- 1Department of Earth and Planetary Sciences, The University of Texas, Austin, TX, United States
- 2Alligator Head Foundation, Port Antonio, Jamaica
- 3Mangroves Plus Project, The Forestry Department, Government of Jamaica, Kingston, Jamaica
- 4Department of Chemistry, The University of the West Indies, Mona, Kingston, Jamaica
The East Portland Special Fishery Conservation Area (EPSFCA) is a no-take marine reserve in northeast Jamaica, established in 2016. The region is historically understudied and lacks ecological data critical for evaluating conservation outcomes. This study uses monitoring data collected with Global Coral Reef Monitoring Network methods to quantify changes in benthic, fish, and invertebrate communities from 2017 to 2024 and to evaluate the influence of herbivores on reef recovery. Results indicate that fish size and abundance increased between 2017 and 2019 following active enforcement, but these gains declined by 2022 as patrols decreased. In contrast, benthic assemblages showed continued degradation; coral cover declined to ~1% by 2024 alongside rising macroalgae and loss of coralline algae, reflecting disease and heat-stress impacts and a shift toward disturbance-tolerant species. Herbivores such as Diadema antillarum and parrotfish were key in limiting macroalgal dominance, although the intensity of their impact differed among sites. Differences in community composition reflect site-specific variation, indicating that local conditions influence recovery dynamics within the sanctuary. Overall, the EPSFCA demonstrates that consistent enforcement and herbivore protection can promote partial but fragile reef recovery, emphasizing the need for sustained management to rebuild resilience. These results provide a rare long-term assessment for Jamaica’s northeast coast and offer a benchmark for evaluating future conservation outcomes.
1 Introduction
Caribbean coral reefs are biodiversity hotspots in the ocean (Roberts et al., 2002); they support high species richness through complex interactions of biotic processes, such as herbivory and competition that vary spatially. Fringing reefs dominate the region, alongside atolls, barrier, bank, and patch reefs (Souter et al., 2021). Important corals in the Caribbean include photosynthetic Scleractinia, such as Acropora spp., Orbicella spp., and Diploria labyrinthiformis, which build structural complexity on reefs (González-Barrios and Álvarez-Filip, 2018).
Caribbean reefs have undergone major ecological reorganizations over recent decades, driven by compounding global and local stressors. Climate-driven warming, coral bleaching, disease outbreaks and overfishing have reduced coral cover and altered the relative abundances of reef-building and algal taxa, leading to widespread phase shifts from coral to algal dominance (Gardner et al., 2003; Bellwood et al., 2004; Jackson et al., 2014; Hughes et al., 2023). The loss of key herbivores, including the 1980’s die-off of Diadema antillarum (Lessios, 1988; Alvarez-Filip et al., 2022) and the continued removal of grazing fish (Jackson et al., 2001) have further weakened top-down control on macroalgae, intensifying competition with corals.
These region-wide ecological shifts are particularly evident in Jamaica, where decades of fishing pressure and storm impacts have led to Jamaica’s reefs becoming among the most degraded in the Caribbean (Gardner et al., 2003; National Environment and Planning Agency, 2021). Most research has centered on Discovery Bay, while other regions remain poorly characterized despite their ecological and socio-economic importance.
Recent conservation initiatives have aimed to reverse reef degradation through locally managed marine protected areas. One such effort is the East Portland Special Fishery Conservation Area (EPSFCA, herein referred to as ‘the sanctuary’) in northeast Jamaica (Figure 1). Baseline assessments before the establishment of the sanctuary (2016) were conducted by The University of the West Indies, Mona to determine the sanctuary location (Buddo et al., 2014). The study found low fish populations due to overfishing, particularly spearfishing, which had resulted in the near complete absence of adult fish. This area is now a “no-take zone”, where no fishing is permitted, and includes a nursery for fish and other marine life (Buddo et al., 2014). Prior to the Buddo et al. (2014) study, there were no coordinated assessments of reef community composition, herbivore populations, or other ecological indicators. This lack of systematic ecological information limits the ability to design effective management strategies for the EPSFCA. Understanding how community composition and herbivore dynamics respond to protection across spatial and temporal scales is critical for guiding restoration.
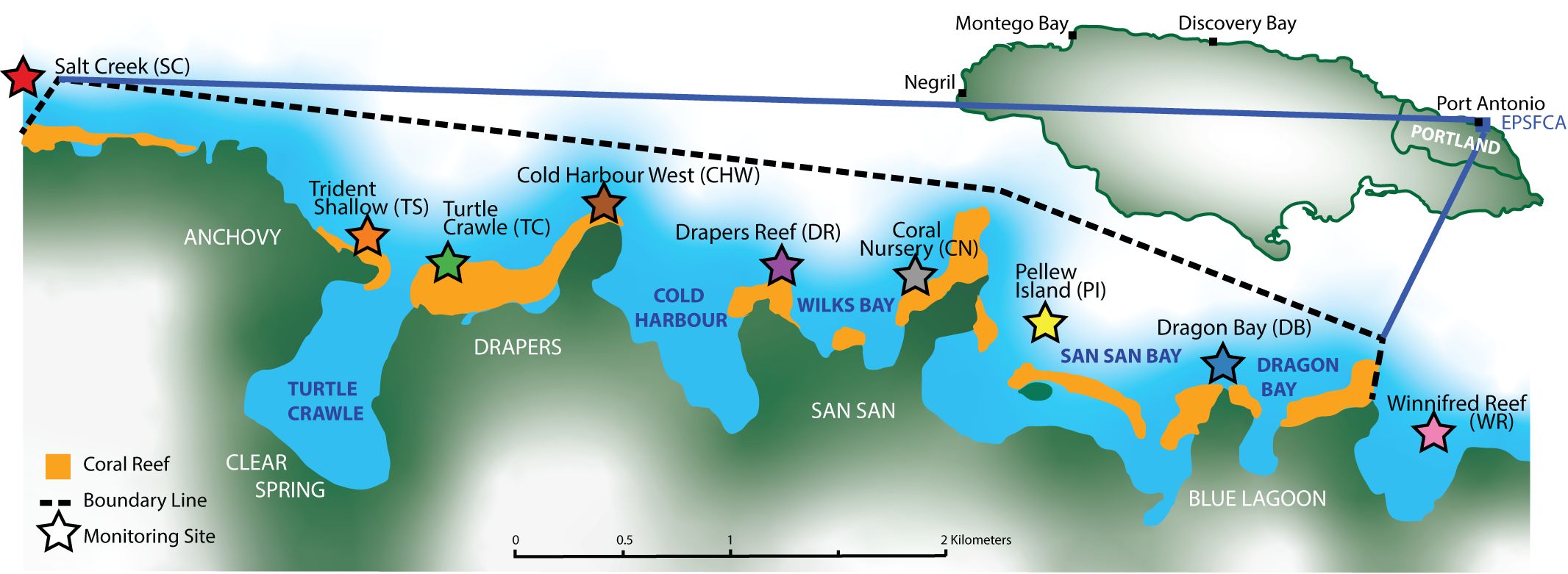
Figure 1. Map of the East Portland Special Fishery Conservation Area (EPSFCA), northeast Jamaica, showing the sanctuary boundary (dashed line) and monitoring sites surveyed in the study (stars; colors correspond to subsequent site-specific figures). Coordinates for the sanctuary are approximately 18°10’49.10”N and 76°24’21.47”W. The scale bar indicates distance in kilometers; north is up. Modified from the Alligator Head Foundation website.
This manuscript synthesizes recent (2017-2024) ecological data to determine the variation of community composition within and outside the EPSFCA. We also document how coral reef communities have changed since the creation of the sanctuary in 2016, with a particular focus on the abundance and importance of herbivores in the region. Specifically, this study addresses the following questions: (1) How have benthic, fish, and invertebrate communities within the EPSFCA changed since the sanctuary’s establishment? (2) How do community compositions differ among sites, and between areas inside and outside the sanctuary? (3) To what extent do herbivorous fish and urchin species influence macroalgal abundance? Together, these questions evaluate whether protection within the EPSFCA is promoting reef recovery and identify the ecological processes shaping community change.
2 Materials and methods
2.1 Study area
The EPSFCA is a 6 km2 area in east Portland (Figure 1) managed by the Alligator Head Foundation (AHF), a non-governmental organization that aims to protect fish stocks, restore habitat, and regenerate local economies. Fishing is an important resource with nearly 2000 fishermen and over 500 fishing vessels active in the area (Wade et al., 2023).
The EPSFCA consists of gently sloping fringing spur and groove reefs on a narrow shelf (Ford et al., 2014). Since the creation of the sanctuary in 2016, nine monitoring sites have been established, Salt Creek (SC), Trident Shallow (TS), Turtle Crawle (TC), Cold Harbour West (CHW), Drapers Reef (DR), Coral Nursery (CN), Pellew Island (PI), Dragon Bay (DB), and Winnifred Reef (WR; Figure 1). Most of the sites are within the sanctuary but Winnifred Reef and Salt Creek are outside of the boundaries (Figure 1). Six of the nine sites have been monitored since 2017, with Trident Shallow, Salt Creek, and Winnifred Reef added in 2018 (Table 1).
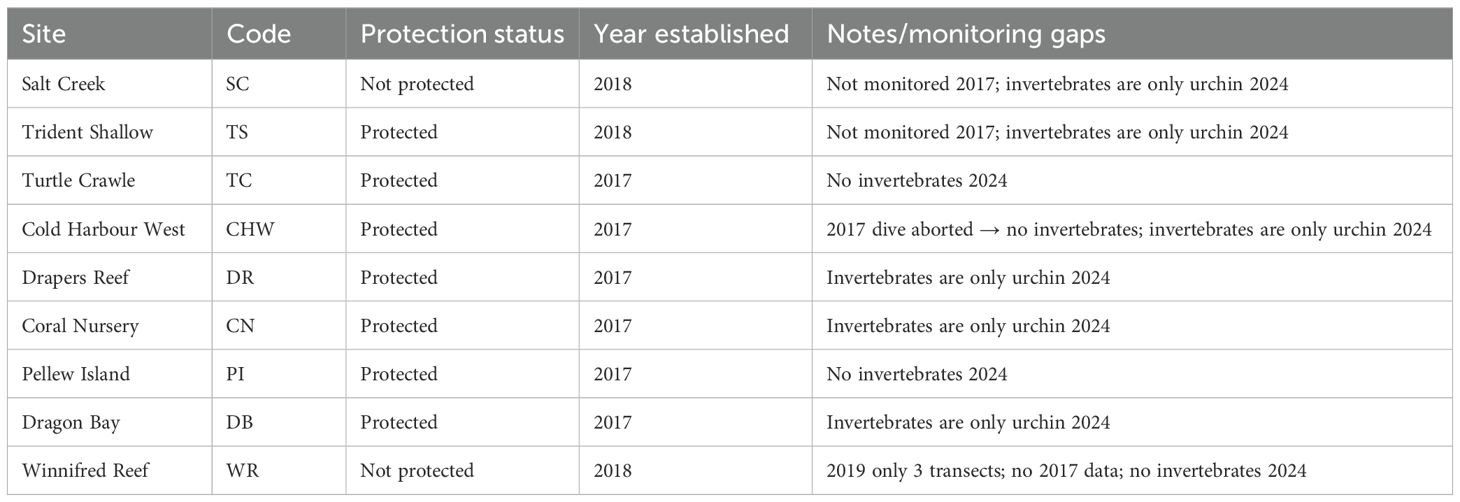
Table 1. Monitoring sites within and adjacent to the East Portland Special Fishery Conservation Area (EPSFCA), showing establishment year and major sampling gaps.
2.2 Field data collection
Community composition data were collected in November 2017, October 2018, May 2019, June 2022, and July 2024; the break in sample collection was due to the COVID-19 pandemic. The methods used herein are based on those used by the Global Coral Reef Monitoring Network (GCRMN). The GCRMN method is used throughout the Caribbean to collect community composition data and describes six elements of the coral reef ecosystem: 1) abundance and biomass of key reef fish taxa, 2) relative cover of reef-building organisms and their dominant competitors, 3) assessment of coral health, 4) recruitment of reef-building corals, 5) abundance of key macroinvertebrate species, and 6) water quality (GCRMN-Caribbean Steering Committee, 2016). The sanctuary specifically collected elements 1 through 5, and to investigate the community composition of this area and how it has changed since the creation of the sanctuary, this project focuses on the data from parts 1, 2 and 5.
Five 30 meter transect lines were laid out at each reef monitoring site (Figure 1). These transects were laid in a straight line by the diver assessing fish abundance (GCRMN Part 1). The diver recorded the type of fish seen, and, when possible, the juvenile stage and size. Data calculated were fish average density (#/100 m2) and average size (cm). Fish surveys were conducted by trained conservation practitioners, including experienced local fishers, under the Atlantic and Gulf Rapid Reef Assessment (AGRRA) protocol. Monitoring priorities within the sanctuary emphasized ecologically and economically important taxa, and observers consistently focused identification effort on these functional groups. As a result, these groups were recorded accurately across years, whereas other species were not consistently enumerated. Therefore, analyses center on the key groups reliably identified with consistent taxonomic resolution (Supplementary Table 4), reflecting both the sanctuary’s management objectives and data comparability across survey years. The fish groups focused on for this study are angelfish, butterflyfish, grunts, parrotfish, snappers, surgeonfish, and wrasse (Supplementary Table 4).
To assess community composition (GCRMN Part 2), a photo quadrat was taken every two meters along each transect. The 0.9 m x 0.6 m photo quadrats were processed using Coral Point Count with Excel extensions (CPCE) software (Kohler and Gill, 2006) where 30 random points were put on each photograph and the benthic species at each point were identified (see Supplementary Tables 1, 2 for list of groups identified). This estimates the percent of the reef bottom with stony corals, gorgonians, sponges, and various types of algae. Potential inconsistencies created by the changeover of personnel performing identifications was mitigated by communication between personnel to ascertain reliably accurate groups and organizing by genera or group level when required for consistency (Supplementary Tables 1, 2).
To maintain consistency in benthic taxa and fish identifications despite personnel changes across survey years, we followed standardized regional protocols (GCRMN for benthic photo quadrats; AGRRA for fish). Prior to each survey season, observers were trained using reference photo quadrats and previous-year datasets under the supervision of the EPSFCA research coordinator (D. Henry). Benthic photo quadrat labels were synchronized across years using a single grouping framework to ensure consistent taxonomic resolution despite occasional differences in species-level identification. When taxa were not recorded consistently or could not be reliably distinguished in imagery, data were aggregated to the genus or functional-group level following GCRMN classification (see Supplementary Table 1).
For the invertebrate analysis (GCRMN Part 5), a diver swam the length of the transect recording how many invertebrates were seen within three meters of the transect. GCRMN recommends recording urchins and sea cucumbers, but the invertebrates recorded for the EPSFCA were increased to include other important invertebrates in the sanctuary. Specifically, conch, long spine urchin (Diadema antillarum), other urchins (predominantly Tripneustes ventricosus, Eucidaris tribuloides, Echinometra lucunter, and Echinometra viridis), lobster, and sea cucumber were recorded. This study focuses on D. antillarum and other urchins. Counts were standardized to density as the number of individuals per 180 m² (the 30 m × 6 m survey belt) to ensure comparability among transects and years.
Because the goal of data collection is monitoring, the transects were conducted in roughly the same location on the reef, but there are differences in the precise start and end position from year to year. Every site consistently had five transects with few exceptions (Table 1): Cold Harbour West had a single transect and recorded no invertebrate data in 2017 (dive had to be aborted), and Winnifred Reef had only three transects in 2019 (camera malfunction). When analyses are run including invertebrates (regressions), Cold Harbour West 2017 is removed. Furthermore, Salt Creek, Winnifred Reef, and Trident Shallow were not monitored in 2017 in any capacity. Fish analyses were not run in 2024 due to a lack of personnel, and urchins (D. antillarum and other) were the only invertebrates monitored at a subset of sites (Salt Creek, Trident Shallow, Cold Harbour West, Drapers Reef, Coral Nursery, and Dragon Bay).
2.3 Variance over time and between sites within the sanctuary
Benthic transect data were summarized into percent cover. This information was compiled into a data frame, and the Bray-Curtis dissimilarity index was calculated between all transects and years (2017, 2018, 2019, 2022, 2024). The Bray and Curtis (1957) metric is particularly appropriate for abundance matrices (Faith et al., 1987; Ricotta and Podani, 2017) and calculates a dissimilarity index ranging from 0 to 1. Prior to ordination, data were Hellinger-transformed to reduce the influence of dominant taxa and make the dataset suitable for Euclidean-based methods (Legendre and Gallagher, 2001). The dissimilarity index was then put into a non-metric multidimensional scaling (NMDS) ordination to determine differences in sanctuary composition between sites and from year to year. NMDS ordinations were run with dimensions from 0–10 to determine the optimal number of dimensions of the NMDS plot, and the stress value was utilized to determine how well the result represented the original dissimilarities. A Detrended Correspondence Analysis (DCA) was run in addition to see if trends were consistent across methods.
Differences between sites and across years were investigated using analysis of variance (ANOVA; Girden, 1992), and the non-parametric ANOVA, Kruskal-Wallis (Kruskal and Wallis, 1952). Dependent variables were fish size and density, benthic community abundance, and invertebrate counts. ANOVA is a robust test that is not strongly influenced by non-normal distributions of data (Blanca et al., 2017), but it does assume normality in the data. ANOVA was used when residuals were approximately normally distributed and variances were homogenous as verified by skewness and kurtosis values between -2 and 2 (Supplementary Table 3). When these assumptions were violated, the Kruskal-Wallis test was used as a distribution-free alternative. Post-hoc tests were conducted on significant findings, using Tukey’s honest significant difference (HSD; Tukey, 1949) for ANOVA, and Dwass-Stell-Crithlow-Fligner (DSCF; Dwass, 1960; Steel, 1960; Critchlow and Fligner, 1991) for Kruskal-Wallis.
2.4 Importance of herbivores
Linear regressions were run to determine if percent cover of macroalgae was correlated with herbivorous fish or invertebrate species (parrotfish, D. antillarum, other urchins, and surgeonfish). Binary (dummy) variables were created to represent categorical variables, to allow site and year to be included. Initially, regressions were run as additive models, and then interactions between two herbivores were considered in subsequent models. Model fit was evaluated using the adjusted coefficient of determination (adj.R²). The change in model explanatory power attributable to herbivores (Δadj.R²) was calculated as the difference in adjusted R² between full models (including herbivores, site, and year) and reduced models containing only site and year. Multicollinearity among predictors was assessed using Variance Inflation Factors (VIF).
3 Results
Data with skew and kurtosis values indicate parametric ANOVA can be conducted on Dictyota abundance, Lobophora abundance, Sargassum abundance, Halimeda abundance, parrotfish count, total fish count, and the sizes of angelfish, butterflyfish, grunt, parrotfish, snapper, and surgeonfish (Supplementary Table 4). The rest of the analyses were done with the nonparametric Kruskal-Wallis analyses.
3.1 Community change since sanctuary creation
The benthic composition of the EPSFCA shifted from year to year since the creation of the sanctuary. The four-dimensional NMDS (stress = 0.082; mean R² = 0.005; Spearman ρ = 0.975), indicates a shift from assemblages with comparatively greater coral and coralline algae in 2017–2019 to assemblages with higher macroalgae, sponges, and gorgonians by 2022–2024 (Figure 2A). These trends were consistent in the DCA plots (Supplementary Figure 1). Year-specific NMDS plots illustrating community composition for each survey year (2017–2024) are provided in Supplementary Figure 2.
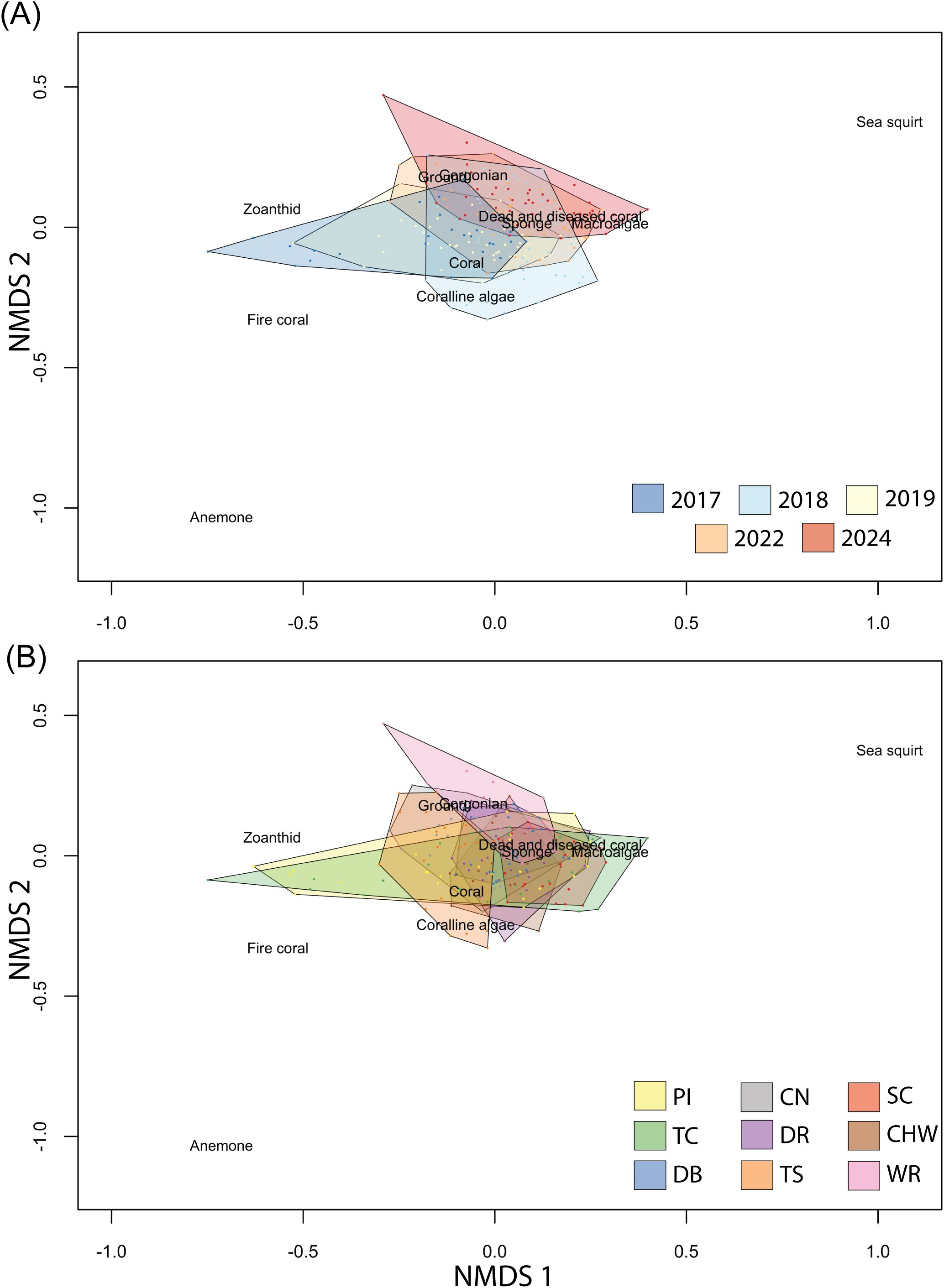
Figure 2. Non-metric multidimensional scaling (NMDS) ordination of reef benthic community composition based on Bray–Curtis dissimilarities of species abundance data. Each point represents a single 30 m transect survey (n = 5 per site per year, except where noted), with spatial proximity in the ordination space reflecting similarity in species composition. Species groupings are overlaid to indicate the relative position of different taxa within the multivariate space. In (A), points are colored by year, illustrating temporal shifts in community structure. In (B), points are colored by site (Figure 1), showing spatial variation in assemblage composition. The NMDS was conducted in four dimensions and yielded a stress value of 0.082, mean R² = 0.005; Spearman ρ = 0.975 indicating a good representation of the original dissimilarities. See Supplementary Files for plots disaggregated by site and year.
The dominant coral taxa in the sanctuary include Agaricia sp., Porites astreoides, Orbicella sp., branching Porites sp., Siderastrea siderea, and Siderastrea radians. Percent abundance of coral cover declined from 7.98 (±5.39) in 2017 to 1.58 (±1.25) in 2024 and significantly varied across years 2017 through 2024 (χ² = 76.08, p < 0.0001). Coral cover in 2018 (p = 0.02), 2022 (p = 0.001) and 2024 (p < 0.0001) were significantly lower than in 2017, whereas 2018 and 2019 did not differ significantly (p > 0.05, Figure 3A). Coralline algae also varied significantly across these years (χ² = 95.56, p < 0.0001). There were decreases from 2017 to 2018 (p = 0.04), increases 2018 to 2019 (p = 0.02), and decreases 2019 to 2022 (p < 0.0001) in coralline algae abundance; abundance remained low into 2024 (Figure 3B).
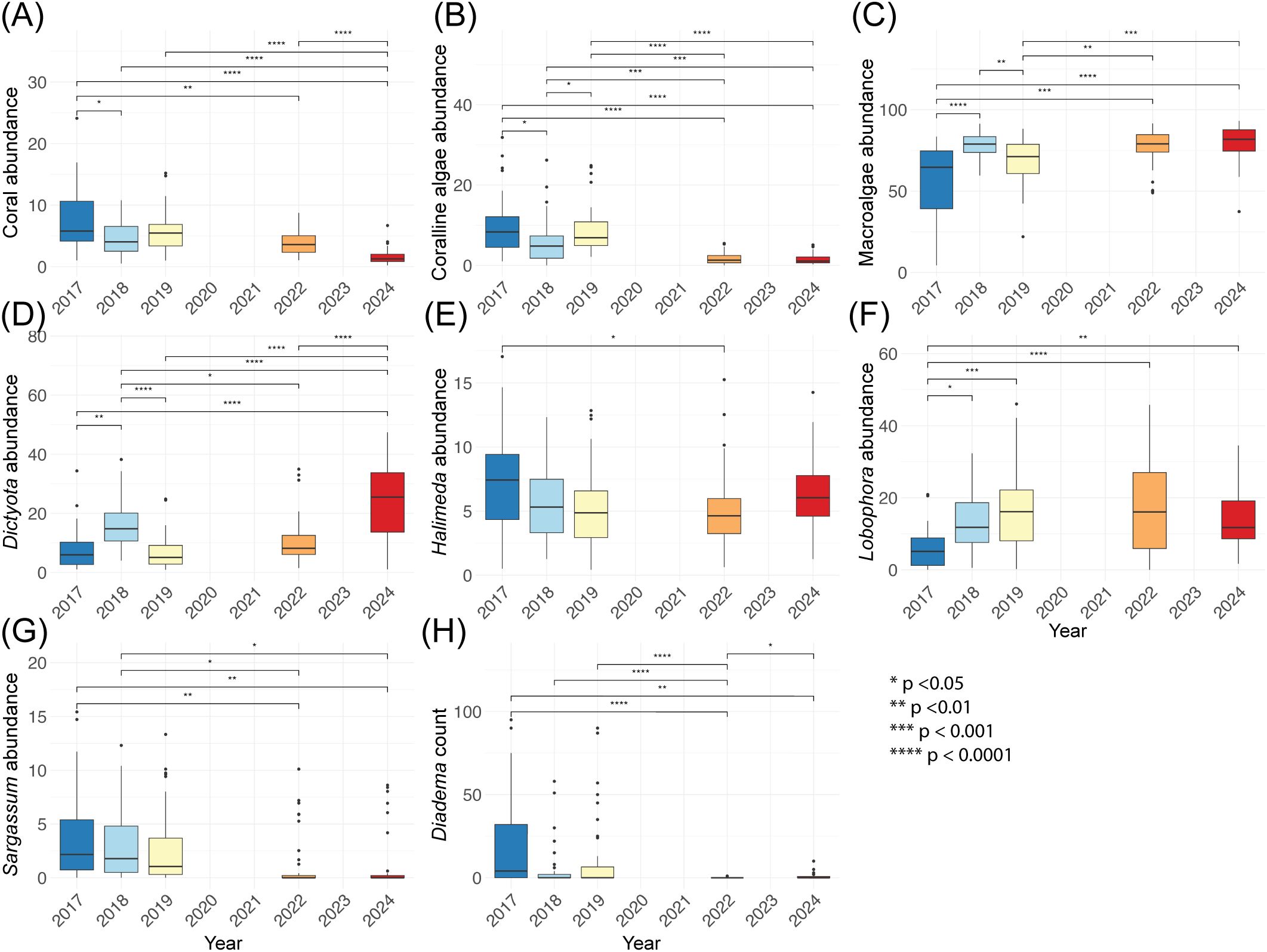
Figure 3. Boxplots of benthic community data across survey years in the East Portland Special Fishery Conservation Area, Jamaica. Panels show percent abundance of (A) coral, (B) coralline algae, (C) macroalgae, (D) Dictyota, (E) Halimeda, (F) Lobophora, (G) Sargassum, and (H) D. antillarum density (individuals per 180 m², the 30 m × 6 m survey belt). Each box summarizes transect-level data from all monitored sites (missing data provided in Table 1). Boxes represent the interquartile range (25th–75th percentile), horizontal lines indicate medians, and whiskers denote 1.5 × the interquartile range (IQR). Significant differences between year, as determined by one-way ANOVA or Kruskal-Wallis with appropriate post hoc comparisons, are indicated with asterisks (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
Total macroalgae varied significantly from 2017 to 2024 (χ² = 44.11, p = < 0.0001). Macroalgae has increased from 56.9% (±23.3) in 2017 to 77.2% (±11.0) in 2022 (p < 0.0001), although there was a small reduction of macroalgae from 2018 to 2019, returning to 2017 levels (p = 0.31). Macroalgae remained high in 2024 at 79.6% (±11.0). Specifically, within algal genera, Dictyota follows the same trend as the grouped macroalgae (F = 29.33, p < 0.0001), Lobophora increased from 2017 to 2018 and remained high over time (F = 6.54, p < 0.0001; 2017<2018 p = 0.033). In contrast, Sargassum abundance declined in 2022 (F = 5.52; p < 0.0001; 2018–2022 p = 0.026). Halimeda exhibited weaker temporal variation (F = 2.73; p = 0.030) and showed no consistent temporal trend (Figures 3D–G).
Examining reef-dwelling urchins, D. antillarum varied from 2017 to 2024 (χ² = 41.85, p < 0.0001) with a loss of D. antillarum in 2022 (2019 > 2022 p < 0.0001); only two urchins were observed in 2022 compared with the hundreds seen in previous years. There is significant recovery of D. antillarum by 2024 (2024 > 2022 p < 0.0001) to values similar to 2018 (p = 0.36; Figure 3H). Other urchins also differed among years (χ² = 10.28, p = 0.036), with lower counts in 2022 relative to 2017 (p = 0.0085) but no significant differences thereafter (2022–2024 p = 0.37).
Fish abundance and count varied strongly among years observed (2017–2022). Overall, fish abundance had no clear recovery after an initial 2017 to 2018 increase. Total fish counts rose from 2017 to 2018 (F = 22.48, p < 0.0001) following sanctuary establishment, declined from 2018 to 2019 (p < 0.0001), and again recovered to 2018 levels in 2022 (2018–2022 p = 0.13). This pattern of initial recovery is seen in many fish groups; parrotfish, surgeonfish, and snapper count increase from 2017 to 2018 (parrotfish F = 33.06; surgeonfish χ² = 61.33, snapper χ² = 9.3, ps <0.0001, parrotfish and surgeonfish 2017–2018 ps <0.0001, snapper 2017–2018 p = 0.03). In 2019, fish group abundances either drop to 2017 levels (parrotfish, surgeonfish, and butterflyfish 2019–2017 ps > 0.05), or remain at 2018 values into 2019 with no further recovery (snapper 2018–2019 ps > 0.05). Of the groups investigated, only wrasse see increase in abundance from 2019 to 2022 (χ² = 64.44 p < 0.0001, 2019–2022 p < 0.0001), despite the overall finding of total fish count increasing from 2019 to 2022. Prior to 2022, wrasse populations had been declining (2017–2019 p < 0.0001). Angelfish and butterflyfish remained rare throughout (Figure 4).
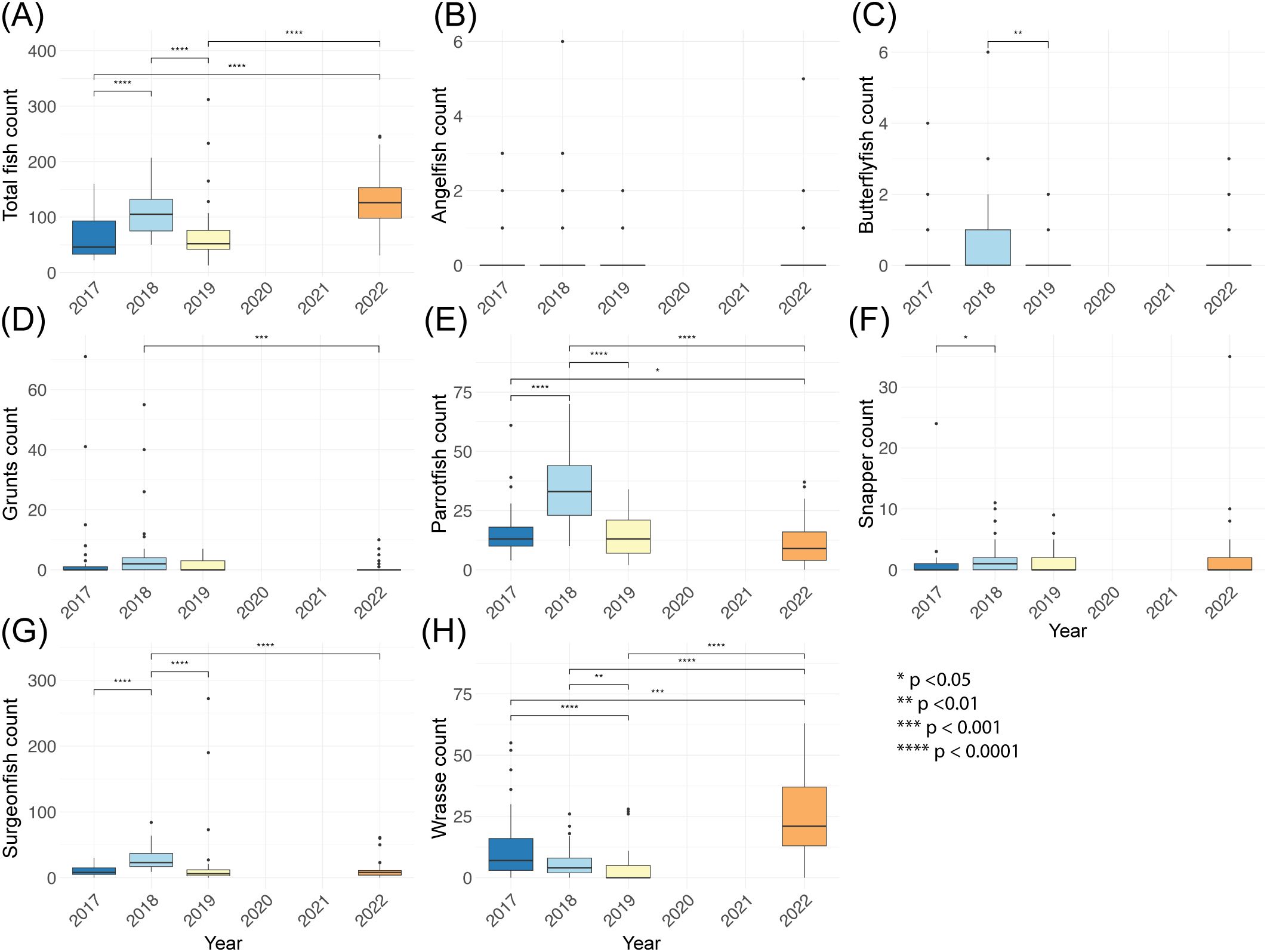
Figure 4. Boxplots of fish average density (#/100 m2) across survey years in the East Portland Special Fishery Conservation Area, Jamaica. Panels show average density (#/100 m2) of (A) total fish, (B) angelfish, (C) butterflyfish, (D) grunt, (E) parrotfish, (F) snapper, (G) surgeonfish, and (H) wrasse. Each box summarizes transect-level data from all monitored sites (missing data provided in Table 1). Boxes represent the interquartile range (25th–75th percentile), horizontal lines indicate medians, and whiskers denote 1.5 × the IQR. Significant differences between year, as determined by one-way ANOVA or Kruskal-Wallis with appropriate post hoc comparisons, are indicated with asterisks (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
In contrast, overall fish size increased from 2017 to 2019 but this gain was lost by 2022. Size increases were significant from 2017 into 2019 for parrotfish (F = 64.55, p < 0.0001; 2017–2019 p < 0.0001), surgeonfish (F = 48.20, p < 0.0001; 2017–2019 p = < 0.0001), grunt (F = 12.35, p < 0.001; 2017–2019 p = 0.002), and wrasse (χ² = 50.97, p < 0.0001; 2017 to 2019 p = 0.006). Fish size is smaller in 2022 than 2019 for almost all observed groups (angelfish, grunt, parrotfish, snapper, surgeonfish, and wrasse ps < 0.005). Butterflyfish size remained constant from 2017 to 2022 (F = 0.45, p = 0.72; Figure 5).
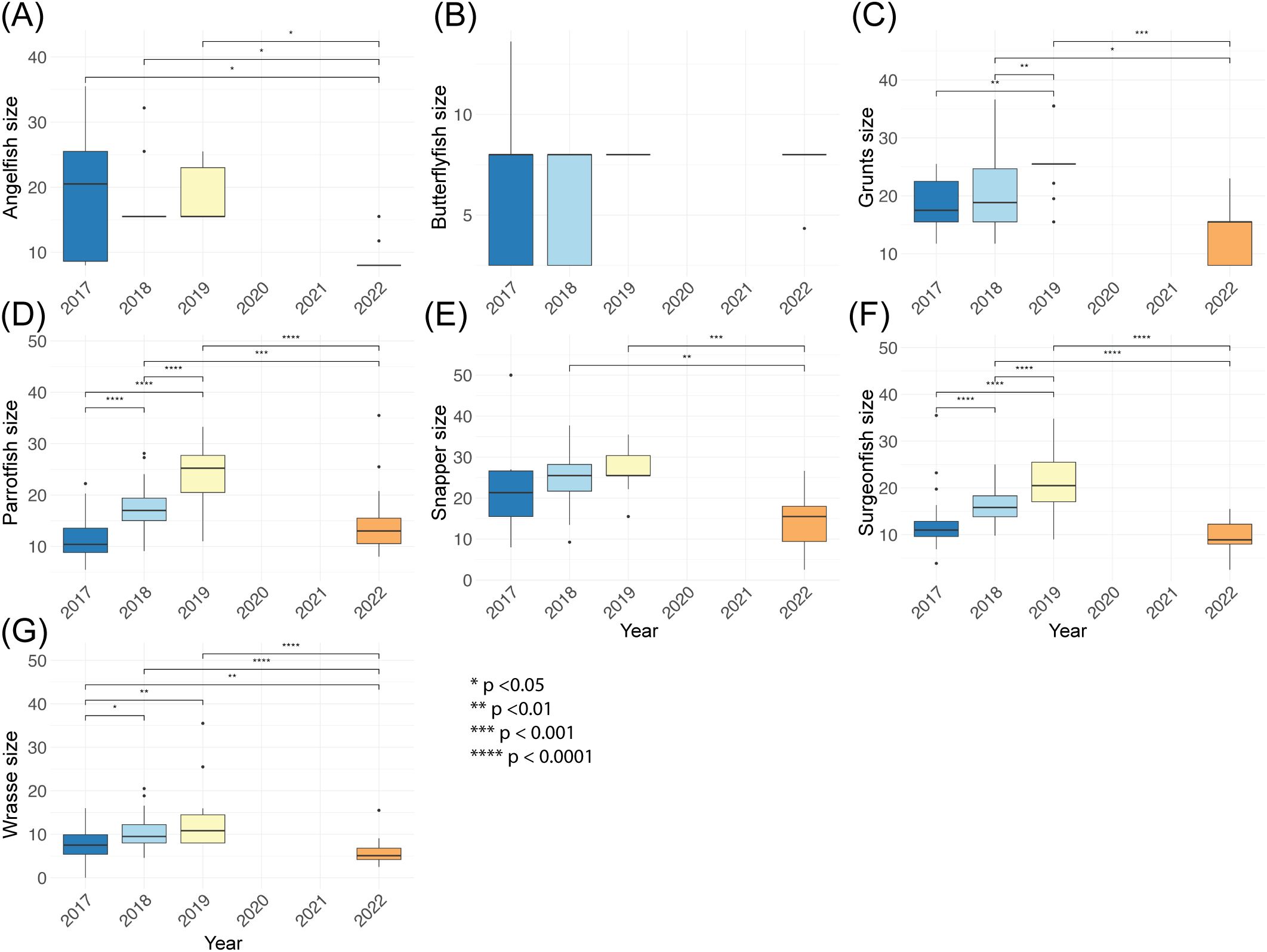
Figure 5. Boxplots of fish size (cm) across survey years in the East Portland Special Fishery Conservation Area, Jamaica. Panels show size of (A) angelfish, (B) butterflyfish, (C) grunt, (D) parrotfish, (E) snapper, (F) surgeonfish, and (G) wrasse. Each box summarizes transect-level data from all monitored years (missing data provided in Table 1). Boxes represent the interquartile range (25th–75th percentile), horizontal lines indicate medians, and whiskers denote 1.5 × the IQR. Significant differences between sites, as determined by one-way ANOVA or Kruskal-Wallis and appropriate post hoc tests, are indicated with asterisks (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
3.1.1 Summary of temporal variability
Since sanctuary establishment, coral and coralline algae cover have declined while macroalgae, particularly Dictyota and Lobophora, expanded to dominate much of the benthos. Urchin populations collapsed in 2022, and the initial increase in fish abundance observed in 2018 and fish size observed in 2019 was not sustained in later years. These temporal patterns were consistent despite variation in site coverage (Table 1). To assess robustness, all analyses were repeated using only sites monitored across all survey years; the direction of effects remained unchanged, with only minor shifts in significance values and effect magnitudes (Supplementary Figures 3–5). For full boxplots of each site for each year, see Supplementary Figure 6.
3.2 Spatial variation and sanctuary effects
In addition to temporal shifts, benthic community composition also varies by geographic location (Figure 2B). Notably, Winnifred Reef has less coral than other sites, and Turtle Crawle and Pellew Island have broader compositions with more zoanthid hexacorals (for individual site NMDS polygons see Supplementary Figure 7). These trends were consistent in the DCA plots (Supplementary Figure 3).
Although there were site-level differences in coral abundance (χ² = 32.05, p < 0.0001), pairwise DSCF tests show no consistent patterns across the sanctuary (most p > 0.05), apart from higher coral cover at Pellew Island, especially compared to Winnifred Reef (p = 0.006; Figure 6A). Within each year, coral cover did not significantly vary between sites (Supplementary Figure 4, p > 0.05). Similarly, coralline algae vary modestly among sites (χ² = 22.19, p = 0.0046) with few consistent differences (most p > 0.1). The only notable contrasts were slightly higher coralline algae cover at Trident Shallow and Turtle Crawle relative to Winnifred Reef (ps = 0.022–0.034; Figure 6B). Also, there are no significant differences in fish communities between sites (angelfish, butterflyfish, grunt, parrotfish, snapper, surgeonfish and wrasse count and size ANOVA and Kruskal-Wallis p > 0.05; Supplementary Figures 8, 9).
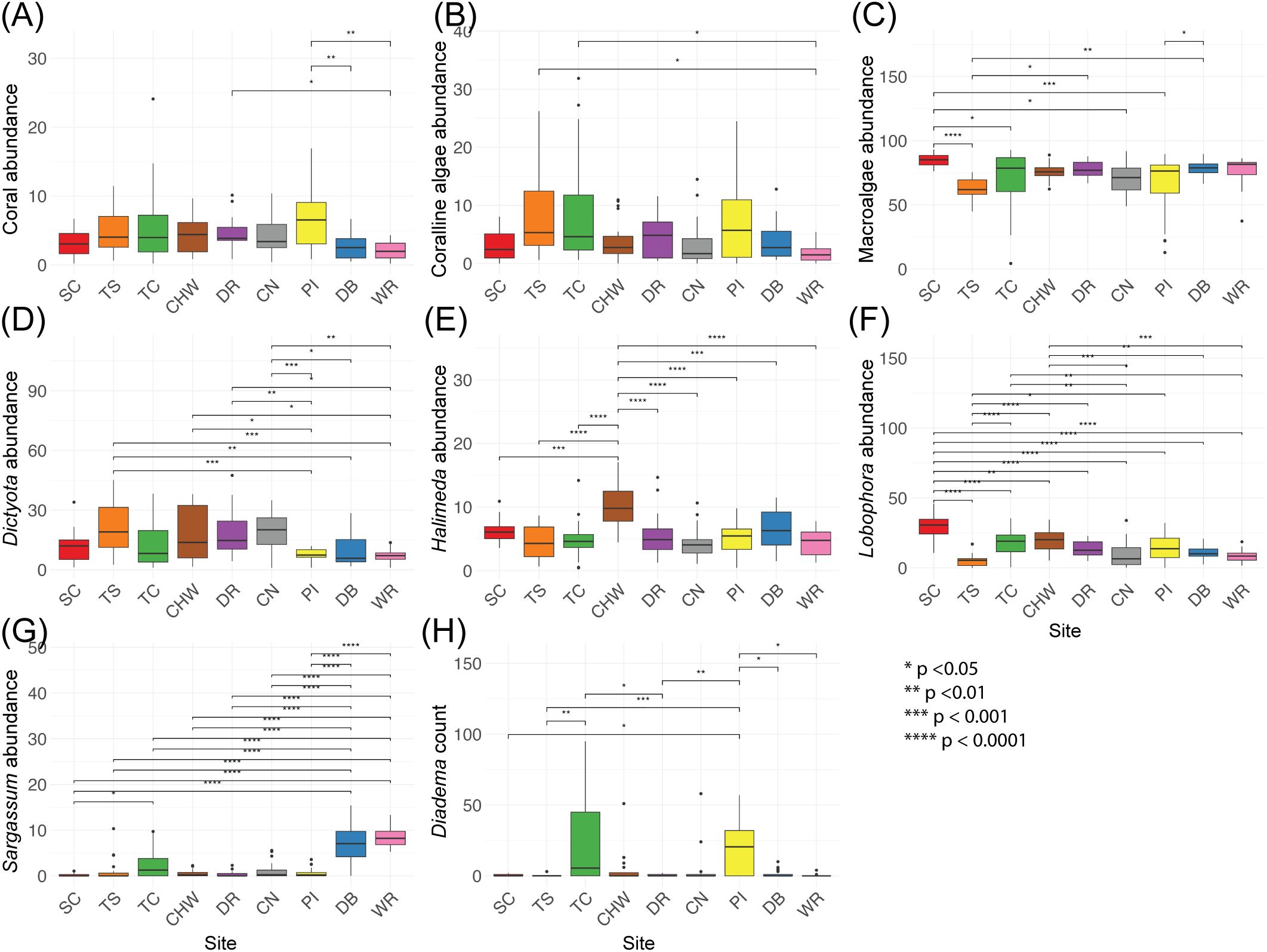
Figure 6. Boxplots of benthic community data across survey sites in the East Portland Special Fishery Conservation Area, Jamaica. Panels show percent abundance of (A) coral, (B) coralline algae, (C) macroalgae, (D) Dictyota, (E) Halimeda, (F) Lobophora, (G) Sargassum, and (H) D. antillarum density (individuals per 180 m², the 30 m × 6 m survey belt). Each box summarizes transect-level data from all monitored years (missing data provided in Table 1). Boxes represent the interquartile range (25th–75th percentile), horizontal lines indicate medians, and whiskers denote 1.5 × the IQR. Significant differences between sites, as determined by one-way ANOVA or Kruskal-Wallis and appropriate post hoc tests, are indicated with asterisks (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
The main group that is driving differences between sites is the macroalgae (χ² = 48.70, p < 0.0001) with the highest values at Salt Creek (p < 0.026 vs. all sites except Turtle Crawle; Figure 6C). Within macroalgae, Lobophora is mainly found at Salt Creek (F = 17.30, p < 0.0001; ps < 0.005 vs. all sites), contributing to the overall trend of high macroalgae cover there (Figures 6C, F). Dictyota cover also varied among sites (F = 6.51, p < 0.0001) and is the lowest at sites on the east side of the sanctuary (Pellew Island, Dragon Bay, and Winnifred Reef; p < 0.03 compared to Trident Shallow and Coral Nursery; Figure 6D). In contrast, Sargassum is found most abundantly at the east side of the sanctuary at Dragon Bay and Winnifred Reef (F = 36.07, p < 0.0001; ps < 0.0001 compared to all sites; Figure 6G). Halimeda is found most abundantly at Cold Harbour West (F = 9.55, p < 0.0001; ps < 0.0004 compared to other sites; Figure 6E).
Urchin abundance also differs between sites (D. antillarum: χ² = 38.81, p < 0.0001; other urchins: χ² = 37.40, p < 0.0001) and mirrored benthic heterogeneity. D. antillarum are most abundant at Pellew Island (ps = 0.01–0.042 vs. Dragon Bay, Drapers Reef, Salt Creek, Trident Shallow, and Winnifred Reef) and Turtle Crawle (ps = 0.005–0.0496 compared to Trident Shallow and Drapers Reef; Figure 6H). Other urchins similarly have highest abundance at Turtle Crawle (ps = 0.003–0.04 vs. Coral Nursery, Dragon Bay, Drapers Reef, and Trident Shallow).
3.2.1 Summary of spatial variability
Overall, areas with more urchins, such as Pellew Island and Turtle Crawle, tended to have less macroalgae and more coral, while macroalgae-dominated sites like Salt Creek and Winnifred Reef supported fewer corals and urchins. Pellew Island stood out for its higher coral and urchin abundance, Trident Shallow and Turtle Crawle for modestly higher coralline algae, and Salt Creek for dense Lobophora. In contrast, Sargassum was concentrated at eastern sites, and Halimeda was most abundant at Cold Harbour West. These patterns remained unchanged when analyses were restricted to the complete survey years (2018–2024; Supplementary Figure 10), indicating that site-level variation is robust to temporal coverage and reflects persistent ecological structure within the sanctuary.
3.3 Ecological interactions
Herbivore abundances were strong predictors of benthic algal composition. Across taxa, D. antillarum consistently reduced macroalgae, whereas parrotfish exerted a mix of suppressive and facilitative effects depending on algal type. For Dictyota, both D. antillarum and parrotfish were significant negative predictors (β = −0.09 ± 0.04, p = 0.02; β = −0.09 ± 0.05, p = 0.04), indicating parallel grazing pressure (adj.R² ≈ 0.4). Variance partitioning of additive models showed that herbivores explained an additional ~2% of Dictyota variance beyond site and year (Δadj.R² = 0.0197). Halimeda declined with D. antillarum (β = −0.06 ± 0.01, p < 0.001) but increased with parrotfish (β = +0.06 ± 0.02, p < 0.001; adj.R² = 0.41), with herbivores accounting for ~14.7% additional variance (Δadj.R² = 0.1473); whereas Lobophora was positively related to parrotfish (β = +0.12 ± 0.05, p = 0.015) and negatively related to D. antillarum (β = −0.10 ± 0.04, p = 0.027; adj.R² ≈ 0.61) but herbivores added a modest ~3.7% beyond site and year (Δadj.R² = 0.0370). Sargassum declined with increasing D. antillarum (β = −0.03 ± 0.01, p = 0.0067; adj.R² = 0.74), with a small herbivore-specific gain of ~2.1% (Δadj.R² = 0.0206), while other herbivores were not significant (Table 2). For all model outputs see Supplementary Tables 5-43. To verify model stability, we assessed multicollinearity; Variance Inflation Factors (VIFs) for additive models were < 3.2; interaction-term VIFs ranged 4.7–7.4, indicating no problematic collinearity (Supplementary Table 44).
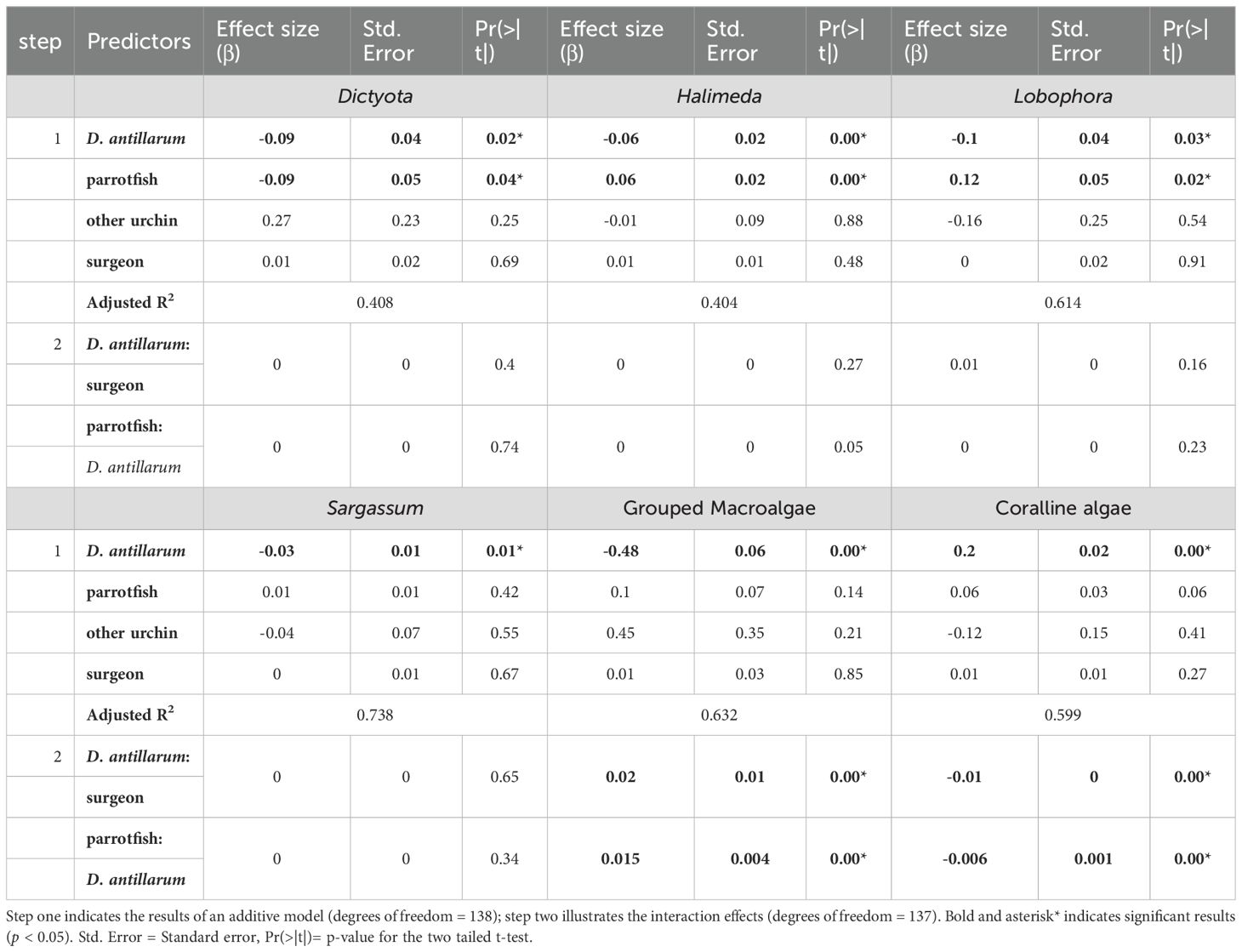
Table 2. Regression results for algae Dictyota, Halimeda, Lobophora, Sargassum, grouped macroalgae, and coralline algae with the predictors of herbivores counts of D. antillarum, parrotfish, other urchin, surgeonfish and their interactions.
At the community level, total macroalgal cover was strongly and inversely related to D. antillarum abundance (β = −0.48 ± 0.06, p < 0.001; adj.R² = 0.63). Interaction models showed that this negative slope became weaker where parrotfish and surgeonfish were more abundant (D. antillarum × parrotfish: β = +0.015 ± 0.004, p = 0.0004; D. antillarum × surgeonfish: β = +0.022 ± 0.006, p = 0.0005; adj.R² ≈ 0.66; Table 2). Models including coral cover also confirmed an inverse relationship between coral and macroalgae (β = −2.69 ± 0.26, p < 0.001; adj.R² = 0.61). In additive models, herbivores explained an additional ~21.4% of total macroalgal variance beyond site and year (Δadj.R² = 0.2140).
In contrast, coralline algae responded positively to herbivory. D. antillarum abundance was a strong positive predictor of coralline algae (β = +0.20 ± 0.02, p < 0.001; adj.R² ≈ 0.60), and models showed no significant parrotfish effect (p = 0.06). Their interaction (parrotfish × D. antillarum: β = −0.006 ± 0.002, p = 0.0009) suggested that where parrotfish were abundant, the positive effect of D. antillarum on coralline algae was slightly dampened (Table 2). Additive-model partitioning indicated that herbivores accounted for an additional ~21.1% of coralline algal variance beyond site and year (Δadj.R² = 0.2110).
Taken together, the additive models show that herbivory contributes an additional ~2–21% of explained variance beyond spatial and temporal structure, strongest for total macroalgae and coralline algae, intermediate for Halimeda, and modest for Lobophora, Dictyota, and Sargassum. Urchin grazing—particularly by D. antillarum—is the dominant herbivorous control on macroalgae and coralline algae alike, while fish herbivory modulates but does not override this pattern. Parrotfish and surgeonfish partly buffer D. antillarum’s suppression of fleshy algae while jointly sustaining high coralline cover.
Regression analyses were constrained to years with complete herbivore and benthic data (2017–2022), as fish data were unavailable in 2024 and several sites lacked full temporal coverage (Table 1). Consequently, models represent the subset of site-years where all variables were measured, ensuring internal consistency but limiting inference to that period. Because site and year were included as covariates, spatial and temporal structure was accounted for within the available dataset.
4 Discussion
4.1 Historical context for the region
Northeast Jamaica has been understudied, but several prior surveys provide insight into long-term trends of coral decline in the region (Table 3). Reef surveys spanning five decades show that coral cover in northeast Jamaica has declined from roughly 50% in the 1970s to less than 1% today (see citations in Table 3). Local studies near Port Antonio and Pellew Island document a steady shift toward macroalgal dominance beginning after the 1980s, with little subsequent recovery. Baseline assessments conducted before the EPSFCA’s creation already recorded low coral cover (~25% at the healthiest site) and macroalgae dominance indicating that the sanctuary was established in an advanced state of degradation (Buddo et al., 2014).
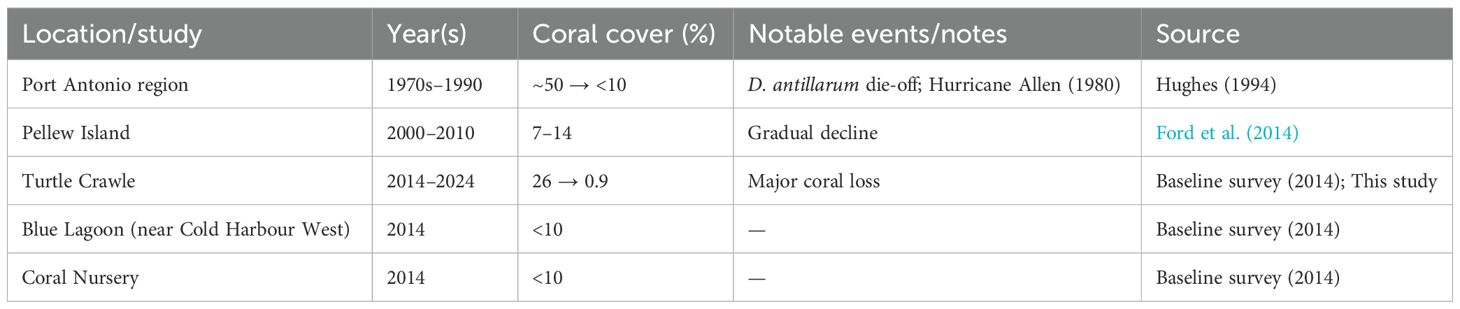
Table 3. Historical coral cover records from sites in and near the East Portland Special Fishery Conservation Area (EPSFCA), Jamaica. Values summarize previously published surveys and the present study, illustrating long-term regional coral decline and major disturbance events since the 1970s.
4.2 Community change since the sanctuary’s establishment
4.2.1 Coral community decline over eight years
One of the major goals of the sanctuary is abundant reefs. Unfortunately, there has been a distinct decrease in coral cover since the sanctuary’s establishment with only around 1% in 2024, indicating the sanctuary is not yet meeting its goal (Figure 6A). At the EPSFCA, the decline in coral cover is concurrent with the rise in macroalgal cover to around 80% in the sanctuary in 2024, particularly in the genera Dictyota, and Lobophora (Figures 6A, D, C, F); this relationship between coral and macroalgae is well established in other locations in Jamaica (Goreau, 1992; Woodley, 1992; Lapointe, 1997; Lapointe et al., 1997; Idjadi et al., 2006). Along with the loss of coral cover, the sanctuary also experienced a decline of coralline algae to 1.6% ± 1.27 in 2024. This further limits the sanctuary’s ability to promote abundant reefs as coralline algae are important reef builders and cementers (Littler and Littler, 2013) and can accelerate colonization of coral and prevent the settlement of fleshy algae (Littler and Littler, 2013).
The coral community in the EPSFCA is dominated by Agaricia sp., Porites astreoides, Orbicella sp., branching Porites sp., and Siderastrea spp. Among these, Agaricia sp. and P. astreoides are considered “weedy” species that recover rapidly after disturbance and tolerate a wide range of environmental stressors (Green et al., 2008; Steneck et al., 2009; Walton et al., 2018). In contrast, Orbicella spp. and Siderastrea sp. are framework-building taxa, consistent with other Caribbean spur-and-groove reefs (Rotjan and Lewis, 2006; Sealey et al., 2019). The prevalence of opportunistic species and relative scarcity of more sensitive reef builders indicate the EPSFCA community reflects disturbance consistent with broader Caribbean trends (Green et al., 2008; Williams et al., 2017).
The low coral cover is reflective of the many regional stressors that impacted the Caribbean from 2017 to 2024, including Stony Coral Tissue Loss Disease (SCTLD). SCTLD was first reported in Jamaica in 2017 (Weil et al., 2019) and reached the EPSFCA by early 2019. Since SCTLD causes rapid mortality, often within six months of infection (Camacho-Vite et al., 2022), infected corals were not captured by annual monitoring intervals (only 3 noted diseased coral during monitoring). The disease preferentially impacts maze and brain corals (family Meandrinadae and the subfamily Faviinae), which likely contributes to the loss of coral cover in the sanctuary after 2019 and the rarity of Diploria and related taxa in our surveys (Alvarez-Filip et al., 2022).
Bleaching is another major driver of coral loss at the EPSFCA. Caribbean reefs have experienced repeated mass bleaching over the past five decades (Goreau, 1992; Wilkinson, 2001; Eakin et al., 2010; Dustan and Lang, 2019; Goreau and Hayes, 2024). The significant declines observed after the bleaching events in 2017 and again following the record 2023 heatwave (Figure 3) suggest that EPSFCA corals follow the same regional pattern of heat stress–driven mortality.
4.2.2 Fish population recovery in the sanctuary
Another goal of the sanctuary is fish filled seas, which is particularly important for east Portland as it has been historically overfished (Buddo et al., 2014). Fish are important food and income sources for the local population, and herbivorous fish, such as parrotfish, play a key role in keeping macroalgae abundance on reefs at a minimum (Bonaldo et al., 2014; Table 2).
This study monitored fish communities in the EPSFCA over six years (2017–2022). In many marine protected areas, particularly in the Pacific, fish biomass and size can recover within five to ten years of protection, but recovery in Caribbean sanctuaries is often slower or incomplete, reflecting cumulative local and regional stressors (Sala and Giakoumi, 2018). Within this context, the EPSFCA showed encouraging but transient improvements. An indication that the sanctuary was achieving their goal of fish restoration was the increase in the size of economically and ecologically important fish groups (e.g., grunt, parrotfish, surgeonfish, wrasse, snapper) from 2017 to 2019 (Figure 5). This is likely a response to the fishing ban enforced through patrolling by AHF wardens. In 2022, however, fish sizes returned to 2017 levels (i.e., parrotfish, surgeonfish, grunts) or lower (snappers, angelfish, wrasse; Figure 5). Conservation experts witnessed more incidents of fishing during the COVID-19 pandemic, likely due to decreased patrolling between 2020 and 2022.
The density of economically and ecologically important fish showed a different pattern; while there were increases in fish counts from 2017 to 2018, they declined from 2018 to 2019 for wrasse, surgeonfish, and parrotfish (Figures 4E–H). This may be indicative of a stressor in and around the sanctuary reducing fish density, while allowing fish size to continue to increase.
One potential stressor is invasive lionfish. The baseline assessment of the sanctuary found lionfish to have a significant presence and concluded more effort needed to be established to lower populations (Buddo et al., 2014). Lionfish are voracious generalist predators that feed heavily on small reef fish, including herbivores such as parrotfish and wrasse (Moonsammy et al., 2011; Jackson et al., 2014; Del Río et al., 2023), and have been shown to reduce herbivore abundance and promote algal dominance in other Jamaican sanctuaries (Chin et al., 2016; May, 2022). Although AHF staff, local fishers, and local divers actively work to remove lionfish (spearing and a campaign to “eat it to beat it” that encourages people to eat lionfish), lionfish are regularly seen by wardens. Since data on lionfish are not collected during monitoring, it is difficult to determine the extent of their impact. Future work in this sanctuary should investigate the abundance of lionfish during monitoring surveys.
In addition, these recoveries in size and abundance were not universal across taxa. Parrotfish showed the strongest and most consistent recovery between 2017 and 2019, followed by more moderate gains in surgeonfish and snappers, while grunts and wrasses exhibited little or no change (Figures 4, 5). These differences likely reflect contrasting life-history traits: shorter-lived, site-attached herbivores, such as parrotfish, responded rapidly to reduced fishing pressure, whereas longer-lived or more mobile species may require larger or more connected refuges to rebuild fully (Pina-Amargós et al., 2014). Considering the sanctuary’s modest size (~6 km²), these results are consistent with broader Caribbean and global patterns showing that small reserves mainly support recovery of resident species, while larger (>100 km²), long-protected sanctuaries achieve more extensive, multi-trophic rebuilding (Pina-Amargós et al., 2014; Sala and Giakoumi, 2018; Mumby et al., 2021).
4.3 Variation of community composition inside and outside the sanctuary
The main differences between sites around the sanctuary are the macroalgae community; coral cover is consistent across the sanctuary, likely due to very low coverage (Figures 2, 6). Lobophora dominates at Salt Creek, consistent with overall high macroalgal abundance there, while Halimeda peaks at Cold Harbour West and Sargassum is most abundant at the eastern sites, particularly Winnifred Reef and Dragon Bay.
Differences between sites were not explained by protection status; reefs inside and outside the sanctuary showed no consistent trends. However, subtle contrasts between protected and neighboring reefs hint that conditions within the sanctuary may be somewhat healthier than adjacent sites (i.e., Salt Creek to the west and Winnifred Reef to the east); for example, Salt Creek had the highest cover of macroalgae (Figure 6C). Although Winnifred Reef does have lower coral cover than some other sites, it does not have higher values of macroalgae abundance, pointing to the likelihood of an acute stressor increasing Salt Creek’s macroalgae cover.
Similar patterns of macroalgal abundance at non-adjacent sites indicate that site-specific stressors may underlie these differences. The reef sites characterized by distinctive algal assemblages—Salt Creek, Cold Harbour West, Dragon Bay, and Winnifred Reef—are all situated near population centers. This pattern points toward the influence of localized anthropogenic stressors, potentially nutrient pollution, sedimentation, or other runoff-related impacts, superimposed on broader reef degradation. Targeted water-quality monitoring and land–sea management will be essential in future studies to evaluate and mitigate these pressures.
Despite the protection status, there were no clear differences for fish between sites inside and outside the boundary. The high mobility of fish, together with the sanctuary’s small size (~6 km²), likely contributes to spillover that blurs contrasts between protected and adjacent reefs. Such spillover effects are common in small, closely connected sanctuaries (Roberts, 1995). Local fishers also report higher catch rates along the border in those adjacent sites, consistent with this pattern of cross-boundary movement.
4.3.1 Management outcomes and future directions for reef recovery in the EPSFCA
Taken together these findings demonstrate that conservation initiatives implemented by the Alligator Head Foundation, including enforcement patrols, have supported partial but fragile recovery of reef communities within the sanctuary, but have not yet been sufficient to overcome reef decline. Maintaining consistent enforcement and considering either expanding the no-take boundary or establishing adjacent protected zones could improve connectivity of communities and spatial coverage, allowing recovery to extend to more mobile and longer-lived fish species, while sustaining herbivore gains. This may be especially important because fishing pressure remains strong overall and is often concentrated near the sanctuary border. Globally, fish reserve performance scales with size, protection age, and enforcement (Sala and Giakoumi, 2018). No-take areas larger than 100 km² and older than ten years typically support three- to five-fold higher fish biomass than small or young reserves (Sala and Giakoumi, 2018; Mumby et al., 2021).
The lack of a benthic response in the EPSFCA over the last eight years is not inconsistent with expected recovery trajectories; ecosystem-level changes such as coral–algal shifts or microbial recovery often require at least a decade to emerge (Sala and Giakoumi, 2018; Mumby et al., 2021). Alarmingly, rather than merely failing to recover, the sanctuary’s coral cover continued to collapse. Weedy, tolerant species will continue to dominate these reefs unless action is taken to improve conditions on the reef and replenish lost species such as Diploria sp. The EPSFCA has a marine laboratory where corals are grown and later outplanted into a coral nursery or on the reef. This avenue of restoration is vital for these reefs to regain some of their lost functionality.
While this study focuses on herbivory and its role in the sanctuary, environmental factors such as heat also play a vital role and an important follow up study to the baseline community composition analysis would be to determine the environmental stressors impacting this area. Historically, it was thought the Portland reefs experienced less anthropogenic stress due to their location in a rural area with little tourism (Goreau, 1992; Ford et al., 2014), but the advanced degradation (i.e. coral cover ~1%) observed here underscores the need to investigate additional stressors—such as thermal anomalies, land-based runoff, and disease dynamics—to fully understand the drivers of reef decline in this region.
4.4 Herbivory in the EPSFCA
4.4.1 Urchin populations are important for macroalgae reduction and increased coralline algae cover
The long spine sea urchin, Diadema antillarum, is a key grazer at the EPSFCA as it is in other Caribbean coral reefs, especially areas that have been historically overfished (Gardner et al., 2003). Recovery of D. antillarum populations has been linked with a reduction of macroalgae cover, and increase of scleractinian corals (Edmunds and Carpenter, 2001; Idjadi et al., 2010) underscoring their continued importance for reef recovery in the sanctuary. In addition, D. antillarum promotes coralline algae cover in the EPSFCA (Table 2).
The differences in macroalgae communities between sites can partially be explained by D. antillarum abundance. D. antillarum are known to be restricted to depths less than 10 meters (Weil et al., 2005; Martín Blanco et al., 2010) and healthy populations were historically identified in shallow areas (Buddo et al., 2014). In contrast, the shallowest sites investigated, Trident Shallow and Coral Nursery, do not have the most D. antillarum (Figure 6H), suggesting the presence of this urchin is not just tied to depth. The sites with the most urchins (Pellew Island and Turtle Crawle) had some of the lowest values of macroalgae seen in the sanctuary, at times near to zero cover in 2017, but this suppression is lost by 2022 likely because herbivory pressure had declined below the level needed to keep macroalgae in check. Our monitoring data show that urchin abundance dropped sharply in 2022, which coincided with the Caribbean-wide resurgence of D. antillarum disease (Levitan et al., 2023). Similar die-offs in the 1980s and sporadic recoveries elsewhere in Jamaica (Edmunds and Carpenter, 2001; Idjadi et al., 2010) demonstrate how vulnerable D. antillarum populations remain. Although we observe a recovery by 2024 (around 10 per 180 m2; Figure 3H), these trends highlight the fragility of this important herbivore feedback.
4.4.2 Herbivore interactions and foraging preferences among algal genera
There are important nuances in algae and herbivore interactions in the EPSFCA (Figures 3D–G). D. antillarum, consistently suppressed fleshy macroalgae, whereas parrotfish exerted a mix of grazing and facultative effects depending on algal type. Dictyota declined with both D. antillarum and parrotfish, suggesting overlapping feeding pressure. In contrast Lobophora and Halimeda showed divergent responses—both increased where parrotfish were abundant but declined with higher urchin densities. These patterns align with prior observations that Dictyota is more frequently grazed than Lobophora (Hay, 1981), whereas Halimeda, which has both chemical and structural defenses, are often avoided by herbivorous reef fish (Spiers and Frazer, 2023). Overall, herbivory strongly structured overall algal cover but explained only modest variation among individual algal genera, underscoring the central role of D. antillarum recovery and balanced fish assemblages in reef resilience.
Complementarity among herbivores on reefs is important as it provides functional redundancy and keeps algal populations from overgrowing corals (Burkepile and Hay, 2008). At the EPSFCA, D. antillarum exerted the strongest overall control on macroalgae, yet this effect weakened where parrotfish and surgeonfish were more abundant. Rather than indicating antagonism, this positive interaction likely reflects overlapping grazing niches. D. antillarum are nocturnal feeders (Tuya et al., 2004), whereas parrotfish are diurnal feeders (Ogden and Buckman, 1973). Herbivorous fish, such as the parrotfish, are known to avoid chemically defended algal species and D. antillarum are known to eat less of the structurally defended algae (Spiers and Frazer, 2023). Such partial redundancy can stabilize grazing pressure even if one group fluctuates.
In addition, these dynamics occurred alongside broader changes in the system: herbivorous fish recovered rapidly following fishing restrictions, while macroalgae increased and coral cover declined, possibly linked to bleaching and disease. Thus, the apparent dampening of D. antillarum’s effect in high-fish areas likely reflects concurrent ecological shifts rather than direct competition. Continued monitoring will help determine whether these interactions remain stable as fish populations and environmental conditions evolve.
4.4.3 The role and limits of herbivory in benthic recovery
Herbivore functional diversity underpins reef resilience in the EPSFCA; D. antillarum strongly suppressed fleshy macroalgae, while parrotfish and surgeonfish exerted selective and sometimes facilitative effects on defended taxa such as Lobophora and Halimeda. These patterns support experimental evidence that no single species or guild can control all algal forms; only functionally diverse assemblages will sustain long-term algal suppression (Burkepile and Hay, 2008). Such complementarity stabilizes grazing pressure but has limits (Mumby et al., 2007; Idjadi et al., 2010; Adam et al., 2015); herbivore recovery alone will likely not fully reverse algal dominance where coral mortality or bleaching continue to erode resilience.
Consistent with these functional limits, model results show that while herbivory explained a meaningful share of benthic variability (adjusted R2 ~2–21%), much of the remaining variation reflected site and year effects, suggesting that broader environmental gradients strongly influence community composition. Total macroalgae and coralline algae were both strongly shaped by herbivory (Δadj.R² ≈ 0.21), but many genera show weaker effects of herbivory, such as Dictyota and Lobophora (Δadj.R² ≈ 0.02–0.04). This suggests that site-level and year-level conditions—such as nutrient enrichment, hydrodynamics, or substrate availability—play a dominant role in determining the distribution of macroalgae, and that herbivory alone is insufficient to regulate these genera within the EPSFCA. Manual removal of these species may be beneficial (Briggs et al., 2018), particularly in locations where they have reached dominance, such as Salt Creek.
Collectively, these findings reveal that the EPSFCA mirrors the reality of many Caribbean reefs (Hughes et al., 2017; Bruno et al., 2019; Johnson et al., 2022): local management can slow degradation and enhance resilience, but without concurrent global action to mitigate climate warming and disease spread, these gains will remain fragile. Effective recovery in the EPSFCA will require an integrated strategy combining fishing enforcement and herbivore protection with coral propagation, and monitoring—but their long-term survival ultimately depends on addressing disease and temperature extremes operating far beyond sanctuary boundaries.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
CW: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Software, Visualization, Writing – original draft, Writing – review & editing, Project administration, Resources. DH: Conceptualization, Investigation, Resources, Writing – review & editing. RM: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. D-AG-S: Conceptualization, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Partial financial support was received from the National Science Foundation Faculty Early Career Development Program (CAREER) Grant No. 1848393, the National Science Foundation Graduate Research Fellowship Program Grant No. DGE 2137420, and the Lerner-Grey Memorial Fund of the American Museum of Natural History Grant.
Acknowledgments
We acknowledge the staff at Alligator Head Foundation for their expertise and data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1684741/full#supplementary-material
References
Adam T., Burkepile D., Ruttenberg B., and Paddack M. (2015). Herbivory and the resilience of Caribbean coral reefs: knowledge gaps and implications for management. Mar. Ecol. Prog. Ser. 520, 1–20.
Alvarez-Filip L., González-Barrios F. J., Pérez-Cervantes E., Molina-Hernández A., and Estrada-Saldívar N. (2022). Stony coral tissue loss disease decimated Caribbean coral populations and reshaped reef functionality. Commun. Biol. 5, 440. doi: 10.1038/s42003-022-03398-6
Bellwood D. R., Hughes T. P., Folke C., and Nyström M. (2004). Confronting the coral reef crisis. Nature 429, 827–833. doi: 10.1038/nature02691
Blanca M. J., Alarcon R., Arnau J., Bono R., and Bendayan R. (2017). Non-normal data: Is ANOVA still a valid option? Psicothema 29, 552. doi: 10.7334/psicothema2016.383
Bonaldo R. M., Hoey A. S., Bellwood D. R., Hughes R. N., Hughes D. J., and Smith I. P. (2014). The ecosystem Roles of Parrotfishes on Tropical Reefs (United Kingdom: CRC Press), 81–132. doi: 10.1201/b17143-3
Bray J. R. and Curtis J. T. (1957). An ordination of the upland Forest communities of southern Wisconsin. Ecol. Monogr. 27, 325–349. doi: 10.2307/1942268
Briggs C. J., Adam T. C., Holbrook S. J., and Schmitt R. J. (2018). Macroalgae size refuge from herbivory promotes alternative stable states on coral reefs. PloS One 13. doi: 10.1371/journal.pone.0202273
Bruno J. F., Côté I. M., and Toth L. T. (2019). Climate Change, Coral Loss, and the Curious Case of the Parrotfish Paradigm: Why Don’t Marine Protected Areas Improve Reef Resilience? Annu. Rev. Mar. Sci. 11, 307–344 doi: 10.1146/annurev-marine-010318-095300
Buddo D., Kyne F., Webber D., Webber M., and Aiken K. (2014). Alligator Head Marine Lab 2014 Annual Report (Kingston, Jamaica: University of the West Indies).
Burkepile D. E. and Hay M. E. (2008). Herbivore species richness and feeding complementarity affect community structure and function on a coral reef. Proc. Natl. Acad. Sci. 105, 16201–16206. doi: 10.1073/pnas.0801946105
Camacho-Vite C., Estrada-Saldívar N., Pérez-Cervantes E., and Alvarez-Filip L. (2022). Differences in the progression rate of SCTLD in Pseudodiploria strigosa are related to colony size and morphology. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.790818
Chin D., Aiken K., and Buddo D. (2016). Lionfish population density in discovery bay, Jamaica. Int. J. Sci. Eng. Res. 7, 1–5.
Critchlow D. E. and Fligner M. A. (1991). On distribution-free multiple comparisons in the one-way analysis of variance. Commun. Stat. Theory Methods 20, 127–139. doi: 10.1080/03610929108830487
Del Río L., Navarro-Martínez Z. M., Cobián-Rojas D., Chevalier-Monteagudo P. P., Angulo-Valdes J. A., and Rodriguez-Viera L. (2023). Biology and ecology of the lionfish Pterois volitans/Pterois miles as invasive alien species: a review. PeerJ San Franc. CA 11, e15728–e15728. doi: 10.7717/peerj.15728
Dustan P. and Lang J. C. (2019). “Discovery bay, Jamaica,” in Mesophotic Coral Ecosystems. Eds. Loya Y., Puglise K. A., and Bridge T. C. L. (Springer International Publishing, Cham), 85–109. doi: 10.1007/978-3-319-92735-0_6
Dwass M. (1960). “Some k-sample rank-order tests,” in Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling. Eds. Olkin I., Ghurye S. G., Hoeffding W., Madow W. G., and Mann H. B. (Stanford University Press, Stanford, CA), 198–202.
Eakin C. M., Morgan J. A., Heron S. F., Smith T. B., Liu G., Alvarez-Filip L., et al. (2010). Caribbean corals in crisis: record thermal stress, bleaching, and mortality in 2005. PloS One 5, e13969. doi: 10.1371/journal.pone.0013969
Edmunds P. J. and Carpenter R. C. (2001). Recovery of Diadema antillarum reduces macroalgal cover and increases abundance of juvenile corals on a Caribbean reef. Proc. Natl. Acad. Sci. 98, 5067–5071. doi: 10.1073/pnas.071524598
Faith D. P., Minchin P. R., and Belbin L. (1987). Compositional dissimilarity as a robust measure of ecological distance. Vegetatio 69, 57–68. doi: 10.1007/BF00038687
Ford M. C., Smith L. J., and Green S. O. (2014). The results of long term coral reef monitoring at three locations in Jamaica: Monkey Island, “Gorgo City” and Southeast Cay. Rev. Biol. Trop. 62, 65–73. doi: 10.15517/rbt.v62i0.15902
Gardner T. A., Côté I. M., Gill J. A., Grant A., and Watkinson A. R. (2003). Long-term region-wide declines in caribbean corals. Sci. Am. Assoc. Adv. Sci. 301, 958–960. doi: 10.1126/science.1086050
GCRMN-Caribbean Steering Committee (2016). GCRMN-Caribbean Guidlines for Coral Reef Biophysical Monitoring (Miami Florida). Available online at: https://gcrmn.net/resources/#res-monitoring-protocols (Accessed July 25, 2022).
González-Barrios F. J. and Álvarez-Filip L. (2018). A framework for measuring coral species-specific contribution to reef functioning in the Caribbean. Ecol. Indic. 95, 877–886. doi: 10.1016/j.ecolind.2018.08.038
Goreau T. J. (1992). Bleaching and Reef community change in Jamaica: 1951–1991. Am. Zool. 32, 683–695. doi: 10.1093/icb/32.6.683
Goreau T. J. F. and Hayes R. L. (2024). 2023 Record marine heat waves: coral reef bleaching HotSpot maps reveal global sea surface temperature extremes, coral mortality, and ocean circulation changes. Oxf. Open Clim. Change 4, kgae005. doi: 10.1093/oxfclm/kgae005
Green D., Edmunds P., and Carpenter R. (2008). Increasing relative abundance of Porites astreoides on Caribbean reefs mediated by an overall decline in coral cover. Mar. Ecol. Prog. Ser. Halstenbek 359, 1–10. doi: 10.3354/meps07454
Hay M. (1981). Spatial patterns of grazing intensity on a caribbean barrier reef: herbivory and algal distribution. Aquat. Bot. 11, 97–109. doi: 10.1016/0304-3770(81)90051-6
Hughes T. P., Kerry J. T., Álvarez-Noriega M., Álvarez-Romero J. G., Anderson K. D., Baird A. H., et al (2017). Global warming and recurrent mass bleaching of corals. Nat. Lond. 543, 373–377. doi: 10.1038/nature21707
Hughes T. P., Baird A. H., Morrison T. H., and Torda G. (2023). Principles for coral reef restoration in the anthropocene. One Earth 6, 656–665. doi: 10.1016/j.oneear.2023.04.008
Idjadi J. A., Haring R., and Precht W. F. (2010). Recovery of the sea urchin Diadema antillarum promotes scleractinian coral growth and survivorship on shallow Jamaican reefs. Mar. Ecol. Prog. Ser. 403, 91–100. Available online at: https://www.int-res.com/abstracts/meps/v403/p91-100/ (Accessed April 14, 2024).
Idjadi J. A., Lee S. C., Bruno J. F., Precht W. F., and Allen-Requa L. (2006). Rapid phase-shift reversal on a Jamaican coral reef. Coral Reefs 25, 209–211. doi: 10.1007/s00338-006-0088-7
Jackson J., Donovan M., Cramer K., and Lam V. (2014). Status and Trends of Caribbean Coral Reefs. Gland, Switzerland: Global Coral Reef Monitoring Network (GCRMN) and International Union for the Conservation of Nature (IUCN). 1970–2012. doi: 10.13140/2.1.4868.6726
Jackson J. B. C., Kirby M. X., Berger W. H., Bjorndal K. A., Botsford L. W., Bourque B. J., et al. (2001). Historical overfishing and the recent collapse of coastal ecosystems. Science 293, 629. doi: 10.1126/science.1059199
Johnson J. V., Dick J. T. A., and Pincheira-Donoso D. (2022). Marine protected areas do not buffer corals from bleaching under global warming. BMC Ecol. Evol. 22, 58. doi: 10.1186/s12862-022-02011-y
Kohler K. E. and Gill S. M. (2006). Coral Point Count with Excel extensions (CPCe): A Visual Basic program for the determination of coral and substrate coverage using random point count methodology. Comput. Geosci. 32, 1259–1269. doi: 10.1016/j.cageo.2005.11.009
Kruskal W. H. and Wallis W. A. (1952). Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 47, 583–621. doi: 10.1080/01621459.1952.10483441
Lapointe B. E. (1997). Nutrient thresholds for bottom-up control of macroalgal blooms on coral reefs in Jamaica and Southeast Florida. Limnol. Oceanogr. 42, 1119–1131. doi: 10.4319/lo.1997.42.5_part_2.1119
Lapointe B. E., Littler M. M., and Littler D. S. (1997). Macroalgal overgrowth of fringing coral reefs at Discovery Bay, Jamaica: bottom-up versus top-down control. Proceedings of the Eighth International Coral Reef Symposium. 1927–932.
Legendre P. and Gallagher E. D. (2001). Ecologically meaningful transformations for ordination of species data. Oecologia 129, 271–280. doi: 10.1007/s004420100716
Lessios H. A. (1988). Mass mortality of diadema antillarum in the caribbean: what have we learned? Annu. Rev. Ecol. Syst. 19, 371–393. doi: 10.1146/annurev.es.19.110188.002103
Levitan D. R., Best R. M., and Edmunds P. J. (2023). Sea urchin mass mortalities 40 y apart further threaten Caribbean coral reefs. Proc. Natl. Acad. Sci. - PNAS 120, e2218901120–e2218901120. doi: 10.1073/pnas.2218901120
Littler M. M. and Littler D. S. (2013). The nature of crustose coralline algae and their interactions on reefs. Smithson. Contrib. Mar. Sci. 39, 199–212. doi: 10.5479/si.1943667X.39.199
Martín Blanco F., González Sansón G., Pina Amargós F., and Clero Alonso L. (2010). Abundance, distribution and size structure of Diadema antillarum (Echinodermata: Diadematidae) in South Eastern Cuban coral reefs Abundance, distribution and size structure of Diadema antillarum (Echinodermata: Diadematidae) in South Eastern Cuban coral reefs. Rev. Biol. Trop. 58, 663–676.
May C. ,. E. A. (2022). “A baseline assessment of lionfish (Pterois spp.) population dynamics, distribution, and diet within the montego bay marine park, Jamaica, in 2018,” in Proceedings of the Inaugural Frontiers of Research in Caribbean Science and Technology (FORECAST) 2022 Conference, Kingston, Jamaica: The University of Technology. 142–161.
Moonsammy S., Buddo D., and Seepersad G. (2011). Assessment of the Economic Impacts of the Lion Fish (Pterois volitans) Invasion in Jamaica. Fort Pierce, Florida, USA: Gulf and Caribbean Fisheries Institute (GCFI).
Mumby P. J., Hastings A., and Edwards H. J. (2007). Thresholds and the resilience of Caribbean coral reefs. Nature 450, 98–101. doi: 10.1038/nature06252
Mumby P. J., Steneck R. S., Roff G., and Paul V. J. (2021). Marine reserves, fisheries ban, and 20 years of positive change in a coral reef ecosystem. Conserv. Biol. 35, 1473–1483. doi: 10.1111/cobi.13738
National Environment and Planning Agency (2021). Coral Reef Health Status Report for Jamaica: 2020 (10 Caledonia Avenue Kingston Jamaica: National Environment and Planning Agency).
Ogden J. C. and Buckman N. S. (1973). Movements, foraging groups, and diurnal migratons of the striped parrotfish scarus croicensis bloch (Scaridae). Ecol. Durh. 54, 589–596. doi: 10.2307/1935344
Pina-Amargós F., González-Sansón G., Martín-Blanco F., and Valdivia A. (2014). Evidence for protection of targeted reef fish on the largest marine reserve in the Caribbean. PeerJ San Franc. CA 2, e274. doi: 10.7717/peerj.274
Ricotta C. and Podani J. (2017). On some properties of the Bray-Curtis dissimilarity and their ecological meaning. Ecol. Complex. 31, 201–205. doi: 10.1016/j.ecocom.2017.07.003
Roberts C. M. (1995). Rapid build-up of fish biomass in a caribbean marine reserve. Conserv. Biol. 9, 815–826. doi: 10.1046/j.1523-1739.1995.09040815.x
Roberts C. M., McClean C. J., Veron J. E.N., Hawkins J. P., Allen G. R., McAllister D. E., et al. (2002). Marine biodiversity hotspots and conservation priorities for tropical reefs. Sci. Am. Assoc. Adv. Sci. 295, 1280–1284. doi: 10.1126/science.1067728
Rotjan R. D. and Lewis S. M. (2006). Parrotfish abundance and selective corallivory on a Belizean coral reef. J. Exp. Mar. Biol. Ecol. 335, 292–301. doi: 10.1016/j.jembe.2006.03.015
Sala E. and Giakoumi S. (2018). No-take marine reserves are the most effective protected areas in the ocean. ICES J. Mar. Sci. 75, 1166–1168. doi: 10.1093/icesjms/fsx059
Sealey K. S., Wood K., and Logan A. (2019). “Chapter 26 - the Turks and Caicos Islands,” in World Seas: an Environmental Evaluation (Second Edition). Ed. Sheppard C. (London, United Kingdom: Academic Press), 617–635. doi: 10.1016/B978-0-12-805068-2.00031-0
Souter D., Planes S., Wicquart J., Obdura D., and Staub F. (2021). Status of Coral Reefs of the World: 2020 report. Global Coral Reef Monitoring Network (GCRMN) and International Coral Reef Initiative (ICRI). doi: 10.59387/WOTJ9184
Spiers L. and Frazer T. K. (2023). Comparison of feeding preferences of herbivorous fishes and the sea urchin Diadema antillarum in Little Cayman. PeerJ San Franc. CA 11, e16264–e16264. doi: 10.7717/peerj.16264
Steel R. G. D. (1960). A rank sum test for comparing all pairs of treatments. Technometrics 2, 197–207. doi: 10.1080/00401706.1960.10489894
Steneck R. S., Paris C. B., Arnold S. N., Ablan-Lagman M. C., Alcala A. C., Butler M. J., et al. (2009). Thinking and managing outside the box: coalescing connectivity networks to build region-wide resilience in coral reef ecosystems. Coral Reefs 28, 367–378. doi: 10.1007/s00338-009-0470-3
Tukey J. W. (1949). Comparing individual means in the analysis of variance. Biometrics 5, 99–114. doi: 10.2307/3001913
Tuya F., Martin J. A., and Luque A. (2004). Patterns of nocturnal movement of the long-spined sea urchin Diadema antillarum (Philippi) in Gran Canaria (the Canary Islands, central East Atlantic Ocean). Helgol. Mar. Res. 58, 26–31. doi: 10.1007/s10152-003-0164-0
Wade E., Alexander S. M., Gerkey D., and Biedenweg K. (2023). Exploring the Relationship Between Fishing Actors and Network Prominence in information-sharing Networks in Jamaican small-scale Fisheries. Hum. Ecol. Interdiscip. J. 51, 877–889. doi: 10.1007/s10745-023-00444-7
Walton C. J., Hayes N. K., and Gilliam D. S. (2018). Impacts of a regional, multi-year, multi-species coral disease outbreak in Southeast Florida. Front. Mar. Sci. 5. doi: 10.3389/fmars.2018.00323
Weil E., Hernández-Delgado E., Gonzalez M., Williams S., Suleimán-Ramos S., Figuerola M., et al. (2019). Spread of the new coral disease “SCTLD. Into Caribbean: Implications Puerto Rico. Reef Encount. 34, 38–43.
Weil E., Torres J. L., and Ashton M. (2005). Population characteristics of the sea urchin Diadema antillarum in La Parguera, Puerto Rico, 17 years after the mass mortality event. Rev. Biol. Trop. 53, 219–231. doi: 10.15517/rbt.v53i3.26778
Wilkinson C. R. (2001). Status of coral reefs of the world 2000 / edited by Clive Wilkinson (Cape Ferguson, Qld: Australian Institute of Marine Science).
Williams S. M., Sánchez-Godínez C., Newman S. P., and Cortés J. (2017). Ecological assessments of the coral reef communities in the Eastern Caribbean and the effects of herbivory in influencing coral juvenile density and algal cover. Mar. Ecol. 38, e12395. doi: 10.1111/maec.12395
Keywords: coral reef monitoring, herbivore abundance, Jamaica, marine protected area, community composition
Citation: Williams CM, Henry D, Martindale RC and Gordon-Smith D-A (2025) Spatiotemporal variability in reef ecology and herbivore impacts in the East Portland Special Fishery Conservation Area, northeast Jamaica (2017-2024). Front. Mar. Sci. 12:1684741. doi: 10.3389/fmars.2025.1684741
Received: 13 August 2025; Accepted: 05 November 2025; Revised: 31 October 2025;
Published: 21 November 2025.
Edited by:
Fraser Januchowski-Hartley, Nova Southeastern University, United StatesReviewed by:
Manuel Olán-González, National Autonomous University of Mexico, MexicoAhmad Faizal, Universitas Hasanuddin, Indonesia
Copyright © 2025 Williams, Henry, Martindale and Gordon-Smith. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claire M. Williams, Y213M0B1dGV4YXMuZWR1
 Claire M. Williams
Claire M. Williams Denise Henry2,3
Denise Henry2,3 Rowan C. Martindale
Rowan C. Martindale Debbie-Ann Gordon-Smith
Debbie-Ann Gordon-Smith