- 1Graduate College, Beijing University of Traditional Chinese Medicine, Beijing, China
- 2Department of Gastroenterology, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 3China Academy of Chinese Medical Sciences, Beijing, China
Background: Irritable bowel syndrome with diarrhea (IBS-D) is a chronic functional gastrointestinal disorder that has a significant impact on quality of life, work productivity, and healthcare resources. External therapy of traditional Chinese medicine (TCM) has positive effects on IBS-D and is simple, convenient, and low-cost. This study aimed to systematically evaluate the efficacy and safety of external therapy of TCM for IBS-D.
Methods: This study was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. The PubMed, Embase, Cochrane Library, Web of Science, China National Knowledge Infrastructure (CNKI), Chinese Scientific Journals (VIP), Wan Fang, and Chinese Biomedical (CBM) databases were electronically searched to collect randomized controlled trials comparing external therapy of TCM with Western medicine for IBS-D from inception to 31 December 2021. Two authors independently screened, extracted, and assessed the selected studies. The Jadad scale and Cochrane Collaboration Risk of Bias tool were used to evaluate study quality. The certainty of evidence was assessed using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE). The meta-analysis was performed using the Review Manager software (version 5.3).
Results: Twenty-one studies involving 1,862 subjects were included. Acupuncture and moxibustion were the most commonly used external therapies. The meta-analysis showed that based on total effective rate with moderate certainty of evidence (n = 21 studies, n = 1,862 participants, RR = 1.25, 95% CI [1.2, 1.31], I2 = 0%, P < 0.00001), clinical cure rate with low certainty of evidence (n = 17 studies, n = 1,502 participants, RR = 1.66, 95% CI [1.4, 1.96], I2 = 1%, P < 0.00001), recurrence rate with very low certainty of evidence (n = 5 studies, n = 260 participants, RR = 0.44, 95% CI [0.34, 0.58], I2 = 0%, P < 0.00001), total symptom score (MD = −4.9, 95% CI [−7.34, −2.47]), and IBS severity scoring system score (IBS-SSS) with moderate certainty of evidence (MD = −52.72, 95% CI [−63.9, −41.53]), the experimental group had significant advantages compared with the control group. The sensitivity analysis further confirmed the robustness of the primary outcomes. The improvement in quality of life associated with IBS (IBS-QOL) was superior in the experimental group compared to the control group, and the difference was statistically significant; however, the clinical heterogeneity was strong. The inverted funnel plot of the included studies indicated a potential publication bias.
Conclusion: External therapy of TCM for IBS-D alleviated abdominal symptoms, improved clinical effectiveness, and reduced recurrence with great safety. However, because of the limitations of publication bias in trials, more rigorous studies with a clinical design are necessary for further verification of the outcomes.
Systematic Review Registration: [https://www.crd.york.ac.uk/PROSPERO/], identifier [CRD42020222993].
Introduction
Irritable bowel syndrome (IBS) is a chronic functional gastrointestinal disorder characterized by recurrent abdominal pain and bloating, altered bowel habits, and stool irregularities without structural or biochemical abnormalities (1). IBS is further categorized into four subtypes depending on stool consistency rather than stool frequency: IBS with constipation (IBS-C), IBS with diarrhea (IBS-D), IBS with a mixed stool pattern (IBS-M), and IBS unsubtyped (IBS-U). However, 40% of all cases are IBS-D (2, 3). Globally, the pooled prevalence of IBS is 10–20% (4). In China, the population of outpatients with IBS comprises more than half of the population attending clinics for digestive system problems, and 75% of these cases are mainly the IBS-D subtype (5). The pathophysiology of IBS is complex and poorly understood; it includes genetic predisposition, the gut-brain axis, visceral hypersensitivity, changes in the gut microbiome, alterations in gastrointestinal motility and intestinal permeability, low-grade mucosal inflammation, and immune system activation (6–10). Antidiarrheal medications, antispasmodic therapy, microecological preparations, and central neuromodulators are commonly used as medical therapies for IBS-D (11). However, their clinical efficacy for intestinal discomfort is unsatisfactory (12). Notably, IBS-D might become increasingly common because of trends in the Westernized diet and lifestyle behavior changes, thus representing a considerable burden to both healthcare service and the society because of costs of diagnosis and treatment (13, 14). IBS-D has a strong impact on the quality of life (QOL), work productivity, healthcare resources, and the society, and it has a strong economic impact because of its refractoriness and recurrence (15, 16).
The external therapy of traditional Chinese medicine (TCM) has a long history and culture. It provides a solid theoretical basis for treating various diseases and has the benefits of simplicity, convenience, and low cost. Additionally, it has been widely used for IBS-D. Several studies have shown that external therapy of TCM, such as acupuncture, moxibustion, and acupoint application, can be used to relieve symptoms and reduce the recurrence rate and adverse reactions associated with IBS-D (17–19). A variety of external therapies involving TCM has gained increasing attention because of their use as an IBS-D treatment. Furthermore, external therapy involving TCM has been included in the consensus on the diagnosis and treatment of IBS (2017 edition) for clinical guidance.
However, the clinical efficacy and safety of various external therapies involving TCM have not yet been statistically and systematically assessed. To more objectively investigate the curative effects of external therapy involving TCM for IBS-D, we collected randomized controlled trials (RCTs) of the IBS-D treatment. Then, we compared the efficacy of the external treatment of TCM with that of conventional Western medicine and conducted a meta-analysis. Additionally, we expected that our results would provide evidence-based suggestions for clinical practice and guide clinical applications more effectively.
Materials and methods
Study protocol
The protocol was registered with the International Prospective Register of Systematic Reviews (registration no. CRD42020222993).1 This study was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines of 2020 (20).
Search strategy and data sources
A comprehensive search strategy for relevant clinical trials was independently performed by two reviewers (YW and YW) using the following eight databases: Web of Science, Embase, PubMed, Cochrane Library, Chinese Biomedical Database (CBM), China National Knowledge Infrastructure (CNKI), Wan Fang, and Chinese Scientific Journals (VIP). Dates ranged from the inception of each of the different databases to 31 December 2021. There were no language restrictions. Because the methods of external therapy of TCM are abundant, we conducted a literature search of the eight aforementioned databases to include the maximum number of clinical trials. Search strategies and specific details are shown in Supplementary materials.
Study selection
Inclusion criteria
The following inclusion criteria for participants, intervention, comparator, study design, and study quality were used:
1) Participants: Patients diagnosed with IBS-D using specific criteria or internationally recognized criteria.
2) Intervention: The experimental group was treated with external therapy of TCM alone without any oral Chinese medicine or Western medicine.
3) Comparator: The control group was treated with conventional Western medicine treatment and without external therapy.
4) Study design: RCTs were designed with a sample size of ≥60. Moreover, treatment duration was ≥ 28 days.
5) Study quality: The methodological quality of each included study was assessed and had a Jadad score ≥3 (indicating a high-quality study) (21).
Exclusion criteria
The following exclusion criteria were used:
1) Duplicate publications (only the earliest publication by the same author was included).
2) Cases were not identified as the IBS-D subtype.
3) Publications were reviews, meta-analyses, animal experiments, conference abstracts, books, theses, or study protocols.
4) The control group was a self-control group or included healthy subjects without intervention.
5) Outcome measures of interest for the meta-analysis were not included.
Primary outcomes
The primary outcomes were total effective rate and clinical cure rate.
Criteria for total efficacy evaluation: IBS-D is a functional gastrointestinal disorder. The treatment goals for IBS-D are alleviation of abdominal pain and abdominal distension, reduction of the frequency of defecation, and improvement in stool form; the achievement of these goals was based on the participants’ self-assessment of diarrhea symptoms. Clinical effect was assessed based on changes in the patients’ self-reported major symptom scores. Total effectiveness rate was assessed using the comprehensive symptom score index (CSSI). The formula for calculating the CSSI was as follows: CSSI (%) = (score before−score after)/score before × 100%. When the CSSI (%) was ≥30%, a clinical effect was considered. If the CSSI (%) reached 90% or higher, then clinical recovery was considered. Treatments were recorded as effective, markedly effective, and clinically curative.
Secondary outcomes
Secondary outcomes were recurrence rate, total symptom score, IBS severity scoring system (IBS-SSS) questionnaire score, and IBS-QOL scale score.
Research screening process
First, duplicate records were eliminated according to the parameters of the title and author information using the NoteExpress software (Beijing Aegean Sea Music Technology Co., Ltd., Beijing, China). Subsequently, the remaining abstracts and full texts were independently reviewed by two authors using the inclusion and exclusion criteria (YW and YW). Disagreements were resolved by negotiation.
Data extraction and management
The two authors (W and YW) separately extracted the following data from each included study using predesigned tables: general information such as title, first author, and year of publication, study features such as method of randomization, allocation sequence generation, assignment concealment, blinding, and withdrawal, and data details such as sample size, age, disease duration, diagnostic criteria, outcome measures, intervention, duration, and adverse events. The included clinical trials were scored using the Jadad scale (22). Discrepancies between the two authors were resolved by a discussion resulting in a consensus, or an assessment by a third reviewer (XM).
Quality assessment
The methodological quality of the trials was assessed independently by two reviewers using the Jadad scale and the Cochrane Collaboration Risk of Bias tool. The quality of the studies used during this review was evaluated using the Jadad scale, which included randomization, blinding, and withdrawals and dropouts (22). Moreover, the risk of bias of the RCTs was appraised using the Cochrane Collaboration tool, which is composed of the following seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of the outcome assessment, incomplete outcome data, selective reporting, and other sources of bias. Three levels of bias, high risk, low risk, and unclear risk, were used to assess each domain, and graphs depicting this information were visualized. A funnel plot was used to assess for publication bias when the pooled analyses included more than 10 studies.
Grading certainty of evidence
The quality of evidence assessment of the primary and secondary outcomes was determined using Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) (23).
Statistical analysis
Data synthesis and statistical analysis using a random effects model were performed using Review Manager (version 5.3). For dichotomous outcomes, the relative risk (RR) and 95% confidence intervals (CIs) were presented as effects measured using the Mantel-Haenszel method. For continuous variable outcomes, weighted mean differences (MDs) and 95% CIs were presented as effect measures. Forest plots were used to display summary statistics. I2 statistics and the chi-square test method were used to statistically evaluate the heterogeneity of the included studies. I2 statistics < 50% and p > 0.1 indicated no significant heterogeneity across the studies. I2 statistics > 50% and p < 0.1 indicated significant heterogeneity across the studies. When the results of the I2 statistics and p-values were inconsistent, I2 statistics evaluation was selected as the main assessment method. Furthermore, a sensitivity analysis was performed to evaluate the robustness of the primary outcomes. Additionally, subgroup analyses were conducted based on the types of external therapy of TCM to explore whether the results had changed.
Results
Search results and study characteristics
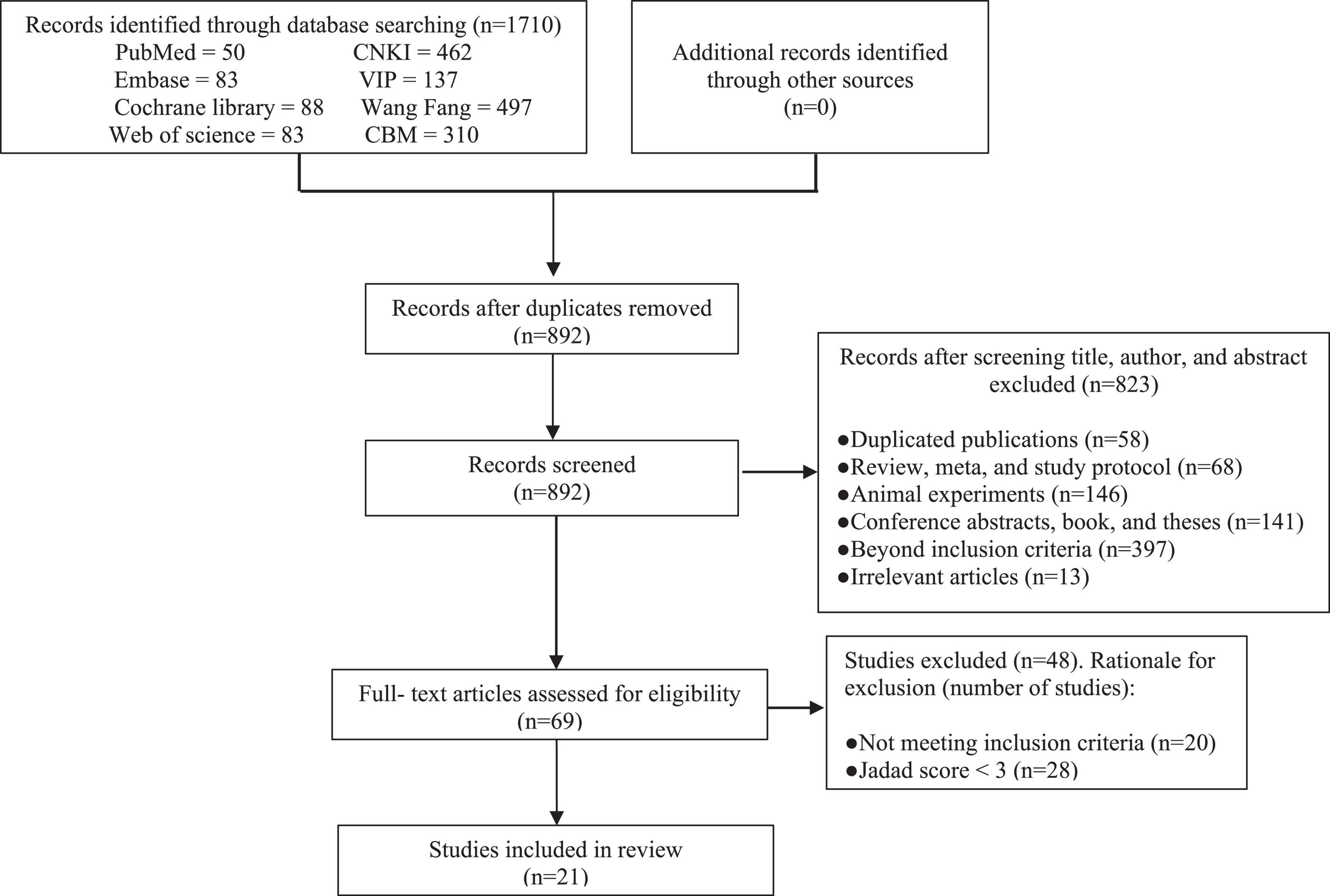
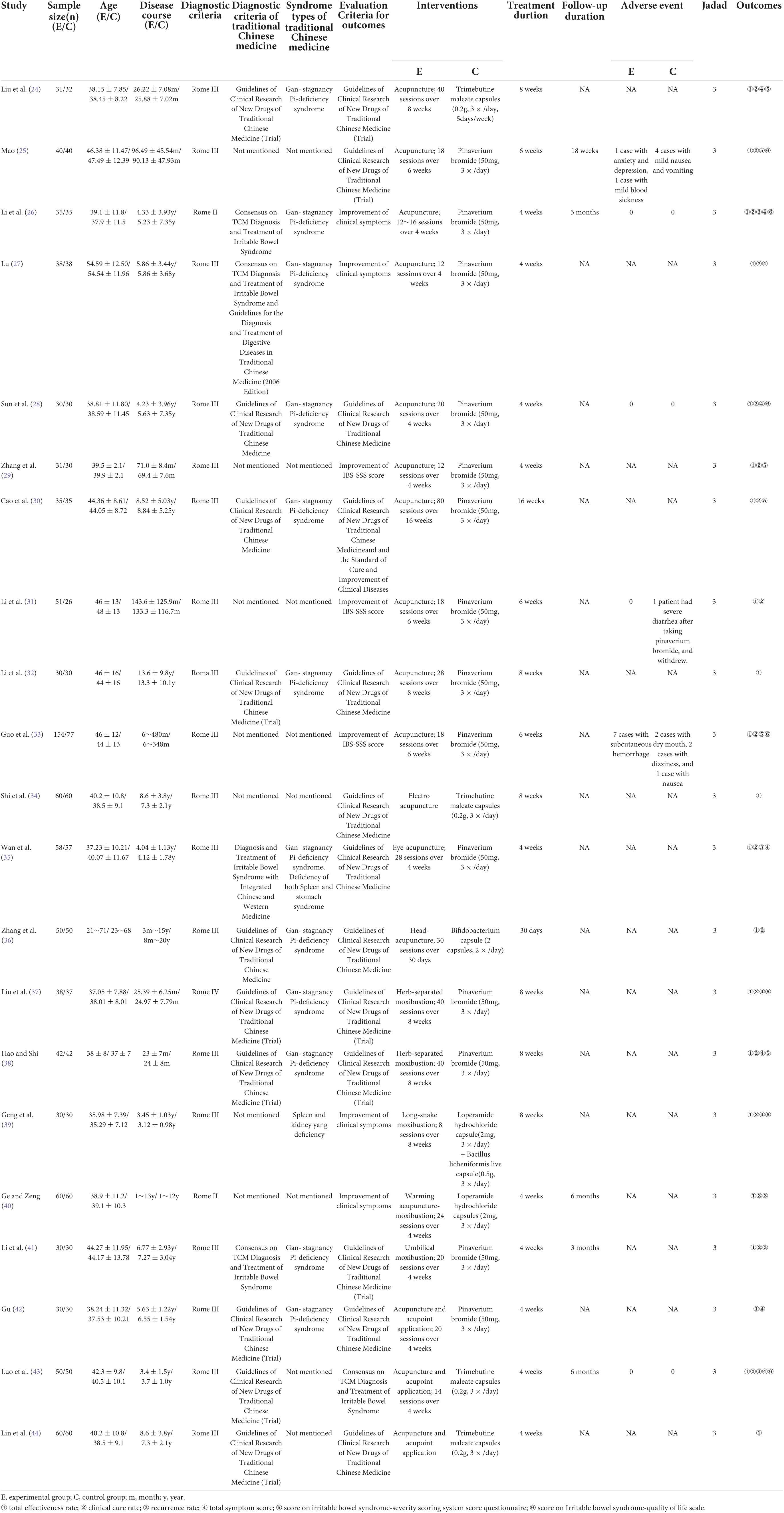
A total of 1,710 relevant RCTs were initially retrieved. After gradual screening, we identified 21 studies involving 1,862 subjects, including 983 in the experimental group and 879 in the control group (Figure 1). The subjects were 17–71 years of age, and disease duration ranged from 3 months to 20 years. The maximum sample size of the included studies was 231. There were 13 studies on acupuncture therapy, including acupuncture, electroacupuncture, eye acupuncture, and head acupuncture (24–36). Five studies on moxibustion therapy included herb-separated moxibustion, long-snake moxibustion, warming acupuncture moxibustion, and umbilical moxibustion (37–41). Three studies on acupuncture included acupoint application (42–44). Acupuncture and moxibustion therapy are the common external treatment methods used in most of the studies. The treatment methods used for the control group were mainly antispasmodic, antidiarrheal, or adjusted intestinal flora. In terms of treatment duration, most of the studies performed treatment for 4 weeks, and only one study performed treatment for up to 16 weeks (30). Only five studies recorded follow-up durations of 3 or 6 months (25, 26, 40, 41, 43). The basic characteristics of the included trials are detailed in Table 1.
Risk of bias and methodological quality assessment
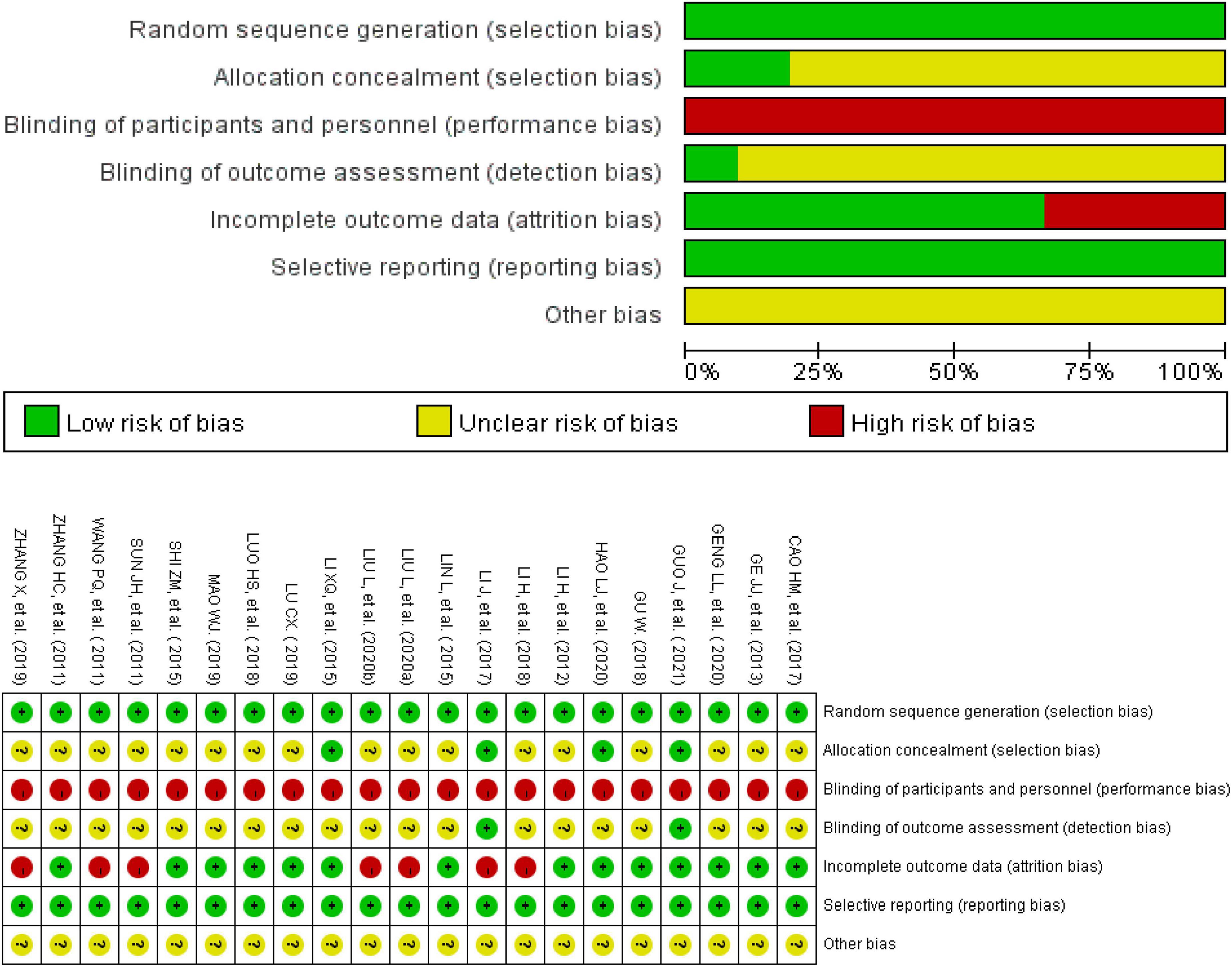
All the trials reported appropriate random sequence generation methods and were rated as low risk. Four studies recorded adequate information about the methods used for allocation concealment and were rated as low risk (31–33, 38). The allocation concealment of the others was not mentioned and rated as unclear. Because of the particularity of the intervention methods, the participants and personnel were not blinded. Therefore, the performance bias of all the trials was rated as high risk. Two trials described the method of blinding of assessors and were rated as low risk (31, 33). Seven studies mentioned that the participants withdrew or dropped out of the trials, and they included abscission data that were not included in the analysis; these were rated as high risk (24, 28, 29, 33, 35, 37, 41). The remaining studies with all data included in the final analysis were rated as low risk. Additionally, it could not be judged whether there was other bias in the 21 studies; therefore, they were rated as unclear. The results of the risk of bias analysis in the included trials are summarized in Figure 2.
Primary outcomes
Total effectiveness rate
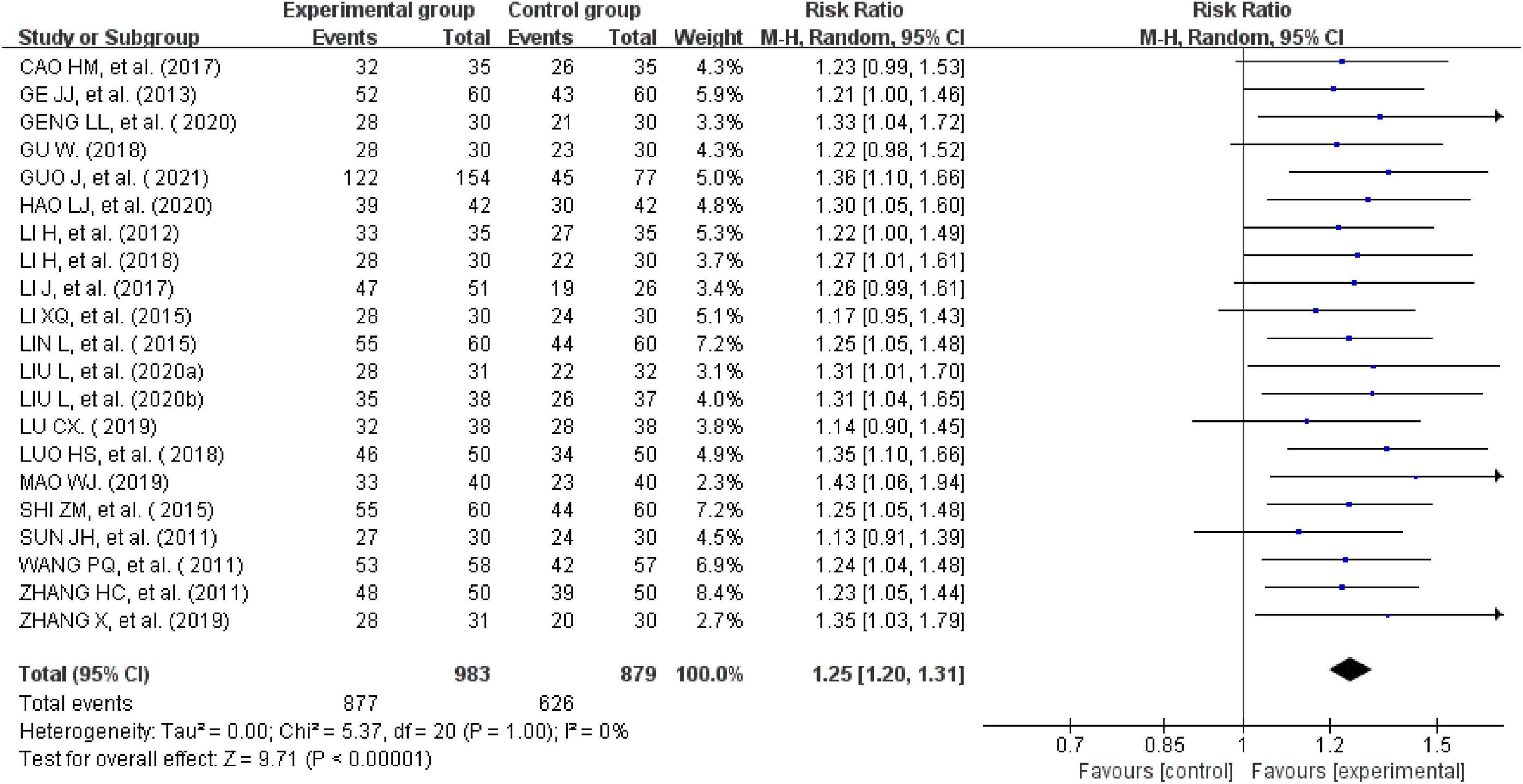
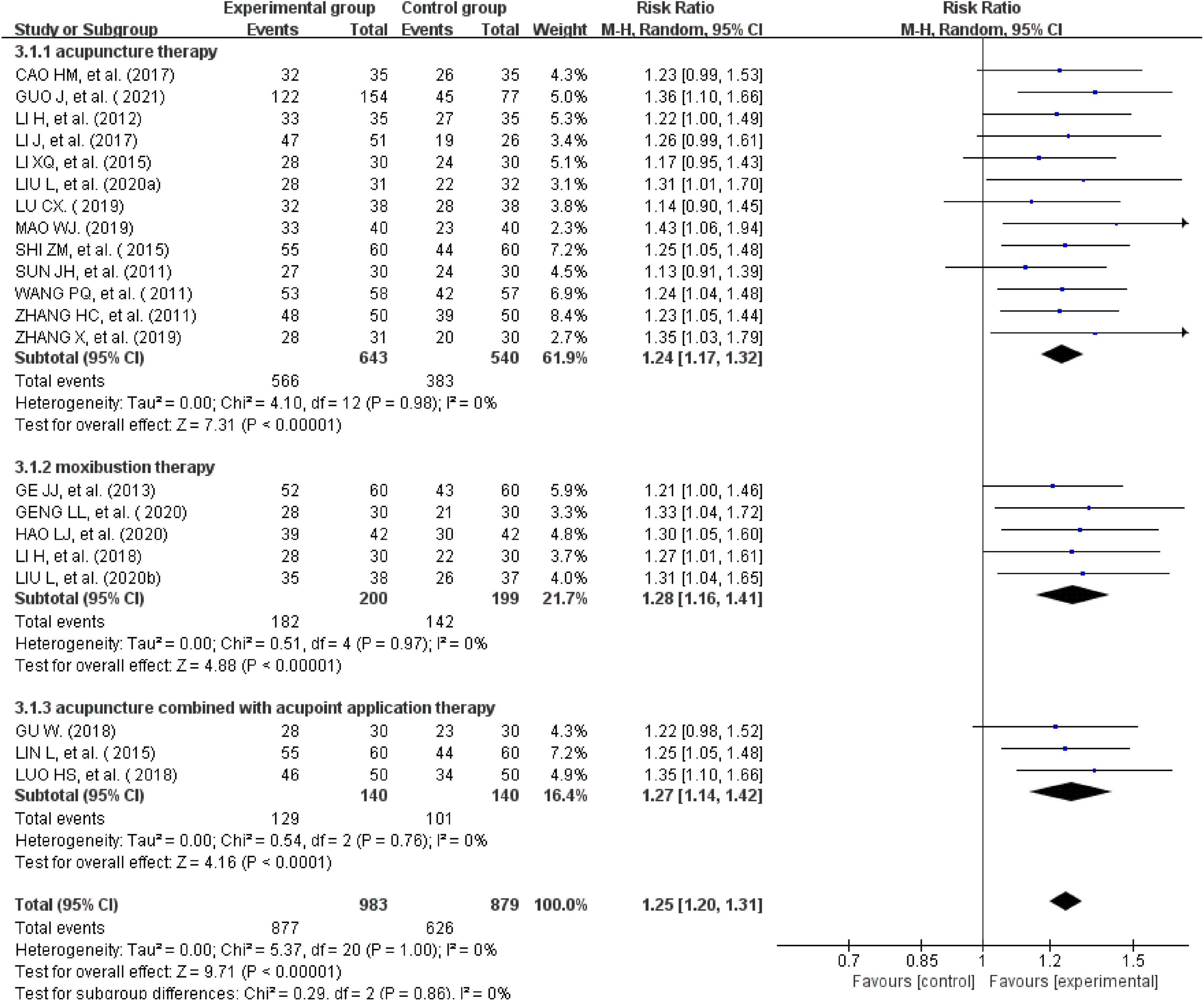
All the 21studies compared the total effectiveness rates of the experimental and control groups. Effectiveness was measured using the scores of the main symptoms. Twelve studies with a CSSI ≥ 30%were evaluated as effective (24, 26, 28, 31, 34–39, 41, 44). One study with a CSSI ≥ 35% was evaluated as effective (27). Additionally, four studies (25, 29, 31, 33) were evaluated using the IBS-SSS score, and four trials (20, 30, 32, 37) were evaluated based on patients’ self-reported scores for symptoms such as abdominal pain, abdominal distension, frequency of defecation, and stool form (30, 40, 42, 43). There was no heterogeneity across the trials when tested using I2statistics (df = 20, I2 = 0%). The meta-analysis showed that external therapy with TCM had a significantly higher total effectiveness rate in the experimental group than in the control group, and that the difference was statistically significant (n = 1,862, RR = 1.25, 95% CI [1.2, 1.31], Z = 9.71, P < 0.00001; Figure 3). The 21 included studies were further removed individually for the sensitivity analysis, which showed that none of the studies significantly affected the results of this analysis, indicating that it had great reliability and stability. This showed that the total clinical effectiveness rate of external therapy with TCM alone for IBS-D was better than that of the control group treated with internal Western medicine. Moreover, a subgroup analysis was conducted after various external treatments. The subgroup analysis showed that compared with the control group, acupuncture therapy (n = 1,183, I2 = 0%, RR = 1.24, 95% CI [1.17, 1.32], Z = 7.31, P < 0.00001), moxibustion therapy (n = 399, I2 = 0%, RR = 1.28, 95% CI [1.16, 1.41], Z = 4.88, P < 0.00001), and acupuncture combined with acupoint application therapy (n = 280, I2 = 0%, RR = 1.27, 95% CI [1.14, 1.42], Z = 4.16, P < 0.00001) had greater total effectiveness rates, indicating that the difference was statistically significant (Figure 4).
Clinical cure rate
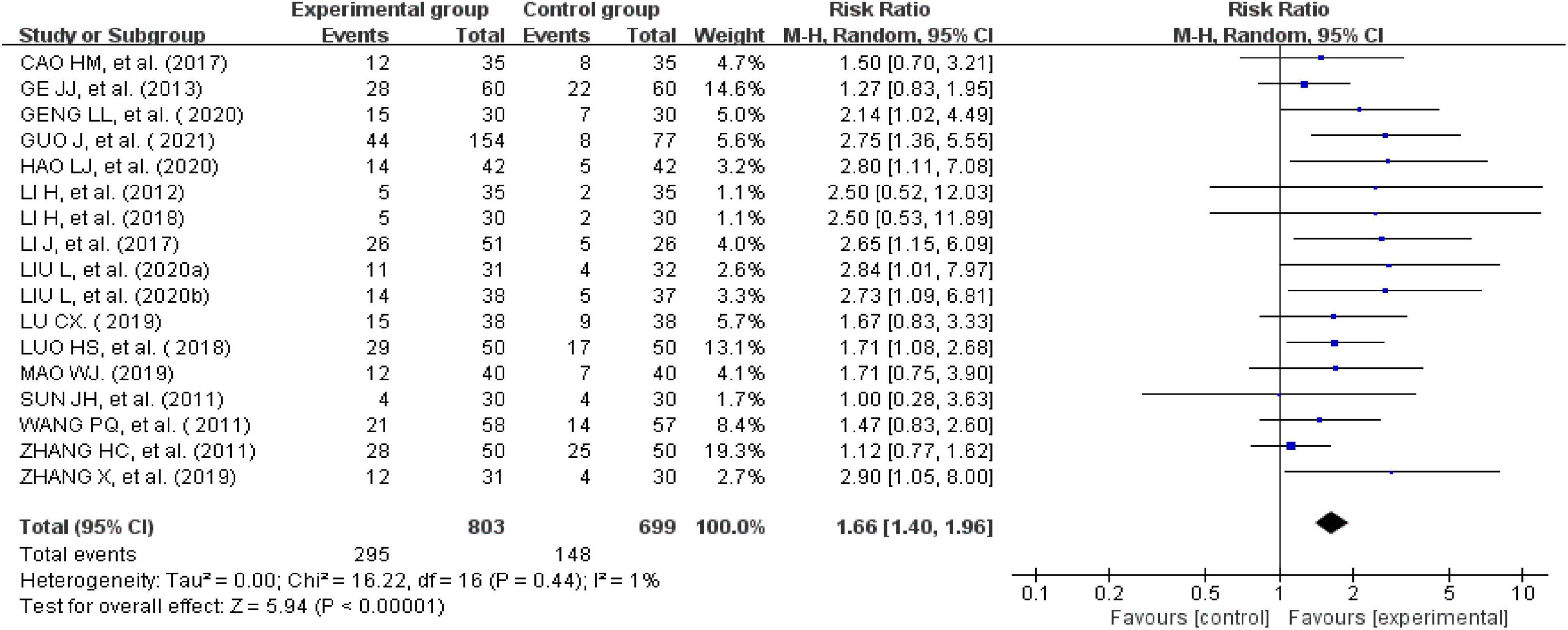
The clinical cure rate was calculated as follows: number of cured cases/total number × 100%. Seventeen studies reported clinical cure rates (24–31, 33, 35–41, 43). There was no significant heterogeneity across the included studies when tested using I2statistics (df = 16; I2 = 1%). The meta-analysis showed that the experimental group receiving external therapy of TCM had a significantly higher clinical cure rate than the control group; this difference was statistically significant (n = 1,502, RR = 1.66, 95% CI [1.4, 1.96], Z = 5.94, P < 0.00001; Figure 5). The sensitivity analysis showed that none of the studies significantly interfered with the results of the analysis, indicating that this study had satisfactory reliability and stability.
Secondary outcomes
Recurrence rate
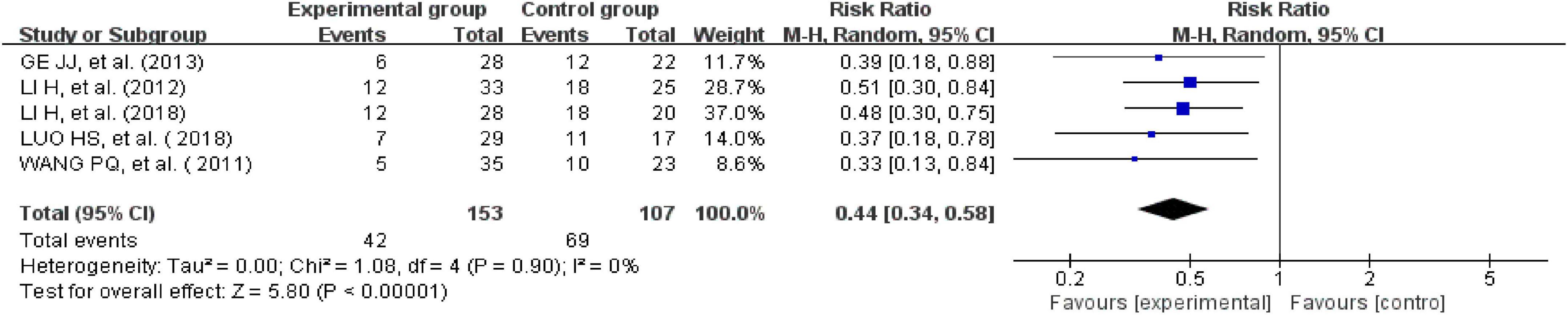
Five studies reported recurrence rates (26, 35, 40, 41, 43). There was no heterogeneity across the included trials when tested using I2 statistics (df = 4; I2 = 0%). The meta-analysis showed that the experimental group receiving external therapy of TCM had a significantly lower clinical cure rate than the control group; this difference was statistically significant (n = 260, RR = 0.44, 95% CI [0.34, 0.58], Z = 5.8, P < 0.00001; Figure 6).
Total symptom score
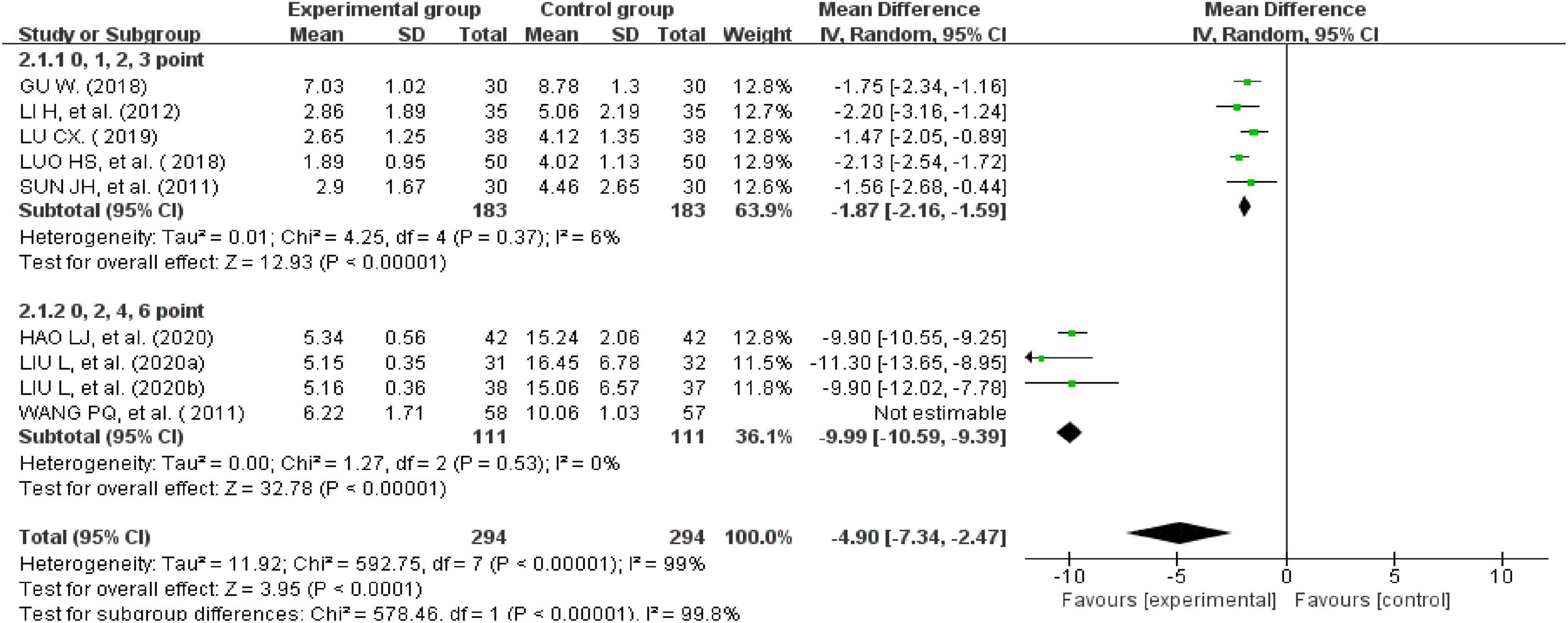
Nine studies recorded total symptom scores (24, 26–28, 35, 37, 38, 42, 43). There was significant heterogeneity across the studies when tested using I2statistics (n = 588, I2 > 50%, MD = -4.9, 95%CI [−7.34, −2.47], Z = 3.95, P < 0.00001). A subgroup analysis was performed according to the different weights given to the clinical symptom scores. The results showed that five studies assessed clinical symptom scores using 0, 1, 2, or 3 points (n = 366, I2 = 6%, MD = −1.87, 95% CI [−2.16, −1.59], Z = 12.93, P < 0.00001) (26–28, 42, 43). The other four studies evaluated clinical symptom scores using 0, 2, 4, or 6 points (P < 0.00001, I2 = 0%), indicating that the heterogeneity was still very high. However, the heterogeneity was significantly reduced after excluding one study (n = 222, I2 = 0%, MD = −9.99, 95% CI [−10.59, −9.39], Z = 32.78, P < 0.00001) (35). This means that the different weights given to the clinical symptom scores affected the results of the analysis. A subgroup analysis showed that the improvement in clinical symptom scores for IBS-D treated with external therapy using TCM alone was better than the improvement in clinical symptom scores for IBS-D in the control group (Figure 7).
Irritable bowel syndrome-severity scoring system score
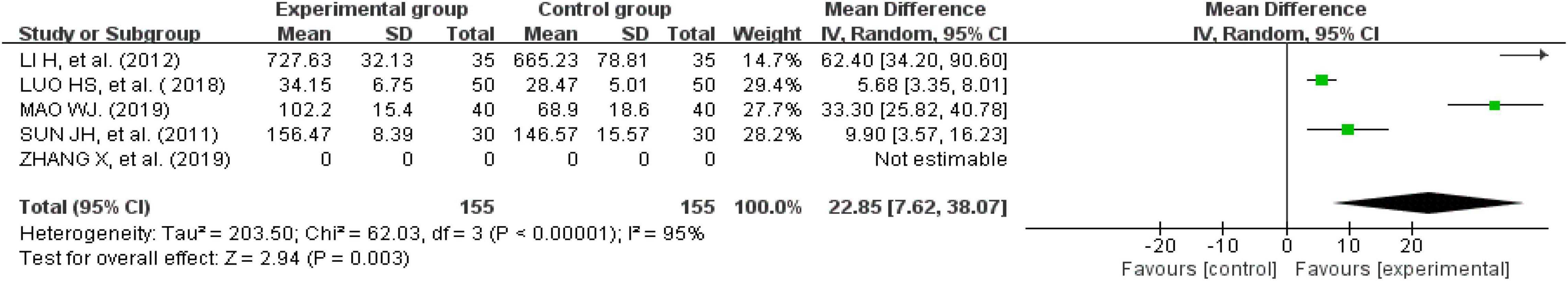
Eight studies reported IBS-SSS questionnaire scores (24, 25, 29, 30, 33, 37–39). One recorded them as the median and quartile; therefore, that study could not be used for this analysis (33). We were unable to consult with the original author to obtain further information. Finally, seven studies were analyzed. There was a significant heterogeneity across the studies when tested using I2 statistics (df = 5, P < 0.00001, I2 = 98%). The heterogeneity was significantly reduced after removing one study (n = 432, I2 = 30%, MD = -52.72, 95% CI [−63.9, −41.53], Z = 9.23, P < 0.00001) (29). The results showed that the improvement in the IBS-SSS questionnaire scores for external therapy of TCM alone was better than the improvement in the IBS-SSS questionnaire scores of the control group (Figure 8).
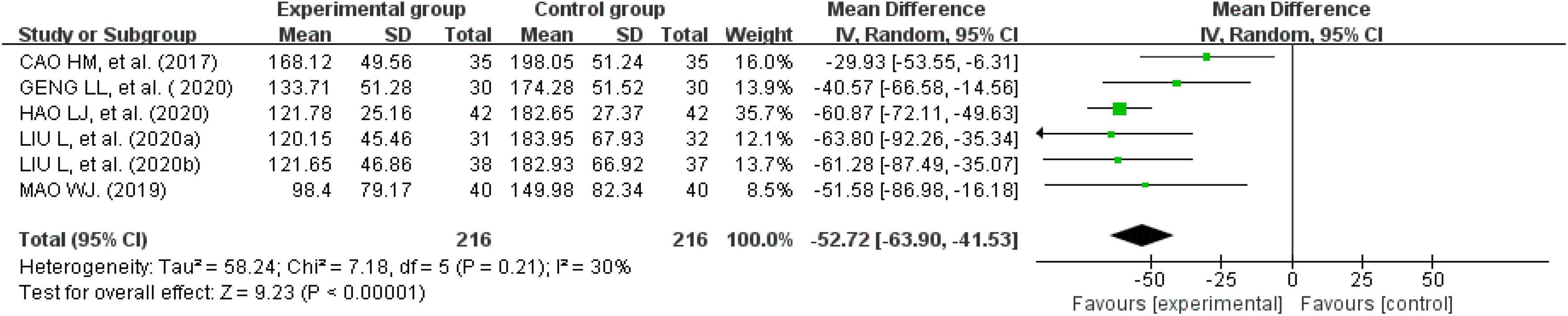
Figure 8. Forest plot of the score on irritable bowel syndrome-severity scoring system score questionnaire.
Irritable bowel syndrome-quality of life
Five studies recorded IBS-QOL questionnaire scores (25, 26, 28, 33, 43). One of these studies (33) reported IBS-QOL questionnaire scores as the median and quartile; therefore, that study could not be used for this analysis. The difference was statistically significant, because the experimental group had a positive effect on improving the IBS-QOL score (P < 0.05). Nevertheless, the heterogeneity was high (I2 > 50%) (Figure 9). A subgroup analysis based on intervention measures and duration showed decreased heterogeneity, but I2 was still > 50%.
Adverse events
Among the 21 included studies, only six mentioned adverse events; among the six studies, three evaluated them as safe (26, 28, 43). One study recorded one case of anxiety and depression experienced by one patient in the experimental group (25). One study reported four cases of mild nausea and vomiting in the control group. The remaining studies reported seven cases of subcutaneous hemorrhage after acupuncture therapy. Correspondingly, two patients in the control group had a dry mouth, two had dizziness, and one had nausea (33). In particular, one patient developed severe diarrhea after using pinaverium bromide and was withdrawn from the study. In contrast, no adverse reactions were observed in the experimental group (31). All adverse reaction symptoms resolved spontaneously.
Publication bias
To detect a possible publication bias, we analyzed the funnel plot of more than 10 studies. The results showed that the morphological distribution on the left and right sides of the midline of the inverted funnel plot in the included studies was not symmetrical, indicating that the included studies had a potential publication bias (Figures 10A–C).
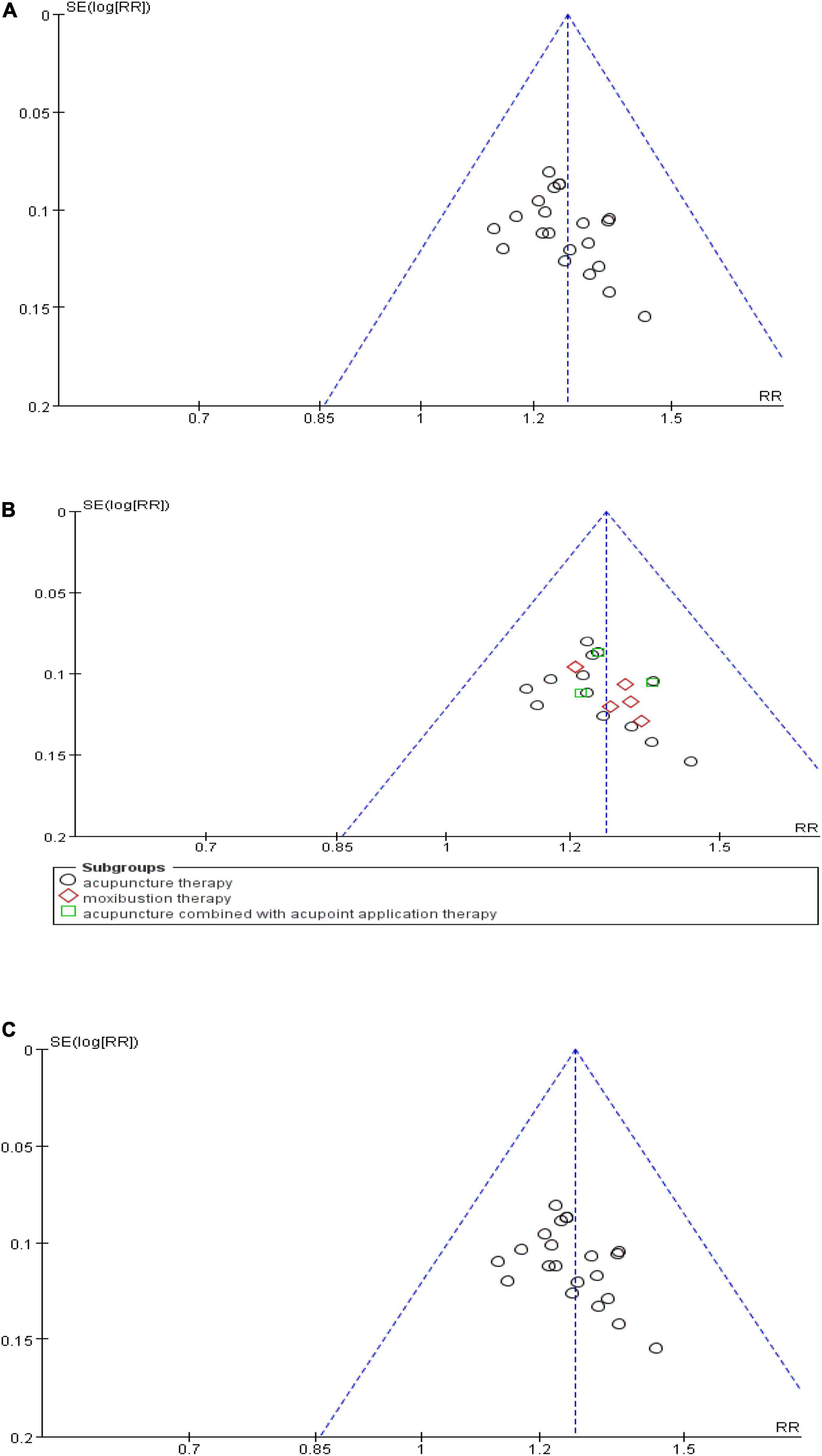
Figure 10. Funnel plot of publication bias. (A) Funnel plot of the total effective rate. (B) Funnel plot of the effective rate of subgroups. (C) Funnel plot of the clinical cure rate.
Grading of recommendations, assessment, development, and evaluations certainty of evidence
The GRADE certainty of evidence for the primary and secondary outcomes is shown in Supplementary materials. The certainty of evidence was moderate for the total effectiveness rate and IBS-SSS score, low for the recurrence rate, and very low for the clinical cure rate, total symptom score, and IBS-QOL.
Discussion
Traditional Chinese medicine, including Chinese herbal medicine and external treatment, can alleviate symptoms, improve cure rate, and reduce the recurrence of IBS-D (45). During this review, the positive effects of 21 external therapies involving TCM on trials involving patients with IBS-D were evaluated. The results showed that external therapy of TCM alone significantly improved the total effectiveness rate and clinical cure rate. Moreover, external therapy of TCM alone can reduce the recurrence rate of and improve both intestinal symptoms and QOL associated with IBS-D, with few side effects. Unlike previous research studies, we comprehensively summarized various external therapies of TCM. Furthermore, all the trials included in this study were high-quality clinical trials with a Jadad score of ≥3, which indicated a high quality of evidence.
Conventional Western medicine has a clear therapeutic mechanism and can temporarily improve the symptoms of diarrhea. However, the long-term effects remain unsatisfactory, and the recurrence rate is still quite high (40%) because of complex and unclear pathological mechanisms of IBS (46). External therapy involving TCM is a therapy that acts on the surface of the body or from outside the body to treat disease through corresponding somatic-visceral reactions. It is simple to use, results in few adverse reactions, and has a quick clinical efficacy; therefore, it is widely used to treat diarrhea. Furthermore, the World Health Organization recommends TCM for treatment of IBS (47). The external therapy of TCM during this study focused on acupuncture, moxibustion, and acupoint application; among these, acupuncture therapy and moxibustion therapy are the most commonly studied and mainly used clinical treatments for IBS-D. Based on the theory of somatic-visceral interactions, each external therapy with TCM has a similar efficacy when used to treat IBS-D. Additionally, several common clinical external treatment methods emphasize the role of stimulation of specific acupoints, and their mechanisms are also similar; therefore, they have similar effects on intestinal symptoms. Moreover, the sensitivity analysis showed that the total effectiveness and clinical cure rates of IBS-D treated with external therapy using TCM were relatively robust. It is noteworthy that the certainty of evidence was moderate for the total effectiveness rate and IBS-SSS. Similarly, the subgroup analysis revealed that the total clinical efficacy rates of acupuncture and moxibustion for treatment of IBS-D were better than that of Western medicine (P < 0.0001). Acupuncture is an important part of external treatment using TCM, which produces somatosensory stimulation at specific acupoints of the human body and releases the whole body to treat the intestinal tract. It induces multifaceted regulation to improve intestinal symptoms through complex mechanisms, such as inhibiting gastrointestinal motility, reducing visceral hypersensitivity, balancing the intestinal-brain axis, and regulating neurotransmitters and the immune system (48). The literature reports no need for acupuncture when the case is suitable for moxibustion. Moxibustion is another commonly used external therapy for patients with IBS-D. It uses the warm and medicinal power of ignited moxa to stimulate acupoints or specific parts of the surface of the body and promote the self-regulation function of the body. Moxibustion regulates intestinal inflammation, alleviates visceral hypersensitivity, and relieves visceral pain to improve functional gastrointestinal disorders (18, 49, 50). Acupoint application is a compound treatment method that integrates acupoints, meridians, and herbs that regulate meridians and improve blood circulation, thus exerting an effect on the intestinal system and improving diarrhea symptoms. Visceral hypersensitivity is the main pathogenesis of abdominal pain and diarrhea for patients with IBS-D; therefore, it has attracted increasing attention (51). Reducing visceral hypersensitivity to alleviate clinical symptoms is an important treatment strategy consistent with the mechanisms of acupuncture and moxibustion.
Unfortunately, the authors of the included studies did not explain the reasons for the lack of current clinical research data. IBS-D is a chronic, recurrent, and functional gastrointestinal disorder with no organic explanation. Its clinical trial is a complex process that involves clinical research and follow-up of participants, which involve high costs. Unfortunately, 20 to 30% of subjects withdrew from study participation (52). This is particularly true of studies with longer trial periods. Lack of long-term follow-up data was one limitation of this study. Another limitation of this study was that the random sequence allocation of the included studies was non-standard according to the summary risk of bias and publication bias graph, which may have caused selection bias. The funnel plot showed a skewed distribution, indicating a publication bias. Moreover, the accuracy of some results may have been affected by differences in the disease, reference standards for efficacy evaluation, and the unequal experience of TCM clinicians. Additionally, because of inevitable problems, such as database permissions, some gray bodies of literature were not retrieved.
External therapy of TCM for the treatment of IBS-D can alleviate abdominal symptoms, improve clinical effectiveness, and reduce recurrence with few side effects. Moreover, external therapy of TCM has a positive effect on improving QOL and can serve as an alternative treatment for IBS-D.
Conclusion
The current evidence indicates that external therapy of TCM for IBS-D has positive efficacy and high safety. It is also simple, convenient, and low-cost. However, because of the limitations of the follow-up period and publication bias of the included trials, more rigorous clinical studies are necessary to further verify the long-term effects of external therapy of TCM.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
XW and XT contributed to the conception and design of the study. YoW and YuW searched the databases and extracted the data. XW, XL, and XM evaluated the studies for inclusion and data analysis. XW conducted the statistical analysis of the data and drafted the manuscript. YoW wrote sections of the manuscript. BZ and XT revised the manuscript. All authors read and approved the final version of the manuscript.
Funding
This study was partially supported by the National Natural Science Foundation of China (No. 81774303) and Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (No. ZYYCXTD-C-202010).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.940328/full#supplementary-material
Footnotes
References
1. Enck P, Aziz Q, Barbara G, Farmer AD, Fukudo S, Mayer EA, et al. Irritable bowel syndrome. Nat Rev Dis Primers. (2016) 2:16014. doi: 10.1038/nrdp.2016.14
2. Lacy BE, Patel NK. Rome criteria and a diagnostic approach to irritable bowel syndrome. J Clin Med. (2017) 6:99. doi: 10.3390/jcm6110099
3. Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. (2012) 10:712–21. doi: 10.1016/j.cgh.2012.02.029
4. Sultan S, Malhotra A. Irritable bowel syndrome. Ann Intern Med. (2017) 166:ITC81–96. doi: 10.7326/AITC201706060
5. Chen YR, Lin LB, Li Y. Research progress of TCM and western medicine on diarrheal irritable bowel syndrome. Mod J Integr Tradit Chin West Med. (2019) 28:2496–500.
6. Waehrens R, Ohlsson H, Sundquist J, Sundquist K, Zöller B. Risk of irritable bowel syndrome in first-degree, second-degree and third-degree relatives of affected individuals: a nationwide family study in Sweden. Gut. (2015) 64:215–21. doi: 10.1136/gutjnl-2013-305705
7. Jin DC, Cao HL, Xu MQ, Wang SN, Wang YM, Yan F, et al. Regulation of the serotonin transporter in the pathogenesis of irritable bowel syndrome. World J Gastroenterol. (2016) 22:8137–48. doi: 10.3748/wjg.v22.i36.8137
8. Tillisch K, Mayer EA, Labus JS. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology. (2011) 140:91–100. doi: 10.1053/j.gastro.2010.07.053
9. Simrén M, Barbara G, Flint HJ, Spiegel BM, Spiller RC, Vanner S, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. (2013) 62:159–76. doi: 10.1136/gutjnl-2012-302167
10. Black CJ, Ford AC. Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nat Rev Gastroenterol Hepatol. (2020) 17:473–86. doi: 10.1038/s41575-020-0286-8
11. Ford AC, Sperber AD, Corsetti M, Camilleri M. Irritable bowel syndrome. Lancet. (2020) 396:1675–88. doi: 10.1016/S0140-6736(20)31548-8
12. Bai JQ, Liu YY, Gao ZF. Research progress on non-drug treatment of irritable bowel syndrome. J Clin Exp Med. (2020) 19:1455–7.
13. Gwee KA. Irritable bowel syndrome in developing countries–a disorder of civilization or colonization? Neurogastroenterol Motil. (2005) 17:317–24. doi: 10.1111/j.1365-2982.2005.00627.x
14. Ford AC. Commentary: estimating the prevalence of IBS globally-past, present and future. Aliment Pharmacol Ther. (2020) 51:198–9. doi: 10.1111/apt.15508
15. Frank L, Kleinman L, Rentz A, Ciesla G, Kim JJ, Zacker C, et al. Health-related quality of life associated with irritable bowel syndrome: comparison with other chronic diseases. Clin Ther. (2002) 24:675–89. doi: 10.1016/S0149-2918(02)85143-8
16. Singh P, Staller K, Barshop K, Dai E, Newman J, Yoon S, et al. Patients with irritable bowel syndrome-diarrhea have lower disease-specific quality of life than irritable bowel syndrome-constipation. World J Gastroenterol. (2015) 21:8103–9. doi: 10.3748/wjg.v21.i26.8103
17. Deng DX, Guo KK, Tan J, Huang GL, Li S, Jiang QR, et al. Acupuncture for diarrhea-predominant irritable bowel syndrome: a meta-analysis. Zhongguo Zhen Jiu. (2017) 37:907–12.
18. Huang H, Xuan YC, Fu Y, Gong HB, Kang MF, Zhang HF. Clinical study on moxibustion therapy in the treatment of irritable bowel syndrome: a systematic review and meta-analysis. Jiangxi J Tradit Chin Med. (2018) 49:55–60.
19. Zhang S, Wang W, Li Z, Cao L, Wang ZR, Shen SW. A systematic review of diarrhea-predominant irritable bowel syndrome with acupoint application. J Hunan Univ Chin Med. (2017) 37:1002–7.
20. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. (2021) 134:178–89.
21. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. (1996) 17:1–12. doi: 10.1016/0197-2456(95)00134-4
22. Berger VW, Alperson SY. A general framework for the evaluation of clinical trial quality. Rev Recent Clin Trials. (2009) 4:79–88. doi: 10.2174/157488709788186021
23. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
24. Liu L, Hao LJ, Shi ZM. Clinical observation of acupuncture at points of Liver Meridian of Foot Jueyin in treating diarrhea-predominant irritable bowel syndrome. J Guangzhou Univ Tradit Chin Med. (2020) 37:279–84.
25. Mao WJ. A clinical study of acupuncture treatment to 40 cases of diarrhea IBS. Jiangsu J Tradit Chin Med. (2019) 51:63–5.
26. Li H, Pei LX, Zhou JL. Comparative observation on therapeutic effects between acupuncture and western medication for diarrhea-predominant irritable bowel syndrome. Zhongguo Zhen Jiu. (2012) 32:679–82.
27. Lu CX. Effect of acupuncture on diarrhea irritable bowel syndrome of liver depression and spleen deficiency. Contemp Med Symp. (2019) 17:43–5.
28. Sun JH, Wu XL, Xia C, Xu LZ, Pei LX, Li H, et al. Clinical evaluation of soothing gan and invigorating Pi acupuncture treatment on diarrhea-predominant irritable bowel syndrome. Chin J Integr Med. (2011) 17:780–5. doi: 10.1007/s11655-011-0875-z
29. Zhang X, Ding M, Feng H. Acupuncture with Du’s heat-reinfbrcing method for diarrhea-predominant irritable bowel syndrome: a randomized controlled trial. J Acupunct Tuina Sci. (2019) 17:124–30. doi: 10.1007/s11726-019-1086-y
30. Cao HM, Zeng DY, Liu SQ. Long term effect of soothing liver and strengthening spleen acupuncture on 35 cases of diarrhea irritable bowel syndrome of liver depression and spleen deficiency. J Gansu Univ Chin Med. (2017) 34:60–3.
31. Li J, Lu J, Sun J, Ruan Z, Xu D, Geng H, et al. Acupuncture with regulating mind and spleen for diarrhea irritable bowel syndrome and sleep quality:a randomized controlled trial. Zhongguo Zhen Jiu. (2017) 37:9–13.
32. Li X, Mu S, Lu X. Therapeutic observation of diarrhea-predominant irritable bowel syndrome majorly treated by acupuncture with Ling Gui Ba Fa. Shanghai J Acupunct Moxibustion. (2015) 34:22–4.
33. Guo J, Sun JH, Chen L, Geng H, Wu XL, Song YF, et al. Correlation between curative effect and 5-HTTLPR polymorphism in treatment of diarrhea-predominant irritable bowel syndrome with acupuncture for regulating shen and strengthening spleen. Chin Acupunct Moxibustion. (2021) 41:365–70.
34. Shi ZM, Li XQ, Liu LN, Liu JP, Guo YJ, Zhou H. ZiwuLiuzhu acupuncture treatment of irritable bowel syndrome. Shaanxi J Tradit Chin Med. (2015) 407:1516–8.
35. Wang PQ, Chen SN, Liu YD, Chen XY, Wang J. Randomize controlled study on the eye-acupuncture for diarrhea-predominant irritable bowel syndrome. J Tradit Chin Med. (2011) 52:1203–6.
36. Zhang HC, Han SK, Tang JL. 50 cases of diarrhea irritable bowel syndrome treated with scalp acupuncture. Chinese Acupunct Moxibustion. (2011) 31:605–6.
37. Liu L, Shi ZM, Hao LJ. Clinical observation of diarrhea-predominant irritable bowel syndrome treated by herb partition moxibustion at abdominal pain. J Guangzhou Univ Tradit Chin Med. (2020) 37:474–9.
38. Hao LJ, Shi ZM. Therapeutic effect of herb-separated moxibustion at Jinsuo (GV 8)-eight-diagram points on diarrhea-type irritable bowel syndrome of liver stagnation and spleen deficiency. Chinese Acupunct Moxibustion. (2020) 40:702–6.
39. Geng LL, Huang H, Jiang XM, Lv MF, Wei GL, Wei HY, et al. Clinical observation on the treatment of diarrhea-predominan irritable bowel of syndrome of yang deficiency of spleen and kidney with long snake moxibustion. Guangming J Chin Med. (2020) 35:3939–41.
40. Ge JJ, Zeng KX. Efficacy observation on warm needling for 60 cases of diarrhea irritable bowel syndrome. World J Acupunct Moxibustion. (2013) 23:43–51. doi: 10.1016/S1003-5257(14)60010-6
41. Li H, Zhou Y, Li Z, Zhu L, Xiong JW, Li YT, et al. Clinical observation on umbilical moxibustion therapy treating 30 cases of diarrhea type irritable bowel syndrome with stagnation of liver qi and spleen deficiency. J Tradit Chin Med. (2018) 59:2034–6.
42. Gu W. Clinical observation on treatment of irritable bowel syndrome by acupoint application combined with acupuncture and its effects on 5-HT and IL-8. Chin J Integr Trad West Med Dig. (2018) 26:261–3.
43. Luo HS, Yang YG, Cai XL. Clinical study on treatment of diarrhea irritable bowel syndrome by acupuncture combined with acupoint application of anchang powder. Int J Tradit Chin Med. (2018) 40:319–22.
44. Lin L, Wang CZ, Shi ZM. Clinical observation of Ziwuliuzhu acupuncture combined with acupoint application in the treatment of diarrhea predominant irritable bowel syndrome. J Chengdu Univ Tradit Chin Med. (2015) 38:59–61.
45. Wan LF. Clinical research progress of diarrhea irritable bowel syndrome treated with traditional Chinese Medicine. J Emerg Tradit Chin Med. (2020) 29:171–3.
46. Triantafillidis JK, Malgarinos G. Long-term efficacy and safety of otilonium bromide in the management of irritable bowel syndrome: a literature review. Clin Exp Gastroenterol. (2014) 7:75–82. doi: 10.2147/CEG.S46291
47. Han JS. Acupuncture analgesia: areas of consensus and controversy. Pain. (2011) 152(3 Suppl.):S41–8. doi: 10.1016/j.pain.2010.10.012
48. Yaklai K, Pattanakuhar S, Chattipakorn N, Chattipakorn SC. The role of acupuncture on the gut-brain-microbiota axis in irritable bowel syndrome. Am J Chin Med. (2021) 49:285–314. doi: 10.1142/S0192415X21500154
49. Chu HR, Wang Y, Tong L, Wu SB, Wu LB, Li N, et al. Effect of moxibustion on TLR4/MyD88/NF-KB signaling pathway in colon of diarrhea-predo-minant irritable bowel syndrome rats. Acupunct Res. (2020) 45:633–9.
50. Qi Q, Wu HG, Jin XM, Jin D, Wang Y, Wang C, et al. Effect of moxibustion on the expression of GDNF and its receptor GFRα3 in the colon and spinal cord of rats with irritable bowel syndrome. Acupunct Med. (2019) 37:244–51. doi: 10.1136/acupmed-2017-011455
51. Ceuleers H, Van Spaendonk H, Hanning N, Heirbaut J, Lambeir AM, Joossens J, et al. Visceral hypersensitivity in inflammatory bowel diseases and irritable bowel syndrome: the role of proteases. World J Gastroenterol. (2016) 22:10275–86. doi: 10.3748/wjg.v22.i47.10275
Keywords: systematic review, meta-analysis, irritable bowel syndrome with diarrhea, external therapy of TCM, randomized controlled trial, complementary therapy
Citation: Wei X, Wen Y, Wei Y, Liang X, Ma X, Zhang B and Tang X (2022) External therapy of traditional Chinese medicine for treating irritable bowel syndrome with diarrhea: A systematic review and meta-analysis. Front. Med. 9:940328. doi: 10.3389/fmed.2022.940328
Received: 10 May 2022; Accepted: 08 July 2022;
Published: 09 August 2022.
Edited by:
Zipeng Gong, Guizhou Medical University, ChinaReviewed by:
Zhipeng Tang, Longhua Hospital Shanghai University of Traditional Chinese Medicine, ChinaLing Hu, Guangzhou University of Chinese Medicine, China
Yun-kai Dai, Shanghai University of Traditional Chinese Medicine, China
Copyright © 2022 Wei, Wen, Wei, Liang, Ma, Zhang and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Beihua Zhang, MTM4MTA4NzQ2MDlAMTYzLmNvbQ==; Xudong Tang, dHhkbHlAc2luYS5jb20=
 Xiuxiu Wei
Xiuxiu Wei Yongtian Wen1,2
Yongtian Wen1,2 Xiangxue Ma
Xiangxue Ma Xudong Tang
Xudong Tang