- 1Fisheries and Aquaculture, Centre for Fisheries and Aquaculture Science (Cefas), Lowestoft, United Kingdom
- 2School of Biological Sciences, The University of Auckland, Auckland, New Zealand
- 3HalieuMer, Arzon, France
- 4School of Science, Auckland University of Technology, Auckland, New Zealand
This study investigates the age-based life-history traits of two filefish species from the Solomon Islands, the Honeycomb filefish (Cantherhines pardalis) and the Broom filefish (Amanses scopas). The research aims to fill a significant gap in our understanding of tropical monacanthid demography by providing an age-based life-history assessment for these species. A total of 201 C. pardalis and 60 A. scopas were collected from the reefs offshore Vavanga Village, Solomon Islands, between January 2016 and February 2017. Age determination was based on thin transverse sagittal otolith sections, revealing that C. pardalis reaches a maximum age of 9 years (n=140, CV=6.7, APE=8.12), while A. scopas of 12 years (n=42, CV=2.5, APE=20.3). The study found marked differences in life-history strategies between the two species, with C. pardalis exhibiting rapid early growth and early maturation within its first year, whereas A. scopas grows more slowly and reaches a larger asymptotic size later in life. Sex-specific patterns in growth and length-weight relationships were observed, indicating differential energy allocation strategies. Mortality estimates align with expectations for small-bodied reef fishes subjected to natural predation and artisanal harvesting. The findings highlight divergent growth and reproductive strategies within a single family in the same reef system, underscoring the ecological plasticity of monacanthids.
Introduction
The Monacanthidae family, commonly referred to as filefish, comprise of 102 species across 27 genera, primarily found in tropical and subtropical regions (Matsuura, 2015; Bray, 2023). Of these, 58 species are found in Australian waters representing a major geographically constrained diversification (Gomon et al., 2008). The widespread distribution of monacanthids across the Pacific, Atlantic and Indian oceans have made them a popular target for subsistence fishing, the ornamental aquarium trade, and the focus of large-scale commercial fisheries (Wood, 2001; Miller and Stewart, 2009; Hinton et al., 2014; Moesinger, 2018). Monacanthids typically display a laterally compressed, slender, and elongated body with a distinctive flat head and snout (Nelson, 1994). They appear to be slow swimmers, have soft caudal and short pectoral fins, lack pelvic fins, and have a long, articulated, sharp spine on their crown (Harmelin-Vivien and Quéro, 1990). They also exhibit a variety of foraging behaviours, adapted to various habitats ranging from coral reefs, seagrass beds, sandy and muddy substrate to the open oceans (Clements and Livingston, 1984; Barlow, 1987; Hobson and Chess, 1996; Ballard and Rakocinski, 2012; Horinouchi et al., 2013; Miyajima-Taga et al., 2016).
Depending on the region, between 30 to 40 species of monacanthids can be globally found on coral reefs (Harmelin-Vivien and Quéro, 1990; Nelson, 1994), ranging from specialist obligate corallivores to generalist omnivores, detritivores, planktivorous and benthivores (Hobson and Chess, 1996; Prado and Heck, 2011; Brooker et al., 2013). In a study by Siqueira et al. (2023) reviewing the evolution of fish–coral interactions, members of the family Monacanthidae were identified as playing a critical functional role in coral reef ecosystems, owing to the strong associations exhibited by several species with coral habitats. A few recent demographic studies of Indo-Pacific coral reef species belonging to acanthurids, labrids and mullids, have also highlighted the importance of the age-based information derived from otoliths not only to inform fisheries managers but also to detect signals of environmental and climatic variability over latitudinal gradients (Pardee et al., 2025; Reed et al., 2025). However, despite the ecological role within coral reef ecosystems of monacanthids, the population dynamics and life history of several tropical species of this family have yet to be documented.
The genus Amanses contains a single Indo-Western Pacific species, A. scopas, whereas the genus Cantherhines comprises 12 tropical species (Matsuura, 2015). Both species are widely distributed across the Indo-Pacific and are typically observed in pairs on outer reef slopes at depths ranging from 2 to 20 meters. Amanses scopas shows a preference for coral-rich areas, reflecting its diet of primarily coral polyps, whereas C. pardalis predominantly feeds on benthic organisms (Harmelin-Vivien and Quéro, 1990; Gomon et al., 2008). The maximum recorded total length (TL) is 25 cm for C. pardalis and 20 cm for A. scopas (Harmelin-Vivien and Quéro, 1990; Hutchins, 2001). Cantherhines pardalis exhibits a range of colour patterns, varying from mottled grey and brown to dark brown, or grey with a network of polygonal spots that confer a leopard-like (pardus) appearance. Despite this variation, a distinctive white spot at the rear base of the second dorsal fin remains a consistent feature that facilitates underwater identification. In contrast, A. scopas displays a more uniform brown body colouration, typically marked by up to 12 narrow dark brown crossbars. Sexual dimorphism can be distinguished by examining the area in front of the caudal peduncle: males possess several long spines, while females display a toothbrush-like mass of setae. Cantherhines pardalis is the only one for which embryonic development has been briefly described from wild-captured eggs (Kawase and Nakazono, 1994), and gonadal and larval development has been examined in captivity, albeit under hormonal induction (Shadrin and Emel’yanova, 2022).
To the best of our knowledge, there are no comprehensive studies on the age-based life-history traits of monacanthids with the exception four species: Meuschenia scaber, Meuschenia australis, Nelusetta ayraudi and Penicipelta vittiger (Barrett, 1995a; Miller et al., 2010; Visconti et al., 2018b). In this context of minimal biological data available for the monacanthid family, we provide the first age-based life-history study of two common coral reef monacanthids from the Solomon Islands: the honeycomb filefish (Cantherhines pardalis) and the broom filefish (Amanses scopas).
Materials and methods
Sample collection
A total of 201 C. pardalis and 60 A. scopas were collected between January 2016 and February 2017 from the reefs of Vavanga Village, located on the southwest coast of Kolombangara Island, Western Province, Solomon Islands (8°03’41” S, 156°58’05” E). Samples were collected from artisanal indigenous spearfishers with no control over the selectivity of size or sex, therefore the age structure presented here is likely to reflect the population in the area sampled. Total length (TL), standard length (SL) and body depth (BD) were recorded to the nearest mm, and total weight (TW) and gutted weight (WG) were measured to the nearest mg. The sex of each specimen was determined during dissection based on external characteristics and macroscopic examination of the gonadal tissue. Both sagittal otoliths were extracted from each specimen (Figures 1A, C), rinsed in 70% ethanol and stored dry in 96-well plates.
Age determination
For each fish, one otolith from the paired sagittal set was randomly selected and used to prepare a thin transverse section, following the methodology described by Visconti et al. (2018b). Otoliths were ground from the posterior and anterior margins to obtain a thin transverse section through the nucleus of approximately 200μm using a combination of Carbimet Silicon carbide P2500 and FiberMet 0.3 μm (Buehler, http://www.buehler.com). Otolith thin sections were subsequently covered with a clear low viscosity epoxy resin (RT310, Resintech, http://www.resintech.co.uk). Otolith sections were viewed under reflected light against a black background with a Leica DM2000 compound microscope (www.leica.com) at ×100 magnification, and pictures were taken for each sample with a GT Vision GXCAM-U3PRO camera (www.gtvision.co.uk) for subsequent analyses. Age determination was conducted using ObjectJ software (www.simon.bio.uva.nl/object) by examining the alternation of opaque and translucent zones along a linear path from the otolith core to its distal edge. The assumption of annual periodicity in the formation of these zones in monacanthid otoliths has been previously proposed (Rogers et al., 2001; Visconti et al., 2020). Each otolith section was read twice by the expert reader (V.V.), with the number of opaque zones counted to estimate age in years. If the two independent counts were identical, the reading was accepted as the final age estimate; otherwise, the individual was excluded from subsequent age-based analyses. A standard birthday of 1 July was assigned to all individuals, based on the assumption of year-round spawning and following the approach of Proctor et al. (2021). In order to examine the precision of the reading, the coefficient of variation (CV) and the average percentage error (APE) were calculated amongst readings (Beamish & Fournier 1981; Campana, 2001). A total of 140 C. pardalis and 42 A. scopas individuals were successfully aged (Figure 1).
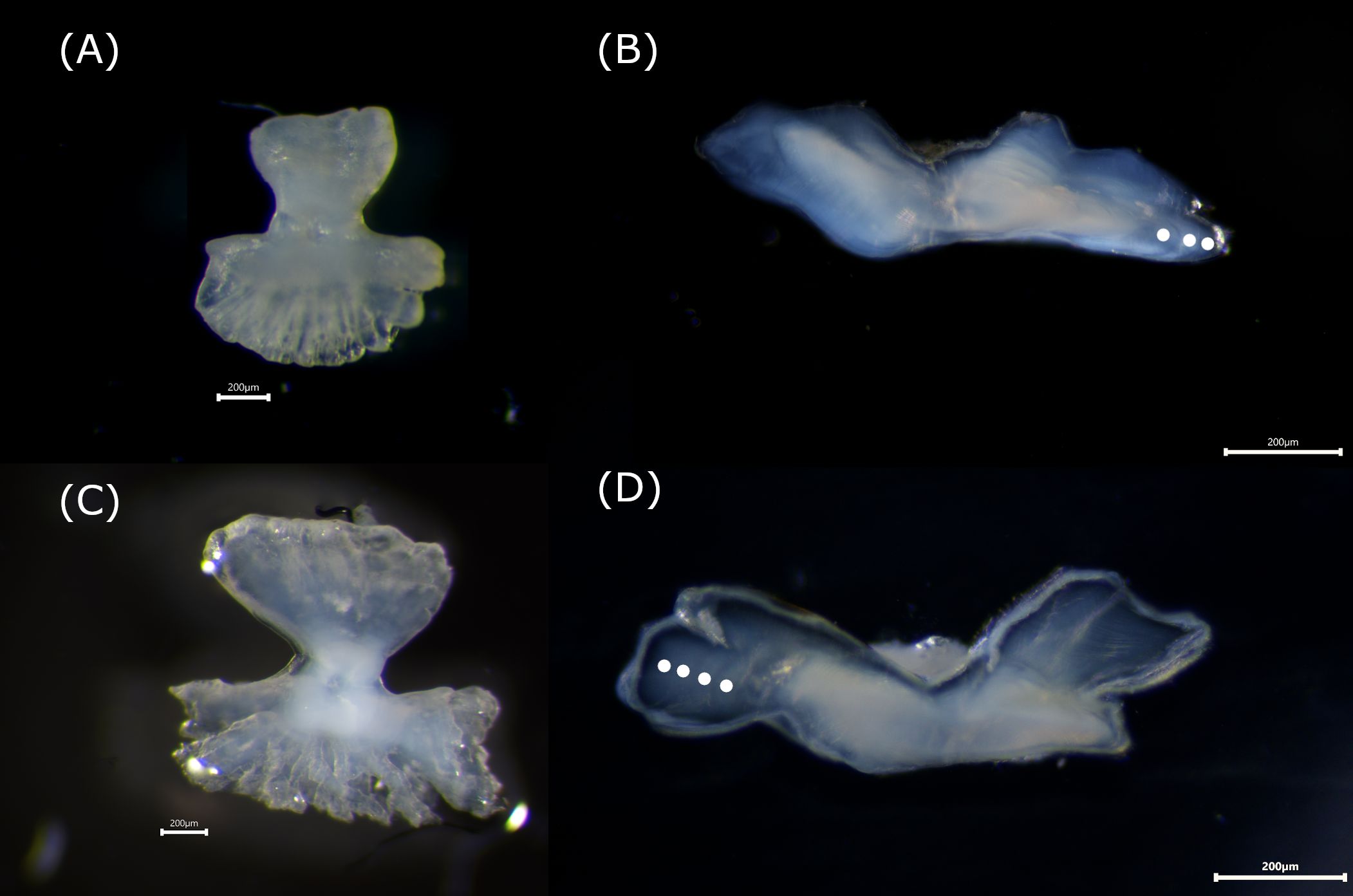
Figure 1. Whole and sectioned otoliths of Cantherhines pardalis (A, B) and Amanses scopas (C, D), viewed under reflected light. Distinct opaque growth increments, interpreted as annual rings, are marked with white dots.
Reproductive parameters
All specimens were eviscerated upon capture, and each pair of gonads was photographed (Supplementary Figure S1), weighed to the nearest 0.01 g, stored in 10% buffered formalin, and histologically processed within six months. All the tissues were processed following Visconti et al. (2018a) where one of the two gonad lobes for each gonad was embedded in paraffin wax, sectioned at 7 μm, and stained using a combination of Ehrlich’s and Gill’s haematoxylin and eosin (H&E). All the gonad sections were observed using a Leica DMRE upright microscope equipped with a colour camera (Leica DC500), and digital images were captured using AnalySIS LifeScience software. Macroscopic and microscopic developmental stages were identified and assigned according to Trip et al. (2011a) and Visconti et al. (2018a) (Supplementary Figure S2).
Population parameters
The distribution of size and age class frequency was analysed to understand the growth and longevity of both species. Instantaneous total mortality rates (Z) were derived from age-based catch curves. A linear regression was applied to the natural logarithm of the descending frequency of individuals per age class, starting from the modal age class (i.e., the age with the highest abundance) and extending to the oldest age class showing a consistent decline in abundance. This included ages 3–9 for C. pardalis and 6–12 for A. scopas.
The relationship between weight and length was described following equation (Ricker, 1973):
where W denotes total weight (g), L represents standard length (mm), ‘a’ is the y-intercept or initial growth coefficient, and ‘b’ is the slope or growth coefficient. For most fish species, growth is isometric, with ‘b’ typically around 3.0. A ‘b’ value less than 3.0 indicates negative allometric growth, while a value greater than 3.0 signifies positive allometric growth.
The relationship between size and age was modelled using the von Bertalanffy growth function (VBGF), fitted for combined sexes, and for males and females separately for both species, following the equation:
where Lt is the estimated mean size-at-age t, and L∞ represents the mean asymptotic size or the maximum average length a fish would theoretically reach if it lived indefinitely. K is the growth coefficient, reflecting the steepness of the ascending portion of the growth curve and indicating how rapidly the fish approaches its asymptotic length, and t0 denotes the theoretical age at which the fish’s length would be zero. t0 is not always an actual observable point but rather a parameter that shapes the growth curve, especially if sufficient young and smaller samples are available. The VBGF was fitted by constraining the curve to a length-at-settlement of 10 mm TL according to previous studies on the Monacanthidae family (Kingsford and Milicich, 1987; Visconti et al., 2020). VBGF growth trajectories were compared between the two species using 95% confidence ellipses surrounding the traditional VBGF estimates of parameters L∞ and K (Kimura, 1980). Mean maximum age Tmax and mean maximum body size Lmax were calculated for each species as the average age (in years) of the 15% oldest individuals, and as the average body size (total length, in mm) of the 15% largest individuals found within each species (Beverton, 1992; Trip et al., 2008).
Age and size at sexual maturity were determined for C. pardalis using immature and mature females collected between 2016 and 2017 (Supplementary Figure S2). Size at maturity was estimated from the size at which 50% of females were sexually mature (L50) and the size at which 95% of females were sexually mature (L95) (Trip et al., 2011b; Visconti et al., 2018a). Age at maturity was estimated from the age at which 50% and 95% of females were sexually mature (T50 and T95, respectively) following Trip et al. (2011b) and Visconti et al. (2018a). The logistic function (the maturity ogive) was fitted to the proportion of mature fish in each year class that were sampled during the spawning season and the ogive was fitted to the mid-point of each age class. The best-fit logistic function was estimated by minimizing the negative log10 of the likelihood based on a probability density function with a binomial distribution (Haddon, 2001). No immature females were present among the A. scopas sampled, which prevented us from calculating the logistic functions and estimating size- and age-at-maturity for this species.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Results
Size, age and growth parameters
The transverse sectioned otoliths showed clear alternating patterns of opaque and translucent zones, with C. pardalis exhibiting better reading patterns (Figure 1B) than A. scopas (Figure 1D). C. pardalis ranged in total length (TL) from 104 mm to a maximum of 163 mm, while A. scopas exhibited a broader size range, from 110 mm up to 188 mm TL (Figure 2A). In terms of age distribution, C. pardalis reached a maximum age of 9 years while A. scopas attained 12 years (Figure 2B). The ageing precision for both species resulted similar to those published for other monacanthids (Visconti et al., 2018a; Miller et al., 2010), with C. pardalis (n=140) scoring a CV=6.7% and APE=8.12, and A. scopas (n=42), a CV=2.5%, APE=20.3.
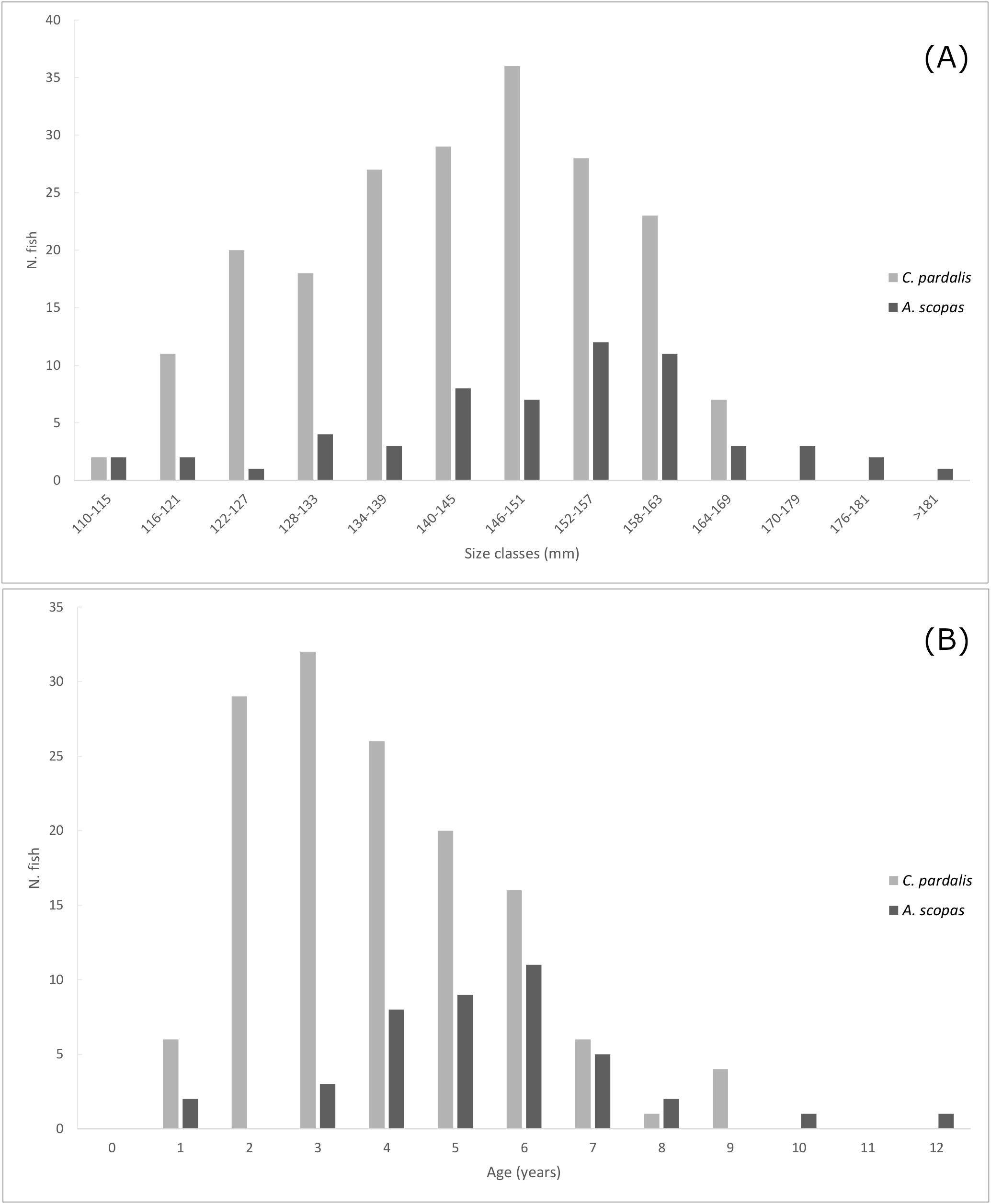
Figure 2. Age (A), and size distribution (B) of Cantherhines pardalis and Amanses scopas from the Solomon Islands.
Mortality estimates for C. pardalis and A. scopas based on age-based catch curves revealed annual mortality rate of 39.25% (yr-1) and 47.74% (yr-1), respectively, reflecting their relatively short life spans (Table 1).
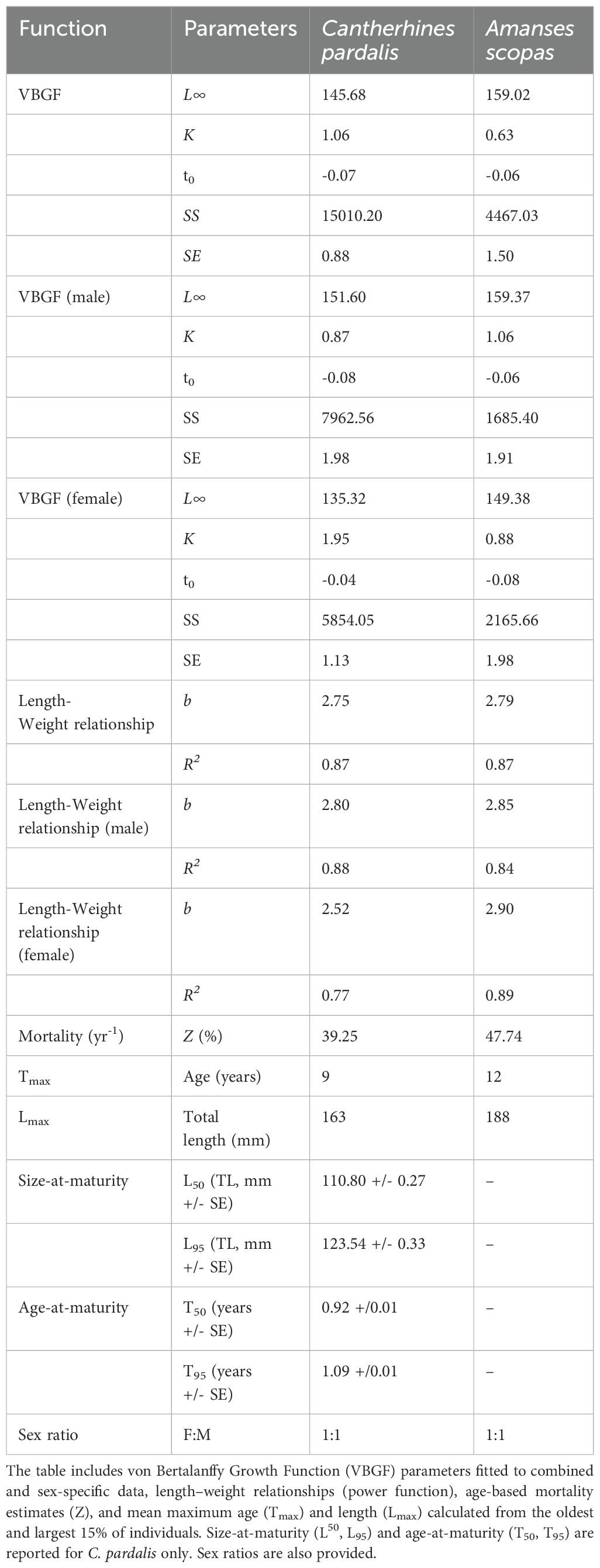
Table 1. Population parameter estimates for Cantherhines pardalis and Amanses scopas from the Solomon Islands.
Analysis of the length (L) and weight (W) relationship for C. pardalis revealed a negatively allometric L-W growth coefficient of 2.75, which indicates a species that prioritises growth over condition (Table 1; Supplementary Figure S3). When partitioned by sex, females exhibited a stronger negative growth coefficient (b=2.52) compared to males (b=2.80), suggesting a behavioural dominance of males over females. The negative allometric relationship between length and weight was the same for A. scopas (b=2.79), however, when partitioned by sex the growth coefficient was similar between males (b=2.85) and females (b=2.90).
Given the 1:1 sex ratio for both species, the length-weight relationships provide an interesting insight into the growth vs condition strategy of this species, which coincides with the results of the VBGF analysis. C. pardalis (Figure 3; Table 1) exhibited fast early growth (K=1.06) in the first two to three years of life, before reaching an asymptotic length of 145.68 mm (TL). In contrast, A. scopas showed comparatively slower and more gradual growth (K=0.63), reaching an asymptotic length of 159.02mm (TL) later in life (Figure 3; Table 1). The comparison of combined growth curves of both species showed C. pardalis to grow faster in their first 3 years of life while A. scopas grow relatively slower but attain bigger asymptotic lengths and older ages. These differences were confirmed by plotting the 95% confidence ellipses surrounding the VBGF estimates of parameters L∞ and K (Figure 3). When partitioned by sex, C. pardalis males grew slower early on but reached a larger asymptotic length (151.60 mm TL) compared to females (135.32 mm TL) (Supplementary Figure S4; Table 1). When partitioned by sex, A. scopas males grew faster than females, and males reached a comparatively larger asymptotic length (159.37 mm TL) than females (149.38 mm TL) (Supplementary Figure S4; Table 1).
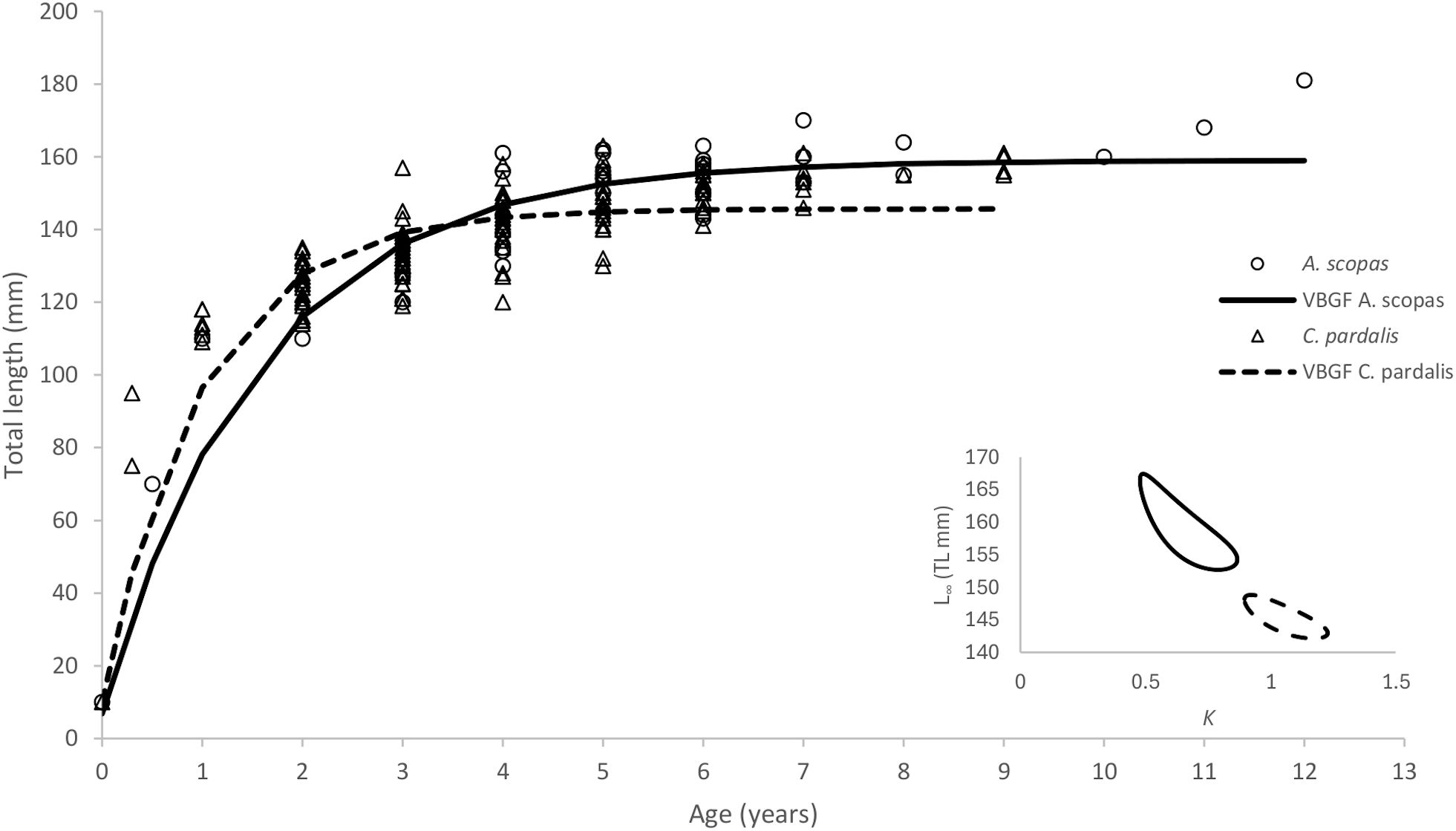
Figure 3. Von Bertalanffy growth trajectories of Cantherhines pardalis (dashed line) and Amanses scopas (continuous line) in the Solomon Islands, and comparison of VBGF parameters K and L∞ using 95% confidence ellipses (inset). Results of VBGF parameter values are presented in Table 1.
Reproductive parameters
Histological analysis identified six reproductive stages that were assigned to females to the gonad histological sections of both species, following the criteria published in Visconti et al. (2018a) (Supplementary Figure S2). Inactive ovaries showed immature characteristics with chromatin nucleolar stage of primary growth cells (pre-vitellogenic oocytes) organised in a compact grid of ovigerous lamellae surrounded by a thin ovarian wall. Ripening ovaries exhibited the developing features during the secondary growth and were divided into two sub-stages: the first (ripening1) displaying early-stage cortical alveolar and primary vitellogenic oocytes, and the second (ripening2) displaying secondary (Vtg2) and tertiary (Vtg3) vitellogenic oocytes. Spawning ovaries contained several full yolk granule oocytes, with most undergoing germinal vesicle migration and breakdown, fully hydrated oocytes were abundant in the central lumen, and postovulatory follicles (POFs) and atretic oocytes were observed in the ovarian tissue. Post-spawning ovaries showed atretic hydrated oocytes and signs of previous spawning (post-ovulatory follicles, intra-lamellar muscle bundles). Resting ovaries (reproductively inactive) had numerous primary growth cells kept together in the lamellae by thick interlamellar muscle bundles and a thick ovary wall (Supplementary Figure S2).
The logistic function for size-at-maturity for C. pardalis showed that maturity is reached at 110mm of FL (L50) with the 95% of the individuals being mature at 123.5mm (L95). The logistic function for age-at-maturity for C. pardalis revealed an early maturation in females at just 1 year of life, with 50% of females becoming mature and active spawners by 0.92 years, 95% of females becoming mature by 1.09 years, and 100% of the individuals becoming mature and active spawners by the second year of life (Supplementary Figure S5). The youngest and smallest A. scopas female (1 year old and 110 mm TL) was diagnosed as being sexually mature with ripened ovaries, suggesting early sexual maturation (in age and body size) in A. scopas, which was also observed in C. pardalis.
Discussion
This study provides the first age-based life-history assessment for C. pardalis and A. scopas from the Solomon Islands, filling a significant gap in our understanding of tropical monacanthid demography. Despite their widespread occurrence and ecological relevance in coral reef habitats, detailed biological data for these species have been absent in the literature to date. Our findings indicate marked differences in life-history strategies between the two species. C. pardalis exhibits rapid early growth, matures within its first year, and reaches a maximum observed age of 9 years. In contrast, A. scopas grows more slowly (K = 0.63), attains a larger asymptotic size, and reaches an older maximum age of 13 years. Sex-specific patterns in growth and length-weight relationships were observed in both species. In C. pardalis, males achieved larger asymptotic lengths but grew more slowly than females, suggesting differential energy allocation strategies—potentially reflecting reproductive roles or territorial behaviours (Afeworki et al., 2014; Olsson and Gislason, 2016). The lack of immature A. scopas females in our sample along with the imbalanced sample-size presented in this study (i.e. C. pardalis n=201 vs A. scopas n=60) complicates direct comparisons, but the presence of mature females at small sizes supports early maturation in this species as well.
The observed negative allometric growth in both species (b< 3) implies prioritisation of somatic growth in length over weight, which may confer hydrodynamic or ecological advantages in structurally complex reef environments (Gust et al., 2002; Dunic and Baum, 2017). Similar patterns have been documented in other monacanthids and may be adaptive for manoeuvrability or habitat use (Ballard and Rakocinski, 2012; Visconti et al., 2020). Mortality estimates (Z = 39.25% for C. pardalis; Z = 47.74% for A. scopas) align with expectations for small-bodied reef fishes subjected to natural predation and artisanal harvesting. Monacanthids have been observed to display a variety of mating behaviours (Kawase, 1998, 2002), and are generally gonochoristic in their reproductive dynamics, with some sex-changing exceptions (Yamaguchi et al., 2013). In our study, both species displayed near 1:1 sex ratios, and gonochorism is consistent with previous reports for monacanthids.
Collectively, these findings support the hypothesis of divergent growth and reproductive strategies within a single family in the same reef system and, particularly, the ecological plasticity of monacanthids. Recent studies and observations have highlighted some peculiar ecological aspects of monacanthids in the coral reef system; an A. scopas individual was recorded to be anchored at the same Acropora spp. colony for several days after a severe storm (Eyal et al., 2011). While this sleeping behaviour reiterates the strong association with coral reef habitats (recently highlighted in Siqueira et al., 2023), on the other hand this species has been seen primarily feeding on coral polyps. If its diet was confirmed, A. scopas could be threatened by the current increase of habitat loss and forced to switch diet towards alternative resources as already documented for another monacanthid, the harlequin filefish Oxymonacanthus longirostris (Hobbs, 2013).
Given their role in reef trophodynamics, further research on their diet, habitat use, and responses to environmental stressors would enhance our understanding of the functional role of monacanthids in Pacific coral reef ecosystems.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by the University of Auckland Animal Ethics for the collection of another monacanthid species and the same guidelines and protocols were used for the collection of the species in this study. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
VV: Project administration, Data curation, Writing – original draft, Formal Analysis, Visualization, Investigation, Resources, Conceptualization, Writing – review & editing, Funding acquisition, Supervision. IW: Writing – review & editing, Investigation, Methodology. ET: Writing – review & editing, Validation, Formal Analysis, Supervision, Visualization, Methodology. AS: Resources, Writing – review & editing, Supervision, Investigation, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The project was funded by the Auckland University of Technology, The Auckland University PhD PReSS Account funding, and CEFAS Science Future funding (project code DP411).
Acknowledgments
The authors would like to thank the local authorities (research permit number 0286418) and field assistants, Woody Green and Nixon Silaspio, for the logistics and sample collection.
Conflict of interest
Author ET was employed by HalieuMer.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1634488/full#supplementary-material
References
Afeworki Y., Videler J. J., Berhane Y. H., and Bruggemann J. H. (2014). Seasonal and life-phase related differences in growth in Scarus ferrugineus on a southern Red Sea fringing reef. J. Fish Biol. 84, 1422–1438. doi: 10.1111/jfb.12372
Ballard S. E. and Rakocinski C. F. (2012). Flexible Feeding Strategies of Juvenile Gray Triggerfish (Balistes capriscus) and Planehead Filefish (Stephanolepis hispidus) Within Sargassum Habitat. GCR 24, 31–40. doi: 10.18785/gcr.2401.05
Barlow G. W. (1987). Spawning, eggs and larvae of the longnose filefish Oxymonacanthus longirostris, a monogamous coralivore. Environ. Biol. Fish 20, 183–194. doi: 10.1007/BF00004953
Barrett N. S. (1995a). Aspects of the biology and ecology of six temperate reef fishes (Families Labridae and Monacanthidae). Hobart, Tasmania: University of Tasmania. PhD Thesis.
Beamish R. J. and Fournier D. A. (1981). A method for comparing the precision of a set of age determinations. Canadian J. Fish. Aquatic Sci. 38 (8), 982–983.
Beverton R. J. H. (1992). Patterns of reproductive strategy parameters in some marine teleost fishes. J. Fish Biol. 41, 137–160. doi: 10.1111/j.1095-8649.1992.tb03875.x
Bray D. J. (2023). “Leatherjackets, MONACANTHIDAE,” in Fishes of Australia. Sydney: Reed New Holland. Available online at: https://fishesofAustralia.net.au/home/family/250.
Brooker R. M., Jones G. P., and Munday P. L. (2013). Prey selectivity affects reproductive success of a corallivorous reef fish. Oecologia 172, 409–416. doi: 10.1007/s00442-012-2521-7
Campana S. E. (2001). Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. J. Fish Biol. 59 (2), 197–242.
Clements W. and Livingston R. (1984). Prey selectivity of the fringed filefish Monacanthus ciliatus (Pisces: Monacanthidae): role of prey accessibility. Mar. Ecol. Prog. Ser. 16, 291–295. doi: 10.3354/meps016291
Dunic J. C. and Baum J. K. (2017). Size structuring and allometric scaling relationships in coral reef fishes. J. Anim. Ecol. 86, 577–589. doi: 10.1111/1365-2656.12637
Eyal G., Eyal-Shaham L., and Loya Y. (2011). Teeth-anchorage”: sleeping behavior of a Red Sea filefish on a branching coral. Coral Reefs 30, 707–707. doi: 10.1007/s00338-011-0766-y
Gomon M. F., Bray D. J., and Kuiter R. H. (2008). Fishes of Australia’s Southern Coast. (Chatswood, Australia: Reed New Holland Publishers Pty Ltd, Sydney).
Gust N., Choat J., and Ackerman J. (2002). Demographic plasticity in tropical reef fishes. Mar. Biol. 140, 1039–1051. doi: 10.1007/s00227-001-0773-6
Haddon M. (2001). Fisheries, population dynamics, and modelling. Modelling and Quantitative Methods in Fisheries. Boca Raton, Florida: Chapman and Hall/CRC, p. 1–18.
Harmelin-Vivien M. L. and Quéro J. C. (1990). “Monacanthidae,” in Check-list of the fishes of the tropical Atlantic, vol. 2 . Eds. Quéro J. C., Hureau J. C., Karrer C., Post A., and Saldanha L. (JNICT, Lisbon, SEI, Paris; and UNESCO, Paris), 1061–1066.
Hinton M. G., Maunder M., Vogel N. W., Olson R., Lennert-Cody C., Aires-da-Silva A., et al. (2014). Stock status indicators for fisheries of the eastern Pacific Ocean. Inter-American Trop. Tuna Commission Stock Assess. Rep. 15, 142–182.
Hobbs J. P. A. (2013). Obligate corallivorous filefish (Oxymonacanthus longirostris) switches diet from Acropora to Pocillopora corals following habitat loss. Mar. Biodiversity 43, 175–176. doi: 10.1007/s12526-013-0155-6
Hobson E. S. and Chess J. R. (1996). Examination of a great abundance of filefish, Pervagor spilosoma, in Hawaii. Environ. Biol. Fishes 47, 269–278. doi: 10.1007/BF00000499
Horinouchi M., Mizuno N., Jo Y., Fujita M., Suzuki Y., Aranishi F., et al. (2013). Habitat preference rather than predation risk determines the distribution patterns of filefish Rudarius ercodes in and around seagrass habitats. Mar. Ecol. Prog. Ser. 488, 255–266. doi: 10.3354/meps10396
Hutchins J. B. (2001). “Monacanthidae. Filefishes (leatherjackets). FAO species identification guide for fishery purposes,” in The living marine resources of the Western Central Pacific Rome: FAO Library, vol. 6, 3929–3947.
Kawase H. (1998). Reproductive behavior and evolution of triggerfish (Balistidae) and filefish (Monacanthidae). Japanese J. Ichthyology 45, 1–19. doi: 10.11369/jji1950.45.1
Kawase K. (2002). Simplicity and diversity in the reproductive ecology of triggerfish (Balistidae) and filefish (Monacanthidae). Fisheries Sci. 68, 119–122. doi: 10.2331/fishsci.68.sup1_119
Kawase H. and Nakazono A. (1994). Reproductive Behavior of the Honeycomb Leatherjacket, Cantherhines pardalis (Monacanthidae), at Kashiwajima, Japan. Japanese J. Ichthyology 41, 80–83. doi: 10.11369/jji1950.41.80
Kimura D. K. (1980). Likelihood methods for the von Bertalanffy growth curve. Fishery Bull. 77, 765–776.
Kingsford M. and Milicich M. (1987). Presettlement phase of Parika scaber (Pisces: Monacanthidae): a temperate reef fish. Mar. Ecol. Prog. Ser. 36, 65–79. doi: 10.3354/meps036065
Matsuura K. (2015). Taxonomy and systematics of tetraodontiform fishes: a review focusing primarily on progress in the period from 1980 to 2014. Ichthyological Res. 62, 72–113. doi: 10.1007/s10228-014-0444-5
Miller M. E. and Stewart J. (2009). The commercial fishery for ocean leatherjackets (Nelusetta ayraudi, Monacanthidae) in New South Wales, Australia. Asian Fisheries Sci. 22, 257–264. doi: 10.33997/j.afs.2009.22.1.024
Miller M. E., Stewart J., and West R. J. (2010). Using otoliths to estimate age and growth of a large Australian endemic monocanthid, Nelusetta ayraudi (Quoy and Gaimard 1824). Environ. Biol. fishes 88, 263–271. doi: 10.1007/s10641-010-9639-4
Miyajima-Taga Y., Masuda R., Morimitsu R., Ishii H., Nakajima K., and Yamashita Y. (2016). Ontogenetic changes in the predator–prey interactions between threadsail filefish and moon jellyfish. Hydrobiologia 772, 175–187. doi: 10.1007/s10750-016-2658-1
Moesinger A. (2018). Catching names: Folk taxonomy of marine fauna on Takuu Atoll, Papua New Guinea. SPC Traditional Mar. Resource Manage. Knowledge Inf. Bull. 39, 2–14.
Olsson K. H. and Gislason H. (2016). Testing reproductive allometry in fish. ICES J. Mar. Sci. 73, 1466–1473. doi: 10.1093/icesjms/fsw017
Pardee C., Wiley J., and Taylor B. M. (2025). Age-based demography of two parrotfish and a goatfish from saipan, Northern Mariana Islands. Fishes 10, 303. doi: 10.3390/fishes10070303
Prado P. and Heck K. (2011). Seagrass selection by omnivorous and herbivorous consumers: determining factors. Mar. Ecol. Prog. Series. 429, 45–55. doi: 10.3354/meps09076
Proctor C., Robertson S., Jatmiko I., and Clear N. (2021). An introductory manual to fish ageing using otoliths 41.
Reed E. M., Fobert E. K., and Taylor B. M. (2025). Within-region differences in growth responses of an herbivorous coral reef fish to local and regional climatic processes. Coral Reefs 44, 99–112. doi: 10.1007/s00338-024-02589-3
Ricker W. E. (1973). Linear regressions in fishery research. J. fisheries board Canada 30, 409–434. doi: 10.1139/f73-072
Rogers J. S., Hare J. A., and Lindquist D. G. (2001). Otolith record of age, growth, and ontogeny in larval and pelagic juvenile Stephanolepis hispidus (Pisces: Monacanthidae). Mar. Biol. 138, 945–953. doi: 10.1007/s002270000521
Shadrin A. M. and Emel’yanova N. G. (2022). Embryo-larval development and some data on the reproductive biology of cantherhines pardalis (Monacanthidae) from the south China sea (Central Vietnam). J. Ichthyology 62, 932–942. doi: 10.1134/S0032945222050150
Siqueira A. C., Muruga P., and Bellwood D. R. (2023). On the evolution of fish–coral interactions. Ecol. Lett. 26, 1348–1358. doi: 10.1111/ele.14245
Trip E. L., Choat J. H., Wilson D. T., and Robertson D. R. (2008). Inter-oceanic analysis of demographic variation in a widely distributed Indo-Pacific coral reef fish. Mar. Ecol. Prog. Ser. 373, 97–109. doi: 10.3354/meps07755
Trip E. L., Clements K. D., Raubenheimer D., and Choat J. H. (2011a). Reproductive biology of an odacine labrid, Odax pullus. J. Fish Biol. 78, 741–761. doi: 10.1111/j.1095-8649.2010.02889.x
Trip E. L., Raubenheimer D., Clements K. D., and Choat J. H. (2011b). Reproductive demography of a protogynous and herbivorous fish, Odax pullus (Labridae, Odacini). Mar. Freshw. Res. 62, 176–186. doi: 10.1071/MF10238
Visconti V., Trip E. D., Griffiths M. H., and Clements K. D. (2018a). Reproductive biology of the leatherjacket, Meuschenia scaber (Monacanthidae)(Forster 1801) in the Hauraki Gulf, New Zealand. New Z. J. Mar. Freshw. Res. 52, 82–99. doi: 10.1080/00288330.2017.1331919
Visconti V., Trip E. D. L., Griffiths M. H., and Clements K. D. (2018b). Life-history traits of the leatherjacket Meuschenia scaber, a long-lived monacanthid. J. Fish Biol. 92, 470–486. doi: 10.1111/jfb.13529
Visconti V., Trip E. D. L., Griffiths M. H., and Clements K. D. (2020). Geographic variation in life-history traits of the long-lived monacanthid Meuschenia scaber (Monacanthidae). Mar. Biol. 167, 1–13. doi: 10.1007/s00227-019-3628-8
Wood E. (2001). Collection of Coral Reef Fish for Aquaria: Global Trade, Conservation Issues and Management Strategies (U.K: Marine Conservation Society).
Keywords: Monacanthidae, otolith, body size, growth, histology, reproductive biology, Pacific Ocean
Citation: Visconti V, Woodgate I, Trip EDL and Sabetian A (2025) Age-based life-history traits of two filefish from the Solomon Islands: the honeycomb (Cantherhines pardalis) and the broom (Amanses scopas) filefishes. Front. Mar. Sci. 12:1634488. doi: 10.3389/fmars.2025.1634488
Received: 24 May 2025; Accepted: 11 July 2025;
Published: 24 July 2025.
Edited by:
Marco Albano, University of Messina, ItalyReviewed by:
Taner Yildiz, Istanbul University, TürkiyeLucrezia Latini, Marche Polytechnic University, Italy
Copyright © 2025 Visconti, Woodgate, Trip and Sabetian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valerio Visconti, dmFsZXJpby52aXNjb250aUBjZWZhcy5nb3YudWs=
 Valerio Visconti
Valerio Visconti Ian Woodgate
Ian Woodgate Elizabeth D. L. Trip
Elizabeth D. L. Trip Armagan Sabetian
Armagan Sabetian