- 1School of Engineering, Edith Cowan University, Joondalup, WA, Australia
- 2College of Materials Science and Engineering, Nanjing Forestry University, Nanjing, China
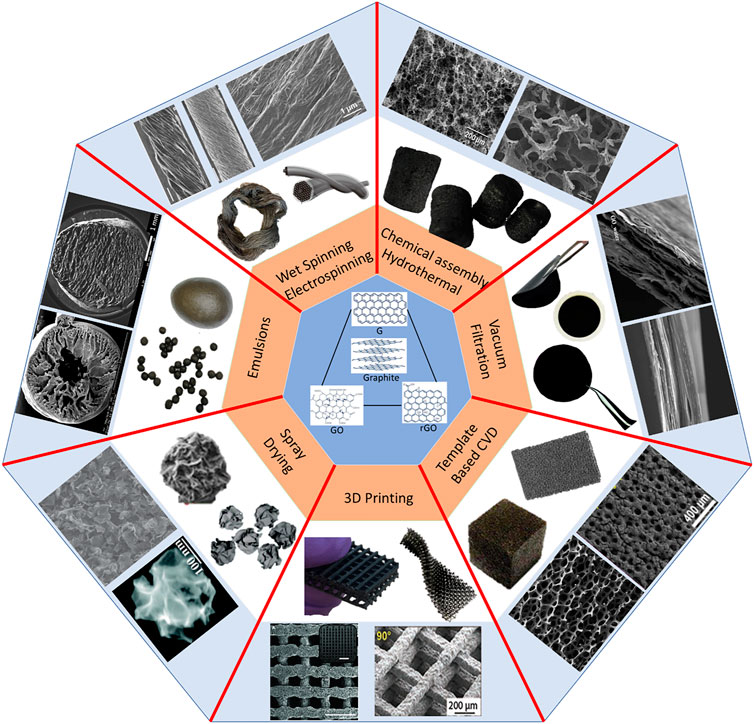
Three-dimensional (3D) graphene-based macrostructures are being developed to combat the issues associated with two-dimensional (2D) graphene materials in practical applications. The 3D macrostructures (3DMs), for example, membranes, fibres, sponges, beads, and mats, can be formed by the self-assembly of 2D graphene-based precursors with exceptional surface area and unique chemistry. With rational design, the 3D macrostructures can then possess outstanding properties and exclusive structures. Thanks to various advantages, these macrostructures are competing in a variety of applications with promising performances unlike the traditional activated carbons, biochars and hydrochars, which have less flexibilities for modifications towards versatile applications. However, despite having such a wide range of applications, 3DMs remain applicable on laboratory scale due to the associated factors like cost and extensive research. This perspective provides an overview of available graphene-based macrostructures and their diverse synthesis protocols. In the synthesis, hydrothermal route, chemical vapor deposition (CVD), wet spinning, 3D printing, vacuum filtration, spray drying and emulsion methods are highlighted. In addition, the physio-chemical properties of these macrostructures are discussed with the relationship among the porosity, surface area and the bulk density. The perspective also highlights the versatile potentials of different 3DMs in wastewater remediation by adsorption, desalination, and catalytic oxidation, etc. Following the concluding remarks, future outlooks on commercial applications of 3DMs are also provided.
Introduction
Because of the exceptionally large theoretical surface area (≈ 2600 m2/g), versatile chemistry and other physicochemical properties, graphene and its derivatives of graphene oxide (GO) and reduced graphene oxide (rGO) have been employed for a vast range of applications, for example, environmental remediation, climate change mitigation, and sustainable energy application (Sun et al., 2014; Yousefi et al., 2019). Since its discovery, graphene in collaboration with various nanotechnologies has demonstrated great success in water and wastewater treatment (Sun et al., 2012; Wang et al., 2013). However, the use of these 2D nanosheets creates various challenges due to their highly hydrophilic nature (Wang et al., 2020). For instance, graphene has a very high colloidal stability in water and therefore, the challenging recovery would form major barrier in practical applications. Over the past few years, extensive efforts have been made to combat this issue. It is demonstrated that 3D graphene macrostructures can be competing alternatives to nanoscale graphene and its compounds (Chowdhury and Balasubramanian, 2017). Graphene macrostructures are a 3-dimensional form of graphene and graphene-based compounds. These macrostructures are very practical and attractive because they not only possess the efficiency of nanomaterials but the ease to manipulate and handle of bulk materials.
In the midst of this emerging research based on graphene macrostructures, we present a perspective on the applications of 3DMs in the vast area of wastewater treatment over the past decade. Rather than being exhaustive, we focus on the recent research trends with a few examples. This research topic is still emerging with very few studies presented in the past few years especially in the degradation of emerging organic contaminants.
Graphene oxide tends to easily form liquid crystal domains in water even at a very low concentration because of its high colloidal stability, high aspect ratio, and geometrical anisotropy (Xu and Gao, 2011). The major driving force of GO nanosheets into the self-assembly of 3D structure is the ordered liquid crystalline arrangement in water. The stable suspension of dispersed graphene oxide has the ability to self-assemble into the light and highly porous sponges by being spun to form fibres, cross-linked to GO beads, pressed to membranes, sprayed to form 3D particles, or even chemically deposited on templates to form foams via hydrothermal or chemical procedures such as chemical vapour deposition (CVD). These synthesis procedures are relatively easy and provide a potential to scale up for commercial applications. In addition, facile and precise control on the porosity and the surface area of the macrostructure is enabled which plays a vital role in their applications. The 3D macrostructure from self-assembly of 2D GO has been applied in various areas such as air and water purification, batteries, and sensors, among which water remediation is of particular interest. 3MDs can be used in various wastewater remediation technologies, for example, adsorption, absorption, catalysis, and desalination. Figure 1 shows the various structures of membranes, fibres, sponges, mats, and beads with their respective synthesis for this purpose (Xu et al., 2015).
Rational Design of Macrostructure Toward Tailored Physiochemical Properties
The self-assembly of 2D GO nanosheets into 3D macrostructures leads to a wide range of properties and environmental performances. Most of the basic characteristics such as porosity, surface area, and bulk density are interrelated, and therefore tend to affect the efficiencies of these 3DMs in their applications. Therefore, it is very important to analyse the trends and to correlate the verse applications of 3DMs to their physiochemical properties.
Pore Structure of 3D Macrostructures
The efficiency of all materials in the removal of pollutants from wastewater highly depends on the surface area and the availability of the active sites. As such it is necessary to study the pore structure and size as they are inversely correlated to surface area and directly correlated to interconnectivity (Yousefi et al., 2019). To balance the surface area and the interconnectivity, Bai et al. suggested the synthesis of a hierarchical pore structure. The hierarchical structure has large pores which are connected with small pores. This avails a large surface area for the reaction and also provides a proper-interconnected network of pores throughout the macrostructure (Bai et al., 2015). The morphology and size of the pores are crucial parameters to consider when designing 3DMs for the removal of viscous oils and other solvents. For this application, structures with a larger pore size are desirable. Chemical vapor deposition (CVD) has been mostly used for the synthesis of sponges with the large pore. These sponges tend to have a more open structure with thin pore edges (Bong et al., 2015).
Another cost-effective method for the synthesis of sponges with large pores is the use of a template. Templates can be dipped in an aqueous dispersion of graphene oxide to coat the outer surface to form microporous graphene oxide coated sponges (Hu et al., 2014). Luo et al. fabricated the graphene sponge using polyurethane as the reinforcement. The sponge formed had a low surface energy with high adsorption capacity (Luo et al., 2017). From the above studies, it can be concluded that the porosity plays a great role since modifications and decorations on the foams affect the surface charges making the foam hydrophilic or hydrophobic. This in turn affects the capillary action of the fluids for instance in the removal of dense and more viscous contaminants than water from narrow channels (Yousefi et al., 2019).
Specific Surface Area and Bulk Density of 3D Macrostructures
Bulk density and the pore volume of 3DMs are interrelated. Generally, when the 3D macrostructure has a high bulk density, the structure is composed of more 2D nanosheets and in turn, it has fewer pores. The effect of density is one of the key considerations in the adsorption of oils and viscous solvents. Theoretically, low bulk density macrostructures should be considered for removal of oil. However, this cannot be implemented practically since contact with water could easily disintegrate these 3DMs due to their low stiffness. Hence, a balance should be considered to ensure that the structure is efficient in the removal of oils and meantime, it does not disintegrate (Yousefi et al., 2019).
The specific surface area of 3DMs determines their efficiency in the removal of the contaminants. 2D nanosheets with a high aspect ratio provide active sites for interaction between the pollutants and catalysts. However, it should be noted that macrostructures with a higher bulk density do not necessarily lead to 3DMs with large specific surface areas (Yousefi et al., 2019). Porosity, interconnectivity and the morphology of the structure jointly determine the specific surface area of 3DMs. In a study based on the removal of methylene blue, the surface area played a great role. Liu et al. prepared a graphene hydrogel with a specific surface area of 450.3 m2/g for the removal of dyes from wastewater. The results showed that the adsorbent was able to remove 177.3 mg/g of MB from the solution (Liu Y. et al., 2017).
From the above study, it can be concluded that specific surface area is a key that controls molecular adsorption on the surface. Among various studies conducted on 3DMs, only a few studies were able to synthesise macrostructures with surface areas larger than 1000 m2/g. Therefore, more researches still need to be done on these macrostructures and new methods, and modifications should be proposed to advance this field. One promising solution to this problem is the rational design of pore architecture, however, this method may form larger pores and hence weaken the whole 3D structure.
Applications in Water Remediation
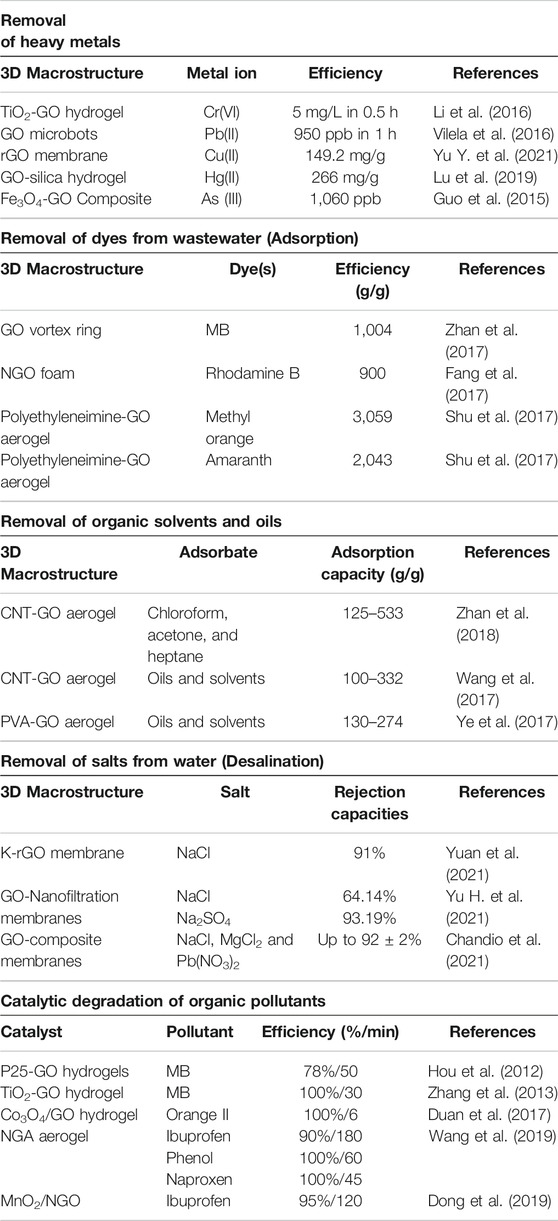
3D macrostructures have versatile applications in the removal of contaminants such as dyes, hazardous oil, organic solvents and heavy metals by adsorption, desalination and catalytic oxidation for wastewater remediation as shown in Table 1.
Removal of Heavy Metals From Wastewater
Heavy metals are frequently discharged to aquatic environments via mining, manufacturing industries, agriculture, automobile among others (Sulaiman et al., 2019). They pose a major hazard to both humans and ecosystems, because the high toxicity of these metals severely threat public health and may cause death in an extreme case. Graphene oxides have ion-chelating negatively charged functional groups which render their 3D macrostructures ideal for the adsorption of the heavy metals from water (Cong et al., 2012; Vilela et al., 2016). The large surface area and the highly porous structure provide ample active sites for adsorption. Yao et al. studied the removal of Cr(IV) using 3D titanium dioxide–graphene hydrogel via adsorption (Li et al., 2016), and showed that oxygen-containing functional groups on the surface of the hydrogel can enhance the adsorption of anionic metal ions by formations of hydrogen bonds. However, it is difficult to remove heavy metals from the wastewater using graphene materials alone. Therefore, external energy is required to boost this process and as such, partial electrical conductivity of graphene oxide macrostructures can be used to tackle this problem. More sophisticated techniques such as the combination of high specific surface area, porosity, introduction of photocatalytic metals oxides such as TiO2 and partial electrical conductivity, which tend to be more advantageous than just adsorption, can be applied (Liu P. et al., 2017). Additionally, metal ions saturated with 3DMs can be separated easily from aqueous solution and can be reclaimed by acid washing to desorb the metallic phase. Further, graphene oxide-based nanocomposite materials consisting of photocatalytic nanomaterials were used to simultaneously adsorb and reduce heavy metal ions thereby quickening the reclamation process (Gao et al., 2013). Hence, it can be concluded that 3DMs are quite efficient in heavy metal removal and could be a revolution in future if this technique is applied in a commercial wastewater treatment plant.
Removal of Dyes From Wastewater
As a result of industrial growth, a wide range of dyes are being discharged to water bodies. Among all 3D macrostructures, GO sponges and beads have shown promising efficiencies in the removal of various dyes from wastewater. The adsorption of dyes on these 3D macrostructures is aided by the electrostatic forces and the π–π interaction (Sui et al., 2013; Wu et al., 2013).
The availability of the active sites on the GO structure plays a vital role in the adsorption of dyes. When GO surface comes in contact with water, the active sites are occupied by the dissolved organic matters such as humic acid which may affect the adsorption performance. However, most studies ignore this impact by the dissolved organic matters and are also limited to a few pollutants. For the commercial application, a better understanding is required on the roles of organic matters, pH of the solution, and the complexity of the multiple pollutants. Further, most of the dye adsorption is conducted in batch other than continuous tests. Also, after the adsorption, regeneration is necessary yet lacks effective methods. Further, most contaminants studied for the adsorption process in the laboratory are made of small molecules and therefore have simple chemistry. In reality, more complex and toxic organic contaminants such as pharmaceuticals and toxins present in the wastewater need to be mineralised (Yousefi et al., 2019). It can therefore be concluded that with the help of advancing technology, 3DMs could be used in commercial wastewater remediation plants to adsorb dyes, and regenerate the adsorbent for multiple uses to be viable economically.
Removal of Organic Solvents and Oils
Oil and organic solvent spills have become a major issue in terms of marine and freshwater pollution. Spills can be cleaned up by dilution, neutralisation, washing, decontamination, and absorption, most of which however are unreliable as they cause secondary pollutions. Absorption can avoid these risks and can remove the secondary contaminants (Bao et al., 2016). Various lightweight and inert traditional adsorbents have been used but the small pores limit the diffusion of more viscous fluids such as crude oil (Muhammad Shafiq et al., 2017). GO macrostructures with a large specific surface area and porosity can be used as an alternative to traditional solutions. Unlike other pollutants such as dyes and metal ions which are removed by adsorption, most hydrocarbons are eliminated from wastewater by physical absorption. This explains why sponges with high porosity are efficient in this application. Nonetheless, seawater may alter the wettability and the stability of the GO sponges upon prolonged contacts. To overcome this issue modification of GO is necessary and previous studies have shown that partial reduction of GO can be used to restore the hydrophobicity and oleophilicity of the sponge. Chemical reduction of the 3D macrostructures can favour the interactions with the target with the π–π bond (Yousefi et al., 2019).
One major advantage of using 3D macrostructures for this purpose is the easy and effective regeneration of the macrostructure. One idea of regenerating the structure is to burn off the oil without damaging the structure. Alternatively, the absorbed solvent can be squeezed out of the structure, however, the efficiency, in this case, will change. Another quite promising technique is to heat the oil-loaded macrostructure to evaporate the oil phase and simultaneously collect it by condensation for efficient recovery of oil and regeneration of the 3D macrostructure (Bi et al., 2012; Li et al., 2014).
Removal of Salts From Water as Desalination
This process is usually based on membrane technology. In recent years, graphene-based membranes have proven to be a potential candidate in the membrane synthesis for the desalination process. Graphene-based nanosheets are usually very thin and smooth which in turn ensure rapid water transportation through the defects or the channels between these sheets (Liu et al., 2016). Among various desalination techniques, two major graphene-based membrane strategies have been proposed for this purpose: highly nanoporous single sheet structures and stacked 3D macrostructures. The nanoporous single sheet membranes have etched defects which can be as a result of plasma etching or ion bombardment (Surwade et al., 2015). However, these membranes cannot be practically applied in the desalination process due to the challenges in precise control of selectivity and scaling up for commercial application (Surwade et al., 2015). As such, 3D macrostructures have been studied since they are feasible and cost-effective for the desalination process.
3D macrostructures are generally applied in the form of pressure-driven membranes. In this case, the interlayer spacing between the stacks is used to determine the selectivity of the materials for separation. Manipulation of the interlayer spacing of the stacks can be used to regulate the materials flowing through the channel of the membrane (Mi, 2014). Unlike nanoporous single-sheet membranes, 3D macrostructures can be scaled up for commercial use by the production of large-area membranes via gravure printing machine. The gravure printing machine is used in this process to align the nematic phase of graphene oxide liquid crystal suspensions for the formation of highly ordered stacks of graphene oxide. This alignment of the GO sheets enhances the stability of the membrane and also sharpens the size of the channel within the membrane to improve the permeability of water and reject the large solutes (Xu et al., 2017).
Despite putting great efforts into the fabrication of pressure-driven membranes, the current 3D macrostructures have yet exceeded the perselectivity of the state of the art thin film composite desalination membranes (Elimelech and Phillip, 2011). These membranes are allowing a higher permeability of water with a poor salt selectivity hence acting as ultrafiltration or nanofiltration processes and not reverse osmosis. Generally, the efficiency of the membrane is determined by the amount of salt it retains rather than the amount of water it permits. Hence, one major challenge with this pressure-driven membrane is to form thins and defect-free nanomaterial-based membranes for laboratory purpose. Besides, other factors such as membrane fouling and decrease in the desalination performance should also be evaluated to ensure long-term stability (Elimelech and Phillip, 2011).
3D Macrostructures for Advanced Oxidation Processes
Recently graphene-based materials have shown excellent efficiencies in advanced oxidation processes (Duan et al., 2015; Duan et al., 2016; Kang et al., 2020; Li et al., 2021). However, one major challenge is the recovery for reusability since the nanoparticles are hard to collect and recycle. One alternative method is the use of 3D macrostructures for the mineralisation of pollutants. The integrated morphology of the 3D macrostructures makes it easy to separate in the practical application. Further, hybridisation with metal oxides such as MnO2 and Co3O4 can be done easily during the synthesis. This results in synergistic charge transfer and enhances mass transport which eventually increases catalytic activity. Besides, these structures also prevent the release of the catalysts in the environment (He et al., 2018).
Wang et al. synthesised N-doped 3D graphene aerogel (NGA) for catalytic oxidation of emerging contaminants like antibiotic from wastewater (Wang et al., 2019). The as-prepared catalyst was used to chemically activate peroxymonosulfate for the degradation of Ibuprofen. The results showed that about 90% of Ibuprofen was removed in 180 min. Stability and reusability were also studied by using the catalyst for three successive runs. It was seen that 90% degradation was achieved after the first-round while 51.8 and 28.4% degradations were achieved after the second and third round.
From Table 1, it can be concluded that graphene-based macrostructures can activate PMS for mineralisation of various pollutants. However, most studies are based on the degradation of dyes and very few studies are done on the degradation of POPs. Further research needs to be done on the degradation of various POPs. Further, stability and the reusability of the catalyst needs to be studied in detail to ensure the efficiency of the degradation not to drop significantly with each cycle. Also, more studies are required to further disclose the catalytic mechanisms of 3D graphene-based macrostructures in verse applications.
Concluding Remarks and Perspectives
Diverse morphological forms of 3-dimensional graphene-based macrostructures can be obtained by rational synthesis and modifications according to the specific application. These macrostructures have proven to be advantageous as they are easy to manipulate and handle than nanoparticles. Furthermore, 3DM’s relatively large specific surface area and porosity would play a vital role in the applications such as adsorption of heavy metals and dyes, organic solvents and oils, and catalytic oxidation thus aiding in the purification and remediation of the wastewater. Despite having such remarkable progress in the past few years, there are a few obstacles that we believe need to be overcome to capitalize on the proposed applications of graphene-based macrostructures for environmental remediation. Based on the above discussion, future outlooks can be based on below points.
1. Further studies should be conducted to investigate how these 3D graphene-based wastewater remediation technologies can be integrated into the existing wastewater treatment plants for promising outcomes. For instance, research should be done on how to integrate this treatment in the ultrafiltration process in case of a 3D graphene membrane.
2. Industrial applications require bulk production of 3DMs and therefore various environmentally friendly and economically viable processes should be developed to magnify the production on a commercial level. This also includes the preparation of the precursor (graphene oxide) as it requires a lot of costly resources hence, a breakthrough in GO synthesis is also required. This, in turn, will help to develop less expensive methods for quality wastewater treatment.
3. The applications of these 3DMs are still limited to simple separation such as oil and water separation, dye and heavy metal removals, and simple catalysis. Applications in the degradation and complete elimination of emerging contaminants such as pharmaceuticals and personal care products (PPCPs) (Asif et al., 2021) and disinfections are still quite scarce.
4. Finally, most current studies are only based on laboratory scale models to evaluate the performance of these macrostructures. With the advancement in technology and the improvement in the research sector, 3DMs applications should be driven to advanced levels such as pilot and full scale, which will be beneficial for industrial applications. This will thereby aid in developing novel wastewater remediation techniques at commercial level.
Author Contributions
RH wrote the draft; AA and NR contribute to data collection and discussion; LS and SZ contribute to revision and supervision; HW contributes to review and discussion; and HS contributes to conception, supervision, and final revision. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Asif, A. H., Wang, S., and Sun, H. (2021). Hematite-based Nanomaterials for Photocatalytic Degradation of Pharmaceuticals and Personal Care Products (PPCPs): A Short Review. Curr. Opin. Green Sustain. Chem. 28, 100447. doi:10.1016/j.cogsc.2021.100447
Bai, J., Zhou, A., Huang, Z., Wu, J., Bai, H., and Li, L. (2015). Ultra-light and Elastic Graphene Foams with a Hierarchical Structure and a High Oil Absorption Capacity. J. Mater. Chem. A. 3 (45), 22687–22694. doi:10.1039/c5ta06204g
Bao, C., Bi, S., Zhang, H., Zhao, J., Wang, P., Yue, C. Y., et al. (2016). Graphene Oxide Beads for Fast Clean-Up of Hazardous Chemicals. J. Mater. Chem. A. 4 (24), 9437–9446. doi:10.1039/c6ta01411a
Bi, H., Xie, X., Yin, K., Zhou, Y., Wan, S., He, L., et al. (2012). Spongy Graphene as a Highly Efficient and Recyclable Sorbent for Oils and Organic Solvents. Adv. Funct. Mater. 22 (21), 4421–4425. doi:10.1002/adfm.201200888
Bong, J., Lim, T., Seo, K., Kwon, C.-A., Park, J. H., Kwak, S. K., et al. (2015). Dynamic Graphene Filters for Selective Gas-Water-Oil Separation. Scientific Rep. 5 (1), 14321. doi:10.1038/srep14321
Chandio, I., Janjhi, F. A., Memon, A. A., Memon, S., Ali, Z., Thebo, K. H., et al. (2021). Ultrafast Ionic and Molecular Sieving through Graphene Oxide Based Composite Membranes. Desalination 500, 114848. doi:10.1016/j.desal.2020.114848
Chowdhury, S., and Balasubramanian, R. (2017). Three-dimensional Graphene-Based Macrostructures for Sustainable Energy Applications and Climate Change Mitigation. Prog. Mater. Sci. 90, 224–275. doi:10.1016/j.pmatsci.2017.07.001
Cong, H.-P., Ren, X.-C., Wang, P., and Yu, S.-H. (2012). Macroscopic Multifunctional Graphene-Based Hydrogels and Aerogels by a Metal Ion Induced Self-Assembly Process. ACS Nano 6 (3), 2693–2703. doi:10.1021/nn300082k
Dong, Q., Wang, J., Duan, X., Tan, X., Liu, S., and Wang, S. (2019). Self-assembly of 3D MnO2/N-Doped Graphene Hybrid Aerogel for Catalytic Degradation of Water Pollutants: Structure-dependent Activity. Chem. Eng. J. 369, 1049–1058. doi:10.1016/j.cej.2019.03.139
Duan, L., Zhou, X., Liu, S., Shi, P., and Yao, W. (2017). 3D-hierarchically Structured Co3O4/graphene Hydrogel for Catalytic Oxidation of Orange II Solutions by Activation of Peroxymonosulfate. J. Taiwan Inst. Chem. Eng. 76, 101–108. doi:10.1016/j.jtice.2017.04.019
Duan, X., Ao, Z., Sun, H., Indrawirawan, S., Wang, Y., Kang, J., et al. (2015). Nitrogen-Doped Graphene for Generation and Evolution of Reactive Radicals by Metal-free Catalysis. ACS Appl. Mater. Inter. 7 (7), 4169–4178. doi:10.1021/am508416n
Duan, X., Sun, H., Ao, Z., Zhou, L., Wang, G., and Wang, S. (2016). Unveiling the Active Sites of Graphene-Catalyzed Peroxymonosulfate Activation. Carbon 107, 371–378. doi:10.1016/j.carbon.2016.06.016
Elimelech, M., and Phillip, W. A. (2011). The Future of Seawater Desalination: Energy, Technology, and the Environment. Science 333 (6043), 712–717. doi:10.1126/science.1200488
Fang, Q., Zhou, X., Deng, W., Liu, Y., Zheng, Z., and Liu, Z. (2017). Nitrogen-Doped Graphene Nanoscroll Foam with High Diffusion Rate and Binding Affinity for Removal of Organic Pollutants. Small 13 (14), 1603779. doi:10.1002/smll.201603779
Gao, H., Sun, Y., Zhou, J., Xu, R., and Duan, H. (2013). Mussel-Inspired Synthesis of Polydopamine-Functionalized Graphene Hydrogel as Reusable Adsorbents for Water Purification. ACS Appl. Mater. Inter. 5 (2), 425–432. doi:10.1021/am302500v
Guo, L., Ye, P., Wang, J., Fu, F., and Wu, Z. (2015). Three-dimensional Fe3O4-Graphene Macroscopic Composites for Arsenic and Arsenate Removal. J. Hazard. Mater. 298, 28–35. doi:10.1016/j.jhazmat.2015.05.011
He, K., Chen, G., Zeng, G., Chen, A., Huang, Z., Shi, J., et al. (2018). Three-dimensional Graphene Supported Catalysts for Organic Dyes Degradation. Appl. Catal. B: Environ. 228, 19–28. doi:10.1016/j.apcatb.2018.01.061
Hou, C., Zhang, Q., Li, Y., and Wang, H. (2012). P25-graphene Hydrogels: Room-Temperature Synthesis and Application for Removal of Methylene Blue from Aqueous Solution. J. Hazard. Mater. 205-206, 229–235. doi:10.1016/j.jhazmat.2011.12.071
Hu, H., Zhao, Z., Gogotsi, Y., and Qiu, J. (2014). Compressible Carbon Nanotube-Graphene Hybrid Aerogels with Superhydrophobicity and Superoleophilicity for Oil Sorption. Environ. Sci. Technol. Lett. 1 (3), 214–220. doi:10.1021/ez500021w
Kang, J., Zhou, L., Duan, X., Sun, H., and Wang, S. (2020). Catalytic Degradation of Antibiotics by Metal-free Catalysis over Nitrogen-Doped Graphene. Catal. Today 357, 341–349. doi:10.1016/j.cattod.2018.12.002
Li, J., Li, J., Meng, H., Xie, S., Zhang, B., Li, L., et al. (2014). Ultra-light, Compressible and Fire-Resistant Graphene Aerogel as a Highly Efficient and Recyclable Absorbent for Organic Liquids. J. Mater. Chem. A. 2 (9), 2934–2941. doi:10.1039/c3ta14725h
Li, J., Zhao, S., Zhang, L., Jiang, S. P., Yang, S.-Z., Wang, S., et al. (2021). Cobalt Single Atoms Embedded in Nitrogen-Doped Graphene for Selective Oxidation of Benzyl Alcohol by Activated Peroxymonosulfate. Small 17, 2004579. doi:10.1002/smll.202004579
Li, Y., Cui, W., Liu, L., Zong, R., Yao, W., Liang, Y., et al. (2016). Removal of Cr(VI) by 3D TiO 2 -graphene Hydrogel via Adsorption Enriched with Photocatalytic Reduction. Appl. Catal. B: Environ. 199, 412–423. doi:10.1016/j.apcatb.2016.06.053
Liu, G., Jin, W., and Xu, N. (2016). Two-Dimensional-Material Membranes: A New Family of High-Performance Separation Membranes. Angew. Chem. Int. Ed. 55 (43), 13384–13397. doi:10.1002/anie.201600438
Liu, P., Yan, T., Zhang, J., Shi, L., and Zhang, D. (2017). Separation and Recovery of Heavy Metal Ions and Salt Ions from Wastewater by 3D Graphene-Based Asymmetric Electrodes via Capacitive Deionization. J. Mater. Chem. A. 5 (28), 14748–14757. doi:10.1039/c7ta03515b
Liu, Y., Gao, T., Xiao, H., Guo, W., Sun, B., Pei, M., et al. (2017). One-pot Synthesis of Rice-like TiO2 /graphene Hydrogels as Advanced Electrodes for Supercapacitors and the Resulting Aerogels as High-Efficiency Dye Adsorbents. Electrochimica Acta 229, 239–252. doi:10.1016/j.electacta.2017.01.142
Lu, J., Wu, X., Li, Y., Liang, Y., and Cui, W. (2019). Facile Fabrication of 3D Graphene–Silica Hydrogel Composite for Enhanced Removal of Mercury Ions. Nanomaterials 9 (3), 314. doi:10.3390/nano9030314
Luo, Y., Jiang, S., Xiao, Q., Chen, C., and Li, B. (2017). Highly Reusable and Superhydrophobic Spongy Graphene Aerogels for Efficient Oil/water Separation. Scientific Rep. 7 (1), 7162. doi:10.1038/s41598-017-07583-0
Mi, B. (2014). Graphene Oxide Membranes for Ionic and Molecular Sieving. Science 343 (6172), 740–742. doi:10.1126/science.1250247
Muhammad Shafiq, Y., Cheong, W. K., and Lau, E. V. (2017). Graphene Aerogel - Recovery of Heavy Crude Oil from Contaminated Sand. J. Environ. Chem. Eng. 5 (2), 1711–1717. doi:10.1016/j.jece.2017.03.009
Shu, D., Feng, F., Han, H., and Ma, Z. (2017). Prominent Adsorption Performance of Amino-Functionalized Ultra-light Graphene Aerogel for Methyl Orange and Amaranth. Chem. Eng. J. 324, 1–9. doi:10.1016/j.cej.2017.04.136
Sui, Z.-Y., Cui, Y., Zhu, J.-H., and Han, B.-H. (2013). Preparation of Three-Dimensional Graphene Oxide-Polyethylenimine Porous Materials as Dye and Gas Adsorbents. ACS Appl. Mater. Inter. 5 (18), 9172–9179. doi:10.1021/am402661t
Sulaiman, M. B., Salawu, K., and Au, B. (2019). Assessment of Concentrations and Ecological Risk of Heavy Metals at Resident and Remediated Soils of Uncontrolled Mining Site at Dareta Village, Zamfara, Nigeria. J. Appl. Sci. Environ. Manage. 23 (1), 187. doi:10.4314/jasem.v23i1.28
Sun, H., Liu, S., Liu, S., and Wang, S. (2014). A Comparative Study of Reduced Graphene Oxide Modified TiO2, ZnO and Ta2O5 in Visible Light Photocatalytic/photochemical Oxidation of Methylene Blue. Appl. Catal. B: Environ. 146, 162–168. doi:10.1016/j.apcatb.2013.03.027
Sun, H., Liu, S., Zhou, G., Ang, H. M., Tadé, M. O., and Wang, S. (2012). Reduced Graphene Oxide for Catalytic Oxidation of Aqueous Organic Pollutants. ACS Appl. Mater. Inter. 4 (10), 5466–5471. doi:10.1021/am301372d
Surwade, S. P., Smirnov, S. N., Vlassiouk, I. V., Unocic, R. R., Veith, G. M., Dai, S., et al. (2015). Water Desalination Using Nanoporous Single-Layer Graphene. Nat. Nanotech 10 (5), 459–464. doi:10.1038/nnano.2015.37
Vilela, D., Parmar, J., Zeng, Y., Zhao, Y., and Sánchez, S. (2016). Graphene-Based Microbots for Toxic Heavy Metal Removal and Recovery from Water. Nano Lett. 16 (4), 2860–2866. doi:10.1021/acs.nanolett.6b00768
Wang, C., Yang, S., Ma, Q., Jia, X., and Ma, P.-C. (2017). Preparation of Carbon Nanotubes/graphene Hybrid Aerogel and its Application for the Adsorption of Organic Compounds. Carbon 118, 765–771. doi:10.1016/j.carbon.2017.04.001
Wang, H., Mi, X., Li, Y., and Zhan, S. (2020). 3D Graphene‐Based Macrostructures for Water Treatment. Adv. Mater. 32 (3), 1806843. doi:10.1002/adma.201806843
Wang, J., Duan, X., Dong, Q., Meng, F., Tan, X., Liu, S., et al. (2019). Facile Synthesis of N-Doped 3D Graphene Aerogel and its Excellent Performance in Catalytic Degradation of Antibiotic Contaminants in Water. Carbon 144, 781–790. doi:10.1016/j.carbon.2019.01.003
Wang, S., Sun, H., Ang, H. M., and Tadé, M. O. (2013). Adsorptive Remediation of Environmental Pollutants Using Novel Graphene-Based Nanomaterials. Chem. Eng. J. 226, 336–347. doi:10.1016/j.cej.2013.04.070
Wu, T., Chen, M., Zhang, L., Xu, X., Liu, Y., Yan, J., et al. (2013). Three-dimensional Graphene-Based Aerogels Prepared by a Self-Assembly Process and its Excellent Catalytic and Absorbing Performance. J. Mater. Chem. A. 1 (26), 7612–7621. doi:10.1039/c3ta10989e
Xu, W. L., Fang, C., Zhou, F., Song, Z., Liu, Q., Qiao, R., et al. (2017). Self-Assembly: A Facile Way of Forming Ultrathin, High-Performance Graphene Oxide Membranes for Water Purification. Nano Lett. 17 (5), 2928–2933. doi:10.1021/acs.nanolett.7b00148
Xu, Y., Shi, G., and Duan, X. (2015). Self-Assembled Three-Dimensional Graphene Macrostructures: Synthesis and Applications in Supercapacitors. Acc. Chem. Res. 48 (6), 1666–1675. doi:10.1021/acs.accounts.5b00117
Xu, Z., and Gao, C. (2011). Aqueous Liquid Crystals of Graphene Oxide. ACS Nano 5 (4), 2908–2915. doi:10.1021/nn200069w
Ye, S., Liu, Y., and Feng, J. (2017). Low-Density, Mechanical Compressible, Water-Induced Self-Recoverable Graphene Aerogels for Water Treatment. ACS Appl. Mater. Inter. 9 (27), 22456–22464. doi:10.1021/acsami.7b04536
Yousefi, N., Lu, X., Elimelech, M., and Tufenkji, N. (2019). Environmental Performance of Graphene-Based 3D Macrostructures. Nat. Nanotech 14 (2), 107–119. doi:10.1038/s41565-018-0325-6
Yu, H., Xiao, G., He, Y., Fan, Y., Mei, X., Li, H., et al. (2021). The Intercalation of Nanoscale Lattices into Micro-sized Graphene Oxide Sheets for Enhancing Pressure-Driven Desalination Performances. Desalination 500, 114868. doi:10.1016/j.desal.2020.114868
Yu, Y., Wang, Z., Sun, R., Chen, Z., Liu, M., Zhou, X., et al. (2021). Self-Supported Reduced Graphene Oxide Membrane and its Cu2+ Adsorption Capability. Materials 14 (1), 146.
Yuan, S., Li, Y., Xia, Y., Selomulya, C., and Zhang, X. (2021). Stable Cation-Controlled Reduced Graphene Oxide Membranes for Improved NaCl Rejection. J. Membr. Sci. 621, 118995. doi:10.1016/j.memsci.2020.118995
Zhan, W., Yu, S., Gao, L., Wang, F., Fu, X., Sui, G., et al. (2018). Bioinspired Assembly of Carbon Nanotube into Graphene Aerogel with “Cabbagelike” Hierarchical Porous Structure for Highly Efficient Organic Pollutants Cleanup. ACS Appl. Mater. Inter. 10 (1), 1093–1103. doi:10.1021/acsami.7b15322
Zhan, Y., Yan, N., Li, Y., Meng, Y., Wang, J., Zhang, N., et al. (2017). Fabrication of Graphene Millimeter-Vortex Ring with Excellent Absorption via Solution Dripping and In-Situ Reduction Method. Chem. Eng. J. 327, 142–149. doi:10.1016/j.cej.2017.06.049
Keywords: 3D macrostructures, graphene, wastewater, remediation, adsorption, advanced oxidation processes
Citation: Hirani RAK, Asif AH, Rafique N, Shi L, Zhang S, Wu H and Sun H (2021) Wastewater Remediation Technologies Using Macroscopic Graphene-Based Materials: A Perspective. Front. Nanotechnol. 3:688552. doi: 10.3389/fnano.2021.688552
Received: 31 March 2021; Accepted: 03 May 2021;
Published: 18 May 2021.
Edited by:
Amitava Mukherjee, VIT University, IndiaReviewed by:
Sasanka Deka, University of Delhi, IndiaIvan Kozyatnyk, Linköping University, Sweden
Wen Da Oh, Universiti Sains Malaysia, Malaysia
Copyright © 2021 Hirani, Asif, Rafique, Shi, Zhang, Wu and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongqi Sun, aC5zdW5AZWN1LmVkdS5hdQ==
 Rajan Arjan Kalyan Hirani
Rajan Arjan Kalyan Hirani Abdul Hannan Asif
Abdul Hannan Asif Nasir Rafique
Nasir Rafique Lei Shi2
Lei Shi2 Shu Zhang
Shu Zhang Hongqi Sun
Hongqi Sun