- 1Department of Pharmacology and Toxicology, School of Pharmacy, University of Kansas, Lawrence, KS, USA
- 2Consortium for Translational Research on Aggression and Drug Abuse, University of Kansas, Lawrence, KS, USA
Schizophrenia is a severe mental disorder, with a highly complex and heterogenous clinical presentation. Our current perspectives posit that the pathogenic mechanisms of this illness lie in complex arrays of gene × environment interactions. Furthermore, several findings indicate that males have a higher susceptibility for schizophrenia, with earlier age of onset and overall poorer clinical prognosis. Based on these premises, several authors have recently begun exploring the possibility that the greater schizophrenia vulnerability in males may reflect specific gene × sex (G×S) interactions. Our knowledge on such G×S interactions in schizophrenia is still rudimentary; nevertheless, the bulk of preclinical evidence suggests that the molecular mechanisms for such interactions are likely contributed by the neurobiological effects of sex steroids on dopamine (DA) neurotransmission. Accordingly, several recent studies suggest a gender-specific association of certain DAergic genes with schizophrenia. These G×S interactions have been particularly documented for catechol-O-methyltransferase (COMT) and monoamine oxidase (MAO), the main enzymes catalyzing DA metabolism. In the present review, we will outline the current evidence on the interactions of DA-related genes and sex-related factors, and discuss the potential molecular substrates that may mediate their cooperative actions in schizophrenia pathogenesis.
Introduction
Schizophrenia is a chronic and severe neurodevelopmental disorder, characterized by a highly complex and heterogeneous set of perceptual, cognitive and emotional deficits (Breier, 1999; Rowley et al., 2001). According to the current diagnostic criteria, the pathognomonic manifestations in schizophrenia are clustered into three groups of symptoms: (1) positive symptoms, which encompass hallucinations and delusions; (2) negative symptoms, including flat affect, alogia, anhedonia and social deficits; and (3) cognitive symptoms, which reflect impairments of attention, memory, perception and thought. Converging evidence has revealed that the primary deficits in schizophrenia are likely mediated by dopamine (DA), in cooperation with other key neurotransmitters, such as glutamate, γ-aminobutyric acid (GABA) and serotonin. Nevertheless, the quest to understand the pathogenic mechanisms of schizophrenia has not yet led to a conclusive theory, and its pathophysiology remains frustratingly elusive.
A wealth of genetic data has identified a number of vulnerability factors that are not inherently pathological, but predispose an individual to develop schizophrenia in the presence of critical environment determinants. These findings have prompted a shift in the conceptual framework of schizophrenia, and underscored the importance of gene-environment (G×E) interactions in this disease (Van Os and Murray, 2008; Van Os et al., 2008, 2010; Van Os and Rutten, 2009).
Multiple lines of evidence have also highlighted that sex-related factors play a potentially important role in shaping the clinical trajectory of schizophrenia. Indeed, males have a higher risk for schizophrenia than females, with earlier age of onset and greater severity of negative and cognitive symptoms (Markham, 2012). Based on these premises, it is possible to theorize the existence of specific gene × sex (G×S) interactions that may also contribute to schizophrenia pathogenesis.
Numerous preclinical studies support that the DAergic system is one of the key mediators of sex differences in schizophrenia (Bay-Richter et al., 2009; Arime et al., 2012; for a detailed presentation of this issue, see Sanchez et al., 2010); accordingly, genetic investigations point to a clear involvement of the key metabolic enzymes of DA, catechol-O-methyltransferase (COMT) and monoamine oxidase (MAO), in the underpinnings of G×S interactions in schizophrenia. In the present review, we will discuss how the emerging evidence on the genes encoding these enzymes and their interactions with sex-related factors may provide fundamental clues to unravel the essence of the biological bases of schizophrenia.
The Role of DA in the Pathophysiology of Schizophrenia
The role of dopamine in the pathogenesis of schizophrenia was originally postulated following the discovery that D2 dopamine receptor antagonism was a fundamental pharmacological requisite of antipsychotic drugs, and that the therapeutic efficacy of these agents was correlated with their inhibitory potency (Seeman and Lee, 1975; Creese et al., 1976). While several studies support the concept that stimulation of D2 receptors in subcortical areas (and particularly striatum and nucleus accumbens) results in psychotic manifestations, other lines of evidence strongly suggest that negative and cognitive symptoms (which are generally not affected by D2 receptor antagonists) may be underpinned by the insufficient activation of D1-like receptors in the prefrontal cortex (PFC) (Goldman-Rakic and Selemon, 1997). These findings have led to the view that schizophrenia may be underpinned by mesolimbic hyperactivity and mesocortical hypoactivity (Weinberger, 1987; Davis et al., 1991). Although studies in the last two decades have documented the fundamental roles of other neurotransmitters in schizophrenia, particularly glutamate and GABA (Benes and Berretta, 2001; Tsai and Coyle, 2002), the DAergic hypothesis still affords the best-validated theoretical framework for this disorder. Recent imaging and post-mortem studies have lead to a refinement of this hypothesis, indicating that the dysregulations of DA neurotransmission in cortex and elevations in presynaptic DA content in the striatum may be the main biological signatures of psychotic disorders (Howes and Kapur, 2009; Fusar-Poli et al., 2011; Howes et al., 2011, 2012, 2013; Allen et al., 2012; Egerton et al., 2013; Stokes et al., 2013; Lataster et al., 2014; see Kuepper et al., 2012 and Smieskova et al., 2013 for more thorough reviews on dopaminergic dysfunctions in brain imaging studies in schizophrenia). The increase in presynaptic striatal DA may disrupt informational salience and help contribute to other schizophrenia symptoms (Rosier et al., 2013; Winton-Brown et al., 2014).
The bulk of the evidence suggests that the DAergic deficits in schizophrenia are underpinned by functional, rather than constitutive, abnormalities. Indeed, the majority of studies on post-mortem tissues have failed to identify consistent alterations in the expression of DAergic targets (Harrison, 2000). Accordingly, multiple large-scale genetic analyses have found no robust association for DAergic genes and schizophrenia (Hoogendoorn et al., 2005; Alvarez et al., 2010), and instead point to a predominant involvement of glutamatergic targets (Collier and Li, 2003). In contrast with this evidence, the notion of functional dysregulation of DAergic circuits in schizophrenia is strongly supported by neuroimaging findings, which point to multiple patterns of dysconnectivity between intracortical and subcortical networks (Laruelle, 2003). These dynamic alterations of DAergic neurotransmission are thought to play a key role in the adaptive and neurodevelopmental processes of this system, which are particularly active throughout childhood and adolescence (Teicher et al., 1995; Spear, 2000). These developmental periods may be especially critical for the interactions of DAergic genes with environmental and sex-related vulnerability factors in schizophrenia. In fact, preclinical experiments have shown that sex hormones have a profound influence on the development of the DAergic system throughout early developmental stages (Anderson et al., 2005).
To establish a conceptual framework for the role of DA in schizophrenia, it is necessary to consider that one of the fundamental functions of this system is the extraction of salient information from the environment, through the stimulation of output neurons of cortical and striatal regions integrated within cortico-striato-thalamo-cortical (CSTC) circuits. In particular, the role of mesocorticolimbic DAergic neurons is consistently influenced by the action of glutamatergic and GABAergic cells, which surround and interface with the somata in the ventral tegmental area, as well as the axons and presynaptic boutons in the efferent areas (nucleus accumbens, striatum and PFC). In line with the role of DAergic pathways as neural mediators of informational salience, both the adaptive plasticity and modalities of neurotransmitter release by these neurons are finely regulated by multiple factors; changes in these variables, particularly if occurring during developmental periods, may therefore have long-standing implications on the integrity and coherence of the perceptual process. The modulatory role of DA on processing informational salience is extremely critical during adolescent stages, in which the DAergic system alters cortical innervation and undergoes synaptic maturation and pruning of its glutamatergic and GABAergic connections (Andersen, 2003; O'Donnell, 2010; Burke and Miczek, 2013; Penzes et al., 2013).
The natural corollary of these premises is that the DAergic system may be directly involved in G×S interactions during postnatal development, while prenatal and inborn elements of predisposition may be more directly related to the glutamatergic system (which in turn governs DAergic function through direct and indirect dynamic interactions). This idea is in line with the “multiple hits” hypothesis for the pathogenesis of schizophrenia, which postulates that the disorder may result from the progressive accumulation of deficits from prenatal to juvenile stages, due to different, yet concurring, causes. In the next sections, we will present an overview of the role of sex hormones on schizophrenia, followed by a detailed discussion on the available evidence on the G×S interactions involving DAergic genes.
The Role of Sex Factors in Schizophrenia
Gender Differences in Schizophrenia
The existence of gender differences in schizophrenia has been recognized since its first nosographic description by Kraepelin. The best-characterized difference concerns arguably the earlier age of onset in male patients, which typically ranges from 15 to 24 years. In comparison, females exhibit their first overt clinical manifestations between 20 and 29 years, with an average difference of 3–5 years from males (Angermeyer and Kuhn, 1988). It is widely assumed that this divergence in age reflects different developmental trajectories in the DAergic system throughout adolescence across both genders.
A comprehensive and systematic analysis of sex differences in schizophrenia is a complicated undertaking, in view of several methodological issues that may generate spurious results, such as recruitment bias, different metabolic responses to antipsychotic drugs and gender diversity in social adjustment with respect to psychiatric disorders (Markham, 2012). The awareness of these issues, and the numerous discrepancies in the literature have led several authors to cast a skeptical eye on other potential sex differences in schizophrenia, such as prevalence and symptomatic presentation (Häfner, 2002; Jablensky, 2003). Nevertheless, more recent studies, performed with more accurate and tighter controls, have actually found that the gender differences in schizophrenia may encompass several aspects of this disorder, including: (1) a higher risk of schizophrenia in males (~40%) (Markham, 2012); (2) poorer premorbid adjustment in males; (3) a greater severity in clinical course in males, characterized by higher frequency and intensity of negative symptoms, as well as more rapid cognitive deterioration and greater predisposition to relapse (Larsen et al., 1996; Markham, 2012). Current studies investigating the role of sex differences in neuropsychiatric disorders have also highlighted the potential impact of stress and hormonal influences on epigenetic phenomena, which may result in enduring behavioral changes across subsequent generations (see Goel and Bale, 2009; McCarthy et al., 2009; Bale, 2011; McCarthy and Nugent, 2013).
Role of Estrogens in Schizophrenia
The prevalent line of interpretation of this sex-related disproportion lies in the neuroprotective role of estrogens in women (Seeman, 1996). Indeed, women display greater severity of their psychotic symptoms in conditions associated with lower concentrations of β-estradiol, the main estrogen hormone, such as fluctuations within the menstrual cycle (Bergemann et al., 2007; Rubin et al., 2010), and menopause (Häfner et al., 1993). Furthermore, plasma β-estradiol levels are reduced in schizophrenia female patients across all phases of the menstrual cycle (Riecher-Rossler et al., 1994), and the age of disease onset in women is inversely related to the age of puberty (Cohen et al., 1999). Accordingly, several clinical trials have shown that additive treatment with estradiol substitutes improves and accelerates the therapeutic response of patients (Kulkarni et al., 1996, 2002; Akhondzadeh et al., 2003; Kulkarni et al., 2008). A number of clinical studies have also shown associations between estrogen receptor polymorphic variants in psychotic-related phenomena (Weickert et al., 2008; Min et al., 2012; Wang et al., 2013). In general, it appears that the sex factors do not induce specific qualitative differences in symptoms, but rather dampen the severity or delay the onset of the same manifestations. The biochemical nature of the neuroprotective effects of estrogens has not been fully qualified yet, but a number of studies point to a direct implication of the DAergic system (Sumner and Fink, 1993; Fink et al., 1998), in addition to glutamate and GABA. In general, the relations between estrogens and DA are supported by a host of clinical and preclinical evidence (for a thorough and detailed presentation of this issue, see Sanchez et al., 2010).
Role of Androgens in Schizophrenia
The involvement of sex steroids in schizophrenia is not likely limited to estrogens, but may also include androgen hormones. These steroids appear to exert a multifaceted influence on the neurobiological substrates of schizophrenia; in particular, the complexity of this role stems from the fact that testosterone, the main gonadal androgen, is also converted into β-estradiol via aromatization. Men with schizophrenia tend to exhibit lower levels of testosterone, and testosterone levels are inversely correlated with the severity of negative symptoms (Akhondzadeh et al., 2006; Ko et al., 2007). Furthermore, this hormone has been shown to exert therapeutic properties for negative symptoms in schizophrenia (Ko et al., 2008). In contrast, the role of other androgens in schizophrenia is less clear. For example, schizophrenia patients exhibit high levels of the adrenal androgens dehydroepiandrosterone (DHEA) and androstenedione (Ritsner and Strous, 2010); in addition, DHEA has been found to attenuate the extrapyramidal symptoms induced by antipsychotic drugs (Ritsner et al., 2010).
In general, it is possible that androgenic metabolites of testosterone may facilitate the development of schizophrenia-related symptoms. The conversion of testosterone and androstenedione into their androgenic metabolites dihydrotestosterone (DHT) and androstanedione, respectively, is mediated by 5α-reductase (Paba et al., 2011). Notably, this process competes with the aromatization of the same substrates to β-estradiol and β-estrone. In males, 5α-reductase activity is enhanced during puberty; thus, it is possible that the increased rate of conversion of testosterone and androstenedione into their 5α-reduced androgenic metabolites (instead of estrogens) may contribute to the greater schizophrenia vulnerability and earlier age of onset in males. Our group has tested this intriguing hypothesis in rodent models of schizophrenia; our results indicate that inhibition of 5α-reductase leads to marked anti-DAergic actions on endophenotypes relevant to schizophrenia, such as sensorimotor gating deficits and stereotyped behavior (Bortolato et al., 2008a; Paba et al., 2011; Devoto et al., 2012; Frau et al., 2013). In addition, we recently found that inhibition of another key androgen-synthetic enzyme, CYP17A1, elicits similar, albeit less potent, anti-DAergic effects in the same schizophrenia-related behavioral paradigms (Frau et al., 2014). Collectively, these findings highlight that, in addition to testosterone, other androgens may have a role in the pathogenesis of schizophrenia-related features, through the mediation of DA neurotransmission (for a more detailed description of this issue and its potential therapeutic implications, see Paba et al., 2011).
The Role of DAergic Genes in G×S Interactions in Schizophrenia
COMT
COMT catalyzes the methylation of the 3O group of catecholamines. The methyl group is donated by S-adenosyl-methionine (SAM), and DA is directly converted by COMT into metanephrine. Other catechol-containing structures are substrates of COMT, including norepinephrine, epinephrine and the DA precursor l-DOPA.
COMT has a soluble form (S-COMT) and a membrane-bound form (MB-COMT), both of which are encoded by the same gene (Lundstrom et al., 1991), located on chromosome 22q11.2. COMT expression is controlled by two promoters in the third exon of the gene (Salminen et al., 1990; Lundstrom et al., 1991). The P1 promoter regulates the expression of a shorter transcript, which can code for S-COMT only (Tenhunen et al., 1993), whereas the more distally located P2 promoter can encode both transcripts. S-COMT is generally dominant in most tissues, with the only exception being in the human brain, where 70% is MB-COMT, and 30% is S-COMT. In the brain, S-COMT is mostly found in the glia and is not likely to serve a primary function in DA metabolism (Rivett et al., 1983; Naudon et al., 1992); conversely, MB-COMT is abundantly localized in postsynaptic terminals of neurons and in perisynaptic locations (Bertocci et al., 1991; Lundstrom et al., 1991; Schott et al., 2010). This form is likely to play a key role in DA degradation, particularly in regions with low DA transporter (DAT) expression, such as the PFC, or, alternatively, in conditions of DAT inhibition (Karoum et al., 1994; Sesack et al., 1998; Huotari et al., 1999; Matsumoto et al., 2003).
Notably, the effect of COMT on DA metabolism may be particularly dominant in males; indeed, only COMT knockout males exhibit a significant (3-fold) increase in DA levels in the PFC (Gogos et al., 1998). MB-COMT is generally localized intracellularly, but not in the cell membrane (Ulmanen et al., 1997). This distribution implies that its function in DA metabolism is secondary to DA uptake in the postsynaptic terminal (Figure 1), which may be served by either the organic cation transporter 3 (OCT3; SLC22A3) or the plasma membrane monoamine transporter (PMAT; SLC29A4) located in the postsynaptic neuron or glia. PMAT is highly expressed in the forebrain (Engel et al., 2004; Dahlin et al., 2007), including brain regions with sparse DAT expression.
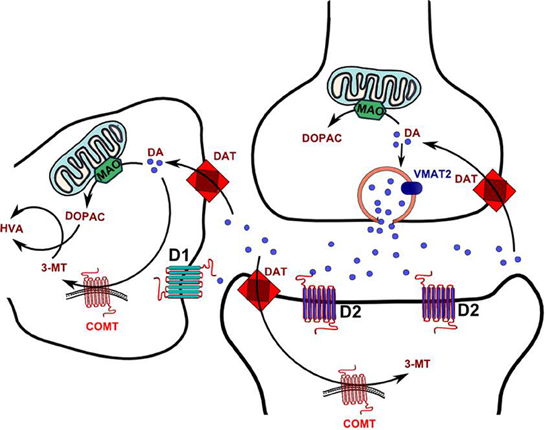
Figure 1. Schematic diagram of DA synaptic metabolism. Abbrevations 3-MT, 3-Methoxytyramine; COMT, Catechol-o-methyltransferase; DA, DA; DAT, DA reuptake transporter; DOPAC, 3,4-Dihydroxyphenylacetic acid; HVA, Homovanillic acid; MAO, Monoamine oxidase.
The perisynaptic location of COMT suggests that this enzyme may be important for volume transmission of DA, which plays an important role in the PFC (Paspalas and Goldman-Rakic, 2004). Given the relevance of volume neurotransmission in the PFC for the acquisition of certain informational aspects, such as the perception of salience and the dynamic regulation of signal-to-noise ratio, alterations in COMT activity may result in cognitive changes, particularly with respect to PFC-mediated functions. In addition, COMT may be a crucial element in differentiating the temporal patterns of tonic and phasic DA action (Bilder et al., 2004).
A host of studies have proposed the COMT gene as a potential candidate for psychosis and related phenomena (Egan et al., 2001; Williams et al., 2007). In particular, numerous investigations have focused on rs4680, one of the best-characterized single-nucleotide polymorphisms of the COMT gene, resulting in the substitution of a valine (Val) for a methionine (Met) residue at position 108 of S-COMT and 158 of MB-COMT (Val-Met) (Lachman et al., 1996; Harris et al., 2005; Wahlstrom et al., 2007). The Val-allele confers a higher intrinsic COMT activity than the Met-allele (Männistö and Kaakkola, 1999), leading to an overall reduction in DA levels in the PFC. Indeed, COMT serves as the primary enzyme for DA metabolism in this region (Egan et al., 2001; Schott et al., 2006; Tan et al., 2007; Diaz-Asper et al., 2008). Accordingly, individuals harboring the Val allele exhibit low DA levels predominantly in the PFC, which may result in a region-specific dysregulation of DA receptors (and particularly D1, the most abundant DA receptor in the cortex). Conversely, striatal DA levels and D2 receptor availability appear to be unaffected by alterations in COMT activity (Yavich et al., 2007; Hirvonen et al., 2010). Moreover, Val-allele carriers have been associated with impaired physiological responses across several functional domains, including cognitive flexibility, working memory, attentional control and emotional resilience (Malhotra et al., 2002; Goldberg et al., 2003; Blasi et al., 2005; Smolka et al., 2005).
The role of COMT in schizophrenia has been extensively studied, yet results have unequivocally shown that neither genetic variants nor the catalytic activity of the enzyme have great intrinsic influence on schizophrenia risk (Chen et al., 1996; Daniels et al., 1996; Riley et al., 1996; Wei et al., 1996; Karayiorgou et al., 1998; Wei and Hemmings, 1999; De Chaldee et al., 2001; Semwal et al., 2001; Strous et al., 2006). Nevertheless, multiple lines of evidence indicate that high-activity COMT variants is robustly associated with a greater severity of negative and cognitive symptoms in schizophrenia patients, as well as specific endophenotypic impairments related to functional deficits of the PFC (Egan et al., 2001; Herken and Erdal, 2001; Weinberger et al., 2001; Weinberger, 2002). Specifically, the Val allele has been associated with poorer performance in schizophrenia patients across several neuropsychological tests for executive functioning (Goldberg et al., 2003; Nolan et al., 2004; Ohnishi et al., 2006; Diaz-Asper et al., 2008; Opgen-Rhein et al., 2008; Ira et al., 2013), as well as sensorimotor gating deficits in comparison to carriers of the Met allele (Quednow et al., 2010). Individuals harboring the Val variant also exhibit greater prefrontal noise, corresponding to the electromagnetic activity in the region (Winterer et al., 2006). In contrast, multiple studies have ascertained that the Met variant is associated with a slightly lower schizophrenia risk, as well as less severity of attentional, cognitive and information-processing deficits (Egan et al., 2001; Bilder et al., 2002; Bray et al., 2003; Gallinat et al., 2003; Tunbridge et al., 2006; Ehlis et al., 2007; Lu et al., 2007).
Although the aforementioned studies indicate that the Val variant confers at best a very modest enhancement of schizophrenia risk, recent investigations suggest that the interaction of this haplotype with other genetic or environmental vulnerability factors may lead to schizophrenia (Schenkel et al., 2005; Stefanis et al., 2007; Collip et al., 2011; Pelayo-Teran et al., 2012). In particular, the interaction of the Val variant with cannabis abuse in adolescence has been shown to increase schizophrenia risk (Caspi et al., 2005; Henquet et al., 2006; Estrada et al., 2011), but the neurobiological bases of this interaction remain poorly understood.
While most of the research on COMT genotypes and schizophrenia has been focused on the impairments associated with the Val variant, emerging lines of evidence have also pointed to the possibility that the Met-variant may predispose schizophrenia patients to aggression and violence (Strous et al., 1997; Lachman et al., 1998; Nolan et al., 2000; Liou et al., 2001; Han et al., 2004, 2006; Kim et al., 2008; Tosato et al., 2011; Bhakta et al., 2012; Singh et al., 2012). Interestingly, this predisposition appears to be specific for males, pointing to a potential G×S interaction (Nolan et al., 2000; Soyka, 2011; Singh et al., 2012).
A summary of the main studies that have identified G×S interactions concerning COMT polymorphisms is reported in Table 1. Although these data should still be regarded as preliminary, several studies suggest that male patients with high-activity COMT may have greater severity of endophenotypes associated with prefrontal deficits in schizophrenia, such as eye movement disturbances (Rybakowski et al., 2002) prefrontal noise (Winterer et al., 2006) and schizotypal traits (Ma et al., 2007). Similarly, Hoenicka et al. (2010) found that the effects of the Val158Met polymorphism on schizophrenia vulnerability are more directly related to male patients, possibly through an epistatic interaction with D1 receptor (Hoenicka et al., 2010) (see below). Other studies indicate that only female carriers of the Val/Met alleles exhibit high propensity to engage in risky behaviors (Amstadter et al., 2012) and alterations in emotional processing (Domschke et al., 2012).
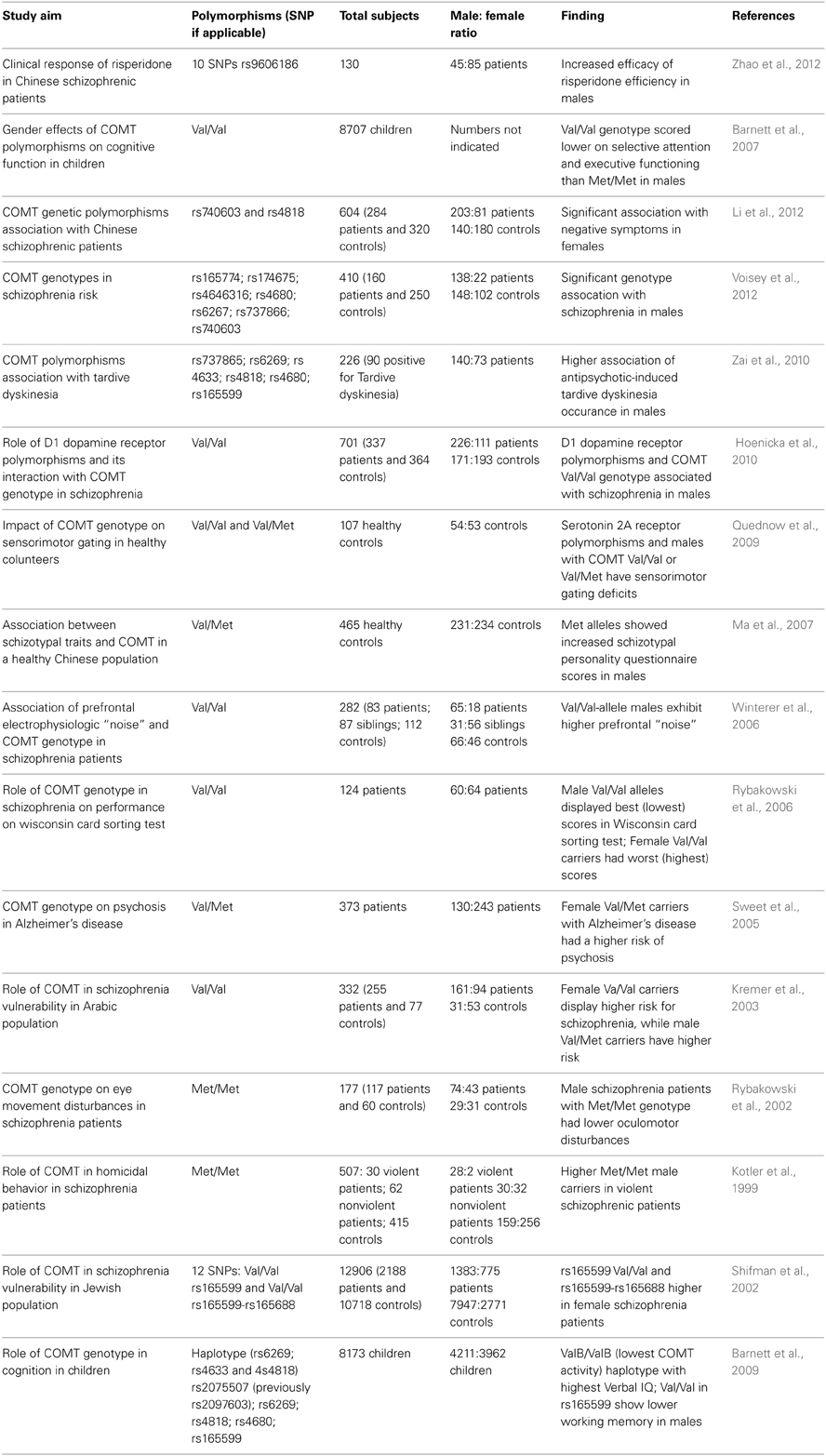
Table 1. List of major studies documenting an interaction between COMT polymorphic variants and sex in schizophrenia and related symptoms.
COMT activity has been reported to be higher in males than females (Boudikova et al., 1990). This gender difference may reflect the ability of testosterone and DHT to increase COMT expression (Purves-Tyson et al., 2012). Alternatively, estrogens have been found to reduce the transcription and expression of COMT (Männistö et al., 1992; Xie et al., 1999). An additional mechanism that may predict a lower COMT activity in females may be afforded by the function of catecholestrogens. These 2- and 4-hydroxylated metabolites of β-estradiol (Ball and Knuppen, 1980; Zhu and Conney, 1998) compete with DA for COMT-mediated metabolism, and may act as inhibitors of the enzyme at high concentrations. Accordingly, catecholestrogens have been shown to modulate the turnover of catecholamines (Parvizi and Wuttke, 1983). While the specific role of catecholestrogens on G×S interactions of COMT in schizophrenia remains to be investigated, the reduction of COMT activity in females may explain the lower susceptibility of this gender for the phenotypic effects of the Val variant on PFC function. At the same time, this mechanism could also account for the higher proclivity of female carriers of the Met allele to engage in risky behaviors (Amstadter et al., 2012).
A number of preclinical studies have found sex-specific neurochemical and behavioral differences associated with COMT (Gogos et al., 1998). In particular, heterozygous male COMT-deficient mice exhibit impaired object recognition (Babovic et al., 2008). Conversely, COMT overexpression was found to be associated with blunted stress responsiveness, as well as impairments in working memory and attentional set-shifting (Papaleo et al., 2008). These data are in agreement with evidence showing that COMT alterations may negatively impact prefrontal functions in both humans and rodents (Papaleo et al., 2012). In a recent study, Risbrough and colleagues found that male mice carrying the COMT158Val-variant exhibit marked reductions in spatial working memory and disruptions in sensorimotor gating; conversely. female mice carrying the COMT158Met-variant displayed alterations in fear-related behavioral responses (Risbrough et al., 2014). Collectively, these preclinical findings further support the role of sex-specific influences of COMT genetic variations on prefrontal DAergic systems.
MAO A and B
MAO A and B are mitochondrial-bound enzymes (Greenawalt and Schnaitman, 1970) differing by substrate affinity. MAO A has high affinity for serotonin and norepinephrine, while MAO B metabolizes the trace amine phenylethylamine. DA can be degraded by both isoforms; however, the primary enzyme differs across species. In humans and primates, MAO B is the major metabolic enzyme of DA, whereas MAO A serves this role in rodents (Garrick and Murphy, 1980; Cases et al., 1995; Fornai et al., 1999). The metabolism of DA mediated by MAOs occurs, for the most part, in the presynaptic terminal, following reuptake by the DAT. The difference between MAO A and MAO B also concerns their anatomical localization. MAO A is localized in catecholaminergic neurons (and in particular in the locus coeruleus, nucleus accumbens, hypothalamus and mammillary complex), whereas MAO B is found in serotonergic neurons, as well as histaminergic cells and astrocytes (Westlund et al., 1988; Saura et al., 1994; Luque et al., 1995, 1996; Jahng et al., 1997; Bortolato et al., 2008b).
Several studies have documented that MAOA gene polymorphisms are associated with different psychiatric disturbances (Ozelius et al., 1988; Black et al., 1991; Hotamisligil and Breakefield, 1991; Hinds et al., 1992; Shih and Thompson, 1999). Most of the genetic studies on MAOA have focused on a variable number tandem repeat (VNTR) polymorphism, which is located 1.2-kilobase upstream of the transcription initiation site and has been associated with changes in gene expression (Sabol et al., 1998). Of the six different allelic variants characterized in humans, the most common display 3 repeats (3R) and 4 repeats (4R) (Sabol et al., 1998; Deckert et al., 1999; Jonsson et al., 2000). The 3R variant has been associated with behavioral features linked to low MAO A activity, such as impulsive aggression and antisocial personality (Oreland et al., 2007; Buckholtz and Meyer-Lindenberg, 2008). In contrast, the 4R variant has been associated with higher MAOA gene transcription and enzyme activity (Sabol et al., 1998; Denney et al., 1999). Neuroimaging imaging studies have found a link between the VNTR variants of MAOA promoter and structural and functional differences in the PFC (Meyer-Lindenberg et al., 2006).
In general, the majority of genetic studies have failed to find a straightforward association between MAOA and schizophrenia (Coron et al., 1996; Sasaki et al., 1998; Syagailo et al., 2001; Norton et al., 2002; Iwata et al., 2003; Li and He, 2008; Wei et al., 2011). Nevertheless, other analyses found preliminary results in support of a sex-specific effect of MAOA with respect to schizophrenia diagnosis or select symptomatic aspects of the disorder (Jonsson et al., 2003; Qiu et al., 2009; Camarena et al., 2012; Sun et al., 2012b).
A summary of the main findings on potential G×S interactions involving the MAOA gene is reported in Table 2. Although the evidence on sex-dependent effects of MAOA is still preliminary and inconclusive, it was recently reported that male schizophrenia patients exhibit abnormal patterns of methylation of the MAOA promoter, pointing to the possibility that the effect of sex may be directly dependent on epigenetic alterations (Chen et al., 2012). In addition to the evidence on schizophrenia, several findings have documented that genetic variations of MAOA may play a central role in neuropsychiatric disorders in a sex-dependent fashion. This concept is best highlighted by the elegant study conducted by Caspi and colleagues, showing that males, but not females, harboring low MAO A activity polymorphic variants and subjected to early childhood maltreatment exhibit a significantly higher vulnerability to develop antisocial and aggressive behaviors in adulthood (Caspi et al., 2002; Foley et al., 2004; Kim-Cohen et al., 2006). Indeed, subsequent studies have found that testosterone levels in the cerebral spinal fluid paralleled aggressive responses in carriers of the low MAO A activity polymorphism (Sjoberg et al., 2008). In contrast, DAergic metabolic levels were inversely associated with testosterone in low MAO A activity carriers (Sjoberg et al., 2008). Although both low-activity MAOA variants and testosterone have been independently shown to affect aggression, it remains unclear how this genotype may predispose individuals to higher androgen synthesis and how these two properties may interact to influence aggression. It is worth noting that males have a markedly higher frequency of low MAO A activity variants than females (Sjoberg et al., 2008).
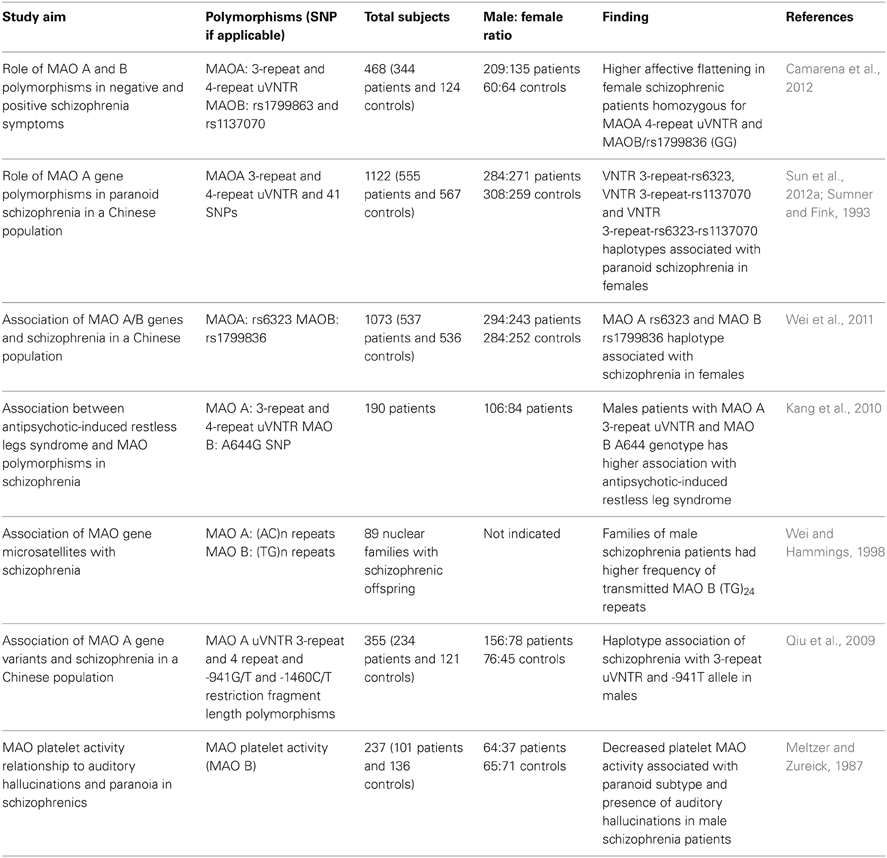
Table 2. List of major studies implicating the interaction between MAO polymorphic variants and sex in schizophrenia and related symptoms.
Although the mechanism is unclear, females harboring the high-activity MAOA variant display higher baseline cortisol levels than males with the same polymorphism than females carrying low-activity alleles (Jabbi et al., 2007). Furthermore, both females and males harboring low-activity MAOA variants exhibited a sexually dimorphic increase in stress response, which was dependent on COMT genotype (Bouma et al., 2012). In particular, male carriers of the Met/Met COMT allele displayed a significantly higher cortisol response to stress than both females with the same allele and males with other genotypes. Conversely, females carrying the Val/Val COMT allele in combination with low-activity MAOA variants showed higher stress responses than their male and female counterparts.
Although no common polymorphisms have been reported in the gene's coding region, MAOB allelic variants may possess different enzymatic activities (Balciuniene et al., 2002; Costa-Mallen et al., 2005). Indeed, several groups have reported the association of polymorphic variants of MAOB gene with several neuropsychiatric disorders characterized by DAergic dysfunction. In particular, MAOB allelic variations have been associated with bipolar disorder (Lin et al., 2000) and higher schizophrenia susceptibility (Hovatta et al., 1999; Gasso et al., 2008; Carrera et al., 2009; Piton et al., 2011); these results, however, have been not been consistently replicated (Coron et al., 1996; Sobell et al., 1997; Matsumoto et al., 2004; Bergen et al., 2009).
A direct implication of MAOB in schizophrenia is supported by several studies (Coron et al., 1996; Bergen et al., 2009; Carrera et al., 2009; Piton et al., 2011; Wei et al., 2011; Sun et al., 2012a) and may be reflective of the greater contribution of this enzyme to the metabolism of DA in humans. In particular (see Table 2), numerous articles have recently reported that different MAOB variants may predispose to schizophrenia in women (Gasso et al., 2008; Wei et al., 2011) or in men (Wei and Hemmings, 1999). In addition, other studies highlighted that MAOB variants may moderate several symptomatic aspects of schizophrenia, including flat affect (Camarena et al., 2012) or paranoid manifestations (Sun et al., 2012a). Although little is currently known on the potential interaction of sex hormones with MAO B, females have been reported to display significantly higher MAO B activity in platelets in comparison with males (Snell et al., 2002).
A plethora of studies has shown that sex hormones differentially affect MAO activity and expression in specific brain regions. Androgens increase MAO transcription in the substantia nigra (Ou et al., 2006; Purves-Tyson et al., 2012) and in the striatum (Thiblin et al., 1999). Chronic administration of anabolic androgenic steroids, however, reduces MAO activity in the caudate and amygdala, as well as DA metabolites in the nucleus accumbens shell (Birgner et al., 2008). Similarly, gonadectomy also increases MAO A activity in the PFC (Meyers et al., 2010), suggesting that acute treatment with androgens may enhance MAO activity, while chronic treatment exerts the opposite effect. In contrast to androgens, estrogen administration to neonatal, but not adult males elicits an increase in hypothalamic MAO activities (Vaccari et al., 1981). In females, estradiol reduces MAO A activity in the hypothalamus and amygdala (Luine et al., 1975; Ma et al., 1993; Gundlah et al., 2002).
Other DAergic Targets
The current evidence on the implication of the other DAergic targets in G×S interactions is scant and mostly limited to DAergic receptors. Interestingly, several studies have shown that polymorphic variants of the genes encoding D1, D2, and D4 receptors are linked to different responses to antipsychotic medications in a gender-sensitive fashion. For example, variants of the DRD2 gene, which codes for the D2 receptor, may predispose females to a greater prolactin increase in response to antipsychotics (Mihara et al., 2000, 2001); however, this difference may not be dependent on an actual G×S interaction, but rather on the higher baseline levels of prolactin in females (Yasui-Furukori et al., 2008). Variants of the DRD1 and DRD4 genes (coding for D1 and D4 receptors) may also predispose to different responses to antipsychotic treatment (including side effects) (Hwu et al., 1998; Potkin et al., 2003; Hwang et al., 2007; Popp et al., 2009). Only few studies have pointed to a direct role of these genes in specific symptomatic aspects. Different variants of the DRD2 gene may be associated with higher perseverative responses in female schizophrenia patients (Rybakowski et al., 2005), while VNTR variants of the DRD4 gene may predict for differences in age of onset in female patients (Goncalves et al., 2012). Notably, the DRD1 gene has been recently found to establish an epistatic interaction with the COMT gene, which predicts schizophrenia risk in males, presumably due to the functional association of D1 receptors and COMT in the PFC (Hoenicka et al., 2010).
Independent investigations have reported that different variants of the DRD3 gene may be associated with schizophrenia predisposition in males (Asherson et al., 1996; Griffon et al., 1996) and females (Aksenova et al., 2004; Godlewska et al., 2010). Interestingly, preliminary results in our animal models suggest that the behavioral responses elicited by agonists of D3, but not D2 receptors, may be under control of neurosteroids with respect to the regulation of sensorimotor gating (Frau et al., submitted). Future work is warranted to establish the nature of this intriguing neurobiological finding.
Concluding Remarks
As mentioned above, the attempts to identify a genetic basis of schizophrenia have revealed a picture of extreme complexity and high heterogeneity of heritable bases. This view has gradually replaced our “genome-centric” perspective with a broader framework, in which genetic vulnerability is a piece of a much greater mosaic, consisting of complex interactions with environmental factors. In this perspective, sex hormones may also play a significant role in shaping the course of schizophrenia and modifying the developmental trajectory of the neurobiological alterations of DA and other neurotransmitter systems underpinning this disorder.
The findings summarized in this review indicate that, although the role of G×S interactions in schizophrenia is still inconclusive, sex hormones might affect brain substrates through a multilayered set of mechanisms, which appear to have a particular impact on the catabolic apparatus of DA.
The diagnostic definition of schizophrenia (as based on the DSM-IV and DSM-5) is only related to symptomatic descriptors, rather than biomarkers and quantitative endophenotypes. This scenario raises the possibility that this disorder may actually correspond to an array of diverse clinical conditions which share a common “final pathway” accounting for the pathognomonic manifestations of schizophrenia. Accordingly, a greater understanding of the role of DA neurotransmission in schizophrenia may have important repercussions also with respect to a better nosographic classification of this disorder.
The integration of preclinical research with neuroimaging and genetic studies will play a critical role in enabling us to identify central neurobiological networks that underpin gender-specific neurobehavioral endophenotypes of schizophrenia. Additionally, the contribution of these studies and a greater understanding of sex-dependent epigenetic mechanisms of transcriptional regulation will be fundamental to qualify premorbid signs and symptoms, and chart the developmental trajectory of psychosis in males and females.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Simone Tambaro for his assistance. This work was supported by grants from the National Institute of Health (R21 HD070611, to Marco Bortolato), the Tourette Syndrome Association (to Marco Bortolato) and the Strategic Initiative of the University of Kansas (to Marco Bortolato). In addition, the study was partially supported by the National Institute of General Medical Sciences of NIH (P20 GM103638) and a NIH Clinical and Translational Science Award grant (UL1 TR000001, formerly UL1RR033179), awarded to the University of Kansas Medical Center.
References
Akhondzadeh, S., Nejatisafa, A. A., Amini, H., Mohammadi, M. R., Larijani, B., Kashani, L., et al. (2003). Adjunctive estrogen treatment in women with chronic schizophrenia: a double-blind, randomized, and placebo-controlled trial. Prog. Neuropsychopharmacol. Biol. Psychiatry 27, 1007–1012. doi: 10.1016/S0278-5846(03)00161-1
Akhondzadeh, S., Rezaei, F., Larijani, B., Nejatisafa, A. A., Kashani, L., and Abbasi, S. H. (2006). Correlation between testosterone, gonadotropins and prolactin and severity of negative symptoms in male patients with chronic schizophrenia. Schizophr. Res. 84, 405–410. doi: 10.1016/j.schres.2006.02.008
Aksenova, M. G., Shestakova Iu, N., Abramova, L. I., Frolova, L. P., Shemiakina, T. K., Lezheiko, T. V., et al. (2004). [D3 dopamine receptor gene Ser9Gly polymorphism in Russian patients with schizophrenia]. Zh. Nevrol. Psikhiatr. Im. S S Korsakova 104, 57–61.
Allen, P., Luigjes, J., Howes, O. D., Egerton, A., Hirao, K., Valli, I., et al. (2012). Transition to psychosis associated with prefrontal and subcortical dysfunction in ultra high-risk individuals. Schizophr. Bull. 38, 1268–1276. doi: 10.1093/schbul/sbr194
Alvarez, S., Mas, S., Gasso, P., Bernardo, M., Parellada, E., and Lafuente, A. (2010). Lack of association between schizophrenia and polymorphisms in dopamine metabolism and transport genes. Fundam. Clin. Pharmacol. 24, 741–747. doi: 10.1111/j.1472-8206.2009.00807.x
Amstadter, A. B., Macpherson, L., Wang, F., Banducci, A. N., Reynolds, E. K., Potenza, M. N., et al. (2012). The relationship between risk-taking propensity and the COMT Val(158)Met polymorphism among early adolescents as a function of sex. J. Psychiatr. Res. 46, 940–945. doi: 10.1016/j.jpsychires.2012.04.010
Andersen, S. L. (2003). Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci. Biobehav. Rev. 27, 3–18. doi: 10.1016/S0149-7634(03)00005-8
Anderson, L. I., Leipheimer, R. E., and Dluzen, D. E. (2005). Effects of neonatal and prepubertal hormonal manipulations upon estrogen neuroprotection of the nigrostriatal dopaminergic system within female and male mice. Neuroscience 130, 369–382. doi: 10.1016/j.neuroscience.2004.09.033
Angermeyer, M. C., and Kuhn, L. (1988). Gender differences in age at onset of schizophrenia. An overview. Eur. Arch. Psychiatr. Neurol. Sci. 237, 351–364. doi: 10.1007/BF00380979
Arime, Y., Kasahara, Y., Hall, F. S., Uhl, G. R., and Sora, I. (2012). Cortico-subcortical neuromodulation involved in the amelioration of prepulse inhibition deficits in dopamine transporter knockout mice. Neuropsychopharmacology 37, 2522–2530. doi: 10.1038/npp.2012.114
Asherson, P., Mant, R., Holmans, P., Williams, J., Cardno, A., Murphy, K., et al. (1996). Linkage, association and mutational analysis of the dopamine D3 receptor gene in schizophrenia. Mol. Psychiatry 1, 125–132.
Babovic, D., O'Tuathaigh, C. M., O'Connor, A. M., O'Sullivan, G. J., Tighe, O., Croke, D. T., et al. (2008). Phenotypic characterization of cognition and social behavior in mice with heterozygous versus homozygous deletion of catechol-O-methyltransferase. Neuroscience 155, 1021–1029. doi: 10.1016/j.neuroscience.2008.07.006
Balciuniene, J., Emilsson, L., Oreland, L., Pettersson, U., and Jazin, E. (2002). Investigation of the functional effect of monoamine oxidase polymorphisms in human brain. Hum. Genet. 110, 1–7. doi: 10.1007/s00439-001-0652-8
Bale, T. L. (2011). Sex differences in prenatal epigenetic programming of stress pathways. Stress 14, 348–356. doi: 10.3109/10253890.2011.586447
Ball, P., and Knuppen, R. (1980). Catecholoestrogens (2-and 4-hydroxyoestrogens): chemistry, biogenesis, metabolism, occurrence and physiological significance. Acta Endocrinol. Suppl. (Copenh). 232, 1–127.
Barnett, J. H., Heron, J., Goldman, D., Jones, P. B., and Xu, K. (2009). Effects of catechol-O-methyltransferase on normal variation in the cognitive function of children. Am. J. Psychiatry 166, 909–916. doi: 10.1176/appi.ajp.2009.08081251
Barnett, J. H., Jones, P. B., Robbins, T. W., and Müller, U. (2007). Effects of the catechol-O-methyltransferase Val158Met polymorphism on executive function: a meta-analysis of the Wisconsin Card Sort Test in schizophrenia and healthy controls. Mol. Psychiatry 12, 502–509. doi: 10.1038/sj.mp.4001973
Bay-Richter, C., O'Tuathaigh, C. M., O'Sullivan, G., Heery, D. M., Waddington, J. L., and Moran, P. M. (2009). Enhanced latent inhibition in dopamine receptor-deficient mice is sex-specific for the D1 but not D2 receptor subtype: implications for antipsychotic drug action. Int. J. Neuropsychopharmacol. 12, 403–414. doi: 10.1017/S1461145708009656
Benes, F. M., and Berretta, S. (2001). GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology 25, 1–27. doi: 10.1016/S0893-133X(01)00225-1
Bergemann, N., Parzer, P., Runnebaum, B., Resch, F., and Mundt, C. (2007). Estrogen, menstrual cycle phases, and psychopathology in women suffering from schizophrenia. Psychol. Med. 37, 1427–1436. doi: 10.1017/S0033291707000578
Bergen, S. E., Fanous, A. H., Walsh, D., O'neill, F. A., and Kendler, K. S. (2009). Polymorphisms in SLC6A4, PAH, GABRB3, and MAOB and modification of psychotic disorder features. Schizophr. Res. 109, 94–97. doi: 10.1016/j.schres.2009.02.009
Bertocci, B., Miggiano, V., Da Prada, M., Dembic, Z., Lahm, H. W., and Malherbe, P. (1991). Human catechol-O-methyltransferase: cloning and expression of the membrane-associated form. Proc. Natl. Acad. Sci. U.S.A. 88, 1416–1420. doi: 10.1073/pnas.88.4.1416
Bhakta, S. G., Zhang, J. P., and Malhotra, A. K. (2012). The COMT Met158 allele and violence in schizophrenia: a meta-analysis. Schizophr. Res. 140, 192–197. doi: 10.1016/j.schres.2012.06.026
Bilder, R. M., Volavka, J., Czobor, P., Malhotra, A. K., Kennedy, J. L., Ni, X., et al. (2002). Neurocognitive correlates of the COMT Val(158)Met polymorphism in chronic schizophrenia. Biol. Psychiatry 52, 701–707. doi: 10.1016/S0006-3223(02)01416-6
Bilder, R. M., Volavka, J., Lachman, H. M., and Grace, A. A. (2004). The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology 29, 1943–1961. doi: 10.1038/sj.npp.1300542
Birgner, C., Kindlundh-Hogberg, A. M., Oreland, L., Alsio, J., Lindblom, J., Schioth, H. B., et al. (2008). Reduced activity of monoamine oxidase in the rat brain following repeated nandrolone decanoate administration. Brain Res. 1219, 103–110. doi: 10.1016/j.brainres.2008.05.014
Black, G. C., Chen, Z. Y., Craig, I. W., and Powell, J. F. (1991). Dinucleotide repeat polymorphism at the MAOA locus. Nucleic Acids Res. 19, 689. doi: 10.1093/nar/19.3.689-a
Blasi, G., Mattay, V. S., Bertolino, A., Elvevag, B., Callicott, J. H., Das, S., et al. (2005). Effect of catechol-O-methyltransferase val158met genotype on attentional control. J. Neurosci. 25, 5038–5045. doi: 10.1523/JNEUROSCI.0476-05.2005
Bortolato, M., Chen, K., and Shih, J. C. (2008b). Monoamine oxidase inactivation: from pathophysiology to therapeutics. Adv. Drug Deliv. Rev. 60, 1527–1533. doi: 10.1016/j.addr.2008.06.002
Bortolato, M., Frau, R., Orru, M., Bourov, Y., Marrosu, F., Mereu, G., et al. (2008). Antipsychotic-like properties of 5-alpha-reductase inhibitors. Neuropsychopharmacology 33, 3146–3156. doi: 10.1038/npp.2008.39
Boudikova, B., Szumlanski, C., Maidak, B., and Weinshilboum, R. (1990). Human liver catechol-O-methyltransferase pharmacogenetics. Clin. Pharmacol. Ther. 48, 381–389. doi: 10.1038/clpt.1990.166
Bouma, E. M., Riese, H., Doornbos, B., Ormel, J., and Oldehinkel, A. J. (2012). Genetically based reduced MAOA and COMT functioning is associated with the cortisol stress response: a replication study. Mol. Psychiatry 17, 119–121. doi: 10.1038/mp.2011.115
Bray, N. J., Buckland, P. R., Williams, N. M., Williams, H. J., Norton, N., Owen, M. J., et al. (2003). A haplotype implicated in schizophrenia susceptibility is associated with reduced COMT expression in human brain. Am. J. Hum. Genet. 73, 152–161. doi: 10.1086/376578
Breier, A. (1999). Cognitive deficit in schizophrenia and its neurochemical basis. Br. J. Psychiatry Suppl. 16–18.
Buckholtz, J. W., and Meyer-Lindenberg, A. (2008). MAOA and the neurogenetic architecture of human aggression. Trends Neurosci. 31, 120–129. doi: 10.1016/j.tins.2007.12.006
Burke, A. R., and Miczek, K. A. (2013). Stress in adolescence and drugs of abuse in rodent models: Role of dopamine, CRF, and HPA axis. Psychopharmacology (Berl). doi: 10.1007/s00213-013-3369-1. [Epub ahead of print].
Camarena, B., Fresan, A., Aguilar, A., Escamilla, R., Saracco, R., Palacios, J., et al. (2012). Monoamine oxidase a and B gene polymorphisms and negative and positive symptoms in schizophrenia. ISRN Psychiatry 2012:852949. doi: 10.5402/2012/852949
Carrera, N., Sanjuan, J., Molto, M. D., Carracedo, A., and Costas, J. (2009). Recent adaptive selection at MAOB and ancestral susceptibility to schizophrenia. Am. J. Med. Genet. B Neuropsychiatr. Genet. 150B, 369–374. doi: 10.1002/ajmg.b.30823
Cases, O., Seif, I., Grimsby, J., Gaspar, P., Chen, K., Pournin, S., et al. (1995). Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science 268, 1763–1766. doi: 10.1126/science.7792602
Caspi, A., McClay, J., Moffitt, T. E., Mill, J., Martin, J., Craig, I. W., et al. (2002). Role of genotype in the cycle of violence in maltreated children. Science 297, 851–854. doi: 10.1126/science.1072290
Caspi, A., Moffitt, T. E., Cannon, M., McClay, J., Murray, R., Harrington, H., et al. (2005). Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol. Psychiatry 57, 1117–1127. doi: 10.1016/j.biopsych.2005.01.026
Chen, C. H., Lee, Y. R., Liu, M. Y., Wei, F. C., Koong, F. J., Hwu, H. G., et al. (1996). Identification of a BglI polymorphism of catechol-O-methyltransferase (COMT) gene, and association study with schizophrenia. Am. J. Med. Genet. 67, 556–559.
Chen, Y., Zhang, J., Zhang, L., Shen, Y., and Xu, Q. (2012). Effects of MAOA promoter methylation on susceptibility to paranoid schizophrenia. Hum. Genet. 131, 1081–1087. doi: 10.1007/s00439-011-1131-5
Cohen, R. Z., Seeman, M. V., Gotowiec, A., and Kopala, L. (1999). Earlier puberty as a predictor of later onset of schizophrenia in women. Am. J. Psychiatry 156, 1059–1064.
Collier, D. A., and Li, T. (2003). The genetics of schizophrenia: glutamate not dopamine? Eur. J. Pharmacol. 480, 177–184. doi: 10.1016/j.ejphar.2003.08.105
Collip, D., Van Winkel, R., Peerbooms, O., Lataster, T., Thewissen, V., Lardinois, M., et al. (2011). COMT Val158Met-stress interaction in psychosis: role of background psychosis risk. CNS Neurosci. Ther. 17, 612–619. doi: 10.1111/j.1755-5949.2010.00213.x
Coron, B., Campion, D., Thibaut, F., Dollfus, S., Preterre, P., Langlois, S., et al. (1996). Association study between schizophrenia and monoamine oxidase A and B DNA polymorphisms. Psychiatry Res. 62, 221–226. doi: 10.1016/0165-1781(96)02933-2
Costa-Mallen, P., Kelada, S. N., Costa, L. G., and Checkoway, H. (2005). Characterization of the in vitro transcriptional activity of polymorphic alleles of the human monoamine oxidase-B gene. Neurosci. Lett. 383, 171–175. doi: 10.1016/j.neulet.2005.04.004
Creese, I., Burt, D. R., and Snyder, S. H. (1976). Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science 192, 481–483. doi: 10.1126/science.3854
Dahlin, A., Xia, L., Kong, W., Hevner, R., and Wang, J. (2007). Expression and immunolocalization of the plasma membrane monoamine transporter in the brain. Neuroscience 146, 1193–1211. doi: 10.1016/j.neuroscience.2007.01.072
Daniels, J. K., Williams, N. M., Williams, J., Jones, L. A., Cardno, A. G., Murphy, K. C., et al. (1996). No evidence for allelic association between schizophrenia and a polymorphism determining high or low catechol O-methyltransferase activity. Am. J. Psychiatry 153, 268–270.
Davis, K. L., Kahn, R. S., Ko, G., and Davidson, M. (1991). Dopamine in schizophrenia: a review and reconceptualization. Am. J. Psychiatry 148, 1474–1486.
De Chaldee, M., Corbex, M., Campion, D., Jay, M., Samolyk, D., Petit, M., et al. (2001). No evidence for linkage between COMT and schizophrenia in a French population. Psychiatry Res. 102, 87–90. doi: 10.1016/S0165-1781(01)00237-2
Deckert, J., Catalano, M., Syagailo, Y. V., Bosi, M., Okladnova, O., Di Bella, D., et al. (1999). Excess of high activity monoamine oxidase A gene promoter alleles in female patients with panic disorder. Hum. Mol. Genet. 8, 621–624. doi: 10.1093/hmg/8.4.621
Denney, R. M., Koch, H., and Craig, I. W. (1999). Association between monoamine oxidase A activity in human male skin fibroblasts and genotype of the MAOA promoter-associated variable number tandem repeat. Hum. Genet. 105, 542–551. doi: 10.1007/s004390051143
Devoto, P., Frau, R., Bini, V., Pillolla, G., Saba, P., Flore, G., et al. (2012). Inhibition of 5alpha-reductase in the nucleus accumbens counters sensorimotor gating deficits induced by dopaminergic activation. Psychoneuroendocrinology 37, 1630–1645. doi: 10.1016/j.psyneuen.2011.09.018
Diaz-Asper, C. M., Goldberg, T. E., Kolachana, B. S., Straub, R. E., Egan, M. F., and Weinberger, D. R. (2008). Genetic variation in catechol-O-methyltransferase: effects on working memory in schizophrenic patients, their siblings, and healthy controls. Biol. Psychiatry 63, 72–79. doi: 10.1016/j.biopsych.2007.03.031
Domschke, K., Baune, B. T., Havlik, L., Stuhrmann, A., Suslow, T., Kugel, H., et al. (2012). Catechol-O-methyltransferase gene variation: impact on amygdala response to aversive stimuli. Neuroimage 60, 2222–2229. doi: 10.1016/j.neuroimage.2012.02.039
Egan, M. F., Goldberg, T. E., Kolachana, B. S., Callicott, J. H., Mazzanti, C. M., Straub, R. E., et al. (2001). Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 98, 6917–6922. doi: 10.1073/pnas.111134598
Egerton, A., Chaddock, C. A., Winton-Brown, T. T., Bloomfield, M. A., Bhattacharyya, S., Allen, P., et al. (2013). Presynaptic striatal dopamine dysfunction in people at ultra-high risk for psychosis: findings in a second cohort. Biol. Psychiatry 74, 106–112. doi: 10.1016/j.biopsych.2012.11.017
Ehlis, A. C., Reif, A., Herrmann, M. J., Lesch, K. P., and Fallgatter, A. J. (2007). Impact of catechol-O-methyltransferase on prefrontal brain functioning in schizophrenia spectrum disorders. Neuropsychopharmacology 32, 162–170. doi: 10.1038/sj.npp.1301151
Engel, K., Zhou, M., and Wang, J. (2004). Identification and characterization of a novel monoamine transporter in the human brain. J. Biol. Chem. 279, 50042–50049. doi: 10.1074/jbc.M407913200
Estrada, G., Fatjo-Vilas, M., Munoz, M. J., Pulido, G., Minano, M. J., Toledo, E., et al. (2011). Cannabis use and age at onset of psychosis: further evidence of interaction with COMT Val158Met polymorphism. Acta Psychiatr. Scand. 123, 485–492. doi: 10.1111/j.1600-0447.2010.01665.x
Fink, G., Sumner, B. E., McQueen, J. K., Wilson, H., and Rosie, R. (1998). Sex steroid control of mood, mental state and memory. Clin. Exp. Pharmacol. Physiol. 25, 764–775. doi: 10.1111/j.1440-1681.1998.tb02151.x
Foley, D. L., Eaves, L. J., Wormley, B., Silberg, J. L., Maes, H. H., Kuhn, J., et al. (2004). Childhood adversity, monoamine oxidase a genotype, and risk for conduct disorder. Arch. Gen. Psychiatry 61, 738–744. doi: 10.1001/archpsyc.61.7.738
Fornai, F., Chen, K., Giorgi, F. S., Gesi, M., Alessandri, M. G., and Shih, J. C. (1999). Striatal dopamine metabolism in monoamine oxidase B-deficient mice: a brain dialysis study. J. Neurochem. 73, 2434–2440. doi: 10.1046/j.1471-4159.1999.0732434.x
Frau, R., Bini, V., Pes, R., Pillolla, G., Saba, P., Devoto, P., et al. (2014). Inhibition of 17alpha-hydroxylase/C17,20 lyase reduces gating deficits consequent to dopaminergic activation. Psychoneuroendocrinology 39, 204–213. doi: 10.1016/j.psyneuen.2013.09.014
Frau, R., Pillolla, G., Bini, V., Tambaro, S., Devoto, P., and Bortolato, M. (2013). Inhibition of 5alpha-reductase attenuates behavioral effects of D1-, but not D2-like receptor agonists in C57BL/6 mice. Psychoneuroendocrinology 38, 542–551. doi: 10.1016/j.psyneuen.2012.07.014
Fusar-Poli, P., Howes, O. D., Allen, P., Broome, M., Valli, I., Asselin, M. C., et al. (2011). Abnormal prefrontal activation directly related to pre-synaptic striatal dopamine dysfunction in people at clinical high risk for psychosis. Mol. Psychiatry 16, 67–75. doi: 10.1038/mp.2009.108
Gallinat, J., Bajbouj, M., Sander, T., Schlattmann, P., Xu, K., Ferro, E. F., et al. (2003). Association of the G1947A COMT (Val(108/158)Met) gene polymorphism with prefrontal P300 during information processing. Biol. Psychiatry 54, 40–48. doi: 10.1016/S0006-3223(02)01973-X
Garrick, N. A., and Murphy, D. L. (1980). Species differences in the deamination of dopamine and other substrates for monoamine oxidase in brain. Psychopharmacology (Berl). 72, 27–33. doi: 10.1007/BF00433804
Gasso, P., Bernardo, M., Mas, S., Crescenti, A., Garcia, C., Parellada, E., et al. (2008). Association of A/G polymorphism in intron 13 of the monoamine oxidase B gene with schizophrenia in a Spanish population. Neuropsychobiology 58, 65–70. doi: 10.1159/000159774
Godlewska, B. R., Olajossy-Hilkesberger, L., Limon, J., and Landowski, J. (2010). Ser9Gly polymorphism of the DRD3 gene is associated with worse premorbid social functioning and an earlier age of onset in female but not male schizophrenic patients. Psychiatry Res. 177, 266–267. doi: 10.1016/j.psychres.2010.02.012
Goel, N., and Bale, T. L. (2009). Examining the intersection of sex and stress in modelling neuropsychiatric disorders. J. Neuroendocrinol. 21, 415–420. doi: 10.1111/j.1365-2826.2009.01843.x
Gogos, J. A., Morgan, M., Luine, V., Santha, M., Ogawa, S., Pfaff, D., et al. (1998). Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc. Natl. Acad. Sci. U.S.A. 95, 9991–9996. doi: 10.1073/pnas.95.17.9991
Goldberg, T. E., Egan, M. F., Gscheidle, T., Coppola, R., Weickert, T., Kolachana, B. S., et al. (2003). Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch. Gen. Psychiatry 60, 889–896. doi: 10.1001/archpsyc.60.9.889
Goldman-Rakic, P. S., and Selemon, L. D. (1997). Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophr. Bull. 23, 437–458. doi: 10.1093/schbul/23.3.437
Goncalves, V. F., Tiwari, A. K., De Luca, V., Kong, S. L., Zai, C., Tampakeras, M., et al. (2012). DRD4 VNTR polymorphism and age at onset of severe mental illnesses. Neurosci. Lett. 519, 9–13. doi: 10.1016/j.neulet.2012.04.027
Greenawalt, J. W., and Schnaitman, C. (1970). An appraisal of the use of monoamine oxidase as an enzyme marker for the outer membrane of rat liver mitochondria. J. Cell Biol. 46, 173–179. doi: 10.1083/jcb.46.1.173
Griffon, N., Crocq, M. A., Pilon, C., Martres, M. P., Mayerova, A., Uyanik, G., et al. (1996). Dopamine D3 receptor gene: organization, transcript variants, and polymorphism associated with schizophrenia. Am. J. Med. Genet. 67, 63–70. doi: 10.1002/(SICI)1096-8628(19960216)67:1%3C63::AID-AJMG11%3E3.0.CO;2-N
Gundlah, C., Lu, N. Z., and Bethea, C. L. (2002). Ovarian steroid regulation of monoamine oxidase-A and -B mRNAs in the macaque dorsal raphe and hypothalamic nuclei. Psychopharmacology (Berl). 160, 271–282. doi: 10.1007/s00213-001-0959-0
Häfner, H. (2002). Prevention and early intervention in schizophrenia: facts and visions. Seishin Shinkeigaku Zasshi 104, 1033–1054.
Häfner, H., Riecher-Rossler, A., An Der Heiden, W., Maurer, K., Fatkenheuer, B., and Loffler, W. (1993). Generating and testing a causal explanation of the gender difference in age at first onset of schizophrenia. Psychol. Med. 23, 925–940. doi: 10.1017/S0033291700026398
Han, D. H., Kee, B. S., Min, K. J., Lee, Y. S., Na, C., Park, D. B., et al. (2006). Effects of catechol-O-methyltransferase Val158Met polymorphism on the cognitive stability and aggression in the first-onset schizophrenic patients. Neuroreport 17, 95–99. doi: 10.1097/01.wnr.0000192740.38653.91
Han, D. H., Park, D. B., Na, C., Kee, B. S., and Lee, Y. S. (2004). Association of aggressive behavior in Korean male schizophrenic patients with polymorphisms in the serotonin transporter promoter and catecholamine-O-methyltransferase genes. Psychiatry Res. 129, 29–37. doi: 10.1016/j.psychres.2004.06.013
Harris, S. E., Wright, A. F., Hayward, C., Starr, J. M., Whalley, L. J., and Deary, I. J. (2005). The functional COMT polymorphism, Val 158 Met, is associated with logical memory and the personality trait intellect/imagination in a cohort of healthy 79 year olds. Neurosci. Lett. 385, 1–6. doi: 10.1016/j.neulet.2005.04.104
Henquet, C., Rosa, A., Krabbendam, L., Papiol, S., Fananas, L., Drukker, M., et al. (2006). An experimental study of catechol-o-methyltransferase Val158Met moderation of delta-9-tetrahydrocannabinol-induced effects on psychosis and cognition. Neuropsychopharmacology 31, 2748–2757. doi: 10.1038/sj.npp.1301197
Herken, H., and Erdal, M. E. (2001). Catechol-O-methyltransferase gene polymorphism in schizophrenia: evidence for association between symptomatology and prognosis. Psychiatr. Genet. 11, 105–109. doi: 10.1097/00041444-200106000-00009
Hinds, H. L., Hendriks, R. W., Craig, I. W., and Chen, Z. Y. (1992). Characterization of a highly polymorphic region near the first exon of the human MAOA gene containing a GT dinucleotide and a novel VNTR motif. Genomics 13, 896–897. doi: 10.1016/0888-7543(92)90181-Q
Hirvonen, M. M., Nagren, K., Rinne, J. O., Pesonen, U., Vahlberg, T., Hagelberg, N., et al. (2010). COMT Val158Met genotype does not alter cortical or striatal dopamine D2 receptor availability in vivo. Mol. Imaging Biol. 12, 192–197. doi: 10.1007/s11307-009-0257-5
Hoenicka, J., Garrido, E., Ponce, G., Rodriguez-Jimenez, R., Martinez, I., Rubio, G., et al. (2010). Sexually dimorphic interaction between the DRD1 and COMT genes in schizophrenia. Am. J. Med. Genet. B Neuropsychiatr. Genet. 153B, 948–954. doi: 10.1002/ajmg.b.31065
Hoogendoorn, M. L., Bakker, S. C., Schnack, H. G., Selten, J. P., Otten, H. G., Verduijn, W., et al. (2005). No association between 12 dopaminergic genes and schizophrenia in a large Dutch sample. Am. J. Med. Genet. B Neuropsychiatr. Genet. 134B, 6–9. doi: 10.1002/ajmg.b.30147
Hotamisligil, G. S., and Breakefield, X. O. (1991). Human monoamine oxidase A gene determines levels of enzyme activity. Am. J. Hum. Genet. 49, 383–392.
Hovatta, I., Varilo, T., Suvisaari, J., Terwilliger, J. D., Ollikainen, V., Arajarvi, R., et al. (1999). A genomewide screen for schizophrenia genes in an isolated Finnish subpopulation, suggesting multiple susceptibility loci. Am. J. Hum. Genet. 65, 1114–1124. doi: 10.1086/302567
Howes, O. D., Bose, S. K., Turkheimer, F., Valli, I., Egerton, A., Valmaggia, L. R., et al. (2011). Dopamine synthesis capacity before onset of psychosis: a prospective [18F]-DOPA PET imaging study. Am. J. Psychiatry 168, 1311–1317. doi: 10.1176/appi.ajp.2011.11010160
Howes, O. D., Kambeitz, J., Kim, E., Stahl, D., Slifstein, M., Abi-Dargham, A., et al. (2012). The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch. Gen. Psychiatry 69, 776–786. doi: 10.1073/pnas.0511311103
Howes, O. D., and Kapur, S. (2009). The dopamine hypothesis of schizophreni: version III – the final common pathway. Schizophr. Bull. 35, 549–562. doi: 10.1093/schbul/sbp006
Howes, O. D., Williams, M., Ibrahim, K., Leung, G., Egerton, A., McGuire, P. K., et al. (2013). Midbrain dopamine function in schizophrenia and depression: a post-mortem and positron emission tomographic imaging study. Brain 136, 3242–3251. doi: 10.1093/brain/awt264
Huotari, M., Gainetdinov, R., and Männistö, P. T. (1999). Microdialysis studies on the action of tolcapone on pharmacologically-elevated extracellular dopamine levels in conscious rats. Pharmacol. Toxicol. 85, 233–238. doi: 10.1111/j.1600-0773.1999.tb02014.x
Hwang, R., Shinkai, T., De Luca, V., Ni, X., Potkin, S. G., Lieberman, J. A., et al. (2007). Association study of four dopamine D1 receptor gene polymorphisms and clozapine treatment response. J. Psychopharmacol. (Oxford) 21, 718–727. doi: 10.1177/0269881106072341
Hwu, H. G., Hong, C. J., Lee, Y. L., Lee, P. C., and Lee, S. F. (1998). Dopamine D4 receptor gene polymorphisms and neuroleptic response in schizophrenia. Biol. Psychiatry 44, 483–487. doi: 10.1016/S0006-3223(98)00134-6
Ira, E., Zanoni, M., Ruggeri, M., Dazzan, P., and Tosato, S. (2013). COMT, neuropsychological function and brain structure in schizophrenia: a systematic review and neurobiological interpretation. J. Psychiatry Neurosci. 38, 366–380. doi: 10.1503/jpn.120178
Iwata, Y., Matsumoto, H., Minabe, Y., Osada, N., Nakamura, K., Sekizawa, T., et al. (2003). Early-onset schizophrenia and dopamine-related gene polymorphism. Am. J. Med. Genet. B Neuropsychiatr. Genet. 116B, 23–26. doi: 10.1002/ajmg.b.10759
Jabbi, M., Korf, J., Kema, I. P., Hartman, C., Van Der Pompe, G., Minderaa, R. B., et al. (2007). Convergent genetic modulation of the endocrine stress response involves polymorphic variations of 5-HTT, COMT and MAOA. Mol. Psychiatry 12, 483–490. doi: 10.1038/sj.mp.4001975
Jablensky, A. (2003). “The epidemiological horizon,” in Schizophrenia, ed S. R. Hirsh, and D. R. Weinberger (Oxford: Blackwell Science), 203–231.
Jahng, J. W., Houpt, T. A., Wessel, T. C., Chen, K., Shih, J. C., and Joh, T. H. (1997). Localization of monoamine oxidase A and B mRNA in the rat brain by in situ hybridization. Synapse 25, 30–36.
Jonsson, E. G., Norton, N., Forslund, K., Mattila-Evenden, M., Rylander, G., Asberg, M., et al. (2003). Association between a promoter variant in the monoamine oxidase A gene and schizophrenia. Schizophr. Res. 61, 31–37. doi: 10.1016/S0920-9964(02)00224-4
Jonsson, E. G., Norton, N., Gustavsson, J. P., Oreland, L., Owen, M. J., and Sedvall, G. C. (2000). A promoter polymorphism in the monoamine oxidase A gene and its relationships to monoamine metabolite concentrations in CSF of healthy volunteers. J. Psychiatr. Res. 34, 239–244. doi: 10.1016/S0022-3956(00)00013-3
Kang, S. G., Park, Y. M., Choi, J. E., Lim, S. W., Lee, H. J., Lee, S. H., et al. (2010). Association study between antipsychotic-induced restless legs syndrome and polymorphisms of monoamine oxidase genes in schizophrenia. Hum. Psychopharmacol. 25, 397–403. doi: 10.1002/hup.1130
Karayiorgou, M., Gogos, J. A., Galke, B. L., Wolyniec, P. S., Nestadt, G., Antonarakis, S. E., et al. (1998). Identification of sequence variants and analysis of the role of the catechol-O-methyl-transferase gene in schizophrenia susceptibility. Biol. Psychiatry 43, 425–431. doi: 10.1016/S0006-3223(97)00202-3
Karoum, F., Chrapusta, S. J., Brinjak, R., Hitri, A., and Wyatt, R. J. (1994). Regional effects of amphetamine, cocaine, nomifensine and GBR 12909 on the dynamics of dopamine release and metabolism in the rat brain. Br. J. Pharmacol. 113, 1391–1399. doi: 10.1111/j.1476-5381.1994.tb17152.x
Kim, Y. R., Kim, J. H., Kim, S. J., Lee, D., and Min, S. K. (2008). Catechol-O-methyltransferase Val158Met polymorphism in relation to aggressive schizophrenia in a Korean population. Eur. Neuropsychopharmacol. 18, 820–825. doi: 10.1016/j.euroneuro.2008.07.009
Kim-Cohen, J., Caspi, A., Taylor, A., Williams, B., Newcombe, R., Craig, I. W., et al. (2006). MAOA, maltreatment, and gene-environment interaction predicting children's mental health: new evidence and a meta-analysis. Mol. Psychiatry 11, 903–913. doi: 10.1038/sj.mp.4001851
Ko, Y. H., Jung, S. W., Joe, S. H., Lee, C. H., Jung, H. G., Jung, I. K., et al. (2007). Association between serum testosterone levels and the severity of negative symptoms in male patients with chronic schizophrenia. Psychoneuroendocrinology 32, 385–391. doi: 10.1016/j.psyneuen.2007.02.002
Ko, Y. H., Lew, Y. M., Jung, S. W., Joe, S. H., Lee, C. H., Jung, H. G., et al. (2008). Short-term testosterone augmentation in male schizophrenics: a randomized, double-blind, placebo-controlled trial. J. Clin. Psychopharmacol. 28, 375–383. doi: 10.1097/JCP.0b013e31817d5912
Kotler, M., Barak, P., Cohen, H., Averbuch, I. E., Grinshpoon, A., Gritsenko, I., et al. (1999). Homicidal behavior in schizophrenia associated with a genetic polymorphism determining low catechol O-methyltransferase (COMT) activity. Am. J. Med. Genet. 88, 628–633. doi: 10.1002/(SICI)1096-8628(19991215)88:6<628::AID-AJMG10>3.0.CO;2-E
Kremer, I., Pinto, M., Murad, I., Muhaheed, M., Bannoura, I., Muller, D. J., et al. (2003). Family-based and case-control study of catechol-O-methyltransferase in schizophrenia among Palestinian Arabs. Am. J. Med. Genet. B Neuropsychiatr. Genet. 119B, 35–39. doi: 10.1002/ajmg.b.20008
Kuepper, R., Skinbjerg, M., and Abi-Dargham, A. (2012). The dopamine dysfunction in schizophrenia revisited: new insights into topography and course. Handb. Exp. Pharmacol. 1–26. doi: 10.1007/978-3-642-25761-2_1
Kulkarni, J., De Castella, A., Fitzgerald, P. B., Gurvich, C. T., Bailey, M., Bartholomeusz, C., et al. (2008). Estrogen in severe mental illness: a potential new treatment approach. Arch. Gen. Psychiatry 65, 955–960. doi: 10.1001/archpsyc.65.8.955
Kulkarni, J., De Castella, A., Smith, D., Taffe, J., Keks, N., and Copolov, D. (1996). A clinical trial of the effects of estrogen in acutely psychotic women. Schizophr. Res. 20, 247–252. doi: 10.1016/0920-9964(96)82949-5
Kulkarni, J., Riedel, A., De Castella, A. R., Fitzgerald, P. B., Rolfe, T. J., Taffe, J., et al. (2002). A clinical trial of adjunctive oestrogen treatment in women with schizophrenia. Arch. Womens Ment. Health 5, 99–104. doi: 10.1007/s00737-002-0001-5
Lachman, H. M., Nolan, K. A., Mohr, P., Saito, T., and Volavka, J. (1998). Association between catechol O-methyltransferase genotype and violence in schizophrenia and schizoaffective disorder. Am. J. Psychiatry 155, 835–837.
Lachman, H. M., Papolos, D. F., Saito, T., Yu, Y. M., Szumlanski, C. L., and Weinshilboum, R. M. (1996). Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics 6, 243–250. doi: 10.1097/00008571-199606000-00007
Larsen, T. K., McGlashan, T. H., Johannessen, J. O., and Vibe-Hansen, L. (1996). First-episode schizophrenia: II. Premorbid patterns by gender. Schizophr. Bull. 22, 257–269. doi: 10.1093/schbul/22.2.257
Laruelle, M. (2003). “Dopamine transmission in the schizophrenic brain,” in Schizophrenia, ed S. R. Hirsh and D. R. Weinberger (Oxford: Blackwell Science), 365–387.
Lataster, J., Collip, D., Ceccarini, J., Hernaus, D., Haas, D., Booij, L., et al. (2014). Familial liability to psychosis is associated with attenuated dopamine stress signaling in ventromedial prefrontal cortex. Schizophr. Bull. 40, 66–77. doi: 10.1093/schbul/sbs187
Li, D., and He, L. (2008). Meta-study on association between the monoamine oxidase A gene (MAOA) and schizophrenia. Am. J. Med. Genet. B Neuropsychiatr. Genet. 147B, 174–178. doi: 10.1002/ajmg.b.30570
Li, W. J., Kou, C. G., Yu, Y., Sun, S., Zhang, X., Kosten, T. R., et al. (2012). Association of catechol-O-methyltransferase gene polymorphisms with schizophrenia and negative symptoms in a Chinese population. Am. J. Med. Genet. B Neuropsychiatr. Genet. 159B, 370–375. doi: 10.1002/ajmg.b.32038
Lin, S., Jiang, S., Wu, X., Qian, Y., Wang, D., Tang, G., et al. (2000). Association analysis between mood disorder and monoamine oxidase gene. Am. J. Med. Genet. 96, 12–14. doi: 10.1002/(SICI)1096-8628(20000207)96:1%3C12::AID-AJMG4%3E3.0.CO;2-S
Liou, Y. J., Tsai, S. J., Hong, C. J., Wang, Y. C., and Lai, I. C. (2001). Association analysis of a functional catechol-o-methyltransferase gene polymorphism in schizophrenic patients in Taiwan. Neuropsychobiology 43, 11–14. doi: 10.1159/000054858
Lu, B. Y., Martin, K. E., Edgar, J. C., Smith, A. K., Lewis, S. F., Escamilla, M. A., et al. (2007). Effect of catechol O-methyltransferase val(158)met polymorphism on the p50 gating endophenotype in schizophrenia. Biol. Psychiatry 62, 822–825. doi: 10.1016/j.biopsych.2006.11.030
Luine, V. N., Khylchevskaya, R. I., and McEwen, B. S. (1975). Effect of gonadal steroids on activities of monoamine oxidase and choline acetylase in rat brain. Brain Res. 86, 293–306. doi: 10.1016/0006-8993(75)90704-0
Lundstrom, K., Salminen, M., Jalanko, A., Savolainen, R., and Ulmanen, I. (1991). Cloning and characterization of human placental catechol-O-methyltransferase cDNA. DNA Cell Biol. 10, 181–189. doi: 10.1089/dna.1991.10.181
Luque, J. M., Bleuel, Z., Hendrickson, A., and Richards, J. G. (1996). Detection of MAO-A and MAO-B mRNAs in monkey brainstem by cross-hybridization with human oligonucleotide probes. Brain Res. Mol. Brain Res. 36, 357–360. doi: 10.1016/0169-328X(96)88407-5
Luque, J. M., Kwan, S. W., Abell, C. W., Da Prada, M., and Richards, J. G. (1995). Cellular expression of mRNAs encoding monoamine oxidases A and B in the rat central nervous system. J. Comp. Neurol. 363, 665–680. doi: 10.1002/cne.903630410
Ma, X., Sun, J., Yao, J., Wang, Q., Hu, X., Deng, W., et al. (2007). A quantitative association study between schizotypal traits and COMT, PRODH and BDNF genes in a healthy Chinese population. Psychiatry Res. 153, 7–15. doi: 10.1016/j.psychres.2007.02.003
Ma, Z. Q., Bondiolotti, G. P., Olasmaa, M., Violani, E., Patrone, C., Picotti, G. B., et al. (1993). Estrogen modulation of catecholamine synthesis and monoamine oxidase A activity in the human neuroblastoma cell line SK-ER3. J. Steroid Biochem. Mol. Biol. 47, 207–211. doi: 10.1016/0960-0760(93)90076-9
Malhotra, A. K., Kestler, L. J., Mazzanti, C., Bates, J. A., Goldberg, T., and Goldman, D. (2002). A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. Am. J. Psychiatry 159, 652–654. doi: 10.1176/appi.ajp.159.4.652
Männistö, P. T., and Kaakkola, S. (1999). Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol. Rev. 51, 593–628.
Männistö, P. T., Ulmanen, I., Lundstrom, K., Taskinen, J., Tenhunen, J., Tilgmann, C., et al. (1992). Characteristics of catechol O-methyl-transferase (COMT) and properties of selective COMT inhibitors. Prog. Drug Res. 39, 291–350.
Markham, J. A. (2012). Sex steroids and schizophrenia. Rev. Endocr. Metab. Disord. 13, 187–207. doi: 10.1007/s11154-011-9184-2
Matsumoto, C., Shinkai, T., Hori, H., Ohmori, O., and Nakamura, J. (2004). Polymorphisms of dopamine degradation enzyme (COMT and MAO) genes and tardive dyskinesia in patients with schizophrenia. Psychiatry Res. 127, 1–7. doi: 10.1016/j.psychres.2004.03.011
Matsumoto, M., Weickert, C. S., Akil, M., Lipska, B. K., Hyde, T. M., Herman, M. M., et al. (2003). Catechol O-methyltransferase mRNA expression in human and rat brain: evidence for a role in cortical neuronal function. Neuroscience 116, 127–137. doi: 10.1016/S0306-4522(02)00556-0
McCarthy, M. M., Auger, A. P., Bale, T. L., De Vries, G. J., Dunn, G. A., Forger, N. G., et al. (2009). The epigenetics of sex differences in the brain. J. Neurosci. 29, 12815–12823. doi: 10.1523/JNEUROSCI.3331-09.2009
McCarthy, M. M., and Nugent, B. M. (2013). Epigenetic contributions to hormonally mediated sexual differentiation in the brain. J. Neuroendocrinol. doi: 10.1111/jne.12072. [Epub ahead of print].
Meltzer, H. Y., and Zureick, J. L. (1987). Relationship of auditory hallucinations and paranoia to platelet MAO activity in schizophrenics: sex and race interactions. Psychiatry Res. 22, 99–109. doi: 10.1016/0165-1781(87)90097-7
Meyer-Lindenberg, A., Buckholtz, J. W., Kolachana, B. R., Hariri, A., Pezawas, L., Blasi, G., et al. (2006). Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc. Natl. Acad. Sci. U.S.A. 103, 6269–6274. doi: 10.1073/pnas.0511311103
Meyers, B., D'Agostino, A., Walker, J., and Kritzer, M. F. (2010). Gonadectomy and hormone replacement exert region- and enzyme isoform-specific effects on monoamine oxidase and catechol-O-methyltransferase activity in prefrontal cortex and neostriatum of adult male rats. Neuroscience 165, 850–862. doi: 10.1016/j.neuroscience.2009.11.013
Mihara, K., Kondo, T., Suzuki, A., Yasui, N., Nagashima, U., Ono, S., et al. (2000). Prolactin response to nemonapride, a selective antagonist for D2 like dopamine receptors, in schizophrenic patients in relation to Taq1A polymorphism of DRD2 gene. Psychopharmacology 149, 246–250. doi: 10.1007/s002139900364
Mihara, K., Suzuki, A., Kondo, T., Yasui-Furukori, N., Ono, S., Otani, K., et al. (2001). Relationship between Taq1 A dopamine D2 receptor (DRD2) polymorphism and prolactin response to bromperidol. Am. J. Med. Genet. 105, 271–274. doi: 10.1002/ajmg.1303
Min, J. A., Kim, J. J., Pae, C. U., Kim, K. H., Lee, C. U., Lee, C., et al. (2012). Association of estrogen receptor genes and schizophrenia: a preliminary study. Prog. Neuropsychopharmacol. Biol. Psychiatry 36, 1–4. doi: 10.1016/j.pnpbp.2011.09.012
Naudon, L., Dourmap, N., Leroux-Nicollet, I., and Costentin, J. (1992). Kainic acid lesion of the striatum increases dopamine release but reduces 3-methoxytyramine level. Brain Res. 572, 247–249. doi: 10.1016/0006-8993(92)90477-Q
Nolan, K. A., Bilder, R. M., Lachman, H. M., and Volavka, J. (2004). Catechol O-methyltransferase Val158Met polymorphism in schizophrenia: differential effects of Val and Met alleles on cognitive stability and flexibility. Am. J. Psychiatry 161, 359–361. doi: 10.1176/appi.ajp.161.2.359
Nolan, K. A., Volavka, J., Czobor, P., Cseh, A., Lachman, H., Saito, T., et al. (2000). Suicidal behavior in patients with schizophrenia is related to COMT polymorphism. Psychiatr. Genet. 10, 117–124. doi: 10.1097/00041444-200010030-00003
Norton, N., Kirov, G., Zammit, S., Jones, G., Jones, S., Owen, R., et al. (2002). Schizophrenia and functional polymorphisms in the MAOA and COMT genes: no evidence for association or epistasis. Am. J. Med. Genet. 114, 491–496. doi: 10.1002/ajmg.10517
O'Donnell, P. (2010). Adolescent maturation of cortical dopamine. Neurotox. Res. 18, 306–312. doi: 10.1007/s12640-010-9157-3
Ohnishi, T., Hashimoto, R., Mori, T., Nemoto, K., Moriguchi, Y., Iida, H., et al. (2006). The association between the Val158Met polymorphism of the catechol-O-methyl transferase gene and morphological abnormalities of the brain in chronic schizophrenia. Brain 129, 399–410. doi: 10.1093/brain/awh702
Opgen-Rhein, C., Neuhaus, A. H., Urbanek, C., Hahn, E., Sander, T., and Dettling, M. (2008). Executive attention in schizophrenic males and the impact of COMT Val108/158Met genotype on performance on the attention network test. Schizophr. Bull. 34, 1231–1239. doi: 10.1093/schbul/sbm155
Oreland, L., Nilsson, K., Damberg, M., and Hallman, J. (2007). Monoamine oxidases: activities, genotypes and the shaping of behaviour. J. Neural Transm. 114, 817–822. doi: 10.1007/s00702-007-0694-8
Ou, X. M., Chen, K., and Shih, J. C. (2006). Glucocorticoid and androgen activation of monoamine oxidase A is regulated differently by R1 and Sp1. J. Biol. Chem. 281, 21512–21525. doi: 10.1074/jbc.M600250200
Ozelius, L., Hsu, Y. P., Bruns, G., Powell, J. F., Chen, S., Weyler, W., et al. (1988). Human monoamine oxidase gene (MAOA): chromosome position (Xp21-p11) and DNA polymorphism. Genomics 3, 53–58. doi: 10.1016/0888-7543(88)90159-0
Paba, S., Frau, R., Godar, S. C., Devoto, P., Marrosu, F., and Bortolato, M. (2011). Steroid 5alpha-reductase as a novel therapeutic target for schizophrenia and other neuropsychiatric disorders. Curr. Pharm. Des. 17, 151–167. doi: 10.2174/138161211795049589
Papaleo, F., Crawley, J. N., Song, J., Lipska, B. K., Pickel, J., Weinberger, D. R., et al. (2008). Genetic dissection of the role of catechol-O-methyltransferase in cognition and stress reactivity in mice. J. Neurosci. 28, 8709–8723. doi: 10.1523/JNEUROSCI.2077-08.2008
Papaleo, F., Erickson, L., Liu, G., Chen, J., and Weinberger, D. R. (2012). Effects of sex and COMT genotype on environmentally modulated cognitive control in mice. Proc. Natl. Acad. Sci. U.S.A. 109, 20160–20165. doi: 10.1073/pnas.1214397109
Parvizi, N., and Wuttke, W. (1983). Catecholestrogens affect catecholamine turnover rates in the anterior part of the mediobasal hypothalamus and medial preoptic area in the male and female castrated rat. Neuroendocrinology 36, 21–26. doi: 10.1159/000123523
Paspalas, C. D., and Goldman-Rakic, P. S. (2004). Microdomains for dopamine volume neurotransmission in primate prefrontal cortex. J. Neurosci. 24, 5292–5300. doi: 10.1523/JNEUROSCI.0195-04.2004
Pelayo-Teran, J. M., Suarez-Pinilla, P., Chadi, N., and Crespo-Facorro, B. (2012). Gene-environment interactions underlying the effect of cannabis in first episode psychosis. Curr. Pharm. Des. 18, 5024–5035. doi: 10.2174/138161212802884609
Penzes, P., Buonanno, A., Passafaro, M., Sala, C., and Sweet, R. A. (2013). Developmental vulnerability of synapses and circuits associated with neuropsychiatric disorders. J. Neurochem. 126, 165–182. doi: 10.1111/jnc.12261
Piton, A., Gauthier, J., Hamdan, F. F., Lafreniere, R. G., Yang, Y., Henrion, E., et al. (2011). Systematic resequencing of X-chromosome synaptic genes in autism spectrum disorder and schizophrenia. Mol. Psychiatry 16, 867–880. doi: 10.1038/mp.2010.54
Popp, J., Leucht, S., Heres, S., and Steimer, W. (2009). DRD4 48 bp VNTR but not 5-HT 2C Cys23Ser receptor polymorphism is related to antipsychotic-induced weight gain. Pharmacogenomics J. 9, 71–77. doi: 10.1038/tpj.2008.5
Potkin, S. G., Basile, V. S., Jin, Y., Masellis, M., Badri, F., Keator, D., et al. (2003). D1 receptor alleles predict PET metabolic correlates of clinical response to clozapine. Mol. Psychiatry 8, 109–113. doi: 10.1038/sj.mp.4001191
Purves-Tyson, T. D., Handelsman, D. J., Double, K. L., Owens, S. J., Bustamante, S., and Weickert, C. S. (2012). Testosterone regulation of sex steroid-related mRNAs and dopamine-related mRNAs in adolescent male rat substantia nigra. BMC Neurosci. 13:95. doi: 10.1186/1471-2202-13-95
Qiu, H. T., Meng, H. Q., Song, C., Xiu, M. H., Chen Da, C., Zhu, F. Y., et al. (2009). Association between monoamine oxidase (MAO)-A gene variants and schizophrenia in a Chinese population. Brain Res. 1287, 67–73. doi: 10.1016/j.brainres.2009.06.072
Quednow, B. B., Schmechtig, A., Ettinger, U., Petrovsky, N., Collier, D. A., Vollenweider, F. X., et al. (2009). Sensorimotor gating depends on polymorphisms of the serotonin-2A receptor and catechol-O-methyltransferase, but not on neuregulin-1 Arg38Gln genotype: a replication study. Biol. Psychiatry 66, 614–620. doi: 10.1016/j.biopsych.2009.05.007
Quednow, B. B., Wagner, M., Mossner, R., Maier, W., and Kuhn, K. U. (2010). Sensorimotor gating of schizophrenia patients depends on Catechol O-methyltransferase Val158Met polymorphism. Schizophr. Bull. 36, 341–346. doi: 10.1093/schbul/sbn088
Riecher-Rossler, A., Hafner, H., Dutsch-Strobel, A., Oster, M., Stumbaum, M., Van Gulick-Bailer, M., et al. (1994). Further evidence for a specific role of estradiol in schizophrenia? Biol. Psychiatry 36, 492–494. doi: 10.1016/0006-3223(94)90649-1
Riley, B., Mogudi-Carter, M., Jenkins, T., and Williamson, R. (1996). No evidence for linkage of chromosome 22 markers to schizophrenia in southern African Bantu-speaking families. Am. J. Med. Genet. 67, 515–522.
Risbrough, V., Ji, B., Hauger, R., and Zhou, X. (2014). Generation and characterization of humanized mice carrying COMT158 Met/Val alleles. Neuropsychopharmacology. doi: 10.1038/npp.2014.29. [Epub ahead of print].
Ritsner, M. S., Gibel, A., Shleifer, T., Boguslavsky, I., Zayed, A., Maayan, R., et al. (2010). Pregnenolone and dehydroepiandrosterone as an adjunctive treatment in schizophrenia and schizoaffective disorder: an 8-week, double-blind, randomized, controlled, 2-center, parallel-group trial. J. Clin. Psychiatry 71, 1351–1362. doi: 10.4088/JCP.09m05031yel
Ritsner, M. S., and Strous, R. D. (2010). Neurocognitive deficits in schizophrenia are associated with alterations in blood levels of neurosteroids: a multiple regression analysis of findings from a double-blind, randomized, placebo-controlled, crossover trial with DHEA. J. Psychiatr. Res. 44, 75–80. doi: 10.1016/j.jpsychires.2009.07.002
Rivett, A. J., Francis, A., and Roth, J. A. (1983). Distinct cellular localization of membrane-bound and soluble forms of catechol-O-methyltransferase in brain. J. Neurochem. 40, 215–219. doi: 10.1111/j.1471-4159.1983.tb12673.x
Rosier, J. P., Howes, O. D., Chaddock, C. A., Joyce, E. M., and McGuire, P. (2013). Neural and behavioral correlates of aberrant salience in individuals at risk for psychosis. Schizophr. Bull. 39, 1328–1336. doi: 10.1093/schbul/sbs147
Rowley, M., Bristow, L. J., and Hutson, P. H. (2001). Current and novel approaches to the drug treatment of schizophrenia. J. Med. Chem. 44, 477–501. doi: 10.1021/jm0002432
Rubin, L. H., Carter, C. S., Drogos, L., Pournajafi-Nazarloo, H., Sweeney, J. A., and Maki, P. M. (2010). Peripheral oxytocin is associated with reduced symptom severity in schizophrenia. Schizophr. Res. 124, 13–21. doi: 10.1016/j.schres.2010.09.014
Rybakowski, J. K., Borkowska, A., Czerski, P. M., Dmitrzak-Weglarz, M., Skibinska, M., Kapelski, P., et al. (2006). Performance on the Wisconsin Card Sorting Test in schizophrenia and genes of dopaminergic inactivation (COMT, DAT, NET). Psychiatry Res. 143, 13–19. doi: 10.1016/j.psychres.2005.10.008
Rybakowski, J. K., Borkowska, A., Czerski, P. M., and Hauser, J. (2002). Eye movement disturbances in schizophrenia and a polymorphism of catechol-O-methyltransferase gene. Psychiatric. Res. 113, 49–57. doi: 10.1016/S0165-1781(02)00245-7
Rybakowski, J. K., Borkowska, A., Czerski, P. M., Kapelski, P., Dmitrzak-Weglarz, M., and Hauser, J. (2005). An association study of dopamine receptors polymorphisms and the Wisconsin Card Sorting Test in schizophrenia. J. Neural Transm. 112, 1575–1582. doi: 10.1007/s00702-005-0292-6
Sabol, S. Z., Hu, S., and Hamer, D. (1998). A functional polymorphism in the monoamine oxidase A gene promoter. Hum. Genet. 103, 273–279. doi: 10.1007/s004390050816
Salminen, M., Lundstrom, K., Tilgmann, C., Savolainen, R., Kalkkinen, N., and Ulmanen, I. (1990). Molecular cloning and characterization of rat liver catechol-O-methyltransferase. Gene 93, 241–247. doi: 10.1016/0378-1119(90)90231-F
Sanchez, M. G., Bourque, M., Morissette, M., and Di Paolo, T. (2010). Steroids-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci. Ther. 16, e43–e71. doi: 10.1111/j.1755-5949.2010.00163.x
Sasaki, T., Hattori, M., Sakai, T., Kato, T., Kunugi, H., Hirose, T., et al. (1998). The monoamine oxidase-A gene and major psychosis in Japanese subjects. Biol. Psychiatry 44, 922–924. doi: 10.1016/S0006-3223(97)00522-2
Saura, J., Richards, J. G., and Mahy, N. (1994). Differential age-related changes of MAO-A and MAO-B in mouse brain and peripheral organs. Neurobiol. Aging 15, 399–408. doi: 10.1016/0197-4580(94)90071-X
Schenkel, L. S., Spaulding, W. D., Dilillo, D., and Silverstein, S. M. (2005). Histories of childhood maltreatment in schizophrenia: relationships with premorbid functioning, symptomatology, and cognitive deficits. Schizophr. Res. 76, 273–286. doi: 10.1016/j.schres.2005.03.003
Schott, B. H., Frischknecht, R., Debska-Vielhaber, G., John, N., Behnisch, G., Duzel, E., et al. (2010). Membrane-bound catechol-O-methyl transferase in cortical neurons and glial cells is intracellularly oriented. Front. Psychiatry 1:142. doi: 10.3389/fpsyt.2010.00142
Schott, B. H., Seidenbecher, C. I., Fenker, D. B., Lauer, C. J., Bunzeck, N., Bernstein, H. G., et al. (2006). The dopaminergic midbrain participates in human episodic memory formation: evidence from genetic imaging. J. Neurosci. 26, 1407–1417. doi: 10.1523/JNEUROSCI.3463-05.2006
Seeman, P., and Lee, T. (1975). Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science 188, 1217–1219. doi: 10.1126/science.1145194
Semwal, P., Prasad, S., Bhatia, T., Deshpande, S. N., Wood, J., Nimgaonkar, V. L., et al. (2001). Family-based association studies of monoaminergic gene polymorphisms among North Indians with schizophrenia. Mol. Psychiatry 6, 220–224. doi: 10.1038/sj.mp.4000839
Sesack, S. R., Hawrylak, V. A., Matus, C., Guido, M. A., and Levey, A. I. (1998). Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J. Neurosci. 18, 2697–2708.
Shifman, S., Bronstein, M., Sternfeld, M., Pisanté-Shalom, A., Lev-Lehman, E., Weizman, A., et al. (2002). A highly significant association between a COMT haplotype and schizophrenia. Am. J. Hum. Genet. 71, 1296–1302. doi: 10.1086/344514
Shih, J. C., and Thompson, R. F. (1999). Monoamine oxidase in neuropsychiatry and behavior. Am. J. Hum. Genet. 65, 593–598. doi: 10.1086/302562
Singh, J. P., Volavka, J., Czobor, P., and Van Dorn, R. A. (2012). A meta-analysis of the Val158Met COMT polymorphism and violent behavior in schizophrenia. PLoS ONE 7:e43423. doi: 10.1371/journal.pone.0043423
Sjoberg, R. L., Ducci, F., Barr, C. S., Newman, T. K., Dell'osso, L., Virkkunen, M., et al. (2008). A non-additive interaction of a functional MAO-A VNTR and testosterone predicts antisocial behavior. Neuropsychopharmacology 33, 425–430. doi: 10.1038/sj.npp.1301417
Smieskova, R., Marmy, J., Schmidt, A., Bendfeldt, K., Riecher-R?ssler, A., Walter, M., et al. (2013). Do subjects at clinical high risk for psychosis differ from those with a genetic high risk?–A systematic review of structural and functional brain abnormalities. Curr. Med. Chem. 20, 467–481.
Smolka, M. N., Schumann, G., Wrase, J., Grusser, S. M., Flor, H., Mann, K., et al. (2005). Catechol-O-methyltransferase val158met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. J. Neurosci. 25, 836–842. doi: 10.1523/JNEUROSCI.1792-04.2005
Snell, L. D., Glanz, J., Tabakoff, B., State, W. I. S. O., Trait Markers Ofalcohol, U., and Dependence, I. (2002). Relationships between effects of smoking, gender, and alcohol dependence on platelet monoamine oxidase-B: activity, affinity labeling, and protein measurements. Alcohol. Clin. Exp. Res. 26, 1105–1113. doi: 10.1111/j.1530-0277.2002.tb02645.x
Sobell, J. L., Lind, T. J., Hebrink, D. D., Heston, L. L., and Sommer, S. S. (1997). Screening the monoamine oxidase B gene in 100 male patients with schizophrenia: a cluster of polymorphisms in African-Americans but lack of functionally significant sequence changes. Am. J. Med. Genet. 74, 44–49.
Soyka, M. (2011). Neurobiology of aggression and violence in schizophrenia. Schizophr. Bull. 37, 913–920. doi: 10.1093/schbul/sbr103
Spear, L. P. (2000). The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 24, 417–463. doi: 10.1016/S0149-7634(00)00014-2
Stefanis, N. C., Henquet, C., Avramopoulos, D., Smyrnis, N., Evdokimidis, I., Myin-Germeys, I., et al. (2007). COMT Val158Met moderation of stress-induced psychosis. Psychol. Med. 37, 1651–1656. doi: 10.1017/S0033291707001080
Stokes, P. R., Shotbolt, P., Mehta, M. A., Turkheimer, E., Benecke, A., Copeland, C., et al. (2013). Nature or nurture? Determining the heritability of human striatal dopamine function: an [18F]-DOPA PET study. Neuropsychopharmacology 38, 485–491. doi: 10.1038/npp.2012.207
Strous, R. D., Bark, N., Parsia, S. S., Volavka, J., and Lachman, H. M. (1997). Analysis of a functional catechol-O-methyltransferase gene polymorphism in schizophrenia: evidence for association with aggressive and antisocial behavior. Psychiatry Res. 69, 71–77. doi: 10.1016/S0165-1781(96)03111-3
Strous, R. D., Lapidus, R., Viglin, D., Kotler, M., and Lachman, H. M. (2006). Analysis of an association between the COMT polymorphism and clinical symptomatology in schizophrenia. Neurosci. Lett. 393, 170–173. doi: 10.1016/j.neulet.2005.09.067
Sumner, B. E., and Fink, G. (1993). Effects of acute estradiol on 5-hydroxytryptamine and dopamine receptor subtype mRNA expression in female rat brain. Mol. Cell. Neurosci. 4, 83–92. doi: 10.1006/mcne.1993.1010
Sun, J., Jayathilake, K., Zhao, Z., and Meltzer, H. Y. (2012a). Investigating association of four gene regions (GABRB3, MAOB, PAH, and SLC6A4) with five symptoms in schizophrenia. Psychiatry Res. 198, 202–206. doi: 10.1016/j.psychres.2011.12.035
Sun, Y., Zhang, J., Yuan, Y., Yu, X., Shen, Y., and Xu, Q. (2012b). Study of a possible role of the monoamine oxidase A (MAOA) gene in paranoid schizophrenia among a Chinese population. Am. J. Med. Genet. B Neuropsychiatr. Genet. 159B, 104–111. doi: 10.1002/ajmg.b.32009
Sweet, R. A., Devlin, B., Pollock, B. G., Sukonick, D. L., Kastango, K. B., Bacanu, S. A., et al. (2005). Catechol-O-methyltransferase haplotypes are associated with psychosis in Alzheimer disease. Mol. Psychiatry 10, 1026–1036. doi: 10.1038/sj.mp.4001709
Syagailo, Y. V., Stober, G., Grassle, M., Reimer, E., Knapp, M., Jungkunz, G., et al. (2001). Association analysis of the functional monoamine oxidase A gene promoter polymorphism in psychiatric disorders. Am. J. Med. Genet. 105, 168–171. doi: 10.1002/ajmg.1193
Tan, H. Y., Callicott, J. H., and Weinberger, D. R. (2007). Dysfunctional and compensatory prefrontal cortical systems, genes and the pathogenesis of schizophrenia. Cereb. Cortex 17(Suppl. 1), i171–i181. doi: 10.1093/cercor/bhm069
Teicher, M. H., Andersen, S. L., and Hostetter, J. C. Jr. (1995). Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain Res. Dev. Brain Res. 89, 167–172. doi: 10.1016/0165-3806(95)00109-Q
Tenhunen, J., Salminen, M., Jalanko, A., Ukkonen, S., and Ulmanen, I. (1993). Structure of the rat catechol-O-methyltransferase gene: separate promoters are used to produce mRNAs for soluble and membrane-bound forms of the enzyme. DNA Cell Biol. 12, 253–263. doi: 10.1089/dna.1993.12.253
Thiblin, I., Finn, A., Ross, S. B., and Stenfors, C. (1999). Increased dopaminergic and 5-hydroxytryptaminergic activities in male rat brain following long-term treatment with anabolic androgenic steroids. Br. J. Pharmacol. 126, 1301–1306. doi: 10.1038/sj.bjp.0702412
Tosato, S., Bonetto, C., Di Forti, M., Collier, D., Cristofalo, D., Bertani, M., et al. (2011). Effect of COMT genotype on aggressive behaviour in a community cohort of schizophrenic patients. Neurosci. Lett. 495, 17–21. doi: 10.1016/j.neulet.2011.03.018
Tsai, G., and Coyle, J. T. (2002). Glutamergic mechanisms in schizophrenia. Annu. Rev. Pharmacol. Toxicol. 42, 165–179. doi: 10.1146/annurev.pharmtox.42.082701.160735
Tunbridge, E. M., Harrison, P. J., and Weinberger, D. R. (2006). Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol. Psychiatry 60, 141–151. doi: 10.1016/j.biopsych.2005.10.024
Ulmanen, I., Peranen, J., Tenhunen, J., Tilgmann, C., Karhunen, T., Panula, P., et al. (1997). Expression and intracellular localization of catechol O-methyltransferase in transfected mammalian cells. Eur. J. Biochem. 243, 452–459. doi: 10.1111/j.1432-1033.1997.0452a.x
Vaccari, A., Caviglia, A., Sparatore, A., and Biassoni, R. (1981). Gonadal influences on the sexual differentiation of monoamine oxidase type A and B activities in the rat brain. J. Neurochem. 37, 640–648. doi: 10.1111/j.1471-4159.1982.tb12535.x
Van Os, J., Kenis, G., and Rutten, B.P. (2010). The environment and schizophrenia. Nature 468, 203–212. doi: 10.1038/nature09563
Van Os, J., and Murray, R. (2008). Introduction. Schizophr. Bull. 34, 1064–1065. doi: 10.1093/schbul/sbn116
Van Os, J., and Rutten, B. P. (2009). Gene-environment-wide interaction studies in psychiatry. Am. J. Psychiatry 166, 964–966. doi: 10.1176/appi.ajp.2008.09060904
Van Os, J., Rutten, B. P., and Poulton, R. (2008). Gene-environment interactions in schizophrenia: review of epidemiological findings and future directions. Schizophr. Bull. 34, 1066–1082. doi: 10.1093/schbul/sbn117
Voisey, J., Swagell, C. D., Hughes, I. P., Lawford, B. R., Young, R. M., and Morris, C. P. (2012). HapMap tag-SNP analysis confirms a role for COMT in schizophrenia risk and reveals a novel association. Eur. Psychiatry 27, 372–376. doi: 10.1016/j.eurpsy.2010.08.004
Wahlstrom, D., White, T., Hooper, C. J., Vrshek-Schallhorn, S., Oetting, W. S., Brott, M. J., et al. (2007). Variations in the catechol O-methyltransferase polymorphism and prefrontally guided behaviors in adolescents. Biol. Psychiatry 61, 626–632. doi: 10.1016/j.biopsych.2006.05.045
Wang, S., Li, W., Zhao, J., Zhang, H., Yang, Y., Wang, X., et al. (2013). Association of estrogen receptor alpha gene polymorphism with age at onset, general psychopathology symptoms, and therapeutic effect of schizophrenia. Behav. Brain Funct. 9, 12. doi: 10.1186/1744-9081-9-12
Wei, J., and Hammings, G. (1998). Allelic association between dinucleotide repeats at the monoamine oxidase loci and schizophrenia. Eur. Psychiatry 13, 407–410. doi: 10.1016/S0924-9338(99)80687-7
Wei, J., and Hemmings, G. P. (1999). Lack of evidence for association between the COMT locus and schizophrenia. Psychiatr. Genet. 9, 183–186. doi: 10.1097/00041444-199912000-00003
Wei, J., Ramchand, C. N., Clark, A. E., and Hemmings, G. P. (1996). A study of enzymes involved in catecholamine metabolism in parents of patients with schizophrenia. Schizophr. Res. 19, 27–32. doi: 10.1016/0920-9964(95)00044-5
Wei, Y. L., Li, C. X., Li, S. B., Liu, Y., and Hu, L. (2011). Association study of monoamine oxidase A/B genes and schizophrenia in Han Chinese. Behav. Brain Funct. 7, 42. doi: 10.1186/1744-9081-7-42
Weickert, C. S., Miranda-Angulo, A. L., Wong, J., Perlman, W. R., Ward, S. E., Radhakrishna, V., et al. (2008). Variants in the estrogen receptor alpha gene and its mRNA contribute to risk for schizophrenia. Hum. Mol. Genet. 17, 2293–2309. doi: 10.1093/hmg/ddn130
Weinberger, D. R. (1987). Implications of normal brain development for the pathogenesis of schizophrenia. Arch. Gen. Psychiatry 44, 660–669. doi: 10.1001/archpsyc.1987.01800190080012
Weinberger, D. R. (2002). Schizophrenia, the prefrontal cortex, and a mechanism of genetic susceptibility. Eur Psychiatry 17(Suppl. 4), 355s–362s. doi: 10.1016/S0924-9338(03)00080-4
Weinberger, D. R., Egan, M. F., Bertolino, A., Callicott, J. H., Mattay, V. S., Lipska, B. K., et al. (2001). Prefrontal neurons and the genetics of schizophrenia. Biol. Psychiatry 50, 825–844. doi: 10.1016/S0006-3223(01)01252-5
Westlund, K. N., Denney, R. M., Rose, R. M., and Abell, C. W. (1988). Localization of distinct monoamine oxidase A and monoamine oxidase B cell populations in human brainstem. Neuroscience 25, 439–456. doi: 10.1016/0306-4522(88)90250-3
Williams, H. J., Owen, M. J., and O'Donovan, M. C. (2007). Is COMT a susceptibility gene for schizophrenia? Schizophr. Bull. 33, 635–641. doi: 10.1093/schbul/sbm019
Winterer, G., Egan, M. F., Kolachana, B. S., Goldberg, T. E., Coppola, R., and Weinberger, D. R. (2006). Prefrontal electrophysiologic “noise” and catechol-O-methyltransferase genotype in schizophrenia. Biol. Psychiatry 60, 578–584. doi: 10.1016/j.biopsych.2006.03.023
Winton-Brown, T. T., Fusar-Poli, P., Ungless, M. A., and Howes, O. D. (2014). Dopaminergic basis of salience dysregulation in psychosis. Trends Neurosci. 37, 85–94. doi: 10.1016/j.tins.2013.11.003
Xie, T., Ho, S. L., and Ramsden, D. (1999). Characterization and implications of estrogenic down-regulation of human catechol-O-methyltransferase gene transcription. Mol. Pharmacol. 56, 31–38.
Yasui-Furukori, N., Saito, M., Tsuchimine, S., Nakagami, T., Sato, Y., Sugawara, N., et al. (2008). Association between dopamine-related polymorphisms and plasma concentrations of prolactin during risperidone treatment in schizophrenic patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 32, 1491–1495. doi: 10.1016/j.pnpbp.2008.05.006
Yavich, L., Forsberg, M. M., Karayiorgou, M., Gogos, J. A., and Männistö, P. T. (2007). Site-specific role of catechol-O-methyltransferase in dopamine overflow within prefrontal cortex and dorsal striatum. J. Neurosci. 27, 10196–10209. doi: 10.1523/JNEUROSCI.0665-07.2007
Zai, C. C., Tiwari, A. K., Müller, D. J., De Luca, V., Shinkai, T., Shaikh, S., et al. (2010). The catechol-O-methyl-transferase gene in tardive dyskinesia. World J. Biol. Psychiatry 11, 803–812. doi: 10.3109/15622975.2010.486043
Zhao, Q. Z., Liu, B. C., Zhang, J., Wang, L., Li, X. W., Wang, Y., et al. (2012). Association between a COMT polymorphism and clinical response to risperidone treatment: a pharmacogenetic study. Psychiatr. Genet. 22, 298–299. doi: 10.1097/YPG.0b013e328358629a
Keywords: schizophrenia, dopamine, catecholamine-O-methyltransferase (COMT), monoamine oxidase (MAO), gene-sex interactions, sex hormones
Citation: Godar SC and Bortolato M (2014) Gene-sex interactions in schizophrenia: focus on dopamine neurotransmission. Front. Behav. Neurosci. 8:71. doi: 10.3389/fnbeh.2014.00071
Received: 20 January 2014; Accepted: 19 February 2014;
Published online: 06 March 2014.
Edited by:
Tim Karl, Neuroscience Research Australia, AustraliaReviewed by:
Tertia Purves-Tyson, Neuroscience Research Australia, AustraliaColm O'Tuathaigh, University College Cork, Ireland
Copyright © 2014 Godar and Bortolato. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marco Bortolato, Department of Pharmacology and Toxicology, School of Pharmacy, University of Kansas, 1251 Wescoe Hall Dr, Malott Hall, Room 5040, Lawrence KS 66045, USA e-mail:Ym9ydG9sYXRvQGt1LmVkdQ==
 Sean C. Godar
Sean C. Godar Marco Bortolato
Marco Bortolato