- 1Department of Gastroenterology, First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, China
- 2Ministry of Health and Family Welfare (OSD-DGHS), Dhaka, Bangladesh
- 3Hubei Key Laboratory of Embryonic Stem Cell Research, Institute of Neuroscience, Hubei University of Medicine, Shiyan, China
- 4Department of Pathology and Pathophysiology, Xi'an Jiaotong University, Xi'an, China
- 5Department of Epidemiology and Health Statistics, Xi'an Jiaotong University, Xi'an, China
Objective: Coronavirus disease 2019 (COVID-19) or severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is a global issue. In addition to managing acute cases, post-COVID-19 persisting symptoms/complaints and different hematological values are of great concern. These have an impact on the patient's well-being and are yet to be evaluated. Therefore, clinical and primary diagnosis based on routine laboratory findings bears high importance during the initial period of COVID-19, especially in regions with fewer diagnostic facilities.
Methods: Clinical information and associated complaints of the COVID-19 illness confirmed by reverse transcription-polymerase chain reaction (RT-PCR) were collected directly from the patients. Regular follow-ups were obtained on the phone every 2 weeks following recovery for 20 weeks. Initial hematological and radiology findings of the hospitalized patients except for intensive care unit (ICU) and high dependency units (HDUs) and a follow-up evaluation after 4 weeks following recovery were analyzed.
Results: The post-COVID-19 persisting symptoms/complaints were found among 21.4% of symptomatic patients, which persisted for ≥20 weeks and had a significant relationship with the duration of COVID-19 illness and the existing comorbidity (p < 0.05). Post-COVID-19 primary type 2 diabetes mellitus (DM, 0.64%) and hypertension (HTN, 1.28%) and unstable DM (54.55%) and HTN (34.78%) to the pre-existing diabetic and hypertensive patients were observed. Post-recovery remarkable changes in the laboratory values included leukocytosis (16.1%), lymphocytosis (14.5%), and an increased prothrombin time (PT, 25.8%). Abnormalities in the D-dimer, serum ferritin, hemoglobin, and erythrocyte sedimentation rate (ESR) levels were present to an extent. Laboratory findings like chest X-ray, ESR, white blood cell (WBC) count, lymphocyte count, C-reactive protein (CRP), serum glutamic pyruvic transaminase (SGPT), serum ferritin, PT, D-dimer, and serum creatinine are important markers for the diagnosis and prognosis of COVID-19 illness (p < 0.05).
Conclusion: Post-COVID-19 persisting symptoms and the changes in the laboratory values need to be considered with importance and as a routine clinical measure. Post-COVID-19 periodic follow-up for evaluating the patient's physical condition and the biochemical values should be scheduled with care and managed accordingly to prevent future comorbidity in patients with the post-COVID-19 syndrome.
Introduction
Coronavirus disease 2019 (COVID-19) or severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is a current global pandemic caused by an RNA virus of the beta Coronavirus family (1). After the first report in the Wuhan province of China, the disease quickly spread all over the globe. However, the presentation of COVID-19 varies depending on the region of origin. Thus, in a region with inadequate facilities, primary identification of SARS-CoV-2 infection remains a challenge. Furthermore, the overlapping clinical presentation of COVID-19 with local viral flu often misleads the diagnosis, thus causing morbidity, mortality, and further spread of the infection.
Additionally, like an emerging disease, managing the persisting symptoms/complaints and the abnormality of the laboratory values following the post-COVID-19 recovery period is complex. Proper understanding of these complaints is necessary for both the healthcare professionals and the patients, as it has a broad impact on patient well-being and health status. The changes in the laboratory values following COVID-19 recovery are yet to be evaluated. This manuscript is intended to investigate the acute and post-recovery manifestations of COVID-19 illness, including the post-COVID-19 persisting symptoms/complaints and changes in the laboratory parameter, namely, post-COVID-19 syndrome among the patients in Bangladesh.
Methods
Study Population and Data Collection
The list of SARS-CoV-2 confirmed cases by reverse transcription-polymerase chain reaction (RT-PCR) was identified and collected from the local health facilities of the Chattogram division in Bangladesh. All the patients took treatments in different COVID-19 designated hospitals. Preliminary information of the symptomatic cases that required treatments regarding the COVID-19 illnesses was collected directly from the outpatient department (OPD) and the hospitalized patients. These included disease symptoms, history, comorbid condition, and associated complaints. In addition, initial radiology and laboratory tests such as chest computed tomography (CT), X-ray chest P/A view, hemoglobin level, erythrocyte sedimentation rate (ESR), total and differential counts of white blood cell (WBC), red blood cell (RBC), platelet, C-reactive protein (CRP), serum glutamic pyruvic transaminase (SGPT), serum ferritin, prothrombin time (PT), D-dimer, and serum creatinine were performed, and the values were noted. In addition, febrile patients were tested for dengue NS1 antigen, dengue immunoglobulin G (IgG) and immunoglobulin M (IgM) antibody, Salmonella Typhi/Para-Typhi IgM and IgG antibody, immunochromatographic test (ICT) for malaria, Widal test to exclude dengue, malaria, and enteric fever.
Ethical Consideration and Consent
Ethical committee approval was taken from Xi'an Jiaotong University, China. Informed written consent was taken in every case. In the case of the below 16 years old participants, written informed consent was obtained from a parent or guardian.
Exclusion Criteria
Critically ill COVID-19 patients in the intensive care unit (ICU) and high dependency units (HDUs); patients who had a preexisting uncontrolled comorbid condition with compromised organ function such as severe chronic obstructive pulmonary disease (COPD) exacerbation, advanced ischemic heart disease, severe uncontrolled diabetes mellitus (DM), advanced renal and hepatic disease, and advanced stage of carcinoma; and prehospitalized (not due to COVID-19), immunocompromised, and pregnant patients were not included in this study. In addition, those who had a recent history of hematological, biochemical, or chest radiograph abnormality within the last 30 days were excluded. Expired patients during this study who missed the regular follow-up schedule twice and who missed the follow-up laboratory evaluation and those who were unwilling to continue participation were also excluded from the study.
Post-COVID-19 Persisting Symptoms and Hematological Manifestations
COVID-19 recovery was defined by two negative PCR 7 days apart. The recovery time was calculated from the date of the first negative PCR. COVID-19 symptoms or newer symptoms, which persisted or appeared following the COVID-19 recovery, were identified as persisting symptoms. Regular follow-ups were obtained on the phone every 2 weeks from each participant to identify and analyze the post-COVID-19 persisting symptoms/complaints. This process continued for 20 weeks. The symptoms that may have been attributed to other illnesses during this study by any current or chronic condition were carefully examined and not included in the study.
An initial laboratory assessment while hospitalized and a post-COVID-19 follow-up evaluation after 4 weeks were done to understand the changes. The follow-up laboratory evaluations included X-ray chest P/A view, a complete blood count (hemoglobin level, ESR, total and differential count of WBC, RBC, platelet), CRP, SGPT, serum ferritin, PT, D-dimer, and serum creatinine. In addition, patients with persisting fever were tested for dengue, malaria, and enteric fever. In the cases of laboratory abnormalities, treatments were given to the patients; therefore, no further laboratory follow-up data were collected.
Data and Statistical Analysis
Data were presented as mean ± standard deviation, and statistical analysis was carried out using SPSS V25.0. Column statistics, chi-square test, and a t-test with a 95% confidence interval were done to see the significance of the necessary values. p < 0.5 was considered statistically significant.
Results
Demographics and Mortality Rate Among the Study Population
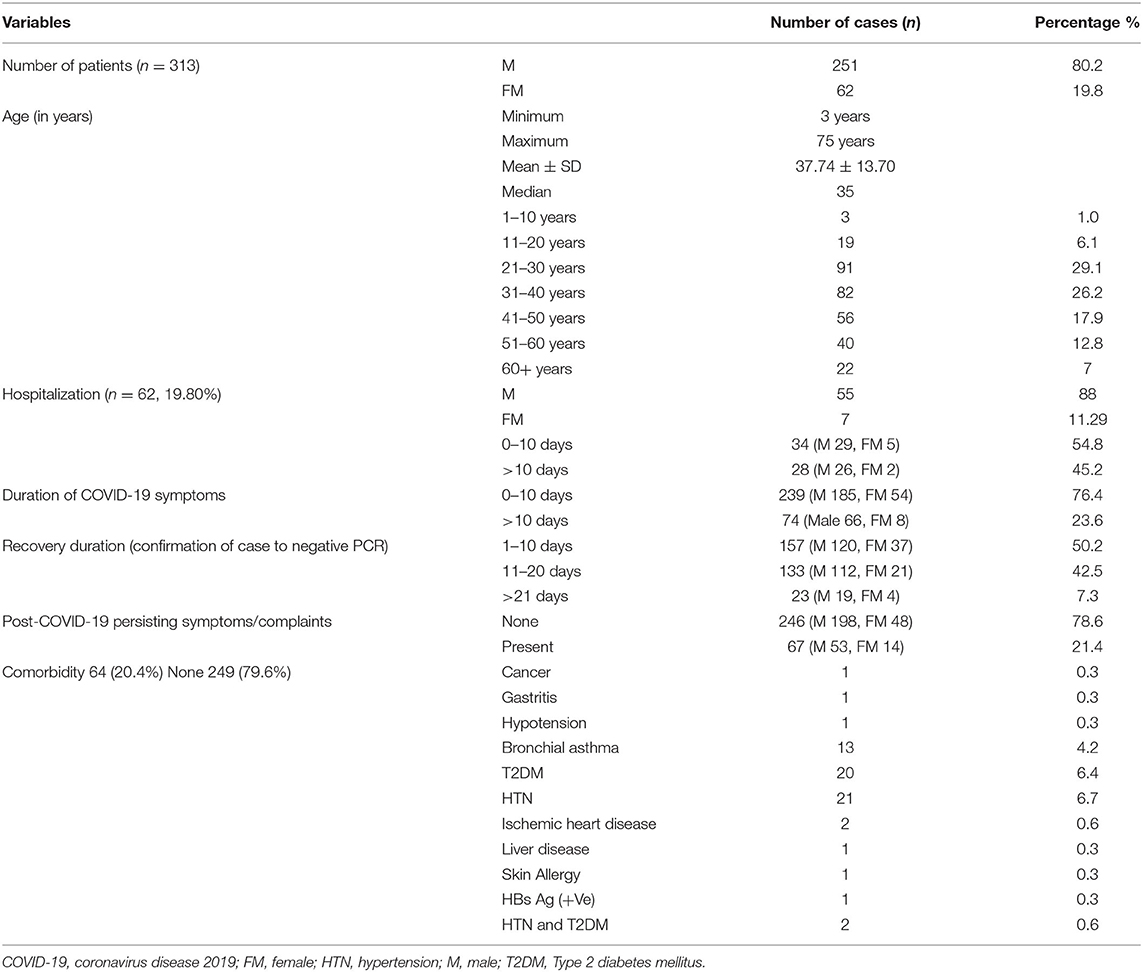
Initially, 512 symptomatic COVID-19 cases were included. Among them, 53 patients refused to participate in the study. Out of the rest, 83 were hospitalized, and 376 were OPD cases. While continuing treatment, 12 OPD patients required hospitalization. Later, they were included among the hospitalized patients. Therefore, 95 hospitalized and 364 OPD cases were enrolled. After enrollment, 33 hospitalized patients were excluded during the study; 11 had preexisting comorbid conditions that fit the exclusion criteria, 10 died during the treatment, and 12 were lost during follow-up. Among the OPD participants, 113 were excluded from the study; 45 patients had preexisting conditions, 48 were unable to maintain regular follow-up, and 20 were unwilling to continue participating. Finally, 313 patients were included in the study for analysis, among whom 62 were hospitalized and 251 were OPD patients. The mortality rate among the COVID-19 patients in our study was 1.96%. The hospitalization rate among the OPD-treated patients was 3.2%.
General Characteristics of the Study Population
The total number of patients was 313; 251 (80.2%) male and 62 (19.8%) female. The mean age was 37.74 years, range from 3 to 75 years. Patients according to the age-groups were 3 (1–10 years), 19 (11–20 years), 91 (21–30 years), 82 (31–40 years), 56 (41–50 years), 40 (51–60 years), and 22 (60+ years). The age-group 21–30 years was the most affected, consisting of 91 (29.1%) patients. Among the hospitalized patients (n = 62), (19.80%), 55 (88%) were male and 7 (11.29%) were female. In addition, 239 (74%) patients experienced <10 days and 74 (23.6%) experienced >10 days of COVID-19-related symptoms. Sixty-seven (21.4%) of the total study population had post-COVID-19 persisting symptoms/complaints. Moreover, 20.4% of the patients had comorbidities such as hypertension (HTN) [21 (6.7%)] and Type 2 diabetes mellitus (T2DM) [20 (6.4%)] (Table 1).
Clinical Characteristics of COVID-19 Patients
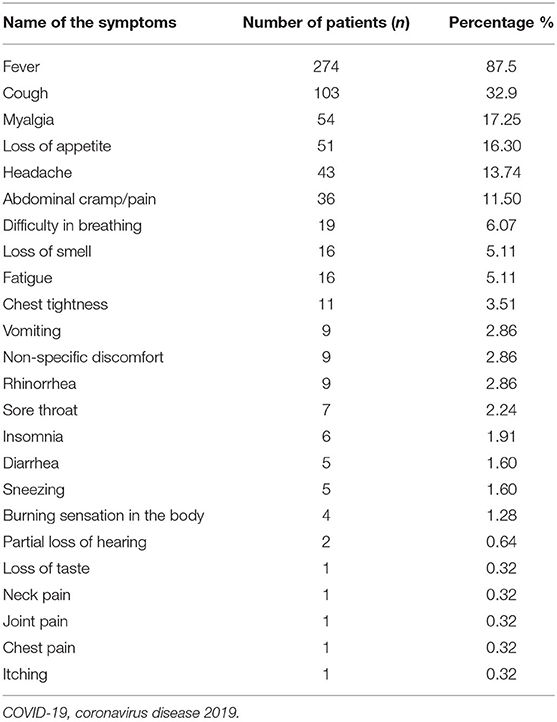
Most COVID-19 patients presented with fever [274 (87.5%)] and cough [103 (32.9%)]. Other symptoms were myalgia [54 (17.25%)], loss of appetite [51 (16.30%)], headache [43 (13.74%)], abdominal cramps/pain [34 (11.50%)], difficulty in breathing [19 (6.07%)], loss of smell [15 (5.11%)], fatigue [16 (5.11%)], chest tightness [11 (3.11%)], vomiting [9 (2.86%)], non-specific discomfort–unable to describe [9 (2.86%)], rhinorrhea [9 (2.86%)], sore throat [7 (2.24%)], insomnia [6 (1.91%)], diarrhea [5 (1.60%)], sneezing [5 (1.60%)], burning sensation in the body [4 (1.28%)], partial loss of hearing [2 (0.64%)], neck pain [1 (0.32%)], joint pain [1 (0.32%)], chest pain [1 (0.32%)], and itching [1 (0.32%)]. Nonetheless, anxiety was experienced by all of these patients at a certain level (Table 2).
A significant difference was found in a t-test among initial radiological and hematological parameters with the post-COVID-19 recovery follow-ups. These were chest X-ray consolidated, ESR, the total count of WBC, lymphocyte, CRP, serum ferritin, PT, D-dimer, and serum creatinine levels (p < 0.05). However, no significant differences were found in the neutrophil, RBC, platelet, and hemoglobin levels (p > 0.05; Table 3).
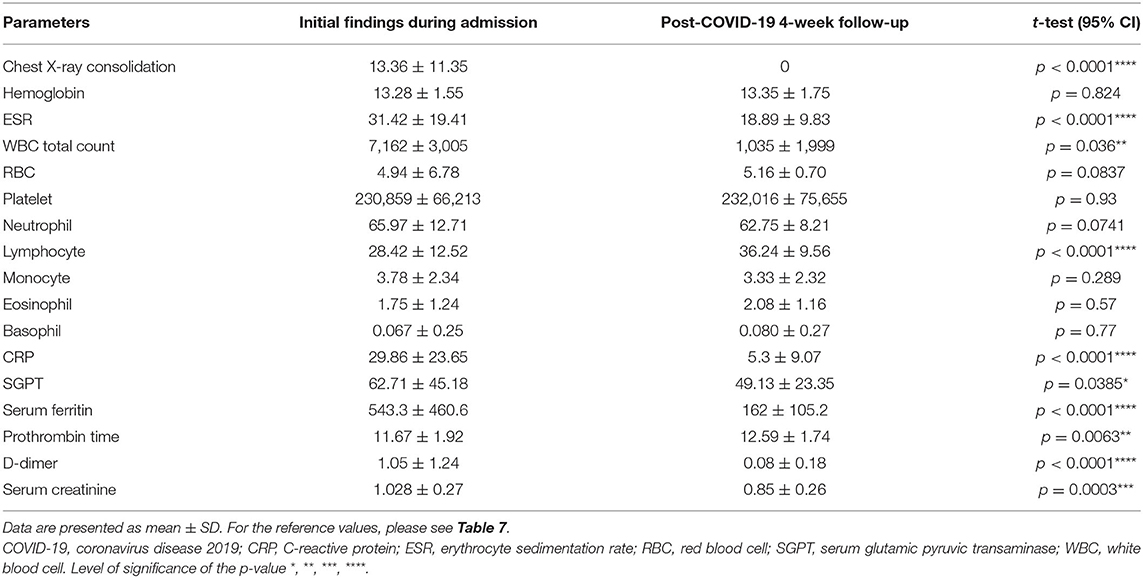
Table 3. Comparison between the initial on admission laboratory and radiology finding and post-COVID-19 recovery (following negative PCR) 4-week follow-up.
Post-COVID-19 Persisting Symptoms/Complaints and Their Characteristics
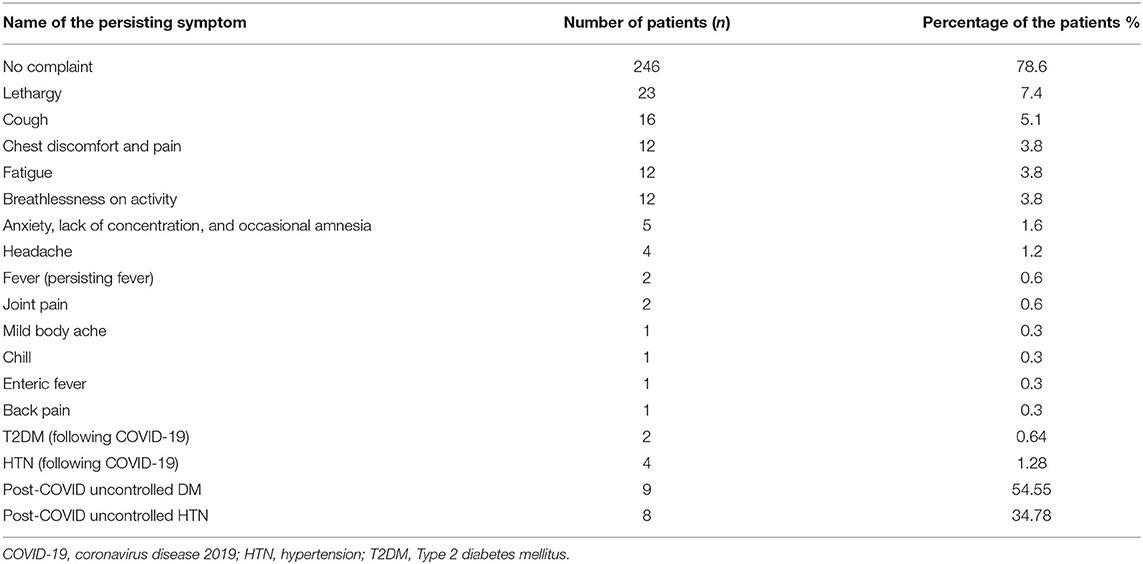
Out of the total, 78.6% (246) patients had no complaints following recovery. The post-COVID-19 persisting symptoms were lethargy [23 (7.4%)], cough [16 (5.1%)], chest discomfort and pain [12 (3.8%)], breathlessness on activity [12 (3.8%)], fatigue [12 (3.8%)], significant anxiety with lack of concentration and occasional amnesia that impaired daily activity [5 (1.6%)], headache [4 (1.2%)], low-grade fever [2 (0.6%)], and joint pain [2 (0.6%)]; mild body ache/chill/back pain was experienced by one (0.3%) each. Following recovery from COVID-19, 2 (0.64%) patients developed T2DM and 4 (1.28%) developed HTN. Nine (55.55%) of the total diabetic patients faced difficulties controlling the glycemic levels and had to take additional hypoglycemic therapy. Eight (34.78%) of the total hypertensive patients had to increase the dose or take additional antihypertensive therapy to achieve proper blood pressure control (Table 4).
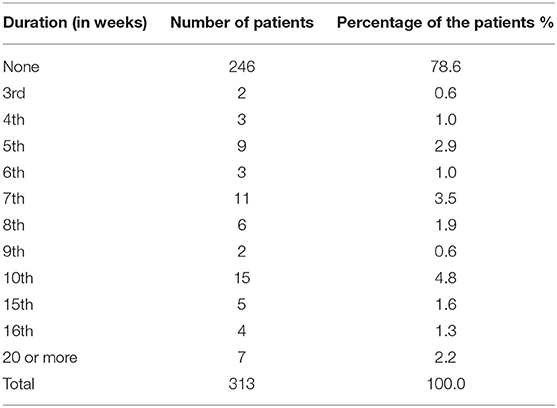
Furthermore, 0.6% of participants had the earliest relief from post-COVID-19 persisting symptoms on the third week from the recovery date. Fifteen (4.8%) recovered on the 10th week. Seven (2.2%) patients experienced 20 weeks or more duration of the persisting complaints (Table 5). However, the correlations between the duration of COVID-19 symptoms of 0–10 days and >10 days and post-COVID-19 persisting symptoms of 0–8 weeks and >8 weeks were statistically significant (**p = 0.00; Table 6).
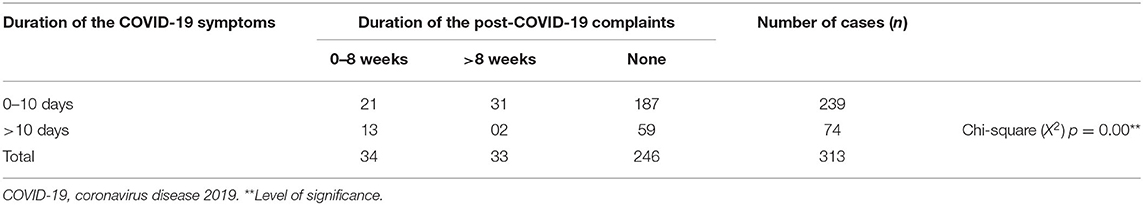
Table 6. Relation between the duration of the COVID-19 symptoms and the duration of post-COVID-19 persisting complaints.
Initial and Post-recovery Laboratory Manifestations of COVID-19 Cases
The mean age was 43.32 years (21–75 years). Forty-three (69.35%) patients had consolidations in the chest X-ray during admission, while at 4-week follow-up, three (0.48%) patients had mild features of pneumonitis. Lymphocytopenia was detected among 19 (30.6%). CRP was increased in all the patients during admission but in 11 (17.7%) during the follow-up. ESR, WBC, neutrophil, serum ferritin, D-dimer, and PT were increased in 43 (69.4%), 8 (12.9%), 11 (17.7%), 28 (45.1%), 31 (50%), and 14 (22.6%) patients during admission, while during follow-up, they were 34 (54.8%), 10 (16.1%), 4 (33.8%), 6 (9.7%), 4 (6.4%), and 16 (25.8%) subsequently. Males had a higher percentage (52.7%) of increased D-dimer during admission. Erythrocytopenia was found only in females. Moreover, females had a greater presentation of an increase in the CRP level (42.8%), serum ferritin (14.2%), PT (42.8%), lymphocyte (28.6%), and X-ray (mild features of pneumonitis) (14.2%) during the follow-up. However, D-dimer (7.3%) and total WBC (16.3%) levels were increased in the males (Table 7).
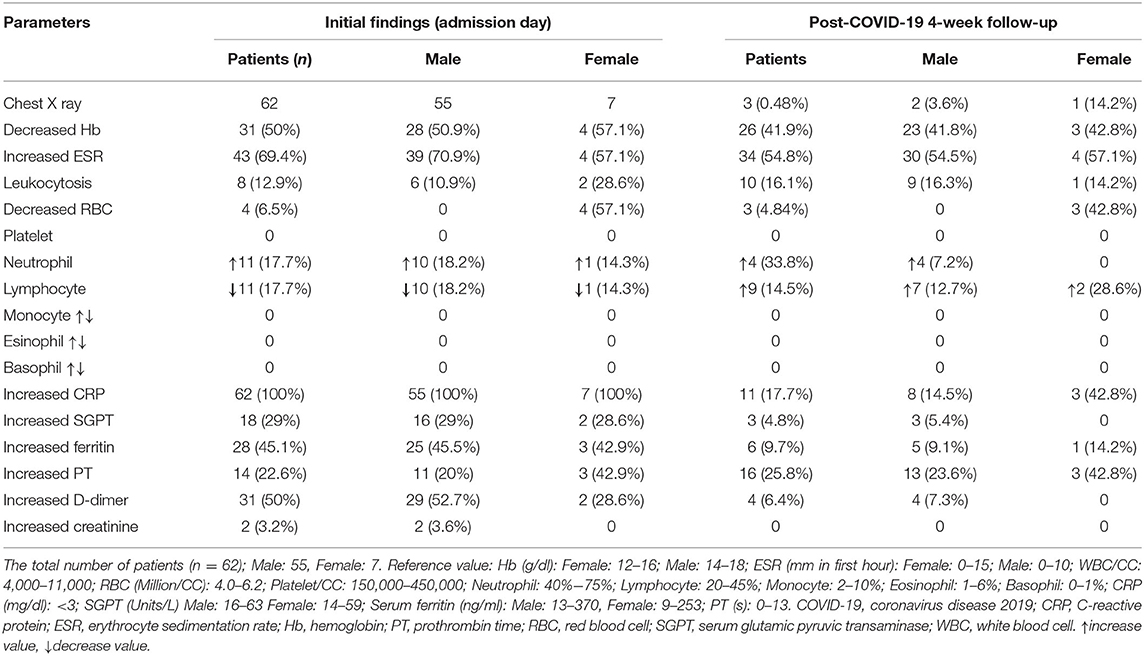
Table 7. Primary (on admission) hematological findings and the post-COVID-19 recovery (following negative PCR) 4-week follow-up of the hospitalized patients.
Subgroup Analysis of the Persisting Symptoms of COVID-19 Cases
Lethargy mainly was seen in age-group 21–30 years (nine patients) and cough (four patients each) in age-groups 21–30, 31–40, 41–50, 51–60 years. Chest pain and breathlessness on activity were dominant in 31–40 years (six patients) and anxiety in 11–20 years (four patients). Four patients 51–60 years of age complained of headache, and two patients each among those aged 41–50 and 31–40 years complained of breathlessness on activity and joint pain. Back pain and myalgia were experienced by two patients of the age-group 21–30 years. In addition, 53 (11.2%) males and 14 (4.5%) females had post-COVID-19 persisting symptoms. Chest pain (12), breathlessness on activity (2), and joint pain (2) were solely complained about by the male participants. On the other hand, six female participants suffered from persisting fever with headache and lethargy. The difference between patients having persisting symptoms according to gender was not statistically significant (p = 0.277) (Supplementary Table 1).
According to age-group, subgroup analyses of patients with persisting symptoms and the duration of the persisting symptoms were done. This revealed that 1–10 years had no complaints, and the 41–50 years age-group had post-COVID-19 complaints that persisted for >20 weeks. The age-groups 21–30 and 41–50 years started to recover the earliest at 3 weeks and the complain persisted up to 16 weeks, and 8 weeks post-recovery respectively; and those were 41–50 years complained up to 20+ weeks (Supplementary Table 2).
Subgroup Analysis of Duration of the Persisting Symptoms Among the Comorbid Patients
A total of 26 patients who experienced post-COVID-19 persisting symptoms had comorbidity. HTN was found in 11 patients; among these patients, four patients experienced a delayed recovery from the post-COVID-19 symptoms/complaints about ≥20 weeks. T2DM and bronchial asthma were found among 12 patients (six each). They had an earlier recovery from the post-COVID-19 persisting symptoms on the 15th and 12th week. Among the persisting symptoms, fever was experienced by hypertensive patients. Lethargy was complained about by 12 patients; among these 12 patients, five had HTN, four had T2DM, two had bronchial asthma, and one had skin allergy. Persisting cough was complained about by five patients, and four who had bronchial asthma suffered from chest pain and breathlessness on activity until the 10th week following a negative PCR. A significant relationship was found between the comorbidity and the duration of the post-COVID-19 persisting symptoms p = 0.000 in the chi-square test (Supplementary Table 3).
Discussion
SARS-CoV-2 is a systemic infection causing a cytotoxic storm that exerts an effect on the homeostatic mechanism (2). The incubation period of COVID-19 can be up to 14 days. Presenting symptoms of COVID-19 may differ from mild to a severe illness expressed by respiratory distress, chest tightness, or pain. COVID-19 symptoms might present with fever, cough, myalgia, chest tightness, breathing difficulty, sore throat, and loss of taste or smell. A deteriorating sign of SARS-CoV-2 infection includes central cyanosis, confusion, respiratory difficulty, persistent chest pain, heaviness in the chest, and inability to stay awake (3). Studies have reported differences in presenting symptoms of COVID-19 illness, depending on regions. However, several symptoms are common among all the reports. Hematological manifestations of COVID-19 are also different depending on the geographical locations and socioeconomic conditions. Moreover, post-COVID-19 persisting symptoms/complaints and associated laboratory manifestations are yet to be evaluated.
The impact of the patient's clinical characteristics and post-recovery manifestations of the COVID-19 patients will shed light on this disease's systematic management (4). Initial investigations from China have shown that clinical symptoms such as fever, cough, dyspnea, and hematological findings of COVID-19 patients are like those in SARS-CoV (5–8). Heterogeneous clinical manifestations have been discovered in COVID-19 patients from different areas of China (9–16), Europe (17, 18), US (19, 20), Malaysia (21), Jordan (22), Kuwait (23), South Korea (24), and Africa (25). Thus, understanding the clinical features can help clinicians predict the disease's progression and apply possible intervention approaches.
This study evaluated the clinical characteristics of COVID-19 illness, post-COVID-19 persisting symptoms/complaints, and changes in the laboratory findings following COVID-19 recovery among the patients in Bangladesh. Our study contains 313 symptomatic patients 3–75 years of age. Among them, 251 (80.2%) were male and 62 (19.8%) were female. The age-groups 20–30 years and 31–40 years were the most affected by SARS-CoV-2 infection, 91 (29.1%) and 82 (26.2%). Overall, 50.2% of the patients recovered within 10 days, 42.5% recovered within 10–20 days, and 7.3% required >21 days to recover. Sixty-seven (21.4%) of the total participants experienced varying degrees of persisting symptoms/complaints following COVID-19 recovery that persisted up to 20 weeks and more. Fifty-three (21.11%) of the males and 14 (22.5%) of the female participants had experienced post-COVID-19 persisting complaints. The distribution of these symptoms was almost similar in both genders. Furthermore, 21.6% of the COVID-19 patients had comorbid conditions, e.g., T2DM, HTN, ischemic heart disease, and bronchial asthma. Diabetic and hypertensive patients were 6.4 and 6.7% (Table 1).
Moreover, in our study, the duration of COVID-19 symptoms and the duration of the post-COVID-19 persisting symptoms/complaints had a significant correlation (****p = 0.00; Table 6). This signifies a longer duration of the illness caused by the prolongation of the post-recovery symptoms.
According to recent studies, COVID-19 symptoms can occasionally continue for months. Thus, the SARS-CoV-2 virus can increase the incidence of long-standing health complications. Carfi et al. (26), in their study, found that in patients who had recovered from COVID-19, 87.4% reported persistence of at least one symptom, especially fatigue and dyspnea. Balachandar et al. (27) also conducted a longitudinal study to evaluate the COVID-19 recovered patient's health status, which revealed that COVID-19 might have the potential impact to cause multiorgan damage. Another study on post-COVID-19 recovery in patients with or without preexisting diabetes by Akter et al. (28) found that people with diabetes have faced severe COVID-19 manifestation and post disease problems. Greenhalgh et al. (29) also reported that nearly 10% of individuals experience persistent illness after COVID-19 infection. Subsequently, another study also reported persisting symptoms of COVID-19 (30). However, studies on persisting symptoms of COVID-19 are scanty. Therefore, it is suggested that follow-up study of COVID-19 recovered patients would be useful to assess any changes in the other organs.
According to this study, COVID-19 patients in this region presented with fever, cough, myalgia, loss of appetite, headache, abdominal cramps, breathing difficulty, loss of smell, fatigue, chest tightness, vomiting, rhinorrhea, sore throat, insomnia, diarrhea, and sneezing (Table 2). However, all participants complained about anxiety, though it was not reported as a symptom in this study. A total of 78% of the participants had no complaints following recovery. Though all the persisting symptoms found in our study caused impairment of routine activity, 2 (0.64%) had developed T2DM and 4 (1.28%) patients developed HTN following recovery. Many preexisting diabetic and hypertensive patients experienced difficulties controlling their normal level and had to receive additional therapy.
The extent of the post-COVID-19 persisting symptoms was at least 3 weeks up to 20 weeks and more. The greatest number of recoveries from post-COVID-19 persisting complaints was on the 10th week, followed by the seventh and fifth weeks among the participants (Table 5). However, the very early age-group of 0–10 years had no report of persisting symptoms (Supplementary Tables 1, 2). According to the initial hematological findings (Table 7), CRP was increased in all the patients. A notable percentage of patients had an increased ESR, WBC, CRP, SGPT, serum ferritin, PT, D-dimer, and serum creatinine level. Approximately 50% of patients had decreased hemoglobin levels, and 6.5% had decreased RBC levels.
An increased percentage of lymphocytosis, leukocytosis, and high PT was observed in the 4-week post-recovery follow-up (Tables 3, 7). No patient had consolidation on chest X-ray during post-COVID-19 4-week follow-up, except three patients who had mild pneumonitis features. However, the new increase in WBC level and PT is a matter of deep concern. There were significant differences in several of these parameters between initial findings during admission and post-COVID-19 4-week follow-up. These parameters are chest X-ray consolidated, ESR, WBC, lymphocyte, CRP, SGPT, serum ferritin, PT, D-dimer, and serum creatinine level (p < 0.05; Table 3). This proves the reliability and high accuracy of these parameters in the diagnosis and prognosis of COVID-19 illness. Evaluation of the underlying cause and management of increased PT, lymphocyte, and WBC demand close attention in the case of post-COVID-19 patients. Though it might be a positive feedback response to hemodilution therapy, further study is required for a better understanding of the cause and management of this condition.
Our study had several limitations. The sample size was relatively small; the follow-up radiology and laboratory values were evaluated only once after 4 weeks; critically ill patients in the ICU were not included. Recovery analysis based on the different drug treatments was not done, and the death cases were not accessed. Despite the limitations, we tried to analyze the clinical features and post-recovery manifestations of the COVID-19 cases without any existing comorbid conditions.
Conclusion
COVID-19 epidemic is a global concern. Primary diagnosis of SARS-CoV2 infection is crucial for the proper management and control of the epidemic, especially in the low facility regions where a PCR and CT chest are not readily available. In our study, 21.4% of the symptomatic COVID-19 patients suffered from persisting symptoms/complaints following recovery, which might persist for more than 20 weeks. They included lethargy, cough, chest discomfort and pain, breathlessness on activity, fatigue, anxiety with lack of concentration and occasional amnesia, headache, persistent low-grade fever, joint pain, body ache, chill, and back pain. Post-COVID-19 development of T2DM (0.64%) and HTN (1.28%) and also the development of unstable DM (54.55%) and HTN (34.78%) to the pre-existing DM and hypertensive patients are a matter of immediate concern. The post-COVID-19 significant changes in the laboratory values included leukocytosis (16.1%), lymphocytosis (14.5%), and an increased PT (25.8%). Besides, an abnormality in the D-dimer, serum ferritin, hemoglobin, and ESR levels was also present to an extent. Moreover, existing comorbidity among the COVID-19 patients increases the risk and duration of the post-COVID-19 persisting symptoms. Management of the post-COVID-19 persisting symptoms and the abnormal laboratory values appeared to be a challenge for healthcare professionals. Better understanding of these conditions is equally important for the patients. A periodic follow-up and evaluation of the patient's physical condition and the biochemical values will help prevent future comorbidity in patients with the post-COVID-19 syndrome.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Ethical Committee approval was taken from Xi'an Jiaotong University, China. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
AM: research concept, data collection, data interpretation, statistical analysis, manuscript writing, and editing. MK: data collection, manuscript writing, and editing. MA: statistical analysis. JI: data collection. YL: interpreted the data of COVID-19 patients. SH: review of the manuscript, maintained supervision, and ensured the quality of the research. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are thankful for the support and cooperation of the First Affiliated Hospital of Xi'an Jiaotong University, Ministry of Health and Family Welfare, Bangladesh, Drs. Mohammad Shahbaz, Wazed Chowdhury, and Hamid Hasan.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.663670/full#supplementary-material
Abbreviations
COVID-19, coronavirus disease 2019; CDC, Centers for Disease Control and Prevention; CRP, C-reactive protein; CT, computed tomography; DM, diabetes mellitus; ESR, erythrocyte sedimentation rate; HTN, hypertension; IgG, immunoglobulin G; IgM, immunoglobulin M; IEDCR, Institute of Epidemiology, Disease Control and Research; LMWH, low-molecular weight heparin; MERS, Middle East respiratory syndrome; OPD, outpatient department; PT, prothrombin time; RBC, red blood cell; RT-PCR, reverse transcription-polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SGPT, serum glutamic pyruvic transaminase; T2DM, Type 2 diabetes mellitus; WBC, white blood cell.
References
1. Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. (2020) 63:457–60. doi: 10.1007/s11427-020-1637-5
2. Thevarajan I, Buising KL, Cowie BC. Clinical presentation and management of COVID-19. Med J Aust. (2020) 213:134–9. doi: 10.5694/mja2.50698
3. Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. (2020) 92:401–2. doi: 10.1002/jmv.25678
4. Zheng XY, Guan WJ, Zhong NS. Clinical characteristics of COVID-19 in developing countries of western pacific: low case-fatality rate unraveled. Lancet Reg Health West Pac. (2021) 6:100073. doi: 10.1016/j.lanwpc.2020.100073
5. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
6. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
7. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019. Novel coronavirus-infected pneumonia in Wuhan, China. JAMA. (2020) 323:1061–9. doi: 10.1001/jama.2020.1585
8. Fu L, Wang B, Yuan T, Chen X, Ao Y, Fitzpatrick T, et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J Infect. (2020) 80:656–65. doi: 10.1016/j.jinf.2020.03.041
9. Li Z, Lijun D, Yangyang Z, Fu W, Gao Y, Zhang Z, et al. Clinical characteristics and risk factors for disease severity and death in patients with coronavirus disease 2019 in Wuhan, China. Front Med. (2020) 7:532. doi: 10.3389/fmed.2020.00532
10. Nie Y, Li J, Huang X, Guo W, Zhang X, Ma Y, et al. Epidemiological and clinical characteristics of 671 COVID-19 patients in Henan Province, China. Int J Epidemiol. (2020) 49:1085–95. doi: 10.1093/ije/dyaa081
11. Zhang SY, Lian JS, Hu JH, Zhang XL, Lu YF, Cai H, et al. Clinical characteristics of different subtypes and risk factors for the severity of illness in patients with COVID-19 in Zhejiang, China. Infect Dis Poverty. (2020) 9:85. doi: 10.1186/s40249-020-00710-6
12. Yao Y, Chen W, Wu X, Shen L, Shen L, Fu Y, et al. Clinical characteristics of COVID-19 patients in three consecutive generations of spread in Zhejiang, China. Clin Microbiol Infect. (2020) 26:1380–5. doi: 10.1016/j.cmi.2020.06.018
13. Jin A, Yan B, Hua W, Feng D, Xu B, Liang L, et al. Clinical characteristics of patients diagnosed with COVID-19 in Beijing. Biosaf Health. (2020) 2:104–11. doi: 10.1016/j.bsheal.2020.05.003
14. Shu L, Wang X, Li M, Chen X, Ji N, Shi L, et al. Clinical characteristics of moderate COVID-19patients aggravation in Wuhan stadium cabin hospital: a 571 cases of retrospective cohort study. J Med Virol. (2021) 93:1133–40. doi: 10.1002/jmv.26414
15. Kaur N, Gupta I, Singh H, Karia R, Ashraf A, Habib A, et al. Epidemiological and clinical characteristics of 6635 COVID-19 patients: a pooled analysis. SN Compr Clin Med. (2020) 2:1–5. doi: 10.1007/s42399-020-00393-y
16. Qian SZ, Hong WD, Mao L, Lin C, Fang Z, Pan JY. Clinical characteristics and outcomes of severe and critical patients with (2019). Novel coronavirus disease (COVID-19) in Wenzhou: a retrospective study. Front Med. (2020) 7:552002. doi: 10.3389/fmed.2020.552002
17. Gil-Rodrigo A, Miró Ò, Piñera P, Burillo-Putze G, Jiménez S, Martín A, et al. Analysis of clinical characteristics and outcomes in patients with COVID-19 based on a series of 1000 patients treated in Spanish emergency departments. Emergencias. (2020) 32:233–41.
18. Alkundi A, Mahmoud I, Musa A, Naveed S, Alshawwaf M. Clinical characteristics and outcomes of COVID-19 hospitalized patients with diabetes in the United Kingdom: a retrospective single centre study. Diabetes Res Clin Pract. (2020) 165:108263. doi: 10.1016/j.diabres.2020.108263
19. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. (2020) 323:2052–9. doi: 10.1001/jama.2020.6775
20. Tusha J, Khanam V, Tegeltija V, Kumar S. Clinical characteristics and outcomes of patients with COVID-19 in a community hospital in Michigan. Chest. (2020) 158:A1175. doi: 10.1016/j.chest.2020.08.1069
21. Sim BLH, Chidambaram SK, Pathmanathan MD, Pathmanathan MD, Peariasamy KM, Hor CP, et al. Clinical characteristics and risk factors for severe COVID-19 infections in Malaysia: a nationwide observational study. Lancet Reg Health West Pac. (2020) 4:100055. doi: 10.1016/j.lanwpc.2020.100055
22. Khraise WN, Khraise TW, Starling Emerald B, Allouh MZ. Epidemiologic and clinical characteristics of COVID-19 patients from a quarantine center in a developing community: a retrospective study. Int J Gen Med. (2020) 13:937–44. doi: 10.2147/IJGM.S276742
23. Alshukry A, Ali H, Ali Y, Al-Taweel T, Abu-Farha M, AbuBaker J, et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) patients in Kuwait. PLoS ONE. (2020) 15:e0242768. doi: 10.1371/journal.pone.0242768
24. Choi MH, Ahn H, Ryu HS, Kim BJ, Jang J, Jung M, et al. Clinical characteristics and disease progression in early-stage COVID-19 patients in South Korea. J Clin Med. (2020) 9:1959. doi: 10.3390/jcm9061959
25. Nachega JB, Ishoso DK, Otokoye JO, Hermans MP, Machekano RN, Sam-Agudu NA, et al. Clinical characteristics and outcomes of patients hospitalized for COVID-19 in Africa: early insights from the democratic republic of the congo. Am J Trop Med Hyg. (2020) 103:2419–28. doi: 10.4269/ajtmh.20-1240
26. Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. (2020) 324:603–5. doi: 10.1001/jama.2020.12603
27. Balachandar V, Mahalaxmi I, Subramaniam M, Kaavya J, Senthil Kumar N, Laldinmawii G, et al. Follow-up studies in COVID-19 recovered patients - is it mandatory? Sci Total Environ. (2020) 729:139021. doi: 10.1016/j.scitotenv.2020.139021
28. Akter F, Mannan A, Mehedi HMH, Rob MA, Ahmed S, Salauddin A, et al. Clinical characteristics and short term outcomes after recovery from COVID-19 in patients with and without diabetes in Bangladesh. Diabetes Metab Syndr. (2020) 14:2031–8. doi: 10.1016/j.dsx.2020.10.016
29. Greenhalgh T, Knight M, A'Court C, Buxton M, Husain L. management of post-acute COVID-19 in primary care. BMJ. (2020) 370:m3026. doi: 10.1136/bmj.m3026
Keywords: COVID-19, SARS-CoV-2, clinical characteristics, post-recovery manifestations, Bangladesh, post-COVID-19 syndrome
Citation: Mohiuddin Chowdhury ATM, Karim MR, Ali MA, Islam J, Li Y and He S (2021) Clinical Characteristics and the Long-Term Post-recovery Manifestations of the COVID-19 Patients—A Prospective Multicenter Cross-Sectional Study. Front. Med. 8:663670. doi: 10.3389/fmed.2021.663670
Received: 03 February 2021; Accepted: 21 June 2021;
Published: 17 August 2021.
Edited by:
Jiufeng Sun, Guangdong Provincial Institute of Public Health, ChinaReviewed by:
Abraham S. Alabi, Centre de Recherche Médicales de Lambaréné, GabonXuhui Chen, Huazhong University of Science and Technology, China
Anda Baicus, Carol Davila University of Medicine and Pharmacy, Romania
Copyright © 2021 Mohiuddin Chowdhury, Karim, Ali, Islam, Li and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuixiang He, ZHl5eWp4a0B4anR1LmVkdS5jbg==; Abu Taiub Mohammed Mohiuddin Chowdhury, ZHJfbW9oaXVkZGluY2h5QHlhaG9vLmNvbQ==
 Abu Taiub Mohammed Mohiuddin Chowdhury
Abu Taiub Mohammed Mohiuddin Chowdhury Md Rezaul Karim
Md Rezaul Karim Md. Ahasan Ali4
Md. Ahasan Ali4