- 1Department of Neuroscience, Imaging, and Clinical Science, Università degli Studi G. d’Annunzio Chieti e Pescara, Chieti, Italy
- 2Department of Neuroscience, Psychology, Drug Area and Child Health, University of Florence, Florence, Italy
- 3Molecular Neurology Unit, Center of Excellence on Aging and Translational Medicine, Università degli Studi G. d’Annunzio Chieti e Pescara, Chieti, Italy
- 4Department of Psychological, Health and Territorial Sciences, School of Medicine and Health Sciences, Università degli Studi G. d’Annunzio Chieti e Pescara, Chieti, Italy
- 5Department of Neurology and Pharmacology, Institute for Memory Impairments and Neurological Disorders, University of California, Irvine, Irvine, CA, United States
- 6School of Human and Social Science, Kore University of Enna, Enna, Italy
- 7Department of Clinical and Behavioral Neurology, Neuropsychiatry Laboratory, IRCCS Santa Lucia Foundation, Rome, Italy
The risk for Alzheimer’s disease (AD) is associated with the presence of the 𝜀4 allele of Apolipoprotein E (APOE) gene and, recently, with a novel genetic variant of the RNF219 gene. This study aimed at evaluating interactions between APOE-𝜀4 and RNF219/G variants in the modulation of behavioral and cognitive features of two cohorts of patients suffering from mild cognitive impairment (MCI) or AD. We enrolled a total of 173 female MCI or AD patients (83 MCI; 90 AD). Subjects were screened with a comprehensive set of neuropsychological evaluations and genotyped for the APOE and RNF219 polymorphic variants. Analysis of covariance was performed to assess the main and interaction effects of APOE and RNF219 genotypes on the cognitive and behavioral scores. The analysis revealed that the simultaneous presence of APOE-𝜀4 and RNF219/G variants results in significant effects on specific neuropsychiatric scores in MCI and AD patients. In MCI patients, RNF219 and APOE variants worked together to impact the levels of anxiety negatively. Similarly, in AD patients, the RNF219 variants were found to be associated with increased anxiety levels. Our data indicate a novel synergistic activity APOE and RNF219 in the modulation of behavioral traits of female MCI and AD patients.
Introduction
Alzheimer’s disease is a complex syndrome characterized by a pleiotropic array of cognitive and behavioral symptoms (Selkoe, 2011). AD is mainly driven by the intraneuronal accumulation of β-amyloid, the extracellular formation of amyloid plaques and the appearance of intracellular neurofibrillary tangles composed of phosphorylated tau proteins. More recent lines of evidence support the idea that imbalance of β-amyloid production and clearance, along with phosphorylated tau and the interplay with other co-morbidity factors (metabolic, vascular, and inflammatory) work synergistically on a permissive condition represented by the aging brain to promote AD (Herrup, 2010; Corona et al., 2011; Selkoe and Hardy, 2016). Genetic and environmental factors also affect the onset and progression of the disease (Tanzi, 2012; Raskin et al., 2015).
Genome-wide-association studies have identified and confirmed more than 20 genetic variants associated with higher susceptibility to develop Late-Onset Alzheimer Disease (LOAD) of the sporadic type (Winblad et al., 2015). Among these, the 𝜀4 allele is a specific variant of the Apolipoprotein E gene (APOE-𝜀4) and a significant risk factor for AD (Saunders et al., 1993; Bertram et al., 2007). The physio-pathological function of APOE is complex (Ossenkoppele et al., 2013; Tai et al., 2015) as the gene can interfere in many ways with the pillars of the disease (Ohm et al., 1999; Tanzi, 2012). As an integral part of cellular membranes, APOE-𝜀4 can influence the amyloidogenic processing of the APP and impair its clearance from the brain (Selkoe and Hardy, 2016). APOE-𝜀4 can also promote tau phosphorylation (Zhou et al., 2016) and affect metabolic and vascular factors such as hypertension, diabetes mellitus, as well as the metabolic syndrome. All these factors synergistically modulate the AD onset and progression (Duron and Hanon, 2008; Toledo et al., 2013). For instance, these factors target the physiological functioning of the neurovascular unit and the BBB integrity. Interestingly, APOE-𝜀4 has been recently shown to converge on this critical step by affecting the operation of the neurovascular unit and promoting the breakdown of proteins responsible for the BBB integrity (Montagne et al., 2015; Zhao et al., 2015). However, despite the growing body of evidence on the APOE-related pathogenic mechanisms, a definitive molecular roadmap on the 𝜀4 haplotype targets remains elusive.
Recent data also indicate that a genetic variant of the RNF219 gene may increase the risk for the AD (Rhinn et al., 2013). The rs2248663 A>G (RNF219/G) polymorphism of the RNF219 gene encoding for a member of the RNF family, has been associated with earlier onset of AD when working in synergy with the APOE-𝜀4 (Rhinn et al., 2013). This accelerating effect is not present in non-𝜀4 and RNF219/A carriers, thereby indicating that the two genes may work on common pathogenic pathways. In the study, we set out to integrate with new evidence the original RNF219 findings (Rhinn et al., 2013) and evaluated whether APOE-𝜀4 and RNF219/G work in synergy or independently to affect the behavioral or cognitive features of patients affected by mild cognitive impairment (MCI) or AD. To that aim, we analyzed a comprehensive set of behavioral and cognitive profiles in two cohorts of female MCI or AD patients that included carriers or non-carriers of APOE-𝜀4 and RNF219/G.
Materials and Methods
Study Population
The study was approved by the Institutional and Ethics Committee of the I.R.C.C.S. Santa Lucia-Rome. All procedures were conducted in accordance with principles expressed in the Helsinki Declaration. We recruited 173 total volunteers (mean age ± standard deviation = 74 ± 7) including 83 MCI and 90 AD patients. All included subjects signed an informed consent form before enrolment. Clinical evaluations were conducted by trained psychologists and AD specialists (neurologists and psychiatrists).
Neuropsychological Assessment
Subjects were assessed with the following neuropsychological tests: MMSE, RAVL, Phonemic Verbal Fluency, CPMs, complex figure of Rey, Stroop test, and NPI. The main functional capacity was assessed by daily non-instrumental (ADL) (Wallace et al., 2007) and instrumental activities (IADL) (Lawton and Brody, 1969).
Mini Mental State Examination defines the global level of cognitive deterioration on a scale of 0–30 and targets general mental abilities, memory, attention, and language. A Score greater than or equal to 24 indicates the absence of cognitive deficits, scores ≤ 9 indicate the presence of severe cognitive deficits, scores between 10 and 18 indicate moderate cognitive deficits, and scores between 19 and 23 indicate mild cognitive deficits (Folstein et al., 1975). RAVL allows a quantitative assessment of the ability of immediate and delayed recall (Snyder and Harrison, 1997). The CPMs measure fluid intelligence (Basso et al., 1987). The complex figure of Rey is a visual-perceptual test that investigates the complex perceptual organization and long-term visual memory (Shin et al., 2006). The Stroop test examines aspects of attention and executive functions (Tremblay et al., 2016). The NPI was developed to provide a way to assess neuropsychiatric symptoms and psychopathology of patients with AD and other neurodegenerative disorders (Cummings et al., 1994). The NPI has been therefore employed to characterize neuropsychiatric profiles and is a structured interview that evaluates the following 12 behavioral domains: delusions, hallucinations, agitation, dysphoria, anxiety, apathy, irritability, euphoria, disinhibition, aberrant motor behavior, night-time behavioral disturbance, eating disorders, and weight changes.
DNA Extraction
For gene variants analysis, genomic DNA was isolated from blood samples by the PureLink Genomic DNA Mini Kit (Life Technologies, Carlsbad, CA, United States), quantified by an Agilent 8453 Spectrophotometer (Agilent, Santa Clara, CA, United States) and stored at -20°C.
APOE Genotyping
APOE genotyping was performed by direct sequencing. PCR amplification of the region containing the rs429358 and rs7412 sites that determine the 𝜀2, 𝜀3, or 𝜀4 variants of the gene was carried out using the primers pair Forward: 5′-TAAGCTTGGCACGGCTGTCCAAGGA-3′ and Reverse: 5′-ACAGAATTCGCCCCGGCCTGGTACAC-3′, resulting in a 244 bp fragment (Hixson and Vernier, 1990). Purified PCR products were sequenced by the BigDye Terminator v3.1 Cycle Sequencing Kit (Life Technologies, Carlsbad, CA, United States) according to the manufacturer protocol. Sequence products were then separated on an ABI 3130xl automatic sequencer (Applied Biosystems, Paisley, United Kingdom) and analyzed using Sequencing Analysis Software (Applied Biosystems, Paisley, United Kingdom).
RNF219 Genotyping
RNF219 genotyping was carried out using High-Resolution Melting Analysis in 48-well plates on a StepOneTM Real-Time PCR System run by StepOne Software v2.2.2 (Applied Biosystems, Paisley, United Kingdom) and analyzed with High-Resolution Melt Software v3.0.1 (Life Technologies, Carlsbad, CA, United States). We amplified a 103 bp fragment using the following primers pair: Forward: 5′-GGAAAAAGACAATGCAGGAAT-3′; Reverse: 5′-TTTTACCAAGGGCAACATTTC-3′. The PCR reaction, containing 20 ng of genomic DNA and the MeltDoctor HRM Master Mix (Applied Biosystems), according to the manufacturer protocols, was run as follow: enzyme activation at 95°C for 10 min; 40 cycles of denaturation and extension at 95°C for 15 s and 60°C for 1 min; melt curve with a denaturation at 95°C for 10 s, annealing at 60°C for 1 min, high resolution melting from 60 to 95°C with a ramp rate of 0.3% and final re-annealing at 60° C for 15 s. Fluorescence signals were measured during the amplification and melting steps.
Statistical Analysis
APOE and RNF219 genotypic and allelic frequencies of female MCI and AD patients were calculated as previously described (Wigginton et al., 2005). For statistical analysis, we separated the MCI and AD cohorts in carriers and non-carriers of the two allelic variants 𝜀4 and G. Allele frequencies of both APOE and RNF219 polymorphisms were assessed for Hardy–Weinberg equilibrium (HWE) using a chi-square (χ2) test with significance set at p < 0.05 (Wigginton et al., 2005).
One-way analysis of variance (ANOVA) followed by Fisher least significant difference (LSD) post hoc test was performed to investigate the significance of differences between age, education levels, MMSE corrected for age and education levels, the reported (by the patient or caregivers) age of appearance of the first symptom for MCI subjects, and age of onset for AD patients. Levene test was performed for assessment of homoscedasticity of the groups. Kruskal–Wallis test followed by multiple comparison of mean ranks was performed when the variances between groups were non-homogeneous.
Analysis of covariance (ANCOVA) was performed using a general linear model (GLM) approach and controlling for age and education level. APOE and RNF219 genotypes were the independent factors, and the neuropsychological scores were the dependent variables. The main and interaction effects of the APOE and RNF219 genotypes were evaluated. The employed ANCOVA model is as follow: Yi = β0 + β1 (agei) + β2 (education leveli) + β3 (APOE genotypei) + β4 (RNF219 genotypei) + β5 (APOE genotypei × RNF219 genotypei) + εi, where Yi indicates the specific ith neuropsychological score, β0 is the intercept, and εi is the error term associated with the model. In the case of ordinal variables or when the assumption of the homogeneity of the variance was rejected by the Levene test, the ART procedure was applied (Wobbrock et al., 2011; Feys, 2016). Multiple comparisons were performed using Fisher LSD post hoc test.
In all cases, p-values were corrected for multiple comparisons using the Benjamini–Hochberg correction at a false discovery rate (FDR) of 5%. p-Values < 0.050 were considered statistically significant. Statistical analysis was performed using Statistica 6.0 software (Statsoft, Tulsa, OK, United States).
Results
Demographic and Clinical Features of MCI and AD Cohorts
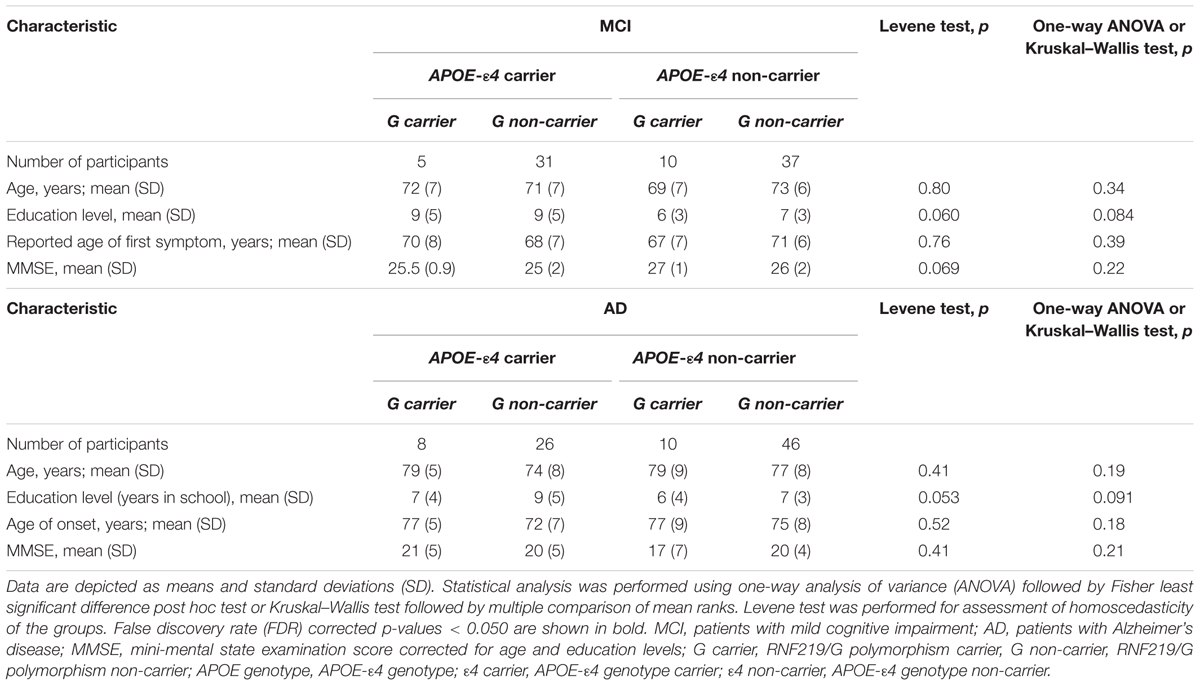
The demographic and clinical characteristic of the study groups in the MCI or AD cohorts are shown in Table 1. The study subgroups were matched for age, education levels, and MMSE scores as well as for the reported age of the first symptoms (in the case of MCI subjects) or age of onset (in the case of AD patients).
Distribution of APOE and RNF219 Genotypes in the MCI and AD Cohorts
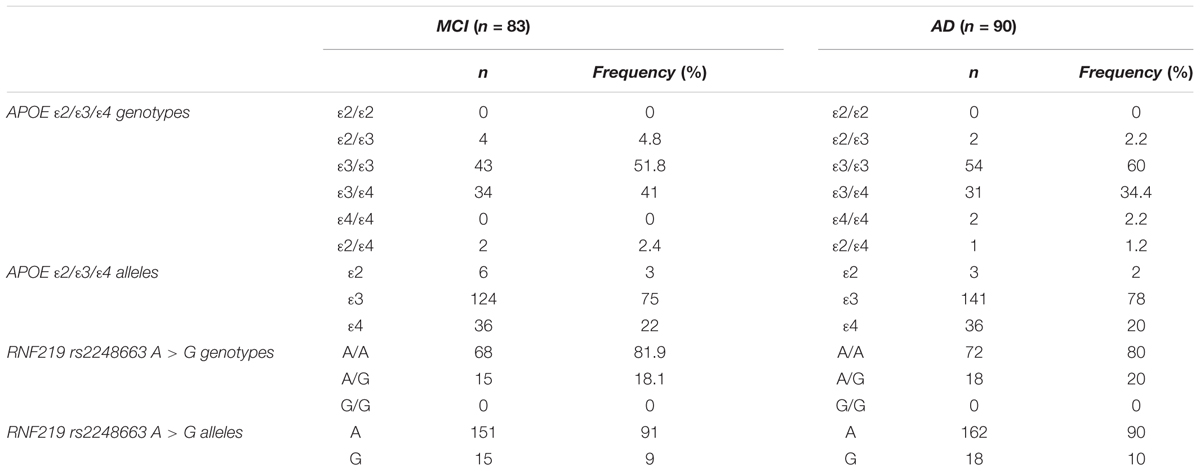
The distribution of APOE and RNF219 genotypes and relative frequencies in MCI and AD patients are shown in Table 2. Genotypes were in the Hardy–Weinberg equilibrium in MCI (APOE p = 0.064; RNF219 p = 0.36) and AD (APOE p = 0.64; RNF219 p = 0.29) patients.
Effects of the APOE and RNF219 Genotypes on Behavioral Features of MCI Subjects
Our study revealed that, in MCI subjects, the anxiety-related NPI score depends on the interaction between APOE and RNF219 genotypes (p = 0.003) (Supplementary Table S1). The APOE genotype alone showed a trend toward significant effect on the same NPI score (p = 0.074) (Supplementary Table S1). In contrast, we did not find significant effects of age or education on the anxiety trait (p = 0.063 and 0.16, respectively).
Post hoc multiple comparisons showed that MCI 𝜀4/G carriers displayed increased levels of anxiety compared to other groups of patients. In fact, MCI patients carrying the 𝜀4/G alleles show higher levels of anxiety [median (interquartile range): 6 (6–9)] compared to MCI 𝜀4/A carriers [median (interquartile range): 2 (0–4); p = 0.009], non-𝜀4/A carriers [median (interquartile range): 2 (0–4); p = 0.017] and non-𝜀4/G carriers [median (interquartile range): 1 (0–2.75); p = 0.009; Figure 1].
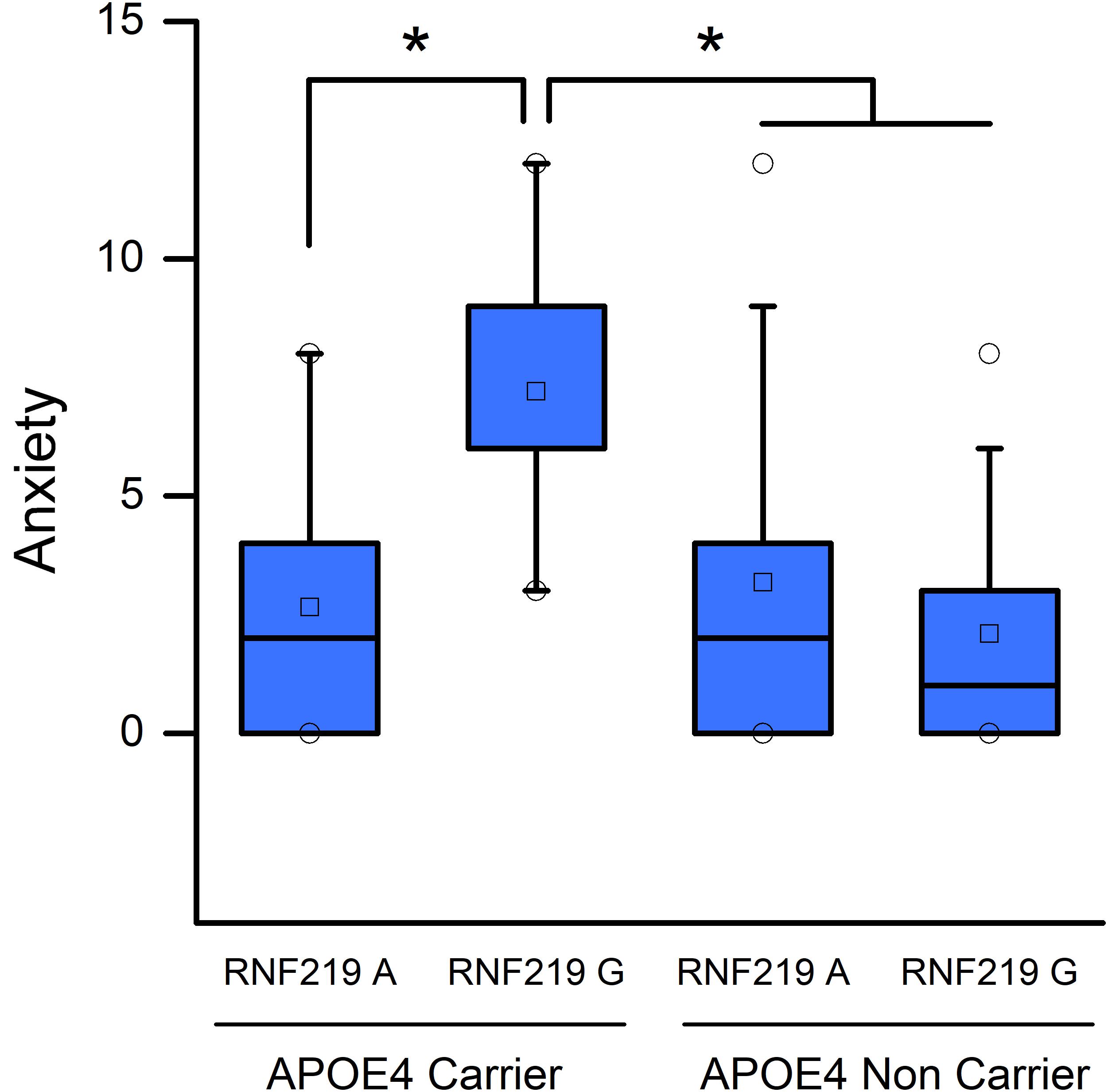
FIGURE 1. Apolipoprotein E (APOE) and RNF219 interaction in the modulation of anxiety of MCI subjects. Box plots show a comparison of anxiety scores and statistical differences set at p < 0.05. Squares depict the mean values. The central horizontal bars represent the median values. The lower and the upper limits of the box represent the first and the third quartiles, respectively. Circles represent the minimum and maximum values of anxiety scores. Note that 𝜀4/G carriers show increased anxiety-related NPI scores compared to 𝜀4/A carriers (p = 0.009), non-𝜀4/A carriers (p = 0.017), and non-𝜀4/G carriers (p = 0.009). ∗Indicate statistically significant differences.
In contrast, we did not find significant main and/or interaction effects of APOE and RNF219 variants on the other neuropsychological scores (Supplementary Table S1).
Effects of the APOE and RNF219 Genotypes on Behavioral Features of AD Patients
In the case of AD patients, we found that RNF219 variants had significant effects on anxiety-related NPI scores (p = 0.015). Similarly to the MCI group, in the AD cohort, we found that 𝜀4/G carriers show higher anxiety-related NPI scores [median (interquartile range): 5.50 (1.75–8.25)] compared to 𝜀4/A [median (interquartile range): 0.5 (0–5.5); p = 0.041; Figure 2] and non-𝜀4/A carriers [median (interquartile range): 0 (0–2.75); p = 0.030; Figure 2].
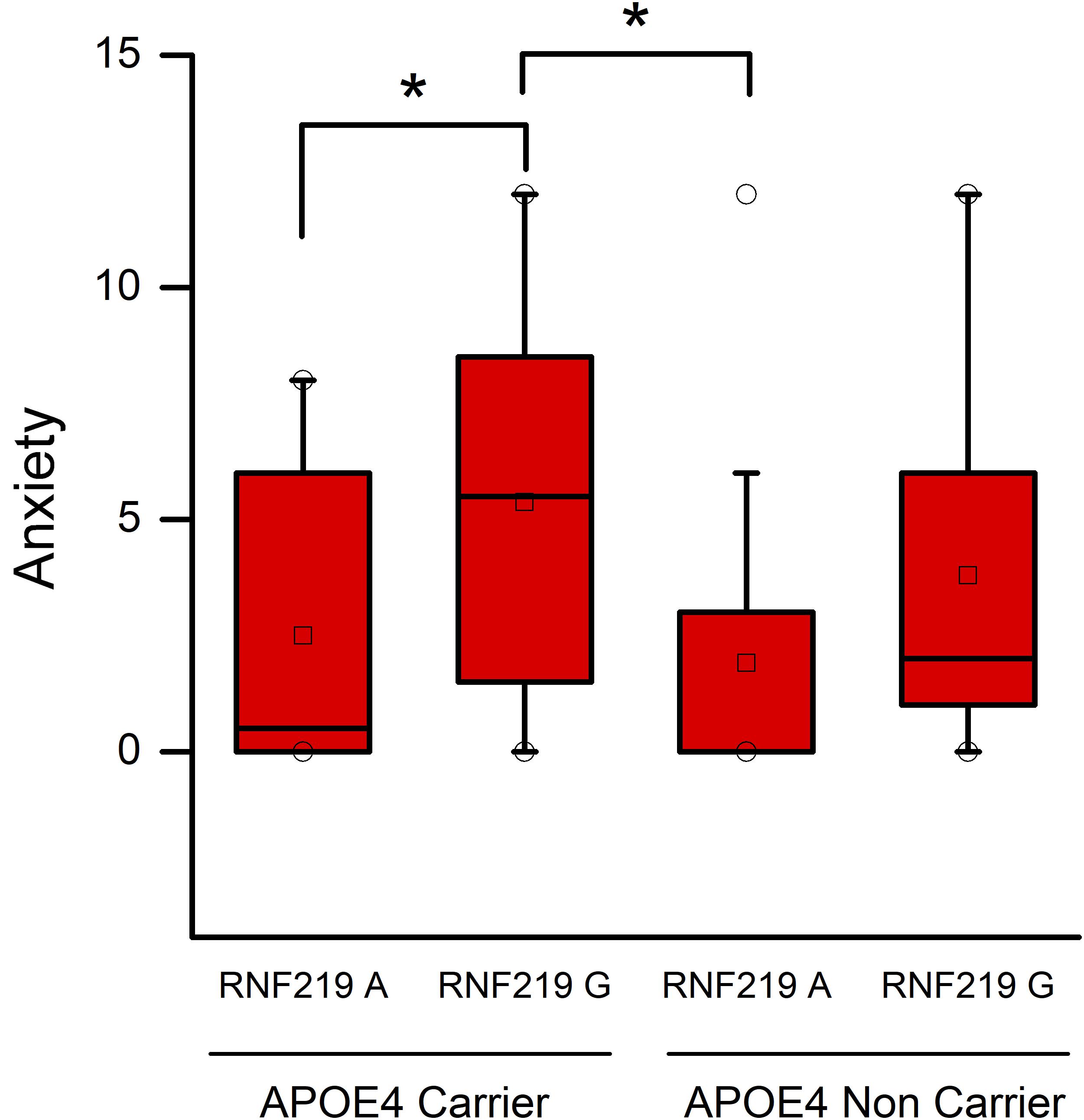
FIGURE 2. Apolipoprotein E and RNF219 interaction in the modulation of anxiety of AD patients. Box plots show a comparison of anxiety NPI scores and statistical differences set at p < 0.05. Squares depict the mean values. The central horizontal bars represent the median values. The lower and the upper limits of the box represent the first and the third quartiles, respectively. Circles represent the minimum and maximum values of anxiety scores. Note that 𝜀4/G carriers show higher anxiety-related NPI scores compared to 𝜀4/A (p = 0.041) and non-𝜀4/A carriers (p = 0.030). ∗Indicate statistically significant differences.
As for MCI subjects, we did not find any significant differences in other neuropsychological scores of the AD cohort (Supplementary Table S2).
Discussion
In the study we explored whether APOE-𝜀4 and RNF219/G work in synergy or independently to affect the behavioral or cognitive features of MCI and AD patients (Rhinn et al., 2013).
In a preliminary phase of the study, we attempted to evaluate the synergistic effects of APOE and RNF219 variants on behavioral and cognitive traits of male and female MCI or AD patients. However, after genotyping, we found that the sample size was too small to evaluate the effects of RNF genotype in males. Therefore, the study was redirected to investigate the impact of APOE-ε4 and RNF219/G only in female patients. We acknowledge that this is a limitation of our study and further studies will need to address effects on male patients.
In the study, we found that the RNF219/G variant, in synergy with the APOE-ε4 allele, amplifies the anxiety-related NPI scores. These scores are higher in APOE-𝜀4 and RNF219/G carriers of the MCI or AD cohorts (Figures 1, 2).
Anxiety disorders are common late-life psychiatric features and have been associated with lower cognitive performance in older adults (Beaudreau and O’Hara, 2008). Several lines of evidence support the modifying effect of the APOE-𝜀4 status on the AD neuropsychiatric symptoms (Ungar et al., 2014). Reports indicate that anxiety and other behavioral symptoms are more prominent and severe in the population of female AD patients who are APOE-𝜀4 carriers (Steinberg et al., 2006; Xing et al., 2015), thereby supporting the notion of a relationship between the interaction of APOE-𝜀4 and gender in the phenotypical shaping of the AD-related behavioral features. The precise biological underpinning of the phenomenon is difficult to be identified. One possibility relies on the role played by estrogens in the disease progression of female patients. These hormones affect the synaptic plasticity of the AD brain as well as shape the response to AD-related pathology (Yaffe et al., 2000; Carroll and Rosario, 2012; Kang and Grodstein, 2012; Kramár et al., 2013). Hormonal changes can act on neurotrophic mechanisms and be responsible for behavioral symptoms. For instance, in females, decreased peri-menopausal levels of estrogens have been suggested to favor the onset and progression of dementia-related depression and anxiety (Aloysi et al., 2006). These estrogen-related effects can amplify the activity of APOE. In fact, it is well-known that APOE-𝜀4 allele acts as a negative modulator of neuropsychiatric features in AD patients (Spalletta et al., 2006; Steinberg et al., 2006; Panza et al., 2012). Moreover, levels of estradiol are known to be influenced by the expression of the APOE-𝜀4 allele and promote a worsening of neuropsychiatric symptoms in female APOE-𝜀4 carriers (Xing et al., 2012). Surprisingly, we did not find significant effects of the APOE-𝜀4 allele on neuropsychological features such as apathy, aggressiveness, and depression. These symptoms have been previously shown in MCI or AD patients (Panza et al., 2011). A possible explanation of these divergent results may depend on the fact that our study has evaluated only female subjects while others have investigated mixed groups that included female and male patients.
The neurobiological effects of RNF219 remain most unexplored. RNF219 belongs to a family of proteins pleiotropically involved in many cellular functions. Some RNF proteins have been shown to modulate myelin formation (Hoshikawa et al., 2008) and the stability of GABAergic synapses (Jin et al., 2014). These proteins interfere with the activation of the ubiquitin system (Joazeiro and Weissman, 2000), a crucial mechanism for neuronal demise (Zheng et al., 2014). A role for selected RNF proteins has also been proposed in neurodegenerative processes (Pranski et al., 2013; Matz et al., 2014). In that regard, several genetic variants at the RNF219 locus have been associated with the presence of cognitive deficits, brain atrophy and lipid deregulation (Barber et al., 2010; Cirulli et al., 2010; Furney et al., 2011). Of note, the RNF219/G variant has been recently associated with an earlier onset of AD (Rhinn et al., 2013).
Interestingly, recent studies in MCI patients have reported a positive relationship between the presence of high levels of anxiety and the likelihood of conversion to AD. Although the issue remains controversial (Devier et al., 2009; Breitve et al., 2016), it has been shown that anxiety is associated with the earlier conversion to AD (Gallagher et al., 2011; Mah et al., 2015). Therefore, our findings allow the speculation of a potential correlation between anxiety, RNF219/G, APOE-𝜀4 and the conversion to AD.
In our study, we did not find any significant correlation between the anxiety levels and an earlier onset age for the first cognitive symptoms for MCI subjects or AD clinical signs (data not shown). RNF219/G has been shown to favor an earlier presentation of the disease in AD patients who are carriers of the polymorphism. The discrepancy with our study may be related to the small sample size of our female study groups and/or a gender effect. Our findings instead show the presence of higher anxiety levels in patients who are carrying APOE-𝜀4 and RNF219/G. This result may support the idea of a synergistic effect of these alleles on the behavioral alteration of the disease. Future studies are needed to clarify whether and how RNF219/G plays in synergy with the gender and APOE-𝜀4 status to affect the neurodegenerative processes underlying dementia.
Author Contributions
SLS and GS: designed the study. AM and SS: performed the experiments. AM, SS, DC, AG, MP, FaP, NB, FeP, FA, VP, LS, CC, GS, and VG: analyzed the data and interpreted the results. SS, AM, and SLS: wrote the paper. All authors approved the final version of the manuscript.
Funding
SLS was supported by research grants from the Italian Department of Education (PRIN 2011; Grant No. 2010M2JARJ_005) and the Italian Department of Health (Grant Nos. RF-2013-02358785 and NET-2011-02346784-1). GS and CC were supported by a grant of the Italian Department of Health (Grant No. NET-2011-02346784-1). AM was supported by a grant of the AIRAlzhOnlus-COOP Italia. FaP and FeP were supported by the Italian Department of Health (Grant No. RF-2013-02359074).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2018.00092/full#supplementary-material
TABLE S1 | Summary data and Analysis of Covariance (ANCOVA) of neuropsychological scores in MCI subjects. ANCOVA was performed in order to evaluate the main and interaction effects of APOE and RNF219 genotype controlling for age and education level. Significant p-values, corrected for multiple comparisons using the Benjamini–Hochberg correction at a false discovery rate (FDR) of 5%, are shown in bold.
TABLE S2 | Summary data and analysis of covariance (ANCOVA) of neuropsychological scores in AD patients. ANCOVA was performed in order to evaluate the main and interaction effects of APOE and RNF219 genotype controlling for age and education level. Significant p-values, corrected for multiple comparisons using the Benjamini–Hochberg correction at a false discovery rate (FDR) of 5%, are shown in bold.
Abbreviations
AD, Alzheimer disease; APOE, apolipoprotein E; APP, amyloid precursor protein; ART, Aligned Rank Transformation; BBB, blood–brain barrier; bp, base pair; CPM, Colored Progressive Matrices; GWASs, genome-wide-association studies; MCI, mild cognitive impairment; MMSE, Mini Mental State Examination; NPI, Neuropsychiatric Inventory; PCR, polymerase chain reaction; RAVL, Rey Auditory Verbal Learning; RNF, Ring Finger Protein.
References
Aloysi, A., Van Dyk, K., and Sano, M. (2006). Women’s cognitive and affective health and neuropsychiatry. Mount Sinai J. Med. 73, 967–975.
Barber, M. J., Mangravite, L. M., Hyde, C. L., Chasman, D. I., Smith, J. D., McCarty, C. A., et al. (2010). Genome-wide association of lipid-lowering response to statins in combined study populations. PLoS One 5:e9763. doi: 10.1371/journal.pone.0009763
Basso, A., Capitani, E., and Laiacona, M. (1987). Raven’s coloured progressive matrices. Funct. Neurol. 2, 189–194. doi: 10.2466/03.04.PR0.115c25z8
Beaudreau, S. A., and O’Hara, R. (2008). Late-life anxiety and cognitive impairment: a review. Am. J. Geriatr. Psychiatry 16, 790–803. doi: 10.1097/JGP.0b013e31817945c3
Bertram, L., McQueen, M. B., Mullin, K., Blacker, D., and Tanzi, R. E. (2007). Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat. Genet. 39, 17–23. doi: 10.1038/ng1934
Breitve, M. H., Hynninen, M. J., Brønnick, K., Chwiszczuk, L. J., Auestad, B. H., Aarsland, D., et al. (2016). A longitudinal study of anxiety and cognitive decline in dementia with Lewy bodies and Alzheimer’s disease. Alzheimers Res. Ther. 8:3. doi: 10.1186/s13195-016-0171-4
Carroll, J. C., and Rosario, E. R. (2012). The potential use of hormone-based therapeutics for the treatment of Alzheimer’s disease. Curr. Alzheimer Res. 9, 18–34. doi: 10.2174/156720512799015109
Cirulli, E. T., Kasperaviciûte, D., Attix, D. K., Need, A. C., Ge, D., Gibson, G., et al. (2010). Common genetic variation and performance on standardized cognitive tests. Eur. J. Hum. Genet. 18, 815–820. doi: 10.1038/ejhg.2010.2
Corona, C., Pensalfini, A., Frazzini, V., and Sensi, S. L. (2011). New therapeutic targets in Alzheimer’s disease: brain deregulation of calcium and zinc. Cell Death Dis. 2:e176. doi: 10.1038/cddis.2011.57
Cummings, J. L., Mega, M., Gray, K., Rosenberg-Thompson, S., Carusi, D. A., and Gornbein, J. (1994). The Neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology 44, 2308–2314. doi: 10.1212/WNL.44.12.2308
Devier, D. J., Pelton, G. H., Tabert, M. H., Liu, X., Cuasay, K., Eisenstadt, R., et al. (2009). The impact of anxiety on conversion from mild cognitive impairment to Alzheimer’s disease. Int. J. Geriatr. Psychiatry 24, 1335–1342. doi: 10.1002/gps.2263
Duron, E., and Hanon, O. (2008). Vascular risk factors, cognitive decline, and dementia. Vasc. Health Risk Manage. 4, 363–381. doi: 10.2147/VHRM.S1839
Feys, J. (2016). New nonparametric rank tests for interactions in factorial designs with repeated measures. J. Mod. Appl. Stat. Methods 15, 78–99. doi: 10.22237/jmasm/1462075500
Folstein, M. F., Folstein, S. E., and McHugh, P. R. (1975). “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198. doi: 10.1016/0022-3956(75)90026-6
Furney, S. J., Simmons, A., Breen, G., Pedroso, I., Lunnon, K., Proitsi, P., et al. (2011). Genome-wide association with MRI atrophy measures as a quantitative trait locus for Alzheimer’s disease. Mol. Psychiatry 16, 1130–1138. doi: 10.1038/mp.2010.123
Gallagher, D., Coen, R., Kilroy, D., Belinski, K., Bruce, I., Coakley, D., et al. (2011). Anxiety and behavioural disturbance as markers of prodromal Alzheimer’s disease in patients with mild cognitive impairment. Int. J. Geriatr. Psychiatry 26, 166–172. doi: 10.1002/gps.2509
Herrup, K. (2010). Reimagining Alzheimer’s disease–an age-based hypothesis. J. Neurosci. 30, 16755–16762. doi: 10.1523/JNEUROSCI.4521-10.2010
Hixson, J. E., and Vernier, D. T. (1990). Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J. Lipid Res. 31, 545–548.
Hoshikawa, S., Ogata, T., Fujiwara, S., Nakamura, K., and Tanaka, S. (2008). A novel function of RING finger protein 10 in transcriptional regulation of the myelin-associated glycoprotein gene and myelin formation in Schwann cells. PLoS One 3:e3464 doi: 10.1371/journal.pone.0003464
Jin, H., Chiou, T. T., Serwanski, D. R., Miralles, C. P., Pinal, N., and De Blas, A. L. (2014). Ring finger protein 34 (RNF34) interacts with and promotes γ-aminobutyric acid type-A receptor degradation via ubiquitination of the γ2 subunit. J. Biol. Chem. 289, 29420–29436. doi: 10.1074/jbc.M114.603068
Joazeiro, C. A. P., and Weissman, A. M. (2000). RING finger proteins?: mediators of ubiquitin ligase activity. Cell 102, 549–552. doi: 10.1016/S0092-8674(00)00077-5
Kang, J. H., and Grodstein, F. (2012). Postmenopausal hormone therapy, timing of initiation, APOE and cognitive decline. Neurobiol. Aging 33, 1129–1137. doi: 10.1016/j.neurobiolaging.2010.10.007
Kramár, E. A., Babayan, A. H., Gall, C. M., and Lynch, G. (2013). Estrogen promotes learning-related plasticity by modifying the synaptic cytoskeleton. Neuroscience 239, 3–16. doi: 10.1016/j.neuroscience.2012.10.038
Lawton, M. P., and Brody, E. M. (1969). Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 9, 179–186. doi: 10.1093/geront/9.3_Part_1.179
Mah, L., Binns, M. A., Steffens, D. C., and Alzheimer’s Disease Neuroimaging Initiative (2015). Anxiety symptoms in amnestic mild cognitive impairment are associated with medial temporal atrophy and predict conversion to Alzheimer disease. Am. J. Geriatr. Psychiatry 23, 466–476. doi: 10.1016/j.jagp.2014.10.005
Matz, A., Lee, S. J., Schwedhelm-Domeyer, N., Zanini, D., Holubowska, A., Kannan, M., et al. (2014). Regulation of neuronal survival and morphology by the E3 ubiquitin ligase RNF157. Cell Death Differ. 22, 626–642. doi: 10.1038/cdd.2014.163
Montagne, A., Toga, A. W., and Zlokovic, B. V. (2015). Blood-brain barrier permeability and gadolinium: benefits and potential pitfalls in research. JAMA Neurol. 73, 13–14. doi: 10.1001/jamaneurol.2015.2960
Ohm, T. G., Scharnagl, H., März, W., and Bohl, J. (1999). Apolipoprotein E isoforms and the development of low and high Braak stages of Alzheimer’s disease-related lesions. Acta Neuropathol. 98, 273–280. doi: 10.1007/s004010051080
Ossenkoppele, R., van der Flier, W. M., Zwan, M. D., Adriaanse, S. F., Boellaard, R., Windhorst, A. D., et al. (2013). Differential effect of APOE genotype on amyloid load and glucose metabolism in AD dementia. Neurology 80, 359–365. doi: 10.1212/WNL.0b013e31827f0889
Panza, F., Frisardi, V., Seripa, D., D’Onofrio, G., Santamato, A., Masullo, C., et al. (2012). Apolipoprotein E genotypes and neuropsychiatric symptoms and syndromes in late-onset Alzheimer’s disease. Ageing Res. Rev. 11, 87–103. doi: 10.1016/j.arr.2011.06.005
Panza, F., Seripa, D., D’Onofrio, G., Frisardi, V., Solfrizzi, V., Mecocci, P., et al. (2011). Neuropsychiatric symptoms, endophenotypes, and syndromes in late-onset Alzheimer’s disease: focus on APOE gene. Int. J. Alzheimers Dis. 2011:721457. doi: 10.4061/2011/721457
Pranski, E. L., Dalal, N. V., Sanford, C. V., Herskowitz, J. H., Gearing, M., Lazo, C., et al. (2013). RING finger protein 11 (RNF11) modulates susceptibility to 6-OHDA-induced nigral degeneration and behavioral deficits through NF-kB signaling in dopaminergic cells. Neurobiol. Dis. 54, 264–279. doi: 10.1016/j.nbd.2012.12.018
Raskin, J., Cummings, J., Hardy, J., Schuh, K., and Dean, R. A. (2015). Neurobiology of Alzheimer’s disease: integrated molecular, physiological, anatomical, biomarker, and cognitive dimensions. Curr. Alzheimer Res. 12, 712–722. doi: 10.1186/s40478-014-0134-6
Rhinn, H., Fujita, R., Qiang, L., Cheng, R., Lee, J. H., and Abeliovich, A. (2013). Integrative genomics identifies APOE 𝜀4 effectors in Alzheimer’s disease. Nature 500, 45–50. doi: 10.1038/nature12415
Saunders, A. M., Strittmatter, W. J., Schmechel, D., George-Hyslop, P. H., Pericak-Vance, M. A., Joo, S. H., et al. (1993). Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology 43, 1467–1472. doi: 10.1212/WNL.43.8.1467
Selkoe, D. J. (2011). Alzheimer’s disease. Cold Spring Harb. Perspect. Biol. 3:a004457. doi: 10.1101/cshperspect.a004457
Selkoe, D. J., and Hardy, J. (2016). The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 8, 595–608. doi: 10.15252/emmm.201606210
Shin, M.-S., Park, S. Y., Park, S. R., Seol, S. H., and Kwon, J. S. (2006). Clinical and empirical applications of the Rey–Osterrieth Complex Figure Test. Nat. Protoc. 1, 892–899. doi: 10.1038/nprot.2006.115
Snyder, K. A., and Harrison, D. W. (1997). The affective auditory verbal learning test. Arch. Clin. Neuropsychol. 12, 477–482. doi: 10.1093/arclin/12.5.477
Spalletta, G., Bernardini, S., Bellincampi, L., Federici, G., Trequattrini, A., and Caltagirone, C. (2006). Delusion symptoms are associated with ApoE epsilon4 allelic variant at the early stage of Alzheimer’s disease with late onset. Eur. J. Neurol. 13, 176–182. doi: 10.1111/j.1468-1331.2006.01165.x
Steinberg, M., Corcoran, C., Tschanz, J. T., Huber, C., Welsh-Bohmer, K., Norton, M. C., et al. (2006). Risk factors for neuropsychiatric symptoms in dementia: the Cache County Study. Int. J. Geriatr. Psychiatry 21, 824–830. doi: 10.1002/gps.1567
Tai, L. M., Ghura, S., Koster, K. P., Liakaite, V., Maienschein-Cline, M., Kanabar, P., et al. (2015). APOE-modulated Aβ-induced neuroinflammation in Alzheimer’s disease: current landscape, novel data, and future perspective. J. Neurochem. 133, 465–488. doi: 10.1111/jnc.13072
Tanzi, R. E. (2012). The genetics of Alzheimer disease. Cold Spring Harb. Perspect. Med. 2:a006296. doi: 10.1101/cshperspect.a006296
Toledo, J. B., Arnold, S. E., Raible, K., Brettschneider, J., Xie, S. X., Grossman, M., et al. (2013). Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain 136(Pt 9), 2697–2706. doi: 10.1093/brain/awt188
Tremblay, M.-P., Potvin, O., Belleville, S., Bier, N., Gagnon, L., Blanchet, S., et al. (2016). The victoria stroop test: normative data in Quebec-French adults and elderly. Arch. Clin. Neuropsychol. doi: 10.1093/arclin/acw029 [Epub ahead of print].
Ungar, L., Altmann, A., and Greicius, M. D. (2014). Apolipoprotein E, gender, and Alzheimer’s disease: an overlooked, but potent and promising interaction. Brain Imaging Behav. 8, 262–273. doi: 10.1007/s11682-013-9272-x
Wallace, M., Shelkey, M., and Hartford Institute for Geriatric Nursing (2007). Katz index of independence in activities of daily living (ADL). Urol. Nurs. 27, 93–94.
Wigginton, J. E., Cutler, D. J., and Abecasis, G. R. (2005). A note on exact tests of Hardy-Weinberg equilibrium. Am. J. Hum. Genet. 76, 887–893. doi: 10.1086/429864
Winblad, B., Amouyel, P., Andrieu, S., Ballard, C., Brayne, C., Brodaty, H., et al. (2015). Defeating Alzheimer’s disease and other dementias: a priority for European science and society. Lancet Neurol. 15, 455–532. doi: 10.1016/S1474-4422(16)00062-4
Wobbrock, J. O., Findlater, L., Gergle, D., and Higgins, J. J. (2011). The Aligned Rank Transform for Nonparametric Factorial Analyses Using Only ANOVA Procedures. Available at: https://faculty.washington.edu/wobbrock/pubs/chi-11.06.pdf [accessed January 27, 2018].
Xing, Y., Qin, W., Li, F., Jia, X. F., and Jia, J. (2012). Apolipoprotein E 𝜀4 status modifies the effects of sex hormones on neuropsychiatric symptoms of Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 33, 35–42. doi: 10.1159/000336600
Xing, Y., Tang, Y., and Jia, J., (2015). Sex differences in neuropsychiatric symptoms of Alzheimer’s disease: the modifying effect of apolipoprotein E 𝜀4 status. Behav. Neurol. 2015:275256. doi: 10.1155/2015/275256
Yaffe, K., Haan, M., Byers, A., Tangen, C., and Kuller, L. (2000). Estrogen use, APOE, and cognitive decline: evidence of gene-environment interaction. Neurology 54, 1949–1954. doi: 10.1212/WNL.54.10.1949
Zhao, Z., Nelson, A. R., Betsholtz, C., and Zlokovic, B. V. (2015). Establishment and dysfunction of the blood-brain barrier. Cell 163, 1064–1078. doi: 10.1016/j.cell.2015.10.067
Zheng, C., Geetha, T., and Babu, J. R. (2014). Failure of ubiquitin proteasome system: risk for neurodegenerative diseases. Neurodegener. Dis. 14, 161–175. doi: 10.1159/000367694
Keywords: dementia, mild cognitive impairment (MCI), Alzheimer disease (AD), APOE, RNF219, genotype
Citation: Mosca A, Sperduti S, Pop V, Ciavardelli D, Granzotto A, Punzi M, Stuppia L, Gatta V, Assogna F, Banaj N, Piras F, Piras F, Caltagirone C, Spalletta G and Sensi SL (2018) Influence of APOE and RNF219 on Behavioral and Cognitive Features of Female Patients Affected by Mild Cognitive Impairment or Alzheimer’s Disease. Front. Aging Neurosci. 10:92. doi: 10.3389/fnagi.2018.00092
Received: 22 November 2017; Accepted: 19 March 2018;
Published: 13 April 2018.
Edited by:
Mohammad Amjad Kamal, King Abdulaziz University, Saudi ArabiaReviewed by:
Khyobeni Mozhui, University of Tennessee Health Science Center, United StatesDavide Ragozzino, Sapienza Università di Roma, Italy
Copyright © 2018 Mosca, Sperduti, Pop, Ciavardelli, Granzotto, Punzi, Stuppia, Gatta, Assogna, Banaj, Piras, Piras, Caltagirone, Spalletta and Sensi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gianfranco Spalletta, Zy5zcGFsbGV0dGFAaHNhbnRhbHVjaWEuaXQ= Stefano L. Sensi, c3NlbnNpQHVjaS5lZHU=
†These authors have contributed equally to this work.
 Alessandra Mosca
Alessandra Mosca Samantha Sperduti1,4†
Samantha Sperduti1,4† Viorela Pop
Viorela Pop Domenico Ciavardelli
Domenico Ciavardelli Nerisa Banaj
Nerisa Banaj Fabrizio Piras
Fabrizio Piras Federica Piras
Federica Piras Carlo Caltagirone
Carlo Caltagirone Gianfranco Spalletta
Gianfranco Spalletta Stefano L. Sensi
Stefano L. Sensi