Abstract
FNDC3B is an endoplasmic reticulum (ER)-anchored transmembrane protein with diverse roles in cell adhesion, migration, and growth signaling. Recognized as multifunctional, it contributes to key cellular processes such as adhesion, proliferation, differentiation, and migration, yet its molecular functions remain largely unannotated in the Gene Ontology database. Initially identified as a regulator of adipogenesis, promoting fat cell differentiation, FNDC3B also facilitates lung cell maturation, which is essential for neonatal survival. Dysregulation of FNDC3B is implicated in cancer progression through the promotion of epithelial–mesenchymal transition (EMT), metastasis, and modulation of multiple oncogenic signaling pathways. Intriguingly, it exhibits dual roles, acting as either an oncogene or a tumor suppressor, depending on the cellular context. However, the mechanistic determinants of this duality remain elusive. Beyond malignancies, FNDC3B also participates in non-cancerous pathologies, underscoring its broad physiological significance. Although 27 phosphosites have been identified in FNDC3B, the associated signaling networks and functional implications of these modifications remain obscure within the dark phosphoproteome. This review comprehensively delineates the structural, functional, and pathological aspects of FNDC3B, emphasizing its role as a molecular bridge between extracellular and intracellular networks and its growing clinical relevance.
1 Introduction
Fibronectin type III domain-containing 3B (FNDC3B), also known as Factor for adipocyte differentiation 104 (FAD104), is an endoplasmic reticulum (ER) transmembrane protein encoded on the long arm (q arm) of chromosome 3, specifically at position 3q26. It has a sequence length of 1,204 amino acids and a molecular weight of 133 kDa (Cai et al., 2012; Fucci et al., 2020). Two major isoforms, arising from alternative splicing, have been reported and differ in sequence length. FNDC3B is one of 11 members of the fibronectin type III domain-containing (FNDC) protein family, characterized by the presence of at least one conserved fibronectin type III (FNIII) domain. FNIII domains are evolutionarily conserved regions of ∼90–100 amino acids, adopting a characteristic β-sandwich fold that contributes to structural stability and protein–protein interactions (Jiang et al., 2022; Ruan et al., 2021). In addition to the FNIII domain, FNDC3B possesses a single transmembrane domain at its C-terminus, which anchors it to the ER membrane and is critical for its function (Nishizuka et al., 2009).
FNDC3B is highly expressed in white adipose tissue, particularly in stromal vascular cells and differentiating adipocytes (Tominaga et al., 2004; Kishimoto et al., 2010), and is moderately expressed in other organ systems, including the endometrium, ovary, and male reproductive tract (Tominaga et al., 2004; Daudon et al., 2022). It was initially recognized for its roles in adipocyte regulation, osteoblast differentiation, and tumor suppression (Kishimoto et al., 2010; Nishizuka et al., 2009). Subsequent studies have implicated FNDC3B in the regulation of cell adhesion, proliferation, migration, signaling, and the epithelial–mesenchymal transition (EMT) (Nishizuka et al., 2009; Han et al., 2020). FNDC3B has been shown to activate several signaling pathways, including integrin, PI3K/Akt, AMPK, TGFβ, and mTOR pathways (Cai et al., 2012). Altered FNDC3B expression has also been reported in several cancers, including pancreatic cancer, cervical cancer, hepatocellular carcinoma, and lung adenocarcinoma (Wang et al., 2024; Han et al., 2020; Lin et al., 2016; Bian et al., 2019). Collectively, these findings highlight FNDC3B as a key component of cellular progression and signaling dynamics.
2 FNDC3B structural and functional characteristics
The fibronectin type III domain-containing (FNDC) protein family consists of 11 members: FNDC1, FNDC3A, FNDC3B, FNDC4, FNDC5, FNDC6 interleukin-20 receptor subunit beta (IL20RB), FNDC7, FNDC8, FNDC9, FNDC10, and FNDC11, each characterized by the presence of at least one conserved fibronectin type III (FNIII) domain, which is illustrated in Figure 1. Although FNDC3B has been implicated in the regulation of adipocyte and osteoblast differentiation, its activation mechanism under physiological conditions remains undefined (Kishimoto et al., 2010). FNDC3B is characterized by nine fibronectin type III (FNIII) domains, a hydrophobic transmembrane segment at its C-terminus, and an N-terminal proline-rich motif (Obholz et al., 2006). Individual FNIII repeats in FNDC3B, approximately 90 amino acids in length, adopt a conserved β-sandwich fold of seven antiparallel β-strands arranged into two sheets. Despite their low sequence identity (∼20–40%), these FNIII domains exhibit high structural homology. FNIII domains constitute one of the largest and most widespread subdomain types in fibronectin, occurring across extracellular matrix proteins, cell-surface receptors, and enzymes (Leahy et al., 1992; Bork and Doolittle, 1992), where they function as structural scaffolds mediating diverse molecular interactions critical for cell adhesion and growth signaling (Lin et al., 2011; Obara et al., 2010). Notably, the first four FNIII domains of FNDC3B have been identified as key mediators of its role in cell migration (Lin et al., 2016).
FIGURE 1

A schematic representation of the Domain architecture of FNDC family members. Domain information was retrieved from the SMART Database.
Considering that, on average any given amino acid makes up about 5% of a protein’s total composition, FNDC3B constitutes ∼13.3% of proline composition in its N-terminal making the N-terminal proline-rich motif (Zhang et al., 2025). This motif imparts structural rigidity through side-chain cyclization with the backbone, thereby stabilizing the three-dimensional conformation and facilitating protein-protein interaction (Adzhubei et al., 2013). At its C-terminus, a hydrophobic transmembrane (TM) domain anchors FNDC3B to the endoplasmic reticulum (ER) membrane, where it participates in essential processes including protein synthesis, folding, trafficking, and ER stress regulation via the unfolded protein response (UPR) (Chi-Rosso et al., 1997; Lin et al., 2016). Both the proline-rich motif and the TM domain are critical for FNDC3B’s metastasis-related function. Notably, deletion of the TM domain shifts its localization from an ER bound cytoplasmic form to the nucleus, resulting in loss of its metastasis-promoting activity (Zhang et al., 2025).
3 FNDC3B evolutionary conservation
FNDC3B shares structural similarity with FNDC3A, containing nine fibronectin type III domains, an N-terminal proline-rich motif, and a hydrophobic C-terminal tail. Multiple sequence alignment using the T-Coffee Expresso tool reveals an alignment score of 95, underscoring their close evolutionary relationship. Both proteins contain a conserved N-terminal PPGY motif within a proline-rich region, which serves as a binding site for WW- and SH3-domain-containing proteins (Daudon et al., 2022; Obholz et al., 2006). FNDC3B is also highly conserved across species, including Homo sapiens, Neanderthals, Macaca mulatta, and Canis lupus, with human and mouse FNDC3B sharing 92.5% amino acid sequence similarity (Zhu et al., 2023; Kishimoto et al., 2010). These conserved features highlight the functional importance of FNDC3B in cellular processes relevant to health and disease.
4 Regulation of FNDC3B by transcription factors and non-coding RNAs
The expression and activity of FNDC3B are tightly regulated by transcriptional and post-transcriptional mechanisms. Transcription factors such as E2F1 directly bind to the FNDC3B promoter, upregulating its expression and facilitating tumor cell migration and metastasis in hepatocellular carcinoma (HCC). Functional assays demonstrate that E2F1 overexpression significantly enhances FNDC3B levels, while knockdown suppresses its expression and reduces migratory capacity (Hua et al., 2024). In addition, non-coding RNAs, including microRNAs and circular RNAs, serve as important regulators of FNDC3B function. For instance, in cervical cancer, hsa_circ_0001627 promotes tumor progression by sponging miR-1225-5p, a microRNA that normally represses FNDC3B expression. Inhibition of miR-1225-5p by this circRNA elevates FNDC3B expression, which in turn activates PI3K/mTOR signaling to enhance malignancy (Li et al., 2023). Similarly, in colorectal cancer, miR-125a-5p and miR-217 regulate FNDC3B-driven mTOR activation, with increased FNDC3B levels being predictive of poor survival outcomes (Li et al., 2020).
5 FNDC3B’s function in cellular dynamics
Dr. Masayoshi Imagawa’s team has extensively studied FNDC3B, where knockdown of its expression was shown to markedly suppress adipogenesis, and FNDC3B-deficient mice died within 1 day of birth, underscoring its essential role in postnatal survival. They further demonstrated that mouse embryonic fibroblasts (MEFs) lacking FNDC3B displayed impaired adipocyte differentiation, reduced proliferation, and compromised cytoskeletal organization, leading to defective stress fibre formation and delayed adhesion, spreading, and migration (Nishizuka et al., 2009). Importantly, FNDC3B was found to reciprocally regulate mesenchymal differentiation, functioning as a positive regulator of adipogenesis and a negative regulator of osteogenesis (Kishimoto et al., 2010). Their phenotypic and morphological analyses revealed that neonatal lethality resulted from cyanosis-associated lung dysplasia, including atelectasis, and immunohistochemical studies identified strong FNDC3B expression in alveolar type II (ATII) cells, confirming its indispensable role in lung maturation and ATII cell differentiation (Kishimoto et al., 2011). In the context of osteogenesis, they showed that FNDC3B disruption caused craniosynostosis-like premature calvarial ossification, and primary calvarial cell analyses established FNDC3B as a negative regulator of the BMP/Smad signaling pathway. Mechanistically, the N-terminal proline-rich motif of FNDC3B directly interacted with Smad1/5/8, thereby attenuating their phosphorylation and downstream transcriptional activity (Chen et al., 2012; Kishimoto et al., 2013).
Extending their research beyond development, Dr. Imagawa’s team demonstrated that FNDC3B is also implicated in tumor progression. In human cervical cancer HeLa cells, FNDC3B expression was upregulated during TGF-β–induced epithelial-to-mesenchymal transition (EMT). Knockdown of FNDC3B enhanced TGF-β–mediated EMT and migration, whereas overexpression suppressed EMT. Mechanistic studies revealed that FNDC3B negatively regulated Smad2/3 phosphorylation but positively regulated Smad1/5/8 phosphorylation during EMT (Goto et al., 2017). In melanoma, FNDC3B was shown to suppress invasion and metastasis by inhibiting STAT3 signaling. Its expression was lower in highly metastatic A375SM cells than in poorly metastatic A375C6 cells, and reduction of FNDC3B enhanced, while overexpression suppressed melanoma cell migration and invasion. Stable expression of FNDC3B further reduced lung colonization in vivo, consistent with its role as a negative regulator of STAT3 phosphorylation and transcriptional activity, without affecting total STAT3 protein levels (Katoh et al., 2015). Mechanistically, FNDC3B was found to interact with the C-terminal region of STAT3, and deletion analysis confirmed that its N-terminal region is indispensable for STAT3 inhibition and suppression of anchorage-independent melanoma growth, thereby establishing FNDC3B as a critical negative regulator of malignant transformation (Katoh et al., 2016).
FNDC3B has also been detected in stress granules that assemble under conditions of cellular stress (Jain et al., 2016). These dense ribonucleoprotein aggregates, composed of mRNAs and proteins, serve as transient sites for translational regulation. Consistent with this, FNDC3B has been identified as a candidate RNA-binding protein (Castello et al., 2012).
6 Cellular signaling associated with FNDC3B
A comprehensive understanding of cellular signaling is fundamental to deciphering protein function, as these networks govern the spatial and temporal regulation of activity, interactions, and downstream outcomes in both physiological and pathological contexts. Among these mechanisms, phosphorylation serves as a dynamic switch that modulates protein activity, interactions, and localization, thereby orchestrating cellular responses to environmental cues. Mapping phosphosignaling patterns is essential to identify functional signaling networks, understand their perturbations in disease, and develop targeted therapeutic strategies (Krug et al., 2019). According to the PhosphoSitePlus database, 27 phosphosites have been identified in human FNDC3B, with the majority located in the N-terminal and C-terminal regions (Hornbeck et al., 2019). The molecular significance of none of these phosphosites is currently known, and the kinases mediating these phosphorylations have also not been identified, rendering FNDC3B an enigmatic protein within the dark phosphoproteome.
Further, based on our research interest, we examined the phosphosites of FNDC3B that were differentially expressed across global cellular phosphoproteomic datasets previously curated and processed in our laboratory (Mahin et al., 2025; Khan et al., 2025). This heterogeneous pool of data was obtained from PubMed-indexed studies conducted under diverse experimental conditions and includes multitemporal datasets generated by researchers worldwide. Across this dataset, we identified 7 phosphosites of FNDC3B that were differentially expressed, with a minimum standard cutoff of a p-value <0.05 and a fold change of ≥1.3 for upregulation and ≤0.76 for downregulation. The phosphosites identified in this dataset exhibited a pattern of occurrence similar to that reported in the PhosphoSitePlus database, with six of the seven sites located in the N-terminal region of FNDC3B. The frequency of their differential expression varied, with phosphosites S211, S260, S257, S254, S238, S393, and S208 identified as differentially expressed in 3, 3, 6, 2, 4, 5, and 74 datasets, respectively (Supplementary Table S1). Interestingly, phosphorylation at S208 of FNDC3B appears to play a predominant role in its signaling compared to other phosphosites, as it is reported to be detected across 45 high-throughput datasets in the PhosphoSitePlus database and was identified to be differentially expressed in 74 cellular phosphoproteomic datasets in our analysis. Differential expression of S208 was observed under diverse experimental conditions, including cancer-associated cell line studies such as lung adenocarcinoma, glioblastoma, breast cancer, squamous cell carcinoma, and colorectal cancer. However, the molecular significance of phosphorylation at S208 and the kinase responsible for this modification remain poorly characterized.
Furthermore, analysis of FNDC3B-associated data from the ActiveDriver database, which integrates genetic variation in human and cancer genomes with post-translational modification sites and signaling networks, revealed 13 mutations located adjacent to phosphosites or within the phosphomotifs of nine FNDC3B phosphosites, which have been implicated in various cancers (Krassowski et al., 2021). These mutations include R202L in uterine corpus endometrial carcinoma, R205C in glioblastoma multiforme, G243E and P242L in head and neck and skin cutaneous melanomas, R252T in pancreatic adenocarcinoma, T489S and T489I in hepatocellular carcinoma and endometrial carcinoma, and L1157R in hepatocellular carcinoma. The schematic overview of phosphosites and the mutation adjacent to phosphosites or within the phosphomotifs, which are associated with various cancers, is represented in Figure 2. These reported variations associated with FNDC3B phosphosites and their clinical relevance warrant detailed analysis of FNDC3B-associated cellular phosphosignaling patterns.
FIGURE 2
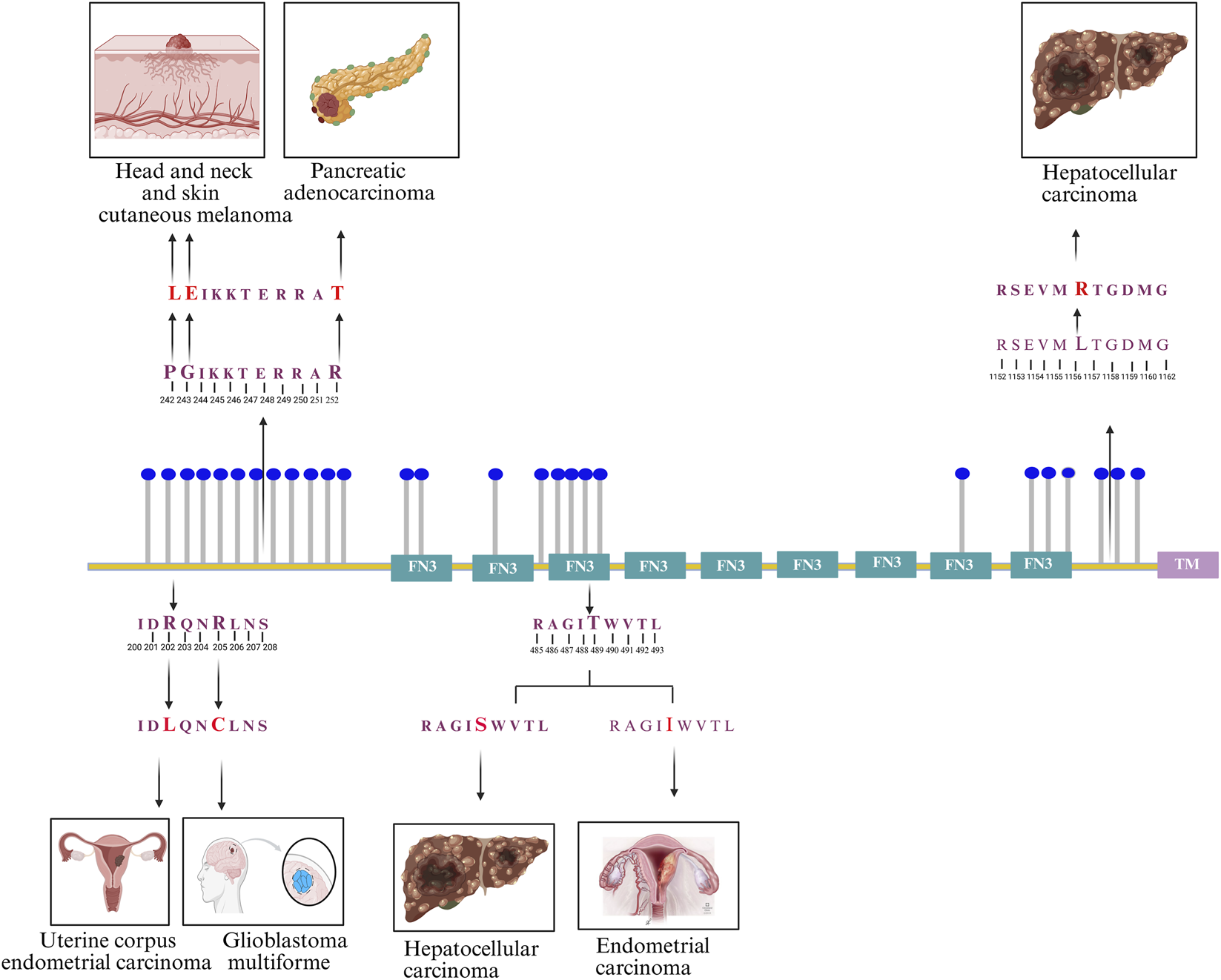
Schematic representation of 27 FNDC3B phosphosites reported in the PhosphoSitePlus database, with depictions of mutations associated with distinct cancer types occurring within phosphomotifs. These include R202L (uterine corpus endometrial carcinoma), R205C (glioblastoma multiforme), G243E and P242L (head and neck and skin cutaneous melanomas), R252T (pancreatic adenocarcinoma), T489S and T489I (hepatocellular and endometrial carcinomas), and L1157R (hepatocellular carcinoma).
7 Pathological significance associated with FNDC3B
FNDC3B has emerged as a context-dependent regulator of cancer biology, displaying both oncogenic and tumor-suppressive properties. Aberrant overexpression of FNDC3B has been documented in multiple malignancies, including hepatocellular carcinoma (HCC), acute myeloid leukemia (AML), tongue squamous cell carcinoma, colorectal, ovarian, breast, cervical, and pancreatic cancers (Han et al., 2020; Chen et al., 2010; Wang X. et al., 2022). Elevated FNDC3B expression is consistently linked to poor prognosis, including reduced progression-free intervals and overall survival. In pancreatic cancer, it is notably proposed as a prognostic biomarker and therapeutic target due to its markedly higher expression in tumor tissues compared with adjacent normal tissues (Wang et al., 2024). In hepatocellular carcinoma, FNDC3B promotes oncogenic signaling by interacting with annexin A2 (ANXA2) to remodel actin cytoskeletal architecture, thereby driving cell motility and metastatic potential (Lin et al., 2016). In gastric cancer, FNDC3B directly interacts with and stabilizes Family with Sequence Similarity 83 Member H (FAM83H), preventing its proteasomal degradation and consequently activating the Snail-driven EMT program to promote invasion and metastasis (Zhang et al., 2025). Conversely, in melanoma, FNDC3B has been reported to exert tumor-suppressive activity by inhibiting STAT3 phosphorylation and downstream transcriptional activation (Kwon et al., 2023).
Beyond cancer, FNDC3B also participates in non-malignant diseases, further underscoring its pleiotropic biological functions. Genome-wide association studies (GWAS) have linked FNDC3B to keratoconus, with the SNP rs4894535 showing a significant association (OR = 1.47, 95% CI = 1.29–1.68, P = 4.9 × 10−9), highlighting its contribution to disease susceptibility (Lu et al., 2013; Sahebjada et al., 2013; Rong et al., 2017). GWAS have also implicated FNDC3B in intraocular pressure regulation and primary open-angle glaucoma, with significant association at chromosome 3q25.31 (rs6445055, P = 4.19 × 10−8), alongside additional loci in ABCA1, ABO, and chromosome 11p11.2 (Hysi et al., 2014; Wiggs and Pasquale, 2017). An association of SNP in FNDC3B (rs7636836) with pseudoexfoliation glaucoma among men, and an increased risk of primary open-angle glaucoma have been reported in the Japanese and Middle-Eastern Saudi cohorts (Shiga et al., 2018; Kondkar et al., 2022). FNDC3B is also linked to sporadic Parkinson’s disease, with GWAS showing strong association (Hu et al., 2016). A rare chromosomal translocation t(3; 17) (q26; q21) gives rise to the FNDC3B-RARA fusion gene in a variant form of acute promyelocytic leukemia (APL). This fusion links exon 24 of FNDC3B with exon 3 of retinoic acid receptor alpha (RARA), producing chimeric transcripts that repress retinoic acid-responsive elements, impair granulocytic differentiation, and promote leukemogenesis (Cheng et al., 2017; Mannan et al., 2020).
FNDC3B is also associated with metabolic and organ specific disorders. Kei Tominaga et al. demonstrated FNDC3B as a positive regulator of adipogenesis. He observed a transient expression of FNDC3B at the earlier stages of adipocyte differentiation. Further, knockdown of FNDC3B by RNAi in 3T3-L1 cells significantly declined its ability to differentiate to mature adipocytes (Tominaga et al., 2004). Moreover, regulation of FNDC3B by miR-215-5p has been reported to inhibit adipocyte differentiation of 3T3-L1 cells (Peng et al., 2016). Although its role in adipocyte differentiation can be considered as a plausible attributing factor to obesity, a thorough characterization of the functional role of FNDC3B in obesity is still missing. However, with the positive energy balance considered as a primary cause of obesity, GWAS of individuals of European ancestry from the Atherosclerosis Risk in Communities and UK Biobank populations analyzed by Gulisija et al, genomic variants in FNDC3B loci were found associated with distinct sets of energy balance-contributing traits such as physical activity and increased hip circumference (rs582780) (Gulisija et al., 2025). Furthermore, FNDC3B has been reported to alleviate hepatic steatosis and ferroptosis via activation of AMP-activated protein kinase (AMPK) (You et al., 2022), whereas in renal ischemia–reperfusion injury, it promotes tubular epithelial apoptosis through TGF-β1 signaling, an effect antagonized by the protective lncRNA XLOC_032768 (Zhou et al., 2020). Despite accumulating reports on the involvement of FNDC3B in various cancers, its role in metabolic diseases and its potential as therapeutic target remains largely unexplored and warrants further investigations. The involvement of FNDC3B in diverse pathological conditions is represented in Figure 3.
FIGURE 3

Diagram illustrating FNDC3B interactions affecting various conditions. Arrows show pathways connecting FNDC3B to acute promyelocytic leukemia, renal injury, hepatocellular carcinoma, gastric cancer, melanoma, glaucoma types, and keratoconus. Elements include chromosomal translocation, cell migration, STAT3 phosphorylation, and genetic variants, emphasizing FNDC3B’s role in diverse diseases.
8 Circular FNDC3B in oncology and beyond
FNDC3B is also identified in the form of circular RNA. circFNDC3B is derived from exons 5 and 6 of the FNDC3B gene that exhibits high cytoplasmic stability with a half-life exceeding 24 h (Zeng et al., 2020). Functionally, it regulates gene expression through diverse mechanisms, including sponging oncogenic or tumour-suppressive miRNAs (Tang et al., 2022) and encoding a 218-amino-acid peptide (circFNDC3B-218aa) that modulates cancer progression and metabolic pathways (Pan et al., 2020). Its role in tumor biology is highly context-dependent. In bladder cancer, circFNDC3B is downregulated and acts as a tumor suppressor by modulating the miR-1178-3p/G3BP2 axis (Liu et al., 2018). Similarly, in colorectal cancer, reduced expression of circFNDC3B exerts inhibitory effects on epithelial–mesenchymal transition (EMT), angiogenesis, and metastasis via the miR-937-5p/TIMP3 pathway, in addition to producing circFNDC3B-218aa with tumor-suppressive activity (Zeng et al., 2020; Zeng et al., 2022). In contrast, circFNDC3B is markedly upregulated in several malignancies, where it assumes oncogenic functions. In gastric cancer, it facilitates migration, invasion, and EMT through the IGF2BP3/CD44 signaling axis (Hong et al., 2019; Zhang et al., 2022). In esophageal squamous cell carcinoma, circFNDC3B promotes proliferation and migration by regulating the miR-490-5p/TXNRD1 and miR-214-3p/CDC25A pathways (Tang et al., 2022; Wang J. et al., 2022). In renal carcinoma, it contributes to curcumin-mediated tumor suppression by modulating the miR-138-5p/IGF2 axis (Xue et al., 2021), while in oral squamous cell carcinoma it enhances malignant phenotypes by suppressing ferroptosis through ceRNA networks such as miR-520d-5p/SLC7A11 and miR-1322/MED1 (Yang et al., 2021).
Emerging evidence also implicates circFNDC3B in non-malignant diseases. In cardiovascular disease, circFNDC3B is significantly downregulated following myocardial infarction, where it promotes cardiac repair by enhancing VEGF-A expression through interaction with the RNA-binding protein FUS (Fused in Sarcoma) (RBP FUS) (Garikipati et al., 2019). Conversely, in abdominal aortic aneurysm, circFNDC3B expression is elevated and contributes to vascular pathology by regulating inflammatory and oxidative stress responses via the miR-143-3p/ADAM10 axis (Liu et al., 2021). Taken together, circFNDC3B acts as a context dependent multifunctional regulator with dual oncogenic and tumor-suppressive properties in cancer, while also exerting critical effects in non-cancerous conditions (Sun et al., 2023).
9 Preclinical and clinical aspects of FNDC3B
To the best of our knowledge, there are no active or completed clinical trials directly targeting FNDC3B. Nevertheless, FNDC3B has become the subject of extensive preclinical investigation, primarily in cell-based systems and animal models, and is increasingly recognized as a potential therapeutic target and prognostic biomarker in multiple malignancies. Genetic knockout studies in mice reveal that loss of FNDC3B results in perinatal lethality accompanied by defects in adipocyte differentiation, underscoring its essential role in development and tissue homeostasis (Kishimoto et al., 2011). A substantial body of preclinical evidence supports a context-dependent oncogenic role for FNDC3B across diverse disease models. Elevated FNDC3B expression has been consistently reported in hepatocellular carcinoma, cervical cancer, gastric cancer, lung adenocarcinoma, and glioma, where it correlates with increased cellular proliferation, migration, invasion, acquisition of EMT like phenotypes, stemness-associated traits, and unfavorable clinical outcomes. A preliminary analysis demonstrated the prognostic as well as therapeutic potential of FNDC3B in pancreatic cancer (Wang et al., 2024) Integrated machine learning approaches revealed that FNDC3B can be used as a prognostic and immunotherapeutic biomarker in glioma (Wang X. et al., 2022). Kwon et al. illustrates that FNDC3B could be a promising therapeutic target in GBM patients (Kwon et al., 2023). FNDC3B was found to interact with FAM83H (a regulatory protein involved in EMT) and prevents the ubiquitin-proteasome degradation of FAM83H which in turn promotes gastric cancer progression (Zhang et al., 2025). The expression of FNDC3B is found enhanced in cancers including cervical cancer as well as hepatocellular carcinoma and leads to its metastasis (Han et al., 2020; Lin et al., 2016). Though several reports highlight the potential of FNDC3B as a biomarker and therapeutic target in diverse cancers, the exact function and mechanism of FNDC3B in cancer progression is still unclear. An integrated bioinformatic study by Wu et al highlights FNDC3B as a key regulatory gene involved ECM-receptor interaction, ECM remodelling and immune infiltration in oral squamous cell carcinoma and periodontal disease (Wu et al., 2025). A comprehensive analysis identified E2F1 as a transcriptional activator of FNDC3B and is involved in cell migration in hepatocellular carcinoma (Hua et al., 2024). Functional studies further demonstrate that genetic silencing or knockdown of FNDC3B significantly suppresses tumor growth, invasive capacity, and metastatic dissemination in both in vitro assays and xenograft or orthotopic animal models, reinforcing its functional relevance in tumor progression (Lin et al., 2016; Bian et al., 2019; Wang J. et al., 2022; Zhang et al., 2025). Fusion of FNDC3B with other genes such as PRKCI, EVI1 and RARA is implicated in multiple cancers and reinstates the significance of FNDC3B in pathophysiological processes (Hua et al., 2017; Wang et al., 2016; Cheng et al., 2017). Analyses of large-scale transcriptomic datasets derived from clinical samples, including those from The Cancer Genome Atlas and the Human Protein Atlas, consistently associate elevated FNDC3B expression with reduced overall and disease-free survival across multiple cancer types. High FNDC3B expression further correlates with adverse clinicopathological features, such as higher tumor grade, advanced disease stage, and lymph node metastasis (Wang X. et al., 2022; Jiang et al., 2022; Kwon et al., 2023).
10 Current strategies for targeting FNDC3B
While FNDC3B represents a potentially attractive therapeutic target, no specific or clinically approved small-molecule inhibitors of FNDC3B are currently available. Moreover, the absence of an experimentally resolved crystal structure for FNDC3B has constrained structure-based drug discovery efforts for candidate small-molecule inhibitors. Consequently, the identification of FNDC3B-directed lead compounds remains a significant unmet challenge. Current preclinical studies have predominantly employed genetic and transcript-level strategies involving siRNA, shRNA, antisense oligonucleotides (ASOs), and CRISPR/Cas9 to study FNDC3B-driven cellular phenotypes (Fan et al., 2013; Liedtke et al., 2019). At the protein level, structural and functional analyses reveal that FNDC3B harbours fibronectin type III domains and a proline-rich N-terminal region that mediate interactions with cytoskeletal and membrane-associated proteins, notably annexin A2 (ANXA2), which are critical for cell motility and invasive behaviour (Lin et al., 2016). Accordingly, disruption of these protein–protein interactions using peptide-based inhibitors, small-molecule PPI inhibitors, or engineered biologics represents a plausible therapeutic strategy.
In parallel, FNDC3B has been linked to activation of the PI3K/AKT/mTOR signaling axis, regulation of epithelial–mesenchymal transition, cytoskeletal remodelling, and endoplasmic reticulum stress responses (Li et al., 2020), indicating that indirect pathway-based interventions may also mitigate FNDC3B-driven phenotypes. In this context, pharmacological inhibitors targeting PI3K, AKT, mTOR, focal adhesion kinase (FAK), or Rho/ROCK signaling pathways offer immediately actionable avenues for therapeutic modulation. Furthermore, emerging proteolysis-targeting strategies, including proteolysis-targeting chimeras (PROTACs) and molecular glue degraders, provide a conceptual framework for selectively eliminating FNDC3B at the protein level, contingent upon the identification of suitable binding ligands, and may offer advantages over functional inhibition alone (Li et al., 2020; Zhao et al., 2022). A summary table depicting the functional role, molecular signaling, pathological relevance and current strategies for targeting FNDC3B is represented in Table 1.
TABLE 1
| Categories of discussion | Biological context | Mechanistic insight | References |
|---|---|---|---|
| Functional roles | Adipogenesis | Positive regulator of adipocyte differentiation | Kishimoto et al. (2010) |
| Osteogenesis | Negative regulator of osteoblast differentiation | Kishimoto et al. (2010) | |
| Cell adhesion and migration | Controls cytoskeletal organization, stress fiber formation, and cell spreading | Lin et al. (2011), Obara et al. (2010), Nishizuka et al. (2009) | |
| Epithelial–mesenchymal transition (EMT) | Context-dependent EMT regulation via TGF-β and Snail-associated pathways | Goto et al. (2017) | |
| Molecular pathways | TGF-β/Smad signaling | FNDC3B negatively regulated Smad2/3 phosphorylation but positively regulated Smad1/5/8 phosphorylation during EMT | Goto et al. (2017) |
| PI3K/Akt/mTOR | Promotes tumor progression in multiple cancers | Cai et al. (2012), Li et al. (2023), Li et al. (2020) | |
| AMPK | Protective role in hepatic steatosis and ferroptosis | You et al. (2022) | |
| Pathologic Conditions | Oncogenic roles | HCC, pancreatic, gastric, colorectal, cervical, ovarian, breast cancers (EMT activation, cytoskeletal remodeling, mTOR signaling) | Lin et al. (2016), Wang et al. (2024), Zhang et al. (2025), Han et al. (2020), Chen et al. (2010), X. Wang J. et al. (2022) |
| Acute Promyelocytic Leukemia | FNDC3B–RARA fusion | Cheng et al. (2017), Mannan et al. (2020) | |
| Tumor-suppressive roles | Melanoma (Inhibits STAT3 phosphorylation and metastasis) | Kwon et al. (2023) | |
| Non-cancer Diseases a. Alcoholic fatty liver disease | Alleviates hepatic steatosis via AMPK | You et al. (2022) | |
| b. Renal injury | Promotes apoptosis via TGF-β1 signaling | Zhou et al. (2020) | |
| c. Eye diseases | Keratoconus, glaucoma (GWAS) | Lu et al. (2013), Sahebjada et al. (2013), Rong et al. (2017), Hysi et al. (2014), Wiggs and Pasquale (2017) | |
| Circular RNA (circFNDC3B) | Oncogenic roles | Gastric, ESCC, oral cancer | Hong et al. (2019), Zhang et al. (2022), Tang et al. (2022), J. Wang X. et al. (2022), Yang et al. (2021) |
| Tumor suppressor roles | Bladder, colorectal cancer | Liu et al. (2018), Zeng et al. (2020), Zeng et al. (2022) | |
| Non-cancer roles | Cardiac repair, vascular inflammation | Garikipati et al. (2019), Liu et al. (2021) | |
| Cellular Signaling | Known phosphosites | 27 sites reported in PhosphoSitePlus | Hornbeck et al. (2019) |
| Predominant phosphosite | S208 is most frequently detected across datasets | Mahin et al. (2025) | |
| Cancer-associated mutations | 13 mutations located adjacent to phosphosites or within the phosphomotifs | Krassowski et al. (2021) | |
| Current strategies for targeting FNDC3B | Genetic and transcript-level strategies | siRNA, shRNA, antisense oligonucleotides (ASOs), and CRISPR/Cas9 | Fan et al. (2013), Liedtke et al. (2019) |
| Protein–protein interaction targeting | Peptide-based inhibitors, small-molecule PPI inhibitors, or engineered biologics | Lin et al. (2016) | |
| Pathway-based therapeutic approaches | PI3K, AKT, mTOR, FAK, Rho/ROCK inhibitors | Li et al. (2020) | |
| Proteolysis-targeting strategies | Proteolysis-targeting chimeras (PROTACs) and molecular glue degraders | Li et al. (2020), Zhao et al. (2022) |
Summary of functional roles, molecular signaling pathways, pathological relevance, and current strategies for targeting FNDC3B is provided in the table.
11 Future directions and conclusion
FNDC3B has emerged as a multifunctional protein with critical roles in cellular processes and diverse pathological conditions, particularly in cancer. However, its precise molecular functions remain insufficiently understood. Defining the mechanisms that govern FNDC3B localization, interactions, and signaling is a key priority. In particular, the transmembrane domain warrants focused investigation, as it anchors FNDC3B to the endoplasmic reticulum, a localization essential for promoting cell migration and epithelial–mesenchymal transition (EMT). Loss of this domain causes nuclear mislocalization and abrogates its migration-inducing activity, underscoring its functional importance. Equally important is mapping the kinase signaling pathways that regulate FNDC3B through phosphorylation, as the kinases responsible for modifications at key sites, such as S208, remain unidentified.
FNDC3B is increasingly recognized as an oncogene driving cancer progression and metastasis across multiple tumor types. Future work should prioritize elucidating its signaling networks, post-translational modifications, and protein–protein interactions, which are crucial for the development of targeted therapies. Additionally, functional characterization of FNDC3B-derived circular RNAs and their encoded peptides will provide insights into their context-dependent tumor-suppressive or oncogenic roles. FNDC3B’s emerging involvement in immune modulation also highlights its potential as an immunotherapeutic target, particularly in reshaping the tumor microenvironment. Finally, a detailed analysis of the phospho-signaling patterns that alter FNDC3B at the molecular level, as well as the kinases that mediate these phosphorylations, needs to be explored to better comprehend FNDC3B for therapeutic and clinical interventions.
Statements
Author contributions
AK: Writing – review and editing, Writing – original draft, Resources, Formal Analysis. AM: Investigation, Writing – review and editing, Conceptualization. FL: Writing – review and editing. AB: Writing – review and editing. AG: Data curation, Writing – review and editing. SS: Writing – review and editing, Supervision. RR: Supervision, Conceptualization, Writing – review and editing, Formal Analysis.
Funding
The author(s) declared that financial support was not received for this work and/or its publication.
Acknowledgments
The authors acknowledge Yenepoya (deemed to be) University, Mangalore, for providing infrastructure for the Centre for Integrative Omics Data Science.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmolb.2026.1741530/full#supplementary-material
Abbreviations
FNDC3B, Fibronectin type III domain-containing 3B; FAD104, Factor for adipocyte differentiation 104; ER, Endoplasmic Reticulum; FNIII, fibronectin type III; EMT, Epithelial–mesenchymal transition; TM, Transmembrane domain; UPR, Unfolded protein response; MEFs, Mouse embryonic fibroblasts; E2F1, E2F transcription factor 1; ANXA2, Annexin A2; FAM83H, Family with sequence similarity 83 member H; STAT3, Signal Transducer and Activator of Transcription 3.
References
1
Adzhubei A. A. Sternberg M. J. E. Makarov A. A. (2013). Polyproline-II helix in proteins: structure and function. J. Molecular Biology425 (12), 2100–2132. 10.1016/j.jmb.2013.03.018
2
Bian T. Zheng L. Jiang D. Liu J. Zhang J. Feng J. et al (2019). Overexpression of fibronectin type III domain containing 3B is correlated with epithelial-mesenchymal transition and predicts poor prognosis in lung adenocarcinoma. Exp. Therapeutic Medicine17 (5), 3317–3326. 10.3892/etm.2019.7370
3
Bork P. Doolittle R. F. (1992). Proposed acquisition of an animal protein domain by bacteria. Proc. Natl. Acad. Sci. U. S. A.89 (19), 8990–8994. 10.1073/pnas.89.19.8990
4
Cai C. Rajaram M. Zhou X. Liu Q. Marchica J. Li J. et al (2012). Activation of multiple cancer pathways and tumor maintenance function of the 3q amplified oncogene FNDC3B. Cell. CycleGeorget. Tex.11 (9), 1773–1781. 10.4161/cc.20121
5
Castello A. Fischer B. Eichelbaum K. Horos R. Beckmann B. M. Strein C. et al (2012). Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell.149 (6), 1393–1406. 10.1016/j.cell.2012.04.031
6
Chen C.-F. Hsu E. C. Lin K. T. Tu P. H. Chang H. W. Lin C. H. et al (2010). Overlapping high-resolution copy number alterations in cancer genomes identified putative cancer genes in hepatocellular carcinoma. Hepatol. Baltim. Md.52 (5), 1690–1701. 10.1002/hep.23847
7
Chen G. Deng C. Li Y.-P. (2012). TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. Journal Biological Sciences8 (2), 272–288. 10.7150/ijbs.2929
8
Cheng C. K. Wang A. Z. Wong T. H. Y. Wan T. S. K. Cheung J. S. Raghupathy R. et al (2017). FNDC3B is another novel partner fused to RARA in the t(3;17)(q26;q21) variant of acute promyelocytic leukemia. Blood129 (19), 2705–2709. 10.1182/blood-2017-02-767707
9
Chi-Rosso G. Gotwals P. J. Yang J. Ling L. Jiang K. Chao B. et al (1997). Fibronectin type III repeats mediate RGD-independent adhesion and signaling through activated beta1 integrins. J. Biological Chemistry272 (50), 31447–31452. 10.1074/jbc.272.50.31447
10
Daudon M. Bigot Y. Dupont J. Price C. A. (2022). Irisin and the fibronectin type III domain-containing family: structure, signaling and role in female reproduction. Reprod. Camb. Engl.164 (1), R1–R9. 10.1530/REP-22-0037
11
Fan X. Chen X. Deng W. Zhong G. Cai Q. Lin T. (2013). Up-regulated microRNA-143 in cancer stem cells differentiation promotes prostate cancer cells metastasis by modulating FNDC3B expression. BMC Cancer13, 61. 10.1186/1471-2407-13-61
12
Fucci C. Resnati M. Riva E. Perini T. Ruggieri E. Orfanelli U. et al (2020). The interaction of the tumor suppressor FAM46C with p62 and FNDC3 proteins integrates protein and secretory homeostasis. Cell. Reports32 (12), 108162. 10.1016/j.celrep.2020.108162
13
Garikipati V. N. S. Verma S. K. Cheng Z. Liang D. Truongcao M. M. Cimini M. et al (2019). Circular RNA CircFndc3b modulates cardiac repair after myocardial infarction via FUS/VEGF-A axis. Nat. Communications10 (1), 4317. 10.1038/s41467-019-11777-7
14
Goto M. Osada S. Imagawa M. Nishizuka M. (2017). FAD104, a regulator of adipogenesis, is a novel suppressor of TGF-β-mediated EMT in cervical cancer cells. Sci. Reports7 (1), 16365. 10.1038/s41598-017-16555-3
15
Gulisija D. Gonzalez-Reymundez A. Fenton J. I. de Los Campos G. Bray M. S. Vazquez A. I. (2025). Uncovering covariance patterns across energy balance traits enables the discovery of new obesity-related genes. Obes. (Silver Spring, Md.)33 (6), 1184–1194. 10.1002/oby.24291
16
Han B. Wang H. Zhang J. Tian J. (2020). FNDC3B is associated with ER stress and poor prognosis in cervical cancer. Oncol. Letters19 (1), 406–414. 10.3892/ol.2019.11098
17
Hong Y. Qin H. Li Y. Zhang Y. Zhuang X. Liu L. et al (2019). FNDC3B circular RNA promotes the migration and invasion of gastric cancer cells via the regulation of E-cadherin and CD44 expression. J. Cellular Physiology234 (11), 19895–19910. 10.1002/jcp.28588
18
Hornbeck P. V. Kornhauser J. M. Latham V. Murray B. Nandhikonda V. Nord A. et al (2019). 15 years of PhosphoSitePlus®: integrating post-translationally modified sites, disease variants and isoforms. Nucleic Acids Research47 (D1), D433–D441. 10.1093/nar/gky1159
19
Hu Y. Deng L. Zhang J. Fang X. Mei P. Cao X. et al (2016). A pooling genome-wide association study combining a pathway analysis for typical sporadic parkinson’s disease in the Han population of Chinese mainland. Mol. Neurobiology53 (7), 4302–4318. 10.1007/s12035-015-9331-y
20
Hua K. Lin C. H. Chen Y. L. Lin C. H. Ping Y. H. Jou Y. S. et al (2017). Identification of novel cancer fusion genes using chromosome breakpoint screening. Oncol. Reports37 (4), 2101–2108. 10.3892/or.2017.5492
21
Hua K. Wu C. T. Lin C. H. Chen C. F. (2024). E2F1 promotes cell migration in hepatocellular carcinoma via FNDC3B. FEBS Open Bio14 (4), 687–694. 10.1002/2211-5463.13783
22
Hysi P. G. Cheng C. Y. Springelkamp H. Macgregor S. Bailey J. N. C. Wojciechowski R. et al (2014). Genome-wide analysis of multi-ancestry cohorts identifies new loci influencing intraocular pressure and susceptibility to glaucoma. Nat. Genetics46 (10), 1126–1130. 10.1038/ng.3087
23
Jain S. Wheeler J. R. Walters R. W. Agrawal A. Barsic A. Parker R. (2016). ATPase-Modulated stress granules contain a diverse proteome and substructure. Cell.164 (3), 487–498. 10.1016/j.cell.2015.12.038
24
Jiang H. Chu B. L. He J. Liu Z. Yang L. (2022). Expression and prognosis analyses of the fibronectin type-III domain-containing (FNDC) protein family in human cancers: a review. Medicine101 (49), e31854. 10.1097/MD.0000000000031854
25
Katoh D. Nishizuka M. Osada S. Imagawa M. (2015). Fad104, a positive regulator of adipocyte differentiation, suppresses invasion and metastasis of melanoma cells by inhibition of STAT3 activity. PloS One10 (2), e0117197. 10.1371/journal.pone.0117197
26
Katoh D. Nishizuka M. Osada S. Imagawa M. (2016). FAD104, a regulator of adipogenesis and osteogenesis, interacts with the C-Terminal region of STAT3 and represses malignant transformation of melanoma cells. Biol. and Pharmaceutical Bulletin39 (5), 849–855. 10.1248/bpb.b15-01026
27
Khan N. A. Fahma A. Mahin A. Gopalakrishnan A. P. Shivamurthy P. B. Rajeev A. C. et al (2025). Integrative phosphoproteomic network analysis identifies CAMK2D as a shared regulator of TPD52 family proteins in cancer. Protoplasma. 10.1007/s00709-025-02145-y
28
Kishimoto K. Kato A. Osada S. Nishizuka M. Imagawa M. (2010). Fad104, a positive regulator of adipogenesis, negatively regulates osteoblast differentiation. Biochem. Biophysical Research Communications397 (2), 187–191. 10.1016/j.bbrc.2010.05.077
29
Kishimoto K. Nishizuka M. Ueda T. Kajita K. Ugawa S. Shimada S. et al (2011). Indispensable role of factor for adipocyte differentiation 104 (fad104) in lung maturation. Exp. Cell Research317 (15), 2110–2123. 10.1016/j.yexcr.2011.06.003
30
Kishimoto K. Nishizuka M. Katoh D. Kato A. Osada S. Imagawa M. (2013). FAD104, a regulatory factor of adipogenesis, acts as a novel regulator of calvarial bone formation. J. Biological Chemistry288 (44), 31772–31783. 10.1074/jbc.M113.452961
31
Kondkar A. A. Sultan T. Azad T. A. Osman E. A. Almobarak F. A. Lobo G. P. et al (2022). Evaluation of ABCA1 and FNDC3B gene polymorphisms associated with pseudoexfoliation glaucoma and primary angle-closure glaucoma in a saudi cohort. Front. Genetics13, 877174. 10.3389/fgene.2022.877174
32
Krassowski M. Pellegrina D. Mee M. W. Fradet-Turcotte A. Bhat M. Reimand J. (2021). ActiveDriverDB: interpreting genetic variation in human and cancer genomes using post-translational modification sites and signaling networks (2021 update). Front. Cell Developmental Biology9, 626821. 10.3389/fcell.2021.626821
33
Krug K. Mertins P. Zhang B. Hornbeck P. Raju R. Ahmad R. et al (2019). A curated resource for phosphosite-specific signature analysis. Mol. and Cellular Proteomics MCP18 (3), 576–593. 10.1074/mcp.TIR118.000943
34
Kwon H. Yun M. Kwon T. H. Bang M. Lee J. Lee Y. S. et al (2023). Fibronectin type III domain containing 3B as a potential prognostic and therapeutic biomarker for glioblastoma. Biomedicines11 (12), 3168. 10.3390/biomedicines11123168
35
Leahy D. J. Hendrickson W. A. Aukhil I. Erickson H. P. (1992). Structure of a fibronectin type III domain from tenascin phased by MAD analysis of the selenomethionyl protein. Sci. (New York, N.Y.)258 (5084), 987–991. 10.1126/science.1279805
36
Li Y. Yang J. Wang H. Qiao W. Guo Y. Zhang S. et al (2020). FNDC3B, targeted by miR-125a-5p and miR-217, promotes the proliferation and invasion of colorectal cancer cells via PI3K/mTOR signaling. OncoTargets Therapy13, 3501–3510. 10.2147/OTT.S226520
37
Li Y. Meng F. Sui C. Wang Y. Cheng D. (2023). CircRNA hsa_circ_0001627 aggravates cervical cancer progression through upregulation of FNDC3B and activating PI3K/mTOR signaling pathway. J. Cell Communication Signaling17 (3), 627–638. 10.1007/s12079-022-00696-w
38
Liedtke D. Orth M. Meissler M. Geuer S. Knaup S. Köblitz I. et al (2019). ECM alterations in Fndc3a (fibronectin domain containing protein 3A) deficient zebrafish cause temporal fin development and regeneration defects. Sci. Reports9 (1), 13383. 10.1038/s41598-019-50055-w
39
Lin F. Ren X. D. Pan Z. Macri L. Zong W. X. Tonnesen M. G. et al (2011). Fibronectin growth factor-binding domains are required for fibroblast survival. J. Investigative Dermatology131 (1), 84–98. 10.1038/jid.2010.253
40
Lin C.-H. Lin Y. W. Chen Y. C. Liao C. C. Jou Y. S. Hsu M. T. et al (2016). FNDC3B promotes cell migration and tumor metastasis in hepatocellular carcinoma. Oncotarget7 (31), 49498–49508. 10.18632/oncotarget.10374
41
Liu H. Bi J. Dong W. Yang M. Shi J. Jiang N. et al (2018). Invasion-related circular RNA circFNDC3B inhibits bladder cancer progression through the miR-1178-3p/G3BP2/SRC/FAK axis. Mol. Cancer17 (1), 161. 10.1186/s12943-018-0908-8
42
Liu Y. Zhong Z. Xiao L. Li W. Wang Z. Duan Z. et al (2021). Identification of Circ-FNDC3B, an overexpressed circRNA in abdominal aortic aneurysm, as a regulator of vascular smooth muscle cells. Int. Heart Journal62 (6), 1387–1398. 10.1536/ihj.21-186
43
Lu Y. Vitart V. Burdon K. P. Khor C. C. Bykhovskaya Y. Mirshahi A. et al (2013). Genome-wide association analyses identify multiple loci associated with central corneal thickness and keratoconus. Nat. Genetics45 (2), 155–163. 10.1038/ng.2506
44
Mahin A. Gopalakrishnan A. P. Ahmed M. Nisar M. John L. Shivamurthy P. B. et al (2025). Orchestrating intracellular calcium signaling cascades by Phosphosite-Centric regulatory network: a comprehensive analysis on kinases CAMKK1 and CAMKK2. Omics A Journal Integrative Biology29 (4), 139–153. 10.1089/omi.2024.0196
45
Mannan A. Muhsen I. N. Barragán E. Sanz M. A. Mohty M. Hashmi S. K. et al (2020). Genotypic and phenotypic characteristics of acute promyelocytic leukemia translocation variants. Hematology/oncology Stem Cell Therapy13 (4), 189–201. 10.1016/j.hemonc.2020.05.007
46
Nishizuka M. Kishimoto K. Kato A. Ikawa M. Okabe M. Sato R. et al (2009). Disruption of the novel gene fad104 causes rapid postnatal death and attenuation of cell proliferation, adhesion, spreading and migration. Exp. Cell Research315 (5), 809–819. 10.1016/j.yexcr.2008.12.013
47
Obara M. Sakuma T. Fujikawa K. (2010). The third type III module of human fibronectin mediates cell adhesion and migration. J. Biochemistry147 (3), 327–335. 10.1093/jb/mvp168
48
Obholz K. L. Akopyan A. Waymire K. G. MacGregor G. R. (2006). FNDC3A is required for adhesion between spermatids and sertoli cells. Dev. Biology298 (2), 498–513. 10.1016/j.ydbio.2006.06.054
49
Pan Z. Cai J. Lin J. Zhou H. Peng J. Liang J. et al (2020). A novel protein encoded by circFNDC3B inhibits tumor progression and EMT through regulating snail in colon cancer. Mol. Cancer19 (1), 71. 10.1186/s12943-020-01179-5
50
Peng Y. Li H. Li X. Yu S. Xiang H. Peng J. et al (2016). MicroRNA-215 impairs adipocyte differentiation and co-represses FNDC3B and CTNNBIP1. International Journal Biochemistry and Cell Biology79, 104–112. 10.1016/j.biocel.2016.08.014
51
Rong S. S. Ma S. T. U. Yu X. T. Ma L. Chu W. K. Chan T. C. Y. et al (2017). Genetic associations for keratoconus: a systematic review and meta-analysis. Sci. Reports7 (1), 4620. 10.1038/s41598-017-04393-2
52
Ruan Y. Chao S. Hu X. Lu L. Lin Y. Wang Q. et al (2021). FN3 domain displaying double epitopes: a cost-effective strategy for producing substitute antigens. Front. Molecular Biosciences8, 742617. 10.3389/fmolb.2021.742617
53
Sahebjada S. Schache M. Richardson A. J. Snibson G. MacGregor S. Daniell M. et al (2013). Evaluating the association between keratoconus and the corneal thickness genes in an independent Australian population. Investigative Ophthalmology and Visual Science54 (13), 8224–8228. 10.1167/iovs.13-12982
54
Shiga Y. Akiyama M. Nishiguchi K. M. Sato K. Shimozawa N. Takahashi A. et al (2018). Genome-wide association study identifies seven novel susceptibility loci for primary open-angle glaucoma. Hum. Molecular Genetics27 (8), 1486–1496. 10.1093/hmg/ddy053
55
Sun K. Yao H. Zhang P. Sun Y. Ma J. Xia Q. (2023). Emerging landscape of circFNDC3B and its role in human malignancies. Front. Oncology13, 1097956. 10.3389/fonc.2023.1097956
56
Tang B. Zhang Q. Liu K. Huang Y. (2022). Exosomal circRNA FNDC3B promotes the progression of esophageal squamous cell carcinoma by sponging miR-490-5p and regulating thioredoxin reductase 1 expression. Bioengineered13 (5), 13829–13848. 10.1080/21655979.2022.2084484
57
Tominaga K. Kondo C. Johmura Y. Nishizuka M. Imagawa M. (2004). The novel gene fad104, containing a fibronectin type III domain, has a significant role in adipogenesis. FEBS Letters577 (1-2), 49–54. 10.1016/j.febslet.2004.09.062
58
Wang H.-Y. McMahon C. Ali S. M. Young L. E. Yekezare S. Ross J. S. et al (2016). Novel FNDC3B and MECOM fusion and WT1 L378fs* 7 frameshift mutation in an acute myeloid leukaemia patient with cytomorphological and immunophenotypic features reminiscent of acute promyelocytic leukaemia. Br. Journal Haematology172 (6), 987–990. 10.1111/bjh.13552
59
Wang J. Li X. Duan C. Jia Y. (2022). CircFNDC3B knockdown restrains the progression of oesophageal squamous cell carcinoma through miR-214-3p/CDC25A axis. Clin. Experimental Pharmacology and Physiology49 (11), 1209–1220. 10.1111/1440-1681.13707
60
Wang X. Huang Y. Li S. Zhang H. (2022). Integrated machine learning methods identify FNDC3B as a potential prognostic biomarker and correlated with immune infiltrates in glioma. Front. Immunology13, 1027154. 10.3389/fimmu.2022.1027154
61
Wang Y. Kong Y. Yang Q. Zhong C. Zhou D. (2024). Identification of fibronectin type III domain containing 3B as a potential prognostic and therapeutic target for pancreatic cancer: a preliminary analysis. Eur. Journal Medical Research29 (1), 221. 10.1186/s40001-024-01823-6
62
Wiggs J. L. Pasquale L. R. (2017). Genetics of glaucoma. Hum. Molecular Genetics26 (R1), R21–R27. 10.1093/hmg/ddx184
63
Wu L. She P. Qiu C. Sun H. Kong F. Wang H. et al (2025). Integrated bioinformatics analysis of common molecular mechanisms and biomarkers in oral squamous cell carcinoma and periodontal disease. Discov. Oncology16 (1), 1891. 10.1007/s12672-025-03728-0
64
Xue L. Tao Y. Yuan Y. Qu W. Wang W. (2021). Curcumin suppresses renal carcinoma tumorigenesis by regulating circ-FNDC3B/miR-138-5p/IGF2 axis. Anti-cancer Drugs32 (7), 734–744. 10.1097/CAD.0000000000001063
65
Yang J. Cao X. H. Luan K. F. Huang Y. D. (2021). Circular RNA FNDC3B protects oral squamous cell carcinoma cells from ferroptosis and contributes to the malignant progression by regulating miR-520d-5p/SLC7A11 axis. Front. Oncology11, 672724. 10.3389/fonc.2021.672724
66
You Y. Liu C. Liu T. Tian M. Wu N. Yu Z. et al (2022). FNDC3B protects steatosis and ferroptosis via the AMPK pathway in alcoholic fatty liver disease. Free Radical Biology and Medicine193 (Pt 2), 808–819. 10.1016/j.freeradbiomed.2022.10.322
67
Zeng W. Liu Y. Li W. T. Li Y. Zhu J. F. (2020). CircFNDC3B sequestrates miR-937-5p to derepress TIMP3 and inhibit colorectal cancer progression. Mol. Oncology14 (11), 2960–2984. 10.1002/1878-0261.12796
68
Zeng W. Zhu J. F. Guo J. Huang G. J. Ai L. S. Zeng Y. et al (2022). mA-modified circFNDC3B inhibits colorectal cancer stemness and metastasis via RNF41-dependent ASB6 degradation. Cell. Death and Disease13 (11), 1008. 10.1038/s41419-022-05451-y
69
Zhang J. Bai J. Zhu H. Li W. An Q. Wang D. (2022). The upregulation of circFNDC3B aggravates the recurrence after endoscopic submucosal dissection (ESD) in early gastric cancer (EGC) patients. Sci. Reports12 (1), 6178. 10.1038/s41598-022-07154-y
70
Zhang Y. Ran L. Liu Y. Li W. Ran A. Li H. et al (2025). FNDC3B promotes gastric cancer metastasis via interacting with FAM83H and preventing its proteasomal degradation. Cell. and Molecular Biology Letters30 (1), 65. 10.1186/s11658-025-00741-7
71
Zhao L. Zhao J. Zhong K. Tong A. Jia D. (2022). Targeted protein degradation: mechanisms, strategies and application. Signal Transduction Targeted Therapy7 (1), 113. 10.1038/s41392-022-00966-4
72
Zhou X. Li Y. Wu C. Yu W. Cheng F. (2020). Novel lncRNA XLOC_032768 protects against renal tubular epithelial cells apoptosis in renal ischemia-reperfusion injury by regulating FNDC3B/TGF-β1. Ren. Failure42 (1), 994–1003. 10.1080/0886022X.2020.1818579
73
Zhu H. Ren X. Ding W. (2023). ‘A pan cancerous analysis of FNDC3B in human multiple tumors’. 10.21203/rs.3.rs-3133850/v1
Summary
Keywords
adipogenesis, FNDC3B, mutation, phosphosites, transmembrane protein
Citation
Khanum A, Mahin A, Lubaba F, Bangera A, Gopalakrishnan AP, Soman S and Raju R (2026) Multifaceted roles of fibronectin type III domain containing 3B (FNDC3B) in cell biology and signaling. Front. Mol. Biosci. 13:1741530. doi: 10.3389/fmolb.2026.1741530
Received
07 November 2025
Revised
30 December 2025
Accepted
19 January 2026
Published
02 February 2026
Volume
13 - 2026
Edited by
Ana Cipak Gasparovic, Rudjer Boskovic Institute, Croatia
Reviewed by
Jyoti Bala Kaushal, University of Nebraska Medical Center, United States
Kirti Singh, University of California, San Francisco, United States
Updates
Copyright
© 2026 Khanum, Mahin, Lubaba, Bangera, Gopalakrishnan, Soman and Raju.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rajesh Raju, rajrrnbt@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.