- 1Department of Respiratory, Zhumadian Central Hospital, Zhumadian, China
- 2Department of Critical Care, The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou, China
- 3Department of Laboratory, The Fifth People’s Hospital of Panyu District, Guangzhou, China
- 4Department of Computer Center, Fujian Provincial Maternity Hospital, Fuzhou, Fujian, China
Background: The relationship between human serum albumin levels and the prognosis of critical care patients with chronic obstructive pulmonary disease (COPD) remains controversial.
Objective: To investigate the relationship between serum albumin levels and in-hospital mortality in critical care patients with COPD. METHODS: This study used a retrospective observational cohort from the Medical Information in Intensive Care database (MIMIC-IV) in the United States. Multivariate Cox regression analysis was used to assess the relationship between serum albumin levels and in-hospital mortality. A restricted cubic spline line was also used to explore nonlinear relationship.
Results: A total of 3,398 critical care patients with COPD were included. The overall in-hospital mortality was 12.4%. We found a negative relationship between human serum albumin and in-hospital mortality (HR = 0.97, 95% CI 0.96–0.99, p = 0.002).
Conclusion: In critical care patients with COPD, there was a negative association between human serum albumin and in-hospital mortality.
1. Introduction
Chronic obstructive pulmonary disease (COPD) is currently a major public health problem worldwide, with a global prevalence of 11.7% in 2010 (1). COPD is also the third leading cause of death worldwide (2). Patients with acute exacerbation of COPD often require hospitalization, or even need intensive care unit admission (2). Identifying the reversible risk factors during hospitalization is therefore essential to help reduce mortality as well as the burden of disease.
Human serum albumin is a multifunctional plasma protein that accounts for more than 50% of the total plasma protein content. Physiologically, human serum albumin, in addition to its antioxidant properties (3), is also an acute-phase response protein whose concentration decreases during the acute-phase response (4). Albumin is also a clinical bio-marker of malnutrition. Hypoproteinemia is associated with prolonged hospital stay during acute exacerbations, acute respiratory failure and increased mortality in COPD patients (3, 5, 6). However, other studies reported no correlation between albumin level and prognosis in COPD patients (7).
In this study, we attempted to identify the dose–response relationship between human serum albumin level and hospital mortality in COPD patients using a large data set of critical illnesses (8).
2. Data and methods
2.1. Methods
This study used a retrospective observational cohort of all patients diagnosed with COPD in the Medical Information in Intensive Care database (8) (MIMIC-IV version 1.0). MIMIC-IV is a large, real-world clinical database of critical care patients from 2008 to 2019 in Beth Israel Deaconess Medical Center (9). One of the authors (Shanglin Chen) was granted access to utilize the database (license number: 10756765). This study was written according to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines (10). All the data accessed complied with relevant data protection and privacy regulations.
2.2. Study population
This study was based on the real-world study concept and included all COPD patients from the MIMIC-IV database. The inclusion criteria: 1. First admission to the intensive care unit. 2. Human serum albumin level measured within 24 h. 3. Age ≥ 18 years.
2.3. Exposure and covariates
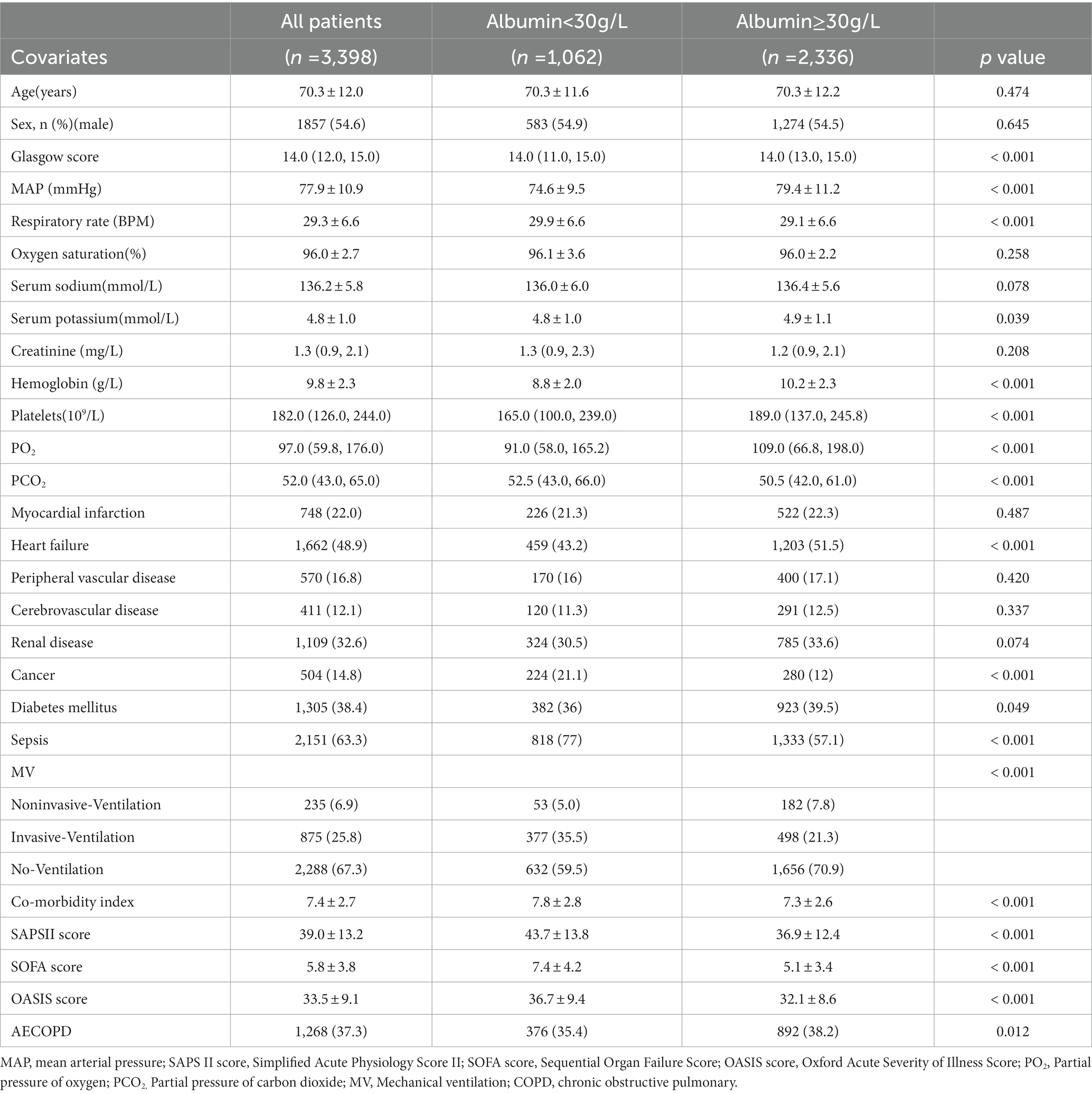
This study focused on assessing the relationship between baseline human serum albumin and hospital mortality in patients with severe COPD. Patients were divided into hypoalbuminemia group (<30 g/L) and hyperalbuminemia group (≥30 g/l) according to previous studies (11, 12). Vital signs and laboratory indicators were analyzed using the worst value within 24 h of ICU admission. Covariates were identified based on previous serum albumin-related studies and clinical relevance (10–13). Covariates in this study including age, sex, Glasgow score, mean arterial pressure, respiratory rate, oxygen saturation, serum sodium, serum potassium, blood creatinine, hemoglobin, platelets, myocardial infarction, heart failure, peripheral vascular disease, cerebrovascular disease, renal disease, cancer, diabetes mellitus, co-morbidity index, SAPSII score, SOFA score, and OASIS score.
2.4. Outcome
The outcome was in-hospital mortality.
2.5. Statistical analysis
Data analysis was performed using R.3.3.2, free statistical software version 1.6.1 (13). Categorical variables were expressed as percentages (%) using the χ2 test; continuous variables with normal distribution were expressed as mean ± standard deviation (x ± s) and t-test were used for comparison between groups. Continuous variables with skewed distribution were expressed as median (quartiles) using Kruskal-Wallis test. Missing data were replaced by dummy variables (14). Multivariable Cox regression analysis was used to analyze the independent association between serum albumin level and in-hospital mortality. A restricted cubic spline function was used to analyze the nonlinear relationship between human serum albumin and hospital mortality, and a log-likelihood ratio test of p < 0.05 was used to consider the relationship as nonlinear. Kaplan–Meier survival curves for low and high human serum albumin were plotted and Multivariable Cox regression analysis was performed. p < 0.05 (two-sided) was considered a statistically significant difference. Propensity-score matching (PSM) was also used to compare the outcomes between two groups.
2.6. Missing data
There are many variables with missing data in the MIMIC IV database. For PaO2/FIO2 ratio, over 20% of the values were missing and were removed from this analysis. In other continuous variables, the missing values were imputed by k-Nearest Neighbors method (15).
3. Results
3.1. Study population
3,398 COPD patients were screened in the MIMIC-IV database who met the inclusion and exclusion criteria of this study.
3.2. Baseline characteristics
Of 3,398 COPD patients, age was 70.3 ± 12.0 years. 1857 (54.6%) were male. The overall in-hospital mortality was 12.4%. 1,062 patients had low human serum albumin (<30 g/L) and 2,336 patients had human serum albumin ≥30 g/L. Glasgow score, mean arterial pressure, respiratory rate, serum potassium, hemoglobin, platelets, PO2, PCO2, heart failure, cancer, diabetes mellitus, sepsis, MV, AECOPD, co-morbidity index, and co-morbidity index SAPSII score, SOFA score, and OASIS score were imbalance in two groups (all p < 0.05) disease.
3.3. Association between baseline human serum albumin and in-hospital mortality
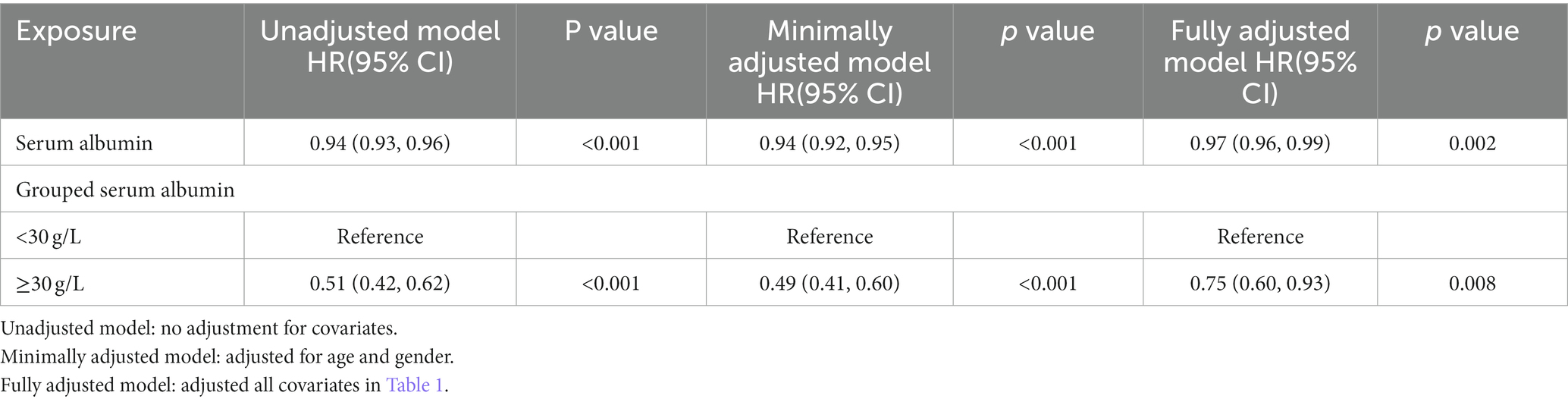
3.3.1. Univariate and multivariate cox regression analyses
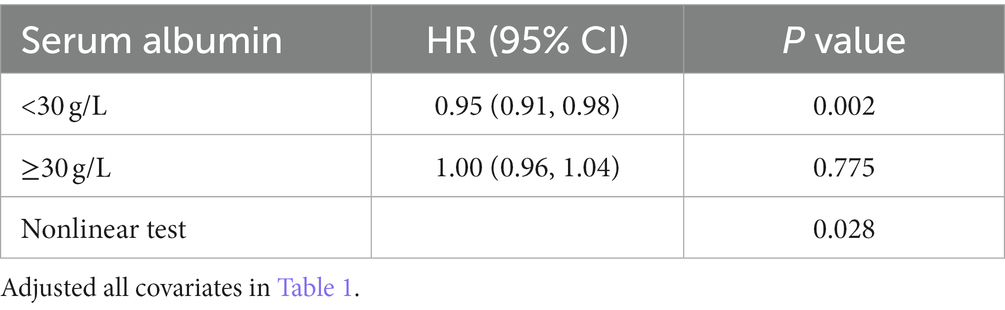
In the extended multivariable Cox models, we observed that the hazard ratios (HRs) of serum albumin were consistently significant in unadjusted, minimally adjusted (adjusted for age and sex), and fully adjusted (adjusted for all covariates in Tables 1, 2; Supplementary Table S1). The fully adjusted model indicated a negative association between serum albumin level and in-hospital mortality (HR = 0.97, 95% CI 0.96–0.99, p = 0.002. Restricted cubic spline analysis (Figure 1) showed nonlinear relationship between human serum albumin and in-hospital mortality (nonlinear test: p = 0.028, Table 3). When human serum albumin was <30 g/L, each 1 g/L increase in human serum albumin was associated with a 5% reduction in hospital mortality (HR = 0.95, 95% CI (0.91, 0.98), p = 0.002). While human serum albumin was ≥30 g/L, increased serum albumin was not associated with changing in hospital mortality (HR = 1.00, 95% CI 0.96–1.04, p = 0.775) (Table 3).
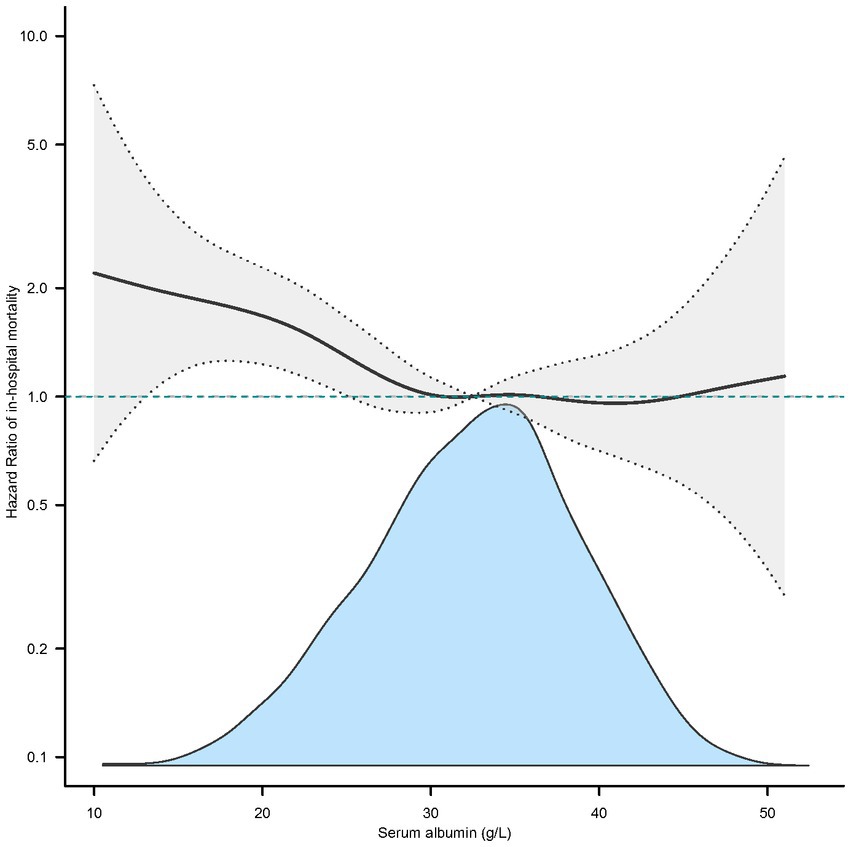
Figure 1. Nonlinear relationship between serum albumin and in-hospital mortality (adjusted all covariates in Table 1).
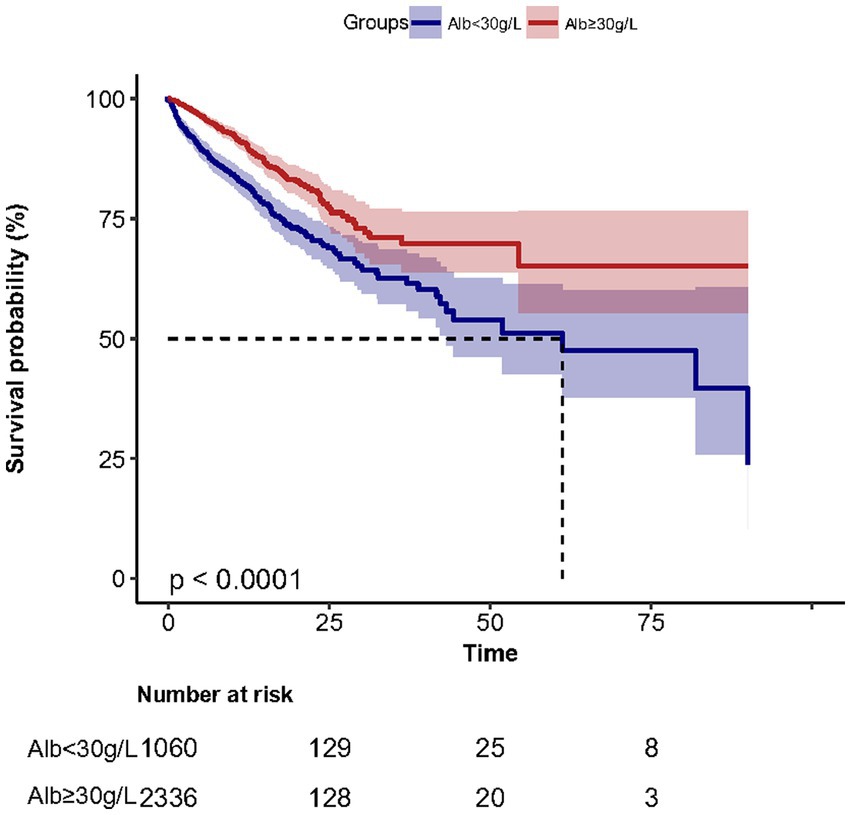
3.3.2. The in-hospital mortality was 21.7% (230/1062) and 8.2% (192/2336) for low and hyperalbuminemia groups, respectively
Kaplan–Meier survival curves (Figure 2) for cumulative hospital survival showed a statistically significant difference between the low and high human serum albumin groups (Log-rank test: p < 0.001). Multivariable Cox regression analysis showed that after adjusting for all confounders in Table 1, the in-hospital mortality in high human serum albumin group was 0.25 times lower than in the low human serum albumin group (HR = 0.75, 95% CI: 0.60 to 0.93, p = 0.008, Table 2). In Multivariable Cox regression analysis, age, Glasgow score, respiratory rate, oxygen saturation, serum potassium, PCO2, peripheral vascular disease, renal disease, diabetes mellitus, co-morbidity index, SOFA score were also associated with in-hospital mortality (Supplementary Table S1).
The distribution of albumin levels in those that survived versus those non-survived was 30.0 ± 6.2 and 28.9 ± 7.0 g/L (Supplementary Figure S3). In subgroup analysis (Figure 3), we found the results were stable in different age, sex, MV, sepsis and AECOPD groups. In multivariable Cox regression models, we evaluating the association between serum albumin and 30-day, 90-day and 1-year mortality, the results remained stable.
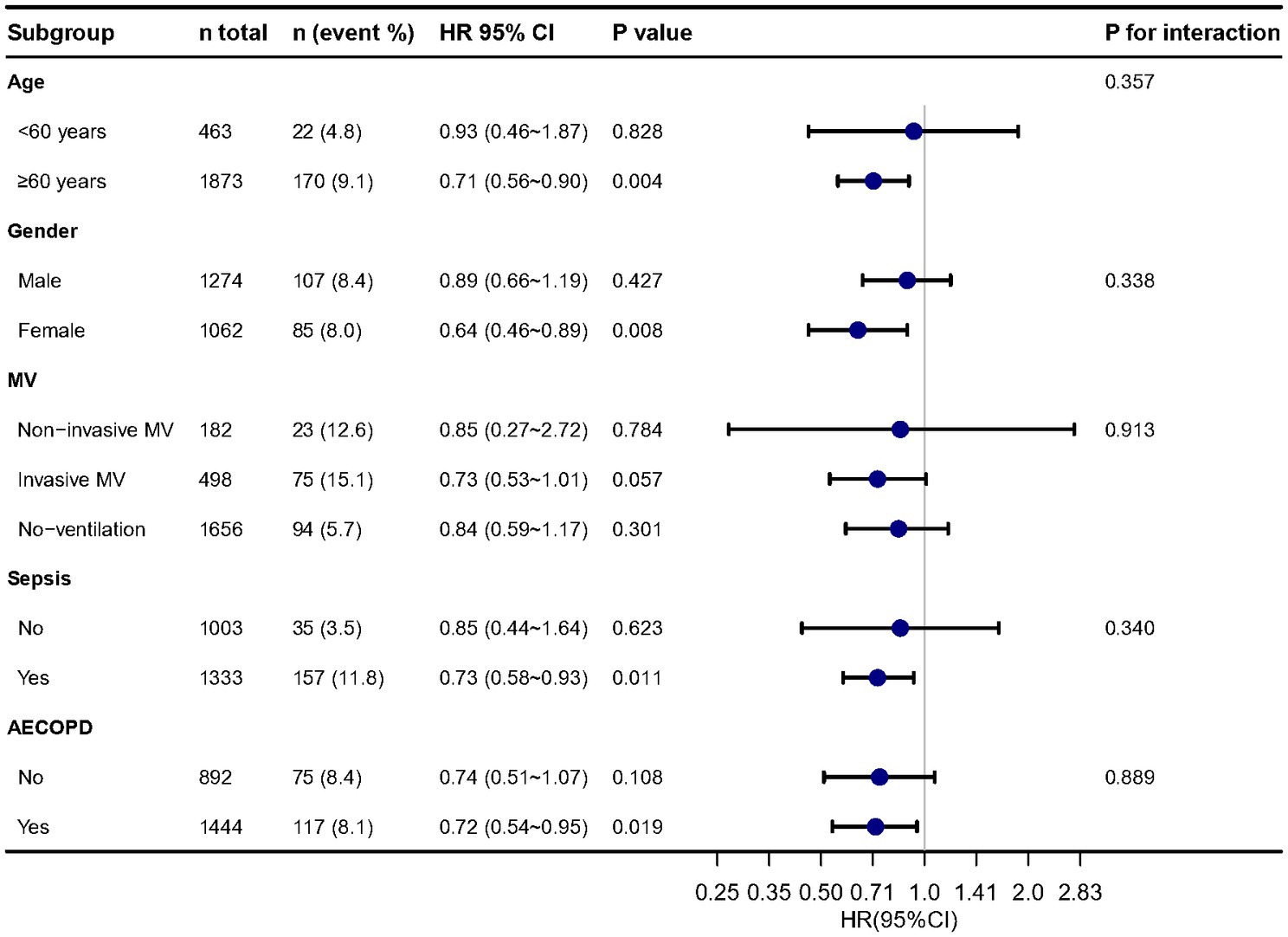
Figure 3. Stratified analysis of relationship between serum albumin and in-hospital mortality. Adjusted for all cofounders in Table 1.
4. Discussion
The results of this study showed that in ICU, lower human serum albumin is negatively associated with higher in-hospital mortality in patients with COPD. Analysis of dose–response relationship using restricted cubic spline functions indicate a nonlinear relationship between human serum albumin and in-hospital mortality. When human serum albumin was below 30 g/L, there was a negative correlation between human serum albumin and in-hospital mortality. When human serum albumin is above or equal to 30 g/L, the correlation was not significant.
As an important biomarker of nutritional status, lower serum albumin was reportedly associated with worsening clinical status during COPD (16, 17). We also found the same effect in COPD patients with critical illness. Previous studies had also reported that low levels of serum albumin could increase in-hospital mortality in COPD patients. In a retrospective (n = 574) cohort study in Spain, serum albumin levels were found to be strongly associated with disease severity and outcome in elderly patients with COPD (6). In another smaller study (n = 20), albumin levels were also indicated to be closely related to the prognosis of COPD patients (18). Similar findings were found in a larger (n = 1,647) cohort (19). However, compared to these studies, the present cohort study had a larger sample size (n = 3,398), adjusted for more confounding factors, and had relatively more stable and reliable results.
It has also been shown that there was no relationship between hypoalbuminemia and patient prognosis in a cohort study (7). However, as a result of the small sample size of this study (n = 431) and the large difference in the distribution of the number of people in the low and high human serum albumin groups in the cohort (1.5% versus 98.5%), the results were not consisting with our study (HR of low albumin versus normal was 1.67 (0.22, 12.57), p = 0.604).
This study also has clinical implications. We found a nonlinear relationship between human serum albumin and hospital mortality in critical care patients with COPD. Our clinical experience also supports this nonlinear relationship. The optimal cut off point for in-hospital mortality was 30 g/L which was similar with previous study (11, 12). Ruiqi Chen et al. found that an albumin level of 30.5 g/l was best to predict the prognosis for in-hospital mortality (11). This cut off point also suggests that clinicians may consider using albumin to increase albumin levels in patients with COPD when human serum albumin is low.
Albumin is a biomarker of malnutrition and frailty in older patients (20). Therefore, we did subgroup analysis based on age groups (>60 years and < 60 years). It seems no different between the two groups. However, for those in the >60 years group higher albumin is associated with lower mortality, while this is not seen in the <60 years group. We looked at the relationship between age and albumin and found that younger patients tended to have higher albumin levels (Supplementary Figure S1A). These patients would fall more on the right side of the curve fit, i.e., after albumin levels above 30 g/L. At this region, the increased serum albumin was not associated with changing in hospital mortality (Supplementary Figure S1B).
Similar to the previous study and our clinical experience, we also found age (21, 22), Glasgow score (23), respiratory rate (24), oxygen saturation (25), serum potassium, PCO2, peripheral vascular disease, renal disease, diabetes mellitus, comorbidity index (26), SOFA score (27) were also associated with in-hospital mortality.
5. Limitations
There are some limitations of this study. First, this is a retrospective observational single-center study and the effect of uncontrolled confounding factors cannot be excluded, however, the large sample size of this study may reduce bias to some extent. Secondly, this study evaluated the most unwell patients (in the ICU setting) and the findings cannot be applied to hospitalized patients who do not end up needing ICU level care. Third, In the study, data on respiratory-related death could not be found in MIMIC-IV database, further studies need to clarify whether albumin is associated with respiratory-related death in COPD patients.
6. Conclusion
In critical care patients with COPD, there was a negatively association between human serum albumin and in-hospital mortality.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ML and LHu wrote the manuscript. BM and CS conducted the data analysis. LHa modified the manuscript and interpreted the analysis. DZ conducted the literature review. WS reviewed the manuscript. BM and LM designed the study and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the Second Affiliated Hospital of Guangzhou Medical University New Technology Clinical Research Project (2020-LCYJ-XJS-08).
Acknowledgments
We thank the team of the Laboratory for Computational Physiology from the Massachusetts Institute of Technology (LCP-MIT) to keep the MIMIC-IV databases available.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1109910/full#supplementary-material
References
1. Adeloye, D, Chua, S, Lee, C, Basquill, C, Papana, A, Theodoratou, E, et al. Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Glob Health. (2015) 5:20415. doi: 10.7189/jogh.05.020415
2. Lozano, R, Naghavi, M, Foreman, K, Lim, S, Shibuya, K, Aboyans, V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet (London, England). (2012) 380:2095–128. doi: 10.1016/S0140-6736(12)61728-0
3. Oettl, K, and Stauber, RE. Physiological and pathological changes in the redox state of human serum albumin critically influence its binding properties. BRIT J PHARMACOL. (2007) 151:580–90. doi: 10.1038/sj.bjp.0707251
4. Gabay, C, and Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. (1999) 340:448–54. doi: 10.1056/NEJM199902113400607
5. Chen, C, Chen, Y, Lu, C, Chen, SC, Chen, Y, Lin, M, et al. Severe hypoalbuminemia is a strong independent risk factor for acute respiratory failure in COPD: a nationwide cohort study. Int J Chronic Obstr. (2015) 10:1147–54. doi: 10.2147/COPD.S85831
6. Tokgoz Akyil, F, Gunen, H, Agca, M, Gungor, S, Yalcinsoy, M, Sucu, P, et al. Patient outcome after chronic obstructive pulmonary disease exacerbations requiring non-invasive ventilation during hospitalization. Arch Bronconeumol. (2016) 52:470–6. doi: 10.1016/j.arbres.2016.01.021
7. Mendy, A, Forno, E, Niyonsenga, T, and Gasana, J. Blood biomarkers as predictors of long-term mortality in COPD. Clin Respir J. (2018) 12:1891–9. doi: 10.1111/crj.12752
8. Johnson, AEW, Pollard, TJ, Shen, L, Lehman, LWH, Feng, M, Ghassemi, M, et al. MIMIC-III, a freely accessible critical care database. ScI Data. (2016) 3:160035. doi: 10.1038/sdata.2016.35
9. Liu, T, Zhao, Q, and Du, B. Effects of high-flow oxygen therapy on patients with hypoxemia after extubation and predictors of reintubation: a retrospective study based on the MIMIC-IV database. BMC Pulm Med. (2021) 21:160. doi: 10.1186/s12890-021-01526-2
10. Elm, EV, Altman, DG, Egger, M, Pocock, SJ, Gøtzsche, PC, and Vandenbroucke, JP. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ-Brit Med J. (2007) 335:806–8. doi: 10.1136/bmj.39335.541782.AD
11. Chen, R, Xing, L, You, C, and Ou, X. Prediction of prognosis in chronic obstructive pulmonary disease patients with respiratory failure: a comparison of three nutritional assessment methods. Eur J Intern Med. (2018) 57:70–5. doi: 10.1016/j.ejim.2018.06.006
12. Luk, JK, Chiu, PK, Tam, S, and Chu, LW. Relationship between admission albumin levels and rehabilitation outcomes in older patients. Arch Gerontol Geriatr. (2011) 53:84–9. doi: 10.1016/j.archger.2010.06.015
13. Yang, Q, Zheng, J, Chen, W, Chen, X, Wen, D, Chen, W, et al. Association between preadmission metformin use and outcomes in intensive care unit patients with sepsis and type 2 diabetes: a cohort study. Front Med-Lausanne. (2021):8. doi: 10.3389/fmed.2021.640785
14. Park, S, Freedman, ND, Haiman, CA, Le Marchand, L, Wilkens, LR, and Setiawan, VW. Association of Coffee Consumption with Total and Cause-Specific Mortality among Nonwhite Populations. Ann Intern Med. (2017) 167:228. doi: 10.7326/M16-2472
15. Chen, J, Fu, C, and Pan, T. Modeling method and miniaturized wavelength strategy for near-infrared spectroscopic discriminant analysis of soy sauce brand identification. Spectrochim Acta A. (2022) 277:121291. doi: 10.1016/j.saa.2022.121291
16. Zisman, DA, Kawut, SM, Lederer, DJ, Belperio, JA, Lynch, JPR, Schwarz, MI, et al. Serum albumin concentration and waiting list mortality in idiopathic interstitial pneumonia. Chest. (2009) 135:929–35. doi: 10.1378/chest.08-0754
17. Weng, C, Hu, C, Yen, T, Hsu, C, and Huang, W. Nutritional predictors of mortality in long term hemodialysis patients. Sci Rep. (2016) 6:35639. doi: 10.1038/srep35639
18. Añón, JM, García de Lorenzo, A, Zarazaga, A, Gómez-Tello, V, and Garrido, G. García de Lorenzo a, Zarazaga a, Gómez-Tello V, Garrido G: mechanical ventilation of patients on long-term oxygen therapy with acute exacerbations of chronic obstructive pulmonary disease: prognosis and cost-utility analysis. Intensive Care Med. (1999) 25:452–7. doi: 10.1007/s001340050879
19. Chen, D, Jiang, L, Li, J, Tan, Y, Ma, M, Cao, C, et al. Interaction of acute respiratory failure and acute kidney injury on in-hospital mortality of patients with acute exacerbation COPD. Int J Chronic Obstr. (2021) 16:3309–16. doi: 10.2147/COPD.S334219
20. Xu, L, Zhang, J, Shen, S, Liu, Z, Zeng, X, Yang, Y, et al. Clinical frailty scale and biomarkers for assessing frailty in elder inpatients in China. J Nutr Health Aging. (2021) 25:77–83. doi: 10.1007/s12603-020-1455-8
21. Easter, M, Bollenbecker, S, Barnes, JW, and Krick, S. Targeting aging pathways in chronic obstructive pulmonary disease. Int J Mol Sci. (2020) 21:6924. doi: 10.3390/ijms21186924
22. Pellicori, P, McConnachie, A, Carlin, C, Wales, A, and Cleland, J. Predicting mortality after hospitalisation for COPD using electronic health records. Pharmacol Res. (2022) 179:106199. doi: 10.1016/j.phrs.2022.106199
23. Zhang, JB, Zhu, JQ, Cao, LX, Jin, XH, Chen, LL, Song, YK, et al. Use of the modified Glasgow coma scale score to guide sequential invasive-noninvasive mechanical ventilation weaning in patients with AECOPD and respiratory failure. Exp Ther Med. (2020) 20:1441–6. doi: 10.3892/etm.2020.8884
24. AbuRuz, S, al-Azayzih, A, ZainAlAbdin, S, Beiram, R, and al Hajjar, M. Clinical characteristics and risk factors for mortality among COVID-19 hospitalized patients in UAE: does ethnic origin have an impact. PLoS One. (2022) 17:e264547. doi: 10.1371/journal.pone.0264547
25. Zhao, Z, Chen, A, Hou, W, Graham, JM, Li, H, Richman, PS, et al. Prediction model and risk scores of ICU admission and mortality in COVID-19. PLoS One. (2020) 15:e236618. doi: 10.1371/journal.pone.0236618
26. Andreen, N, Andersson, L, Sundell, N, Gustavsson, L, and Westin, J. Mortality of COVID-19 is associated with comorbidity in patients with chronic obstructive pulmonary disease. Infect Dis. (2022) 54:508–13. doi: 10.1080/23744235.2022.2050422
Keywords: severe chronic obstructive pulmonary disease, serum albumin, in-hospital mortality, ICU - intensive care unit, relationship
Citation: Ling M, Huiyin L, Shanglin C, Haiming L, Zhanyi D, Shuchun W, Meng B and Murong L (2023) Relationship between human serum albumin and in-hospital mortality in critical care patients with chronic obstructive pulmonary disease. Front. Med. 10:1109910. doi: 10.3389/fmed.2023.1109910
Edited by:
Justin Garner, Royal Brompton Hospital, United KingdomReviewed by:
Corrado Pelaia, Magna Græcia University, ItalyZhe Wu, Royal Brompton Hospital, United Kingdom
Copyright © 2023 Ling, Huiyin, Shanglin, Haiming, Zhanyi, Shuchun, Meng and Murong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bai Meng, YmFpbWVuZzQ0NTBAMTI2LmNvbQ==; Lu Murong, bHVjZW5jeTE2M0AxNjMuY29t
†These authors have contributed equally to this work
 Ma Ling1†
Ma Ling1† Bai Meng
Bai Meng Lu Murong
Lu Murong