- Australian Institute of Marine Science, Townsville, QLD, Australia
Early post-settlement mortality is a major bottleneck in larval-based coral restoration, largely driven by competitive overgrowth from benthic fouling organisms. Non-biocidal fouling-release coatings (FRCs) may reduce fouling pressure and enhance spat survival, but their efficacy in situ remains poorly quantified. We evaluated whether a commercial FRC could reduce benthic fouling and improve survival of Acropora loripes spat on a mid-shelf Great Barrier Reef. Larvae were settled onto ceramic seeding devices containing either FRC-treated or untreated (control) cores. Devices were deployed on the reef and monitored for fouling cover and spat survival over 46 weeks (~12 months). Relationships between spat survival, fouling, and benthic community composition were assessed. Fouling was substantially lower on FRC-treated devices, with only 25% fouling cover, compared to near-total overgrowth on controls. Importantly, spat survival remained consistently higher on FRC devices (68%) compared to controls (59%) at 46 weeks. Spat survival was negatively associated with device fouling, independent of immediate benthic community composition. This study provides the first in situ mechanistic evidence that FRCs indirectly enhance coral spat survival by mitigating competitive fouling pressure during the critical early growth period. Although the greatest benefit occurred in the first six months, fouling protection persisted throughout the deployment, suggesting that FRCs could provide a scalable solution to improve restoration outcomes. Integration of FRCs into seeding device design represents a promising strategy to support large-scale coral reef restoration under ongoing climate stress.
1 Introduction
Increasingly frequent and severe marine heatwaves are causing extensive coral mortality and leading to a widespread loss of biodiversity (Henley et al., 2024; Hughes et al., 2017). Even under scenarios of full global climate mitigation, projections indicate continued degradation of coral reefs, highlighting the urgent need for complementary strategies to help preserve reef structure and function (Kleypas et al., 2021; Condie et al., 2021). In response, active coral reef restoration is gaining momentum as a strategy to enhance resilience, replenish coral populations, and restore ecological processes (Duarte et al., 2020; Bostrom-Einarsson et al., 2020; Fischer et al., 2021). While reducing carbon emissions remains critical, the strategic development and scaling of restoration technologies, particularly those suited to large reef systems like Australia’s Great Barrier Reef (GBR), will be essential to support biodiversity and ecosystem function in the face of ongoing climate pressures (Suggett et al., 2024; Bayraktarov et al., 2019).
Coral reef restoration initiatives have typically involved outplanting clonal fragments from donor colonies, often by hand (Edwards, 2010; Evans et al., 2021; Schmidt-Roach et al., 2023). However, widespread adoption of this approach depends on ensuring adequate biological supply, which may be limited in both fragment numbers and genetic diversity (Randall et al., 2020). Mass larval collection, either by capturing wild coral slicks (Heyward et al., 2002; Harrison et al., 2021) or production in aquaria (Severati et al., 2024), offers a means of generating large numbers of sexually-derived recruits with high genetic diversity, potentially enhancing resilience to future environmental stressors (Baums et al., 2022). Coral larvae can be settled onto seeding devices made from a range of materials, often featuring structures to improve their retention and stability (Chamberland et al., 2017; Randall et al., 2022; Ramsby et al., 2025). The deployment of seeded devices in large numbers from surface vessels represents a strategy particularly well suited to restoration programs targeting expansive ecosystems, such as the GBR (Suggett et al., 2024).
Devices are commonly deployed when coral spat (settled coral larvae) are just ~1 mm in diameter; however, survival during this early phase is low, often only 10–30% in the first year (Chamberland et al., 2017; Doropoulos et al., 2012; Edwards et al., 2015; Wilson and Harrison, 2005). While physical design features have been added to seeding devices to reduce sediment accumulation, grazing, and predation (Whitman et al., 2024), competition by benthic organisms, including macroalgae, represents a persistent threat (Box and Mumby, 2007; Vermeij, 2005). Until coral recruits reach a size threshold of approximately 1 cm², they remain vulnerable to being outcompeted, smothered, or killed by surrounding algal and invertebrate communities (Doropoulos et al., 2012; Tebbett and Bellwood, 2019), which rapidly colonize artificial surfaces deployed in tropical marine environments (Antunes et al., 2019; Birrell et al., 2008).
Efforts to mitigate fouling on artificial substrates have drawn from developments in the maritime industry. Conventional antifouling coatings (AFCs), which release biocides such as copper, diuron, or dichlorooctylisothiazolinone (DCOIT) have proven effective in deterring colonization (Amara et al., 2018), and copper-based formulations are still used in some coral aquaculture contexts (Shafir et al., 2010). However, these compounds are generally toxic to corals and other non-target reef organisms (Negri and Heyward, 2001; Weber and Esmaeili, 2023), making them unsuitable for use in restoration settings. In response, biocide-free fouling release coatings (FRCs) have emerged as a more environmentally sustainable alternative. These hydrophobic or amphiphilic poly(dimethylsiloxane) (PDMS)-based formulations have very low surface free energy, reducing the adhesion strength of fouling organisms and enabling their removal by natural water movement (Upadhyay et al., 2017; Zhang and Chiao, 2015).
FRCs have been successfully used in aquaculture to reduce maintenance costs (Bannister et al., 2019), and several recent studies have investigated the use of FRCs to mitigate biofouling on coral restoration substrates. The earliest study applied an FRC paraffin wax modified with 0.1% silicone oil to coral settlement surfaces (Tebben et al., 2014). Coral larvae settled near the wax, with the wax-treated surfaces showing significantly reduced fouling and higher coral survival over 39 days under aquarium conditions compared to uncoated surfaces. Subsequent studies tested innovative FRCs (silica-based sol gel with/without cerium dioxide nanoparticles) on ceramic settlement tabs (Roepke et al., 2022b, 2022a). These coatings did not hinder coral settlement but only moderately reduced fouling over 37 days. A third experiment demonstrated that lubricant-infused polydimethylsiloxane (PDMS) substrates reduced fouling by ~70% over three months in both aquarium and field conditions (Karimi et al., 2025). Despite the slippery nature of PDMS, Stylophora pistillata fragments successfully grew tissue directly over the PDMS, supporting their compatibility for coral restoration (Karimi et al., 2025). Most recently, two commercial non-biocidal FRCs and an FRC wax significantly reduced fouling on coral seeding devices deployed for 46 weeks in situ (Montalvo-Proano et al., 2025). These coatings maintained up to 10 times more clear surface area than uncoated devices, without impairing the growth or survival of Acropora millepora microfragments. Coral tissue was again observed overgrowing the coated surfaces.
These findings indicate that FRCs may offer an effective means of protecting vulnerable coral spat from algal overgrowth, thereby reducing early-stage mortality until a size refuge. To test this, the present study deployed Acropora loripes spat on ceramic seeding devices at a shallow mid-shelf reef site. Each device featured a 4-cm diameter central core adjacent to the coral spat, which was either treated with a commercially available hydrophobic silicone FRC or left uncoated as a control. Fouling, spat survival, and surrounding benthic composition (e.g., marine invertebrate cover, algal cover, sediments) were monitored across four time points over a 46-week period. The primary aim was to evaluate the long-term efficacy of FRCs in reducing colonization by benthic competitors and thereby enhancing the survival of coral spat on seeding devices under natural field conditions.
2 Materials and methods
2.1 Seeding devices and settlement surfaces
Coral seeding devices were star-shaped, with three 5-cm arms (Supplementary Figure S1). Each device featured a ~4 cm diameter core with three slots designed to hold 14 × 14 mm concrete settlement tabs with coral spat. The core design featured two triangular side protrusions on each side of the slots, designed to limit grazing of the corals by corallivores (Whitman et al., 2024). The concrete tabs were secured in place by a ceramic spindle. The devices, made from 95% alumina ceramic (i.e., fully sintered aluminium oxide, Al2O3) were manufactured at Shanghai Gongtao Ceramics CO., Ltd. PRC (www.gongtaoceramics.com) (see detailed specifications in (Ramsby et al., 2025).
Devices were assigned to two treatments: FRC and control. FRC-treated devices were hand painted using the hydrophobic silicone coating Hempasil 77300 (Supplementary Table S1) (Hempel, 2024), with the coating applied to the core and spindle while deliberately leaving the arms uncoated. This allowed for natural benthic algal colonization to assist retention of devices to the reef substratum. Hempasil 77300 was used due to its ability to prevent fouling on device surfaces for almost a year (Montalvo-Proano et al., 2025), its biocide-free formulation, and its compliance with the International Convention on the Control of Harmful Antifouling Systems on Ships (IMO October 2001 (Champ, 2001)).
2.2 Sexual propagation: coral spat
Twelve gravid coral colonies (Acropora loripes) were collected from Davies Reef (18.82°S, 147.65°E; GBRMPA permit No. G12/35236.1) in November 2022. Colonies were transferred to 1700 L semi-recirculating tanks at the National Sea Simulator (SeaSim), Australian Institute of Marine Science, Townsville, and maintained at reef-matching temperature (28.5°C). On the night of spawning, gametes were collected, fertilized (Severati et al., 2024) and allocated into two 500 L batch larval culture tanks. When settlement competency reached >75% (after five days), approximately 4000 larvae were settled onto two concrete tiles (28 × 28 x 0.6 cm) within each of eight 50 L acrylic flow-through tanks (inflow rate: 2 L min-1; with 120 µm mesh at the outflow). Concrete tiles were pre-conditioned for 8 weeks to promote settlement cues (i.e., CCA and biofilm formation). They were designed to be broken into individual small tiles (“tabs”, 14 x 14 x 0.6 cm), each with a consistent surface topography resulting from the curing process in purpose made- molds (See Ramsby et al. (2025 for specific details). Spat were held in the same tanks for ~20 days, with the addition of three representative broodstock fragments to facilitate natural infection by symbionts (e.g., Symbiodiniaceae). During this period, conditions were maintained at 28.5°C, with a light intensity of 20 µmol photons m−2 s−1 photosynthetically active radiation (PAR) and an increased flow rate of 8 L min-1. Larvae were fed daily with a mixed algal diet (2000 cells mL−1 of a mix of Tisochrysis lutea, Nannochloropsis oceanica, Pavlova lutheri, Dunaliella sp.) and Artemia salina nauplii (1.5 mL−1). Large tiles were carefully split into tabs by adding pressure from top to bottom with a guillotine system without negatively affecting coral spat within the tab, apart for a few casualties on the breaking points that were not included in survival assessments.
2.3 Assembly, design and reef deployment
Individual settlement tabs were inspected to confirm a density of five to ten coral spat per tab prior to device assembly. Tabs were randomly assigned and inserted into each seeding device. In total, 216 devices were assembled – 108 FRC-treated and 108 control – arranged into nine groups of 24 devices (12 of each treatment). These devices were represented in replicated transects across three sites (Figure 1, Supplementary Table S2). Sites were pre-selected via snorkel surveys to represent an environmental gradient in coral, crustose coralline algae (CCA) and macro/turf algal abundance. Each site had three transects of approximately 20 m separated by a 20-m gap. Twenty-four devices were deployed along each transect in pairs, one of each treatment.
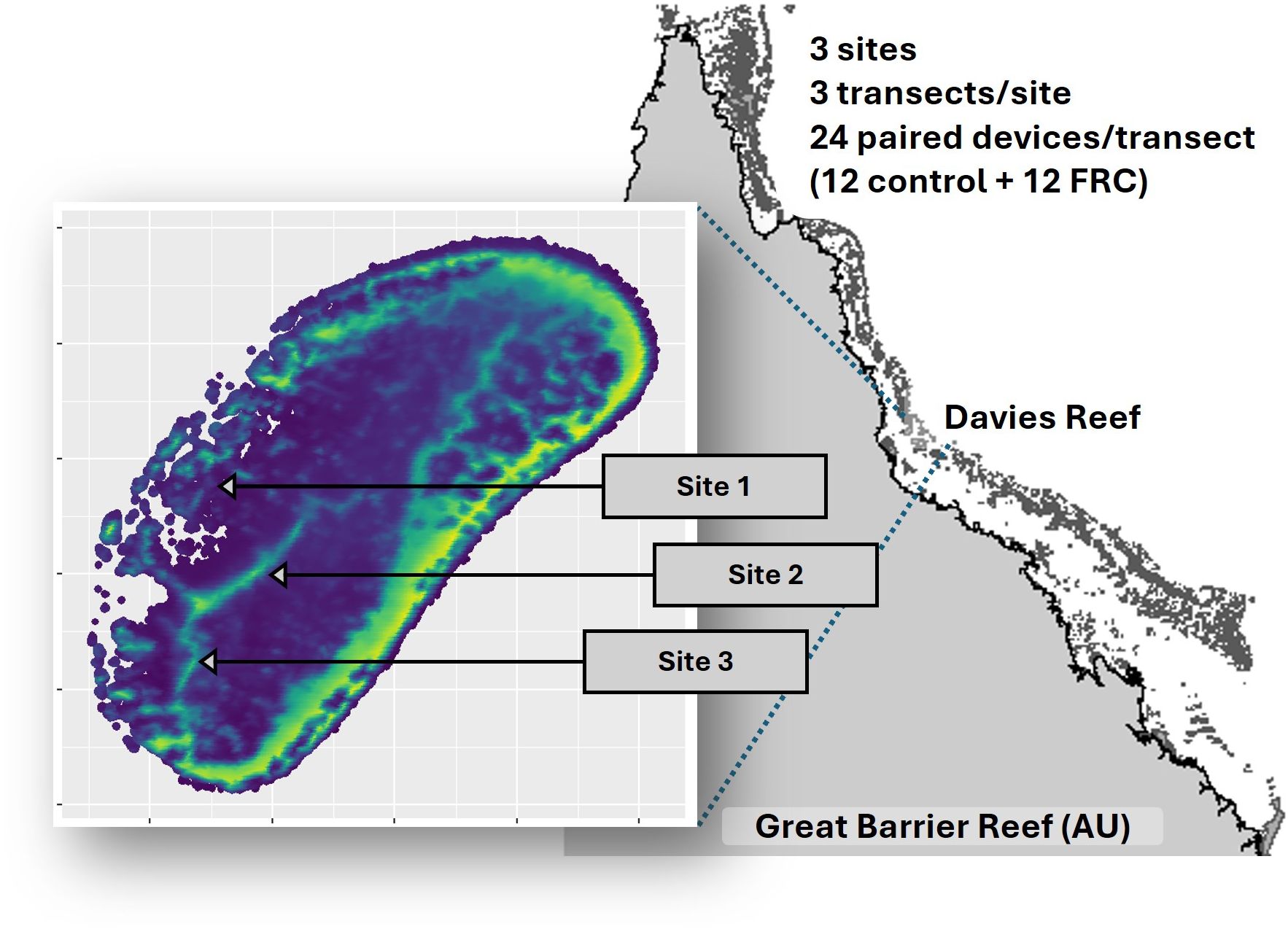
Figure 1. Experimental design and geographic location of sites within the GBR (grey map). The inset shows Davies Reef with estimated routine horizontal water velocities at the seabed, ranging from low (dark blue; 0 ms-1) to high (yellow; 0.6 ms-1), based on publicly available wave models (Callaghan, 2023).
Pre-assembled transect lines were deployed by SCUBA in December 2022 at depths of 4–6 m. Treatment pairs were placed at 1–2 m intervals along each transect (i.e., considering topography and suitable area available), ensuring both devices experienced similar benthic surroundings. To secure devices in place, they were threaded with a 2 mm nylon rope, which was anchored to the reef with nails every three meters. Transects were marked with steel reinforcing bars at the start and end with the corresponding transect label (GBRMPA SAP Approved for permit G21/45348.1).
2.4 Trait assessment and statistical approach
Devices were left in situ for almost 12 months, with censuses conducted at 13, 27, 38 and 46 weeks. Device and tab censuses were performed on SCUBA and involved photographing (1) the benthic community within 50 × 50 cm quadrats surrounding each device, (2) each device to quantify fouling, and (3) individual tabs to track spat survival over time (Montalvo-Proano et al., 2025). Images were taken using an Olympus TG-6 camera with Ikelite® housing using the underwater HDR mode (1:1 frame), while individual tabs were imaged using the underwater microscope function. A SeaLife/SeaDragon 2500 Lm diving light (intensity level 1; 33%) was mounted on the housing to improve image quality.
2.4.1 Surrounding benthos
Quadrat images were uploaded to ReefCloud (González-Rivero et al., 2020), a machine-learning artificial intelligence platform for benthic image classification. Classification was based on a custom category label set derived from the ReefCheck (GBRMPA) database (Done et al., 2017), with additions to include relevant taxa of interest and equipment (e.g., alumina device) (Supplementary Table S3). Each image was annotated with 20 randomly placed points, of which 32% were manually (human) classified to train the algorithm as per ReefCloud guidelines. Machine classifications were validated through random checks to confirm sufficient accuracy (>95%).
The annotated dataset was exported and processed using R (version 4.0.3). Several taxa were consolidated into broader categories to facilitate clearer ecological descriptions and analyses (Supplementary Table S4), including coral cover, macroalgae, invertebrates, CCA, turf algae, sediments, soft coral, sponges. Annotation points placed over non-target life forms or equipment, or over unclear or unknown surfaces were excluded from the analyses. We excluded any categories included in the ReefCheck labels for which there were no observations in our dataset. To explore spatial and temporal patterns in benthic composition, nonmetric multidimensional scaling of variance (NMDS) was performed using Bray-Curtis dissimilarity in the R package ‘vegan’ (Oksanen et al., 2013).
Benthic community composition can be influenced by reef hydrodynamics (Roberts et al., 2015). Horizontal water velocity (ms−1) at the seabed was extracted from publicly available wave modelling predictions for Davies Reef (Callaghan et al., 2015; Callaghan, 2023) (available at https://espace.library.uq.edu.au/view/UQ:8246441). Water velocity data was used to characterize the site exposure but was not included in the statistical analysis.
2.4.2 Device fouling
Images of the exposed device cores and arms were assessed with ImageJ (Rasband, 2012) following the process followed in Montalvo-Proano et al. (2025). Fouling classification involved aggregating pixels of similar color using the eyedropper tool and versatile wand plug-ins to streamline image processing. Major fouling types were color-coded: pink for CCA, green for green algae or brown/red for brown/red algae and light grey for sediments or dead CCA (included under ‘other’). Low-coverage features, such as live coral tissue or invertebrates (e.g., bryozoans, sponges), required manual tracing due to color similarity. Fouling categories were grouped into broader classifications for analysis (Supplementary Table S5). Non-fouled areas (e.g., visible ceramic or FRC coating) were classified as ‘clear’.
Fouling data were converted into the proportion of device surface area covered. Total fouling was calculated as the device proportion of all CCA and green/brown algae coverage. Statistical analyses were performed in R version 4.0.3 (R Core Team, 2020). To quantify fouling on device cores, we fitted a generalized linear mixed effect model. Fixed effects included the interaction of time and treatment, and NMDS1 and NMDS2 scores representing benthic community gradients – aiming to assess the influence of the surrounding benthos on device core fouling. The model also included a random effect of transect nested in site. Because fouling proportions were constrained between 0 and 1, a zero-one inflated beta distribution was used.
2.4.3 Spat survival
Images of the individual tabs were assessed to quantify spat survival and scored as either alive (1) or dead (0). Spat survival was modelled using a set of generalized linear mixed effects models. To test how core fouling affected spat survival, and whether its effect differed across time or with benthic composition, we fitted and compared eight models. The baseline model included device core fouling, time, NMDS1 and NMDS2 scores as predictor variables, without any interactions. Then, one at a time, we tested whether including a two-way interaction between core fouling and each of the remaining predictors improved model fit. The additional models included three models with a single two-way interaction with core fouling (i.e., one for each of the remaining predictors), three models with two two-way interactions with core fouling (i.e., one for every possible combination), and one model with three two-way interactions (i.e., all predictors interacting with core fouling) (Supplementary Table S6). All models included a random effect of transect nested in site. Survival models assumed a binomial distribution (logit link) with the number of tabs representing the number of trials. Models were compared using leave-one-out cross-validation (LOO) with the ‘loo’ package (Vehtari et al., 2016). All mixed effect models were fitted using a Bayesian framework in the ‘brms’ package (Bürkner, 2017), with four chains of 2000 iterations each (half of which were discarded during warm-up).
To provide a more ecologically relevant assessment of FRC application for restoration purposes, our models included device fouling in substitution of treatment as explanatory variable for spat survival. This approach quantifies the effect of fouling coverage on spat survival while still capturing the benefits of FRC treatments relative to controls. Moreover, this generates a more informative outcome, where survival–competition patterns are not tied to specific FRCs, thereby facilitating the transfer of this approach to other studies or locations where alternative techniques may be evaluated.
3 Results and discussion
Fouling protection and spat survival were higher on FRC-treated devices compared to uncoated controls (Figure 2). The cores of control devices became rapidly fouled, reaching ~90% coverage by week 13 and exceeding ~97% by week 27 (Figure 2). In contrast, fouling was strongly inhibited on FRC-treated cores, with coverage increasing only from 20% to 42% over the 13 to 46 week period. Similarly, spat survival on FRC-treated devices was consistently higher than on controls, starting at 88% versus 76% at 13 weeks. The largest difference occurred at approximately 6 months, with survival of 83% on FRC devices compared to 65% on controls. By the end of the experiment at 46 weeks, spat survival remained higher on FRC-treated devices (67% versus 58%) and spat had grown to over 1 cm in diameter, covering the settlement tile and expanding across the FRC surface of the seeding device. In this experiment, FRC treatment was applied to the device cores, approximately 5 to 10 mm away from the spat, suggesting that its influence on survival was indirect; instead, acting through a reduction in fouling competition within the immediate benthic habitat. The following sections explore the influence of benthic habitat and fouling pressure on spat survival over the duration of the deployments.
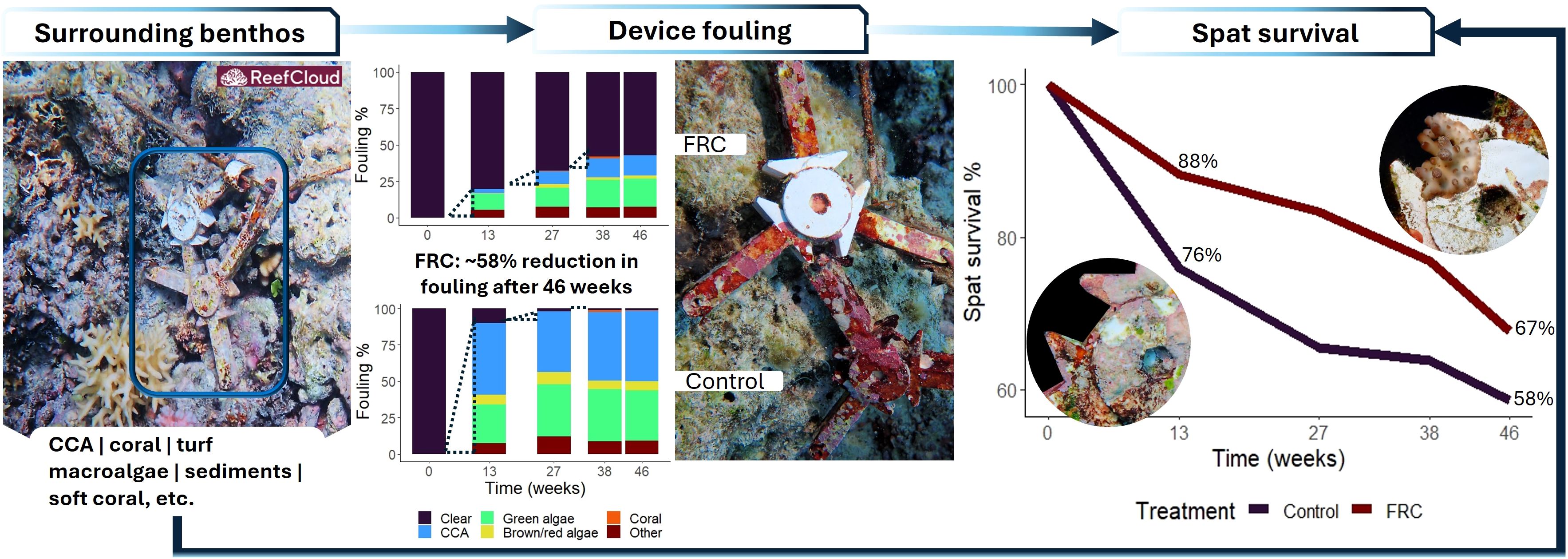
Figure 2. Surface fouling and spat survival on seeding devices with and without core FRC-treatments over 46-week deployments at Davies Reef. From left to right: typical quadrat image including seeding devices, mean fouling in broad categories, close-up image of fouling (FRC core is white) and mean spat survival. Model estimates of % fouling and spat survival, along with confidence intervals, are provided in Figures 4, 5. Arrows represent potential pathways explaining spat survival outcomes across experimental traits.
3.1 Benthic composition across sites
The benthic community assessment revealed that coral, CCA and turf algae were the most abundant taxa across sites (Figure 3A). This pattern was consistent across all time points, although site-level differences were apparent. The most evident variation was higher coral cover at Site 1 (28%) followed by Site 2 (~16%) and Site 3 (~8.3%). CCA abundance was similar across sites, ranging from 30 to 37%, whereas turf algae were most abundant at Site 3 (~40%), followed by Site 2 (~26%) and Site 1 (~18%). Remaining categories, such as macroalgae and other benthic taxa, were less common. Sediment cover was predominantly observed at Site 3 (~6.9%) and <1% at Sites 1 and 2.
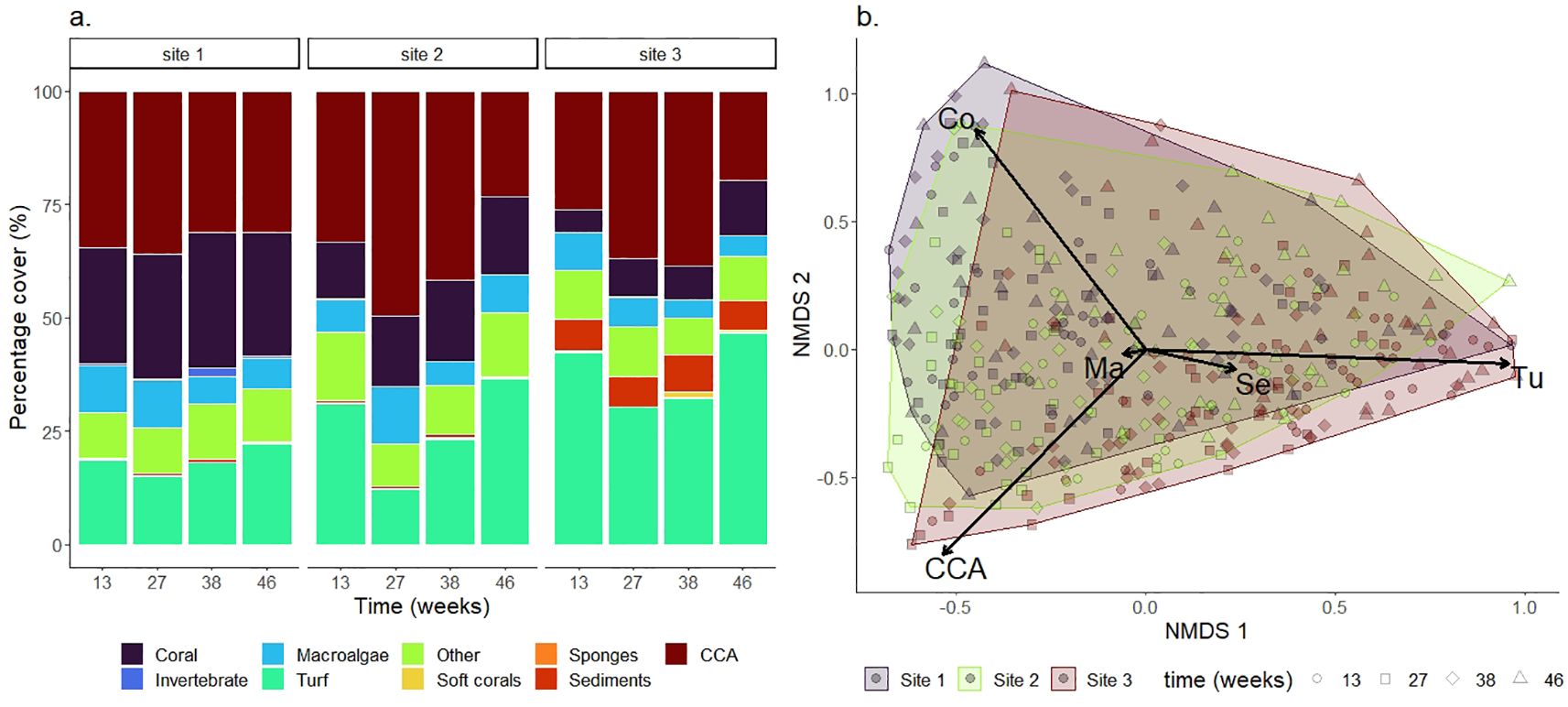
Figure 3. Benthic composition across sites. (A) Proportion of benthic categories obtained from quadrat photos (50 × 50 cm) surrounding pairs of devices across time and grouped by site. (B) NMDS ordination plot of the quadrat images collected from all sites and experimental time points (n=432; stress=0.08; R2 > 0.95). Scaled benthic categories included CCA (CCA), Co (coral cover), Tu (turf), Ma (macroalgae), and Se (sediments).
NMDS ordination of benthic categories did not reveal a clear clustering of sites (Figure 3B). However, coral cover scaled positively with NMDS2, while CCA cover showed a negative relationship. CCA also scaled negatively with NMDS1, in contrast to turf algae, which scaled strongly and positively with NMDS1. This indicates that NMDS1 represents a gradient from turf dominated to CCA- and coral- dominated quadrats. Meanwhile, NMDS2 captures partitioning between CCA and coral dominance. These NMDS axes provided meaningful summary variables to include as potential explanatory predictors of spat survival on both FRC and control devices.
We hypothesized that the benthic community surrounding seeding devices would influence fouling patterns and spat survival. However, the limited variability in benthic composition across sites constrained our ability to identify significant relationships between these variables, suggesting that the benthic composition varies more among quadrats within sites, than among sites. For instance, water velocity at the seabed, as estimated by wave model outputs across Davies Reef (Figure 1), was consistent among sites, although empirical validation of this assumption was lacking. Moreover, a distinct algal gradient was not evident across these mid-shelf reef sites compared to inshore locations, where macroalgal dominance (exceeding 50% of benthic cover) is prevalent, particularly on shallow inshore reefs (Fabricius et al., 2023; Gruber et al., 2024). The detrimental effects of macroalgae on coral settlement and survival are well documented, with studies showing lower settlement and survival (Box and Mumby, 2007, McCook et al., 2014, Smith et al., 2022; Morrow et al., 2012), in addition to reduced coral growth (Suzuki et al., 2018). The specific species of macroalgae also play a critical role in these interactions (Page et al., 2024). Furthermore, areas dominated by crustose coralline algae (CCA), turf algae, or other colonial invertebrates, such as zoanthids and bryozoans, are likely to facilitate rapid colonization of seeding devices and overgrowth of slower-growing coral spat (McCook et al., 2014; Tebbett and Bellwood, 2019; Diaz-Pulido et al., 2009). While benthic community structure can exhibit significant variation across mid-shelf reef locations, potentially influencing coral survival on restoration devices (Randall et al., 2022), incorporating a more contrasting benthic environment would likely enhance the sensitivity of the FRC devices to variations in fouling and spat survival, thus better elucidating the role of algal-dominated areas in shaping these ecological dynamics.
3.2 Device fouling
Device fouling was consistently over two-fold lower on FRC-treated devices than on controls (Figure 4, Supplementary Table S7). In fact, FRC-treatment was the only predictor with a posterior distribution that did not overlap zero (95% CI: -2.82– -2.23), indicating a strong effect on reducing fouling. In contrast, the 95% CIs for all other predictors, including time, NMDS1, NMDS2, and the treatment × time interaction overlapped zero, suggesting no clear effect (Supplementary Table S7). The effect size of the interaction term between treatment and time suggested a modest decrease in the difference between treatments over time. At 13 weeks, control devices had 47% more total fouling than FRC devices (CI: 0.33–0.52). By 46 weeks, this difference declined slightly, with control devices exhibiting 39% more total fouling than FRC devices (CI: 0.30–0.42).
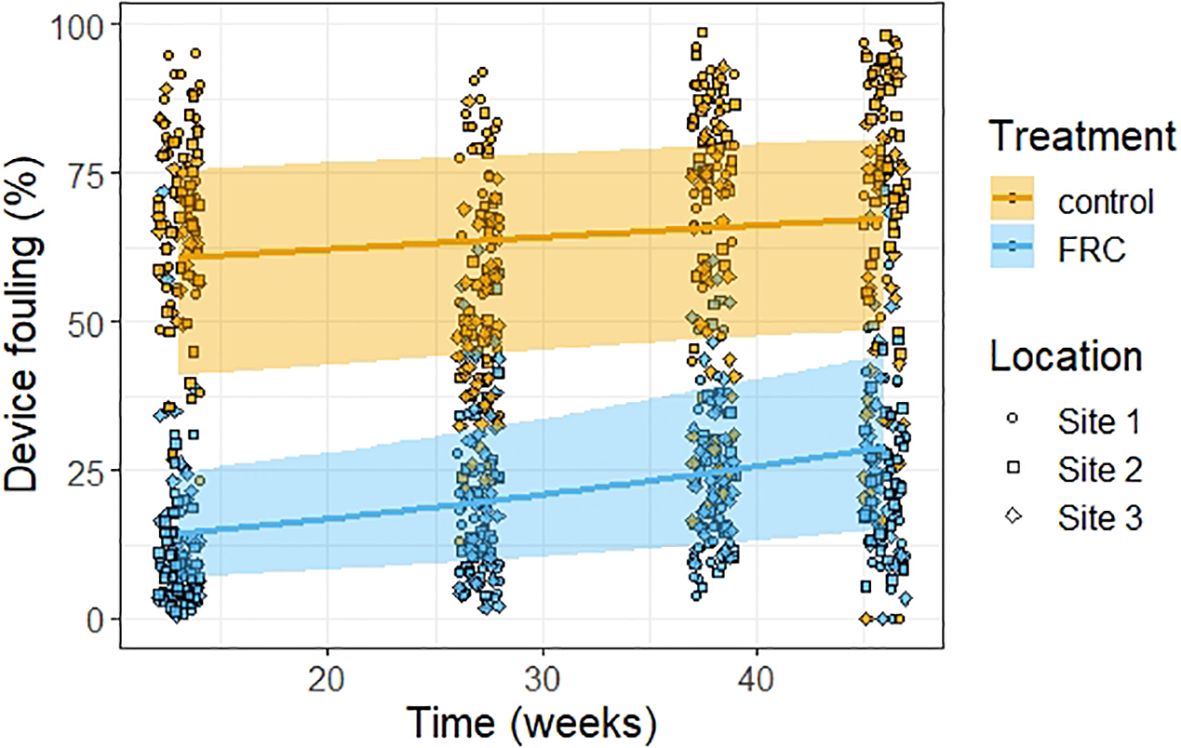
Figure 4. Estimates of device fouling observed over time according to presence (FRC) or absence (control) of FRC core treatment. Raw data for total fouling on device cores is represented in the background at each timepoint as well as its corresponding deployment site. The solid lines show the predictions of the fitted model, the ribbons show the 95% credible intervals, and the points show the raw data. The colors indicate device treatments, and the shape of the points indicate the sites.
The colonization of all uncoated device surfaces by benthic algae was rapid and nearly complete by 13 weeks (Figure 2 and Figure 4), highlighting both the potential for aggressive overgrowth of deployed spat and the potential for FRCs to mitigate this threat. The non-biocidal FRC-coating (Hempasil 77300) reduced fouling on device cores by 58% at week 46, a performance comparable to that achieved by the same FRC (50–75%) and by Intersleek 1001 (40–50%) during similar deployments with coral microfragments at Davies Reef (Montalvo-Proano et al., 2025). Similar reductions were also observed with lubricant-infused PDMS devices seeded with larger coral fragments during a shorter three-month field deployment (Karimi et al., 2025). Other FRCs, including wax-based and silica-based sol-gels (with or without cerium dioxide additives), appeared less effective in field and aquarium settings (Tebben et al., 2014; Montalvo-Proano et al., 2025; Roepke et al., 2022a). However, the composition of the surrounding benthic habitat, which differed among studies, may influence the efficacy of FRC treatments. In this study, habitat composition did not vary substantially among sites, and the immediate benthic environment had no detectable effect on fouling of either FRC-treated or control devices (Figure 2). Future research should evaluate the performance of FRCs across broader environmental gradients, particularly in relation to algal dominance, which is a major pressure on recruit survival (Ceccarelli et al., 2018; Smith et al., 2022). FRCs generally perform best under high-flow conditions (Dafforn et al., 2011). In this study, device shapes, and therefore their fine-scale hydrodynamics, were equivalent across all treatments. However, performance may be enhanced by designing device geometries that increase local shear stress to help dislodge algal fouling. Fine-scale hydrodynamic conditions at reef sites may also influence fouling patterns; although such differences can occur among sites and treatments, our fouling assessments indirectly captured any resulting variation.
The FRC treatment remained durable, with the white coating clearly visible after the 46-week deployment (Figure 2). However, the greatest relative protection from fouling occurred early in the deployment period (Figure 4). This finding is important, as protection of spat from fouling may only be required for a limited period. The use of a reduced volume of FRC (i.e., a thinner coating) or the application of a less durable FRC, such as wax (Montalvo-Proano et al., 2025), may therefore be more efficient or optimal, provided it maintains fouling protection until spat reach the critical escape-size threshold.
3.3 Spat survival
Spat survival was primarily influenced by the extent of device fouling, time, and benthic NMDS2 scores (Figure 5; Supplementary Table S8). In contrast, no relationship was detected between NMDS1 score and spat survival. Device fouling had a strong negative effect on spat survival at the device level, with an estimated ~26% reduction in survival from clean to fully fouled devices (Figure 5A). Marginal means indicated survival of 82% at 0% fouling (95% CI: 0.55–0.95), compared to 61% at 100% fouling (CI: 0.27–0.86). This result, coupled with the strong effect of FRC-treatment on fouling reduction (Figure 2), provides the first mechanistic evidence demonstrating that FRC application indirectly improves spat survival in situ by reducing fouling on seeding devices. Previously, no direct positive or negative effect of FRC treatment was observed on the survival of field-deployed microfragments, whose outcomes were more strongly influenced by genotype than by FRC treatment or benthic habitat (Montalvo-Proano et al., 2025). However, supporting our findings, positive effects of wax FRC treatments on spat survival have been reported in aquarium-based studies (Tebben et al., 2014), indicating FRC seeding device treatments are likely more beneficial to spat than larger corals. In the studies by Tebben et al. (2014) and Karimi et al. (2025), coral spat and fragments (respectively) were positioned closer to the FRC-treated surfaces than in our experiments, potentially receiving greater protection from benthic competitors. The benefits of near-field FRC application to spat survival should therefore be tested in situ over longer deployment periods. Additionally, while our settlement tabs contained mature biofilms, including CCA to promote larval settlement, these biofilms can overgrow spat as they continue to mature (Fong et al., 2024; Ramsby et al., 2024). Spat survival could potentially be improved further by using biologically inert tabs for larval settlement, thereby enhancing the protective effects of FRC-coated device cores.
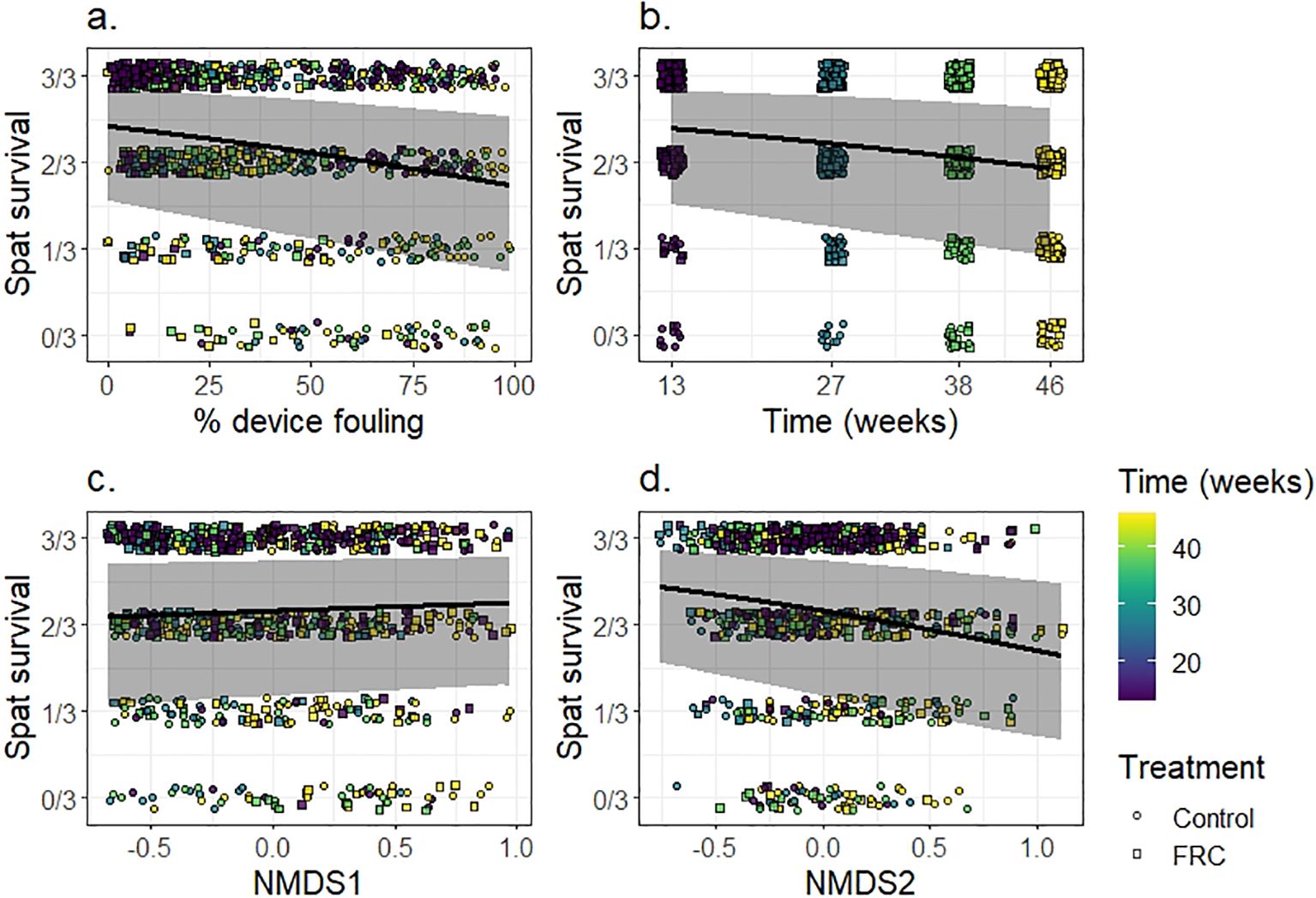
Figure 5. Outcome of spat survival estimates relative to individual explanatory variables, including: (A) proportion of device fouling, (B) experimental timepoints, (C) surrounding benthos NMDS1, and (D) NMDS2. The solid black line represents the predictions of the fitted model, and the ribbons show the 95% credible intervals. Colored shapes in the background represent the distribution of survival raw data according to time (weeks) and treatment.
Spat survival at the tab level (3 tabs per device) also declined over time, with an ~18% decrease from 13 to 46 weeks. At 13 weeks, the marginal mean survival was 81% (C.I. 0.52–0.94), which dropped to 66% by week 46 (C.I. 0.32–0.88). This decline was expected, as fouling pressure is likely to have increased with time. Although, the increase in surface area fouling coverage only marginally increased after 13 weeks on FRC-treated and control devices, more mature algal crusts are more likely to outcompete and overgrow smaller spat as they thicken over time (Brunner et al., 2021; Ramsby et al., 2024). Other reef pressures, such as incidental grazing (Whitman et al., 2024, 2025) and sediment deposition (Jones et al., 2015), were not measured in this study and likely also contributed to the declines in survival. Interestingly, spat survival was lower on devices located in areas with high coral dominance. For example, devices with surrounding benthos represented by an NMDS2 score of 1.11 (coral-dominated) had a marginal mean of 60% (C.I. 0.26–0.85), compared to 84% (C.I. 0.58–0.96) for devices in areas dominated by CCA, represented by an NMDS2 score of -0.76. A negative relationship between coral survival and branching coral abundance has been previously reported (Page et al., 2024). Importantly, the lower spat survival observed in coral-dominated habitats, even in the absence of increased fouling, points to other competitive mechanisms, such as allelopathy or space limitation (Roth et al., 2018; Page et al., 2024), which warrant further investigation.
3.4 Outlook for FRC application in reef seeding
This study provides the first mechanistic field evidence that non-biocidal FRCs can substantially enhance early post-settlement survival of coral spat by reducing competitive overgrowth from benthic fouling organisms. By limiting early-stage fouling, FRCs mitigate space competition and associated stressors during the critical period before coral recruits reach a size refuge, significantly improving survival rates. These local-scale survival benefits could translate into proportional increases in seeding efficiency, either by boosting the number of surviving corals per device or reducing the number of devices required to restore a given area.
The successful implementation of large-scale larval-based restoration projects using FRC-treated seeding devices will require the deployment of high numbers of coated units. Silicone-based FRCs provide effective antifouling performance at a relatively low cost (approximately $US0.10 per device; Montalvo-Proano et al. (2025)), but the continued development of more sustainable and cost-effective alternatives remains important. Paraffin-based waxes represent one such option (Tebben et al., 2014), though they offer lower fouling resistance compared to silicone-based coatings (Montalvo-Proano et al., 2025). Future research should prioritize the development of FRCs that retain antifouling efficacy during the critical early stages of coral spat development, while also degrading safely without releasing harmful residues. Scalability and economic feasibility could also be improved by integrating industrial application methods such as compressed air, airless, or electrostatic spray systems, which may reduce coating thickness, material usage, and application time. Overall, the results of this study demonstrate that FRCs offer a scalable, low-impact strategy to improve coral spat survival and support the effectiveness of field-based reef restoration programs.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
JM-P: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. MA-N: Formal analysis, Validation, Writing – review & editing. FF: Writing – review & editing. AS: Resources, Writing – review & editing. AN: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the Reef Restoration and Adaptation Program, which aims to develop effective interventions to help the Reef resist, adapt and recover from the impacts of climate change, and which is funded by the partnership between the Australian Governments Reef Trust and the Great Barrier Reef Foundation.
Acknowledgments
We thank the staff of SeaSim for their help during experimental set ups at AIMS and the RRAP-CAD field team for their help during coral collections and censusing. The foul release coatings were kindly donated by Hempel (Wattyl) Australia Pty Ltd (David Neumann and Evert Hut). We thank David and Evert for their expert advice on the use and application of the respective coatings. We would also like to acknowledge and pay our respects to the Bindal and Wulgurukaba people, the Traditional Custodians of the land and sea country on which this research was performed.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1684011/full#supplementary-material.
References
Amara I., Miled W., Slama R. B., and Ladhari N. (2018). Antifouling processes and toxicity effects of antifouling paints on marine environment. A review. Environ. Toxicol. Pharmacol. 57, 115–130. doi: 10.1016/j.etap.2017.12.001
Antunes J., Leao P., and Vasconcelos V. (2019). Marine biofilms: Diversity of communities and of chemical cues. Environ. Microbiol. Rep. 11, 287–305. doi: 10.1111/1758-2229.12694
Bannister J., Sievers M., Bush F., and Bloecher N. (2019). Biofouling in marine aquaculture: A review of recent research and developments. Biofouling 35, 631–648. doi: 10.1080/08927014.2019.1640214
Baums I. B., Chamberland V. F., Locatelli N. S., and Conn T. (2022). Coral Reef Conservation and Restoration in the Omics Age. In: van Oppen M. J.H. and Aranda Lastra M. (eds), 35–53. Springer International Publishing, Cham, Switzerland. doi: 10.1007/978-3-031-07055-6_3
Bayraktarov E., Stewart-Sinclair P. J., Brisbane S., Boström-Einarsson L., Saunders M. I., Lovelock C. E., et al. (2019). Motivations, success, and cost of coral reef restoration. Restor. Ecol. 27, 981–991. doi: 10.1111/rec.12977
Birrell C., Mccook L., Willis B., and Diaz-Pulido G. (2008). “Effects of benthic algae on the replenishment of corals and the implications for the resilience of coral reefs,” in Oceanography and marine biology, vol. 46. (Boca Raton, USA: CRC Press), 31–70.
Bostrom-Einarsson L., Babcock R. C., Bayraktarov E., Ceccarelli D., Cook N., Ferse S. C. A., et al. (2020). Coral restoration - a systematic review of current methods, successes, failures and future directions. PloS One 15, e0226631. doi: 10.1371/journal.pone.0226631
Box S. J. and Mumby P. J. (2007). Effect of macroalgal competition on growth and survival of juvenile caribbean corals. Mar. Ecol. Prog. Ser. 342, 139–149. doi: 10.3354/meps342139
Brunner C. A., Uthicke S., Ricardo G. F., Hoogenboom M. O., and Negri A. P. (2021). Climate change doubles sedimentation-induced coral recruit mortality. Sci. Total Environ. 768, 143897. doi: 10.1016/j.scitotenv.2020.143897
Bürkner P. (2017). Brms: An r package for bayesian multilevel models using stan. J. Stat. Software 80, 1–28. doi: 10.18637/jss.v080.i01
Callaghan D. P. (2023). Great barrier reef non-cyclonic and on-reef wave model predictions (The university of queensland). Available online at: https://espace.Library.Uq.Edu.Au/view/uq:8246441 (Accessed November 19, 2025).
Callaghan D. P., Leon J. X., and Saunders M. I. (2015). Wave modelling as a proxy for seagrass ecological modelling: Comparing fetch and process-based predictions for a bay and reef lagoon. Estuarine Coast. Shelf Sci. 153, 108–120. doi: 10.1016/j.ecss.2014.12.016
Ceccarelli D. M., Loffler Z., Bourne D. G., Al Moajil-Cole G. S., Boström-Einarsson L., Evans-Illidge E., et al. (2018). Rehabilitation of coral reefs through removal of macroalgae: State of knowledge and considerations for management and implementation. Restor. Ecol. 26, 827–838. doi: 10.1111/rec.12852
Chamberland V. F., Petersen D., Guest J. R., Petersen U., Brittsan M., and Vermeij M. J. A. (2017). New seeding approach reduces costs and time to outplant sexually propagated corals for reef restoration. Sci. Rep. 7, 18076. doi: 10.1038/s41598-017-17555-z
Champ M. (2001). New imo convention to control anti-fouling systems on ships:Imo adopted a treaty for the control of harmful anti-fouling systems on ships to elimate the use of anti-fouling paint containing tbt after january1, 2003. Sea Technol. 42, 48–50.
Condie S. A., Anthony K. R. N., Babcock R. C., Baird M. E., Beeden R., Fletcher C. S., et al. (2021). Large-scale interventions may delay decline of the great barrier reef. R. Soc. Open Sci. 8, 201296. doi: 10.1098/rsos.201296
Dafforn K. A., Lewis J. A., and Johnston E. L. (2011). Antifouling strategies: History and regulation, ecological impacts and mitigation. Mar. pollut. Bull. 62, 453–465. doi: 10.1016/j.marpolbul.2011.01.012
Diaz-Pulido G., Mccook L. J., Dove S., Berkelmans R., Roff G., Kline D. I., et al. (2009). Doom and boom on a resilient reef: Climate change, algal overgrowth and coral recovery. PloS One 4, e5239. doi: 10.1371/journal.pone.0005239
Done T., Roelfsema C., Harvey A., Schuller L., Hill J., Schlappy M. L., et al. (2017). Reliability and utility of citizen science reef monitoring data collected by reef check Australia 2002-2015. Mar. pollut. Bulletinl 117, 148–155. doi: 10.1016/j.marpolbul.2017.01.054
Doropoulos C., Ward S., Marshell A., Diaz-Pulido G., and Mumby P. J. (2012). Interactions among chronic and acute impacts on coral recruits: The importance of size-escape thresholds. Ecology 93, 2131–2138. doi: 10.1890/12-0495.1
Duarte C. M., Agusti S., Barbier E., Britten G. L., Castilla J. C., Gattuso J. P., et al. (2020). Rebuilding marine life. Nature 580, 39–51. doi: 10.1038/s41586-020-2146-7
Edwards A. (2010). Reef rehabilitation manual. Available online at: https://gefcoral.Org/portals/53/downloads/reef%20rehabilitation%20manual_web.Pdf (Accessed November 19, 2025).
Edwards A. J., Guest J. R., Heyward A. J., Villanueva R. D., Baria M. V., Bollozos I. S. F., et al. (2015). Direct seeding of mass-cultured coral larvae is not an effective option for reef rehabilitation. Mar. Ecol. Prog. Ser. 525, 105–116. doi: 10.3354/meps11171
Evans A. J., Lawrence P. J., Natanzi A. S., Moore P. J., Davies A. J., Crowe T. P., et al. (2021). Replicating natural topography on marine artificial structures – a novel approach to eco-engineering. Ecol. Eng. 160, 106144. doi: 10.1016/j.ecoleng.2020.106144
Fabricius K. E., Crossman K., Jonker M., Mongin M., and Thompson A. (2023). Macroalgal cover on coral reefs: Spatial and environmental predictors, and decadal trends in the great barrier reef. PloS One 18, e0279699. doi: 10.1371/journal.pone.0279699
Fischer J., Riechers M., Loos J., Martin-Lopez B., and Temperton V. M. (2021). Making the un decade on ecosystem restoration a social-ecological endeavour. Trends Ecol. Evol. 36, 20–28. doi: 10.1016/j.tree.2020.08.018
Fong J., Ramsby B. D., Flores F., Dada T., Antunes E., Abdul Wahab M. A., et al. (2024). Effects of material type and surface roughness of settlement tiles on macroalgal colonisation and early coral recruitment success. Coral Reefs 43, 1083–1096. doi: 10.1007/s00338-024-02526-4
González-Rivero M., Beijbom O., Rodriguez-Ramirez A., Bryant D. E. P., Ganase A., Gonzalez-Marrero Y., et al. (2020). Monitoring of coral reefs using artificial intelligence: A feasible and cost-effective approach. Remote Sens. 12, 489. doi: 10.3390/rs12030489
Gruber R., Waterhouse J., Petus C., Howley C., Lewis S., Moran D., et al. (2024). Marine monitoring program: Annual report for inshore water quality monitoring 2022-23. Great Barrier Reef Marine Park Authority, Townsville. Available online at: https://hdl.handle.net/11017/4047 (Accessed November 19, 2025).
Harrison P. L., Dela Cruz D. W., Cameron K. A., and Cabaitan P. C. (2021). Increased coral larval supply enhances recruitment for coral and fish habitat restoration. Front. Mar. Sci. 8, 750210. doi: 10.3389/fmars.2021.750210
Hempel (2024). Hempasil 77300. Available online at: https://www.Hempel.Com/products/hempasil-77300-77300 (Accessed November 19, 2025).
Henley B. J., Mcgregor H. V., King A. D., Hoegh-Guldberg O., Arzey A. K., Karoly D. J., et al. (2024). Highest ocean heat in four centuries places great barrier reef in danger. Nature 632, 320–326. doi: 10.1038/s41586-024-07672-x
Heyward A. J., Smith L. D., Rees M., and Field S. N. (2002). Enhancement of coral recruitment by in situ mass culture of coral larvae. Mar. Ecol. Prog. Ser. 230, 113–118. doi: 10.3354/meps230113
Hughes T. P., Kerry J. T., Alvarez-Noriega M., Alvarez-Romero J. G., Anderson K. D., Baird A. H., et al. (2017). Global warming and recurrent mass bleaching of corals. Nature 543, 373–377. doi: 10.1038/nature21707
Jones R., Ricardo G. F., and Negri A. P. (2015). Effects of sediments on the reproductive cycle of corals. Mar. pollut. Bull. 100, 13–33. doi: 10.1016/j.marpolbul.2015.08.021
Karimi Z., Flores I., Kolle S., Kundu S., Walton E., Badder L., et al. (2025). Mitigating algal competition with fouling-prevention coatings for coral restoration and reef engineering. ACS Sustain. Chem. Eng. 13, 5808–5817. doi: 10.1021/acssuschemeng.4c07508
Kleypas J., Allemand D., Anthony K., Baker A. C., Beck M. W., Hale L. Z., et al. (2021). Designing a blueprint for coral reef survival. Biol. Conserv. 257, 109107. doi: 10.1016/j.biocon.2021.109107
Mccook L., Jompa J., and Diaz-Pulido G. (2014). Competition between corals and algae on coral reefs: A review of evidence and mechanisms. Coral Reefs 19, 400–417. doi: 10.1007/s003380000129
Montalvo-Proano J., Flores F., Severati A., and Negri A. P. (2025). Fouling release coatings reduce colonisation of coral seeding devices. Sci. Rep. 15, 24023. doi: 10.1038/s41598-025-08268-9
Morrow K. M., Ritson-Williams R., Ross C., Liles M. R., and Paul V. J. (2012). Macroalgal extracts induce bacterial assemblage shifts and sublethal tissue stress in caribbean corals. PloS One 7, e44859. doi: 10.1371/journal.pone.0044859
Negri A. P. and Heyward A. J. (2001). Inhibition of coral fertilisation and larval metamorphosis by tributyltin and copper. Mar. Environ. Res. 51, 17–27. doi: 10.1016/S0141-1136(00)00029-5
Oksanen J., Blanchet F. G., Kindt R., Legendre P., Minchin P. R., O’hara R., et al. (2013). Package ‘vegan’. Community Ecol. package version 2, 1–295.
Page C. A., Giuliano C., and Randall C. J. (2024). Benthic communities influence coral seeding success at fine spatial scales. Restor. Ecol. 32, e14212. doi: 10.1111/rec.14212
Ramsby B. D., Emonnot F., Flores F., Schipper S., Diaz-Pulido G., Abdul Wahab M. A., et al. (2024). Low light intensity increased survival of coral spat in aquaculture. Coral Reefs 43, 627–640. doi: 10.1007/s00338-024-02489-6
Ramsby B. D., Forster R., Ferguson S. N., Haikola P., Randall C. J., Abdul Wahab M. A, et al. (2025). Developing coral seeding devices and rapid deployment methods to scale up reef restoration. Restor. Ecology. doi: 10.1111/rec.70206
Randall C. J., Giuliano C., Allen K., Bickel A., Miller M., and Negri A. P. (2022). Site mediates performance in a coral-seeding trial. Restor. Ecol. 31, e13745.
Randall C. J., Negri A. P., Quigley K. M., Foster T., Ricardo G. F., Webster N. S., et al. (2020). Sexual production of corals for reef restoration in the anthropocene. Mar. Ecol. Prog. Ser. 635, 203–232. doi: 10.3354/meps13206
R Core Team (2020). R: A language and environment for statistical computing. Vienna, Austria. Available online at: https://www.r-project.org/ (Accessed November 19, 2025).
Roberts T. E., Moloney J. M., Sweatman H. P. A., and Bridge T. C. L. (2015). Benthic community composition on submerged reefs in the central great barrier reef. Coral Reefs 34, 569–580. doi: 10.1007/s00338-015-1261-7
Roepke L. K., Brefeld D., Soltmann U., Randall C. J., Negri A. P., and Kunzmann A. (2022a). Antifouling coatings can reduce algal growth while preserving coral settlement. Sci. Rep. 12, 15935. doi: 10.1038/s41598-022-19997-6
Roepke L. K., Brefeld D., Soltmann U., Randall C. J., Negri A. P., and Kunzmann A. (2022b). Applying behavioral studies to the ecotoxicology of corals: A case study on acropora millepora. Front. Mar. Sci. 9, 1002924. doi: 10.3389/fmars.2022.1002924
Roth F., Saalmann F., Thomson T., Coker D. J., Villalobos R., Jones B. H., et al. (2018). Coral reef degradation affects the potential for reef recovery after disturbance. Mar. Environ. Res. 142, 48–58. doi: 10.1016/j.marenvres.2018.09.022
Schmidt-Roach S., Klaus R., Al-Suwailem A. M., Prieto A. R., Charrière J., Hauser C. A. E., et al. (2023). Novel infrastructure for coral gardening and reefscaping. Front. Mar. Sci. 10, 1110830. doi: 10.3389/fmars.2023.1110830
Severati A., Nordborg F. M., Heyward A., Abdul Wahab M. A., Brunner C. A., Montalvo-Proano J., et al. (2024). The autospawner system - automated ex situ spawning and fertilisation of corals for reef restoration. J. Environ. Manage. 366, 121886. doi: 10.1016/j.jenvman.2024.121886
Shafir S., Edwards A., Bongiorni L., Levy G., and Shaish L. (2010). “Constructing and managing nurseries for asexual rearing of corals,” in Reef rehabilitation manual. Coral reef targeted research and capacity building for management program. The Coral Reef Targeted Research & Capacity Building for Management Program, St Lucia, Australia. Available online at: https://gefcoral.Org/portals/53/downloads/reef%20rehabilitation%20manual_web.Pdf (Accessed November 19, 2025).
Smith H. A., Brown D. A., Arjunwadkar C. V., Fulton S. E., Whitman T., Hermanto B., et al. (2022). Removal of macroalgae from degraded reefs enhances coral recruitment. Restor. Ecol. 30 553: 151762. doi: 10.1111/rec.13624
Suggett D. J., Guest J., Camp E. F., Edwards A., Goergen L., Hein M., et al. (2024). Restoration as a meaningful aid to ecological recovery of coral reefs. NPJ Ocean Sustainability 3, 20. doi: 10.1038/s44183-024-00056-8
Suzuki G., Okada W., Yasutake Y., Kai S., Fujikura Y., Tanita I., et al. (2018). Interspecific differences in the post-settlement survival of acropora corals under a common garden experiment. Fisheries Sci. 84, 849–856. doi: 10.1007/s12562-018-1230-5
Tebben J., Guest J. R., Sin T. M., Steinberg P. D., and Harder T. (2014). Corals like it waxed: Paraffin-based antifouling technology enhances coral spat survival. PloS One 9, e87545. doi: 10.1371/journal.pone.0087545
Tebbett S. B. and Bellwood D. R. (2019). Algal turf sediments on coral reefs: What’s known and what’s next. Mar. pollut. Bulletinl 149, 110542. doi: 10.1016/j.marpolbul.2019.110542
Upadhyay V., Galhenage T., Battocchi D., and Webster D. (2017). Amphiphilic icephobic coatings. Prog. Organic Coatings 112, 191–199. doi: 10.1016/j.porgcoat.2017.07.019
Vehtari A., Gelman A., and Gabry J. (2016). Practical bayesian model evaluation using leave-one-out cross-validation and waic. Stat Computing 27, 1413–1432. doi: 10.1007/s11222-016-9696-4
Vermeij M. J. A. (2005). Early life-history dynamics of caribbean coral species on artificial substratum: The importance of competition, growth and variation in life-history strategy. Coral Reefs 25, 59–71. doi: 10.1007/s00338-005-0056-7
Weber F. and Esmaeili N. (2023). Marine biofouling and the role of biocidal coatings in balancing environmental impacts. Biofouling 39, 661–681. doi: 10.1080/08927014.2023.2246906
Whitman T. N., Hoogenboom M. O., Negri A. P., and Randall C. J. (2024). Coral-seeding devices with fish-exclusion features reduce mortality on the great barrier reef. Sci. Rep. 14, 13332. doi: 10.1038/s41598-024-64294-z
Whitman T. N., Jurriaans S., Lefevre C., Sims C. A., Radford B., Puotinen M., et al. (2025). Seeded acropora digitifera corals survive best on wave-exposed reefs with grazing from small fishes. Restor. Ecol. e70016, e70016. doi: 10.1111/rec.70016
Wilson J. and Harrison P. (2005). Post-settlement mortality and growth of newly settled reef corals in a subtropical environment. Coral Reefs 24, 418–421. doi: 10.1007/s00338-005-0033-1
Keywords: coral restoration, antifouling, fouling-release, Great Barrier Reef, coral reef, competition, recruit
Citation: Montalvo-Proano J, Alvarez-Noriega M, Flores F, Severati A and Negri AP (2025) Fouling-release coatings enhance Acropora loripes coral spat survival by limiting algal competition on seeding devices. Front. Mar. Sci. 12:1684011. doi: 10.3389/fmars.2025.1684011
Received: 12 August 2025; Accepted: 11 November 2025; Revised: 04 November 2025;
Published: 01 December 2025.
Edited by:
Jesús Ernesto Arias González, National Polytechnic Institute of Mexico (CINVESTAV), MexicoReviewed by:
Maggy Nugues, Université Paris Sciences et Lettres, FranceZahra Karimi, Cedars-Sinai, United States
Copyright © 2025 Montalvo-Proano, Alvarez-Noriega, Flores, Severati and Negri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jose Montalvo-Proano, ai5tb250YWx2b3Byb2Fub0BhaW1zLmdvdi5hdQ==
 Jose Montalvo-Proano
Jose Montalvo-Proano Mariana Alvarez-Noriega
Mariana Alvarez-Noriega Florita Flores
Florita Flores Andrea Severati
Andrea Severati Andrew P. Negri
Andrew P. Negri