- People's Hospital of Guangxi Zhuang Autonomous Region, Nanning, China
Leptospirosis is a zoonotic disease with diverse clinical manifestations, and its severe form can lead to life-threatening complications such as pulmonary hemorrhage. We present a novel case of a 57-year-old woman with leptospirosis who developed severe pulmonary hemorrhage and acute respiratory distress syndrome (ARDS) and was successfully managed with early venovenous extracorporeal membrane oxygenation (VV-ECMO), minimal anticoagulation, and lymphoplasmacyte exchange (LPE). This case highlights the importance of early ECMO initiation, individualized anticoagulation, and immunomodulatory therapy in improving outcomes for patients with leptospiral pulmonary hemorrhage syndrome (LPHS), a condition with mortality exceeding 50%. To our knowledge, this is the first reported case combining VV-ECMO with LPE in LPHS, offering a new therapeutic paradigm for critically ill patients at the intersection of infection and autoimmunity.
Introduction
Leptospirosis is a globally prevalent zoonosis caused by pathogenic Leptospira species. It is primarily transmitted to humans through contact with water or soil contaminated by the urine of infected animals, including rodents and livestock (1, 2). The clinical spectrum of leptospirosis ranges from a mild, flu-like illness to severe forms involving multiple organ systems, such as the lungs, liver, kidneys, and central nervous system (3). Pulmonary involvement in leptospirosis can present as mild pneumonia or, in severe cases, as massive pulmonary hemorrhage, which is associated with a high mortality rate (4, 5). While ECMO has been used in severe leptospirosis, the combination with immunomodulatory techniques such as LPE remains unexplored.
Case presentation
Initial presentation
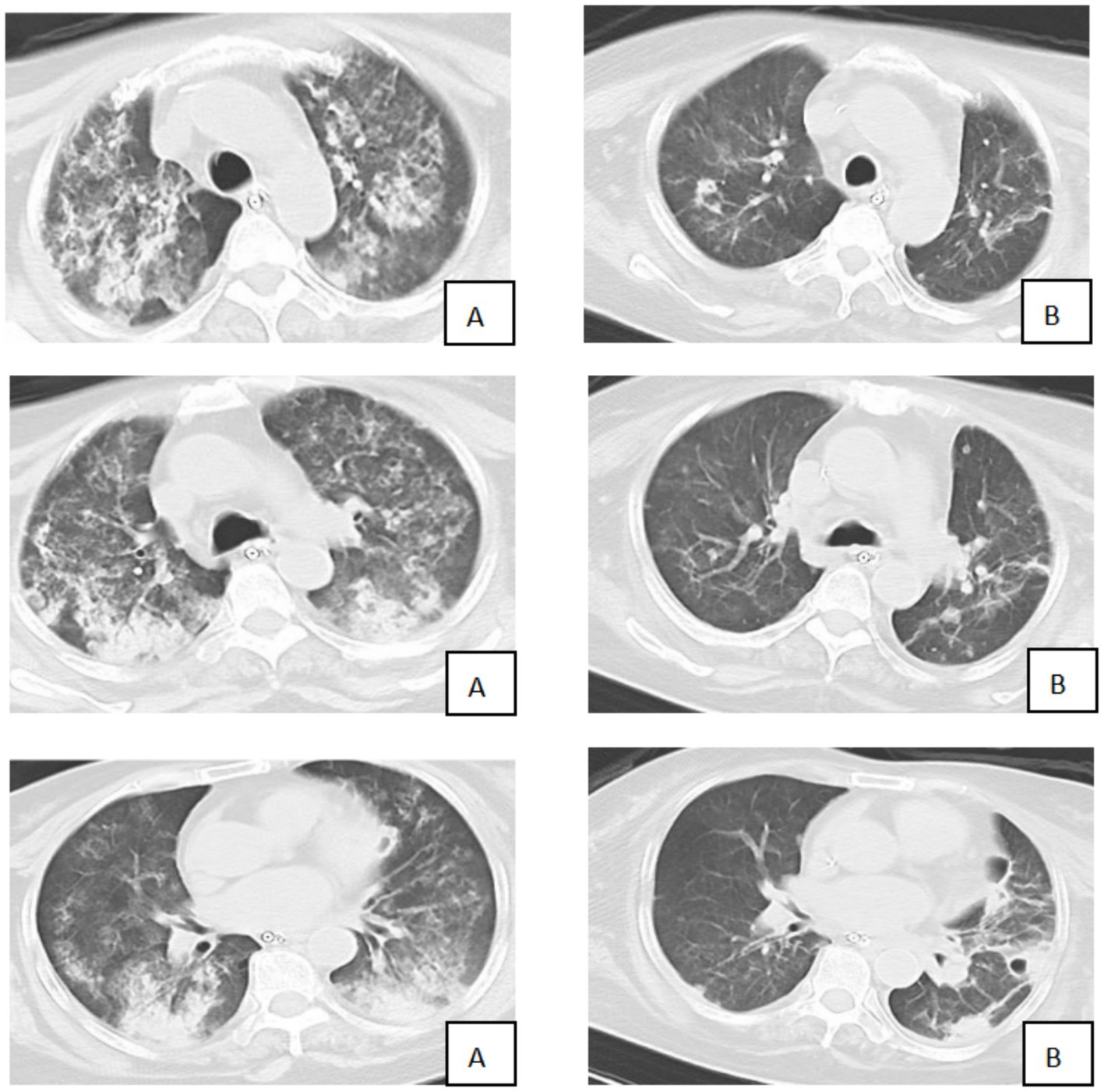
A 57-year-old female farmer was admitted to our hospital with a 3-day history of fever, fatigue, myalgia, and headache. The patient denied recent travel outside the local area but reported frequent work in rice paddy fields. On admission, the patient was febrile, with a body temperature of 39.5 °C, a heart rate of 110 beats per minute, a respiratory rate of 30 breaths per minute, and a blood pressure of 100/60 mmHg. Physical examination revealed scattered rales on the auscultation of both lungs and mild jaundice. Laboratory investigations showed leukocytosis (white blood cell count:15 × 10^9/L), thrombocytopenia (platelet count: 80 × 10^9/L), elevated liver enzymes (alanine aminotransferase: 200 U/L, aspartate aminotransferase: 250 U/L), and elevated creatinine (150 μmol/L). C-reactive protein was significantly elevated at 120 mg/L. The myositis-specific antibody panel revealed the presence of anti-Ro-52 and low-titer anti-Mi-2β antibodies. Bedside transthoracic echocardiography revealed impaired left ventricular systolic function (ejection fraction, 36%) and mild-to-moderate tricuspid regurgitation. A chest CT scan was performed and showed diffuse bilateral ground-glass opacities and consolidation, consistent with alveolar hemorrhage and ARDS (Figure 1).
Clinical deterioration
Over the subsequent 24 h, the patient’s condition deteriorated rapidly. Progressive dyspnea developed, and arterial blood gas analysis indicated severe hypoxemia (partial pressure of arterial oxygen/fraction of inspired oxygen ratio [PaO₂/FiO₂] = 107 mmHg) despite optimal mechanical ventilation settings. Chest radiography demonstrated diffuse bilateral infiltrates, consistent with acute respiratory distress syndrome (ARDS) (Figure 2). Bronchoscopy revealed massive endobronchial bleeding, indicative of severe pulmonary hemorrhage (Figure 3). Considering the patient’s rapidly worsening respiratory status and presence of multiorgan dysfunction, a diagnosis of severe leptospirosis with pulmonary hemorrhage was suspected. As this pathogen is not commonly encountered in our hospital, specific antigens for the microscopic agglutination test (MAT) were not available. However, microscopic examination of bronchoalveolar lavage fluid collected via bronchoscopy revealed Leptospira (as shown in Figure 4). After 48 h, polymerase chain reaction (PCR) results from the patient’s blood and alveolar lavage fluid samples confirmed the presence of Leptospira DNA and additional Mycobacterium chelonae and Aspergillus flavus. The diagnosis of leptospirosis with hemorrhagic lung manifestation was thus confirmed.
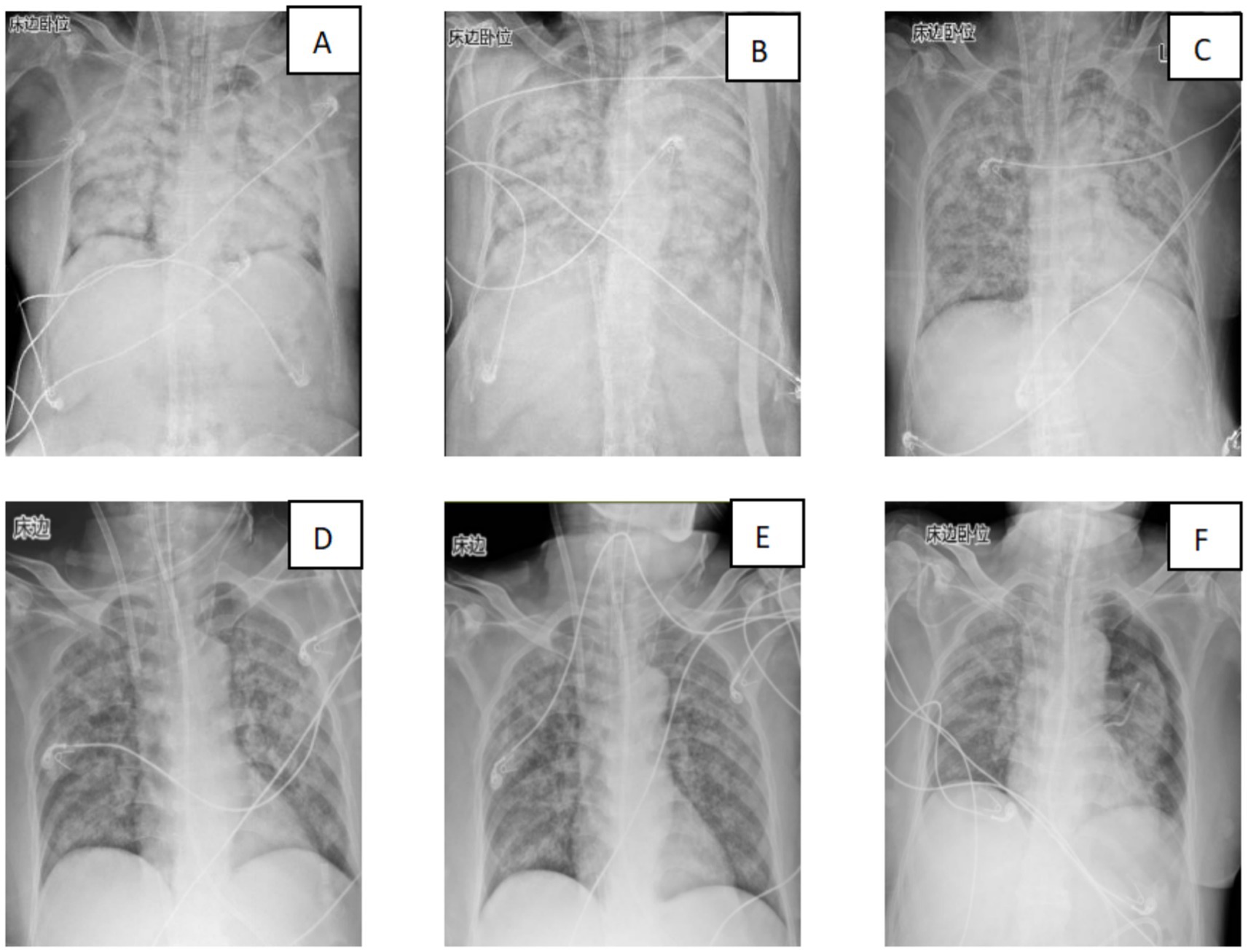
Figure 2. Changes in the patient’s lung imaging (A: 29th July; B: 30th July 30; C: 31st July; D: 2nd August; E: 3rd August; and F: 11th August).
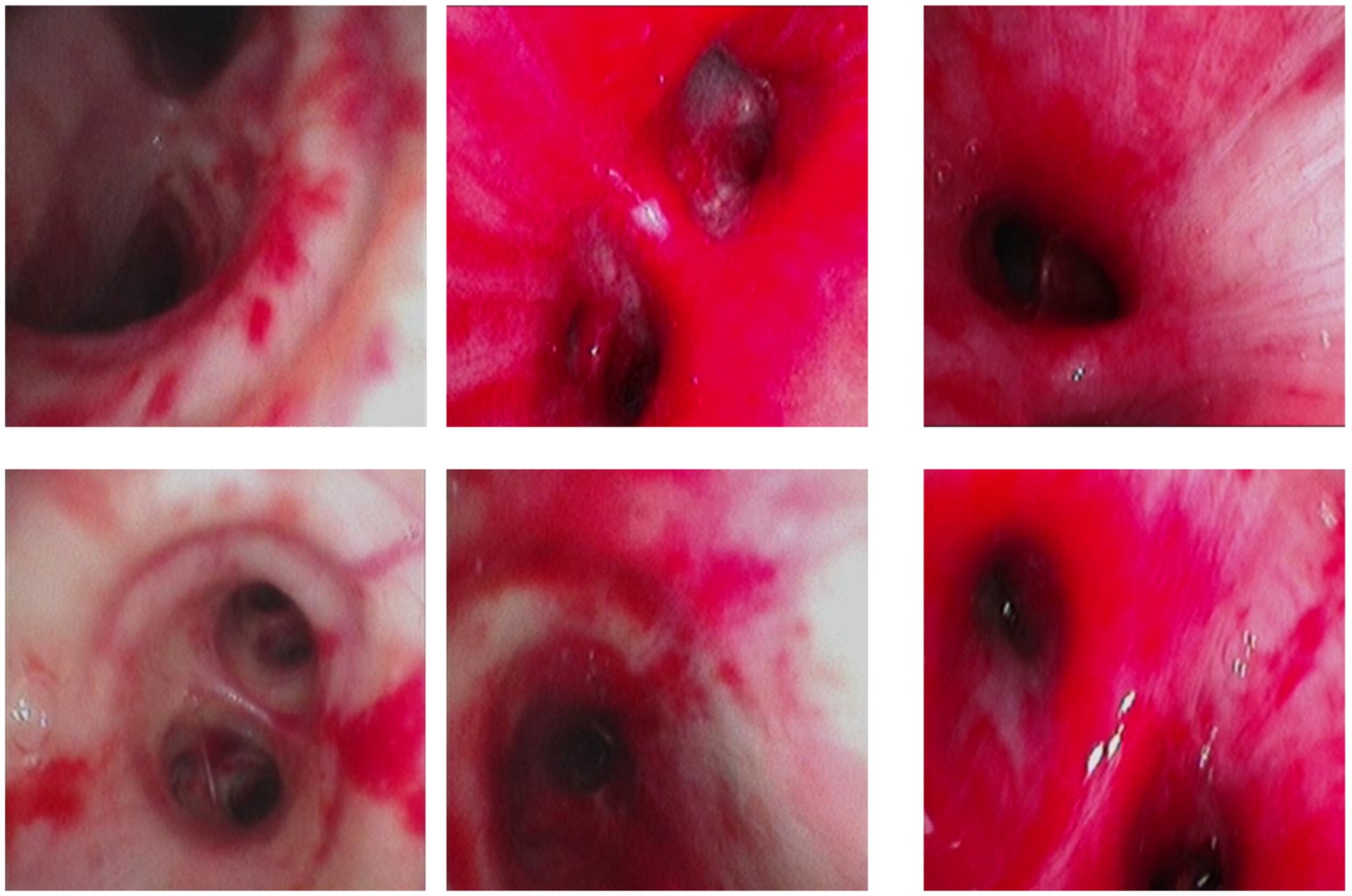
Figure 3. Diffuse redness, swelling, and bleeding from the airway in the patient under bronchoscopy.
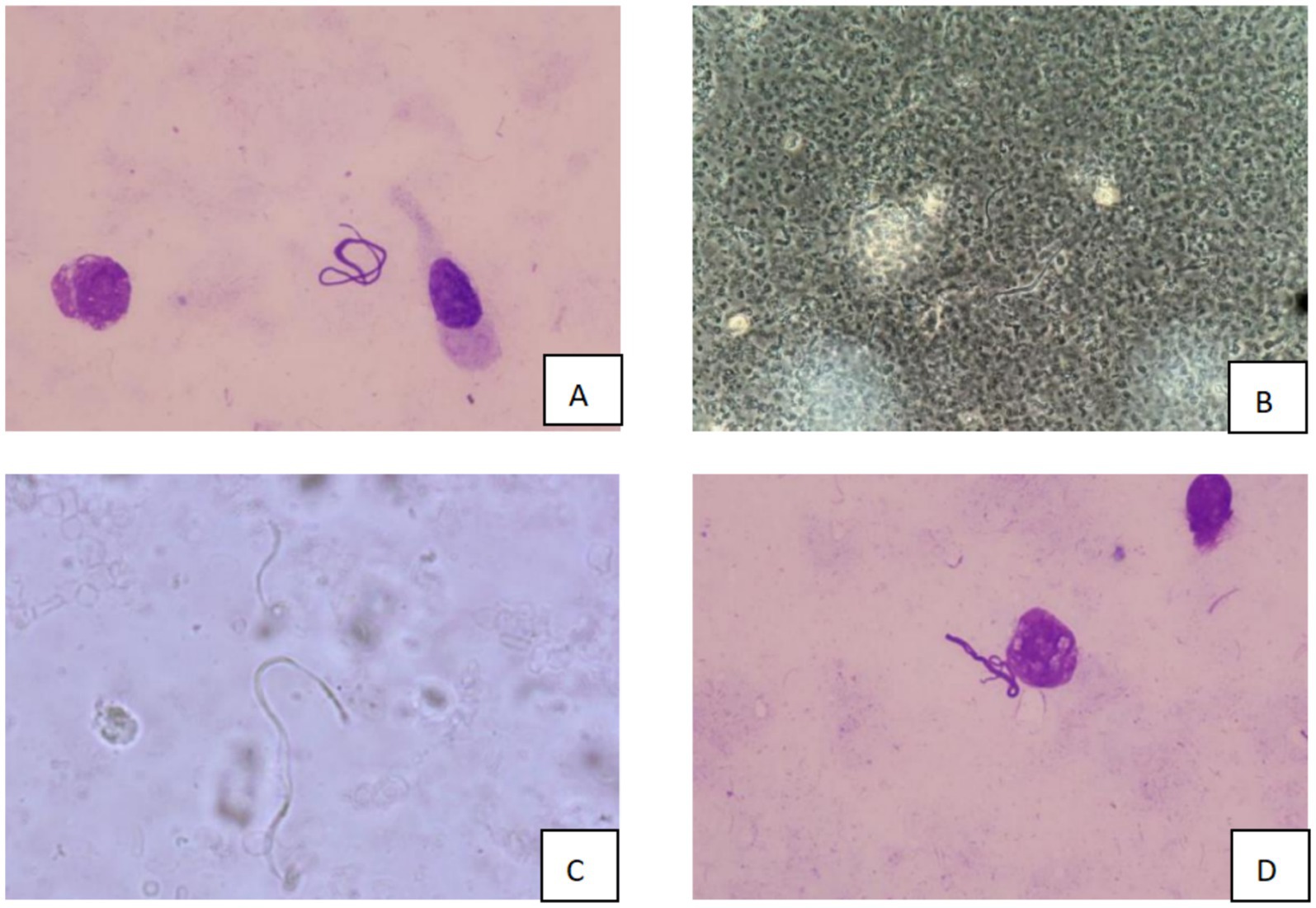
Figure 4. Microscopic examination results of bronchoalveolar lavage fluid (A/D: Wright’s staining, 10 × 100 magnification; B/C: dark field microscopy, 10 × 40 magnification).
Management
Initiation of ECMO
Due to refractory hypoxemia and failure to maintain adequate oxygenation with conventional mechanical ventilation, a decision was made to initiate VV-ECMO. Venovenous extracorporeal membrane oxygenation (VV-ECMO) was established via cannulation of the right internal jugular vein and femoral vein. The cannulation procedure involved ultrasound-guided placement of a 21-French (Fr) multistage drainage cannula into the right femoral vein, advanced to a depth of 40 cm at the junction of the inferior vena cava and right atrium. A 17-Fr return cannula was placed in the right internal jugular vein to a depth of 14 cm. The circuit consisted of a Maquet Rotaflow® centrifugal pump with Bioline coating and a Maquet Quadrox® polymethylpentene oxygenator. Initial ECMO settings included a pump speed of 2,500 revolutions per minute (rpm), generating a blood flow rate of 3.5 liters per minute (L/min), and a sweep gas (oxygen) flow rate of 4 L/min. Following ECMO initiation, the patient demonstrated significant improvement in oxygenation, with the ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO₂/FiO₂ ratio) increasing to 250 mmHg.
Antibiotic therapy
Based on the diagnosis of leptospirosis, intravenous penicillin G (2 million units every 4 h) was immediately administered. Given the polymicrobial infection with Mycobacterium chelonae and Aspergillus flavus, combination therapy with intravenous amikacin (10–15 mg/kg once daily) and amphotericin B lipid complex (3–5 mg/kg/day) was administered.
Supportive therapies
In addition to ECMO and antibiotic treatment, the patient received comprehensive supportive care. Continuous renal replacement therapy (CRRT) was initiated due to acute kidney injury. Fluid management was optimized to maintain hemodynamic stability while avoiding fluid overload. Platelet and fresh-frozen plasma transfusions were administered as needed to correct thrombocytopenia and coagulopathy. Given the presentation of pulmonary hemorrhage and the specific autoantibody profile, the differential diagnosis included connective tissue disease-associated vasculitis. This finding warranted antibody-removing therapy; hence, lymphoplasmacyte exchange (LPE) was performed on 31st July, 1st August, and 3rd August. The patient was also placed in the prone position to improve oxygenation and recruit collapsed alveoli. Notably, corticosteroids were not administered due to active pulmonary hemorrhage and concern about exacerbating bleeding, as well as the availability of a more targeted immunomodulatory approach via LPE.
Clinical course
Over the following days, the patient’s condition gradually improved. The volume of endobronchial bleeding decreased, and the oxygenation index continued to improve. On day 4 of ECMO support, the patient’s lung exudation had decreased compared to before, and oxygenation had begun to improve. On day 8, following a comprehensive assessment of respiratory function, oxygenation status, and overall clinical condition, the decision was made to wean the patient from ECMO. The weaning process was uneventful, and the ECMO circuit was successfully removed. The patient was then gradually weaned off mechanical ventilation over the next 3 days.
Outcome
The patient was transferred to the general ward on day 15 after admission. Follow-up laboratory tests showed normal liver and kidney function, and the complete blood count had returned to normal. The patient was discharged on day 18 with a prescription for oral doxycycline for a total of 14 days. At the 2-week follow-up visit, the patient reported no residual symptoms, and chest radiography showed complete resolution of pulmonary infiltrates.
Discussion
Leptospiral pulmonary hemorrhage syndrome (LPHS), the most severe complication of leptospirosis, is characterized by toxin-mediated endothelial damage and cytokine storms that lead to diffuse alveolar hemorrhage (DAH) and refractory hypoxemia, with mortality exceeding 50% (6). Our patient presented with an oxygenation index (PaO₂/FiO₂) of 107 mmHg that persisted despite mechanical ventilation and prone positioning, meeting criteria for severe ARDS (7). Venovenous extracorporeal membrane oxygenation (VV-ECMO) served as a salvage therapy, providing critical time for lung recovery through extracorporeal gas exchange. Notably, our early ECMO initiation within 24 h of mechanical ventilation contrasts with conventional practice. Trejnowska et al. (8) demonstrated that delayed ECMO initiation (>48 h) in LPHS increased mortality 2-fold (OR 2.1, 95% CI 1.3–3.4), aligning with Extracorporeal Life Support Organization (ELSO) guidelines advocating prompt intervention in severe ARDS (9).
The application of ECMO in active pulmonary hemorrhage presents a therapeutic paradox: insufficient anticoagulation increases thrombotic risk (peak D-dimer: 10.40 mg/L), while excessive anticoagulation exacerbates bleeding (10). We implemented a minimal effective anticoagulation strategy (target activated partial thromboplastin time (APTT) 40–50 s) with fibrinogen maintenance >1.5 g/L, combined with topical hemostasis (bronchoscopic lavage + carbazochrome). This approach diverged significantly from published protocols: Suvadeep Sen et al. reported fatalities in both of their two LPHS-ECMO patients, attributing the outcomes to hemorrhage aggravated by conventional anticoagulation (targeting an APTT of 60–80 s) (11). Our patient achieved thromboprophylaxis without new hemorrhagic complications, supporting ELSO’s recommendation for reduced anticoagulation in hemorrhagic infectious disease (12). On the 10th day after admission, follow-up echocardiography showed that the patient’s cardiac function had significantly improved, with a left ventricular ejection fraction (LVEF) of 66% and complete resolution of tricuspid regurgitation. Furthermore, VV-ECMO support improved systemic oxygenation, which in turn mitigated hypoxic pulmonary vasoconstriction and secondary pulmonary hypertension, leading to improved tricuspid regurgitation and reduced right ventricular afterload. This mechanism disrupted the self-perpetuating cycle of hypoxia, pulmonary vasoconstriction, and right heart failure, which was particularly beneficial given the patient’s pre-existing impairment of left ventricular systolic function (13).
Antimicrobial stewardship constituted another cornerstone of recovery. While penicillin remains the gold standard for leptospirosis (>95% susceptibility) (14), Jarisch–Herxheimer reactions may exacerbate pulmonary hemorrhage during treatment initiation (15). Under ECMO support, we administered high-dose penicillin (4.8 million units Q6H vs. conventional 1.6–2.4 million units) without hemodynamic compromise, with alveolar hemorrhage significantly diminishing within 48 h. This corroborates Brett-Major’s meta-analysis, wherein early high-dose penicillin reduced leptospirosis mortality by 79% (RR 0.21, 95% CI 0.08–0.55) (16). The patient subsequently developed a life-threatening polymicrobial co-infection with Mycobacterium chelonae and Aspergillus flavus in the bronchoalveolar lavage fluid, a consequence of dysbiosis from broad-spectrum antibiotics. To address this and the attendant risk of ECMO circuit biofilm formation (17), we instituted a targeted antimicrobial regimen: amphotericin B lipid complex (for deep tissue penetration) and amikacin (M. chelonae). This intervention was critical, as polymicrobial infections have been reported to increase mortality in LPHS to 80% (18), making the insights from this case particularly valuable.
The most innovative intervention was lymphoplasmacyte exchange (LPE). Positive anti-Mi-2β and anti-Ro-52 antibodies suggested autoimmune involvement (e.g., ANCA-associated vasculitis) in lung injury (19). Conventional plasma exchange would deplete coagulation factors and increase bleeding risk. LPE selectively removes pathogenic lymphocytes and antibodies while preserving coagulation function—particularly advantageous for ECMO patients with hemorrhage (19). Post-LPE (three sessions), oxygenation index improved from 107 mmHg to >200 mmHg, and IL-6 normalized from 103.0 pg/mL without bleeding complications. Lymphocytapheresis reduces the IL-6/TNF-α load by depleting cytokine-producing lymphocytes, thereby attenuating C5a generation and its downstream endothelial injury pathways (oxidative stress, apoptosis, and barrier dysfunction) (20, 21). To our knowledge, this represents the first successful combination of LPE with ECMO in LPHS with autoimmune seropositivity, offering a novel strategy for critical illnesses at the intersection of infection and immunity.
Critical deviations from conventional management protocols were implemented in this case, with comparative analysis revealing significant associations with improved outcomes. First, VV-ECMO was initiated within 24 h of mechanical ventilation—markedly earlier than the mean 72-h window reported in comparable cohorts (22). This expedited intervention correlated with patient survival, contrasting sharply with the 80% mortality observed under delayed initiation. Second, anticoagulation targeting an APTT of 40–50 s combined with fibrinogen monitoring was used, substantially lower than the standard 60–80 s range. This conservative strategy resulted in zero hemorrhagic complications, compared to a 60% hemorrhage rate associated with conventional intensity anticoagulation. Third, immunomodulation utilizing three sessions of LPE was administered instead of high-dose corticosteroid regimens. This approach demonstrated superior efficacy in normalizing interleukin-6 (IL-6) levels, contrasting with persistent inflammatory responses documented with steroid-based protocols. Compared to Umei N’s ECMO-only approach (reporting 20% survival in LPHS with co-infections) (23), our LPE-ECMO-antimicrobial triad achieved survival where literature predicts <20% success. Weaning followed international consensus: ECMO discontinued when oxygenation index exceeded 200 mmHg with bronchoscopic confirmation of hemorrhage cessation (13).
Limitations and future directions
Three limitations warrant mention: Diagnostic certainty: Leptospira identification initially relied on morphology rather than the microscopic agglutination test (MAT) or PCR (6); LPE quantification: The efficiency of antibody clearance (e.g., anti-Ro-52) was not measured. Long-term outcomes: Neurological recovery requires extended follow-up. Future studies should establish leptospirosis-ECMO registries to optimize anticoagulation (e.g., thromboelastography-guided) and biomarker-directed LPE indications.
Conclusion
This case report demonstrates the successful use of VV-ECMO in combination with antibiotics and supportive therapies for a patient with severe leptospirosis complicated by life-threatening pulmonary hemorrhage. Early disease recognition, prompt ECMO initiation, and comprehensive management are key factors in improving prognosis in such patients. Further studies are needed to better define optimal management strategies for leptospirosis-related severe pulmonary hemorrhage and to enhance outcomes in critically ill patients.
Patient perspective
The patient provided written consent for publication. In follow-up interviews, she expressed gratitude for the “second chance” but highlighted the need for psychological support post-ICU.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of Guangxi Zhuang Autonomous Region People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LY: Conceptualization, Data curation, Investigation, Writing – original draft, Formal Analysis. XT: Formal Analysis, Methodology, Writing – review & editing. BX: Writing – review & editing. YZ: Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Clinical Research Center for Critical Treatment of Major Infectious Diseases (No. Guike AD22035101) and the Guangxi Key Laboratory of Diagnosis and Treatment of Acute Respiratory Distress Syndrome (No. ZZH2020013). We declare that the funders had no involvement in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1696005/full#supplementary-material
References
1. Tazerji, SS, Nardini, R, Safdar, M, Shehata, AA, and Duarte, PM. An overview of anthropogenic actions as drivers for emerging and re-emerging zoonotic diseases. Pathogens. (2022) 11:1376. doi: 10.3390/pathogens11111376
2. Sayanthi, Y, and Susanna, D. Pathogenic Leptospira contamination in the environment: a systematic review. Infect Ecol Epidemiol. (2024) 14:2324820. doi: 10.1080/20008686.2024.2324820
3. Sethi, A, Kumar, TP, Vinod, KS, Boodman, C, Bhat, R, Ravindra, P, et al. Kidney involvement in leptospirosis: a systematic review and meta-analysis. Infection. (2025) 53:785–96. doi: 10.1007/s15010-025-02492-1
4. Priyankara, D, Ruwanpathirana, P, Rambukwella, R, and Perera, N. Sequential pulmonary functions in survivors of leptospirosis pulmonary haemorrhage syndrome: a prospective cohort study. Trop Med Health. (2024) 52:96. doi: 10.1186/s41182-024-00665-6
5. Wickramasinghe, M, Chandraratne, A, Doluweera, D, Weerasekera, MM, and Perera, N. Predictors of severe leptospirosis: a review. Eur J Med Res. (2025) 30:445. doi: 10.1186/s40001-025-02518-2
6. Haake, DA, and Levett, PN. Leptospirosis in humans. Curr Top Microbiol Immunol. (2015) 387:65–97. doi: 10.1007/978-3-662-45059-8_5
7. Ranieri, VM, Rubenfeld, GD, Thompson, BT, Ferguson, ND, Caldwell, E, Fan, E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. (2012) 307:2526–33. doi: 10.1001/jama.2012.5669
8. Trejnowska, E, Drobiński, D, Knapik, P, Wajda-Pokrontka, M, Szułdrzyński, K, Staromłyński, J, et al. Extracorporeal membrane oxygenation for severe COVID-19-associated acute respiratory distress syndrome in Poland: a multicenter cohort study. Crit Care. (2022) 26:97. doi: 10.1186/s13054-022-03959-5
9. Barbaro, RP, MacLaren, G, Boonstra, PS, Iwashyna, TJ, Slutsky, AS, Fan, E, et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the extracorporeal life support organization registry. Lancet. (2020) 396:1071–8. doi: 10.1016/S0140-6736(20)32008-0
10. Smith, DE, Chang, SH, Geraci, TC, James, L, Kon, ZN, Carillo, JA, et al. One-year outcomes with Venovenous extracorporeal membrane oxygenation support for severe COVID-19. Ann Thorac Surg. (2022) 114:70–5. doi: 10.1016/j.athoracsur.2022.01.003
11. Sen, S, Goyal, A, and Lokhande, V. Extracorporeal membrane oxygenation in severe pulmonary forms of leptospirosis: a report of two cases. Indian J Crit Care Med. (2022) 26:966–9. doi: 10.5005/jp-journals-10071-24286
12. McMichael, A, Ryerson, LM, Ratano, D, Fan, E, Faraoni, D, and Annich, GM. 2021 ELSO adult and pediatric anticoagulation guidelines. ASAIO J. (2022) 68:303–10. doi: 10.1097/MAT.0000000000001652
13. Schmidt, M, Hajage, D, Lebreton, G, Monsel, A, Voiriot, G, Levy, D, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: a retrospective cohort study. Lancet Respir Med. (2020) 8:1121–31. doi: 10.1016/S2213-2600(20)30328-3
14. Win, TZ, Han, SM, Edwards, T, Maung, HT, Brett-Major, DM, Smith, C, et al. Antibiotics for treatment of leptospirosis. Cochrane Database Syst Rev. (2024) 2024 3:CD014960. doi: 10.1002/14651858.CD014960.pub2
15. Wimalawansa, SJ. Infections and autoimmunity—the immune system and vitamin D: a systematic review. Nutrients. (2023) 15:3842. doi: 10.3390/nu15173842
16. Brett-Major, DM, and Coldren, R. Antibiotics for leptospirosis. Cochrane Database Syst Rev. (2012) 2012:CD008264. doi: 10.1002/14651858.CD008264.pub2
17. Ramanathan, K, Shekar, K, Ling, RR, Barbaro, RP, Wong, SN, Tan, CS, et al. Extracorporeal membrane oxygenation for COVID-19: a systematic review and meta-analysis. Crit Care. (2021) 25:211. doi: 10.1186/s13054-021-03634-1
18. Lippi, I, Puccinelli, C, Perondi, F, Ceccherini, G, Pierini, A, Marchetti, V, et al. Predictors of fatal pulmonary Haemorrhage in dogs affected by leptospirosis approaching Haemodialysis. Veterinary Sciences. (2021) 8:25. doi: 10.3390/vetsci8020025
19. Goldschmied, A, and Riessen, R. New aspects of therapeutic plasma exchange in critical care medicine. Dtsch Med Wochenschr. (2021) 146:167–70. doi: 10.1055/a-1340-3256
20. Montrucchio, G, Traversi, R, Arrigo, G, Bonetto, C, Sales, G, Busca, A, et al. Hemophagocytic Lymphohistiocytosis in the adult critically ill: a narrative review of case reports and case series. Front Med (Lausanne). (2025) 12:1622770. doi: 10.3389/fmed.2025.1622770
21. Kanai, T, Makita, S, Kawamura, T, Nemoto, Y, Kubota, D, Nagayama, K, et al. Extracorporeal elimination of TNF-alpha-producing CD14(dull)CD16(+) monocytes in leukocytapheresis therapy for ulcerative colitis. Inflamm Bowel Dis. (2007) 13:284–90. doi: 10.1002/ibd.20017
22. Cantwell, T, Ferre, A, Van Sint Jan, N, Blamey, R, Dreyse, J, Baeza, C, et al. Leptospirosis-associated catastrophic respiratory failure supported by extracorporeal membrane oxygenation. J Artif Organs. (2017) 20:371–6. doi: 10.1007/s10047-017-0998-x
Keywords: leptospirosis, pulmonary hemorrhage, venovenous extracorporeal membrane oxygenation (VV-ECMO), acute respiratory distress syndrome (ARDS), lymphoplasmacyte exchange (LPE)
Citation: Yang LW, Tang XG, Xiong B and Zhang Y (2025) A case report of leptospirosis complicated by severe pulmonary hemorrhage treated with venovenous extracorporeal membrane oxygenation. Front. Med. 12:1696005. doi: 10.3389/fmed.2025.1696005
Edited by:
Pavlo Petakh, Uzhhorod National University, UkraineReviewed by:
Mariam Mohammed Thalji, Al-Quds University, PalestineYash Kedia, Vardhman Mahavir Medical College and Safdarjung Hospital, India
Copyright © 2025 Yang, Tang, Xiong and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Wen Yang, eWxpd2VuMjAyNUAxNjMuY29t; Xiao Gang Tang, NjY2MTY2MGJveUAxNjMuY29t
 Li Wen Yang
Li Wen Yang Xiao Gang Tang*
Xiao Gang Tang*