Abstract
Introduction:
Pearl oyster ecosystems have played a central role in Bahrain’s marine economy and cultural heritage for centuries. Despite their ecological importance, systematic research on Bahrain’s pearl oyster beds is lacking since the early 1990s. In this study a detailed assessment of the benthic composition, pearl oyster community structure, population size, and pearl yield on pearl oyster beds is provided for Bahraini waters.
Methods:
Between January and May 2024, field surveys were conducted at ten sites across Bahrain’s northern, eastern, and western waters to assess benthic composition, pearl oyster community structure, population size structure, and pearl yield. A total of 1,973 pearl oysters were collected and studied.
Results:
Nine species of pearl oysters were recorded, with Pinctada radiata dominating across all sites. Pinctada species richness was highest in the north (six species) with greater oyster densities (34 ± 4.9 oysters/m2) compared to the east (6 ± 2.7 oysters/m2) and west (10 ± 2.9 oysters/m2). Oyster beds were associated with coral in the north (up to 22.6 ± 2.3% cover), algae in the upper eastern (75.6% ± 4.4%) and western (65.8% ± 4.2%) sites, and seagrass mixed with algae in the lower eastern (27.9% ± 4.9% seagrass) and western (33.1% ± 4.2% seagrass) sites. Pearl yield was highest in the Northern and western sites, with Hayr Bu Amamah (14.3%) and Hayr Bul Thamah (11.1%) showing the highest incidence of pearls.
Discussion/Conclusion:
This study provides the first comprehensive quantitative assessment of Bahrain’s pearl oyster beds in over three decades. Given that these oyster beds inhabit some of the most extreme marine environments on the planet, where temperatures fluctuate annually between 16 °C to 36 °C and salinity exceed 50 ppt in certain areas. These findings provide critical baseline data to inform sustainable management, conservation planning and marine heritage protection in Bahrain and the wider Gulf region.
1 Introduction
Pearl oyster ecosystems have been central for the marine economy and cultural heritage of the Kingdom of Bahrain for centuries resulting in them being deeply intertwined with the history of the region. The pearling industry, which flourished in Bahrain, and the surrounding Arabian Gulf during the pre-modern era, once served as the foundation of the country’s prosperity (Carter, 2018). However, with the introduction of cultured pearls in the 20th century added to the discovery of oil and other factors, the natural pearls industry experienced a sharp decline, leading to the eventual collapse of the industry (Burdett, 1995; Carter, 2005). Despite this significant reduction, pearl oyster ecosystems remain crucial due to their valuable ecological services as they contribute towards sustaining marine biodiversity, improve water quality, act as essential habitat for fisheries species, and contribute towards the stability of the islands and their coastal habitats (Hemraj et al., 2022; Grabowski and Peterson, 2007; Coen and Luckenbach, 2000). In addition, these oyster beds exist in the hottest sea in the world harboring a uniquely extreme ecological niche, where temperatures fluctuate annually between 16 °C to 36 °C and salinity exceed 50 ppt in certain areas (AlMealla et al., 2024; Hume et al., 2015). Such harsh conditions surpass those experienced by most marine bivalve habitats worldwide, positioning Bahrain’s pearl oyster beds, as a natural laboratory for studying species resilience and adaption. However, despite their historical and cultural significance, pearl oyster beds in the island nation of Bahrain and the populations they host remain poorly studied, especially in the context of modern ecological pressures (Ali, 2022).
Pearl oysters are broadcast spawners with a planktonic larval phase that rely on successful recruitment and settlement on hard substrates for population persistence (Lal et al., 2020). Their dynamics are shaped by a combination of environmental stressors, predation, harvesting pressure and disease (Johnson and Smee, 2014; Kuchel et al., 2011). They provide critical ecosystem services including water filtration, nutrient cycling, shoreline stabilization, and biodiversity support through their ability to create complex benthic habitats as reef-forming bivalves (Thomas et al., 2022; Richardson et al., 2022). Recent studies have also shown that pearl oysters exhibit physiological plasticity and genetic variability that confer resilience to extreme temperature and salinity regimes, traits that are particularly relevant for populations in the Arabian Gulf (Navarro et al., 2020; Li et al., 2018).
As climate change intensifies and marine ecosystems worldwide face increasing thermal stress compounded by other environmental factors, understanding the status and ecological roles of species living in extreme habitats, like pearl oysters, is more urgent than ever. Although, oysters contribute to the structure of marine habitats, particularly through their interaction with other species and their role in sediment stabilization and nutrient cycling (Koch et al., 2015), ecosystems such as mangroves and coral reefs have recently become the focus of scientific inquiry and conservation efforts within the Gulf region. Moreover, the number of species of pearl oysters present in the region adds to the challenges posed. The lack of consensus among scientists stems from the difficulty in distinguishing between species solely based on morphological characteristics and lack of regional studies that utilize modern technological genetic tools. One of the first attempts to establish a comprehensive list of species in Bahrain was conducted three decades ago by Khamdan (1988). However, this study is considered outdated for several reasons including, the study sites where samples were sourced do not represent the current delimitation of Bahrain’s waters and the latest taxonomy. For example, Pinctada anomioides is now accepted and considered as Pinctada maxima (as reported by the WoRMS database – World Register of Marine Species).
The need for contemporary and accurate research is further emphasized by the fact that no scientific articles have been published on pearl oysters in Bahrain in the past two decades (Supplementary Material 1). This absence of current data highlights a critical gap in understanding pearl oyster populations in Bahrain’s territorial waters. Establishing baseline survey data on biodiversity and habitat composition is essential not only for assessing the health of these beds but also for supporting the Kingdom’s strategy and effort to transition towards sustainable development. As a means of regulating the trade, Bahrain, as per our knowledge, remains the only nation in the world to ban the trade of cultured pearls, as governed by royal decree (10) of 1990 regarding the regulations of pearls and gemstones, later superseded by decision (65) of 2014, in an effort to preserve the legacy of natural pearls in the Kingdom. Moreover, three of Bahrain’s largest oyster beds (Hayr Shtayyah, Hayr Bu Amamah, and Hayr Bul Thamah), known as the “Northern Hayrat” were inscribed as a UNESCO world heritage site in 2012 (Table 1; UNESCO, 2025). In 2017, the Northern Hayrat were officially declared as a Marine Protected Area (MPA) as per Ministerial Order (3) of 2017. While Bahrain’s national plan for the revival of the pearling trade mandates the sustainable management of these protected beds, pearl oyster populations remain largely overlooked despite their ecological importance, underscording the urgent need to better understand their dynamics including their seasonal distribution, habitat preferences, and vulnerability to environmental change.
Table 1
| Local term | Definition |
|---|---|
| Fasht | A shallow area in the sea, typically less than 10 meters in depth. While commonly associated with coral reef habitats, Fasht’s can also host pearl oyster, seagrass, and sandy beds. |
| Najwa(t)* | Shallow peak of a sea mound that is typically less than 10 meters deep. Differs from a Fasht as it resembles the sample of an underwater mountain surrounded by deep water. Strong currents contribute to high levels of biodiversity of Najwa’s, usually associated with thriving fish communities as well as pearl oyster beds and coral reefs. |
| Hayr(at)* | A flat, rocky seabed exceeding 10 meters in depth that is dominated by pearl oyster bed habitat. |
Definition of local terminologies used to refer to oyster bed areas (*the letters in parentheses indicates pluralNB: Although there is some debate among seafarers regarding the origins of these terms, the definitions provided here are derived from a combination of local knowledge and the geological characteristics of the areas).
The present study performed in 2024 provides the first comprehensive data driven study on the ecological characteristics of pearl oyster beds in the Kingdom of Bahrain. The specific objectives were to assess the: (1) benthic composition of the pearl oyster beds, (2) pearl oyster community structure by providing an overview of the species of pearl oysters based on morphology, (3) pearl oyster densities, (4) population size structure and (5) incidence of pearls. This manuscript provides contemporary baseline data on the ecological characteristics of pearl oyster habitats, filling a significant knowledge gap in our understanding of pearl oyster populations in Bahrain and offering insights that can be extrapolated to the wider region of the Arabian Gulf. They also provide valuable insights that will enable stakeholders to make informed, evidence-based, decisions for the sustainable management and conservation of these species, further addressing both ecological and cultural aspects of pearl oyster populations in Bahrain. Moreover, since pearl oysters are globally distributed, insights into their ecology and reef dynamics can be useful on a wider scale.
2 Materials and methods
2.1 Study sites
Bahrain is a small archipelago nation in the Arabian Gulf made up of 33 natural islands, located off the eastern coast of Saudi Arabia and western coast of Qatar. It has a total land area of 786.8 km2 and a territorial water area of 7482.2 km2 (Bahrain National Portal, 2025). In this study ten sites were surveyed between January and May 2024 across Bahrain’s Northern, eastern, and western waters were surveyed (Figure 1). These sites were purposively selected based on their ecological, historical, and management significance. This ensures representation across different environmental gradients including topography, depth, distance from shore, and protection status (i.e., within or outside a MPA). Local ecological knowledge and historical records were also considered in the selection process, particularly with respect to sites known to be actively targeted by traditional pearl divers and artisanal fishers.
Figure 1
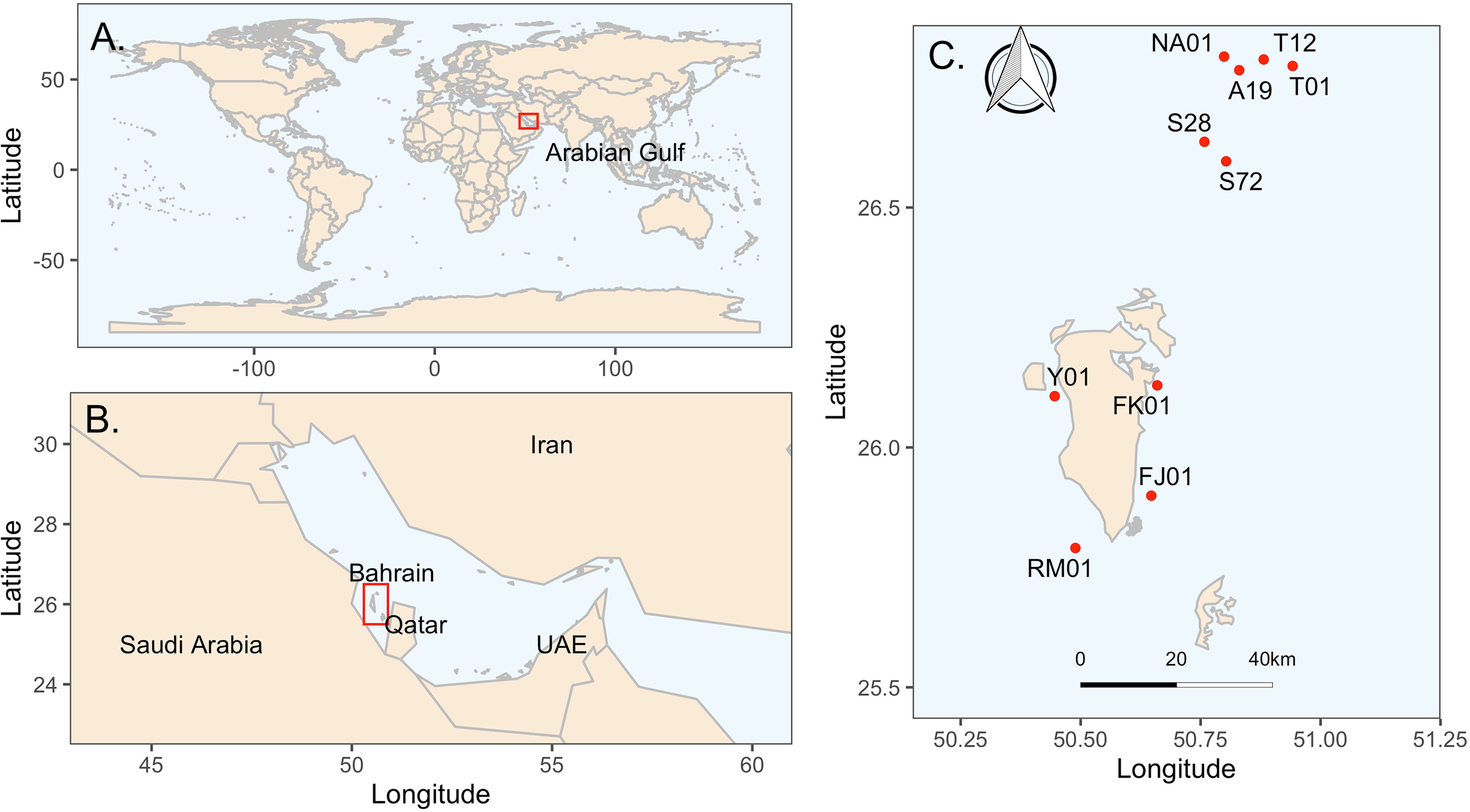
Location of study sites (A) Location of Arabian Gulf (AG); (B) Location of Bahrain within the AG; (C) Location of selected study sites within Bahrain [Site codes: Northern Hayrat Waters: (1) Hayr Shtayyah (S28); (2) Hayr Shtayyah (S72); (3) Hayr Bu Amamah (NA01); (4) Hayr Bu Amamah (A19); (5) Hayr Bul Thamah (T01); (6) Hayr Bul Thamah (T12); (B) Eastern Waters: (7) Falkland, Fasht Al Adhm (FK01); (8) Fasht Al Jabbari (FJ01); (C) Western Waters (9) Yassouf (Y01); (10) Ras Al Mumtallah (RM01)].
Six of the surveyed sites are located within the Northern Hayrat (Figure 1) - specifically, Hayr Shtayyah (S28, S72), Hayr Bu Amamah (NA01, A19), and Hayr Bul Thamah (T01, T12) which in terms of area represents double the size of Bahrain’s land mass. These beds are among the most historically important oyster habitats in Bahrain, having served as primary sources of natural pearls for centuries. Together they represent one of the country’s most biodiverse and productive marine regions, which is recognized for their importance to artisanal fisheries. Notably, these sites are part of a designated MPA (Ministerial Order (3) of 2017) and were inscribed as a UNESCO World Heritage Site in 2012, underscoring their cultural and ecological value.
Two additional sites were selected in Bahrain’s eastern waters: Falkland, Fasht Al Adhm (FK01) and Fasht Al Jabbari (FJ01). These areas are shallower, located closer to shore, and are known to contain mixed habitats that include seagrass beds, sand patches, and coral communities. The assessment of these sites allow the understanding of oyster bed dynamics outside the jurisdiction of the MPA and which are frequently used by artisanal fishers.
Lastly, Yassouf (Y01) and Ras Al Mumtallah (RM01) sites, located in western Bahrain, offer a distinct environmental gradient due to their proximity to the Saudi border and slightly higher turbidity. These areas have historically supported both oyster and finfish fisheries, although they remain comparatively under-studied.
In addition, at each site, we conducted benthic surveys (photoquadrat and transect assessments of benthic composition) and ecological surveys (in situ observations of pearl oyster populations in relation to benthic features, combined with measurements of water parameters. This integrated approach was adopted to assess both habitat composition and ecological associations.
These in situ water quality measurements were conducted at each site, approximately 1 meter below the surface using a portable ThermoScientific Orion Star A329 pH meter, A323 DO meter which also measured water temperature while salinity was measured using a refractometer.
2.2 Habitat characteristics and benthic community composition
At each site, scientific dives were performed at depths ranging from 3.6 to 20.6m, with the exception of Yassouf and Falkland, which were done through wading and supported by a scuba tank respectively. During each dive, 6 x 20m underwater transects were placed parallel, with a 4m distance between each transect. Along each transect, five 0.25 m² photoquadrats were systematically placed at intervals of 0, 5, 10, 15, and 20 meters, resulting in a total of 30 quadrats per site and covering an area of 120 m² at each site. This systematic design follows standard methods in ecological surveys, ensuring comparability across sites while minimizing potential spatial autocorrelation by spacing transects and quadrats evenly within the survey area.
During the dives, both qualitative and quantitative observations of the epibiota were made within each quadrat and along the transects at each site. These observations were documented through photographs to assess benthic community composition which was classified into major life form groups: hard corals, soft corals, seagrasses, macroalgae, sponge, sand, and others (includes living and non-living categories such as rock, rubble, ascidians, hydroids, crustose coralline algae and bivalves). For the purposes of this study, the term benthic hereafter refers specifically to these defined life form groups.
2.3 Pearl oyster community structure
From within each quadrat (i.e. a total of 30 quadrats per site defining the survey area representing 120 m2 at each site), all pearl oysters were collected regardless of size or species. After collection, the oysters were: (1) allocated a specimen number; (2) photographed; (3) opened for pearl search and extraction – if a pearl was found, it was photographed, recorded, following which the pearl and shell would be stored separately; (4) identified to species level where possible; and (5) shell measurements were taken. The pearl community structure was determined based on the number of different species classified based on morphological characteristics (Supplementary Material 2).
Due to large local and global variation within species, a consensus regarding morphological traits was required for Pinctada radiata, Pinctada maculata, Pinctada nigra, Pinctada maxima, and Pinctada sugillata. For the purposes of this study, the primary references used were “The Gulf Encyclopedia in Pearls and Oysters” by Khamdan (2004) and the World Register of Marine Species (WoRMS). Refer to Supplementary Material 2 for the developed identification approach agreed upon by the co-authors as a first attempt to provide an overview of species composition of pearl oysters in Bahrain.
2.4 Pearl oyster abundance & population size structure
Although abundance and size structure are recognized as integral components of community structure, they are presented separately here to ensure clarity of data presentation to highlight detailed patterns in each. The number of pearl oysters belonging to the Pinctada genus within each quadrat were counted regardless of species present to assess abundance. To determine the population size structure of pearl oysters at each site, external shell measurements of all pearl oysters collected were recorded as an indicator of oyster size during specimen processing at the lab. Measurements include: (1) dorsal-ventral measurement (DVM) commonly referred to as shell length/height (SL), (2) anterior-posterior measurement (APM) commonly referred to as shell width (SW), (3) hinge length (HL) (4) oyster thickness (OT) which is measured at the thickest point of a whole unopened oysters and (5) shell thickness (ST) which is measured on the thickest shell. Measurements were taken in millimeters (mm) using a 0–150 mm digital calipers (model HDCD01150, INGCO) calibrated daily and were accurate to 0.01mm. The specific size parameters recorded for this subsection were limited to Pinctada spp.
For the purposes of defining pearl oyster size structure, the oysters were grouped into six size ranges based on their DVM as outlined in Table 2. This was used to gauge the estimated age of pearl oysters in order to group and analyze population size structure at each site.
Table 2
| Oyster age | < 1 year (spat) | 1–2 years | 2–3 years | 3–4 years | 4–5 years | > 5 years |
|---|---|---|---|---|---|---|
| Estimated size range (cm) | 0.1-3 | 3-5 | 5-6.5 | 6.5-7.5 | 7.5-7.75 | > 7.75 |
Estimated oyster age based on size range (Mohammed, 1994; Mohammed and Yassien, 2003; Nayar and Al-Rumaidh, 1993a, b).
2.5 Pearl yield
After species identification and measurement collection, all pearl oysters were meticulously searched for pearls through a visual inspection. The oyster surface flesh inspection was done following which a thumb was run across the mantle to feel for any pearls, then a shucker is used to flip over the muscle and search underneath. This was done for both sides of the oyster. In the case a pearl was found, a photo was taken in its place of discovery before being transferred into a glass bottle (Figure 2). The presence of pearls, number of pearls found in the specimen, the specimen number, site, date collected and date processed were recorded.
Figure 2
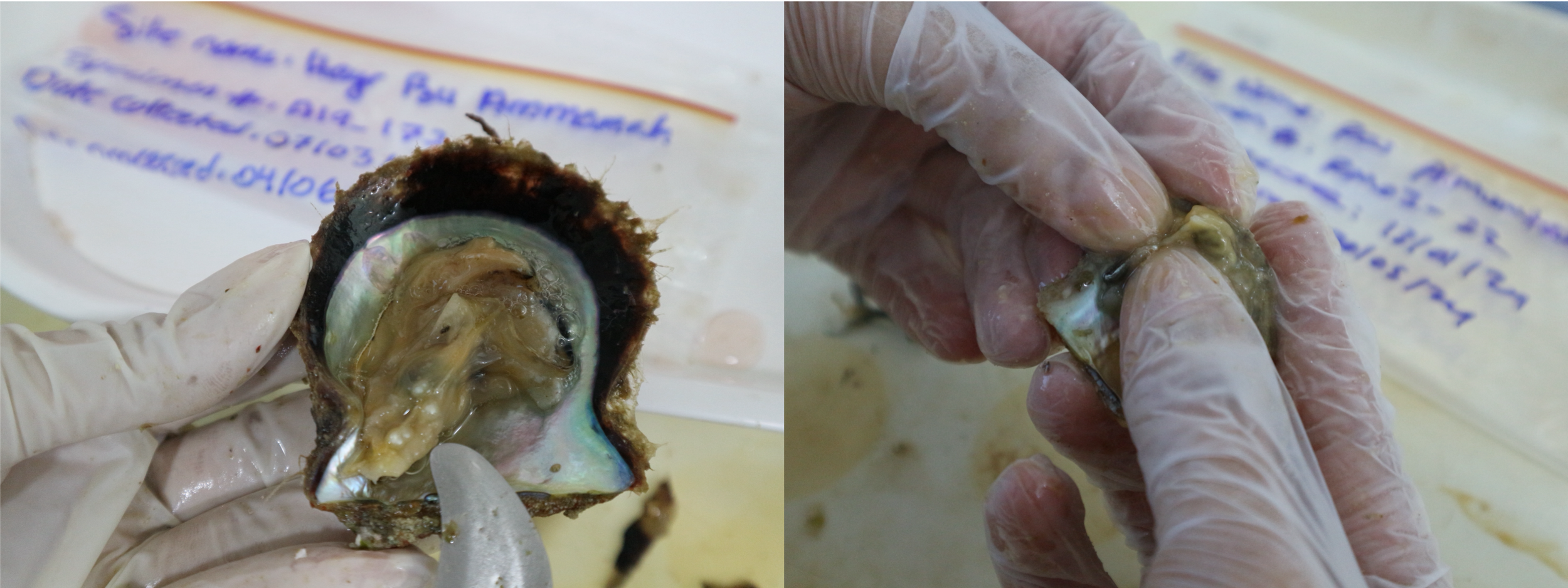
Naturally occurring pearls found inside pearl oysters during specimen examination.
2.6 Data analysis
All data analyses were conducted using the software “R” and “RStudio” version 12.1 (2024). The benthic community composition was assessed by calculating the percent relative cover of major life form categories (HC = hard corals, SC = soft corals, SG = seagrasses, MAC = macroalgae, SP = sponge, SD = sand, and Others = includes rock, rubble, ascidians, hydroids, crustose coralline algae and bivalves). Multivariate analyses were performed on these relative abundance data using the Bray–Curtis similarity index to quantify and visualize differences in benthic communities between sites and areas. To test for statistically significant differences in benthic community composition, a one-way permutational analysis of variance (PERMANOVA) was conducted. Additionally, non-metric multidimensional scaling (NMDS) was used to visually represent patterns in community similarity, while similarity percentage (SIMPER) analysis identified which lifeforms contributed most to dissimilarities between groups. The assumptions of homogeneity of multivariate dispersions were also validated.
To evaluate differences in oyster density, Generalized Linear Models (GLMs) with a negative binomial distribution and a log link function were fitted to account for overdispersion in count data (Bolker, 2008). Two models were constructed: one testing the effect of site and another examining the effect of area.
To examine differences in oyster morphology across sites and areas, a series of Generalized Linear Models (GLMs) were applied to each measured morphological trait (shell length, shell width, shell thickness, oyster thickness, and hinge length), using the Gaussian family with an identity link function (Bolker, 2008). Separate models were fitted for site and area to test their effects on each trait, with the general model expressed as
Where β0 is the intercept, β1 is the effect of the categorical predictor (site or area), and ϵ is the normally distributed error term.
Model fit was assessed using analysis of deviance with an F-test to determine whether morphological measurements differed significantly among sites or areas. Statistical significance was evaluated at α=0.05. Diagnostic plots (residuals vs. fitted values and Q–Q plots) were used to verify model assumptions, and data transformations (e.g., log or square root) were applied when necessary to improve model fit and meet assumptions.
To further classify oysters based on morphometric traits, hierarchical clustering analysis was performed. All morphometric variables were first standardized (z-score normalization) to account for differences in scale. A Euclidean distance matrix was computed, and clustering was carried out using the complete linkage method, which defines inter-cluster distance as the maximum distance between points from different clusters. The resulting dendrogram was delimitated to form three distinct clusters (i.e. small, medium, and large), selected based on prior biological knowledge and visual inspection of clustering patterns.
To assess whether the distribution of oyster size categories and oysters species differed significantly among sites, a Chi-square test of independence was applied to the contingency tables. Because some cells had expected counts less than five, which violates the assumptions of the traditional Chi-square test, a simulation-based approach was used to approximate the p-value. A significance level of α = 0.05 was used throughout. Pearl yield was calculated as the proportion of oysters with pearls at each site, defined as the number of pearl-containing oysters divided by the total number of oysters sampled. These percentages were used to assess spatial variation in pearl formation across sites.
To examine the relationship between pearl incidence (presence/absence) and oyster morphometrics, we performed multiple logistic regression using the glm() function in R. The binary response variable was Presence (1 = pearl present, 0 = absent), and candidate predictors included shell length (SL, mm), shell width (SW, mm), oyster thickness (OT, mm), hinge length (HL, mm), and shell thickness (ST, mm). We first fitted a full model containing all five predictors:
To identify the most parsimonious model, we sequentially compared nested models by likelihood ratio tests using the anova function and by evaluating the Akaike Information Criterion (AIC). Starting from the full model, we removed non-significant predictors one at a time to create reduced models, retaining variables that improved model fit (lowest AIC and significant likelihood ratio tests). The final selected model included shell length, oyster thickness, and hinge length as predictors. Model coefficients were interpreted as log-odds, and odds ratios (OR) with 95% confidence intervals (CI) were derived to quantify the effect of each morphometric variable on the probability of pearl presence. We did not develop any new models in this study but applied well-established statistical approaches widely recognized and validation in ecological and population studies.
3 Results
3.1 Habitat characteristics, benthic community composition & environmental characteristics
Environmental parameters varied across the surveyed sites (Table 3). The Northern Hayrat (all sites are within a MPA) were characterized by deeper waters (~ 11–21 m), with moderate salinity (40–42 ppt) and stable pH (8.1-8.2), with water temperatures generally between 21-23 °C except for T01 where 25.1 °C was recorded (Table 3). These values are typical of the winter to early spring season in Bahrain, when sea surface temperatures are at their lower annual range. On the contrary, the Eastern oyster beds (FK01 and FJ01) were shallow (1-4m) with slightly higher salinity levels (43–45 ppt) and similar temperatures (~21 °C). The Western sites (Y01 and RM01) were also shallow in nature (<4m) and exhibited extreme salinity (54 ppt), with temperatures of 21-22 °C. Dissolved oxygen (DO) values were relatively consistent across sites (~ 6.4-6.9 mg/L. Overall, the Northern Hayrat were observed to harbor clear and deeper waters while the eastern and western oyster beds live in opposite conditions as they exist in shallow and more environmentally extreme turbid waters particularly in terms of salinity (Table 3). Benthic communities also varied across sites with sand as the dominant (>50%) feature across all sites except for FK01 (15.5 ± 4.4%), Yassouf (33.1 ± 4.2%) and FJ01 (39.4 ± 3.0%) (Figures 3, 4). The majority of the Northern pearl oyster sites were associated with hard corals (e.g., Coscinaraea monile, Porites lutea, Dipsastraea speciosa), specifically Hayr Shtayyah (site S28) whereby corals (22.6 ± 2.3%) are the second most dominant benthic group at this site followed by others (including rock, rubble, ascidians, hydroids and bivalves) (Figure 3). Sites NA01, T01, T12 and S72 all had significantly lower coral cover (3.1 ± 0.9%, 1.0 ± 0.8%, 0.2 ± 0.1% and 0.1 ± 0.1% respectively) with no coral cover observed at site A19 (Figures 3, 4).
Table 3
| Site | Site code | Site depth (m) | Temp. (°C) | Salinity (ppt) | DO | pH | Protected area |
|---|---|---|---|---|---|---|---|
| Northern Hayrat | |||||||
| Hayr Shtayyah | S28 | 12.1 | 21.5 | 41.2 | 6.8 | 8.2 | Y |
| S72 | 10.9 | 21.5 | 41.6 | 6.9 | 8.1 | Y | |
| Hayr Bu Amamah | NA01 | 16.9 | 21.0 | 40.8 | 6.7 | 8.2 | Y |
| A19 | 15.9 | 21.4 | 40.7 | 6.8 | 8.2 | Y | |
| Hayr Bul Thamah | T01 | 20.6 | 25.1 | 40.7 | 6.9 | 8.1 | Y |
| T12 | 16.2 | 23.2 | 40.6 | 6.9 | 8.1 | Y | |
| Eastern Oyster beds | |||||||
| Falkland, Fasht Al Adhm | FK01 | 1.3 | 21.6 | 43.2 | 6.4 | 8.3 | N |
| Fasht Jabbari | FJ01 | 3.6 | 21.0 | 44.7 | 6.8 | 8.2 | N |
| Western Oyster beds | |||||||
| Yassouf | Y01 | 0.5 | 20.7 | 53.8 | 6.7 | 8.3 | N |
| Ras Al Mumtallah | RM01 | 4.1 | 22.0 | 54.5 | 6.6 | 8.2 | N |
Environmental characteristics of the study sites.
Figure 3
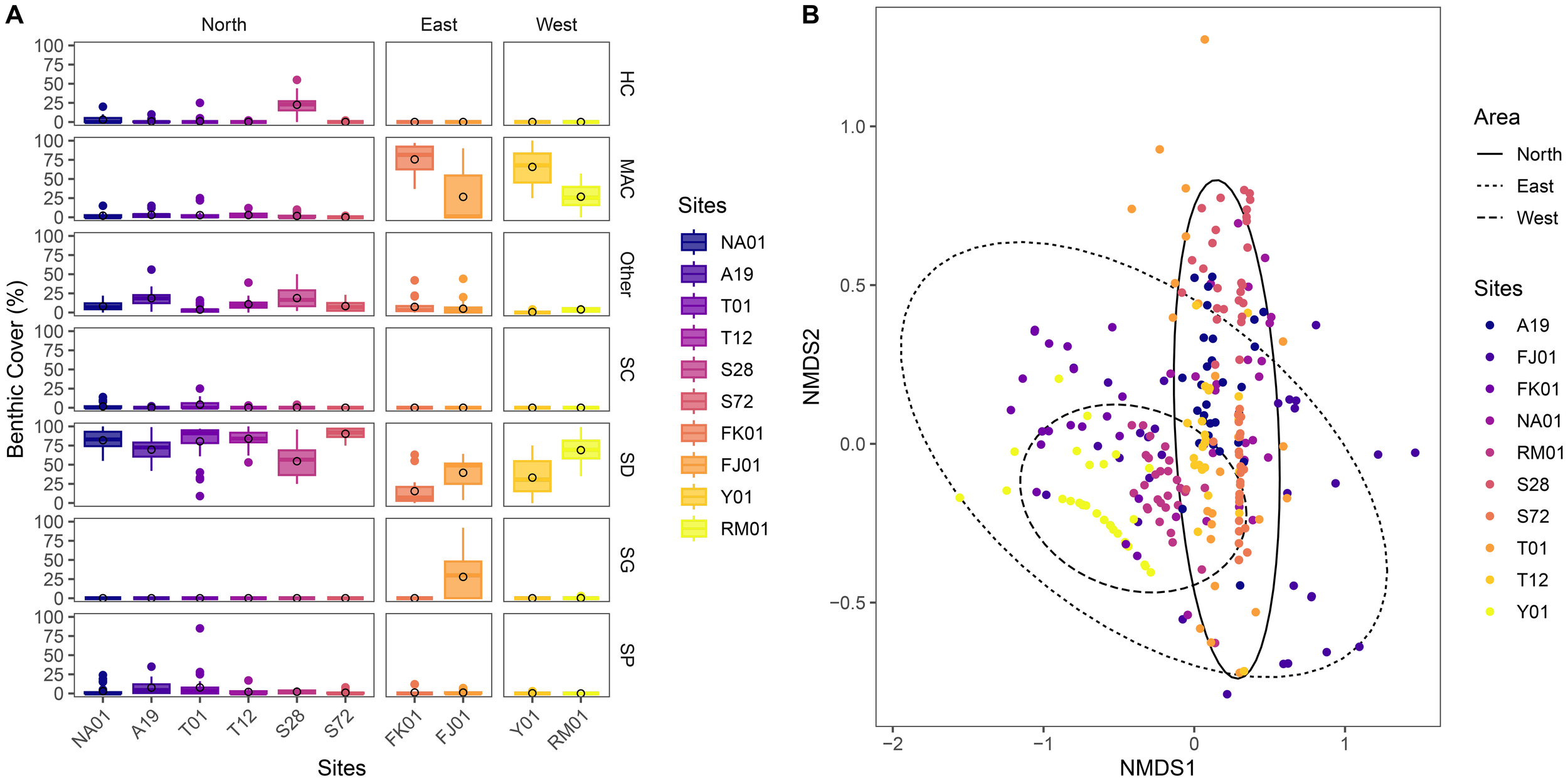
Benthic composition of the pearl oyster beds in the kingdom of bahrain. (A) Box plots showing the distribution of the percent cover of major benthic groups (i.e. HC = hard corals, SC = soft corals, SG = seagrasses, MAC = macroalgae, SP = sponge, SD = sand, and Others = includes rock, rubble, ascidians, hydroids, crustose coralline algae and bivalves) across ten reef sites - (I) Northern Area are represented by sites: NA01 and A19 (Hayr Bu Amamah); T01 and T12 (Hayr Bul Thamah); S28 and S72 (Hayr Shtayyah); (ii) Eastern Area: FK01 (Falkland, Fasht Al Adhm) and FJ01 (Fasht Al Jabbari); (iii) Western Area: Y01 (Yassouf) and RM01 (Ras Al Mumtallah). The unfilled circles within each plot represent the mean percent cover for that benthic group at the site. (B) Non-metric multidimensional scaling (NMDS) plot visualizing the differences in benthic community structure among Eastern, Northern, and Western oyster beds. Each point represents a quadrat, coloured by site, and ellipses indicate 95% confidence intervals around area groupings, highlighting spatial separation in benthic composition.
Figure 4
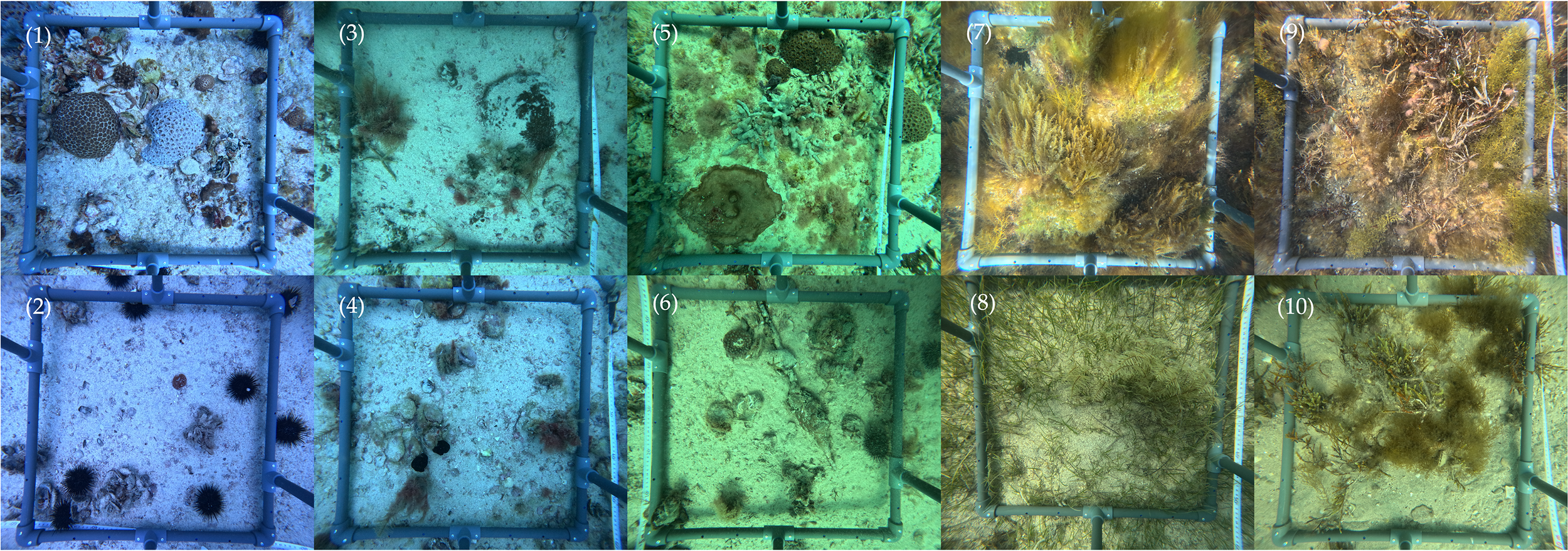
Benthic features of pearl oyster beds in the kingdom of Bahrain. Northern Hayrat Sites (1) Hayr Shtayyah (S28); (2) Hayr Shtayyah (S72); (3) Hayr Bu Amamah (NA01); (4) Hayr Bu Amamah (A19); (5) Hayr Bul Thamah (T01); (6) Hayr Bul Thamah (T12); Eastern Sites (7) Falkland, Fasht Al Adhm (FK01); (8) Fasht Al Jabbari (FJ01); Western Sites (9) Yassouf (Y01); (10) Ras Al Mumtallah (RM01).
In general, amongst the three Hayrs (i.e. Hayr Bu Amamah, Hayr Bul Thamah and Hayr Shtayyah), Hayr Bu Amamah was characterized with bedrock covered with coarse grained sand, broken shells and scattered rubble. Site A19 located on the eastern side of the Hayr exhibited low biodiversity with scattered rubble, sponge, and ascidian colonies present, whereas in the upper western side of the Hayr (Site NA01) additional taxa was supported including solitary or individual colonies of hard coral (e.g., Turbinaria peltata), soft coral (e.g., Junceella sp.), sponges, ascidians, anemones (e.g., Stichodactyla haddoni) and urchins (e.g., Prionocidaris baculosa).
Hayr Bul Thamah also characterized with bedrock covered with coarse grained sand, scattered rubble and broken shells. Biodiversity at sites T01 and T12 was similar to Hayr Bu Amamah with individual coral colonies (e.g. Porites lutea, Turbinaria peltata and Favites chinesis) scattered across the sites along with sponges, ascidians and soft corals (e.g., Antipatharia spp. (black coral)). On the contrary, Hayr Shtayyah, supported the highest observed biodiversity with abundant hard coral (e.g., Cyphastrea microphthalma, Porites lobata, Platygyra daedalea) and soft coral colonies, sponges, and anemones (e.g., Entacmaea quadricolor).
The general benthic composition of the nearshore sites Y01, RM01, FK01 and FJ01, were fairly similar. These sites were dominated by coarse sand (Y01 had fine sand). Broken shells were recorded at RM01 and scattered rubble was abundant at FJ01. While the sites host similar benthic characteristics, the species of macroalgae were diverse across sites. In the western (Y01, RM01) and eastern (FK01) sites, macroalgae was seen to be the dominant benthic group (65.8 ± 4.2%, 69.0 ± 2.9.0% and 75.6 ± 4.4% respectively) associated with pearl oysters while the benthic composition of site FJ01 was observed to be dominated by a mixture of seagrass (27.9 ± 4.9%) and algae (26.6 ± 5.5%) (Figures 3, 4). Lastly, sponges were also observed to be present at all sites except for RM01 (Figure 3).
Overall, there is a significant difference in the benthic composition among sites (PERMANOVA: F9,285 = 55.17, R² = 0.6353, p< 0.01) and areas (PERMANOVA: F2,292 = 96.49, R² = 0.3979, p< 0.01) (Figure 3). Results illustrate that the Northern oyster beds are associated with coral while the western and eastern beds are associated with algal beds and the south-east, a mixture of seagrass and algal beds. SIMPER analysis revealed that differences in benthic composition were primarily driven by macroalgae, sand, and seagrass. Between eastern and western oyster beds, macroalgae (19.09%, p = 0.001), sand (16.35%, p = 0.261), and seagrass (8.89%, p = 0.001) accounted for 92.6% of dissimilarity. The difference in the benthic composition between eastern and Northern oyster beds were explained by sand (24.04%, p = 0.001), macroalgae (21.88%, p = 0.001), and seagrass (8.87%, p = 0.001), totaling 84.1%. Macroalgae (22.22%, p = 0.001) and sand (16.89%, p = 0.020) contributed 79.8% of the dissimilarity in the benthic composition between western and Northern oyster beds.
3.2 Pearl oyster community structure
A total of 1,973 pearl oysters were collected from the quadrats across the eastern, Northern, and western sites. In total nine pearl oyster species were identified by morphology within the quadrat-transect area: (1) P. radiata; (2) P. nigra; (3) P. maculata; (4) P. maxima; (5) P. sugillata; (6) P. spp.; (7) Malleus regula; (8) Isognomon legumen and (9) Pinna bicolour (Figures 5, 6).
Figure 5
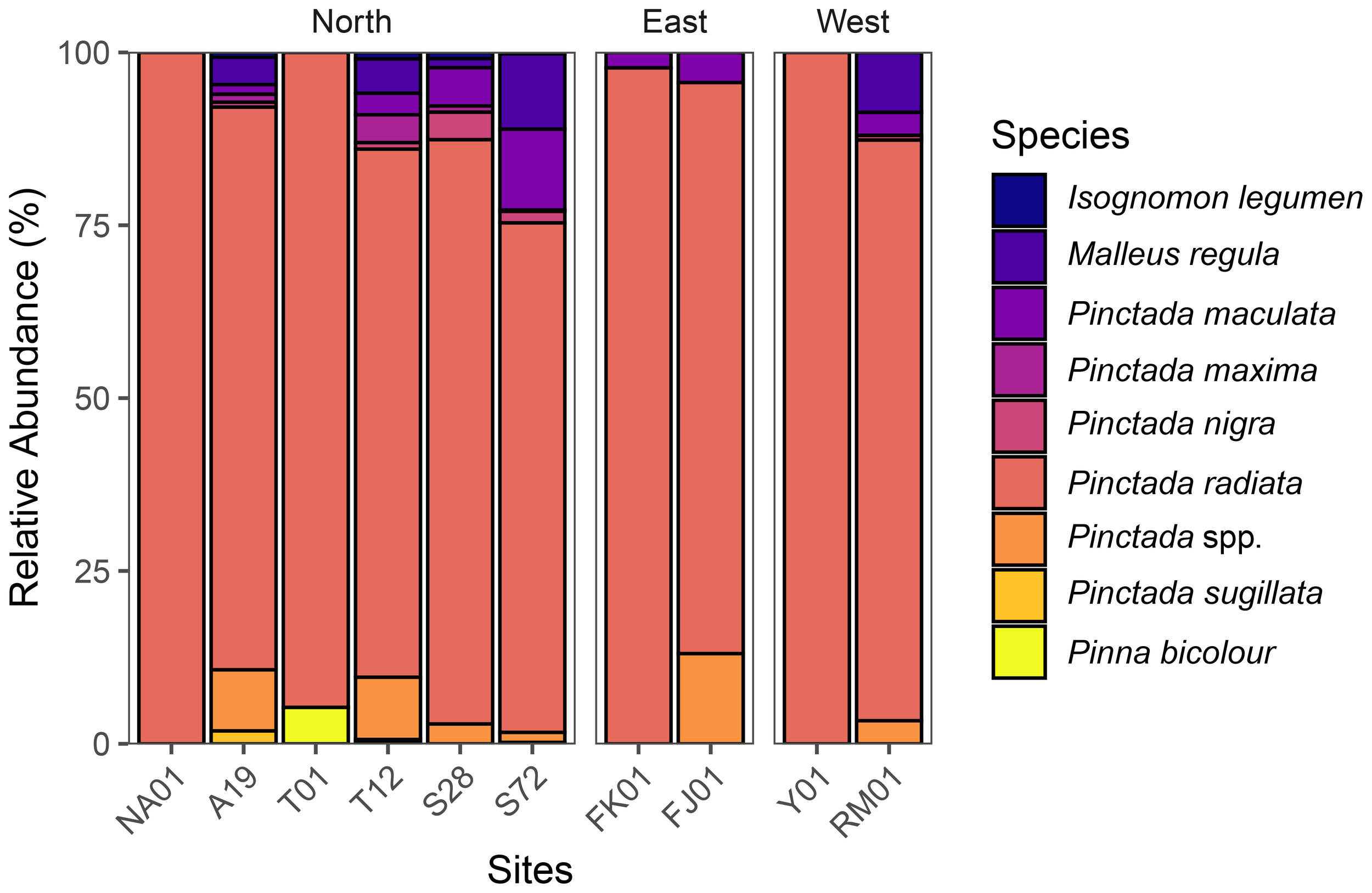
Relative abundance of pearl oyster community structure across all sites in Bahraini waters, where Northern Waters are represented by sites: NA01 and A19 (Hayr Bu Amamah); T01 and T12 (Hayr Bul Thamah); S28 and S72 (Hayr Shtayyah); Eastern Waters: FK01 (Falkland, Fasht Al Adhm) and FJ01 (Fasht Al Jabbari); Western Waters: Y01 (Yassouf) and RM01 (Ras Al Mumtallah).
Figure 6
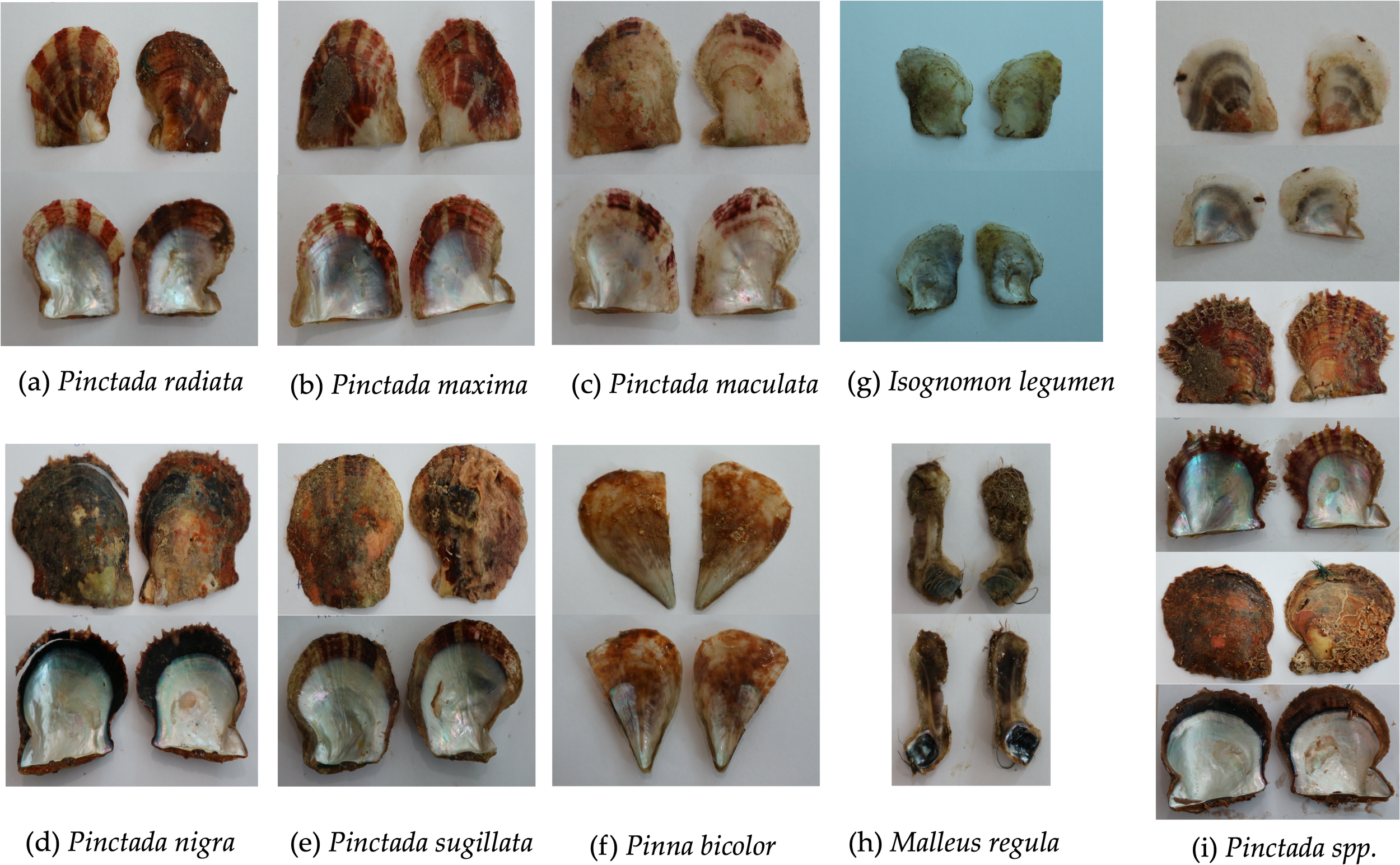
Specimens of pearl oysters recorded and grouped based on morphological characteristics in the Kingdom of Bahrain. Specimens include: (a) Pinctada radiata; (b) Pincatada maxima; (c) Pincatada maculata; (d) Pinctada nigra; (e) Pinctada sugillata; (f) Pinna bicolor; (g) Isognomon legumen; (h) Malleus regula; (i) Pincatada spp.
Oyster species composition differed significantly across sites (χ² = 387.1, p< 0.001) and areas (χ² = 300.19, p< 0.001) indicating non-uniform distribution of size classes by geographic location. The Northern sites exhibited the highest species richness, with six species of Pinctada identified, whereas the eastern and western sites were primarily dominated by P. radiata, P. maculata and an unidentified P. sp (Figure 5).
In the eastern waters, P. radiata had the highest relative abundance at site FK01 (97.8%) and FJ01 (82.6%), however, the remaining species were noted to be absent from these sites with the exception of Pinctada maculata which was present at both sites but in much lower numbers (2.2% at FK01 and 4.3% at FJ01) (Figure 5). In addition, an unidentified Pinctada sp. was recorded at FJ01, contributing 13.0% to the community (Figure 5). In the western waters, site RM01 had the highest relative abundance of M. regula (8.7%), P. maculata (3.3%) while all pearl oyster species recorded at Yassouf were P. radiata (100%) (Figure 5).
In terms of species richness, the Northern Hayrat sites hosted the highest species richness (Figure 5). Site A19 in Hayr Bu Amamah had the second highest relative abundance recorded for Malleus regula (4.0%), and P. maculata (1.4%) after the western waters. P. maxima (1.2%), P. nigra (0.7%) and an unidentified Pinctada sp. (8.8%) were also recorded in this site with P. radiata (81.3%) having the highest relative abundance.
Moreover, P. sugillata occurred at very low abundance and recorded only at Hayr Shtayyah at site S72 (0.2%) and Hayr Bu Amamah at site A19 (1.9%) (Figure 5). Lastly, I. legumen was only observed at site A19 (0.7%) while, P. bicolour was recorded at only one out of the ten surveyed sites i.e., at Hayr Bul Thamah, site T12 (5%).
3.3 Pearl oyster density
A total of 1,855 Pinctada spp. oysters were collected from all quadrats across all sites, 1,612 from the Northern Hayrat, 68 from the eastern waters and 175 from the western waters. Comparing across the Northern sites, Hayr Shtayyah was noted to have the largest density of pearl oysters (S28: mean = 67 ± 8.6 oysters/m2, N=6; S72: mean = 54 ± 11.5 oysters/m2, N=6; Figure 7A). Densities within Hayr Bul Thamah (T12: mean = 30 ± 3.4 oysters/m2, N=6; T01: mean = 2 ± 1 oysters/m2, N=6) and Hayr Bu Amamah (A19: mean = 47 ± 4.5 oysters/m2, N=6; NA01: mean = 1 ± 0.4 oysters/m2, N=6) were variable depending on the area (Figure 7A).
Figure 7
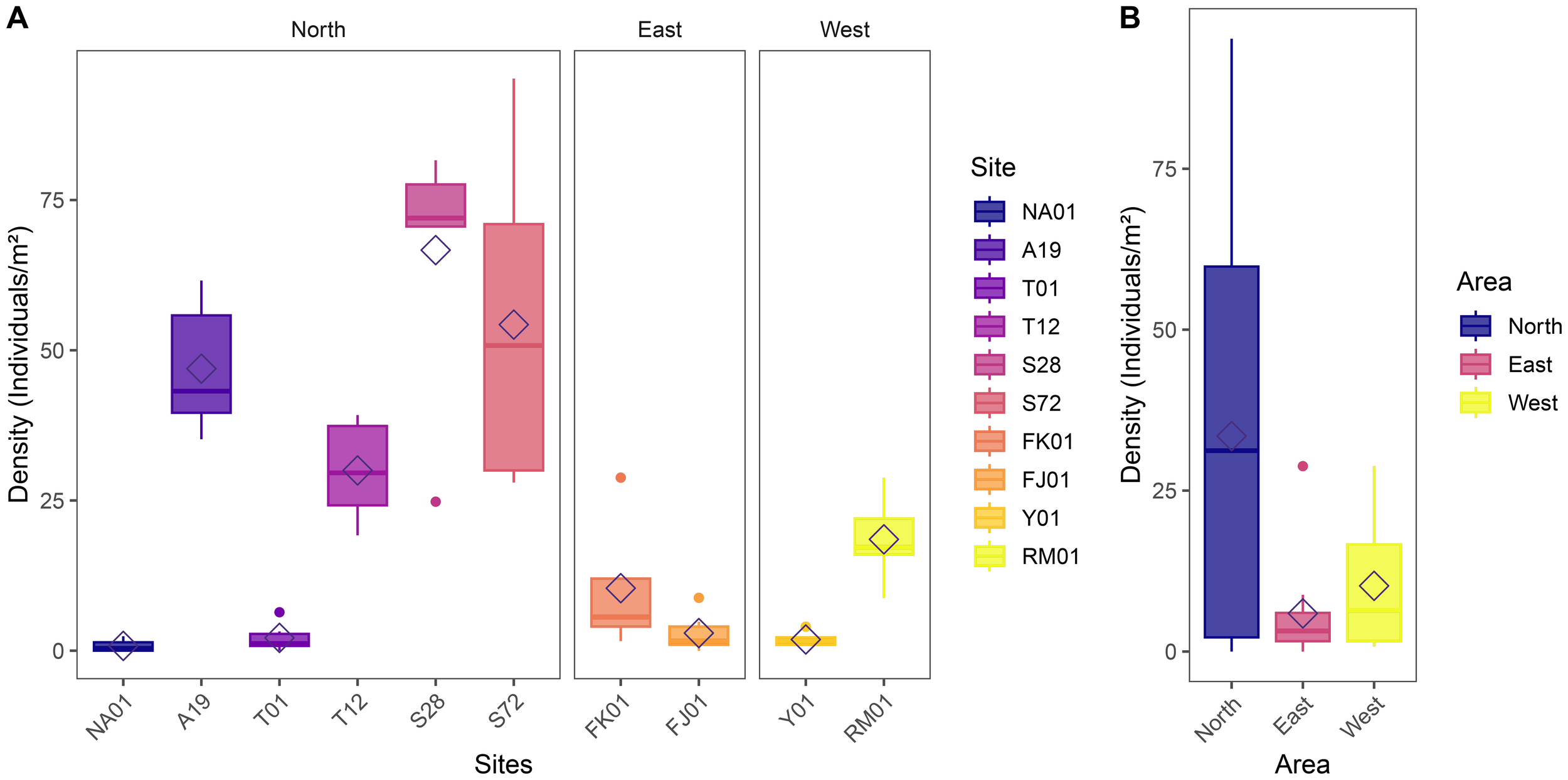
Pearl oyster (pinctada spp.) densities in the kingdom of bahrain (A) At each study site (n=6; area=20 m2 per transect) and (B) Between areas (Eastern Waters: FK01 (Falkland, Fasht Al Adhm) and FJ01 (Fasht Al Jabbari); Northern Waters are represented by sites: NA01 and A19 (Hayr Bu Amamah); T01 and T12 (Hayr Bul Thamah); S28 and S72 (Hayr Shtayyah); Western Waters: Y01 (Yassouf) and RM01 (Ras Al Mumtallah)).
Densities varied across sites with higher densities observed in the south-west site of RM01 (mean = 19 ± 2.8 oysters/m2, N=6) and north-east site of FK01 (mean =10 ± 6.2 oysters/m2, N=4) while the opposite was observed in the north-west site (Y01: mean = 2 ± 0.5 oysters/m2, N=6) and south-east site (FJ01: mean = 3 ± 1.2 oysters/m2, N=6) which were recorded to have lower densities of oysters (Figure 7A).
In general, the highest pearl oyster density was found in the Northern areas with densities of 34 ± 4.9 oysters/m2 (N=36). Areas in the West were the second most dense with 10 ± 2.9 oysters/m2 (N=12) and the Eastern areas had the lowest densities of 6 ± 2.7 oysters/m2 (N=10)(Figure 7B). Generalized linear models using a negative binomial distribution were fitted to assess differences in oyster density across sites and areas. The analysis of deviance indicated a statistically significant effect of site (χ²9,48 = 403.63, p< 0.001) and area (χ²2,55 = 69.44, p< 0.001) on oyster density. This suggests that oyster density varied significantly among the different sites and areas.
3.4 Pearl oyster population size structure & distribution
3.4.1 Morphometric trait variation
Overall, oyster morphometric traits showed significant variation across both sites and areas (Figure 8). GLM analyses revealed strong spatial effects across all five traits. Shell length differed significantly across sites (F9,1845 = 47.86, p<2.2 × 10-16) and areas (F2,1852 = 16.98, p=4.95 × 10-8). Similarly, shell width differed significantly among sites (F9,1845 = 48.78, p<2.2 × 10-16) and areas (F2,1852 = 20.69, p=1.30 × 10-9). Significant differences were also observed in shell thickness across sites (F9,1845 = 16.65, p<2.2 × 10-16) and areas (F2,1852 = 34.52, p=1.92 × 10-15). Oyster thickness showed significant variation by site (F9,1845 = 36.66, p<2.2 × 10-16) and by area (F2,1852 = 18.74, p=8.74 × 10-9). Lastly, hinge length significantly differed among sites (F9,1845 = 62.37, p<2.2 × 10-16) and areas (F2,1852 = 29.46, p=2.54 × 10-13). These patterns demonstrate that population-level morphological variability extends beyond size classes to other shell dimensions, reflecting local environmental or ecological influences.
Figure 8
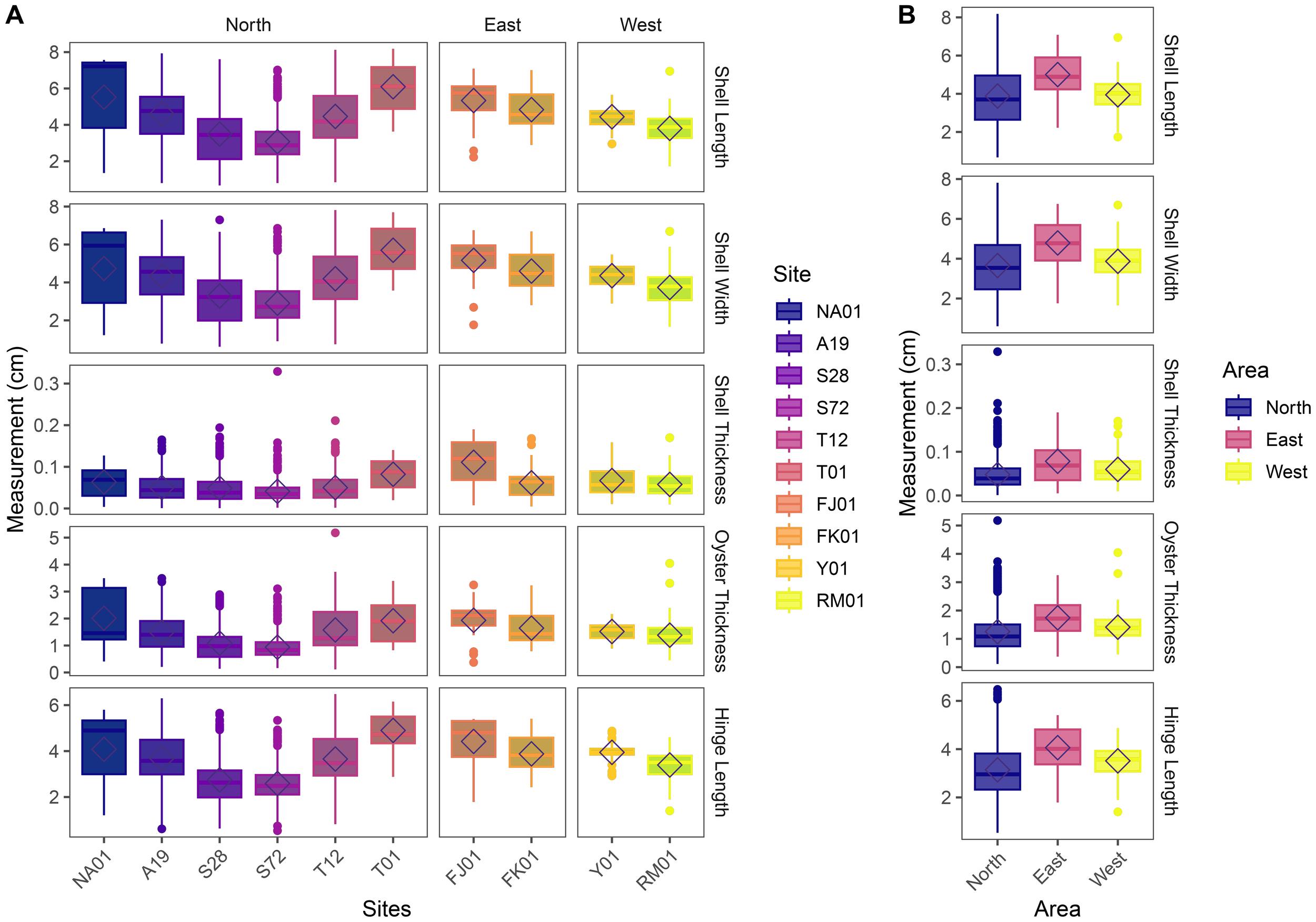
Variation in oyster morphometric traits. Boxplots showing the distribution of five oyster morphological traits—Shell Length, Shell Width, Shell Thickness, Oyster Thickness, and Hinge Length—measured in millimeters across different (A) sampling sites and (B) areas (Eastern Waters: FK01 (Falkland, Fasht Al Adhm) and FJ01 (Fasht Al Jabbari); Northern Waters are represented by sites: NA01 and A19 (Hayr Bu Amamah); T01 and T12 (Hayr Bul Thamah); S28 and S72 (Hayr Shtayyah); Western Waters: Y01 (Yassouf) and RM01 (Ras Al Mumtallah)). Diamonds indicate group means; boxes represent the interquartile range, and whiskers extend to 1.5× IQR.
3.4.2 Population size structure and age inference
Results illustrated that there is variability in the population size structure of Pinctada spp. across the study sites, with age inferred from shell length following the size-age categories in Table 2 (Figure 9; Supplementary Material 3). The Northern pearl oyster beds exhibit a broader range of size classes compared to the eastern and western beds, with individuals spanning a more diverse range of age (Figure 9; Supplementary Material 3). Notably, the Northern sites of Hayr Bul Thamah (Sites: T01 and T12), contain the largest individuals recorded in our dataset (8.2cm and 8.1cm respectively which correspond to >5 years old), followed by Hayr Bu Amamah (Sites: A19 and NA01) suggesting the presence of older cohorts and potentially more stable environmental conditions (Figure 9; Table 2; Supplementary Material 3). In addition, site T01 in Hayr Bul Thamah was recorded to have the largest mean oyster length (6.1 ± 0.3cm (N=18) corresponding to ~3 years old) while Hayr Shtayyah was noted to have the smallest mean oyster length (Sites: S28 = 3.5 ± 0.1cm (N=442) and S72 = 3.1± 0.1cm (N=433), corresponding to ~1 years old) indicating younger cohorts (Figure 9; Table 2; Supplementary Material 3).
Figure 9
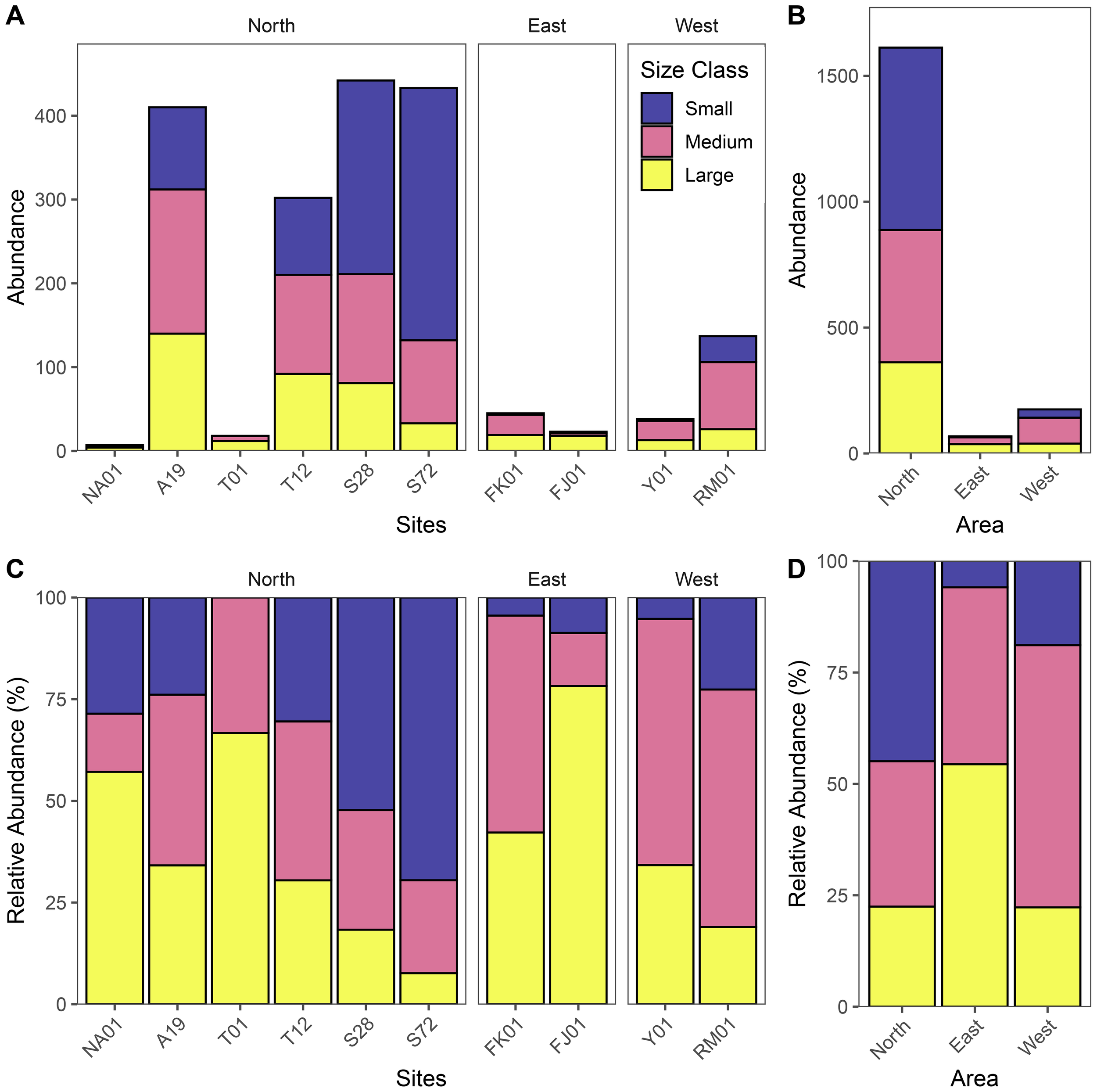
Size Class Composition of Oysters. Stacked bar plots showing (A, B) the absolute abundance and (C, D) the relative abundance (%) of oysters classified into size classes (Small, Medium, Large) across sampling sites (A, C) and areas (B, D) - Eastern Waters: FK01 (Falkland, Fasht Al Adhm) and FJ01 (Fasht Al Jabbari); Northern Waters are represented by sites: NA01 and A19 (Hayr Bu Amamah); T01 and T12 (Hayr Bul Thamah); S28 and S72 (Hayr Shtayyah); Western Waters: Y01 (Yassouf) and RM01 (Ras Al Mumtallah). These visualizations highlight spatial variation in oyster population structure based on shell size distribution.
In contrast, the eastern and western oyster beds support a younger oyster population compared to the Northern oyster beds. Both areas predominantly consist of juvenile and sub-adult oysters (mean shell length: FK01 (4.8 ± 0.2cm, N=45); FJ01 (5.3 ± 0.3cm, N=23); Y01 (4.4 ± 0.1cm, N=38); RM01 (3.8 ± 0.1cm, N=137) (Figure 9; Table 2; Supplementary Material 3). It is worth noting that the largest individuals recorded in the eastern sites were 7.1cm and 7.0cm at FJ01 and FK01 respectively while the western sites had the smallest adult individuals at 6.9cm and 5.7cm at RM01 and Y01 indicating that, while considered adults, the cohorts do not exceed the age of 3.5 years as per the size-age estimation based on shell length (Figure 9; Table 2; Supplementary Material 3). Interestingly, the age structure across the sites, indicates that the largest percentage of spats (<3cm) are at Hayr Shtayyah (Sites: S72 = 55% and S28 = 41.9%), while Yassouf (Y01) had the lowest amount of spats (2.6%). Lastly, no spats were recorded at Site T01.
3.4.3 Size class composition
Further confirmation of significant differences in oyster size class composition among sites (χ² = 387.1, p< 0.001) and areas (χ² = 106.82, p< 0.001) (Figure 9). Sites in the north supported the greatest number of individuals (n=1612), with large oysters making up a substantial proportion particularly at A19, T12 and S28, where medium and large size classes each accounted for ~30-70% of the population. On the contrary, S28 and S72 were strongly dominated by smaller size classes, i.e. >50% of the oysters (Figure 9). The eastern sites supported small populations (n=68 combined), dominated by large size classes (~42% at FK01 and ~78% at FJ01), with only a small fraction of small individuals (<8%). The western sites exhibited a mixed structure (n=175 combined) dominated by medium sized oysters (~58% at RM01 and ~60% at Y01). When comparing across areas, the Northern Hayrat were characterized by a broader and more balanced spread across small, medium and large size classes (Figures 9C, D), thereby supporting both higher abundance and more diverse size structures reflecting both ongoing recruitment (e.g. spats at S28 and S72) alongside persistence of older cohorts (e.g. A19 and T12). In contrast, both the eastern and western beds were dominated by sub-adults and adults, with small size classes comprising<22% of the total) suggesting limited recent recruitment and a more mature population structure (Figure 9).
3.5 Incidence of pearls
Out of the 1,855 Pinctada spp. oysters collected from across the ten study sites in Bahrain, 3.6% [95% CI: 2.8–4.6%] of oysters had pearls in them. The proportion of oysters with pearls varied across sampling sites (Figure 10). The highest percentage of pearl presence was observed in Hayr Bu Amamah at site NA01 (14.3% [95% CI: 0.4–57.9%]) followed by Hayr Bul Thamah at site T01 (11.1% [95% CI: 1.4–34.7%]) and T12 (6.3% [95% CI: 3.8–9.7%]), while other sites such as FJ01, S28, A19, and RM01 exhibited moderate pearl yields ranging between 3.5% and 4.5%. In contrast, Y01, S72, and FK01 showed the lowest proportions, with FK01 recording no pearl incidence. These findings indicate variability in pearl occurrence across different oyster bed regions, with selected sites within the Northern Hayrat beds showing generally higher pearl percentages compared to the Eastern and Western beds. In addition, significant differences in the pearl yield across sites (χ² = 80.094, df = 9, p< 0.001), and areas (χ² = 96.608, df = 2, p< 0.001) were detected.
Figure 10
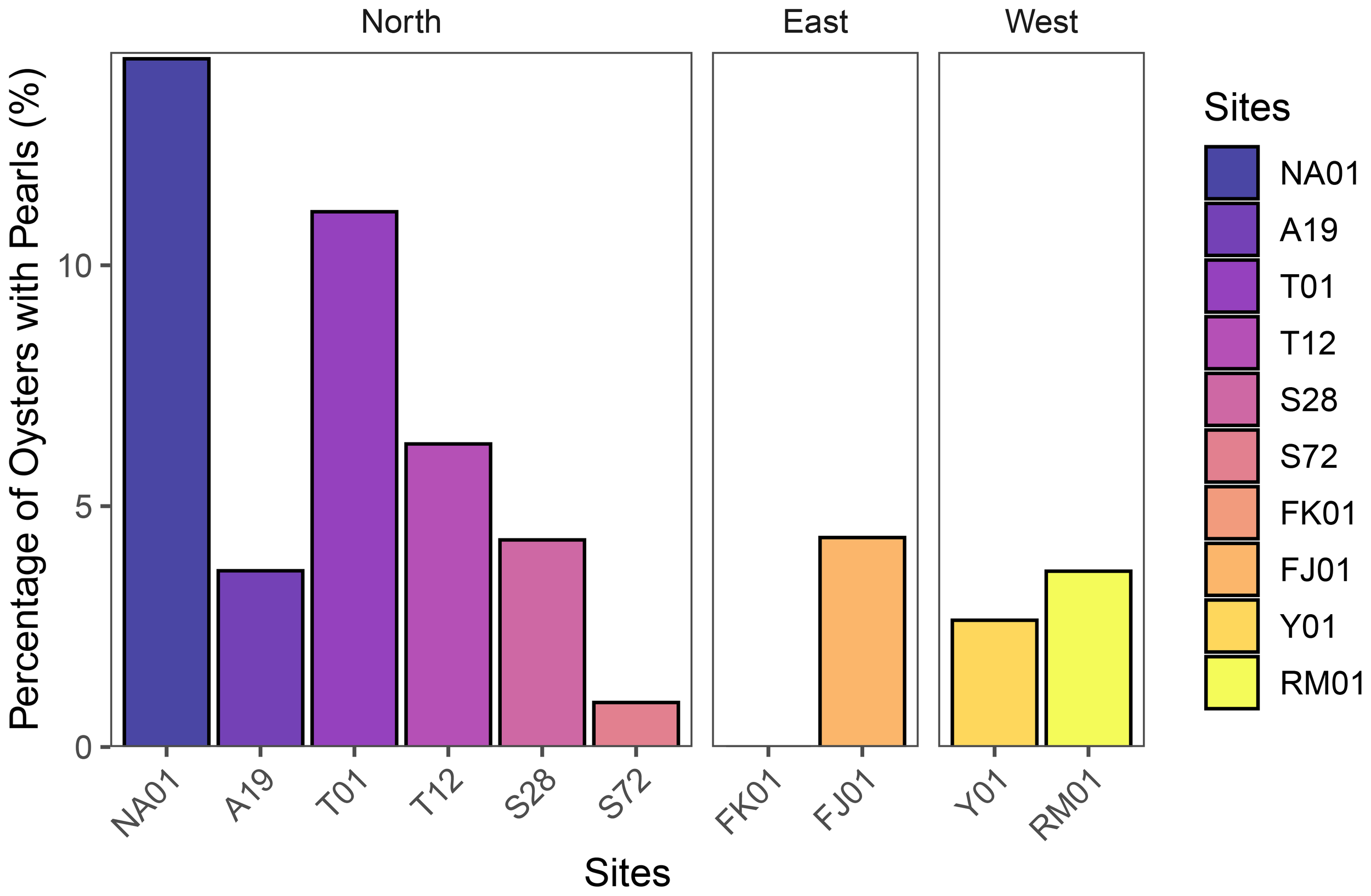
Percentage of oysters with pearls indicating incidence of pearls in Bahraini waters, where Northern Waters are represented by sites: NA01 and A19 (Hayr Bu Amamah); T01 and T12 (Hayr Bul Thamah); S28 and S72 (Hayr Shtayyah); Eastern Waters: FK01 (Falkland, Fasht Al Adhm) and FJ01 (Fasht Al Jabbari); Western Waters: Y01 (Yassouf) and RM01 (Ras Al Mumtallah).
The logistic regression analysis showed that oyster morphometrics were significant predictors of pearl incidence. The final model, selected based on likelihood ratio tests and the lowest AIC, retained shell length, oyster thickness, and hinge length as key variables. Pearl presence increased significantly with greater shell length (OR=1.07, 95% CI: 1.02–1.12, p<0.05) and greater oyster thickness (OR=1.15, 95% CI: 1.07–1.24, p<0.01). In contrast, hinge length was negatively associated with pearl presence (OR=0.92, 95% CI: 0.88–0.97, p<0.05), indicating that oysters with shorter hinge lengths were more likely to contain pearls. The model intercept (OR=0.003, 95% CI: 0.001–0.010) reflects the low baseline probability of pearl formation when morphometric predictors are at their reference values. These findings suggest that oysters with longer shells and thicker bodies, but relatively shorter hinges, have a higher likelihood of pearl formation.
4 Discussion
Pearl oyster beds in the hottest sea on the planet were investigated to assess their ecological characteristics providing insights into the benthic composition, oyster community structure, pearl yield, and population size structure across multiple sites and geographic areas. The pearl oyster beds around Bahrain were shown to harbor different and distinct ecological habitats, which are clearly influenced by their location, depth, and other environmental factors. Sand was the dominant benthic feature across all oyster bed areas, with the exception of Falkland (FK01) in the northeast and Yassouf (Y01) in the northwest. On the other hand, the eastern and western waters were seen to be more dominated by algal beds and, to a lesser extent, seagrass. Sponges were observed across several sites but played a relatively minor role in the benthic community composition. This aligns with previous findings that report the ecological role of sponges is context-dependent, and in some systems, they strongly contribute to nutrient cycling and habitat structure while in other, they may play a relatively minor role (Kandler et al., 2019; Ruzicka and Gleason, 2009). This pattern reinforces the broader latitudinal gradient observed, with higher coral cover in the north and increased algal and seagrass dominance in the east and west with extensive seagrass areas existing around Fasht Al Jabbari (FJ01) in addition to areas surrounding Yassouf (Y01) and Ras Al Mumtallah (RM01). Coral was observed to play a significant role in shaping the benthic environment at the Northern sites, with a high percentage of coral association observed at Hayr Shtayyah (site S28: mean = 23.4%), indicating strong reef cover in an area dominated by pearl oysters. This could be influenced by ecological connectivity to a coral reef locally known as Najwat Bul Thamah (Reef Bul Thamah) (AlMealla and Hepburn, 2024; Ministerial Order (3) of 2017). While coral associations with oyster species have been reported previously (Richardson et al., 2022; Kleemann et al., 1992), the ecosystems in the Arabian Gulf differ from the traditional perspective, reflecting the unique environmental pressures and adaptations in this region (Coles and Riegl, 2013). Understanding population dynamics in extreme environments such as the Arabian Gulf may offer broader lessons for managing oyster populations facing climatic stressors globally.
4.1 Environmental gradients and pearl oyster beds
Regional (i.e., north, east and west) differences in benthic composition are closely tied to underlying environmental gradients (Table 3). The Northern Hayrat occur in deeper (~ 11–21 m) and clearer waters with moderate salinity (40–42 ppt) in addition to stable conditions which likely facilitate greater habitat complexity and higher oyster densities. In contrast, the Eastern and Western oyster beds are much shallower (<5m) and more environmentally extreme with elevated salinity (43–45 ppt in the east; >53 ppt in the west). Differences in depth, water clarity and salinity regimes are important drivers of both habitat composition and oyster population structure (Tolley et al., 2005).
Salinity differences across the study sites may also explain the observed patterns in benthic composition and oyster community structure (Mrozińska et al., 2021; Broman et al., 2019; Kennish et al., 2009). In particular, the western waters of Bahrain, Yassouf (Y01) and Ras Al Mumtallah (RM01), exhibited extremely high salinity levels i.e., >50 ppt. This could be attributed to the enclosed nature of these areas, which resemble bay environments. Their shallow depth, combined with higher evaporation rates (1.5–2 m/yr) and limited water exchange (Ghazi et al., 2017; Xue and Eltahir, 2015), likely contributes to increased salinity. Such hypersaline conditions not only shape benthic composition but also directly affect oyster populations by limiting larval settlement success and favoring smaller-bodied, stress-tolerant cohorts (Pérez-Velasco et al., 2022; Marshall et al., 2021). This may explain why these sites were dominated by juvenile or ‘dwarf’ oysters and exhibited lower overall densities compared to the Northern Hayrat, where salinity is relatively lower. Nevertheless, the variation observed in community structure of pearl oyster beds across Bahraini waters reflected clear regional differences in species composition and relative abundance, which similar to the benthic composition may be influenced by a latitudinal gradient and local environmental factors on oyster community structure.
It is important to note that the temperatures recorded during our surveys (21-25 °C) reflect the winter to early spring season (January–April). Although winter temperatures drop annually to as low as 16 °C (late December–February), the Gulf summer peaks of 34–36 °C (July–September) combined with hypersaline conditions, define it as the hottest sea on the planet and exert a strong influence on oyster population dynamics.
4.2 Diversity patterns in pearl oyster community structure
Despite ongoing differences regarding the number of pearl oyster species present in Bahraini waters (Ali, 2022; Khamdan, 2004; Nayar and Al-Rumaidh, 1993a), an effort was made to document the species of pearl oysters encountered at the study sites based on their morphological features. The community structure of the pearl oyster beds across all sites revealed a total of nine pearl oyster species within the quadrat-transect areas. These species included P. radiata, P. nigra, P. maculata, P. maxima, P. sugillata, Pinctada spp., M. regula, I. legumen, and P. bicolour. Khamdan (2004) previously reported eight species in Bahraini waters, and our results are broadly consistent with his findings while also indicating the possible presence of additional taxa.
The Northern sites exhibited the highest species richness, with six species of Pinctada identified, suggesting greater habitat complexity and potentially more favorable environmental conditions for oyster diversity. Notably, P. sugillata was among the least abundant species across all sites, recorded only in the Northern areas at site S72 in Hayr Shtayyah (0.2%) and site A19 in Hayr Bu Amamah (1.9%). Similarly, I. legumen and P. bicolour were recorded at single locations: site A19 (0.7%) and site T12 in Hayr Bul Thamah (5%), respectively. This localized presence of rare species highlights the importance of specific microhabitats and environmental conditions in supporting unique oyster assemblages.
In contrast, the eastern and western sites were dominated by three species (P. radiata, P. maculata, and an unidentified species of Pinctada), indicating a more homogenous community structure in these areas. Notably, P. radiata dominated the community structure across all sites, underscoring its role as a foundational species in the Gulf. Its ability to tolerate wide salinity and temperature ranges likely provides a competitive advantage under extreme conditions allowing it to monopolize spaces and responses (Mohammed and Yassien, 2003; Al-Wedaei et al., 2011). This ecological dominance has cascading implications, while it supports bed persistence, it may also reduce overall diversity by competitively excluding less tolerant species. Understanding the balance between resilience and homogenization is therefore central to managing Gulf oyster beds under climate stress.
In addition, in the eastern waters, P. radiata exhibited the highest relative abundance at site FK01 (97.8%) and FJ01 (82.6%), underscoring its dominance in this region. However, species diversity was low in the eastern sites, with P.maculata being the only other species recorded at both sites, albeit in much lower numbers (2.2% at FK01 and 4.3% at FJ01). In addition, an unidentified Pinctada species was recorded at FJ01, contributing 13.0% to the community, which suggests the presence of cryptic diversity or potentially misidentified species in this area. In the western waters, site RM01 demonstrated a more diverse community structure, with M. regula (8.7%) and P. maculata (3.3%) contributing to the community alongside P. radiata. However, at Yassouf (Y01) P. radiata dominated the entire community structure (100%), suggesting environmental factors or competitive advantages favor this species in the western sites. Unfortunately, to date, no study has specifically focused on species richness in Bahrain thereby limiting direct comparison of our findings with historical data.
4.3 Patterns in pearl oyster association with coral, algal and seagrass
Coral structures in the Northern sites may offer suitable substrate for larval settlement and their complex habitat provide protection to juveniles from predation and support higher long-term survival (Bartol et al., 1999), thus supporting higher oyster densities and greater species richness. On the other hand, algal and seagrass-prevalent sites like those in the eastern and western waters may offer different ecological niches that could host less stable substrate and higher competition for space, favoring fast-growing, stress tolerant species such as P. radiata thereby, influencing the distribution and abundance of oysters. The variation in benthic composition and environmental conditions across sites reinforces the importance of localized management strategies that account for habitat-specific dynamics. Similar latitudinal and environmental patterns have been observed in other oyster systems worldwide. For example, Pinctada margaritifera and P. maxima in the Great Barrier Reef and French Polynesia are strongly influenced by temperature and food availability with the greatest abundance occurring in deeper, clearer coral reef and atoll lagoon habitats (Yukihira et al., 2006, 2000, 1999, 1998; Pouvreau et al., 1999). In addition, previous studies have showcased that the role of oysters as ecosystem engineers varies with habitat setting, with stronger facilitative effects on rocky shores under greater thermal stress compared to mangroves (McAfee et al., 2016). These global observations are mirrored in Bahrain’s oyster systems whereby the Northern sites with the higher species richness and oyster densities are in coral-associated areas in comparison to the algal and seagrass dominated eastern and western sites. The coral habitats in the north likely provide more structurally complex and stable habitats that support diverse oyster assemblages, whereas the shallower hypersaline and turbid conditions of the east and west select for stress-tolerant species such as P. radiata. These findings highlight that both latitude and habitat type interact with environmental stressors to shape oyster community structure, a pattern consistent with ecological theory (Bertness and Callaway, 1994) and global oyster studies (Kimbro et al., 2020; McAfee et al., 2016; Yukihira et al., 2006).
4.4 Densities of pearl oysters: the present & historical comparison
Variations in pearl oyster densities across Bahraini waters highlights distinct regional differences in habitat suitability and oyster aggregation patterns. A total of 1,855 individuals of the genus Pinctada were collected across all surveyed sites, with the Northern Hayrat contributing the majority (1,612 individuals), followed by the western waters (175 individuals) and the eastern waters (68 individuals). The highest pearl oyster densities were recorded in the Northern sites, particularly at Hayr Shtayyah, also considered the largest Hayr amongst the Northern Hayrat.
When compared to historical estimates from the survey report produced by Arfa et al. (2012), our results show both consistencies and notable changes. For example, at Hayr Shtayyah, site S28, the present density of 67 ± 8.6 oysters/m² aligns well with what was previously reported (i.e. Abundant category using the SACFOR method, characterized by >100 oysters/m²). This consistency suggests that S28 continues to function as one of Bahrain’s most productive oyster habitats, providing highly favorable conditions for pearl oyster settlement and survival including optimal water temperatures, appropriate salinity levels, food availability, clearer waters and depth (Khalifa et al., 2024; Kimbro et al., 2020; Ishikawa et al., 2020; Tolley et al., 2005). In contrast, site S72, classified previously as Occasional (≤1 oysters/m²), now supports a much higher densities (54 ± 11.5 oysters/m²) indicating a possible recovery or local recruitment event. In Hayr Bu Amamah (the smallest Hayr), site A19 was previously categorized as Common (9–100 oysters/m²) while our present measured density of 47 ± 4.5 oysters/m² falls within this range, suggesting relative stability in oyster abundance. Similarly, although NA01 was not surveyed in 2012, a site close to its location was classified as Frequent but locally Common (1–100 oysters/m²) and our recorded density of 1 ± 0.4 oysters/m² indicates persistence but at the lower end of this range. In Hayr Bul Thamah, both sites surveyed were historically categorized as Frequent but locally Common (T01) and Common (T12) while our recorded densities showed them at only 2 ± 1 oysters/m² suggesting localized declines. Overall, these historical comparisons show that some Northern sites illustrate persistence of high-density oyster populations such as S28 and A19 while some indicate potential declines or shifts (e.g. T01 and T12). This variability underscores the importance of localized environmental conditions, recruitment dynamics and harvesting pressures in shaping present-day oyster population structures in Bahrain.
In the western waters, densities were highest at site RM01 (mean = 19 ± 2.8 oysters/m²), which may reflect more stable environmental conditions or greater availability of suitable substrate compared to the eastern sites, where lower densities were observed at FK01 (mean = 10 ± 6.2 oysters/m²) and FJ01 (mean = 3 ± 1.2 oysters/m²). The lowest recorded density was at Yassouf (Y01) in the northwest (mean = 2 ± 0.5 oysters/m²), further supporting the pattern of reduced oyster aggregation in more exposed or environmentally extreme locations. The overall trend of higher densities in the Northern sites (mean = 34 ± 4.9 oysters/m²), followed by the western sites (mean = 10 ± 2.9 oysters/m²), and the lowest in the eastern sites (mean = 6 ± 2.7 oysters/m²) suggests that environmental gradients, such as substrate availability, hydrodynamics, and possibly food availability, may be influencing pearl oyster distribution. The significant difference in oyster densities across sites and areas, as indicated by the GLM analysis, revealed the importance of regional environmental conditions and localized habitat features in shaping oyster community structure.
4.5 Size-structure of pearl oysters
While density comparisons provide insight into temporal shifts, our population size-structure data illustrate an additional dimension of population dynamics. Hayr Bu Amamah supports both stable densities and older cohorts, whereas Hayr Bul Thamah, despite reduced densities, still harbors long-lived individuals indicative of residual population stability. The broader range of size classes at these two sites, including individuals >8cm in length (corresponding to >5 years of age) suggests the persistence of older cohorts and relative population stability. However, when this is considered alongside historical density estimates (Arfa et al., 2012), a more informative picture emerges. While Hayr Bu Amamah appears relatively stable in both size, structure and density, Hayr Bul Thamah shows evidence of containing large and older individuals despite an overall decline in density. This divergence between density and size structure demonstrates that population stability is multi-dimensional, requiring both abundance and age structure to be assessed together. Declining densities may mask the presence of older residual cohorts, while high densities alone may not guarantee long-term persistence-underscoring the importance of integrating both metrics in evaluating recovery potential.
In contrast, the eastern and western sites were dominated by smaller oysters, with mean shell lengths corresponding to younger cohorts (<3.5 years), highlighting that these populations are primarily composed of juvenile and sub-adult oysters. The higher proportion of younger oysters in the eastern and western sites could be indicative of recent recruitment or higher turnover rates, possibly due to increased environmental stress or harvesting pressure. Hayr Shtayyah had the highest percentage of spats (<3cm), suggesting that this site may serve as an important recruitment ground, whereas the absence of spats at site T01 could indicate limited recruitment or high post-settlement mortality. The significant variation in shell length across sites, emphasizes the spatial differences in population structure, but the lack of significant differences between broader areas suggests that local-scale factors such as habitat quality, recruitment success, and harvesting pressure are more influential in shaping size structure. These results examining population size structure reiterate the importance of the need for localized management strategies. Previous studies in neighboring countries such as in Qatari waters, have reported Pinctada radiata shell length to be between 5.6 to 8.4cm (Mohammed and Yassien, 2003).
4.6 Pearl yield
The overall pearl occurrence of 3.6 ± 0.4% across all sites is consistent with historical reports of 3.3% in 2012 exclusively for the Northern Hayrat sites (Arfa et al., 2012) and 4.6% in 1993 from 15 sites including Hayr Bul Thamah, Hayr Bu Amamah, Hayr Shtayyah and the areas in close proximity to Falkland (Nayar and Al-Rumaidh, 1993a). Pearl yield was highest at Hayr Bu Amamah (NA01: 14.3%) and Hayr Bul Thamah (T01: 11.1%) likely reflecting the presence of older cohorts, since larger, long-lived oysters are more prone to pearl oyster formation under prolonged exposure to environmental stressors that trigger nacre deposition (Ishikawa et al., 2020). In contrast, Hayr Shtayyah exhibited the lowest pearl yield, particularly at S72 (0.9%), consistent with its younger size structure, indicating that younger oysters are less likely to produce pearls (Ishikawa et al., 2020). The absence of pearls at FK01 (eastern site) could be due to the small sample size at the historically heavily targeted and overharvested site while the low yield at Y01 (north-west site) could be a reflection of environmental or genetic factors influencing pearl production, such as water quality, nutrient availability, or disease pressure since this site endures extreme conditions such as high salinity levels (55.1ppt), shallow waters (<2m) and large annual temperatures fluctuations (16-36 °C) (Al-Wedaei et al., 2011). Oysters from the western waters as mentioned previously are also notably smaller (“dwarf oysters”, ≤3.5 years old at RM01 and Y01), a trait local scientists attribute to genetic adaptation to extreme conditions.
Overall, pearl yield was higher in the western sites (3.4%) compared to the eastern sites (1.5%) suggesting site-specific factors, particularly environmental stability, population structure, harvesting intensity, all of which shape pearl formation potential. These findings also reflect the historical importance of Hayr Bu Amamah, Hayr Bul Thamah, and Falkland as highly productive pearl beds and have been heavily targeted by pearl divers for generations (Nayar and Al-Rumaidh, 1993a). The accessibility and environmental conditions of these sites likely amplify differences in both oyster densities and yield. Hayr Bu Amamah, Hayr Bul Thamah, and Hayr Shtayyah are located approximately 58km, 57km and 30km respectively from the mainland (Arfa et al., 2012), and are >10m deep, making them difficult to access during rough weather conditions. Harvesting at these sites requires larger boats and specialized diving equipment, which may reduce the frequency and intensity of pearling activity. It is however important to note that when pearl divers do access these sites, harvesting is practiced heavily. In contrast, Falkland is located less than 2km from the mainland and at a shallow depth of less than 2 meters, allowing for easy access and pearling without the need for dive equipment. This ease of access likely increases harvesting pressure, which could explain the low oyster densities recorded at this site. The combined effects of historical overharvesting and varying accessibility may be key factors driving the observed differences in oyster densities across these traditionally productive beds.
4.7 Policy recommendations
To enhance the sustainability of Bahrain’s pearl oyster beds, particularly in the Northern Hayrat which are already designated as MPAs, more targeted regulatory measures and on-field monitoring are recommended. Our findings support the following measures to ensure the recovery of oyster populations where relevant and maintain the ecological and economic value of Bahrain’s pearl beds:
-
Adoption of a Rotational Zonation Strategy: The persistence of high densities at sites such as in Hayr Shtayyah (e.g. site S28) and Hayr Bu Amamah (e.g. site A19), alongside declines at Hayr Bul Thamah (e.g. sites T01 and T12), underscores the importance of reducing localized harvesting pressure. Rotational zonation would allow exploited areas to recover while maintaining access to stable sites.
-
Designation of New Protected Areas: Sites in the Northern Hayrat area, especially the coral-associated sites, showed higher species richness and oyster densities compared to the eastern and western sites. Protecting ecologically, historically and economically important sites such as Falkland and Fasht Al Jabbari would conserve productive habitats and reduce harvesting pressure.
-
Establish a National Oyster Database & Monitoring: Comparisons with Arfa et al. (2012) revealed both consistencies (e.g. S28, A19) and declines (e.g. T01 and T12) thereby highlighting the importance of comparative data built through a long-term monitoring program. The establishment of a centralized national database to track oyster catch data, including daily harvest records and site-specific trends will enable early detection of overharvesting and shifts in population structure, supporting adaptive management.
-
Habitat-Specific Management Strategies: Our findings of stress-tolerant dwarf oysters in hypersaline western sites versus older, stable cohorts in the Northern sites highlight the need for strategies tailored to local ecological conditions.
-
Strengthen Enforcement and Compliance: Current harvesting regulations covered under Ministerial Order (3) of 2017 regarding designating Hayr Bul Thamah, Hayr Shattayah, and Hayr Bu Amamah as Marine Protected Areas dictate that only those holding a pearling permit issued by the Directorate of Marine Wealth and the Directorate of Reserves at the Supreme Council for Environment are allowed to harvest oysters. However, there is no catch limit or regulation to track landing data or site-specific harvesting rates. Given observed site-specific declines, introducing catch limits and mandatory reporting of landing data would improve monitoring and resource management thereby ensuring sustainability.
-
Introduce Seasonal Bans: While our study did not assess seasonal variability, evidence from other oyster fisheries shows that temporary closures during spawning seasons support recruitment and stock recovery (Marquardt et al., 2025; Beentjes, 2023; van Overzee and Rijnsdorp, 2015). Similar approaches could be explored in Bahrain especially noting that pearl diving historically was based on a pearl diving season not all year harvesting.
-
Regulate Tourist Harvesting: Currently, tourists are allowed to collect up to 60 oysters per person when accompanied by authorized dive centers based on Decision No. (43) of 2017 on regulating pearl fishing and extraction. Although not assessed in our study, tourist collection may add pressure to already stressed beds. Lessons from global fisheries suggest reviewing and adjusting recreational harvest limits (e.g. daily bag limits, size thresholds, harvest seasons, or closures) to reflect local sustainability thresholds (Fisheries New Zealand, 2025; Hilker and Liz, 2020; Peters et al., 2017).
4.8 Limitations
While this study provides an initial overview of species composition based on morphological traits, it is important to acknowledge the inherent risk of misidentification when relying on shell morphology alone. Pearl oyster species in the Gulf are known for considerable phenotypic plasticity, and the presence of cryptic species cannot be ruled out. Moreover, regional endemism may contribute to morphotypes that do not align neatly with existing taxonomic descriptions. For these reasons, future work should incorporate molecular validation (e.g., DNA barcoding and genomic approaches) to confirm species identities and resolve uncertainties in regional taxonomy. Such approaches are critical to disentangle cryptic diversity, refine species boundaries, and provide a more robust basis for biodiversity assessments and management. Similar challenges in other regions have benefited with important insights derived from molecular techniques (Lal et al., 2018; Sun et al., 2016; Arnaud-Haond et al., 2008). It is important to acknowledge that our sampling was limited to the period between January and May, and therefore does not capture potential seasonal or interannual variation in pearl oyster populations. The patterns presented here should thus be interpreted as a temporal snapshot rather than representing year-round dynamics. Multi-seasonal studies from multiple regions have shown that density, reproductive activity and size structure of pearl oysters can vary seasonally, often linked to spawning cycles and temperature fluctuations (e.g. Khalifa et al., 2024; Pafras et al., 2024; Gómez-Robles et al., 2023; Yigitkurt, 2021). Furthermore, monitoring across multiple seasons in Bahrain will be essential to determine whether similar temporal trends occur in this extreme environment.
5 Conclusion and future research
Our results highlight that oyster densities are not shaped by environmental conditions alone but by the interaction of ecology and human pressure. Sites with favorable environmental conditions, such as the deeper and more stable Northern Hayrat sites can sustain higher oyster densities when accessibility limits harvesting frequency. On the contrary, shallow and easily accessible sites like Falkland show low densities despite seemingly suitable substrates, illustrating how harvesting pressure can override environmental suitability. This interplay between natural and anthropogenic drivers is critical for understanding present-day oyster distributions in Bahrain. Beyond the local context, these findings also hold broader relevance for oyster population resilience in extreme environments worldwide. Similar patterns of environmental stress shaping oyster community structure, recruitment, and persistence have been documented in regions such as the Great Barrier Reef, French Polynesia, and intertidal oyster reefs in Australia (Leong et al., 2024; McAfee et al., 2016; Yukihira et al., 2006). Bahrain’s pearl oyster beds therefore serve as a natural laboratory for testing global predictions on how foundation species respond to combined environmental extremes and harvesting pressures, offering insights for the conservation and management of oyster systems under future climate change.
The ecological patterns observed in Bahrain’s pearl oyster beds ranging from their association with coral, algal, and seagrass habitats to their responses under hypersaline and thermally extreme conditions offer insights of global importance. As pearl oysters are distributed across tropical and subtropical regions, understanding their ecological dynamics in the world’s hottest sea provides a natural laboratory for predicting how similar ecosystems may respond to future climate extremes. Moreover, the dynamic between traditional resource use, cultural heritage, and conservation in Bahrain mirrors challenges faced by coastal communities worldwide.
This study is the first to quantify and define the benthic composition, community structure, population size, and pearl yield of Pinctada spp. across Bahrain’s pearl oyster beds. The patterns observed here provides important ecological insights into the distribution of oyster beds and the associated benthic communities, highlighting that Northern sites exhibit higher species richness and larger oyster sizes, likely due to more stable environmental conditions and association with coral structures. In contrast, variation in oyster densities and pearl yield across sites reflects localized environmental factors and harvesting pressures. These findings add to the growing recognition that site-specific ecological data, particularly from underrepresented regions, are critical for informing global models of marine ecosystem resilience and guiding adaptive management in light of climate change. Future research should focus on investigating the drivers of these patterns, including the role of temperature, salinity, and nutrient availability, to better understand the resilience of pearl oyster populations in a rapidly changing environment. Integrating molecular tools such as DNA barcoding and population genomics would help clarify species boundaries, detect cryptic diversity and assess adaptive potential under extreme environmental stressors.
Statements
Data availability statement
The data, including benthic composition, pearl oyster community structure, oyster density, pearl yield, and population size structure, as well as the R scripts used for data processing, analysis, and visualization, are accessible at https://zenodo.org/records/17271892. All datasets generated and analyzed during this study are openly available under a Creative Commons Attribution 4.0 International (CC BY 4.0) license. Users are free to share and adapt the data provided appropriate credit is given.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
RA: Writing – review & editing, Investigation, Supervision, Formal analysis, Resources, Data curation, Project administration, Methodology, Funding acquisition, Writing – original draft, Conceptualization. BE: Writing – original draft, Formal analysis, Visualization, Writing – review & editing, Data curation. MA: Writing – review & editing, Investigation. ZM: Data curation, Writing – review & editing, Investigation. MK: Writing – review & editing, Investigation. MS: Writing – review & editing, Investigation. AT: Writing – review & editing, Investigation. NA: Investigation, Writing – review & editing. FO: Writing – review & editing, Investigation. MH: Investigation, Writing – review & editing. LH: Writing – review & editing. AK: Writing – review & editing, Supervision. EA: Writing – review & editing. TD: Writing – review & editing, Investigation, Methodology, Writing – original draft, Data curation, Formal analysis.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Funding for this project was made possible thanks to the support of BNP Paribas, ITRI-NY, and Nuwat for Environmental Research & Education, which enabled both the field and laboratory work.
Acknowledgments
The authors would like to thank Jaber Hasan for his invaluable support in the field and for generously sharing his local knowledge, which greatly enriched this study. Further thanks go to the Supreme Council for Environment for providing the laboratory facilities necessary to process the samples, and to the Arab Regional Center for World Heritage (ARC-WH) for facilitating the logistics, including securing the research permits. Gratitude is extended to Sh. Isa D. AlKhalifa and Nawaf Al Rumaihi for providing support when required during lab processing. Further gratitude goes to Dr. Mikey Mohan for his valuable feedback that helped improve the presentation of the paper. The first author extends her heartfelt gratitude to Mohamed Slaise for his guidance over the last 13 years on cultivating a relationship with the native oyster beds and for teaching her the traditional methods of finding pearls using ancestral tools. The authors would like to thank the reviewers for their suggestions which has greatly improved the manuscript. Lastly, the authors acknowledge that ChatGPT (OpenAI, San Francisco, CA, USA) was used for language editing to improve grammar and fluency.
Conflict of interest
The authors declare no conflict of interest, except for RKA, who served as the Research Manager at Bahrain’s Institute for Pearls and Gemstones DANAT from 2020 to 2022. During this period, DANAT partnered with the Supreme Council for Environment and RKA led the scientific dive team in surveying the Northern oyster beds. The aim was to conduct a temporal comparison of the population size structure and pearl incidence using data plus method of 2012 Arfa et al., 2012. Both datasets i.e., data collected during the period 2020-2022 and 2012, to date remain unpublished and undisclosed, data for this study was collected independently in 2024 as part of the research led and executed by Nuwat for Environmental Research & Education.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. The authors acknowledge that ChatGPT (OpenAI, San Francisco, CA, USA) was used for language editing to improve grammar and fluency. No AI tools were used for data analysis, interpretation of results, or drafting of scientific content. The responsibility for the accuracy and integrity of the manuscript rests solely with the authors.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Correction note
This article has been corrected with minor changes. These changes do not impact the scientific content of the article.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1667575/full#supplementary-material
References
1
Ali T. S. (2022). Marine biotopes of Askar coastal area, east of Bahrain. Saudi J. Biol. Sci.29, 103364. doi: 10.1016/j.sjbs.2022.103364
2
AlMealla R. K. Edullates Hepburn L. J. (2024). Bleaching threatens positive carbonate budgets on Bahraini reefs. Mar. Biol.171, 39. doi: 10.1007/s00227-023-04351-9
3
AlMealla R. Hepburn L. (2024). “ Coral reefs in the pearl of the gulf—Bahrain,” in Coral reefs and associated marine fauna around the Arabian Peninsula. Eds. RasulN. M. A.StewartI. C. F. (London, UK: Taylor & Francis).
4
Al-Sayed H. Al-Rumaidh M. J. Nayar K. N. (1997). Spat settlement and growth of yearling of the pearl oyster, Pinctada radiata, in Bahrain waters. Arab Gulf J. Sci. Res.15, 467–480.
5
Al-Wedaei K. Naser H. Al-Sayed H. Khamis A. (2011). Assemblages of macro-fauna associated with two seagrass beds in Kingdom of Bahrain: Implications for conservation. J. Assoc. Arab Universities Basic Appl. Sci.10, 1–7. doi: 10.1016/j.jaubas.2011.06.004
6
Arfa S. B. AlMealla R. Edwards D. Cruz E. (2012). “ Monitoring programme for the oyster bed sites,” in Environment Arabia ( Technical Report, Manama, Kingdom of Bahrain).
7
Arnaud-Haond S. Vonau V. Rouxel C. Bonhomme F. Blanc F. Prou J. et al . (2008). Genetic structure at different spatial scales in the pearl oyster (Pinctada margaritifera cumingii) in French Polynesian lagoons: Beware of sampling strategy and genetic patchiness. Mar. Biol.155, 147–157. doi: 10.1007/s00227-008-1013-0
8
Bahrain National Portal (2025). Facts & figures: Area of the Kingdom of Bahrain (Kingdom of Bahrain: Information & eGovernment Authority). Available online at: https://www.Bahrain.bh (Accessed March 28, 2025).
9
Bartol I. K. Mann R. Luckenbach M. (1999). Growth and mortality of oysters (Crassostrea virginica) on constructed intertidal reefs: Effects of tidal height and substrate level. J. Exp. Mar. Biol. Ecol.237, 157–184. doi: 10.1016/S0022-0981(98)00175-0
10
Beaumont A. R. Khamdan S. A. A. (1991). Electrophoretic and morphometric characters in population differentiation of the pearl oyster, Pinctada radiata (Leach), from around Bahrain. J. Molluscan Stud.57, 433–441. doi: 10.1093/mollus/57.4.433
11
Beentjes M. P. (2023). Are marine reserves and temporary closed areas effective in enhancing blue cod (Parapercis colias) sub-populations? New Z. J. Mar. Freshw. Res.58, 541–576. doi: 10.1080/00288330.2023.2277766
12
Bertness M. D. Callaway R. (1994). Positive interactions in communities. Trends Ecol. Evol.9, 191–193. doi: 10.1016/0169-5347(94)90088-4
13
Bolker B. M. (2008). Ecological models and data in R (Princeton, USA: Princeton University Press).
14
Broman E. Raymond C. Sommer C. Gunnarsson J. S. Creer S. Nascimento F. J. A. (2019). Salinity drives meiofaunal community structure dynamics across the Baltic ecosystem. Mol. Ecol.28, 3813–3829. doi: 10.1111/mec.15179
15
Burdett A. (1995). Records of the Persian Gulf pearl fisheries 1857–1962. Cambridge Archive Editions Ltd., Farnham Common, UK.
16
Burt J. A. Al-Khalifa K. Khalaf E. AlShuwaikh B. Abdulwahab A. (2013). The continuing decline of coral reefs in Bahrain. Mar. pollut. Bull.72, 357–363. doi: 10.1016/j.marpolbul.2012.08.022
17
Carter R. (2005). The history and prehistory of pearling in the Persian Gulf. J. Economic Soc. History Orient48, 139–209. doi: 10.1163/1568520054127149
18
Carter R. (2018). “ Pearl fishing and globalisation: From the Neolithic to the twentieth century CE,” in The Gulf in world history: Arabia at the global crossroads (Edinburgh, UK: Edinburgh University), 239–261. doi: 10.1515/9781474430678-017
19
Coen L. D. Luckenbach M. W. (2000). Developing success criteria and goals for oyster habitat restoration. Ecol. Eng.15, 323–343. doi: 10.1016/S0925-8574(00)00084-7
20
Coles S. L. Riegl B. M. (2013). Thermal tolerances of reef corals in the Gulf: A review of the potential for increasing coral survival and adaptation to climate change through assisted translocation. Mar. pollut. Bull.72, 323–332. doi: 10.1016/j.marpolbul.2012.09.006
21
Fisheries New Zealand (2025). Review of sustainability measures for green-lipped mussels (GLM 7A), horse mussels (HOR 7), and dredge oysters (OYS 7) for 2025/26 (Discussion Paper No. 2025/14). Fisheries New Zealand. Available online at: https://www.mpi.govt.nz/dmsdocument/70086/direct/.
22
Ghazi E. Bidokhti A. Ezam M. Azad M. Hassanzadeh S. (2017). Physical properties of Persian Gulf outflow thermohaline intrusion in the Oman Sea. Open J. Mar. Sci.7, 169–190. doi: 10.4236/ojms.2017.71013
23
Gómez-Robles E. Acosta-Salmón H. Mazón-Suástegui J. M. Saucedo P. E. (2023). Seasonal variation in the reproductive and larval performance of the winged pearl oyster Pteria sterna associated with anomalous environmental conditions. Cienc. Marinas49. doi: 10.7773/cm.y2023.3353
24
Grabowski J. H. Peterson C. H. (2007). “ Restoring oyster reefs to recover ecosystem services,” in Theoretical ecology, vol. 4 . Eds. CuddingtonK.ByersJ. E.WilsonW. G.HastingsA. (the Netherlands: Academic Press, Elsevier, Inc.), 281–298. doi: 10.1016/S1875-306X(07)80017-7
25
Hazeem L. J. Yusuf M. Al-Sayed H. (2024). “ Pearl oysters in Bahrain: Economic, heritage, and biological aspects,” in Coral reefs and associated marine fauna around the Arabian PeninsulaLondon, UK, 1st ed, 8. doi: 10.1201/9781003321392
26
Hemraj D. A. Bishop M. J. Hancock B. Minuti J. J. Thurstan R. H. Zu Ermgassen P. S. E. et al . (2022). Oyster reef restoration fails to recoup global historic ecosystem losses despite substantial biodiversity gain. Sci. Adv.8, eabp8747. doi: 10.1126/sciadv.abp8747
27
Hilker F. M. Liz E. (2020). Threshold harvesting as a conservation or exploitation strategy in population management. Theor. Ecol.13, 519–536. doi: 10.1007/s12080-020-00465-8
28
Hume B. C. D’Angelo C. Smith E. G. Stevens J. R. Burt J. Wiedenmann J. (2015). Symbiodinium thermophilum sp. nov., a thermotolerant symbiotic alga prevalent in corals of the world’s hottest sea, the Persian/Arabian Gulf. Sci. Rep.5, 8562. doi: 10.1038/srep08562
29
Ishikawa T. Wada K. T. Komaru A. Kobayashi T. Kondo M. (2020). The influence of water temperature, salinity, and food availability on nacre deposition rates in shells and pearls of Japanese and hybrid pearl oyster, Pinctada fucata. Mar. pollut. Bull.161, 111722. doi: 10.1016/j.marpolbul.2020.111722
30
Johnson K. D. Smee D. L. (2014). Predators influence the tidal distribution of oysters (Crassostrea virginica). Mar. Biol.161, 1557–1564. doi: 10.1007/s00227-014-2440-8
31
Kandler N. M. Wooster M. K. Leray M. Knowlton N. de Voogd N. J. Paulay G. et al . (2019). Hyperdiverse macrofauna communities associated with a common sponge, Stylissa carteri, shift across ecological gradients in the central Red Sea. Diversity11, 18. doi: 10.3390/d11020018
32
Kennish M. J. Haag S. M. Sakowicz G. P. Durand J. B. (2009). Benthic macrofaunal community structure along a well-defined salinity gradient in the Mullica River–Great Bay Estuary. J. Coast. Res.10045, 209–226. doi: 10.2112/SI45-209.1
33
Khalifa R. Hamadou R. B. Giraldes B. W. Joaquim S. Hizam Z. Hamza S. et al . (2024). The impact of increasing seawater temperatures over the last 30 years on the reproductive cycle of the pearl oyster Pinctada radiata (Leach 1814) in the Arabian Gulf. J. Mar. Sci. Eng.12, 2180. doi: 10.3390/jmse12122180
34
Khamdan S. (1988). Bahrain pearl oyster, genetics and systematic. U.C.N.W. (Bangor), UK: University College of North Wales (U.C.N.W).
35
Khamdan S. A. (1998). “ Aspects of reproduction in the pearl oyster, Pinctada radiata (Leah),” in Offshore environment of the ROPME Sea Area after the war-related oil spill. Eds. OtsukiA.AbdulraheemM. Y.ReynoldsR. M. ( Terra Scientific Publishing Company), 203–214. Available online at: https://www.terrapub.co.jp/e-library/ropme/index.html (Accessed April 05, 2025).
36
Khamdan S. (2004). The Gulf encyclopedia for pearls and oysters (Manama, Kingdom of Bahrain: Bahrain Centre for Studies and Research).
37
Kimbro D. L. Scherer A. E. Byers J. E. Grabowski J. H. Hughes A. R. Piehler M. F. et al . (2020). Environmental gradients influence biogeographic patterns of nonconsumptive predator effects on oysters. Ecosphere11, e03260. doi: 10.1002/ecs2.3260
38
Kleemann K. (1992). Coral communities and coral-bivalve associations in the northern Red Sea at Safaga, Egypt. Facies26 (1), 1–9. doi: 10.1007/BF02539790
39
Koch E. W. Barbier E. B. Silliman B. R. Reed D. J. Perillo G. M. E. (2015). The role of oysters in marine ecosystems. Mar. Ecol. Prog. Ser.518, 191–205. doi: 10.3354/meps11073
40
Kuchel R. P. O’Connor W. A. Raftos D. A. (2011). Environmental stress and disease in pearl oysters, focusing on the Akoya pearl oyster (Pinctada fucata Gould 1850). Rev. Aquaculture3, 138–154. doi: 10.1111/j.1753-5131.2011.01051.x
41
Lal M. M. Bosserelle C. Kishore P. Southgate P. C. (2020). Understanding marine larval dispersal in a broadcast-spawning invertebrate: A dispersal modelling approach for optimising spat collection of the Fijian black-lip pearl oyster Pinctada margaritifera. PLoS One15, e0234605. doi: 10.1371/journal.pone.0234605
42
Lal M. M. Southgate P. C. Jerry D. R. Zenger K. R. (2018). Genome-wide comparisons reveal evidence for a species complex in the black-lip pearl oyster Pinctada margaritifera (Bivalvia: Pteriidae). Sci. Rep.8, 191. doi: 10.1038/s41598-017-18602-5
43
Leong R. C. Bugnot A. B. Ross P. M. Erickson K. R. Gibbs M. C. Marzinelli E. M. et al . (2024). Recruitment of a threatened foundation oyster species varies with large and small spatial scales. Ecol. Appl.34, e2968. doi: 10.1002/eap.2968
44
Li A. Li L. Wang W. Song K. Zhang G. (2018). Transcriptomics and fitness data reveal adaptive plasticity of thermal tolerance in oysters inhabiting different tidal zones. Front. Physiol.9. doi: 10.3389/fphys.2018.00825
45
Mahasneh A. M. Al-Sayed H. Al-Saaed (1993). “ Impact of some organic and inorganic pollutants of pearl oyster (Pinctada radiata) in the Bahrain environment,” in Bahrain Centre for Studies and Research ( Technical Report, Manama, Kingdom of Bahrain), 78pp.
46
Marquardt A. R. Southworth M. Scheld A. M. Button A. Mann R. (2025). Oyster reef recovery: Impacts of rotational management and restoration efforts on public fishing grounds. J. Environ. Manage.375, 124179. doi: 10.1016/j.jenvman.2025.124179
47
Marshall D. A. Casas S. M. Walton W. C. Rikard F. S. Palmer T. A. Breaux N. et al . (2021). Divergence in salinity tolerance of Northern Gulf of Mexico eastern oysters under field and laboratory exposure. Conserv. Physiol.9, coab065. doi: 10.1093/conphys/coab065
48
McAfee D. Cole V. J. Bishop M. J. (2016). Latitudinal gradients in ecosystem engineering by oysters vary across habitats. Ecology97, 929–939. doi: 10.1890/15-0651.1
49
Mohammed S. Z. (1994). Pearl oyster project. Phase 1: Survey and ecological studies on Qatari pearl oyster beds, pilot investigation report (Doha, Qatar: University of Qatar).
50
Mohammed S. Z. Yassien M. H. (2003). Population parameters of the pearl oyster Pinctada radiata (Leach) in Qatari waters, Arabian Gulf. Turkish J. Zoology27, 339–343. Available online at: https://journals.tubitak.gov.tr/zoology/vol27/iss4/10 (Accessed January 15, 2025).
51
Mrozińska N. Glińska-Lewczuk K. Obolewski K. (2021). Salinity as a key factor on the benthic fauna diversity in the coastal lakes. Animals11, 3039. doi: 10.3390/ani11113039
52
Navarro J. M. Villanueva P. Rocha N. Torres R. Chaparro O. R. Benítez S. et al . (2020). Plastic response of the oyster Ostrea Chilensis to temperature and pCO2 within the present natural range of variability. PloS One15, e0234994. doi: 10.1371/journal.pone.0234994
53
Nayar K. N. Al-Rumaidh M. J. (1993a). “ Studies on the pearl oysters of Bahrain waters,” in Bahrain Centre for Studies and Research ( Technical Report, Manama, Kingdom of Bahrain), 333 pp.
54
Nayar K. N. Al-Rumaidh M. J. (1993b). The rate of growth of spat and yearlings of the pearl oyster Pinctada radiata (Leach) of Bahrain waters. J. Mar. Biol. Assoc. India35, 1–14. Available online at: https://mbai.org.in/uploads/manuscripts/Article%201(1-14)1042249499.pdf (Accessed October 10, 2025).
55
Pafras D. Apostologamvrou C. Balatsou A. Theocharis A. Lolas A. Hatziioannou M. et al . (2024). Reproductive biology of pearl oyster (Pinctada radiata, Leach 1814) based on microscopic and macroscopic assessment of both sexes in the Eastern Mediterranean (South Evia Island). J. Mar. Sci. Eng.12, 1259. doi: 10.3390/jmse12081259
56
Pérez-Velasco R. Manzano-Sarabia M. Hurtado-Oliva M.Á. (2022). Effect of hypo- and hypersaline stress conditions on physiological, metabolic, and immune responses in the oyster Crassostrea corteziensis (Bivalvia: Ostreidae). Fish Shellfish Immunol.120, 252–260. doi: 10.1016/j.fsi.2021.11.033
57
Peters J. W. Eggleston D. B. Puckett B. J. Theuerkauf S. J. (2017). Oyster demographics in harvested reefs vs. no-take reserves: Implications for larval spillover and restoration success. Front. Mar. Sci.4. doi: 10.3389/fmars.2017.00326
58
Pouvreau S. Jonquières G. Buestel D. (1999). Filtration by the pearl oyster Pinctada margaritifera under conditions of low seston load and small particle size in tropical lagoon habitat. Aquaculture176, 295–314. doi: 10.1016/S0044-8486(99)00104-3
59
Richardson M. A. Zhang Y. Connolly R. M. Gillies C. L. McDougall C. (2022). Some like it hot: The ecology, ecosystem benefits and restoration potential of oyster reefs in tropical waters. Front. Mar. Sci.9. doi: 10.3389/fmars.2022.873768
60
Ruzicka R. Gleason D. F. (2009). Sponge community structure and anti-predator defenses on temperate reefs of the South Atlantic Bight. J. Exp. Mar. Biol. Ecol.380, 36–46. doi: 10.1016/j.jembe.2009.08.011
61
Sun S. Li Q. Kong L. Yu H. Zheng X. Yu R. et al . (2016). DNA barcoding reveal patterns of species diversity among northwestern Pacific molluscs. Sci. Rep.6, 33367. doi: 10.1038/srep33367
62
Thomas S. Collins K. Hauton C. Jensen A. (2022). A review of the ecosystem services provided by the native oyster (Ostrea edulis): Implications for restoration. IOP Conf. Series: Materials Sci. Eng.1245, 12010. doi: 10.1088/1757-899X/1245/1/012010
63
Tolley S. G. Volety A. K. Savarese M. (2005). Influence of salinity on the habitat use of oyster reefs in three southwest Florida estuaries. J. Shellfish Res.24, 127–137. doi: 10.2983/0730-8000(2005)24[127:IOSOTH]2.0.CO;2
64
UNESCO (2025). Bahrain: Pearling, testimony of an island economy (UNESCO: UNESCO World Heritage Centre). Available online at: https://whc.unesco.org/en/list/1364/ (Accessed February 10, 2025).
65
van Overzee H. M. J. Rijnsdorp A. D. (2015). Effects of fishing during the spawning period: Implications for sustainable management. Rev. Fish Biol. Fisheries25, 65–83. doi: 10.1007/s11160-014-9370-x
66
Xue P. Eltahir E. A. B. (2015). Estimation of the heat and water budgets of the Persian (Arabian) Gulf using a regional climate model. J. Climate28, 5041–5062. doi: 10.1175/JCLI-D-14-00189.1
67
Yigitkurt S. (2021). Reproductive biology of the rayed pearl oyster (Pinctada imbricata radiata, Leach 1814) in Izmir Bay. Oceanological Hydrobiological Stud.50, 87–97. doi: 10.2478/oandhs-2021-0009
68
Yukihira H. Klumpp D. W. Lucas J. S. (1998). Effects of body size on suspension feeding and energy budgets of the pearl oysters Pinctada margaritifera and P. maxima. Mar. Ecol. Prog. Ser.170, 119–130. doi: 10.3354/meps170119
69
Yukihira H. Klumpp D. W. Lucas J. S. (1999). Feeding adaptations of the pearl oysters Pinctada maxima and P. margaritifera to variations in natural particulates. Mar. Ecol. Prog. Ser.182, 161–173. doi: 10.3354/meps182161
70
Yukihira H. Lucas J. S. Klumpp D. W. (2000). Comparative effects of temperature on suspension feeding and energy budgets of the pearl oysters Pinctada maxima and P. margaritifera. Mar. Ecol. Prog. Ser.195, 179–188. doi: 10.3354/meps195179
71
Yukihira H. Lucas J. S. Klumpp D. W. (2006). The pearl oysters, Pinctada maxima and P. margaritifera, respond in different ways to culture in dissimilar environments. Aquaculture252, 208–224. doi: 10.1016/j.aquaculture.2005.06.032
Summary
Keywords
Arabian Gulf, GCC, Hayr, benthic composition, Bahrain, ecosystems
Citation
AlMealla RK, Edullantes B, Ali M, Mardhi Z, Khan M, Sharif M, Tyabji A, AlMousa N, Omran F, Hasan M, Hazeem L, Khamis AS, Al Khalifa E and Dabruzzi T (2025) Comprehensive associations between pearl oyster beds and coral, seagrass, and algal habitats in the hottest sea on the planet. Front. Mar. Sci. 12:1667575. doi: 10.3389/fmars.2025.1667575
Received
16 July 2025
Accepted
29 September 2025
Published
27 October 2025
Corrected
15 December 2025
Volume
12 - 2025
Edited by
Hongqiang Yan, Chinese Academy of Sciences (CAS), China
Reviewed by
Gulnihal Ozbay, Delaware State University, United States
Bingpeng Xing, State Oceanic Administration, China
Updates
Copyright
© 2025 AlMealla, Edullantes, Ali, Mardhi, Khan, Sharif, Tyabji, AlMousa, Omran, Hasan, Hazeem, Khamis, Al Khalifa and Dabruzzi.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Reem K. AlMealla, reem@nuwat.org
ORCID: Reem K. AlMealla, orcid.org/0000-0002-2693-8641; Brisneve Edullantes, orcid.org/0000-0003-4998-5018
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.