- 1Food4Future (F4F), c/o Leibniz Institute of Vegetable and Ornamental Crops (IGZ), Grossbeeren, Germany
- 2Department Plant Quality and Food Security, Leibniz Institute of Vegetable and Ornamental Crops, Großbeeren, Germany
- 3Institute of Nutritional Science, University of Potsdam, Nuthetal, Germany
- 4Food Metabolome, Faculty of Life Science: Food, Nutrition and Health, University of Bayreuth, Kulmbach, Germany
Rich in nutritional proteins and health-promoting compounds, edible macroalgae, known as “sea vegetables,” provide a valuable and needed alternative food source for a growing human population. Controlled environmental cultivation is a promising approach to provide freshly harvested macroalgal biomass in inland areas. In this context, natural saline groundwater (brine) offers an innovative solution for land-based, urban indoor cultivation of marine organisms. Here, we investigated the suitability of regional brine from the Brandenburg area (Central Europe) for the indoor cultivation of the three different macroalgae: Ulva compressa (“flat gut weed”), Ulva fenestrata (“sea salad”), and Cladophora sp. (Chlorophyta). For the evaluation of brine as cultivation media, we investigated biomass growth (e.g., specific growth rate, SGR) and pigment composition (carotenoids, chlorophylls) at different life stages (e.g., germlings and reproductive thalli). Responses toward the brine media varied between species. Ulva compressa showed comparable growth and elevated chlorophylls when cultivated in brine media, whereas Ulva fenestrata was negatively affected by the brine-based media and stagnated in its reproduction and growth. Also, Cladophora sp. was initially hampered by the brine media but showed a positive shift in growth after 45 days of exposure. For all taxa, high levels of chlorophylls and some of the carotenoids were measured in brine media compared to control media. Assuming that this reflects a stress response during acclimatization to a new cultivation environment, the study provides a promising strategy for macroalgal cultivation in saline groundwater with enhanced levels of photopigments. Given the rising demand for fresh-harvested algal biomass, we suggest fostering the initiated research to further develop “sea vegetable” production in inland areas.
1 Introduction
As outlined in the UN Sustainable Development Goal Zero Hunger (SDG 2), one major challenge of this century is to create a world free from hunger and malnutrition through sustainable consumption and production patterns (SDG 12) (United Nations, 2015). The world’s population is growing rapidly, expected to increase by nearly 2 billion persons, from the current 8 billion to 9.7 billion in 2050 (Nations, 2024). Adjacent environmental alterations are already becoming visible, affecting food production by reducing crop yields and their nutritional content. Increasing awareness of these impacts is raising efforts to identify (or innovate) new food sources and develop food production systems that are more sustainable than those conventionally available. Rapid urbanization and the expansion of cities across the globe are placing urban food systems in a unique position to help shape the overall transformation of agrifood systems (Cai, 2021). In this context, these key factors are driving the growing interest in the utilization of edible macroalgae: rising attention to food sources that are nutritious, healthy, and sustainable. Additionally, the versatility of seaweeds in terms of applications in several industries, such as pharmaceuticals, cosmetics, and animal feed, contributes further to this interest (Cai et al., 2021).
Edible macroalgae (sea vegetables) provide a traditional marine food source in many coastal areas worldwide (Pereira, 2016) and are widely commercially processed as food additives, like monosodium glutamate, agar, carrageenan, and alginate. Rich in proteins, dietary fibers, and micronutrients, edible macroalgae (sea vegetables) are treated as low-calorie food (because of low fat content), particularly favored by people who prefer low-carbohydrate (but high in fiber) or plant-based diets (FAO, 2021). In addition, its partly high content of bioactive compounds provides additional health-supporting benefits for human nutrition and can be used as food supplements for health benefits (Guo et al., 2022; FAO, 2021).
The increasing interest in algal biomass applicable for many industries led to an increased demand in macroalgal cultivation, enabling constant and high-quality delivery of fresh harvested material (Fricke et al., 2024). Traditional coastal-bound methods of seaweed cultivation, such as raft or tube net-based onshore methods, are not recommended throughout the year due to seasonal changes in seawater composition and temperature, as well as unpredictable weather events in coastal areas. To address these challenges, a standardized land-based cultivation system is proposed as a viable solution to ensure consistent production of quality seaweeds year-round (Suthar et al., 2019).
Algae can thrive in various conditions, including brackish water, seawater, and non-arable lands, which conserves freshwater and land resources. New initiatives, promoting algae farming in urban and peri-urban areas, are arising, offering sustainable development for those areas. So, it is concluded that cultivating algae in urban and peri-urban areas can have significant benefits such as pollution mitigation, biofiltering, and improving water quality and concomitantly provide feedstock to small and medium enterprises. Furthermore, they can offer sustainable solutions to urban ecosystems, provide job opportunities to unemployed agricultural laborers, and stimulate economic growth through algae-based entrepreneurial initiatives in peri-urban systems (Tahir et al., 2024).
A more recent approach involves the use of saline groundwater to enable macroalgal cultivation in inland areas (Fricke et al., 2023a). This regional approach brings several advantages, such as reducing fresh water (to dilute salt solution) and minimizing distance for the transport of the media to the cultivation site. Moreover, regional brine water from the region of Brandenburg is iodine free, which potentially eliminates the toxicological issue of seaweed as a food source. Recent findings showed that regional brine can be utilized for the cultivation of Ulva compressa, enhancing levels of carotenoids, chlorophylls, and MUFA (Fricke et al., 2023b). Based on the reported cultivation success for green macroalgae, two additional green seaweeds were selected to test their potential cultivability in natural brines: Ulva fenestrata (Ulvales, Ulvaceae) and Cladophora sp. (Cladophorales, Cladophoracea). Given the known difficulties of species identification in the Ulva genus (Steinhagen et al., 2019), U. fenestrata, together with other similar species, was for a long time considered as its sister species, Ulva lactuca, under which name it is also still registered in the novel food catalogue (https://ec.europa.eu/food/food-feed-portal/screen/novel-food-catalogue/search). Thus, Ulva fenestrata (aka Ulva lactuca) is already commercially applied for food purposes and included in many products. Also, the genus Cladophora comprises many edible species, consumed as food in different parts of the world, which are rich in phytochemical compounds that can be exploited for sustaining both human and animal health (Pereira, 2016). Moreover, it has been shown that during cultivation, the productivity of Cladophora spp. can reach higher values than in nature: up to 57 mg L−1 day−1 dry weight with a daily growth rate of up to 11% (Ross et al., 2018).
To develop a suitable protocol for land-based macroalgae cultivation, the present work studied the growth of the macroalgae Ulva fenestrata and Cladophora sp. in different types of brine-based cultivation media. In this context, the research aims are as follows:
i. to test the cultivability in terms of survival and growth of Ulva fenestrata and Cladophora sp. in two different types of brine media under laboratory conditions,
ii. to validate earlier results by conducting repetitive growth experiments with Ulva compressa, and
iii. to analyze the harvested biomass for potential changes in its pigment composition (e.g., carotenoids, chlorophylls) at the end of the growth experiments.
In this context, the present study will evaluate the cultivability of three edible macroalgae in brine-based cultivation media and study the potential impact on their nutritional composition.
2 Materials and methods
2.1 Experimental organisms and cultivation conditions
Three macroalgae were selected to test their potential cultivability in natural brines: (i) Ulva compressa (Ulvales, Ulvaceae; known under the trivial term “flat gut weed,” formerly named Enteromorpha compressa), (ii) Ulva fenestrata (Ulvales, Ulvaceae; known under the trivial name “sea lettuce”), and (iii) Cladophora sp. (Cladophorales, Cladophoracea).
The algal cultivars originated from field material collected and isolated by Fricke in November 2019 in the intertidal area of the North Sea Island of Helgoland (GPS: 54°11′N, 7°53′E; Schleswig-Holstein, Germany).
2.2 Experimental approach
To test the feasibility of regional saline groundwater (brine) as media for the inland cultivation of selected macroalgae, different multifactorial experiments were designed. An overview of the experiments is given in Table 1. A total of 10 experiments (EXP 1- EXP 10) were performed between 20 and 73 days in duration in the cultivation room. For Ulva compressa and Cladophora sp., the first two experiments were short term (up to 38 days), whereas the last experiment for each species was prolonged to >60 days in order to observe changes over time. Ulva fenestrata was tested in long-term experiments, where in EXP 6, the design was changed from testing thallus growth to testing germling growth after a sporulation event.
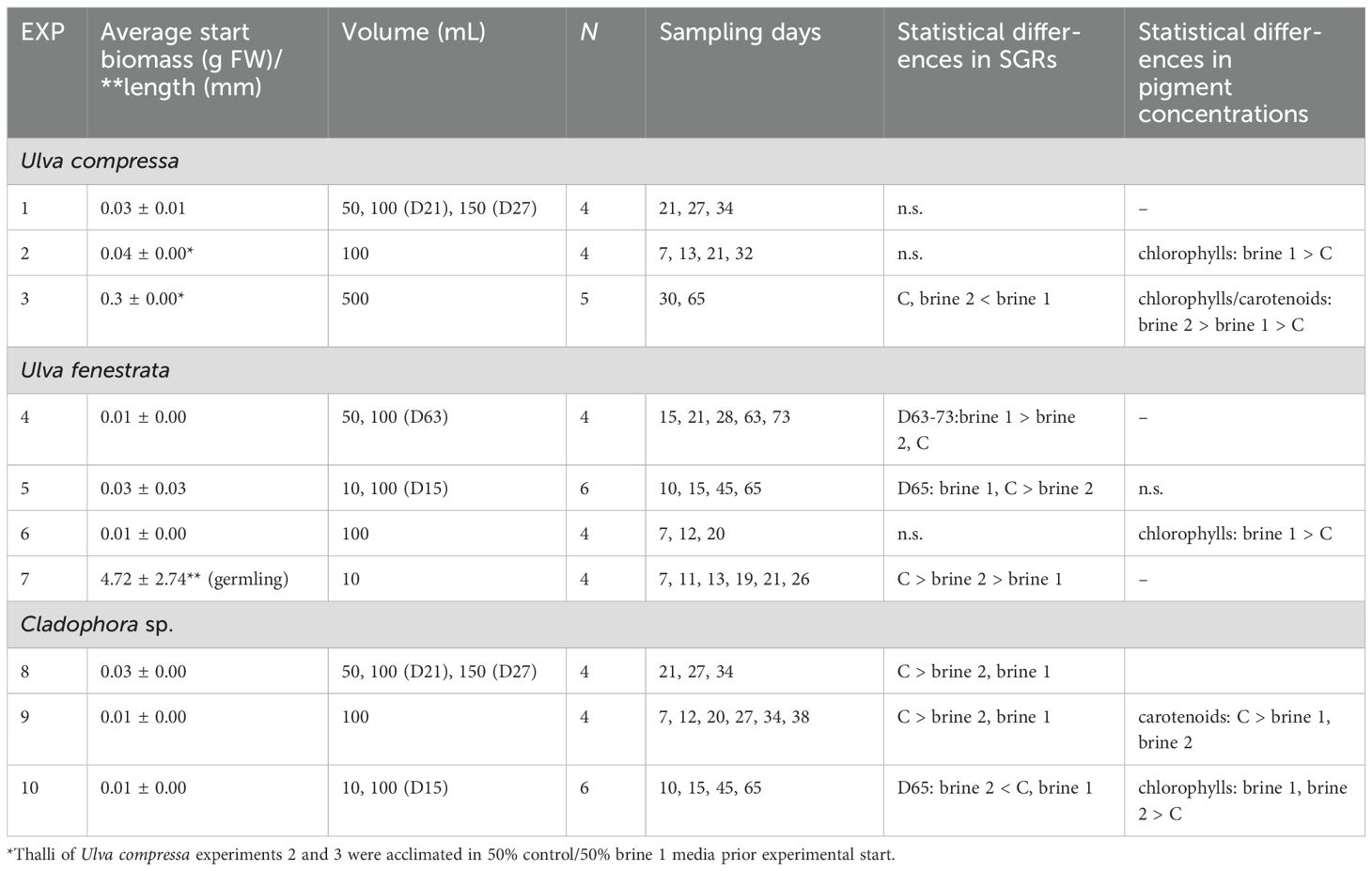
Table 1. Overview of 10 different experiments (EXP) for the macroalgae Ulva compressa, Ulva fenestrata and Cladophora sp. exposed to different amounts of biomass or germling lengths (**) (mean ± SD), to different volumes (Vol; in mL) of media (brackets indicated days of volume change), measured at different sampling times to analyse differences between tested cultivation media in chemical composition (pigments) at the experimental end. Statistical differences were analysed with analyses of variance (ANOVA) or non-parametrical Kruskal-Wallis test. n.s. not significant.
2.3 Experimental media
At each experimental run, experimental units were filled with one of three different cultivation media: (i) brine 1: brine, originated from the Katharine spring (GPS: N 52° 17.47 E 14° 3.62; Brandenburg, Germany) was purified by the ozone plant of the thermal bath Bad Saarow (Typ: OZ 50/80 V2-2K; Kaufmann Umwelttechnik, Germany) and stored in opaque 60 L barrels until experimental start, (ii) brine 2: brine 1 used for thermal business (e.g., mineral bath treatments) and filtered over GF/F to exclude potential particles ≥0.7 µm and stored in opaque 60 L barrels until experimental start; (iii) artifical seawater (Tropic Marin Classic, Dr. Biener GmbH, Wartenberg, Germany) at 2.4% salinity, was applied as control media (control). To determine the mineral composition of the applied cultivation media, anion concentrations were measured using an ion chromatograph 930 Compact IC Flex equipped with a conductivity detector with a suppression system (Metrohm AG, Herisau, Swisse). One hundred twenty microliters of samples was diluted up to 12 mL using ultrapure water, including 400 µL sodium bromide (0.6 mg mL−1) as an internal standard. An injection volume of 20 µL at a flow rate of 0.7 mL min−1 was used with an eluent consisting of sodium carbonate (3.2 mmol L−1) and sodium bicarbonate (1 mmol L−1) and a Metrosep a Supp5 column (Metrohm AG, Hersiau, Swisse). The final concentrations of the anions fluoride (F-), chloride (Cl-), sulfate (SO42-), nitrate (NO3-), and phosphate (PO43-) were calculated with external calibrations using MagIC Net 3.2 software (Metrohm AG, Swisse). Salinity (PSU NaCl) was calculated from the measured chloride concentration. In addition, the cations (boron, calcium, copper, iron, potassium, magnesium, Manganese, sodium, phosphorus, sulfur, and zinc) were measured using inductively coupled plasma—optical emission spectrometry (ICP-OES) (iCAP 7400, Thermo Fisher Scientific, Henningsdorf, Germany).
2.4 Cultivation conditions
Until the experimental start, the macroalgae were cultivated under stable artificial conditions in a cultivation room at 18°C water temperature and illuminated using a 10h photoperiod of LED lights, emitting photosynthetically active radiation (PAR, 400–700 nm) with an intensity of 35 µmol m²s−1. The light source was placed above the cultivation units. Cladophora sp. and U. fenestrata were cultivated in artificial seawater (Tropic Marin Classic, Dr. Biener GmbH, Wartenberg, Germany) at 2.4%. To test possible effects of cultivation media independent from thallus size, different start weights were implemented. Dependent on the organism size, different experimental units were applied, namely six-well plates (individual wells filled with 10 mL media), 200 mL polysterol container (filled with 50 to 150 mL media, volume increased with biomass growth), and 500 mL glass bottles (filled with 500 mL media), which were considered as experimental units. U. compressa was acclimated in two different media prior to the experiments: (i) in the control media for the first experiment and (ii) in a mixed media of control media with brine 1 (50/50), as it was already known to be capable of growing in brine 1 (Fricke et al., 2023a, b). Here, thalli were grown in a 50/50 mix of control media and brine 1 before the experimental start to investigate if this prior acclimatization might ease the further growth in the brine 1 media. To maintain a constant biomass growth, all algae were fertilized weekly by applying 1.25 mL/L of the fertilizer Algal (AQUALGAE SL, Coruna, Spain).
2.5 Differences in algal growth
Growth was measured, either as biomass or germling length (EXP 7), for all experimental runs, whereas pigment concentration was determined only for six experiments.
To determine algal growths, fresh weight was measured at different times using a microanalytic balance (XPE26, Mettler Toledao, USA) with a readability of 1 µg. Algal growth was calculated as specific growth rates (SGR) based on changes in fresh weights following (Pérez-Mayorga et al., 2011), expressed as % increase per day according to the formula:
whereas x1 and x2 are fresh weights measured at time 1 and time 2, expressed as days.
In addition, according to (Malta and de Nys, 2015) stocking density (SD) of the algal thalli was calculated at the time point of harvest in order to evaluate light access of the harvested biomass:
At the experimental end, weighted samples were immediately stored in liquid nitrogen, freeze-dried, and stored at −80°C for later analysis. Information on chemicals used during the experimental approach is listed in Supplementary Table S1. In experiment 6, Ulva fenestrata thalli began to sporulate in all media after day 20, visible as a greenish coloration. After 27 days, germlings became visible in all dishes. At this point it was decided to measure the growth of germlings. For that, an average single germling from each plate was transferred into a six-well plate, each well filled with 10 mL of media. This experiment is presented as EXP 7. For EXP 7, growth of germlings of U. fenestrata was determined using differences in thalli length. To document thallus length, photos were taken with a stereomicroscope (Axioscope, Zeiss, Germany) equipped with an attached smartphone and object millimeter scale. Photos were subsequently scaled and analyzed using the ImageJ software (version 1.53e).
2.6 Determination of concentrations of carotenoids and chlorophylls
To investigate potential alterations in the macroalgal biochemistry, carotenoids and chlorophylls, known as key components for photosynthetic activity and valuable bioactives (e.g., lutein, β-carotene), were analyzed at the experimental end. Carotenoid and chlorophyll concentrations were determined for all three algal taxa based on the methodology described by Fricke et al. (2023a). About 5 mg of lyophilized algal material was homogenized to a fine powder and extracted three times in 0.5 mL of methanol/tetrahydrofuran (1:1, v/v). Samples were dried under a nitrogen stream, re-dissolved in 50 µL dichloromethane and 200 µL isopropanol, and filtered through PTFE-filter tubes (0.2 µm, Thermo Fischer Scientific, Wilmington, USA) before they were transferred to HPLC vials. The chromatographic separation of the different analytes was performed with an Agilent Technologies 1290 Infinity UHPLC (Agilent Technologies Sales and Services, GmbH & Co. KG, Waldbronn, Germany) on a C30 Carotenoid column (YMC Co. Ltd., Kyoto, Japan; 100 × 2.1 mm, 3 µm) at 15°C. The analytes were detected using a DAD detector (UV/VIS spectra) and mass spectrometry (HR-MS data) using a coupled Agilent Technologies 6230 ToF LC/MS. Identification and quantification were achieved by external calibration with carotenoid standards of all-trans-isomers from β-carotene, lutein, zeaxanthin, and (9Z)-neoxanthin, as well as chlorophyll a and b standards at the wavelength of 450 nm. Data analysis was performed using the software package Mass Hunter (Agilent, Santa Clara, USA). Total chlorophyll and total carotenoid content were calculated based on the summed concentrations of single analytes.
2.7 Data transformation and statistical analyses
All statistical analyses were performed in Statistica v10. Data were checked for homogeneity using the Cochran test and analyzed with analyses of variance (ANOVA) when homogeneity was detected, followed by Tukey’s honestly significant difference (Tukey’s HSD) test to identify significant differences (p < 0.05) posteriori. In cases where homogeneity was not detected, the significance of intergroup differences was tested with the nonparametrical Kruskal–Wallis test, followed by multiple comparisons of p-values for between three groups. Data were visualized using GraphPad Prism v.10.
3 Results
3.1 Differences in cultivation media composition
Data on the mineral composition of applied media is presented in Supplementary Table S11. Differences between both brine media and the control were manifested in sulfate levels (control > brine 1, brine 2). Potassium levels were lower in both brine media compared to the control, where brine 2 had the lowest concentration (control > brine 1 > brine 2). For magnesium levels were comparably higher in brine 1 and the control, but again the lowest in brine 2 (control, brine 1 > brine 2). Also, the lowest value of sulfur was detected in brine 2 (control > brine 1 > brine 2). In contrast, calcium levels were lower in the control and brine 1 and higher in brine 2 (brine 2 > brine 1, control). Nitrate, nitrite, and phosphate were not detected in all cultivation media and were added by fertilizer.
3.2 Responses of different macroalgal taxa
The responses of the three macroalgal taxa to brine-based cultivation media are presented in the following. Statistical data on macroalgal biomass and growth are documented in Supplementary Tables S2–S4, whereas data for pigments are documented in Supplementary Tables S5–S10.
3.2.1 Ulva compressa
Thalli of U. compressa showed no significant differences in biomass and specific growth rates (SGR) between the different treatments after an exposure of about 30 days (EXP 1 and EXP 2), independent of their prior acclimatization to different media (Figure 1; Supplementary Table S2). Nevertheless, an elevated initial growth rate in thalli of the control media led to a measured maximum biomass of 770 ± 540 mg FW after 27 days of exposure in EXP 1. Significant differences became visible during the longest–lasting (65 days) EXP 3, whereas brine 1 yielded the highest biomass over the whole experimental time. Significant highest growth rates of about 3.21 ± 0.21% d-1 were reached in brine 1 over the initial 30 days and decreased afterwards in all media to < 0.5% d-1.
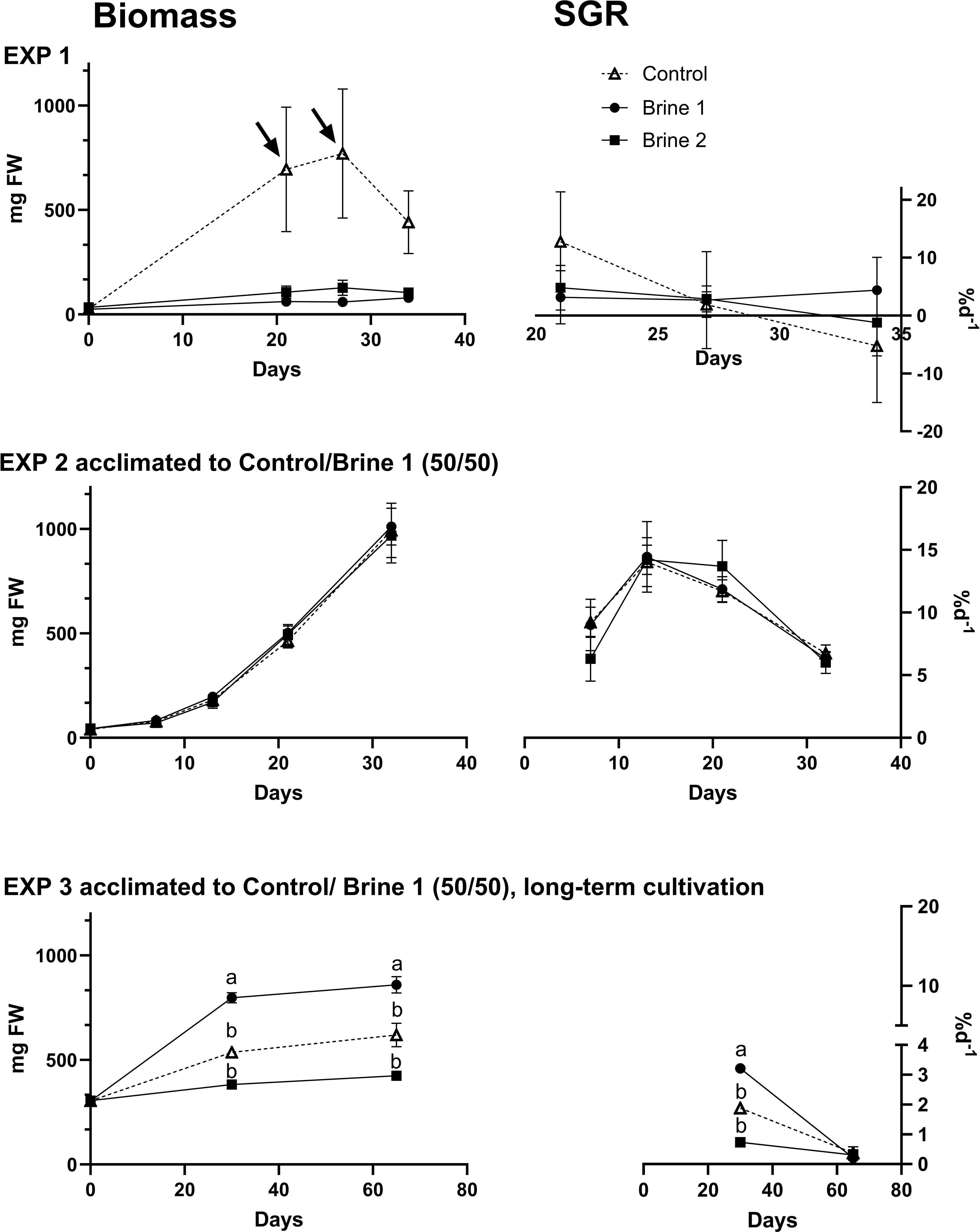
Figure 1. Specific growth rate SGR (%d−1) and biomass growth (mg FW) for Ulva compressa exposed to different cultivation media (control, brine 1, brine 2), based on thalli acclimated to control (EXP 1) and 50% brine 1/50% control media (EXP 2 and EXP 3) prior experimental start. Arrows indicate time of change in media volume (50 to 100 to 150 mL, EXP 1). Effect directions are indicated with small letters based on Tukey’s HSD post-hoc test or non-parametrical multiple comparisons of p-values. Data are showing mean ± SEM of EXP 1: n = 4; EXP 2: n = 4; EXP 3: n = 5.
Analysis of pigment concentrations revealed that both types of brine media led to increased carotenoid and chlorophyll concentrations in Ulva compressa. In EXP 2, measured total chlorophyll concentrations of thalli exposed to brine 1 were significant (12 times higher) as in the control medium (Figure 2; Supplementary Table S5). Notably, chlorophyll a was very low, and chlorophyll b was not detected in the thalli exposed to the control media. No differences were observed in total carotenoids, but alpha -carotene was significantly higher in the control media compared to brine 1 (Figure 2; Supplementary Table S6).
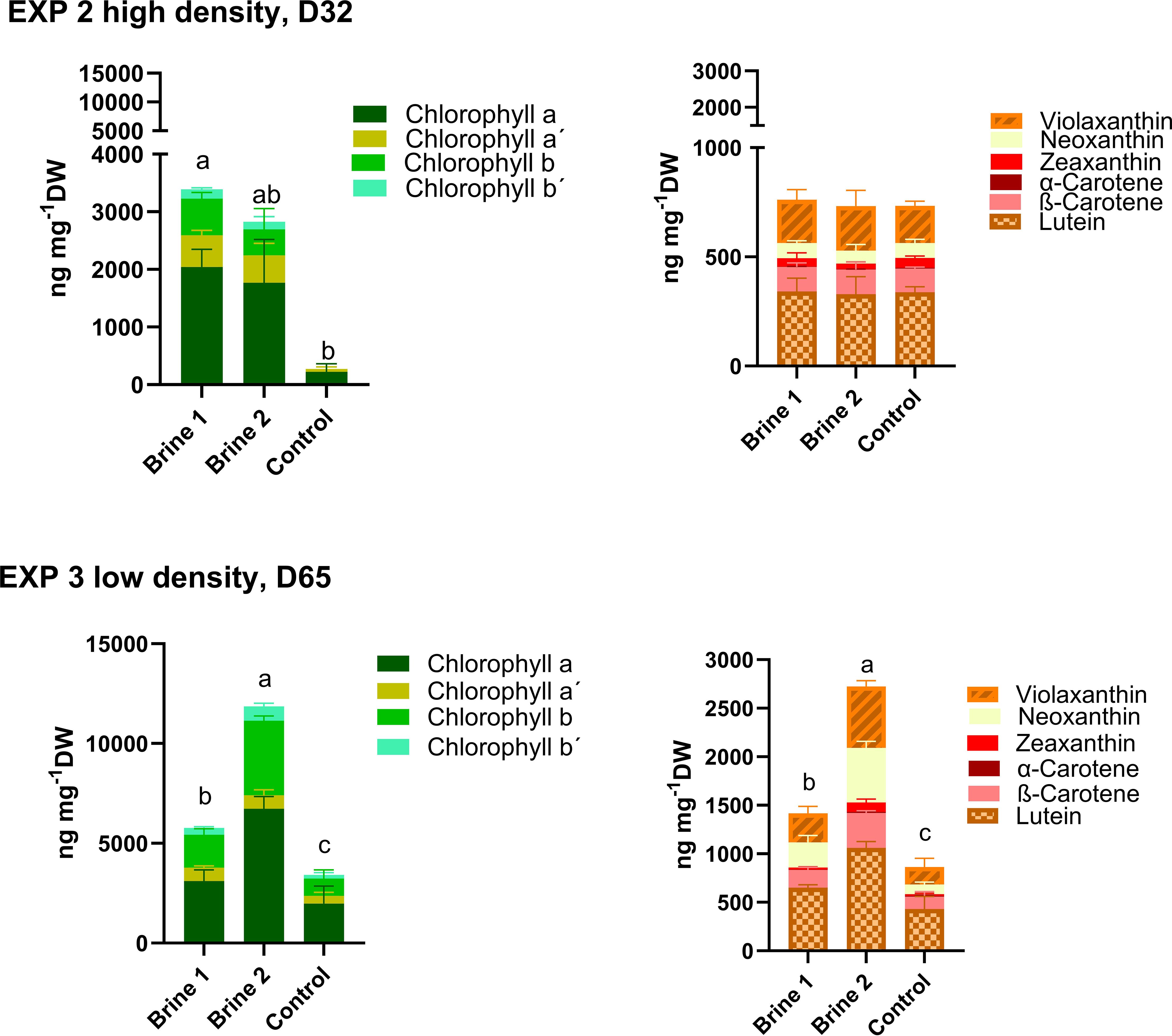
Figure 2. Concentrations of chlorophylls and carotenoids measured in thalli of Ulva compressa exposed for a period 32 days (EXP 2) and 65 days (EXP 3) to different cultivation media. Control—artificial seawater with 2,4% salinity; Brine 1—ozone-treated brine, Brine 2—used ozone-treated brine. Stagger plots showing averages of pigment concentrations. Small letters indicate significant differences of total chlorophyll and carotenoids between treatments, analyzed by one-way ANOVA or non-parametrical Kruskal–Wallis test (p < 0.05). Data showing means ± SD of total numbers, n = 4 (EXP 2) and n = 5 (EXP 3). Backtick signs indicate chlorophyll isomers.
Also, in the longer lasting EXP 3, where overall higher carotenoid and chlorophyll concentrations were measured compared to EXP 1, differences were observed in the pigment concentrations. Here, total chlorophylls, as well as total carotenoids, were significantly higher in thalli exposed to the two brine-based media, brine 1 and brine 2, compared to the control media (Figure 2; Supplementary Tables S5, S6).
Apart from cultivation media, other factors, like the stocking density, also seem to affect the observed pattern. Thus, thalli exposed to brine 2 in EXP 3 were characterized by the lowest biomass growth (Figure 1) and lowest stocking density (Table 2) and showed even significantly higher total chlorophyll (11859.87 ± 941.53 ng mg-1 DW) and carotenoid concentrations (2722.99 ± 151.33 ng mg-1 DW) than brine 1. Whereas in EXP 2, thalli exposed to brine 2 under higher biomass growth (7% d-1 SGR, Figure 1) and thallus stocking density (9.7 mg mL-1, Table 2) showed about four times lower chlorophyll concentrations of 2827 ± 1222 ng mg-1 DW than measured in EXP 3 (Figure 2).
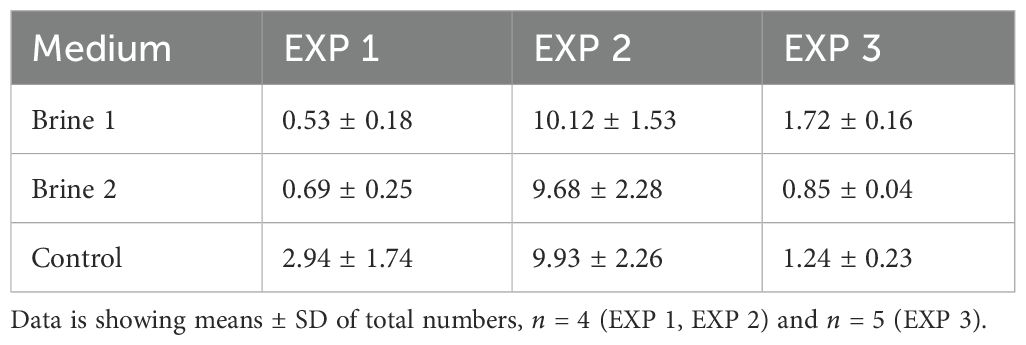
Table 2. Stocking density (mg/mL) of the thalli of Ulva compressa grown in different cultivation media (brine 1, brine 2 and control) at the end of the different experiments (EXP 1, EXP 2, EXP 3).
3.2.2 Ulva fenestrata
During EXP 4, U. fenestrata was stagnated in its biomass development during initial 63 days in all media (Figure 3A; Supplementary Table S3). After a change of cultivation media volume, germlings in both brine media reached exceptionally high SGR values, significantly peaking with 33.0 ± 1.4% d-1 in brine 1. Interestingly, after being detached and transferred to the experimental units, the U. fenestrata germlings stopped their lateral growth and formation of a blade and started instead with the formation of additional branchlets, which provide them a more tufted appearance (Figure 3B).
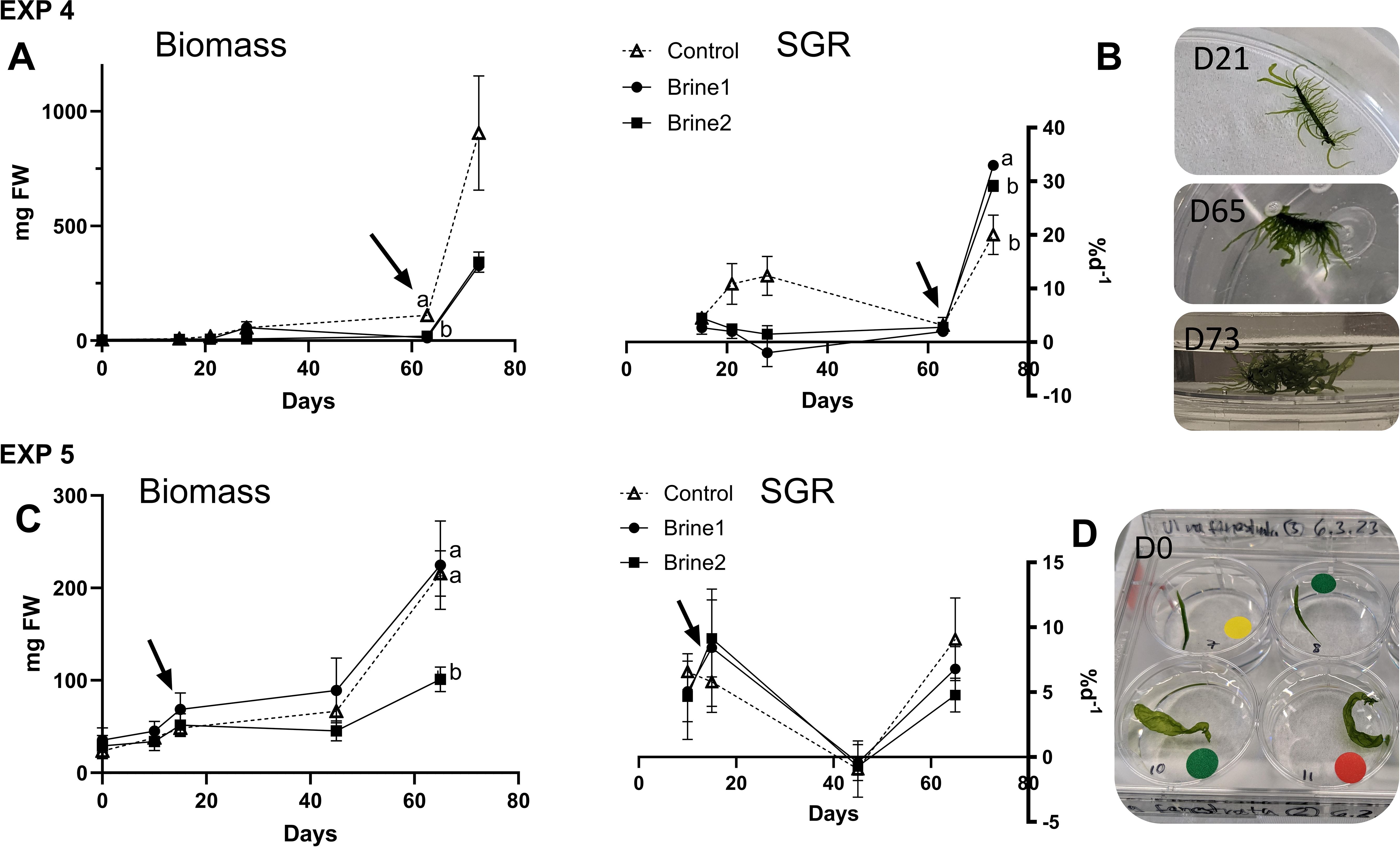
Figure 3. Macroalgal growth shown as changes in fresh weight (mg FW) and calculated specific daily growth (%d−1) of (A) EXP 4 and (C) EXP 5 of Ulva fenestrata exposed to cultivation media (control, brine 1, brine 2) over a period of 73 days (EXP 4) and 65 days (EXP 5). Arrows indicate time of change in media volume (EXP 4–50 to 100 mL, EXP5–10 to 100 mL). (B) Photos of thallus in control media on different days of EXP 4. (D) Photos of thallus at the start of EXP 5 in all cultivation media (green label-control, yellow label-brine 1, red label-brine 2). Effect directions are indicated with small letters based on Tukey’s HSD posthoc test or non-parametrical multiple comparisons of p-values. Control—artificial seawater with 2.4% salinity. Data are showing mean ± SEM of n = 4 (EXP 4), n = 6 (EXP 5).
In EXP 5, no differences were observed in the biomass of thalli exposed to the control medium or brine 1, whereas the growth in biomass of thalli exposed to brine 2 was clearly decreased at the end of the experimental exposure (Figure 3C; Supplementary Table S3).
In EXP 6, no difference was observed in the biomass between the treatments, which was about six times higher at the end of the study (Figures 4A, B; Supplementary Table S3). Ulva fenestrata thalli began to sporulate after day 20 in all treatments, recognizable by greenish coloration of the media. Consequently, the experiment was stopped and the thalli removed, but the spores were further cultivated in the dish. After 27 days, germlings became visible and were individually transferred into wells of a six-well plate as EXP 7. Already after 7 days of exposure to different media, strong differences became visible between the treatments (Figures 4C, D; Supplementary Table S3). Germlings exposed to the control media showed significantly higher length growth than specimens exposed to the different brine media, whereas brine 1 showed the lowest growth. These differences stayed constant over the experimental period of 19 days, when germlings in the control reached about six times higher lengths (24 ± 4.5 mm) compared to germlings in brine 1 (4.6 ± 0.74 mm). About four weeks in well plates, germlings in both brine media (brine 1 and brine 2) started to sporulate. After transfer of germlings into fresh media (100 ml), the germlings in both brine media (but not in the control) sporulated again and showed no further biomass growth after 22 days (Supplementary Figure S1). Examination of spores from brine 1 under a microscope (Axioscope, Zeiss) revealed a disturbed germination pattern, as instead of expected initial filamentous germlings, spores attached to clumps (Figure 4E).
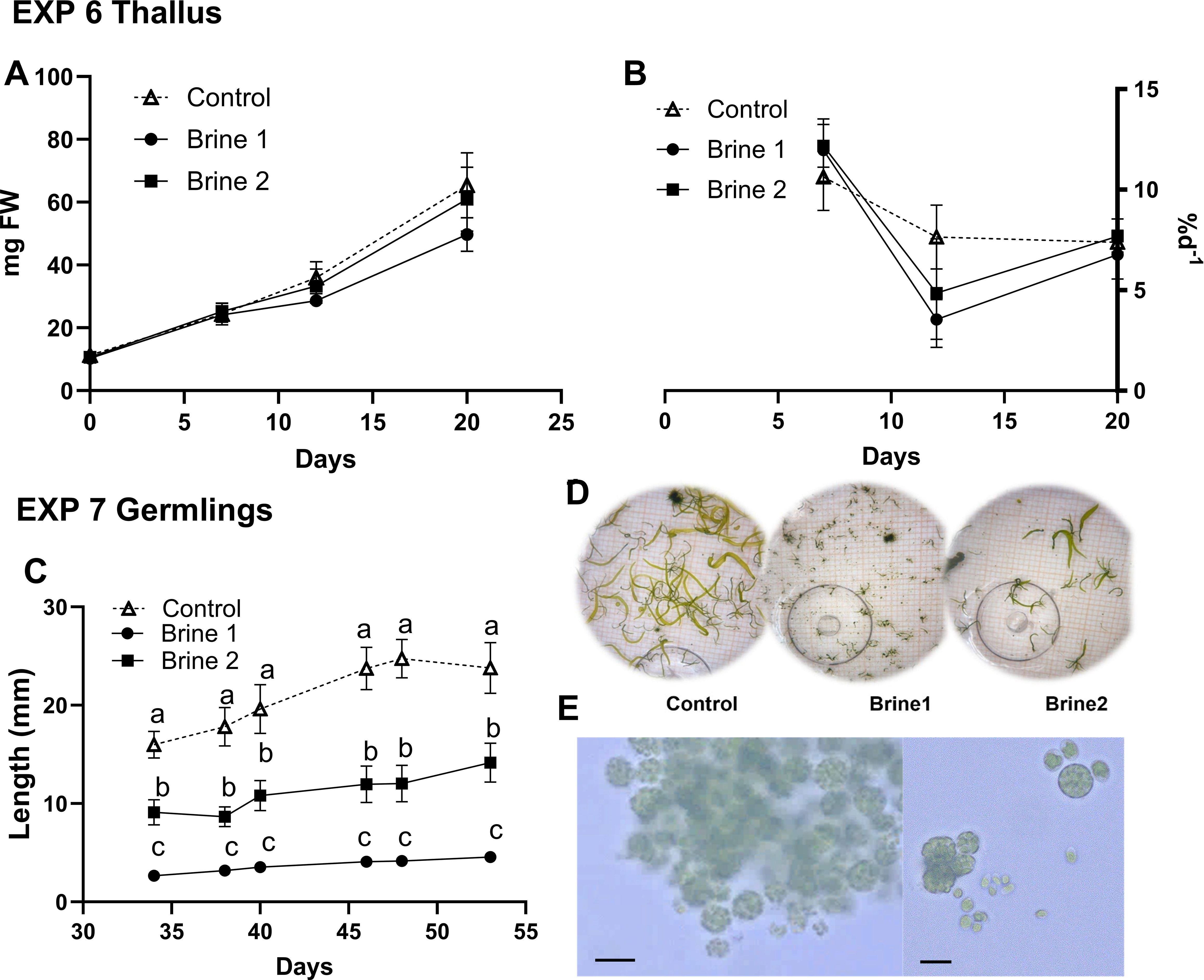
Figure 4. Growth of Ulva fenestrata thalli (EXP 6; (A, B)) and germlings [EXP 7; (C–E)] exposed to cultivation media (control, brine 1, brine 2). (A) Experiment 6, biomass growth (mg FW); (B) experiment 6, SGR (%d−1); (C) biomass growth (mm), (D) germlings of U. fenestrata, D20, EXP 6, (E) microscope image of Ulva fenestrata spores in brine 1 (scale bar refers to 10 µm). Effect directions are indicated with small letters based on Tukey’s HSD posthoc test or non-parametrical multiple comparisons of p-values. Data are showing mean ± SEM of EXP 6: n = 4; EXP 7: n = 4.
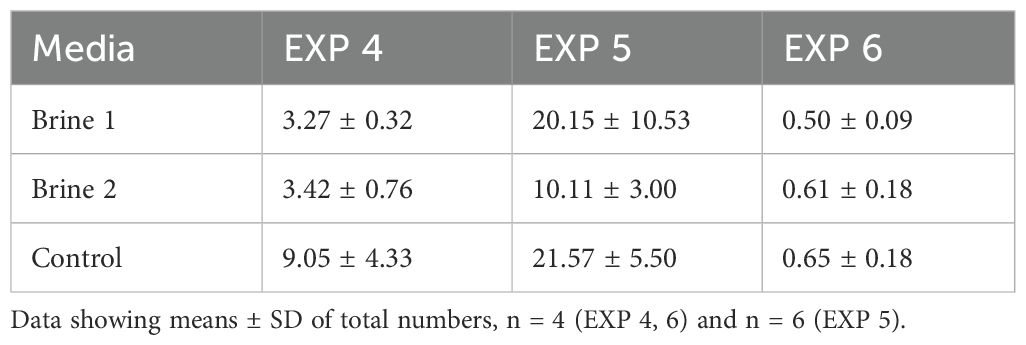
In samples of Ulva fenestrata during EXP 5, slightly elevated but not significantly different levels of carotenoids and chlorophylls were detected in thalli exposed to brine 1 and brine 2 (Figure 5; Supplementary Tables S7, S8).
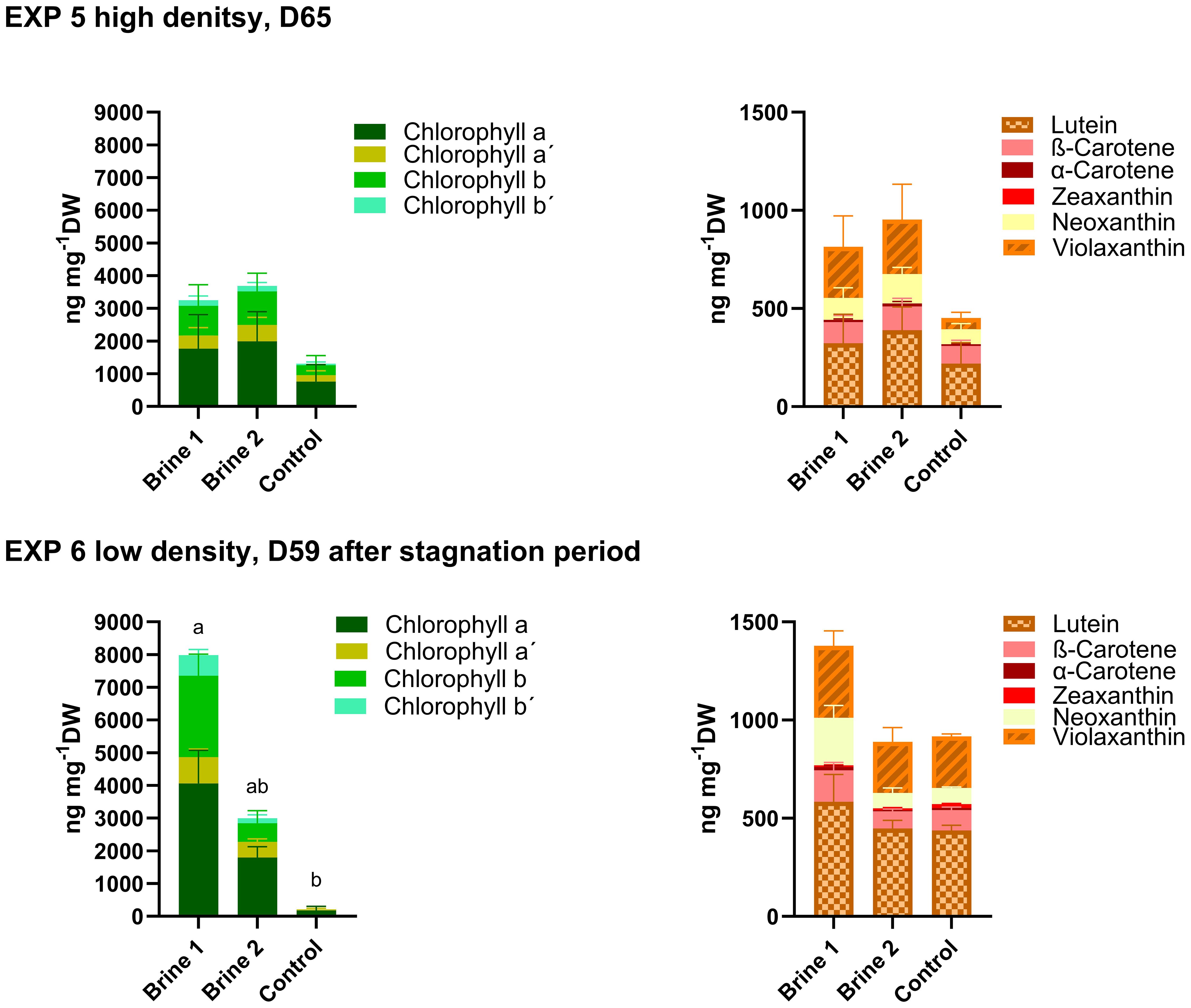
Figure 5. Concentrations of chlorophylls and carotenoids measured in thalli of Ulva fenestrata exposed for a period 59 days (20 days growth, 39 days stagnation EXP 5) and 65 days (EXP 6) to different cultivation media. Control—artificial seawater with 2,4% salinity; Brine 1—ozone-treated brine, Brine 2—used ozone-treated brine. Stagger plots showing averages of pigment concentrations. Small letters indicate significant differences of total chlorophyll and carotenoids between treatments, analyzed by one-way ANOVA or non-parametrical Kruskal–Wallis test (p < 0.05). Data showing means ± SD of total numbers, n = 6 (EXP 5) and n = 4 (EXP 6). Backtick signs indicate chlorophyll isomers.
For EXP 6, thalli exposed to brine 1 showed the highest values of both carotenoids (not statistically significant) and chlorophylls (significant) (Figure 5; Supplementary Tables S7, S8).
Once again, the smallest SGR values and stocking density were associated with the highest levels of carotenoids and chlorophylls: brine 2 in EXP 5 (953.51 ± 313.16 ng mg−1DW and 3687.48 ± 1639.12 ng mg−1DW, respectively) and brine 1 in EXP 6 (1377.86 ± 276.99 ng mg−1DW and 7984.38 ± 1738.59 ng mg−1DW, respectively) (Table 3; Figure 5).
Analysis from EXP 6 revealed again very low levels of chlorophyll a and no detectable chlorophyll b in the control media (Figure 5; Supplementary Table S7). Comparing concentrations from both runs, EXP 6 with low density showed overall higher values of pigments than EXP 5 with high density (Table 3; Figure 5).
3.2.3 Cladophora sp.
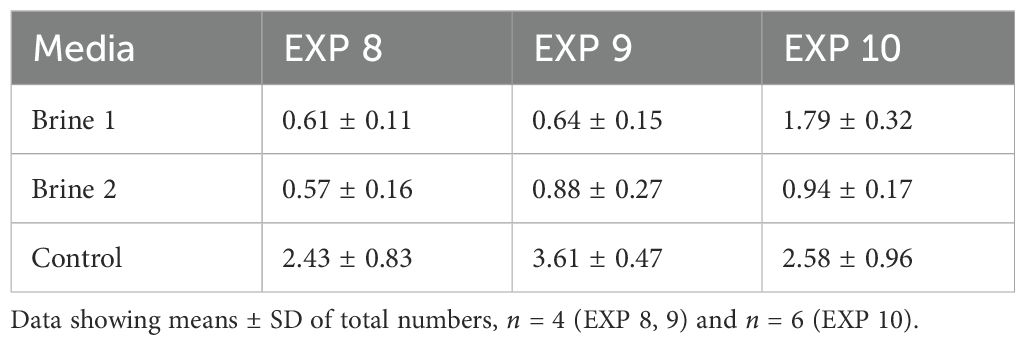
Cladophora sp. exhibited significantly higher growth in control media compared to both brine media across all experiments (Figure 6; Supplementary Table S4). The highest SGR value was 16.68 ± 5.60 % d−1 in EXP 9 during the first week of growth. Although negative SGR values were recorded in all experiments at some point of development, the longest EXP 10, showed a shift to a positive SGR after day 45 in all treatments (9.01 ± 3.60% d−1).
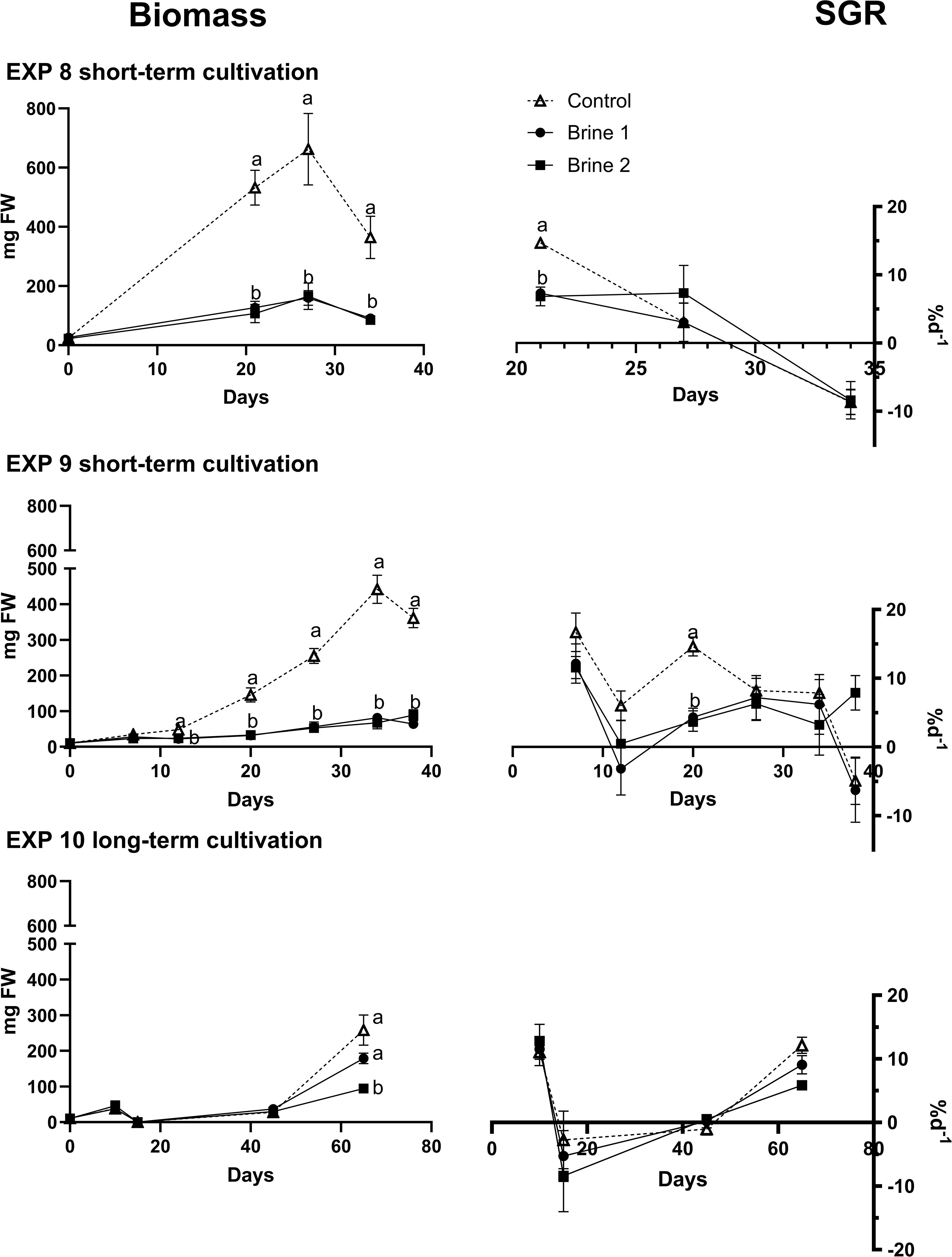
Figure 6. Exposure of Cladophora sp. to different cultivation media (control, brine 1, brine 2). SGR (%d−1) and biomass growth (mg FW) in different cultivation media during three experiments (EXP 8, EXP 9, EXP 10). Arrows indicate time of change in media volume (EXP 10 - 10 to 100 mL). Effect directions are indicated with small letters based on Tukey’s HSD posthoc test or non-parametrical multiple comparisons of p-values. Data showing mean ± SEM of EXP 8: n = 4; EXP 9: n = 4; EXP 10: n = 6.
As a result of higher growth, stocking density was higher in control samples in all runs at the moment of harvest (Table 4).
Cladophora sp. was observed to have higher concentrations of total chlorophylls in both brine media during EXP 9 and EXP 10, but only significant for EXP 10, reaching the highest level in brine 2 (3492.20 ± 638.62 ng mg−1 DW) (Figure 7; Supplementary Table S9).
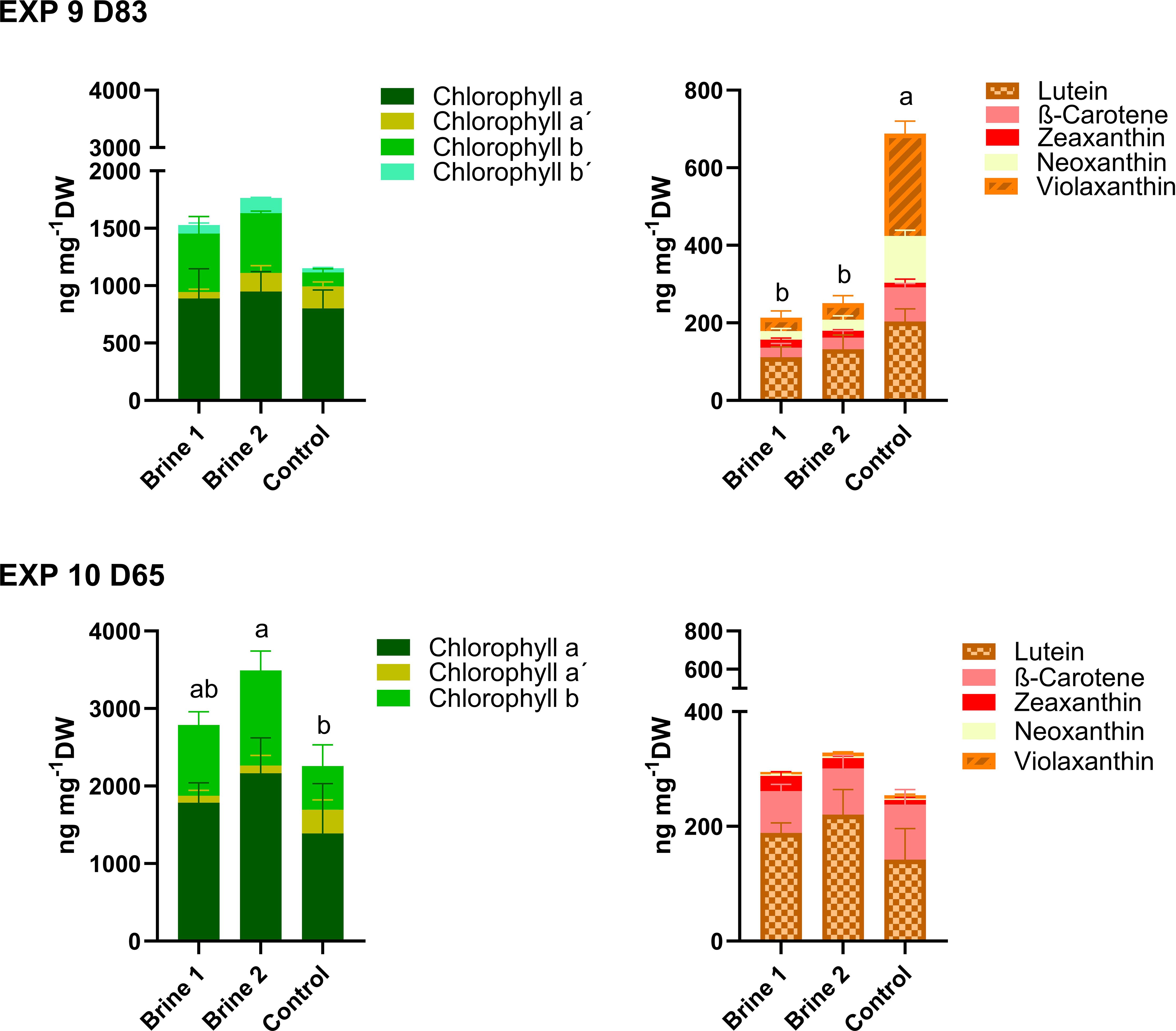
Figure 7. Concentrations of chlorophylls and carotenoids measured in thalli of Cladophora sp. exposed for a period 38 days (EXP 9) and 65 days (EXP 10) to different cultivation media. Control—artificial seawater with 2,4% salinity; Brine 1—ozone-treated brine, Brine 2—used ozone-treated brine. Stagger plots showing averages of pigment concentrations. Small letters indicate significant differences of total chlorophyll and carotenoids between treatments, analyzed by one-way ANOVA or non-parametrical Kruskal–Wallis test (p < 0.05). Data showing means ± SD of total numbers, n = 4 (EXP 9) and n = 6 (EXP 10). Backtick signs indicate chlorophyll isomers.
As for total carotenoid levels, no significant difference was detected in EXP 10 (Figure 7), but most carotenoids were lower in EXP 9 for both brine media (Supplementary Table S10).
4 Discussion
4.1 Macroalgal growth in brine media
As shown in the present study, macroalgal growth response toward the brine media exposition is species-specific. As expected from former studies (Fricke et al., 2023b, a), Ulva compressa reacted positively to the brine water exposure, showing comparable growth rates and elevated pigment concentrations. Interestingly, the macroalgae seem to cope quickly with the new cultivation media, as no difference in growth was measured independent from the applied acclimatization steps. In contrast, the other Ulva species, U. fenestrata seems to be more affected by the change in cultivation media. Despite the highest pigment concentrations being found likewise in the brine-based treatments, the growth and development of the macroalgae were strongly hampered. This became visible in increased sporulation activity, decreased growth, and disturbed germling development over the experimental period. As the developed germlings were not able to grow or reproduce (as they did in the control media), the use of brine-based media led to a stagnation in the cultivation of U. fenestrata. Also, Cladophora sp. was negatively affected by the brine media, visible in an overall decrease of biomass growth. Nevertheless, the observed positive trend toward brine 1 at the end of the long-lasting (>60 days) experiment could indicate a possible acclimatization potential for this macroalga toward the brine-based cultivation. Known for its high acclimatization potential toward different environmental stressors, members of the ubiquitous genus Cladophora are known to grow in different saline to limnic water sources and are even able to adapt and grow in wastewaters (Wang et al., 2024). As the chemistry of brine 2 can be additionally affected by the human activities in the thermal bath, its composition might differ from brine 1, as visible for concentrations of some minerals (Supplementary Table S11). This might explain the observed discrepancies between the treatments during our experiment. Thus, for further investigations, additional chemical analyses need to be included.
4.2 Chlorophyll levels in relation to growth and storage strategies
Interestingly, despite the observed differences in growth, the pattern of increased chlorophyll concentrations in thalli exposed to brine-based media was observed in all three taxa. Based on a review of (da Silva Ferreira and Sant’Anna, 2017) on the impact of different cultivation factors on chlorophyll concentrations, reduced levels of key nutrients, like magnesium or nitrogen, would be expected to decrease chlorophyll production. However, contrary to these expectations, the comparatively lower magnesium content in the brine media (Fricke et al., 2023a) has not resulted in lower chlorophyll concentrations for all three species studied.
Known to be essential for the synthesis of proteins, nucleic acids, and chlorophyll molecules, nitrogen is an important nutrient for macroalgae. Depending on nutrient availability but also algal life stage, different nitrogen accumulation strategies are described (da Silva Ferreira and Sant’Anna, 2017). Moreover, as known from studies on land plants, chloroplasts can act as effective storage units for the nitrogen-rich chlorophyll compounds (Han et al., 2016).
In the current experiments, the highest chlorophyll concentrations were measured in conditions when Ulva species showed the lowest growth and lowest stocking density.
Also for Cladophora sp., thalli with the highest growth rates harvested in control media showed the lowest levels of chlorophyll. (Lourenço et al., 2004) observed a trend in various microalgae of reduced chlorophyll content during the stationary growth phase. Likewise (Piorreck and Pohl, 1984), found that, supported by nitrogen exposure, algal biomass increased while total chlorophyll content decreased. In this respect, the pattern observed in this study of high chlorophyll concentrations correlated with decreased growth can be explained by redistribution of N sources. Chlorophyll-bound N typically changes in response to macroalgal N status. It is largely considered as a metabolically active pool, but because of its small size, it is not considered to be significant for N storage (McGlathery et al., 2008; Buapet et al., 2008).
4.3 Stress responses in macroalgae
Apart from possible relations between chlorophyll concentrations and algal growth dynamics, the observed elevated levels of chlorophylls in brine-treated thalli might also be explained by additional aspects. Any changes in environmental conditions can be considered as stressors, as they necessitate acclimatization strategies in algae (Kaur et al., 2022). Thus, brine media can be considered as a stress factor for macroalgae that were adapted to artificial seawater. In the study by Gao et al. (2016), the conspecific Ulva prolifera increased its pigment concentrations, including chlorophyll a, chlorophyll b, and carotenoids, when exposed to altered salinities but showed no alteration in its growth.
The authors suggested that a portion of the energy generated from photosynthesis might be used to mitigate the negative effects of stress (Gao et al., 2016). This is further supported by observations in higher plants, where increased chlorophyll concentration in response to low-level stress may enhance the plant’s defense capacity against environmental challenges (Agathokleous et al., 2020).
It remains unclear which factor in brine media can trigger the decrease in growth and enhance the production of photopigments. One possible explanation for the higher chlorophyll concentrations in macroalgal thalli could be related to the ozone treatment of the brine media. Research conducted by (Weidlich, 2025) indicated that ozonation negatively affected Ulva sp. biomass productivity, altered its morphology, and affected its metabolism (chlorophyll, phenolic, and soluble sugar contents), leading to a darker, fringier, and more rigid appearance. This finding was related to a direct response toward so-called ozone-produced oxidants (OPOs) (Weidlich, 2025). In marine and brackish water, ozone quickly reacts with halogen ions to form secondary oxidants (OPOs). Brominated oxidants, including hypobromous acid, hypobromite, and bromamines, are the primary species in ozonated saline waters. These OPO species are strong oxidizing agents that serve as secondary disinfectants (Wuertz et al., 2023). These products are also unstable and may decay over time or react with ammonia/ammonium present in the water (Perrins et al., 2006). In general, xanthophylls and chlorophylls as radical scavengers are increased under oxidative stress by defense of the algae (Cikoš et al., 2022).
Also in our study, supporting this hypothesis, altered morphology (darker, fringier, but also tended to fragmentation) was observed for U. fenestrata exposed to ozone-treated brine media. Moreover, this thallus seemed to be hampered in their reproduction, as spores remain clumped together, and compared with the stages of ontogenetic development of Ulva gametophytes described by Steinhagen et al. (2022), they stagnate before the “germination-tube formation” stage without further development.
But more interestingly, this pattern was not observed in the other two studied macroalgal taxa, suggesting further underlying and, in the case of U. compressa even species-specific mechanisms.
Next to a direct effect on the algal thallus, OPOs appear to also negatively impact the abundance of the microbiome of the investigated Ulva (Weidlich, 2025). Macroalgae, like Ulva, are known to strongly depend on their associated microbiome, as different bacteria shape their morphology and development by releasing different types of phytochemicals (Wichard, 2023).
4.4 Acclimatization to new conditions
Overall, both the measured higher chlorophyll concentrations and the observed elevated sporulation events could be considered as possible stress responses during acclimatization to the new cultivation media. Different microalgal species and strains are known to produce varying metabolite contents to maintain or enhance their survival in different growth conditions (Japar et al., 2021). This hypothesis was tested and supported in an experiment by (Japar et al., 2021) on different strains of microalgae. Following inoculation in a new medium, acclimatization was induced, resulting in an initial decrease in biomass production. However, subsequent generations exhibited higher biomass yields in the new media (Japar et al., 2021). Also, lipids, chlorophylls, and carotenoids were enhanced in some species during the acclimatization (Japar et al., 2021). Authors explain that the cells of the tested microalgae absorbed more light for their survival and improved their growth in each generation until the cells reached the saturation level where the cells are fully acclimatized (Japar et al., 2021). This effect could also apply to the tested macroalgae, as we observed better biomass growth in U. compressa in brine media after acclimatization in a 50/50 brine water and artificial seawater mixture. Increased SGRs in brine water were also observed in Cladophora sp. and U. fenestrata during the longest runs (after days 45 or 63).
The need for acclimatization is further supported by the study of Chieti et al., 2024, where acclimated cells (10 generations) showed similar growth in a new medium compared to the control cultures in terms of growth rate, cell density, and biomass. Interestingly, acclimated green microalgae showed lower photosynthetic activity (Chieti et al., 2024), which is the opposite of the observation in our study.
The acclimatization process indicates a potential for the cultivation of green macroalgae in brine water for enhanced levels of valuable compounds. In addition to chlorophylls, lutein was measured in high levels in all three species. Lutein serves as an essential antioxidant, food coloring agent, and nutraceutical, offering health benefits. It shows potential in reducing the risks of cancer and cardiovascular diseases, highlighting its overall importance (Ribaya-Mercado and Blumberg, 2004). Although lutein is usually obtained from marigolds, the production of lutein that is extracted from algal sources is being actively researched. Still, the highest contents in macroalgae named are around 248.67 μg g–1 in Cladophora sp (Bhat et al., 2021), which is higher than measured in the investigated Cladophora in the present study but lower compared to both Ulva sp. in this study. For example, Ulva compressa cultivated in brine 2 reached lutein concentrations between 0.33 ± 0.07 and 1.06 ± 0.06 and 0.34 ± 0.05 – 0.65 ± 0.03 mg g–1. Lutein content measured in German marigold flowers ranged between 0.77 ± 0.251 and 14.4 ± 0.234 mg g–1 (Khalil et al., 2012). Upper levels are much higher than those in brine-cultivated U. compressa, but lutein from marigold consumes more land and water as if it were compared to microalgae (Lin et al., 2015). This offers the potential of using Ulva cultivated in brine water as a lutein source, at least as a part of the biorefinery process.
5 Conclusion and outlook
This study presents a potential approach to cultivating macroalgae in regional brine water. During acclimatization to the new cultivation medium, higher concentrations of chlorophyll and some carotenoids were measured in all three species. However, the biomass response depended strongly on the taxon investigated. As demonstrated by the present study, taxon-specific stress adaptation mechanisms appear to play a significant role and must be considered in future research. By enabling the growth of marine organisms in inland areas without requiring valuable freshwater (e.g., for diluting artificial salts) and by re-evaluating thermal bath side-streams through the use of recirculating cultivation, the presented approach provides a sustainable, regional solution for producing fresh biomass and bioactive compounds.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Author contributions
VD: Formal analysis, Investigation, Writing – review & editing, Writing – original draft. MS: Funding acquisition, Project administration, Resources, Writing – review & editing. SB: Validation, Resources, Writing – review & editing, Funding acquisition, Formal analysis, Methodology. AF: Methodology, Data curation, Conceptualization, Writing – original draft, Supervision, Writing – review & editing, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The work was part of the project food4future funded by the German Federal Ministry of Education and Research (031B0730 A, 031B1525A and 031B1525D).
Acknowledgments
For helping hands in cultivation and laboratory we thank Andrea Maikath and the members of the IGZ science support platform Annette Platalla and Andrea Jankowski. The article is also based upon work from COST Action CA20106 “Tomorrow’s wheat of the sea’: Ulva, a model for an innovative mariculture”, supported by COST (European Cooperation in Science and Technology, www.cost.eu).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1630093/full#supplementary-material
References
Agathokleous E., Feng Z., and Penuelas J. (2020). Chlorophyll hormesis: Are chlorophylls major components of stress biology in higher plants? Sci. Total Environ. 726, 138637. doi: 10.1016/j.scitotenv.2020.138637
Bhat I., Haripriya G., Jogi N., and Mamatha B. S. (2021). Carotenoid composition of locally found seaweeds of Dakshina Kannada district in India. Algal Res. 53. doi: 10.1016/j.algal.2020.102154
Buapet P., Hiranpan R. J., Ritchie R., and Prathep A. (2008). Effect of nutrient inputs on growth, chlorophyll, and tissue nutrient concentration of Ulva reticulata from a tropical habitat. ScienceAsia 34, (2). doi: 10.2306/scienceasia1513-1874.2008.34.245
Cai J., Lovatelli A., Aguilar-Manjarrez J., Cornish L., Dabbadie L., Desrochers A., et al. (2021). Seaweeds and microalgae: an overview for unlocking their potential in global aquaculture development. FAO, Rome, Italy. doi: 10.4060/cb5670en
Chieti M. G., Petrucciani A., Mollo L., Gerotto C., Eusebi A. L., Fatone F., et al. (2024). Acclimated green microalgae consortium to treat sewage in an alternative urban WWTP in a coastal area of Central Italy. Sci. Total Environ. 945, 174056. doi: 10.1016/j.scitotenv.2024.174056
Cikoš A.-M., Šubarić D., Roje M., Babić J., Jerković I., and Jokić S. (2022). Recent advances on macroalgal pigments and their biological activities, (2016–2021. Algal Res. 65. doi: 10.1016/j.algal.2022.102748
da Silva Ferreira V. and Sant’Anna C. (2017). Impact of culture conditions on the chlorophyll content of microalgae for biotechnological applications. World J. Microbiol. Biotechnol. 33, 20. doi: 10.1007/s11274-016-2181-6
Fricke A., Capuzzo E., Bermejo R., Hofmann L., Hernández I., Pereira R., et al. (2024). Ecosystem services provided by seaweed cultivation: state of the art, knowledge gaps, constraints and future needs for achieving maximum potential in europe. Rev. Fisheries Sci. Aquaculture 33, 1–19. doi: 10.1080/23308249.2024.2399355
Fricke A., Harbart V., Schreiner M., and Baldermann S. (2023a). A proof of concept for inland production of the “sea-vegetable” Ulva compressa in Brandenburg (Central Europe) using regional saline groundwater. Algal Res. 74, 103226. doi: 10.1016/j.algal.2023.103226
Fricke A., Harbart V., Schreiner M., and Baldermann S. (2023b). Study on the nutritional composition of the sea vegetable Ulva compressa in a brine-based cultivation system. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1292947
Gao G., Zhong Z., Zhou X., and Xu J. (2016). Changes in morphological plasticity of Ulva prolifera under different environmental conditions: A laboratory experiment. Harmful Algae 59, 51–58. doi: 10.1016/j.hal.2016.09.004
Guo J., Qi M., Chen H., Zhou C., Ruan R., Yan X., et al. (2022). Macroalgae-derived multifunctional bioactive substances: the potential applications for food and pharmaceuticals. Foods 11, 3455. doi: 10.3390/foods11213455
Han Y.-L., Song H.-X., Liao Q., Yu Y., Jian S.-F., Lepo J. E., et al. (2016). Nitrogen use efficiency is mediated by vacuolar nitrate sequestration capacity in roots of brassica napus. Plant Physiol. 170, 1684–1698. doi: 10.1104/pp.15.01377
Japar A. S., Takriff M. S., and Mohd Yasin N. H. (2021). Microalgae acclimatization in industrial wastewater and its effect on growth and primary metabolite composition. Algal Res. 53. doi: 10.1016/j.algal.2020.102163
Kaur M., Saini K. C., Ojah H., Sahoo R., Gupta K., Kumar A., et al. (2022). Abiotic stress in algae: response, signaling and transgenic approaches. J. Appl. Phycology 34, 1843–1869. doi: 10.1007/s10811-022-02746-7
Khalil M., Raila J., Ali M., Islam K. M. S., Schenk R., Krause J.-P., et al. (2012). Stability and bioavailability of lutein ester supplements from Tagetes flower prepared under food processing conditions. J. Funct. Foods 4, 602–610. doi: 10.1016/j.jff.2012.03.006
Lin J. H., Lee D. J., and Chang J. S. (2015). Lutein production from biomass: marigold flowers versus microalgae. Bioresour Technol. 184, 421–428. doi: 10.1016/j.biortech.2014.09.099
Lourenço S. O., Barbarino E., Lavín P. L., Lanfer Marquez U. M., and Aidar E. (2004). Distribution of intracellular nitrogen in marine microalgae: Calculation of new nitrogen-to-protein conversion factors. Eur. J. Phycology 39, 17–32. doi: 10.1080/0967026032000157156
Malta E.-J. and de Nys R. (2015). The effect of short-term preharvest strategies on the carbon constituents of Ulva ohnoi M. Hiraoka et S. Shimada. J. Appl. Phycology 28, 555–565. doi: 10.1007/s10811-015-0543-3
McGlathery K. J., Pedersen M. F., and Borum J. (2008). Changes in intracellular nitrogen pools and feedback controls on nitrogen uptake in chaetomorpha linum (Chlorophyta)1. J. Phycology 32, 393–401. doi: 10.1111/j.0022-3646.1996.00393.x
Nations U. (2024). World population prospects 2024: summary of results. UN DESA/POP/2024/TR/NO. 9 (United Nations, NewYork).
Pérez-Mayorga D. M., Ladah L., Zertuche J., Leichter J. J., Filonov A., and Lavín M. F. (2011). Nitrogen uptake and growth by the opportunistic macroalga Ulva lactuca (Linnaeus) during the internal tide. J. Exp. Mar. Biol. Ecol. 406, 108–115. doi: 10.1016/j.jembe.2011.05.028
Perrins J. C., Cooper W. J., van Leeuwen J., and Herwig R. P. (2006). Ozonation of seawater from different locations: formation and decay of total residual oxidant–implications for ballast water treatment. Mar. pollut. Bull. 52, 1023–1033. doi: 10.1016/j.marpolbul.2006.01.007
Piorreck M. and Pohl P. (1984). Formation of biomass, total protein, chlorophylls, lipids and fatty acids in green and blue-green algae during one growth phase. Phytochemistry 23, 217–223. doi: 10.1016/s0031-9422(00)80305-2
Ribaya-Mercado J. D. and Blumberg J. B. (2004). Lutein and zeaxanthin and their potential roles in disease prevention. J. Am. Coll. Nutr. 23, 567S–587S. doi: 10.1080/07315724.2004.10719427
Ross M. E., Davis K., McColl R., Stanley M. S., Day J. G., and Semião A. J. C. (2018). Nitrogen uptake by the macro-algae Cladophora coelothrix and Cladophora parriaudii: Influence on growth, nitrogen preference and biochemical composition. Algal Res. 30, 1–10. doi: 10.1016/j.algal.2017.12.005
Steinhagen S., Karez R., and Weinberger F. (2019). Cryptic, alien and lost species: molecular diversity of Ulva sensu lato along the German coasts of the North and Baltic Seas. Eur. J. Phycol 54, 466–483. doi: 10.1080/09670262.2019.1597925
Steinhagen S., Larsson K., Olsson J., Albers E., Undeland I., Pavia H., et al (2022) Closed life-cycle aquaculture of sea lettuce (Ulva fenestrata): performance and biochemical profile differ in early developmental stages. Front. Mar. Sci. 9, 942679. doi: 10.3389/fmars.2022.942679
Suthar P., Gajaria T. K., and Reddy C. R. K. (2019). Production of quality seaweed biomass through nutrient optimization for the sustainable land-based cultivation. Algal Res. 42. doi: 10.1016/j.algal.2019.101583
Tahir F., Ashfaq H., Khan A. Z., Amin M., Akbar I., Malik H. A., et al. (2024). Emerging trends in algae farming on non-arable lands for resource reclamation, recycling, and mitigation of climate change-driven food security challenges. Rev. Environ. Sci. Bio/Technology 23, 869–896. doi: 10.1007/s11157-024-09697-0
United Nations (2015). Transforming our world: the 2030 Agenda for Sustainable Development. Resolution adopted by the General Assembly, A/RES/70/1.
Wang Y., Wang K., Bing X., Tan Y., Zhou Q., Jiang J., et al. (2024). Influencing factors for the growth of cladophora and its cell damage and destruction mechanism: implication for prevention and treatment. Water 16, (13). doi: 10.3390/w16131890
Weidlich S. (2025). Bioremediation of ozone-produced oxidants in marine RAS using Ulva sp.: benefits and impacts on seaweed physiology and associated microbiomes (Bremen, Germany: Leibniz Centre for Tropical Marine Research (ZMT) GmbH). Available online at: https://www.leibniz-zmt.de/en/news-at-zmt/events/bioremediation-of-ozone-produced-oxidants-in-marine-ras-using-ulva-sp-benefits-and-impacts-on-seaweed-physiology-and-associated-microbiomes.html (Accessed April 15, 2025).
Wichard T. (2023). From model organism to application: Bacteria-induced growth and development of the green seaweed Ulva and the potential of microbe leveraging in algal aquaculture. Semin. Cell Dev. Biol. 134, 69–78. doi: 10.1016/j.semcdb.2022.04.007
Keywords: saline agriculture, carotenoids, Ulva compressa, Ulva fenestrata, macroalgae cultivation
Citation: Denisova V, Schreiner M, Baldermann S and Fricke A (2025) Sea vegetables for brine-based inland cultivation. Front. Mar. Sci. 12:1630093. doi: 10.3389/fmars.2025.1630093
Received: 16 May 2025; Accepted: 20 June 2025;
Published: 31 July 2025.
Edited by:
Lior Guttman, University of Haifa, IsraelReviewed by:
Leonel Pereira, University of Coimbra, PortugalEitan Salomon, National Center for Mariculture (NCM), Israel
Copyright © 2025 Denisova, Schreiner, Baldermann and Fricke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna Fricke, ZnJpY2tlQGlnemV2LmRl
 Valeriya Denisova
Valeriya Denisova Monika Schreiner
Monika Schreiner Susanne Baldermann1,2,4
Susanne Baldermann1,2,4 Anna Fricke
Anna Fricke