- 1School of Biological Sciences and Environment Institute, University of Adelaide, Adelaide, SA, Australia
- 2The Institute of Marine and Antarctic Studies, University of Tasmania, Hobart, TAS, Australia
- 3Centre for Nature Positive Solutions, School of Science, Royal Melbourne Institute of Technology (RMIT) University, Melbourne, VIC, Australia
- 4AUSMAP, Total Environment Centre, Surry Hills, NSW, Australia
- 5Australian Laboratory for Emerging Contaminants, School of Chemistry, The University of Melbourne, Melbourne, VIC, Australia
- 6School of Biological Sciences, Oceans Graduate School, and The Oceans Institute, The University of Western Australia, Crawley, WA, Australia
- 7Centre for Sustainable Materials Research and Technology, SMaRT@University of New South Wales (UNSW), School of Materials Science and Engineering, University of New South Wales (UNSW) Sydney, Sydney, NSW, Australia
- 8College of Science and Engineering, James Cook University, Townsville, QLD, Australia
- 9Commonwealth Scientific and Industrial Research Organisation (CSIRO) Environment, Hobart, TAS, Australia
- 10Gulbali Institute, Charles Sturt University, Wagga Wagga, NSW, Australia
- 11Australian Research Council (ARC) Training Centre for Biofilm Research and Innovation, Flinders University, Bedford Park, SA, Australia
- 12Australian Rivers Institute, School of Environment and Science, Griffith University, Southport, QLD, Australia
- 13NSW Department of Climate Change, Energy, The Environment and Water, Lidcombe, NSW, Australia
- 14College of Science and Engineering, Flinders University, Bedford Park, SA, Australia
- 15Australian Institute of Marine Science, Townsville, QLD, Australia
- 16AIMS@JCU, Division of Research and Innovation, James Cook University, Townsville, QLD, Australia
- 17Aquatic Assessments, Adelaide, SA, Australia
- 18School of Biosciences, University of Melbourne, VIC, Australia
- 19College of Engineering, Science and Environment (CESE), The University of Newcastle, Newcastle, NSW, Australia
- 20Queensland Alliance for Environmental Health Sciences (QAEHS), The University of Queensland, Brisbane, QLD, Australia
- 21Centre for Marine Socioecology, University of Tasmania, Hobart, TAS, Australia
- 22Natural History Museum, London, United Kingdom
- 23The Institute for Marine and Antarctic Studies, University of Tasmania, Hobart, TAS, Australia
- 24School of Natural Sciences, Macquarie University, Sydney, NSW, Australia
Global interest in microplastics is increasing, with numerous organisations collecting data on microplastics in the environment. However, disparate sampling, analysis, and reporting methods limit our ability to integrate data, hindering a global understanding of microplastic occurrence, effects and dynamics. Drawing on international directives and collaborations, we present a comprehensive guideline of harmonised and standardised field and laboratory approaches for microplastics in marine and coastal environments. We aim to ensure data consistency and comparability, incorporating the latest methodological developments for investigating and monitoring microplastics in four environmental matrices: sediment, water, biota, and air. A participatory approach brought together 40 researchers with diverse experience, reflecting a broad range of regional and international research. We provide best practice recommendations for sample processing to isolate, quantify and characterise microplastics, along with effective quality assurance and quality control measures. We also include reporting and data release recommendations, to ensure consistency and comparability across datasets. This guideline is endorsed by Ocean Best Practices System. By following these guidelines, and incorporating workflows supporting Findable, Accessible, Interoperable, and Reusable (FAIR) data, diverse stakeholders and practitioners can generate harmonised data essential for decision-making, facilitating a collective ability to synthesise global datasets and support action on microplastics.
1 Introduction
Plastic pollution is a pervasive and complex global issue, impacting terrestrial, freshwater, coastal, and marine ecosystems. Originating from various sources and stages of plastic production, consumption and disposal, the presence of plastic in the environment leads to long-lasting environmental, economic and social consequences (Diggle and Walker, 2022; Joshi and Vashishth, 2024; Li et al., 2023; Murphy et al., 2022). In recent years, microplastics, defined as plastic particles between 1 μm and 5 mm in size (Frias and Nash, 2019; Hartmann et al., 2019; Rochman et al., 2019; Thompson et al., 2009), have entered the publics consciousness and are now considered a contaminant of grave concern due to their ubiquity, persistence and ability to enter food webs, posing potential risks to biodiversity, food security, and human health (de Jersey et al., 2025; Thornton Hampton et al., 2022; Xu et al., 2022; Zhu et al., 2025). In response to this, many organisations are undertaking research, monitoring, and data assessments to quantify the level of microplastics in the environment (e.g., Cowger et al., 2020; Jenkins et al., 2022). These data are crucial for identifying indicators and setting targets to mitigate plastic pollution (Munhoz et al., 2022). Despite this, many datasets are collected in a non-standardised or irreproducible manner, often leading to fragmented and non-comparable information (Halfar et al., 2021; Wootton et al., 2021) and hindering the development of new research that adheres to established literature standards.
The global scientific community has faced many challenges in standardising sampling and laboratory methodologies for plastics and microplastics (Mitrano et al., 2023; Thompson et al., 2024). This difficulty arises not only from the diversity of plastic types, polymer composition, behaviours and land- or seascape contexts, but also due to the need to adapt to different scientific, logistical, environmental and ethical constraints (Galgani et al., 2024). This complicates the establishment of uniform standardised protocols. However, if data is to be fit-for-purpose, with meaningful comparisons, then harmonised and consistent approaches are essential. Many groups have created frameworks, protocols and guidelines to improve consistency and accuracy, including regional initiatives [e.g., OSPAR commission (OSPAR, 2025), Arctic Monitoring and Assessment Programme (AMAP, 2025)], research consortiums [e.g., in the European Union - Defining the Baseline and Standards for Microplastics Analyses in European Waters, BASEMAN (JPI Oceans, 2019); or Australia - Nano and Microplastic Research Consortium, NMRC (NMRC, 2025)] and intergovernmental organisations [e.g., the Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection, GESAMP (GESAMP, 2019)]. Despite these efforts, significant variability remains in how plastics and microplastics are sampled, processed, analysed and, crucially, reported worldwide (e.g., Cowger et al., 2020; Hermsen et al., 2018; Serra-Gonçalves et al., 2019; Wootton et al., 2024). Many studies also lack robust quality assurance and quality control (QA/QC) procedures to validate method performance (Dawson et al., 2023), allowing questionable data to enter the literature (e.g., Worthington and Cockburn, 2025), undermining the reliability of research findings and limiting the fields capacity to inform effective policy and management. This challenge is now being addressed to improve the accuracy of data generated and how it is reported.
Existing protocols offer insights into the methods used for sampling microplastics (e.g., Burgess et al., 2021; European Commission, 2023; GESAMP, 2019; International Organization for Standardization, 2023). However, they often fall into two categories: (1) broad frameworks that are difficult to operationalise or (2) highly specific protocols tailored to particular environmental compartments (e.g., abiotic: air, water, sediment, ice or biotic: species, wildlife exposure, wildlife ingestion, trophic transfer), sampling techniques (e.g., surface tows, Niskin bottles, sediment grabs), or microplastic type (e.g., morphology, size, or polymer). In general protocols do not clearly define reporting parameters. This disparity in methods and reporting can make it challenging for researchers and practitioners to identify and apply current guidelines across different contexts. While the need for specificity in standardisation is well recognised (Przeslawski et al., 2019), there remains a gap in widely accessible, comprehensive guidance that consolidates and harmonises sampling and processing methodologies for microplastics. Our approach builds upon and integrates existing efforts, drawing from internationally recognised best practices (e.g., AMAP, 2025; GESAMP, 2019) and Australian applications (e.g., Crutchett and Bornt, 2024; Okoffo et al., 2022; Santana et al., 2022; Schlawinsky et al., 2022) to develop a cohesive framework for sampling in marine and coastal environments. Overall, consolidating methodologies and ensuring clear reporting standards is key to support comparability, interoperability, and informed decision-making across scientific, regulatory, and environmental management sectors.
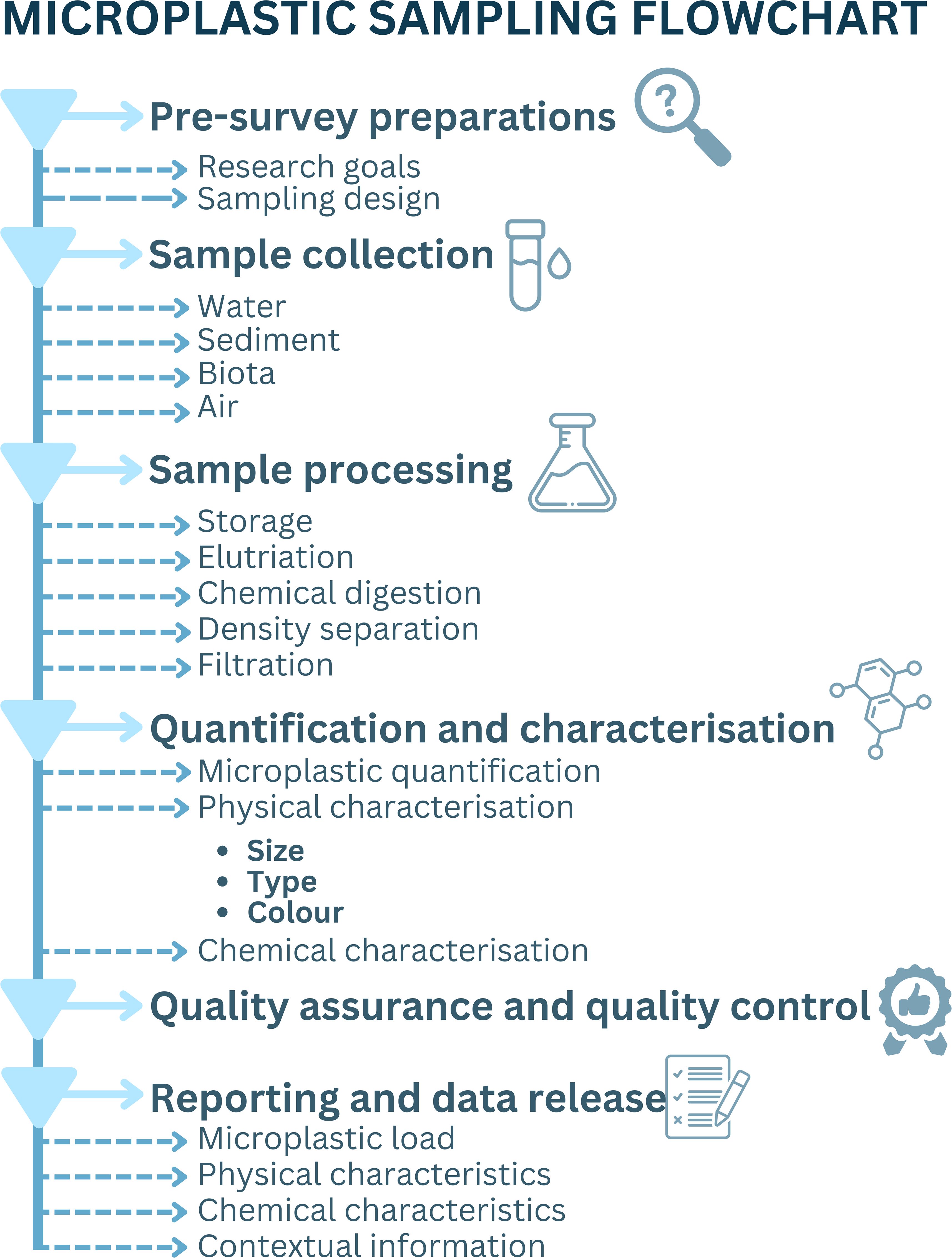
Here we present protocols for harmonising and standardising microplastics sampling, processing, analysis, and reporting, catering to the needs of diverse stakeholders across academia, industry, government, and non-government organisations (NGOs). This paper serves as an introduction and an abridged version of a comprehensive microplastics manual developed in a collaborative effort across different research institutes and organisations, that is an endorsed Ocean Best Practice System (OBPS), a secure, international, permanent, digital repository for ocean research, operations, data management, and applications (Ocean Best Practices System, 2024). This paper provides an overview of the key issues and needs, acting as a first point of reference and a step-by-step workflow of the essential components of sampling, processing, analysis and reporting of microplastics in water, sediment, biota, and air for coastal and marine environments (Figure 1).
With a strong emphasis on harmonised approaches, and adherence to Findable, Accessible, Interoperable, and Reusable principles (FAIR; Wilkinson et al., 2016) as well as best practice development guidelines (Przeslawski et al., 2023), this paper provides a framework to reduce methodological variations, minimise bias, enhance data accessibility, and facilitate dataset synthesis and comparison. It consolidates and details accepted methods in microplastic research to ensure consistent and comparable datasets now and into the future, supporting the synthesis of regional and global information. Covering microplastics in coastal and marine waters, sediment, biota, and air matrices, this paper spans sampling design, collection, processing, laboratory procedures, plastics characterisation, QA/QC and data reporting (Figure 1). Distinctions are made between essential and desirable reporting parameters, with the essential reporting parameters ensuring, at a minimum, accurate, efficient, and standardised microplastic analysis approaches. These are critical for establishing guidelines across diverse environments. Moreover we provide a checklist for reporting microplastic datasets, essential to supporting scientifically robust and interoperable data comparisons necessary for informed management and regulatory action (e.g., Lusher et al., 2021; Omeyer et al., 2022; Wootton et al., 2024).
The development of the paper was driven by the need to consolidate and refine widely used methodologies into a practical, field-tested set of guidelines, rather than proposing novel techniques. While many microplastic sampling and analysis methods exist, they are often disparate, inconsistently applied, and challenging to compare across studies. The approach taken here aimed to synthesise existing best practices worldwide into a cohesive, practical, and field-tested set of guidelines and protocols that reflect a broad expert consensus and are widely adopted in microplastic research. This process aligns with the validation of robust methodologies that have been extensively used, ensuring that the protocols presented are both practical and reliable.
2 Materials and methods
A collaborative project involving over 40 researchers from 21 institutions/organisations from Australia was established to deliver a comprehensive paper for harmonising sampling, processing, analysis and reporting of microplastics in marine and coastal environments (Wootton et al., 2024). The researchers contributed diverse backgrounds (academic, government, NGOs) and extensive global experience and research in microplastics, spanning disciplines from marine science, chemistry, ecology, materials science, to environmental management and policy making. This diversity enriched this work's development by integrating expertise in microplastic detection, analysis and policy, ensuring the recommendations are scientifically rigorous, globally relevant and applicable across various environmental contexts.
We followed the workflow of Przeslawski et al. (2023) to develop best practices, employing a participatory approach to define and refine the proposed protocols. A participatory approach brings together researchers and stakeholders to collaboratively develop methods, ensuring diverse expertise is considered (Kapoor, 2001; Vaidya and Mayer, 2014). In this context, it facilitates the creation of microplastic protocols that are scientifically robust, practical to implement, and broadly applicable across different research and environmental settings. Briefly, experts in the field of microplastics were invited to join a working group and contribute to the content of the paper through a series of online workshops, systematic evaluations, and iterative revisions. Experts were identified through an initial review of the literature (conducted by Wootton, Reis-Santos, Przeslawski, Gillanders), via communications with key professional societies and institutions, and peer engagement and recommendations. Over seven months, the working group convened remotely five times, with each meeting (duration of approximately 1 hour) focused on different aspects of microplastic research (e.g., current methodologies in use, sampling design, microplastic size categories and terminology). Discussions were guided by targeted questions designed to critically assess existing methodologies, resolve inconsistencies, and build consensus on best practices (See Materials Supplementary Table S1 for details of working group activities). During each meeting, targeted questions were discussed in turns, with discussions mediated by two researchers (Wootton and Reis-Santos) and information discussed collated using online, real time, collaborative whiteboard platform (Miro Board) and/or drafts of the guideline. After each meeting, researchers also had a period of ~ one month before the next meeting to revise the topics discussed and add in more information into the live documents if needed.
Through this iterative and consensus-driven process, the working group systematically evaluated sampling, processing, analysis, and reporting methodologies, ensuring alignment with the most widely accepted and field-validated techniques, including international directives and global literature (e.g., from regions such as Australia, Brazil, Canada, China, India, the EU, the UK, and the US). Where discrepancies or gaps existed in the literature, discussions were informed by collective experience, methodological precedence, and practical feasibility. This approach guaranteed that the final recommendations resulting from this collaborative effort reflect a widely supported and standardised approach.
The resulting manual and associated reporting checklist provides a structured framework for microplastic research, ensuring methodological consistency across studies while allowing for flexibility in application to different environmental settings. This manual sits within a broader suite of best-practice sampling methods established by the Australian Governments National Environmental Science Program (NESP) and is endorsed by Ocean Best Practices System (OBPS) - https://microplastics-field-manual.github.io. In this paper, we summarise the findings and recommendations provided in the best practice manual (Wootton et al., 2024).
3 Guidelines
3.1 Pre-survey preparations
3.1.1 Research goals
Microplastic contamination crosses both disciplinary and geographic boundaries, requiring a collaborative approach to research, involving not only research scientists but also government scientists and managers, community organisations, and citizen scientists (Arciszewski et al., 2023; Bakir et al., 2024; Forrest et al., 2019; Setälä et al., 2022). Therefore, it is essential to clearly define the goals of a research or monitoring project and identify the likely end users of the generated data. Immediate goals may include assessing the accumulation or sources of microplastics, or understanding their impact on organisms. There are three main types of research goals in microplastic sampling; monitoring planning, abundance assessments and impact assessments (Provencher et al., 2022). Monitoring planning involves the strategic design of research protocols that outline specific objectives, methodologies, and data collection timelines to systematically track changes in microplastic levels over time. Potential users of this systematic data include research teams, environmental managers and government agencies seeking to establish environmental risk assessments for ecosystem management. In contrast, a general abundance assessment focuses on quantifying the overall presence and concentration of microplastics in a particular area without a detailed framework for ongoing evaluation, often serving as a snapshot that may not account for temporal fluctuations or source identification. Potential users of this data include research teams, government agencies, community groups, and businesses seeking to drive change or assess the success of contamination management efforts. Impact assessments focus on determining the biological, ecological, or socio-economic effects of microplastic pollution on organisms, ecosystems, or human communities, with end users often including policymakers, conservation organisations, and industries aiming to mitigate environmental and health risks.
Given the diversity of goals, habitats, and end users, a lack of harmonisation in sampling methodologies, quality control, and data reporting can limit the value and comparability of research (Gimiliani and Izar, 2022; Wootton et al., 2024). While the research goal will influence post-survey needs [e.g., processing time, microplastic analysis, statistical analysis, and reporting units (Cowger et al., 2020)], harmonisation offers a solution by ensuring that, while methodological differences may exist, essential benchmarks and standards are met to allow for broader spatial and temporal comparisons (de Ruijter et al., 2020; Koelmans et al., 2020).
3.1.2 Sampling design
First, the spatial and temporal scope of the research question should be defined. Spatial studies can reveal changes in microplastics spatial distribution (load and characteristics), requiring a sampling design that considers the number of collection sites, their geographical location, accessibility, and environmental characteristics of the sampling sites, including permanent (e.g., presence of river mouth or urbanisation), semi-permanent (e.g., shifting sand dunes) and temporary characteristics (e.g., weather conditions at the time of the sampling event). Temporal studies assess shifts in microplastic distribution over time, which calls for considerations of site accessibility across seasons and under different weather conditions (e.g., Miller et al., 2022b). Documenting environmental characteristics at the time of the sampling event is critical. Recording (semi-) permanent environmental characteristics is recommended as they can also be relevant for temporal analysis (An et al., 2024; Kurniawan and Imron, 2019; Lyu et al., 2022). We recommend stratified sampling designs for spatial and temporal studies with replicate samples taken randomly within different groups, where a group refers to a location or time period (Quinn and Keough, 2023).
Overall, sample size is guided by the research question, budget, logistics, and organisational capability or resources, with a larger number of samples improving accuracy and statistical robustness (Underwood et al., 2017). We strongly recommend undertaking a pre-survey, or pilot study to test the methodological techniques, and help determine the number (both count and volume) of samples required to address the question or research outcome desired. Estimates of background variation from the pilot study, or other published data, can be used to establish an appropriate level of replication, for instance, through power analysis, using tools such as G*Power (Faul et al., 2007), the pwr package in R (Champley et al., 2020) or a simulation-based approach for complex designs (Kumle et al., 2021). Understanding these requirements and following reporting recommendations promotes interoperability and harmonisation of datasets, thus ensuring data comparability when sampling efforts vary.
3.2 Sample collection
Sample collection and processing procedures depend on the specific environmental matrix and, potentially, the research question being addressed. Although collection methods may vary, it is important to harmonise practices, and ensure clear reporting of the study design and how the samples were collected. Here we provide a summary of field sampling procedures for nearshore coastal water, sediment, biota, and air matrices, as a guide to developing the research plan (Figure 2). We recommend that users review the section of the manual (https://microplastics-field-manual.github.io/) relating to the specific matrix being investigated to ensure collection methods are applicable, practical and implementable, and determine whether modifications or further development are required.
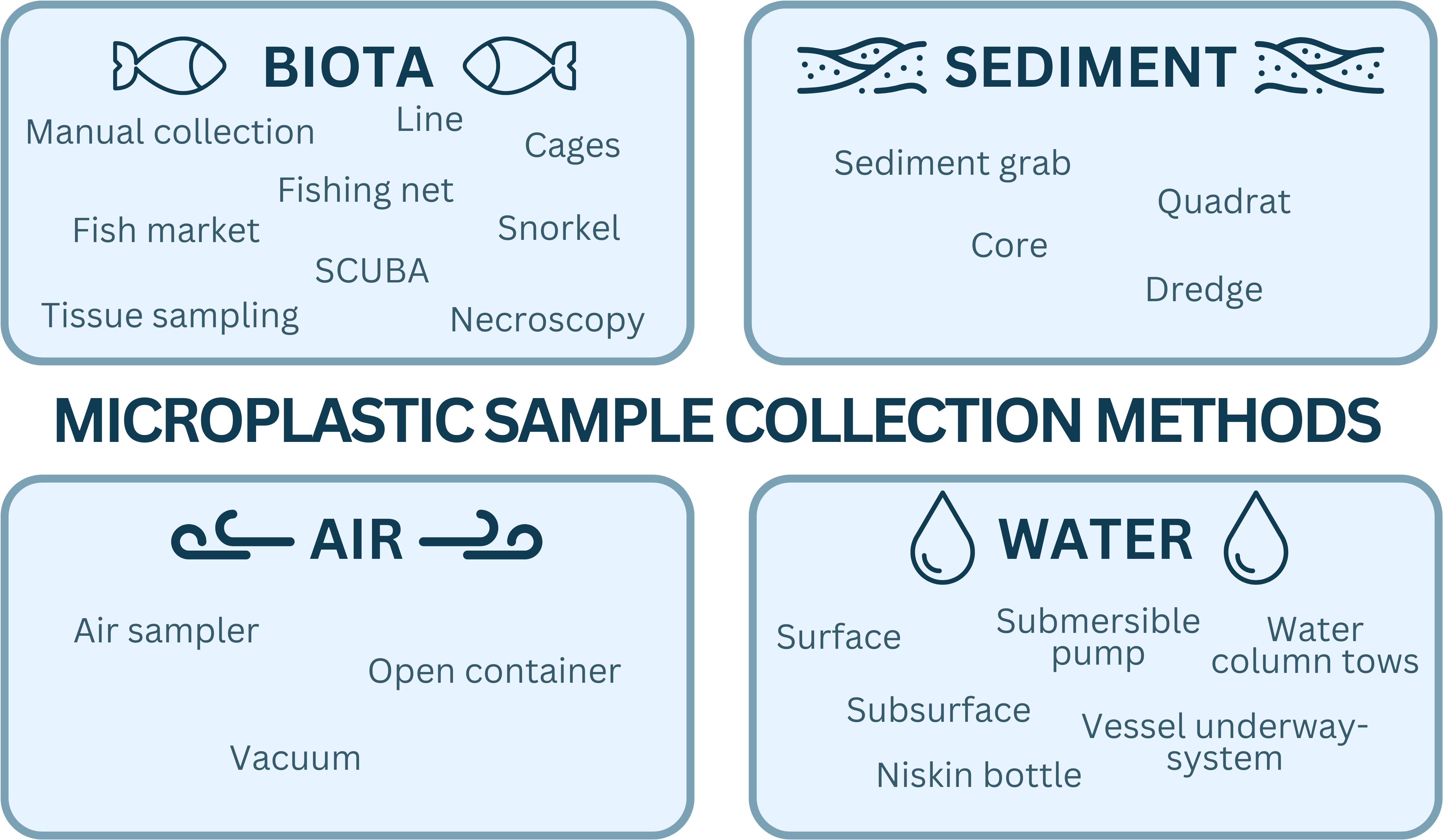
Figure 2. An example of the diversity of collection methods for water, sediment, biota, and air that can be sampled for microplastic analysis.
In all cases, and independent of the matrix type, it is essential to recognise the dynamic nature of coastal and marine environments. Replication is important, and if possible, periodic, repeated sampling is recommended to observe seasonal/temporal shifts (Morrisey et al., 1992). When repeated sampling is not feasible, meticulous records of environmental conditions at the time of collection become vital and will enable long-term comparisons. It is essential that volume or quantity of the sample collected is reported (Supplementary materials Supplementary Table S2). Of note, during field sampling, contamination should be purposefully minimised by implementing rigorous QA/QC procedures. These include, the imperative use of blanks and avoiding the use of plastic gear when possible (Noonan et al., 2023) (see QA/QC section below for further details).
3.3 Sample processing
The microplastic sample processing workflow (primarily laboratory-based) must be tailored to the research question, collection and preservation methods, available equipment, expertise level, and reporting requirements. Different methods are recommended for microplastics that are visible to the naked eye (1 mm - 5 mm) and those that are microscopic (1 μm - 1 mm). This manual focuses on accurately detecting microplastics > 20 μm in size. While most methods are, in theory, applicable to the finer size fractions between 1 μm and 20 μm, their practical application, at this time, is more challenging due to technological limitations, methodological constraints, risk of contamination, and the complexity of environmental matrices. Therefore, our recommendations do not extend to microplastics < 20 μm. All sample processing must undergo strict QA/QC procedures (see QA/QC section below for further details).
3.3.1 Storage
Regardless of the collection method, samples must undergo immediate processing or be appropriately preserved to prevent the decomposition of co-occurring organic material, which, if left unchecked, can impact microplastic retrieval, identification, and subsequent chemical analysis (Phan et al., 2022). Samples are best preserved at low temperatures (< 4°C) to minimise bacteria, fungal, or algal growth. Samples should be stored in non-plastic containers (ideally made of glass or metal or on/in chemical-free paper (suitable for short-term storage only); (see QA/QC section below). Chemical solutions (e.g., 20% ethanol) are acceptable as preservation methods if demonstrated not to impact the integrity of the plastic polymer for subsequent identification (Schrank et al., 2022). Preliminary processing of samples (e.g., filtering of sea water) is recommended prior to storage. All sample storage containers should undergo appropriate QA/QC procedures (see QA/QC section below).
3.3.2 Chemical digestion
Chemical digestion is recommended to remove organic matter from samples, allowing for better microplastic recovery and facilitating their instrumental analysis. Chemical digestion is commonly used in biota and sediment samples but is generally only necessary in water or air if there is a large amount of organic content. A wide range of different chemicals, from solvents to peroxides, acids, alkalis and enzymes, have been used (e.g., Di Fiore et al., 2024) (Table 1), with digestion efficiency dependent on sample composition, reagent concentration, temperature, activity period (e.g., enzymes), and treatment time. Thus, the type of chemical digestion chosen is often a compromise between multiple factors, including the complexity of the sample matrix and the efficiency of the digestion, its cost, health and safety risks, as well as the potential for physical and/or chemical degradation of different microplastics types and sizes (Di Fiore et al., 2024; Lavers et al., 2019; Miller et al., 2017; Pfeiffer and Fischer, 2020; Santana et al., 2022; Tuuri et al., 2024). It is particularly important to limit the use of heat for the digestion, following the recommended temperatures seen in Table 1, as the chemical composition of microplastics can be altered.
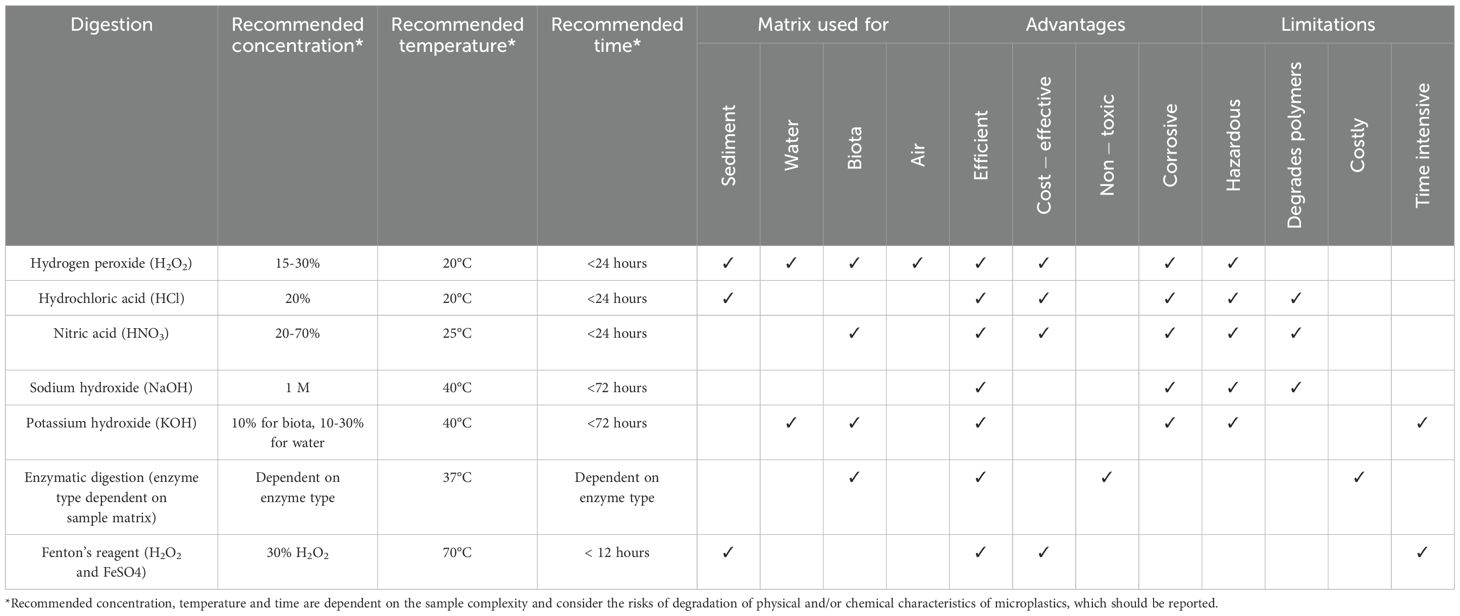
Table 1. Summary table of reagents for chemical digestion, their recommended concentrations, temperatures, times, advantages and limitations; adapted from Pfeiffer & Fischer (2020) and Di Fiore et al. (2024).
3.3.3 Elutriation
For sediment samples, an elutriation pre-treatment can be used to separate microplastics from sediment particles and larger organic items. Elutriation is designed to separate lighter particles from heavier ones, using an upward stream of gas or liquid (Claessens et al., 2013; Hengstmann et al., 2018; Zhu, 2015). This reduces the sample volume requiring further processing with, for example, density separation or, if organic content is high, chemical digestion. The elutriation step is not always used, nor necessary, and its application and effectiveness will vary depending on the type of sediment and specifications of the elutriation equipment and technique applied (Forsythe et al., 2024).
3.3.4 Density separation
Density separation can be used to isolate microplastics from neat water samples, or post elutriation or chemical digestion in a stepwise approach. Samples are mixed with the density separation reagent (i.e., brine solution) and left to settle, allowing particles lighter than the solution (e.g., microplastics) to float and denser materials (e.g., sediment) to sink. Various brine solutions can be used, each having a specific density and thereby separation efficiency (e.g., depending on the density of microplastics and materials in the sample), but also differing in toxicity and cost (Table 2). Some brine solutions can be reused after filtration, which will reduce costs over time. Measuring and reporting the final solution density is crucial, as it dictates the plastic polymers that are recoverable (Rani et al., 2023). For example, microplastic recovery is improved when using reagents with higher densities (e.g., ZnCl2 and KI) in comparison to lower-density solutions (e.g., NaCl), yet high-density solutions are more expensive and in some instances toxic. The choice of reagent for density separation will depend on the expected properties of microplastics, your matrix, as well as specific target particles of interest (e.g., if sampling for microplastics in surface seawater, using a reagent that recovers low-density microplastics can reduce costs while still effectively extracting the pieces). Sample solutions can be poured over a filter or sieve, allowed to drain by gravity or under vacuum, which can expedite the process for high particulate content. Filters or sieves can vary in pore aperture size and from single to tiered filtration systems (Schlawinsky et al., 2022). Recording the smallest pore aperture size is essential, as these parameters will determine the minimum size and potentially the shape and tactility of the microplastics collected, impacting reported concentrations. For example, a 100 μm sieve is unlikely to capture individual microplastic items < 100 μm (though larger fibres can slip through depending on the angle). We recommend using a metal mesh, glass microfibre or silicon-coated filter (Forsythe et al., 2024).
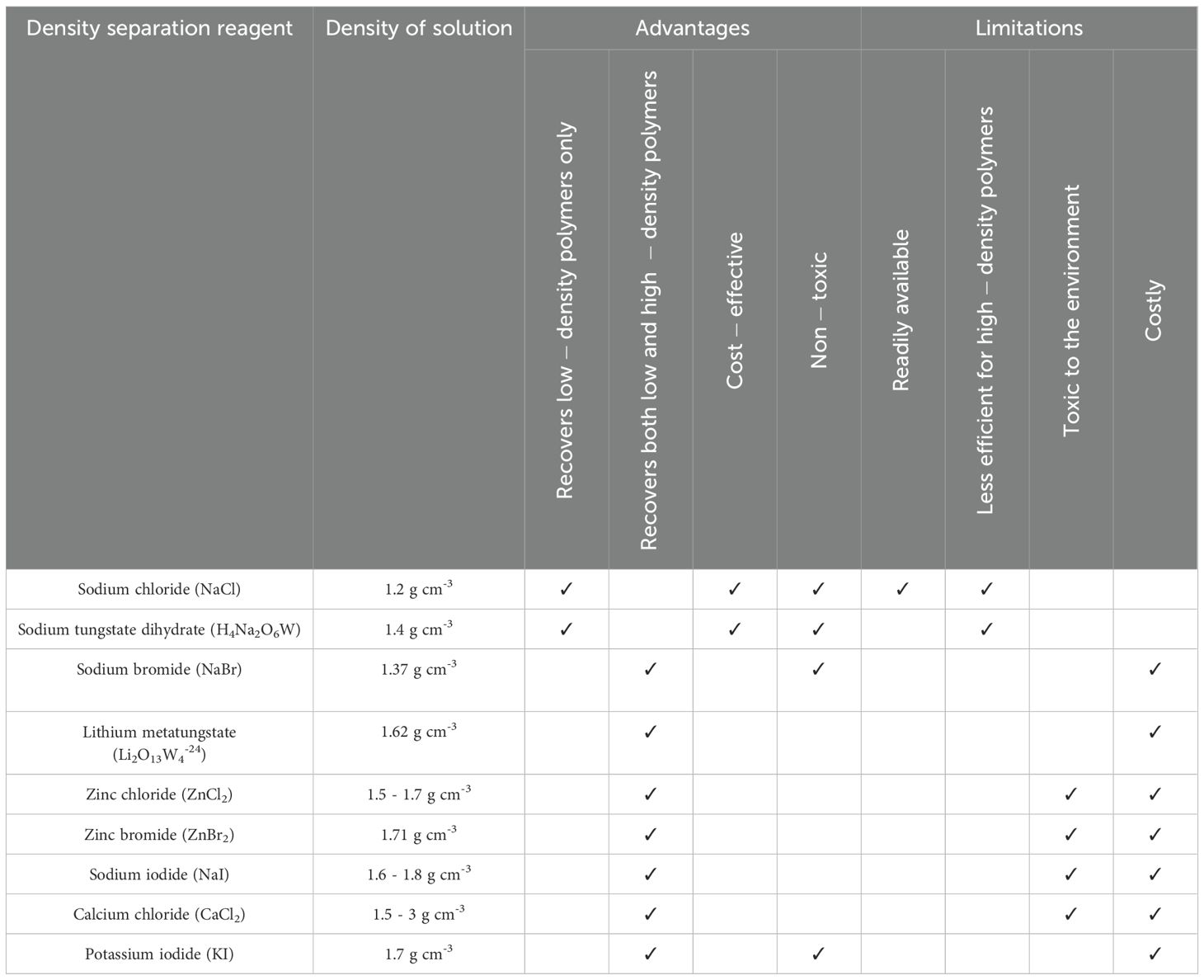
Table 2. Commonly used density separation reagents for microplastics analysis, including their density, main advantages and limitations.
3.4 Quantification and characterisation
3.4.1 Microplastic quantification
Identifying and counting microplastics > 1 mm can often be done without a microscope, either by direct visual observation or weighing [although polymer confirmation is still required (refer to section 3.4.3)]. However, a microscope is strongly recommended, particularly where further morphological information is required (i.e., texture, surface uniformity). Microplastics not visible to the naked eye can be quantified using methods like manual counting under a microscope or semi-automated counting with microphotography and specialised software (e.g., Razzell Hollis et al., 2024b), however chemical polymer confirmation is still required. It is recommended that, if microplastics are identified under the microscope, all particles should be counted without using fluorescent dyes. However, depending on the research question some alternative method (e.g., gridded method, fluorescent dyes) may still be appropriate.
3.4.1.1 Gridded method
Under a microscope, the gridded method involves examining and counting microplastics within squares of a real or virtual grid (Brandt et al., 2021). If the microplastics are too numerous a subset could be counted in each square of two diagonals throughout the filter, though this is not recommended as environmental microplastic samples are not homogenised.
3.4.1.2 Fluorescent dyes
Fluorescent dyes are selected based on properties like compatibility with microplastics, stability, and fluorescence. Nile red is commonly used, however, the success of the dye binding to the microplastics can vary (Meyers et al., 2022; Stanton et al., 2019). Other options include rhodamine B, acridine orange, and propidium iodide (e.g., Tong et al., 2021). Dyes are mixed with the sample and then processed as described above. When illuminated at a specific wavelength the adsorbed dye re-emits light at a longer wavelength, making microplastics evident. The suitability of the fluorescent dye depends on both the chemical nature and size of the item, and caution must be taken with any subsequent chemical analysis for polymer identification. For example heavily pigmented microplastics can exhibit lower fluorescence intensity, causing difficulty in detection and quantification (Gao et al., 2022). Consideration should also be given to non-plastic natural chemistries that might be present, and which may also be dyed and inadvertently counted as plastics (Shruti et al., 2022; Stanton et al., 2019).
3.4.1.3 Image analysis software
Software such as ImageJ (Fiji), a free Java-based image processing program (U.S. NIH, MD, USA https://imagej.nih.gov/ij), the open access computer vision Segmentation Model from The Ocean Cleanup (Royer et al., 2024), CellSens, and the commercially available Saturna Imaging System and camera (https://oceandiagnostics.com/microplastics-imaging-technology) are examples of tools that support semi-automated counting of microplastics. These can expedite the counting of microplastics and return comparable data on the 2D characteristics of microplastic size, shape, and colour, which can be automatically exported into a spreadsheet or database. Yet, underestimations can occur, especially when microplastics are in contact or overlapping, leading to a miscount and skewing data towards fewer counts and greater particle size (Boyle and Örmeci, 2024). To ensure count accuracy, images should be checked for QA/QC before data reporting.
3.4.2 Physical characterisation
Microplastics are characterised by size distribution and other key physical traits such as morphology (defined by apparent shape, texture, and tactility) and colour. Accurate reporting of these traits enables researchers to investigate the complexities of microplastic contamination. At a minimum, standardising physical data by size, shape, and colour is essential, and assignments should be made with reference to category definitions, i.e., size ranges, morphological profiles, and colour charts. Where possible, photographs of microplastics should include scale bars and colour charts to allow for future comparability, especially with longstanding datasets.
3.4.2.1 Size
Classifying plastics by size is essential, as size influences both ecological impacts (e.g., effects on organisms) and methodological approaches. Size is a continuous variable, yet categorisation is necessary for standardised reporting, facilitating comparability across studies and policy frameworks.
Size is typically measured as the length of the longest axis or the maximum Feret diameter, and objects are usually grouped into different size ranges (Table 3). The simplest method is to categorise the plastic object using visual assessment against a scale bar. However, if microscopy images are available, processing with (semi-) automated image analysis software is recommended to produce a faster, more accurate estimation of visible dimensions (e.g., Razzell Hollis et al., 2024b). When reporting size parameters, we recommend specifying which parameters were measured (e.g., maximum Feret diameter, or major diameter from elliptical approximation) and present size distributions as well as category counts. Ideally if using image analysis software (Image J) collect as much data as possible. It is essential to report the minimum size category that can be accurately detected by the collection and processing techniques used, in line with the size categories in Table 3.
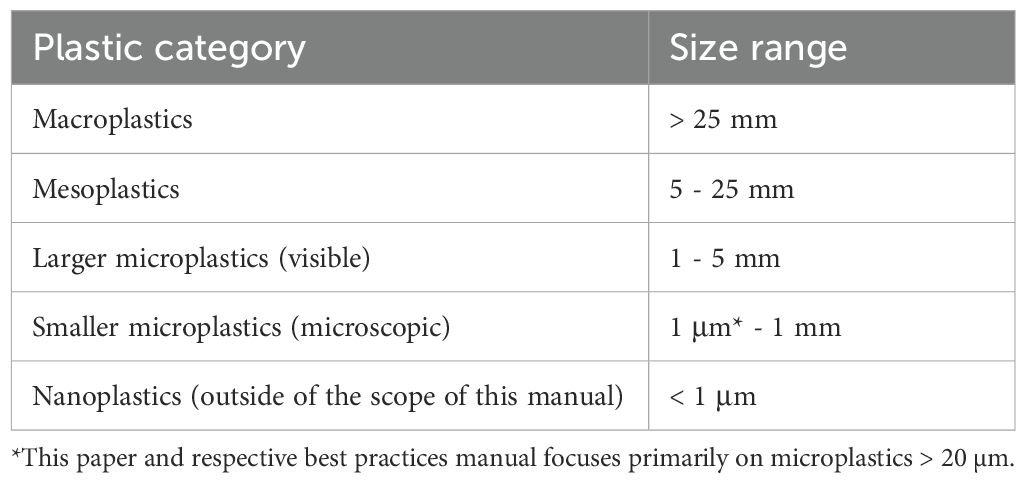
Table 3. Size classifications are used to categorise plastic, from macroplastics (> 25 mm) to microplastics (1 μm* - 5 mm), as well as nanoplastics (< 1 μm), which require specialised procedures for detection.
3.4.2.2 Morphology
Microplastic morphology is the least consistently reported characteristic across existing literature but can provide some indication of an object's original manufacture, source and history. The apparent shape (e.g., round, nurdle, irregular) and texture (i.e., rough, smooth) are both visually assessed, whereas tactility is assessed by applying pressure (Table 4). Microplastics should be categorised into one of the most common overarching plastic morphologies (commonly defined by shape), and if more details of the source are evident this should also be reported (e.g., artificial turf, tyre wear particles). If photographs/images are available, automated image analysis software can provide some indication by measuring various shape parameters (e.g., elliptical eccentricity, roundness) of each object (Razzell Hollis et al., 2024b; Valente et al., 2023).

Table 4. Description and classification of various plastic morphologies found in environmental samples, including pellets, fragments, filaments, foams, and films.
3.4.2.3 Colour
Colour can be altered by chemical agents, strong acids, or high temperatures during sample processing, which may bias interpretation. Where possible, record colour before digestion and report processing methods to provide context for any changes. When photographs/images are available, we recommend that the average colour of a plastic object is measured in red, green, and blue (RGB) values or hue saturation value (HSV) as acquired by a calibrated camera. RGB and HSV are more precise and less subjective than colour categories and provide semi-continuous data (e.g., suitable for studying trends such as discolouration). Alternatively, images of the plastic pieces can also be captured using conventional photography with a photographic colour reference card imaged alongside the plastics to provide a point of calibration and ensure greater consistency in colour reproduction (e.g., ColorChecker Classic mini reference chart in Razzell Hollis et al. (2024b) or Pantone colours in Martí et al. (2020)). If a colour reference card cannot be included, it is essential to report the lighting used (e.g., source, colour, temperature).
3.4.3 Chemical characterisation
Chemical analysis is crucial to verify the visual identification of microplastics, especially for small or neutral-coloured items. The best practice is to analyse 100% of the items when investigating and reporting polymer composition. Subsampling is recommended only to confirm the synthetic nature of the material, and to validate extraction and identification processes. Various spectroscopy and spectrometry methods (e.g., de los Santos‐Villarreal and Elizalde, 2013; Samandra et al., 2025; Vlnieska et al., 2024; Wesdemiotis et al., 2024) are suitable to characterise polymer composition and confirm plastic identification, with Fourier-Transform Infrared Spectroscopy (FTIR) and Raman Spectroscopy routinely used (International Organization for Standardization, 2023) (Table 5). Commercial, collaborative, and custom-built reference libraries facilitate the chemical assignment of each item. Yet, weathering and or the presence of additives, dyes and biofilm material can affect polymer signatures or introduce secondary signatures that may potentially result in incorrect assignments (Fernández-González et al., 2021; Phan et al., 2022; Razzell Hollis et al., 2024a). Therefore, libraries including spectra of aged/weathered polymers (De Frond et al., 2021; Miller et al., 2022a; Nava et al., 2021) are recommended, and expert assessment of spectra is necessary to identify any potential mismatches. Spectra matching with library references must be done with caution to ensure accurate identification. A match score of 70% or above should be used as the threshold for an accepted spectral match, while spectra with match scores between 60 and 70% should be further examined (Kroon et al., 2018; Wong and Coffin, 2021). Particles with a match score between 30% to 60% should be flagged as “possible” and require further examination.
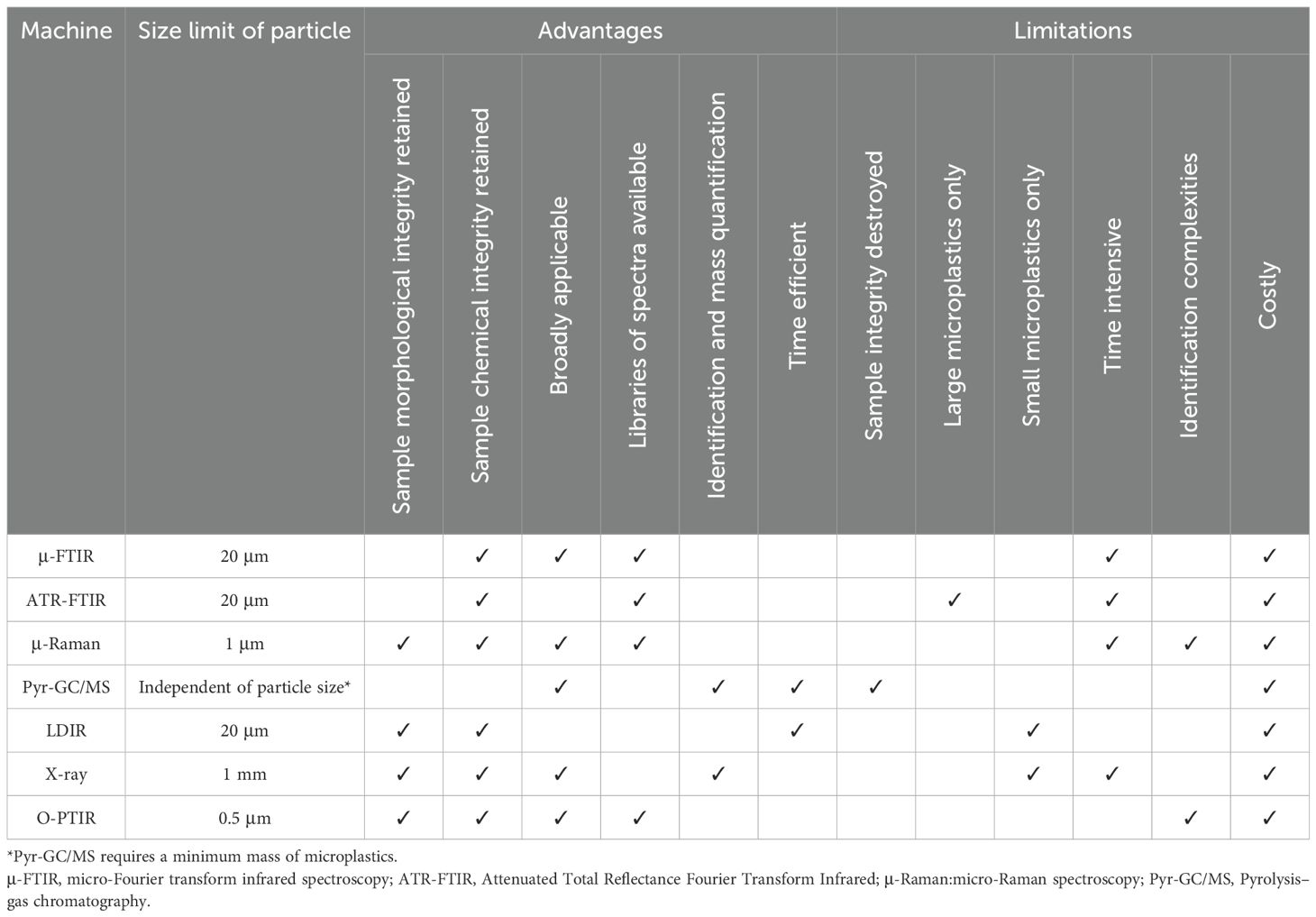
Table 5. Summary table of polymer identification instrumentation and their advantages, limitations, and minimum size that can be analysed.
3.4.4 Quality assurance and quality control
Minimising and addressing microplastic contamination are essential during sample collection and processing (Prata et al., 2021; Primpke et al., 2023). Researchers should acquaint themselves with contamination mitigation methods and reporting requirements [see below, and recommendations in Jones et al. (2024)].
During collection, at a minimum, plastic gear should be avoided. When this is not possible, plastic gear (e.g., tow nets, collection containers, etc) should be cleaned and regularly inspected for degradation, and if needed, replaced. Pieces from used plastic gear should also be collected for physical and chemical characterisation and this information added to a reference library against which microplastics found in samples can be cross-checked (i.e., field and laboratory blanks; Table 6).
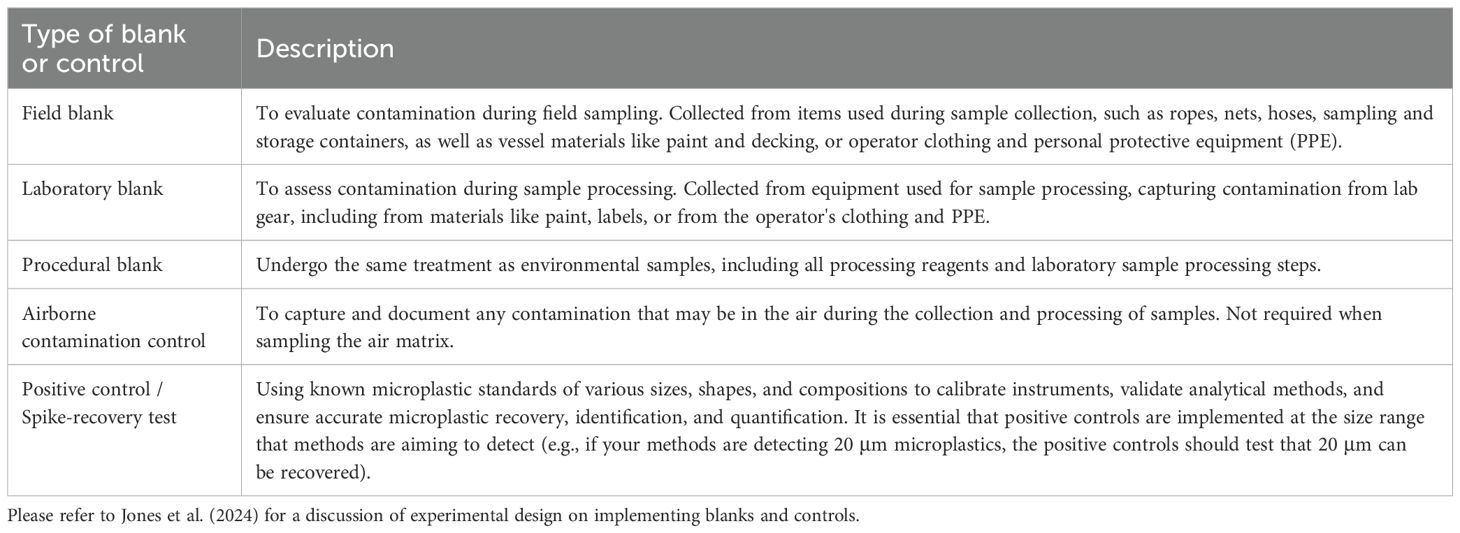
Table 6. A description of the type of blanks and controls that are recommended throughout microplastic sampling and quantification.
Sample processing should occur in a plastic-free laboratory environment regularly cleaned with filtered ethanol and lint-free cloths. Preferably, samples should be processed in a biological safety cabinet or laminar flow cabinet, but not a fume hood. The use of glass and metal equipment is advised, avoiding aluminium foil to cover vials (Jones et al., 2024) and opting for glass lids. All equipment should be rinsed with filtered water (e.g., ultrapure) three times before use, with glassware undergoing further acid wash decontamination. Further to this, the ultrapure water should be regularly tested for contamination, see Prata et al. (2021); Jones et al. (2024). To further prevent contamination, reagents used throughout the workflow should be filtered if safe to do so. It is also recommended to use a sticky mat, limit traffic, avoid synthetic clothing, and where possible, wear brightly coloured cotton clothing (e.g., lab coat) so that any extraneous contamination from the operator can be readily identified.
It is essential to include both blanks and controls throughout the sample processing to ensure data integrity and to allow detecting contamination levels (e.g., Barrett et al., 2020; Dawson et al., 2023; Noonan et al., 2023) (Table 6). Blank correction should also be performed, e.g., limit of detection (LOD) or limit of quantification (LOQ) if possible (Brander et al., 2020; Dawson et al., 2023; Waddell et al., 2020).
3.6 Reporting and data release
Transparent and coherent reporting is essential for interoperable and reusable data. All data should be acquired and collated in its raw form, with the aim to be publicly released on an open access platform, unless circumstances restrict this (e.g., confidentiality or embargo, grant agreement, Indigenous data sovereignty). Making raw data publicly available is crucial, as it enhances the potential for broader use and reanalysis of collected data. Repositories for microplastic-specific raw data are becoming increasingly common and accessible (Table 7). However, many of these platforms still focus on seawater only. Importantly, these repositories often impose requirements such as common definitions, standardised reporting units or minimum QA/QC procedures to ensure consistency, interoperability and comparability across various data sources. This reflects the need for standardised and rigorous methodologies (Bakir et al., 2024; Van Mourik et al., 2021). In situations where data cannot be shared, comprehensive metadata should be made available (Serra-Gonçalves et al., 2019). We strongly recommend adopting the microplastic data collection checklist (see below, and Supplementary materials Supplementary Table S2) and providing this alongside reported results. Ideally, five key metrics and a suite of parameters should be reported for microplastic results (Table 8). If subsampling occurred (e.g., during polymer identification), it is essential to clearly indicate whether the reported results are based on the subsample data or extrapolated to represent the entire dataset (e.g., if 40% of the items were identified as polyethylene, does this percentage reflect the entire dataset or just the subsample).
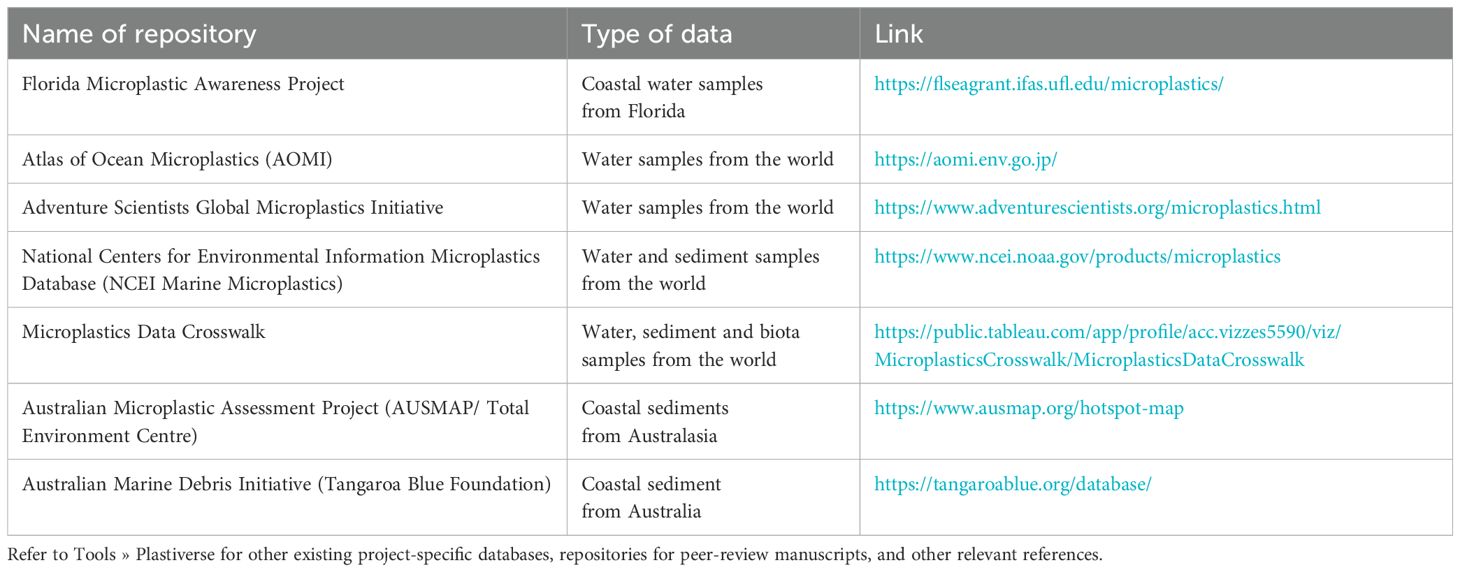
Table 7. Example databases established to collate and disseminate environmental information on the abundance and/or characteristics of microplastics. Repositories listed allow for the inclusion of microplastics data by researchers and other stakeholders for public access and use that is not platform-specific.
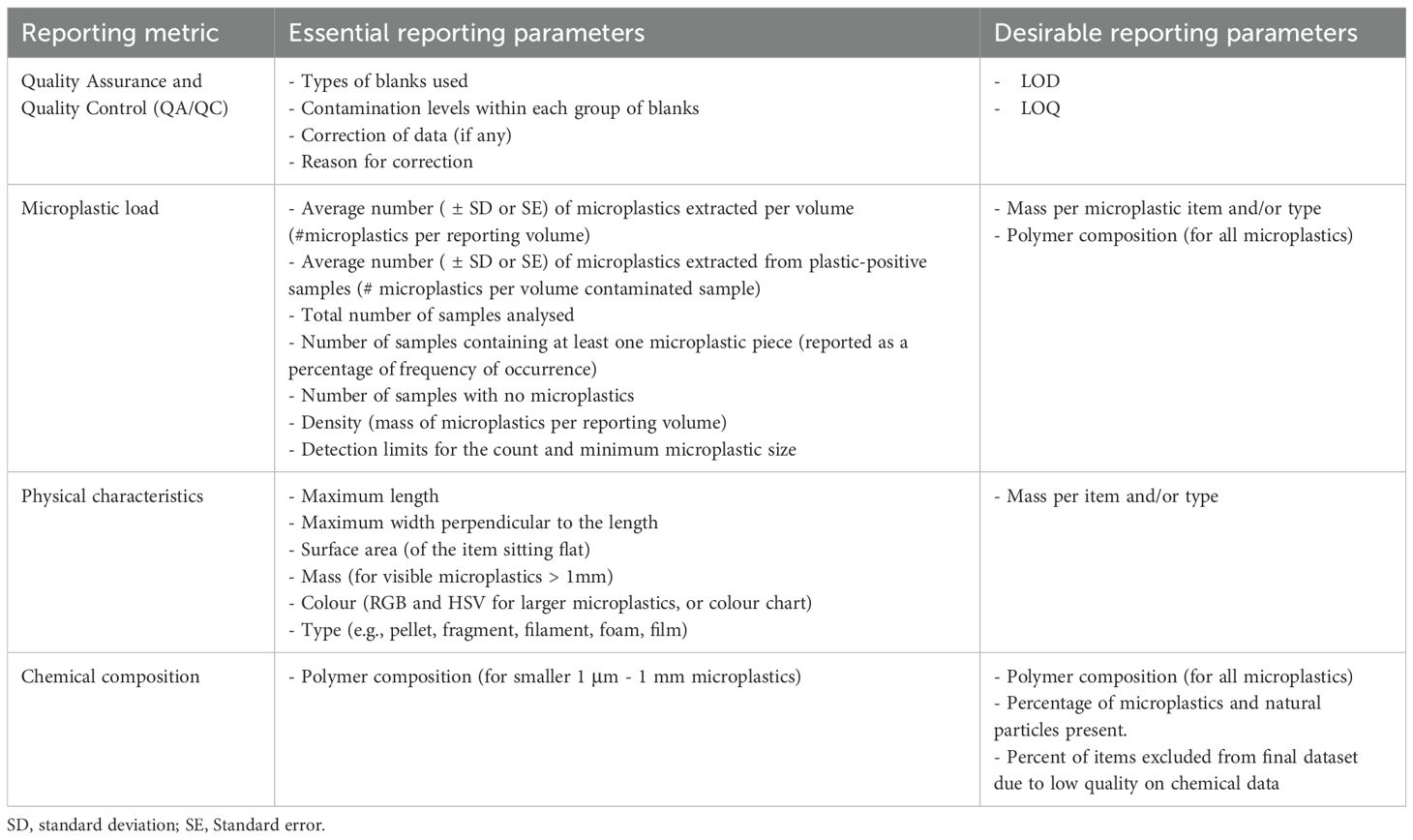
Table 8. Essential and desirable reporting parameters for microplastic load, physical characteristics and chemical composition.
The following key data and information metrics need to be reported (see also Table 7, and checklist in Supplementary materials, Supplementary Table S2).
Data should be reported in the following way:
1. Quality assurance and quality control (QA/QC).
This section ensures the reliability and accuracy of the reported data by detailing the QA/QC measures applied throughout the sampling and analysis process. It includes reporting the type of blanks and controls used to detect contamination levels, along with data correction procedures, such as adjustments for LOD or LOQ. Documenting contamination levels from the blanks, data correction, and the rationale for any corrections is recommended.
2. Load.
This reports the load, or amount, of microplastic found in the chosen matrix. When designing sampling regimes, consider appropriate reporting units (e.g., average number per sample, weight or area) relevant to the research question. Microplastics should be reported in number per sample/weight/area/volume, including by physical and chemical characteristics (see below). When feasible, report data for larger microplastics in mass, acknowledging challenges in weighing small microplastics (< 1 mm). Ensure any variability in microplastic load is reported along with sample replication. Providing raw data or transformed data in complementary units as supplementary information is highly recommended to allow broad-scale comparisons among studies.
3. Physical characteristics.
This information identifies and describes the microplastics found in the environment and includes data on the morphology (e.g., filament, fragment), size, and colour of each item. This information is crucial due to the diversity and heterogeneity of microplastics and can help in the assessment of changes over time and space.
4. Chemical composition.
This provides information on polymer composition and the presence of additives or other associated chemicals, as well as potential evidence of any weathering (e.g., age, biofouling, degradation). If spectral libraries are used for matching, any commercial libraries should be explicitly named and custom-built libraries summarised in terms of their composition and method of data collection.
5. Contextual information.
Information describing environmental (e.g., rain, wind, oceanographic features) and biotic variables (e.g., sex, size or life stage of individuals) that can help researchers make like-for-like data comparisons should be reported. Where possible, this information should be collected at the time of sampling, although some data can be calculated or assessed retrospectively (e.g., GPS location data).
4 Discussion
The variability in sampling methodologies and inconsistent data reporting have limited the effectiveness of current datasets and hindered broadscale, long-term comparisons (Halfar et al., 2021; Wootton et al., 2021). Existing protocols, although valuable, often target specific environments, polymers, or particle sizes, making it difficult to compare datasets collected under different frameworks. Furthermore, finding standardised methods often requires researchers to sift through thousands of peer-reviewed publications and grey literature before identifying a few studies to use as guidelines. Harmonisation offers a path forward, enabling us to adapt to diverse contexts, from scientific to environmental or logistical, while still maintaining best practices. By aligning methods, we improve our ability to synthesise data across regions and time, which is crucial for demonstrating important patterns in microplastic pollution and informing policy development. This not only strengthens the quality of research, ensuring data is accurate and trustworthy (Van Rensburg and Head, 2017) but also enhances our capacity for a coordinated and more effective response to plastic contamination. However, efforts towards method standardisation should focus on important methodological aspects while also allowing for flexibility in research design which is needed to accommodate various research goals and logistical considerations (e.g., matrix and environments being sampled).
This paper addresses these challenges by offering recommendations for harmonised and standardised approaches that consolidate practices across various environmental matrices, including marine and coastal waters, sediments, air, and biota. In particular, the manual stands out by providing a framework designed to meet the needs of diverse stakeholders. By following FAIR principles (Wilkinson et al., 2016) and incorporating guidance from best practice development (Przeslawski et al., 2023), this project ensures data generated using these methods can be easily shared, compared, and integrated.
What makes this paper and the accompanying best practices manual particularly useful is its broad scope, collaborative foundation, and global perspective. Unlike many existing protocols, which are often developed in isolation and tailored to specific compartments or particle sizes, this guideline integrates methodologies across multiple environmental matrices and sampling modes. By doing so, it provides a versatile framework that can be adapted for a wide range of research contexts, from coastal monitoring to open ocean surveys and from macroplastic assessments to near-detection-level microplastics analysis. Developed with input from over 40 researchers across 21 research institutes, the manual reflects multidisciplinary expertise and diverse international experiences, building on contributions and ongoing collaborations in Australia, Brazil, China, EU, India, UK and US. This global approach ensures alignment with international directives while addressing critical gaps in existing guidelines. Our collaborative effort aimed to enhance the real-world applicability of actionable recommendations, providing a comprehensive, accessible primer to serve as a key reference for harmonising and standardising methods.
While we recommend standardised approaches for all sample matrices in terms of collection and processing, we acknowledge the need for some flexibility, as methodologies must often align with the specific hypothesis and research goals—there is no one-size-fits-all solution for microplastic studies. Our recommendations aim to balance harmonisation with adaptability, ensuring consistency in data quality while allowing for methodological adjustments across different research contexts. A key focus of our manual is the importance of QA/QC and comprehensive data reporting, both of which are crucial for promoting data comparability. We also emphasise the need for detailed metadata and thorough descriptions of methodologies in publications to enhance transparency and reproducibility. This balance between structured workflow, adaptability and harmonising field approaches, makes the manual applicable to a wide range of monitoring programs, whether led by government agencies, academic researchers, or non-government organisations. By reinforcing a harmonised approach, we maintain flexibility while ensuring that core standards are followed, providing important benchmarks for adaptability across various research contexts. This ensures data consistency and reliability, supporting the integrity of microplastic monitoring worldwide. By promoting clear and consistent reporting, the manual supports research development, facilitates cross-study comparability, and strengthens confidence in microplastic data among scientists, policymakers, and the public.
In conclusion, the development and dissemination of this manual represents a critical step toward harmonising microplastic research in Australia and globally. We encourage researchers, government agencies, and organisations involved in microplastic monitoring to adopt these standardised approaches and utilise the reporting checklist provided (Supplementary materials, Supplementary Table S2) to ensure consistent data generation. By working together to align methods and reporting standards, the scientific community can generate high-quality, interoperable data that supports meaningful comparisons, long-term monitoring, and informed management decisions. Furthermore, the manuals open-access nature (https://microplastics-field-manual.github.io) ensures that it remains a living document, open to updates and improvements as the field of microplastic research evolves.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
NW: Conceptualization, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. PR-S: Conceptualization, Methodology, Investigation, Funding acquisition, Writing – original draft, Writing – review & editing. RP: Conceptualization, Funding acquisition, Writing – review & editing. TA: Writing – review & editing. MB: Writing – review & editing. BC: Writing – review & editing. TC: Writing – review & editing. AG: Writing – review & editing. SH: Writing – review & editing. MH: Writing – review & editing. BH: Writing – review & editing. RH: Writing – review & editing. JL: Writing – review & editing. SL: Writing – review & editing. FL: Writing – review & editing. SL: Writing – review & editing. MM: Writing – review & editing. CM: Writing – review & editing. WN: Writing – review & editing. AOB: Writing – review & editing. TP: Writing – review & editing. EO: Writing – review & editing. KP: Writing – review & editing. PP: Writing – review & editing. JH: Writing – review & editing. LR: Writing – review & editing. VS: Writing – review & editing. MS: Writing – review & editing. AS: Writing – review & editing. ET: Writing – review & editing. SW: Writing – review & editing. SZ: Writing – review & editing. BG: Writing – review & editing, Conceptualization, Funding acquisition.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Marine and Coastal Hub, a collaborative partnership supported through funding from the Australian Governments National Environmental Science Program (NESP). TMA thanks the support of an Australian Research Council Discovery Early Career Researcher Award (DECRA, DE240100633) grant.
Acknowledgments
We acknowledge the Traditional Custodians of the lands on which this research was conducted. We thank all those who contributed to the workshops and discussions, and whose feedback was invaluable.
Conflict of interest
Author WN was employed by Aquatic Assessments.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1674412/full#supplementary-material
References
AMAP (2025). Arctic monitoring and assessment programme. Available online at: https://www.amap.no (Accessed 28th of March 2025).
An X., et al. (2024). Natural factors of microplastics distribution and migration in water: A review. Water 16, 1595. doi: 10.3390/w16111595
Arciszewski T. J., et al. (2023). Distinguishing between research and monitoring programs in environmental science and management. J. Environ. Stud. Sci. 13, 674–681. doi: 10.1007/s13412-023-00859-0
Bakir A., et al. (2024). Creation of an international laboratory network towards global microplastics monitoring harmonisation. Sci. Rep. 14, 12714. doi: 10.1038/s41598-024-62176-y
Barrett J., et al. (2020). Microplastic pollution in deep-sea sediments from the Great Australian Bight. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.576170
Boyle K. and Örmeci B. (2024). Beta testing an AI-based physical analysis technology for microplastic quantification and characterization. Water 16, 2518. doi: 10.3390/w16172518
Brander S. M., et al. (2020). Sampling and quality assurance and quality control: A guide for scientists investigating the Occurrence of microplastics across matrices. Appl. Spectrosc. 74, 1099–1125. doi: 10.1177/0003702820945713
Brandt J., et al. (2021). Assessment of subsampling strategies in microspectroscopy of environmental microplastic samples. Front. Environ. Sci. 8, 2296–665X. doi: 10.3389/fenvs.2020.579676
Burgess H. K., et al. (2021). NOAA marine debris monitoring and assessment project shoreline survey guide. NOAA Technical Memorandum NOS OR&R ; 56. doi: 10.25923/g720-2n18
Claessens M., et al. (2013). New techniques for the detection of microplastics in sediments and field collected organisms. Mar. pollut. Bull. 70, 227–233. doi: 10.1016/j.marpolbul.2013.03.009
Cowger W., et al. (2020). Reporting guidelines to increase the reproducibility and comparability of research on microplastics. Appl. Spectrosc. 74, 1066–1077. doi: 10.1364/AS.74.001066
Crutchett T. W. and Bornt Katrina R. (2024). A simple overflow density separation method that recovers> 95% of dense microplastics from sediment. MethodsX 102638. doi: 10.1016/j.mex.2024.102638
Dawson A. L., et al. (2023). Taking control of microplastics data: a comparison of control and blank data correction methods. J. Hazardous Materials 443, 130218. doi: 10.1016/j.jhazmat.2022.130218
De Frond H., Rubinovitz R., and Rochman Chelsea M. (2021). μATR-FTIR spectral libraries of plastic particles (FLOPP and FLOPP-e) for the analysis of microplastics. Analytical Chem. 93, 15878–15885. doi: 10.1021/acs.analchem.1c02549
de Jersey A. M., et al. (2025). Seabirds in crisis: Plastic ingestion induces proteomic signatures of multiorgan failure and neurodegeneration. Sci. Adv. 11, eads0834. doi: 10.1126/sciadv.ads0834
de los Santos-Villarreal G. and Elizalde Luis E. (2013). Polymer spectroscopy and compositional analysis. Handb. Polymer Synthesis Characterization Process., 335–354.
de Ruijter V. N., et al. (2020). Quality criteria for microplastic effect studies in the context of risk assessment: A critical review. Environ. Sci. Technol. 54, 11692–11705. doi: 10.1021/acs.est.0c03057
Di Fiore C., Ishikawa Y., and Wright S. L. (2024). A review on methods for extracting and quantifying microplastic in biological tissues. J. Hazardous Materials 464, 132991. doi: 10.1016/j.jhazmat.2023.132991
Diggle A. and Walker ,.T. R. (2022). Environmental and economic impacts of mismanaged plastics and measures for mitigation. Environments 9, 15. doi: 10.3390/environments9020015
European Commission (2023). Guidance on the monitoring of marine litter in European seas – An update to improve the harmonised monitoring of marine litter under the Marine Strategy Framework Directive (Publications Office of the European Union).
Faul F., et al. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191. doi: 10.3758/BF03193146
Fernández-González V., et al. (2021). Impact of weathering on the chemical identification of microplastics from usual packaging polymers in the marine environment. Analytica Chimica Acta 1142, 179–188. doi: 10.1016/j.aca.2020.11.002
Forrest S. A., et al. (2019). Citizen science sampling programs as a technique for monitoring microplastic pollution: results, lessons learned and recommendations for working with volunteers for monitoring plastic pollution in freshwater ecosystems. Environ. Monit. Assess. 191, 1–10. doi: 10.1007/s10661-019-7297-3
Forsythe K., et al. (2024). Viability of elutriation for the extraction of microplastics from environmental soil samples. Environ. Science: Adv. 3, 1039–1047. doi: 10.1039/D4VA00087K
Frias J. P. G. L. and Nash R. (2019). Microplastics: Finding a consensus on the definition. Mar. pollut. Bull. 138, 145–147. doi: 10.1016/j.marpolbul.2018.11.022
Galgani F., et al. (2024). Revisiting the strategy for marine litter monitoring within the european marine strategy framework directive (MSFD). Ocean Coast. Manage. 255, 107254. doi: 10.1016/j.ocecoaman.2024.107254
Gao Z., Wontor K., and Cizdziel J. V. (2022). Labeling microplastics with fluorescent dyes for detection, recovery, and degradation experiments. Molecules 27. doi: 10.3390/molecules27217415
GESAMP (2019). Guidelines for the monitoring and assessment of plastic litter in the ocean, in GESAMP reports and studies no.99. 123. http://www.gesamp.org/publications/guidelines-for-the-monitoring-and-assessment-of-plastic-litter-in-the-ocean.
Gimiliani Giovana T. and Izar G. (2022). Difficulties in comparison among different microplastic studies. Environ. Toxicol. Chem. 41: 820–821. doi: 10.1002/etc.5237
Halfar J., et al. (2021). Disparities in methods used to determine microplastics in the aquatic environment: A review of legislation, sampling process and instrumental analysis. Int. J. Environ. Res. Public Health 18, 1660–4601. doi: 10.3390/ijerph18147608
Hartmann N. B., et al. (2019). Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environ. Sci. Technol. 53, 1039–1047. doi: 10.1021/acs.est.8b05297
Hengstmann E., et al. (2018). Microplastic in beach sediments of the Isle of Rügen (Baltic Sea) - Implementing a novel glass elutriation column. Mar. pollut. Bull. 126, 263–274. doi: 10.1016/j.marpolbul.2017.11.010
Hermsen E., et al. (2018). Quality criteria for the analysis of microplastic in biota samples: a critical review. Environ. Sci. Technol. 52, 10230–10240. doi: 10.1021/acs.est.8b01611
International Organization for Standardization (2023). Principles for the analysis of microplastics present in the environment, (ISO/TC 61/SC 14), Vol. 21. ISO/TC 61/SC 14.
Jenkins T., et al. (2022). Current state of microplastic pollution research data: trends in availability and sources of open data. Front. Environ. Sci. 10, 2296–665X. doi: 10.3389/fenvs.2022.912107
Jones N. R., et al. (2024). Identifying laboratory sources of microplastic and nanoplastic contamination from the air, water, and consumables. J. Hazardous Materials 465, 133276. doi: 10.1016/j.jhazmat.2023.133276
Joshi S. K. and Vashishth A. (2024). Plastonomics: impact of plastic on ecosystem and the world economy. Advanced Strategies Biodegradation Plast. Polymers, 405–420.
Kapoor I. (2001). Towards participatory environmental management? J. Environ. Manage. 63, 269–279. doi: 10.1006/jema.2001.0478
Koelmans A. A., et al. (2020). Solving the nonalignment of methods and approaches used in microplastic research to consistently characterize risk. Environ. Sci. Technol. 54, 12307–12315. doi: 10.1021/acs.est.0c02982
Kroon F., et al. (2018). A workflow for improving estimates of microplastic contamination in marine waters: A case study from North-Western Australia. Environ. pollut. 238, 26–38. doi: 10.1016/j.envpol.2018.03.010
Kumle L., Võ Melissa L. H., and Draschkow D. (2021). Estimating power in (generalized) linear mixed models: An open introduction and tutorial in R. Behav. Res. Methods 53, 2528–2543. doi: 10.3758/s13428-021-01546-0
Kurniawan S. B. and Imron M. F. (2019). Seasonal variation of plastic debris accumulation in the estuary of Wonorejo River, Surabaya, Indonesia. Environ. Technol. Innovation 16, 100490. doi: 10.1016/j.eti.2019.100490
Lavers J. L., et al. (2019). Detection of ultrafine plastics ingested by seabirds using tissue digestion. Mar. pollut. Bull. 142, 470–474. doi: 10.1016/j.marpolbul.2019.04.001
Li Y., et al. (2023). Potential health impact of microplastics: a review of environmental distribution, human exposure, and toxic effects. Environ. Health 1, 249–257. doi: 10.1021/envhealth.3c00052
Lusher A. L., et al. (2021). Moving forward in microplastic research: A Norwegian perspective. Environ. Int. 157, 106794. doi: 10.1016/j.envint.2021.106794
Lyu C., Paterson H. L., and Fogarty J. (2022). The spatiotemporal dynamics, distribution, and characteristics of beached plastics along the remote south coast of Western Australia. Mar. pollut. Bull. 184, 114126. doi: 10.1016/j.marpolbul.2022.114126
Martí E., et al. (2020). The colors of the ocean plastics. Environ. Sci. Technol. 54, 6594–6601. doi: 10.1021/acs.est.9b06400
Meyers N., et al. (2022). Microplastic detection and identification by Nile red staining: Towards a semi-automated, cost- and time-effective technique. Sci. Total Environ. 823, 153441. doi: 10.1016/j.scitotenv.2022.153441
Miller E. A., et al. (2022a). A Raman spectral reference library of potential anthropogenic and biological ocean polymers. Sci. Data 9, 780. doi: 10.1038/s41597-022-01883-5
Miller M. E., et al. (2022b). Temporal patterns of plastic contamination in surface waters at the SS Yongala shipwreck, Great Barrier Reef, Australia. Environ. pollut. 307, 119545. doi: 10.1016/j.envpol.2022.119545
Miller M. E., Kroon Frederieke J., and Motti C. A. (2017). Recovering microplastics from marine samples: A review of current practices. Mar. pollut. Bull. 123, 6–18. doi: 10.1016/j.marpolbul.2017.08.058
Mitrano D. M., et al. (2023). Balancing new approaches and harmonized techniques in nano-and microplastics research Vol. 11 (ACS Publications), 8702–8705.
Morrisey D. J., et al. (1992). Spatial variation in soft-sediment benthos. Mar. Ecol. Prog. Ser., 197–204. doi: 10.3354/meps081197
Munhoz D. R., et al. (2022). Microplastics: a review of policies and responses. Microplastics 2, 1–26. doi: 10.3390/microplastics2010001
Murphy E. L., et al. (2022). A decision framework for estimating the cost of marine plastic pollution interventions. Conserv. Biol. 36, e13827. doi: 10.1111/cobi.13827
Nava V., Frezzotti M. L., and Leoni B. (2021). Raman spectroscopy for the analysis of microplastics in aquatic systems. Appl. Spectrosc. 75, 1341–1357. doi: 10.1177/00037028211043119
Noonan M. J., et al. (2023). Microplastics analytics: why we should not underestimate the importance of blank controls. Microplastics Nanoplastics 3, 17. doi: 10.1186/s43591-023-00065-3
Ocean Best Practices System. (2024). Ocean Best Practices System (OBPS). Available online at: https://www.oceanbestpractices.org/ (Accessed July 23, 2024).
JPI Oceans (2019). BASEMAN: Microplastics analyses in European waters. Available online at: https://www.jpi-oceans.eu/archive/baseman/main-page.html (Accessed March 25, 2025).
Okoffo E. D., et al. (2022). Identification and quantification of micro-bioplastics in environmental samples by pyrolysis–gas chromatography–mass spectrometry. Environ. Sci. Technol. 56, 13774–13785. doi: 10.1021/acs.est.2c04091
Omeyer L. C. M., et al. (2022). Priorities to inform research on marine plastic pollution in Southeast Asia. Sci. Total Environ. 841, 156704. doi: 10.1016/j.scitotenv.2022.156704
OSPAR. (2025). Original Oslo and Paris Conventions Commission. Available online at: https://www.ospar.org (Accessed March 25, 2025).
Pfeiffer F. and Fischer E. K. (2020). Various digestion protocols within microplastic sample processing—Evaluating the resistance of different synthetic polymers and the efficiency of biogenic organic matter destruction. Front. Environ. Sci. 8, 2296–665X. doi: 10.3389/fenvs.2020.572424
Phan S., Padilla-Gamiño Jacqueline L., and Luscombe Christine K. (2022). The effect of weathering environments on microplastic chemical identification with Raman and IR spectroscopy: Part I. polyethylene and polypropylene. Polymer Testing 116, 107752. doi: 10.1016/j.polymertesting.2022.107752
Prata J. C., et al. (2021). Contamination issues as a challenge in quality control and quality assurance in microplastics analytics. J. Hazardous Materials 403, 123660. doi: 10.1016/j.jhazmat.2020.123660
Primpke S., et al. (2023). Monitoring of microplastic pollution in the Arctic: recent developments in polymer identification, quality assurance and control, and data reporting. Arctic Sci. 9, 176–197. doi: 10.1139/as-2022-0006
Provencher J., et al. (2022). An ecosystem-scale litter and microplastics monitoring plan under the Arctic Monitoring and Assessment Programme (AMAP). Arctic Sci. 8, 1067–1081. doi: 10.1139/as-2021-0059
Przeslawski R., et al. (2019). A suite of field manuals for marine sampling to monitor Australian waters. Front. Mar. Sci. 6. doi: 10.3389/fmars.2019.00177
Przeslawski R., et al. (2023). Developing an ocean best practice: A case study of marine sampling practices from Australia. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1173075
Quinn G. P.a. K. and Michael J. (2023). Experimental design and data analysis for biologists. 2 (Cambridge: Cambridge University Press).
Rani M., et al. (2023). A complete guide to extraction methods of microplastics from complex environmental matrices. Molecules 28, 1420–3049. doi: 10.3390/molecules28155710
Razzell Hollis J., et al. (2024b). Quantitative photography for rapid, reliable measurement of marine macro-plastic pollution. Methods Ecol. Evol. 15, 227–243. doi: 10.1111/2041-210X.14267
Razzell Hollis J., Lavers Jennifer L., and Bond Alexander L. (2024a). The use of vibrational spectroscopy and supervised machine learning for chemical identification of plastics ingested by seabirds. J. Hazardous Materials 476, 134996. doi: 10.1016/j.jhazmat.2024.134996
Rochman C. M., et al. (2019). Rethinking microplastics as a diverse contaminant suite. Environ. Toxicol. Chem. 38, 703–711. doi: 10.1002/etc.4371
Royer S.-J., et al. (2024). Computer vision segmentation model—deep learning for categorizing microplastic debris. Front. Environ. Sci. 12, 2296–665X. doi: 10.3389/fenvs.2024.1386292
Samandra S., et al. (2025). Identification of microplastics using spectroscopic methods. Anal. Microplastics Nanoplastics (Elsevier), 183–205. doi: 10.1016/B978-0-443-15779-0.00020-1
Santana M., et al. (2022). An assessment workflow to recover microplastics from complex biological matrices. Mar. pollut. Bull. 179, 113676. doi: 10.1016/j.marpolbul.2022.113676
Schlawinsky M., et al. (2022). Improved microplastic processing from complex biological samples using a customized vacuum filtration apparatus. Limnology Oceanography: Methods 20, 553–567. doi: 10.1002/lom3.10504
Schrank I., et al. (2022). Microplastic sample purification methods - Assessing detrimental effects of purification procedures on specific plastic types. Sci. Total Environ. 833, 154824. doi: 10.1016/j.scitotenv.2022.154824
Serra-Gonçalves C., Lavers Jennifer L., and Bond Alexander L. (2019). Global review of beach debris monitoring and future recommendations. Environ. Sci. Technol. 53, 12158–12167. doi: 10.1021/acs.est.9b01424
Setälä O., Tirroniemi J., and Lehtiniemi M. (2022). Testing citizen science as a tool for monitoring surface water microplastics. Environ. Monit. Assess. 194, 851. doi: 10.1007/s10661-022-10487-w
Shruti V. C., et al. (2022). Analyzing microplastics with Nile Red: Emerging trends, challenges, and prospects. J. hazardous materials 423, 127171. doi: 10.1016/j.jhazmat.2021.127171
Stanton T., et al. (2019). Exploring the efficacy of nile red in microplastic quantification: A costaining approach. Environ. Sci. Technol. Lett. 6, 606–611. doi: 10.1021/acs.estlett.9b00499
Thompson R. C., et al. (2009). Plastics, the environment and human health: current consensus and future trends. Philos. Trans. R. Soc. B: Biol. Sci. 364, 2153–2166. doi: 10.1098/rstb.2009.0053
Thompson R. C., et al. (2024). Twenty years of microplastic pollution research—what have we learned? Science 386, eadl2746. doi: 10.1126/science.adl2746
Thornton Hampton L. M., et al. (2022). A living tool for the continued exploration of microplastic toxicity. Microplastics Nanoplastics 2, 13. doi: 10.1186/s43591-022-00032-4
Tong H., et al. (2021). Rhodamine B dye staining for visualizing microplastics in laboratory-based studies. Environ. Sci. pollut. Res. 28, 4209–4215. doi: 10.1007/s11356-020-10801-4
Tuuri E. M., Gascooke Jason R., and Leterme Sophie C. (2024). Efficacy of chemical digestion methods to reveal undamaged microplastics from planktonic samples. Sci. Total Environ. 174279. doi: 10.1016/j.scitotenv.2024.174279
Underwood A. J., Chapman M. G., and Browne M. A. (2017). Some problems and practicalities in design and interpretation of samples of microplastic waste. Analytical Methods 9, 1332–1345. doi: 10.1039/C6AY02641A
Vaidya A. and Mayer A. L. (2014). Use of the participatory approach to develop sustainability assessments for natural resource management. Int. J. Sustain. Dev. World Ecol. 21, 369–379. doi: 10.1080/13504509.2013.868376
Valente T., et al. (2023). Image processing tools in the study of environmental contamination by microplastics: reliability and perspectives. Environ. Sci. pollut. Res. 30, 298–309. doi: 10.1007/s11356-022-22128-3
Van Mourik L. M., et al. (2021). Results of WEPAL-QUASIMEME/NORMANs first global interlaboratory study on microplastics reveal urgent need for harmonization. Sci. Total Environ. 772, 145071. doi: 10.1016/j.scitotenv.2021.145071
Van Rensburg W. and Head B. W. (2017). Climate change scepticism: reconsidering how to respond to core criticisms of climate science and policy. SAGE Open 7, 2158244017748983. doi: 10.1177/2158244017748983
Vlnieska V., et al. (20241771). Polypy: Aframework to interpret polymer properties from mass spectroscopy data. Polymers 16. doi: 10.3390/polym16131771
Waddell E. N., Nigel L., and Conkle Jeremy L. (2020). Microplastic contamination in Corpus Christi Bay blue crabs. Limnology Oceanography Lett. 5, 92–102. doi: 10.1002/lol2.10142
Wesdemiotis C., et al. (2024). Mass spectrometry of polymers: A tutorial review. Mass Spectrometry Rev. 43, 427–476. doi: 10.1002/mas.21844
Wilkinson M. D., et al. (2016). The FAIR guiding principles for scientific data management and stewardship. Sci. Data 3, 160018. doi: 10.1038/sdata.2016.18
Wong C. S. and Coffin S. (2021). Standard operating procedures for extraction and measurement by infrared spectroscopy of microplastic particles in drinking water (California, USA: Southern California Coastal Water Research Project Authority).
Wootton N., et al. (2024). Research priorities on microplastics in marine and coastal environments: An Australian perspective to advance global action. Mar. pollut. Bull. 205, 116660. doi: 10.1016/j.marpolbul.2024.116660
Wootton N., Reis-Santos P., Adyel T., Blewitt M., Clarke B., Crutchett T., et al. (2024). Marine sampling field manual for microplastics, (In Field Manuals for Marine Sampling to Monitor Australian Waters, Version 3. Eds. Przeslawski R. and Foster S. (National Environmental Science Programme (NESP).
Wootton N., Reis-Santos P., and Gillanders B. M. (2021). Microplastic in fish – A global synthesis. Rev. Fish Biol. Fisheries 31, 753–771. doi: 10.1007/s11160-021-09684-6
Worthington E. and Cockburn P. (2025). Health risk over black kitchen utensils revisited after scientists discover error in study. ABC News Online.
Xu J.-L., et al. (2022). A review of potential human health impacts of micro-and nanoplastics exposure. Sci. Total Environ. 851, 158111. doi: 10.1016/j.scitotenv.2022.158111
Zhu X. (2015). Optimization of elutriation device for filtration of microplastic particles from sediment. Mar. pollut. Bull. 92, 69–72. doi: 10.1016/j.marpolbul.2014.12.054
Keywords: polymer, plastic, monitoring, best practices, marine debris, marine sampling
Citation: Wootton N, Reis-Santos P, Przeslawski R, Adyel TM, Blewitt M, Clarke B, Crutchett T, Ghose A, Hajbane S, Hamann M, Hardesty BD, Hossain R, Lavers JL, Leterme SC, Leusch FDL, Lynch SK, MacGregor M, Motti CA, Noble W, OBrien A, Palanisami T, Okoffo ED, Perera K, Puskic P, Hollis JR, Roman L, Sahajwalla V, Santana MFM, Snigirova A, Tuuri EM, Wilson SP, Ziajahromi S and Gillanders BM (2025) A field and laboratory manual for sampling, processing and reporting microplastics in coastal and marine environments. Front. Mar. Sci. 12:1674412. doi: 10.3389/fmars.2025.1674412
Received: 28 July 2025; Accepted: 20 August 2025;
Published: 25 September 2025.
Edited by:
David Alberto Salas de León, National Autonomous University of Mexico, MexicoReviewed by:
Erik Coria-Monter, National Autonomous University of Mexico, MexicoHannah Hapich, University of California, Riverside, United States
Copyright © 2025 Wootton, Reis-Santos, Przeslawski, Adyel, Blewitt, Clarke, Crutchett, Ghose, Hajbane, Hamann, Hardesty, Hossain, Lavers, Leterme, Leusch, Lynch, MacGregor, Motti, Noble, OBrien, Palanisami, Okoffo, Perera, Puskic, Hollis, Roman, Sahajwalla, Santana, Snigirova, Tuuri, Wilson, Ziajahromi and Gillanders. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nina Wootton, TmluYS53b290dG9uQGFkZWxhaWRlLmVkdS5hdQ==
†Present address: Michelle Blewitt, Marine Environmental Research Consultants, Adelaide, SA, Australia
‡These authors share first authorship
 Nina Wootton
Nina Wootton Patrick Reis-Santos
Patrick Reis-Santos Rachel Przeslawski
Rachel Przeslawski Tanveer M. Adyel3
Tanveer M. Adyel3 Bradley Clarke
Bradley Clarke Sara Hajbane
Sara Hajbane Mark Hamann
Mark Hamann Britta Denise Hardesty
Britta Denise Hardesty Rumana Hossain
Rumana Hossain Cherie A. Motti
Cherie A. Motti Allyson OBrien
Allyson OBrien Thava Palanisami
Thava Palanisami Elvis D. Okoffo
Elvis D. Okoffo Peter Puskic
Peter Puskic Lauren Roman
Lauren Roman Veena Sahajwalla
Veena Sahajwalla Marina F. M. Santana
Marina F. M. Santana Bronwyn M. Gillanders
Bronwyn M. Gillanders