- 1College of Economics and Management, Tianjin Agricultural University, Tianjin, China
- 2Tianjin Fisheries Research Institute, Tianjin, China
- 3Key Laboratory of Aquatic Ecology and Aquaculture, College of Fisheries, Tianjin Agricultural University, Tianjin, China
Introduction: As an ecologically and economically significant bivalve species, the Ruditapes philippinarum plays a vital role in carbon sequestration and marine ecosystem restoration. However, its ecological carrying capacity and carbon sequestration potential remain relatively underexplored.In this study, we further incorporated carbon budget estimation, Mixed Trophic Impact (MTI) analysis, and pedigree analysis to provide a comprehensive system-wide evaluation.
Methods: The Dashentang Marine Ranch in Tianjin, China, covering 23.6 km² (13.6 km² artificial reefs), served as the study area. We constructed an EwE food-web model based on two seasonal surveys (May and August 2023), including 20 functional groups such as Oratosquilla oratoria, Dorippe japonica, Sebastes schlegelii, Lateolabrax japonicus, Apostichopus japonicus, and Ruditapes philippinarum. Biomass, production/consumption parameters, and diet compositions were used to simulate ECC and carbon dynamics. The carbon budget of R. philippinarum was quantified, MTI analysis was conducted to assess its trophic interactions, and a pedigree index was applied to evaluate model reliability.
Results and Discussion: The EwE model estimated the ECC of R. philippinarum at 208.1 t/km², far above its current biomass of 6.3 t/km², indicating substantial aquaculture potential. Carbon sequestration assessment showed that at ECC, R. philippinarum contributes 21, 500 tonnes of carbon uptake, 10, 300 tonnes of benthic deposition, 280, 000 tonnes of harvestable biomass, and 870, 000 tonnes of carbon release via respiration.MTI analysis revealed that increasing R. philippinarum biomass exerts strong positive impacts on demersal fish (e.g., Lateolabrax japonicus) and benthic habitat quality, but negative impacts on benthic predators (e.g., Sebastes schlegelii, Decapoda), reflecting trade-offs in trophic competition.Pedigree analysis yielded an index of 0.716, suggesting a moderate-to-high level of model reliability, supporting confidence in the results.Overall, ecological network analysis demonstrated that higher clam biomass enhances ecosystem maturity and stability while providing significant carbon sink and economic benefits.
Conclusion: Therefore, this study suggests that increasing the cultivation of Ruditapes philippinarum within its ecological carrying capacity can enhance the carbon sequestration potential of marine ranch ecosystems.The integration of carbon budget estimation, MTI analysis, and pedigree validation highlights both the ecological trade-offs and reliability of these findings, providing robust evidence that effective management of R. philippinarum can simultaneously improve carbon sink function and economic efficiency in marine ranches.
1 Introduction
The ocean, covering over 70% of the Earth’s surface, is a cornerstone of global economic development. It plays a crucial role in providing ecosystem goods and services. Not only does the ocean sustain life on a global scale, but it also supports the well-being of billions of people (Gaines et al., 2023; Teh and Sumaila, 2013; Food and Agriculture Organization of the United Nations (FAO), 2018; Hoegh-Guldberg et al., 2019; Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES), 2019). Given the vast resources and opportunities the ocean holds, it is essential to adopt a coordinated and sustainable approach to its utilization in order to minimize risks, promote social equity, ensure human well-being, and preserve environmental health (Sumaila et al., 2023). Marine ecosystems harbor some of the most diverse forms of life on Earth, encompassing a wide range of biological communities from microorganisms, plankton, and algae to fish, marine mammals, and large apex predators. These organisms sustain complex food web structures and regulate global ecological balance through nutrient cycling, carbon sequestration, and energy transfer (Costello and Chaudhary, 2017; Worm et al., 2006). For instance, phytoplankton contribute approximately half of the world’s oxygen production through photosynthesis, while critical habitats such as coral reefs and seagrass meadows provide essential spawning and nursery grounds for numerous fish and invertebrate species (Moberg and Folke, 1999).
Marine biological resources have been a cornerstone of human culture for thousands of years, providing not only an essential source of protein but also forming the foundation of maritime commerce and trade (Fogarty, 2002). Marine ecosystems and their biodiversity sustain life on Earth and possess intrinsic value. Their key ecosystem services include maintaining global oxygen and carbon cycles, supporting food and energy production, and contributing to overall human well-being. However, due to the unsustainable exploitation of marine resources and the accelerating impacts of climate change, marine ecosystems are undergoing rapid degradation. R. philippinarum, a filter-feeding bivalve of marine origin, plays a pivotal role in maintaining regional ecological carrying capacity by improving water quality, regulating nutrient cycling, and sustaining benthic ecosystem stability (Bartoli et al., 2001; Nizzoli et al., 2001). Meanwhile, through the incorporation of organic carbon into soft tissues and the deposition of inorganic carbon as calcium carbonate in shells during growth, this species exhibits considerable carbon sequestration potential, thereby serving as an important contributor to marine carbon sinks (Filgueira et al., 2022; Zhang et al., 2024). Importantly, the role of bivalve aquaculture as a potential carbon sink remains debated internationally, given concerns about CO2 release during calcium carbonate precipitation and uncertainties regarding the ultimate fate of shells. R. philippinarum is considered a highly sustainable aquaculture species, as it feeds naturally on phytoplankton without requiring external feed inputs (Dumbauld et al., 2009). Its stocking can enhance water quality, stabilize sediment, and promote nutrient cycling through filter-feeding, while also improving benthic biodiversity and overall biological productivity and ecosystem stability in marine ranching systems. However, excessive stocking density may introduce ecological risks. On one hand, the breakdown of species barriers increases the likelihood of cross-species disease transmission (Zhang et al, 2019); on the other hand, large-scale feeding and dredging activities may lead to bioturbation, disturbing sediment stratification and altering organic matter decomposition and microbial community composition (Wang et al., 2022). To mitigate the biomass decline of other functional groups and to enhance primary productivity, the controlled proliferation of macroalgae as an auxiliary food source may be a viable strategy (Li et al., 2023).
Marine ranching is designed to enable the sustainable utilization of fishery resources as an artificial model of fishery development. Consequently, ensuring the sustainable yield of target species within marine ranches has remained a central concern among stakeholders (Wang and Zhang, 2021). At the same time, marine ranch products carry both economic and ecological value, and their environmentally friendly attributes meet consumer demand for sustainable and eco-friendly goods (Wan et al., 2023). Over the past four decades, China has invested substantial resources in developing marine ranches as a strategy to restore wild fisheries and increase seafood production. However, the long-term effectiveness of this approach remains contested, and its future growth and optimization are subjects of ongoing debate (Liu et al., 2022). Against this backdrop, ecosystem-based management approaches are increasingly seen as essential for maintaining the ecological health of marine ranches and achieving maximum sustainable fisheries production (Lee and Zhang, 2018).
Carrying capacity is generally defined as the maximum population size of a species that an environment can sustain over the long term under the constraints of food, habitat, water, and other resources (Odum, 1983). In fisheries, carrying capacity refers to the maximum biomass of a given population that an ecosystem can maintain without compromising its ability to support that population in the future (Food and Agriculture Organization of the United Nations (FAO), 1999). More specifically, ecological carrying capacity (ECC) refers to the maximum level of aquaculture or harvesting that a system can sustain without causing significant alterations to ecological processes, community structure, or ecosystem stability (McKindsey et al., 2006). Accordingly, ECC reflects the threshold at which the balance between human use and ecological integrity is maintained. Carbon sequestration, as a key ecosystem service, is closely linked to the carrying thresholds of ECC. Incorporating carbon sequestration potential into the ECC assessment framework can therefore provide a more comprehensive understanding of the ecological functions and sustainability of marine ranching. However, existing studies have largely focused on either ECC or carbon sequestration in isolation, while integrated frameworks that simultaneously evaluate both remain scarce, despite their importance for systematically assessing the sustainability and multifunctionality of marine ranching systems.
The EwE model, as a mature ecosystem modeling tool, provides quantitative ecological structure analysis and dynamic simulation capabilities, widely applied in various ecosystem studies (Christensen et al., 2005). Specifically, the EwE model integrates key ecological indicators such as phytoplankton ecological efficiency (EE), offering scientific basis for assessing the ecological carrying capacity of marine ranches (Polovina, 1984). Moreover, the modular structure of the EwE model (including Ecopath, Ecosim, and Ecospace) endows it with high flexibility and adaptability in spatiotemporal analyses, suitable for dynamic analysis of complex ecosystems (Walters et al., 1997). This study employed the Ecopath with Ecosim (EwE) modeling framework to systematically evaluate the ecosystem structure and functional status of the Dashiangtang National Marine Ranch in Bohai Bay, Tianjin, China. Specifically, the analysis focused on the current status of R. philippinarum, its ecological carrying capacity (ECC), and the spatiotemporal dynamics under scenarios exceeding 120% of the ECC. In this study, ECC is defined as the biomass of R. philippinarum when the ecological nutritional efficiency (EE) of phytoplankton is equal to 1. The results revealed key characteristics of ecosystem functioning, trophic utilization efficiency, and system productivity, thereby providing critical support for integrated ecosystem assessment and science-based management. Building on this, the study further incorporated carbon sequestration potential into the evaluation of ECC, establishing a unified framework that offers new perspectives and theoretical foundations for advancing the understanding of sustainability and multifunctionality in marine ranching systems.
2 Materials and methods
2.1 Study area and period
Tianjin Dashentang Marine Ranching is one of the first national-level marine ranch demonstration zones. Located in the northwestern part of Bohai Bay, it covers an area of approximately 23.6 square kilometers, with 13.6 square kilometers designated as artificial reefs areas. The study was conducted in May and August 2023 within the Dashentang National Marine Ranching Demonstration Area, Tianjin. Specific coordinates of the study area are provided in Figure 1. Fifteen sampling stations were established within the artificial reefs zone of the marine ranch, and three additional stations were designated as controls on the periphery of the reef area. The spatial distribution of these stations is illustrated in Figure 2. The region has a water depth ranging from 3 to 6 meters, an average flood tide current velocity of 0.221 m/s, an average ebb tide current velocity of 0.166 m/s, and stable tidal directions (280° during flood tide and 82° during ebb tide), providing favorable hydrodynamic conditions for the development of marine ranches.
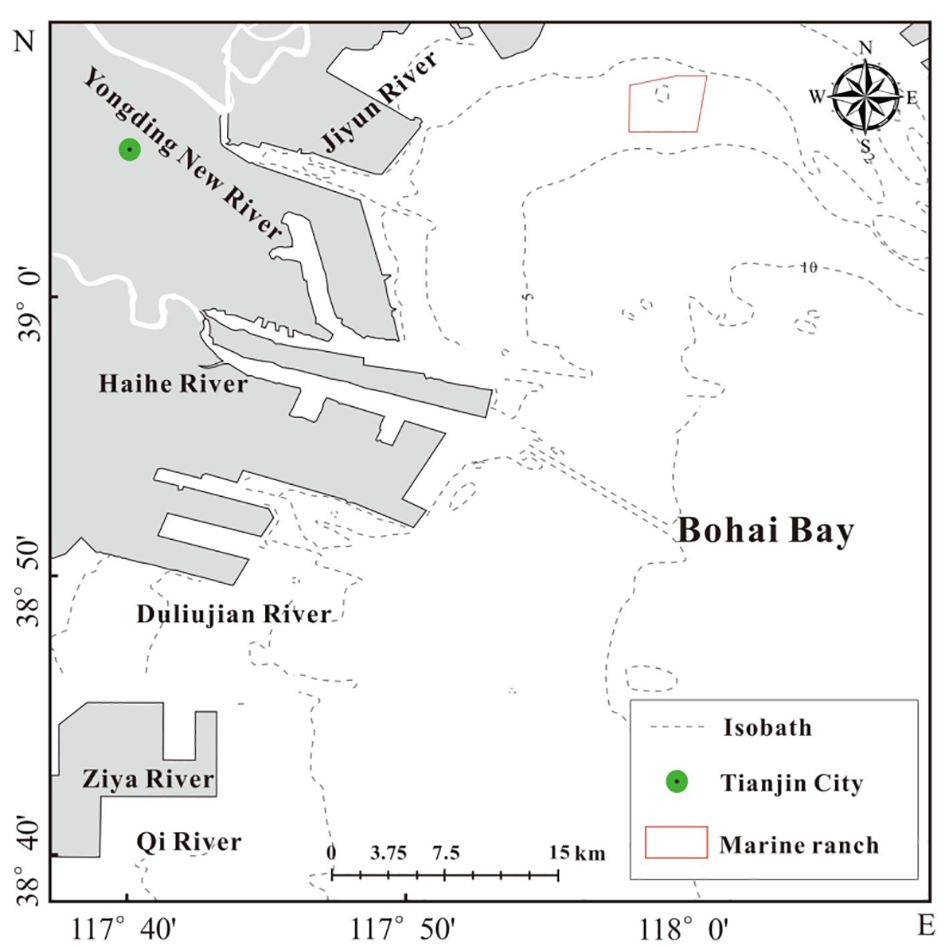
Figure 1. Schematic diagram of the national-level marine pasture demonstration area in the Dashentang Marine Ranch in Tianjin Bohai Bay, China.
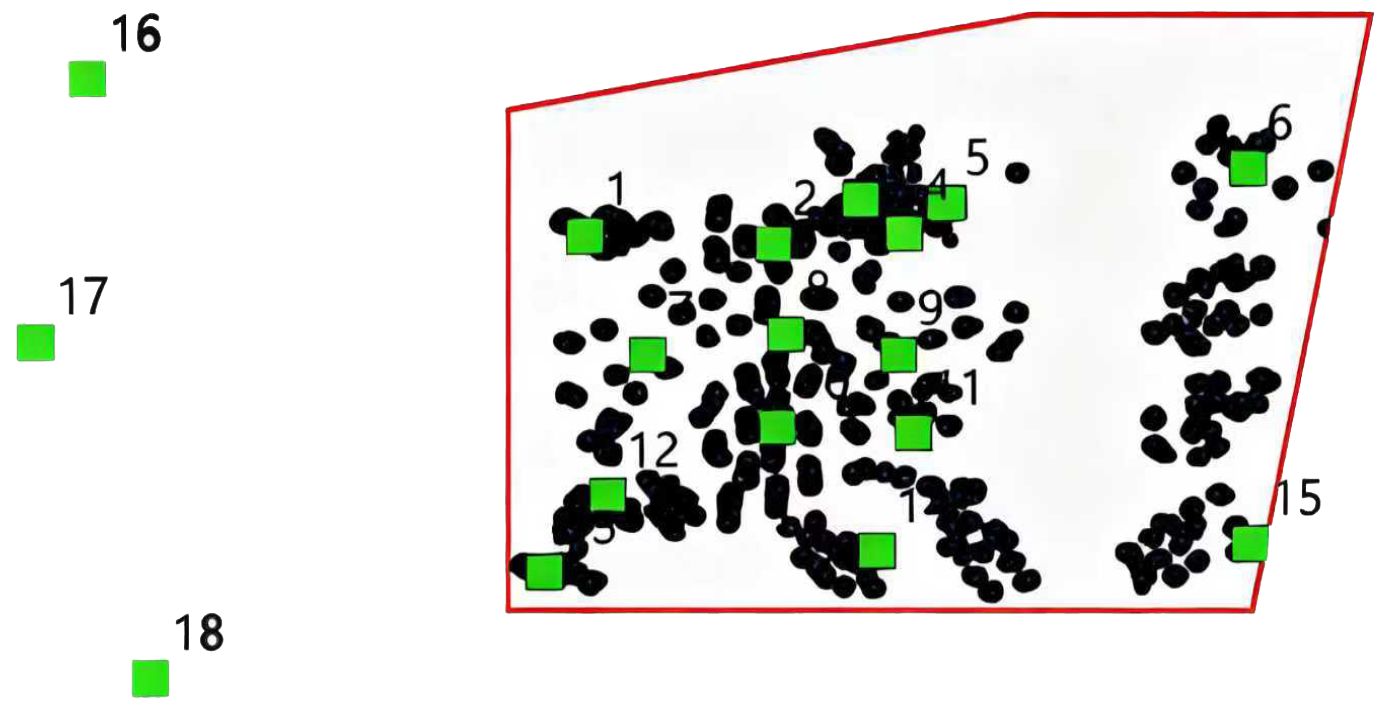
Figure 2. Schematic diagram of routine monitoring sampling stations in the Dashentang Marine Ranch in Tianjin.
2.2 Ecopath ecological modeling
2.2.1 Model description
Ecopath with Ecosim (EwE) is a widely applied modeling framework for evaluating the structure, function, and dynamics of aquatic ecosystems. The suite originated from the Ecopath module, initially developed by Jeffrey Polovina at the U.S. National Oceanic and Atmospheric Administration (NOAA) in the early 1980s to assess energy flow in Hawaiian coral reef ecosystems (Grigg et al., 1984; Moreau, 1995). Building upon this foundation, scholars such as Daniel Pauly and Villy Christensen expanded the model to include Ecosim for temporal dynamic simulation (Pauly et al., 1984; Walters et al., 1997; Dalsgaard and Christensen, 1997), Ecospace for spatial dynamics (Walters et al., 1999), and Ecotracer for contaminant tracking (Christensen et al., 2005). The EwE modeling suite simulates energy transfer among trophic levels, species interactions, and anthropogenic impacts on ecosystems (Coll et al., 2009). It has been widely applied in fisheries management, ecosystem assessment, and the design of marine protected areas (Morissette, 2007). In recognition of its scientific impact, NOAA listed the Ecopath module as one of the top ten scientific breakthroughs in its 200-year history in 2007 (National Oceanic and Atmospheric Administration (NOAA), 2010), highlighting its transformative role in ecosystem-based management. Today, EwE is employed globally as a fundamental tool for ecosystem modeling and decision-making in marine resource management (Coll et al., 2015; Philippsen et al., 2019).
Ecopath with Ecosim (EwE) integrates a static mass-balance module (Ecopath) with a temporal dynamic module (Ecosim) and an optional spatial component (Ecospace) to quantify energy flows, characterize trophic interactions, and assess the ecological impacts of fishing and management interventions. Functional groups—producers, consumers, and decomposers—are parameterized using biomass estimates and diet composition matrices. The Ecopath module establishes trophic structure by balancing these inputs, while Ecosim simulates temporal dynamics of ecosystem components to inform sustainable management strategies.
The core routines of Ecopath were derived from Polovina (1984)’s Ecopath program and subsequently updated and modified, rendering its original steady-state assumption redundant. Ecopath no longer assumed steady-state conditions but parameterized the model based on mass balance assumptions over arbitrary time periods (often one year) (but see also the discussion on seasonal modeling). In its current implementation, Ecopath was parameterized based on two main equations, one describing production conditions and the other describing energy balance for each group.
The first module of Ecopath serves to identify and characterize the production flows among the functional groups within the ecosystem. Equation 1 can be expressed as:
I is a functional group of biological species, is the total production of functional group (I), Yi is the total catch rate of functional group (I), M2i is the total predation rate of functional group (I), BiBi is the biomass of functional group (I), is the net migration rate (inflow - outflow), is the biomass accumulation rate of functional group (I), and is the ‘other mortality rate’ of functional group (I).
Equation 2 can also be expressed as Equation 3:
Or, Equation 4 can be expressed as:
Where is the production-to-biomass ratio of functional group (I), is the consumption-to-biomass ratio of functional group (I), is the proportion of prey (I) in the diet of predator (J), is the ecological efficiency (proportion of production utilized in the system), is the fishing mortality rate, is the biomass accumulation rate, and is the net migration rate.
The second module was used to determine the internal mass balance of functional groups (5), where the consumption of each functional group () equaled the sum of its production (), respiration (), and unassimilated food (). The equation is as follows. Equation 5 can be expressed as:
Where is respiration, and U is unassimilated food.
Ecopath requires the use of at least three out of these four parameters ( , , ) in the module. Mandatory fields include , , , (). If one of the first three values is missing, it can be specified accordingly. Additionally, fishing mortality and are also required.
2.2.2 Ecosim model dynamic simulation
Ecosim is a time-scale-based dynamic module based on ecopaths (Christensen and Walters, 2004). It incorporates aspects of temporal dynamics to simulate changes in system response over a time series by changing the Ecopath module incrementally over time. Equation 6 can be expressed as:
where is the rate of change in biomass, is the net growth efficiency, is the rate of depletion of functional group (J) on functional group (I), is the rate of migration, is the fishing mortality rate, is the nonpredatory natural mortality rate, is the rate of immigration, and is the biomass of functional group (I) (Christensen and Walters, 2004).
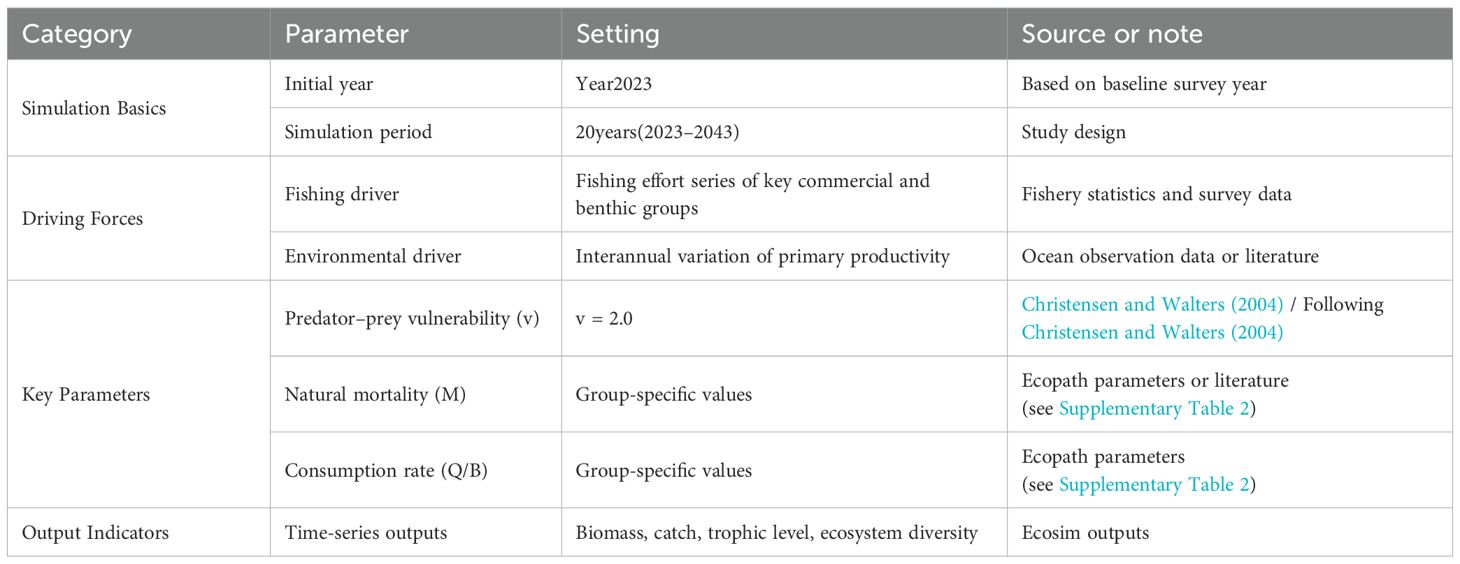
Based on field investigations in the Dashentang National Marine Ranch, we identified the major functional groups of the ecosystem and collected biomass data derived from in situ sampling, fishery catch statistics, and relevant model outputs. These data were used to construct the trophic structure of functional groups within the Ecopath model, with biomass and trophic interactions incorporated into the Ecopath with Ecosim (EwE, version 6.6) platform. The model, grounded in theoretical ecology, was applied to quantitatively analyze the flows of matter and energy within the ecosystem. Building upon this, the Ecosim time-dynamic module was introduced, with 2023 set as the initial year and a 20-year simulation period defined (2023–2043). Driving variables included the time series of fishing effort for the main functional groups and interannual variation in primary productivity as an environmental forcing factor. Key parameterization followed established practice, with the predator–prey vulnerability coefficient set at v = 2.0 (Christensen and Walters, 2004), and natural mortality rates as well as consumption rates assigned to each functional group based on published literature. Through this simulation framework, we systematically evaluated the dynamic characteristics of the Dashentang marine ranch ecosystem, providing a scientific basis for the sustainable utilization of fishery resources and the ecological management of the marine environment (see Table 1).
2.3 Ecopath model construction
2.3.1 Functional group construction
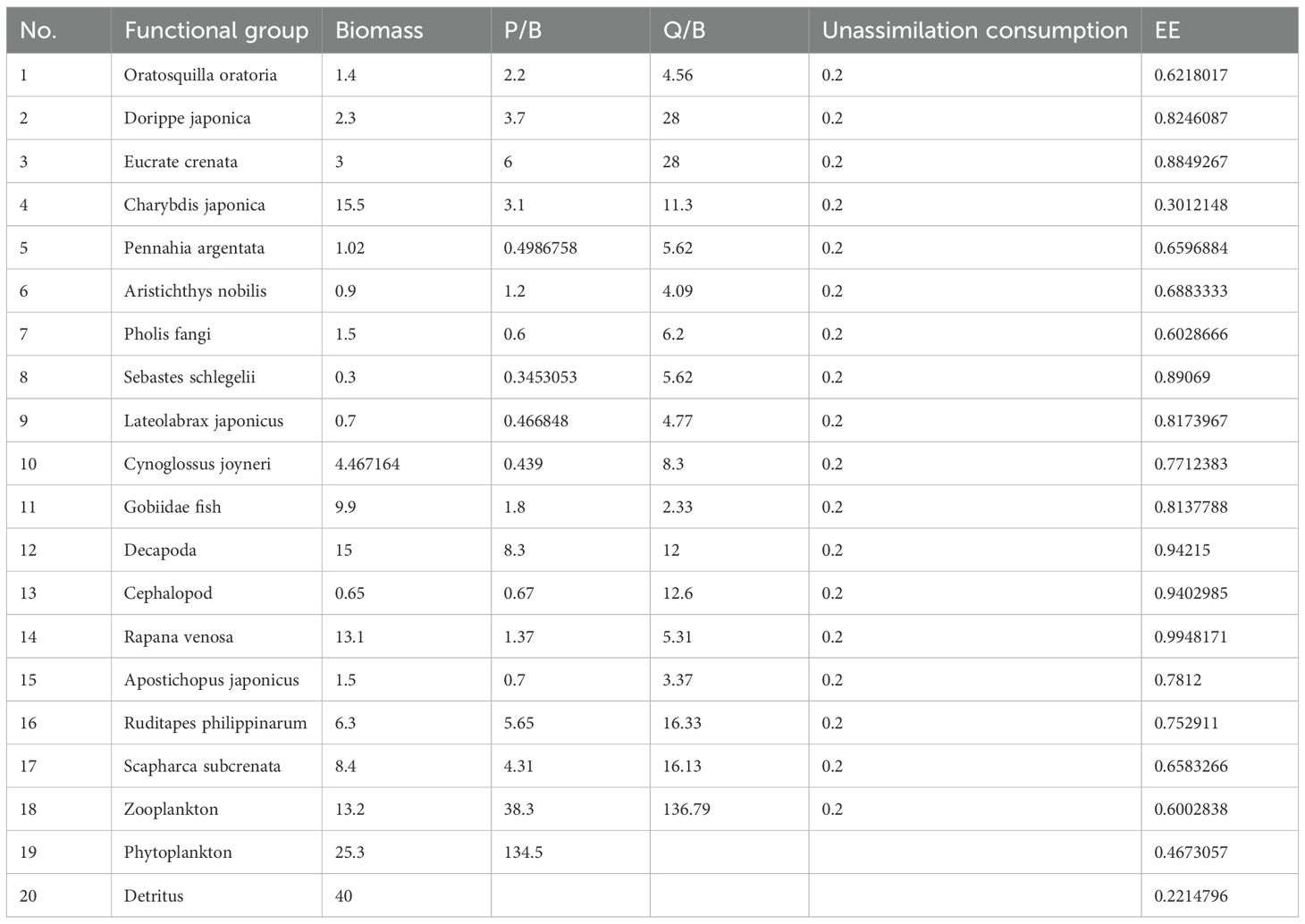
Based on the ecological characteristics, economic importance, and species’ functional roles in the Dashentang Marine Ranch, 20 functional groups were defined, as listed in Table 2. These include:Oratosquilla oratoria, Dorippe japonica, Eucrate crenata, Charybdis japonica, Pennahia argentata, Aristichthys nobilis, Pholis fangi, Sebastes schlegelii, Lateolabrax japonicus, Cynoglossus joyneri, Gobiidae fish, Decapoda, Cephalopod, Rapana venosa, Apostichopus japonicus, Ruditapes philippinarum, Scapharca, Zooplankton, Phytoplankton and Detritus.
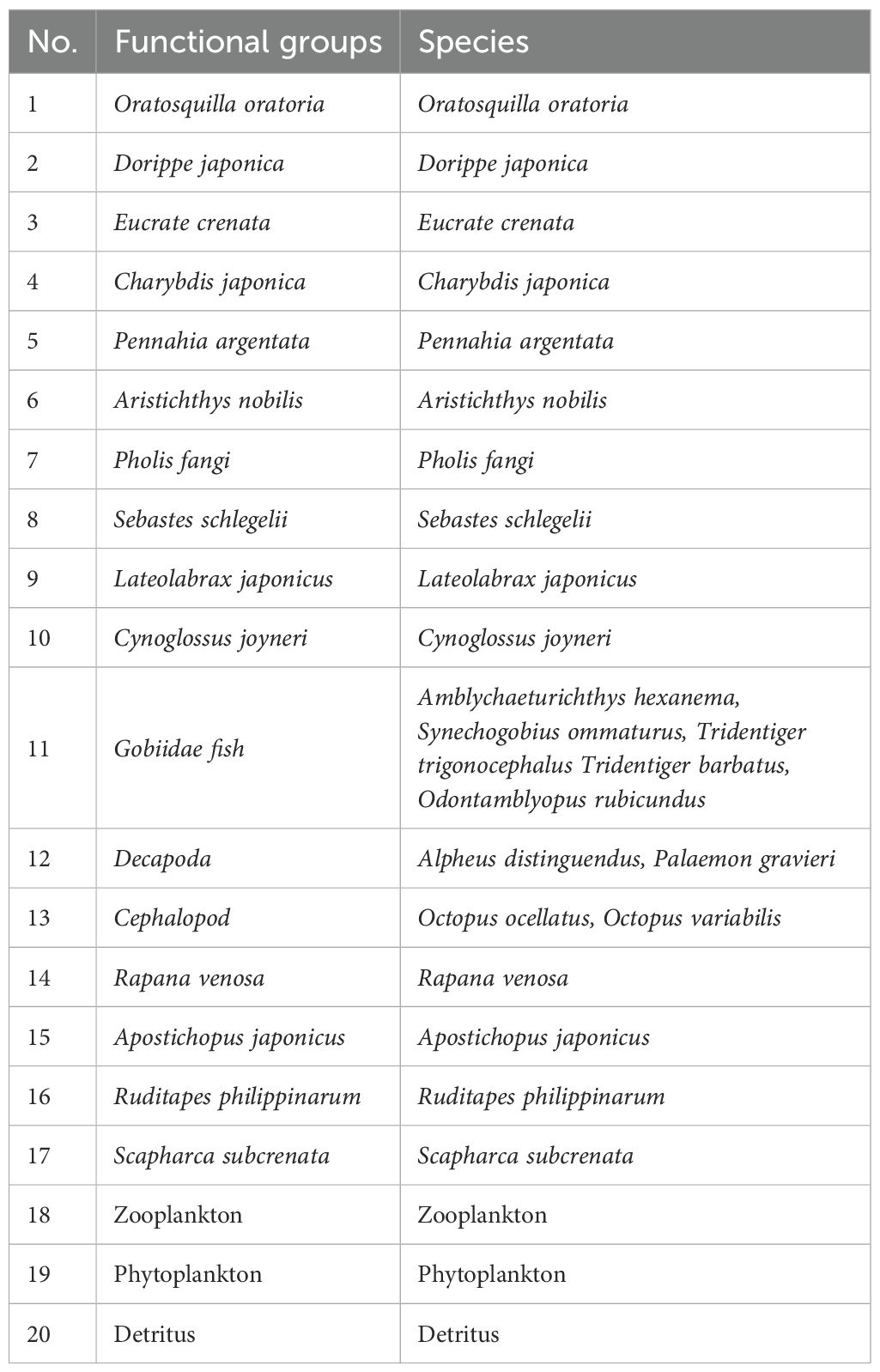
Table 2. Division of functional groups and their species composition in the ecosystem model of Dashentang Marine Ranch in Tianjin Bohai Bay, China.
2.3.2 Source of parameters
Model parameters were derived from field experiments conducted in May and August 2023 at the Dashentang Marine Ranch in Tianjin, while functional group biomass was averaged from the two sampling values.The functional group biomass ratio P/B is equal to total mortality (Z).The mortality rate (Z) is equal to the sum of the fishing mortality rate (F) and the natural mortality rate (M). Equation 7 can be expressed as:
The natural mortality rate (M) was estimated using the Equation 8:
where M is the natural mortality rate (year-1); is the asymptotic body length (total length, cm); and is the average water temperature (). For unexploited fish stocks, total mortality (Z) equals natural mortality (M), so that the P/B is set equal to Z. Accordingly, P/B ratios were derived by applying this formula to the biomass and temperature data collected during our field surveys. Q/B ratios and diet compositions were obtained primarily from Fish-base (https://www.fishbase.se). For functional groups whose P/B and Q/B could not be directly estimated, we adopted recent estimates from analogous ecosystem models in this and adjacent regions (Li et al., 2023); see the Supplementary Table for details.
2.4 Model equilibrium and uncertainty analysis
During the model balancing process, it is essential to ensure that the ecotrophic efficiency (EE) for each functional group remains below 1.0, as values exceeding this threshold indicate violations of mass-balance constraints and thermodynamic inconsistencies (Christensen and Walters, 2004). If EE exceeds 1.0, adjustments should be made to the diet composition of the functional group in question.Subsequently, the internal consistency of functional groups is evaluated. If imbalances are detected, adjustments to the P/B or Q/B ratios are made to achieve model equilibrium (Christensen and Walters, 2004; Link, 2010; Heymans et al., 2016). Model quality is commonly assessed via the pedigree index, which evaluates the confidence in model input parameters by assigning uncertainty scores according to their provenance. Model quality is commonly assessed via the pedigree index, which assigns uncertainty scores to model input parameters based on their provenance, thereby quantifying confidence in those parameters (Xiao et al., 2024). To further validate the model, we applied the PREBAL routine (Link, 2010) to conduct pre-balance diagnostics of P/B and Q/B ratios for each functional group and trophic level. We applied the PREBAL diagnostics (Link, 2010)to verify four criteria: (1) Biomass Range: Functional group biomass spans 5–7 orders of magnitude. (2) Biomass Slope: On a log-log plot of biomass versus trophic level, the slope declines by 5-10% per level. (3) Transfer Ratios: P/B and Q/B ratios decrease with increasing trophic level. (4) Monte Carlo Uncertainty: We ran 500 Monte Carlo simulations, varying B, P/B, and Q/B by ± 10%, to assess the sensitivity of Ecosim outputs to input uncertainty.
2.5 Ecological network analysis
Ecological Network Analysis (ENA) is a foundational, systems-oriented methodology for quantifying ecosystem structure, function, and energy flows, playing an essential role in elucidating ecosystem complexity, dynamics, and resilience (Dunne et al., 2002).
Ecopath with Ecosim (EwE) features an integrated Ecological Network Analysis (ENA) plugin, equipping researchers with effective tools to quantify ecosystem network structure and function (Christensen and Walters, 2004; Heymans et al., 2016; Piroddi et al., 2015).
Ecological Network Analysis (ENA) serves as a robust tool for quantifying energy and material flows within ecosystems through the calculation of essential indices, including Total System Throughput (TST), Total Primary Production (TPP), Total Biomass (TB), and Total Respiration (TR). By utilizing these indices, ENA offers valuable insights into the stability, maturity, and complexity of ecosystems. Among these indices, the TPP/TR (Total Primary Production/Total Respiration) and TPP/TB (Total Primary Production/Total Biomass) ratios stand out as crucial indicators. Higher values of these ratios typically reflect increased productivity in relation to system respiration or biomass, highlighting the intricate dynamics of ecosystems and the necessity of maintaining balance for sustainable functioning (Ulanowicz, 1986; Li et al., 2015).
The Finn Cycling Index (FCI) is a powerful metric that quantifies the proportion of system throughflow that is recycled, highlighting the network’s resilience and cycling complexity (Finn, 1976; Borrett and Osidele, 2007). The Connectance Index (CI) and System Omnivory Index (SOI) together provide a nuanced understanding of food-web complexity by revealing both the density and breadth of trophic linkages (Dunne et al., 2002; Allesina and Bondavalli, 2003). The Finn Mean Path Length (FMPL) offers critical insight into the energy flow within a network, measuring the average number of compartments energy traverses and showcasing the interconnectedness of the system (Finn, 1976; Fath et al., 2007). Trophic Transfer Efficiency (TE), which represents the ratio of energy flux between successive trophic levels, is a direct measure of energy transfer efficiency; notably, it tends to decline with each ascending trophic level (Dunne et al., 2002; Williams and Martinez, 2004). Finally, the Shannon Diversity Index (SDI) evaluates species diversity and evenness, providing an essential perspective on the stability of ecosystems, underscoring their complexity and richness (Magurran, 2004; Tuomisto, 2010).
The ratios TPP/TR and TPP/TB are key indicators of ecosystem stability and maturity, reflecting energy utilization efficiency and the relationship between biomass accumulation and primary production, respectively (Ulanowicz, 1986). Additional indices, including the Finn Cycling Index (FCI), Connectance Index (CI), System Omnivory Index (SOI), and Finn Mean Path Length (FMPL), provide insights into the complexity of energy cycling and food web topology (Finn, 1976). Trophic Transfer Efficiency (TE) is a key metric that quantifies the proportion of energy transferred between successive trophic levels, providing insights into the efficiency and structure of energy flow within the ecosystem (Dunne et al., 2002). The Shannon Diversity Index (SDI) is also widely used to assess biodiversity, contributing to evaluations of ecosystem stability (Magurran, 2004).
Lindeman energy flow diagrams provide a visual representation of trophic energy transfer and network index dynamics, thereby facilitating a deeper understanding of the functional relationships between trophic structure and ecosystem processes (Lindeman, 1942). Together, these analyses offer an integrated framework to support ecosystem-based management and conservation planning.
2.6 Carbon budget and emissions assessment
The carbon budget was quantified by evaluating both carbon inputs and carbon outputs, with explicit consideration of the pathways of carbon storage and release. The analytical framework integrated ingestion, assimilation, and incorporation of carbon into soft tissues and calcareous shells, as well as carbon losses via respiration, excretion, and biodeposition.
i. Carbon input and assimilation. Carbon input was estimated from clearance and filtration rates, combined with measurements of particulate organic carbon (POC) in the ambient water. Assimilation efficiency was determined by comparing the organic carbon content of ingested material with that of biodeposits, allowing for partitioning between assimilated and rejected fractions. Assimilated carbon was subsequently allocated to growth of soft tissue biomass and shell carbonate deposition.
ii. Carbon release. Carbon efflux was resolved into three primary pathways:
iii. Respiration, quantified as oxygen consumption rates and converted to carbon equivalents using a respiratory quotient (RQ) of 1.0.
iv. Excretion, assessed by collecting filtered water samples and analyzing dissolved organic carbon (DOC) concentrations.
v. Biodeposition, estimated from feces and pseudofeces production, with organic carbon content determined via elemental analysis or loss-on-ignition (LOI).
Carbon budget calculation. The net carbon budget at the individual level was calculated as: Carbon incorporated into tissues and shells was regarded as the effective storage (sequestration) component, whereas respiration, excretion, and biodeposition represented carbon release pathways. Scaling from individuals to the population level was achieved using field-estimated clam densities (ind. M-2), and ecosystem-level fluxes were derived.
(iii)Temporal and spatial scales. Measurements were conducted under controlled international conditions, with rates standardized to daily fluxes (mg C ind-1 d-1). Population and ecosystem budgets were expressed on an annual basis (g C m-2 yr-1), enabling direct comparison with published estimates of bivalve carbon cycling and fisheries carbon footprints.
For ecosystem-scale calculations, empirical parameters from the Dashentang Marine Ranch were applied. The ranch covers an area of 13.6 km2, with a current standing biomass of 0.511 t km-2 and an estimated ecological carrying capacity (ECC) biomass of 208 t km-2. These values were used to scale individual-level measurements of carbon budget and emissions to population and ecosystem levels, thereby enabling assessment of both the present carbon fluxes and the potential carbon sequestration capacity under maximum ecological carrying capacity scenarios.
3 Results
3.1 Ecological model quality analysis
The confidence index for the input parameters B, P/B, and Q/B in this study is 0.716 (Feng et al., 2021), indicating that the reliability of the model’s input data sources is at a medium-to-high level. This result implies that the model’s energy balance is well-assured, and the estimation of trophic level efficiency (EE) is robust.Model results revealed that Charybdis japonica, shrimp species, Rapana venosa, and Gobiidae fish rank among the highest, followed by R. philippinarum and Scapharca subcrenata.Phytoplankton exhibited a high P/B, and their rapid growth and reproduction provided a substantial energy source for shrimps, crabs, and bivalves.
3.2 Evaluation of the ECC of Ruditapes philippinarum
Model outputs Table 3 indicated that C. japonica (15.5 t km²), R.venosa(13.1t/km²), Gobiidae fish(9.9 t km²), S. subcrenata(8.4t/km²), and R.philippinarum (6.3t/km²) exhibited the highest biomass densities and represented the primary ecological and commercial functional groups in the model area. The analysis of the aquaculture potential of R. philippinarum (Table 4) indicates that the ecological carrying capacity in the Dashentang National Marine Ranch, Tianjin, is 208.1 t/km², determined at the Ecopath model equilibrium threshold where EE = 1. When clam biomass exceeds this threshold, the system becomes unsustainable (EE > 1); thus, 208.1 t/km² represents the maximum carrying level.
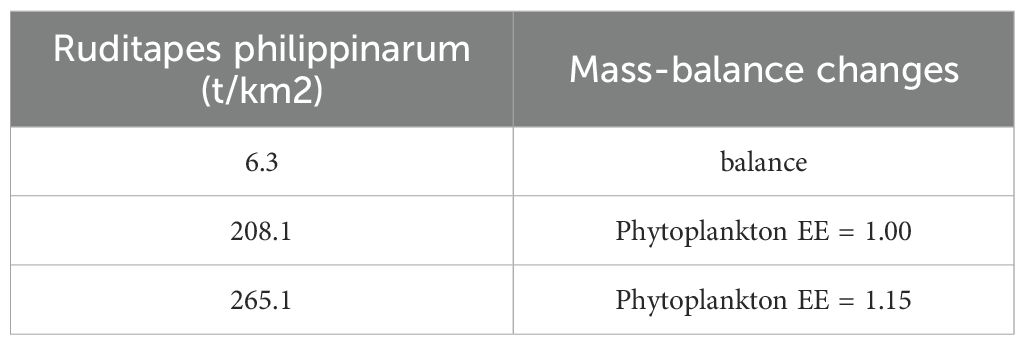
Table 4. Variation of other parameters in estimating the ecological carrying capacity of Ruditapes philippinarum using the Ecoapth model.
3.3 Analysis of the overall characteristics of the study marine ranching ecosystem
According to the Figure 3 Mixed Trophic Impact (MTI) matrix outputs of the Ecopath model, the direct and indirect interactions among functional groups in the system are generally weak, with most interaction values approaching zero. This indicates that the marine ecosystem is in a relatively stable state, where energy flows and regulatory relationships are mainly realized through the grazing and filter-feeding chains. Negative impacts are predominantly manifested as predation-driven suppression, whereas positive impacts arise primarily from nutrient recycling and indirect energy transfer. As a benthic filter-feeding bivalve, R. philippinarum exerts a slight negative regulatory effect on phytoplankton through grazing and competes to some extent with Scapharca subcrenata for shared resources. In addition, R. philippinarum exhibits very weak positive effects on zooplankton, crustaceans, and cephalopods, while showing no significant direct relationships with higher-trophic-level fish groups.
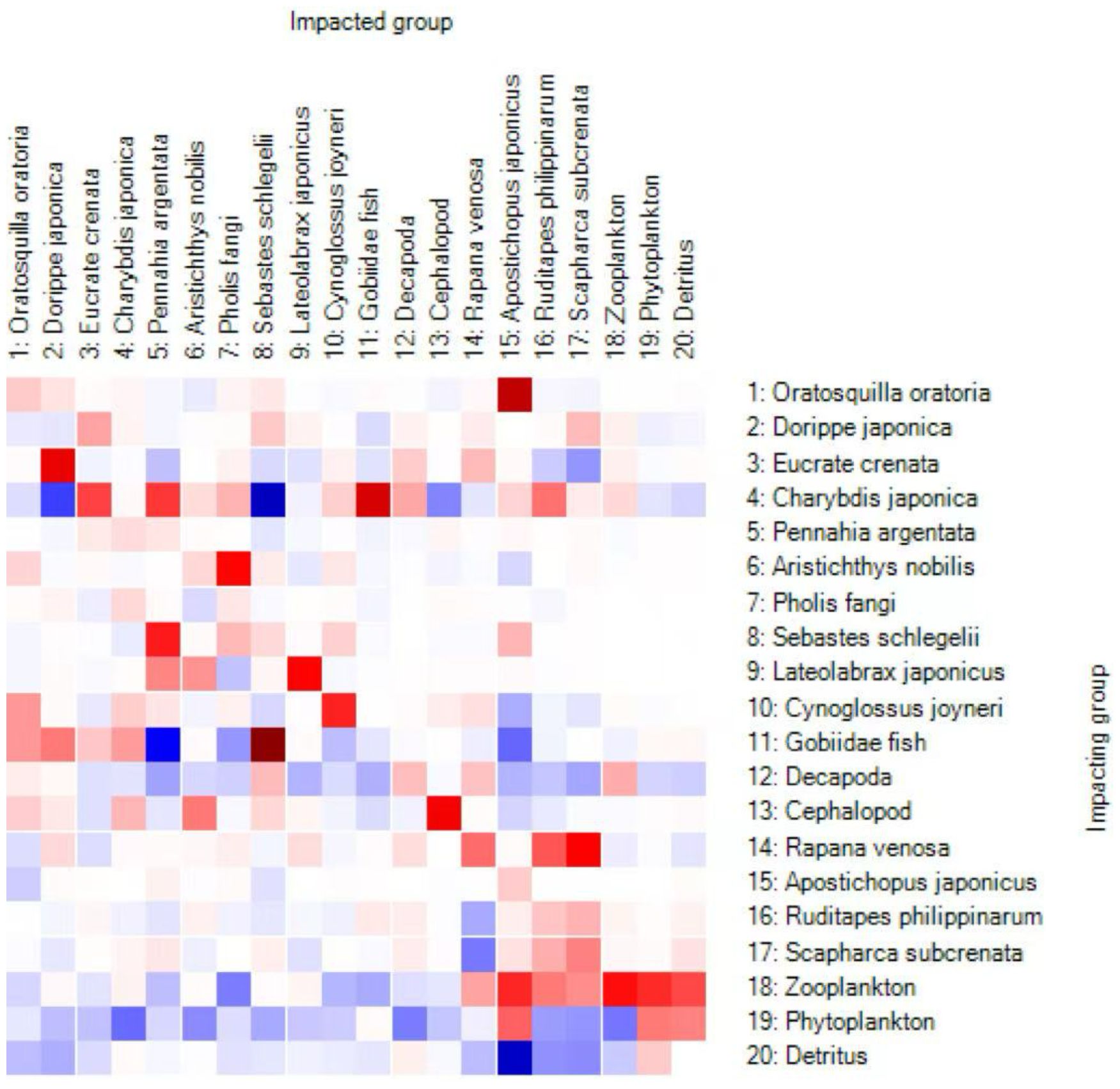
Figure 3. Mixed Trophic Impact (MTI) heatmap showing positive (red) and negative (blue) effects among functional groups.
The trophic flow matrix of the Dashentang National Marine Ranch ecosystem further revealed the trophic levels (TL) of each functional group (Table 5; Figure 4). Cephalopods exhibited the highest TL (4.01), followed by Lateolabrax japonicus (3.84) and Sebastes schlegelii (3.82). Energy transfer was concentrated mainly at TL I and TL II: TL I was dominated by contributions from phytoplankton and detritus, while TL II was characterized primarily by bivalves (S. subcrenata and R. philippinarum), followed by zooplankton and Acipenser dabryanus. With increasing biomass of R. philippinarum, significant changes in system-wide energy and matter cycling were observed. Table 6 shows that, Total System Throughput (TST), Total Biomass (TB), and Total Respiration (TR) all increased, whereas Total Primary Production (TPP) remained largely stable, with TB showing the most pronounced increase.Indicators reflecting system maturity and stability also exhibited distinct responses: TPP/TR, TPP/TB, the Shannon diversity index (H, SDI), the System Omnivory Index (SOI), A/C, and Average Mutual Information (AMI) all declined; O/C increased slightly; the Finn’s Mean Path Length (FMPL) and the Food Chain Index (FCI) rose substantially; while the Connectance Index (CI) remained largely unchanged. Transfer Efficiency (TE) decreased, and biodiversity (SDI) was also reduced. At the functional-group level, the short-term increase in clam biomass suppressed certain benthic predators.
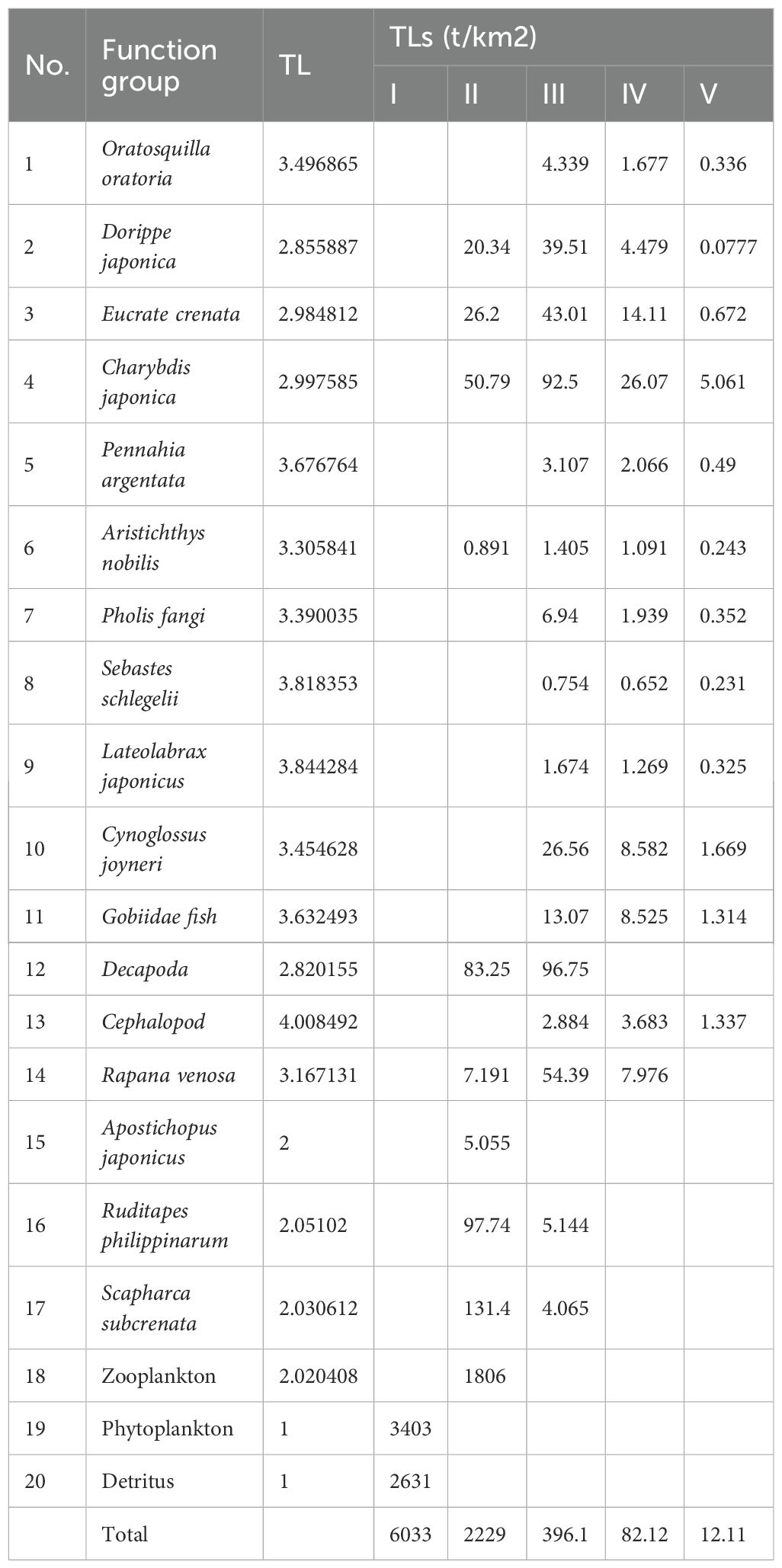
Table 5. Matrix of nutrient flows in studied marine rangeland ecosystems according to functional groups and TLs.
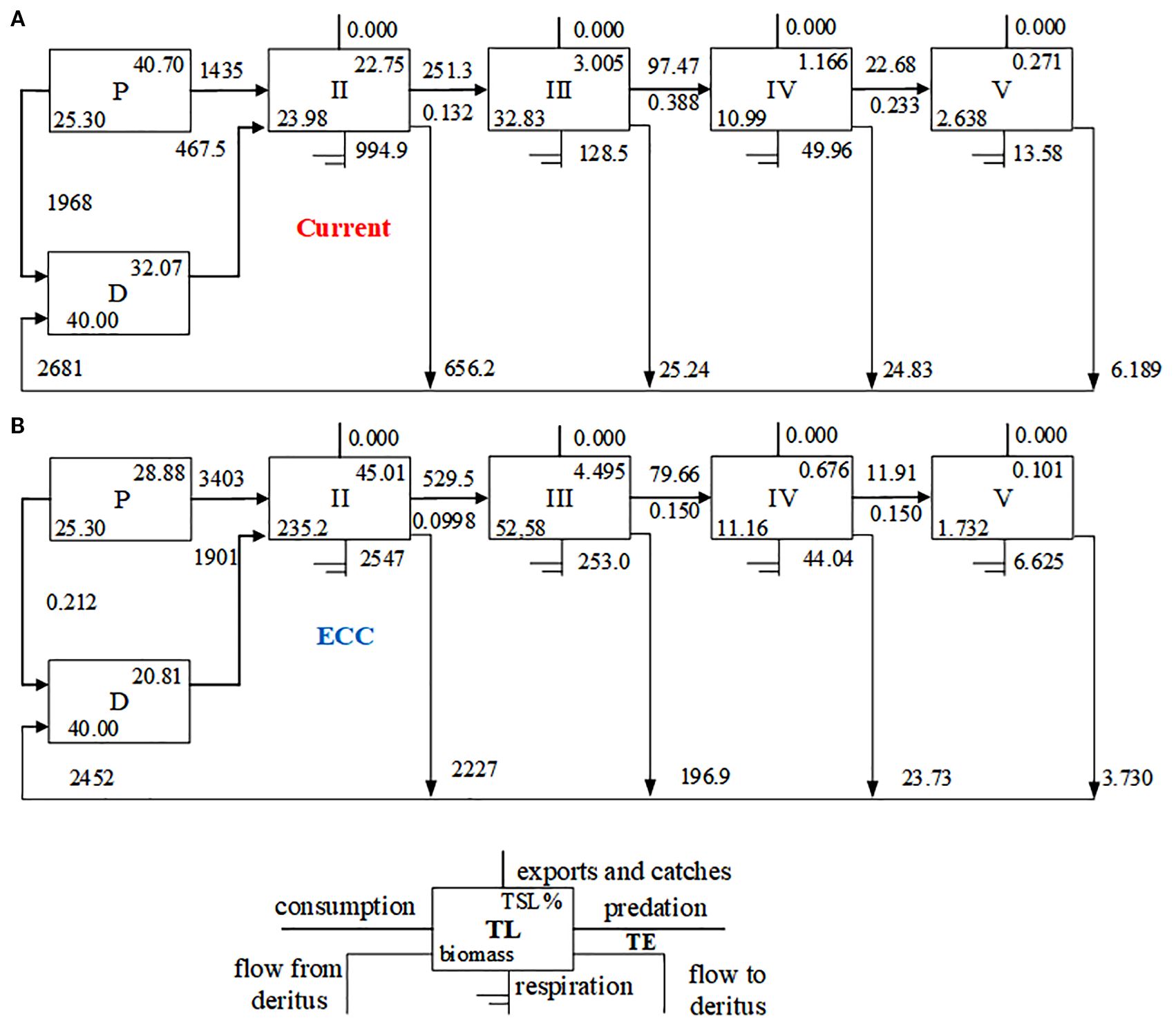
Figure 4. Lindeman energy flow diagram between trophic levels for two scenarios for (A) current and (B) ECC. ECC, ecological carrying capacity; D, detritus; P, primary producers; TL, trophic level; TE, transfer efficiency; TST, total system throughput.
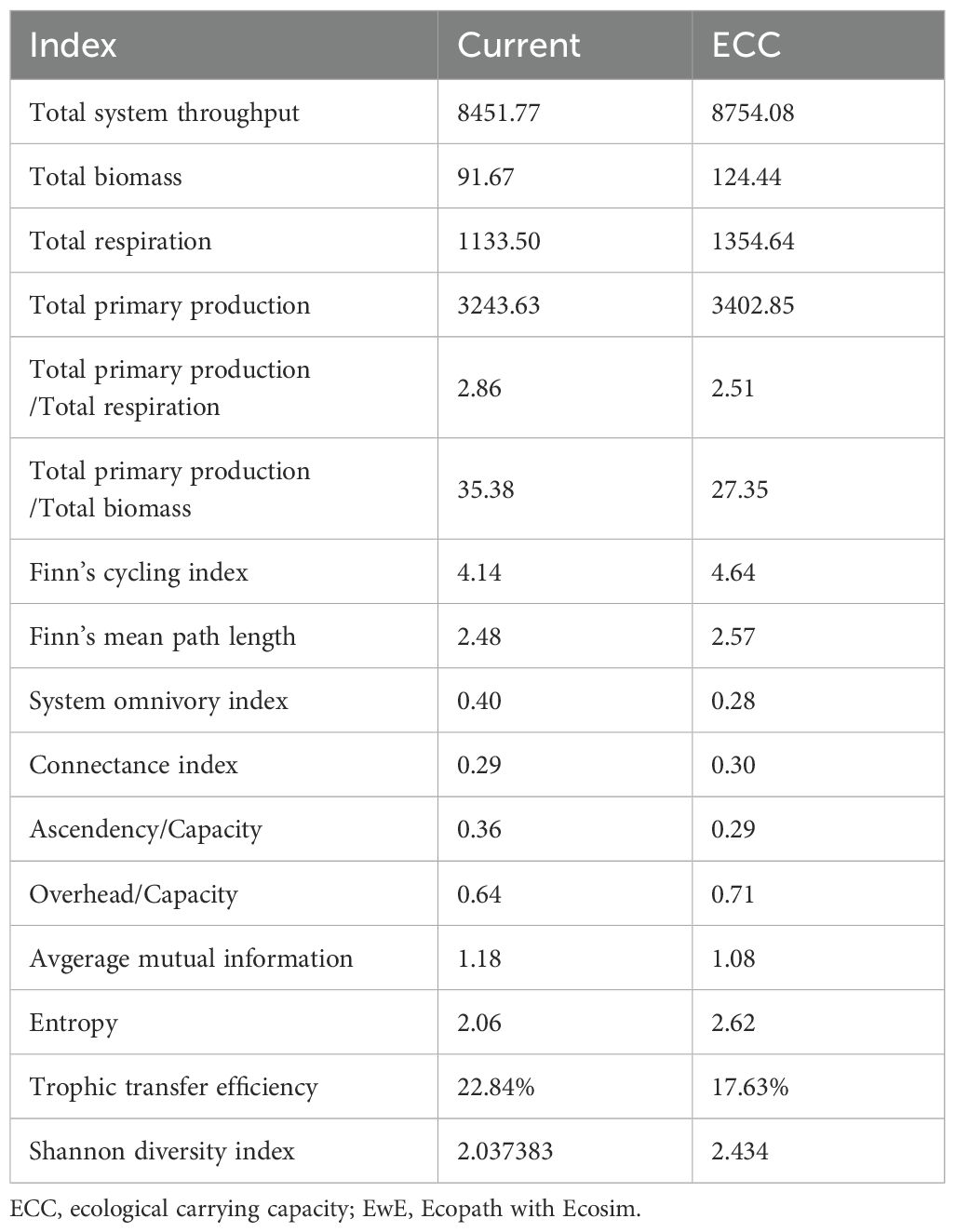
Table 6. The relative changes in the analyzed indices of ecological forgetfulness for the two scenarios (Current, ECC) predicted according to the EwE model.
4 Discussion
4.1 Overall maturity and stability of marine rangeland ecosystems
In this study, the overall maturity and stability of the marine rangeland ecosystem was analyzed, specifically covering ecosystem size, maturity, stability, and trophic transfer, in order to predict the potential impacts of increasing R. philippinarum biomass on the system. TST, TB, and TPP of the ecosystem tended to increase with increasing R. philippinarum biomass, indicating a gradual increase in the overall size of the ecosystem. According to Odum (1969) and Christensen and Walters (2004), the ratios of TPP/TR and TPP/TB are the key parameters reflecting the maturity of the ecosystem. When the ecosystem is mature, both tend to be close to 1. In this study, TPP/TR and TPP/TB showed a decreasing trend as the biomass of R. philippinarum increased, and when R. philippinarum reached the ECC, they were 1.19 and 10.43, respectively, suggesting that the ecosystem is maturing with the increase in the number of R. philippinarum. However, the seasonal resolution of the dataset may constrain the accuracy of short-term predictions, while its influence on long-term trends and the main conclusions is limited. Future studies incorporating higher temporal resolution data could further improve the robustness of the model.
In natural ecosystems, a certain degree of reticulation is usually evident even in the early stages of development, largely due to resource overlap and the diversity of trophic interactions among species. As ecosystems mature, their complexity and organizational levels progressively increase, with food chains evolving from relatively linear forms into more intricate food-web structures. Our results show that with the increase in R. philippinarum biomass, both the Finn’s Mean Path Length (FMPL) and the Food Chain Index (FCI) rose significantly. The rates of change were comparable to those reported by Heymans et al. (2014) for ecosystems within the 1–10 km² range, suggesting that detrital energy utilization efficiency improved and that material cycling pathways became more extended. On the other hand, when compared with the global food-web models synthesized by Heymans et al. (2014), both the System Omnivory Index (SOI) and the Connectance Index (CI) in the Dashentang marine ranch remained at relatively low levels, regardless of the extent of clam enhancement. This indicates that, at a global scale, the system remains relatively simple and linear in structure, with limited complexity.It should be emphasized that these results reflect the characteristics of interaction strength among functional groups within the system, rather than serving as the sole criterion for overall ecosystem stability.
The ratios A/C and O/C serve as important indicators of ecosystem stability and maturity. According to Ulanowicz (1986, 1997), Christensen (1995), and subsequent empirical research based on Ecological Network Analysis (ENA), A/C values for natural, healthy ecosystems typically fall within 0.25–0.45 (25%–45%). Values exceeding 50% suggest high efficiency but insufficient redundancy, implying greater vulnerability to external disturbances; following perturbation, A/C should ideally recover to within ±20–25% of its reference mean. In the study area, the initial A/C value was 36.52%, indicating high efficiency but elevated vulnerability. With the increase in clam biomass, A/C decreased to 26.57%, a range considered more appropriate, signifying a transition from an “artificially efficient” state toward a “naturally robust” condition. Concurrently, O/C increased from 63.65% to 72.77%, suggesting enhanced resilience against disturbance (Liu et al., 2022; Wang et al., 2024). However, the continued decline in the Shannon diversity index (SDI) indicates that energy flows became increasingly concentrated within a few dominant species, potentially undermining system resilience (Odum, 1969; Holling, 1973). This not only reflects a potential simplification of community structure under the dominance of a few species, but may also imply a reduced capacity of the ecosystem to withstand external disturbances. Therefore, a decline in SDI can be interpreted both as a sign of energy efficiency concentration and system maturity, and as an indication of biodiversity loss and increased ecological vulnerability.
Our findings underscore that while the proliferation of R. philippinarum enhances system-level energy utilization efficiency and ecological carrying capacity, it simultaneously poses a risk of reduced biodiversity. These insights provide important guidance for future marine ranch management, highlighting the need to balance efficient energy cycling with the maintenance of community diversity in order to ensure the long-term robustness and sustainability of the ecosystem.
4.2 tapes philippinarum proliferation on other functional groups
The results of the Ecosim simulation (Figure 3) suggest that an increase in R. philippinarum biomass initiates a cascading effect throughout the trophic network. This species filters large amounts of phytoplankton and organic particulate matter, altering the energy flow pathways. This process has both direct inhibitory and indirect facilitative effects on associated functional groups, such as fish and crustaceans, exhibiting gradual and multidimensional complexity. These results suggest that increases in R. philippinarum biomass under static equilibrium conditions may exert competitive pressure on certain benthic predators, leading to a decline in their biomass.
The study examined the changes in other functional groups by simulating an increase in R. philippinarum biomass from its current level to the ECC. Since R. philippinarum primarily feeds on phytoplankton, the availability of phytoplankton is a key limiting factor for bivalve populations.As shown in the Figure 3, when the biomass of Ruditapes philippinarum approaches its ecological carrying capacity (ECC), the biomass of phytoplankton exhibits a slight initial decline, though the variation remains negligible. This suggests that the system maintains a high phytoplankton productivity, allowing R. philippinarum to proliferate without exerting significant pressure on its primary food source.As time progressed, the biomass of different species exhibited varying degrees of increase or decrease. The biomass of S. schlegelii and decapoda declined in the later stages.Overall, R. philippinarum primarily functions as a primary consumer in the system, with key ecological roles in regulating phytoplankton abundance, participating in material cycling, and contributing to water quality maintenance, thereby supporting ecosystem stability.Phytoplankton biomass declined by approximately 30%, and shrimp larval survival decreased when Ruditapes philippinarum culture density exceeded 500 individuals/m² (Zhang et al., 2024). In the Bohai Bay aquaculture region, decapoda larval abundance is estimated to decrease by 0.8% for every 1 g/m² increase in clam biomass (Liu et al., 2023). The Figure 3 MTI results further corroborated these relationships: overall interactions within the system were weak, with Ruditapes philippinarum exerting a slight negative regulatory effect on phytoplankton, competing with Scapharca subcrenata, and showing very weak positive effects on zooplankton, crustaceans, and cephalopods, while exhibiting no significant interactions with higher-trophic-level fishes. Overall, R. philippinarum functions as a primary consumer, playing an important role in stabilizing the system by regulating phytoplankton and contributing to material cycling, although its proliferation may intensify competition with certain detritus-dependent groups. Building on Ecosim outputs of primary productivity, functional group biomass, and trophic energy flows, we quantitatively assessed the system’s carbon sequestration potential by applying biomass-to-carbon conversion factors, with particular emphasis on the roles of benthic organisms and long-lived fish species in carbon storage. Finally, ecosystem-level indicators derived from Ecosim—including trophic structure, diversity indices, and total energy throughput—were integrated as core metrics for evaluating ecological health and sustainability, thereby providing scientific support for fisheries resource management and ecological compensation decisions.R. philippinarum attaches to the substrate by secreting byssal threads, which can reduce the available burrowing space for benthic decapod species, leading to localized habitat overlap (Liu et al., 2024). From the perspective of the trophic flow matrix (Figure 4; Table 5), filter-feeding bivalves under the ECC scenario substantially increased their direct interception of primary production, thereby diminishing the efficiency of stepwise energy transfer to higher trophic levels. This “bottom-up competitive effect” resulted in insufficient energy supply to intermediate trophic levels, ultimately driving declines in the biomass of higher-trophic-level fishes such as Sebastes schlegelii. Consistent with the Figure 3 MTI matrix analysis, Ruditapes philippinarum exerted only very weak positive effects on zooplankton and crustacean groups, but through competition (e.g., with Scapharca subcrenata) and the consumption of phytoplankton, it imposed an “energy squeeze effect” on intermediate trophic pathways.As a result, an increase in R. philippinarum biomass is linked to a decline in the biomass of other species.Secondly, R. philippinarum and S. schlegelii exhibited indirect competition through trophic cascades and habitat disturbance, resulting in a negative correlation between their biomasses (Chen et al., 2023; Wang et al., 2025). The high filtration efficiency of R. philippinarum (one individual can filter up to 20 L of water per day) significantly reduced phytoplankton biomass, which subsequently hindered zooplankton reproduction—the primary food source for S.schlegelii—and ultimately led to reduced growth rates in S.schlegelii (Zhang et al., 2024; Li et al., 2025).The growth rate of S. schlegelii larvae was 18% lower in R. philippinarum farming areas compared to non-farming zones (Liu and Zhou, 2024). High densities of clam excreta significantly increased concentrations of ammonia (NH3–N) and phosphate (PO4³-) in the water column, leading to localized hypoxic conditions (dissolved oxygen < 3 mg/L), which in turn induced habitat avoidance and migration of adult S. schlegelii (Wang et al., 2023; Sun et al., 2025). A threshold effect was observed: when clam density exceeded 800 individuals/m², bottom water dissolved oxygen levels declined by 40%, and the occurrence of S. schlegelii decreased by 55% during summer months (Zhang et al., 2025). Moreover, clam aquaculture zones were mainly located in intertidal and shallow waters (<10 m), spatially overlapping with the rocky reef spawning habitats preferred by adult S. schlegelii, thereby compressing reproductive habitats and reducing reproductive success (Guo et al., 2024; Huang et al., 2025). The expansion of clam farming has also led to a 32% reduction in effective ecosystem area and diminished population resilience (Dalian Fisheries Research Institute, 2025).
According to Figure 5, As R. philippinarum biomass was increased to 120% of its ecological carrying capacity, the responses of co-occurring functional groups became more pronounced. Under this scenario, benthic predators—such as S.schlegelii and various decapods—experienced significant biomass declines, likely due to competition for food and habitat resources under elevated clam densities Frontiers. Conversely, filter-feeding and associated benthic taxa, notably C. japonica, exhibited biomass increases, reflecting the release of benthic resources and enhanced habitat complexity created by dense clam aggregations Frontiers (Yan et al., 2025). These divergent responses highlight the multidimensional and species-specific nature of trophic interactions in response to shellfish enhancement, with potential benefits for economically valuable species and local fisheries livelihoods.
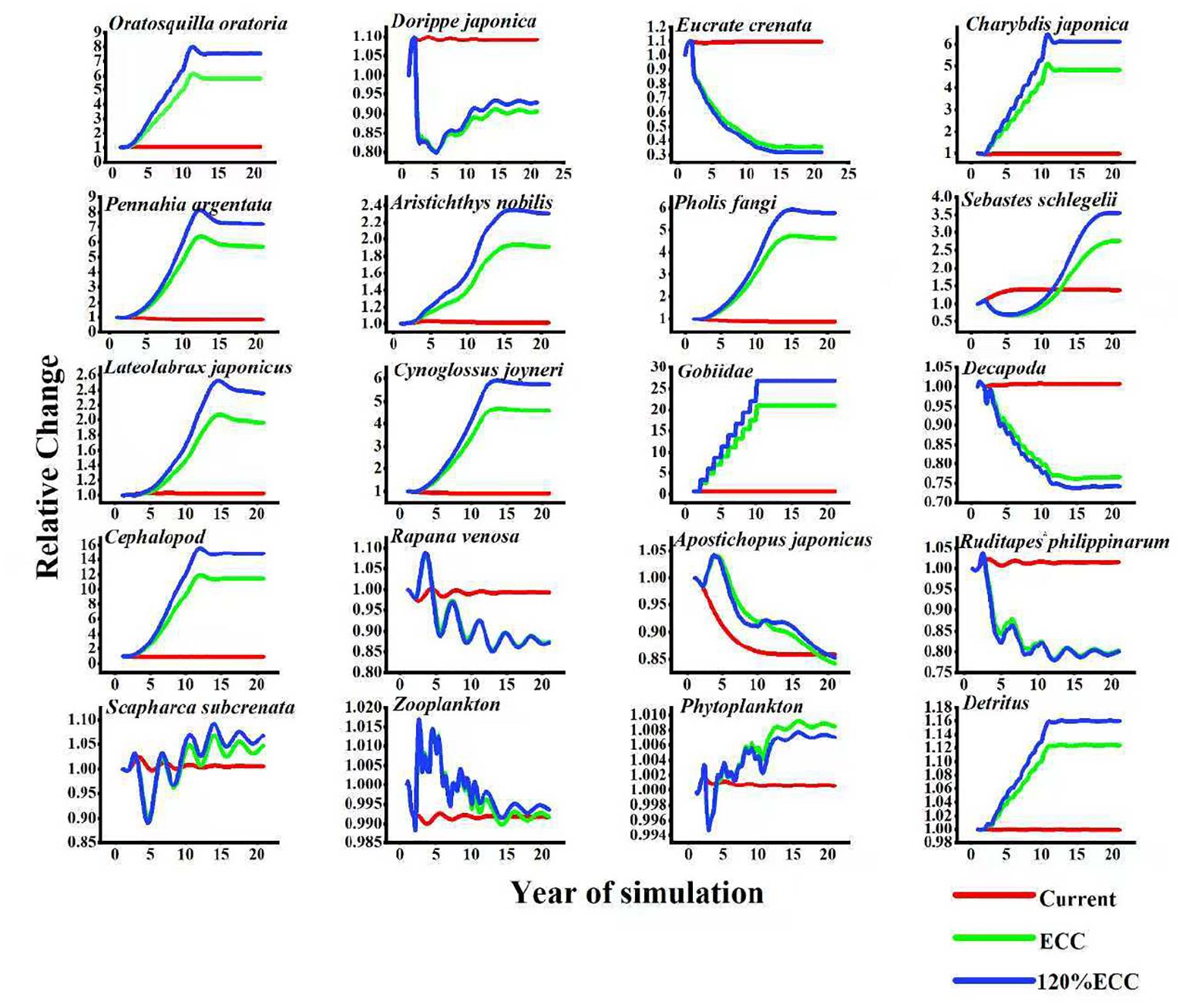
Figure 5. The relative changes in biomass of each functional group under three scenarios (Current, ECC, and 120% ECC) of Ruditapes philippinarum biomass augmentation in the study marine ranching ecosystem model were predicted. ECC, ecological carrying capacity; 120%ECC, 120% ecological carrying capacity.
4.3 Measurement of Ruditapes philippinarum population levels in the sea ranch study
Ecosim simulation results showed a continuous decrease in decapoda biomass following increased R.philippinarum stocking, culminating in an approximately 70% decline by the end of the simulation period. This is likely due to significant dietary overlap, as shared prey items constitute approximately 35–40% of the total food intake of both groups (Wang et al., 2019). In summary, while moderate increases in R.philippinarum biomass can yield positive ecological engineering outcomes within benthic habitats, excessive densities may impair benthic ecosystem functioning and reduce overall system resilience. R. philippinarum is a key economic species in the region, and its cultivation contributes to a moderate increase in the biomass of other economically valuable species through habitat modification and enhanced trophic support. Ecosim simulation results suggest that the biomass of R.philippinarum could increase to approximately 265.1 t/km² under optimized ecological conditions. The construction of artificial reefs may provide relatively suitable habitats for filter-feeding bivalves and could contribute to the growth of R. philippinarum populations to some extent.
R.philippinarum, an important marine-cultured bivalve, plays a significant role in carbon sequestration through both inorganic and organic pathways. During shell formation, it absorbs bicarbonate (HCO3-) and calcium ions (Ca²+) from seawater to synthesize calcium carbonate (CaCO3), thereby achieving long-term inorganic carbon storage. This process sequesters approximately 0.35 kg of CO2-equivalent per kilogram of biomass, with shell carbon accounting for 65–75% of the total (Zhang et al., 2012). While the soft tissue also stores organic carbon, it is less stable due to susceptibility to predation and decomposition.In addition, the species exhibits pronounced filter-feeding activity, removing suspended organic particles and phytoplankton from the water column. These materials are deposited in sediments, promoting benthic carbon sequestration (Xu et al., 2018). The estimated blue carbon storage capacity of R.philippinarum is about 5–6 t CO2-eq per hectare per year-approximately 70% of the sequestration capacity of mangrove forests of the same area (Zhang et al., 2012; Guo et al., 2020)-and can offset up to 40% of the carbon emissions generated by fishing vessel fuel consumption (Wang et al., 2016). Its filter-feeding behavior also reduces phytoplankton concentrations by ~20% and total suspended solids by ~38%, consequently improving water quality and enhancing carbon deposition. Clam beds further contribute by providing substrate for sessile organisms and enhancing benthic biodiversity and associated ecosystem carbon sequestration.Based on the time-series of functional group biomass derived from Ecosim outputs and in conjunction with fishing effort levels, the ecological carrying capacity (ECC) of the system was estimated, enabling a quantitative assessment of its carbon sequestration potential, as illustrated in Figure 5. At the ecological carrying capacity (ECC) level of R.philippinarum in the Dashentang Marine Ranch, Figure 6 shows that the total carbon uptake was calculated to be 21, 500 t, with benthic biomass deposition of 10, 300 t, harvestable biomass of 280, 000 t, and respiratory CO2 release of 870, 000 t. In the figure, red lines indicate carbon input pathways (including ingestion and inorganic carbon uptake), whereas black lines represent carbon output pathways (including deposition, respiration and calcification, and removal through harvest). Enhancement and release strategies—such as the use of habitat-assisted methods like caisson placement and artificial reef construction—have been implemented in this ranch (Liu, 2011; Kang et al., 2017; Wada et al., 2015), marking the first global initiative to stock clams in a marine ranching context. Artificial reefs significantly enhance the carbon sequestration efficiency of R. philippinarum by creating complex, three-dimensional habitats that provide attachment substrates and shelter, optimize nutrient flow, improve survival and biomass, and amplify both calcification-based carbon storage and benthic deposition through filter-feeding (Zhang et al., 2012; Xu et al., 2018). These interventions ultimately contribute to dual benefits: reducing carbon emissions in the fishery sector and promoting ecological restoration of marine ranches.Through improvements in habitat quality and resource availability, artificial reefs may support higher sustainable biomass of Ruditapes philippinarum, providing a potential management tool for enhancing ecological carrying capacity in marine ranching systems. Nevertheless, these inferences require further validation through long-term monitoring and experimental comparisons to clarify the specific contribution of artificial reefs to the ecological carrying capacity of R. philippinarum.
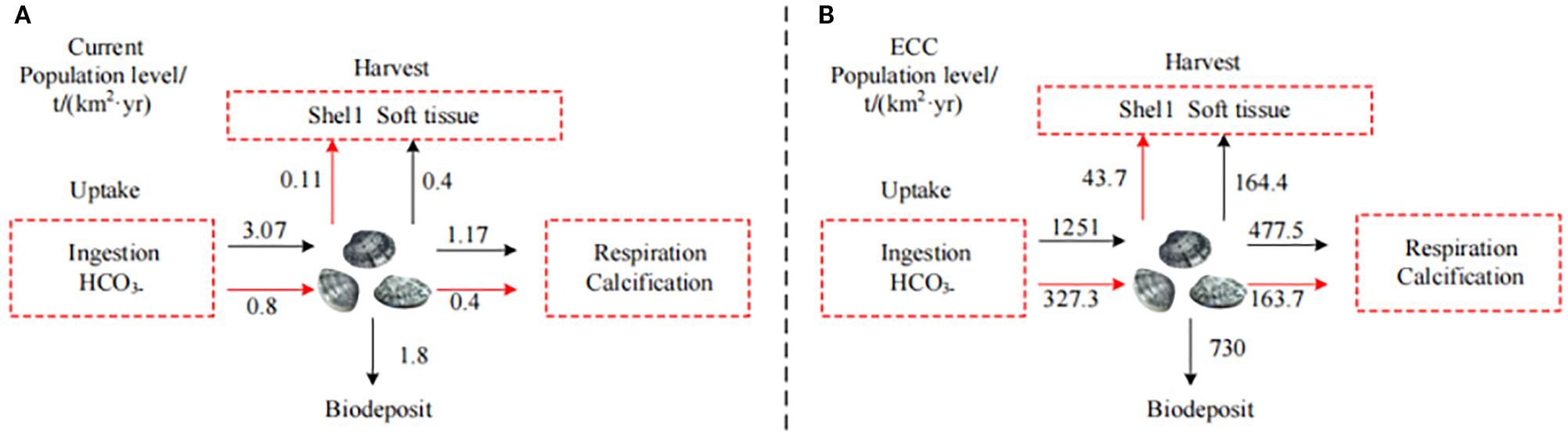
Figure 6. Carbon balance at the population level of Ruditapes philippinarum in Dashentang National Marine Ranch in TianJin. Red lines indicate carbon input pathways (including ingestion and inorganic carbon uptake), while black lines represent carbon output pathways (including deposition, respiration and calcification, and removal through harvest).
5 Conclusion
Against the backdrop of increasingly degraded marine environments, the Tianjin Dashentang National Marine Ranch has implemented multiple ecological restoration measures in recent years, including the construction of artificial reefs and the stocking and release of marine species, aiming to conserve fishery resources and restore ecosystem services. Ecosystem analysis based on the EwE model demonstrated that the combined implementation of R.philippinarum stocking and artificial reefs deployment improved the structural integrity and functional performance of the ranch ecosystem to some extent. the structural integrity and functional performance of the ranch ecosystem. The model estimated the ecological carrying capacity of R. philippinarum in the Dashentang Marine Ranch to be 208.1 t/km².Under simulated scenarios, increases in the biomass of R. philippinarum potentially accompanied by rises in the biomass of key functional groups, including filter-feeding bivalves, benthic fishes, cephalopods, and benthic predators, suggesting a potential enhancement of secondary productivity and overall ecosystem carrying capacity.Within the food web structure, R. philippinarum functioned as a key energy node, dominating the primary trophic pathway from phytoplankton to benthic carnivores, thereby improving primary production utilization and streamlining energy transfer routes.Under static equilibrium conditions in Ecopath, the expansion of R. philippinarum aquaculture may impose competitive pressure on benthic predators. However, with the enhancement of habitat structure and energy flow provided by artificial reefs, the overall system carrying capacity appears to increase, allowing key functional groups—including benthic predators—to still potentially exhibit synergistic growth in the long-term dynamics simulated by Ecosim. This indicates that the sustainability of clam aquaculture depends not only on the scale of stocking itself but also on the integration of ecological engineering measures, thereby optimizing both ecosystem structure and function.Ecosystem stability and maturity showed a rising trend, as indicated by a 35.75% increase in TB, along with rises in TST and FCI.Nevertheless, it should be noted that the decline in Shannon Diversity Index (SDI) does not necessarily only represent improved stability or maturity, but may also indicate potential risks, and thus should be interpreted with caution.In terms of carbon sink capacity, each square kilometer of R. philippinarum can sequester approximately 7.6 tons of carbon annually, resulting in an estimated system-wide increase of about 10, 300 tons of carbon fixed per year. Meanwhile, artificial reefs further contributed to blue carbon enhancement by improving hydrodynamic conditions, increasing filter-feeding efficiency, and promoting benthic carbon deposition.The synergistic effect of clam stocking and reef deployment not only boosted carbon sequestration capacity, but also appears to facilitate biodiversity recovery and improvements in water quality.It is important to note, however, that the role of bivalve aquaculture as a carbon sink remains debated internationally; for example, CaCO3 precipitation may release CO2, and the ultimate fate of shells is uncertain. Therefore, such conclusions should be treated cautiously.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
YZ: Conceptualization, Writing – review & editing, Software, Investigation, Methodology, Formal analysis, Writing – original draft, Data curation, Visualization. DZ: Writing – review & editing, Supervision, Methodology, Resources, Investigation. YG: Formal analysis, Funding acquisition, Investigation, Writing – review & editing, Supervision, Methodology. SL: Investigation, Formal analysis, Supervision, Methodology, Writing – review & editing, Funding acquisition. BG: Supervision, Methodology, Investigation, Resources, Writing – review & editing, Funding acquisition. ML: Methodology, Supervision, Investigation, Resources, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by the National Key R&D Program of China (Grant numbers:2023YFD2401103), Tianjin Fisheries Green Development Project (YYLSFZ202501) and Tianjin Seawater Aquaculture Industry Technology System Innovation Team Project (ITTMRS 2021013).
Acknowledgments
We gratefully acknowledge Yaxin Shang, Bingqian Liu and Lingyun Zhang, for their assistance during the fieldwork. We also thank Yuxuan Fan and Wenqing Cui for their helpful discussion and proofreading in the course of writing articles.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1640824/full#supplementary-material
References
Allesina S. and Bondavalli C. (2003). Topological indicators for food web models: A graph-theoretic approach. Ecol. Model. 169, 1–25. doi: 10.1016/S0304-3800(03)00179-3
Bartoli M., Nizzoli D., Viaroli P., Turolla E., Castaldelli G., Fano E. A., et al. (2001). Impact of Tapes philippinarum farming on nutrient dynamics and benthic respiration in the Sacca di Goro. Hydrobiologia 455, 203–212. doi: 10.1023/A:1011994620914
Borrett S. R. and Osidele O. O. (2007). Cycling in ecological networks: Finn’s index revisited. Ecol. Model. 203, 439–453. doi: 10.1016/j.ecolmodel.2007.03.011
Chen L., Li R., and Sun J. (2023). Indirect competition mediated by bivalve aquaculture: A case study on Sebastiscus marmoratus. J. Exp. Mar. Biol. Ecol. 567, 102345. doi: 10.1016/j.jembe.2023.102345
Christensen V. (1995). Ecosystem maturity—towards quantification. Ecol. Model. 77, 3–32. doi: 10.1016/0304-3800(93)E0073-C
Christensen V., Walters C. J., and Pauly D. (2005). Ecopath with Ecosim: Methods, capabilities and limitations. Fisheries Centre Research Reports 12 (2), 1–154. Vancouver: University of British Columbia. Retrieved from https://www.researchgate.net/publication/267193103_Ecopath_with_Ecosim_A_User%27s_Guide.
Christensen V., Walters C. J., and Pauly D. (2005). Ecopath with Ecosim: Methods, capabilities and limitations. Fisheries Centre Res. Rep. 12, 1–154. Vancouver: University of British Columbia. Available online at: https://www.researchgate.net/publication/267193103_Ecopath_with_Ecosim_A_User%27s_Guide
Coll M., Bundy A., and Shannon L. J. (2009). “Ecosystem modelling using the Ecopath with Ecosim approach,” in Computers in fisheries research. Eds. Megrey B. A. and Moksness E. (Springer, Dordrecht), 225–291. doi: 10.1007/978-1-4020-8636-6_8
Coll M., Palomera I., Tudela S., and Dowd M. (2015). Ecological indicators to capture the effects of fishing on biodiversity and conservation status of marine ecosystems. Ecol. Indic. 60, 947–962. doi: 10.1016/j.ecolind.2015.08.036
Costello M. J. and Chaudhary C. (2017). Marine biodiversity, biogeography, deep-sea gradients, and conservation. Curr. Biol. 27, R511–R527. doi: 10.1016/j.cub.2017.04.060
Dalian Fisheries Research Institute (2025). Annual report on the ecological impacts of Manila clam aquaculture in Changhai County (Dalian: China Ocean Press).
Dalsgaard J. P. T. and Christensen V. (1997). Flow modelling with ECOPATH: Providing insights on the ecological state of agroecosystems (Dordrecht: Springer). doi: 10.1007/978-94-011-5416-1_16
Dumbauld B. R., Ruesink J. L., and Rumrill S. S. (2009). The ecological role of bivalve shellfish aquaculture in the estuarine environment: A review with application to oyster and clam culture in West Coast (USA) estuaries. Aquaculture 290, 196–223. doi: 10.1016/j.aquaculture.2009.02.033
Dunne J. A., Williams R. J., and Martinez N. D. (2002). Network structure and robustness of marine food webs. Proc. R. Soc. B: Biol. Sci. 269, 1420–1426. doi: 10.1098/rspb.2002.2001
Fath B. D., Scharler U. M., Ulanowicz R. E., and Hannon B. (2007). Network analysis of ecosystems: How pathways and cycling shape network properties. Ecol. Complexity 4, 37–43. doi: 10.1016/j.ecocom.2007.02.001
Feng X., Fan J., and San X. (2021). The stock enhancement effect evaluation of artificial reef in Wailingding, Zhuhai. J. South Agric. Sci. 52, 3228–3236. doi: 10.3969/j.issn.2095-1191.2021.12.005
Filgueira R., Peteiro L. G., Labarta U., Fernández-Reiriz M. J., and King J. (2022). Manila clam and Mediterranean mussel aquaculture is sustainable and a net carbon sink. Sci Total Environ. 848, 157508. doi: 10.1016/j.scitotenv.2022.157508
Finn J. T. (1976). Measures of ecosystem structure and function derived from analysis of flows. J. Theor. Biol. 56, 363–380. doi: 10.1016/0022-5193(76)90126-2
Fogarty M. J. (2002). “Climate variability and ocean ecosystem dynamics: Implications for sustainability,” in Challenges of a changing Earth. Eds. Steffen W., Jäger J., Carson D. J., and Bradshaw C. (Springer, Berlin, Heidelberg), 43–48. doi: 10.1007/978-3-642-19016-2_4
Food and Agriculture Organization of the United Nations (FAO) (1999). Indicators for sustainable development of marine capture fisheries. FAO Technical Guidelines for Responsible Fisheries No. 8 (Rome: FAO).
Food and Agriculture Organization of the United Nations (FAO) (2018). The state of world fisheries and aquaculture 2018: Meeting the sustainable development goals (Rome: FAO). Available online at: http://www.fao.org/3/i9540en/i9540en.pdf (Accessed October 15, 2024).
Gaines S., Lubchenco J., and Haugan P. M. (2023). “The expected impacts of climate change on the ocean economy,” in The Blue Compendium. Eds. Lubchenco J. and Haugan P. M. (Springer, Cham). doi: 10.1007/978-3-031-16277-0_2
Grigg R. W., Polovina J. J., and Atkinson M. J. (1984). Model of a coral reef ecosystem. Coral Reefs 3, 23–27. doi: 10.1007/BF00306137
Guo B., Ma X., and Song C. (2020). Blue carbon potential of bivalve aquaculture. Ecol. Eng. 150, 105756. doi: 10.1016/j.ecoleng.2020.105756
Guo Y., Zhang Y., and Li M. (2024). Spatial conflicts between bivalve farms and fish spawning grounds in coastal China. Fisheries Res. 265, 106732. doi: 10.1016/j.fishres.2024.106732
Heymans J. J., Coll M., Libralato S., and Pauly D. (2014). Global patterns in ecological indicators of marine food webs: A modelling approach. PloS One 9, e95207. doi: 10.1371/journal.pone.0095207
Heymans J. J., Coll M., Link J. S., Mackinson S., Steenbeek J., Walters C., et al. (2016). Best practice in Ecopath with Ecosim food-web models for ecosystem-based management. Ecol. Model. 331, 173–184. doi: 10.1016/j.ecolmodel.2015.12.007
Hoegh-Guldberg O., Northrop E., and Lubchenco J. (2019). The ocean as a solution to climate change: Five opportunities for action (Washington, DC: World Resources Institute). Available online at: https://oceanpanel.org/sites/default/files/2019-10/HLP_Report_Ocean_Solution_Climate_Change_final.pdf (Accessed October 15, 2024).
Holling C. S. (1973). Resilience and stability of ecological systems. Annu. Rev. Ecol. Systematics 4, 1–23. doi: 10.1146/annurev.es.04.110173.000245
Huang X., Wang Q., and Zhang Y. (2025). Restoring rocky reef habitats for Sebastiscus marmoratus: A case study in the Yellow Sea. ICES J. Mar. Sci 82, 1123–1135. doi: 10.1093/icesjms/fsad045
Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES) (2019). Global assessment report on biodiversity and ecosystem services (Bonn: IPBES Secretariat). Available online at: https://ipbes.net/global-assessment (Accessed October 15, 2024).
Kang D., Kim S., Park J., and Lee C. (2017). Artificial habitat construction improves survival and settlement of Manila clam (Ruditapes philippinarum) in coastal restoration projects. Mar. Ecol. Prog. Ser. 573, 101–115. doi: 10.3354/meps12145
Lee S. I. and Zhang C. I. (2018). Evaluation of the effect of marine ranching activities on the Tongyeong marine ecosystem. Ocean Sci J. 53, 557–582. doi: 10.1007/s12601-018-0045-8
Li Z., Chen Y., Wang G., Mu J., Sun Y., Yu H., et al. (2023). Ecological carrying capacity and carbon sequestration potential of bivalve shellfish in marine ranching: A case study in Bohai Bay, China. Front. Mar. Sci 10. doi: 10.3389/fmars.2023.1174235
Li Y., Sun J., and Zhang Y. (2015). Comparative analysis of ecosystem maturity indices in Lake Taihu. Ecol. Model. 317, 107–116. doi: 10.1016/j.ecolmodel.2015.07.014
Li M., Wang Q., and Zhang Y. (2025). Larval fish growth inhibition under bivalve-dominated planktonic communities. Limnology Oceanography 70, 789–801. doi: 10.1002/lno.12456
Lindeman R. L. (1942). The trophic-dynamic aspect of ecology. Ecology 23, 399–418. doi: 10.2307/1930126
Link J. S. (2010). Ecosystem-based fisheries management: Confronting tradeoffs (Cambridge: Cambridge University Press). doi: 10.1017/CBO9780511667091
Liu X. (2011). Marine ranching and shellfish stocking in Bohai Bay: Case studies and management implications. Chin. J. Appl. Ecol. 22, 2103–2110.
Liu X., Li J., Sun T., and Zhao R. (2023). Trophic cascades induced by Manila clam (Ruditapes philippinarum) farming in Bohai Bay: Implications for crustacean recruitment. Mar. pollut. Bull. 195, 115432. doi: 10.1016/j.marpolbul.2023.115432
Liu H., Wang Q., and Zhang Y. (2022). Energy cycling and disturbance resistance in marine ecosystems: The role of filter-feeding organisms. Mar. Ecol. Prog. Ser. 688, 1–14. doi: 10.3354/meps14156
Liu X., Zhang Y., and Wang H. (2024). Habitat overlap between Manila clams and burrowing crustaceans in intertidal zones. Mar. Ecol. Prog. Ser. 689, 45–58. doi: 10.3354/meps14256
Liu S. and Zhou W. (2024). Impacts of clam aquaculture on juvenile Sebastiscus marmoratus in Rongcheng Bay. Chin. J. Oceanology Limnology 42, 234–245. doi: 10.1007/s00343-024-3056-9
Liu S. R., Zhou X. J., Zeng C., Frankstone T., and Cao L. (2022). Characterizing the development of sea ranching in China. Rev. Fish Biol. Fisheries 32, 783–803. doi: 10.1007/s11160-022-09709-8
Magurran A. E. (2004). Measuring biological diversity (Wiley-Blackwell, Oxford, UK: Blackwell Publishing).
McKindsey C. W., Thetmeyer H., Landry T., and Silvert W. (2006). Review of recent carrying capacity models for bivalve culture and recommendations for research and management. Aquaculture 261, 451–462. doi: 10.1016/j.aquaculture.2006.06.044
Moberg F. and Folke C. (1999). Ecological goods and services of coral reef ecosystems. Ecol. Econ 29, 215–233. doi: 10.1016/S0921-8009(99)00009-9
Moreau J. (1995). “Analysis of species changes in Lake Victoria using ECOPATH, a multispecies trophic model,” in The Impact of Species Changes in African Lakes. Chapman & Hall Fish and Fisheries Series, vol 18. Eds. Pitcher T. J. and Hart P. J. B. (Springer, Dordrecht). doi: 10.1007/978-94-011-0563-7_8
Morissette L. (2007). Complexity, cost and quality of ecosystem models and their impact on resilience: A comparative analysis, with emphasis on marine mammals and the Gulf of St (Modulo, Montréal, QC, Canada: University of British Columbia). doi: 10.14288/1.0074903
National Oceanic and Atmospheric Administration (NOAA) (2010). Ecopath modeling: Precursor to an ecosystem approach to fisheries management (U.S. Department of Commerce). Available online at: https://spo.nmfs.noaa.gov/ (Accessed October 15, 2024).
Nizzoli D., Welsh D. T., Bartoli M., and Viaroli P. (2001). Denitrification, nitrate recycling and organic matter oxidation in sediments underlying Tapes philippinarum (Manila clam) culture. Mar. Ecol. Prog. Ser. 215, 237–249. doi: 10.3354/meps215237
Odum E. P. (1969). The strategy of ecosystem development. Science 164, 262–270. doi: 10.1126/science.164.3877.262
Pauly D., Christensen V., and Dalsgaard J. (1984). Fisheries impacts on marine ecosystems: A global perspective (Manila: International Center for Living Aquatic Resources Management).
Philippsen J. S., Minte-Vera C. V., Coll M., and Angelini R. (2019). Assessing fishing impacts in a tropical reservoir through an ecosystem modeling approach. Rev. Fish Biol. Fisheries 29, 125–146. doi: 10.1007/s11160-018-9539-9
Piroddi C., Coll M., and Christensen V. (2015). Global patterns in ecological indicators of marine food webs: A modelling approach. PloS One 10, e0123763. doi: 10.1371/journal.pone.0123763
Polovina J. J. (1984). Model of a coral reef ecosystem. J. Fisheries Res. Board Canada 41, 1305–1311. doi: 10.1139/f84-149
Sumaila U. R., Virdin J., and Lubchenco J. (2023). “Ocean finance: Financing the transition to a sustainable ocean economy,” in The Blue Compendium. Eds. Lubchenco J. and Haugan P. M. (Springer, Cham), 135–150. doi: 10.1007/978-3-031-16277-0_9
Sun H., Zhang J., Li M., and Wang Q. (2025). Ammonia and phosphate accumulation in bivalve aquaculture zones: Thresholds for fish avoidance. Mar. pollut. Bull. 201, 116789. doi: 10.1016/j.marpolbul.2025.116789
Teh L. C. L. and Sumaila U. R. (2013). Contribution of marine fisheries to worldwide employment. Fish Fisheries 14, 77–88. doi: 10.1111/j.1467-2979.2011.00450.x
Tuomisto H. (2010). A consistent terminology for quantifying species diversity? Oecologia 164, 853–860. doi: 10.1007/s00442-010-1812-5
Ulanowicz R. E. (1986). Growth and development: Ecosystem phenomenology (New York: Springer-Verlag). doi: 10.1007/978-1-4612-4916-0
Wada T., Tanaka Y., and Morioka S. (2015). Effectiveness of bottom sowing and anti-predator devices in Manila clam (Ruditapes philippinarum) stock enhancement. Fisheries Res. 164, 76–85. doi: 10.1016/j.fishres.2014.12.010
Walters C., Christensen V., and Pauly D. (1997). Structuring dynamic models of exploited ecosystems from trophic mass-balance assessments. Rev. Fish Biol. Fisheries 7, 139–172. doi: 10.1023/A:1018479526149
Walters C., Pauly D., and Christensen V. (1999). Ecospace: Prediction of mesoscale spatial patterns in trophic relationships of exploited ecosystems, with emphasis on the impacts of marine protected areas. Ecosystems 2, 539–554. doi: 10.1007/s100219900101
Wan X., Zhang G., and Li Q. (2023). Marine ranching engineering collaborative innovation from the perspective of ecological environment. J. Ocean Univ. China 22, 1151–1163. doi: 10.1007/s11802-023-5567-8
Wang Q., Liu H., and Zhang Y. (2024). Enhancing ecosystem resilience through biodiversity and functional redundancy. Ecol. Evol. 14, 1234–1245. doi: 10.1002/ece3.9876
Wang Y. and Zhang W. (2021). Maximum sustainable yield estimation of enhancement species with the characteristics of movement inside and outside marine ranching. J. Oceanology Limnology 39, 2380–2387. doi: 10.1007/s00343-020-0288-y
Wang X., Zhang Y., Li J., and Chen W. (2022). Assessing unintended ecological consequences of Manila clam proliferation in coastal marine ecosystems. Mar. Environ. Res. 178, 105634. doi: 10.1016/j.marenvres.2022.105634
Wang T., Zhang Y., Li M., and Zhao X. (2023). Hypoxia-driven habitat shifts in coastal fish populations. Environ. Res. Lett. 18, 104023. doi: 10.1088/1748-9326/acf0d2
Wang Q., Zhang Y., Li M., and Zhao X. (2025). Trophic cascades and habitat degradation in coastal ecosystems: Synergistic effects of Ruditapes philippinarum. Ecol. Appl. 35, e2895. doi: 10.1002/eap.2895
Wang Y., Zhang H., and Zhao F. (2016). Role of bivalve calcification in blue carbon sequestration. Mar. pollut. Bull. 110, 9–14. doi: 10.1016/j.marpolbul.2016.06.001
Wang Y., Zhang H., and Zhao F. (2019). Diet overlap and resource partitioning between Manila clam and shrimp in coastal ecosystems. Aquaculture 503, 201–209. doi: 10.1016/j.aquaculture.2019.01.027
Williams R. J. and Martinez N. D. (2004). Stabilization of chaotic and non-permanent food-web dynamics. Eur. Phys. J. B 38, 297–303. doi: 10.1140/epjb/e2004-00204-1
Worm B., Barbier E. B., Beaumont N., Duffy J. E., Folke C., Halpern B. S., et al. (2006). Impacts of biodiversity loss on ocean ecosystem services. Science 314, 787–790. doi: 10.1126/science.1132294
Xiao S., Liu X., and Zhou R. (2024). Energy flow analysis of grass carp pond system based on Ecopath model. Environ. Sci pollut. Res. 31, 10184–10197. doi: 10.1007/s11356-023-27154-3
Xu J., Chen L., and Li X. (2018). Carbon sequestration potential of Ruditapes philippinarum populations. J. Shellfish Res. 37, 45–56. doi: 10.2983/035.037.0105
Yan J., Chen Y., Cao Y., Sun J., Wen B., Gao X., et al. (2025). Marine ranching enhances ecosystem stability and biological carbon sequestration potential: Insights from Ecopath with Ecosim model simulation of 30-year ecological path of a national marine ranching in China. Front. Mar. Sci 12. doi: 10.3389/fmars.2025.1583896
Zhang X., Li Y., Wang Q., and Chen J. (2019). Ecosystem risks of high-density bivalve release: Disease transmission and biosecurity concerns in marine ranching. Mar. pollut. Bull. 148, 123–135. doi: 10.1016/j.marpolbul.2019.07.041
Zhang Y., Wang L., and Chen H. (2024a). Bivalve aquaculture density thresholds for balancing ecosystem services and disservices in Jiaozhou Bay, China. Ecol. Indic. 158, 109876. doi: 10.1016/j.ecolind.2023.109876
Zhang Y., Wang Q., and Li M. (2024b). Filtration capacity and phytoplankton suppression by Manila clams in intensive mariculture. Aquaculture Environ. Interact. 16, 89–102. doi: 10.3354/aei00478
Zhang J., Wang Q., and Li M. (2025). Density thresholds for hypoxia induction in Manila clam farming areas. J. Mar. Syst. 248, 103212. doi: 10.1016/j.jmarsys.2025.103212
Zhang Y., Wang X., Xu M., and Chen Y. (2024c). Assessment of carbon sink capacity and its value accounting for a farmed shellfish in the coastal wetland of the Yalu River Estuary. Front. Aquaculture 1. doi: 10.3389/faquc.2024.1355741
Keywords: Ruditapes philippinarum, marine ranching, ecopath with ecosim, ecological carrying capacity, carbon budget
Citation: Zhang Y, Zheng D, Guo Y, Liang S, Guo B and Li M (2025) Ecological carrying capacity and carbon sequestration potential of Ruditapes philippinarum: a case study in Dashentang national marine ranch in TianJin Bohai Bay, China. Front. Mar. Sci. 12:1640824. doi: 10.3389/fmars.2025.1640824
Received: 04 June 2025; Accepted: 15 September 2025;
Published: 29 September 2025.
Edited by:
Shulei Cheng, Southwestern University of Finance and Economics, ChinaReviewed by:
Chunyu Lin, Ocean University of China, ChinaPo-Yuan Hsiao, National Taiwan Ocean University, Taiwan
Copyright © 2025 Zhang, Zheng, Guo, Liang, Guo and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Muhan Li, bGltdWhhbkB0amF1LmVkdS5jbg==; Biao Guo, b3VjZ3VvYmlhb0AxNjMuY29t
 Yue Zhang
Yue Zhang Debin Zheng2
Debin Zheng2 Yongjun Guo
Yongjun Guo Shuang Liang
Shuang Liang Biao Guo
Biao Guo Muhan Li
Muhan Li