- 1Haide College, Ocean University of China, Qingdao, Shandong, China
- 2First Institute of Oceanography, Ministry of Natural Resources, Qingdao, Shandong, China
- 3Qingdao Key Laboratory of Coastal Ecological Restoration and Security, Marine Science Research Institute of Shandong Province, Qingdao, Shandong, China
- 4The Second Geological Exploration Institute of China Metallurgical Geology Administration, Fuzhou, Fujian, China
- 5Fuzhou Dongxin Mining Technology Co., Ltd., Fuzhou, Fujian, China
The YanTai HY-Nuclear Power Plant (HYNPP) is a newly constructed nuclear power plant that entered operation after 2021. To establish a preoperational ecological baseline for the HYNPP, this study examined macrobenthic community structure and its relationships with multiple environmental variables using year-round field surveys conducted from 2016 to 2017. Eighty-five species from eight phyla were recorded in total, with winter showing the highest species number and spring exhibiting peak biomass. Species composition displayed pronounced seasonal turnover, with replacement rates exceeding 93% between adjacent seasons. Principal coordinates analysis (PCoA) and hierarchical clustering confirmed significant seasonal variation and the localized aggregation of species near the HYNPP. Diversity indices (S, H′, D′, and J′) varied across seasons and spatial gradients, strongly influenced by sea bottom temperature (SBT), salinity, dissolved oxygen (DO), and nutrient concentrations. Spearman correlation analysis and random forest (RF) modeling revealed SBT, DO, phosphate, and phytoplankton cell as dominant factors shaping macrobenthic diversity. RF models provided key insights into nonlinear interactions and variable importance across seasons. Leveraging the dependence-preserving power of copulas, Copula-Based Random Forest (CBRF) models were further developed under a +4°C warming scenario to simulate post-operational thermal-discharge effects; the CBRF framework captured complex spatial responses, predicting localized biomass increases in sheltered muddy areas and biomass reductions in the outer bay. Mollusk biomass was projected to peak in spring near mixed-substrate habitats, while annelids and arthropods showed variable responses linked to sediment type and nutrient availability. These findings highlight strong spatiotemporal coupling between environmental parameters and macrobenthic assemblages, emphasizing the roles of SBT and phytoplankton-driven organic inputs in modulating community structure. The predictive framework built here supports long-term ecological risk assessments and management strategies for mitigating thermal discharge impacts in the Yellow Sea region.
1 Introduction
Nuclear power plants, as vital artificial facilities, have become an integral part of modern energy infrastructure, providing significant benefits in terms of power generation. In China, nuclear power plants have been constructed in many regions to meet rising energy demands while reducing reliance on fossil resources (Geng et al., 2023). In recent years, the construction of thermal and nuclear power plants has been essential to support societal growth. The YanTai HY-nuclear power plant (HYNPP), as one of China’s most advanced nuclear power projects, consists of six nuclear power units constructed in batches. Among these, Units 3 and 4 of the HYNPP are located on the coast of the Yellow Sea in the Jiaodong Peninsula, Shandong Province, China, and the total investment is expected to exceed 100 billion yuan. To satisfy their requirements for water intake and thermal discharge, a growing number of plants have been developed in coastal regions, bringing the environmental implications of nuclear power plants to the forefront of marine and ecological conservation (Lin et al., 2018). The environmental impacts of these plants, particularly the thermal discharge associated with cooling systems, cannot be overlooked, as well as the risk of blockage. The temperature of the surrounding water environment increases, potentially causing ecological problems. Thermal discharge significantly affects seawater quality, marine biodiversity, and the occurrence of red tides, as shown by previous reviews and comprehensive studies (Nie et al., 2021; Teixeira et al., 2009; Leng et al., 2024a). Temperature fluctuations can induce modifications in the distribution of macrobenthic communities, affecting the biodiversity and ecological health of neighboring marine ecosystems. In addition, the cold-water intake system near the NYNPP faces the risk of blockage due to recurrent outbreaks and accumulations of drifting marine organisms e.g. Nemopilema nomurai (Liu, 2018).
Macrobenthic organisms live on or in bottom sediments. Macrobenthos are key to ecosystem functions such as nutrient cycling, carbon mineralization, and maintaining coastal productivity (Griffiths et al., 2017). Habitat changes from environmental and human impacts can alter their physiology and community structure. For example, artificial reefs increase habitat complexity and biodiversity (Bohnsack and Sutherland, 1985). Furthermore, macrobenthic organisms are long-lived, relatively slow-moving, sensitive to environmental disturbances, and capable of causing bioturbation through feeding, burrowing, tube-building, and other behaviors that can alter the biogeochemical cycle in sediment (Somerfield et al., 2006; Li et al., 2022b; Yan et al., 2017). Hence, macrobenthos are widely employed as indicators in short- and long-term environmental studies and monitoring programs (Trannum et al., 2010; Han and Han, 2024). Macrobenthic organisms are a crucial component of marine macrobenthic ecosystems, playing a fundamental role in maintaining energy flow and material cycling (Snelgrove, 1999; Danovaro et al., 2008). As key intermediaries in the food web, macrobenthic organisms facilitate the vertical transfer of energy and nutrients across the water–sediment interface through their feeding and bioturbation activities (Mermillod-Blondin and Rosenberg, 2006). These organisms are essential for ecosystem services such as nutrient cycling, carbon mineralization, and coastal productivity (Griffiths et al., 2017). Due to their relatively long lifespans, limited mobility, and sensitivity to environmental changes, macrobenthic communities are frequently utilized as indicators of marine ecological health (Trannum et al., 2018; Borja et al., 2000). Numerous studies have demonstrated that variations in macrobenthic community structure and function are effective indicators of pollution levels and ecological disturbances that reflect the overall integrity and stability of marine ecosystems (Dauer, 1993; Warwick, 1993).
Decades of research on the impacts of thermal discharge on macrobenthic communities in oceans suggest that thermal discharge can disrupt macrobenthic community structure, potentially leading to a decrease in species diversity and an increase in the dominance of certain species due to changes in habitat suitability and temperature-induced stress (Lin et al., 2018). The thermal discharge associated with the cooling systems of coastal nuclear power plants significantly alters local water temperatures, impacting the structure and function of adjacent macrobenthic communities (Hung et al., 1998; Yu et al., 2013). Early studies reported that heated effluents caused coral bleaching and mortality in tropical coral reef ecosystems (Jokiel and Coles, 1974), while estuarine environments experienced shifts in the macrobenthic invertebrate community composition and declines in sensitive species (Loi and Wilson, 1979). In subtropical regions, long-term thermal discharge has been shown to decrease overall biodiversity and favor opportunistic, thermally tolerant species (Teixeira et al., 2009; Lin et al., 2018). For example, in the coastal waters adjacent to nuclear power plants in Korea, Yu et al. (2013) observed spatial variations in macrobenthic communities that were directly related to the volume of thermal discharge. Studies from Taiwan (Hung et al., 1998) and Brazil (Teixeira et al., 2009) similarly confirm that thermal effluents cause declines in species richness and biomass. The mechanisms underlying these impacts include direct thermal stress exceeding species’ physiological tolerances, reduced dissolved oxygen (DO) content, and altered sediment characteristics (Leng et al., 2024a; Hung et al., 1998). Therefore, establishing baseline data before the operation of nuclear power plants, such as the HYNPP, is crucial to subsequent environmental impact assessments and ecological management. Obtaining this data requires investigations and analyses of seasonal changes in the macrobenthos and ecological groupings. Moreover, predictive modeling can be utilized, such as the prediction and analysis of the potential effects of thermal discharge from the HYNPP on adjacent macrobenthic communities and ecological groupings after the HYNPP begins operation conducted in the present study.
The Yellow Sea is a large inlet of the western Pacific Ocean that connects mainland China with the Korean peninsula (approximately 600 miles north to south and 435 miles east to west). The Yellow Sea is rich in marine biological resources and essential for aquaculture, particularly shellfish and fish (Zhou, 2012). Therefore, the environmental quality of the Yellow Sea is the focus of extensive attention. However, in recent years the ecological quality of coastal waters has entered a sub-healthy state due to various factors, including climate change leading to rising water temperatures in coastal waters and shelf seas, which have affected the spatial and temporal distribution of marine species (Poloczanska et al., 2016), as well as human activities, such as ongoing urbanization, pollution from terrestrial sources, and artificial island projects (Li et al., 2022a). Jellyfish blooms and green tides, particularly those driven by Ulva prolifera, are significant factors influencing species’ ecology and the environment. Since 2007, large-scale blooms of U. prolifera have periodically occurred in the Yellow Sea from May to July, severely impacting the coastal ecosystem, including the structure and functional groups of benthic communities (Qu et al., 2020). In recent years, many studies have investigated macrobenthic communities in the Yellow Sea, focusing on various factors and spatiotemporal distribution. For example, Jiang et al. (2023) surveyed the spatiotemporal distribution of macrobenthos and the macrobenthic ecological health status in the Yellow Sea and the Bohai Sea. The macrobenthic communities in many coastal regions have been reported to display significant seasonal variations in terms of the number of species, density, and diversity, as well as regional differences in biomass and density (Shou et al., 2018). Kim and Yu (2022) investigated the structure and feeding guilds of macrobenthic polychaete communities in the Yellow Sea bottom cold water zone, focusing on the impact of water temperature, depth, and sediment composition on macrobenthos distribution. This study also offers valuable insights into the dynamics of macrobenthic communities in the ecological context surrounding the nuclear power plant, focusing on how environmental factors such as sediment composition and water temperature influence species diversity and distribution, and the predictive effects of thermal discharge. The coupling between water column primary production and macrobenthic community structure is increasingly recognized as a major ecological link in coastal ecosystems (Graf, 1992; Ruhl and Smith, 2004). Changes in phytoplankton productivity directly affect the macrobenthic food supply, regulating macrobenthic abundance, biomass, and reproduction (McQuatters-Gollop et al., 2007; Josefson and Conley, 1997). Increased phytoplankton production enhances organic matter deposition, which can favor deposit feeders and restructure macrobenthic communities, but excessive blooms followed by decay may cause hypoxia or anoxia, leading to mass mortality and reduced diversity (Graf, 1992; Diaz and Rosenberg, 2008). Changes in phytoplankton community structure—such as shifts between diatoms and dinoflagellates—can further influence the quality and availability of organic matter reaching the macrobenthos (Turner et al., 1998). Studies have thus incorporated primary production metrics as critical environmental variables when modeling macrobenthic community dynamics (Lin et al., 2018). Phytoplankton cell abundance is a key driver of macrobenthic community structure through macrobenthic–pelagic coupling, as higher surface productivity increases the organic matter flux to the seafloor, thereby enhancing the macrofaunal biomass—a relationship confirmed by global studies across diverse regions (Graf, 1989; Grebmeier et al., 2006; Wei et al., 2010; Yool et al., 2017). Given this strong empirical foundation, incorporating phytoplankton abundance into macrobenthic ecological models is ecologically justified and essential for predicting community responses to environmental change (Wei et al., 2010; Grebmeier et al., 2006). Furthermore, random forest (RF) modeling can be applied to explore the relationships between diversity indices and environmental variables, enhancing the understanding of how abiotic factors shape macrobenthic community dynamics. Investigating the relationships between macrobenthic diversity indices and environmental factors is essential for understanding community dynamics (Gray, 2002). Numerous studies have explored how variables such as bottom water temperature, salinity, DO, and nutrient concentrations affect macrobenthic diversity. Spearman correlation analysis is commonly performed to reveal linear or monotonic relationships between environmental parameters and diversity metrics (Bremner et al., 2006; Jiang et al., 2023). More advanced modeling approaches, such as generalized additive models (GAMs), have been employed to characterize the nonlinear responses of macrobenthic communities to environmental gradients (Jiang et al., 2023). Recently, machine learning methods such as RF have been utilized to identify the most important environmental drivers of macrobenthic diversity while effectively handling multicollinearity and nonlinearity (Lin et al., 2024). For example, Lin et al. (2024) applied RF analysis to macrobenthic datasets along the Taiwan coast to identify key predictors affecting macrobenthic assemblages, such as light intensity, inorganic nitrogen concentrations, and land-use patterns. This study was conducted in the South Yellow Sea, near the HYNPP, which began operation in 2021. Year-round sampling of macrobenthic communities and environmental parameters was carried out in 2016–2017 to establish a baseline before the plant’s operation. These data provide a foundation for future ecological and safety assessments of potential impacts, such as biofouling risks to nuclear facilities (Zhang et al., 2017). By addressing this gap, the study contributes to understanding macrobenthic–environment interactions and offers a predictive framework for assessing thermal discharge and habitat changes associated with HYNPP operations. The goals of this study were to (1) describe the composition and seasonal variations of macrobenthic communities; (2) investigate the relationships between macrobenthos and environmental parameters, thereby providing baseline ecological information and identifying key environmental drivers to support future impact assessments and ecological management; and (3) develop a predictive framework linking environmental factors to macrobenthic community structure that can be used to anticipate and manage potential ecological shifts following the operation of the HYNPP.
2 Materials and methods
2.1 Survey area
The study area is located near Units 3 and 4 of the HYNPP (Figure 1), a coastal nuclear power plant situated at the eastern tip of a cape surrounded by the sea on three sides, with Rushan Bay to the northeast, Fengcheng Port to the southwest, and the vast Yellow Sea to the east and south. This area, located on China’s east coast, is a typical shallow epicontinental sea with a mean depth of less than 50 m. It is characterized by a monsoon wind regime, featuring strong north-northwest winds during winter and weaker south-southeast winds in spring and summer. The investigation of the environmental parameters was conducted in the vicinity of the construction site of Units 3 and 4 of the HYNPP. The project involves a seawater consumption of over 5 million m³ per day (specifically, approximately 5.61 million m³/day for Units 3 and 4).
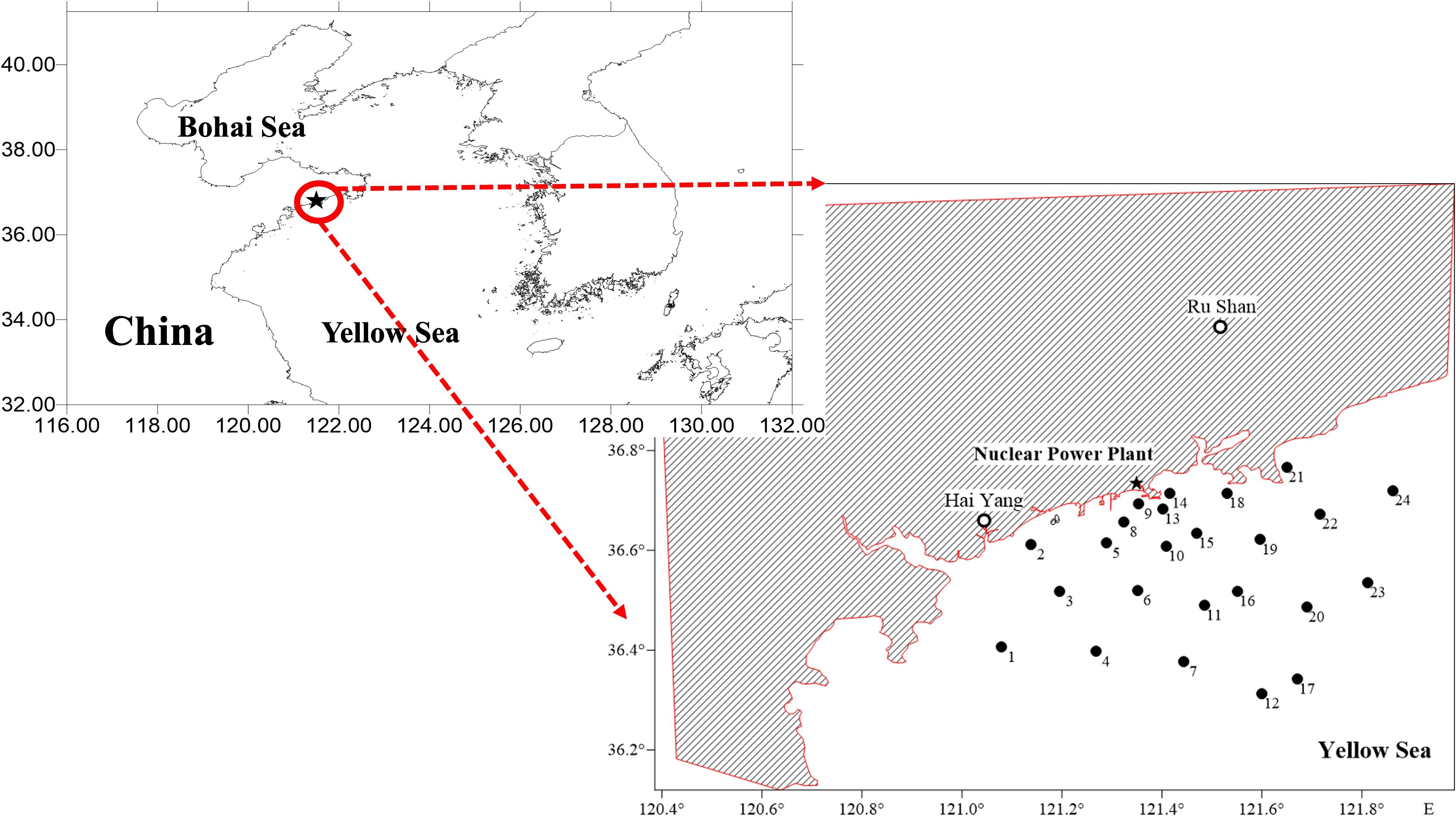
Figure 1. Study area and sampling stations (1–24) along the coastal Yellow Sea near the nuclear power plant.
2.2 Sampling and laboratory analysis
A total of 24 sampling stations (Figure 1) were established within a 50 km radius from the planned discharge outlet. The same set of stations was consistently sampled during each season—spring, summer, autumn, and winter—during a one-year period from 2016 to 2017 (May 2016, August 2016, November 2016, and February 2017, respectively).
Macrobenthic samples were collected using a Box Corer at each station. The Box Corer is a device used to collect undisturbed sediment samples that is suitable for marine and freshwater environmental studies. Its working principle involves vertically inserting a metal box into the sediment, then sealing the bottom with a mechanical closure to prevent sample loss or disturbance. The device, with a sampling area of 0.1 m², was used to collect surface sediment samples at each station. At each site, three replicate samples were randomly taken: two were used as parallel samples for macrobenthic organisms, and one was used for sediment analysis. After sampling, the sediment was sieved through a series of mesh screens with decreasing pore sizes (2.0–5.0 mm, 1.0 mm, and 0.5 mm). The collected macrobenthic organisms were washed and sorted using a 0.5-mm sieve, then preserved in 5%–7% neutral formaldehyde solution for subsequent species identification and biomass measurement. The sediment samples collected in this study were consistently processed through sequential sieving, washing, and preservation in 5%–7% neutral formaldehyde, followed by microscopic analysis to identify the species and measure the biomass of macrobenthic organisms.
Seawater collection was conducted using a kick-open water sampler following standard protocols outlined in the GB12763.4–2007 and the GB17378.4-2007 (Standardization Administration of China, 2007). Water samples were collected using double-capped, high-density polyethylene bottles to assess the DO, nutrients, and other parameters, including the nitrate (NO3--N), nitrite (NO2--N), ammonium (NH4--N), phosphate (PO4³--P), and silicate (SiO3²--Si) contents. Nutrient analysis was performed by filtering the water samples through a 0.45-μm acetate fiber filter membrane. A 100-cm³ portion of the filtrate was then stored in high-pressure polyethylene bottles and frozen for laboratory analysis. In the laboratory, according to the GB17378.4–2007 standard (Standardization Administration of China, 2007), DO was measured using the iodometric method (APHA, 2017) and nutrient levels were analyzed using spectrophotometric techniques: ammonia was measured with indophenol blue (APHA, 2017), NO2--N with N-(1-naphthyl) ethylenediamine (APHA, 2017), and NO3--N via cadmium reduction (APHA, 2017). The reactive PO4³--P and SiO3²--Si contents were determined following the molybdenum blue and silicomolybdenum blue methods (APHA, 2017), respectively, while the total nitrogen and phosphorus were assessed using potassium persulfate oxidation (APHA, 2017). To explore the relationship between macrobenthos and phytoplankton in the study area, phytoplankton, which play a crucial role in the marine ecosystem as primary producers, were collected from 2 m above the seabed through vertical tows using a shallow-water type-III plankton net (net length = 140 cm; mouth inner diameter = 37 cm; stainless-steel net ring Φ 10 mm). The collected net samples were fixed in 5% neutral formalin for identification, while 1 L of seawater was preserved in 1%–1.5% Lugol’s solution and concentrated via sedimentation. Sediment was gathered using a grab sampler. After draining the overlying water, the redox potential (Eh) of the sediment was measured, which was determined using a platinum electrode with a calomel electrode as the reference. The top 0–2 cm of sediment was carefully extracted using a plastic knife or spoon, and for gravel layers, the 0–3 cm layer was sampled. A wet sample weighing 500–600 g was stored in a 500-cm³ glass bottle for moisture content and grain size analysis. Sediment samples were stored at approximately 4°C before laboratory analysis. In the lab, moisture content was determined by drying the sediment at 105°C ± 1°C to a constant weight, while grain size analysis was performed by mixing the sediment with purified water and 0.5 mol/L sodium hexametaphosphate, followed by examination using a laser particle size analyzer. After analysis, all collected data were verified and recalculated by a second person to ensure accuracy before the final datasets were prepared for analysis.
2.3 Data analysis and statistical methods
In this study, seasonal changes in the macrobenthic biomass and environmental parameters of the marine ecosystem near the HYNPP were described separately based on observational data. Surfer (v21.1.158) was employed to map the distribution of environmental factors, including the sea bottom temperature (SBT), salinity, DO, water depth, sediment parameters including mean grain size (Mz), moisture content (MC) and sediment pH, and inorganic nutrient salts (NO3--N, NO2--N, NH4--N, SiO3²--Si, and PO4³--P), along with the biomass of various macrobenthic phyla. The relationships between environmental factors and the macrobenthos community were further analyzed by visualizing biomass and species distribution. Cumulative bar charts and boxplots were generated using the “ggplot2()” function from the ggplot2 package in R (v4.4.3). Additionally, hierarchical clustering analysis was conducted to assess the similarity of species composition among stations, and the Euclidean distance matrix was computed using the “dist()” function (stats package), followed by clustering with the “hclust()” function (stats package) using the average linkage method. The resulting dendrograms were converted into a dendrogram object using “as.dendrogram()” (dendextend package) and plotted with the “plot()” function (graphics package). Principal coordinates analysis (PCoA) was conducted to explore differences in species composition across seasons. A binary dissimilarity matrix was calculated using “vegdist()” (vegan package) following the binomial method, and PCoA was performed using “cmdscale()” (stats package) to extract the first two principal coordinates. Seasonal variations in species composition were tested with permutational multivariate analysis of variance (PERMANOVA) using the “adonis2()” function in the vegan package. The PCoA results were visualized with “ggplot()” (ggplot2 package). The R (Lian et al., 1990) was calculated for the assessment of macrobenthos species replacement:
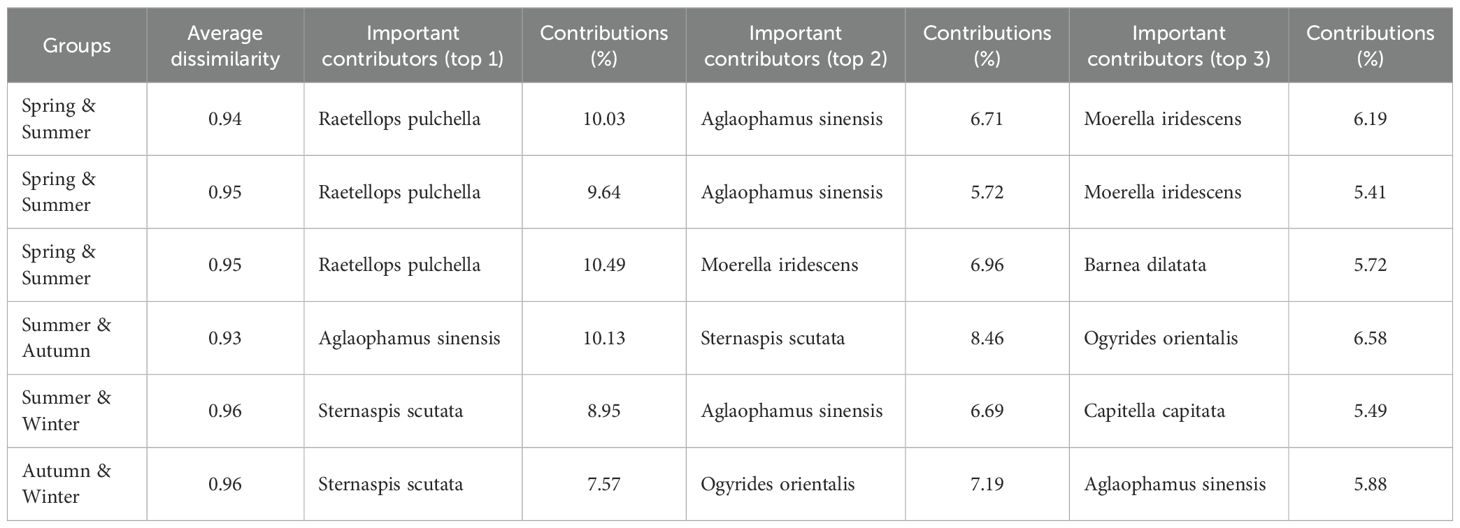
where R is the species replacement rate; a and b are the numbers of species observed in two consecutive seasons; and c represents the number of species common to both seasons. When the R value was 100%, all species were different between the two periods (complete replacement); when the R value was 0%, the species composition was identical (no replacement). The macrobenthic species replacement rate (Equation 1) between adjacent seasons was calculated by comparing species composition at each of the 24 stations. The average replacement rate across all stations was then utilized to represent each seasonal transition. After calculating the R for each of the 24 stations, we used a fourth-root–transformed species-biomass matrix to compute Bray–Curtis dissimilarities and ran average-linkage clustering. The resulting dendrogram allowed us to see whether samples from the same season truly grouped together, thus verifying the high turnover suggested by the R-values. We then applied SIMilarity PERcentage analysis (SIMPER) with season as the grouping factor to quantify the mean assemblage dissimilarity between successive seasons and to identify the top three species contributing most to each difference.
Ecological indices, including the number of species (S), Shannon–Wiener diversity index (H′), Simpson index (D’), and Pielou’s evenness index (J), were calculated to assess macrobenthos status (Equations 2–4). These indices provide key insights into species diversity and community structure, which are essential for understanding the region’s ecological dynamics (Shannon, 1948; Smith and Wilson, 1996). These indices are calculated as follows:
where S is the total number of macrobenthos species in each sample and is the relative proportion of each species i.
To visualize the spatial distribution of diversity indices, contour maps were generated based on data from 24 stations, and boxplots were used to illustrate their seasonal variation. This study then investigated the influence of environmental factors, including temperature, salinity, DO, water depth, sediment parameters (moisture content, Mz, and pH), the contents of nutrient salts, and the phytoplankton cell count, on these indices. Spearman correlation analysis was conducted using “cor()” to compute correlation coefficients, and “cor.test()” was applied to assess significance. The correlation matrix was visualized using “qcorrplot()” (linkET package). To address the issue of multiple comparisons, Bonferroni correction was applied by adjusting the significance threshold according to , where is significant level and represents the total number of pairwise tests (with 17 variables in total). Then we had a corrected significant threshold of , which was used to evaluate the significance of the correlation results in spearman. In order to further explore the relationship between environmental variables and diversity indices, all four seasonal surveys were pooled and a RF regression (randomForest package) was fitted with 13 hydro-environmental factors, providing a year-round, integrated depiction of how environmental factors responds to the macrobenthic species diversity near the HYNPP. This model was used to assess the impact of these factors on species diversity indices, with model performance evaluated based on the percentage of explained variance, where higher values indicated a better fit. The RF algorithm offers significant advantages in handling high-dimensional data, bypassing the need for multicollinearity testing and variable screening required in traditional nonlinear regression methods. RF models outperform GAMs in predictive accuracy for macrobenthic assessments, particularly when dealing with complex ecological datasets characterized by strong multicollinearity, high frequencies of zeros, and overfitting. Studies by Kosicki (2020) and Colombelli et al. (2022) show that the RF approach reduces prediction error, especially in scenarios with numerous significant predictors. RF’s ability to rank predictor importance helps identify key environmental factors affecting macrobenthic diversity, making the RF method invaluable for studying ecosystems in dynamic, anthropogenically impacted environments, such as those near nuclear power plants. Given RF’s ability to handle multicollinearity, traditional stepwise regression was not employed in the current study. The importance of each factor was determined using the mean decrease accuracy metric extracted via “importance()”. Partial effect plots were generated by predicting responses with “predict()” and visualized using “ggplot()” (ggplot2 package), employing “geom_smooth()” for continuous variables. All mean values are presented in this manuscript as the mean ± standard deviation (± SD), with data ranges displayed in tables. The top five most important factors for diversity indices were selected for further analysis.
Finally, a Copula-Based Random Forest (CBRF) model was constructed to estimate the potential changes in the macrobenthic community under scenarios of elevated seawater temperature around the HYNPP. A study by Kim (2025) shows that the CBRF explicitly models the dependence structure among predictors through a Gaussian copula transformation before tree growing, thereby retaining the full joint behavior of highly collinear environmental variables while still leveraging the non-parametric, ensemble strengths of random forests. Key target variables remained the total biomass, annelid biomass, mollusk biomass, arthropod biomass. To generate spatially continuous input data, ordinary kriging was first performed on each season’s observations of the nine predictive variables (SBT, DO, NO3--N, NO2--N, NH4--N, SiO3²--Si, PO4³--P, phytoplankton cells, and Mz) and the four response variables (total biomass, annelid biomass, mollusk biomass and arthropod biomass). All raw point data were projected from WGS84 (EPSG:4326) to Web Mercator (EPSG:3857), and the autoKrige() function in the R automap package was used to fit the semivariogram automatically and project the results onto a 30×30 grid. The gridded results were then re-projected back to latitude/longitude and merged into a single seasonal dataset. Using these gridded data, this study then built 16 independent RF regression models (one for each combination of four seasons—spring, summer, autumn, and winter—and five response variables) in R with the caret package. Each model was trained via the train() function with 15-fold cross-validation [trainControl (method=“cv”,number=15)] and the automatic tuning of mtry (tuneLength=5). For each model, the seasonal grid was randomly split into 70% training/30% testing, the hold-out mean squared error (MSE) and R² of the test set were recorded, and the final fitted forest was retained. To predict the thermal-discharge scenario (+4°C sea-bottom temperature, SBT; Shandong Nuclear Power Company, 2013), we inserted a Gaussian-copula conditioning step between the temperature shift and the random-forest inference. For every site and season we proceeded as follows: 1) Temperature shift: the measured sea-bottom temperature was increased by four degrees Celsius to represent the thermal discharge condition. 2) Copula conditioning: a season-specific Gaussian copula—previously fitted to the nine environmental variables (temperature, dissolved oxygen, inorganic nutrients, phytoplankton cell density and suspendedktonusl content)—was used to derive the expected values and the joint variability of the eight non-temperature variables, conditional on the shifted temperature. This step preserves the historical correlations among predictors. 3) Monte-Carlo resampling: five hundred synthetic covariate vectors were generated by drawing from the above conditional distribution and then combining each draw with the fixed, elevated temperature. The resulting sample cloud represents physically plausible “on-manifold” environmental states under the +4 °C scenario. 4) Random-forest inference: each of the 500 covariate vectors was passed through the pre-trained season–variable random-forest models. The arithmetic mean of the 500 outputs served as the best estimate for the scenario, while the 2.5% and 97.5% percentiles provided an empirical 95% confidence interval for every macrobenthic response variable. The resulting predictions were utilized for subsequent ecological impact assessment and visualization.
3 Results
3.1 Hydrographic conditions and water quality
The SBT and DO content exhibited significant spatiotemporal variability. SBT varied significantly between seasons and stations. The annual average SBT was 14.88 ± 8.17°C, with peak temperatures occurring in summer (up to 28.99°C) and minimum temperatures in winter, ranging from 3.64 °C to 5.11°C (Table 1). Elevated temperatures were noted in proximity to the coast, particularly adjacent to the HYNPP. In summer, SBTs were elevated in offshore regions, whereas in winter, the lowest temperatures exhibited a more uniform distribution. The yearly average DO concentration was 8.38 ± 1.83 mg/L, peaking in winter at 12.11 mg/L and declining in summer to a range of 5.06 mg/L to 7.15 mg/L (Table 1). Salinity displayed minimal seasonal fluctuation, sustaining a consistent annual average of 31.91 ± 0.18‰. Salinity levels exhibited a spatial gradient, progressively rising from southwest to northeast, with peak concentrations noted at the shorelines and around the HYNPP, especially during autumn and winter (Table 1; Figure 2). The sediment parameters displayed considerable stability over the year, with an annual average of Mz (6.26 ± 0.42 mm), Mc (43.73 ± 4.93%) and sediment pH (8.52 ± 0.11) and no significant spatiotemporal variation (Table 1; Figure 2). The distribution of the sea depth was rather consistent throughout the research area, varying from 7 to 32 m, with negligible seasonal or locational fluctuation.
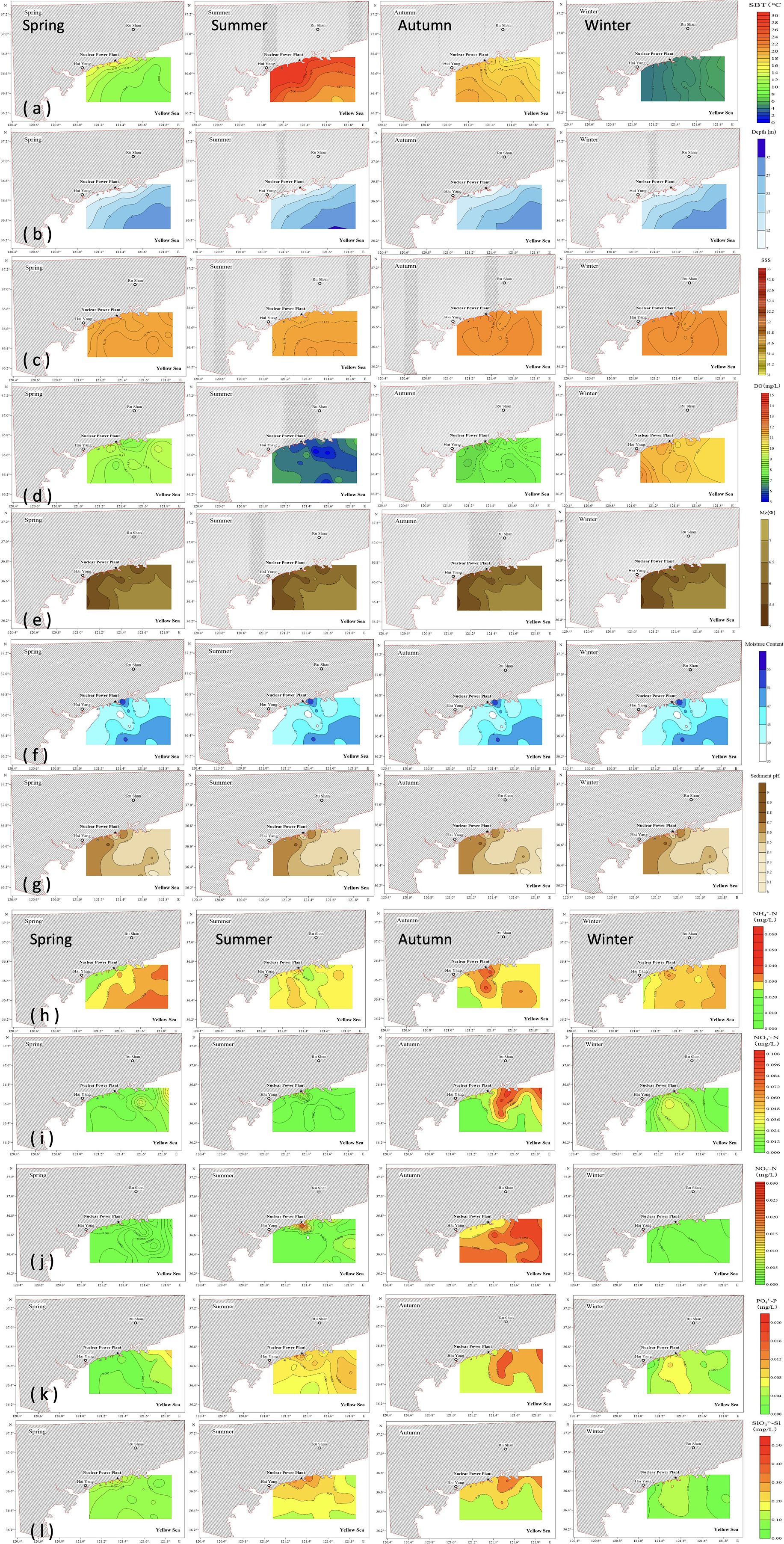
Figure 2. Distributions of environmental factors during the four seasons along the area. (a–g) present the factors of sea bottom temperature (SBT), depth, salinity, dissolved oxygen (DO), and sediment parameters including mean grain size (Mz), moisture content (MC) and sediment pH. (h–l) present the factors of nitrate (NO3--N), nitrite (NO2--N), ammonium (NH4--N), silicate (SiO3²--Si), and phosphate (PO4³--P).
Nutrient concentrations, specifically SiO3²--Si, NO3--N, and NH4+-N, demonstrated significant seasonal fluctuations. The concentrations of SiO3²--Si reached their peak in autumn, averaging 0.23 ± 0.08 mg/L, concurrently with elevated levels of NO3−-N and NH4+-N during this season (Table 1; Supplementary Table 1). Conversely, the lowest nutrient concentrations were found in spring, with NO3−-N averaging 0.011 ± 0.008 mg/L and NH4+-N averaging 0.030 ± 0.010 mg/L (Table 1). The N/P ratio exhibited significant seasonal variation, with spring values (Table 1: 106.47 ± 77.67) being notably higher than those in other seasons (Table 1: summer: 15.76 ± 4.41; autumn: 27.67 ± 7.94; and winter: 37.10 ± 11.72). Notably, regions adjacent to the HYNPP and major river estuaries displayed heightened nutrient levels, including elevated NH4+-N in autumn and increased levels of NO3−-N, NO2−-N, PO43−-P, and SiO32−-Si in summer near the HYNNP, with the accumulation of NO3−-N, PO43−-P, and SiO32−-Si downstream along the river estuary’s outflow (Figure 2).
3.2 Seasonal changes in macrobenthos
3.2.1 Seasonal variation in macrobenthic species composition and distribution
A total of eight macrobenthic phyla and 85 macrobenthic species were classified and identified across the four seasons in the study area (Figure 3). During the spring, 37 species from six phyla were collected and identified; in summer, 23 species from six phyla; in autumn, 27 species from five phyla; and in winter, 47 species from seven phyla (Figure 3A). Spring communities were dominated by annelids, molluscs and arthropods, with single-station biomass peaks of Barnea dilatata 128.56 g/m² (station 19), gobiids 122.46 g/m² (station 2), Glycera chirori 99.22 g/m² (station 3) and Raetellops pulchella 43.06 g/m² (station 5). In summer, coelenterates and hemichordates disappeared; biomass was instead carried by Protankyra bidentata (91.08 g/m², station 23), Aglaophamus sinensis (20.22, 24.24, 24.92 g/m², at stations 6, 7, 22 respectively), nemerteans and gobiids (Figure 3B). Autumn retained a similar species set (27 species) but with P. bidentata 106.74 g/m² (station 10), Nitidotellina minuta 26.38 g/m² (station 7) and Heteromacoma irus dominating. In winter, the number of species increased yet individual biomasses were modest: P. bidentata 8.92 g/m² (station 5), Ctenotrypauchen chinensis 2.02 g/m² (station 13), Sternaspis scutata broadly distributed, and Odontamblyopus rubicundus 1.78 g/m² (station 21). Spatial patterns were highly patchy—P. bidentata formed isolated hotspots in three seasons, while annelid biomass peaked at station 3 in spring (100.28 g/m²), mollusc biomass at most stations in spring, and arthropod biomass at station 10 in autumn (6.58 g/m², chiefly Hemigrapsus penicillatus). Box-plots (Figure 4) show median values for annelids, molluscs and arthropods dropping markedly after spring; most stations otherwise remained below 5 g/m².
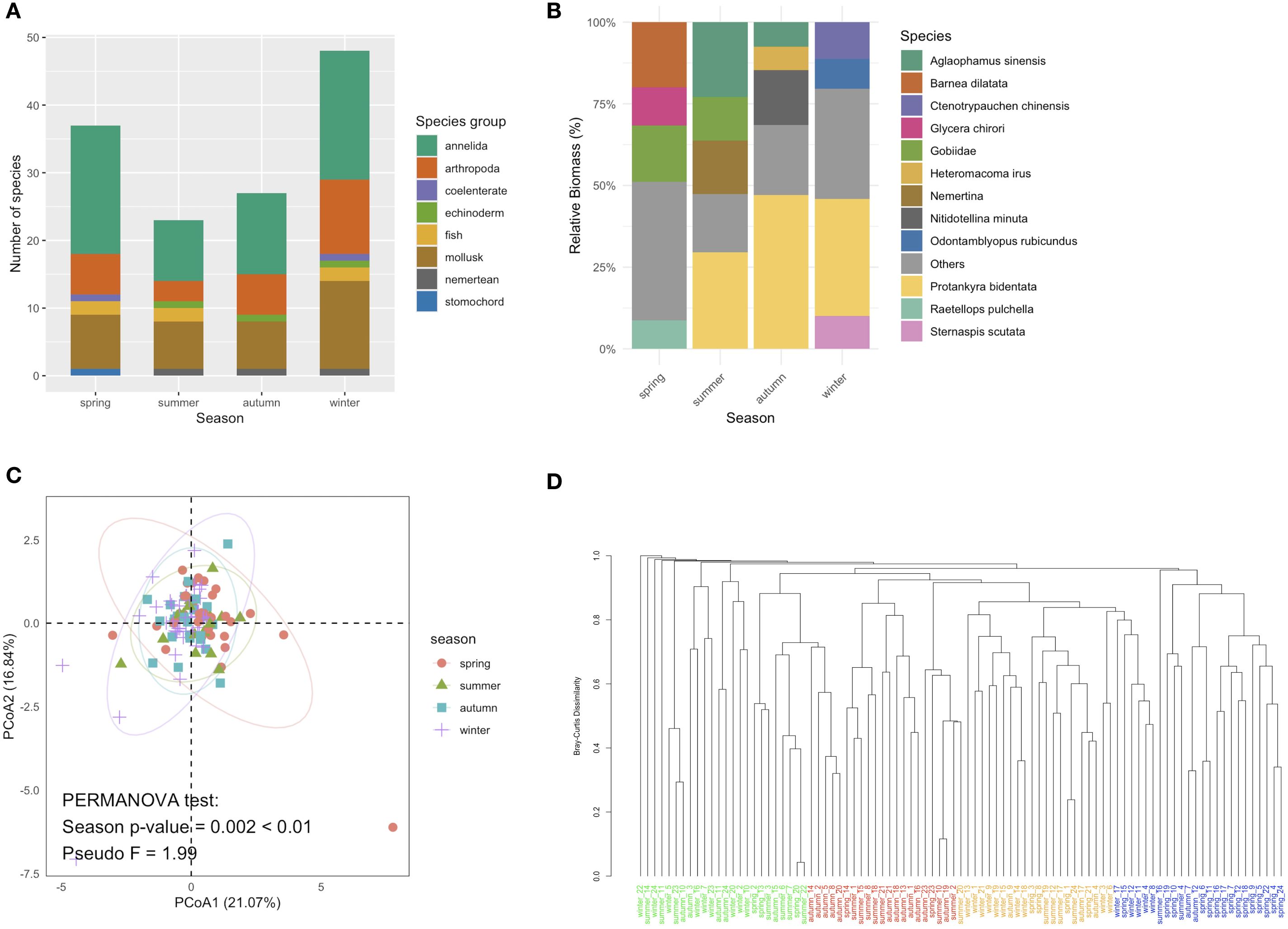
Figure 3. Seasonal variation in species composition in the study area. (A) Number of species within the macrobenthic communities in the four seasons; (B) relative biomass of major species within the macrobenthic communities in the four seasons; (C) principal coordinates analysis (PCoA) of significant differences in macrobenthic species composition across seasons; and (D) cluster analysis of macrobenthos biomass at each stations during the four seasons.
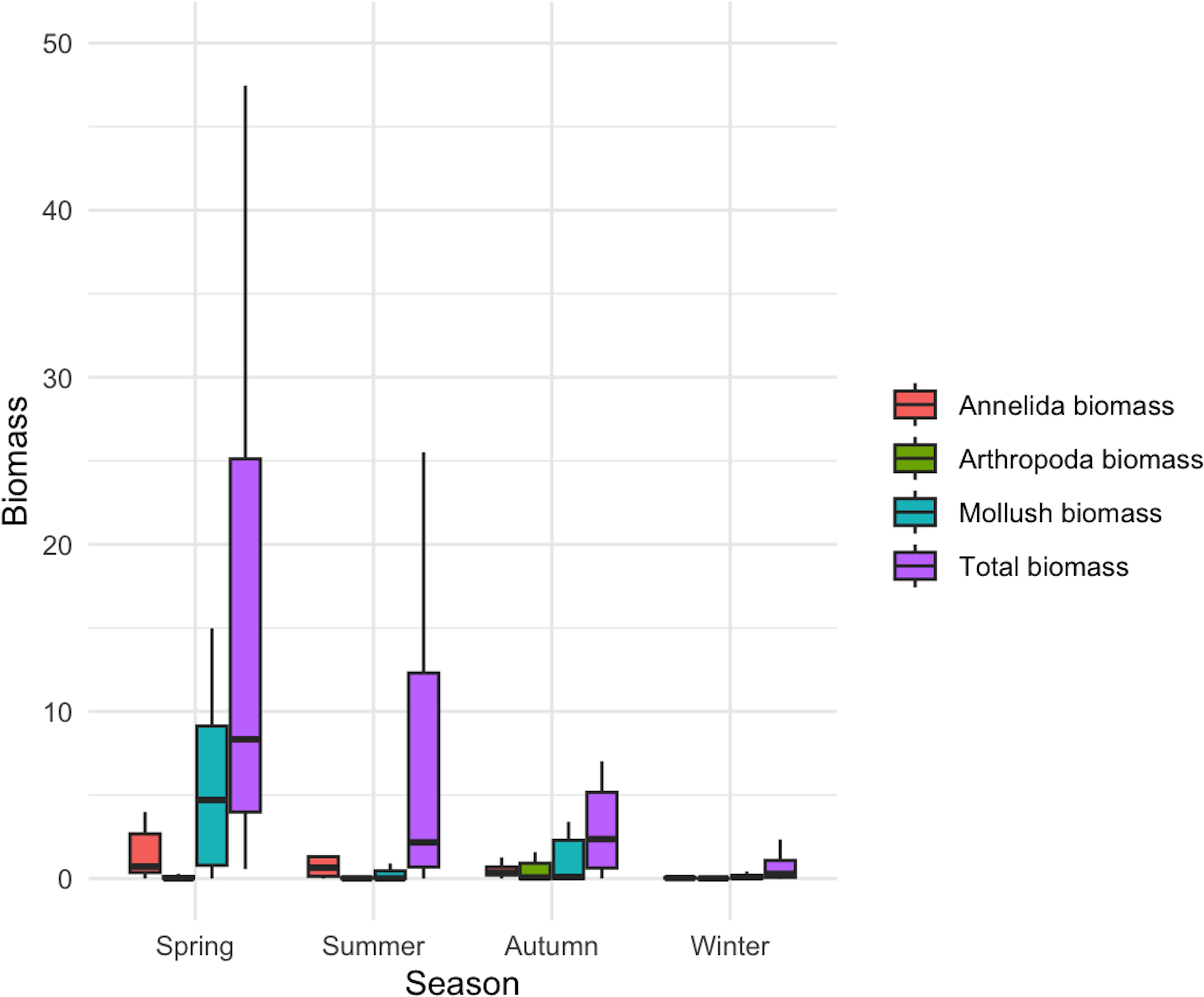
Figure 4. Seasonal distribution of biomass. The box plots illustrate the biomass distribution of three phyla, namely, Annelida (red), Arthropoda (green), and Mollusca (blue), and the total biomass (purple) across different seasons (spring, summer, autumn, and winter).
The very high seasonal-replacement indices (R from 93.6% to 95.1%) during the four seasons are corroborated by the multivariate results. The hierarchical dendrogram (Figure 3D) clusters the 96 station-samples almost exclusively by season, forming four groups and indicates Bray–Curtis dissimilarities of ≥0.90 between any two seasons. SIMPER (Table 2) quantifies these dissimilarities (0.93–0.96) and shows that only a few taxa drive most of the turnover. In spring–summer and spring–autumn contrasts, the polychaete Raetellops pulchella alone explains ~10% of the change, with Aglaophamus sinensis and the bivalve Moerella iridescens contributing a further 5–7%. From summer to winter the community is re-structured by the rise of Sternaspis scutata (8.95%) together with Aglaophamus sinensis (6.69%) and Capitella capitata (5.49%). Autumn shows the smallest average dissimilarity and the dendrogram branch-lengths within this cluster are the shortest, confirming that autumn is the most compositionally stable season. PCoA analysis further validated significant differences in macrobenthic species composition between seasons (PERMANOVA test: p < 0.01; Figure 3C). The Pseudo F value of 1.99 indicated that seasonal variations influenced the structure of the macrobenthic ecosystem, driving the observed patterns alongside other contributing factors. Dendrograms were generated for each season to analyze these patterns via hierarchical clustering based on species biomass distribution (Figure 5). During spring, stations 3 and 2 formed a distinct cluster, indicating notable similarity in species composition and biomass, while other stations displayed more gradual differentiation. Summer clustering indicated a significant connection among stations 7, 6, and 22 (Figure 5). In autumn, stations 19, 9, and 21 were clustered together, revealing a comparable macrobenthic community structure in these locations, which indicated a significant tendency for species to aggregate near the nuclear power plant, whereas station 10 remained relatively isolated (Figure 5). Winter dendrogram analysis demonstrated that stations 21 and 1 formed a distinct cluster, while stations 5 and 13 exhibited considerable divergence from the others (Figure 5).
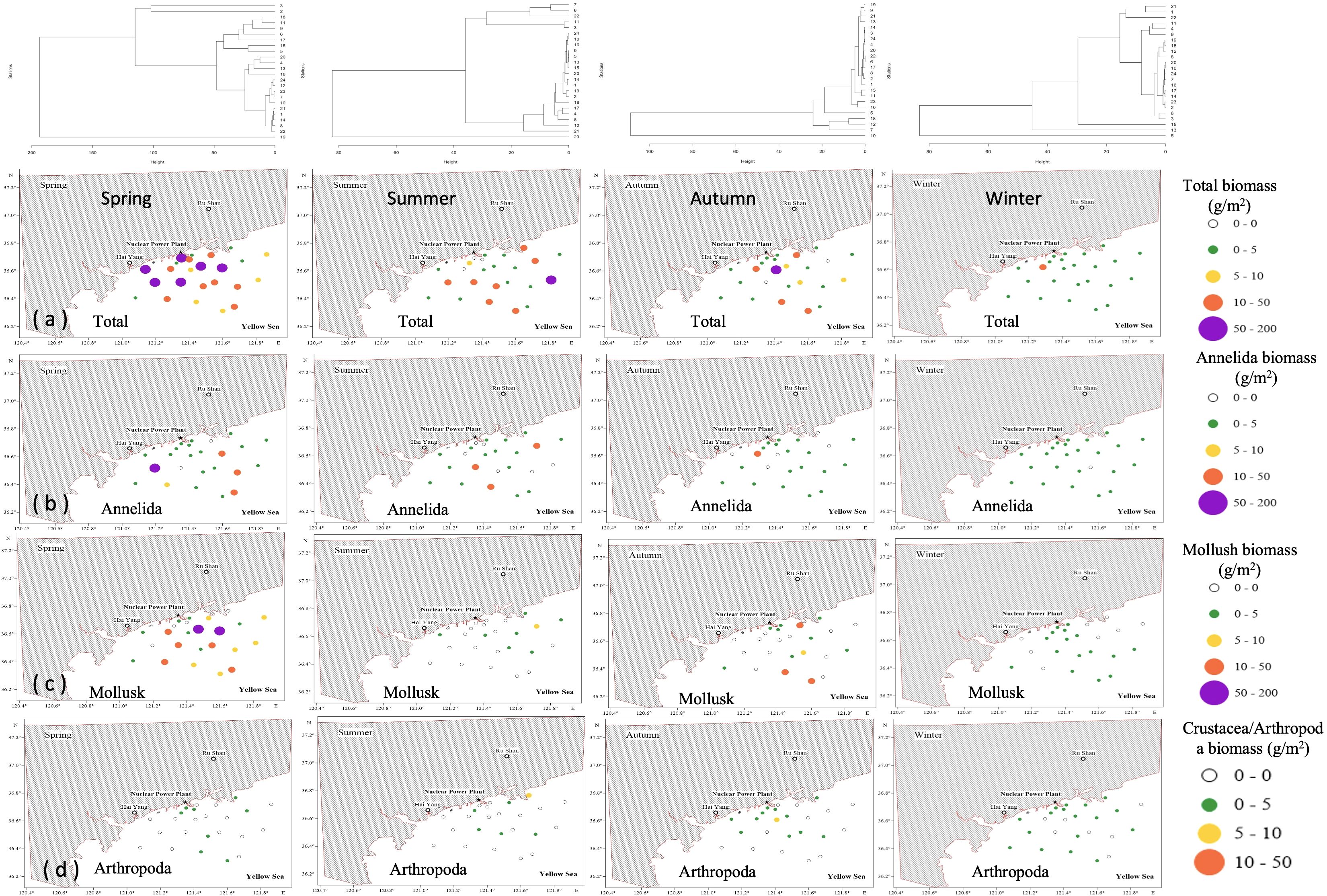
Figure 5. Spatial variations of the biomass of different types of macrobenthic organisms in spring, summer, autumn, and winter in the study area. (a) Distribution of the total biomass (g/m2); (b) distribution of Annelida biomass (g/m2); (c) distribution of mollusk biomass (g/m2); and (d) distribution of crustacean/arthropod biomass (g/m2).
3.2.2 Diversity indices and distribution
The macrobenthic community structure in the coastal Yellow Sea was evaluated using four key diversity indices: the number of species (S), Simpson’s diversity index (D’), Shannon–Wiener diversity index (H’), and Pielou’s evenness index (J’). These indices reflected seasonal variability in macrobenthic community structure, with several indices exhibiting changes across various seasons and stations. The number of species peaked in winter (S = 5.29 ± 0.51; maximum = 9; Table 3; Figure 6), especially at offshore stations and those adjacent to the HYNPP, whereas summer showed the lowest values (S = 2.08 ± 0.29) and coastal sites remained species-poor through summer and autumn. Simpson’s index (D′) was highest in spring (0.41 ± 0.05; 0.78 at station 23) (Figure 6) and again at offshore station 17 in winter (0.80), but fell to 0.24 ± 0.04 in summer and 0.35 ± 0.05 in autumn, indicating stronger dominance at inshore stations during the warmer months. Shannon–Wiener (H′) followed a similar pattern, with a winter maximum (0.88 ± 0.11; 1.82 at station 17), moderate spring values (0.78 ± 0.09; 1.63 at station 22) and marked reductions along the coast in summer and autumn (Figure 6). Pielou’s evenness (J′) was greatest in spring (0.56 ± 0.06), reaching 0.96 at station 10 and 0.95 at station 8 near the HYNPP, 0.91 at inshore station 22 and 0.94 at offshore station 4 (Figure 6). Evenness declined slightly in winter (0.54 ± 0.06) and reached an annual minimum in summer (0.45 ± 0.08). Overall, offshore and HYNPP-adjacent sites supported higher diversity and evenness in winter–spring, while coastal zones were dominated by a few taxa during the summer–autumn low-diversity period.
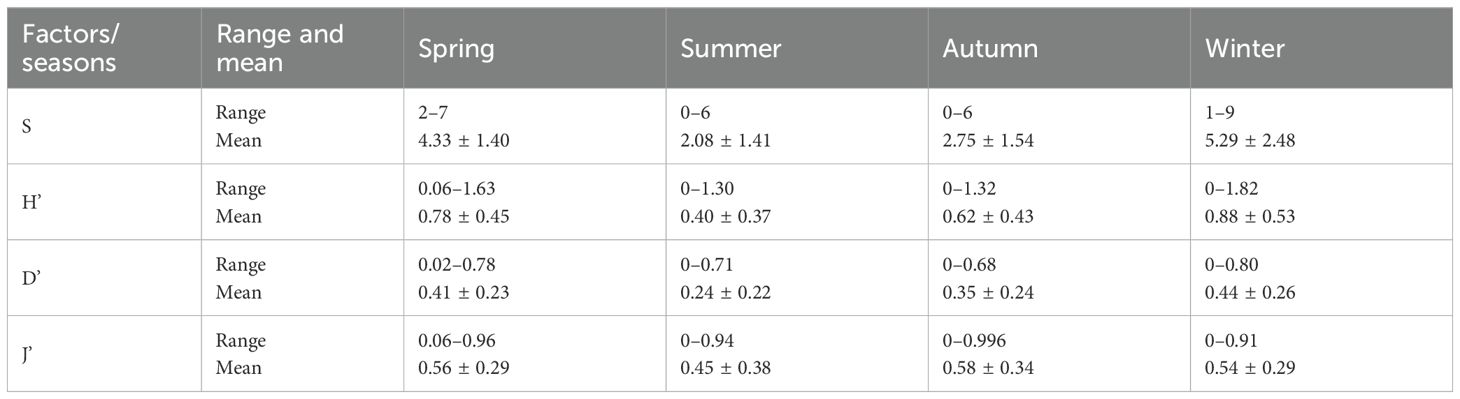
Table 3. Range and mean (± SD) values of macrobenthic S, H’, D’, and J’ indices during the four seasons.
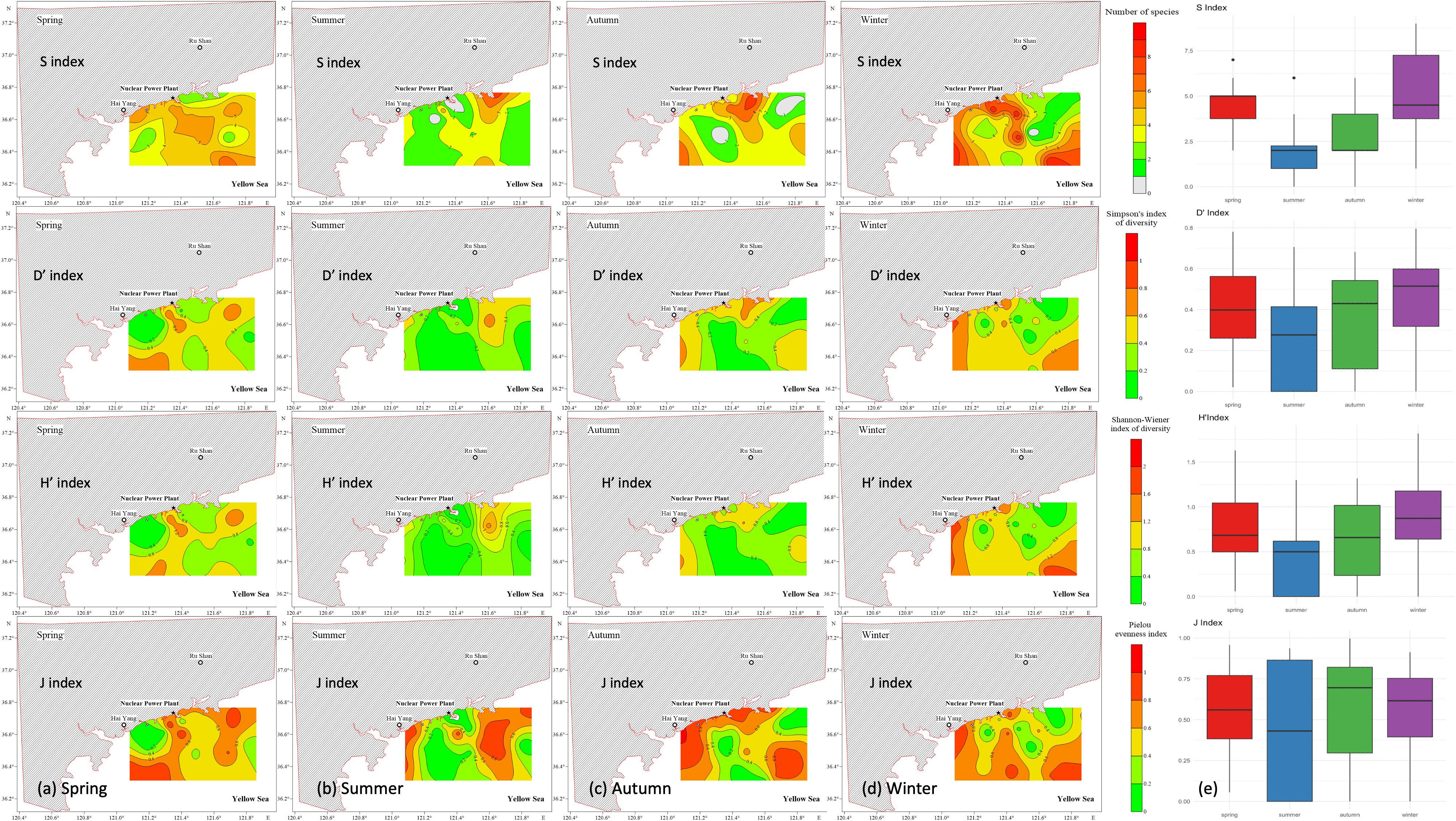
Figure 6. Spatial variations of the four diversity indices (S, H’, D’, and J’) in spring, summer, autumn, and winter in the study area.
3.3 Seasonal dynamics of macrobenthic communities in relation to various environmental variables
3.3.1 Correlations between environmental parameters and diversity indices
The relationship between environmental factors and macrobenthic diversity indices (S, H’, D’, and J’) was analyzed using Spearman correlation analysis. This revealed significant correlations between various factors (temperature, salinity, depth, DO, sedimental parameters, nutrients, and phytoplankton) and diversity indices, highlighting their impact on macrobenthic community structure in the coastal Yellow Sea. Temperature was negatively correlated with the number of species (S: r=−0.606, p < ; Figure 7), and the Shannon–Wiener diversity index (H’: r=−0.416, p < ; Figure 7). Salinity was positively correlated with the number of species (S: r=0.233, p = 0.022; Figure 7) and the Shannon–Wiener index (H’: r=0.209, p = 0.041; Figure 7), which suggests that consistent salinity conditions may enhance species variety through creating a more conducive environment for macrobenthic organisms. DO exhibited positive associations with the number of species (S: r=0.587, p < ; Figure 7), and the Shannon–Wiener index (H’: r=0.381, p < ; Figure 7). These findings indicate that oxygen availability is essential for sustaining diverse and balanced macrobenthic ecosystems. The Mz was negatively correlated with Simpson’s diversity index (D’: r=−0.206, p = 0.044 < 0.05; Figure 7) and Pielou’s evenness index (J’: r=−0.220, p = 0.031 < 0.05), which indicates that sediment size may facilitate greater diversity via offering more stable and resource-abundant microhabitats. The phytoplankton cell count displayed a significant correlation with the number of species (S: r=0.389, p < ; Figure 7) and the Shannon–Wiener diversity index (H’: r=0.239, p = 0.019; Figure 7). This implies that elevated phytoplankton abundance may increase macrobenthic diversity, possibly through enhanced organic matter deposition that supports macrobenthic species.
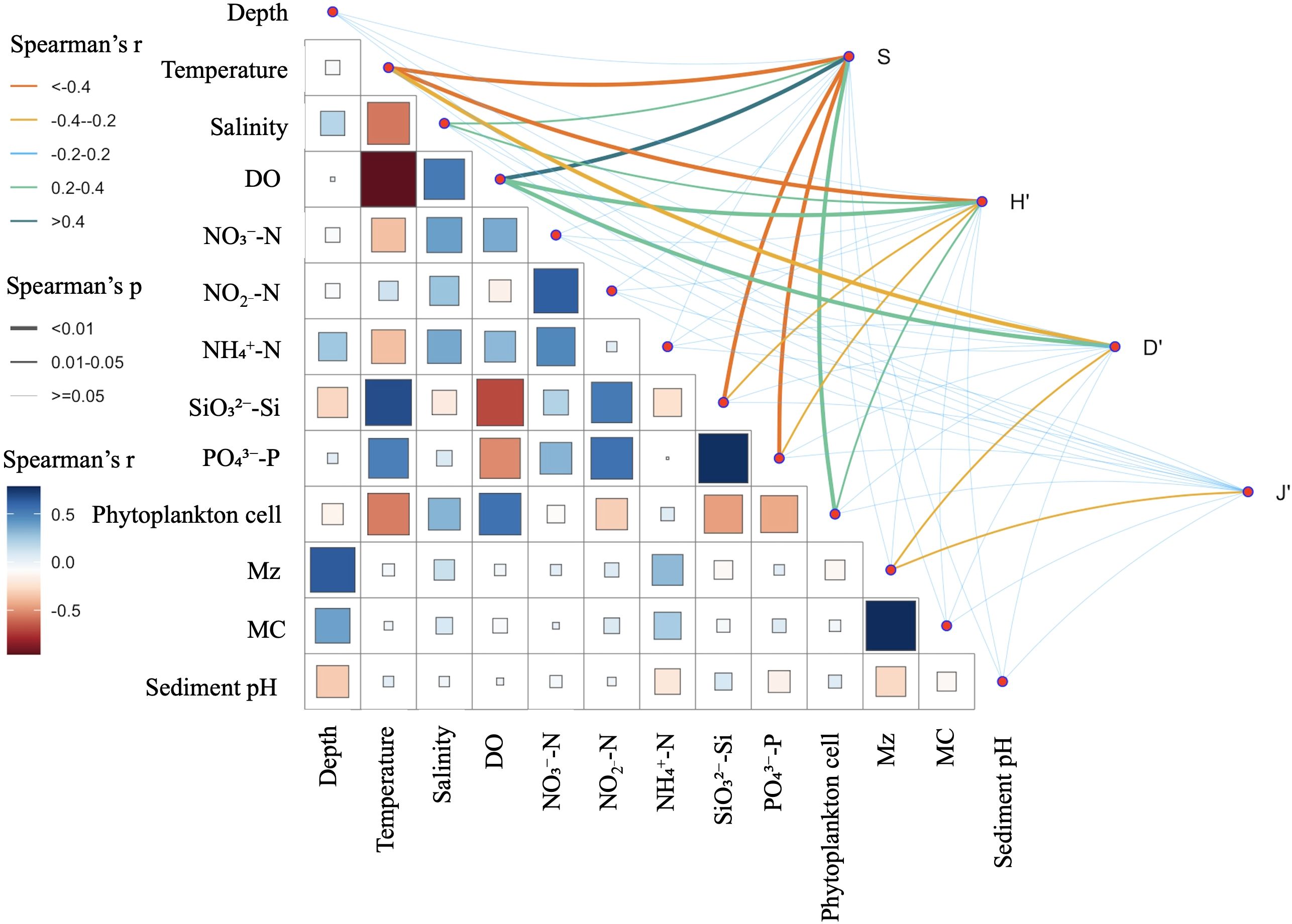
Figure 7. Spearman correlations between environmental variables and diversity indices (S, H′, D′, and J′). The color of the matrix squares represents the correlation coefficient (Spearman’s p), with blue representing positive correlations and red representing negative correlations. The intensity of the color corresponds to the strength of the correlation, as shown in the color bar on the left. The line thickness indicates the p-value of the correlation, with thick lines representing significant correlations (p < 0.01), medium lines indicating 0.01 < p < 0.05, and thin lines indicating p ≥ 0.05.
3.3.2 Identification of key predictors and simulation of the effect of thermal discharge
The RF model was utilized to assess the relative importance of environmental factors in influencing the four diversity indices (S, H’, D’, and J’) and reveal their complex relationships, thereby obtaining valuable insights to support improved environmental monitoring and management. The top five most important environmental factors for each index were determined, in addition to their corresponding relationships (Figure 8). The top five most influential variables for the number of species (S) were DO (11.22%; Table 4A), SBT (9.62%; Table 4A), PO4³--P (8.01%; Table 4A), salinity (6.49%; Table 4A), and SiO3²--Si (5.03%; Table 4A). The results demonstrated that DO was the most important positive driver of the number of species. Additionally, the influencing factors for H’ and D’ showed similar patterns in terms of their effects on diversity indices (Figure 8). Moreover, the analysis showed that J’ was strongly influenced by sediment parameters, particularly Mz and sediment pH (Figure 8). Temperature was the most important predictor for H’ (7.21%; Table 4A), D’ (6.58%; Table 4A), and J’ (3.08%; Table 4A), showing a consistently negative relationship with diversity. Salinity also emerged as an important factor for H’ (3.21%; Table 4A) and D’ (2.55%; Table 4A), which suggests that stable salinity conditions can support more balanced macrobenthic communities. In addition, DO played a crucial role in influencing S and J’, while Mz had a negative impact on H’ and D’ (Figure 8). The phytoplankton cell count exhibited important correlations with the number of species (S: 9.05%; Table 4A), H’ (3.65%; Table 4A), and D’ (2.51%; Table 4A), reinforcing its role as a primary driver of macrobenthic diversity. The S, H’, and D’ all increased as the phytoplankton cell count rose before stabilizing (Figure 8). In addition to performing in-depth analysis of the relationships between macrobenthic diversity indices, this study employed RF modeling to explore the relationship between phytoplankton and nutrient concentrations, with a variance explained (VAR) of 53.89%; in RF regression, VAR quantifies how much of the variability in the target variable is captured by the model. While direct correlations between nutrients and macrobenthic diversity indices were weaker, nutrients were found to influence phytoplankton, which in turn affected macrobenthic communities. The RF model revealed that NO2- (21.43%; Table 4B), SiO3²- (17.42%; Table 4B), and PO4³- (16.25%; Table 4B) were the most influential nutrients. PO4³- and SiO3²- had a direct and important influence on macrobenthic diversity through regulating the availability of organic matter (Figure 8). These findings suggest that nutrients primarily affect macrobenthic diversity through their effects on primary production rather than direct interactions.
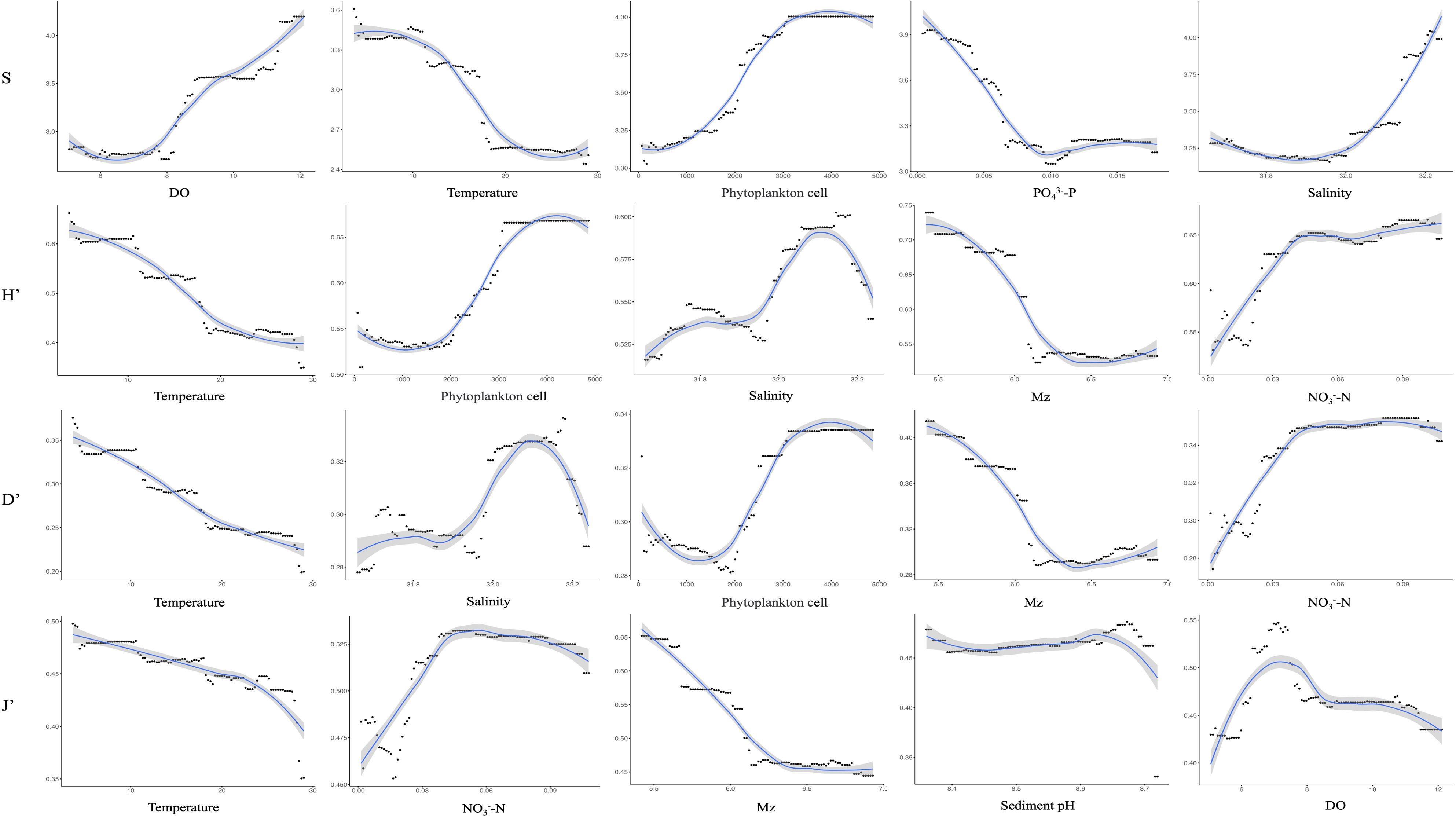
Figure 8. Relationship between the four diversity indices (S, H’, D’, and J’) and the top five environmental factors ranked by the corresponding importance indices through random forest modeling.
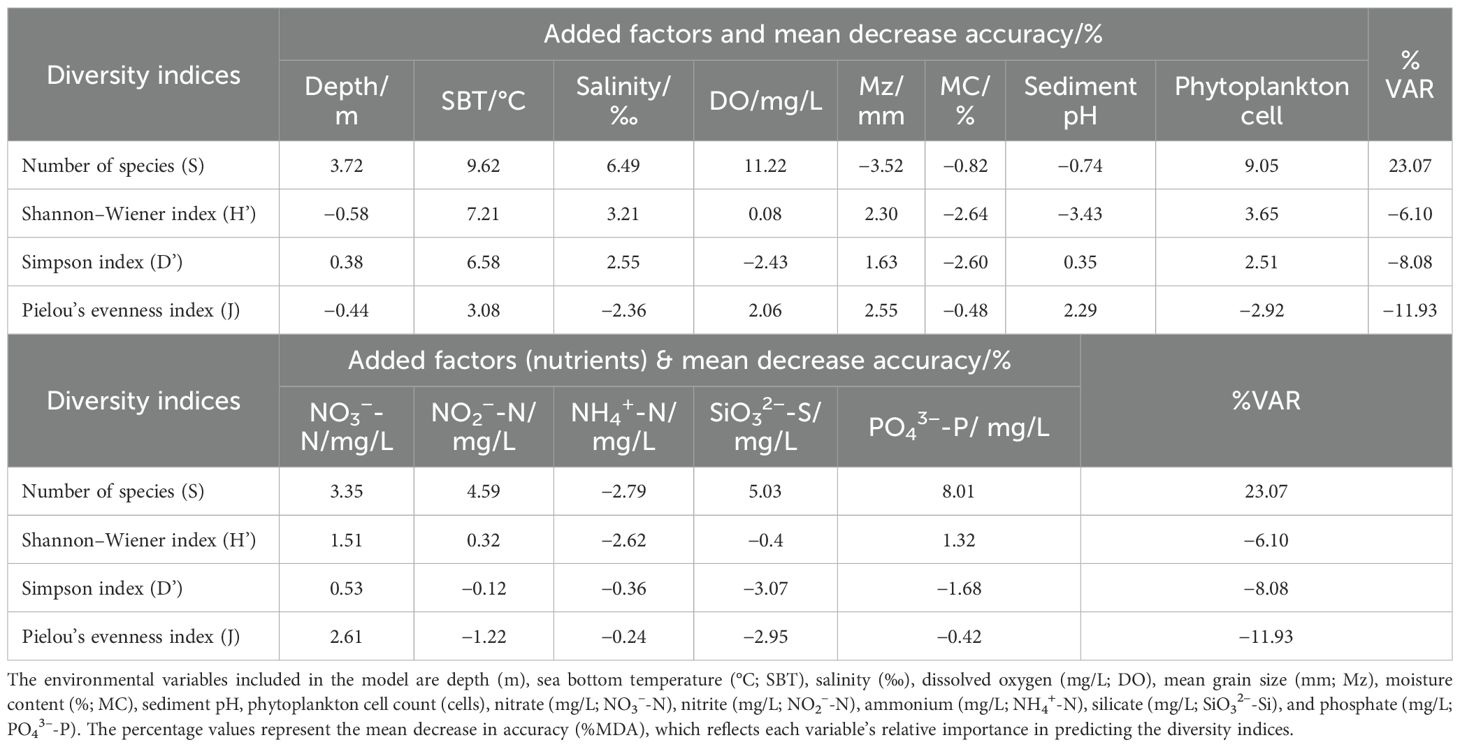
Table 4A. Random Forest model output identifying the top environmental predictors for macrobenthic diversity indices (S: number of species, H′: Shannon-Wiener index, D′: Simpson index, J′: Pielou’s evenness index) in the offshore region near the YanTai HY-Nuclear Power Plant (HYNPP) in Shandong, China.
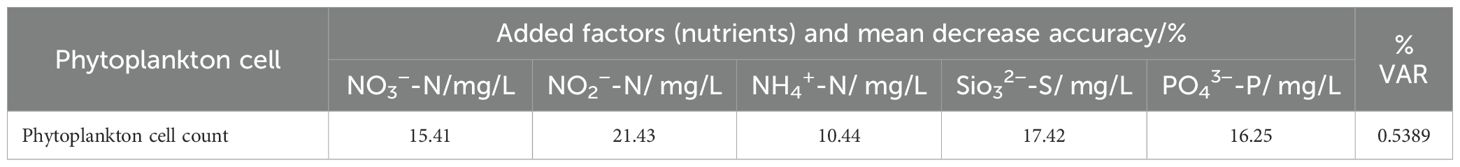
Table 4B. Random forest model of the phytoplankton cell count in offshore Shandong near the YanTai HY-nuclear power plant (HYNPP).
CBRF models were used to simulate the spatial variability of the macrobenthic community after the operation of HYNPP under the effects of thermal discharge. In the model training phase, all summer models achieved R2 ≥ 0.89 and MSEs ≤ 12.0 for the total biomass and ≤ 3.2 for the biomass of Annelida (Table 5), indicating excellent explanatory power. Similarly, autumn predictions for mollusk biomass were strong, with R2 = 0.92 and MSE of 2.4 (Table 5). By contrast, the total biomass in winter showed the weakest fit (R2 = 0.55, MSE = 1.93; Table 5), and the spring total biomass, while retaining a high R2 = 0.89, exhibited a very large error spread (MSE = 187; Table 5), suggesting sensitivity to a small number of extreme observations and possible over-fitting. During the winter, the Annelida biomass also remained comparatively uncertain (R2 = 0.68; Table 5). When the CBRF models were forced with a uniform +4 °C rise in SBT, they generated a coherent pattern of hot and cold spots across the Haiyang bay (Figure 9). The largest response was predicted for the total biomass in spring, which increased to 128.15 g/m² at station 16—a muddy, weak-flushing inner-bay site (Figure 9). Conversely, the lowest total biomass under the warming scenario was projected to occur during summer for station 24 (1.72 g/m²; Figure 9). Among taxonomic groups, annelid biomass peaked at station 15 in spring (44.67 g/m²; Figure 9), which was consistent with the occurrence of fine sediments, whereas mollusk biomass reached a maximum of 81.26 g/m² in spring at station 4 (Figure 9), which was close to patchy hard substrate. The highest arthropod biomass (6.9 g/m²) appeared during the summer at station 10, while the minimum value (≈0.1 g/m²) occurred in spring across the whole area (Figure 9).
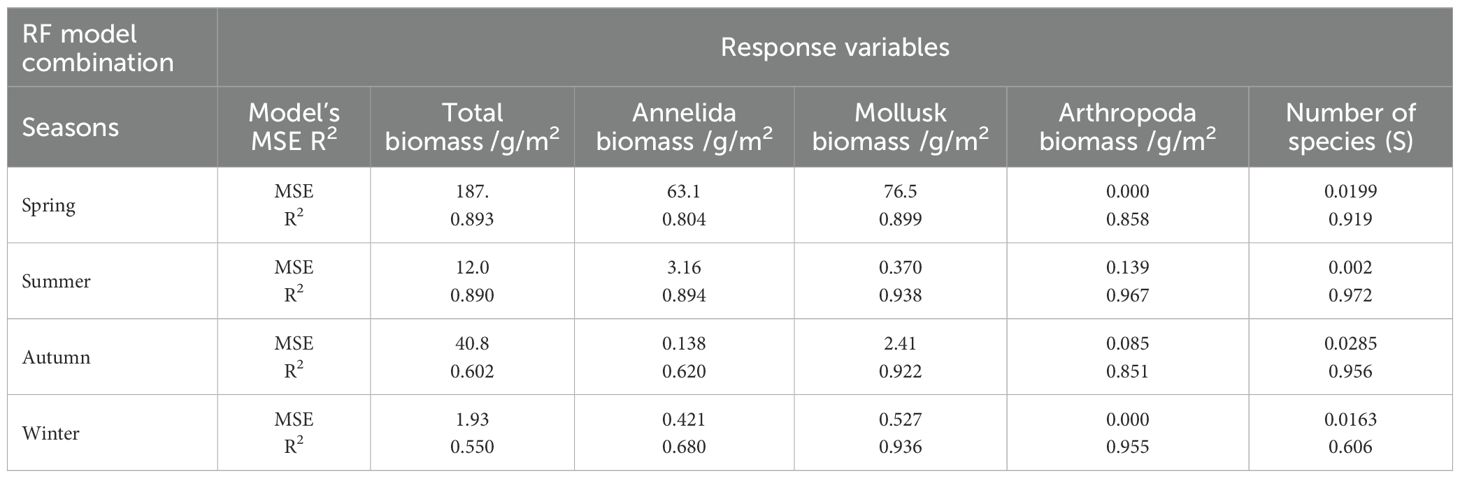
Table 5. Performance metrics (mean squared error (MSE) and R2) of random forest (RF) predictive models for macrobenthic biomass and the number of species (S) across the season.
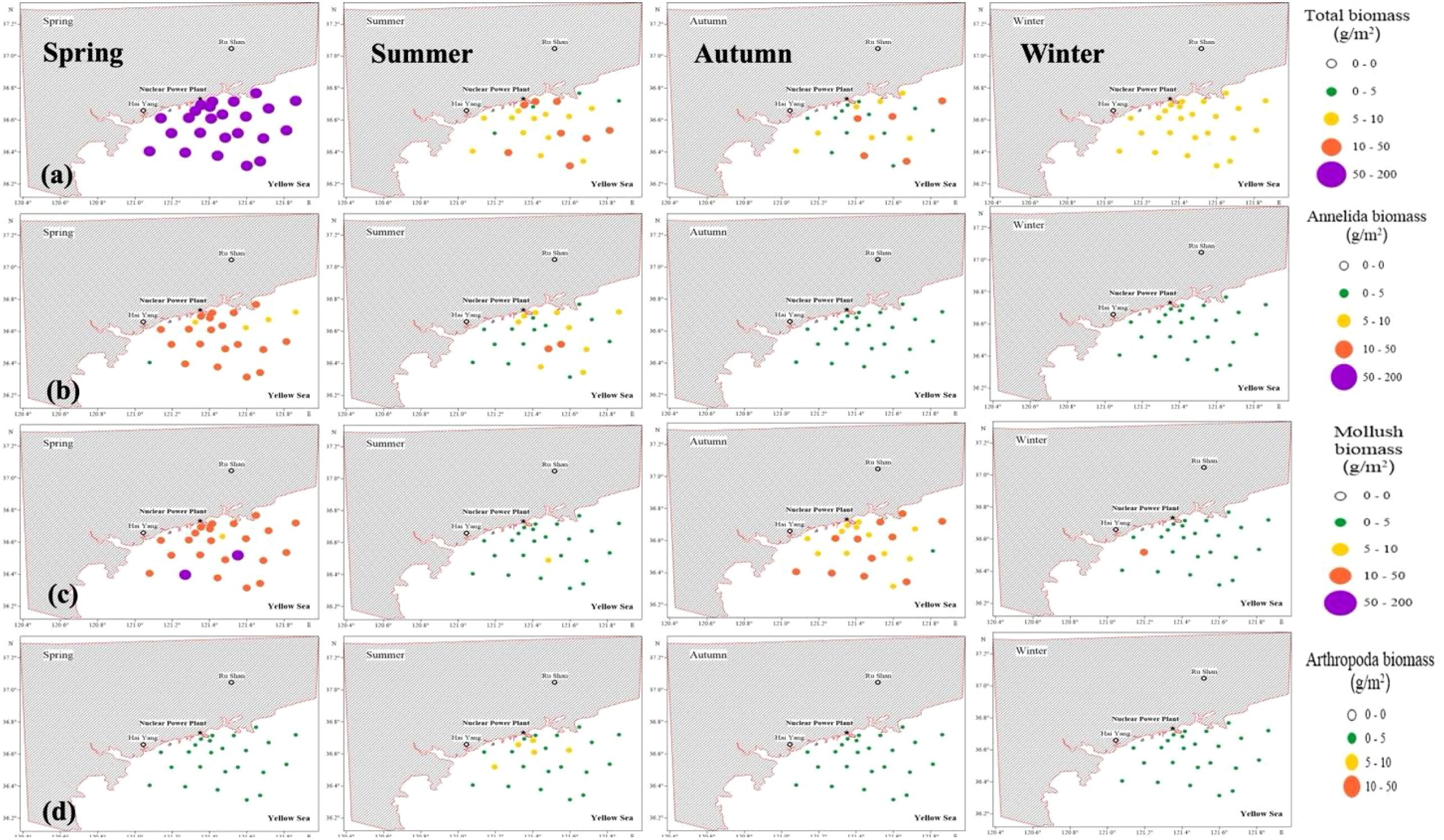
Figure 9. Simulation of thermal discharge-induced temperature increases following YanTai HY-nuclear power plant (HYNPP) operation. Random forest predictive models were used to predict the spatial variations of the biomass of different macrobenthic organisms in spring, summer, autumn, and winter in the study area. (a) Distribution of the total biomass (g/m2); (b) distribution of Annelida biomass (g/m2); (c) distribution of mollusk biomass (g/m2); and (d) distribution of arthropod biomass (g/m2).
4 Discussion
4.1 Relationship between environmental variables and macrobenthos in seasonal variation
This section discusses the major influence of environmental factors, including temperature, DO, sediment characteristics, salinity, and nutrients, on the composition and diversity of macrobenthic communities near the HYNPP, highlighting seasonal variations and their ecological consequences. The temperature and salinity ranges recorded in the Yellow Sea during this study were in accordance with previously reported findings (Fu et al., 2024). The results showed that key environmental factors such as water temperature, DO, salinity, and nutrient levels played a crucial role in shaping the composition and diversity of the macrobenthic community. These factors affected species distribution and ecosystem functioning via direct and indirect pathways, with their interconnections influencing biogeochemical cycling and macrobenthos composition (Ge et al., 2024b). Changes in these characteristics can result in substantial alterations in community structure, frequently resulting in reduced species diversity and abundance, particularly in vulnerable marine habitats. Consequently, monitoring these factors is essential for the efficient management and conservation of marine biodiversity (Rajapaksha et al., 2024). In the Yellow Sea, bottom water temperatures reach their annual peak during summer, particularly in areas outside the influence of the Yellow Sea Cold Water Mass, where temperatures can rise to ~20°C (Kim and Yu, 2022). This seasonal warming significantly affects macrobenthic community distribution, biomass, and reproduction, as higher temperatures elevate metabolic rates and feeding activity, often resulting in greater biomass and species richness in warmer, stratified zones (Shou et al., 2018; Kim and Yu, 2022). Warmer conditions also accelerate gametogenesis and spawning in many temperate macrobenthic invertebrates, thus synchronizing reproduction with periods of high food availability (Shou et al., 2018). However, excessively high temperatures may exceed the thermal tolerance of cold-adapted species, leading to range contractions or migration into deeper, cooler waters (Kim and Yu, 2022). These thermally induced distributional shifts are part of a broader global pattern: as ocean temperatures rise, many macrobenthic organisms have moved poleward or downslope to maintain their thermal niches (Poloczanska et al., 2013). For example, long-term studies in the North Sea indicate that macrobenthic macrofauna have deepened their distributions at a rate of several meters per decade in response to bottom warming (Hiddink et al., 2015). Such vertical and latitudinal migrations result in the reassembly of macrobenthic communities, with warm-affinity species expanding and cold-affinity species retreating (Bianchi et al., 2023). In constrained environments such as semi-enclosed seas, where poleward shifts are limited, depth migration is the primary strategy utilized by macrobenthic communities (Hiddink et al., 2015). These shifts not only alter species distributions but also impact macrobenthic ecosystem functioning, highlighting the ecological importance of summer bottom temperature maxima and the need for macrobenthic management strategies to account for climate-driven range shifts (Poloczanska et al., 2013; Bianchi et al., 2023). Prolonged exposure to elevated temperatures results in reduced macrobenthic diversity as the metabolic rates, feeding behaviors, and reproductive cycles of species are altered (Li et al., 2019), consistent with the present findings (Figures 7, 9). Elevated temperatures modify nutrient cycling through influencing the availability of essential nutrients such as nitrogen and phosphorus, which are vital for primary production in marine ecosystems. Furthermore, elevated water temperatures diminish the solubility of oxygen, thereby intensifying hypoxic conditions in nutrient-dense coastal regions. Conversely, oxygen levels decline in warmer months, driven by increased biological oxygen demand from organic matter decomposition and macrobenthic metabolic activity. Anthropogenic nutrient inputs, such as sewage and agricultural runoff, exacerbate these oxygen-depleting processes, leading to eutrophication (Xu et al., 2022b). During summer, elevated water temperatures reduce oxygen solubility and strengthen stratification, hindering surface-to-bottom oxygen exchange (Breitburg et al., 2018). Simultaneously, increased organic input from spring blooms and river runoff undergoes microbial degradation, leading to elevated biochemical oxygen demand in bottom waters (Diaz and Rosenberg, 2008). These processes commonly lead to seasonal hypoxia, especially by late summer, when decomposition peaks and re-aeration is minimal. Globally, coastal systems such as the Gulf of Mexico and Baltic Sea exhibit recurring summer hypoxia due to stratification and organic loading (Diaz and Rosenberg, 2008). Eutrophication induced by agricultural and urban nutrient inputs exacerbates this through stimulating algal blooms, which further deplete oxygen as they decay (Rabalais et al., 2010). Warmer temperatures also enhance microbial respiration, consequently accelerating oxygen consumption (Breitburg et al., 2018). These mechanisms contribute to the global expansion of hypoxic zones, including intensifying oxygen minimum zones in the open ocean due to reduced ventilation and warming-driven decomposition (Breitburg et al., 2018). In sum, a combination of thermal, biological, and anthropogenic factors drives summer oxygen declines, with significant implications for macrobenthic ecosystems and biogeochemical cycles (Diaz and Rosenberg, 2008; Breitburg et al., 2018).
In addition to temperature and DO, the sediment characteristics, salinity, and depth significantly affect macrobenthic diversity. Sediment characteristics, particularly grain size, play a key role in shaping macrobenthic community composition via influencing habitat suitability (Sanders, 1958). Coarse sediments generally support higher macrobenthic abundance due to better habitat conditions and nutrient availability (Long and Lewis, 1987; Coleman et al., 2007; Shou et al., 2018). Studies in the Bohai Sea and off the coast of Yantai have confirmed positive correlations between macrobenthic abundance and sand content, while silt and clay negatively affect macrobenthic communities (Li et al., 2022a; Li et al., 2020). In the present study, the notable correlation identified between macrobenthic communities and Mz may have been influenced by the limited spatial scale of the data and the relatively homogeneous sediment characteristics. A similar influence was observed for the sea bottom depth. Although the scale of the Mz data was small, its pronounced seasonal distribution pattern (Figure 2e) likely drove the correlation between Mz and macrobenthic communities, in line with previous research demonstrating that macrobenthic community distribution was primarily associated with sediment grain size (Li et al., 2022a). Salinity levels in the Yellow Sea exhibit seasonal change, with elevated levels noted in coastal regions. Elevated salinity levels can induce stress in macrobenthic organisms, especially those accustomed to lower salinity conditions. During summer, increased salinity resulting from evaporation and diminished freshwater intake may diminish species diversity and alter community composition (Lai et al., 2014). Stenohaline species are particularly susceptible to these fluctuations, resulting in localized population declines. Salinity plays a pivotal role in modulating nutrient chemistry and availability, which cascades through primary production to shape macrobenthic ecosystems. Variations in salinity influence the solubility and cycling of nutrients such as phosphorus and nitrogen, thereby altering their availability to phytoplankton (Sklar and Browder, 1998). In estuaries, low salinity levels due to riverine input can desorb PO4³--P from particles and reduce stratification, consequently enhancing nutrient mixing in surface waters, whereas high salinity may reduce nutrient bioavailability through chemical sequestration (Sklar and Browder, 1998). Observations across tropical and temperate estuaries reveal that lower salinity often correlates with higher nutrient levels and phytoplankton biomass (Bharathi et al., 2022). Freshwater inflows thus stimulate phytoplankton growth at intermediate salinities, increasing the organic matter supply to macrobenthos. Global studies show that salinity gradients indirectly shape macrobenthic biomass and community composition via nutrient-driven productivity (Montagna et al., 2025). For example, in the Gulf of Mexico and the Baltic Sea, freshwater pulses have been found to elevate nutrient inputs and phytoplankton blooms, leading to enhanced macrobenthic secondary production and changes in oxygen conditions (Montagna et al., 2025; Bharathi et al., 2022). Overall, research indicates that salinity acts as a key driver of estuarine nutrient regimes and macrobenthic ecological responses (Sklar and Browder, 1998; Bharathi et al., 2022; Montagna et al., 2025). Regions that exhibit increased salinity during summer, attributable to diminished freshwater influx, frequently experience a decrease in brackish species, but species resilient to heightened salinity may flourish (Ge et al., 2024b). The findings of these previous studies highlight the necessity of regulating nutrient and freshwater inputs to sustain balanced ecosystems capable of supporting varied macrobenthic communities. Moreover, alterations in nutrient cycling under the influence of temperature and hydrodynamic phenomena such as upwelling exacerbate these correlations. Winter upwelling events in the Yellow Sea bring nutrient-dense deep waters to the surface, promoting phytoplankton proliferation and providing essential organic matter to macrobenthic species (Ge et al., 2024a). However, the timing and intensity of these upwelling events fluctuate periodically, profoundly affecting the quality and quantity of food accessible to macrobenthic communities.
4.2 Phytoplankton and macrobenthos
The results of the present study demonstrated that phytoplankton directly influenced the macrobenthic community. Given the significant relationship between macrobenthic organisms and phytoplankton observed in the results of the current work and previous studies, it is essential to separately discuss their interplay. A recent study (Cecchetto et al., 2024) presents a new perspective, suggesting that the seasonality of primary productivity may affect the species richness of macrobenthic communities via altering the seasonal dynamics of planktonic communities, thereby influencing the distribution and availability of organic matter. In highly seasonal ecosystems, primary productivity reaches its peak during specific seasons (e.g., spring or summer), resulting in increases in phytoplankton and organic matter, while phytoplankton and organic matter exhibit considerable declines in other seasons, hence constraining resource availability over time (Cecchetto et al., 2024). In the current study area, which displays a somewhat seasonal environment at approximately 36°N latitude, the present findings indicate that phytoplankton positively impacts macrobenthic diversity indices, with nutrients predominantly influencing macrobenthic populations indirectly via regulating phytoplankton dynamics. The seasonality of primary productivity presumably modulates this relationship because seasonal nutrient variations may exert greater control over macrobenthic diversity at different times of the year, consistent with findings from Cecchetto et al. (2024). Nutrients are essential in altering macrobenthic ecosystems, particularly through influencing phytoplankton communities that provide organic material and nutrients to macrobenthic species through sedimentation. According to the current results, the concentrations of nutrients such as NO3--N, PO4³--P, and SiO3²--Si, indirectly yet substantially influence macrobenthic communities. The RF model showed that SiO3²--Si was a pivotal factor in promoting phytoplankton growth, which in turn fostered a more productive macrobenthic environment (Table 4). The presence of these nutrients governed the growth and composition of phytoplankton, including diatoms, which serve as a fundamental supply of organic matter for macrobenthic organisms. Elevated SiO3²--Si levels have been demonstrated to stimulate diatom blooms, hence substantially increasing the sedimentary organic matter accessible to macrobenthic species (Xu et al., 2024; Fu et al., 2024). Diatoms are abundant in silica and prevail in the phytoplankton community under elevated SiO32−-Si conditions, hence sustaining the macrobenthic food web by continuously providing organic material through sedimentation. Phytoplankton, especially diatoms and dinoflagellates, constitute the basis of the aquatic food web and are propelled by hydrological alterations, including water mass movements and upwelling. These organisms supply abundant nourishment for macrobenthic species such as annelids and mollusks, which flourish on organic debris (Rajapaksha et al., 2024; Ge et al., 2024a, 2024b). Their periodic blooms are governed by nutrient availability and exert cascading impacts on macrobenthic communities. During periods of elevated nutrient input, such as spring, phytoplankton biomass increases, leading to elevated sedimentation rates. This process provides macrobenthic species with an increased supply of organic material, enhancing species richness and biomass during these seasons (Xu et al., 2024; Dory et al., 2024). However, nutrient depletion during the summer or excessive nutrient levels leading to eutrophication disrupt this balance, adversely impacting macrobenthic diversity (Table 3; Guo et al., 2020; Ge et al., 2024b). Notably, imbalances in nutrient inputs, particularly excessive nitrogen (NO3−-N, NO2−-N, and NH4+-N), can precipitate hazardous algal blooms, diminish the quality of organic matter reaching the macrobenthos, induce hypoxic conditions, and decrease biodiversity (Xu et al., 2022a; Ge et al., 2024b). Moreover, human activities such as intensive agriculture and urban wastewater discharge have significantly increased nitrogen loading to coastal waters, thereby accelerating sedimentary nitrification–denitrification processes, with widespread impacts on macrobenthic ecosystems (Galloway et al., 2008; Seitzinger et al., 2006; Li et al., 2015; Guo et al., 2020). Over the past century, anthropogenic nitrogen inputs—via fertilizer runoff, manure, and sewage—have approximately doubled, altering the coastal nitrogen cycle (Galloway et al., 2008). This excess reactive nitrogen fuels microbial transformations in sediments: NH4--N stimulates nitrification, while the resulting NO3--N promotes denitrification under low-oxygen conditions (Seitzinger et al., 2006). Although denitrification helps remove excess nitrogen, it can also emit nitrous oxide (N2O), a potent greenhouse gas, highlighting the global ramifications of nutrient pollution (Galloway et al., 2008). For macrobenthic communities, this intensified microbial activity is a double-edged sword—beneficial for nutrient removal, yet harmful under conditions of organic overload and hypoxia, which degrade macrobenthic habitats (Diaz and Rosenberg, 2008). Excess nutrients often drive phytoplankton blooms and oxygen depletion, resulting in stress or the collapse of macrobenthic fauna and feedback loops that favor microbial over animal dominance (Diaz and Rosenberg, 2008). Globally, regions including the Gulf of Mexico, Baltic Sea, and East China Sea illustrate how human-driven nitrogen enrichment alters macrobenthic biodiversity and function through enhanced microbial processing and hypoxia (Rabalais et al., 2010; Breitburg et al., 2018). In summary, anthropogenic nutrient inputs intensify sedimentary nitrogen cycling, with cascading ecological consequences for macrobenthic systems worldwide (Seitzinger et al., 2006; Diaz and Rosenberg, 2008; Galloway et al., 2008; Breitburg et al., 2018).
4.3 Seasonal changes in macrobenthic community composition with dominant taxa and species
The macrobenthic communities in the Yellow Sea display considerable seasonal variations in species composition that are affected by factors such as water temperature, salinity, sediment type, and nutrient levels (Jiang et al., 2023; Hu et al., 2024; Ji et al., 2024; Xie et al., 2024). In addition, the seasonal dynamics of the Yellow Sea Cold Water Mass significantly influence the distribution and diversity of macrobenthic species (Wang et al., 2011). In the last decade, the species composition and community structure of macrobenthic fauna in the Yellow Sea have undergone alterations. Peng et al. (2017) observed that diminutive polychaetes, such as Notomastus latericeus and Ninöe palmata, have emerged as dominant species in coastal regions, while species in cold-water-mass areas, such as Ophiura sarsii vadicola and Thyasira tokunagai, have maintained stability. In the present study, the R-index captures the magnitude of species replacement, while the cluster-SIMPER combination demonstrates that seasonal assemblages are statistically distinct and a handful of polychaetes and molluscs account for more than one-fifth of the seasonal shifts, providing an ecological explanation for the high turnover values (Figure 3D; Table 2). The PCoA and Spearman correlation analyses conducted indicate that these variations are closely connected to seasonal fluctuations, sampling stations, and specific variables near the HYNPP (Figures 3c, 8). In the present study, temperature, salinity, and DO levels were found to be significant factors shaping macrobenthic communities, while the relatively consistent sea bottom depth had a minimal influence. Interestingly, P. bidentata, a widely distributed echinoderm with known anti-tumor properties (Shen et al., 2013), dominates coastal areas of the Yellow Sea and offshore regions of the Yangtze River estuary (Sun et al., 2007), Jiaozhou Bay (Liao, 2004), and the shallow seas around Dalian City (Wu et al., 2021). The presence of these rich germplasm resources in the study area aligns with previous findings. Depth, particularly in the 30–50 m range, is an important environmental factor for species distribution. P. bidentata adapts well to temperature changes, expanding its range as temperatures rise (Xu et al., 2023), which explains its clustering during summer and autumn in the study area (Figure 3B). Generally, declining DO levels, which are negatively correlated with water temperature, further impact species that require high-oxygen environments, especially during summer. However, some species, such as Capitella capitata, are less affected due to their high tolerance for low-oxygen conditions. This species is well known for colonizing disturbed and polluted environments with high organic content, such as sewage outfalls and harbors, which are often hypoxic (Silva et al., 2017; Checon et al., 2021).
During the spring, the macrobenthic communities in the study area exhibited increased diversity and biomass, with dominant groups including annelids and crustaceans. This pattern was linked to advantageous environmental conditions, such as moderate temperatures and enhanced food availability during this period. Nemertines exhibited increased biomass during the summer in the study area because higher temperatures enhanced their metabolic rates and activity levels. Nemertines, being primarily carnivorous, can restrict the growth and reproduction of smaller invertebrates such as crustaceans, mollusks, and annelids (Brockington and Clarke, 2001). Seagrass meadows (especially dense stands of species like Zostera marina in temperate regions) are well-known as marine foundation habitats that enhance biodiversity. Their complex underwater canopy structure provides refuge and nursery grounds for many organisms, especially for arthropods such as shrimps and crabs (McCloskey and Unsworth, 2015; Park et al., 2020). The total species count was highest in winter; however, the composition changes and the total biomass reached the lowest point in the year. This reflected the dominance of low-trophic-level species in macrobenthic niches, as evidenced by the comparison of trophic-level species between winter and other seasons in the present study. This observation aligned with previous studies (Rautio et al., 2011; Grebmeier et al., 2015) and was primarily attributed to bottom water temperatures. Similarly, Quan et al. (2020) demonstrated that a decrease in water temperature resulted in diminished primary productivity and biological density, consequently leading to reduced macrobenthic abundance during winter. Conversely, numerous macrobenthos larvae emerge in autumn, aligning with the breeding season of annelids, mollusks, and arthropods (Mao et al., 2023), potentially leading to increased diversity in winter despite their minimal contribution to the total biomass. According to the research of Jiang et al. (2023), the niche breadth during summer in the Yellow Sea is narrow, implying that food resources are sufficiently abundant in both quality and quantity to satisfy energy requirements. Consequently, feeding habits become more specialized, leading to reduced competition. The diversity of food sources, including plankton, decreases in summer, which further narrows the niche breadth (Du et al., 2013; Fu et al., 2021; Li et al., 2022a). Seasonal variations in food availability and competition affect niche breadth and the dynamics of macrobenthic communities throughout different seasons.
4.4 Implications for monitoring and marine management near the HYNPP
This study highlights the necessity to utilize an integrated approach for managing macrobenthic diversity in coastal ecosystems, supported by insights gained through RF analysis. The impact of thermal discharge on plankton was found to be significant for evaluating the effects of thermal discharge on macrobenthic ecosystems in the future, given the critical relationship between phytoplankton and macrobenthic communities. Elevated water temperature and residual chlorine (Cl) represent significant hazards posed by thermal discharge from coastal nuclear power plants. Thermal discharge can affect phytoplankton communities through increasing the seawater temperature and altering vertical mixing, which in turn may influence macrobenthic ecosystems through altering primary productivity (Langford, 1990; Krishnakumar et al., 1991; Chen, 1992; Pane et al., 2001; Poornima et al., 2005; Zeng, 2008). Thermal discharge also affects mmacrobenthic ecosystems. Previous studies have reported declines in taxa such as Polychaeta, Mollusca, Arthropoda, and Echinodermata under conditions of thermal discharge, with species from these taxa declining during community succession (Lin et al., 2018; Guimarães et al., 2023). Research indicates that some Mollusca species are being gradually replaced by higher-biomass species due to the thermal discharge from power plants, reflecting a general pattern of macrobenthic community succession that is often associated with environmental disturbances or habitat changes (Lin et al., 2018; Guimarães et al., 2023). Alterations in macrobenthic species distributions may lead to extensive community alterations, with considerable consequences for ecosystem functionality (Kirby et al., 2007; Neumann et al., 2008; Neumann and Kröncke, 2011). Studies suggest that phytoplankton and zooplankton communities, rather than temperature alone, are the primary regulators of macrobenthic abundance and structure (Lin et al., 2018). Thus, thermal discharge influences the entire marine ecosystem via altering phytoplankton and zooplankton abundance, subsequently affecting macrobenthic growth.
In the present study, the predictive models under a +4 °C warming scenario indicated pronounced changes in the macrobenthic community, including an overall increase in biomass and shifts toward greater spatial heterogeneity in terms of organism distribution. The total macrofaunal biomass was predicted to rise broadly across the area, suggesting enhanced secondary production or the expansion of warm-tolerant species. This aligns with observations that moderate temperature elevation can stimulate macrobenthic invertebrate growth and recruitment, especially in nutrient-rich environments (Yang et al., 2020; Yanygina, 2015). For example, a shallow lake study found that macrobenthic abundances peaked in cooler spring conditions and declined during hot summers, underscoring that warming can boost productivity up to an optimal threshold (Yang et al., 2020). Similarly, in a Siberian power plant cooling reservoir, the presence of warmer water during late winter promoted the early reproduction and rapid population expansion of a macrobenthic amphipod (a Baikalian endemic), demonstrating a positive biomass response to moderate heating in spring (Yanygina, 2015). The results of the present study further showed that the seafloor became more “patchy,” with the emergence of distinct high-biomass hotspots and low-biomass cold spots. Such increased spatial heterogeneity is a common ecological outcome of environmental stress; thermal disturbances often create refugia where tolerant taxa accumulate in high density, while other areas become depauperate (Link et al., 2013; Leng et al., 2024b). This patchiness has been noted in Arctic macrobenthos, where sites with locally favorable food or temperature conditions sustain a much higher biomass (“hotspots”) than surrounding areas (Link et al., 2013). Under the warming scenario in the present study, the magnified contrast between hotspots and coldspots implies that macrobenthic productivity and ecosystem functions may become concentrated in fewer areas, potentially affecting nutrient cycling and food web dynamics in a spatially uneven manner. The convergence of the model results with the existing literature increases confidence in the projections, while highlighting key uncertainties.
4.5 Limitations and future directions
This study provides valuable insights into the spatiotemporal dynamics of macrobenthic communities near the HYNPP. However, several limitations must be acknowledged to contextualize the findings and guide future research. First, all sampling was conducted within a single year (2016–2017), which limited the ability of this work to capture interannual variability in macrobenthic communities and environmental conditions. Many marine ecosystems exhibit significant year-to-year fluctuations due to climatic oscillations, hydrodynamic changes, and anthropogenic influences. As such, while the seasonal comparisons in the present study provide a robust snapshot, longer-term monitoring is essential to determine the stability and generalizability of the observed patterns.
Second, although RF modeling is effective for handling complex, nonlinear ecological data, this technique has inherent limitations. RF models are prone to overfitting, particularly when applied to datasets with many predictors and limited temporal coverage, such as the dataset in the current study. While RF excels at matching existing data, its predictions for unobserved regions are often conservative and carry a higher probability of omission errors (Breiman, 2001; Elith and Graham, 2009; Li, 2019). Although cross-validation and performance metrics indicated that the RF model had relatively high predictive power (R² > 0.85 for many seasonal models), some seasonal models—especially the winter total biomass—exhibited lower performance and higher error variance. This highlights the model’s sensitivity to uneven data distribution and extreme values. Moreover, RF models provide variable importance rankings but do not infer causality or directly reveal ecological mechanisms. The spatial predictive models constructed to simulate thermal discharge in the present study (+4 °C) were based on the assumption that other environmental parameters would remain constant, potentially oversimplifying the actual ecological dynamics.
Future studies should incorporate multi-year, continuous time series data to better evaluate long-term ecological trends and variability. Additionally, integrating mechanistic or process-based models (e.g., structural equation modeling or coupled physical–biological models) may help disentangle causal relationships among environmental drivers and macrobenthic dynamics. The validation of predictive models using independent post-operation data from the HYNPP would further enhance their applicability in ecological management and impact assessment.
5 Conclusion
This study provides a baseline assessment of macrobenthic community dynamics and their relationships with environmental factors in the coastal waters near the HYNPP before its operation. The findings suggest that temperature, DO, and salinity are the principal abiotic factors influencing macrobenthic diversity in the study area, while nutrient levels exert indirect effects through phytoplankton dynamics. Seasonal variation in community composition was observed, with species richness being highest in winter and lowest in summer, and the dominant taxa shifting across seasons. Based on the results and the anticipated influence of thermal discharge, future changes in bottom water temperature and DO concentrations are likely to reshape macrobenthic communities near the HYNPP, potentially leading to declines in cold-adapted species and favoring tolerant, opportunistic taxa. Given the sensitivity of macrobenthic organisms to such fluctuations, a targeted long-term monitoring strategy should be established. This should include seasonal surveys of temperature, DO, and salinity, alongside biological metrics such as biomass, diversity indices, and species turnover rates. Particular attention should be paid to indicator species such as P. bidentata, whose abundance may reflect broader ecological responses. The insights gained through RF modeling further support the design of adaptive management strategies, helping predict community shifts and providing early warning signals for ecological risks around coastal nuclear infrastructure.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The study involved macrobenthic invertebrates, which are not classified as vertebrate or higher-order animals requiring ethical approval under standard research guidelines. The samples were preserved and analyzed solely for ecological assessment purposes, with no procedures involving pain, harm, or experimental manipulation. Therefore, ethical approval was not required.
Author contributions
YX: Formal analysis, Software, Visualization, Investigation, Writing – review & editing, Validation, Methodology, Data curation, Writing – original draft. XY: Validation, Resources, Visualization, Data curation, Methodology, Investigation, Writing – review & editing. XL: Writing – original draft, Methodology, Investigation, Visualization, Writing – review & editing, Formal analysis, Data curation. MX: Visualization, Formal analysis, Data curation, Writing – review & editing, Methodology. DW: Investigation, Data curation, Visualization, Writing – review & editing, Methodology. QL: Writing – review & editing, Methodology, Formal analysis, Visualization, Writing – original draft, Data curation. WC: Writing – review & editing, Investigation, Methodology, Formal analysis, Data curation. HW: Investigation, Writing – review & editing, Methodology, Formal analysis, Visualization, Data curation.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by the Natural Science Foundation of Shandong Province (Grant Nos. ZR2022MD009), the R & D Project of Marine Science Research Institute of Shandong Province (2024SDMSRI0401) and the Qingdao Natural Science Foundation (23-2-1-47- zzyd-jch).
Acknowledgments
We thank LetPub (www.letpub.com.cn) for its linguistic assistance during the preparation of this manuscript.
Conflict of interest
Author DW was employed by Fuzhou Dongxin Mining Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Editing and language polishing support. Specifically, generative AI tools (such as ChatGPT) were used to refine the clarity, structure, and grammar of certain sections (e.g., the abstract, figure captions, and responses to reviewer comments). All scientific content, analysis, interpretation, and conclusions were generated and verified independently by the authors.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1637335/full#supplementary-material
References
APHA, (American Public Health Association), AWWA, (American Water Works Association), WEF, and (Water Environment Federation) (2017). Standard methods for the examination of water and wastewater. 23rd (Washington, DC: American Public Health Association).
Bharathi, M. D., Venkataramana, V., and Sarma, V. V. S. S. M. D., Venkataramana V., and Sarma V. V. S. S. (2022). Phytoplankton community structure is governed by salinity gradient and nutrient composition in the tropical estuarine system. Continental Shelf Research 234, 104643. doi: 10.1016/j.csr.2021.104643
Bianchi T. S., Brown C. J., Snelgrove P. V. R., and Stanley R. R. E. (2023). Benthic invertebrates on the move: A tale of ocean warming and sediment carbon storage. Limnology Oceanography Bull. 32, 13–20. doi: 10.1002/lob.10544
Bohnsack J. A. and Sutherland D. L. (1985). Artificial reef research: a review with recommendations for future priorities. Bull. Mar. Sci 37, 11–39.
Borja Á., Franco J., and Pérez V. (2000). A marine biotic index to establish the ecological quality of soft-bottom benthic communities in estuarine and coastal environments. Mar. pollut. Bull. 40, 1100–1114. doi: 10.1016/S0025-326X(00)00061-8
Breiman L. (2001). Random forests, machine learning 45. J. Clin. Microbiol. 2, 199–228. doi: 10.1023/A:1010933404324
Breitburg D., Levin L. A., Oschlies A., Grégoire M., Chavez F. P., Conley D. J., et al. (2018). Declining oxygen in the global ocean and coastal waters. Science 359, eaam7240. doi: 10.1126/science.aam7240
Bremner J., Rogers S. I., and Frid C. L. J. (2006). Methods for describing ecological functioning of marine benthic assemblages using biological traits analysis (BTA). Ecol. Indic. 6, 609–622. doi: 10.1016/j.ecolind.2005.08.026
Brockington S. and Clarke A. (2001). The relative influence of temperature and food on the metabolism of a marine invertebrate. J. Exp. Mar. Biol. Ecology. 258, 87–99. doi: 10.1016/S0022-0981(00)00347-6
Cecchetto M., Dettai A., Gallut C., Obst M., Kuklinski P., Balazy P., et al. (2024). Seasonality of primary production explains the richness of pioneering benthic communities. Nat. Commun. 15, 8340. doi: 10.1038/s41467-024-52673-z
Checon H. H., Corte G. N., Silva C. F., Bícego M. C., and Amaral A. C. Z. (2021). Using the Capitella complex to investigate the effects of sympatric cryptic species distinction on ecological and monitoring studies in coastal areas. Mar. Biodiversity. 51, 48. doi: 10.1007/s12526-021-01185-w
Chen Y. L. (1992). Summer phytoplankton community structure in the Kuroshio current-related upwelling northeast of Taiwan. Terrestrial Atmospheric Oceanic Sci. 3, 305–319. doi: 10.3319/TAO.1992.3.3.305(KEEP)
Coleman N., Cuff W., Moverley J., Anne S., and Gason H. (2007). Depth, sediment type, biogeography and high species richness in shallow-water benthos. Mar. Freshw. Res. 58, 293–305. doi: 10.1071/MF06098
Colombelli A., Pulcinella J., Bonanomi S., Notti E., Moro F., and Sala A. (2022). Fishing the waves: Comparing GAMs and random forest to evaluate the effect of changing marine conditions on the energy performance of vessels. Mediterr. Mar. Sci. 23, 935–951. doi: 10.12681/mms.29721
Danovaro R., Gambi C., Dell’Anno A., and Corinaldesi C. (2008). Exoenzyme activity and organic matter composition in sediments of the Ross sea (Antarctica). Appl. Environ. Microbiol. 74, 1164–1172. doi: 10.1016/j.cub.2007.11.056
Dauer D. M. (1993). Biological criteria, environmental health and estuarine macrobenthic community structure. Mar. pollut. Bull. 26, 249–257. doi: 10.1016/0025-326X(93)90063-P
Diaz R. J. and Rosenberg R. (2008). Spreading dead zones and consequences for marine ecosystems. Science 321, 926–929. doi: 10.1126/science.1156401
Dory F., Nava V., Spreafico M., Orlandi V., Soler V., and Leoni B. (2024). Interaction between temperature and nutrients: How does the phytoplankton community cope with climate change? Sci Total Environment. 906, 167566. doi: 10.1016/j.scitotenv.2023.167566
Du M. M., Liu Z. S., Wang C. S., Zhang D. S., and Zhang Q. (2013). The seasonal variation and community structure of zooplankton in China Sea. Acta Ecologica Sinica. 33, 5407–5418. doi: 10.5846/stxb201206080828
Elith J. and Graham C. H. (2009). Do they? How do they? Why do they differ? on finding reasons for differing performances of species distribution models. Ecography. 32, 66–77. doi: 10.1111/j.1600-0587.2008.05505.x
Fu X., Shi W., Liu Z., Wang X., Zhang G., and Sun J. (2024). Impact of environmental variables on the distribution of phytoplankton communities in the Southern Yellow Sea. Environ. Res. 243, 117862. doi: 10.1016/j.envres.2023.117862
Fu X. T., Sun J., Wei Y. Q., Liu Z. S., Xin Y. H., Guo Y., et al. (2021). Seasonal shift of a phytoplankton (>5 µm) community in Bohai Sea and the adjacent Yellow Sea. Diversity. 13, 65. doi: 10.3390/d13020065
Galloway J. N., Townsend A. R., Erisman J. W., Bekunda M., Cai Z., Freney J. R., et al. (2008). Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320, 889–892. doi: 10.1126/science.1136674
Ge R., Chen Y., Chen H., Zhang X., Shi J., Li H., et al. (2024a). Effects of Yellow Sea warm current on zooplankton community composition and functional groups in winter. Mar. Environ. Res. 202, 106715. doi: 10.1016/j.marenvres.2024.106715
Ge Y., Zhang R., Zhu Z., Zhao J., Zhu Z., Li Z., et al. (2024b). Distributions of nutrients, trace metals, phytoplankton composition, and elemental consumption in the Ross and Amundsen Seas. Mar. Chem. 265-266. doi: 10.1016/j.marchem.2024.104436
Geng B., Lu L., Cao Q., Zhou W., Li S., Wen D., et al. (2023). Three-dimensional numerical study of cooling water discharge of Daya Bay Nuclear Power Plant in southern coast of China during summer. Front. Mar. Sci. 9, 1012260. doi: 10.3389/fmars.2022.1012260
Graf G. (1989). Benthic–pelagic coupling in a deep-sea benthic community. Nature 341, 437–439. doi: 10.1038/341437a0
Graf G. (1992). Benthic-Pelagic Coupling: A benthic view. Oceanography Mar. Biology: Annu. Rev. 30, 149–190.
Gray J. S. (2002). Species richness of marine soft sediments. Mar. Ecol. Prog. Ser. 244, 285–297. doi: 10.3354/meps244285
Grebmeier J. M., Bluhm B. A., Cooper L. W., Danielson S. L., Arrigo K. R., Blanchard A. L., et al. (2015). Ecosystem characteristics and processes facilitating persistent macrobenthic biomass hotspots and associated benthivory in the Pacific Arctic. Progress in Oceanography 136, 92–114. doi: 10.1016/j.pocean.2015.05.006
Grebmeier J. M., Cooper L. W., Feder H. M., and Sirenko B. I. (2006). Ecosystem dynamics of the pacific-influenced northern Bering and Chukchi Seas in the spring and summer. Prog. Oceanography 71, 331–361. doi: 10.1016/j.pocean.2006.10.001
Griffiths J. R., Kadin M., Nascimento F. J. A., Tamelander T., Tornroos A., Bonaglia S., et al. (2017). The importance of benthic-pelagic coupling for marine ecosystem functioning in a changing world. Global Change Biol. 23, 2179–2196. doi: 10.1111/gcb.13642
Guimarães L. S. F., Carvalho-Junior L., Façanha G. L., Resende N. S., Neves L. M., and Cardoso S. J. (2023). Meta-analysis of the thermal pollution caused by coastal nuclear power plants and its effects on marine biodiversity. Mar. pollut. Bulletin. 195, 115452. doi: 10.1016/j.marpolbul.2023.115452
Guo J., Wang Y., Lai J., Pan C., Wang S., Fu H., et al. (2020). Spatiotemporal distribution of nitrogen biogeochemical processes in the coastal regions of northern Beibu Gulf, South China Sea. Chemosphere. 239, 124803. doi: 10.1016/j.chemosphere.2019.124803
Han W. and Han Q. (2024). Macrobenthic indicator species: From concept to practical applications in marine ecology. Global Ecol. Conserv. 55, e03262. doi: 10.1016/j.gecco.2024.e03262
Hiddink J. G., Burrows M. T., and Garcia Molinos J. (2015). Temperature tracking by North Sea benthic invertebrates in response to climate change. Global Change Biol. 21, 117–129. doi: 10.1111/gcb.12726
Hu L. S., Liu J. H., Yang Z., Zhang Y., Gao J., Wu Z., et al. (2024). Water-sediment regulation affects community structures of macrobenthic mollusks in the Yellow River estuary and adjacent waters. Regional Studies in Marine Science 73, 103282. doi: 10.1016/j.rsma.2024.103282
Hung T. C., Huang C. C., and Shao K. T. (1998). Ecological survey of coastal water adjacent to nuclear power plants in Taiwan. Chem. Ecology. 15, 129–142. doi: 10.1080/02757549808037625
Ji Y., Wang C., Zhang C., Gao S., Li H., Zhang L., et al. (2024). Distribution and influencing factors of macrobenthos on three seagrass beds in the intertidal zone of Shandong Province, China. Front. Mar. Sci. 11, 1349131. doi: 10.3389/fmars.2024.1349131
Jiang S., Fan W., Chen L., Chen J., and Li B. (2023). Spatio-temporal distribution of macrobenthos and benthic ecological health status in the Bohai Sea and the northern Yellow Sea, China. Mar. pollut. Bulletin. 196, 115671. doi: 10.1016/j.marpolbul.2023.115671
Jokiel P. L. and Coles S. L. (1974). Effects of heated effluent on hermatypic corals at Kahe point, Oahu. Pacific Sci 28, 1–18.
Josefson A. B. and Conley D. J. (1997). Benthic response to a pelagic front. Mar. Ecol. Prog. Ser. 147, 49–62. doi: 10.3354/meps147049
Kim J.-M. (2025). Integrating copula-based random forest and deep learning approaches for analyzing heterogeneous treatment effects in survival analysis. Mathematics 13, 1659. doi: 10.3390/math13101659
Kim S. L. and Yu O. H. (2022). Benthic polychaete community structure in the Yellow Sea Bottom Cold Water zone: Species diversity, temporal-spatial distribution, and feeding guilds. Mar. pollut. Bulletin. 183, 114071. doi: 10.1016/j.marpolbul.2022.114071
Kirby R. R., Beaugrand G., Lindley J. A., Richardson A. J., Edwards M., and Reid P. C. (2007). Climate effects and benthic-pelagic coupling in the North Sea. Mar. Ecol. Prog. Series. 330, 31–38. doi: 10.3354/meps330031
Kosicki J. Z. (2020). Generalised additive models and random forest approach as effective methods for predictive species density and functional species richness. Environ. Ecol. Statistics. 27, 273–292. doi: 10.1007/s10651-020-00445-5
Krishnakumar V., Sastry J. S., and Swamy G. N. (1991). Implication of thermal discharges into the sea: a review. Indian J. Environ. Protection. 11, 525–527.
Lai J., Jiang F., Ke K., Xu M., Lei F., and Chen B. (2014). Nutrients distribution and trophic status assessment in the northern Beibu Gulf, China. Chin. J. Oceanology Limnology. 32, 1128–1144. doi: 10.1007/s00343-014-3199-y
Langford T. E. L. (1990). Ecological effects of thermal discharges (London: Elsevier Applied Science), 28–103.
Leng Q., Mohamat-Yusuff F., Mohamed K. N., Zainordin N. S., and Hassan M. Z. (2024a). Impacts of thermal and cold discharge from power plants on marine benthos and its mitigation measures: a systematic review. Front. Mar. Sci 11, 1465289. doi: 10.3389/fmars.2024.1465289
Leng Q., Mohamat F., Talib A., Kumar R. R., and Yoshida T. (2024b). Impacts of thermal and cold discharge from power plants on marine benthos and its mitigation measures: a systematic review. Front. Mar. Sci 11, 1465289. doi: 10.3389/fmars.2024.1465289
Li X. (2019). Random forest is a specific algorithm, not omnipotent for all datasets. Chinese. J. Appl. Entomology. 56, 170–179. doi: 10.7679/j.issn.2095-1353.2019.022
Li S. W., Li F., Song X. K., and Zhang M. L. (2020). The influence of water-sediment regulation on macrobenthic community structures in the huanghe River (Yellow river) estuary during 2012–2016. Acta Oceanologica Sinica. 39, 120–128. doi: 10.1007/s13131-020-1664-3
Li Z., Li X., Sun L., Liu Z., Gu Y., Zhai F., et al. (2019). Analysis of dissolved oxygen concentration in bottom seawater of dagu estuary periodical of ocean university of China. Periodical Ocean Univ. China. 49, 23–33.
Li F., Ma Y. Q., Song X. K., Li S. W., Zhang X. M., Wang X. X., et al. (2022a). Community structure and ecological quality assessment of macrobenthos in the coastal sea areas of Northern Yantai, China. Front. Mar. Sci. 9, 989034. doi: 10.3389/fmars.2022.989034
Li D. Y., Wen Y. J., Zhao G. D., Zhang G. C., Sun J., and Xu W. Z. (2022b). Effects of terrestrial inputs on mesozooplankton community structure in Bohai Bay, China. Diversity. 14, 410. doi: 10.3390/d14050410
Li P., Xue R., Wang Y., Zhang R., and Zhang G. (2015). Influence of anthropogenic activities on PAHs in sediments in a significant gulf of low-latitude developing regions, the Beibu Gulf, South China Sea: Distribution, sources, inventory and probability risk. Mar. pollut. Bulletin. 90, 218–226. doi: 10.1016/j.marpolbul.2014.10.048
Liao Y. (2004). “Fauna sinica: invertebrata,” in Ophiuroidea, vol. 40. (Beijing, China: Science Press).
Lin Y. V., Chateau P.-A., Nozawa Y., Wei C.-L., Wunderlich R. F., and Denis V. (2024). Drivers of coastal benthic communities in a complex environmental setting. Mar. Pollut. Bull. 203, 116462. doi: 10.1016/j.marpolbul.2024.116462
Lin J., Zou X., and Huang F. (2018). Effects of the thermal discharge from an offshore power plant on plankton and macrobenthic communities in subtropical China. Mar. pollut. Bulletin. 131, 106–114. doi: 10.1016/j.marpolbul.2018.04.005
Link H., Archambault P., and Piepenburg D. (2013). Are hotspots always hotspots? The relationship between diversity, resource availability and ecosystem function in the Arctic. PloS One 8, e74077. doi: 10.1371/journal.pone.0074077
Liu Q. (2018). Genetic diversity and population genetic structure of two jellyfish species in Chinese coastal waters. Master’s Thesis, University of Chinese Academy of Sciences. Yantai Coastal Zone Research Institute, Chinese Academy of Sciences, Yantai, China.
Loi T. N. and Wilson B. J. (1979). Macroinfaunal structure and effects of thermal discharges in a mesohaline habitat of Chesapeake Bay, near a nuclear power plant. Mar. Biol. 55, 3–16. doi: 10.1007/BF00391711
Long B. and Lewis J. B. (1987). Distribution and community structure of the benthic fauna of the north shore of the gulf of st. Lawrence described by numerical methods of classification and ordination. Mar. Biol. 95, 93–101. doi: 10.1007/BF00447490
Mao C. Z., Zhang Y., Wei A. H., Peng M., Cui C. X., Jiao X. M., et al. (2023). Seasonal variation of macrobenthos community structure in Qinshan Inland Sea Area. Administrative Technol. Environ. Monitoring. 35, 39–44.
McCloskey R. M. and Unsworth R. K. F. (2015). Decreasing seagrass density negatively influences associated fauna. PeerJ 3, e1053. doi: 10.7717/peerj.1053
McQuatters-Gollop A., Raitsos D. E., Edwards M., and Reid P. C. (2007). Spatial patterns of diatom and dinoflagellate seasonal cycles in the NE atlantic ocean and their relation to interannual climate variability. Mar. Ecol. Prog. Ser. 339, 301–306. doi: 10.3354/meps339301
Mermillod-Blondin F. and Rosenberg R. (2006). Ecosystem engineering: The impact of bioturbation on biogeochemical processes in marine and freshwater benthic habitats. Aquat. Sci. 68, 434–442. doi: 10.1007/s00027-006-0858-x
Montagna P. A., Kalke R. D., and Hyde L. J. (2025). “Effect of freshwater inflow on benthic infauna,” in Freshwater inflows to Texas bays and estuaries: A regional-scale review, synthesis, and recommendations. Eds. Montagna P. A. and Douglas A. R. (Cham, Switzerland: Springer), 259–293.
Neumann H., Ehrich S., and Kroncke I. (2008). Effects of cold winters and climate on the temporal variability of an epibenthic community in the German Bight. Climate Res. 37, 241–251. doi: 10.3354/cr00769
Neumann H. and Kroncke I. (2011). The effect of temperature variability on ecological functioning of epifauna in the German Bight. Mar. Ecology. 32, 49–57. doi: 10.1111/j.1439-0485.2010.00420.x
Nie P., Wu H., Xu J., Wei L., Zhu H., and Ni L. (2021). Thermal pollution monitoring of Tianwan nuclear power plant for the past 20 years based on landsat remote sensed data. IEEE J. Selected Topics Appl. Earth Observations Remote Sensing. 14, 6146–6155. doi: 10.1109/JSTARS.2021.3088529
Pane L., Franceschi E., and Nuccio L. D. (2001). Applications of thermal analysis on the marine phytoplankton, Tetraselmis suecica. J. Thermal Anal. Calorimetry. 66, 145–154. doi: 10.1023/A:1012443800271
Park J. M., Kwak S. N., and Riedel R. (2020). Crustacean decapod assemblage associated with seagrass (Zostera marina) beds in southern waters of Korea. Diversity 12, 89. doi: 10.3390/d12030089
Peng S. Y., Li X. Z., Xu Y., Wang H. F., and Zhang B. L. (2017). Variation of macrobenthos in the Yellow Sea in past 10 years. Oceanologia Limnologia Sinica. 48, 537–542. doi: 10.11693/hyhz20160900200
Poloczanska E. S., Brown C. J., Sydeman W. J., Kiessling W., Schoeman D. S., Moore P. J., et al. (2013). Global imprint of climate change on marine life. Nat. Climate Change 3, 919–925. doi: 10.1038/nclimate1958
Poloczanska E. S., Burrows M. T., Brown C. J., Molinos J. G., Halpern B. S., HoeghGuldberg O., et al. (2016). Responses of marine organisms to climate change across oceans. Front. Mar. Sci. 3, 62. doi: 10.3389/fmars.2016.00062
Poornima E. H., Rajadurai M., Rao T. S., Anupkumarb B., Rajamohanb R., Narasimhanb S. V., et al. (2005). Impact of thermal discharge from a tropical coastal power plant on phytoplankton. J. Thermal Biol. 30, 307–316. doi: 10.1016/j.jtherbio.2005.01.004
Qu T., Hou C., Yu Z., Wang Y., and Tang X. (2020). Ecological effects of Ulva prolifera green tide on zoobenthos in the Qingdao intertidal area. Periodical Ocean Univ. China (Natural Sci Edition). 50, 59–69. doi: 10.16441/j.cnki.hdxb.20200086
Quan Q. M., Xiao Y. Y., Xu S. N., Fu F. F., Li C. H., and Liu Y. (2020). Seasonal variation in macrobenthos community structure and its relation to environmental factors in Jiaozhou Bay. Chin. J. Ecology. 39, 4110–4120. doi: 10.13292/j.1000-4890.202012.027
Rabalais N. N., Turner R. E., and Díaz R. J. (2010). Global change and eutrophication of coastal waters. ICES J. Mar. Sci 66, 1528–1537. doi: 10.1093/icesjms/fsp047
Rajapaksha R. P., Wu M.-L., Wang Y.-T., Bandara G., Atapaththu K. S. S., and Wang Y.-S. (2024). Long-term alterations of nutrient dynamics and phytoplankton communities in Daya Bay, South China Sea. Mar. pollut. Bulletin. 208, 116955. doi: 10.1016/j.marpolbul.2024.116955
Rautio M., Dufresne F., Laurion I., Bonilla S., Vincent W. F., and Christoffersen K. S. (2011). Shallow freshwater ecosystems of the circumpolar Arctic. Écoscience. 18, 204–222. doi: 10.2980/18-3-3463
Ruhl H. A. and Smith K. L. (2004). Shifts in deep-sea community structure linked to climate and food supply. Science 305, 513–515. doi: 10.1126/science.1099759
Sanders H. (1958). Benthic studies in buzzards bay. i. animal-sediment relationships. Limnology Oceanography. 3, 245–258. doi: 10.4319/lo.1958.3.3.0245
Seitzinger S., Harrison J. A., Böhlke J. K., Bouwman A. F., Lowrance R., Peterson B., et al. (2006). Denitrification across landscapes and waterscapes: A synthesis. Ecol. Appl. 16, 2064–2090. doi: 10.1890/1051-0761(2006)016[2064:DALAWA]2.0.CO;2
Shandong Nuclear Power Company (2013). Environmental impact report for units 3 and 4 of haiyang nuclear power project (Site selection stage), summary version. (Haiyang, Shandong, China: Shanghai Nuclear Engineering Research & Design Institute).
Shannon C. E. (1948). A mathematical theory of communication. Bell System Tech. J. 27, 379–423. doi: 10.1002/j.1538-7305.1948.tb01338.x
Shen X., Luan J., Jiang D., Chen W., Liu L., and Jia F. (2013). Purification and antitumor effect evaluation of Protankyrabidentata antitumor factor. Chin. J. Mar. Drugs 32, 1–6. doi: 10.13400/j.cnki.cjmd.2013.01.001
Shou L., Liao Y., Tang Y., Chen J., Jiang Z., Gao A., et al. (2018). Seasonal distribution of macrobenthos and its relationship with environmental factors in the Yellow Sea and East China Sea. J. Oceanology Limnology. 36, 772–782. doi: 10.1007/s00343-018-6271-1
Silva C. F., Seixas V. C., Barroso R., Di Domenico M., Amaral A. C. Z., and Paiva P. C. (2017). Demystifying the Capitella capitata complex (Annelida, Capitellidae) diversity by morphological and molecular data along the Brazilian coast. PloS One 12, e0177760. doi: 10.1371/journal.pone.0177760
Sklar F. H. and Browder J. A. (1998). Coastal environmental impacts brought about by alterations to freshwater flow in the Gulf of Mexico. Environ. Manage. 22, 547–562. doi: 10.1007/s002679900127
Smith B. and Wilson J. B. (1996). A consumer’s guide to evenness indices. Oikos. 76, 70–82. doi: 10.2307/3545749
Snelgrove P. V. R. (1999). Getting to the bottom of marine biodiversity: Sedimentary habitats. BioScience 49, 129–138. doi: 10.2307/1313538
Somerfield P. J., Cochrane S. J., Dahle S., and Pearson T. H. (2006). Free-living nematodes and macrobenthos in a high-latitude glacial fjord. J. Exp. Mar. Biol. Ecology. 330, 284–296. doi: 10.1016/j.jembe.2005.12.034
Standardization Administration of China (2007). Specification for oceanographic survey — Part 4: Marine Hydrological Observation (GB 12763.4-2007) (Beijing: Standards Press of China).
Sun Y., Cao L., Wang J., Qin Y., and Cheng X. (2007). Analysis of macrobenthos community structure in the adjacent sea area of Changjiang River estuary. Mar. Sci Bull. (Tianjin). 26, 66–70. doi: 10.1002/cem.1038
Teixeira T. P., Neves L. M., and Araújo F. G. (2009). Effects of a nuclear power plant thermal discharge on habitat complexity and fish community structure. Mar. Environ. Res. 68, 188–195. doi: 10.1016/j.marenvres.2009.06.004
Trannum H. C., Gundersen H. E., Rygg O. B., and Norderhaug K. M. (2018). Soft bottom benthos and responses to climate variation and eutrophication in Skagerrak. J. Sea Res. 141, 83–98. doi: 10.1016/j.seares.2018.08.007
Trannum H. C., Nilsson H. C., Schaanning M. T., and Øxnevad S. (2010). Effects of sedimentation from water-based drill cuttings and natural sediment on benthic macrofaunal community structure and ecosystem processes. J. Exp. Mar. Biol. Ecol. 383, 111–121. doi: 10.1016/j.jembe.2009.12.004
Turner J. T., Tester P. A., and Hansen P. J. (1998). Interactions between toxic marine phytoplankton and zooplankton grazers. Mar. Ecol. Prog. Ser. 169, 103–120.
Wang J. D., Lei Y. L., Xu K. D., and Du Y. F. (2011). An investigation on the biomass, abundance and distribution of meiofauna under the cold water mass and its surrounding areas of the Yellow Sea. Oceanologia Limnologia Sinica. 42, 360–366. doi: 10.11693/hyhz201103004004
Warwick R. M. (1993). Environmental impact studies on marine communities: Pragmatical considerations. Aust. J. Ecol. 18, 63–80. doi: 10.1111/j.1442-9993.1993.tb00435.x
Wei C.-L., Rowe G. T., Escobar-Briones E., Boetius A., Soltwedel T., Caley M. J., et al. (2010). Global patterns and predictions of seafloor biomass using random forests. PloS One 5, e15323. doi: 10.1371/journal.pone.0015323
Wu J., Song G., Song L., Wang B., Zhang Y., Yin M., et al. (2021). Investigation and analysis on death of marine life in local area of Dalian Shallow Sea in spring 2020. Fisheries Sci. 40, 537–543. doi: 10.16378/j.cnki.1003-1111.20041
Xie G., Sun C., Wang Y., Ma H., Li W., and Zhao L. (2024). Biodiversity and distribution of zoobenthos in the ecological water-replenishment area of the Yellow River Estuary. PLOS ONE 19 (12), e0315346. doi: 10.1371/journal.pone.0315346
Xu S., Liu Y., Fan J., Xiao Y., Qi Z., and Lakshmikandan M. (2022b). Impact of salinity variation and silicate distribution on phytoplankton community composition in Pearl River estuary, China. Ecohydrology Hydrobiology. 22, 466–475. doi: 10.1016/j.ecohyd.2022.01.004
Xu Y., Ma L., Sui J., Li X., Wang H., and Zhang B. (2023). Potential impacts of climate change on the distribution of echinoderms in the Yellow Sea and East China Sea. Mar. pollut. Bull. 194, 115246. doi: 10.1016/j.marpolbul.2023.115246
Xu S., Xiao Y., Xu Y., Su L., Cai Y., Qi Z., et al. (2024). Effects of seasonal variations and environmental factors on phytoplankton community structure and abundance in Beibu Gulf, China. Ocean Coast. Management. 248, 106982. doi: 10.1016/j.ocecoaman.2023.106982
Xu M., Zhang C., Xue Y., Xu B., Ji Y., and Ren Y. (2022a). Relationship between species diversity and environmental factors in the fishery community of Shandong coastal waters. J. Fisheries China. 46, 1008–1017. doi: 10.11964/jfc.20201012468
Yan J., Xu Y., Sui J. X., Li X. Z., Wang H. F., and Zhang B. L. (2017). Long-term variation of the macrobenthic community and its relationship with environmental factors in the Yangtze River estuary and its adjacent area. Mar. pollut. Bulletin. 123, 339–348. doi: 10.1016/j.marpolbul.2017.09.023
Yang Y., Yi Y., Zhou Y., Wang X., Zhang S., and Yang Z. (2020). Spatio-temporal variations of benthic macroinvertebrates and driving environmental variables in a shallow lake. Ecol. Indic. 110, 105948. doi: 10.1016/j.ecolind.2019.105948
Yanygina L. V. (2015). Spatial distribution of Gmelinoides fasciatus in thermally polluted water (Belovo Reservoir, Southwest Siberia). Int. J. Environ. Res. 9, 877–884.
Yool A., Martin A. P., Anderson T. R., Bett B. J., Jones D. O. B., and Ruhl H. A. (2017). Big in the benthos: Future change of seafloor community biomass in a global, body size-resolved model. Global Change Biol. 23, 3554–3566. doi: 10.1111/gcb.13680
Yu O. H., Lee H. G., Lee J. H., Kim K. T., Myung C. S., Moon H. T., et al. (2013). Spatial variation in macrobenthic communities affected by the thermal discharge volumes of a nuclear power plant on the east coast of korea. Ocean Polar Res. 35, 299–312. doi: 10.4217/OPR.2013.35.4.299
Zeng J. N. (2008). Ecological effect by thermal discharged water from subtropical coastal power plants (Hangzhou: Dissertation for the doctoral degree. Zhejiang University).
Zhang F., Li C., Sun S., Wei H., and Wang Y. (2017). Progress on studying jellyfish bloom, and the monitoring and control. Oceanol. Limnol. Sin. 48, 1187–1195. doi: 10.11693/hyhz20171000258
Keywords: macrobenthos, seasonal variability, Copula-Based Random Forest, Yellow Sea, nuclear power plant, thermal discharge
Citation: Xia Y, Yin X, Liu X, Xu M, Weng D, Liu Q, Chi W and Wu H (2025) One-year assessment and predictive modeling of macrobenthic communities under thermal discharge and environmental influences near the preoperational YanTai HY-Nuclear Power Plant in the Yellow Sea. Front. Mar. Sci. 12:1637335. doi: 10.3389/fmars.2025.1637335
Received: 29 May 2025; Accepted: 08 September 2025;
Published: 30 September 2025.
Edited by:
Stelios Katsanevakis, University of the Aegean, GreeceReviewed by:
Nosad Sahu, Centre for Marine Living Resources and Ecology (CMLRE), IndiaQingxi Han, Ningbo University, China
Copyright © 2025 Xia, Yin, Liu, Xu, Weng, Liu, Chi and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qian Liu, cmF1bGxpdXFpYW5AMTYzLmNvbQ==; Wendan Chi, dmFlZGFuQDE2My5jb20=
†These authors have contributed equally to this work
 Yu Xia
Yu Xia Xiaofei Yin2†
Xiaofei Yin2† Xiaohui Liu
Xiaohui Liu Qian Liu
Qian Liu