- 1Division of Medical Biology, Jan Kochanowski University in Kielce, Kielce, Poland
- 2Faculty of Technology and Bionics, Rhine-Waal University of Applied Science, Kleve, Germany
- 3Department of Basic Medical Sciences, Faculty of Medical Sciences and Health Sciences, Casimir Pulaski University of Radom, Radom, Poland
- 4EA 6295 Nanomédicaments et Nanosondes, Faculté de pharmacie, Université de Tours, Tours, France
Background: Electrospun nanofibers, which are becoming increasingly popular in biomedicine, can directly or indirectly affect the properties and formation of the edothelial layer. This effect can be both toxic and pro-stimulatory. Therefore, in this study, electrospun nanofibers PLCL/PVP composed of biodegradable and biocompatible L-lactide-block-ϵ-caprolactone copolymer (PLCL, 70:30) blended with polyvinylpyrrolidone (PVP), containing in situ synthesized PVP different types of nanoparticles - gold (AuNPs), cadmium selenide (CdSe QDs) or tantalum (TaNPs), were investigated. Understanding how different modifications of nanofibers can affect the formation of the endothelial layer is crucial to using them as tools in tissue regeneration.
Methods: electrospun nanofibers with gold (AuNPs), cadmium selenide (CdSe QDs) or tantalum (TaNPs), were synthesized and physico-chemical characteristic were caried out. Cytotoxicity and prostimulatory effect of nanofibers on Primary Human Umbilical Vein Endothelial Cells were tested by microscopic and spectrofluorescence techniques.
Results: The endothelial layer forms to 75% confluence (after 24 h) and reaches 100% after 72 h when no nanofibers are present. A slower formation of the endothelial layer is seen in the presence of PLCL/PVP nanofibers (60%) and (80%) after 72 h. The introduction of various nanoparticles into the nanofibers caused changes in the morphology and rate of endothelial layer formation. In the presence of nanofibers modified with AuNPs after 72 h it reached only 40%. A similar effect was obtained for PLCL/PVP-CdSe QDs. In the case of PLCL/PVP-TaNPs, after 48 h 90% and after 72 h 100%. The tested nanofibers did not show toxic behavior towards the formed HUVEC cell monolayer. All of the tested nanofibers, except PLCL/PVP-TaNPs, induced increased HUVEC cells layer permeability, which resulted in increased translocation of fluorescently labeled dextran from 20% to 50%.
Conclusion: It was estimated that the effect of nanofibers on the formation of the endothelial layer can be direct, where cells contact the nanofibers and thus the growth of the endothelium is hindered. Additionally, the uptake of biological fluid components can have an indirect effect on endothelial cells, their adhesion and growth. Among the tested nanofibers, non-toxic PLCL/PVP-TaNPs seem to be particularly promising due to safety issues and the possibility of using them as effective scaffolds.
1 Introduction
Since Nanotechnology has become more popular in many fields of science, especially in biology and medicine, the market is flooded with more and more medical products based on nanomaterials. In 2022 alone, according to the European Observatory for Nanomaterials (EUON), 2,200 nanomaterials-based products were already available on the European market (Study of the EU market for nanomaterials, including substances, uses, volumes and key operators; https://euon.echa.europa.eu/reports). In order to develop effective nanotools for use in anticancer therapies (Shi et al., 2017; van der Meel et al., 2019; Wang et al., 2024), antibacterial (Gold et al., 2018; Saravanan et al., 2023; Skrzyniarz et al., 2023) and diagnostics (Tang et al., 2024; Wang et al., 2024) research is being conducted on nanomaterials and their modifications. Despite numerous reports, knowledge about the toxicity of nanocompounds and their impact on the human body is still limited. Nanomaterials interact with body’s cells and tissues, which can lead to various, often uncontrolled reactions, inducing damage or inflammation of the organism (Hirai et al., 2016; Egbuna et al., 2021; Wu et al., 2021; Kuc-Ciepluch et al., 2022; El-Kady et al., 2023; Märkl et al., 2024). However, it is important, that regardless of the route of administration of nanocompounds, they eventually enter the bloodstream, interacting with blood components, as well as with endothelial cells (ECs) (Setyawati et al., 2013; Ni et al., 2022; Lasak and Ciepluch, 2023a; Huang et al., 2024).
The vascular endothelium, as a single layer of mesenchymal cells lining the inner layer of blood vessels, plays a key role in our body. This highly selective cells monolayer acts as a permeability barrier between blood and tissues, controlling the bidirectional transport of molecules and ions circulating in the blood and protecting tissues from the penetration of dangerous substances (Díaz-Coránguez et al., 2017; Rahimi, 2017; Claesson-Welsh et al., 2021). Endothelial cells also perform an endocrine function, and the mediators secreted by them regulate the vessel tension and processes such as blood clotting, angiogenesis and fibrinolysis. Therefore, the proper functioning of the endothelium is essential for maintaining the homeostasis of the entire organism, and its dysfunction can cause various pathological conditions, including atherosclerosis, stroke, diabetes and neurodegenerative diseases (Wang and He, 2024).
One of the nanomaterials that, due to their purpose, can influence the function and properties of the endothelial layer are nanofibers (NF). These materials have a number of advantages in biomedical applications. Firstly, nanofibers are characterized by a high surface-to-volume ratio and ease of functionalization, which allows for efficient loading of nanofibers matrix with various biologically active substances, such as antibacterial, including metal nanoparticles in wound disinfection and regeneration (Yan et al., 2020; Maliszewska and Czapka, 2022; Reyes-guzmán et al., 2024; Xin et al., 2024; Zhao et al., 2024) and anticancer agents (Bonan et al., 2019), growth factors (Jiang et al., 2018; Asiri et al., 2021), drugs (Cleeton et al., 2019) or vitamins (Cheng et al., 2024), thus broadening the spectrum of their action. Secondly, their high porosity, structure mimicking the external cellular matrix (ECM) and good mechanical strength provide adequate structural support for vascularization, cell adhesion and migration (Abadi et al., 2022; Al-Abduljabbar and Farooq, 2023). Hence, these features make them attractive candidates for wound dressing materials, biosensors for detecting biological compounds, as drug delivery systems or scaffolds in tissue engineering (Dutta et al., 2019; Kulkarni et al., 2022; Nirwan et al., 2023; Lasak et al., 2024).
Polymer nanoscaffolds offer a wide range of both natural and synthetic materials for biomedical applications. Unfortunately, they are not without disadvantages. Natural polymers, such as chitosan or collagen, are biocompatible and biodegradable, but their poor mechanical properties and processability raise concerns. Another problem is the risk of transferring microorganisms together with the natural polymer to the patient’s body. Synthetic polymers, including poly (lactic acid) (PLA) or poly (lactic-co-glycolic acid) (PGA), are characterized by increased mechanical strength, but their low interaction with cells is a problem (Anjum et al., 2022). For this reason, attempts are being made to mix different polymers and modify them (e.g., with nanoparticles) in order to obtain effective scaffolds with the desired properties (Behere et al., 2021).
Until now, it has been reported that nanoparticles (NPs) exhibit cytotoxic and genotoxic effects on endothelial cells, which are mainly associated with inflammatory mechanisms and oxidative stress (Fattahi et al., 2023; Cong et al., 2024). Moreover, nanomaterials can directly induce endothelial leakiness (NanoEL effect, Nanomaterials-Induced Endothelial Leakiness), through interaction with VE-cadherin, which is crucial for maintaining adherens junctions between endothelial cells (Setyawati et al., 2023). However, there is little information on the direct or indirect interaction of nanofibers with ECs, which may be due to the relatively early stage of research on them compared to other nanocompounds. Nevertheless, caused by their great potential in the field of medicine, as well as the increasingly common use of nanomaterials in daily life in commercially available products, it is necessary to determine their effect on endothelial cells.
Herein, electrospun PLCL/PVP nanofibers composed of biodegradable and biocompatible copolymer of L-lactide-block-ϵ-caprolactone (PLCL, 70:30) blended with polyvinylpyrrolidone (PVP), containing synthesized in situ PVP different types of nanoparticles - gold (AuNPs), cadmium selenide (CdSe QDs) or tantalum (TaNPs), were investigated. Electrospinning is relatively cheap and highly reproducible method of nanofibers fabrication, while in situ PVP synthesis of NPs provided controlled size distribution of nanoparticles. Three different types of nanoparticles with various potential for biomedical application were selected in order to provide a wider spectrum of information on their effect on endothelial cells. Gold nanoparticles are known for their antibacterial properties (Li et al., 2014), while CdSe QDs exhibit high toxicity towards cancer cells and unique optical properties (Nirwan et al., 2023). In turn, tantalum compounds, appear as ideal radiosensitizers, increasing the sensitivity of cancer cells to radiotherapy (Ji et al., 2022). In this study, the produced nanomats - PLCL/PVP-AuNPs, PLCL/PVP- CdSe QDs, PLCL/PVP- TaNPs, were physicochemically characterized and their effect on endothelial cells was determined.
2 Materials and methods
2.1 Polymers and nanoparticles
For the fabrication of nanofibers, poly (L-lactide-co-ε-caprolactone) (PLCL) (av. Mw 200 kg) polymer was bought from Purasorb®, Corbion, Netherlands. Polyvinylpyrrolidone (PVP) (Mw 250 kg), analytical grade chloroform, ethanol, gold precursor, HCl, and Cadmium acetate were purchased from Carl Roth, Karlsruhe, Germany. Sodium borohydride and Ta nanoparticles were purchased from Sigma Aldrich. And selenium powder from Alfa Aesar.
2.2 Synthesis of PVP templated nanoparticles
The syntheses of nanoparticles were performed in situ using a polymer template strategy. PVP polymer was chosen due to its good solubility in both chloroform and ethanol. Moreover, it has the ability to produce solutions with better electrospinnability. Additionally, PVP was used here as co co-spinning agent to assist the generation of nanofibers using PLCL as the matrix. For the synthesis of PVP-templated AuNPs, 0.01 mM HAuCl4· 3 H2O was solubilized in ethanol, and 3.6 g of PVP was added once the solution was stable. The resulting solution was kept at 30°C and reduced using a freshly prepared 0.1 mM concentration of NaBH4. The mixture was stirred for a couple of hours (pink solution) to ensure complete reduction, and the ethanol was evaporated using an oven, and the particles were resuspended in 10 mL of chloroform (Lasak et al., 2024).
The preparation of Cadmium selenide nanoparticles was performed using the methodology described elsewhere (Nirwan et al., 2023). Briefly, Cd acetate was solubilized in PVP ethanol solution under N2 purge. NaHSe solution prepared simultaneously from Se powder and NaBH4 was added dropwise to it until the solution turned yellowish. The resulting CdSe NPs were re-dispersed in chloroform before being suspended in electrospinning solution.
To prepare a suspension from Ta powder, 9 mg powder was added to 5 mL of chloroform and 200 µL 37% HCl. The suspension was kept under the ultrasonic bath for 2 h to ensure a stable suspension. A black colored suspension was obtained that was used to functionalize the electrospinning solution.
The nanoparticles were analyzed using TEM (Transimsion electron microscopy), and the resulting hybrid nanofibers were analyzed using SEM (scaning electron microscopy).
2.3 Fabrication of the nanofibers
The generation of nanofibers using electrospinning from various solutions (coelectrospining) was done at optimized parameters already described elsewhere (Nirwan et al., 2022; 2023; Lasak et al., 2024; Kulkarni et al., 2025). For the fabrication of PLCL/PVP fibers, an electrospinning solution of 0.425g PVP added to 1.7g PLCL dissolved in chloroform was used. The electrospinning solution for AuNPs loaded PLCL/PVP fibers was formed by dissolving 0.425 g PVP in 8 mL AuNPs chloroform suspension and 1.7 g PLCL in 8 mL chloroform. The two solutions were blended before electrospinning. Similarly, CdSe NPs loaded fibers were obtained from a blend of solutions by dissolving 0.425 g PVP in 8 mL CdSe NPs chloroform suspension and 1.7 g PLCL in 8 mL chloroform. Finally, Ta NPs loaded fibers were obtained from the solution by dissolving 0.425 g PVP in 3 mL chloroform, adding to it 5 mL Ta NPs suspension and 1.7 g PLCL in 8 mL chloroform. The optimized parameters used for electrospinning and generation of nanofibers are described in Table 1.

Table 1. Optimized parameters for the generation of pristine and nanoparticle functionalized hybrid nanofibers.
2.4 Physicochemical characterization of fabricated nanofibers
Physicochemical characterization of the nanofibers has already been performed and can be found in the following articles (Nirwan et al., 2023; Lasak et al., 2024). The morphologies of the unmodified and modified nanofibers were determined using scanning electron microscopy (SEM, JSM-IT 100 InTouchScope™, Freising, Germany) at an accelerating voltage of 15 kV.
The samples were placed on carbon-coated 300 mesh copper grids, left to dry completely, coated with gold spray using a JEOL JFC 110E Fine Coat Ion Sputter, and analyzed by SEM. The hydrodynamic diameter of AuNPs, QDs and TaNPs was measured using dynamic light scattering (DLS) in a photon correlation spectrometer (Anton Paar Ligth sizer 500, Austria). The refraction factor was assumed to 1.33 while detection angles were 15°, 90° and 175°, and the wavelength 658 nm. The data were analyzed using Anton Paar software. PBS was used as a solvent. Additionally, Transmission Electron Microscopy TEM microscopy was also done to confirm the size distribution of nanoparticles.
2.5 Preparation and sterilization of nanofibers for biological tests
For biological tests, the UV sterilization method of nanofibers was used, as the most preferred and effective according to the literature data (Evrova et al., 2019a). For this purpose, nanofibers were cut into appropriately diameter disks: 6, 22, 35 mm for 96-, 12- and 6-well plates, respectively, to cover the well surface. Then, the disks were exposed to UV light (λ = 254 nm) for 30 min each side. The effect of UV radiation (0.5 h and 24 h) on the morphology of nanofibers was assessed using a Confocal Raman Microscope (LabRam, Horiba) (exc 691 nm; objx50 lwd; 36 × 5s; bin = 1). All spectra were normalized to the same band intensity at 1,435 cm-1. Offset for clarity.
2.6 Effect of nanofibers on endothelial cells
2.6.1 Cell growth and morphology in the presence of nanofibers
In vitro tests were performed using the HUVEC cell line (Primary Human Umbilical Vein Endothelial Cells, PromoCell). Cells were cultured in complete Endothelial Cell Growth Medium (Cell Applications, Inc.) at 37ᵒC in a humidified atmosphere and 5% CO2. The culture medium was changed every 2 days. To assess cell growth and morphology, HUVEC cells were seeded in 6-well plates and cultured with different types of nanofibers for 24, 48, and 72 h and observed under an optical microscope (64x) at each time point.
2.6.2 Cytotoxic effect of nanofibers on endothelial cells
The viability of HUVEC cells treated with nanofibers was assessed using the MTS Cell Proliferation Assay Kit (Colorimetric) (Abcam). Briefly, cells were cultured in a 96-well plate until the appropriate cell confluence was reached and then treated with nanofibers for 24 h. After that, the MTS test was used according to the manufacturer’s recommendations. Absorbance was measured at 490 nm.
2.6.3 Stability of endothelial monolayer treated with nanofibers
The integrity of the endothelial monolayer treated with nanofibers was assessed using a transwell system equipped with 0.4 μm pore inserts (Corning). For this purpose, HUVEC cells were seeded into the upper chamber of the transwell inserts and cultured until a compact cell monolayer was achieved. After that, nanofibers were added to the lower compartment and incubated for 24 h at 37°C in a humidified atmosphere containing 5% CO2. Then, the nanofibers were removed and replaced with a solution of fluorescein isothiocyanate–dextran (FITC-Dextran, 40 kDa, Sigma-Aldrich) at a final concentration of 1 mg/mL. The plate was incubated in the dark for 20 min at room temperature. The medium from the upper chamber was transferred to a 96-well plate and fluorescence measurement was performed at Ex/Em = 485 nm/525 nm.
3 Results
3.1 Physicochemical characterization of fabricated nanofibers
Various compositions as described in the methodology were electrospun using the optimized parameters to generate nanofibers. The use of chloroform as the solvent here was prohibited to obtain an interruption free and high-yielding electrospinning process. Due to the very high vapor pressure of the chloroform, constant clogging of the capillary at the spinneret was observed. This led to interruptions during the process as the capillary had to be cleaned, and the clogged polymer needed constant attention. This problem has been described well, and using a solvent mixture should be able to help minimize this issue and improve the electrospinning process (Maduna and Patnaik, 2024; Xing et al., 2024).
As observed from Figure 1, the morphological analysis of fibers under SEM confirmed the generation of cylindrical fibril structures with dimensions from submicron to a few microns. There was not much change in the diameter distribution of the nanofibers after functionalization with inorganic moieties. The average diameter of pristine nanofibers, as measured using ImageJ, was 2.1 ± 1.4 µm, followed by CdSe functionalized nanofibers at 2.2 ± 2.4 µm. The TaNPs functionalized nanofibers showed an average diameter of 1.82 ± 1 μm and 1.5 ± 0.9 µm was the average diameter measure for AuNPs functionalized nanofibers. The surface of the fibers showed textured topology with variation in depth among various types of fibers. Here, the use of high humidity while performing electrospinning is attributed to the presence of these structures (Xue et al., 2017; Liu et al., 2022). The micro- and macroporous structures observed on the surface of the nanofibers are highly beneficial in the case of biological applications (Leal et al., 2024). These structures provide roughness to the otherwise smooth nanofiber surface, which can improve cell adhesion in the case of tissue regeneration scaffolds. Moreover, these porous structures can act as drug loading sites for the nanofibers, enhancing their loading capacity (Nirwan et al., 2023). Furthermore, via SEM, it was possible to observe the CdSe NPs and AuNPs on the surface of the nanofibers. The surface of TaNPs nanofibers did not show nanoparticles at magnification. The size of the nanoparticles and their morphology were measured using the Dynamic light scattering method and TEM microscopy (S7-S9).
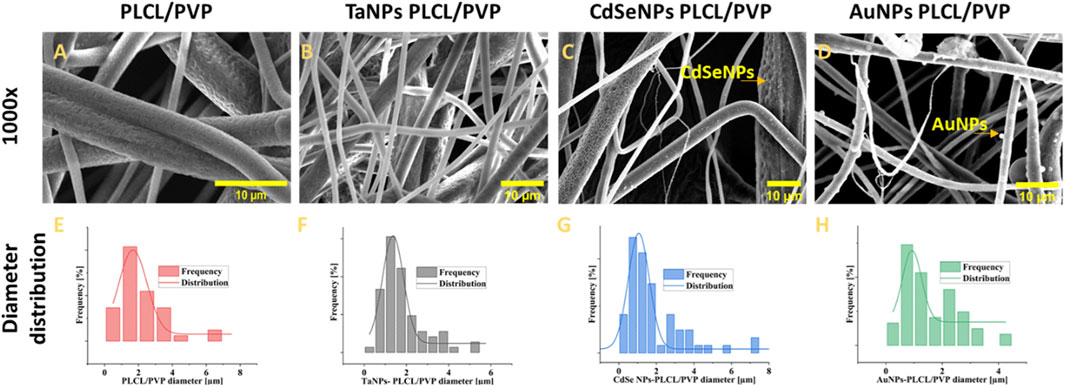
Figure 1. The morphological analysis of fibers under SEM with diameter distribution;, nonfunctionalized (A,E) TaNPs functionalized nanofibers, (B,F) CdSeNPs functionalized nanofibers (C,G), AuNPs functionalized nanofibers (D,H).
For biological tests, all nanofibers were previously subjected to a UV lamp sterilization procedure. Taking into account the fact that the selected sterilization method may affect the properties of the produced nanoscaffolds, their stability after the sterilization process was assessed. Figure 2 shows the effect of UV radiation on the morphology of nanofibers after 0.5 h and 24 h of treatment. As an example, the results for PLCL/PVP and PLCL/PVP - AuNPs nanofibers are presented. As observed, even 24 h exposure of nanofibers to UV light did not cause significant changes in their structure. As expected, a similar result was also observed for 30-min UV treatment of nanofibers.
Figure 2. The effect of UV radiation on the morphology of nanofibers after 0.5 h and 24 h of treatment.
3.2 Effect of nanofibers on endothelial layer formation
The growth and morphology of cells in subsequent days of incubation with different types of nanofibers are shown in Figure 3. The endothelial layer forms to 75% confluence (after 24 h) and reaches 100% after 72 h when no nanofibers are present. A slightly slower formation of the endothelial layer is seen in the presence of PLCL/PVP nanofibers. After 24 h, the plate overgrowth is around 60% and after 72 h it reaches only 80%. The introduction of various nanoparticles into the nanofibers caused changes in the morphology and rate of endothelial layer formation (Figure 4). In the presence of nanofibers modified with AuNPs, the cells had problems with attachment to the substrate and did not show the typical morphology of endothelial cells. After 48 h, the plate overgrowth was only 10% and after 72 h it reached only 40%. A similar effect was obtained for PLCL/PVP-CdSe QDs nanofibers. In this case, after 24 h, the plate overgrowth was 10% and after 72 h 40%. In the case of PLCL/PVP-TaNPs, after 24 h the confluence was 40%, after 48 h 90% and after 72 h 100%.
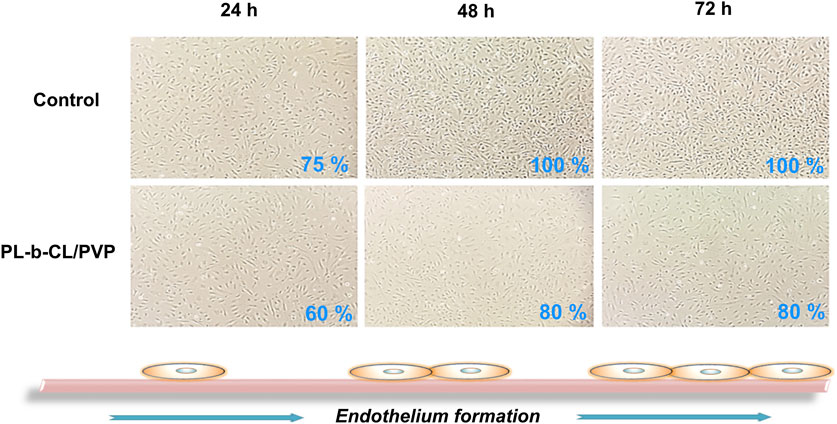
Figure 3. The formation of the endothelial layer in the presence of PLCL/PVP nanofibers compare to cells incubated without nanofibers, after 24, 48 and 72 h.
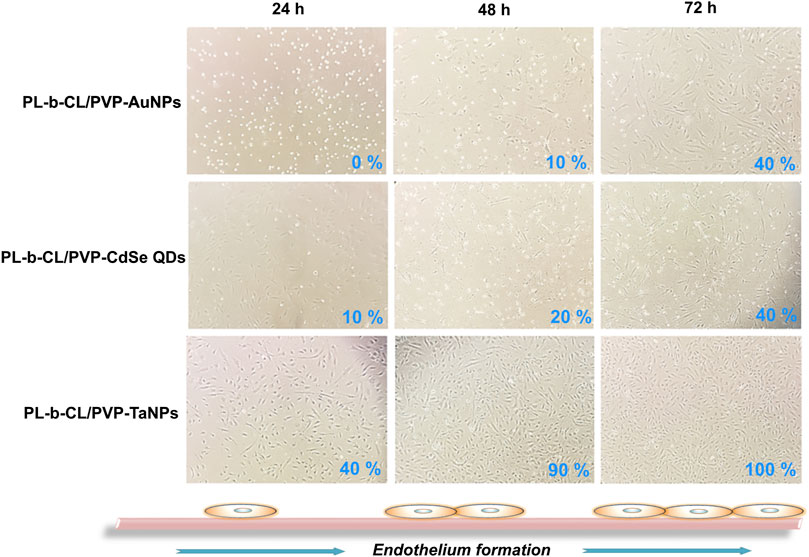
Figure 4. The formation of the endothelial layer in the presence of AuNPs functionalized nanofibers, CdSeNPs functionalized nanofibers and TaNPs functionalized nanofibers after 24, 48 and 72 h.
3.3 Cytotoxicity of nanofibers on endothelial layer
The cytotoxic properties of nanofibers on endothelial cells were studied using the MTS Proliferation Assay. As shown in Figure 5, the tested nanofibers did not show toxic behavior towards the formed HUVEC cell monolayer after 24 h of incubation with different nanofibers, which may suggest their safety in medical applications.
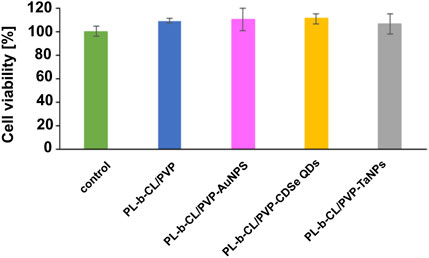
Figure 5. Cell viability of endothelial cells after 24 incubation with different nanofibers measured by MTS Proliferation Assay.
3.4 Stability of endothelial layer in presence of nanofibers
The next step, the effect of fabricated nanofibers on the endothelial monolayer integrity was evaluated. All of the tested nanofibers, except PLCL/PVP-TaNPs, induced increased HUVEC cells layer permeability, which resulted in increased translocation of fluorescently labeled dextran (FITC-Dextran) from 20% to 50% (Figure 6), which confirms the leakiness effect dependent on the type of nanoparticles used. The most significant permeabilization effect is observed in presence of PLCL/PVP alone (up to 50%). The lowest or even no effect was visible for PLCL/PVP modified with TaNPs.
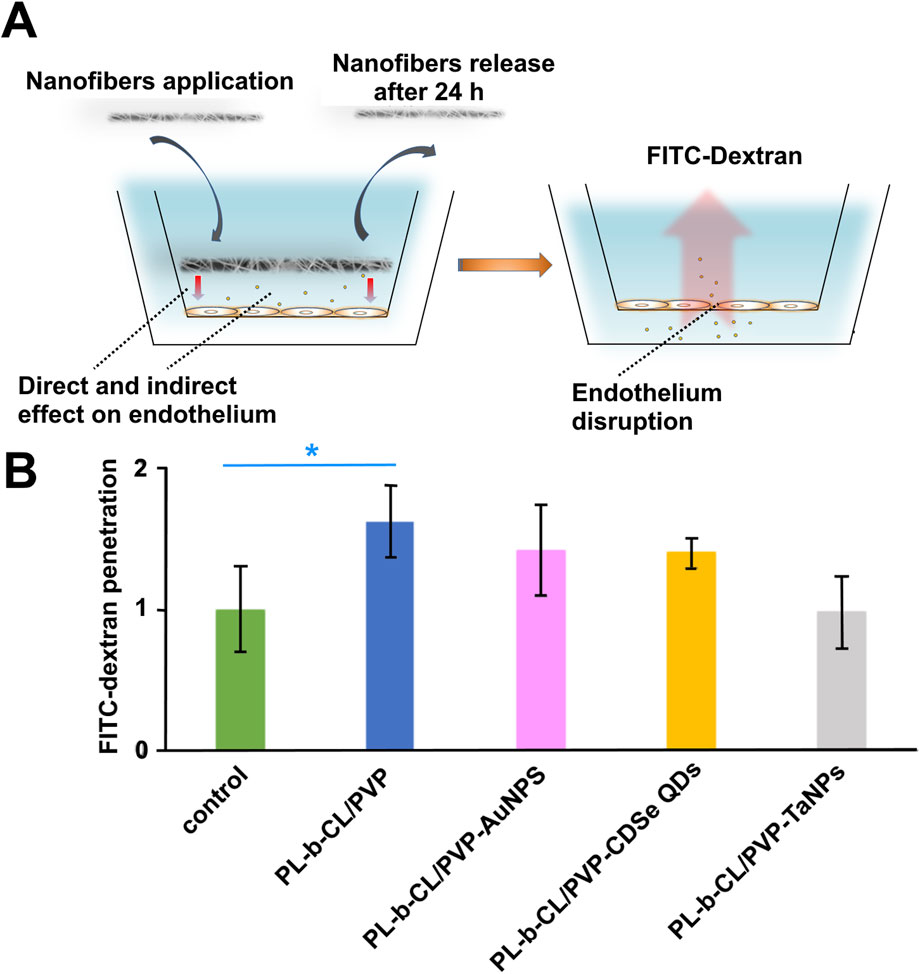
Figure 6. The effect of fabricated nanofibers on the endothelial monolayer. The scheme of experiment (A), HUVEC cell layer permeability caused by increased translocation of fluorescently labeled dextran (FITC-Dextran) (B).
4 Discussion
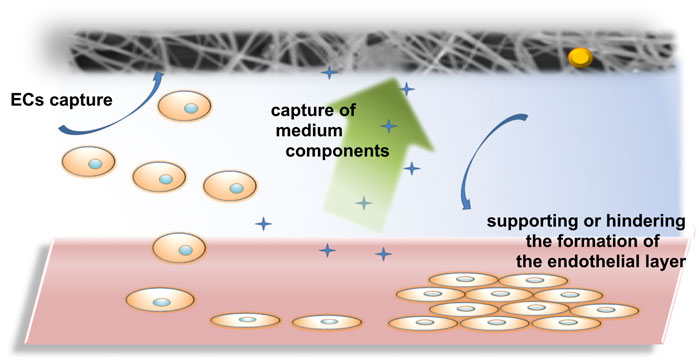
Nanofibers are gaining increasing importance in biomedical applications, including drug delivery, antibacterial and anticancer therapies or effective tissue engineering scaffolds (Chen et al., 2023), which makes their interaction with endothelial cells inevitable (Jiang et al., 2018; Chen et al., 2019; Song et al., 2024). This influence can be both direct, in contact with the endothelial layer, or in the case of new vessel formation, and indirect, when nanofiber components can interact with the endothelial layer or have a real impact on the composition of biological fluids necessary for endothelial formation. Therefore, in this study, we investigated the effect of electrospun PLCL/PVP nanofibers modified with different nanoparticles (gold, cadmium selenide or tantalum) with different potential applications in biomedicine on endothelial cells HUVEC.
For biological tests, all nanofibers were previously subjected to a UV lamp sterilization procedure. Maintaining the correct morphology and structure in potential medical applications is crucial. Available studies show a strong correlation between the type of polymer used and the stability of nanofibers exposed to UV light (Elashnikov et al., 2019; Evrova et al., 2019b; Tort et al., 2020). Considering the fact that the selected sterilization method can affect the properties of the produced nanofibers, their stability was assessed after the sterilization process. Figure 2 shows the effect of UV radiation on the morphology of nanofibers after 0.5 h and 24 h of treatment. As an example, the results for PLCL/PVP and PLCL/PVP - AuNPs nanofibers are presented. As observed, even 24-h exposure of nanofibers to UV light did not cause significant changes in their structure. As expected, a similar result was also observed after 30-min UV treatment of nanofibers.
As mentioned earlier, UV sterilization is crucial and only such nanofibers can be used in biomedicine. The growth and morphology of cells in the subsequent days of incubation with different types of nanofibers after UV sterilization are shown in Figures 3, 4. Cells exposed to PLCL/PVP and PLCL/PVP-TaNPs nanomats maintained normal morphology and growth comparable to the control group, in contrast to HUVEC cells exposed to CdSe QDs nanofibers. The limited growth of endothelial cells exposed to PLCL/PVP-CdSe QDs nanofibers observed in this study may be due to the toxic effects of cadmium compounds (Hu et al., 2021; Rodríguez-Fragoso et al., 2024). However, the application of PVP coating as well as the immobilization of nanoparticles in the nanofiber matrix reduced the toxic effects of CdSe quantum dots while maintaining, albeit limited, cell growth.
In turn, tantalum-based materials are very common in bone tissue engineering, as scaffolds supporting cell growth, with very good mechanical properties and biocompatibility (Zhao et al., 2021; Nan et al., 2022). Nan et al. showed that electrospun scaffolds made of poly-ε-caprolactone (PCL) with tantalum nanoparticles (TaNPs) and magnesium nanooxide (MgO), due to their high osteogenic activity, can be ideal candidates in the treatment of bone defects, which was confirmed by in vitro and in vivo tests. Moreover, the developed scaffolds stimulated the proliferation of endothelial progenitor cells (EPCs) and angiogenesis, indicating their possible application in vascular tissue engineering (Nan et al., 2022). Fortunately, our studies also showed that cells treated with PLCL/PVP-AuNPs nanofibers needed a longer adaptation time to the substrate than cells treated with other nanofibers. Cell adhesion occurred only after 48 h, and after 72 h, their significant growth with typical morphology was observed. Mohamed et al., studying gold nanoparticles coated with polyvinylpyrrolidone, observed that they inhibited the viability and proliferation of bovine aortic endothelial cells (BAECs) and the phosphorylation of the ERK1/2 pathway, which is important in the regulation of endothelial homeostasis (Mohamed et al., 2017). Among other reasons for reduced HUVEC cell viability in response to gold nanoparticles, disruption of important pathways for endothelial function, such as VEGF-A/VEGFR, has also been indicated (Darweesh et al., 2019). In summary, the incorporation of nanoparticles with different properties into nanofibers affects the biological activity of the entire scaffold and thus induces a different behavior of endothelial cells in the presence of the applied nanofibers. Therefore, different rates of cell proliferation and endothelial layer formation may result from both cell contact with nanofibers, interception of medium components (biological fluid), which may affect the condition of cells, and possible release of nanoparticles from nanofibers. The cytotoxic properties of nanofibers on endothelial cells were studied using the MTS Proliferation Assay. As shown in Figure 5, the tested nanofibers did not show toxic behavior towards the formed HUVEC cell monolayer after 24 h of incubation with different nanofibers, which may suggest their safety in medical applications. These results are consistent with our previous studies, which confirmed the lack of cytotoxic effect of PLCL/PVP and PLCL/PVP-AuNPs nanofibers on human fibroblast cell line (VH10) (Lasak et al., 2024). However, PLCL/PVP-CdSe QDs nanoscaffolds, which showed high cytotoxicity against human lung carcinoma A549 cells in our prior tests (Nirwan et al., 2023), in this study, were not toxic to endothelial cells. Several studies have been conducted to confirm that the toxicity of CdSeQDs is dose, size and surface chemistry dependent, and the mechanism of their toxicity is based on the generation of ROS (reactive oxygen species) and the induction of oxidative stress in cells. Nevertheless, due to their great potential in cancer therapies or bioimaging, various strategies are being undertaken to reduce their toxicity (Kumari et al., 2018; Lin and Chen, 2023; Rodríguez-Fragoso et al., 2024). This was studied by Tang et al. who showed that modification of CdSe/ZnS core–shell QDs with polyethylene glycol (PEG) significantly reduces their in vitro and in vivo toxicity compared to QDs coated with the cationic polymer polydiallyldimethylammonium chloride (PDDA) (Tang et al., 2013). Our proposed method of in situ PVP nanoparticles synthesis, as well as immobilization in a nanofiber matrix, also works in a similar way, reducing the cytotoxic effect on endothelial cells. However, the decreased sensitivity of HUVEC to the toxic effects of PLCL/PVP-CdSe QDs scaffolds observed in this study, compared to other cell types, may also result from the increased resistance of endothelial cells to oxidative stress with subsequent subcultures. It has been known, the decreased sensitivity of HUVEC to oxidative damage correlates with a decrease in cellular iron content, which mediates oxidative stress (Qian et al., 2010). Freshly harvested human umbilical vein endothelial cells contain a significant amount of iron, but the level decreases with subsequent passages, causing increased cell resistance to oxidative damage (Sullivan, 2005).
Nanofibers may be ideal scaffolds for blood vessel regeneration and repair, as developed by Kong et al. biomimetic gelatin (Gt)/polycaprolactone (PCL) composite nanofibers containing chondroitin sulfate (CS) (Kong et al., 2021). However, exposure of cells to nanoparticles incorporated into nanomats can lead to destabilization of cell-cell interactions and increased monolayer permeability. It is also possible that nanoparticles will be released from the nanomats and interact directly or indirectly with blood vessels, as well as the nanofibers topography itself may influence on the interaction with endothelial cells (Cao, 2018; Liu et al., 2018; Lasak and Ciepluch, 2023b). Therefore, in the next step, we investigated the effect of fabricated nanofibers on the endothelial monolayer integrity. All of the tested nanofibers, except PLCL/PVP-TaNPs, induced increased HUVEC cells layer permeability, which resulted in increased translocation of fluorescently labeled dextran (FITC-Dextran) (Figure 6), which confirms the leakiness effect dependent on the type of nanoparticles used. Liu et al. reported a size-dependent effect of gold nanoparticles on vascular endothelial leakiness. They confirmed that 20 nm gold nanoparticles had no significant effect on cell viability, but induced more than 50% increase in HUVEC cell layer permeability due to changes in the cytoskeletal structure, including actin rearrangement, stress fiber formation, and actomyosin contraction (Liu et al., 2017). These results were consistent also in their subsequent work, where they evaluated titanium dioxide, silicon dioxide and polystyrene (NP) nanoparticles with sizes in the range of 20–30 nm, thus confirming the permeabilization effect dependent on the nanoparticle size (Liu et al., 2018).
5 Conclusion
The biological tests performed allowed us to assess the effect of electrospun PLCL/PVP nanofibers modified with nanoparticles on HUVEC cells, which sheds new light on the toxicity of the nanomaterials used and their interactions with the endothelium, which can be used to develop new solutions in nanomedicine. The incorporation of nanoparticles into the nanofiber matrix can affect many biological aspects, including cell growth, proliferation or adhesion, as well as mechanical properties of the scaffolds. It was estimated that the effect of nanofibers on the formation of the endothelial layer can be direct, where cells contact the nanofibers and thus the growth of the endothelium is hindered. Additionally, the uptake of biological fluid by nanofibers can have an indirect effect on endothelial cells, their adhesion and growth (Figure 7). Among the tested nanofibers, non-toxic PLCL/PVP-TaNPs nanomats seem to be particularly promising due to safety issues and the possibility of using them as effective scaffolds. Moreover, the lack of influence of the remaining nanomats on the viability of endothelial cells, together with the possibility of causing leakage of the cell monolayer, can be used in the delivery of drugs to target sites, to which access is difficult. Therefore, our presented results are not only a source of information on the cytocompatibility of the developed nanofibers, but also demonstrate their great potential as nanobiomedical materials.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the studies on humans in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used.
Author contributions
ML: Investigation, Methodology, Visualization, Writing – original draft. VN: Investigation, Methodology, Visualization, Writing – original draft. DK-C: Writing – original draft, Validation. RT: Funding acquisition, Validation, Writing – review and editing. IC: Methodology, Writing – review and editing. AF: Writing – review and editing, Supervision, Funding acquisition, Conceptualization. KC: Project administration, Supervision, Conceptualization, Writing – review and editing, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was funding by Jan Kochanowski University in Kielce and Casimir Pulaski University of Radom.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmolb.2025.1638442/full#supplementary-material
References
Abadi, B., Goshtasbi, N., Bolourian, S., Tahsili, J., Adeli-Sardou, M., and Forootanfar, H. (2022). Electrospun hybrid nanofibers: fabrication, characterization, and biomedical applications. Front. Bioeng. Biotechnol. 10, 986975. doi:10.3389/fbioe.2022.986975
Al-Abduljabbar, A., and Farooq, I. (2023). Electrospun polymer nanofibers: processing, properties, and applications. Polym. (Basel) 15, 65. doi:10.3390/polym15010065
Anjum, S., Rahman, F., Pandey, P., Arya, D. K., Alam, M., Rajinikanth, P. S., et al. (2022). Electrospun biomimetic nanofibrous scaffolds: a promising prospect for bone tissue engineering and regenerative medicine. Int. J. Mol. Sci. 23, 9206. doi:10.3390/ijms23169206
Asiri, A., Saidin, S., Sani, M. H., and Al-Ashwal, R. H. (2021). Epidermal and fibroblast growth factors incorporated polyvinyl alcohol electrospun nanofibers as biological dressing scaffold. Sci. Rep. 11, 5634. doi:10.1038/s41598-021-85149-x
Behere, I., Pardawala, Z., Vaidya, A., Kale, V., and Ingavle, G. (2021). Osteogenic differentiation of an osteoblast precursor cell line using composite PCL-gelatin-nHAp electrospun nanofiber mesh. Int. J. Polym. Mater. Polym. Biomaterials 70, 1281–1295. doi:10.1080/00914037.2020.1767619
Bonan, R. F., Mota, M. F., da Costa Farias, R. M., da Silva, S. D., Bonan, P. R. F., Diesel, L., et al. (2019). In vitro antimicrobial and anticancer properties of TiO2 blow-spun nanofibers containing silver nanoparticles. Mater. Sci. Eng. C 104, 109876. doi:10.1016/j.msec.2019.109876
Cao, Y. (2018). “The toxicity of nanoparticles to human endothelial cells,” in Cellular and molecular toxicology of nanoparticles. Editors Q. Saquib, M. Faisal, A. A. Al-Khedhairy, and A. A. Alatar (Cham: Springer International Publishing), 59–69. doi:10.1007/978-3-319-72041-8_4
Chen, D., Zhu, T., Fu, W., and Zhang, H. (2019). Electrospun polycaprolactone/collagen nanofibers cross-linked with 1-ethyl-3-(3- dimethylaminopropyl) Carbodiimide/nhydroxysuccinimide and genipin facilitate endothelial cell regeneration and May be a promising candidate for vascular scaffolds. Int. J. Nanomedicine 14, 2127–2144. doi:10.2147/IJN.S192699
Chen, K., Li, Y., Li, Y., Tan, Y., Liu, Y., Pan, W., et al. (2023). Stimuli-responsive electrospun nanofibers for drug delivery, cancer therapy, wound dressing, and tissue engineering. J. Nanobiotechnology 21, 237. doi:10.1186/s12951-023-01987-z
Cheng, E., Geng, Z., Xiang, L., Zhao, X., Xiang, A., and Tian, H. (2024). Development of vitamin C-Loaded electrospun nanofibers of mixture of polysaccharides of pullulan/xanthan gum for fast dissolving oral film applications. Materials 17, 861. doi:10.3390/ma17040861
Claesson-Welsh, L., Dejana, E., and McDonald, D. M. (2021). Permeability of the endothelial barrier: identifying and reconciling controversies. Trends Mol. Med. 27, 314–331. doi:10.1016/j.molmed.2020.11.006
Cleeton, C., Keirouz, A., Chen, X., and Radacsi, N. (2019). Electrospun nanofibers for drug delivery and biosensing. ACS Biomater. Sci. Eng. 5, 4183–4205. doi:10.1021/acsbiomaterials.9b00853
Cong, X., Zhang, Z., Li, H., Yang, Y. G., Zhang, Y., and Sun, T. (2024). Nanocarriers for targeted drug delivery in the vascular system: focus on endothelium. J. Nanobiotechnology 22, 620. doi:10.1186/s12951-024-02892-9
Darweesh, R. S., Ayoub, N. M., and Nazzal, S. (2019). Gold nanoparticles and angiogenesis: molecular mechanisms and biomedical applications. Int. J. Nanomedicine 14, 7643–7663. doi:10.2147/IJN.S223941
Díaz-Coránguez, M., Ramos, C., and Antonetti, D. A. (2017). The inner blood-retinal barrier: cellular basis and development. Vis. Res. 139, 123–137. doi:10.1016/j.visres.2017.05.009
Dutta, S. D., Patel, D. K., Seo, Y. R., Park, C. W., Lee, S. H., Kim, J. W., et al. (2019). In vitro biocompatibility of electrospun poly(ϵ-Caprolactone)/Cellulose nanocrystals-nanofibers for tissue engineering. J. Nanomater 2019, 1–11. doi:10.1155/2019/2061545
Egbuna, C., Parmar, V. K., Jeevanandam, J., Ezzat, S. M., Patrick-Iwuanyanwu, K. C., Adetunji, C. O., et al. (2021). Toxicity of nanoparticles in biomedical application: Nanotoxicology. J. Toxicol. 2021, 9954443. doi:10.1155/2021/9954443
El-Kady, M. M., Ansari, I., Arora, C., Rai, N., Soni, S., Verma, D. K., et al. (2023). Nanomaterials: a comprehensive review of applications, toxicity, impact, and fate to environment. J. Mol. Liq. 370, 121046. doi:10.1016/j.molliq.2022.121046
Elashnikov, R., Rimpelová, S., Vosmanská, V., Kolská, Z., Kolářová, K., Lyutakov, O., et al. (2019). Effect of sterilization methods on electrospun cellulose acetate butyrate nanofibers for SH-SY5Y cultivation. React. Funct. Polym. 143, 104339. doi:10.1016/j.reactfunctpolym.2019.104339
Evrova, O., Kellenberger, D., Scalera, C., Calcagni, M., Giovanoli, P., Vogel, V., et al. (2019a). Impact of UV sterilization and short term storage on the in vitro release kinetics and bioactivity of biomolecules from electrospun scaffolds. Sci. Rep. 9, 15117–11. doi:10.1038/s41598-019-5513-1
Evrova, O., Kellenberger, D., Scalera, C., Calcagni, M., Giovanoli, P., Vogel, V., et al. (2019b). Impact of UV sterilization and short term storage on the in vitro release kinetics and bioactivity of biomolecules from electrospun scaffolds. Sci. Rep. 9, 15117. doi:10.1038/s41598-019-51513-1
Fattahi, P., Yeh, Y. T., Zhao, T., Younesi, M., Huang, C., Terrones, M., et al. (2023). Endothelial cell selectivity to nanoparticles depends on mechanical phenotype. Adv. Mater Interfaces 10, 2300137. doi:10.1002/admi.202300137
Gold, K., Slay, B., Knackstedt, M., and Gaharwar, A. K. (2018). Antimicrobial activity of metal and metal-oxide based nanoparticles. Adv. Ther. (Weinh) 1, 1700033–15. doi:10.1002/adtp.201700033
Hirai, T., Yoshioka, Y., Izumi, N., Ichihashi, K. I., Handa, T., Nishijima, N., et al. (2016). Metal nanoparticles in the presence of lipopolysaccharides trigger the onset of metal allergy in mice. Nat. Nanotechnol. 11, 808–816. doi:10.1038/nnano.2016.88
Hu, L., Zhong, H., and He, Z. (2021). Toxicity evaluation of cadmium-containing quantum dots: a review of optimizing physicochemical properties to diminish toxicity. Colloids Surf. B Biointerfaces 200, 111609. doi:10.1016/j.colsurfb.2021.111609
Huang, Y., Huang, B., Ye, D., Luo, X., Xiong, X., Xiong, H., et al. (2024). Nano-induced endothelial leakiness-reversing nanoparticles for targeting, penetration and restoration of endothelial cell barrier. Acta Biomater. 175, 226–239. doi:10.1016/j.actbio.2023.12.040
Ji, C., Zhao, M., Wang, C., Liu, R., Zhu, S., Dong, X., et al. (2022). Biocompatible tantalum nanoparticles as radiosensitizers for enhancing therapy efficacy in primary tumor and metastatic sentinel lymph nodes. ACS Nano 16, 9428–9441. doi:10.1021/acsnano.2c02314
Jiang, Y. C., Wang, X. F., Xu, Y. Y., Qiao, Y. H., Guo, X., Wang, D. F., et al. (2018). Polycaprolactone nanofibers containing vascular endothelial growth factor-encapsulated gelatin particles enhance mesenchymal stem cell differentiation and angiogenesis of endothelial cells. Biomacromolecules 19, 3747–3753. doi:10.1021/acs.biomac.8b00870
Kong, X., He, Y., Zhou, H., Gao, P., Xu, L., Han, Z., et al. (2021). Chondroitin sulfate/polycaprolactone/gelatin electrospun nanofibers with antithrombogenicity and enhanced endothelial cell affinity as a potential scaffold for blood vessel tissue engineering. Nanoscale Res. Lett. 16, 62. doi:10.1186/s11671-021-03518-x
Kuc-Ciepluch, D., Gorzkiewicz, M., Pędziwiatr-Werbicka, E., Barrios-Gumiel, A., Lasak, M., Mariak, A., et al. (2022). The effect of surface modification of dendronized gold nanoparticles on activation and release of pyroptosis-inducing pro-inflammatory cytokines in presence of bacterial lipopolysaccharide in monocytes. Colloids Surf. B Biointerfaces 217, 112652. doi:10.1016/j.colsurfb.2022.112652
Kulkarni, D., Musale, S., Panzade, P., Paiva-Santos, A. C., Sonwane, P., Madibone, M., et al. (2022). Surface functionalization of nanofibers: the multifaceted approach for advanced biomedical applications. Nanomaterials 12, 3899–35. doi:10.3390/nano12213899
Kulkarni, D., Giram, P., Mahore, J., Kapare, H., and Panzade, P. (2025). Electrospun nanofibers: a promising paradigm for biomedical applications. Int. J. Polym. Mater. Polym. Biomaterials 74, 403–423. doi:10.1080/00914037.2024.2335173
Kumari, A., Sharma, A., Malairaman, U., and Singh, R. R. (2018). Proficient surface modification of CdSe quantum dots for highly luminescent and biocompatible probes for bioimaging: a comparative experimental investigation. J. Lumin 199, 174–182. doi:10.1016/j.jlumin.2018.03.032
Lasak, M., and Ciepluch, K. (2023a). Overview of mechanism and consequences of endothelial leakiness caused by metal and polymeric nanoparticles. Beilstein J. Nanotechnol. 14, 329–338. doi:10.3762/BJNAN.14.28
Lasak, M., and Ciepluch, K. (2023b). Overview of mechanism and consequences of endothelial leakiness caused by metal and polymeric nanoparticles. Beilstein J. Nanotechnol. 14, 329–338. doi:10.3762/BJNANO.14.28
Lasak, M., Nirwan, V. P., Kuc-Ciepluch, D., Lysek-Gladysinska, M., Javier de la Mata, F., Gomez, R., et al. (2024). Dendronized Ag/Au nanomats: antimicrobial scaffold for wound healing bandages. Macromol. Biosci. 24, e2300513–e2300516. doi:10.1002/mabi.202300513
Leal, F., Viraj, N., Andreia, M. G., Nikolai, P., Eva, F., Amir, F., et al. (2024). Bio-inspired nanoporous scaffold: electrospun hybrid fibers based on self-assembled block copolymer mineralized with inorganic nanoparticles for bone tissue engineering. Int. J. Polym. Mater. Polym. Biomaterials 73, 1054–1067. doi:10.1080/00914037.2023.2243369
Li, X., Robinson, S. M., Gupta, A., Saha, K., Jiang, Z., Moyano, D. F., et al. (2014). Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria. ACS Nano 8, 10682–10686. doi:10.1021/nn5042625
Lin, X., and Chen, T. (2023). A review of in vivo toxicity of quantum dots in animal models. Int. J. Nanomedicine 18, 8143–8168. doi:10.2147/IJN.S434842
Liu, Y., Rogel, N., Harada, K., Jarett, L., Maiorana, C. H., German, G. K., et al. (2017). Nanoparticle size-specific actin rearrangement and barrier dysfunction of endothelial cells. Nanotoxicology 11, 846–856. doi:10.1080/17435390.2017.1371349
Liu, Y., Yoo, E., Han, C., Mahler, G. J., and Doiron, A. L. (2018). Endothelial barrier dysfunction induced by nanoparticle exposure through actin remodeling via caveolae/raft-regulated calcium signalling. NanoImpact 11, 82–91. doi:10.1016/j.impact.2018.02.007
Liu, R., Hou, L., Yue, G., Li, H., Zhang, J., Liu, J., et al. (2022). Progress of fabrication and applications of electrospun hierarchically porous nanofibers. Adv. Fiber Mater. 4, 604–630. doi:10.1007/s42765-022-00132-z
Maduna, L., and Patnaik, A. (2024). Challenges associated with the production of nanofibers. Processes 12, 2100. doi:10.3390/pr12102100
Maliszewska, I., and Czapka, T. (2022). Electrospun polymer nanofibers with antimicrobial activity. Polym. (Basel) 14, 1661–32. doi:10.3390/polym14091661
Märkl, S., Przybilla, F., Rachel, R., Hirsch, T., Keller, M., Witzgall, R., et al. (2024). Impact of surface chemistry of upconversion nanoparticles on time-dependent cytotoxicity in non-cancerous epithelial cells. Sci. Rep. 14, 30610–30611. doi:10.1038/s41598-024-83406-3
Mohamed, T., Matou-Nasri, S., Farooq, A., Whitehead, D., and Azzawi, M. (2017). Polyvinylpyrrolidone-coated gold nanoparticles inhibit endothelial cell viability, proliferation, and ERK1/2 phosphorylation and reduce the magnitude of endothelial-independent dilator responses in isolated aortic vessels. Int. J. Nanomedicine 12, 8813–8830. doi:10.2147/IJN.S133093
Nan, J., Liu, W., Zhang, K., Sun, Y., Hu, Y., and Lei, P. (2022). Tantalum and magnesium nanoparticles enhance the biomimetic properties and osteo-angiogenic effects of PCL membranes. Front. Bioeng. Biotechnol. 10, 1038250. doi:10.3389/fbioe.2022.1038250
Ni, N., Wang, W., Sun, Y., Sun, X., and Leong, D. T. (2022). Inducible endothelial leakiness in nanotherapeutic applications. Biomaterials 287, 121640. doi:10.1016/j.biomaterials.2022.121640
Nirwan, V. P., Kowalczyk, T., Bar, J., Buzgo, M., Filová, E., and Fahmi, A. (2022). Advances in electrospun hybrid nanofibers for biomedical applications. Nanomaterials 12, 1829. doi:10.3390/nano12111829
Nirwan, V. P., Lasak, M., Ciepluch, K., and Fahmi, A. (2023). Hybrid nanomat: copolymer template CdSe quantum dots in situ stabilized and immobilized within nanofiber matrix. Nanomaterials 13, 630. doi:10.3390/nano13040630
Qian, J., Jiang, F., Wang, B., Yu, Y., Zhang, X., Yin, Z., et al. (2010). Ophiopogonin D prevents H2O2-induced injury in primary human umbilical vein endothelial cells. J. Ethnopharmacol. 128, 438–445. doi:10.1016/j.jep.2010.01.031
Rahimi, N. (2017). Defenders and challengers of endothelial barrier function. Front. Immunol. 8, 1847–10. doi:10.3389/fimmu.2017.01847
Reyes-guzmán, V. L., Villarreal-gómez, L. J., Vázquez-mora, R., Méndez-ramírez, Y. I., Paz-gonzález, J. A., Zizumbo-lópez, A., et al. (2024). Integrating an antimicrobial nanocomposite to bioactive electrospun fibers for improved wound dressing materials. 1–21.
Rodríguez-Fragoso, L., González De La Cruz, G., Rodríguez-López, A., and Rodríguez-Fragoso, P. (2024). “Toxicity of quantum dots,” in Toxicity of nanoparticles - recent advances and new perspectives. Editors M. M. Rahman, J. Uddin, A. M. Mohammed Asiri, and M. R. Rahman (Croatia, Rijeka: IntechOpen). doi:10.5772/intechopen.112073
Saravanan, H., Subramani, T., Rajaramon, S., David, H., Sajeevan, A., Sujith, S., et al. (2023). Exploring nanocomposites for controlling infectious microorganisms: charting the path forward in antimicrobial strategies. Front. Pharmacol. 14, 1282073–17. doi:10.3389/fphar.2023.1282073
Setyawati, M. I., Tay, C. Y., Chia, S. L., Goh, S. L., Fang, W., Neo, M. J., et al. (2013). Titanium dioxide nanomaterials cause endothelial cell leakiness by disrupting the homophilic interaction of VE-cadherin. Nat. Commun. 4, 1673–12. doi:10.1038/ncomms2655
Setyawati, M. I., Wang, Q., Ni, N., Tee, J. K., Ariga, K., Ke, P. C., et al. (2023). Engineering tumoral vascular leakiness with gold nanoparticles. Nat. Commun. 14, 4269. doi:10.1038/s41467-023-40015-4
Shi, J., Kantoff, P. W., Wooster, R., and Farokhzad, O. C. (2017). Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Cancer 17, 20–37. doi:10.1038/nrc.2016.108
Skrzyniarz, K., Kuc-Ciepluch, D., Lasak, M., Arabski, M., Sanchez-Nieves, J., and Ciepluch, K. (2023). Dendritic systems for bacterial outer membrane disruption as a method of overcoming bacterial multidrug resistance. Biomater. Sci. 11, 6421–6435. doi:10.1039/d3bm01255g
Song, E., Chen, K. M., Margolis, M. S., Wungcharoen, T., Koh, W. G., and Myung, D. (2024). Electrospun nanofiber membrane for cultured corneal endothelial cell transplantation. Bioengineering 11, 54. doi:10.3390/bioengineering11010054
Sullivan, J. L. (2005). Stored iron and vascular reactivity. Arterioscler. Thromb. Vasc. Biol. 25, 1532–1535. doi:10.1161/01.ATV.0000174124.20147.22
Tang, Y., Han, S., Liu, H., Chen, X., Huang, L., Li, X., et al. (2013). The role of surface chemistry in determining in vivo biodistribution and toxicity of CdSe/ZnS core–shell quantum dots. Biomaterials 34, 8741–8755. doi:10.1016/j.biomaterials.2013.07.087
Tang, C., Zhou, K., Wu, D., and Zhu, H. (2024). Nanoparticles as a novel platform for cardiovascular disease diagnosis and therapy. Int. J. Nanomedicine 19, 8831–8846. doi:10.2147/IJN.S474888
Tort, S., Demiröz, F. T., Yıldız, S., and Acartürk, F. (2020). Effects of UV exposure time on nanofiber wound dressing properties during sterilization. J. Pharm. Innov. 15, 325–332. doi:10.1007/s12247-019-09383-7
van der Meel, R., Sulheim, E., Shi, Y., Kiessling, F., Mulder, W. J. M., and Lammers, T. (2019). Smart cancer nanomedicine. Nat. Nanotechnol. 14, 1007–1017. doi:10.1038/s41565-019-0567-y
Wang, X., and He, B. (2024). Endothelial dysfunction: molecular mechanisms and clinical implications. MedComm (2020). 5, e651–27. doi:10.1002/mco2.651
Wang, B., Hu, S., Teng, Y., Chen, J., Wang, H., Xu, Y., et al. (2024). Current advance of nanotechnology in diagnosis and treatment for malignant tumors. Signal Transduct. Target Ther. 9, 200. doi:10.1038/s41392-024-01889-y
Wu, L. P., Ficker, M., Christensen, J. B., Simberg, D., Trohopoulos, P. N., and Moghimi, S. M. (2021). Dendrimer end-terminal motif-dependent evasion of human complement and complement activation through IgM hitchhiking. Nat. Commun. 12, 4858–13. doi:10.1038/s41467-021-24960-6
Xin, H., Liu, Y., Xiao, Y., Wen, M., Sheng, L., and Jia, Z. (2024). Design and nanoengineering of photoactive antimicrobials for bioapplications: from fundamentals to advanced strategies. Adv. Funct. Mater 34, 2402607. doi:10.1002/adfm.202402607
Xing, J., Zhang, W., Sun, S., and Liu, Z. (2024). Preparation of porous polylactic acid nanofibers and application in non-electret high-efficiency filtration composites. RSC Adv. 14, 14857–14867. doi:10.1039/D4RA01278J
Xue, J., Xie, J., Liu, W., and Xia, Y. (2017). Electrospun nanofibers: new concepts, materials, and applications. Acc. Chem. Res. 50, 1976–1987. doi:10.1021/acs.accounts.7b00218
Yan, D., Yao, Q., Yu, F., Chen, L., Zhang, S., Sun, H., et al. (2020). Surface modified electrospun poly(lactic acid) fibrous scaffold with cellulose nanofibrils and Ag nanoparticles for ocular cell proliferation and antimicrobial application. Mater. Sci. Eng. C 111, 110767. doi:10.1016/j.msec.2020.110767
Zhao, Z., Wang, M., Shao, F., Liu, G., Li, J., Wei, X., et al. (2021). Porous tantalum-composited gelatin nanoparticles hydrogel integrated with mesenchymal stem cell-derived endothelial cells to construct vascularized tissue in vivo. Regen. Biomater. 8, rbab051. doi:10.1093/rb/rbab051
Keywords: nanofibers, gold nanoparticles, quantum dots, tantalum nanoparticles, endothelium
Citation: Lasak M, Nirwan VP, Kuc-Ciepluch D, Tomasiuk R, Chourpa I, Fahmi A and Ciepluch K (2025) The endothelial layer formation in the presence of AuNPs/CdSe/TaNPs-loaded PLCL/PVP-based electrospun nanofibers. Front. Mol. Biosci. 12:1638442. doi: 10.3389/fmolb.2025.1638442
Received: 30 May 2025; Accepted: 28 July 2025;
Published: 18 August 2025.
Edited by:
Chao Wang, University of Illinois Chicago, United StatesReviewed by:
Deepak Kulkarni, Srinath College of Pharmacy, IndiaLiyuan Sheng, Peking University, India
Copyright © 2025 Lasak, Nirwan, Kuc-Ciepluch, Tomasiuk, Chourpa, Fahmi and Ciepluch. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karol Ciepluch, ay5jaWVwbHVjaEB1cmFkLmVkdS5wbA==
 Magdalena Lasak1
Magdalena Lasak1 Amir Fahmi
Amir Fahmi Karol Ciepluch
Karol Ciepluch