- Molecular Modelling and Drug Design Laboratory, Department of Chemistry, University of Delhi, New Delhi, India
DPP-4 inhibition is an interesting line of therapy for treating Type 2 Diabetes Mellitus (T2DM) and is based on promoting the incretin effect. Here, the authors have presented a brief appraisal of DPP-4 inhibitors, their modes of action, and the clinical efficiency of currently available drugs based on DPP-4 inhibitors. The safety profiles as well as future directions including their potential application in improving COVID-19 patient outcomes have also been discussed in detail. This review also highlights the existing queries and evidence gaps in DPP-4 inhibitor research. Authors have concluded that the excitement surrounding DPP-4 inhibitors is justified because in addition to controlling blood glucose level, they are good at managing risk factors associated with diabetes.
1 Introduction
The success and ever increasing interest in incretin-based therapies and drugs especially sitagliptin, vildagliptin and exenatide depict their immense importance in improving patient outcomes in T2DM (Nasr and Sadek, 2022). Incretin effect is the enhancement in the secretion of insulin as a function of increase in oral intake of glucose. For a given rise in plasma glucose concentration, the increase in plasma insulin is roughly threefold times greater, if glucose is administered orally compared to intravenous route (Perley and Kipnis, 1967). The main incretin hormones secreted by the epithelial cells of the gastrointestinal track are glucagon-like peptide-1 (GLP)-1 and glucose-dependent insulinotropic peptide, also known as Gastric Inhibitory Polypeptide (GIP), which stimulate the pancreatic cells (α and β cells) and regulate insulin secretion with respect to blood glucose concentration (Drucker, 2006).
The first incretin to be reported was GIP. It is secreted in a single biologically active form by the K-cells in the duodenum and jejunum upon consumption of carbohydrates or lipids. Its main functions are stimulation of insulin secretion in a glucose dependent manner and contribution to fat metabolism in adipocytes. It also has proliferative effect on β-cells. The second important incretin hormone is GLP-1, released from L-cells in the distal ileum and colon (Baggio and Drucker, 2007; Gautier et al., 2008). GLP-1 is similar to but unlike GIP, actions of GLP-1 are better preserved in type 2 diabetes mellitus (T2DM) patients, which makes it a highly desirable target for drug discovery for T2DM (Sharma and Bhatia, 2020); (Nauck et al., 1993).
However, both GLP-1 and GIP are quickly degraded by dipeptidyl peptidase 4 (DPP-4) enzyme (Figure 1). It cleaves the oligo-peptides after the 2nd amino acid from the N terminal proline or alanine sequences, thus rendering GLP-1 and GIP inactive (Deacon et al., 1995). In addition to that, DPP-4 enzyme also deactivates more than 40 physiologically active hormones, neuropeptides, cytokines, and other proteins in vivo through dipeptide cleavage (Mentlein et al., 1993). This very short half-life (<2 min) of GLP-1 due to the action of DPP-4 is a formidable challenge in the incretin-based drug development for T2DM.
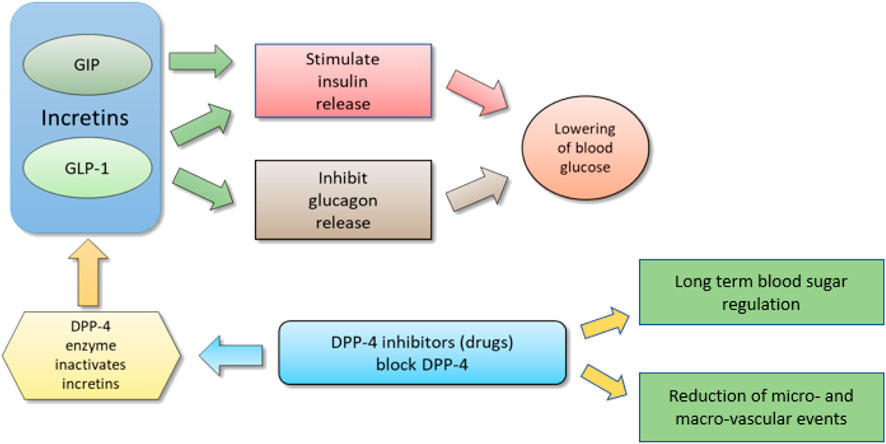
FIGURE 1. Schematic diagram to show relation between incretins and DPP-4 inhibitors; By I. Karonen under CCA-SA (Karonen, 2007).
DPP-4 is member of a family of proteases which also includes dipeptidyl-peptidase-8 (DPP-8), dipeptidylpeptidase-9 (DPP-9) and fibroblast activation protein (FAP) (Ajami et al., 2004). DPP-4 enzyme is a 766 amino acid trans membrane glycoprotein, also known as adenosine deaminase complexing protein 2 or CD26 (Jose and Inzucchi, 2012). It is a serine amino peptidase enzyme, which exists on the superficial side of epithelial and endothelial cells (involving monocytes and lymphocytes) and propagates in plasma (Fadini and Avogaro, 2011). These enzymes are broadly distributed in various tissues (brain, lungs, kidneys), T-cell, B-cell and natural killer cells (Lambeir et al., 2003). DPP-4 has two mechanisms of action: first as a membrane spanning protein, where it binds and activates adenosine deaminase complexing protein and transfers intracellular signals via dimerization; and second as an enzyme (Lambeir et al., 2003). Thus, a DPP-4 can circulate freely or can exist in the membrane bound form.
1.1 Incretin effect in T2DM: GLP-1 receptor agonists vs DPP-4 inhibitors
Broadly, there are two strategies in the drug design for T2DM that make use of the incretin effect. First is the use of GLP-1 receptor agonists (GLP-1RAs) also known as GLP-1 mimetics that enhance GLP-1 action in vivo (Parker et al., 2010). These agonists possess sequence similarities to native GLP-1 and can bind and stimulate GLP-1 receptor. Also, they are resistant to DPP-4 action. The second approach is inhibiting the DPP-4 enzyme itself, due to which levels of active GLP-1 increases (Drucker, 2007). The active GLP-1 consists of only one-third to one-half of the postprandial GLP-1 in the plasma and the rest being the inactive fragment (GLP-1 (9–36)amide) formed by truncation. DPP-4 inhibitors raise only the proportion of active GLP-1 rather than total GLP-1 concentration after a meal (Herman et al., 2006a), resulting in elevated plasma levels of GLP-1 at a concentration that does not produce GLP-1-related side effects (Vilsbøll et al., 2001; Sharma and Bhatia, 2021). This is described in Figure 2.
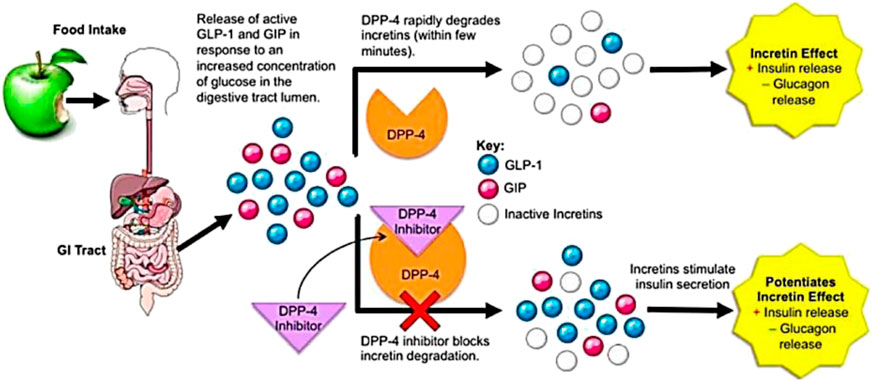
FIGURE 2. DPP-4 function and mechanism of DPP-4 inhibitors’ action; under CCA-SA; taken from (Makrilakis, 2019).
The inhibition of DPP-4 not only elevates the prandial GLP-1 levels but also alters the 24-h pattern of GLP-1 levels, including fasting levels (Mari et al., 2005). This protracted circadian rhythm at a higher level is important for the physiology of incretin receptors (Ahrén, 2007). Hsieh et al. discovered the complimentary action of DPP-4 inhibition and pharmacological enhancement of GLP-1 receptor (GLP-1R) signalling. Multiple studies apart from these, have shown promising positive effects of significant magnitude on blood glucose as well as heart and coronary function (Dicker, 2011). Multiple differences exist between the physiological effects GLP-1 receptor agonists and DPP-4 inhibitors. These are summarised in Table 1.
Notably, in a clinical trial which compared short-term 2-week treatment with exenatide (GLP-1RA) versus sitagliptin (DPP-4 inhibitor), patient outcomes were better after treatment with exenatide. It was more effective in reducing postprandial glucose, increasing insulin levels, lowering glucagon levels and decreasing caloric intake (DeFronzo et al., 2008a). Despite the apparent better results for GLP-1 RAs, their safety profiles remains a concern. For instance, both exenatide and liraglutide have black box warnings from FDA due to risk of medullary thyroid cancer and pancreatitis in case of exenatide and thyroid C-cell tumours and for patients with multiple endocrine neoplasia syndrome type 2 in case of liraglutide (Pozo et al., 2019). Crucially, DPP-4 inhibitors do not cause nausea or vomiting, which are common side effects of GLP-1 agonists (Ahrén, 2007). Since the DPP-4 inhibitors promote physiological incretin concentrations unlike incretin mimetics which induce pharmacological incretin concentrations. The key advantages of DPP-4 inhibitors are summarized in Figure 3.
One needs to keep in mind that the latest research points that SGLT-2 inhibitors and GLP-1 receptor agonists both reduce cardiovascular events (no study has compared their respective potency in this respect), whereas DPP-4 inhibitors are neutral. However, all over the globe, many research groups are investigating the potential of DPP-4 inhibitors, and so many studies are being published, we felt this topic merited a comprehensive review. Supported by satisfactory performance, obvious gains in the quality of life of a diabetic patient and a robust safety profile, we shall focus on exploring the role of DPP-4 inhibitors as a novel and effective therapeutic strategy for treating T2DM.
2 The key interactions between the ligand and DPP-4 complex
As shown in Figure 4, the β-propeller domain is packing against the α/β hydrolase domain. In addition to that, at the interface of β-propeller domain and α/β hydrolase domain, catalytic triad (residues Ser630, His740 and Asp708) is located (Aertgeerts et al., 2004), with both these domains participating in the binding of the inhibitor. In the DPP4-like protein family, glutamic acid-rich loop (Glu205/Glu206) is highly conserved and is responsible for substrate recognition. The catalytic serine S630 falls within the pentapeptide sequence Gly628-X-Ser630-Tyr631-Gly632, which is highly conserved across the α/β hydrolase protein family and is indispensable for catalytic activity.
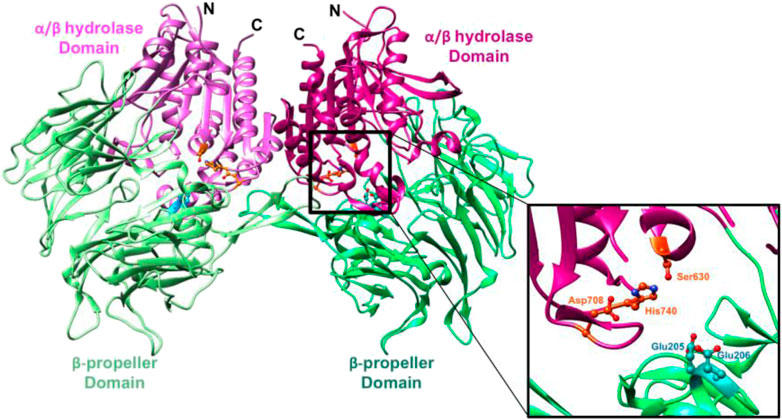
FIGURE 4. (Metzler et al., 2008): Ribbon representation of the structure of the symmetric DPP-4 homodimer. “α/β hydrolase domain and the β-propeller domain of each monomer unit are coloured in two shades of pink and green respectively (PDB ID 1r9m). The inset shows a closeup of the DPP-4 active site (corresponding to the right half) of the DPP-4 homodimer. The catalytic triad (Ser630, His740 and Asp708) is shown in orange and the anchoring Glu motif (Glu205/Glu206) in cyan”.
Studying the binding interactions of DPP-4 inhibitors at the binding site are crucial in understanding their performance and also for guiding research into novel drug candidates. The DPP-4 binding site can be divided into two pockets viz. S1 and S2 and a hydrophobic sub-S1 or sub-S3 sub-pocket. Figure 5 represents a general DPP-4 ligand. It contains an aromatic group, a heterocycle, a primary amine, as well as a nitrile group. S1 pocket is usually occupied by the aromatic ring or the saturated heterocycle, whereas the primary amine group forms hydrogen bond with Glu205/206 and Tyr662. S2 site binds with the aromatic heterocycle or fused rings present in the inhibitor (Metzler et al., 2008). The hydrophobic nature of the sub-pockets dictate that these are occupied by an aromatic group, and linker is necessary between the S1 and sub-S1 binding ligands (Deng et al., 2018). A common feature amongst most DPP-4 inhibitors approved by regulatory authorities is the presence of a cyanopyrrolidine moiety. The presence of the nitrile group allows the ligand to catalytically bind to the active serine hydroxyl group (Kumar et al., 2021).
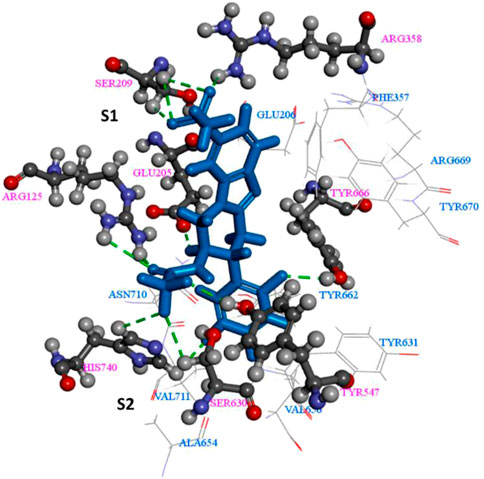
FIGURE 5. The key interactions between the ligand and DPP-4 complex (Meduru et al., 2016).
Their main interaction is with the DPP-4 complex which are recognized for competitive inhibition (Jose and Inzucchi, 2012). DPP-4 enzyme inhibition also regulates the action of various cardio active elements, neuropeptide Y and stromal cell derived factor-1 (SDF-1) (Drucker, 2007). Fibroblast activation protein, DPP-2, DPP-8 and DPP-9 show DPP-4 enzyme like activity and therefore DPP-4 inhibitors must be selective (Green et al., 2006).
3 DPP-4 inhibitors: current status
DPP-4 inhibitors are already being employed in T2DM treatment globally. Table 2 lists all the DPP-4 inhibitors till date in various stages of drug development (Kumar et al., 2021).
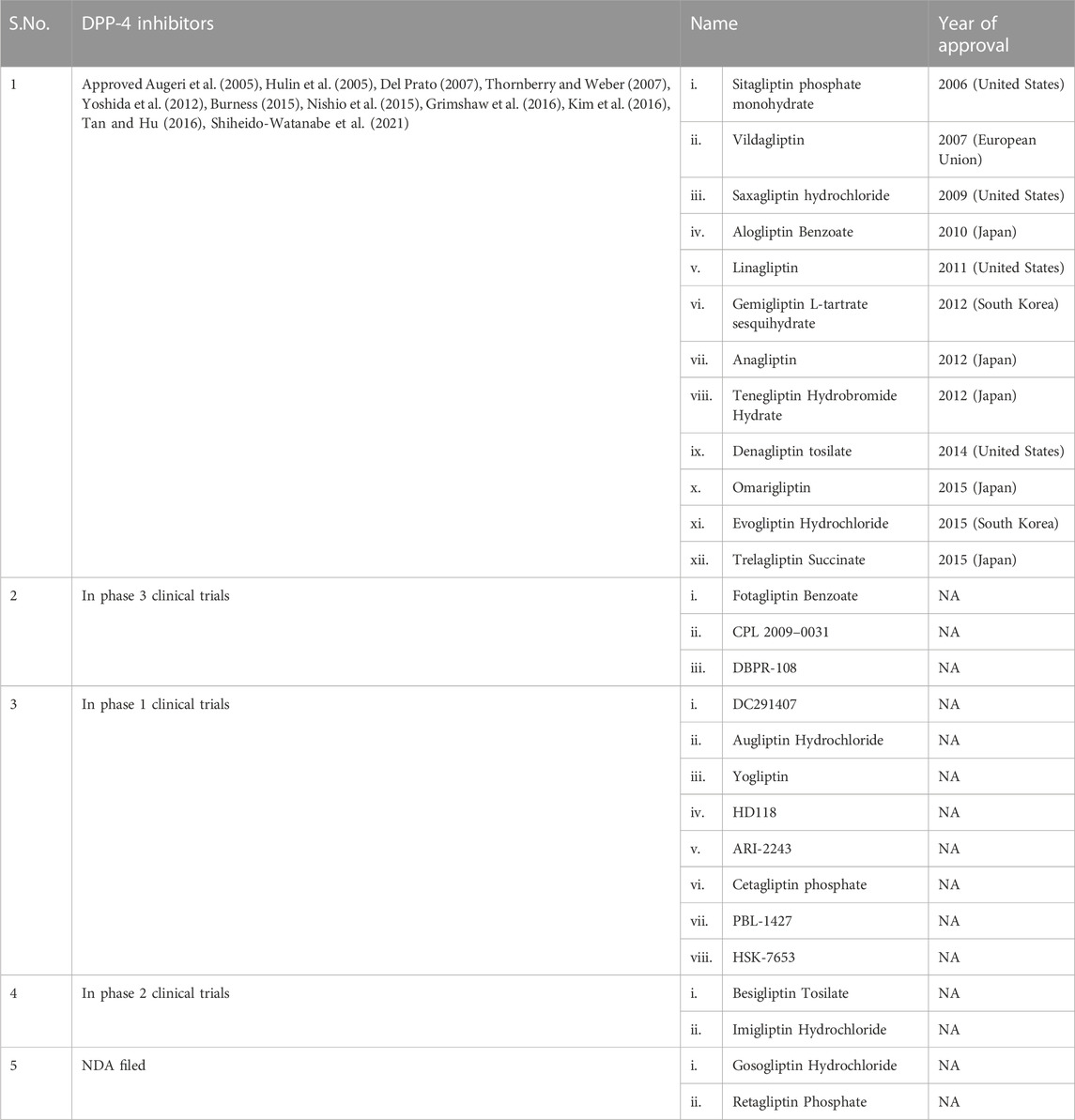
TABLE 2. List of DPP-4 inhibitors (Kumar et al., 2021).
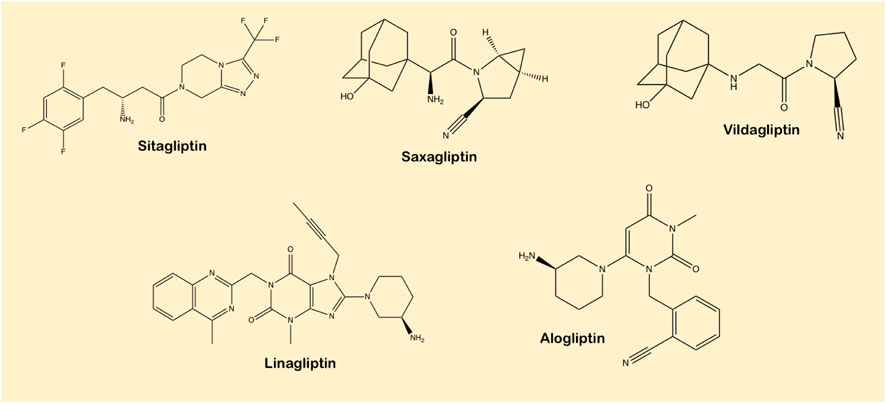
DPP-4 inhibitors are characterized into three different classes based on their binding sites (refer to Figure 5 from Section 2) (Tomovic et al., 2018). Vildagliptin and saxagliptin make up Class 1 where binding occurs only on the S1 and S2 binding sites between the nitrile group of the cyanopyrrolidine group and Ser630 of the active site on DPP-4. Saxagliptin is five times more active in blocking DPP4 than vildagliptin. Class 2 is composed of linagliptin and alogliptin where binding interaction with the S1 sub pocket or in case of the former with S2 sub pocket as well, giving it eightfold higher activity than alogliptin. The uracil rings in both ligands force a conformational alteration of Tyr547 within the S2 sub pocket. Class 3 consists of ligands binding with the S2-extensive site. Sitagliptin and tenegliptin form this class which has the highest inhibitory capability among gliptins (Röhrborn et al., 2015).
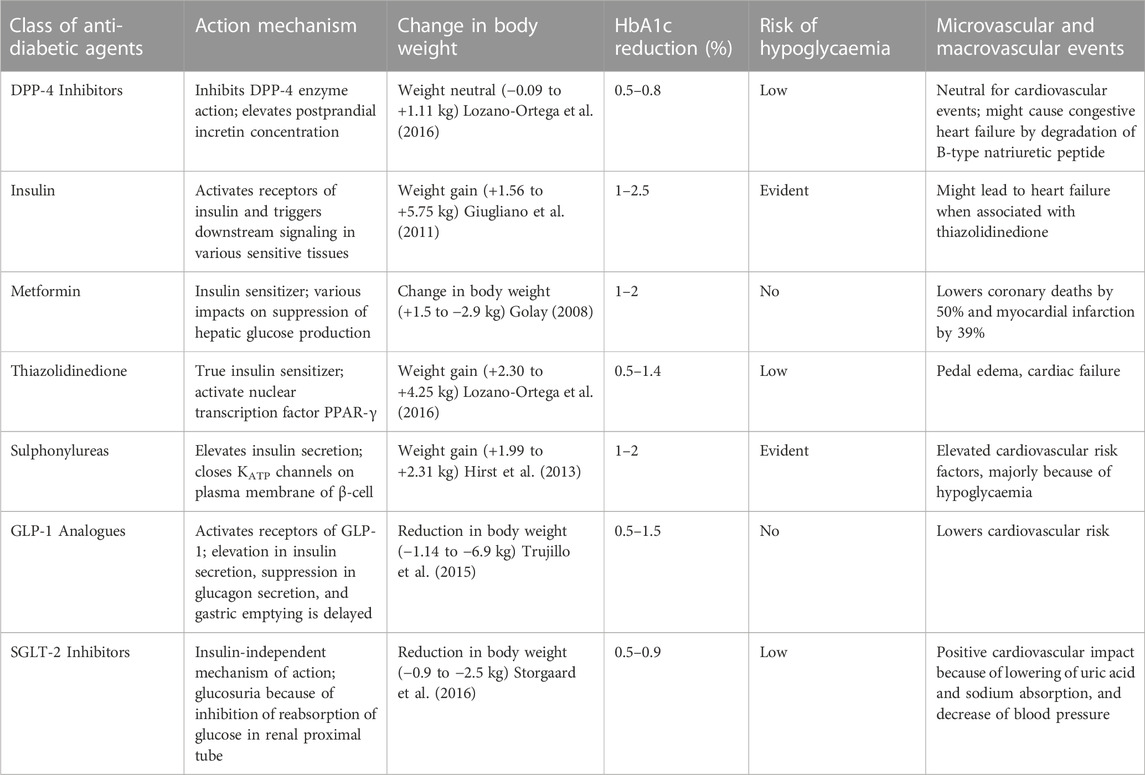
Sitagliptin, alogliptin and linagliptin possess high selectivity for DPP-4 due to strong binding with S2-extensive site which is absent in related peptidases like DPP-8, DPP-9, and FAP, wherein vildagliptin seems less selective. These inhibitors are appropriate for once or twice daily dosage, orally active are absorbed relatively fast. They are all excreted renally after negligible metabolism, except linagliptin which is eliminated via the biliary pathway (Deacon, 2019; Gallwitz, 2019). Insulin secretion can be enhanced by sulphonylureas independently and therefore introduce diabetic patients to a heightened risk of hypoglycaemia. DPP-4 inhibitors are thus added to the sub-maximal limit of sulphonylurea to achieve tighter glycaemic control (Gerich, 2010). In fact, DPP-4 inhibitors are now replacing the use of sulphonylureas as insulin-releasing agents due to their insulin-tropic effect, and notably because DPP-4 inhibitors reduce the intrinsic risk of low blood sugar or hypoglycaemia. Also, DPP-4 inhibitors are body weight neutral, in contrast to sulphonylurea therapy which is linked with body weight gain (Palmer et al., 2016). Differences between DPP-4 inhibitors with various classes of antidiabetic agents are shown in Table 3. Prior experiments in vitro have exhibited that DPP-4 inhibitors impede T-cell proliferation and cytokine expression and these agents have also been examined in animal models, showing potential anti-inflammatory effects (Yazbeck et al., 2009).
Sitagliptin was the first commercialized, orally active and selective DPP-4 inhibitor (Dhillon, 2010), which was capable of stimulating a 2-fold increase in post-meal active plasma GLP-1 levels with respect to placebo (Lim et al., 2009). It is presently available as a single agent or in fixed-dose association with metformin (Reynolds et al., 2008). A 50 mg daily dosage produces 80% inhibition of DPP-4 activity in healthy subjects and type 2 diabetic patients (Herman et al., 2006a). It is extremely selective for DPP-4 (upto 2600 times higher than other DPP family enzymes (Richter et al., 2008a). Additionally, sitagliptin improves surrogate markers of β-cell function in humans and enhanced β-cell mass in animal studies (Palalau et al., 2009).
Saxagliptin is an incredibly selective and reversible DPP-4 inhibitor (Tahrani et al., 2009a). It can increase the GLP-1 levels to 1.5 to 3-fold with a single dose of 2.5–400 mg which inhibits DPP-4 activity between 50% and 79% (Chacra, 2010). Saxagliptin is tenfold more effective than vildagliptin and sitagliptin. Its pharmacokinetics permit once-daily administration and are not influenced by age or gender. The pharmacokinetics of other drugs are also not affected by combination of saxagliptin with some other (oral antidiabetic agents) OADs such as metformin, pioglitazone, or glyburide in a remarkable way (Tahrani et al., 2009a). Saxagliptin improved β-cell function when employed as mono-therapy or in combination with metformin (Jadzinsky et al., 2009). However the similar improvement was not seen when combined with suboptimal glyburide (Chacra et al., 2009). The FDA endorsed saxagliptin for its use as a supplement to diet and exercise to improve glycaemic control in adults for curing type 2 diabetes (Anz et al., 2014). Saxagliptin can be employed in mono-therapy or in combination with other OADs including metformin, sulphonylureas or TZDs (Karyekar et al., 2011).
Vildagliptin is another selective DPP-4 inhibitor that can produce 1.5- to 3-fold increase in post meal active plasma GLP-1 levels vis-à-vis placebo (Hu et al., 2009). A 100-mg vildagliptin dose is enough to completely suppress the activity of DPP-4 in patients with T2DM (Balas et al., 2007). Vildagliptin also improved β-cell replacement function in humans and stimulated β-cell mass increase in animal studies (Mari et al., 2008). It improved HbA1c and reduced FPG and PPG in type 2 diabetic patients when used in mono-therapy or in combination with some other OADs. With vildagliptin the improvement of glycaemic control lasts at least 2 years (Scherbaum et al., 2008), and is better in patients with greater initial HbA1c levels (Rosenstock et al., 2007). Vildagliptin has also been identified to be effective in the older population (Das et al., 2021). Due to their compatible mechanisms of action and low risk of hypoglycaemia, vildagliptin and metformin have been formulated in a single tablet (Tahrani et al., 2009b).
Alogliptin is another effective, highly selective DPP-4 inhibitor approved in 2010. It can be employed in mono-therapy or in combination with metformin or glyburide for T2DM patients. The improvements in fasting glycaemia and HbA1c are maintained for at least 26 weeks. Alogliptin possesses good gastrointestinal tolerability, is weight neutral and has a very low degree of hypoglycaemia risk (Nauck et al., 2009). Administration of alogliptin with metformin, pioglitazone or glibenclamide in healthy patients suggests no significant drug-drug interactions (Karim et al., 2009). Alogliptin in combination with a specific dose of insulin, improved glycaemic control without elevating hypoglycaemia rates and without magnifying weight gain (Rosenstock et al., 2009a). Notably, greater reductions in HbA1c levels were identified when alogliptin was combined with pioglitazone, without elevating the possibility of hypoglycaemic events (Deacon, 2011). The therapeutic effectiveness of alogliptin-pioglitazone combination deserves attention in the future management of T2DM (Argyrakopoulou and Doupis, 2009).
Other important approved DPP-4 inhibitors as listed in Table 2 are Linagliptin, Gemigliptin L-tartrate sesquihydrate, Anagliptin, Tenegliptin Hydrobromide Hydrate, Denagliptin tosilate, Omarigliptin, Evogliptin Hydrochloride and Trelagliptin Succinate.
4 Pharmacological variation among different DPP-4 inhibitors
As can be seen from Figure 6, DPP-4 inhibitors constitute a heterogeneous class of small molecules with diversity and variations in chemistry, in pharmacokinetic properties such as absorption, distribution, metabolism and excretion routes, and in pharmacodynamic components such as potency and selectivity of DPP-4 inhibition (Scheen, 2012a). From a pharmacological point of view, tight-binding inhibitors are crucial as once they are bound to their target, inhibition of the enzyme operation continues even after the loose drug has been removed from circulation or from the vicinity of the site of action, a profile which is ideal for once-daily dosing. Currently available DPP-4 inhibitors display slow, tight-binding inhibition kinetics and are reversible competitive inhibitors of DPP-4 (Villhauer et al., 2003).
Despite clinical dissimilarities within the group, all DPP-4 inhibitors have a common working action. Firstly, all DPP-4 inhibitors possess pronounced structural heterogeneity which may account for differences in the pharmacokinetic properties. Linagliptin, for instance, possesses distinctive xanthine-based structure (Deacon and Holst, 2010). Its terminal half-life of 184 h (Hüttner et al., 2008), is substantially longer than that of sitagliptin (10–12 h) (Neumiller, 2009). Saxagliptin assimilation by CYP3A4/5 produces an active metabolite, however metabolites of linagliptin, vildagliptin and sitagliptin seem to be inactive. Also, terminal half-life of saxagliptin is 2.5 h, notably less than the mean half-life of 26.9 h for plasma DPP-4 inhibitors. A direct consequence is that if patients miss a dose, the long terminal half-life of linagliptin would predictably retain effectual glycaemic control whereas the short half-lives of sitagliptin and saxagliptin indicate that patients need to strictly follow the dosing interval (Gerich, 2010). When handling T2DM patients with renal dysfunction, the differences in the path of metabolism of action may also prove pivotal. Linagliptin is unlikely to accrue in renally impaired T2DM patients unlike other DPP-4 inhibitors that are removed renally. Due to the strong binding capacity of linagliptin with DPP-4, it rapidly saturates at low doses (Fuchs et al., 2009) and therefore any free linagliptin not bound to DPP-4 is quickly eliminated (Ahmad, 2007). Linagliptin is primarily excreted unchanged by enterohepatic mechanism and thus is appropriate in renally impaired patients. Vildagliptin, however, is excreted via the kidney by instant breakdown into an active metabolite, with an absolute bioavailability being 85% (He, 2012).
4.1 Pharmacokinetic profile
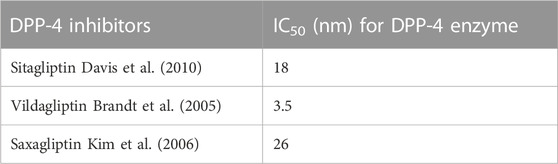
DPP-4 inhibitors and other oral hypoglycaemics such as metformin, sulphonylureas or thiazolidinediones have not exhibited any concerted pharmacokinetics (Graefe-Mody et al., 2011). There are no prominent interactions with lipid reducing agents (Scheen, 2012b) or with hormonal contraception (Friedrich et al., 2011). Anticoagulation potency of warfarin is not affected (Wright et al., 2009). Dose adjustment of digoxin is not recommended for administration of DPP-4 inhibitors (He et al., 2007a). The pharmacokinetic properties of DPP-4 inhibitors qualify for a once-daily dosing regimen (Herman et al., 2005), although greater glycaemic effect is present if given twice a day. In type 2 diabetes patients and healthy volunteers, studies have shown that these inhibitors are rapidly absorbed (cmax is 1–2 h) with explicit oral bioavailability of 80%–85% (Herman et al., 2005). Modest hepatic insufficiency has no clinical effect on the pharmacokinetic properties of sitagliptin (Zerilli and Pyon, 2007). The pharmacokinetic properties of vildagliptin and sitagliptin are independent of BMI. And the IC50 value for sitagliptin, vildagliptin and saxagliptin for inhibiting DPP-4 enzyme is displayed in Table 4.
No clinically apparent pharmacokinetic effects were displayed by linagliptin vis-à-vis commonly prescribed oral hypoglycaemics such as metformin, pioglitazone, glibenclamide or with medications commonly employed for patients with cardiac disorders such as warfarin, digoxin (Neumiller, 2012). The differences in the binding and metabolic characteristics of DPP-4 inhibitors partly explains the dosing frequency of all of them, except vildagliptin. Saxagliptin is advised because of the existence of its active metabolite, which continues to have half the potential of saxagliptin. Whereas, vildagliptin requires twice daily administration. These drugs possess reversible and low binding to plasma proteins, with the exception of linagliptin (70%) and alogliptin (∼100%) and the latter are poorly metabolized (Scheen, 2012a). For saxagliptin, the metabolism is mediated in liver by CYP3A4 and CYP3A5 isoenzymes. Thus, strong inhibitors and prompters of these isoenzymes can affect the pharmacokinetic features of saxagliptin (Scheen, 2010a). Vildagliptin undergoes liver metabolism and produces multiple inactive metabolites but is not mediated by CYP (Deacon, 2011). And linagliptin is eliminated mostly in the faeces, a dose adjustment is not required according to the stage of creatinine clearance (Ceriello et al., 2014). Pharmacokinetic outline of DPP-4 inhibitors depicts negligible risk of drug interactions (Scheen, 2010b), which is significantly advantageous in patients undergoing treatment with complex drug systems (Deacon, 2011). Also metformin did not alter the pharmacokinetics of sitagliptin (Herman et al., 2006b). It is possible that potential cytochrome P450 3A4 (CYP3A4) inhibitors such as ketoconazole, itraconazole, ritonavir and clarithromycin could affect the pharmacokinetics of sitagliptin in severely renal insufficient patients (Fasanya, 2021). In case of saxagliptin, significant pharmacodynamics interactions were not observed between saxagliptin and its metabolite. However, when co-administered with ketoconazole and diltiazem, interactions were observed and so dose adjustment is required (Tahrani et al., 2009a). When vildagliptin was used with other OADs such as digoxin, warfarin, simvastatin, amlodipine, ramipril or valsartan no clinically pertinent pharmacokinetics interactions were observed (Ayalasomayajula et al., 2007). Since vildagliptin is employed in combination with metformin in patients with T2DM, either independently or in a fixed-dose association, it is necessary to analyse the pharmacokinetic inter-effects of these two compounds, as they may significantly influence glycaemic control (Tahrani et al., 2009b). Also, the combination of DPP-4 inhibitors with pioglitazone was well tolerated. Therefore, vildagliptin may be advised in association with metformin, thiazolidinedione or sulphonylurea for the management of T2DM patients when monotherapy is insufficient and not satisfactory (Banerjee et al., 2009).
4.2 Pharmacodynamic profile
In accordance with their chemical structure, DPP-4 inhibitors are categorized into peptidomimetics like sitagliptin, saxagliptin and vildagliptin and non-peptidomimetics including linagliptin and alogliptin (Scheen, 2010b). Sitagliptin possess a triazolopiperazine-based structure; vildagliptin and saxagliptin have cyanopyrrolidine-based structure, whereas alogliptin and linagliptin have pyrimidinedione-based and imidazole-based scaffolds, respectively (Havale and Pal, 2009). Linagliptin is most effective in the inhibition of enzyme as compared to other DPP-4 inhibitors. The prolonged action is directly related to the strength and reversibility of binding to the enzyme (Ligueros-Saylan et al., 2010). DPP-4 inhibitors bind at a similar site as the fragment of biochemically relevant substrate (Berger et al., 2018). Sitagliptin is classified as a ‘competitive enzyme inhibitor’ that inhibits the enzyme in a dose-dependent mode and its dissociation is instant, whereas vildagliptin and saxagliptin inhibit the DPP-4 enzyme in a biphasic process, where the initial formation of the reversible covalent enzyme-inhibitor complex is pursued by a slow dissociation. In this case, they act as a ‘substrate-blocker’. As a repercussion, the enzyme inhibition is sustained even after elimination of the free drug and explains the longer duration of action of vildagliptin and saxagliptin in spite of their short half–life (Deacon, 2011).
4.3 Other pharmacological differences
Differences among all DPP-4 inhibitors have been observed in the effects on DPP-4 inhibition, GLP-1, insulin and glucagon levels and in the antioxidant activity.
4.3.1 DPP-4 inhibition
The administration of single-dose DPP-4 inhibitors provides long-lasting effects in both healthy volunteers and type 2 diabetes patients (Ahrén et al., 2004). The inhibition of DPP-4 increases with dose. The extent and duration of inhibition is not altered by age, gender or body mass index.
4.3.2 Antioxidant activity
Matsui et al. highlighted that vildagliptin downregulates generation of oxidative stress and reduces vascular injury in thoracic aorta of fatty rats, but there is no explanation for the glucose-reducing-independent effects of vildagliptin on vascular injury (Matsui et al., 2011). Linagliptin is also known to propagate pleiotropic vasodilatory, antioxidant and anti-inflammatory effects in animal models. Similar results have also been observed in a randomised trial in humans, where linagliptin improved vascular function and decreased inflammation markers (Baltzis et al., 2016).
4.3.3 GLP-1
One of the important roles of DPP-4 is to inactivate GLP-1. GLP-1 lowers post prandial glucose (PPG) by stimulating secretion of insulin, inhibiting release of glucagon and delaying gastric emptying (Nicolaus et al., 2011). Vildagliptin and sitagliptin studies have reported increased levels of active GLP-1 both at baseline and in reaction to a meal (during postprandial period) which suggests that the effect of DPP-4 inhibitors on glucose tolerance is interspersed by increased levels of active GLP-1 (Herman et al., 2006a).
4.3.4 Insulin and glucagon
Improved metabolic control by DPP-4 inhibition in T2DM patients is linked with reduced glucagon levels and, in spite of the lower glycaemia, unaltered insulin levels (Ahren et al., 2004). A greater suppression of glucagon was observed with vildagliptin than with sitagliptin in the course of inter-prandial periods, however these differences were not noteworthy in postprandial periods (Marfella et al., 2010).
4.3.5 β-cell function
Many investigators have concluded that DPP-4 inhibitors can improve β-cell function in humans as evidenced by increased insulin secretion with DPP-4 inhibitors. It is reported that vildagliptin and sitagliptin significantly increased the insulin secretion rate as compared to placebo (Barnett, 2006). This is because both these agents are very effective in preventing the degradation of endogenous GIP and GLP-1. Thereby improving the levels of active incretin, which further stimulates pancreatic insulin secretion and inhibits glucagon release.
4.3.6 Microvascular and macrovascular events
Microvascular events are nephropathy, retinopathy, diabetic ulcers, and peripheral and autonomic neuropathy. Diabetes might cause serious issues like cardiovascular effects (cardiovascular mortality, stroke, myocardial infarction), renal disorder and lower-extremity detachment. FDA completed cardiovascular outcomes trials for all four US-approved DPP-4 inhibitors and it was found that all DPP-4 inhibitors possess a neutral impact on microvascular and macrovascular events except saxagliptin, which might elevate the heart failure risk. Also, few research have displayed that linagliptin and saxagliptin might slow down the albuminuria progression in T2DM patients (Taylor and Lam, 2020).
4.3.7 Sarcopenia and other muscle disorders
Sarcopenia is described by a reduction in muscle strength, mass, and quality. It is commonly attributed to ageing and various chronic disorders like T2DM. A study shows that insulin is capable of lowering sarcopenia risk, whereas DPP-4 inhibitors show a neutral effect (Massimino et al., 2021). However, in another study, DPP-4 inhibitors show a positive impact on the reduction of various sarcopenic parameters like gait speed, skeletal muscle strength, muscle mass, and associated indices (Rizzo et al., 2016).
5 Safety profile of DPP-4 inhibitors
DPP-4 inhibitors exhibit appreciable safety and tolerance profile. Some recurrent adverse effects are reported such as nasopharyngitis and skin lesions but these are not serious enough to lead to discontinuation of the treatment.
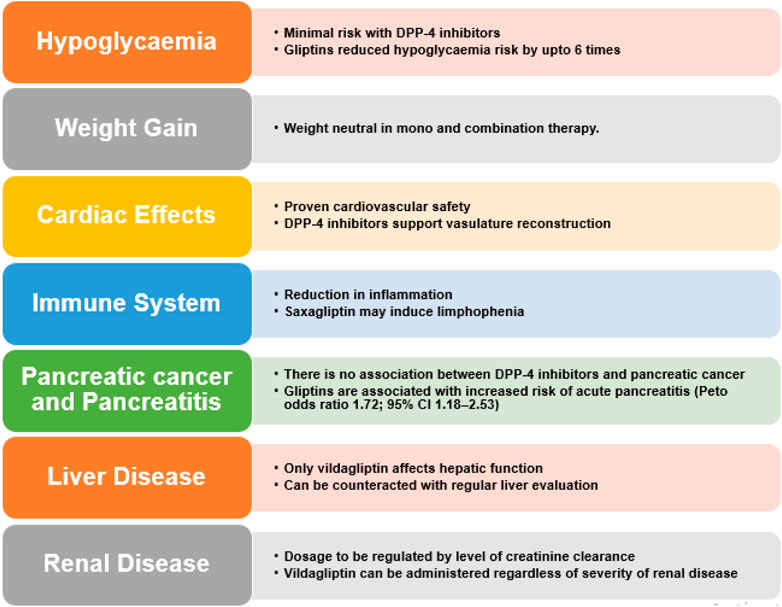
The effectiveness and safety profile of these inhibitors manifests a favourable scenario particularly for elderly subjects, but only when any influence on complications is not needed (Gallwitz, 2016; Sesti et al., 2019). Other side effects include infection in upper respiratory tract, urinary tract infection and headache. Urticarial dermatological reactions and angioedema have also been stated. Also, T2DM patients face twice the risk of acute pancreatitis and various retroactive studies and meta-analysis indicate that DPP-4 inhibitor therapy actually lowers said risk (Pinto et al., 2018). These safety profile of DPP-4 inhibitors is summarized in Figure 7.
DPP-4 has consequences beyond its proteolytic action which includes T-cell activation and proliferation (Lambeir et al., 2003). DPP-4 inhibitors may prolong the action of various hormones. The potential side-effects associated with the action of these hormones include neurogenic inflammation, elevated blood pressure and magnified general inflammation and allergic reactions (chemokines). Such side-effects have not been observed in preclinical or clinical research yet.
Linagliptin (Forst et al., 2010), saxagliptin (Rosenstock et al., 2009b), vildagliptin (Pi-Sunyer et al., 2007), sitagliptin (Aschner et al., 2006), and alogliptin (DeFronzo et al., 2008b) shows mild tolerability with adverse event profiles compared to placebo. Currently, linagliptin is the only DPP-4 inhibitor that showed improvement in wound healing in preclinical studies (Gupta and Kalra, 2011). The wide distribution of DPP-4 in various tissues has led to concern that they may have increased risk of infectious and inflammatory processes. DPP-4 adjusts levels of various mediators required in immune modulation and a meta-analysis report identifies elevated risk of infections with sitagliptin, but not with vildagliptin (Richter et al., 2009). A systematic review disclosed that DPP-4 inhibitors slightly increase the risk of nasopharyngitis and urinary tract infections in contrast with non–incretin-based placebo or OAD (Amori et al., 2007). Nasopharyngitis is detected in ≥5% of sitagliptin cured patients (Fasanya, 2021).
5.1 Hypoglycaemia
As the glucose reducing effects of GLP-1 are dependent on elevated blood glucose that fall back to normal, the hypoglycaemia risk during DPP-4 inhibitor dosing is minimal. They follow a glucose-dependent mechanism, i.e., they enhance insulin secretion only during hyperglycaemia. When incretin-based therapies are used alone or with metformin, they possess lower risk of hypoglycaemia. In spite of inhibiting glucagon secretion during hyperglycemia, Vildagliptin is not known to compromise glucagon counterregulatory response during hypoglycemia in T1DM (Farngren et al., 2012). This is an advantageous characteristic of incretin mimetic and DPP-4 inhibitors, in contradiction to sulphonylureas, which promote insulin secretion irrespective of ambient glucose concentrations. In monotherapy trials, the overall extent of hypoglycaemia was akin to placebo for both vildagliptin and sitagliptin (Aschner et al., 2006). Sitagliptin also showed similar effects in combination with metformin and TZD. An exhaustive meta-analysis concluded that the sitagliptin is safe and was found to tolerable up to 2 years in clinical trials (Herman et al., 2010). Saxagliptin was responsible for reduced rates of hypoglycaemia, specifically when used in mono-therapy or in combination with metformin or TZD (Rosenstock et al., 2009b). In a study with vildagliptin supplemented insulin therapy, hypoglycaemic events were rare and less intense as compared to those receiving placebo plus insulin therapy (Fonseca et al., 2007). In another study, sitagliptin (100 mg once daily) reduced the risk of hypoglycaemia by six fold as compared to glipizide (Grunberger, 2014).
5.2 Weight gain
Contrary to the results acquired with GLP-1 mimics, no remarkable decrease in body weight has been observed with DPP-4 inhibitors either in monotherapy or combination therapy. These inhibitors seem to be body weight neutral (Ahren et al., 2004). DPP-4 inhibitors are associated with a very low risk of hypoglycaemia (DeFronzo et al., 2008b) and are not linked with weight gain, gastrointestinal symptoms or peripheral oedema. In mono-therapy or in combination with metformin, saxagliptin is weight neutral (Jadzinsky et al., 2009), (DeFronzo et al., 2009). And a combination of saxagliptin with glyburide display small but notable weight gain as compared with glyburide alone, along with improved glycaemic control (Chacra et al., 2009). No weight gain and decreased HbA1c levels were noted with vildagliptin also (Göke et al., 2008).
5.3 Effects on the immune system
In preclinical studies, it is evident that DPP-4 inhibitors can reduce inflammation (Shah et al., 2011a). Saxagliptin may also cause lymphopenia (Dhillon, 2015). There was minute reduction in the lymphocyte number induced by saxagliptin, yet mice medicated with saxagliptin and vildagliptin had no anomaly in their innate immune response fostered by Toll-like receptor ligands; cytokine creation, immune cell activation and lymphocyte trafficking were normal (Anz et al., 2014). Linagliptin treatment reduced inflammation and elevated epithelialization of wounds of diabetic mice (Schürmann et al., 2012). In human studies, evidence exists that sitagliptin possesses anti-inflammatory properties (Satoh-Asahara et al., 2013).
5.4 Cardiovascular effects
In terms of cardiovascular results, DPP-4 inhibitors are comparable to placebo (Fei et al., 2019). The previous studies did indicate cardiovascular benefits for DPP-4 inhibitors and a probable cause for this discrepancy is differences in characteristics of patients enrolled probably for the CV safety trials (Mannucci et al., 2016).
One such previous study indicated possible cardiac benefits (possibly mediated through GLP-1) and showed that DPP-4 inhibitors may also have direct effects on the heart and on cardiovascular risk factors (such as hypertension and hyperlipidaemia), independent of the incretin system (Zhang et al., 2019). The mechanism proposed was improved signalling via bone marrow chemokine which is known as SDF-1α, also named as pre-B cell growth stimulating factor (PBSF). Endothelial progenitor cells (EPCs), which are obtained from the bone marrow, are known to stimulate vascular repair and neo-angiogenesis. When vascular damage occurs, local growth factors and cytokines are convoluted which eventually signal the bone marrow to discharge EPCs targeted to the injured sites. EPCs develop into mature endothelial cells and support in vasculature reconstruction (Fadini and Avogaro, 2011). SDF-1α is one of the chief managers of EPCs stimulating their mobilisation (Heissig et al., 2002). Because SDF-1α is a well-known substrate for DPP-4, DPP-4 inhibition will increase the concentration of SDF-1α, potentially intensifying the transport of EPCs to injured endovascular sites (Packer, 2018). Encouraged by this concept, another likely mechanism is through a reduction in inflammation which is recognised as a main contributor to the atherosclerotic process. A few animal studies have also indicated that DPP-4 inhibition may lessen inflammatory cells within visceral adipose tissue and halt the progression of atherosclerosis via immunomodulation (Shirakawa et al., 2011).
In another study, DPP-4 treatment of both diabetic and non-diabetic hypertensive patients demonstrated lowering of blood pressure (Mistry et al., 2008). Sitagliptin helps the myocardium function after an ischemic episode and also improves overall cardiac performance in patients with coronary artery disease in the course of dobutamine stress echocardiography (Read et al., 2010). There is no cardiovascular risk with saxagliptin. On the contrary, it has potential for reduction in cardiovascular events (Frederich et al., 2010). Yet, these indications are preliminary, as the trials were not formulated to assess cardiovascular safety initially, even though the data indicates otherwise.
In total 20 clinical trials were analysed to reveal no enhanced risk of adverse cardiovascular effects or of heart failure in saxagliptin-treated patients (Iqbal et al., 2014). A study claims that T2DM patients with previous heart failure instances who were administered sitagliptin were more likely to be hospitalized for subsequent heart failure (Weir et al., 2014). Shah et al. revealed that DPP-4 inhibition within microcirculation mitigates vascular tone by direct mediation of the nitric oxide system (Shah et al., 2011b). The investigators proposed this drug promotes better control of blood pressure, which is a crucial cardiovascular risk factor. Also, DPP-4 inhibitors have not been found to have any noticeable effects on circulating lipid concentrations in human clinical trials. However, Boschman et al. (Boschmann et al., 2009) and Matikainen et al. (Matikainen et al., 2006) have reported the effects of vildagliptin therapy on post-prandial lipid mobilization, oxidation and lipoprotein metabolism in patients with T2DM, where DPP-4 inhibitor course ameliorated triglyceride and apolipoprotein B-48 particle digestion after a fat-rich meal. Thus it is clear that more studies need to elucidate the overall effects of DPP-4 inhibition on cardiovascular risk factors as the findings are limited and should be regarded as highly preliminary. In 2012 at Scientific Sessions of the American Diabetes Association (ADA), Johansen et al. presented the link between linagliptin and cardiovascular outcomes (Johansen et al., 2012).
5.5 Concern for pancreatitis and pancreatic cancer
A latest meta-analysis supervised by Monami et al. did not record any elevated risk of pancreatitis associated with DPP-4 inhibitors (Monami et al., 2014). No relationship between DPP-4 inhibitors and GLP-1RAs and pancreatitis and pancreatic cancer incidence has been established. This is supported by evidence from U.S. FDA and the European Medicines Agency (Egan et al., 2014).
5.6 Skin toxicity
After the approval of sitagliptin, serious allergic and hypersensitivity reactions were reported such as anaphylaxis, angioedema and exfoliated skin conditions like the Stevens–Johnson syndrome. In comparison to placebo, alogliptin-treated patients were more likely to report dermal side effects including pruritus (Rendell et al., 2012).
5.7 Outcomes in patients suffering from liver disease
An elevated DPP-4 mRNA expression in the livers of patients suffering from non-alcoholic fatty liver disease (NAFLD) is observed. Vildagliptin alone has shown hepatotoxicity in clinical trials. So far, hepatic impairment has not influenced vildagliptin pharmacokinetics (He et al., 2007b). To counteract this, it is advised that evaluation of liver enzymes be carried out within 3 months of initial vildagliptin treatment, and its usability is contraindicated in patients with serious liver disease. Dosage adjustment of linagliptin, alogliptin, saxagliptin, or sitagliptin is not recommended in patients with liver disease (Tella and Rendell, 2015).
5.8 Renal outcomes
Again, parallel to the cardiovascular effects, among the latest antidiabetic drug classes SGLT-2 inhibitors, GLP-1 RAs and DPP-4 inhibitors, SGLT-2 inhibitors are superior in terms of renal outcomes followed by GLP-1 RAs. DPP-4 inhibitors donot exhibit any beneficial renal outcomes (Fei et al., 2019). However, previous studies indicated no clear effect on kidney health (O’Hara et al., 2021). Except linagliptin, they are all eliminated renally and have been ascertained to accumulate in individuals with renal insufficiency. Sitagliptin is eliminated through urine via active tubular secretion and glomerular filtration in humans (Lyseng-williamson, 2007), (Scheen, 2010b). Patients with slight renal insufficiency did not demonstrate any clinically notable increase in the plasma concentration of sitagliptin. Sitagliptin was also moderately removed by haemodialysis. The US Food and Drug Administration (FDA) suggests the reduction of sitagliptin dose in patients with renal impairment, whereas the European Medicines Agency (EMA) advices to avoid sitagliptin in patients with moderate-to-severe or end-stage renal failure (Fasanya, 2021). Although there are no reports on the effect of saxagliptin on the renal system, the FDA suggests that renal function should be checked prior to and regularly after initiating saxagliptin therapy (Dhillon, 2015), (Food and Drug Administration, 2009). The pharmacokinetic properties of five DPP-4 inhibitors have been investigated in subjects with varying degrees of renal impairment (RI). Depending on the creatinine clearance they were classed as mild (50–80 mL/min), moderate (30–50 mL/min) and severe patients with end-stage renal disease (<30 mL/min) (Scheen, 2012a), (Scheen, 2010b).
• sitagliptin (25 mg and 50 mg—both moderate and severe renal impairment) (JANUVIA, 2020),
• saxagliptin (2.5 mg - both moderate and severe renal impairment) (OPDIVO, 2021),
• alogliptin (6.25 mg - severe renal disease including dialysis patients) (NESINA, 2013) and
• vildagliptin (50 mg - both moderate and severe renal impairment) (Novartis, 2012).
Linagliptin possesses a non-renal route of excretion and so can be employed without dose adjustment at all stages in patients with renal disease.
5.9 Other side effects
The most common side effects reported with sitagliptin include runny nose and sore throat, headache, diarrhoea, upper respiratory infection, joint pain and urinary tract infection. Hypersensitivity reactions such as Stevens-Johnson syndrome were also reported with sitagliptin (Fasanya, 2021). The following is a list of other adverse effects observed when sitagliptin was used in combination with.
• Metformin and rosiglitazone - headache, diarrhoea, and vomiting;
• Metformin - nausea;
• Pioglitazone - flatulence;
• Sulphonylurea and metformin - constipation; and
• Rosiglitazone or pioglitazone - peripheral edema.
When vildagliptin is combined with an angiotensin-converting enzyme inhibitor, some cases of angioedema have also been reported (Fasanya, 2021). The most common side effects seen were cold/flu-like symptoms, headaches and dizziness.
5.10 Gliptins in special populations
DPP-4 inhibitors are considered good options for older patients because of their efficacy and low risk of hypoglycaemia. Although, findings of these new agents are still scarce in this population area due to lack of proper representation in start of clinical trials, underlining the need for additional targeted studies (Scheen, 2012a). For elderly patients suffering from T2DM, lowering of HbA1c levels after treatment with DPP-4 inhibitors were not notably different from those observed in younger patients (Baron et al., 2006).
6 Implementing DPP-4 inhibitors in clinical practice
Utilising DPP-4 inhibitors in clinical practice is comparatively easy than other OADs as there is no need of dose titration and adjustment when used with other prescribed medications. In monotherapy, they could challenge traditional OADs. Saxagliptin is associated with appreciable drug–drug interactions, whereas both sitagliptin and vildagliptin demonstrate minor inclination for drug–drug interactions. As DPP-4 inhibition mainly supports physiological roles of endogenous GLP-1, these inhibitors may be of significant relevance in curing T2DM or even pre-diabetes, but this remains to be confirmed in comprehensive clinical trials. A review of Cochrane mentions the evaluation of sitagliptin and vildagliptin over a period of 12 and 52 weeks. The report disclosed lowering of HbA1c levels approximately 0.7% and 0.6%, respectively, in contrast to placebo (Richter et al., 2008b). Monami et al. carried out a meta-analysis comparing the surplus glycaemic effects of DPP-4 inhibitors and numerous anti-hyperglycaemic agents on the lipid profile in T2DM patients. It was observed that DPP-4 inhibitors were slightly more effective in lowering total cholesterol and triglycerides vis-à-vis other agents (Monami et al., 2012). DPP-4 inhibitors have a unique action pathway vis-à-vis other existing class of glucose lowering agents taken orally (Krentz et al., 2008). They enhance β-cell proliferation and neogenesis and also inhibit apoptosis (Butler et al., 2003). Apart from mono-therapy, DPP-4 inhibitors have shown promise in several combinations with other glucose-lowering agents (Neumiller et al., 2010). Combination of DPP-4 inhibitors with existing drugs could be a welcome supplement. The most favoured combination of DPP-4 inhibitors is with metformin (Dahut and Madan, 2010). Combining metformin/DPP-4 inhibitor resulted in higher GLP-1 concentrations compared to DPP-4 inhibitor mono-therapy (Vardarli et al., 2014). Rather in one of the study, metformin itself acts as a DPP-4 inhibitor by inhibiting the DPP-4 enzyme in the fasting state (Cuthbertson et al., 2009). Moreover, with this dual therapy (metformin/DPP-4 inhibitor), patients can also receive additional treatment intensification with sodium-glucose cotransporter-2 (SGLT-2) inhibitor or thiazolidinedione (TZD) (Inzucchi et al., 2015). Other combinations include sulphonylurea, thiazolidinedione or metformin and sulphonylurea combined therapy (Drucker and Nauck, 2006).
Combination of GLP-1RAs with DPP-4 inhibitors is not advisable, because no clinically significant auxiliary benefit is predicted, although there are no concerns about adverse effects (Davies et al., 2018); (American Diabetes Association, 2019). Patients unable to tolerate GLP-1RAs treatment are recommended for DPP-4 inhibitor therapy. Also, it is advised that DPP-4 inhibitor therapy should cease when treatment is taken forward with an injectable (GLP-1RAs) therapy, at later stages (American Diabetes Association, 2019).
6.1 Monotherapy
Metformin has solidified its place as the first-line drug for treating T2DM (Rodbard et al., 2009). Predominantly, metformin exhibited slight but notable reductions in both HbA1c and body weight. On the other hand, the DPP-4 inhibitor demonstrated superior gastrointestinal tolerability as compared to metformin. Overall, the reduction in HbA1c was same with both the pharmacological approaches, with low likelihood of hypoglycaemic events (Scheen, 2012c).
6.1.1 Vildagliptin monotherapy
A 12-week investigation was designed to ascertain a dosage of vildagliptin which was effective in lowering down HbA1c levels and was safe and well tolerated in type 2 diabetic patients (baseline HbA1c 7.6%–7.8% and fasting plasma glucose 9.2–9.4 mmol/L for vildagliptin vs placebo, respectively). There was statistically notable reduction in HbA1c levels in the vildagliptin 50 and 100 mg/day groups as compared to placebo (Ristic et al., 2005).
6.1.2 Sitagliptin mono-therapy
The clinical inquiry of sitagliptin followed a similar design to the vildagliptin study. In two 12-week dose-range studies, all sitagliptin doses markedly reduced HbA1c compared with baseline. In another study of diabetic patients who had also undergone coronary heart disease, it was demonstrated that treatment with sitagliptin improved their heart function and coronary artery perfusion, validated by echo-debutamin tests (Read et al., 2010). For coronary heart disease threat factors, DPP-4 may rise in response to fall in blood pressure. Mistry et al. displayed that sitagliptin produced little but statistically notable reductions in blood pressure (Mistry et al., 2008). Marney et al. hypothesised that the combination of sitagliptin with high-dose angiotensin-converting enzyme (ACE) inhibition leads to activation of sympathetic tone and thus attenuates blood pressure reduction process (Marney et al., 2010).
6.1.3 Saxagliptin mono-therapy
Saxagliptin showed significant reduction in HbA1c levels for 2.5, 5 and 10 mg vs placebo in a main treatment cohort of 401 patients over a period of 24 weeks. Likelihood of adverse reactions was similar in both placebo and saxagliptin treated patients. No weight gain or hypoglycaemia cases were observed (Rosenstock et al., 2009b). Another study focusing cardiovascular effects concluded that although no increased risk of ischemic stroke was related to saxagliptin use, hospitalisation rate due to heart failure did increase. The study recommended other approaches to minimise this cardiovascular risk (Scirica et al., 2013).
6.2 Gliptins in special combinations
The combination treatment of DPP-4 inhibitors with some other anti-hyperglycaemic drugs may be worthwhile as these agents target contrasting pathophysiological processes and would be anticipated to have additional benefits on measures of glycaemic control (Barnett, 2006). Because of cost, lack of familiarity and no endpoint data, they ought to be employed mainly in combination treatment, in the initial years.
6.2.1 Gliptins combined with sulphonylureas
While the combination of gliptin–metformin did not result in hypoglycaemia, such events may arise with the gliptin–sulphonylurea combination. Therefore, in patients with T2DM characterised with moderately elevated HbA1c levels taking sulphonylurea as monotherapy, it would be safer to reduce the sulphonylurea dose when a DPP-4 inhibitor is added on. This would reduce the risk of hypoglycaemia, especially in elderly patients (Scheen, 2012c).
6.2.2 Gliptins combined with thiazolidinediones
The impact of combination of sitagliptin-metfromin vs pioglitazone monotherapy was studied in a controlled trial. The subjects were patients with poorly controlled T2DM. Improvements in HbA1c, fasting blood glucose and postprandial glucose levels were noted. Metformin caused body weight reduction, and swift improvement of insulin resistance and inflammation parameters. In contrast, sitagliptin performed better on the conservation of β-cell function. This research suffered a drawback since the dose of pioglitazone employed was non-identical between the two trial arms, i.e. 15 mg with metformin and 30 mg with sitagliptin (Derosa et al., 2010a). In another study, pioglitazone was taken in combination with vildagliptin and it was even more effective in preserving β-cell function and reducing insulin resistance and inflammation parameters. Similar improvements in glucose control parameters as compared to glimepiride-vildagliptin combination was observed (Derosa et al., 2010b). DPP-4 inhibitor with pioglitazone combination thus is a promising avenue for patients who cannot tolerate either metformin or sulphonylurea as an effective and safe therapeutic approach (Mikhail, 2008).
6.2.3 Gliptins as oral triple therapy
Initial intervention with a combination therapy of vildagliptin plus metformin delivers better and robust longstanding benefits in comparison to the present standard-of-care initial metformin monotherapy for patients with newly diagnosed T2DM as proved by the VERIFY study (Matthews et al., 2019). The advent of DPP-4 inhibitors offered new candidates for oral triple therapy at a time when the metformin-sulphonylurea-TZD combination was the only available option (Scheen et al., 2009).
• Sitagliptin daily dosage remarkably improved glycaemic control and β-cell function in T2DM patients who had ineffective glycaemic control with glimepiride and metformin therapy (Hermansen et al., 2007).
• Combining linagliptin with metformin and with sulphonylurea separately, significatly improved glycaemic control in patients with T2DM and was well tolerated (Owens et al., 2011).
• Combining alogliptin with metformin–pioglitazone combination stimulated better glycaemic control and potentially improved β-cell function (Bosi et al., 2011).
6.2.4 Gliptins combined with insulin in type 2 diabetes mellitus
DPP-4 inhibitors have advantages such as lack of hypoglycaemia and weight gain which justifies combining DPP-4 inhibitors with insulin. In addition to that, a DPP-4 inhibitor decreases glycaemic excursions that are not appropriately addressed by basal insulin therapy even after optimization of the titration of the basal insulin. Combining a DPP-4 inhibitor with insulin therapy may be useful in patients with T2DM for improving glucose control without prompting hypoglycaemia and likely suitable for restricting weight gain. Further studies are desirable to explore the role of DPP-4 inhibitor added to optimized insulin scheme, as the available research only involved patients using basal insulin therapy (Charbonnel et al., 2013).
6.2.5 Gliptins in initial combination therapy
Gliptins as initial combinations may offer a complementary initial treatment option for T2DM, especially in cases where assigning metformin is precarious such as in patients with renal impairment.
6.3 Head-to-head trials comparing incretin-based therapies
6.3.1 DPP-4 inhibitors vs GLP-1 receptor agonists
GLP-1 RAs mimic endogenous GLP-1, while DPP-4 inhibitors block their degradation (Neumiller, 2009). Thus both these approaches elevate GLP-1 activity and improve plasma glucose control. Though, head-to-head comparisons of the two therapies are scant and only one DPP-4 inhibitor, i.e., sitagliptin has been so evaluated, in meta-analyses of randomized controlled trials, GLP-1 receptor agonists were better in reducing blood glucose and resulted in substantial weight loss, whereas DPP-4 inhibitors reduces blood glucose levels to a lesser extent and are weight-neutral (Fakhoury et al., 2010). GLP-1 receptor agonists showed superiority over DPP-4 inhibitors, yet the average moderate differences in HbA1c and weight reductions may be counteracted by numerous disadvantages of GLP-1RAs (Gilbert and Pratley, 2020). However, generally GLP-1 RA’s are preferred over DPP-4 inhibitors.
7 DPP-4 inhibitors - Future prospects
New incretin-based therapies provide numerous options for glycaemic control in patients with T2DM and have clear advantages over other classical glucose-lowering agents (Phung et al., 2010), yet estimated cost of the therapy is an essential factor when making global comparisons for clinical usage (Waugh et al., 2010). DPP-4 inhibitors undoubtedly are more expensive than sulphonylureas, but more affordable than GLP-1 receptor agonists. At the moment, only sparse preliminary data are available (Schwarz et al., 2008). Thus, further economic analyses are necessary to support the switch from sulphonylureas to DPP-4 inhibitors (Waugh et al., 2010). In addition to this, further studies are needed to explore the long-term safety and efficacy of DPP-4 inhibitors. This will help establish their role in the management of T2DM and provide clinicians with valuable information to make informed treatment decisions. Additionally, identifying patient populations that are most likely to benefit from DPP-4 inhibitor therapy is crucial for personalized treatment plans. This can be achieved through ongoing clinical trials and real-world evidence studies. Furthermore, research is needed to investigate the potential benefits of combination therapy with DPP-4 inhibitors and other glucose-lowering agents. This could help improve overall glycemic control and reduce the risk of adverse effects associated with higher doses of single agents. Overall, ongoing research in the field of DPP-4 inhibitors holds promise for improved management of T2DM and better patient outcomes.
8 Conclusion
Leveraging incretin effect for treating T2DM is a relatively novel line of treatment. DPP-4 inhibitors are rapidly catching attention as they not only complement but also extend the traditional therapeutic avenues to treat T2DM. Improvement in T2DM in terms of delay in gastric emptying, reduction in postprandial glucose levels, and inhibition of glucagon secretion is observed with DPP-4 inhibitors. As recounted above, they can be efficiently used in combination therapy. Moreover, they have the advantage of meeting medical needs in metabolic disorders, which is especially significant for elderly diabetic patients or patients having obesity problems along with T2DM. In addition to that, evidence increasingly points towards reversal of dysfunction of pancreatic β cells. It is believed that the anti-apoptotic and proliferative characteristics of DPP-4 inhibitors can help in the regeneration of pancreatic β cells. Research also needs to be focused on finding the correct intervention point to mitigate progressive loss of βcells mass and function—the hallmark of prediabetes.
Though many questions have been raised regarding the safety profile of DPP-4 inhibitors particularly in areas of pancreatitis and pancreatic cancer, not much evidence is available for it. One should be cautious nonetheless and further research is needed in this regard. GLP-1 stimulation, at this stage should prove beneficial but again little evidence exists to support this hypothesis and more studies are needed for clarification in this regard.
The most disappointing result is its negligible favourable effect on cardiovascular and renal outcomes compared to other new antidiabetic agents like SGLT-2 inhibitors or GLP-1 RAs.
Apart from the cyanopyrollidine scaffolds mentioned above, research groups and pharmaceutical companies all over the world are developing multiple new candidates, many of which are already undergoing clinical trials. This is testament to the importance of harnessing the incretin effect as an attractive approach for T2DM therapy, as well as the role of DPP-4 as a drug target in achieving this goal.
The best treatment option for diabetes would not only control blood sugar levels but would also help in managing overall risk factors. In this context, DPP4 inhibitors seem to be very good candidates as are not known to cause hypoglycemia or weight gain, no requirement of dose escalation and have a favorable anti-inflammatory and safety profiles. Also, they are relatively safer for elderly diabetic patients and kidney disease patients with diabetes. They have been used quite extensively and thus there is lot of experience in their use. All these factors make them excellent choices and it can be safely said that all the hype surrounding DPP-4 inhibitors is very well justified.
Author contributions
SS conceptualized and wrote the paper. KS did the literature survey and wrote the paper. YK edited and also wrote a section of the paper. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aertgeerts, K., Ye, S., Tennant, M. G., Kraus, M. L., Rogers, J., Sang, B., et al. (2004). Crystal structure of human dipeptidyl peptidase IV in complex with a decapeptide reveals details on substrate specificity and tetrahedral intermediate formation. Protein Sci. 13 (2), 412–421. doi:10.1110/ps.03460604
Ahmad, A. M. (2007). Recent advances in pharmacokinetic modeling. Biopharm. Drug Dispos. 28 (3), 135–143. doi:10.1002/bdd.540
Ahrén, B. (2007). DPP-4 inhibitors. Best Pract. Res. Clin. Endocrinol. Metabolism 21 (4), 517–533. doi:10.1016/j.beem.2007.07.005
Ahren, B., Gomis, R., Standl, E., Mills, D., and Schweizer, A. (2004). Twelve- and 52-week efficacy of the dipeptidyl peptidase IV inhibitor LAF237 in metformin-treated patients with type 2 diabetes. Diabetes Care 27 (12), 2874–2880. doi:10.2337/diacare.27.12.2874
Ahrén, B., Landin-Olsson, M., Jansson, P. A., Svensson, M., Holmes, D., and Schweizer, A. (2004). Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J. Clin. Endocrinol. Metabolism 89 (5), 2078–2084. doi:10.1210/jc.2003-031907
Ajami, K., Abbott, C. A., McCaughan, G. W., and Gorrell, M. D. (2004). Dipeptidyl peptidase 9 has two forms, a broad tissue distribution, cytoplasmic localization and DPIV-like peptidase activity. Biochimica Biophysica Acta - Gene Struct. Expr. 1679 (1), 18–28. doi:10.1016/j.bbaexp.2004.03.010
American Diabetes Association (2019). Pharmacologic approaches to glycemic treatment: Standards of medical Care in diabetes—2019. Diabetes Care 42 (1), 90–102. doi:10.2337/dc19-s009
Amori, R. E., Lau, J., and Pittas, A. G. (2007). Efficacy and safety of incretin therapy in type 2 diabetes: Systematic review and meta-analysis. J. Am. Med. Assoc. 298 (2), 194–206. doi:10.1001/jama.298.2.194
Anz, D., Kruger, S., Haubner, S., Rapp, M., Bourquin, C., and Endres, S. (2014). The dipeptidylpeptidase-IV inhibitors sitagliptin, vildagliptin and saxagliptin do not impair innate and adaptive immune responses. Diabetes Obes. Metab. 16 (6), 569–572. doi:10.1111/dom.12246
Argyrakopoulou, G., and Doupis, J. (2009). DPP4 inhibitors: From sitagliptin monotherapy to the new alogliptin-pioglitazone combination therapy. Adv. Ther. 26 (3), 272–280. doi:10.1007/s12325-009-0009-6
Aschner, P., Kipnes, M. S., Lunceford, J. K., Sanchez, M., Mickel, C., Williams-Herman, D. E., et al. (2006). Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 29 (12), 2632–2637. doi:10.2337/dc06-0703
Augeri, D. J., Robl, J. A., Betebenner, D. A., Magnin, D. R., Khanna, A., Robertson, J. G., et al. (2005). Discovery and preclinical profile of saxagliptin (BMS-477118): A highly potent, long-acting, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J. Med. Chem. 48 (15), 5025–5037. doi:10.1021/jm050261p
Ayalasomayajula, S. P., Dole, K., He, Y. L., Ligueros-Saylan, M., Wang, Y., Campestrini, J., et al. (2007). Evaluation of the potential for steady-state pharmacokinetic interaction between vildagliptin and simvastatin in healthy subjects. Curr. Med. Res. Opin. 23 (12), 2913–2920. doi:10.1185/030079907X233296
Baggio, L. L., and Drucker, D. J. (2007). Biology of incretins: GLP-1 and GIP. Gastroenterology 132 (6), 2131–2157. doi:10.1053/j.gastro.2007.03.054
Balas, B., Baig, M. R., Watson, C., Dunning, B. E., Ligueros-Saylan, M., Wang, Y., et al. (2007). The dipeptidyl peptidase IV inhibitor vildagliptin suppresses endogenous glucose production and enhances islet function after single-dose administration in type 2 diabetic patients. J. Clin. Endocrinol. Metabolism 92 (4), 1249–1255. doi:10.1210/jc.2006-1882
Baltzis, D., Dushay, J. R., Loader, J., Wu, J., Greenman, R. L., Roustit, M., et al. (2016). Effect of linagliptin on vascular function: A randomized, placebo-controlled study. J. Clin. Endocrinol. Metab. 101 (11), 4205–4213. doi:10.1210/jc.2016-2655
Banerjee, M., Younis, N., and Soran, H. (2009). Vildagliptin in clinical practice: A review of literature. Expert Opin. Pharmacother. 10 (16), 2745–2757. doi:10.1517/14656560903302265
Barnett, A. (2006). DPP-4 inhibitors and their potential role in the management of type 2 diabetes. Int. J. Clin. Pract. 60 (11), 1454–1470. doi:10.1111/j.1742-1241.2006.01178.x
Baron, M., Dejager, S., Chang, I., Foley, J., Schweizer, A., Duggal, A., et al. (2006). Efficacy and tolerability of the DPP-4 inhibitor vildagliptin in drug-naive patients with type 2 diabetes aged 65 and older: P805. Diabet. Med. 23, 295–296.
Berger, J. P., Sinharoy, R., Pocai, A., Kelly, T. M., Scapin, G., Gao, Y. D., et al. (2018). A comparative study of the binding properties, dipeptidyl peptidase- 4 (DPP- 4) inhibitory activity and glucose-lowering efficacy of the DPP-4 inhibitors alogliptin, linagliptin, saxagliptin, saxagliptin, sitagliptin and vildagliptin in mice. Endocrinol. Diabetes Metab. 1, e00002. doi:10.1002/edm2.2
Boschmann, M., Engeli, S., Dobberstein, K., Budziarek, P., Strauss, A., Boehnke, J., et al. (2009). Dipeptidyl-peptidase-IV inhibition augments postprandial lipid mobilization and oxidation in type 2 diabetic patients. J. Clin. Endocrinol. Metabolism 94 (3), 846–852. doi:10.1210/jc.2008-1400
Bosi, E., Ellis, G. C., Wilson, C. A., and Fleck, P. R. (2011). Alogliptin as a third oral antidiabetic drug in patients with type 2 diabetes and inadequate glycaemic control on metformin and pioglitazone: A 52-week, randomized, double-blind, active-controlled, parallel-group study. Diabetes Obes. Metab. 13 (12), 1088–1096. doi:10.1111/j.1463-1326.2011.01463.x
Brandt, I., Joossens, J., Chen, X., Maes, M. B., Scharpé, S., De Meester, I., et al. (2005). Inhibition of dipeptidyl-peptidase IV catalyzed peptide truncation by Vildagliptin ((2S)-{[(3-hydroxyadamantan-1-yl)amino]acetyl}-pyrrolidine-2- carbonitrile). Biochem. Pharmacol. 70 (1), 134–143. doi:10.1016/j.bcp.2005.04.009
Burness, C. B. (2015). Omarigliptin: First global approval. Drugs 75 (16), 1947–1952. doi:10.1007/s40265-015-0493-8
Butler, A. E., Janson, J., Bonner-Weir, S., Ritzel, R., Rizza, R. A., and Butler, P. C. (2003). Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52, 102–110. doi:10.2337/diabetes.52.1.102
Ceriello, A., Sportiello, L., Rafaniello, C., and Rossi, F. (2014). DPP-4 inhibitors: Pharmacological differences and their clinical implications. Expert Opin. Drug Saf. 13 (1), 57–68. doi:10.1517/14740338.2014.944862
Chacra, A. R. (2010). Saxagliptin for type 2 diabetes. Diabetes Metab. Syndr. Obes. 3, 325–335. doi:10.2147/DMSOTT.S12241
Chacra, A. R., Tan, G. H., Apanovitch, A., Ravichandran, S., List, J., Chen, R., et al. (2009). Saxagliptin added to a submaximal dose of sulphonylurea improves glycaemic control compared with uptitration of sulphonylurea in patients with type 2 diabetes: A randomised controlled trial. Int. J. Clin. Pract. 63 (9), 1395–1406. doi:10.1111/j.1742-1241.2009.02143.x
Charbonnel, B., Schweizer, A., and Dejager, S. (2013). Combination therapy with DPP-4 inhibitors and insulin in patients with type 2 diabetes mellitus: What is the evidence? Hosp. Pract. 41 (2), 93–107. doi:10.3810/hp.2013.04.1059
Cuthbertson, J., Patterson, S., O’Harte, F. P. M., and Bell, P. M. (2009). Investigation of the effect of oral metformin on dipeptidylpeptidase-4 (DPP-4) activity in Type 2 diabetes. Diabet. Med. 26 (6), 649–654. doi:10.1111/j.1464-5491.2009.02748.x
Dahut, W. L., and Madan, R. A. (2010). Addition of incretin therapy to metformin in type 2 diabetes. Lancet 375 (9724), 1410–1412. doi:10.1016/S0140-6736(10)60399-6
Das, S., Gupta, A. K., Bandyopadhyaya, B., Darla, B. H., Arya, V., Abhyankar, M., et al. (2021). Data on vildagliptin and vildagliptin plus metformin combination in type-2 diabetes mellitus management. Bioinformation 17 (3), 413–423. doi:10.6026/97320630017413
Davies, M. J., Alessio, D. A. D., Fradkin, J., Kernan, W. N., Mathieu, C., and Mingrone, G. (2018). Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American diabetes association (ADA) and theEuropeanAssociation for the study of diabetes (easd). Diabetes Care 41, 2669–2701. doi:10.2337/dci18-0033
Davis, J., Singh, S., Sethi, S., Roy, S., Mittra, S., Rayasam, G., et al. (2010). Nature of action of sitagliptin, the dipeptidyl peptidase-IV inhibitor in diabetic animals. Indian J. Pharmacol. 42 (4), 229–233. doi:10.4103/0253-7613.68425
Deacon, C. F. (2011). Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: A comparative review. Diabetes Obes. Metab. 13, 7–18. doi:10.1111/j.1463-1326.2010.01306.x
Deacon, C. F., and Holst, J. J. (2010). Linagliptin, a xanthine-based dipeptidyl peptidase-4 inhibitor with an unusual profile for the treatment of type 2 diabetes. Expert Opin. Investig. Drugs 19 (1), 133–140. doi:10.1517/13543780903463862
Deacon, C. F., Nauck, M. A., Toft-Nielsen, M., Pridal, L., Willms, B., and Holst, J. J. (1995). Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes 44 (9), 1126–1131. doi:10.2337/diab.44.9.1126
Deacon, C. F. (2019). Physiology and pharmacology of DPP-4 in glucose homeostasis and the treatment of type 2 diabetes. Front. Endocrinol. (Lausanne) 10, 80–14. doi:10.3389/fendo.2019.00080
DeFronzo, R. A., Fleck, P. R., Wilson, C. A., and Mekki, Q. (2008a). Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes and inadequate glycemic control: A randomized, double-blind, placebo-controlled study. Diabetes Care 31 (12), 2315–2317. doi:10.2337/dc08-1035
DeFronzo, R. A., Hissa, M. N., Garber, A. J., Gross, J. L., Duan, R. Y., Ravichandran, S., et al. (2009). The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care 32 (9), 1649–1655. doi:10.2337/dc08-1984
DeFronzo, R. A., Okerson, T., Viswanathan, P., Guan, X., Holcombe, J. H., and MacConell, L. (2008b). Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: A randomized, cross-over study. Curr. Med. Res. Opin. 24 (10), 2943–2952. doi:10.1185/03007990802418851
Del Prato, S. (2007). Dipeptidyl peptidase 4 inhibition and vildagliptin therapy for type 2 diabetes. Int. J. Clin. Pract. 61 (154), 38–48. doi:10.1111/j.1742-1241.2007.01439.x
Deng, X., Shen, J., Zhu, H., Xiao, J., Sun, R., Xie, F., et al. (2018). Surrogating and redirection of pyrazolo [1, 5-a] pyrimidin-7 (4H)-one core, a novel class of potent and selective DPP-4 inhibitors. Bioorg Med. Chem. 26 (4), 903–912. doi:10.1016/j.bmc.2018.01.006
Derosa, G., Maffioli, P., Ferrari, I., Mereu, R., Ragonesi, P. D., Querci, F., et al. (2010b). Effects of one year treatment of vildagliptin added to pioglitazone or glimepiride in poorly controlled type 2 diabetic patients. Hormone Metabolic Res. 42 (9), 663–669. doi:10.1055/s-0030-1255036
Derosa, G., Maffioli, P., Salvadeo, S. A. T., Ferrari, I., Ragonesi, P. D., Querci, F., et al. (2010a). Effects of sitagliptin or metformin added to pioglitazone monotherapy in poorly controlled type 2 diabetes mellitus patients. Metabolism 59 (6), 887–895. doi:10.1016/j.metabol.2009.10.007
Dhillon, S. (2015). Saxagliptin: A review in type 2 diabetes. Drugs 75 (15), 1783–1796. doi:10.1007/s40265-015-0473-z
Dhillon, S. (2010). Sitagliptin: A review of its use in the management of type 2 diabetes mellitus. Drugs 70 (4), 489–512. doi:10.2165/11203790-000000000-00000
Dicker, D. (2011). DPP-4 Inhibitors: Impact on glycemic control and cardiovascular risk factors. Diabetes Care 34 (2), 276–278. doi:10.2337/dc11-s229
Drucker, D. J. (2007). Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes: Preclinical biology and mechanisms of action. Diabetes Care 30 (6), 1335–1343. doi:10.2337/dc07-0228
Drucker, D. J., and Nauck, M. A. (2006). The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368 (9548), 1696–1705. doi:10.1016/S0140-6736(06)69705-5
Drucker, D. J. (2006). The biology of incretin hormones. Cell Metab. 3, 153–165. doi:10.1016/j.cmet.2006.01.004
Egan, A. G., Blind, E., Dunder, K., de Graeff, P. A., Hummer, B. T., Bourcier, T., et al. (2014). Pancreatic safety of incretin-based drugs — FDA and EMA assessment. N. Engl. J. Med. 370 (9), 794–797. doi:10.1056/NEJMp1314078
Fadini, G. P., and Avogaro, A. (2011). Cardiovascular effects of DPP-4 inhibition: Beyond GLP-1. Vasc. Pharmacol. 55, 10–16. doi:10.1016/j.vph.2011.05.001
Fakhoury, W. K. H., Lereun, C., and Wright, D. (2010). A meta-analysis of placebo-controlled clinical trials assessing the efficacy and safety of incretin-based medications in patients with type 2 diabetes. Pharmacology 86 (1), 44–57. doi:10.1159/000314690
Farngren, J., Persson, M., Schweizer, A., Foley, J. E., and Ahreń, B. (2012). Vildagliptin reduces glucagon during hyperglycemia and sustains glucagon counterregulation during hypoglycemia in type 1 diabetes. J. Clin. Endocrinol. Metabolism 97 (10), 3799–3806. doi:10.1210/jc.2012-2332
Fasanya, O. (2021). Summary of product characteristics. Amsterdam, Netherlands: European Medicines Agency.
Fei, Y., Tsoi, M. F., Man, B., and Cheung, Y. (2019). Cardiovascular outcomes in trials of new antidiabetic drug classes: A network meta - analysis. Cardiovasc Diabetol. 18 (112), 112–113. doi:10.1186/s12933-019-0916-z
Fonseca, V., Schweizer, A., Albrecht, D., Baron, M. A., Chang, I., and Dejager, S. (2007). Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes. Diabetologia 50 (6), 1148–1155. doi:10.1007/s00125-007-0633-0
Food and Drug Administration (2009). Onglyza (saxagliptin): US prescribing information. United States: Food and Drug Administration.
Forst, T., Uhlig-Laske, B., Ring, A., Graefe-Mody, U., Friedrich, C., Herbach, K., et al. (2010). Linagliptin (BI 1356), a potent and selective DPP-4 inhibitor, is safe and efficacious in combination with metformin in patients with inadequately controlled Type 2 diabetes. Diabet. Med. 27 (12), 1409–1419. doi:10.1111/j.1464-5491.2010.03131.x
Frederich, R., Alexander, J. H., Fiedorek, F. T., Donovan, M., Berglind, N., Harris, S., et al. (2010). A systematic assessment of cardiovascular outcomes in the saxagliptin drug development program for type 2 diabetes. Postgrad. Med. 122 (3), 16–27. doi:10.3810/pgm.2010.05.2138
Friedrich, C., Port, A., Ring, A., Graefe-Mody, U., Giessmann, T., Iovino, M., et al. (2011). Effect of multiple oral doses of linagliptin on the steady-state pharmacokinetics of a combination oral contraceptive in healthy female adults: An open-label, two-period, fixed-sequence, multiple-dose study. Clin. Drug Investig. 31 (9), 643–653. doi:10.2165/11590240-000000000-00000
Fuchs, H., Binder, R., and Greischel, A. (2009). Tissue distribution of the novel DPP-4 inhibitor BI 1356 is dominated by saturable binding to its target in rats. Biopharm. Drug Dispos. 30, 229–240. doi:10.1002/bdd.662
Gallwitz, B. (2019). Clinical use of DPP-4 inhibitors. Front. Endocrinol. (Lausanne) 10, 1–10. doi:10.3389/fendo.2019.00389
Gallwitz, B. (2016). Novel therapeutic approaches in diabetes. Endocr. Dev. 31, 43–56. doi:10.1159/000439372
Gautier, J. F., Choukem, S. P., and Girard, J. (2008). Physiology of incretins (GIP and GLP-1) and abnormalities in type 2 diabetes. Diabetes Metab. 34 (2), 65–72. doi:10.1016/S1262-3636(08)73397-4
Gerich, J. (2010). DPP-4 inhibitors: What may be the clinical differentiators? Diabetes Res. Clin. Pract. 90 (2), 131–140. doi:10.1016/j.diabres.2010.07.006
Gilbert, M. P., and Pratley, R. E. (2020). GLP-1 analogs and DPP-4 inhibitors in type 2 diabetes therapy: Review of head-to-head clinical trials. Front. Endocrinol. (Lausanne) 11, 178–213. doi:10.3389/fendo.2020.00178
Giugliano, D., Maiorino, M. I., Bellastella, G., Chiodini, P., Ceriello, A., and Esposito, K. (2011). Efficacy of insulin analogs in achieving the hemoglobin A 1c target of <7% in type 2 diabetes: Meta-analysis of randomized controlled trials. Diabetes Care 34 (2), 510–517. doi:10.2337/dc10-1710
Göke, B., Hershon, K., Kerr, D., Calle Pascual, A., Schweizer, A., Foley, J., et al. (2008). Efficacy and safety of vildagliptin monotherapy during 2-year treatment of drug-naïve patients with type 2 diabetes: Comparison with metformin. Hormone Metabolic Res. 40 (12), 892–895. doi:10.1055/s-0028-1082334
Golay, A. (2008). Metformin and body weight. Int. J. Obes. 32 (1), 61–72. doi:10.1038/sj.ijo.0803695
Graefe-Mody, U., Rose, P., Ring, A., Zander, K., Iovino, M., and Woerle, H. J. (2011). Assessment of the pharmacokinetic interaction between the novel DPP-4 inhibitor linagliptin and a sulfonylurea, glyburide, in healthy subjects. Drug Metab. Pharmacokinet. 26 (2), 123–129. doi:10.2133/dmpk.dmpk-10-rg-091
Green, B. D., Flatt, P. R., and Bailey, C. J. (2006). Dipeptidyl peptidase IV (DPP IV) inhibitors: A newly emerging drug class for the treatment of type 2 diabetes. Diab Vasc. Dis. Res. 3 (3), 159–165. doi:10.3132/dvdr.2006.024
Grimshaw, C. E., Jennings, A., Kamran, R., Ueno, H., Nishigaki, N., Kosaka, T., et al. (2016). Trelagliptin (syr-472, zafatek), novel once-weekly treatment for type 2 diabetes, inhibits dipeptidyl peptidase-4 (dpp-4) via a non-covalent mechanism. PLoS One 11 (6), e0157509–e0157514. doi:10.1371/journal.pone.0157509
Grunberger, G. (2014). Clinical utility of dipeptidyl peptidase-4 inhibitors: A descriptive summary of current efficacy trials. Eur. J. Clin. Pharmacol. 70 (11), 1277–1289. doi:10.1007/s00228-014-1727-5
Gupta, V., and Kalra, S. (2011). Choosing a gliptin. Indian J. Endocrinol. Metab. 15 (4), 298–308. doi:10.4103/2230-8210.85583
Havale, S. H., and Pal, M. (2009). Medicinal chemistry approaches to the inhibition of dipeptidyl peptidase-4 for the treatment of type 2 diabetes. Bioorg Med. Chem. 17 (5), 1783–1802. doi:10.1016/j.bmc.2009.01.061
He, Y. L. (2012). Clinical pharmacokinetics and pharmacodynamics of vildagliptin. Clin. Pharmacokinet. 51 (3), 147–162. doi:10.2165/11598080-000000000-00000
He, Y. L., Sabo, R., Campestrini, J., Wang, Y., Ligueros-Saylan, M., Lasseter, K. C., et al. (2007b). The influence of hepatic impairment on the pharmacokinetics of the dipeptidyl peptidase IV (DPP-4) inhibitor vildagliptin. Eur. J. Clin. Pharmacol. 63 (7), 677–686. doi:10.1007/s00228-007-0312-6
He, Y. L., Sabo, R., Sunkara, G., Bizot, M. N., Riviere, G. J., Leon, S., et al. (2007a). Evaluation of pharmacokinetic interactions between vildagliptin and digoxin in healthy volunteers. J. Clin. Pharmacol. 47 (8), 998–1004. doi:10.1177/0091270007301802
Heissig, B., Hattori, K., Dias, S., Friedrich, M., Ferris, B., Hackett, N. R., et al. (2002). Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of Kit-ligand. Cell 109 (5), 625–637. doi:10.1016/s0092-8674(02)00754-7
Herman, D. W., Engel, S. S., Round, E., Johnson, J., Golm, G. T., Guo, H., et al. (2010). Research article safety and tolerability of sitagliptin in clinical studies: A pooled analysis of data from 10,246 patients with type 2 diabetes. BMC Endocr. Disord. 10 (7), 1–21.
Herman, G. A., Bergman, A., Stevens, C., Kotey, P., Yi, B., Zhao, P., et al. (2006a). Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J. Clin. Endocrinol. Metabolism 91 (11), 4612–4619. doi:10.1210/jc.2006-1009
Herman, G. A., Bergman, A., Yi, B., and Kipnes, M. (2006b). Tolerability and pharmacokinetics of metformin and the dipeptidyl peptidase-4 inhibitor sitagliptin when co-administered in patients with type 2 diabetes. Curr. Med. Res. Opin. 22 (10), 1939–1947. doi:10.1185/030079906X132587
Herman, G. A., Stevens, C., van Dyck, K., Bergman, A., Yi, B., de Smet, M., et al. (2005). Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: Results from two randomized, double-blind, placebo-controlled studies with single oral doses. Clin. Pharmacol. Ther. 78 (6), 675–688. doi:10.1016/j.clpt.2005.09.002
Hermansen, K., Kipnes, M., Luo, E., Fanurik, D., Khatami, H., Stein, P., et al. (2007). Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes. Metab. 9 (5), 733–745. doi:10.1111/j.1463-1326.2007.00744.x
Hirst, J. A., Farmer, A. J., Dyar, A., Lung, T. W. C., and Stevens, R. J. (2013). Estimating the effect of sulfonylurea on HbA1c in diabetes: A systematic review and meta-analysis. Diabetologia 56 (5), 973–984. doi:10.1007/s00125-013-2856-6
Hu, P., Yin, Q., Deckert, F., Jiang, J., Liu, D., Kjems, L., et al. (2009). Pharmacokinetics and pharmacodynamics of vildagliptin in healthy Chinese volunteers. J. Clin. Pharmacol. 49 (1), 39–49. doi:10.1177/0091270008325152
Hulin, B., Cabral, S., Lopaze, M. G., Van Volkenburg, M. A., Andrews, K. M., and Parker, J. C. (2005). New fluorinated pyrrolidine and azetidine amides as dipeptidyl peptidase IV inhibitors. Bioorg Med. Chem. Lett. 15 (21), 4770–4773. doi:10.1016/j.bmcl.2005.07.026
Hüttner, S., Graefe-Mody, E. U., Withopf, B., Ring, A., and Dugi, K. A. (2008). Safety, tolerability, pharmacokinetics, and pharmacodynamics of single oral doses of BI 1356, an inhibitor of dipeptidyl peptidase 4, in healthy male volunteers. J. Clin. Pharmacol. 48 (10), 1171–1178. doi:10.1177/0091270008323753
Inzucchi, S. E., Bergenstal, R. M., Buse, J. B., Diamant, M., Ferrannini, E., Nauck, M., et al. (2015). Management of hyperglycemia in type 2 diabetes, 2015: A patient-centered approach: Update to a position statement of the American diabetes association and the European association for the study of diabetes. Diabetes Care 38 (1), 140–149. doi:10.2337/dc14-2441
Iqbal, N., Parker, A., Frederich, R., Donovan, M., and Hirshberg, B. (2014). Assessment of the cardiovascular safety of saxagliptin in patients with type 2 diabetes mellitus: Pooled analysis of 20 clinical trials. Cardiovasc Diabetol. 13, 33–39. doi:10.1186/1475-2840-13-33
Jadzinsky, M., Pfützner, A., Paz-Pacheco, E., Xu, Z., Allen, E., Chen, R., et al. (2009). Saxagliptin given in combination with metformin as initial therapy improves glycaemic control in patients with type 2 diabetes compared with either monotherapy: A randomized controlled trial. Diabetes Obes. Metab. 11 (6), 611–622. doi:10.1111/j.1463-1326.2009.01056.x
Johansen, O. E., Neubacher, D., von Eynatten, M., Patel, S., and Woerle, H. J. (2012). Cardiovascular safety with linagliptin in patients with type 2 diabetes mellitus: A pre-specified, prospective, and adjudicated meta-analysis of a phase 3 programme. Cardiovasc Diabetol. 11 (3), 3–10. doi:10.1186/1475-2840-11-3
Jose, T., and Inzucchi, S. E. (2012). Cardiovascular effects of the DPP-4 inhibitors. Diab Vasc. Dis. Res. 9 (2), 109–116. doi:10.1177/1479164111436236
Karim, A., Laurent, A., Munsaka, M., Wann, E., Fleck, P., and Mekki, Q. (2009). Coadministration of pioglitazone or glyburide and alogliptin: Pharmacokinetic drug interaction assessment in healthy participants. J. Clin. Pharmacol. 49 (10), 1210–1219. doi:10.1177/0091270009338938
Karonen, I. (2007). Action of GLP-1 and DPP-4 inhibitors. Available at: https://commons.wikimedia.org/wiki/File:Incretins_and_DPP_4_inhibitors.svg.
Karyekar, C., Donovan, M., Allen, E., Fleming, D., Ravichandran, S., and Chen, R. (2011). Efficacy and safety of saxagliptin combination therapy in US patients with type 2 diabetes. Postgrad. Med. 123 (4), 63–70. doi:10.3810/pgm.2011.07.2305
Kim, S. H., Yoo, J. H., Lee, W. J., and Park, C. Y. (2016). Gemigliptin: An update of its clinical use in the management of type 2 diabetes mellitus. Diabetes Metab. J. 40 (5), 339–353. doi:10.4093/dmj.2016.40.5.339
Kim, Y. B., Kopcho, L. M., Kirby, M. S., Hamann, L. G., Weigelt, C. A., Metzler, W. J., et al. (2006). Mechanism of Gly-Pro-pNA cleavage catalyzed by dipeptidyl peptidase-IV and its inhibition by saxagliptin (BMS-477118). Arch. Biochem. Biophys. 445 (1), 9–18. doi:10.1016/j.abb.2005.11.010
Krentz, A. J., Patel, M. B., and Bailey, C. J. (2008). New drugs for type 2 diabetes mellitus. Drugs 68 (15), 2131–2162. doi:10.2165/00003495-200868150-00005
Kumar, S., Mittal, A., and Mittal, A. (2021). A review upon medicinal perspective and designing rationale of DPP-4 inhibitors. Bioorg Med. Chem. 46, 116354. doi:10.1016/j.bmc.2021.116354
Lambeir, A. M., Durinx, C., Scharpé, S., and De Meester, I. (2003). Dipeptidyl-peptidase IV from bench to bedside: An update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit. Rev. Clin. Lab. Sci. 40 (3), 209–294. doi:10.1080/713609354
Ligueros-Saylan, M., Foley, J. E., Schweizer, A., Couturier, A., and Kothny, W. (2010). An assessment of adverse effects of vildagliptin versus comparators on the liver, the pancreas, the immune system, the skin and in patients with impaired renal function from a large pooled database of Phase II and III clinical trials. Diabetes Obes. Metab. 12, 495–509. doi:10.1111/j.1463-1326.2010.01214.x
Lim, K. S., Cho, J. Y., Kim, B. H., Kim, J. R., Kim, H. S., Kim, D. K., et al. (2009). Pharmacokinetics and pharmacodynamics of LC15-0444, a novel dipeptidyl peptidase IV inhibitor, after multiple dosing in healthy volunteers. Br. J. Clin. Pharmacol. 68 (6), 883–890. doi:10.1111/j.1365-2125.2009.03376.x
Lozano-Ortega, G., Goring, S., Bennett, H. A., Bergenheim, K., Sternhufvud, C., and Mukherjee, J. (2016). Network meta-analysis of treatments for type 2 diabetes mellitus following failure with metformin plus sulfonylurea. Curr. Med. Res. Opin. 32 (5), 807–816. doi:10.1185/03007995.2015.1135110
Lyseng-williamson, K. A. (2007). Sitagliptin. Drugs. 67 (4), 587–597. doi:10.2165/00003495-200767040-00007
Makrilakis, K. (2019). The role of dpp-4 inhibitors in the treatment algorithm of type 2 diabetes mellitus: When to select, what to expect. Int. J. Environ. Res. Public Health 16 (15), 2720. doi:10.3390/ijerph16152720
Mannucci, E., Mosenzon, O., and Avogaro, A. (2016). Analyses of results from cardiovascular safety trials with DPP-4 inhibitors: Cardiovascular outcomes, predefined safety outcomes, and pooled analysis and meta-analysis. Diabetes c. 39, 196–204. doi:10.2337/dcS15-3024
Marfella, R., Barbieri, M., Grella, R., Rizzo, M. R., Nicoletti, G. F., and Paolisso, G. (2010). Effects of vildagliptin twice daily vs. sitagliptin once daily on 24-hour acute glucose fluctuations. J. Diabetes Complicat. 24 (2), 79–83. doi:10.1016/j.jdiacomp.2009.01.004
Mari, A., Sallas, W. M., He, Y. L., Watson, C., Ligueros-Saylan, M., Dunning, B. E., et al. (2005). Vildagliptin, a dipeptidyl peptidase-IV inhibitor, improves model-assessed β-cell function in patients with type 2 diabetes. J. Clin. Endocrinol. Metabolism 90 (8), 4888–4894. doi:10.1210/jc.2004-2460
Mari, A., Scherbaum, W. A., Nilsson, P. M., Lalanne, G., Schweizer, A., Dunning, B. E., et al. (2008). Characterization of the influence of vildagliptin on model-assessed β-cell function in patients with type 2 diabetes and mild hyperglycemia. J. Clin. Endocrinol. Metabolism 93 (1), 103–109. doi:10.1210/jc.2007-1639
Marney, A., Kunchakarra, S., Byrne, L., and Brown, N. J. (2010). Interactive hemodynamic effects of dipeptidyl peptidase-IV inhibition and angiotensin-converting enzyme inhibition in humans. Hypertension 56 (4), 728–733. doi:10.1161/HYPERTENSIONAHA.110.156554
Massimino, E., Izzo, A., Riccardi, G., and Pepa, G. D. (2021). The impact of glucose-lowering drugs on sarcopenia in type 2 diabetes: Current evidence and underlying mechanisms. Cells 10, 1958. doi:10.3390/cells10081958
Matikainen, N., Mänttäri, S., Schweizer, A., Ulvestad, A., Mills, D., Dunning, B. E., et al. (2006). Vildagliptin therapy reduces postprandial intestinal triglyceride-rich lipoprotein particles in patients with type 2 diabetes. Diabetologia 49 (9), 2049–2057. doi:10.1007/s00125-006-0340-2
Matsui, T., Nishino, Y., Takeuchi, M., and Yamagishi, S. I. (2011). Vildagliptin blocks vascular injury in thoracic aorta of diabetic rats by suppressing advanced glycation end product-receptor axis. Pharmacol. Res. 63 (5), 383–388. doi:10.1016/j.phrs.2011.02.003
Matthews, D. R., Paldánius, P. M., Proot, P., Chiang, Y. T., Stumvoll, M., del Prato, S., et al. (2019). Glycaemic durability of an early combination therapy with vildagliptin and metformin versus sequential metformin monotherapy in newly diagnosed type 2 diabetes (VERIFY): A 5-year, multicentre, randomised, double-blind trial. Lancet 394 (10208), 1519–1529. doi:10.1016/S0140-6736(19)32131-2
Meduru, H., Wang, Y. T., Tsai, J. J. P., and Chen, Y. C. (2016). Finding a potential dipeptidyl peptidase-4 (DPP-4) inhibitor for type-2 diabetes treatment based on molecular docking, pharmacophore generation, and molecular dynamics simulation. Int. J. Mol. Sci. 17 (6), 920. doi:10.3390/ijms17060920
Mentlein, R., Gallwitz, B., and Schmidt, W. E. (1993). Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7–36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur. J. Biochem. 214 (3), 829–835. doi:10.1111/j.1432-1033.1993.tb17986.x
Metzler, W. J., Yanchunas, J., Weigelt, C., Kish, K., Klei, H. E., Xie, D., et al. (2008). Involvement of DPP-IV catalytic residues in enzyme–saxagliptin complex formation. Protein Sci. 17 (2), 240–250. doi:10.1110/ps.073253208
Mikhail, N. (2008). Combination therapy with DPP-4 inhibitors and pioglitazone in type 2 diabetes: Theoretical consideration and therapeutic potential. Vasc. Health Risk Manag. 4 (6), 1221–1227. doi:10.2147/vhrm.s3374
Mistry, G. C., Maes, A. L., Lasseter, K. C., Davies, M. J., Gottesdiener, K. M., Wagner, J. A., et al. (2008). Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on blood pressure in nondiabetic patients with mild to moderate hypertension. J. Clin. Pharmacol. 48 (5), 592–598. doi:10.1177/0091270008316885
Monami, M., Dicembrini, I., and Mannucci, E. (2014). Dipeptidyl peptidase-4 inhibitors and pancreatitis risk: A meta-analysis of randomized clinical trials. Diabetes Obes. Metab. 16 (1), 48–56. doi:10.1111/dom.12176
Monami, M., Vitale, V., Ambrosio, M. L., Bartoli, N., Toffanello, G., Ragghianti, B., et al. (2012). Effects on lipid profile of dipeptidyl peptidase 4 inhibitors, pioglitazone, acarbose, and sulfonylureas: Meta-analysis of placebo-controlled trials. Adv. Ther. 29 (9), 736–746. doi:10.1007/s12325-012-0045-5
Nasr, N. E., and Sadek, K. M. (2022). Role and mechanism(s) of incretin-dependent therapies for treating diabetes mellitus. Environ. Sci. Pollut. Res. 29, 18408–18422. doi:10.1007/s11356-022-18534-2
Nauck, M. A., Ellis, G. C., Fleck, P. R., Wilson, C. A., and Mekki, Q. (2009). Efficacy and safety of adding the dipeptidyl peptidase-4 inhibitor alogliptin to metformin therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: A multicentre, randomised, double-blind, placebo-controlled study. Int. J. Clin. Pract. 63 (1), 46–55. doi:10.1111/j.1742-1241.2008.01933.x
Nauck, M. A., Heimesaat, M. M., 0rskov, C., Holst, J. J., Ebert, R., and Creutzfeldt, W. (1993). Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J. Clin. Invest. 91, 301–307. doi:10.1172/JCI116186
Neumiller, J. J. (2009). Differential chemistry (structure), mechanism of action, and pharmacology of GLP-1 receptor agonists and DPP-4 inhibitors. J. Am. Pharm. Assoc. 49 (5), S16–S29. doi:10.1331/JAPhA.2009.09078
Neumiller, J. J. (2012). Pharmacology, efficacy, and safety of linagliptin for the treatment of type 2 diabetes mellitus. Ann. Pharmacother. 46 (3), 358–367. doi:10.1345/aph.1Q522
Neumiller, J. J., Wood, L., and Campbell, R. K. (2010). Dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes mellitus. Pharmacotherapy 30 (5), 463–484. doi:10.1592/phco.30.5.463
Nicolaus, M., Brödl, J., Linke, R., Woerle, H. J., Göke, B., and Schirra, J. (2011). Endogenous GLP-1 regulates postprandial glycemia in humans: Relative contributions of insulin, glucagon, and gastric emptying. J. Clin. Endocrinol. Metabolism 96 (1), 229–236. doi:10.1210/jc.2010-0841
Nishio, S., Abe, M., and Ito, H. (2015). Anagliptin in the treatment of type 2 diabetes: Safety, efficacy, and patient acceptability. Diabetes Metab. Syndr. Obes. 8, 163–171. doi:10.2147/DMSO.S54679
Novartis (2012). Summary of product characteristics. Amsterdam, Netherlands: European Medicines Agency, 183–226.
O’Hara, D. v., Parkhill, T. R., Badve sunil, v., Jun, M., Jardine, M. J., and Perkovic, V. (2021). The effects of dipeptidyl peptidase-4 inhibitors on kidney outcomes. Diabetes Obes. Metab. 23 (3), 763–773. doi:10.1111/dom.14281
OPDIVO (2021). Opdivo (nivolumab) injection, for intravenous use. New York, United States: Bristol-Myers Squibb, 1–43.
Owens, D. R., Swallow, R., Dugi, K. A., and Woerle, H. J. (2011). Efficacy and safety of linagliptin in persons with Type2 diabetes inadequately controlled by a combination of metformin and sulphonylurea: A 24-week randomized study. Diabet. Med. 28 (11), 1352–1361. doi:10.1111/j.1464-5491.2011.03387.x
Packer, M. (2018). Do DPP-4 inhibitors cause heart failure events by promoting adrenergically mediated cardiotoxicity. Circ. Res. 122, 928–932. doi:10.1161/CIRCRESAHA.118.312673
Palalau, A. I., Tahrani, A. A., Piya, M. K., and Barnett, A. H. (2009). DPP-4 inhibitors in clinical practice. Postgrad. Med. 121 (6), 70–100. doi:10.3810/pgm.2009.11.2079
Palmer, S. C., Mavridis, D., Nicolucci, A., Johnson, D. W., Tonelli, M., Craig, J. C., et al. (2016). Comparison of clinical outcomes and adverse events associated with glucose-lowering drugs in patients with type 2 diabetes a meta-analysis. JAMA - J. Am. Med. Assoc. 316 (3), 313–324. doi:10.1001/jama.2016.9400
Parker, H. E., Reimann, F., and Gribble, F. M. (2010). Molecular mechanisms underlying nutrient-stimulated incretin secretion. Expert Rev. Mol. Med. 12, 1–17. doi:10.1017/S146239940900132X
Perley, M. J., and Kipnis, D. M. (1967). Plasma insulin responses to oral and intravenous glucose: Studies in normal and diabetic sujbjects. J. Clin. Invest. 46 (12), 1954–1962. doi:10.1172/JCI105685
Phung, O. J., Scholle, J. M., Talwar, M., and Coleman, C. I. (2010). Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. J. Am. Med. Assoc. 303 (14), 1410–1418. doi:10.1001/jama.2010.405
Pi-Sunyer, F. X., Schweizer, A., Mills, D., and Dejager, S. (2007). Efficacy and tolerability of vildagliptin monotherapy in drug-naïve patients with type 2 diabetes. Diabetes Res. Clin. Pract. 76 (1), 132–138. doi:10.1016/j.diabres.2006.12.009
Pinto, L. C., Rados, D. v., Barkan, S. S., Leitão, C. B., and Gross, J. L. (2018). Dipeptidyl peptidase-4 inhibitors, pancreatic cancer and acute pancreatitis: A meta-analysis with trial sequential analysis. Sci. Rep. 8, 782–811. doi:10.1038/s41598-017-19055-6
Pozo, L., Bello, F., Suarez, A., Ochoa-Martinez, F. E., Mendez, Y., Chang, C. H., et al. (2019). Novel pharmacological therapy in type 2 diabetes mellitus with established cardiovascular disease: Current evidence. World J. Diabetes 10 (5), 291–303. doi:10.4239/wjd.v10.i5.291
Read, P. A., Khan, F. Z., Heck, P. M., Hoole, S. P., and Dutka, D. P. (2010). DPP-4 inhibition by sitagliptin improves the myocardial response to dobutamine stress and mitigates stunning in a pilot study of patients with coronary artery disease. Circ. Cardiovasc Imaging 3 (2), 195–201. doi:10.1161/CIRCIMAGING.109.899377
Rendell, M., Drincic, A., and Andukuri, R. (2012). Alogliptin benzoate for the treatment of type 2 diabetes. Expert Opin. Pharmacother. 13 (4), 553–563. doi:10.1517/14656566.2012.656088
Reynolds, J. K., Neumiller, J. J., and Campbell, R. K. (2008). Janumet: A combination product suitable for use in patients with type 2 diabetes. Expert Opin. Investig. Drugs 17 (10), 1559–1565. doi:10.1517/13543784.17.10.1559
Richter, B., Bandeira-Echtler, E., Bergerhoff, K., and Lerch, C. (2008a). Emerging role of dipeptidyl peptidase-4 inhibitors in the management of type 2 diabetes. Vasc. Health Risk Manag. 4 (4), 753–768. doi:10.2147/vhrm.s1707
Richter, B., Bergerhoff, K., and Lerch, C. (2008b). Dipeptidyl peptidase-4 (DPP-4) inhibitors for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2008 (2), CD006739. doi:10.1002/14651858.CD006739.pub2
Richter, B., Bergerhoff, K., and Lerch, C. (2009). Dipeptidyl peptidase-4 (DPP-4) inhibitors for type 2 diabetes mellitus (Review). Cochrane Database Syst. Rev. 4 (3), 1–76.
Ristic, S., Byiers, S., Foley, J., and Holmes, D. (2005). Improved glycaemic control with dipeptidyl peptidase-4 inhibition in patients with type 2 diabetes: Vildagliptin (LAF237) dose response. Diabetes Obes. Metab. 7 (6), 692–698. doi:10.1111/j.1463-1326.2005.00539.x
Rizzo, M. R., Barbieri, M., Fava, I., Desiderio, M., Coppola, C., Marfella, R., et al. (2016). Sarcopenia in elderly diabetic patients: Role of dipeptidyl peptidase 4 inhibitors. J. Am. Med. Dir. Assoc. 17 (10), 896–901. doi:10.1016/j.jamda.2016.04.016
Rodbard, H. W., Jellinger, P. S., Davidson, J. A., Einhorn, D., Garber, A. J., Grunberger, G., et al. (2009). Statement by an American association of clinical endocrinologists/American college of endocrinology consensus panel on type 2 diabetes mellitus: An algorithm for glycemic control. Endocr. Pract. 15 (6), 540–559. doi:10.4158/EP.15.6.540
Röhrborn, D., Wronkowitz, N., and Eckel, J. (2015). DPP4 in diabetes. Front. Immunol. 6, 386. doi:10.3389/fimmu.2015.00386
Rosenstock, J., Aguilar-Salinas, C., Klein, E., Nepal, S., List, J., Chen, R., et al. (2009b). Effect of saxagliptin monotherapy in treatment-naïve patients with type 2 diabetes. Curr. Med. Res. Opin. 25 (10), 2401–2411. doi:10.1185/03007990903178735
Rosenstock, J., Baron, M. A., Camisasca, R. P., Cressier, F., Couturier, A., Dejager, S., et al. (2007). Efficacy and tolerability of initial combination therapy with vildagliptin and pioglitazone compared with component monotherapy in patients with type 2 diabetes. Diabetes Obes. Metab. 9 (2), 175–185. doi:10.1111/j.1463-1326.2006.00698.x
Rosenstock, J., Rendell, M. S., Gross, J. L., Fleck, P. R., Wilson, C. A., and Mekki, Q. (2009a). Alogliptin added to insulin therapy in patients with type 2 diabetes reduces HbA1c without causing weight gain or increased hypoglycaemia. Diabetes Obes. Metab. 11 (12), 1145–1152. doi:10.1111/j.1463-1326.2009.01124.x
Satoh-Asahara, N., Sasaki, Y., Wada, H., Tochiya, M., Iguchi, A., Nakagawachi, R., et al. (2013). A dipeptidyl peptidase-4 inhibitor, sitagliptin, exerts anti-inflammatory effects in type 2 diabetic patients. Metabolism 62 (3), 347–351. doi:10.1016/j.metabol.2012.09.004
Scheen, A. J. (2012a). A review of gliptins in 2011. Expert Opin. Pharmacother. 13 (1), 81–99. doi:10.1517/14656566.2012.642866
Scheen, A. J. (2010a). Dipeptidylpeptidase-4 inhibitors (gliptins) focus on drug-drug interactions. Clin. Pharmacokinet. 49 (9), 573–588. doi:10.2165/11532980-000000000-00000
Scheen, A. J. (2012c). DPP-4 inhibitors in the management of type 2 diabetes: A critical review of head-to-head trials. Diabetes Metab. 38 (2), 89–101. doi:10.1016/j.diabet.2011.11.001
Scheen, A. J. (2012b). Pharmacokinetic evaluation of atorvastatin and sitagliptin in combination for the treatment of type 2 diabetes. Expert Opin. Drug Metab. Toxicol. 8 (6), 745–758. doi:10.1517/17425255.2012.686603
Scheen, A. J. (2010b). Pharmacokinetics of dipeptidylpeptidase-4 inhibitors. Diabetes Obes. Metab. 12 (8), 648–658. doi:10.1111/j.1463-1326.2010.01212.x
Scheen, A. J., Tan, M. H., Betteridge, D. J., Birkeland, K., Schmitz, O., Charbonnel, B., et al. (2009). Long-term glycaemic control with metformin-sulphonylurea-pioglitazone triple therapy in PROactive (PROactive 17). Diabet. Med. 26 (10), 1033–1039. doi:10.1111/j.1464-5491.2009.02816.x
Scherbaum, W. A., Schweizer, A., Mari, A., Nilsson, P. M., Lalanne, G., Wang, Y., et al. (2008). Evidence that vildagliptin attenuates deterioration of glycaemic control during 2-year treatment of patients with type 2 diabetes and mild hyperglycaemia. Diabetes Obes. Metab. 10 (11), 1114–1124. doi:10.1111/j.1463-1326.2008.00875.x
Schürmann, C., Linke, A., Engelmann-Pilger, K., Steinmetz, C., Mark, M., Pfeilschifter, J., et al. (2012). The dipeptidyl peptidase-4 inhibitor linagliptin attenuates inflammation and accelerates epithelialization in wounds of diabetic ob/ob mice. J. Pharmacol. Exp. Ther. 342 (1), 71–80. doi:10.1124/jpet.111.191098
Schwarz, B., Gouveia, M., Chen, J., Nocea, G., Jameson, K., Cook, J., et al. (2008). Cost-effectiveness of sitagliptin-based treatment regimens in European patients with type 2 diabetes and haemoglobin A1c above target on metformin monotherapy. Diabetes Obes. Metab. 10 (1), 43–55. doi:10.1111/j.1463-1326.2008.00886.x
Scirica, B. M., Bhatt, D. L., Braunwald, E., Steg, P. G., Davidson, J., Hirshberg, B., et al. (2013). Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med. 369 (14), 1317–1326. doi:10.1056/NEJMoa1307684
Sesti, G., Avogaro, A., Belcastro, S., Bonora, B. M., Croci, M., Daniele, G., et al. (2019). Ten years of experience with DPP-4 inhibitors for the treatment of type 2 diabetes mellitus. Acta Diabetol. 56 (6), 605–617. doi:10.1007/s00592-018-1271-3
Shah, Z., Kampfrath, T., Deiuliis, J. A., Zhong, J., Pineda, C., Ying, Z., et al. (2011a). Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation 124 (21), 2338–2349. doi:10.1161/CIRCULATIONAHA.111.041418
Shah, Z., Pineda, C., Kampfrath, T., Maiseyeu, A., Ying, Z., Racoma, I., et al. (2011b). Acute DPP-4 inhibition modulates vascular tone through GLP-1 independent pathways. Vasc. Pharmacol. 55, 2–9. doi:10.1016/j.vph.2011.03.001
Sharma, S., and Bhatia, V. (2021). Drug design of GLP-1 receptor agonists: Importance of in silico methods. Curr. Pharm. Des. 27, 1015–1024. doi:10.2174/1381612826666201118094502
Sharma, S., and Bhatia, V. (2020). Treatment of type 2 diabetes mellitus (T2DM): Can GLP-1 receptor agonists fill in the gaps? Chem. Biol. Lett. 7 (4), 215–224.
Shiheido-Watanabe, Y., Maejima, Y., Kasama, T., Tamura, N., Nakagama, S., Ito, Y., et al. (2021). Linagliptin, A xanthine-based dipeptidyl peptidase-4 inhibitor, ameliorates experimental autoimmune myocarditis. JACC Basic Transl. Sci. 6 (6), 527–542. doi:10.1016/j.jacbts.2021.04.006
Shirakawa, J., Fujii, H., Ohnuma, K., Sato, K., Ito, Y., Kaji, M., et al. (2011). Diet-induced adipose tissue inflammation and liver steatosis are prevented by DPP-4 inhibition in diabetic mice. Diabetes 60 (4), 1246–1257. doi:10.2337/db10-1338
Storgaard, H., Gluud, L. L., Bennett, C., Grøndahl, M. F., Christensen, M. B., Knop, F. K., et al. (2016). Benefits and harms of sodium-glucose co-transporter 2 inhibitors in patients with type 2 diabetes: A systematic review and meta-analysis. PLoS One 11 (11), 01661255–e166223. doi:10.1371/journal.pone.0166125
Tahrani, A. A., Piya, M. K., and Barnett, A. H. (2009b). Drug evaluation: Vildagliptin-metformin single-tablet combination. Adv. Ther. 26 (2), 138–154. doi:10.1007/s12325-009-0010-0
Tahrani, A. A., Piya, M. K., and Barnett, A. H. (2009a). Saxagliptin: A new DPP-4 inhibitor for the treatment of type 2 diabetes mellitus. Adv. Ther. 26 (3), 249–262. doi:10.1007/s12325-009-0014-9
Tan, X., and Hu, J. (2016). Evogliptin: A new dipeptidyl peptidase inhibitor for the treatment of type 2 diabetes. Expert Opin. Pharmacother. 17 (9), 1285–1293. doi:10.1080/14656566.2016.1183645
Taylor, O. M., and Lam, C. (2020). The effect of dipeptidyl peptidase-4 inhibitors on macrovascular and microvascular complications of diabetes mellitus: A systematic review. Curr. Ther. Res. - Clin. Exp. 93, 100596. doi:10.1016/j.curtheres.2020.100596
Tella, S. H., and Rendell, M. S. (2015). DPP-4 inhibitors: Focus on safety. Expert Opin. Drug Saf. 14 (1), 127–140. doi:10.1517/14740338.2015.977863
Thornberry, N., and Weber, A. E. (2007). Discovery of JANUVIATM (sitagliptin), a selective dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. Curr. Top. Med. Chem. 7, 557–568. doi:10.2174/156802607780091028
Tomovic, K., Lazarevic, J., Kocic, G., Deljanin-Ilic, M., Anderluh, M., and Smelcerovic, A. (2018). Mechanisms and pathways of anti-inflammatory activity of DPP-4 inhibitors in cardiovascular and renal protection. Med. Res. Rev. 1–19, 404–422. doi:10.1002/med.21513
Trujillo, J. M., Nuffer, W., and Ellis, S. L. (2015). GLP-1 receptor agonists: A review of head-to-head clinical studies. Ther. Adv. Endocrinol. Metab. 6 (1), 19–28. doi:10.1177/2042018814559725
Vardarli, I., Arndt, E., Deacon, C. F., Holst, J. J., and Nauck, M. A. (2014). Effects of sitagliptin and metformin treatment on incretin hormone and insulin secretory responses to oral and isoglycemic intravenous glucose. Diabetes 63 (2), 663–674. doi:10.2337/db13-0805
Villhauer, E. B., Brinkman, J. A., Naderi, G. B., Burkey, B. F., Dunning, B. E., Prasad, K., et al. (2003). 1-[[(3-Hydroxy-1-adamantyl)amino]acetyl]-2-cyano-(S)-pyrrolidine: A potent, selective, and orally bioavailable dipeptidyl peptidase IV inhibitor with antihyperglycemic properties. J. Med. Chem. 46 (13), 2774–2789. doi:10.1021/jm030091l
Vilsbøll, T., Krarup, T., Deacon, C. F., Madsbad, S., and Holst, J. J. (2001). Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes 50 (3), 609–613. doi:10.2337/diabetes.50.3.609
Waugh, N., Cummins, E., Royle, P., Clar, C., Marien, M., Richter, B., et al. (2010). Newer agents for blood glucose control in type 2 diabetes: Systematic review and economic evaluation. Health Technol. Assess. (Rockv) 14 (36), 1–248. doi:10.3310/hta14360
Weir, D. L., McAlister, F. A., Senthilselvan, A., Minhas-Sandhu, J. K., and Eurich, D. T. (2014). Sitagliptin use in patients with diabetes and heart failure: A population-based retrospective cohort study. J. Am. Coll. Cardiol. Heart Fail. 2 (6), 573–582. doi:10.1016/j.jchf.2014.04.005
Wright, D. H., Herman, G. A., Maes, A., Liu, Q., Johnson-Levonas, A. O., and Wagner, J. A. (2009). Multiple doses of sitagliptin, a selective DPP-4 inhibitor, do not meaningfully alter pharmacokinetics and pharmacodynamics of warfarin. J. Clin. Pharmacol. 49 (10), 1157–1167. doi:10.1177/0091270009341653
Yazbeck, R., Howarth, G. S., and Abbott, C. A. (2009). Dipeptidyl peptidase inhibitors, an emerging drug class for inflammatory disease? Trends Pharmacol. Sci. 30 (11), 600–607. doi:10.1016/j.tips.2009.08.003
Yoshida, T., Akahoshi, F., Sakashita, H., Kitajima, H., Nakamura, M., Sonda, S., et al. (2012). Discovery and preclinical profile of teneligliptin (3-[(2S,4S)-4-[4-(3- methyl-1-phenyl-1H-pyrazol-5-yl)piperazin-1-yl]pyrrolidin-2-ylcarbonyl] thiazolidine): A highly potent, selective, long-lasting and orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. Bioorg Med. Chem. 20 (19), 5705–5719. doi:10.1016/j.bmc.2012.08.012
Zerilli, T., and Pyon, E. Y. (2007). Sitagliptin phosphate: A DPP-4 inhibitor for the treatment of type 2 diabetes mellitus. Clin. Ther. 29 (12), 2614–2634. doi:10.1016/j.clinthera.2007.12.034
Zhang, J., Chen, Q., Zhong, J., Liu, C., Zheng, B., Hamilton, R. M., et al. (2019). DPP-4 inhibitors as potential candidates for antihypertensive therapy: Improving vascular inflammation and assisting the action of traditional antihypertensive drugs. Front. Immunol. 10, 1050–1112. doi:10.3389/fimmu.2019.01050
Keywords: T2DM, incretin effect, DPP-4 enzyme, DPP-4 inhibitors, (GLP)-1, insulin
Citation: Saini K, Sharma S and Khan Y (2023) DPP-4 inhibitors for treating T2DM - hype or hope? an analysis based on the current literature. Front. Mol. Biosci. 10:1130625. doi: 10.3389/fmolb.2023.1130625
Received: 28 December 2022; Accepted: 08 May 2023;
Published: 23 May 2023.
Edited by:
Hem Chandra Jha, Indian Institute of Technology Indore, IndiaReviewed by:
Finbarr P. M. O'Harte, Ulster University, United KingdomJobichen Chacko, Australian National University, Australia
Sapana Kushwaha, National Institute of Pharmaceutical Education and Research, India
Copyright © 2023 Saini, Sharma and Khan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Smriti Sharma, c21yaXRpLmNoZW1pc3RyeUBnbWFpbC5jb20=
 Kunika Saini
Kunika Saini Smriti Sharma
Smriti Sharma