killer whales are attracted to pelagic herring purse seine vessels and often approach during the hauling or pumping process (Similä, 2005; Mul et al., 2020). Despite frequent entrapments, nearly all killer whales are released from nets, and overall mortality is estimated to be less than one whale per year (Bjorge et al., 2023). Similar low mortality rates have been documented for cetacean species in other purse seine fisheries in the Atlantic and Indian Oceans (Escalle et al., 2015). Successful avoidance of fatal bycatch depends on multiple factors, including the extent to which whales interact with the fishing gear, fishers’ ability to promptly detect and respond to entrapped whales, and the ability of whales to reach the surface to breathe while entrapped.
Ship strikes are another risk for killer whales interacting with fisheries. Trawl fisheries tend to concentrate mammals near the stern where the net is being towed, increasing the chance of propeller strikes (Bonizzoni et al., 2022). In Alaska, at least ten killer whales were struck and killed by propellers on trawl vessels from 1998 through 2016 (Dahlheim et al., 2022). Some killer whales associated with these vessels display severe injuries caused by previous ship strikes, such as severed dorsal fins, suggesting that some individuals may continue to engage in high-risk, high-reward behaviors around fisheries despite prior negative experiences (Dahlheim et al., 2022).
Killer whales may be left with scars or injuries caused by interactions with net gear such as purse seines (Similä, 2005). Other depredating odontocetes sustain significant injuries during fishery interactions. For example, the insular Hawaiian Islands false killer whale population, known to interact frequently with pelagic longline fisheries, has a high rate of dorsal fin disfigurements caused by longlines and hooks (Baird and Gorgone, 2005). Analyses of dorsal fin disfigurations in killer whales are limited. However, research from Iceland suggests that a small percentage of killer whales have sustained superficial to moderate injuries through interactions with fishing gear (Lionnet, 2020).
4.3 Management and deterrence
Interactions with killer whales are a significant concern in many fisheries, particularly when depredation is involved, as it can result in steep monetary losses caused by lost or damaged catch and gear; risk bycatch and injury of killer whales, which are protected in some regions; and impact stock assessments and fisheries management.
Various strategies have been tested across different fisheries to mitigate killer whale interactions. Most entail changes to fishing practices or technological interventions.
4.3.1 Changes to fishing practices
When faced with high levels of depredation, fishers may attempt to reduce interactions by altering their fishing practices. Mitigation measures include moving to different fishing grounds, increasing distances between sets, changing gear length, changing hauling speed, or fishing in tandem with other vessels (Dahlheim, 1988; Guinet et al., 2015; Peterson and Carothers, 2013; Tixier et al., 2015c). These measures have variable efficacy rates and often come with their own costs. For example, because killer whales can pursue fishing vessels across large areas (300–1000 km; Cieslak et al., 2021; Tixier et al., 2015c; Towers et al., 2019), fishers must travel long distances to reduce the chance of being followed, which increases fishing time and associated costs. Fishers’ options may also be limited if fishing activity is constrained to specific areas.
While these measures are specifically aimed at reducing depredation, changing fishing practices may also prove useful for mitigating commensal interactions. While commensalism does not negatively affect fisheries as extensively as depredation, limiting commensal interactions may prevent depredation from developing by reducing the reinforcement of associations between fishing activity and foraging opportunities. Some fisheries may benefit from limiting discards of unwanted catch, bycatch, or offal in the presence of killer whales. However, discard retention within individual fisheries is often subject to capacities in vessel holds, quality of catch, and prevailing fisheries regulations (Suuronen and Gilman, 2020).
Management decisions can also influence killer whale interactions through spatiotemporal aspects of fisheries. For example, before 1995, halibut and sablefish longline fisheries in the Bering Sea and Gulf of Alaska were managed as open-access, derby-style fisheries, resulting in intense competition (Peterson and Carothers, 2013; Warpinski et al., 2016). Introducing individual fishing quotas reduced vessel numbers and extended fishing seasons, but also coincided with increased reports of killer whale depredation (Peterson and Carothers, 2013). The increase may be due to two factors: longer fishing seasons likely provided killer whales with more reliable feeding opportunities, while fewer vessels on the fishing grounds probably increased the chances of individual vessels being targeted by killer whales. For comparison, in the Crozet Islands, vessels fishing alone faced higher depredation levels than the average depredation levels experienced when multiple vessels fished near each other, suggesting a dilution or satiation effect (Tixier et al., 2015c).
4.3.2 Technological interventions
Technological interventions aim to reduce or prevent depredation by rendering the catch inaccessible to whales or deterring whales from depredating—for example, by switching from unprotected gear, such as baited hooks, to protected gear, like pots or traps. Federal regulations for sablefish fisheries in the Bering Sea/Aleutian Islands region and the Gulf of Alaska were changed in 2008 and 2017, respectively, to permit the use of pot gear in place of hook-and-line gear to mitigate killer whale and sperm whale depredation (NOAA, 2008; 2016). In the Crozet Islands, switching to pot gear also proved effective against killer whale depredation (Guinet et al., 2015). Iceland’s fishers switched from longlines to trawl gear after killer whales significantly reduced catches of Greenland and Atlantic halibut in the 1970s, which largely eliminated killer whale depredation (Samarra et al., 2018). However, switching between gear types, such as from longlines to trawl, can be complicated by competing operational restraints and fishery management objectives, including complex bottom topography, vulnerable fish habitat, bycatch concerns, regulatory constraints, or economic considerations. Even when gear changes effectively reduce killer whale depredation, considerations such as vessel size, handling, costs, target catch rate, and adherence to relevant fishing regulations affect their uptake. For example, although pots reduce killer whale depredation in toothfish fisheries, they have not been adopted due to low CPUE, increased crab bycatch, biased catch of large female toothfish, and crew safety concerns (Guinet et al., 2015).
Alternatively, gear can be modified to obscure the catch from whales. For example, filamentous “umbrella” shrouding devices combined with shortened gangions resulted in a 68% reduction in killer whale depredation in blue-eyed trevalla longline fisheries (IPHC, 2022). Similarly, the “cachelotera” device, developed for Chilean toothfish fisheries, covers each hooked fish with a cone-shaped rigid net (Moreno et al., 2008; IPHC, 2022). Gear modifications also include bycatch excluder devices in fisheries where marine mammals may become entrapped in trawl nets. A gear modification to reduce the likelihood that killer whales will enter the trawl mouth is being investigated in deep-water flatfish trawl nets in the Bering Sea, with promising initial results (Myers et al., 2025).
Acoustic deterrent devices, which include pingers and acoustic harassment devices (AHDs), have been tested on odontocetes in a variety of fisheries and fish farming applications with mixed results (Dawson et al., 2013; Elmegaard et al., 2023; Kolipakam et al., 2022; López and Mariño, 2011; Palka et al., 2008). These devices are placed on or near fishing gear and emit sounds intended to alert cetaceans to the gear’s presence and/or discourage them from depredating or feeding around fishing operations (FAO, 2021). An AHD device designed specifically to deter killer whales, the OrcaSaver, was tested in the Crozet Islands toothfish fishery in 2011 (Tixier et al., 2015b). While killer whales initially departed when the device was activated, they demonstrated habituation (i.e., a reduced or nonexistent response) after fewer than ten exposures. Moreover, Tixier et al. (2015b) advised against using this device in longline fisheries due to the potential adverse effects of increased noise exposure on killer whales. Some scholars have also critiqued using these acoustic devices to keep whales away from fishing gear while simultaneously provisioning them (Lucas and Berggren, 2023).
Other potential techniques for deterring killer whales from interacting with fisheries include electric shocks, rubber bullets, bubble screens, noxious chemicals, and previously discussed injurious deterrents (Dahlheim, 1988). However, most of these methods are ineffective or untested, may raise ethical concerns, or may violate marine mammal protection statutes in some countries (Visser, 2000).
4.4 Policy frameworks to address interactions
Policy frameworks in the U.S. and internationally provide a basis for addressing marine mammal conservation issues. In the U.S., the Marine Mammal Protection Act (MMPA; 1972) does not directly address marine mammal depredation but does establish a framework for managing marine mammal bycatch in fisheries. Management actions addressing bycatch of marine mammals in the U.S. include gear modifications (Willse et al., 2022) and spatiotemporal fishing restrictions (Bisack and Magnusson, 2021). These techniques can be employed to lower the incidence of bycatch of depredating marine mammals. For example, “weak hook” requirements have been implemented in Hawaiian and Atlantic longline fisheries to reduce serious injury and mortality in depredating false killer whales and short-finned pilot whales (Globicephala macrorhynchus), respectively (Fader et al., 2021). When fishery takes of a marine mammal stock pose a conservation concern (e.g., they exceed Potential Biological Removal, the maximum number of animals that can be removed by human activities while allowing the population to maintain or recover a sustainable size), further actions are often triggered, including the creation of Take Reduction Teams. As a caveat, these metrics rely on accurate stock assessments, many of which are limited by data availability and funding (Hilt, 2023). In addition, in December 2014, the National Marine Fisheries Service requested input on national guidelines for deterring marine mammals under Section 101(a)(4) of the MMPA, which authorizes fishers to deter marine mammals from fishing gear in a manner that does not result in serious injury or death (Long et al., 2015). However, these guidelines have not been finalized as of 2024.
In international law, marine mammal conventions are typically limited in scope and primarily address direct harvest; they do not provide direct authority to regulate fishery interactions with killer whales or other marine mammals (e.g., International Convention for the Regulation of Whaling, 1946; United Nations Law of the Sea Convention, 1982). However, RFMOs may provide an avenue for addressing interactions between high-seas fisheries and killer whales. Currently, only one RFMO, the Southern Indian Ocean Fisheries Agreement (SIOFA), addresses killer whale interactions in its conservation and management plans (Elliott et al., 2023). The SIOFA’s Conservation and Management Measure for the Management of Demersal Stocks in the Agreement Area (Management of Demersal Stocks) encourages fisheries to cease longline hauling in the presence of killer whales and to set longlines at depths over 1000 m (SIOFA, 2024), consistent with research on documented diving depths in depredating killer whales (Towers et al., 2019). However, international law has limited power with non-party states and largely relies on self-enforcement by states party to relevant agreements.
Human dimensions are an important aspect of policy considerations regarding killer whale interactions. Social factors, such as personal experiences and cultural norms, play an important role in shaping perceptions of human-wildlife conflict (Dickman, 2010). These may be particularly critical to consider when addressing conflicts with killer whales due to the species’ broad global distribution and capacity to interact with different fisher socio-cultural groups. Of the papers reviewed here, only one focused on the perspectives and opinions of fishers facing killer whale depredation. Peterson and Carothers (2013) found that most respondents in their study were frustrated with fisheries managers and highlighted the disproportionate attention killer whales receive as charismatic megafauna. Some fishers have also expressed a reluctance to share their knowledge regarding depredation with managers (Dracott et al., 2019). Trust-building between stakeholders and managers is crucial for resolving conservation conflicts, particularly when facilitating knowledge-sharing (Young et al., 2016).
Programs and policies implemented in terrestrial environments to address the depredation of crops and livestock may also be instructive for fisheries managers and policymakers focused on mitigating killer whale interactions. For example, compensatory programs aim to reduce the lethal take of predators, such as wolves (Canis spp.) or mountain lions (Puma concolor), by compensating ranchers for financial losses due to livestock depredation (Jacobs and Main, 2015; Morehouse et al., 2018). While marine systems and fisheries differ in many ways from terrestrial farming and ranching, programs to reduce the financial impacts of killer whale interactions could be construed. Compensatory programs could alleviate monetary losses for small-scale commercial fishing operations that sometimes lose substantial portions of catch, and thus income, due to killer whale depredation. Such programs could also reduce financial pressure on fishers who incur costs while avoiding or limiting depredation, such as the time and fuel costs involved in move-on strategies, or incentivize fishers to cease harmful retaliation or deterrence methods. Subsidies for alternative gear or gear modifications could also relieve the up-front financial costs to limiting killer whale interactions.
4.5 Knowledge gaps
4.5.1 Underreporting, lack of monitoring, and biases
A major challenge for assessing operational fishery interactions with killer whales is underreporting and a lack of monitoring in many regions. Observer presence on vessels can be useful in documenting interactions. For example, in the Southern Ocean toothfish fishery, 100% observer coverage is required for all vessels fishing in waters managed through the Convention for the Conservation of Antarctic Living Marine Resources. This results in detailed data collection on interactions with killer whales (FAO, 2024). The deepwater flatfish trawl fishery in the Bering Sea and Aleutian Islands also operates with 100% observer coverage (every haul is observed). However, observer coverage can be uneven across fisheries (e.g., Somers et al., 2022), and in those with less than full coverage, fishing behavior may change when observers are present (Duarte and Cadrin, 2024).
Additionally, research efforts are unequally distributed across areas where fisheries interactions occur. The studies reviewed here showed a strong bias towards the Southern Ocean and North Pacific, with notable gaps in tropical and subtropical regions. The majority of the literature on killer whale depredation comes from studies conducted on toothfish fisheries in the Southern Ocean (e.g., Faure et al., 2021; Tixier et al., 2017, 2016), followed by research carried out in Alaska (Supplementary Table 1). Consequently, the patterns we present in this review may overrepresent certain gear types and fisheries while underrepresenting others. Additionally, this unequal coverage, which may stem from differences in research capacity and fisheries management structures, makes evaluating the status and trends of killer whale interactions in equatorial areas difficult.
Furthermore, depredation may be overrepresented in studies compared to behaviors like commensalism. Depredation can have both immediate and long-term consequences for fisheries (e.g., damaged gear, increased expenditures to avoid depredating animals, and decreased catches), likely resulting in increased attention from fishers, managers, and researchers. In contrast, commensal interactions, which do not impact fishery yields, may be less likely to attract notice or be formally reported. Literature on human-predator relations tends to emphasize negative outcomes (Pooley et al., 2016), and this bias may also be present in fishery interaction literature. For instance, behaviors like foraging on discards are intentionally excluded in some analyses of fishery interactions (Perez, 2006), which may reinforce emphasis on high-conflict behaviors like depredation. Managers may consider incorporating observations of other behaviors, such as foraging on discards, into monitoring to obtain a more robust understanding of all types of interactions and impacts to fisheries.
Another challenge in assessing interaction levels is that data from individual fisheries is often contained within RFMO reports and other gray literature and requires a high level of searching to collate.
4.5.2 Distribution and ecology
Killer whale behavior and ecology are poorly understood in many world regions where fishery interactions occur, especially in the tropics. While some studies have described seasonal trends in fisheries interactions, few have examined possible driving factors. For instance, in southeastern Brazil, killer whale depredation of tuna and swordfish longlines occurred primarily between June and October (Secchi and Vaske, 1998). However, killer whale movements are largely unknown outside of that timeframe. In southern Australia, killer whales may naturally forage in areas where they also depredate blue-eyed trevalla fisheries (Cieslak et al., 2021), but their dietary habits outside of fisheries interactions remain unstudied.
Spatiotemporal closures have been proposed as a way to reduce contact between fishing operations and depredating marine mammals (Fader et al., 2021), but implementation requires a robust understanding of habitat use and movement patterns, and such data are lacking for many regions where killer whales depredate. Additionally, as fisheries can alter prey availability for marine mammals and may influence dependency on fisheries (Esteban et al., 2016a; Tixier et al., 2019), further ecological and distribution research on less well-studied killer whale populations would also support a better understanding of fishery interactions.
4.5.3 Behavior
Details on killer whale behavior during fishery interactions are limited because individuals spend most of the time underwater when interacting with or around fishing gear. Thus, many aspects of these interactions remain unknown, such as how individuals access catch or become entangled. These knowledge gaps may reduce fishers’ and researchers’ ability to effectively prevent depredation and bycatch, and can also have implications for fish stock assessments and management. For example, research by Richard et al. (2022); Cieslak et al. (2021); Towers et al. (2019), and Tixier et al. (2019) in longline fisheries from the Southern Ocean and southern Australia demonstrated that killer whales are capable of diving to sufficient depths to access soaking demersal longlines and may actively depredate from them. However, it is unknown if demersal longline depredation in other areas is limited to when the gear is being hauled or if killer whales also depredate while it soaks.
Additionally, depredation behavior can vary across killer whale matrilines within a population, and some social groups engage in interactions more frequently than others (Tixier et al., 2015a, 2017; Auguin et al., 2024). Understanding social networks within populations may help explain how behaviors spread among individuals, especially in populations where interactions with fisheries are not widespread. Additional variables that may be relevant include competition with other depredating odontocetes, natural prey availability, foraging conditions, and overall population size.
5 Conclusion
Killer whale interactions with commercial fisheries are a marked example of human-wildlife conflict in marine ecosystems. By synthesizing literature in a global context, this review highlighted important behavioral patterns and potential underlying mechanisms that may facilitate killer whale interactions with fisheries. Depredation is more frequently documented and can lead to greater economic losses for fisheries, which heightens conflict and the potential for retaliatory measures. In contrast, the impacts of commensalism are less substantial, but interactions are probably underreported. In either case, killer whale bycatch remains a fishery management concern. Developing effective management and mitigation methods has proven challenging, as there is no one-size-fits-all solution for addressing these interactions; building trust between fishers and managers and considering social factors within fisher communities is important to resolving these conflicts.
At the same time, the current body of research remains biased towards specific fisheries and geographic regions, which hinders our ability to fully elucidate global trends. Other gaps in knowledge, such as limited research on killer whale ecology and behavior in key areas, further restrict our understanding of how interactions occur or develop over time. More systematic monitoring would help address these information deficits.
Recognizing the full spectrum of operational interactions advances behavioral insights and opportunities to tailor best practices in monitoring and mitigation. By doing so, fishers, managers, and researchers can work towards developing measures that support both fisheries and killer whale populations.
Author contributions
EL: Conceptualization, Investigation, Visualization, Writing – original draft, Writing – review & editing. HM: Writing – review & editing. KRC: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The University of Alaska Fairbanks, Pollock Conservation Cooperative Research Center, provided financial support for the publication of this article.
Acknowledgments
We would like to thank the reviewers for their comments, which substantially improved this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1629516/full#supplementary-material
References
Alvarez-León R. (2002). Capturas comerciales con palangre en la zona económica exclusiva frente a la Guajira, Caribe de Colombia. Rev. Biol. Trop. 50, 237–231. Available online at: https://archivo.revistas.ucr.ac.cr/index.php/rbt/article/view/16253.
Auguin E., Guinet C., Mourier J., Clua E., Gasco N., and Tixier P. (2024). Behavioural heterogeneity across killer whale social units in their response to feeding opportunities from fisheries. Ecol. Evol. 14, e11448. doi: 10.1002/ece3.11448
Baird R. W., Anderson D. B., Kratofil M. A., and Webster D. L. (2021). Bringing the right fishermen to the table: Indices of overlap between endangered false killer whales and nearshore fisheries in Hawai’i. Biol. Conserv. 255, 108975. doi: 10.1016/j.biocon.2021.108975
Baird R. W. and Gorgone A. W. (2005). False killer whale dorsal fin disfigurement as possible indicator of long-line fishery interactions in Hawaiian waters. Pac. Sci. 59, 593–601. doi: 10.1353/psc.2005.0042
Baird R. W., Hanson M. B., and Dill L. M. (2005). Factors influencing the diving behavior of fish-eating killer whales: sex differences and diel and interannual variation in diving rates. Can. J. Zool. 83, 257–267. doi: 10.1139/z05-007
Baird R. W., Mahaffy S. D., Gorgone A. M., Cullins T., McSweeney D. J., Oleson E. M., et al. (2015). False killer whales and fisheries interactions in Hawaiian waters: Evidence for sex bias and variation among populations and social groups. Mar. Mamm. Sci. 31, 579–590. doi: 10.1111/mms.12177
Ballance L. T., Gerrodette T., Lennert-Cody C. E., Pitman R. L., and Squires D. (2021). A history of the tuna-dolphin problem; successes, failures, and lessons learned. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.754755
Ban N. C., Eckert L., McGreer M., and Frid A. (2017). Indigenous knowledge as data for modern fishery management: a case study of Dungeness crab in Pacific Canada. Ecosyst. Health Sustain. 3, 1379887. doi: 10.1080/20964129.2017.1379887
Barlow J. and Cameron G. A. (2003). Field experiments show that acoustic pingers reduce marine mammal bycatch in the California drift gill net fishery. Mar. Mammal. Sci. 19, 265–283. doi: 10.1111/j.1748-7692.2003.tb01108.x
Barry K. P., Mullin K. D., Maze-Foley K., Wilcox Talbot L. A., Rosel P. E., Soldevilla M. S., et al. (2024). Killer whales in the Gulf of Mexico and North Atlantic off the southeastern United States. Front. Mar. Sci. 11. doi: 10.3389/fmars.2024.1460314
Bell C., Shaughnessy P., Morrice M., and Stanley B. (2006). Marine mammals and Japanese long-line fishing vessels in Australian waters: interactions and other sightings. Pac. Conserv. Biol. 12, 31–39. doi: 10.1071/PC060031
Belonovich O. A., Agafonov S. V., Matveev A. A., and Kalugin A. A. (2021). Killer whale (Orcinus orca) depredation on longline groundfish fisheries in the northwestern Pacific. Polar. Biol. 44, 2235–2242. doi: 10.1007/s00300-021-02948-8
Belonovich O. A. and Burkanov V. N. (2012). Killer whale (Orcinus orca) depredation on the Greenland halibut (Reinhardtius hippoglossoides) in long-line fishery in the Sea of Okhotsk (Russia: Suzda).
Belonovich O. A., Novikov R. N., and Terentev D. A. (2019). First records of killer whales (Orcinus orca) depredation on Greenland turbot (Reinhardtius hippoglossoides) and Pacific halibut (Hippoglossus stenolepis) fisheries in Western Bering Sea. Abstract retrieved from PICES 2019 Annual Meeting Presentations (Poster W2-P8) (Victoria, BC, Canada: North Pacific Marine Science Organization (PICES)).
Bisack K. D. and Magnusson G. M. (2021). Spatial management to reduce entanglement risk to North Atlantic right whales in fishing gear: a case study of U.S. northeast lobster fishery 2002-2009. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.540966
Bjorge A., Moan A., Ryeng K. A., and Wiig J. R. (2023). Low anthropogenic mortality of humpback (Megaptera novaeangliae) and killer (Orcinus orca) whales in Norwegian purse seine fisheries despite frequent entrapments. Mar. Mamm. Sci. 39, 481–491. doi: 10.1111/mms.12985
Bjørndal T. (2023). The Northeast Atlantic and Mediterranean bluefin tuna fishery: Back from the brink. Mar. Policy 157, 105848. doi: 10.1016/j.marpol.2023.105848
Bloch D. and Lockyer C. (1988). Killer whales (Orcinus orca) in Faroese waters. Rit. Fiskid. 11, 55–64. Available online at: https://www.hafogvatn.is/static/research/files/rit_fisk_1988_xi_04.pdf.
Bolaños-Jiménez J., Henriquez A., Sánchez-Criollo L., Briceño B., Trujillo F., Esteban R., et al. (2024). “Closer encounters between Orcinus orca and man-made objects: an emerging issue in the Caribbean Sea. 25th Biennial Conference on the Biology of Marine Mammals,” in Abstract retrieved from 25th Biennial Conference on the Biology of Marine Mammals Book of Abstracts, Perth, Australia: Society for Marine Mammalogy. 124–125.
Bolling Z. M., Wright S. K., Teerlink S. S., and Lyman E. G. (2023). Killer Whale Entanglements in Alaska Summary Report: 1991-2022 (Juneau, Alaska, USA: U.S. Department of Commerce). Available online at: https://repository.library.noaa.gov/view/noaa/56185.
Bonizzoni S., Hamilton S., Reeves R. R., Genov T., and Bearzi G. (2022). Odontocete cetaceans foraging behind trawlers, worldwide. Rev. Fish. Biol. Fish. 32, 827–877. doi: 10.1007/s11160-022-09712-z
Branson J. (1971). Killer whales pursue sea lions in Bering Sea drama. Commer. Fish. Rev. 33, 39–40.
Burdin A. M., Hoyt E., Filatova O. A., Ivkovich T., Tarasyan K., and Sato H. (2007). Status of killer whales (Orcinus orca) in Eastern Kamchatka (Russian Far East) based on photo-identification and acoustic studies: Preliminary results. IWC. Rep. SC/59/SM4, 1–11. Available online at: http://www.russianorca.org/Doc/Science/SC59SM4.pdf.
Busson M., Authier M., Barbraud C., Tixier P., Reisinger R. R., Janc A., et al. (2019). Role of sociality in the response of killer whales to an additive mortality event. Proc. Natl. Acad. Sci. 116, 11812–11817. doi: 10.1073/pnas.1817174116
Carretta J. V., Oleson E. M., Forney K. A., Weller D. W., Lange A., Baker J., et al. (2023). U.S. Pacific marine mammal stock assessments: 2022 (West Coast Region: U.S. Department of Commerce). Available online at: https://repository.library.noaa.gov/view/noaa/51022.
Charles W. D., Hazin H., and Travassos P. (2020). Interactions between cetaceans and the tuna/swordfish pelagic longline fishery in the tropical western Atlantic Ocean. Fish. Res. 226, 105530. doi: 10.1016/j.fishres.2020.105530
Chilvers B. L., Corkeron P. J., and Puotinen M. L. (2003). Influence of trawlilng on the behaviour and spatial distribution of Indo-Pacific bottlenose dolphins (Tursiops aduncus) in Moreton Bay, Australia. Can. J. Zool. 81, 1947–1955. doi: 10.1139/z03-195
Cieslak M., Tixier P., Richard G., Hindell M., Arnould J. P. Y., and Lea M. A. (2021). Acoustics and photo-identification provide new insights on killer whale presence and movements when interacting with longline fisheries in South East Australia. Fish. Res. 233, 105748. doi: 10.1016/j.fishres.2020.105748
Clark J. M. and Agnew J. D. (2010). Estimating the impact of depredation by killer whales and sperm whales on longline fishing for toothfish (Dissostichus eleginoides) around South Georgia. CCAMLR. Sci. 17, 163–178. Available online at: https://www.ccamlr.org/fr/system/files/science_journal_papers/09moir-clark-and-agnew.pdf.
Couperus A. S. (1994). Killer whales (Orcinus orca) scavenging on discards of freezer trawlers northeast of the Shetland Islands. Aquat. Mamm. 20, 47–51. Available online at: https://www.aquaticmammalsjournal.org/wp-content/uploads/2009/12/20-01_Couperus.pdf.
Dahlheim M. E. (1988). Killer whale (Orcinus orca) depredation on longline catches of sablefish (Anoplopoma fimbria) in Alaskan waters (Seattle, Washington: National Marine Fisheries Service, Northwest and Alaska Fisheries Center). Available online at: https://apps-afsc.fisheries.noaa.gov/Publications/ProcRpt/PR%2088-14.pdf.
Dahlheim M. E., Cahalan J., and Breiwick J. M. (2022). Interactions, injuries, and mortalities of killer whales (Orcinus orca) observed during fishing operations in Alaska. Fish. Bull. 120, 79–94. doi: 10.7755/fb.120.1.8
Dahlheim M. E. and White P. A. (2010). Ecological aspects of transient killer whales Orcinus orca as predators in southeastern Alaska. Wildl. Biol. 16, 308–322. doi: 10.2981/09-075
Dawson S., Northridge S., Waples D., and Read A. (2013). To ping or not to ping: the use of active acoustic devices in mitigating interactions between small cetaceans and gillnet fisheries. Endanger. Species. Res. 19, 201–221. doi: 10.3354/esr00464
DeMaster D. P., Perry S. L., and Richlen M. F. (2001). Predation and competition: the impact of fisheries on marine mammal populations over the next one hundred years. J. Mammal. 82, 641–651. doi: 10.1644/1545-1542(2001)082<0641:PACTIO>2.0.CO;2
De Stephanis R., Pérez Gimeno N., Salazar Sierra J., Poncelot E., and Guinet C. (2002). “Interactions between killer whales (Orcinus orca) and red tuna (Thunnus thynnus) fishery in the Strait of Gibraltar,” in Proceedings of the Fourth International Orca Symposium, Villiers en Bois, France: CEBC-CNRS. 138–142.
Dickman A. J. (2010). Complexities of conflict: the importance of considering social factors for effectively resolving human-wildlife conflict. Anim. Conserv. 13, 458–466. doi: 10.1111/j.1469-1795.2010.00368.x
Donoghue M., Reeves R. R., and Stone G. S. (2003). Report on the workshop on interactions between cetaceans and longline fisheries (Apia, Samoa: New England Aquarium).
Dracott K., Visona B., and Barrett-Lennard L. G. (2019). Whale Depredation Workshop Summary Report (British Columbia, Canada: OceanWise).
Duarte D. and Cadrin S. X. (2024). Review of methodologies for detecting an observer effect in commercial fisheries data. Fish. Res. 274, 107000. doi: 10.1016/j.fishres.2024.107000
Elliott B., Tarzia M., and Read A. J. (2023). Cetacean bycatch management in regional fisheries management organizations: Current progress, gaps, and looking ahead. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.1006894
Elmegaard S. L., Teilmann J., Rojano-Doñate L., Brennecke D., Mikkelsen L., Balle J. D., et al. (2023). Wild harbour porpoises startle and flee at low received levels from acoustic harassment device. Sci. Rep. 13, 16691. doi: 10.1038/s41598-023-43453-8
Engelbrecht T. M., Kock A. A., and O’Riain. M. J. (2019). Running scared: when predators become prey. Ecosphere 10, e02531. doi: 10.1002/ecs2.2531
Escalle L., Capietto A., Chavance P., Dubroca L., Molina A. D. D., Murua H., et al. (2015). Cetaceans and tuna purse seine fisheries in the Atlantic and Indian Oceans: interactions but few mortalities. Mar. Ecol. Prog. Ser. 522, 255–268. doi: 10.3354/meps11149
Esteban R., Verborgh P., Gauffier P., Giménez J., Foote A. D., and de Stephanis R. (2016a). Maternal kinship and fisheries interaction influence killer whale social structure. Behav. Ecol. Sociobiol. 70, 111–122. doi: 10.1007/s00265-015-2029-3
Esteban R., Verborgh P., Gauffier P., Giménez J., Guinet C., and de Stephanis R. (2016b). Dynamics of killer whale, bluefin tuna and human fisheries in the Strait of Gibraltar. Biol. Conserv. 194, 31–38. doi: 10.1016/j.biocon.2015.11.031
Fader J. E., Elliot B. W., and Read A. J. (2021). The challenges of managing depredation and bycatch of toothed whales in pelagic longline fisheries: two U.S. case studies. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.618031
FAO (2021). Guidelines to prevent and reduce bycatch of marine mammals in capture fisheries (Rome: FAO). Available online at: https://www.bmis-bycatch.org/index.php/references/4dvz8u9u.
FAO (2024). Commission on the Conservation of Antarctic Marine Living Resources - Regional Fishery Bodies (RFB). Available online at: https://www.fao.org/fishery/en/organization/rfb/ccamlr (Accessed October 15, 2024).
Faure J., Péron C., Gasco N., Massiot-Granier F., Spitz J., Guinet C., et al. (2021). Contribution of toothfish depredated on fishing lines to the energy intake of killer whales off the Crozet Islands: a multi-scale bioenergetic approach. Mar. Ecol. Prog. Ser. 668, 149–161. doi: 10.3354/meps13725
Fertl D., Acevedo-Gutierrez A., and Darby F. L. (1996). A report of killer whales (Orcinus orca) feeding on a carcharhinid shark in Costa Rica. Mar. Mamm. Sci. 12, 606–611. doi: 10.1111/j.1748-7692.1996.tb00075.x
Fertl D. and Leatherwood S. (1997). Cetacean interactions with trawls: a preliminary review. J. Northw. Atl. Fish. Sci. 22, 219–248. doi: 10.2960/J.v22.a17
Filatova O. A., Fedutin I. D., Belonovich O. A., Borisova E. A., Volkova E. V., Ivkovich T. V., et al. (2023). Differences in the diet of reproductively isolated ecotypes of killer whales (Orcinus orca Linnaeus 1758) in the Seas of the Russian Far East. Russ. J. Mar. Biol. 49, 477–487. doi: 10.1134/S1063074023060032
Finley C. (2016). “The industrialization of commercial fishing 1930–2016,” in Oxford Research Encyclopedia of Environmental Science (Oxford, United Kingdom: Oxford University Press). Available online at: https://oxfordre.com/environmentalscience/display/10.1093/acrefore/9780199389414.001.0001/acrefore-9780199389414-e-31.
Ford J. K. B. (2018). “Killer whale: Orcinus orca,” in Encyclopedia of Marine Mammals, 3rd ed. Eds. Würsig B., Thewissen J. G. M., and Kovacs K. M. (Cambridge, MA, USA: Academic Press), 531–537. doi: 10.1016/B978-0-12-804327-1.00010-8
Ford J. K. B. and Ellis G. M. (2006). Selective foraging by fish-eating killer whales Orcinus orca in British Columbia. Mar. Ecol. Prog. Ser. 316, 185–199. doi: 10.3354/meps316185
Ford J. K. B., Stredulinsky E. H., Ellis G. M., Durban J. W., and Pilkington J. F. (2014). Offshore killer whales in Canadian Pacific waters: distribution, seasonality, foraging ecology, population status and potential for recovery. DFO Canadian Sci. Adv. Sec. Res. Doc., vii + 55. Available online at: https://publications.gc.ca/site/archivee-archived.html?url=https://publications.gc.ca/collections/collection_2014/mpo-dfo/Fs70-5-2014-088-eng.pdf.
Ford J. K. B., Ellis G. M., Barrett-Lennard L. G., Morton A. B., Palm R. S., and Balcomb K. C. III (1998). Dietary specialization in two sympatric populations of killer whales (Orcinus orca) in coastal British Columbia and adjacent waters. Can. J. Zool. 76, 1456–1471. doi: 10.1139/cjz-76-8-1456
Ford M. J., Hempelmann J., Hanson M. B., Ayres K. L., Baird R. W., Emmons C. K., et al. (2016). Estimation of a killer whale (Orcinus orca) population's diet using sequencing analysis of DNA from feces. PLoS ONE 11, e0144956. doi: 10.1371/journal.pone.0144956
Forney K. A. and Wade P. R. (2006). “Worldwide distribution and abundance of killer whales,” in Whales, Whaling, and Ocean Ecosystems (Berkeley, CA, USA: University of California Press), 145–163.
Fraker M. A. (2013). Killer whale (Orcinus orca) deaths in Prince William Sound, Alaska 1985–1990. Hum. Ecol. Risk Assess. Int. J. 19, 28–52. doi: 10.1080/10807039.2012.719385
Gasco N., Tixier P., Duhamel G., and Guinet C. (2015). Comparison of two methods to assess fish losses due to depredation by killer whales and sperm whales on demersal longlines. CCAMLR. Sci. 22, 1–14 Available online at: https://www.ccamlr.org/ru/system/files/science_journal_papers/Gasco%20et%20al_0.pdf.
Gimonkar Y., Lea M. A., Burch P., Arnould J. P. Y., Sporcic M., and Tixier P. (2022). Quantifying killer whale depredation in the blue-eye trevalla commercial fisheries of south-east Australia. Ocean. Coast. Manage. 221, 106114. doi: 10.1016/j.ocecoaman.2022.106114
Gormley G. (1990). Orcas of the Gulf: A Natural History (San Francisco, California, USA: Sierra Book Club).
Grandi M. F., de Castro R. L., and Crespo E. A. (2012). Killer whales attack on South American sea lion associated with a fishing vessel: predator and prey tactics. Lat. Am. J. Aquat. Res. 40, 1072–1076. doi: 10.3856/vol40-issue4-fulltext-22
Gray C. A. and Kennelly S. J. (2018). Bycatches of endangered, threatened and protected species in marine fisheries. Rev. Fish. Biol. Fish. 28, 521–541. doi: 10.1007/s11160-018-9520-7
Griffin L. L., Haigh A., Amin B., Faull J., Corcoran F., Baker-Horne C., et al. (2023). Does artificial feeding impact neonate growth rates in a large free-ranging mammal? R. Soc Open Sci. 10, 221386. doi: 10.1098/rsos.221386
Griffin L. L., Haigh A., Amin B., Faull J., Norman A., and Ciuti S. (2022). Artificial selection in human-wildlife feeding interactions. J. Anim. Ecol. 91, 1892–1905. doi: 10.1111/1365-2656.13771
Guinet C., Domenici P., Stephanis R., Barrett-Lennard L., Ford J. K. B., and Verborgh P. (2007). Killer whale predation on bluefin tuna: exploring the hypothesis of the endurance-exhaustion technique. Mar. Ecol. Prog. Ser. 347, 111–119. doi: 10.3354/meps07035
Guinet C., Tixier P., Gasco N., and Duhamel G. (2015). Long-term studies of Crozet Island killer whales are fundamental to understanding the economic and demographic consequences of their depredation behaviour on the Patagonian toothfish fishery. ICES. J. Mar. Sci. 72, 1587–1597. doi: 10.1093/icesjms/fsu221
Hallier J. P. and Gaertner D. (2008). Drifting fish aggregation devices could act as an ecological trap for tropical tuna species. Mar. Ecol. Prog. Ser. 353, 255–265. doi: 10.3354/meps07180
Hanson M. B., Emmons C. K., Ford M. J., Everett M., Parsons K., Park L. K., et al. (2021). Endangered predators and endangered prey: seasonal diet of southern resident killer whales. PloS One 16, e0247031. doi: 10.1371/journal.pone.0247031
Herman D. P., Burrows D. G., Wade P. R., Durban J. W., Matkin C. O., LeDuc R. G., et al. (2005). Feeding ecology of eastern North Pacific killer whales Orcinus orca from fatty acid, stable isotope, and organochlorine analyses of blubber biopsies. Mar. Ecol. Prog. Ser. 302, 275–291. doi: 10.3354/meps302275
Hilt R. (2023). The evolution of stock definitions and management implications under the U.S. Marine Mammal Protection Act (Duke University). Available online at: https://dukespace.lib.duke.edu/items/57d2f88c-26ae-4d9b-b106-31cce931ce8d.
Hucke-Gaete R., Moreno C. A., and Arata J. (2004). Operational interactions of sperm whales and killer whales with the Patagonian toothfish industrial fishery off southern Chile. CCAMLR. Sci. 11, 127–140. Available online at: https://www.ccamlr.org/en/system/files/science_journal_papers/08huckegaete-etal.pdf.
Hückstädt L. and Antezana T. (2004). Behaviour of Southern sea lions in presence of killer whales during fishing operations in Central Chile. Sci. Mar. 68, 295–298. doi: 10.3989/scimar.2004.68n2295
International Convention for the Regulation of Whaling (1946). Available online at: https://treaties.un.org/pages/showDetails.aspx?objid=0800000280150135.
IPHC (2022). Report of the 1st international workshop on protecting fishery catches from whale depredation (WS001). IPHC-2022-WS0010-R. Available online at: https://www.iphc.int/uploads/pdf/ws/ws001/iphc-2022-ws001-r.pdf?_t=1699037697 (Accessed March 12, 2025).
Iwashita M., Inoue M., and Iwasaki Y. (1963). On the distribution of Orcinus in the northern and southern Pacific equatorial waters as observed from reports on Orcinus predation. Rep. Fish. Res. Lab. Tokai. Univ. 1, 16.
Jacobs C. E. and Main M. B. (2015). A conservation-based approach to compensation for livestock depredation: The Florida panther case study. PloS One 10, e0139203. doi: 10.1371/journal.pone.0139203
Jin-gu K. (2022). Killer whale, a marine protected species, caught by accident in the sea off Guryongpo, Pohang (Seoul, South Korea: Newsis). Available online at: https://mobile.newsis.com/view.html?ar_id=NISX20220305_0001782647.
Jog K., Sutaria D., Diedrich A., Grech A., and Marsh H. (2022). Marine mammal interactions with fisheries: review of research and management trends across commercial and small-scale fisheries. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.758013
Jones I. M. (2006). A northeast Pacific offshore killer whale (Orcinus orca) feeding on a Pacific halibut (Hippoglossus stenolepis). Mar. Mamm. Sci. 22, 198–200. doi: 10.1111/j.1748-7692.2006.00013.x
Jourdain E., Ugarte F., Víkingsson G. A., Samarra F. I. P., Ferguson S. H., Lawson J., et al. (2019). North Atlantic killer whale Orcinus orca populations: a review of current knowledge and threats to conservation. Mamm. Rev. 49, 384–400. doi: 10.1111/mam.12168
Kissui B. M. (2008). Livestock predation by lions, leopards, spotted hyenas, and their vulnerability to retaliatory killing in the Maasai steppe, Tanzania. Anim. Conserv. 11, 422–432. doi: 10.1111/j.1469-1795.2008.00199.x
Kiszka J. J., Caputo M., Méndez-Fernandez P., and Fielding R. (2021). Feeding ecology of elusive Caribbean killer whales inferred from Bayesian stable isotope mixing models and whalers’ ecological knowledge. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.648421
Kock K. H., Purves M. G., and Duhamel G. (2006). Interactions between cetacean and fisheries in the Southern Ocean. Polar. Biol. 29, 379–388. doi: 10.1007/s00300-005-0067-4
Kolipakam V., Jacob M., Gayathri A., Deori S., Sarma H., Tasfia S. T., et al. (2022). Pingers are effective in reducing net entanglement of river dolphins. Sci. Rep. 12, 9382. doi: 10.1038/s41598-022-12670-y
Kornev S. I., Belonovich O. A., and Nikulin S. V. (2014). Killer whales (Orcinus orca) and Greenland halibut (Reinhardtius hippoglossoides) fishery in the Sea of Okhotsk. Res. Aquat. Biol. Resour. Kamchatka. Northwest. Part Pac. Ocean. 34, 35–50. Available online at: https://www.researchgate.net/publication/311296970_KILLER_WHALES_ORCINUS_ORCA_AND_GREENLAND_HALIBUT_REINHARDTIUS_HIPPOGLOSSOIDES_FISHERY_IN_THE_SEA_OF_OKHOTSK_SI_Kornev_OA_Belonovich_SV_Nikulin.
Lawson J., Stevens T., and Snow D. (2007). Killer whales of Atlantic Canada, with Particular reference to the Newfoundland and Labrador Region (St. John's, Newfoundland, Canada: Department of Fisheries and Oceans Canada). Available online at: https://www.dfo-mpo.gc.ca/csas-sccs/publications/resdocs-docrech/2007/2007_062-eng.htm.
Lennert A. E. and Richard G. (2017). At the cutting edge of the future: Unravelling depredation, behaviour and movement of killer whales in the act of flexible management regimes in Arctic Greenland. Ocean. Coast. Manage. 148, 272–281. doi: 10.1016/j.ocecoaman.2017.08.016
Lionnet L. A. M. G. (2020). Measuring potential anthropogenic impacts on Icelandic killer whales (Orcinus Orca) through scar-based analysis. Ísafjörður, Iceland: University Centre of the Westfjords. Available online at: https://skemman.is/handle/1946/36275.
Long S., Blicher M. E., Arboe N. H., Fuhrmann M., Darling M., Kemp K. M., et al. (2021). Deep-sea benthic habitats and the impacts of trawling on them in the offshore Greenland halibut fishery, Davis Strait, west Greenland. ICES. J. Mar. Sci. 78, 2724–2744. doi: 10.1093/icesjms/fsab148
Long K. J., DeAngelis M. L., Engleby L. K., Fauquier D. A., Johnson A. J., Kraus S. D., et al. (2015). Marine mammal non-lethal deterrents: summary of the technical expert workshop on marine mammal non-lethal deterrents. NOAA Tech. Memo. NMFS-OPR-50 Seattle, Washington, USA: NOAA Fisheries. 38. doi: 10.7289/V5/TM-NMFS-OPR-50
López B. D. and Mariño F. (2011). A trial of acoustic harassment device efficacy on free-ranging bottlenose dolphins in Sardinia, Italy. Mar. Freshw. Behav. Physiol. 44, 197–208. doi: 10.1080/10236244.2011.618216
Lucas S. and Berggren P. (2023). A systematic review of sensory deterrents for bycatch mitigation of marine megafauna. Rev. Fish. Biol. Fish. 33, 1–33. doi: 10.1007/s11160-022-09736-5
Luque P. L., Davis C. G., Reid D. G., Wang J., and Pierce G. J. (2006). Opportunistic sightings of killer whales from Scottish pelagic trawlers fishing for mackerel and herring off North Scotland (UK) between 2000 and 2006. Aquat. Living. Resour. 19, 403–410. doi: 10.1051/alr:2007009
Matkin C. O., Saulitis E. L., Ellis G. M., Olesiuk P., and Rice S. D. (2008). Ongoing population-level impacts on killer whales Orcinus orca following the ‘Exxon Valdez’ oil spill in Prince William Sound, Alaska. Mar. Ecol. Prog. Ser. 356, 269–281. doi: 10.3354/meps07273
Miller P. J. O., Shapiro A. D., and Deecke V. (2010). The diving behavior of mammal-eating killer whales (Orcinus orca): variations with ecological not physiological factors. Can. J. Zool. 88, 1103–1112. doi: 10.1139/Z10-080
Mitani Y., Otuski Y., Nakahara F., Ohizumi H., and Kita. Y. (2024). “Interaction between flat fish bottom gillnet and killer whales Orcinus orca in Hokkaido, Japan. 25th Biennial Conference on the Biology of Marine Mammals,” in 25th Biennial Conference on the Biology of Marine Mammals Book of Abstracts, Perth, Australia: Society for Marine Mammalogy. 296.
Morehouse A. T., Tigner J., and Boyce M. S. (2018). Coexistence with large carnivores supported by a predator-compensation program. Environ. Manage. 61, 719–731. doi: 10.1007/s00267-017-0994-1
Moreno C. A., Castro R., Mújica L. J., and Reyes P. (2008). Significant conservation benefits obtained from the use of a new fishing gear in the Chilean Patagonian toothfish fishery. CCAMLR. Sci. 15, 79–91. Available online at: https://www.ccamlr.org/en/system/files/science_journal_papers/04moreno-et-al.pdf.
Morrice M. G. (2004). Killer whales in Australian territorial waters. Report for the Whale and Dolphin Conservation Society (Narrawong, Australia: Deakin University).
Mucientes G. and Gonzalez-Pestana A. (2020). Depredation by killer whales (Orcinus orca) on a blue shark (Prionace glauca) in Northeastern Atlantic. Aquat. Mamm. 46, 478–482. doi: 10.1578/AM.46.5.2020.478
Mul E., Blanchet M. A., McClintock B. T., Grecian W. J., Biuw M., and Rikardsen A. (2020). Killer whales are attracted to herring fishing vessels. Mar. Ecol. Prog. Ser. 652, 1–13. doi: 10.3354/meps13481
Myers H., Olsen D., Van Cise A., Parsons K., Wells A., and Matkin C. O. (2024). The diverse diet of southern Alaska resident killer whales changes across spatiotemporally distinct foraging aggregations. bioRxiv 1–17. Available online at: https://www.biorxiv.org/content/10.1101/2024.09.12.612612v1 (Accessed March 10, 2025). Preprint.
Myers H., Webster S., Gauvin J., and Concepcion B. (2025). “Reducing killer whale bycatch in the Amendment 80 trawl fishery: lessons learned and the path ahead,” in Alaska Marine Science Symposium, Anchorage, Alaska, North Pacific Research Board.
Narayama M. (2023). Pursuing orcas, a nuisance for fishermen; Kyoto University launches crowdfunding campaign to clarify ecology and take measures (Japan: The Asahi Shimbun). Available online at: https://www.asahi.com/articles/ASR1N5RSXR1JIIPE00L.html.
Nishiwaki M. and Handa C. (1958). Killer whales caught in the coastal waters off Japan for recent 10 years. Sci. Rep. Whales. Res. Inst. 13, 85–96. Available online at: https://www.icrwhale.org/pdf/SC01385-96.pdf.
NOAA Fisheries (2008). Fisheries of the Exclusive Economic Zone Off Alaska; Individual Fishing Quota Program; Community Development Quota Program. Federal Register 73. Available online at: https://www.federalregister.gov/documents/2008/05/19/E8-11183/fisheries-of-the-exclusive-economic-zone-off-alaska-individual-fishing-quota-program-community.
NOAA Fisheries (2016). Fisheries of the Exclusive Economic Zone Off Alaska; Allow the Use of Longline Pot Gear in the Gulf of Alaska Sablefish Individual Fishing Quota Fishery; Amendment 101. Federal Register 81. Available online at: https://www.federalregister.gov/documents/2017/01/31/2017-02055/fisheries-of-the-exclusive-economic-zone-off-alaska-allow-the-use-of-longline-pot-gear-in-the-gulf.
NOAA Fisheries (2024). Western Atlantic Bluefin Tuna. Available online at: https://www.fisheries.noaa.gov/species/western-atlantic-bluefin-tuna (Accessed September 23, 2024).
NOAA Fisheries (2023). “Cause of Death determined for 11 killer whales incidentally caught in fishing gear in Alaska in 2023,” in NOAA Fish Seattle, Washington, USA: Alaska Fisheries Science Center. Available online at: https://www.fisheries.noaa.gov/feature-story/cause-death-determined-11-killer-whales-incidentally-caught-fishing-gear-alaska-2023.
Nolan C. P., Liddle G. M., Elliot J., Nolan C. P., Liddle G. M., and Elliot J. (2000). Interactions between killer whales (Orcinus orca) and sperm whales (Physeter macrocephalus) with a longline fishing vessel. Mar. Mamm. Sci. 16, 658–664. doi: 10.1111/j.1748-7692.2000.tb00961.x
Northridge S. (2018). “Fisheries interactions,” in Encyclopedia of Marine Mammals, 3rd ed. Eds. Würsig B., Thewissen J. G. M., and Kovacs K. M. (Cambridge, MA, USA: Academic Press), 375–383. doi: 10.1016/B978-0-12-804327-1.00130-8
Nøttestadd L., Sivle L. D., Krafft B. A., Langård L., Anthonypillai V., Bernasconi M., et al. (2014). Prey selection of offshore killer whales Orcinus orca in the Northeast Atlantic in late summer: spatial associations with mackerel. Mar. Ecol. Prog. Ser. 499, 275–283. doi: 10.3354/meps10638
Palka D. L., Rossman M. C., VanAtten A. S., and Orphanides C. D. (2008). Effect of pingers on harbour porpoise (Phocoena phocoena) bycatch in the US Northeast gillnet fishery. J. Cetacean. Res. Manage. 10, 217–226. doi: 10.47536/jcrm.v10i3.638
Passadore C., Domingo A., and Secchi E. R. (2015). Depredation by killer whale (Orcinus orca) and false killer whale (Pseudorca crassidens) on the catch of the Uruguayan pelagic longline fishery in Southwestern Atlantic Ocean. ICES. J. Mar. Sci. 72, 1653–1666. doi: 10.1093/icesjms/fsu251
Perez M. A. (2006). Analysis of marine mammal bycatch data from the trawl, longline, and pot groundfish fisheries of Alaska 1998-2004, defined by geographic area, gear type, and catch target groundfish species. U.S. Department of Commerce, NOAA Technical Memorandum NMFS-AFSC-167. Available online at: https://repository.library.noaa.gov/view/noaa/22882 (Accessed April 30, 2025).
Peterson M. J. and Carothers C. (2013). Whale interactions with Alaskan sablefish and Pacific halibut fisheries: Surveying fishermen perception, changing fishing practices and mitigation. Mar. Policy 42, 315–324. doi: 10.1016/j.marpol.2013.04.001
Peterson M. J. and Hanselman D. (2017). Sablefish mortality associated with whale depredation in Alaska. ICES. J. Mar. Sci. 74, 1382–1394. doi: 10.1093/icesjms/fsw239
Peterson M. J., Mueter F., Criddle K., and Haynie A. C. (2014). Killer whale depredation and associated costs to Alaskan sablefish, Pacific halibut and Greenland turbot longliners. PloS One 9, e88906. doi: 10.1371/journal.pone.0088906
Peterson M. J., Mueter F., Hanselman D., Lunsford C., Matkin C., and Fearnbach H. (2013). Killer whale (Orcinus orca) depredation effects on catch rates of six groundfish species: implications for commercial longline fisheries in Alaska. ICES. J. Mar. Sci. 70, 1220–1232. doi: 10.1093/icesjms/fst045
Pinfield R., Enright J., McCarthy A., and Rogan E. (2012). “Interactions between killer whales (Orcinus orca) and the Irish pelagic trawl fisheries during the winter 2010/2011 fishing season,” in 26th Annual Conference of the European Cetacean Society, Galway, Ireland, European Cetacean Society.
Pitcher T. J. and Lam M. E. (2015). Fish commoditization and the historical origins of catching fish for profit. Marit. Stud. 14, 2. doi: 10.1186/s40152-014-0014-5
Poncelet E., Barbraud C., and Guinet C. (2010). Population dynamics of killer whales (Orcinus orca) in the Crozet Archipelago, southern Indian Ocean: a mark-recapture study from 1977 to 2002. J. Cetacean. Res. Manage. 11, 41–48. doi: 10.47536/jcrm.v11i1.629
Pooley S., Barua M., Dickman A., Holmes G., Lorimer J., Loveridge A. J., et al. (2016). An interdisciplinary review of current and future approaches to improving human-predator relations. Conserv. Biol. 31, 513–523. doi: 10.1111/cobi.12859
Purves M. G., Agnew D. J., Balguerías E., Moreno C. A., and Watkins B. (2004). Killer whale (Orcinus orca) and sperm whale (Physeter macrocephalus) interactions with longline vessels in the Patagonian toothfish fishery at South Georgia, South Atlantic. CCAMLR. Sci. 11, 111–126. Available online at: https://aobacwebpage.s3.us-east-2.amazonaws.com/260.pdf.
Read A. J. (2008). The looming crisis: interactions between marine mammals and fisheries. J. Mammal. 89, 541–548. doi: 10.1644/07-MAMM-S-315R1.1
Read A. J., Drinker P., and Northridge S. (2006). Bycatch of marine mammals in U.S. and global fisheries. Conserv. Biol. 20, 163–169. doi: 10.1111/j.1523-1739.2006.00338.x
Reeves R. R., McClellan K., and Werner T. B. (2013). Marine mammal bycatch in gillnet and other entangling net fisheries 1990 to 2011. Endanger. Species. Res. 20, 71–97. doi: 10.3354/esr00481
Reeves I. M., Totterdell J. A., Betty E. L., Donnelly D. M., George A., Holmes S., et al. (2023). Ancestry testing of “Old Tom,” a killer whale central to mutualistic interactions with human whalers. J. Hered. 114, 598–611. doi: 10.1093/jhered/esad058
Reeves I. M., Weeks A. R., Towner A. V., Impey R., Fish J. J., Clark Z. S. R., et al. (2025). Genetic evidence of killer whale predation on white sharks in Australia. Ecol. Evol. 15, 70786. doi: 10.1002/ece3.70786
Reid A. J., Eckert L. E., Lane J. F., Young N., Hinch S. G., Darimont C. T., et al. (2021). Two-Eyed Seeing”: An Indigenous framework to transform fisheries research and management. Fish. Fish. 22, 243–261. doi: 10.1111/faf.12516
Reisinger R. R., Gröcke D. R., Lübcker N., McClymont E. L., Hoelzel A. R., and de Bruyn P. J. N. (2016). Variation in the diet of killer whales Orcinus orca at Marion Island, Southern Ocean. Mar. Ecol. Prog. Ser. 549, 263–274. doi: 10.3354/meps11676
Reisinger R. R., Keith M., Andrews R. D., and De Bruyn N. (2015). Movement and diving behavior of killer whales (Orcinus orca) at a Southern Ocean archipelago. J. Exp. Mar. Biol. Ecol. 473, 90–102. doi: 10.1016/j.jembe.2015.08.008
Remili A., Dietz R., Sonne C., Samarra F. I. P., Rikardsen A. H., Kettemer L. E., et al. (2023). Quantitative fatty acid signature analysis reveals a high level of dietary specialization in killer whales across the North Atlantic. J. Anim. Ecol. 92, 1216–1229. doi: 10.1111/1365-2656.13920
Richard G., Bonnel J., Beesau J., Calvo E., Cassiano F., Dramet M., et al. (2022). Passive acoustic monitoring reveals feeding attempts at close range from soaking demersal longlines by two killer whale ecotypes. Mar. Mamm. Sci. 38, 304–325. doi: 10.1111/mms.12860
Richard G., Bonnel J., Tixier P., Arnould J. P. Y., Janc A., and Guinet C. (2019). Evidence of deep-sea interactions between toothed whales and longlines. Ambio 49, 173–186. doi: 10.1007/s13280-019-01182-1
Roche C. and Guinet C. (2007). Marine mammals and demersal longlines fishery interactions in Crozet and Kerguelen Exclusive Economic Zones: an assessment of the depredation level. CCAMLR. Sci. 14, 67–82. Available online at: http://archive.ccamlr.org/ccamlr_science/Vol-14-2007/04roche-etal.pdf.
Rosa L. D. and Secchi E. R. (2007). Killer whale (Orcinus orca) interactions with the tuna and swordfish longline fishery off southern and south-eastern Brazil: a comparison with shark interactions. J. Mar. Biol. Assoc. U. K. 87, 135–140. doi: 10.1017/S0025315407054306
Rudd J. L., Abel G., Baringo F., Birch S., Block B. A., Collins M. A., et al. (2024). High-resolution biologging of an Atlantic bluefin tuna captured and eaten by a supposed orca. Sci. Rep. 14, 29352. doi: 10.1038/s41598-024-80744-0
Samarra F. I. P., Bassoi M., Béesau J., Elíasdóttir M. Ó., Gunnarsson K., Mrusczok M. T., et al. (2018). Prey of killer whales (Orcinus orca) in Iceland. PloS One 13, e0207287. doi: 10.1371/journal.pone.0207287
Saulitis E., Matkin C. O., Barrett-Lennard L., Heise K., and Ellis G. (2000). Foraging strategies of sympatric killer whale (Orcinus orca) populations in Prince William Sound, Alaska. Mar. Mamm. Sci. 16, 94–109. doi: 10.1111/j.1748-7692.2000.tb00906.x
Schorr G. S., Hanson M. B., Falcone E. A., Emmons C. K., Jarvis S. M., Andrews R. D., et al. (2022). Movement and diving behavior of the eastern North Pacific offshore killer whale (Orcinus orca). Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.854893
Secchi E. and Vaske T. (1998). Killer whale (Orcinus orca) sightings and depredation on tuna and swordfish longline catches in southern Brazil. Aquat. Mamm. 24, 117–122. Available online at: https://www.researchgate.net/profile/Eduardo-Secchi/publication/263203187_Killer_whale_Orcinus_orca_sightings_and_depredation_on_tuna_and_swordfish_longline_catches_in_southern_Brazil/links/5b42541b0f7e9bb59b17a88b/Killer-whale-Orcinus-orca-sightings-and-depredation-on-tuna-and-swordfish-longline-catches-in-southern-Brazil.pdf.
Silva M. A., Machete M., Reis D., Santos M., Prieto R., Dâmaso C., et al. (2011). A review of interactions between cetaceans and fisheries in the Azores. Aquat. Conserv.: Mar. Freshw. Ecosyst. 21, 17–27. doi: 10.1002/aqc.1158
Similä T. (2005). Interactions between herring fishery and killer whales in northern Norway (Aberdeen, Scotland: ICES). Available online at: https://www.ices.dk/sites/pub/CM%20Doccuments/2005/R/R0305.pdf.
Similä T. and Ugarte F. (1993). Surface and underwater observations of cooperatively feeding killer whales in northern Norway. Can. J. Zool. 71, 1494–1499. doi: 10.1139/z93-210
SIOFA (2024). Conservation and Management Measure for the Management of Demersal Stocks in the Agreement Area (Management of Demersal Stocks). Available online at: https://siofa.org/sites/default/files/documents/cmm/CMM-15%282024%29-Management-of-demersal-stocks.pdf (Accessed October 12, 2024).
Sivasubramaniam K. (1964). Predation of tuna longline catches in the Indian Ocean by killer whales and sharks. Bull. Fish. Res. Stn. Ceylon. 17, 221–236. Available online at: https://core.ac.uk/download/pdf/33720395.pdf.
Soede L. P., Natasasmita D., Mahendra I. G., and Rizki W. (2019). Marine mammal interactions with tuna fishing activities in Indonesian seas. IOP. Conf. Ser.: Earth Environ. Sci. 399, 12128. doi: 10.1088/1755-1315/399/1/012128
Somers K. A., Richerson K. E., Tuttle V. J., and McVeigh J. T. (2022). Fisheries Observation Science Program Coverage Rates 2002-21. U. S. Department of Commerce, NOAA Data Report NMFS-NWFSC-DR-2022-02. Available online at: https://repository.library.noaa.gov/view/noaa/47111 (Accessed May 15, 2025).
Suuronen P. and Gilman E. (2020). Monitoring and managing fisheries discards: new technologies and approaches. Mar. Pol. 116, 103554. doi: 10.1016/j.marpol.2019.103554
Tennessen J. B., Holt. M. M., Ward E. J., Hanson M. B., Emmons C. K., Giles D. A., et al. (2019). Hidden Markov models reveal temporal patterns and sex differences in killer whale behavior. Sci. Rep. 9, 14951. doi: 10.1038/s41598-019-50942-2
Thode A., Straley J., Tiemann C. O., Folkert K., and O’Connell V. (2007). Observations of potential acoustic cues that attract sperm whales to longline fishing in the Gulf of Alaska. J. Acoust. Soc Am. 122, 1265–1277. doi: 10.1121/1.2749450
Tixier P., Authier M., Gasco N., and Guinet C. (2015a). Influence of artificial food provisioning from fisheries on killer whale reproductive output. Anim. Conserv. 18, 207–218. doi: 10.1111/acv.12161
Tixier P., Barbraud C., Pardo D., Gasco N., Duhamel G., and Guinet C. (2017). Demographic consequences of fisheries interaction within a killer whale (Orcinus orca) population. Mar. Biol. 164, 170. doi: 10.1007/s00227-017-3195-9
Tixier P., Gasco N., Duhamel G., and Guinet C. (2015b). Habituation to an acoustic harassment device (AHD) by killer whales depredating demersal longlines. ICES. J. Mar. Sci. 72, 1673–1681. doi: 10.1093/icesjms/fsu166
Tixier P., Gasco N., Duhamel G., and Guinet C. (2016). Depredation of Patagonian toothfish (Dissostichus eleginoides) by two sympatrically occurring killer whale (Orcinus orca) ecotypes: insights on the behavior of the rarely observed type D killer whales. Mar. Mamm. Sci. 32, 983–1003. doi: 10.1111/mms.12307
Tixier P., Gasco N., Duhamel G., and Vivant M. (2010). Interactions of Patagonian toothfish fisheries with killer and sperm whales in the Crozet Islands Exclusive Economic Zone: an assessment of depredation levels and insights on possible mitigation strategies. CCAMLR. Sci. 17, 179–195. Available online at: https://aobacwebpage.s3.us-east-2.amazonaws.com/263.pdf.
Tixier P., Gasco N., Towers J. R., and Guinet C. (2021b). Killer whales of the Crozet Archipelago and adjacent waters: photo-identification catalogue, population status and distribution in 2020 (Villiers en Bois, France: Centre d’Etudes Biologiques de Chizé; La Rochelle Université; Muséum National d’Histoire Naturelle; Bay Cetology). doi: 10.6084/m9.figshare.13677145.v1
Tixier P., Giménez J., Reisinger R. R., Méndez-Fernandez P., Arnould J. P. Y., Cherel Y., et al. (2019). Importance of toothfish in the diet of generalist subantarctic killer whales: implications for fisheries interactions. Mar. Ecol. Prog. Ser. 613, 197–210. doi: 10.3354/meps12894
Tixier P., Lea M., Hindell M. A., Guinet C., Gasco N., Duhamel G., et al. (2018). Killer whale (Orcinus orca) interactions with blue-eye trevalla (Hyperoglyphe Antarctica) longline fisheries. PeerJ 6, e5306. doi: 10.7717/peerj.5306
Tixier P., Lea M. A., Hindell M. A., Welsford D., Mazé C., Gourguet S., et al. (2021a). When large marine predators feed on fisheries catches: Global patterns of the depredation conflict and directions for coexistence. Fish. Fish. 22, 31–53. doi: 10.1111/faf.12504
Tixier P., Vacquie Garcia J., Gasco N., Duhamel G., and Guinet C. (2015c). Mitigating killer whale depredation on demersal longline fisheries by changing fishing practices. ICES. J. Mar. Sci. 72, 1610–1620. doi: 10.1093/icesjms/fsu137
Torres D. F., Oliveira E. S., Alves R. R. N., and Alves R. R. N. (2018). “Understanding human-wildlife conflicts and their implications,” in Ethnozoology: Animals in Our Lives. Eds. Alves R. R. N. and Albuquerque U. P. (Cambridge, MA, USA: Academic Press), 421–425. doi: 10.1016/B978-0-12-809913-1.00022-3
Towers J. R., Tixier P., Ross K. A., Bennett J., Arnould J. P. Y., Pitman R. L., et al. (2019). Movements and dive behaviour of a toothfish-depredating killer and sperm whale. ICES. J. Mar. Sci. 76, 298–311. doi: 10.1093/icesjms/fsy118
Towner A. V., Kock A. A., Stopforth C., Hurwitz D., and Elwen S. H. (2022). Direct observation of killer whales preying on white sharks and evidence of a flight response. Ecol 104, e3875. doi: 10.1002/ecy.3875
United Nations Law of the Sea Convention (1982). Available online at: http://treaties.un.org/doc/Publication/UNTS/Volume%201833/volume-1833-A-31363-English.pdf (Accessed February 12, 2025).
Van Cise A. M., Hanson M. B., Emmons C., Olsen D., Matkin C. O., Wells A. H., et al. (2024). Spatial and seasonal foraging patterns drive diet differences among North Pacific resident killer whale populations. R. Soc Open Sci. 11, rsos240445. doi: 10.1098/rsos.240445
Visser I. (2000). Killer whale (Orcinus orca) interactions with longline fisheries in New Zealand waters. Aquat. Mamm. 26, 241–252. Available online at: https://www.orcaresearch.org/wp-content/uploads/2024/12/Visser-2000-orca-longlines-NZ.pdf.
Visser I. N., Berghan J., Van Meurs R., and Fertl D. (2000). Killer whale (Orcinus orca) predation on a shortfin mako shark (Isurus oxyrinchus) in New Zealand waters. Aquat. Mamm. 26, 229–231.
Warpinski S., Herrmann M., Greenberg J. A., and Criddle K. R. (2016). Alaska’s sablefish fishery after individual fishing quota (IFQ) implementation: an international economic market model. N. Am. J. Fish. Manage. 36, 864–875. doi: 10.1080/02755947.2016.1165766
Werner T. B., Northridge S., McClennan Press K., and Young N. (2015). Mitigating bycatch and depredation of marine mammals in longline fisheries. ICES J. Mar. Sci. 72, 1576–1586. doi: 10.1093/icesjms/fsv092
Whitehead H. and Reeves R. (2005). Killer whales and whaling: the scavenging hypothesis. Biol. Lett. 1, 415–418. doi: 10.1098/rsbl.2005.0348
Whitehead H., Rendell L., Osborne R. W., and Würsig B. (2004). Culture and conservation of non-humans with reference to whales and dolphins: review and new directions. Biol. Conserv. 120, 427–437. doi: 10.1016/j.biocon.2004.03.017
Willse N., Summers E., and Chen Y. (2022). Vertical line requirements and North Atlantic right whale entanglement risk reduction for the Gulf of Maine American lobster fishery. Mar. Coast. Fish. 14, e210203. doi: 10.1002/mcf2.10203
Woodroffe R., Thirgood S., and Rabinowitz A. (2005). People and Wildlife: Conflict or Co-Existence? (Cambridge, United Kingdom: Cambridge University Press).
Wright B. M., Olsen D., Gisborne B., Schulze A. D., Wetklo M. H., Ellis G., et al. (2025). Dietary specialization on elasmobranchs and seasonal foraging patterns of offshore killer whales Orcinus orca in the northeastern Pacific. Mar. Ecol. Prog. Ser. 763, 171–196. doi: 10.3354/meps14876
Yano K. and Dahlheim M. E. (1995). Behavior of killer whales Orcinus orca during longline fishery interactions in the Southeastern Bering Sea and adjacent waters. Fish. Sci. 61, 584–589. doi: 10.2331/fishsci.61.584
Young N. C., Brower A. A., Muto M. M., Freed J. C., Angliss R. P., Friday N. A., et al. (2024). Alaska marine mammal stock assessments 2023. U.S. Department of Commerce, NOAA Technical Memorandum NMFS-AFSC-493. Available online at: https://www.fisheries.noaa.gov/s3/2024-12/Alaska_SARs_Final_2023.pdf (Accessed March 12, 2025).
Keywords: odontocete, depredation, bycatch, fisheries management, human-wildlife conflict
Citation: Luck E, Myers H and Criddle KR (2025) A global review of operational fishery interactions with killer whales (Orcinus orca): dynamics, impacts, and management strategies. Front. Mar. Sci. 12:1629516. doi: 10.3389/fmars.2025.1629516
Received: 15 May 2025; Accepted: 15 September 2025;
Published: 26 September 2025.
Edited by:
Xuelei Zhang, Ministry of Natural Resources, ChinaReviewed by:
Raquel Puig-Lozano, University of Las Palmas de Gran Canaria, SpainKetki Jog, James Cook University, Australia
Copyright © 2025 Luck, Myers and Criddle. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emma Luck, ZWtsdWNrQGFsYXNrYS5lZHU=
 Emma Luck
Emma Luck Hannah Myers
Hannah Myers Keith R. Criddle
Keith R. Criddle