- 1Department of Earth and Marine Sciences, University of Palermo, Palermo, Italy
- 2NBFC, National Biodiversity Future Center, Palermo, Italy
- 3National Center for Scientific Research, Research and Service Units (USR) 3278 - Centre de Recherches Insulaires et Observatoire de l’environnement (CRIOBE) - École Pratique des Hautes Etudes (EPHE)- University of Perpignan Via Domitia (UPVD)-CNRS, Perpignan, France
Biomass is an essential indicator for assessing the quality, stability, productivity, and health of ecosystems and it is increasingly used as a practical monitoring parameter within the Essential Ocean Variables (EOV) framework. In the Mediterranean Sea, vermetid reefs - unique bioconstructions structurally comparable to fringing coral reefs - play a significant role in coastal dynamics yet remain understudied. This study aimed to quantify the biomass distribution of the main structural species Dendropoma cristatum, seaweeds, and associated fauna found across three distinct reef zones: the inner edge (adjacent to the mainland with limited water movement), the cuvette (a central zone characterized by shallow, sheltered pools), and the outer edge (directly exposed to constant wave action). Rather than testing for a causal “zone effect”, we provide a site-specific quantitative baseline that can be used to track future change and to generate hypotheses on the environmental drivers of biomass patterns. Samples were collected from a vermetid reef in north-western Sicily, Italy, and biomass was estimated as ash-free dry weight (AFDW; a measure of organic matter excluding mineral content). This exploratory study of spatial patterns within one reef site revealed an average biomass of 321.4 g AFDW m−2, with values that increased from the inner edge to the outer zone within the single site studied. D. cristatum biomass was highest at the outer edge (likely reflecting its preference for stronger water movement and higher food supply), while seaweed biomass peaked in the outer edge and cuvette, where macroalgal diversity was greatest. Phytal fauna biomass was strongly correlated with seaweed biomass (R² = 0.68), emphasizing the role of macroalgae as ecosystem engineers, whereas vagile fauna were more abundant in the inner edge and cuvette, possibly benefiting from increased structural complexity and reduced predation. These site-specific findings illuminate spatial patterns of biomass distribution within this vermetid reef and provide essential baseline data for monitoring this location, whilst generating hypotheses for testing at other Mediterranean vermetid reef sites.
1 Introduction
Biomass, or body mass - the weight of all living organisms in a given area or volume in terrestrial or aquatic habitats - is an essential quantitative indicator for assessing ecosystems’ quality reflecting its stability, productivity, and health (Bar-On et al., 2018; Kissling et al., 2018; Miloslavich et al., 2018). Research in the marine environment led to identify biomass as one of the Essential Ocean Variables (EOV), being a valid indicator of oceanic health and productivity (Constable et al., 2016; Levin et al., 2019; Miloslavich et al., 2018). This variable supports the assessment and monitoring of the material and energy flows in ecosystems (Tomlinson et al., 2014), the study of ecosystem structure and functioning (Bar-On et al., 2018), and the quantification of many ecosystem services (Benoist et al., 2019). Biomass census represents a first step in the investigation of the marine environment, providing pivotal information, especially for habitats where data are scarce or perhaps non-existent. These data are essential for understanding the role these habitats play at a large ecosystem scale, which is crucial for planning management and conservation actions (Benoist et al., 2019).
In the Mediterranean Sea, vermetid reefs are a unique bioconstruction superficially comparable in structure to fringing coral reefs in tropical areas (Safriel and Ben-Eliahu, 1991; Antonioli et al., 1999; Chemello and Silenzi, 2011). They are built by the gregarious gastropods belonging to the genus Dendropoma and the coralline alga Neogoniolithon brassica-florida (Harvey) Setchell & L.R.Mason that cements the tubular shells of the gastropod (Milazzo et al., 2016). The resulting structures are horizontal platforms that extend from land to sea in the lower intertidal zone, composed by three distinct zones: an inner edge (IE), the border between the reef and the mainland; a cuvette (CV), a central area characterized by shallow pools; and an outer edge (OE), which separates the structure from the sea (Chemello and Silenzi, 2011).
Vermetid reefs provide important regulating ecosystem services such as coastal stabilization and protection against erosion (Chemello and Silenzi, 2011; Milazzo et al., 2016); mediating sediment transport (Milazzo et al., 2016); providing refuge from predation to interstitial organisms (Chemello and Milazzo, 2002; Danovaro and Fraschetti, 2002; Frame et al., 2007; Gee and Warwick, 1994; Milazzo et al., 2016) and maintaining nursery populations for many species (Franzitta et al., 2016; Ingrosso et al., 2018; Milazzo et al., 2016). In addition, the presence of the vermetid reefs in the coastal seascape increases its structural complexity (Picone and Chemello, 2023), creating different micro-habitats that promote high biodiversity levels, including numerous sessile organisms. Among these, macroalgae play an important role as they further increase the complexity of the structure and provide additional refuge from predation.
Different studies report an overall deterioration status of vermetid reefs attributable to several stressors between global warming and local impacts occurring in the last few decades in the region (Badreddine et al., 2019; Bisanti et al., 2024, 2022; Di Franco et al., 2011; Rilov, 2016). Despite their vulnerability, vermetid reefs are only generically protected under the European Habitat Directive (92/43/EEC, code 1170) and not explicitly considered in many conservation management plans. Despite being acknowledged as a bioconstruction to be protected, vermetid reefs remain a neglected habitat, with a general lack of scientific interest and of data on their status and spatial distribution (Picone et al., 2022). Information on benthic assemblages inhabiting vermetid reefs is scarce or absent, and many references are sourced from grey literature. Previous studies on benthic communities such as gastropods (Chemello et al., 1997), polychaetes (Safriel and Ben-Eliahu, 1991), macroalgae (Mannino, 1992) and meiofaunal organisms (Ape et al., 2018) have shown a high diversity and abundance of species at a small spatial scale with differences in distribution along the vermetid reef. However, a quantitative census of biomass within vermetid reef zones and their associated assemblages is still lacking, creating a knowledge gap that this baseline study seeks to address.
This study therefore offers a first site-specific census of biomass partitioning across the three morphological zones (IE, CV, OE) of a Mediterranean vermetid reef, focusing on the reef-builder Dendropoma cristatum (Biondi-Giunti, 1859), macroalgae and their associated fauna. The results are intended as site-specific baseline data rather than as generalized evidence of a zone effect, providing a foundation for hypothesis generation and future comparative studies across multiple vermetid reef sites.
2 Materials and methods
2.1 Study area and sample collection
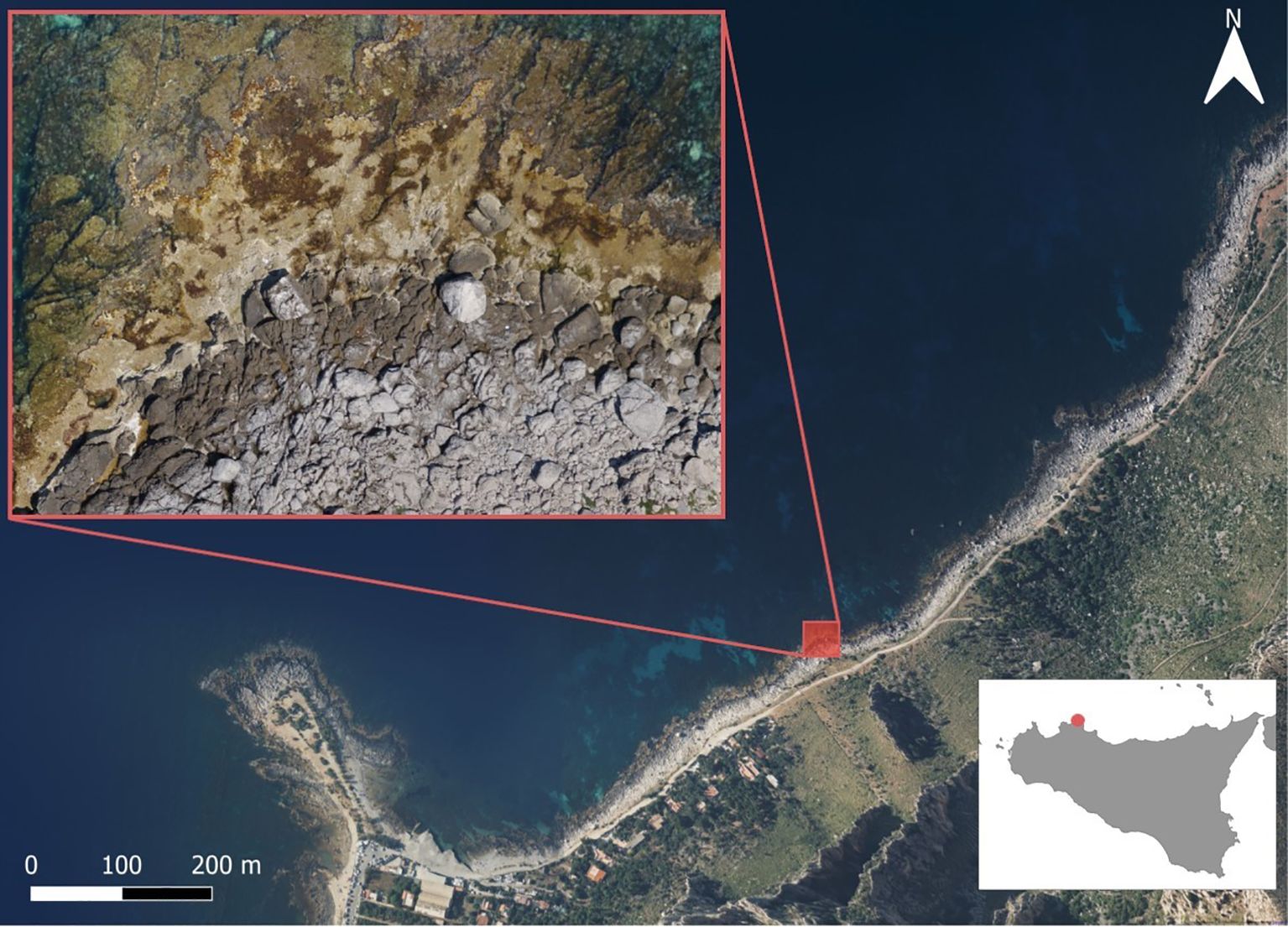
The study was carried out on the rocky shore of the AMP “Capo Gallo - Isola delle Femmine”, Palermo (north-western Sicily, Italy; 38.211566, 13.288540) during the summers (July) of 2018 and 2019. Data from both years were pooled for analysis; we acknowledge that interannual variability was not explicitly tested, which represents a limitation of this baseline study. The coastline is characterized by dolomitic limestone substrates from which extensive vermetid reefs built by the mollusk D. cristatum (Biondi-Giunti, 1859) develop (Chemello and Silenzi, 2011) (Figure 1). In this area, the seaweed community growing on the vermetid reefs is mainly composed of species of the Laurencia complex (Martin-Lescanne et al., 2010), which are uniformly distributed across the reef. The brown seaweeds Halopteris filicina (Grateloup) Kützing, Padina pavonica (Linnaeus) Thivy 1960 and Dyctiota spp. are mainly present in the cuvette area, along with some species of the Cystoseira sensu lato complex (Molinari Novoa and Guiry, 2020; Orellana et al., 2019), such as Ericaria amentacea (C. Agardh) Molinari & Guiry, Cystoseira compressa (Esper) Gerloff & Nizamuddin 1975, Ericaria brachycarpa (J.Agardh) Molinari & Guiry 2020, and C. humilis Schousboe ex Kützing 1860. The Corallinales Jania rubens (Linnaeus) J.V. Lamoroux and Ellisolandia elongata (J.Ellis & Solander) K.R.Hind & G.W. Saunders 2013 are found, respectively, as epiphyte or over the substrate and underneath the brown algae canopy. The outer edge of the vermetid reef is often characterized by the presence of E. amentacea, forming a continuous fringe in optimal environmental conditions (Milazzo et al., 2016).
Because conservation constraints prevented destructive sampling at multiple reefs, the three zones were treated as sub-areas of a single 50m reef section, and subsequent analyses are therefore exploratory and descriptive rather than inferential. Samples were collected randomly on the inner edge (IE), cuvette (CV) and outer edge (OE) along a 50 m vermetid reef section during low-tide conditions. To minimize disturbance to this sensitive habitat, spatial replication was intentionally limited to a single reef section. Different structuring taxa were considered for biomass analysis, with 10 replicates collected for each taxon: the reef builder D. cristatum, the dominant seaweeds C. compressa, E. amentacea, E. elongata, H. filicina, J. rubens, Lithophyllum byssoides (Lamarck) Foslie 1900, P. pavonica (Linnaeus), and algae belonging to the Laurencia complex. Moreover, the main macrofauna (Crustacea, Mollusca, Polychaeta, Sipunculoidea and Echinodermata) was collected and categorized into phytal and vagile assemblages.
Within each vermetid reef section (IE, CV, OE), the biomass of D. cristatum was estimated by taking the individuals present in quadrates of 10 x 10 cm (n = 3). This sample surface was chosen to minimize the effect of the sampling phase on this vulnerable species. Seaweed taxa and associated macrofauna were sampled using quadrats of 20 x 20 cm (n = 3) placed on homogeneous algal patches, with this larger quadrat size selected to ensure representative sampling while still limiting habitat disturbance. The quadrats were entirely scraped and quickly placed into plastic bags to avoid loss of organisms. Additionally, five visual census transects (5 m long and 1 m wide; n = 5) were randomly performed to sample vagile assemblages; all censused organisms were weighed on field with an analytical balance. For these organisms, biomass was estimated indirectly by collecting at least five individuals of each identified species and using them to calculate weight-to-weight conversion factors such as DW/WW (dry weight/wet weight), AFDW/WW (ash-free dry weight/wet weight) and AFDW/DW for all taxa (Van der Meer et al., 2005). All the collected samples were stored at -20°C for further analysis.
2.2 Biomass estimation
The collected seaweeds were rinsed under tap water and the associated fauna was sieved through a 0.5 mm mesh, after a previous sieving through 2 mm mesh. Phytal fauna were sorted into taxonomic macro-groups using a stereomicroscope. For the biomass estimation, all samples (D. cristatum, macroalgae, macrofauna) were weighed to obtain the wet weight (WW) and oven-dried for 48 hours at 80°C to reach the dry weight (DW). After drying, the ash mass was obtained by burning samples in a muffle furnace at 550°C for 4 hours. Finally, biomass was calculated as ash-free dry weight (AFDW) by subtracting ash mass from DW (Van der Meer et al., 2005). Biomass values were standardized in g AFDW m-2 before data analysis.
2.3 Statistical analysis
Zone-level biomass was summarized as means ± SE and explored with one-way ANOVA; full outputs are provided in the Supplementary Material and serve only to visualize within-reef heterogeneity, so post-hoc Tukey contrasts are omitted from the main text. Differences in phytal-fauna biomass among host seaweeds (seven seaweed species/groups: E. elongata, H. filicina, J. rubens, P. pavonica, C. compressa, E. amentacea and the Laurencia complex of species) were explored with PERMANOVA (Permutational multivariate analysis of variance). The analysis was performed using a Bray-Curtis distance matrix based on square-root transformed epifaunal biomass, using 9999 permutations. Homogeneity of multivariate dispersion was checked with PERMDISP (Anderson et al., 2008), and pairwise tests were used to determine which seaweed species drove any significant differences. Variation of the phytal fauna community structure was visualized with a principal coordinate analysis (PCoA) plot (based on a Bray-Curtis distance matrix). Taxon vectors were overlaid (using Pearson correlation) to highlight taxa most responsible for the ordination. All statistical analyses are treated as exploratory and descriptive tools to visualize within-reef heterogeneity and generate hypotheses for future studies, rather than as inferential tests of ecological patterns, given the spatial dependence of zones within a single site. Statistical analyses were performed using R open access statistical software 4.1.2 (R Core Team, 2021).
3 Results
The biomass of D. cristatum changed within the single vermetid reef examined, with an overall average value of 43.6 g AFDW m-2. D. cristatum showed the highest biomass in the outer edge (OE = 60.5 ± 10.9 g AFDW m-2), roughly 1.8-fold higher biomass than the inner edge (IE = 33.0 ± 7.1 g AFDW m-2) and cuvette (CV = 37.3 ± 10.9 g AFDW m-2) (Figure 2a, see Supplementary Material). These zone-wise means are presented descriptively to illustrate spatial patterns within this single reef site; exploratory statistical outputs are supplied in the Supplementary Material but are not interpreted as evidence of a replicated habitat-zone effect.
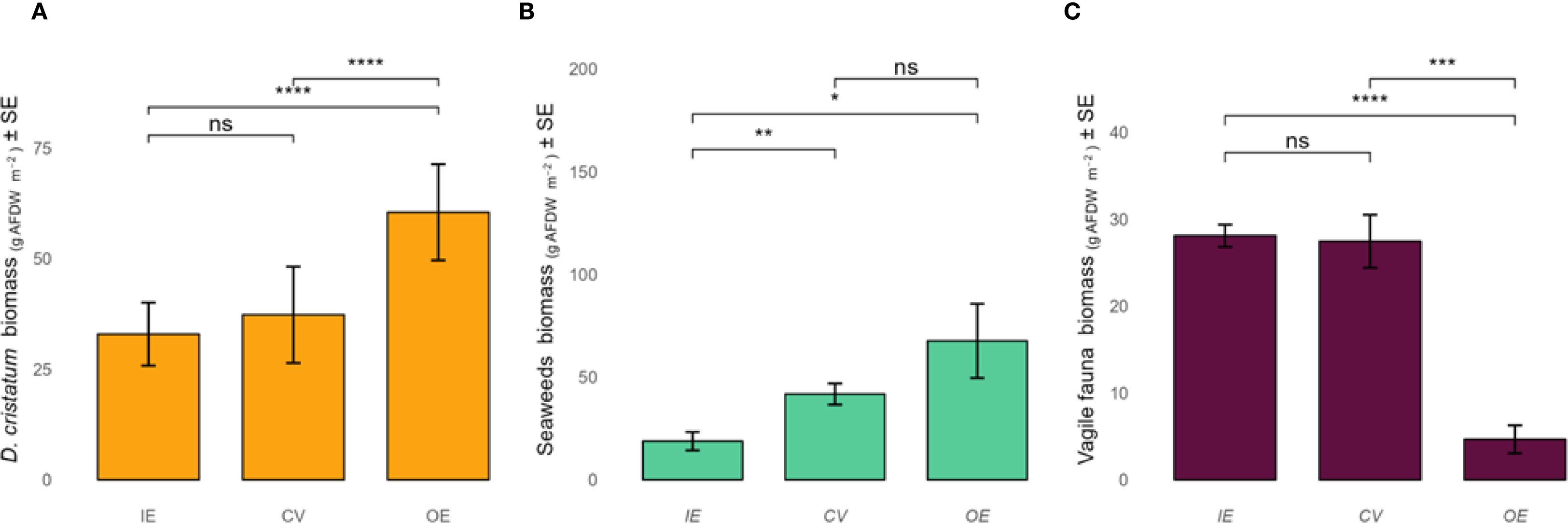
Figure 2. Average biomass (± SE) of Dendropoma cristatum (A), seaweeds (B), and vagile fauna (C) across the three zones (inner edge IE, cuvette CV and outer edge OE) of the vermetid reef. Note the high variability and small sample sizes inherent in this single-site exploratory study.
The overall average seaweeds biomass for a square meter (g AFDW m-2 representing organic biomass) of vermetid reef was 46 ± 31. Within the study site, organic biomass values were 42 ± 18 g AFDW m-2 for the cuvette (CV), 68 ± 44 g AFDW m-2 for the outer edge (OE), and 19 ± 8 g AFDW m-2 for the inner edge (IE) (Figure 2b). The outer edge thus showed the greatest seaweed biomass, whereas the inner edge supported the lowest. Four seaweeds species were found in the cuvette (C. compressa, H. filicina, J. rubens, P. pavonica), while two (E. amentacea and species of the Laurencia complex) and one (E. elongata) species were recorded, respectively, in the outer and inner edges (Figure 2b).
In total, 13 species of vagile macrofauna were identified in the samples. Specifically, 6 crustacean species (Clibanarius erythropus (Latreille, 1818), Eriphia verrucosa (Forskål, 1775), Pachygrapsus marmoratus (Fabricius, 1787), Pachygrapsus maurus (Lucas, 1846), Pachygrapsus transversus (Gibbes, 1850), Palaemon serratus (Pennant, 1777)), and 7 mollusc gastropod species (Cerithium lividulum Risso, 1826, Patella ulyssiponensis Gmelin, 1791, Patella caerulea Linnaeus, 1758, Phorcus turbinatus (Born, 1778), Pisania striata (Gmelin, 1791), Stramonita haemastoma (Linnaeus, 1767), Steromphala divaricata (Linnaeus, 1758)) were found. The overall biomass of the vagile fauna was higher in the IE (10 species, 28.1 ± 1.3 g AFDW m-2) and CV (8 species, 27.5 ± 3 g AFDW m-2) and markedly lower in the OE (4 species, 5 ± 1.6 g AFDW m-2) (Supplementary Tables S5, S6, Figure 2c).
Phytal fauna biomass was most abundant in the OE compared with the other two zones (IE and CV) (Figure 3a, Supplementary Tables S7, S8) and was positively correlated with seaweed biomass (R2 = 0.68). The composition of the phytal fauna community did not change among zones (Figure 3b) or seaweeds (Figures 4a, b), and it was composed by five taxa overall (i.e., Crustacea, Echinodermata, Mollusca, Polychaeta e Sipunculoidea). Sipunculoidea accounted for around 50% of total biomass, followed by Polychaeta (ranging from 10 to 30%), Mollusca, Echinodermata and Crustacea (Figures 4a, b).
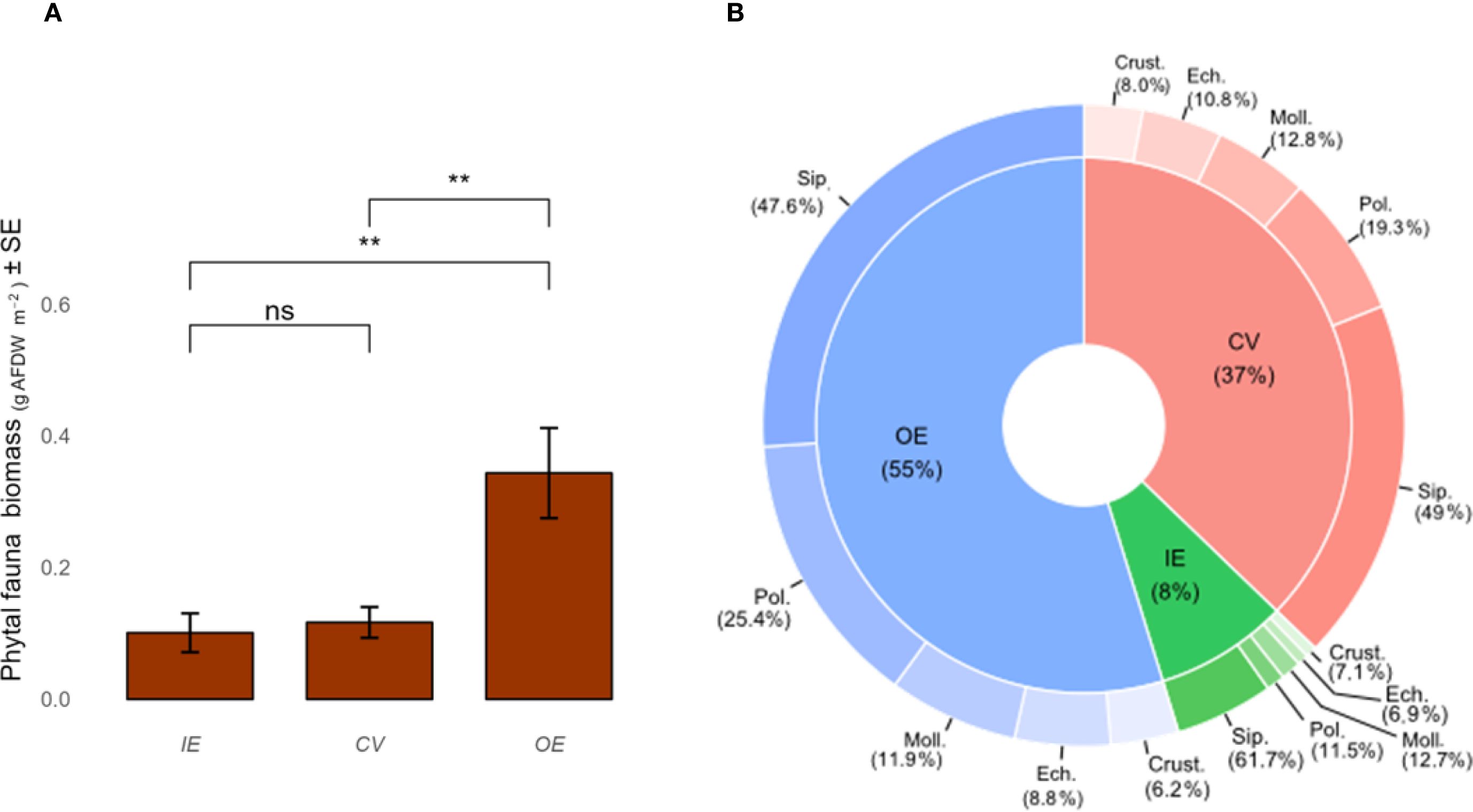
Figure 3. Average biomass (± SE) (A) and percentage of each taxonomic group (B) of the seaweeds and associated fauna in the three portions of the vermetid reef (inner edge IE, cuvette CV and outer edge OE). High variability reflects the exploratory nature of this single-site study.
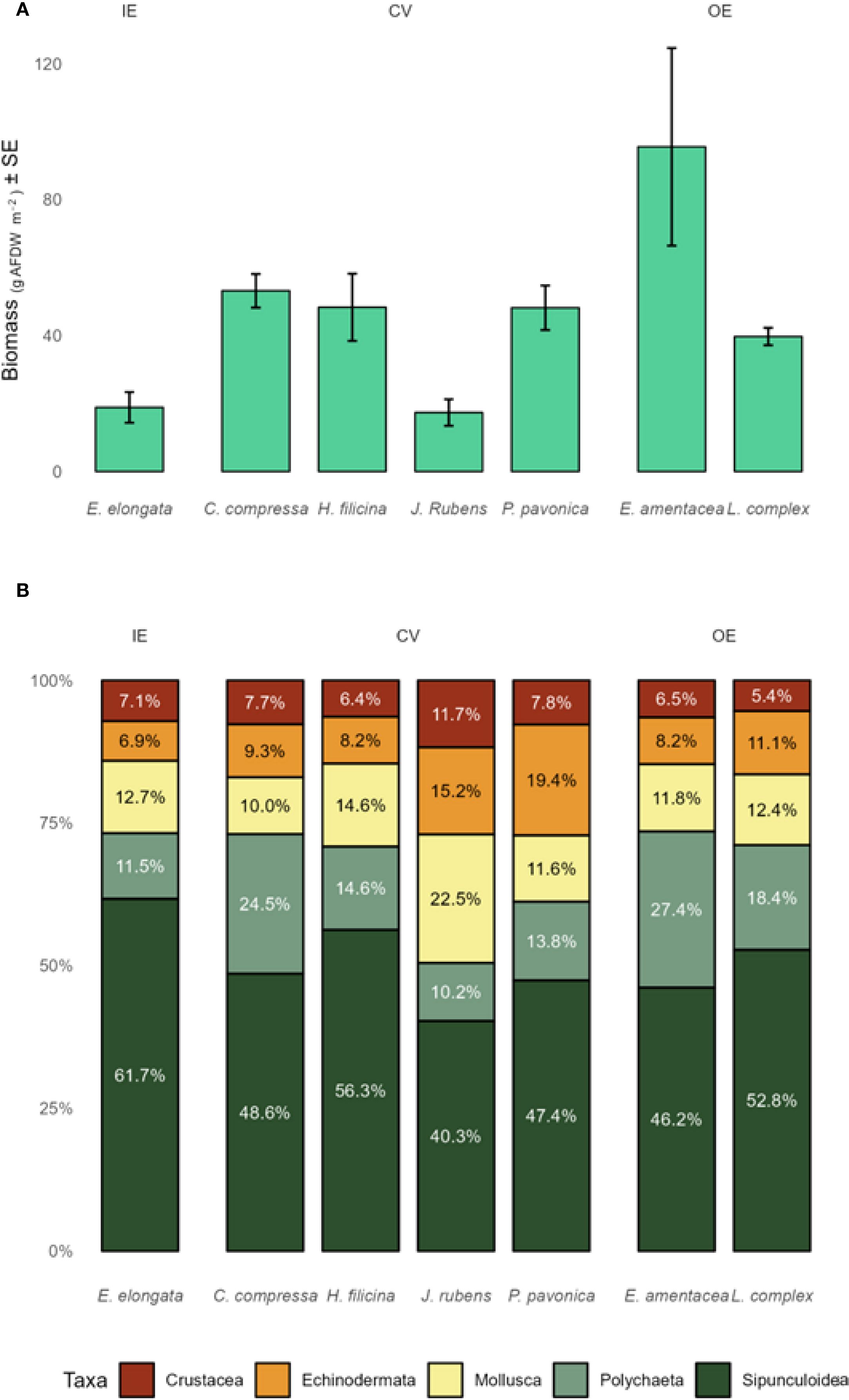
Figure 4. Average seaweeds biomass (± SE) (A) and composition of the associated fauna (B) associated with the macroalgal species colonizing the vermetid reef. IE, inner edge; CV, cuvette; OE, outer edge. Note the considerable variability among samples within this single reef site.
Exploratory PERMANOVA indicated that phytal faunal biomass structure differed among seaweeds hosts (p < 0.001; see Supplementary Table S9); pairwise contrasts showed that E. amentacea and C. compressa supported higher biomass across taxa than the other seaweeds (Figure 5, see Supplementary Table S10), though these patterns should be viewed as hypothesis-generating observations for future multi-site studies rather than as robust statistical inferences.
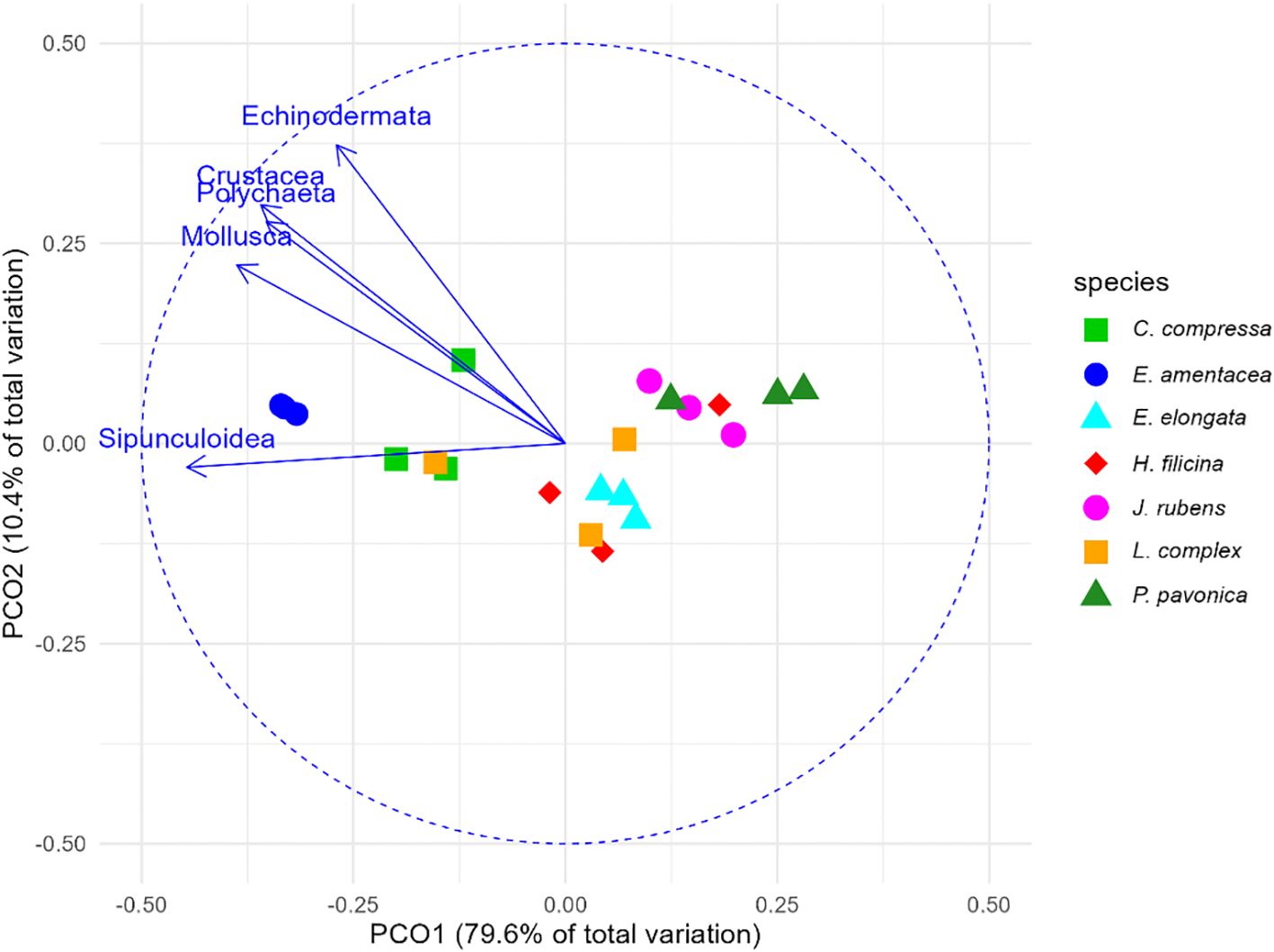
Figure 5. Principal-coordinate analysis (PCoA) of phytal-fauna biomass associated with the seven host seaweeds. Points are individual samples (Bray–Curtis distances on square-root–transformed biomass) colored by algal species; ellipses delimit 95% confidence regions. Vectors represent taxa whose biomass is strongly correlated with the ordination space, highlighting those that drive the largest differences among seaweeds. This exploratory analysis generates hypotheses about host-specific patterns for testing in future multi-site studies.
4 Discussion
This study provides a first site-specific baseline of biomass distribution patterns across the three zones of a Mediterranean vermetid reefs, offering crucial insights into the structure and functioning of these unique intertidal ecosystems. Our single-reef baseline reveals a total biomass like values reported for other intertidal systems (321 g m−2), suggesting that vermetid reefs may act as localized hotspots of biomass (Figure 6).
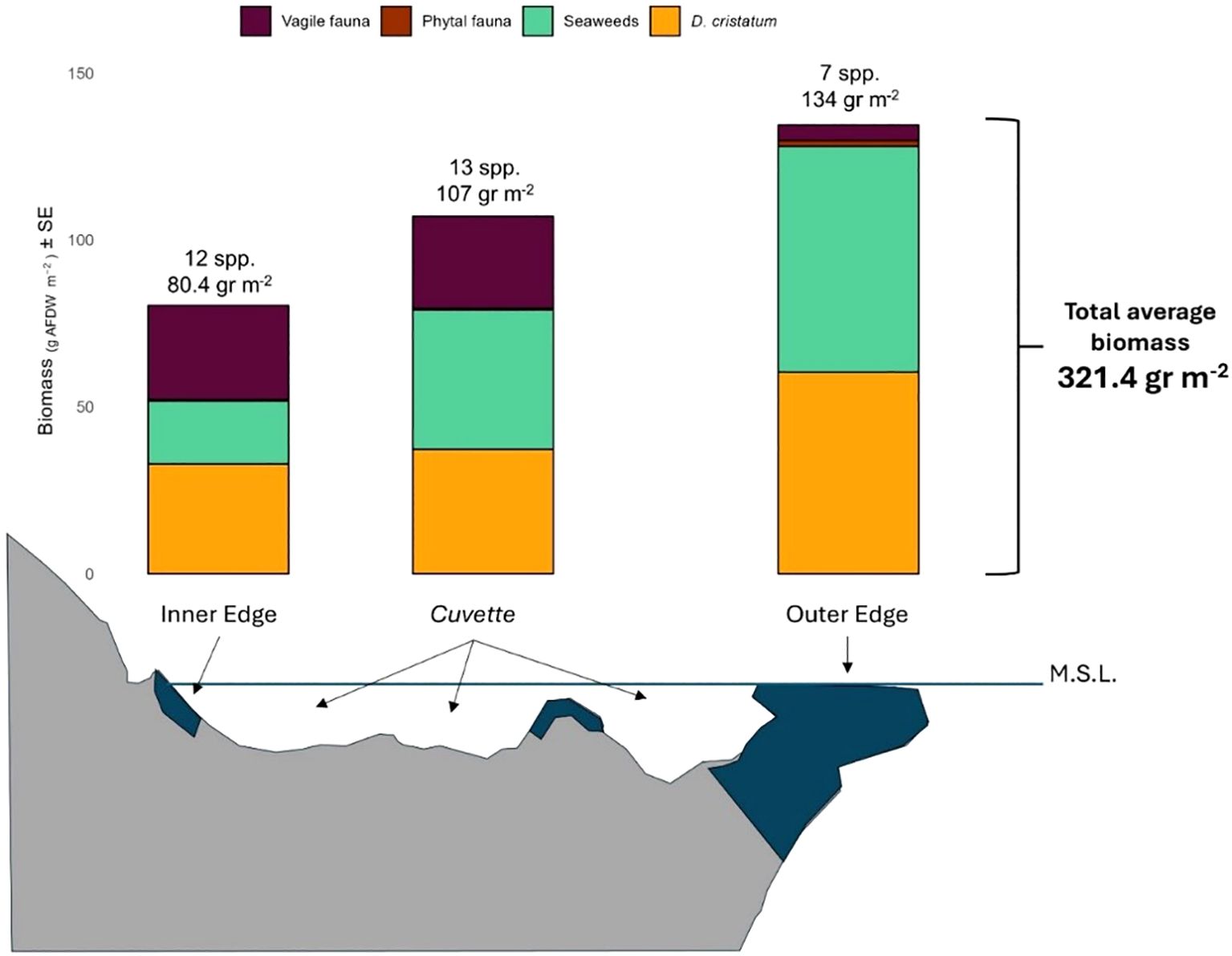
Figure 6. Total number of species and average biomass of each group among the vermetid zones. M.L.S. = mean sea level. Data are specific to the single 50-m reef section studied.
This value is comparable to other rocky intertidal systems in temperate regions 100–500 g AFDW m−2 (Ricciardi and Bourget, 1999), as well as coral reefs in the Red Sea 240 g m−2 (Sawall et al., 2015). While the ecological roles and environmental contexts differ, this comparison underscores the potential significance of vermetid reefs as biomass hotspots in the Mediterranean and highlights the significant contribution of these ecosystems to coastal productivity at local scales.
Within this single reef site, biomass is unevenly distributed both spatially, across the three vermetid zones, and functionally, among different biotic groups, with a clear increasing trend from the inner edge to the outer edge. Using the average biomass values obtained here, we produced a back-of-the-envelope estimate of the total organic matter contained in the 50 m reef section sampled (~93 kg AFDW, ca 32 g m−2). This calculation is meant solely as a contextual figure for this particular site and should not be extrapolated to larger spatial scales (Figure 7).
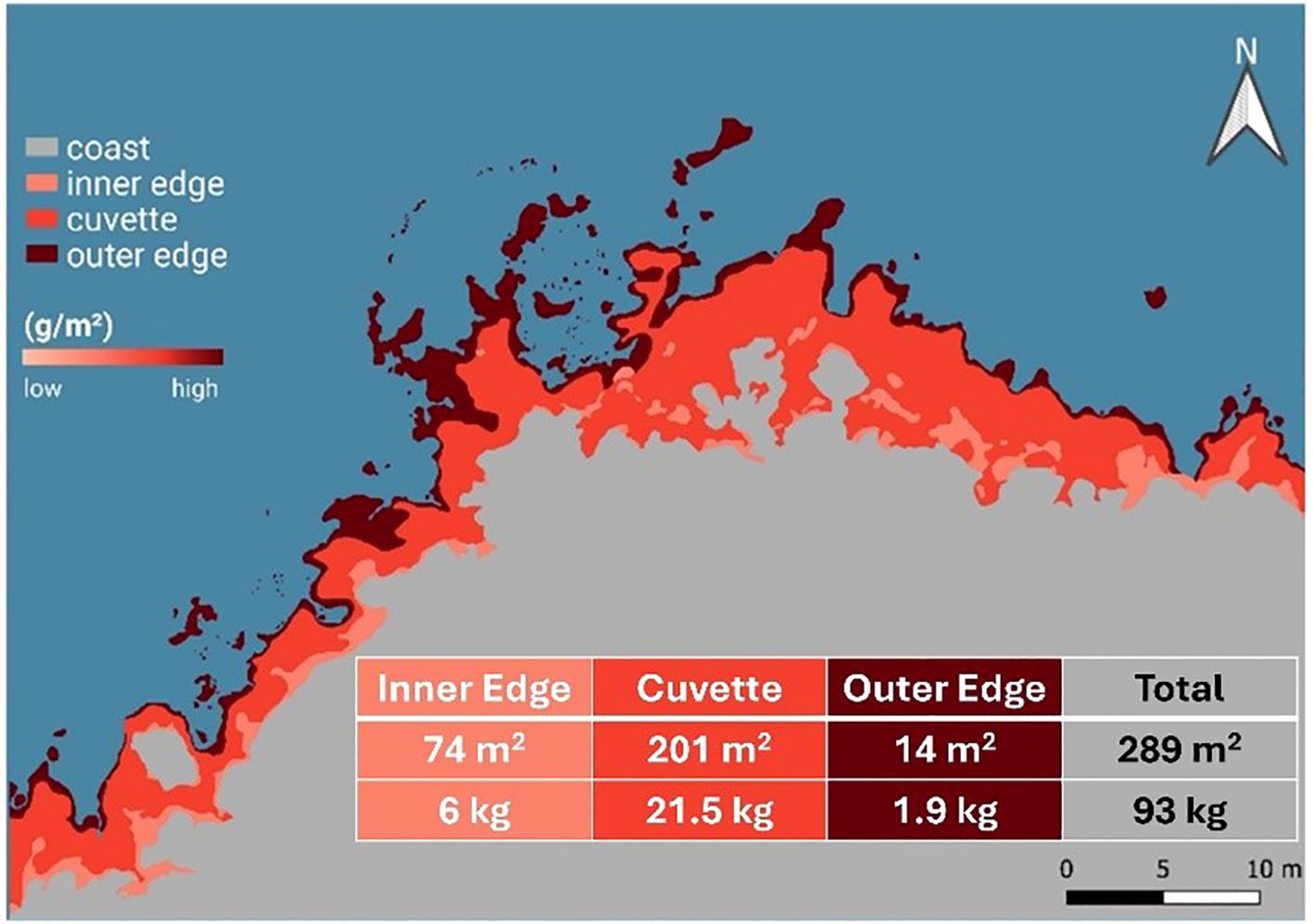
Figure 7. Biomass estimation of the investigated vermetid reef in this study. This schematic representation applies only to the specific 50-m reef section studied and is not intended as an extrapolation across broader reef systems.
The higher biomass of D. cristatum observed in the outer edge relative to the inner edge and cuvette at small spatial scale reflects the species’ ecological preferences and the reef-formation processes. This spatial distribution pattern likely indicates the gastropod’s need for constant water movement and food supply, which are more pronounced at the reef’s seaward margin (Chemello and Silenzi, 2011; Milazzo et al., 2016). The observed gradient of gradual increase in D. cristatum biomass from the inner edge to the outer edge within this reef is likely driven by the environmental gradients of wave exposure, water quality, and food availability.
The seaweeds community showed a distinct zonation pattern at small spatial scale site, with higher biomass in the outer edge and cuvette, while being markedly lower in the inner edge. However, the biomass distribution of macroalgae did not directly reflect species richness, as diversity was highest in the cuvette. This discrepancy suggests that while the cuvette zone may host a greater variety of algal species due to its intermediate environmental conditions, the outer edge provides more favorable conditions for biomass accumulation at this location. The distinct environmental stressors across zones, particularly desiccation and wave action (Milazzo et al., 2016), are likely key factors shaping these patterns.
The phytal fauna biomass was highest in the outer edge, closely correlating with macroalgal biomass at small spatial scale. This relationship underscores the importance of macroalgae as ecosystem engineers, providing habitat and resources for associated fauna (Gee and Warwick, 1994; Mancuso et al., 2023). Among seaweeds, E. amentacea and C. compressa hosted highly abundant fauna compared to other macroalgae in our samples. This result aligns with previous findings on Sicilian vermetid bioconstruction, which reported a greater abundance and diversity of phytal organisms associated with Cystoseira sensu lato species compared to other algae harbored by the vermetid reefs (Ape et al., 2018; Chemello and Milazzo, 2002; Mancuso et al., 2023). Interestingly, despite similar biomass levels of C. compressa and other seaweeds in the cuvette zone at our site, C. compressa hosted significantly higher phytal fauna biomass. This highlights the structural role of Cystoseira sensu lato species in supporting biodiversity (Chemello and Milazzo, 2002; Mancuso et al., 2023, 2021). The consistent proportion of fauna across reef zones and seaweeds, dominated by Sipunculoidea and Polychaeta, suggests a degree of spatial consistency in community structure despite varying environmental conditions within this reef site. Rather than inferring community stability, we refer to this spatial consistency as a pattern that warrants investigation through temporal studies. A more detailed taxonomic identification would allow for a better understanding of differences in phytal fauna composition among seaweeds.
The biomass of mobile macrofauna was greatest in the inner edge and cuvette and much lower in the outer edge, suggesting that the more sheltered zones provide better refuge from wave action and predation (Donnarumma et al., 2021; Chemello et al., 1997; Safriel and Ben-Eliahu, 1991).
The observed patterns of biomass distribution small spatial scale have significant implications for the stability and resilience of vermetid reef ecosystems. In this context, we define stability as the persistence of community composition and structure over time, while resilience refers to the system’s capacity to recover from disturbances. The spatial and functional partitioning of biomass across reef zones suggests that resource availability and habitat complexity are key factors influencing these ecosystem properties locally. The high biomass of D. cristatum in the outer edge suggests that the vermetid reef is well-structured and in a healthy state, which allows the bioconstruction to keep providing the regulating services of coastal protection and sediment stabilization (Chemello and Silenzi, 2011; Milazzo et al., 2016). Meanwhile, the diverse macroalgal community, particularly in the cuvette, enhances habitat complexity, increasing the reef’s potential to support biodiversity and act as a nursery ground at local scale.
The distribution of faunal biomass across the reef suggests a functional redundancy among species utilizing different zones small spatial scale, which could enhance resilience to environmental changes. For example, the strong association between phytal fauna and macroalgal biomass indicates that alterations in algal communities due to anthropogenic stressors (e.g., climate change-induced heatwaves) could trigger cascading effects throughout the ecosystem (Badreddine et al., 2019; Bisanti et al., 2022). Recently, Bisanti et al. (2024) reported drastic biomass reductions of key macroalgal species in Sicilian vermetid reefs, leading to the collapse of associated faunal communities during summer periods in terms of both abundance and species richness. This highlights the vulnerability of the reef ecosystem to temperature-driven shifts in algal assemblages.
Given the threats faced by vermetid habitats and their diversified algal and faunal assemblages due to global and local stressors, establishing baseline data for monitoring future changes and informing conservation efforts is crucial. The distinct biomass distribution pattern across reef zones observed reinforces the need for a seascape approach (integration of spatial complexity and geomorphological features at landscape scales) in vermetid reef ecology. Considering the peculiar role of vermetid reefs in shaping the coastal seascape and enhancing its structural complexity (Picone and Chemello, 2023), it is essential to account for the spatial heterogeneity of these bioconstructions in assessment and monitoring studies. Future research on vermetid reefscapes (as defined by Picone and Chemello, 2023) will need to explore species assemblage composition and biomass distributions in relation to the structural complexity of the reefs, assessing how structural changes might influence biodiversity across multiple spatial scales.
This baseline study, whilst providing the first quantitative biomass assessment for Mediterranean vermetid reefs, has several limitations that should be acknowledged. Our study focuses on a single 50-m vermetid reef section, with the three zones (IE, CV, OE) representing spatial sub-areas rather than independent replicates, which limits broader spatial generalizations but allows for detailed characterization of within-reef patterns. Sampling was conducted during summer months (July 2018 and 2019), providing a snapshot of peak biomass conditions but leaving seasonal variability unexplored. Due to conservation restrictions on this protected habitat, statistical analyses should be interpreted as exploratory tools for pattern detection and hypothesis generation rather than definitive tests of ecological processes. These constraints mean that our results provide valuable site-specific baseline data and generate testable hypotheses for future comparative studies across multiple vermetid reef sites and temporal scales.
Future research should consider annual temporal variations and broader geographical scales to capture the full range of variability in these marine ecosystems. By improving our understanding of biomass distribution patterns at the site scale, this study supports targeted conservation and management of these unique and vulnerable Mediterranean habitats, while generating hypotheses for testing across additional sites and temporal scales in future research.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
FM: Visualization, Writing – original draft, Data curation, Supervision, Writing – review & editing. LB: Writing – review & editing, Supervision, Writing – original draft, Data curation, Visualization. FP: Visualization, Supervision, Data curation, Writing – original draft, Writing – review & editing. RC: Visualization, Data curation, Writing – original draft, Supervision, Funding acquisition, Methodology, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.4 - Call for tender No. 3138 of 16 December 2021, rectified by Decree n.3175 of 18 December 2021 of Italian Ministry of University and Research funded by the European Union - NextGenerationEU; Project code CN_00000033, Concession Decree No. 1034 of 17 June 2022 adopted by the Italian Ministry of University and Research, CUP B73C22000790001, Project title “National Biodiversity Future Center - NBFC”.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1656826/full#supplementary-material.
References
Anderson M. J., Gorley R. N., and Clarke K. R. (2008). PERMANOVA+ for PRIMER: guide to software and statistical methods (Plymouth, UK: PRIMER-E, Plymouth). doi: 10.13564/j.cnki.issn.1672-9382.2013.01.010
Antonioli F., Chemello R., Improta S., and Riggio S. (1999). Dendropoma lower intertidal reef formations and their palaeoclimatological significance, NW Sicily. Mar. Geol. 161 (2–4), 155–170. doi: 10.1016/S0025-3227(99)00038-9
Ape F., Gristina M., Chemello R., Sarà G., and Mirto S. (2018). Meiofauna associated with vermetid reefs: the role of macroalgae in increasing habitat size and complexity. Coral Reefs 37, 875–889. doi: 10.1007/s00338-018-1714-x
Badreddine A., Milazzo M., Abboud-Abi Saab M., Bitar G., and Mangialajo L. (2019). Threatened biogenic formations of the Mediterranean: Current status and assessment of the vermetid reefs along the Lebanese coastline (Levant basin). Ocean Coast. Manage. 169, 137–146. doi: 10.1016/j.ocecoaman.2018.12.019
Bar-On Y. M., Phillips R., and Milo R. (2018). The biomass distribution on Earth. Proc. Natl. Acad. Sci. United States America 115, 6506–6511. doi: 10.1073/pnas.1711842115
Benoist N. M. A., Bett B. J., Morris K. J., and Ruhl H. A. (2019). A generalised volumetric method to estimate the biomass of photographically surveyed benthic megafauna. Prog. Oceanography 178, 102188. doi: 10.1016/j.pocean.2019.102188
Bisanti L., Turco G., and Chemello R. (2024). Signals of loss, part two: A phytal community collapsing under extreme-climate conditions on a Mediterranean vermetid reef. Mar. pollut. Bull. 209, 117223. doi: 10.1016/j.marpolbul.2024.117223
Bisanti L., Visconti G., Scotti G., and Chemello R. (2022). Signals of loss: Local collapse of neglected vermetid reefs in the western Mediterranean Sea. Mar. pollut. Bull. 185, 114383. doi: 10.1016/j.marpolbul.2022.114383
Chemello R., Cìuna I., Pandolfo A., and Riggio S. (1997). Molluscan assemblages associated with intertidal vermetid formations: a morpho-functional approach. Bollettino Malacologico 3, 105–114.
Chemello R. and Milazzo M. (2002). Effect of algal architecture on associated fauna: Some evidence from phytal molluscs. Mar. Biol. 140, 981–990. doi: 10.1007/s00227-002-0777-x
Chemello R. and Silenzi S. (2011). Vermetid reefs in the Mediterranean Sea as archives of sea-level and surface temperature changes. Chem. Ecol. 27, 121–127. doi: 10.1080/02757540.2011.554405
Constable A. J., Costa D. P., Schofield O., Newman L., Urban Jr E. R., Fulton, et al. (2016). Developing priority variables (“ecosystem Essential Ocean Variables” - eEOVs) for observing dynamics and change in Southern Ocean ecosystems. J. Mar. Syst. 161, 26–41. doi: 10.1016/j.jmarsys.2016.05.003
Danovaro R. and Fraschetti S. (2002). Meiofaunal vertical zonation on hard-bottoms: Comparison with soft-bottom meiofauna. Mar. Ecol. Prog. Ser. 230, 159–169. doi: 10.3354/meps230159
Di Franco A., Graziano M., Franzitta G., Felline S., Chemello R., and Milazzo M. (2011). Do small marinas drive habitat specific impacts? A case study from Mediterranean Sea. Mar. pollut. Bull. 62, 926–933. doi: 10.1016/j.marpolbul.2011.02.053
Donnarumma L., D’Argenio A., Sandulli R., Russo G. F., and Chemello R. (2021). Unmanned aerial vehicle technology to assess the state of threatened biogenic formations: the vermetid reefs of mediterranean intertidal rocky coasts. Estuarine Coast. Shelf Sci. 251. doi: 10.1016/j.ecss.2021.107228
Frame K., Hunt G., and Roy K. (2007). Intertidal meiofaunal biodiversity with respect to different algal habitats: A test using phytal ostracodes from Southern California. Hydrobiologia 586, 331–342. doi: 10.1007/s10750-007-0707-5
Franzitta G., Capruzzi E., La Marca E. C., Milazzo M., and Chemello R. (2016). Recruitment patterns in an intertidal species with low dispersal ability: the reef-building Dendropoma cristatum (Biondi 1859) (Mollusca: gastropoda). Ital. J. Zoology 83, 400–407. doi: 10.1080/11250003.2016.1205152
Gee J. and Warwick R. (1994). Body-size distribution in a marine metazoan community and the fractal dimensions of macroalgae. J. Exp. Mar. Biol. Ecol. 178, 247–259. doi: 10.1016/0022-0981(94)90039-6
Ingrosso G., Abbiati M., Badalamenti F., Bavestrello G., Belmonte G., Cannas R., et al. (2018). “Chapter three - mediterranean bioconstructions along the italian coast,” in Advances in marine biology. Ed. Sheppard C. (Academic Press), 61–136. doi: 10.1016/bs.amb.2018.05.001
Kissling W. D., Walls R., Bowser A., Jones M. O., Jens K., Donat A., et al. (2018). Towards global data products of Essential Biodiversity Variables on species traits. Nat. Ecol. Evol. 2, 1531–1540. doi: 10.1038/s41559-018-0667-3
Levin L. A., Bett B. J., Gates A. R., Heimbach P., Howe B. M., Janssen F., et al. (2019). Global observing needs in the deep ocean. Front. Mar. Sci. 6. doi: 10.3389/fmars.2019.00241
Mancuso F. P., D’Agostaro R., Milazzo M., and Chemello R. (2021). The invasive Asparagopsis taxiformis hosts a low diverse and less trophic structured molluscan assemblage compared with the native Ericaria brachycarpa. Mar. Environ. Res. 166, 105279. doi: 10.1016/j.marenvres.2021.105279
Mancuso F. P., Lo Brutto S., Chemello R., Sarà G., and Mannino A. M. (2023). Effects of structural complexity on epifaunal assemblages associated with two intertidal Mediterranean seaweeds. Plant Biosyst. - Int. J. Dealing all Aspects Plant Biol. 157, 812–820. doi: 10.1080/11263504.2023.2200775
Mannino A. M. (1992). Studio fitosociologico della vegetazione mesolitorale a Lithophyllum lichenoides Philippi (Rhodophyceae, Corallinales). Naturalista siciliano 16, 3–25.
Martin-Lescanne J., Rousseau F., de Reviers B., Payri C., Couloux A., and Le Gall L. (2010). Phylogenetic analyses of the Laurencia complex (Rhodomelaceae, Ceramiales) support recognition of five genera: Chondrophycus, Laurencia, Osmundea, Palisada and Yuzurua stat. nov. Eur. J. Phycology 45, 51–61. doi: 10.1080/09670260903314292
Milazzo M., Fine M., La Marca E. C., Alessi C., and Chemello R. (2016). “Drawing the line at neglected marine ecosystems: ecology of vermetid reefs in a changing ocean,” in Marine animal forests (Springer International Publishing, Cham), 1–23. doi: 10.1007/978-3-319-17001-5_9-1
Miloslavich P., Bax N. J., Simmons S. E., Klein E., Appeltans W., Aburto‐Oropeza O., et al. (2018). Essential Ocean variables for global sustained observations of biodiversity and ecosystem changes. Global Change Biol. 24, 2416–2433. doi: 10.1111/gcb.14108
Molinari Novoa E. A. and Guiry M. D. (2020). Reinstatement of the genera gongolaria boehmer and ericaria stackhouse (Sargassaceae, phaeophyceae). Not Algarum 172, 1–10.
Orellana S., Hernández M., and Sansón M. (2019). Diversity of Cystoseira sensu lato (Fucales, Phaeophyceae) in the eastern Atlantic and Mediterranean based on morphological and DNA evidence, including Carpodesmia gen. emend. and Treptacantha gen. emend. Eur. J. Phycology 54, 447–465. doi: 10.1080/09670262.2019.1590862
Picone F. and Chemello R. (2023). Seascape characterization of a Mediterranean vermetid reef: a structural complexity assessment. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1134385
Picone F., Sottile G., Fazio C., and Chemello R. (2022). The neglected status of the vermetid reefs in the Mediterranean Sea: A systematic map. Ecol. Indic. 143, 109358. doi: 10.1016/j.ecolind.2022.109358
Ricciardi A. and Bourget E. (1999). Global patterns of macroinvertebrate biomass in marine intertidal communities. Mar. Ecol. Prog. Ser. 185, 21–35. doi: 10.3354/meps185021
Rilov G. (2016). Multi-species collapses at the warm edge of a warming sea. Sci. Rep. 6, 1–14. doi: 10.1038/srep36897
Safriel U. N. and Ben-Eliahu M. N. (1991). “The influence of habitat structure and environmental stability on the species diversity of polychaetes in vermetid reefs,” in Habitat structure, 349–369. Dordrecht: Springer Netherlands. doi: 10.1007/978-94-011-3076-9_17
Sawall Y., Al-Sofyani A., Hohn S., Banguera-Hinestroza E., Voolstra C. R., and Wahl M. (2015). Extensive phenotypic plasticity of a Red Sea coral over a strong latitudinal temperature gradient suggests limited acclimatization potential to warming. Sci. Rep. 5. doi: 10.1038/srep08940
Tomlinson S., Arnall S. G., Munn A., Bradshaw S. D., Maloney S. K., Dixon K. W., et al. (2014). Applications and implications of ecological energetics. Trends Ecol. Evol. 29, 280–290. doi: 10.1016/j.tree.2014.03.003
Keywords: bioconstruction, vermetid reefs, biomass distribution, Dendropoma cristatum, seaweeds, Mediterranean Sea
Citation: Mancuso FP, Bisanti L, Picone F and Chemello R (2025) Within-reef biomass partitioning in a Mediterranean vermetid reef: a baseline for future monitoring. Front. Mar. Sci. 12:1656826. doi: 10.3389/fmars.2025.1656826
Received: 30 June 2025; Accepted: 22 September 2025;
Published: 06 October 2025.
Edited by:
Luigia Donnarumma, University of Naples Parthenope, ItalyReviewed by:
Fabio Favoretto, University of California, San Diego, United StatesAdriana Giangrande, University of Salento, Italy
Copyright © 2025 Mancuso, Bisanti, Picone and Chemello. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luca Bisanti, bHVjYS5iaXNhbnRpQHVuaXBhLml0
 Francesco Paolo Mancuso
Francesco Paolo Mancuso Luca Bisanti
Luca Bisanti Flavio Picone3
Flavio Picone3 Renato Chemello
Renato Chemello