- 1School of Health Sciences, University of Petroleum and Energy Studies, Dehradun, India
- 2School of Applied Sciences, KIIT Deemed to be University, Bhubaneswar, India
- 3School of Basic Sciences, Indian Institute of Technology Bhubaneswar, Bhubaneswar, India
Adenosine triphosphate (ATP) is an important fuel of life for humans and Mycobacterium species. Its potential role in modulating cellular functions and implications in systemic, pulmonary, and ocular diseases is well studied. Plasma ATP has been used as a diagnostic and prognostic biomarker owing to its close association with disease’s progression. Several stresses induce altered ATP generation, causing disorders and illnesses. Small heat shock proteins (sHSPs) are dynamic oligomers that are dominantly β-sheet in nature. Some important functions that they exhibit include preventing protein aggregation, enabling protein refolding, conferring thermotolerance to cells, and exhibiting anti-apoptotic functions. Expression and functions of sHSPs in humans are closely associated with several diseases like cataracts, cardiovascular diseases, renal diseases, cancer, etc. Additionally, there are some mycobacterial sHSPs like Mycobacterium leprae HSP18 and Mycobacterium tuberculosis HSP16.3, whose molecular chaperone functions are implicated in the growth and survival of pathogens in host species. As both ATP and sHSPs, remain closely associated with several human diseases and survival of bacterial pathogens in the host, therefore substantial research has been conducted to elucidate ATP-sHSP interaction. In this mini review, the impact of ATP on the structure and function of human and mycobacterial sHSPs is discussed. Additionally, how such interactions can influence the onset of several human diseases is also discussed.
Introduction
ATP is termed as energy currency of cells owing to its high energy phosphate bonds. It is used by several enzymes and structural proteins to mediate cellular processes. Besides energy production, ATP plays a pivotal role in synthesis of several macromolecules which are essential for cell survival. It acts as a switch to regulate chemical reactions and send messages. Mitochondria plays a key role in ATP synthesis by regulating oxidative phosphorylation (Bulthuis et al., 2019). Nitric oxide also regulates ATP synthesis by inhibiting cytochrome oxidase (Zhao et al., 2009). Low ATP synthesis is reported to correlate with faster tumor growth and its high invasive behavior (Granchi and Minutolo 2012). Although energy related dysfunction is not usually correlated with common diseases, but evidence suggests existence of such links in some disorders. Muscle, brain, liver, heart, and kidney that are primary energy consuming organs in human are often affected by mitochondrial dysfunction, which is a common cause for lower ATP levels (Kishikawa et al., 2018). Recent studies have demonstrated strategies to elevate levels of ATP by xanthine oxidoreductase inhibitors (Hosoyamada et al., 2016; Johnson et al., 2019; Kamatani et al., 2019) to treat disorders with ATP deficiency, associated with brain, heart, skeletal muscle, etc. (Ansari-Ramandi et al., 2017; Singh et al., 2017; Bredemeier et al., 2018; El-Bassossy et al., 2018; Ferrando et al., 2018; Singh and Cleveland 2018). Altogether, ATP is an important molecule that regulates metabolic processes and is closely associated with human diseases.
Small heat shock proteins (sHSPs) are the most strongly induced molecular chaperones under stress (Liu et al., 2015). It constitutes a divergent group within the class of HSPs characterized by a conserved “α-crystallin domain” (ACD) (Basha et al., 2012). The molecular mass of sHSPs ranges between 12–43 kDa and it can assemble into large, dynamic oligomers upto 1 MDa (Sharma and Santhoshkumar 2009). sHSPs are molecular chaperones that prevent stress induced aggregation of partially denatured proteins (Horwitz 1992; Raju et al., 2011). Some of the best explored sHSPs are archeal sHSPs such as HSP16.5 from Methanococcus jannaschii, HSP26 from Saccharomyces cerevisiae, α-crystallin and HSP27 (mammalian sHSPs), plant sHSP (HSP16.9 from wheat) and mycobacterial sHSP (HSP16.3 from Mycobacterium tuberculosis) (Horwitz 1992; Kim et al., 1998; Haslbeck et al., 1999; van Montfort et al., 2001; Fu et al., 2005; Lelj-Garolla and Mauk 2006). Besides the aggregation prevention ability, they also exhibit refolding ability like large heat shock proteins but are ATP hydrolysis independent (Jakob et al., 1993; Biswas and Das 2004). sHSPs confer thermotolerance to cells in vivo (Lavoie et al., 1995; Muchowski and Clark 1998; Valdez et al., 2002). Besides, sHSPs are over-expressed, which protect organisms and substrate proteins from other stress conditions such as oxidative and nitrosative stress (Wang and Spector 1995; Garbe et al., 1999). sHSPs exhibits anti-apoptotic function. They are also used to develop DNA vaccines which help in prevention and cure of infectious disease such as tuberculosis (Shi et al., 2010). Therefore, it is quite rational that these sHSPs can be used therapeutically in prevention of protein aggregation, apoptosis, and diseases.
In rat models, intravenously injected α-crystallin protects the retinal ganglion cells from apoptosis and promoted axonal regeneration after optic nerve crush (Ying et al., 2008; Wang et al., 2012; Wu et al., 2014). The retinal degeneration in the early phase of the autoimmune disease uveoretinitis can be prevented by systematic administration of αA (Saraswathy et al., 2010; Rao et al., 2012). In diabetic retinopathy, delivery of αA into the eyes of the mice decreased the vascular leakage and pericyte apoptosis, which is useful to stop the early lesions in the eyes (Kim et al., 2012). Delivery of cell penetration peptide tagged to α-crystallin into the cells exhibits improved protection against oxidative stress in lens epithelial cells (Mueller et al., 2013; Christopher et al., 2014). Apart from this, peptides derived from the sHSP (α-crystallin), act as mini chaperone and inhibit epithelial cell apoptosis and prevent cataract in experimental rat models, which can be of immense therapeutic use (Nahomi et al., 2013). Therefore, from the above discussion, it is reasonable to propose that sHSPs like α-crystallin and its peptides can be utilized as therapeutic agents.
On the contrary, several reports are available in the literature which demonstrates the detrimental effect of the over-expression of sHSPs in many diseases. For example, the over-expression of αB-crystallin in breast tumors leads to a shorter lifetime of the patients (Moyano et al., 2006). Subsequently, a recent study has identified a small molecule inhibitor for αB-crystallin, which binds to the ACD domain of the protein and inhibits the tumor growth in human breast cancer xenografts in mice (Chen et al., 2014). Similarly, the over-expression of HSP27 in breast cancer cells confers resistance to anti-cancer agents like doxorubicin (Oesterreich et al., 1993). Subsequently, attempts have also been made to inhibit the over-expression of HSP27 by using anti-sense or nucleotide-based therapies (Arrigo et al., 2007; Jego et al., 2013).
Vaccination is often used as a preventive therapeutic against pathogenic diseases. For example, Mycobacterium bovis Bacillus Calmette–Guérin (BCG), a live attenuated strain of Mycobacterium bovis is widely used as a vaccine against tuberculosis (Fine 1995). There are several reports which show that the use of M. tuberculosis HSP16.3, increases the efficacy of the BCG vaccination (Shi et al., 2010; Marongiu et al., 2013). Another report in the literature has showed that HSP16.3 and its T-cell epitope synthetic peptide could induce specific antibodies remarkably better than classical tuberculosis vaccine i.e., BCG (Shi et al., 2009). Single or multi-subunit DNA vaccines, over-expressing antigenic proteins from M. tuberculosis including HSP16.3 are used to improve the efficacy of BCG in tuberculosis (Shi et al., 2010). Small heat shock protein is also used as carrier protein to develop effective second-generation vaccine (Costa et al., 1998). This approach has been widely used for vaccine development against leprosy, where HSP18 has been used as a carrier protein for the development of second-generation vaccine (Costa et al., 1998). Vaccination is often considered as a safe and effective method to prevent the occurrence of diseases. Altogether, from all the above discussions, it is quite evident that sHSPs have tremendous therapeutic potential (as an agent or a target). This further reinforces the fact that sHSPs are intrinsically involved with the onset of or prevention of several human diseases. Keeping in view the role of ATP and sHSP in the cellular processes of the human body, the role of ATP-sHSP interaction in human diseases is discussed below.
ROLE OF ATP-SHSP INTERACTION WITH PROLIFERATION OR PREVENTION OF HUMAN DISEASES
Cardiovascular Disease
Role of HSP27 and HSP20 in cardiovascular disease: sHSPs protect cells against ischemia or reperfusion injuries, as evidenced from gene deletion experiments (Sun and MacRae 2005). Over-expressed wild type and non-phosphorylated HSP27 are effective in safeguarding contractile activity and cell integrity, as determined by retention of creatine kinase activity in transgenic mice hearts during ischemia/reperfusion (Hollander et al., 2004). During atrial fibrillation (AF) human body can show response by over-expression of HSP27 to handle the rapid atrial pacing. Mechanism behind this may be the inhibitory effect of angiotensin on atrial remodeling (Wang et al., 2018). HSP27 can also help in prediction of reoccurrence of AF (Marion et al., 2020). A study by Traxler et al. demonstrated that HSP27 can be an independent biomarker for prognosis in chronic heart failure (HF) (Traxler et al., 2017). Wang and others studied the effect of HSP27 on myocardial infarction (MI). They found that deficiency of HSP27 which is specific to cardiomyocytes, can alter the cardiac function negatively like increment in cardiac dysfunction, mortality, and cardiac rupture.
In another example, hearts of double knockout mice that lacked abundant sHSPs like HSP20, showed normal contractility (Morrison et al., 2004). In contrast, hearts of these animals exhibited reduced contractility accompanied by enhanced necrosis and apoptosis when being exposed to ischemia and reperfusion. Thus, HSP20 is essential for optimal recovery from heart attack. Phosphorylation of HSP20 inhibits caspase-3 activation, which arrest apoptosis induced by β-agonist (Morrison et al., 2003). Overall, HSP20 and HSP27 are found to be involved in increased cardiomyocytes contractility, vasorelaxation, smooth muscle relaxation, apoptosis, myocardial contraction, glucose transport, platelet aggregation and ischemia/reperfusion injury (Yu et al., 2019; Zhang et al., 2019; Shan et al., 2021).
Effect of ATP on HSP27 and HSP20: Implications on Cardiovascular Disease
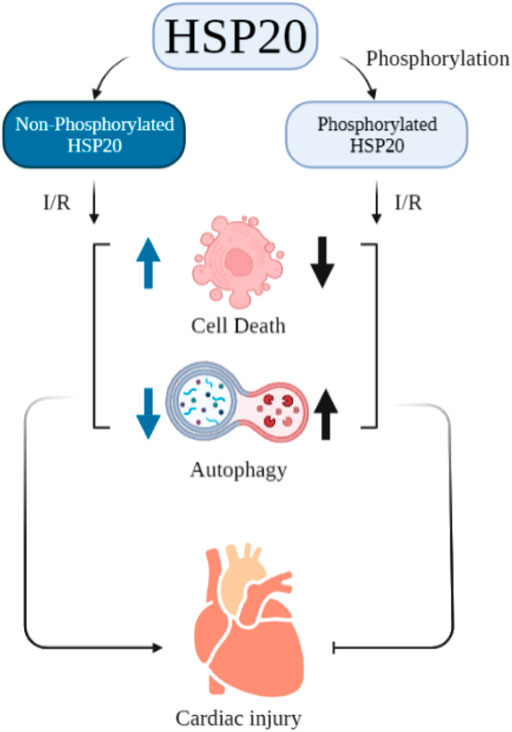
The impact of ATP on structure and function of HSP20 and HSP27 are sparsely studied. ATP depletion in endothelial cells resulted in dephosphorylation of HSP27 which caused its translocation into insoluble cellular fraction with altered functional activity towards actin (Loktionova et al., 1996). In contrast, in tubular epithelial cells, ATP depletion caused increased phosphorylation of HSP27 that triggered its migration from cytoskeleton to cytoplasm and promoted actin polymerization (Du et al., 2010). In vitro assay using γ-32P-ATP revealed that HSP20 is phosphorylated at Ser16 (Sin and Baillie 2015). Ser16 phosphorylation of HSP20 has an impact on cardiac injury. Blocking Ser16 phosphorylation, resulted in increased cell death and reduced autophagy, thereby promoting cardiac injury. A schematic representation of this study is given in Figure 1 (Qian et al., 2009).
Burniston studied the effect of tolerance exercise on the hearts of rats. He claimed that this exercise can help in improving cardiac function and cardiac protection. In this experiment, he also stated that this exercise leads to an increment in phosphorylation of HSP20 at Ser16 (Burniston 2009). Furthermore, the substitution of proline 20 with leucine in HSP20 can diminish the cardio protective activity of its Ser16 (Nicolaou et al., 2008). Guo-Chang Fan and co-workers demonstrated that β-agonist stimulation can lead to phosphorylation of HSP20 which then binds with actin. This binding results in cytoskeleton stabilization and inhibition of apoptosis. Altogether, it can be inferred that ATP possibly controls the phosphorylation of these two sHSPs which influences cardiovascular disease (Figure 1). However, binding affinity of ATP to these two important sHSPs and its effect needs to be assessed carefully.
Cataract
Role of αA and αB in cataract: Mutation and post-translational modifications in sHSP contribute to the cataract formation in mammalian lens (Panda et al., 2015; Hafizi et al., 2021; Khoshaman et al., 2021; Sprague-Piercy et al., 2021). α-crystallin, a major vertebrate eye lens protein is believed to have a chaperone function which plays a major role in maintaining lens transparency, thereby preventing the formation of cataract. Scientists revealed that several post translational modification processes including truncation (Takeuchi et al., 2004), deamidation (Gupta and Srivastava 2004), glycation (Seidler et al., 2004) and phosphorylation (Kamei et al., 2004) decreased the chaperone function of α-crystallin which may be the basis for cataract formation in human lens. The mutations are responsible for autosomal dominant congenital cataract, a common cause of infant blindness, is localized to the arginine 116 (R116) in the αA gene (CRYAA) (Litt et al., 1998). The R116C mutation in αA destroyed its chaperone function (Cobb and Petrash 2000). When a series of transgenic mouse models were created to express R116C mutated αA, it induced lens opacity and structural defects (Hsu et al., 2006). Several other point mutations in αA with autosomal dominant congenital cataracts are: R12C, R21L, R21W, R49C, R54C, G98R and R116H which are well known to impair the structure and function of the protein, thereby inducing cataract formation in human eye lens (Singh et al., 2006; Raju and Abraham 2011). An autosomal recessive congenital cataract causing mutation in αA, W9X, has been also reported in the literature (Pras et al., 2000). Mutations in αB gene have been also reported. Three arginine mutations (R11H, R69C and R120G) were found in the αB gene, which are associated with autosomal dominant congenital cataract in human (Panda et al., 2015). Apart from these arginine mutations, there are other point mutations and truncations in the lens α-crystallin which leads to the formation of the cataract.
Effect of ATP on α-crystallin’s Chaperone Function
A human lens generally contains 3 mM ATP. Thus, the interaction between α-crystallin and ATP inside lens is highly probable. In fact, ATP binds to β4-β8 groove ACD of αB (Ghosh et al., 2006). The β4-β8 domains are known to interact with C-terminal extension of αB and these domains also interact with stress prone substrate proteins. Thus, ATP binding to αB has altered the chaperone function of the protein. However, ATP hydrolysis is not required for the same (Biswas and Das 2004). ATP enhances the association between chaperone and client proteins, thereby improving the chaperone activity of αA and αB (Muchowski and Clark 1998). ATP binding also improves αB mediated refolding of denatured client proteins like lactate dehydrogenase (Biswas and Das 2004). α-crystallin binds molten globule state of protein like xylanase II that can be refolded in presence of ATP (Nath et al., 2002). The structural stability of α-crystallin is increased in the presence of ATP. In another independent study, it is demonstrated that chaperone-client complexes of α-crystallin remains stable in presence of ATP for 2 weeks under in vitro conditions, supporting the notion that ATP promotes association of chaperone-client protein complexes of α-crystallin (Nandi et al., 2020b). In contrast to all these findings, Wang et al. demonstrated that ATP induces conformational changes in α-crystallin that triggers dissociation of chaperone-client protein complexes of α-crystallin (Wang and Spector 2001). This causes release of denatured client protein from α-crystallin, which is then taken up by large heat shock protein to refold. Altogether, it can be inferred that ATP can efficiently regulate chaperone function of α-crystallin and such regulation may be helpful for delaying cataract formation in human. However, the underlying mechanism needs to be further investigated to understand the impact of “α-crystallin-ATP interaction” on the onset of cataract formation.
Leprosy
Role of Mycobacterium leprae HSP18 in leprosy: Among the various antigens over-expressed inside Mycobacterium leprae, the etiological pathogen of leprosy, the 18 kDa antigenic protein is an important one. The 18 kDa protein of M. leprae is specifically expressed during intracellular growth and may be involved in the survival of M. leprae pathogen within macrophages (Dellagostin et al., 1995). Study indicates that the 18 kDa gene may be useful in providing expression signals for foreign gene expression in recombinant BCG vaccines (Dellagostin et al., 1995). Identification of such a gene which is selectively expressed during intracellular growth in macrophages and helps in growth and survival of the pathogen, hinted towards providing a new target for chemotherapy or immunotherapy in the context of the effective treatment of leprosy. Owing to the presence of the “α-crystallin domain” and its sequence identity, this 18 kDa protein is classified as a member of the small heat shock protein family, hence also known as HSP18. Several reports indicate that similar to other well-known sHSPs, HSP18 also exhibits chaperone function by preventing enzymes from thermal inactivation, protecting several thermally and chemically stressed client proteins from aggregation and preventing thermal killing of E. coli cells (Lini et al., 2008; Nandi et al., 2013; Nandi et al. 2015a; Nandi et al. 2015b; Nandi et al. 2016; Chakraborty et al., 2018; Nandi et al., 2018; Nandi et al., 2020a; Chakraborty and Biswas 2020; Chakraborty et al., 2021). It has also been found that the over-expression of M. leprae HSP18 might facilitate the survival of M. leprae under various stressed conditions (Maheshwari and Dharmalingam 2013). In order to find out the molecular basis behind the chaperone function of HSP18, a number of studies have been carried out which includes studies under various thermal and stressed conditions as well as studies in the presence of metal ions and small molecules (Nandi et al., 2015a; Nandi et al., 2015b; Nandi et al., 2016; Chakraborty et al., 2018; Nandi et al., 2018; Nandi et al., 2020a; Chakraborty and Biswas 2020). All these reports indicated that HSP18 is an important leprotic drug target and its chaperoning property is one of the important factors behind controlling the survivability of M. leprae pathogen inside the infected hosts.
Effect of ATP on the Chaperone Function of HSP18
The nutritional requirements and energy metabolism revealed that unlike other obligatory parasitic microorganisms, M. leprae does not uptake exogenous ATP from the host species, rather generates its own ATP for energy and other biochemical activities (Lee and Colston 1985; Lee and Colston 1986; Rosa et al., 2021). Aside from energy requirements, ATP is also found to interact with an important antigenic protein HSP18 from M. leprae (Nandi et al., 2015a). ATP mostly binds to the β4-β8 strand of HSP18 having binding affinity in sub-micromolar range. In fact, this is the first report which showed that ATP interacts with an antigenic protein of M. leprae pathogen. The reversible binding of ATP to M. leprae HSP18 enhances its chaperone function without any significant alteration in its conformations. Moreover, ATP is also reported to be involved in the autophosphorylation of HSP18 (Maheshwari and Dharmalingam 2013). Thus, increased chaperone function as a result of HSP18-ATP association along with the autophosphorylation activity in turn may be of significant importance in order to help in the growth and survival of the pathogen M. leprae under various physiologically stressed conditions. These findings also indicate that M. leprae possesses an ATP binding protein, which evokes the possibility of using ATP competitive antibiotics/inhibitors in the context of effective treatment of leprosy.
Tuberculosis
Role of Mycobacterium tuberculosis HSP16.3 in tuberculosis: Over the years, tuberculosis (TB) remains as one of the major infectious afflictions worldwide, with rising cases of human mortality and morbidity (Preneta et al., 2004; Soong et al., 2018). Mycobacterium tuberculosis is the etiological agent of this disease. The characteristic feature of this involved pathogen is that it can remain as a stable dormant bacilli inside the host for years before emerging into active TB (Muchowski et al., 2002). It is possible for the pathogen to remain stable in the hostile environment of host only because of secretion of different immuno-dominant antigens. HSP16.3 is a pivotal one amongst them. This protein was previously known as a 14 kDa antigen, later denoted as HSP16.3 (Panda et al., 2017). It possesses a complex oligomeric assembly of dodecamer (Preneta et al., 2004). HSP16.3 is believed to be overproduced during the latency of M. tuberculosis infection and serves as an important diagnostic marker for pleural tuberculosis (Limongi et al., 2011; Zhang et al., 2018; Huang et al., 2021). Garcia et al. and Yang et al. have observed that, mycobacteria engulfed by the macrophages, remain in the form of granulomas and produce various mycobacterial products, especially peptides derived from HSP16.3 which act as a vital biomarker for latent tuberculosis and active tuberculosis (Kruh-Garcia et al., 2014; Yang et al., 2018).
HSP16.3 is considered as an important immuno-dominant antigen, which belongs to the family of small heat shock protein and exhibits chaperone activity (Verbon et al., 1992; Chang et al., 1996; Zhang et al., 2018). This protein is highly expressed in the stationary phase of M. tuberculosis (Lee et al., 1992). In other words, the molecular chaperone function plays an important role in the growth and survival of M. tuberculosis during the latent phase of infection (Yuan et al., 1996). Several attempts have been executed to understand how this immuno-dominant antigen favors the growth and survivability of this pathogen. The studies from Yuan et al. have revealed a slower decline in the cell viability in M. tuberculosis which are over-expressed with HSP16.3 (Yuan, Crane and Barry third 1996). It also leads to long-term viability during latency and plays an important role in the replication during the initial phase of infection (Yuan et al., 1998). Garbe et al. have explored that HSP16.3 plays a prominent role in the survival of this pathogen under nitric oxide stress condition (Garbe et al., 1999). In two independent studies, Timm et al. and Hu et al. have demonstrated that this antigen is dispensable for the bacterial growth as the multidrug resistant Acr1/HSP16.3 deficient clinical isolate of M. tuberculosis do not show impaired replication in macrophages and also exhibit an enhanced rate of growth of the bacilli in vivo (Hu et al., 2006; Timm et al., 2006). Also, some studies have emphasized the important role of HSP16.3 in maintaining the dormancy of M. tuberculosis during prolonged periods of infection (Hu and Coates 1999, Yuan, Crane and Barry third 1996).
Extensive research is also being conducted to evaluate the potential of this mycobacterial peptide as a successful candidate for developing vaccines. It has been found that the recombinant BCG harboring multistage antigens including HSP16.3 provides long-term protection and increased immune response against the infection caused by M. tuberculosis as compared to wild-type BCG vaccine (Shi et al., 2010; Liang et al., 2015). Moreover, Tyagi et al. demonstrated that superior booster vaccine can be developed by using these latent antigens such as HSP16.3 which is capable of reducing the risk of developing active tuberculosis by reactivating the latent infection mode (Dey et al., 2011). It has also been observed that the chaperoning ability of HSP16.3 towards the mycobacterial molecules increases the immune response as well as BCG boosting efficacy, which makes it a promising candidate for developing better vaccines for tuberculosis (Taylor et al., 2012). It exhibits this chaperoning activity in an ATP independent manner (Preneta et al., 2004).
Effect of ATP on the Chaperone Function of HSP16.3
A strong interaction between ATP and HSP16.3 is well established from UV cross-linking experiments and proteolytic studies of HSP16.3 (Muchowski et al., 2002). HSP16.3 has autophosphorylation property in vitro, but whether ATP triggers the phosphorylation in HSP16.3 is still unclear. A comparative study revealed the effect of ATP on the recombinant HSP16.3 and human αB to be similar and in both the cases the chaperone activity is significantly increased (Muchowski et al., 2002). In addition to this, from studies of Valdez et al., it is evident that the presence of ATP also prevented the mycobacterial protein from the proteolytic digestion of chymotrypsin (Muchowski et al., 2002). In fact, Dobos and coworkers have identified 122 ATP binding proteins in M. tuberculosis and HSP16.3 is one of them (Wolfe et al., 2013). In recent times, ATP competitive inhibitors are being used for the treatment of tuberculosis (Gordon et al., 2015), these inhibitors may affect the “HSP16.3-ATP interaction” which may possibly affect the growth and survival of M. tuberculosis in the infected hosts.
Conclusion
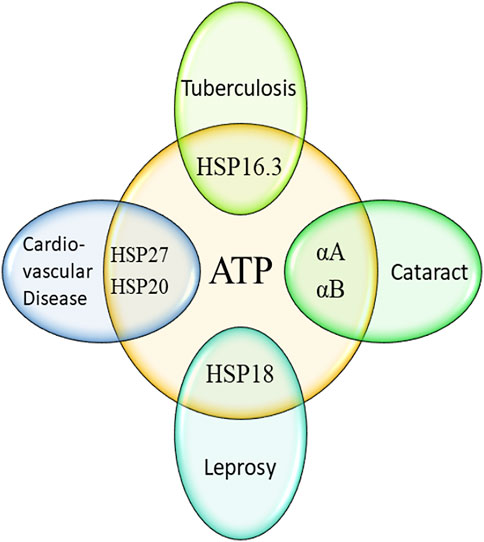
This short review clearly depicted that both ATP and different sHSPs play important role in various human diseases (Figure 2). In most cases, the chaperone function of sHSPs is enhanced by the interaction with ATP. The improved chaperone function of many sHSPs in presence of ATP eventually helps in controlling various important diseases. But the improved chaperone function of different mycobacterial sHSPs (HSP18 and HSP16.3) may assist the pathogens (M. leprae and M. tuberculosis) to survive more in infected hosts. Therefore, these two sHSPs may be a potent target for the development of ATP competitive inhibitors. Interestingly, ATP levels are increased in both leprosy and tuberculosis. Also, the levels of HSP18 and HSP16.3 is elevated in leprosy and tuberculosis, respectively. These sHSPs along with ATP are often used as biomarkers for these two diseases. But, whether the over-expression of these two sHSPs is due to increased levels of ATP is far from clear. Such aspect needs to be explored for the better understanding of host-pathogen interaction.
Author Contributions
SKN and AB conceived the idea for the review article. All authors contributed in writing the mini-review.
Funding
This work was supported by UPES-SEED grant program - UPES/R&D-HS/12032021/02 (SKN); SERB DST extramural fund - SRG/2021/001149 (SKN); SERB DST extramural fund -EMR/2017/002160 (AB).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
IR and SB acknowledge the receipt of an Institute Fellowship from Indian Institute of Technology Bhubaneswar. SSM acknowledges UGC, New Delhi, India, for providing fellowship. SR acknowledges the receipt of an Institute Fellowship from University of Petroleum and Energy Studies Dehradun.
Abbreviations
ATP, Adenosine triphosphate; sHSPs, Small heat shock proteins; ACD, α-crystallin domain; αA, αA-crystallin; αB, αB-crystallin; AF, Atrial fibrillation; MI, Myocardial infarction.
References
Ansari-Ramandi, M. M., Maleki, M., Alizadehasl, A., Amin, A., Taghavi, S., Alemzadeh-Ansari, M. J., et al. (2017). Safety and Effect of High Dose Allopurinol in Patients with Severe Left Ventricular Systolic Dysfunction. J. Cardiovasc. Thorac. Res. 9, 102–107. doi:10.15171/jcvtr.2017.17
Arrigo, A. P., Simon, S., Gibert, B., Kretz-Remy, C., Nivon, M., Czekalla, A., et al. (2007). Hsp27 (HspB1) and alphaB-Crystallin (HspB5) as Therapeutic Targets. FEBS Lett. 581, 5813665–5813674. doi:10.1016/j.febslet.2007.04.033
Basha, E., O’Neill, H., and Vierling, E. (2012). Small Heat Shock Proteins and α-crystallins: Dynamic Proteins with Flexible Functions. Trends Biochem. Sci. 37, 106–117. doi:10.1016/j.tibs.2011.11.005
Biswas, A., and Das, K. P. (2004). Role of ATP on the Interaction of Alpha-Crystallin with its Substrates and its Implications for the Molecular Chaperone Function. J. Biol. Chem. 279, 27942648–27942657. doi:10.1074/jbc.M404444200
Bredemeier, M., Lopes, L. M., Eisenreich, M. A., Hickmann, S., Bongiorno, G. K., d'Avila, R., et al. (2018). Xanthine Oxidase Inhibitors for Prevention of Cardiovascular Events: a Systematic Review and Meta-Analysis of Randomized Controlled Trials. BMC Cardiovasc. Disord. 18, 1824. doi:10.1186/s12872-018-0757-9
Bulthuis, E. P., Adjobo-Hermans, M. J. W., Willems, P. H. G. M., and Koopman, W. J. H. (2019). Mitochondrial Morphofunction in Mammalian Cells. Antioxid. Redox Signaling 30, 2066–2109. doi:10.1089/ars.2018.7534
Burniston, J. G. (2009). Adaptation of the Rat Cardiac Proteome in Response to Intensity-Controlled Endurance Exercise. Proteomics 9, 106–115. doi:10.1002/pmic.200800268
Chakraborty, A., Ghosh, R., and Biswas, A. (2021). Interaction of Constituents of MDT Regimen for Leprosy with Mycobacterium leprae HSP18: Impact on its Structure and Function. FEBS J. Sep. 23.
Chakraborty, A., and Biswas, A. (2020). Structure, Stability and Chaperone Function of Mycobacterium leprae Heat Shock Protein 18 Are Differentially Affected upon Interaction with Gold and Silver Nanoparticles. Int. J. Biol. macromolecules 152, 250–260. doi:10.1016/j.ijbiomac.2020.02.182
Chakraborty, A., Nandi, S. K., Panda, A. K., Mahapatra, P. P., Giri, S., and Biswas, A. (2018). Probing the Structure-Function Relationship of Mycobacterium leprae HSP18 under Different UV Radiations. Int. J. Biol. macromolecules Nov 119, 604–616. doi:10.1016/j.ijbiomac.2018.07.151
Chang, Z., Primm, T. P., Jakana, J., Lee, I. H., Serysheva, I., Chiu, W., et al. (1996). Mycobacterium tuberculosis 16-kDa Antigen (Hsp16.3) Functions as an Oligomeric Structure In Vitro to Suppress Thermal Aggregation. J. Biol. Chem. 271, 7218–7223. doi:10.1074/jbc.271.12.7218
Chen, Z., Ruan, Q., Han, S., Xi, L., Jiang, W., Jiang, H., et al. (2014). Discovery of Structure-Based Small Molecular Inhibitor of αB-crystallin against Basal-Like/triple-Negative Breast Cancer Development In Vitro and In Vivo. Breast Cancer Res. Treat. 145, 45–59. doi:10.1007/s10549-014-2940-8
Christopher, K. L., Pedler, M. G., Shieh, B., Ammar, D. A., Petrash, J. M., and Mueller, N. H. (2014). Alpha-crystallin-mediated protection of Lens Cells against Heat and Oxidative Stress-Induced Cell Death. Biochim. Biophys. Acta (Bba) - Mol. Cell Res. 1843, 309–315. doi:10.1016/j.bbamcr.2013.11.010
Cobb, B. A., and Petrash, J. M. (2000). Structural and Functional Changes in the Alpha A-Crystallin R116C Mutant in Hereditary Cataracts. Biochemistry 39 (39), 15791–15798. doi:10.1021/bi001453j
Costa, M. H. B., Sant’Anna, O. A., de Araujo, P. S., Sato, R. A., Quintilio, W., Silva, L. V. N., et al. (1998). Conformational Stability and Antibody Response to the 18 kDa Heat- Shock Protein Formulated into Different Vehicles. Appl. Biochem. Biotechnol. 73, 19–28. doi:10.1007/bf02788830
Dellagostin, O. A., Esposito, G., Eales, L. J., Dale, J. W., and McFadden, J. (1995). Activity of Mycobacterial Promoters during Intracellular and Extracellular Growth. Microbiology (Reading) 141 (Pt 8), 1785–1792. doi:10.1099/13500872-141-8-1785
Dey, B., Jain, R., Khera, A., Gupta, U. D., Katoch, V. M., Ramanathan, V. D., et al. (2011). Latency Antigen α-Crystallin Based Vaccination Imparts a Robust Protection against TB by Modulating the Dynamics of Pulmonary Cytokines. PloS one 6, e18773. doi:10.1371/journal.pone.0018773
Du, J., Zhang, L., Yang, Y., Li, W., Chen, L., Ge, Y., et al. (2010). ATP Depletion-Induced Actin Rearrangement Reduces Cell Adhesion via P38 MAPK-HSP27 Signaling in Renal Proximal Tubule Cells. Cell Physiol Biochem 25, 501–510. doi:10.1159/000303055
El-Bassossy, H. M., Mahmoud, M. F., and Eid, B. G. (2018). The Vasodilatory Effect of Allopurinol Mediates its Antihypertensive Effect: Effects on Calcium Movement and Cardiac Hemodynamics. Biomed. Pharmacother. 100, 381–387. doi:10.1016/j.biopha.2018.02.033
Ferrando, B., Gomez-Cabrera, M. C., Salvador-Pascual, A., Puchades, C., Derbré, F., Gratas-Delamarche, A., et al. (2018). Allopurinol Partially Prevents Disuse Muscle Atrophy in Mice and Humans. Sci. Rep. 8 (8), 3549. doi:10.1038/s41598-018-21552-1
Fine, P. E. M. (1995). Variation in protection by BCG: Implications of and for Heterologous Immunity. The Lancet 18346, 1339–1345. doi:10.1016/s0140-6736(95)92348-9
Fu, X., Zhang, H., Zhang, X., Cao, Y., Jiao, W., Liu, C., et al. (2005). A Dual Role for the N-Terminal Region of Mycobacterium tuberculosis Hsp16.3 in Self-Oligomerization and Binding Denaturing Substrate Proteins. J. Biol. Chem. 280 (280), 6337–6348. doi:10.1074/jbc.M406319200
Garbe, T. R., Hibler, N. S., and Deretic, V. (1999). Response to Reactive Nitrogen Intermediates in Mycobacterium tuberculosis : Induction of the 16-Kilodalton α-Crystallin Homolog by Exposure to Nitric Oxide Donors. Infect. Immun. 67, 460–465. doi:10.1128/iai.67.1.460-465.1999
Ghosh, J. G., Houck, S. A., Doneanu, C. E., and Clark, J. I. (2006). The β4-β8 Groove is an ATP-Interactive Site in the α Crystallin Core Domain of the Small Heat Shock Protein, Human αB Crystallin. J. Mol. Biol. 364, 364–375. doi:10.1016/j.jmb.2006.09.003
Gordon, S., Simithy, J., Goodwin, D. C., and Calderón, A. I. (2015). Selective Mycobacterium tuberculosis Shikimate Kinase Inhibitors as Potential Antibacterials. Perspect. Medicin Chem. 7, 9–20. doi:10.4137/PMC.S13212
Granchi, C., and Minutolo, F. (2012). Anticancer Agents that Counteract Tumor Glycolysis. ChemMedChem 7, 1318–1350. doi:10.1002/cmdc.201200176
Gupta, R., and Srivastava, O. P. (2004). Deamidation Affects Structural and Functional Properties of Human alphaA-Crystallin and its Oligomerization with alphaB-Crystallin. J. Biol. Chem. 279, 27944258–27944269. doi:10.1074/jbc.M405648200
Hafizi, M., Chebotareva, N. A., Ghahramani, M., Moosavi-Movahedi, F., Khaleghinejad, S. H., Kurganov, B. I., et al. (2021). Structural and Functional Studies of D109A Human αB-crystallin Contributing to the Development of Cataract and Cardiomyopathy Diseases. PloS one 16, e0260306. doi:10.1371/journal.pone.0260306
Haslbeck, M., Walke, S., Stromer, T., Ehrnsperger, M., White, H. E., Chen, S., et al. (1999). Hsp26: a Temperature-Regulated Chaperone. EMBO J. 18, 6744–6751. doi:10.1093/emboj/18.23.6744
Hollander, J. M., Martin, J. L., Belke, D. D., Scott, B. T., Swanson, E., Krishnamoorthy, V., et al. (2004). Overexpression of Wild-type Heat Shock Protein 27 and a Nonphosphorylatable Heat Shock Protein 27 Mutant Protects against Ischemia/reperfusion Injury in a Transgenic Mouse Model. Circulation 110, 3544–3552. doi:10.1161/01.cir.0000148825.99184.50
Horwitz, J. (1992). Alpha-crystallin Can Function as a Molecular Chaperone. Proc. Natl. Acad. Sci. 89, 10449–10453. doi:10.1073/pnas.89.21.10449
Hosoyamada, M., Tsurumi, Y., Hirano, H., Tomioka, N. H., Sekine, Y., Morisaki, T., et al. (2016). Urat1-Uox Double Knockout Mice are Experimental Animal Models of Renal Hypouricemia and Exercise-Induced Acute Kidney Injury. Nucleosides, Nucleotides & Nucleic Acids 35, 543–549. doi:10.1080/15257770.2016.1143559
Hsu, C.-D., Kymes, S., and Petrash, J. M. (2006). A Transgenic Mouse Model for Human Autosomal Dominant Cataract. Invest. Ophthalmol. Vis. Sci. 47, 2036–2044. doi:10.1167/iovs.05-0524
Hu, Y., and Coates, A. R. M. (1999). Transcription of the Stationary-Phase-Associated hspX Gene of Mycobacterium tuberculosis is Inversely Related to Synthesis of the 16-kilodalton Protein. J. Bacteriol. 181, 1380–1387. doi:10.1128/jb.181.5.1380-1387.1999
Hu, Y., Movahedzadeh, F., Stoker, N. G., and Coates, A. R. M. (2006). Deletion of the Mycobacterium tuberculosis α-Crystallin-Like hspX Gene Causes Increased Bacterial Growth In Vivo. Infect. Immun. 74, 861–868. doi:10.1128/iai.74.2.861-868.2006
Huang, C., Pan, L., Shen, X., Tian, H., Guo, L., Zhang, Z., et al. (2021). Hsp16.3 of Mycobacterium tuberculosis in Exosomes as a Biomarker of Tuberculosis. Eur. J. Clin. Microbiol. Infect. Dis. 40, 2427–2430. doi:10.1007/s10096-021-04246-x
Jakob, U., Gaestel, M., Engel, K., and Buchner, J. (1993). Small Heat Shock Proteins are Molecular Chaperones. J. Biol. Chem. Jan 25, 2681517–2681520. doi:10.1016/s0021-9258(18)53882-5
Jego, G., Hazoumé, A., Seigneuric, R., and Garrido, C. (2013). Targeting Heat Shock Proteins in Cancer. Cancer Lett. 332 (332), 275–285. doi:10.1016/j.canlet.2010.10.014
Johnson, T. A., Jinnah, H. A., and Kamatani, N. (2019). Shortage of Cellular ATP as a Cause of Diseases and Strategies to Enhance ATP. Front. Pharmacol. 10, 98. doi:10.3389/fphar.2019.00098
Kamatani, N., Kushiyama, A., Toyo-Oka, L., and Toyo-Oka, T. (2019). Treatment of Two Mitochondrial Disease Patients with a Combination of Febuxostat and Inosine that Enhances Cellular ATP. J. Hum. Genet. 64, 351–353. doi:10.1038/s10038-018-0558-0
Kamei, A., Takamura, S., Nagai, M., and Takeuchi, N. (2004). Phosphoproteome Analysis of Hereditary Cataractous Rat Lens Alpha-crystallin. Biol. Pharm. Bull. 27, 1923–1931. doi:10.1248/bpb.27.1923
Khoshaman, K., Ghahramani, M., Shahsavani, M. B., Moosavi-Movahedi, A. A., Kurganov, B. I., and Yousefi, R. (2021). Myopathy-associated G154S Mutation Causes Important Changes in the Conformational Stability, Amyloidogenic Properties, and Chaperone-like Activity of Human αB-crystallin. Biophys. Chem. 282, 282106744. doi:10.1016/j.bpc.2021.106744
Kim, R., Kim, K. K., Yokota, H., and Kim, S.-H. (1998). Small Heat Shock Protein of Methanococcus janaschii, a Hyperthermophile. Pnas 95, 9129–9133. doi:10.1073/pnas.95.16.9129
Kim, Y. H., Park, S. Y., Park, J., Kim, Y. S., Hwang, E. M., Park, J. Y., et al. (2012). Reduction of Experimental Diabetic Vascular Leakage and Pericyte Apoptosis in Mice by Delivery of αA-crystallin with a Recombinant Adenovirus. Diabetologia 55, 2835–2844. doi:10.1007/s00125-012-2625-y
Kishikawa, J.-i., Inoue, Y., Fujikawa, M., Nishimura, K., Nakanishi, A., Tanabe, T., et al. (2018). General Anesthetics Cause Mitochondrial Dysfunction and Reduction of Intracellular ATP Levels. PloS one 13, e0190213. doi:10.1371/journal.pone.0190213
Kruh-Garcia, N. A., Wolfe, L. M., Chaisson, L. H., Worodria, W. O., Nahid, P., Schorey, J. S., et al. (2014). Detection of Mycobacterium tuberculosis Peptides in the Exosomes of Patients with Active and Latent M. tuberculosis Infection Using MRM-MS. PloS one 9, e103811. doi:10.1371/journal.pone.0103811
Lavoie, J. N., Lambert, H., Hickey, E., Weber, L. A., and Landry, J. (1995). Modulation of Cellular Thermoresistance and Actin Filament Stability Accompanies Phosphorylation-Induced Changes in the Oligomeric Structure of Heat Shock Protein 27. Mol. Cel. Biol. 15, 505–516. doi:10.1128/mcb.15.1.505
Lee, B. Y., Hefta, S. A., and Brennan, P. J. (1992). Characterization of the Major Membrane Protein of Virulent Mycobacterium tuberculosis. Infect. Immun. 60, 2066–2074. doi:10.1128/iai.60.5.2066-2074.1992
Lee, Y. N., and Colston, M. J. (1985). Measurement of ATP Generation and Decay in Mycobacterium leprae In Vitro. Microbiology 131, 3331–3337. doi:10.1099/00221287-131-12-3331
Lee, Y. N., and Colston, M. J. (1986). Adenylate Kinase Activity in Mycobacterium leprae. Microbiology 132, 561–563. doi:10.1099/00221287-132-2-561
Lelj-Garolla, B., and Mauk, A. G. (2006). Self-association and Chaperone Activity of Hsp27 Are Thermally Activated. J. Biol. Chem. 281, 2818169–2818174. doi:10.1074/jbc.M512553200
Liang, J., Teng, X., Yuan, X., Zhang, Y., Shi, C., Yue, T., et al. (2015). Enhanced and Durable Protective Immune Responses Induced by a Cocktail of Recombinant BCG Strains Expressing Antigens of Multistage of Mycobacterium tuberculosis. Mol. Immunol. 66, 392–401. doi:10.1016/j.molimm.2015.04.017
Limongi, L. C. S. A., Olival, L., Conde, M. B., and Junqueira-Kipnis, A. P. (2011). Determination of Levels of Specific IgA to the HspX Recombinant Antigen of Mycobacterium Tuberculosis for the Diagnosis of Pleural Tuberculosis. Jornal Brasileiro de Pneumologia 37, 302–307.
Lini, N., Rehna, E. A., Shiburaj, S., Maheshwari, J. J., Shankernarayan, N. P., and Dharmalingam, K. (2008). Functional Characterization of a Small Heat Shock Protein from Mycobacterium leprae. BMC Microbiol. 8 (8), 208. doi:10.1186/1471-2180-8-208
Litt, M., Kramer, P., LaMorticella, D. M., Murphey, W., Lovrien, E. W., and Weleber, R. G. (1998). Autosomal Dominant Congenital Cataract Associated with a Missense Mutation in the Human Alpha Crystallin Gene CRYAA. Hum. Mol. Genet. Mar. 7, 471–474. doi:10.1093/hmg/7.3.471
Liu, L., Chen, J., Yang, B., and Wang, Y. (2015). Oligomer‐dependent and ‐independent Chaperone Activity of sHsps in Different Stressed Conditions. FEBS Open Bio 5, 155–162. doi:10.1016/j.fob.2015.02.006
Loktionova, S. A., Ilyinskaya, O. P., Gabai, V. L., and Kabakov, A. E. (1996). Distinct Effects of Heat Shock and ATP Depletion on Distribution and Isoform Patterns of Human Hsp27 in Endothelial Cells. FEBS Lett. 392, 392100–392104. doi:10.1016/0014-5793(96)00792-2
Maheshwari, J. J., and Dharmalingam, K. (2013). Protective Role of Mycobacterium leprae Small Heat-Shock Protein in Heterologous Hosts, Escherichia coli and Mycobacterium smegmatis, Grown under Stress. J. Med. Microbiol. 62, 959–967. doi:10.1099/jmm.0.057851-0
Marion, D. M. S. V., Lanters, E. A. H., Ramos, K. S., Li, J., Wiersma, M., Baks-Te Bulte, L., et al. (2020). Evaluating Serum Heat Shock Protein Levels as Novel Biomarkers for Atrial Fibrillation. Cells 9, 9. doi:10.3390/cells9092105
Marongiu, L., Donini, M., Toffali, L., Zenaro, E., and Dusi, S. (2013). ESAT-6 and HspX Improve the Effectiveness of BCG to Induce Human Dendritic Cells-dependent Th1 and NK Cells Activation. PloS one 8, e75684. doi:10.1371/journal.pone.0075684
Morrison, L. E., Hoover, H. E., Thuerauf, D. J., and Glembotski, C. C. (2003). Mimicking Phosphorylation of AlphaB-crystallin on Serine-59 is Necessary and Sufficient to Provide Maximal protection of Cardiac Myocytes from Apoptosis. Circ. Res. 92 (92), 203–211. doi:10.1161/01.res.0000052989.83995.a5
Morrison, L. E., Whittaker, R. J., Klepper, R. E., Wawrousek, E. F., and Glembotski, C. C. (2004). Roles for αB-crystallin and HSPB2 in Protecting the Myocardium from Ischemia-Reperfusion-Induced Damage in a KO Mouse Model. Am. J. Physiology-Heart Circulatory Physiol. 286, H847–H855. doi:10.1152/ajpheart.00715.2003
Moyano, J. V., Evans, J. R., Chen, F., Lu, M., Werner, M. E., Yehiely, F., et al. (2006). AlphaB-crystallin is a Novel Oncoprotein that Predicts Poor Clinical Outcome in Breast Cancer. J. Clin. Invest. 116, 261–270. doi:10.1172/JCI25888
Muchowski, P. J., and Clark, J. I. (1998). ATP-enhanced Molecular Chaperone Functions of the Small Heat Shock Protein Human AlphaB-crystallin. Proc. Natl. Acad. Sci. 95, 1004–1009. doi:10.1073/pnas.95.3.1004
Mueller, N. H., Ammar, D. A., and Petrash, J. M. (2013). Cell Penetration Peptides for Enhanced Entry of αB-Crystallin into Lens Cells. Invest. Ophthalmol. Vis. Sci. 54, 2–8. doi:10.1167/iovs.12-10947
Nahomi, R. B., Wang, B., Raghavan, C. T., Voss, O., Doseff, A. I., Santhoshkumar, P., et al. (2013). Chaperone Peptides of α-crystallin Inhibit Epithelial Cell Apoptosis, Protein Insolubilization, and Opacification in Experimental Cataracts. J. Biol. Chem. 288, 28813022–28813035. doi:10.1074/jbc.M112.440214
Nandi, S. K., Nahomi, R. B., Rankenberg, J., Glomb, M. A., and Nagaraj, R. H. (2020). Glycation-mediated Inter-protein Cross-Linking Is Promoted by Chaperone-Client Complexes of α-crystallin: Implications for Lens Aging and Presbyopia. J. Biol. Chem. 295 (295), 5701–5716. doi:10.1074/jbc.RA120.012604
Nandi, S. K., Chakraborty, A., Panda, A. K., and Biswas, A. (2016). Conformational Perturbation, Hydrophobic Interactions and Oligomeric Association Are Responsible for the Enhanced Chaperone Function of Mycobacterium leprae HSP18 under Pre-thermal Condition. RSC Adv. 6, 62146–62156. doi:10.1039/c6ra00167j
Nandi, S. K., Chakraborty, A., Panda, A. K., and Biswas, A. (2020). M. leprae HSP18 Suppresses Copper (II) Mediated ROS Generation: Effect of Redox Stress on its Structure and Function. Int. J. Biol. macromolecules 146, 648–660. doi:10.1016/j.ijbiomac.2019.12.215
Nandi, S. K., Chakraborty, A., Panda, A. K., Kar, R. K., Bhunia, A., and Biswas, A. (2018). Evidences for Zinc (II) and Copper (II) Ion Interactions with Mycobacterium leprae HSP18: Effect on its Structure and Chaperone Function. J. Inorg. Biochem. 188, 62–75. doi:10.1016/j.jinorgbio.2018.08.010
Nandi, S. K., Chakraborty, A., Panda, A. K., Sinha Ray, S., Kar, R. K., Bhunia, A., et al. (2015a). Interaction of ATP with a Small Heat Shock Protein from Mycobacterium leprae: Effect on its Structure and Function. Plos Negl. Trop. Dis. 9, e0003661. doi:10.1371/journal.pntd.0003661
Nandi, S. K., Panda, A. K., Chakraborty, A., Ray, S. S., and Biswas, A. (2015). Role of Subunit Exchange and Electrostatic Interactions on the Chaperone Activity of Mycobacterium leprae HSP18. PloS one 10, e0129734. doi:10.1371/journal.pone.0129734
Nandi, S. K., Rehna, E. A. A., Panda, A. K., Shiburaj, S., Dharmalingam, K., and Biswas, A. (2013). A S52P Mutation in the ‘α-crystallin Domain’ of Mycobacterium leprae HSP18 Reduces its Oligomeric Size and Chaperone Function. Febs J. 280, 5994–6009. doi:10.1111/febs.12519
Nath, D., Rawat, U., Anish, R., and Rao, M. (2002). Alpha-crystallin and ATP Facilitate the In Vitro Renaturation of Xylanase: Enhancement of Refolding by Metal Ions. Protein Sci. 11, 2727–2734. doi:10.1110/ps.0213802
Nicolaou, P., Knöll, R., Haghighi, K., Fan, G. C., Dorn, G. W., Hasenfub, G., et al. (2008). Human Mutation in the Anti-apoptotic Heat Shock Protein 20 Abrogates its Cardioprotective Effects. J. Biol. Chem. 283 (283), 33465–33471. doi:10.1074/jbc.M802307200
Oesterreich, S., Weng, C. N., Qiu, M., Hilsenbeck, S. G., Osborne, C. K., and Fuqua, S. A. (1993). The Small Heat Shock Protein Hsp27 is Correlated with Growth and Drug Resistance in Human Breast Cancer Cell Lines. Cancer Res. 53, 4443–4448.
Panda, A. K., Nandi, S. K., Chakraborty, A., Nagaraj, R. H., and Biswas, A. (2015). Differential Role of Arginine Mutations on the Structure and Functions of Alpha-Crystallin. Biochim. Biophys. Acta 1860, 199–210. doi:10.1016/j.bbagen.2015.06.004
Panda, A. K., Chakraborty, A., Nandi, S. K., Kaushik, A., and Biswas, A. (2017). The C‐terminal Extension of Mycobacterium tuberculosis Hsp16.3 Regulates its Oligomerization, Subunit Exchange Dynamics and Chaperone Function. Febs J. 284, 277–300. doi:10.1111/febs.13975
Pras, E., Frydman, M., Levy-Nissenbaum, E., Bakhan, T., Raz, J., Assia, E. I., et al. (2000). A Nonsense Mutation (W9X) in CRYAA Causes Autosomal Recessive Cataract in an Inbred Jewish Persian Family. Invest. Ophthalmol. Vis. Sci. 41, 3511–3515.
Preneta, R., Papavinasasundaram, K. G., Cozzone, A. J., and Duclos, B. (2004). Autophosphorylation of the 16 kDa and 70 kDa Antigens (Hsp 16·3 and Hsp 70) of Mycobacterium tuberculosis. Microbiology 150, 2135–2141. doi:10.1099/mic.0.26789-0
Qian, J., Ren, X., Wang, X., Zhang, P., Jones, W. K., Molkentin, J. D., et al. (2009). Blockade of Hsp20 Phosphorylation Exacerbates Cardiac Ischemia/Reperfusion Injury by Suppressed Autophagy and Increased Cell Death. Circ. Res. 105, 1051223–1051231. doi:10.1161/CIRCRESAHA.109.200378
Raju, I., and Abraham, E. C. (2011). Congenital Cataract Causing Mutants of αA-Crystallin/sHSP Form Aggregates and Aggresomes Degraded through Ubiquitin-Proteasome Pathway. PloS one 6, e28085. doi:10.1371/journal.pone.0028085
Raju, I., Kumarasamy, A., and Abraham, E. C. (2011). Multiple Aggregates and Aggresomes of C-Terminal Truncated Human αA-Crystallins in Mammalian Cells and Protection by αB-Crystallin. PloS one 6, e19876. doi:10.1371/journal.pone.0019876
Rao, N. A., Saraswathy, S., Pararajasegaram, G., and Bhat, S. P. (2012). Small Heat Shock Protein αA-Crystallin Prevents Photoreceptor Degeneration in Experimental Autoimmune Uveitis. PloS one 7, e33582. doi:10.1371/journal.pone.0033582
Rosa, T. L. S. A., Marques, M. A. M., DeBoard, Z., Hutchins, K., Silva, C. A. A., Montague, C. R., et al. (2021). Reductive Power Generated by Mycobacterium leprae through Cholesterol Oxidation Contributes to Lipid and ATP Synthesis. Front. Cel. Infect. Microbiol. 11, 709972. doi:10.3389/fcimb.2021.709972
Saraswathy, S., Nguyen, A. M., and Rao, N. A. (2010). The Role of TLR4 in Photoreceptor αA Crystallin Upregulation during Early Experimental Autoimmune Uveitis. Invest. Ophthalmol. Vis. Sci. 51, 3680–3686. doi:10.1167/iovs.09-4575
Seidler, N. W., Yeargans, G. S., and Morgan, T. G. (2004). Carnosine Disaggregates Glycated α-crystallin: an In Vitro Study. Arch. Biochem. Biophys. 427, 110–115. doi:10.1016/j.abb.2004.04.024
Shan, R., Liu, N., Yan, Y., and Liu, B. (2021). Apoptosis, Autophagy and Atherosclerosis: Relationships and the Role of Hsp27. Pharmacol. Res. 166, 105169. doi:10.1016/j.phrs.2020.105169
Sharma, K. K., and Santhoshkumar, P. (2009). Lens Aging: Effects of Crystallins. Biochim. Biophys. Acta (Bba) - Gen. Subjects 1790, 1095–1108. doi:10.1016/j.bbagen.2009.05.008
Shi, C., Chen, L., Chen, Z., Zhang, Y., Zhou, Z., Lu, J., et al. (2010). Enhanced protection against Tuberculosis by Vaccination with Recombinant BCG Over-expressing HspX Protein. Vaccine 28, 5237–5244. doi:10.1016/j.vaccine.2010.05.063
Shi, C., Zhang, H., Zhang, T., Wang, X., Bai, B., Zhao, Y., et al. (2009). New Alternative Vaccine Component against Mycobacterium tuberculosis- Heat Shock Protein 16.3 or its T-Cell Epitope. Scand. J. Immunol. Nov 70, 465–474. doi:10.1111/j.1365-3083.2009.02325.x
Sin, Y. Y., and Baillie, G. S. (2015). Heat Shock Protein 20 (HSP20) Is a Novel Substrate for Protein Kinase D1 (PKD1). Cell Biochem Funct 33, 421–426. doi:10.1002/cbf.3147
Singh, D., Raman, B., Ramakrishna, T., and Rao, Ch. M. (2006). The Cataract-Causing Mutation G98R in Human alphaA-Crystallin Leads to Folding Defects and Loss of Chaperone Activity. Mol. Vis. 12, 1372–1379.
Singh, J. A., and Cleveland, J. D. (2018). Comparative Effectiveness of Allopurinol versus Febuxostat for Preventing Incident Dementia in Older Adults: a Propensity-Matched Analysis. Arthritis Res. Ther. 20, 20167. doi:10.1186/s13075-018-1663-3
Singh, J. A., Ramachandaran, R., Yu, S., and Curtis, J. R. (2017). Allopurinol Use and the Risk of Acute Cardiovascular Events in Patients with Gout and Diabetes. BMC Cardiovasc. Disord. 17, 1776. doi:10.1186/s12872-017-0513-6
Soong, J. X., Lim, T. S., and Choong, Y. S. (2018). The Structural Insights of 16.3 kDa Heat Shock Protein (HSP16.3) from Mycobacterium tuberculosis via in silico Study. Mol. Simulation 44, 117–127. doi:10.1080/08927022.2017.1346254
Sprague-Piercy, M. A., Rocha, M. A., Kwok, A. O., and Martin, R. W. (2021). Alpha-Crystallins in the Vertebrate Eye Lens: Complex Oligomers and Molecular Chaperones. Annu. Rev. Phys. Chem. Apr 20 (72), 143–163. doi:10.1146/annurev-physchem-090419-121428
Sun, Y., and MacRae, T. H. (2005). The Small Heat Shock Proteins and Their Role in Human Disease. FEBS J. Jun 272, 2613–2627. doi:10.1111/j.1742-4658.2005.04708.x
Takeuchi, N., Ouchida, A., and Kamei, A. (2004). C-terminal Truncation of .ALPHA.-Crystallin in Hereditary Cataractous Rat Lens. Biol. Pharm. Bull. 27, 308–314. doi:10.1248/bpb.27.308
Taylor, J. L., Wieczorek, A., Keyser, A. R., Grover, A., Flinkstrom, R., Karls, R. K., et al. (2012). HspX‐mediated protection against Tuberculosis Depends on its Chaperoning of a Mycobacterial Molecule. Immunol. Cell Biol 90, 945–954. doi:10.1038/icb.2012.34
Timm, J., Kurepina, N., Kreiswirth, B. N., Post, F. A., Walther, G. B., Wainwright, H. C., et al. (2006). A Multidrug‐Resistant,acr1‐Deficient Clinical Isolate of Mycobacterium tuberculosis is Unimpaired for Replication in Macrophages. J. Infect. Dis. 193, 1703–1710. doi:10.1086/504526
Traxler, D., Lainscak, M., Simader, E., Ankersmit, H. J., and Jug, B. (2017). Heat Shock Protein 27 Acts as a Predictor of Prognosis in Chronic Heart Failure Patients. Clinica Chim. Acta 473, 127–132. doi:10.1016/j.cca.2017.08.028
Valdez, M. M., Clark, J. I., Wu, G. J., and Muchowski, P. J. (2002). Functional Similarities between the Small Heat Shock Proteins Mycobacterium tuberculosis HSP16.3 and Human alphaB-Crystallin. Eur. J. Biochem. 269, 1806–1813. doi:10.1046/j.1432-1033.2002.02812.x
Valdez, M. M., Clark, J. I., Wu, G. J. S., and Muchowski, P. J. (2002). Functional Similarities between the Small Heat Shock Proteins Mycobacterium tuberculosis HSP16.3 and Human αB-crystallin. Eur. J. Biochem./FEBS 269, 1806–1813. doi:10.1046/j.1432-1033.2002.02812.x
van Montfort, R. L. M., Basha, E., Friedrich, K. L., Slingsby, C., and Vierling, E. (2001). Crystal Structure and Assembly of a Eukaryotic Small Heat Shock Protein. Nat. Struct. Biol. 8, 1025–1030. doi:10.1038/nsb722
Verbon, A., Hartskeerl, R. A., Schuitema, A., Kolk, A. H., Young, D. B., and Lathigra, R. (1992). The 14,000-Molecular-Weight Antigen of Mycobacterium tuberculosis is Related to the Alpha-Crystallin Family of Low-Molecular-Weight Heat Shock Proteins. J. Bacteriol. 174, 1352–1359. doi:10.1128/jb.174.4.1352-1359.1992
Wang, K., and Spector, A. (1995). Alpha-crystallin Can Act as a Chaperone under Conditions of Oxidative Stress. Invest. Ophthalmol. Vis. Sci. 36, 311–321.
Wang, K., and Spector, A. (2001). ATP Causes Small Heat Shock Proteins to Release Denatured Protein. Eur. J. Biochem./FEBS. Dec 268, 6335–6345. doi:10.1046/j.0014-2956.2001.02580.x
Wang, X., Shangguan, W., and Li, G. (2018). Angiotensin-(1-7) Prevents Atrial Tachycardia Induced-Heat Shock Protein 27 Expression. J. Electrocardiol. 51, 117–120. doi:10.1016/j.jelectrocard.2017.08.015
Wang, Y. H., Wang, D. W., Wu, N., Wang, Y., and Yin, Z. Q. (2012). Alpha-Crystallin Promotes Rat Axonal Regeneration through Regulation of RhoA/rock/cofilin/MLC Signaling Pathways. J. Mol. Neurosci. 46, 138–144. doi:10.1007/s12031-011-9537-z
Wolfe, L. M., Veeraraghavan, U., Idicula-Thomas, S., Schürer, S., Wennerberg, K., Reynolds, R., et al. (2013). A Chemical Proteomics Approach to Profiling the ATP-Binding Proteome of Mycobacterium tuberculosis. Mol. Cell Proteomics 12, 1644–1660. doi:10.1074/mcp.m112.025635
Wu, N., Yu, J., Chen, S., Xu, J., Ying, X., Ye, M., et al. (2014). α-Crystallin Protects RGC Survival and Inhibits Microglial Activation after Optic Nerve Crush. Life Sci. 94 (94), 17–23. doi:10.1016/j.lfs.2013.10.034
Yang, L., Zhang, C., Zhao, Y., Zhao, N., Wu, P., Zhang, H., et al. (2018). Effects of Mycobacterium tuberculosis Mutant Strain Hsp16.3 Gene on Murine RAW 264.7 Macrophage Autophagy. DNA Cell Biol. 37, 7–14. doi:10.1089/dna.2016.3599
Ying, X., Zhang, J., Wang, Y., Wu, N., Wang, Y., and Yew, D. T. (2008). α-Crystallin Protected Axons from Optic Nerve Degeneration after Crushing in Rats. J. Mol. Neurosci. 35, 253–258. doi:10.1007/s12031-007-9010-1
Yu, D. W., Ge, P. P., Liu, A. L., Yu, X. Y., and Liu, T. T. (2019). HSP20-mediated Cardiomyocyte Exosomes Improve Cardiac Function in Mice with Myocardial Infarction by Activating Akt Signaling Pathway. Eur. Rev. Med. Pharmacol. Sci. 23, 4873–4881. doi:10.26355/eurrev_201906_18075
Yuan, Y., Crane, D. D., and Barry, C. E. (1996). Stationary Phase-Associated Protein Expression in Mycobacterium tuberculosis: Function of the Mycobacterial Alpha-Crystallin Homolog. J. Bacteriol. 178, 4484–4492. doi:10.1128/jb.178.15.4484-4492.1996
Yuan, Y., Crane, D. D., Simpson, R. M., Zhu, Y., Hickey, M. J., Sherman, D. R., et al. (1998). The 16-kDa α-crystallin (Acr) Protein of Mycobacterium tuberculosis is Required for Growth in Macrophages. Pnas 95, 9578–9583. doi:10.1073/pnas.95.16.9578
Zhang, C., Yang, L., Zhao, N., Zhao, Y., and Shi, C. (2018). Insights into Macrophage Autophagy in Latent Tuberculosis Infection: Role of Heat Shock Protein 16.3. DNA Cell Biol. 37, 442–448. doi:10.1089/dna.2017.4066
Zhang, H. L., Jia, K. Y., Sun, D., and Yang, M. (2019). Protective Effect of HSP27 in Atherosclerosis and Coronary Heart Disease by Inhibiting Reactive Oxygen Species. J. Cell Biochem 120, 2859–2868. doi:10.1002/jcb.26575
Zhao, Z., Zhao, C., Zhang, X. H., Zheng, F., Cai, W., Vlassara, H., et al. (2009). Advanced Glycation End Products Inhibit Glucose-Stimulated Insulin Secretion through Nitric Oxide-dependent Inhibition of Cytochrome C Oxidase and Adenosine Triphosphate Synthesis. Endocrinology 150, 2569–2576. doi:10.1210/en.2008-1342
Keywords: sHSPs, ATP, cataract, cardiovascular diseases, tuberculosis, leprosy
Citation: Nandi SK, Panda AK, Chakraborty A, Rathee S, Roy I, Barik S, Mohapatra SS and Biswas A (2022) Role of ATP-Small Heat Shock Protein Interaction in Human Diseases. Front. Mol. Biosci. 9:844826. doi: 10.3389/fmolb.2022.844826
Received: 28 December 2021; Accepted: 18 January 2022;
Published: 16 February 2022.
Edited by:
Sofia R. Pauleta, New University of Lisbon, PortugalReviewed by:
Ranjeet Kumar, Banaras Hindu University, IndiaCopyright © 2022 Nandi, Panda, Chakraborty, Rathee, Roy, Barik, Mohapatra and Biswas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sandip K. Nandi, c2FuZGlwLm5hbmRpQGRkbi51cGVzLmFjLmlu; Ashis Biswas, YWJpc3dhc0BpaXRiYnMuYWMuaW4=
†Present address: Ayon Chakraborty, Advanced Centre for Treatment, Research and Education in Cancer (ACTREC), Tata Memorial Centre, India
 Sandip K. Nandi
Sandip K. Nandi Alok Kumar Panda
Alok Kumar Panda Ayon Chakraborty
Ayon Chakraborty Shivani Rathee1
Shivani Rathee1 Ipsita Roy
Ipsita Roy Subhashree Barik
Subhashree Barik