- State Key Laboratory for Managing Biotic and Chemical Threats to the Quality and Safety of Agro-products, School of Marine Sciences, Ningbo University, Ningbo, China
Organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) are pollutants of global concern due to their toxicity, bioaccumulation, and long-term ecological consequences in marine ecosystems. Their presence and associated hazards were investigated in the water–sediment system of the Xiangshan Harbour Watershed, a zone influenced by both industrial and agricultural activities. Samples were obtained from 19 key locations during autumn (October 2020) and summer (July 2021). OCP concentrations ranged from 0.03–231.52 ng/L in water and 0.28–804.27 μg/kg in sediment, while PCBs ranged from 0.01–7.35 ng/L in water and 0.35–3.77 μg/kg in sediment. The lower section of the XianXiang River (XX-DOWN) was the hotspot for OCPs, and the Huangduan Harbor lower section (HDG-DOWN) was the hotspot for PCBs. Temperature and salinity were positively correlated with pollutant concentrations. Source identification using diagnostic ratios indicated OCPs mainly originated from lindane and technical DDT, while PCA–MLR analysis showed PCBs were sourced from e-waste (15.3%), thermoplastics (57.1%), and pigments (27.6%). Risk quotient (RQ) analysis revealed ecological risks from hexachlorobenzene, PCB 138, and DDT metabolites. Total non-carcinogenic hazard quotient (TnHQ) values remained below 1 for all assessed age groups—infants, children, teens, and adults—indicating low risks. Continuous surveillance along with targeted pollution control strategies in the watershed are encouraged.
1 Introduction
The presence and bioaccumulation of environmental pollutants in marine ecosystems is a growing global concern (Pescatore et al., 2025). Among these, organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) are especially problematic because they resist degradation and are widely detected in marine environments long after their use has been banned. These pollutants have been linked to endocrine disruption, immune system dysfunction, neonatal health complications, and neurotoxicity (Encarnação et al., 2019; Li et al., 2006). Despite global regulatory efforts under the Stockholm Convention, these pollutants persist within environmental matrices worldwide, such as the sediments of Paranagua Bay (Rizzi et al., 2017), the Karun River (Behfar et al., 2013), and the Shanghai river network (Qadeer et al., 2019).
China, with its rapidly urbanizing coastal zones, faces intensified risks from such pollutants, especially in semi-enclosed and economically strategic areas like the Xiangshan Harbor Watershed (Zhang and Ning, 2024). This watershed supports vital mariculture and agricultural activities but is increasingly affected by pollutant loads from industrial and domestic sources (Chen et al., 2018). While studies have assessed POP contamination within the inner bay (e.g., Wang et al. (2022a)), the rivers feeding into the bay remain understudied.
These pollutants are persistent and can travel long distances through urban runoff, ultimately reaching riverine ecosystems. For example, Abdulnaser and Ibrahim (2009) detected DDT in three rivers in Southern Thailand, which was linked to historical vector control usage. Ogola et al. (2024) found aldrin in the Kibos-Nyamasaria River due to agricultural runoff, while Cui et al. (2020) attributed PCB contamination in the Yangtze River to historical residues. A previous study on the inner Xiangshan Bay (Wang et al., 2022a) identified sources of Aroclor mixtures. Yet, the specific sources in the rivers flowing into the bay, remain unexplored. Furthermore, there is a significant knowledge gap regarding the source contributions within riverine ecosystems.
Accordingly, the formulated questions are as follows: (1) What are the spatial and seasonal patterns of pollutants within the watershed? (2) What are their major sources? (3) What ecological and human health risks do they pose?
To answer these questions, we employed land-use pattern analysis, diagnostic ratios, together with Principal Component Analysis–Multiple Linear Regression (PCA–MLR) to apportion sources (Liu et al., 2023; Rachdawong and Christensen, 1997). We examined 50 OCPs and 34 PCBs in a semi-enclosed watershed known for aquaculture, agriculture, and shipbuilding, yet lacking centralized wastewater treatment. The study focuses on the water–sediment system to assess pollutant occurrence, identify sources, and evaluate ecological and human health risks.
2 Methodology
2.1 Reagents
Chromatography-grade solvents such as methanol, acetone, as well as dichloromethane were sourced through Sino-pharm Chemical Reagent Corporation (China), while n-hexane came from Merck Co. (Germany). A total of 50 OCPs and 34 PCBs with a purity of at least 97% (listed in Supplementary Table S1) were acquired from o2si (USA). Internal standards, including 13C12 decachlorobiphenyl (13C12 PCB 209) and 13C6 hexachlorobenzene (13C6 HCB), were provided by Supelco (USA), with a purity of ≥ 97.6% and 99%, respectively. Florisil (1 g, 6 mL) along with C18 (1 g, 6 mL), were acquired from Waters (Milford, USA).
2.2 Sampling
River water and sediment samples were systematically obtained from 19 key locations across the watershed (Supplementary Table S2, Supplementary Figure S1) during autumn (October 2020) and summer (July 2021). These locations exhibit potential for OCP and PCB emissions, including aquaculture sites, Tianming Gate, industrial areas, textile factories, pesticide storage barrels, orange groves, sewage outlets, and scenic spots. Detailed sampling procedures are outlined in Section S1: Sampling and Sample Treatment.
2.3 GC–MS/MS analysis
OCP and PCB residues were quantified using a gas chromatograph coupled with tandem mass spectrometry (GC-MS/MS, 7890B-7000D, Agilent, USA), incorporating a DB-5 MS UI capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness). The column temperature program started at 80°C for 1 min, elevated to 150°C at 20°C/min, then raised to 300°C at 5°C/min, and held at 300°C for 5 min. An inlet temperature of 280°C was used. Acquisition and quality control parameters followed routine methods from our previous research (Wang et al., 2022a), with minor adjustments.
Target analytes were determined through the internal standard peak area method (n = 8). Matrix spike recoveries (n = 6) were also used to assess method accuracy, as outlined in our previous research by Wang et al. (2022a). The limits of detection, with a signal-to-noise ratio of 3, were 0.01-0.15 ng/L for river water and 0.02-0.31 μg/kg for sediment. OCP recoveries ranged from 85.5% to 102.4% in river water and 81.5% to 115.4% in sediment. PCB recoveries varied from 78.5% to 103.2% in river water and 82.1% to 116.4% in sediment. The relative standard deviations for river water and sediment analyses were below 7.8% and 9.1%, respectively.
2.4 Source analysis methods
Diagnostic ratios (DRs) are widely applied in source apportionment (Pokhrel et al., 2018; Zhang et al., 2021). They provide a simple, straightforward, and effective method for distinguishing between different pollutant sources based on their relative concentrations (Dvorská et al., 2011). HCHs primary sources were identified using the following ratio in Equation 1:
where 3< DR1< 7 indicates the presence of technical HCHs, and DR1 ≤ 1 suggests lindane usage (Qiu et al., 2019).
The sources of DDT were distinguished based on the following ratio in Equation 2:
A 0.2< < 0.3 reflects technical DDT, while values near 7.0 suggests dicofol usage (Fujii et al., 2010; Wang et al., 2022a).
To assess DDT degradation conditions, the following ratio was used in Equation 3:
A < 1 reflects aerobic conditions, while a value greater than 1 reflects anaerobic conditions (Hiller et al., 2011). Additionally, PCA-MLR was applied for PCB source apportionment, following methodologies outlined in previous studies (Rachdawong and Christensen, 1997; Xu et al., 2013). PCA-MLR was chosen over other multivariate tools due to its ability to handle large, complex datasets with multicollinearity, and its superior accuracy in source apportionment, making it more suitable for analyzing overlapping pollutant sources in the water-sediment system (Fahmi et al., 2011).
2.5 Evaluation of ecological and health risk
The ecological risk of target contaminants in water was assessed via the risk quotient (RQ) approach in Equations 4, 5:
Here, signifies the ambient level of OCPs or PCBs in the river water sample, while is the predicted non-effect concentration (Liu et al., 2024b). AF denotes the assessment factor (Gan et al., 2024). values of<0.01; 0.01–0.1; 0.1–1; ≥1, denote insignificant; low; moderate; and severe hazard, respectively (Liu et al., 2024a; Wu et al., 2022).
Given that the watershed includes human activities such as boating, fishing, tourism, and orange farming, the non-carcinogenic human health risk from oral ingestion of target pollutants was evaluated. The risk was evaluated through average daily intakes (ADI) coupled with chronic reference dose (RfDo) pertaining to four age groups, based on data from published literature (Li et al., 2023b), as detailed in Supplementary Table S3. Additionally, the watershed’s proximity to populations relying on groundwater for drinking and irrigation further justifies the need for this health risk assessment, as the potential for contamination of groundwater could pose significant risks to human health.
2.6 Data analysis
Graphs were generated by Origin 2022. Kriging interpolation maps were generated using ArcMap 10.8.
3 Results and discussion
3.1 Occurrence and levels of OCPs and PCBs in river water
The statistical data and detection frequencies of OCPs and PCBs detected during autumn as well as summer can be found in Supplementary Tables S4 and Supplementary Tables S5. Individual OCP levels varied from 0.03 to 98.91 ng/L (average = 10.00 ± 0.09 ng/L, n = 15) in autumn and from 0.04 to 231.52 ng/L (average = 21.81 ± 2.41 ng/L, n = 19) in summer. Individual PCB levels varied between 0.01 and 1.83 ng/L (average = 0.13 ± 0.01 ng/L, n = 1) in autumn, and from 0.10 to 7.35 ng/L (average = 1.06 ± 0.04 ng/L, n = 4) in summer. Dicloran, hexachlorobenzene (HCB), and PCB 8 were detected at 100% frequency in both seasons, reflecting their ubiquity in the watershed.
In autumn, the aggregate OCP concentrations were approximately two times lower (p< 0.05) (144.76 ng/L) than in summer (310.71 ng/L), while the aggregate PCB concentrations were about eight times lower in autumn (1.83 ng/L) compared to summer (14.80 ng/L). This seasonal difference is likely attributed not only to increased riverine input and the desorption of pollutants from suspended particles into the water column during the summer months, but also to several biogeochemical processes. In summer, higher temperatures and increased microbial activity may accelerate the degradation of certain OCP and PCB compounds (Leigh et al., 2006), yet their net concentrations may still rise due to enhanced pollutant release from sediments and reduced adsorption under lower dissolved oxygen conditions (Defeo et al., 2024). Conversely, in autumn, cooler temperatures, reduced biological activity (Alver et al., 2025), and enhanced particle settling may contribute to lower concentrations. Wang et al. (2022b) also noted that monthly fluctuations in pollutant concentrations entering the bay region were driven by river runoff. Similar seasonal patterns for OCPs have been observed in Izmir Bay (Odabasi et al., 2008) and for PCBs within the Chahe River (Zhao et al., 2016). The OCP hotspot was located in the XianXiang River (XX-DOWN) region during summer (219.41 ng/L), associated with industrial parks and textile factories, while the PCB hotspot was in the XX-UP region during summer (2.87 ng/L), linked to a sewage outlet and a pond bank, as indicated by land use patterns (Supplementary Table S2).
Dicloran concentrations were significantly higher in summer (231.52 ng/L) compared to autumn (98.91 ng/L), reflecting its widespread use as a fungicide and in dye production (Figures 1a, b). Its percentage contributions were 74.51% in summer and 68.32% in autumn, showing a clear seasonal variation. The dominance of dicloran in this region is likely linked to its historical and ongoing use in local agriculture, particularly for controlling fungal diseases in vegetables, wheat, and melons, which are widely cultivated in the surrounding areas (Fu et al., 2022). Additionally, dicloran may have been used in textile and dye manufacturing processes by upstream industries such as Ningbo Sanyou Printing and Dyeing Co., Ltd., which remains active and may contribute to environmental levels through industrial discharge (Wang et al., 2022b). This dominance of dicloran was also observed by de Oliveira et al (2009) in the Cristais River. For chlorobenzenes, HCB concentrations were higher in summer (1.95 ng/L) than in autumn (1.17 ng/L), with HCB being used as a fungicide before its global ban in 2004 (Mahalingaiah et al., 2012). PCB 149, which was heavily used in industrial mixtures before its ban in 1979, dominated in summer (49.66%) with a concentration of 7.35 ng/L, although PCB 8 was detected at 100% in both seasons. Further investigation into the contamination levels and associated risks of dicloran, HCB, and PCB 149 is recommended.
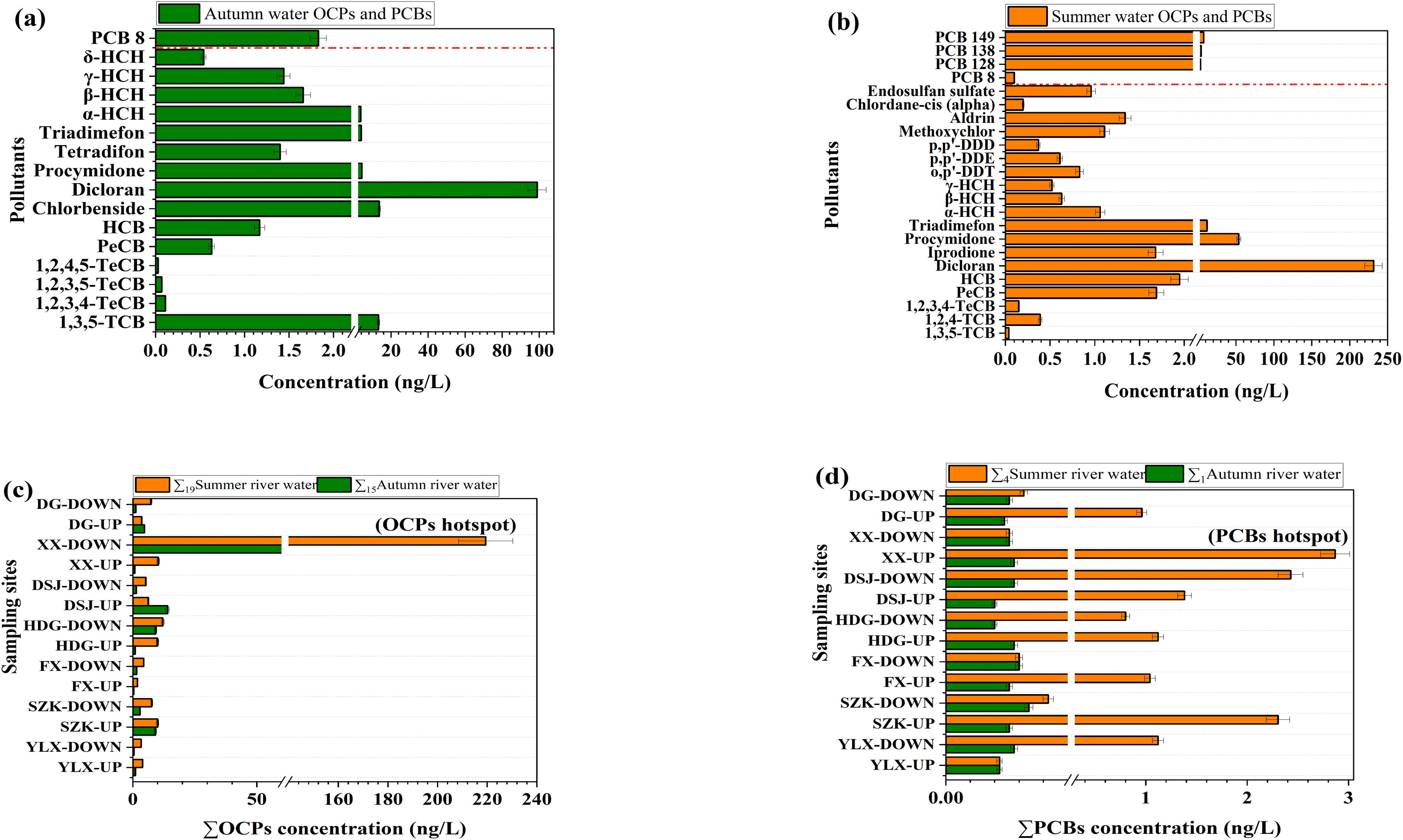
Figure 1. Concentrations of OCPs and PCBs in river water during autumn (a), n = 16) and summer (b), n = 23). Seasonal variation of OCPs (c) and PCBs (d) in the rivers that flow into the Xiangshan Harbour Watershed.
The locational trends of OCPs and PCBs in river water exhibited seasonal differences (Figures 1c, d). In autumn, OCP hotspots were identified at industrial parks and residential sites such as XX-DOWN and Dasongjiang River (DSJ-UP) (92.01 ng/L and 14.04 ng/L, respectively). During summer, hotspots shifted to industrial parks and dumping sites like XX-DOWN and Huangduan Harbor (HDG-DOWN) (219.41 ng/L and 12.10 ng/L, respectively). Summer PCB hotspots were found at sewage outlets and industrial sites, such as XX-UP (2.87 ng/L) and DSJ-DOWN (2.43 ng/L). The pollutant gradient was found to correlate with proximity to industrial emissions, similar to findings in Baiyangdian Lake (Dai et al., 2011).
The comparative analysis of pollutant levels between watershed rivers and the inner bay revealed significant differences (p< 0.05) (Supplementary Figures S2, S3). In autumn, dicloran concentration in the watershed rivers was about 14 times higher (98.91 ng/L) than in the bay (6.73 ng/L), while HCB was about 35 times lower in watershed rivers (1.17 ng/L) compared to the bay (41.43 ng/L). In summer, dicloran concentrations were about 111 times higher in watershed rivers (231.52 ng/L) than in the bay (2.08 ng/L), while HCB levels were about twice as high in the bay (4.2 ng/L) compared to the rivers. These differences may be influenced by several factors, including the bay’s semi-enclosed morphology, the dominance of the semi-diurnal M2 tide, stratification patterns, and biogeochemical cycling processes, all of which can significantly affect pollutant mixing, distribution, and residence times (Kong et al., 2022; Li et al., 2023a; Pimenta et al., 2023; Shaikh et al., 2024). For instance, previous studies conducted in Xiangshan Bay have shown that the fast and turbulent tidal currents in the central section play a key role in shaping pollutant distribution. Higher concentrations have been observed near river mouths and estuarine zones, where the interplay between freshwater inflow and marine dynamics enhances mixing and retention of contaminants (Wang et al., 2022b; Xu et al., 2023). Although HCB and PCB concentrations remained below China’s surface water guidelines of 5000 ng/L and 20 ng/L, respectively, assessing riverine inputs and the marine dynamics driving these patterns is warranted (Bao et al., 2012; Wang et al., 2016).
Among local watercourses, Liu et al. (2015) detected OCPs in autumn (16.7–249.2 ng/L) and summer (118.0–188.9 ng/L) in the Sichuan Basin, with higher levels than those found in the Xiangshan Harbor Watershed. Zhou et al. (2006) reported OCP levels in the Qiantang River (28.30–238.7 ng/L during autumn, alongside 9.61–269.4 ng/L over summer), which were also higher. Zhang et al. (2011b) detected PCBs within Yangtze River Delta (1.23–16.6 ng/L), which exceeded the levels in this study. On a global scale, Yahaya et al. (2017) observed OCP levels in the Buffalo River (autumn:<LOD–313 ng/L, summer:<LOD–4403 ng/L), which were higher, and Oliveira et al. (2011) detected PCBs in Lake Ontario (0.31–42.75 ng/L), also higher than in this study.
3.2 Occurrence and levels of OCPs and PCBs in sediment
The statistical values for sediment pollutants in autumn are presented in Supplementary Table S6. Individual OCP concentrations ranged from 0.28 to 804.27 μg/kg (average = 186.53 ± 633.26 μg/kg, n = 16), while individual PCB concentrations spanned 0.35–3.77 μg/kg (mean = 1.03 ± 0.01 μg/kg, n = 10). Although we did not measure pollutant concentrations in benthic organisms, elevated levels of p,p’-DDT and PCB 156, could lead to their accumulation in the food web, affecting ecosystem health. This has been observed in similar studies (Qin and Yan, 2006; Voorspoels et al., 2004). Future research measuring bioaccumulation in benthic organisms would provide a more direct link between sediment contamination and ecological impacts.
The detection frequencies of OCPs (0.18-100%) and PCBs (42-100%) ranked higher (p< 0.05) in sediment relative to water. To better visualize the data spread and minimize the impact of outliers, boxplots were constructed to show the distribution of pollutant concentrations (Figure 2a). The OCP hotspot was identified at XX-DOWN (914.07 μg/kg), linked to industrial park emissions. OCP contamination resulting from industrial activities has also been noted within the Olt River sediments (Ciucure et al., 2023). The PCB hotspot was located at HDG-DOWN (2.23 μg/kg), associated with dumping activities. Similar PCB contamination from dumping has been reported in the Pilica River (Urbaniak et al., 2019).
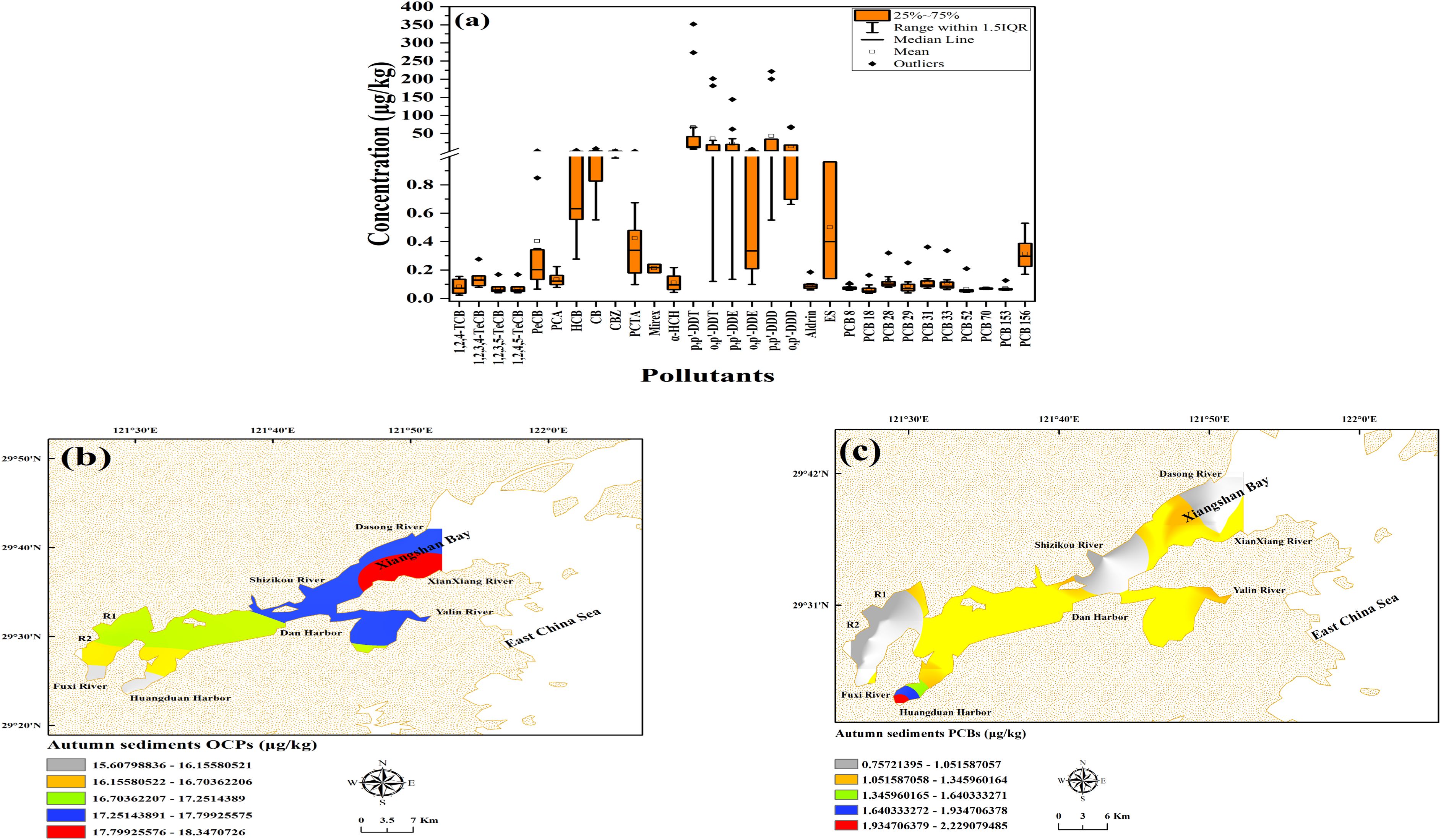
Figure 2. Boxplot of OCPs (n = 16) and PCBs (n = 10) concentrations in sediments during autumn (a). Kriging interpolation maps showing the distribution of OCPs (b) and PCBs (c) in the sediments of the Xiangshan Harbor Watershed.
The sediment OCP profiles revealed that p,p’-DDT dominated, accounting for 35.93%, with p,p’-DDD as well as o,p’-DDT being significant compounds (Supplementary Table S6). The p,p’-DDT levels peaked at 804.27 μg/kg. Due to their hydrophobicity and high log KOW (6.02-6.91), DDTs tend to strongly adsorb to sediment particles, making them less bioavailable in the water column (Placencia and Contreras, 2018). However, under certain environmental variations in temperature and salinity, or through bioturbation, these pollutants can be desorbed from sediments and re-enter the water column (Zeng and Venkatesan, 1999).
Historically, p,p’-DDT was used as an insecticide in the 1940s and in malaria control (Persson et al., 2012). α-HCH, at 0.05%, also played a dominant role, with concentrations up to 1.23 μg/kg. α-HCH is a degradation product of lindane (Karadeniz and Yenisoy-Karakaş, 2015). PCB 156 dominated the PCB profile, accounting for 30.53%, with concentrations reaching 3.77 μg/kg. PCB 156 was historically used in coolants (Chen et al., 2020).
Spatial distribution of ΣOCPs, mapped by Kriging interpolation, showed higher concentrations in industrial and agricultural areas, such as XX-DOWN and Shizikou (SZK-UP) (914.07 μg/kg and 48.72 μg/kg, respectively) (Figure 2b). For ΣPCBs, higher concentrations were found in dumping sites and electrical appliance factory areas, such as HDG-DOWN (2.23 μg/kg) and Fuxi (FX-DOWN) (0.75 μg/kg) (Figure 2c). The pollutant gradients likely correlate with human activities (Zhang et al., 2024).
A comparative analysis between watershed sediments and the inner bay revealed marked disparities (p< 0.05) (Supplementary Figure S4). Within watershed rivers, p,p’-DDT concentrations were approximately 80 times higher (804.27 μg/kg) than in the bay (10.05 μg/kg), while α-HCH concentrations were slightly lower in the watershed rivers (1.23 μg/kg) compared to the bay (1.55 μg/kg). PCB 156 levels were approximately 12 times higher in the watershed rivers (3.77 μg/kg) compared to the bay (0.31 μg/kg). Since p,p’-DDT, α-HCH, and PCB 156 are included in the EU POPs Control List, it is recommended that their levels be regularly monitored both inside and outside the bay (Guo et al., 2019).
OCP levels in the Xiangshan Harbor Watershed were higher than those in Xinghua Bay (1.84-80.46 μg/kg) (Zhang et al., 2011a) and Lake Gucheng (9.01-35.34 μg/kg) (Kan et al., 2020), but lower compared to the Niger River (4672-7009 μg/kg) (Unyimadu et al., 2019). PCB concentrations within Xiangshan Harbor Watershed were lesser than the Lagos Lagoon (273.46-6757.61 μg/kg) (Unyimadu and Benson, 2023) and Santos Estuary (0.125-70.6 μg/kg) (de Souza et al., 2018). This study also observed reduced PCB contamination compared to previous research in the Xiangshan region (2011–2016) (Lin et al., 2020). The COVID-19 pandemic likely contributed to reduced pollutant emissions owing to diminished industrial activity (Forster et al., 2020; Xu et al., 2024).
3.3 Correlation analysis
Pearson correlation analysis (Supplementary Figure S5) demonstrated a notable association of DDTs and HCHs (p< 0.05, r = 1). This correlation is likely due to similar sources, such as historical agricultural applications, as noted by Devi et al. (2013) in northeastern India. OCPs also exhibited positive correlations with PCBs, HCHs, and DDTs (p< 0.05, r = 0.17, 1, and 0.2). Olatunji (2019) suggested that the co-existence of these compounds is due to their widespread use and resistance to degradation.
In summer river water, temperature indicated a positive correlation with pollutants (p< 0.05, r = 0.19 for OCPs and r = 0.81 for PCBs) (Supplementary Figure S6). Prokeš et al. (2012) reported that high temperatures can enhance the desorption of pollutants, as observed within the Morava River. Additionally, salinity exhibited a positive correlation with pollutants (p< 0.05, r = 0.10 for OCPs and r = 0.19 for PCBs) (Supplementary Figure S7). Wang et al. (2022a) also noted a positive correlation between salinity and these pollutants in the East China Sea. Our field investigations recorded dissolved oxygen levels between 4.00 and 8.80 mg/L, indicating good water quality for aquatic life in the Xiangshan Harbor Watershed (Mainali and Chang, 2021).
3.4 Source apportionment
The use of diagnostic ratios offers benefits such as simplicity and cost-effectiveness (Dvorská et al., 2011). Several sites showed an ratio of less than 1, including HDG-UP, HDG-DOWN, XX-DOWN, and DSJ-DOWN, suggesting the presence of lindane, while SZK-UP (with a ratio of 3 to 7) indicated the use of technical HCH (Figure 3a). Similar patterns of lindane dominance have been observed in the Yellow River (Gao et al., 2008) and the Peshawar valley (Khan et al., 2023). At Residential area (R2-UP) and DSJ-DOWN (industrial and breeding areas), the ratio spanned 0.2–0.3, indicating industrial DDT, while no values approached 7, indicating the absence of dicofol in the watershed (Figure 3b).
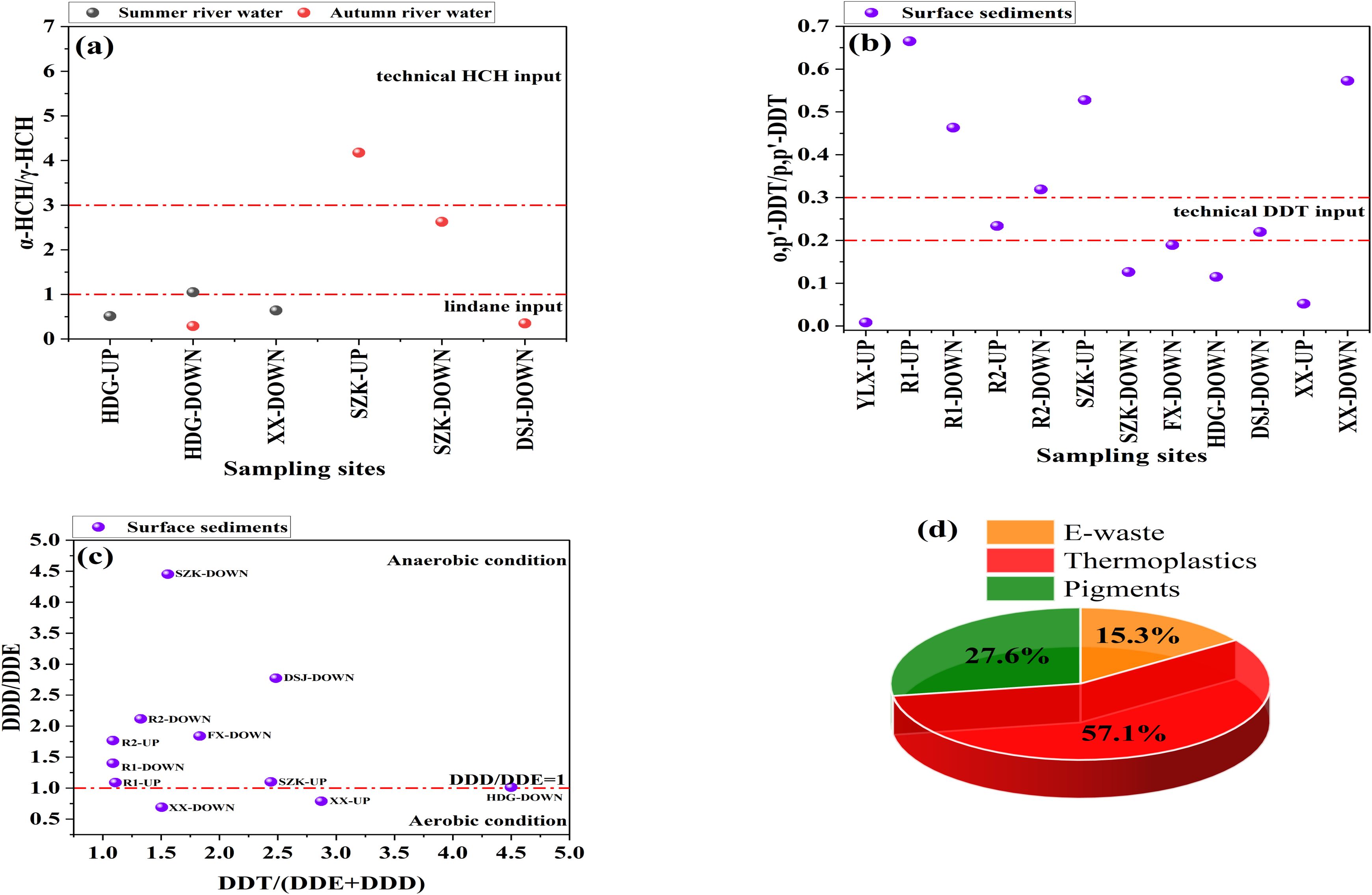
Figure 3. Diagnostic ratio scatter plots showing HCHs (a) and DDTs (b, c). PCA-MLR derived contributing factors for PCBs in the rivers flowing into the Xiangshan Harbor Watershed (d).
Several sites, including SZK-DOWN, DSJ-DOWN, R2-DOWN, and FX-DOWN, exhibited a ratio surpassing 1, indicating anaerobic conditions. In contrast, XX-DOWN and XX-UP had ratios below 1, indicating aerobic conditions (Figure 3c). The dominance of anaerobic conditions may be linked to eutrophication-related algal blooms, similar to conditions observed in Taiwan sediments (Chiu et al., 2004). Additionally, the majority of ratios in our investigation were ≥1, suggesting fresh DDT inputs (Figure 3c) (Hitch and Day, 1992).
The sources of PCBs were determined through PCA-MLR factor analysis (Supplementary Table S7). PC1 accounted for 18.2% of the total variance, with high loadings of PCB 28 + 31. Zhao et al. (2020) noted that these species are predominantly associated with e-waste, while PCB 31 is also found in industrial Clophen. PC2 explained 43.4% of the variance, with high loadings of PCB 8, which is commonly found in thermoplastics, as reported by Megson et al. (2019). PC3 accounted for 38.4% of the variance, with high loadings of PCB 18, which Anezaki et al. (2015) identified as a pigment byproduct and a component of industrial PCB mixtures containing more than 1%. Multiple linear regression of PCA-derived factor scores against the normalized sum of 10 PCBs revealed their contributions as follows: e-waste (15.3%), thermoplastics (57.1%), and pigments (27.6%) (Figure 3d). These contributions were lower than those predicted by the national source inventory from 2019 (Zhao et al., 2020). Other potential sources of PCBs in the watershed could include sewage outlets, shipyards, and fire-extinguishing foams, but further investigation is needed.
3.5 Evaluation of pollutants’ ecological and health risks
The RQ calculations for the pollutants detected in autumn and summer are provided in Supplementary Tables S8, S9, respectively. In both seasons, most pollutants, including pentachlorobenzene, α-HCH, β-HCH, and PCB 8, exhibited low risk levels (< 0.1) to fish, crustaceans, and algae. However, hexachlorobenzene showed moderate risk levels (0.1-1) in both seasons, while PCB 138 exhibited high risk levels (> 1) during summer (Figures 4a, b). Overall, the risk levels were higher (p< 0.05) in summer compared to autumn, likely due to increased aquaculture activities and riverine runoff. This seasonal variation in risk is consistent with findings in Lake Vela (Abrantes et al., 2010).
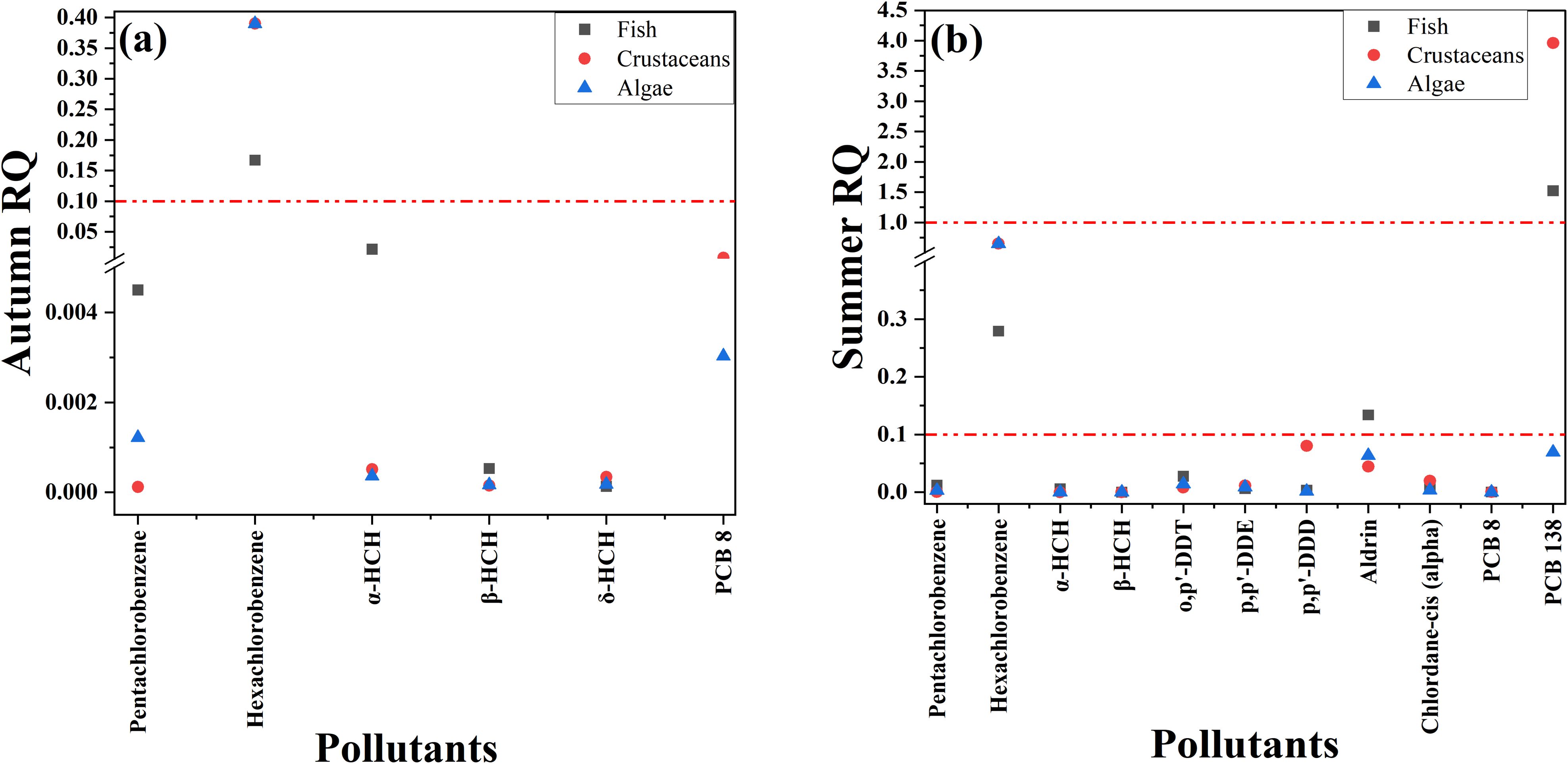
Figure 4. Risk quotient (RQ) values for OCPs and PCBs in the river water of the Xiangshan Harbor Watershed during autumn (a) and summer (b).
The pollutant concentrations were assessed against the Sediment Quality Guidelines (SQG) (Supplementary Table S10). Aldrin concentrations were below the ERL value of 1.98 and the ERM value of 13.89, indicating low risk. However, the concentration of endosulfan sulfate exceeded both the ERL (0.19) and ERM (1.33), indicating potential adverse biological effects. Levels of DDTs, DDEs, and DDDs exceeded the SQG guidelines, indicating a high risk, while total PCBs were below the guidelines, indicating low risk (p< 0.05) (Supplementary Table S10). The elevated levels of DDT metabolites are likely due to intensive agricultural activities and historical residues within the watershed. High DDT risk has also been documented in the Ortega River (Ouyang et al., 2003) and Rhône River (Liber et al., 2019).
To assess potential non-carcinogenic risks from oral intake of pollutants, the total non-carcinogenic risk hazard quotient (TnHQ) was estimated among four age groups (Supplementary Figure S8). RfDo data from published sources were used for the analysis, including 6.3E-0 mg/kg/day for α-HCH (Bradley et al., 2016) and 6.56E-1 mg/kg/day for PCB 149 (Ermler and Kortenkamp, 2022; Eze et al., 2023). PCB 149 had higher TnHQ values (ranging from 2.20E-11 to 2.50E-11) compared to α-HCH (ranging from 3.51E-16 to 1.04E-15) across all age groups, including infants, children, teenagers, and adults (Supplementary Figure S8). Overall, the TnHQ values were well below the USEPA criterion of 1, indicating low non-carcinogenic risk (Sultana et al., 2014). This is in line with results from the Chenab River (Siddique et al., 2023) and Taihu Lake (Wang et al., 2018), where TnHQs of detected pollutants were also below 1. While the non-carcinogenic risks are within acceptable limits, further comprehensive analysis of the synergistic effects of these pollutants is recommended.
4 Conclusion
The research assessed the presence, sources, along with hazards of pollutants in the Xiangshan Harbour Watershed, influenced by industrial and agricultural activities. OCP concentrations were higher in summer, with dicloran being the dominant congener. PCB concentrations were also higher in summer, with PCB 149 being the dominant congener. Elevated levels of dicloran were found in watershed rivers, while HCB was lower. In sediments, p,p’-DDT and PCB 156 were more concentrated in the watershed rivers, while α-HCH was lower. The XX-DOWN area was identified as the OCP hotspot in the water phase, and XX-UP as the PCB hotspot, with XX-DOWN and HDG-DOWN as hotspots in sediments.
The primary sources of OCPs were lindane and technical DDT, while e-waste, thermoplastics, and pigments were the main PCB sources. Risk quotient analysis indicated moderate and high risks for HCB and PCB 138, respectively. SQG guidelines showed high risks for DDTs, DDEs, and DDDs in sediments. However, the non-carcinogenic risk to humans was low. Recommendations include stricter pesticide regulations, promoting e-waste recycling, long-term monitoring, raising public awareness, and future research on bioaccumulation and pollutant interactions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
LC: Visualization, Writing – original draft, Data curation, Investigation, Conceptualization. RZ: Data curation, Software, Methodology, Writing – review & editing. XS: Supervision, Writing – review & editing, Conceptualization, Formal Analysis. ZZ: Methodology, Visualization, Conceptualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research project was supported by the National Natural Science Foundation of China (32303005), the Zhejiang Provincial Natural Science Foundation of China (No. LQ24C190002) and the Natural Science Foundation of Ningbo (No. 2023J107).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. The author(s) used ChatGPT to enhance grammar and clarity. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1630605/full#supplementary-material
References
Abdulnaser H. and Ibrahim S. (2009). Organochlorine pesticide residues in the major rivers of southern Thailand. Malaysian J. Analytical Sci. 12, 280–284.
Abrantes N., Pereira R., and Gonçalves F. (2010). Occurrence of pesticides in water, sediments, and fish tissues in a lake surrounded by agricultural lands: concerning risks to humans and ecological receptors. Water Air Soil pollut. 212, 77–88. doi: 10.1007/s11270-010-0323-2
Alver A., Baştürk E., and Kılıç A. (2025). Impact of seasonal variations and water quality parameters on the formation of trihalomethanes and haloacetic acids in drinking water treatment processes. J. Environ. Manage. 384, 125567. doi: 10.1016/j.jenvman.2025.125567
Anezaki K., Kannan N., and Nakano T. (2015). Polychlorinated biphenyl contamination of paints containing polycyclic-and Naphthol AS-type pigments. Environ. Sci. pollut. Res. 22, 14478–14488. doi: 10.1007/s11356-014-2985-6
Bao L.-J., Maruya K., Snyder S., and Zeng E. (2012). China’s water pollution by persistent organic pollutants. Environ. pollut. (Barking Essex 1987) 163, 100–108. doi: 10.1016/j.envpol.2011.12.022
Behfar A., Nazari Z., Rabiee M. H., Raeesi G., Oveisi M. R., Sadeghi N., et al. (2013). The organochlorine pesticides residue levels in karun river water. Jundishapur J. Nat. Pharm. Prod 8, 41–46. doi: 10.17795/jjnpp-6783
Bradley A. E., Shoenfelt J. L., and Durda J. L. (2016). Carcinogenicity and mode of action evaluation for alpha-hexachlorocyclohexane: Implications for human health risk assessment. Regul. Toxicol. Pharmacol. 76, 152–173. doi: 10.1016/j.yrtph.2015.12.007
Chen M., Jin M., Tao P., Wang Z., Xie W., Yu X., et al. (2018). Assessment of microplastics derived from mariculture in Xiangshan Bay, China. Environ. pollut. 242, 1146–1156. doi: 10.1016/j.envpol.2018.07.133
Chen N., Shan Q., Qi Y., Liu W., Tan X., and Gu J. (2020). Transcriptome analysis in normal human liver cells exposed to 2, 3, 3′, 4, 4′, 5 - Hexachlorobiphenyl (PCB 156). Chemosphere 239, 124747. doi: 10.1016/j.chemosphere.2019.124747
Chiu T. C., Yen J. H., Liu T. L., and Wang Y. S. (2004). Anaerobic degradation of the organochlorine pesticides DDT and heptachlor in river sediment of Taiwan. Bull. Environ. Contam Toxicol. 72, 821–828. doi: 10.1007/s00128-004-0318-z
Ciucure C. T., Geana E.-I., Arseni M., and Ionete R. E. (2023). Status of different anthropogenic organic pollutants accumulated in sediments from Olt River Basin, Romania: From distribution and sources to risk assessment. Sci. Total Environ. 886, 163967. doi: 10.1016/j.scitotenv.2023.163967
Cui X., Dong J., Huang Z., Liu C., Qiao X., Wang X., et al. (2020). Polychlorinated biphenyls in the drinking water source of the Yangtze River: characteristics and risk assessment. Environ. Sci. Europe 32, 29. doi: 10.1186/s12302-020-00309-6
Dai G., Liu X., Liang G., Han X., Shi L., Cheng D., et al. (2011). Distribution of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) in surface water and sediments from Baiyangdian Lake in North China. J. Environ. Sci. (China) 23, 1640–1649. doi: 10.1016/S1001-0742(10)60633-X
Defeo S., Beutel M. W., Rodal-Morales N., and Singer M. (2024). Sediment release of nutrients and metals from two contrasting eutrophic California reservoirs under oxic, hypoxic and anoxic conditions. Front. Water Volume 6, 2024. doi: 10.3389/frwa.2024.1474057
de Oliveira D. P., Sakagami M., Warren S., Kummrow F., and Umbuzeiro G. (2009). Evaluation of dicloran’s contribution to the mutagenic activity of Cristais river, Brazil, water samples. Environ. Toxicol. Chem. 28, 1881–1884. doi: 10.1897/09-103.1
de Souza A. C., Taniguchi S., Lopes Figueira R. C., Montone R. C., Caruso Bícego M., and Martins C. C. (2018). Historical records and spatial distribution of high hazard PCBs levels in sediments around a large South American industrial coastal area (Santos Estuary, Brazil). J. Hazard. Mater. 360, 428–435. doi: 10.1016/j.jhazmat.2018.08.041
Devi N. L., Chakraborty P., Shihua Q., and Zhang G. (2013). Selected organochlorine pesticides (OCPs) in surface soils from three major states from the northeastern part of India. Environ. Monit. Assess. 185, 6667–6676. doi: 10.1007/s10661-012-3055-5
Dvorská A., Lammel G., and Klanova J. (2011). Use of diagnostic ratios for studying source apportionment and reactivity of ambient polycyclic aromatic hydrocarbons over Central Europe. Atmospheric Environ. 45, 420–427. doi: 10.1016/j.atmosenv.2010.09.063
Encarnação T., Pais A. A., Campos M. G., and Burrows H. D. (2019). Endocrine disrupting chemicals: Impact on human health, wildlife and the environment. Sci. Prog. 102, 3–42. doi: 10.1177/0036850419826802
Ermler S. and Kortenkamp A. (2022). Systematic review of associations of polychlorinated biphenyl (PCB) exposure with declining semen quality in support of the derivation of reference doses for mixture risk assessments. Environ. Health 21, 94. doi: 10.1186/s12940-022-00904-5
Eze V. C., Onwukeme V. I., Ogbuagu J. O., and Aralu C. C. (2023). Toxicity and risk evaluation of polychlorinated biphenyls in River Otamiri, Imo State. Sci. Afr. 22, e01983. doi: 10.1016/j.sciaf.2023.e01983
Fahmi M., Mohd Nasir M. F., Samsudin M., Mohamad I., Roshide M., Awaluddin A., et al. (2011). River water quality modeling using combined principle component analysis (PCA) and multiple linear regressions (MLR): A case study at klang river, Malaysia. World Appl. Sci. J. 14.
Forster P. M., Forster H. I., Evans M. J., Gidden M. J., Jones C. D., Keller C. A., et al. (2020). Current and future global climate impacts resulting from COVID-19. Nat. Climate Change 10, 913–919. doi: 10.1038/s41558-020-0883-0
Fu L., Mao X., Mao X., and Wang J. (2022). Evaluation of agricultural sustainable development based on resource use efficiency: empirical evidence from zhejiang province, China. Front. Environ. Sci. 10. doi: 10.3389/fenvs.2022.860481
Fujii Y., Haraguchi K., Harada K., Hitomi T., Inoue K., Itoh Y., et al. (2010). Detection of dicofol and related pesticides in human breast milk from China, Korea and Japan. Chemosphere 82, 25–31. doi: 10.1016/j.chemosphere.2010.10.036
Gan W., Zhang R., Cao Z., Liu H., Fan W., Sun A., et al. (2024). Unveiling the hidden risks: Pesticide residues in aquaculture systems. Sci. Total Environ. 929, 172388. doi: 10.1016/j.scitotenv.2024.172388
Gao J., Liu L., Liu X., Lu J., Zhou H., Huang S., et al. (2008). Occurrence and distribution of organochlorine pesticides – lindane, p,p′-DDT, and heptachlor epoxide – in surface water of China. Environ. Int. 34, 1097–1103. doi: 10.1016/j.envint.2008.03.011
Guo W., Pan B., Sakkiah S., Yavas G., Ge W., Zou W., et al. (2019). Persistent organic pollutants in food: contamination sources, health effects and detection methods. Int. J. Environ. Res. Public Health 16. doi: 10.3390/ijerph16224361
Hiller E., Sirotiak M., Tatarková V., and Jurkovic L. (2011). Occurrence of selected organochlorine pesticide residues in surface sediments from the Velke Kozmalovce, Ruzin, and Zemplinska Sirava water reservoirs, Slovakia. J. Hydrology Hydromechanics 59, 51–59. doi: 10.2478/v10098-011-0004-x
Hitch R. K. and Day H. R. (1992). “Unusual persistence of DDT in some western USA soils,” in Bulletin of environmental contamination and toxicology;(United states), 48.
Kan K. C., Gu X. H., Li H. M., Chen H. H., Mao Z. G., and Zeng Q. F. (2020). Distribution and risk assessment of OCPs in surface water, sediments, and fish from lake gucheng and inflow and outflow rivers. Huan Jing Ke Xue 41, 1346–1356.
Karadeniz H. and Yenisoy-Karakaş S. (2015). Spatial distributions and seasonal variations of organochlorine pesticides in water and soil samples in Bolu, Turkey. Environ. Monit. Assess. 187, 4329. doi: 10.1007/s10661-015-4329-5
Khan S., Khan J. A., Shah N. S., Sayed M., Ateeq M., Ansar S., et al. (2023). Determination of lindane in surface water samples and its degradation by hydrogen peroxide and persulfate assisted TiO(2)-based photocatalysis. RSC Adv. 13, 20430–20442. doi: 10.1039/D3RA03610C
Kong G., Li L., and Guan W. (2022). Influences of tidal flat and thermal discharge on heat dynamics in xiangshan bay. Front. Mar. Sci. Volume 9, 2022. doi: 10.3389/fmars.2022.850672
Leigh M. B., Prouzová P., Macková M., Macek T., Nagle D. P., and Fletcher J. S. (2006). Polychlorinated biphenyl (PCB)-degrading bacteria associated with trees in a PCB-contaminated site. Appl. Environ. Microbiol. 72, 2331–2342. doi: 10.1128/AEM.72.4.2331-2342.2006
Li Q. Q., Loganath A., Chong Y. S., Tan J., and Obbard J. P. (2006). Persistent organic pollutants and adverse health effects in humans. J. Toxicol. Environ. Health A 69, 1987–2005. doi: 10.1080/15287390600751447
Li W., Wang B., Yuan Y., and Wang S. (2023b). Spatiotemporal distribution patterns and ecological risk of multi-pesticide residues in the surface water of a typical agriculture area in China. Sci. Total Environ. 870, 161872. doi: 10.1016/j.scitotenv.2023.161872
Li L., Xu J., Kong G., Li P., Ren Y., and Wang H. (2023a). Hydrodynamics in the tidal flat in semi-enclosed Xiangshan Bay. Front. Mar. Sci. Volume 10, 2023. doi: 10.3389/fmars.2023.1073254
Liber Y., Mourier B., Marchand P., Bichon E., Perrodin Y., and Bedell J.-P. (2019). Past and recent state of sediment contamination by persistent organic pollutants (POPs) in the Rhône River: Overview of ecotoxicological implications. Sci. Total Environ. 646, 1037–1046. doi: 10.1016/j.scitotenv.2018.07.340
Lin S., Zhao B., Ying Z., Fan S., Hu Z., Xue F., et al. (2020). Residual characteristics and potential health risk assessment of polychlorinated biphenyls (PCBs) in seafood and surface sediments from Xiangshan Bay, Chin–2016). Food Chem. 327, 126994. doi: 10.1016/j.foodchem.2020.126994
Liu Y., Chen L., Li H., Song Y., Yang Z., and Cui Y. (2024b). Occurrence of organophosphorus flame retardants in Xiangjiang River: Spatiotemporal variations, potential affecting factors, and source apportionment. Chemosphere 355, 141822. doi: 10.1016/j.chemosphere.2024.141822
Liu X., Dong Z., Baccolo G., Gao W., Li Q., Wei T., et al. (2023). Distribution, composition and risk assessment of PAHs and PCBs in cryospheric watersheds of the eastern Tibetan Plateau. Sci. Total Environ. 890, 164234. doi: 10.1016/j.scitotenv.2023.164234
Liu H., Hu Y., Qi S., Xing X., Zhang Y., Yang D., et al. (2015). Organochlorine pesticide residues in surface water from Sichuan Basin to Aba Prefecture profile, east of the Tibetan Plateau. Front. Earth Sci. 9. doi: 10.1007/s11707-014-0451-x
Liu H., Li R., Hu W., Jian L., Huang B., Zhao Y., et al. (2024a). Multi-medium residues and ecological risk of herbicides in a typical agricultural watershed of the Mollisols region, Northeast China. Sci. Total Environ. 937, 173507. doi: 10.1016/j.scitotenv.2024.173507
Mahalingaiah S., Missmer S. A., Maity A., Williams P. L., Meeker J. D., Berry K., et al. (2012). Association of hexachlorobenzene (HCB), dichlorodiphenyltrichloroethane (DDT), and dichlorodiphenyldichloroethylene (DDE) with in vitro fertilization (IVF) outcomes. Environ. Health Perspect. 120, 316–320. doi: 10.1289/ehp.1103696
Mainali J. and Chang H. (2021). Environmental and spatial factors affecting surface water quality in a Himalayan watershed, Central Nepal. Environ. Sustainability Indic. 9, 100096. doi: 10.1016/j.indic.2020.100096
Megson D., Benoit N. B., Sandau C. D., Chaudhuri S. R., Long T., Coulthard E., et al. (2019). Evaluation of the effectiveness of different indicator PCBs to estimating total PCB concentrations in environmental investigations. Chemosphere 237, 124429. doi: 10.1016/j.chemosphere.2019.124429
Odabasi M., Cetin B., Demircioglu E., and Sofuoglu A. (2008). Air–water exchange of polychlorinated biphenyls (PCBs) and organochlorine pesticides (OCPs) at a coastal site in Izmir Bay, Turkey. Mar. Chem. 109, 115–129. doi: 10.1016/j.marchem.2008.01.001
Ogola J. O., Olale K., Mogwasi R., and Mainya O. (2024). Organochlorine pesticide residues in water and sediments in river Kibos-Nyamasaria in Kisumu County: An inlet river of Lake Victoria, Kenya. Sci. Afr. 23, e02094. doi: 10.1016/j.sciaf.2024.e02094
Olatunji O. S. (2019). Evaluation of selected polychlorinated biphenyls (PCBs) congeners and dichlorodiphenyltrichloroethane (DDT) in fresh root and leafy vegeta bles using GC-MS. Sci. Rep. 9, 538. doi: 10.1038/s41598-018-36996-8
Oliveira T., Santacroce G., Coleates R., Hale S., Zevin P., and Belasco B. (2011). Concentrations of polychlorinated biphenyls in water from US Lake Ontario tributaries between 2004 and 2008. Chemosphere 82, 1314–1320. doi: 10.1016/j.chemosphere.2010.12.012
Ouyang Y., Nkedi-Kizza P., Mansell R. S., and Ren J. Y. (2003). Spatial distribution of DDT in sediments from estuarine rivers of central Florida. J. Environ. Qual. 32, 1710–1716. doi: 10.2134/jeq2003.1710
Persson E. C., Graubard B. I., Evans A. A., London W. T., Weber J. P., LeBlanc A., et al. (2012). Dichlorodiphenyltrichloroethane and risk of hepatocellular carcinoma. Int. J. Cancer 131, 2078–2084. doi: 10.1002/ijc.27459
Pescatore T., Rauseo J., Spataro F., Calace N., and Patrolecco L. (2025). Persistent organic pollutants (POPs) in marine sediments of the Arctic fjord Kongsfjorden, Svalbard Islands. Mar. pollut. Bull. 211, 117407. doi: 10.1016/j.marpolbul.2024.117407
Pimenta A. R., Oczkowski A., McKinney R., and Grear J. (2023). Geographical and seasonal patterns in the carbonate chemistry of Narragansett Bay, RI. Regional Stud. Mar. Sci. 62, 102903. doi: 10.1016/j.rsma.2023.102903
Placencia J. A. and Contreras S. (2018). Organochlorine pesticides in surface waters from Reloncaví Fjord and the inner sea of Chiloé (~39.5°S - 43°S), Chilean Patagonia. Mar. pollut. Bull. 126, 389–395. doi: 10.1016/j.marpolbul.2017.11.053
Pokhrel B., Gong P., Wang X., Chen M., Wang C., and Gao S. (2018). Distribution, sources, and air–soil exchange of OCPs, PCBs and PAHs in urban soils of Nepal. Chemosphere 200. doi: 10.1016/j.chemosphere.2018.01.119
Prokeš R., Vrana B., and Klánová J. (2012). Levels and distribution of dissolved hydrophobic organic contaminants in the Morava river in Zlín district, Czech Republic as derived from their accumulation in silicone rubber passive samplers. Environ. pollut. 166, 157–166.
Qadeer A., Liu S., Liu M., Liu X., Ajmal Z., Huang Y., et al. (2019). Historically linked residues profile of OCPs and PCBs in surface sediments of typical urban river networks, Shanghai: Ecotoxicological state and sources. J. Cleaner Production 231, 1070–1078. doi: 10.1016/j.jclepro.2019.05.203
Qin G. Q. and Yan C. L. (2006). The research progress of organic pollutants ecology on the benthic organisms. Acta Ecologica Sin. 26, 914–922.
Qiu Y.-W., Qiu H.-L., Zhang G., and Li J. (2019). Bioaccumulation and cycling of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) in three mangrove reserves of south China. Chemosphere 217, 195–203. doi: 10.1016/j.chemosphere.2018.10.188
Rachdawong P. and Christensen E. (1997). Determination of PCB sources by a principal component method with nonnegative constraints. Environ. Sci. Technol. - Environ. Sci. Technol. 31. doi: 10.1021/es970107v
Rizzi J., Pérez-Albaladejo E., Fernandes D., Contreras J., Froehner S., and Porte C. (2017). Characterization of quality of sediments from Paranaguá Bay (Brazil) by combined in vitro bioassays and chemical analyses. Environ. Toxicol. Chem. 36, 1811–1819. doi: 10.1002/etc.3553
Shaikh A., Kurian S., Shenoy D. M., Pratihary A. K., and Shetye S. S. (2024). Biogeochemical cycling of iodine in the Bay of Bengal: A comparison with the Arabian Sea. Mar. pollut. Bull. 209, 117329. doi: 10.1016/j.marpolbul.2024.117329
Siddique S., Chaudhry M. N., Ahmad S. R., Nazir R., Zhao Z., Javed R., et al. (2023). Ecological and human health hazards; integrated risk assessment of organochlorine pesticides (OCPs) from the Chenab River, Pakistan. Sci. Total Environ. 882, 163504. doi: 10.1016/j.scitotenv.2023.163504
Sultana J., Syed J. H., Mahmood A., Ali U., Rehman M. Y. A., Malik R. N., et al. (2014). Investigation of organochlorine pesticides from the Indus Basin, Pakistan: Sources, air–soil exchange fluxes and risk assessment. Sci. Total Environ. 497, 113–122. doi: 10.1016/j.scitotenv.2014.07.066
Unyimadu J. P. and Benson N. U. (2023). Polychlorinated biphenyls (PCBs) in intertidal sediment and water from Lagos lagoon: Baseline report on occurrence, distribution and ecotoxicological risk assessment. J. Hazardous Materials Adv. 12, 100372. doi: 10.1016/j.hazadv.2023.100372
Unyimadu J. P., Osibanjo O., and Babayemi J. O. (2019). Concentration and distribution of organochlorine pesticides in sediments of the Niger river, Nigeria. J. Health pollut. 9, 190606. doi: 10.5696/2156-9614-9.22.190606
Urbaniak M., Kiedrzyńska E., Wyrwicka A., Zieliński M., Mierzejewska E., Kiedrzyński M., et al. (2019). An ecohydrological approach to the river contamination by PCDDs, PCDFs and dl-PCBs – concentrations, distribution and removal using phytoremediation techniques. Sci. Rep. 9, 19310. doi: 10.1038/s41598-019-55973-3
Voorspoels S., Covaci A., Maervoet J., De Meester I., and Schepens P. (2004). Levels and profiles of PCBs and OCPs in marine benthic species from the Belgian North Sea and the Western Scheldt Estuary. Mar. pollut. Bull. 49, 393–404. doi: 10.1016/j.marpolbul.2004.02.024
Wang D., Wang Y., Singh V. P., Zhu J., Jiang L., Zeng D., et al. (2018). Ecological and health risk assessment of PAHs, OCPs, and PCBs in Taihu Lake basin. Ecol. Indic. 92, 171–180. doi: 10.1016/j.ecolind.2017.06.038
Wang D., Yang S., Wang G., Gao L., Wang Y., Jiang Q., et al. (2016). Residues and distributions of organochlorine pesticides in China’s weihe river. Polish J. Environ. Stud. 25, 1285–1292. doi: 10.15244/pjoes/61821
Wang X., Zhang Z., Zhang R., Huang W., Dou W., You J., et al. (2022a). Occurrence, source, and ecological risk assessment of organochlorine pesticides and polychlorinated biphenyls in the water–sediment system of Hangzhou Bay and East China Sea. Mar. pollut. Bull. 179, 113735. doi: 10.1016/j.marpolbul.2022.113735
Wang X., Zhu H., Geng Y., Ding K., and Ye L. (2022b). Investigation the effect of the main land-based pollutants in xiangshan bay. Ecol. Chem. Eng. S 29, 27–38. doi: 10.2478/eces-2022-0004
Wu S., Hua P., Gui D., Zhang J., Ying G., and Krebs P. (2022). Occurrences, transport drivers, and risk assessments of antibiotics in typical oasis surface and groundwater. Water Res. 225, 119138. doi: 10.1016/j.watres.2022.119138
Xu J., Guan Y., Oldfield J., Guan D., and Shan Y. (2024). China carbon emission accounts 2020-2021. Appl. Energy 360, 122837. doi: 10.1016/j.apenergy.2024.122837
Xu J., Tian Y.-Z., Zhang Y., Guo C.-S., Shi G.-L., Zhang C.-Y., et al. (2013). Source apportionment of perfluorinated compounds (PFCs) in sediments: Using three multivariate factor analysis receptor models. J. Hazard. Mater. 260, 483–488. doi: 10.1016/j.jhazmat.2013.06.001
Xu Y., Wang Y., Hu S., Zhu Y., Zuo J., and Zeng J. (2023). Study on the impact of the coastline changes on hydrodynamics in xiangshan bay. Appl. Sci. 13, 8071. doi: 10.3390/app13148071
Yahaya A., Okoh O. O., Okoh A. I., and Adeniji A. O. (2017). Occurrences of organochlorine pesticides along the course of the buffalo river in the eastern cape of South Africa and its health implications. Int. J. Environ. Res. Public Health 14. doi: 10.3390/ijerph14111372
Zeng E. Y. and Venkatesan M. I. (1999). Dispersion of sediment DDTs in the coastal ocean off southern California. Sci. Total Environ. 229, 195–208. doi: 10.1016/S0048-9697(99)00064-9
Zhang F., Cui K., Yuan X., Huang Y., Yu K., Li C.-X., et al. (2024). Differentiated cognition of the effects of human activities on typical persistent organic pollutants and bacterioplankton community in drinking water source. Environ. Res. 252, 118815. doi: 10.1016/j.envres.2024.118815
Zhang Q., Meng J., Su G., Liu Z., Shi B., and Wang T. (2021). Source apportionment and risk assessment for polycyclic aromatic hydrocarbons in soils at a typical coking plant. Ecotoxicol. Environ. Saf. 222, 112509. doi: 10.1016/j.ecoenv.2021.112509
Zhang X. and Ning J. (2024). Land use change in coastal zones of China from 1985 to 2020. Front. Mar. Sci. Volume 11, 2024. doi: 10.3389/fmars.2024.1323032
Zhang J., Qi S., Xing X., Tan L., Gong X., Zhang Y., et al. (2011a). Organochlorine pesticides (OCPs) in soils and sediments, southeast China: A case study in Xinghua Bay. Mar. pollut. Bull. 62, 1270–1275. doi: 10.1016/j.marpolbul.2011.03.010
Zhang L., Shi S., Dong L., Zhang T., Zhou L., and Huang Y. (2011b). Concentrations and possible sources of polychlorinated biphenyls in the surface water of the Yangtze River Delta, China. Chemosphere 85, 399–405. doi: 10.1016/j.chemosphere.2011.07.064
Zhao S., Jones K. C., Li J., Sweetman A. J., Liu X., Xu Y., et al. (2020). Evidence for major contributions of unintentionally produced PCBs in the air of China: implications for the national source inventory. Environ. Sci. Technol. 54, 2163–2171. doi: 10.1021/acs.est.9b06051
Zhao Z., Sun J., Fang X.-K., Xia L.-L., and Hussain J. (2016). Impacts of channel morphology on residues and ecological risks of polychlorinated biphenyls in water and sediment in Chahe River. Water Sci. Eng. 9, 300–311. doi: 10.1016/j.wse.2017.01.006
Keywords: OCPs, PCBs, source apportionment, Xiangshan harbour watershed, environmental pollution
Citation: Chidewe L, Zhang R, Shi X and Zhang Z (2025) Occurrence and ecological risks of organochlorine pesticides and polychlorinated biphenyls in a semi-enclosed urban watershed. Front. Mar. Sci. 12:1630605. doi: 10.3389/fmars.2025.1630605
Received: 18 May 2025; Accepted: 01 August 2025;
Published: 20 August 2025.
Edited by:
Xiutang Yuan, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Sundhar Shanmugam, Fisheries College and Research Institute, IndiaLufeng Chen, Jianghan University, China
Copyright © 2025 Chidewe, Zhang, Shi and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zeming Zhang, emhhbmd6ZW1pbmdAbmJ1LmVkdS5jbg==
 Liberty Chidewe
Liberty Chidewe Rongrong Zhang
Rongrong Zhang Xizhi Shi
Xizhi Shi Zeming Zhang
Zeming Zhang