- Neurobiology of Adolescent Drinking in Adulthood Consortium (NADIA), Developmental Exposure Alcohol Research Center (DEARC), Department of Psychology, Binghamton University, Binghamton, NY, United States
Adolescence is a developmental period associated with rapid age-specific physiological, neural, and hormonal changes. Behaviorally, human adolescents are characterized by age-typical increases in novelty-seeking and risk-taking, including the frequent initiation of alcohol and drug use. Alcohol use typically begins during early adolescence, and older adolescents often report high levels of alcohol consumption, commonly referred to as high-intensity drinking. Early-onset and heavy drinking during adolescence are associated with an increased risk of developing alcohol use disorders later in life. Yet, long-term behavioral consequences of adolescent alcohol use that might contribute to excessive drinking in adulthood are still not well understood. Recent animal research, however, using different exposure regimens and routes of ethanol administration, has made substantial progress in identifying the consequences of adolescent ethanol exposure that last into adulthood. Alterations associated with adolescent ethanol exposure include increases in anxiety-like behavior, impulsivity, risk-taking, and ethanol intake, although the observed alterations differ as a function of exposure regimens and routes of ethanol administration. Rodent studies have also shown that adolescent ethanol exposure produces alterations in sensitivity to ethanol, with these alterations reminiscent of adolescent-typical ethanol responsiveness. The goal of this mini-review article is to summarize the current state of animal research, focusing on the long-term consequences related to adolescent ethanol exposure, with a special emphasis on the behavioral alterations and changes to ethanol sensitivity that can foster high levels of drinking in adulthood.
Introduction
Initiation of alcohol use is commonly reported during early (11–15 years of age) adolescence (Faden, 2006; Masten et al., 2009; Morean et al., 2018), and this early initiation is frequently associated with the development of alcohol abuse/dependence later in life (Kuntsche et al., 2016). Several researchers have reported that adolescents who begin drinking at or before the age of 14 are at an elevated risk of becoming alcohol-dependent compared to those who initiate alcohol use at the age of 19 or later (DeWit et al., 2000; Ehlers et al., 2006; Dawson et al., 2008). Likewise, a fast progression from first drink to the first intoxication is a strong predictor and indicator of binge and high-intensity drinking among adolescents (Morean et al., 2014, 2018; Kuntsche et al., 2016; Patrick et al., 2019).
The developing adolescent brain is thought to be particularly vulnerable to alcohol (Olsson et al., 2016; Silveri et al., 2016; Spear, 2018). Thus, early initiation of alcohol use together with high levels of drinking during adolescence (Patrick et al., 2013) can potentially disrupt maturational changes occurring in the brain (Blakemore, 2012; Mills et al., 2014). Therefore, investigations of the consequences of ethanol exposure on the adolescent brain and behavior are critical for understanding the relationship between adolescent drinking and the development of alcohol use disorders later in life.
Rodent models of adolescence allow researchers to determine the consequences of adolescent ethanol exposure that may contribute to the development of alcohol use disorders in adulthood. Behaviorally, consequences of adolescent alcohol exposure include reductions in cognitive flexibility, as well as increases in risk-taking and anxiety, which are associated with multiple neural alterations reviewed in several recent publications (Pascual et al., 2009; Crews et al., 2016, 2019; Spear, 2018). The focus of this mini-review article is on the specific consequences of adolescent alcohol exposure that might foster high levels of ethanol intake later in life and, therefore, become risk factors for the development of alcohol use disorders.
Adolescent Ethanol Exposure: Impact on Ethanol Intake in Adulthood
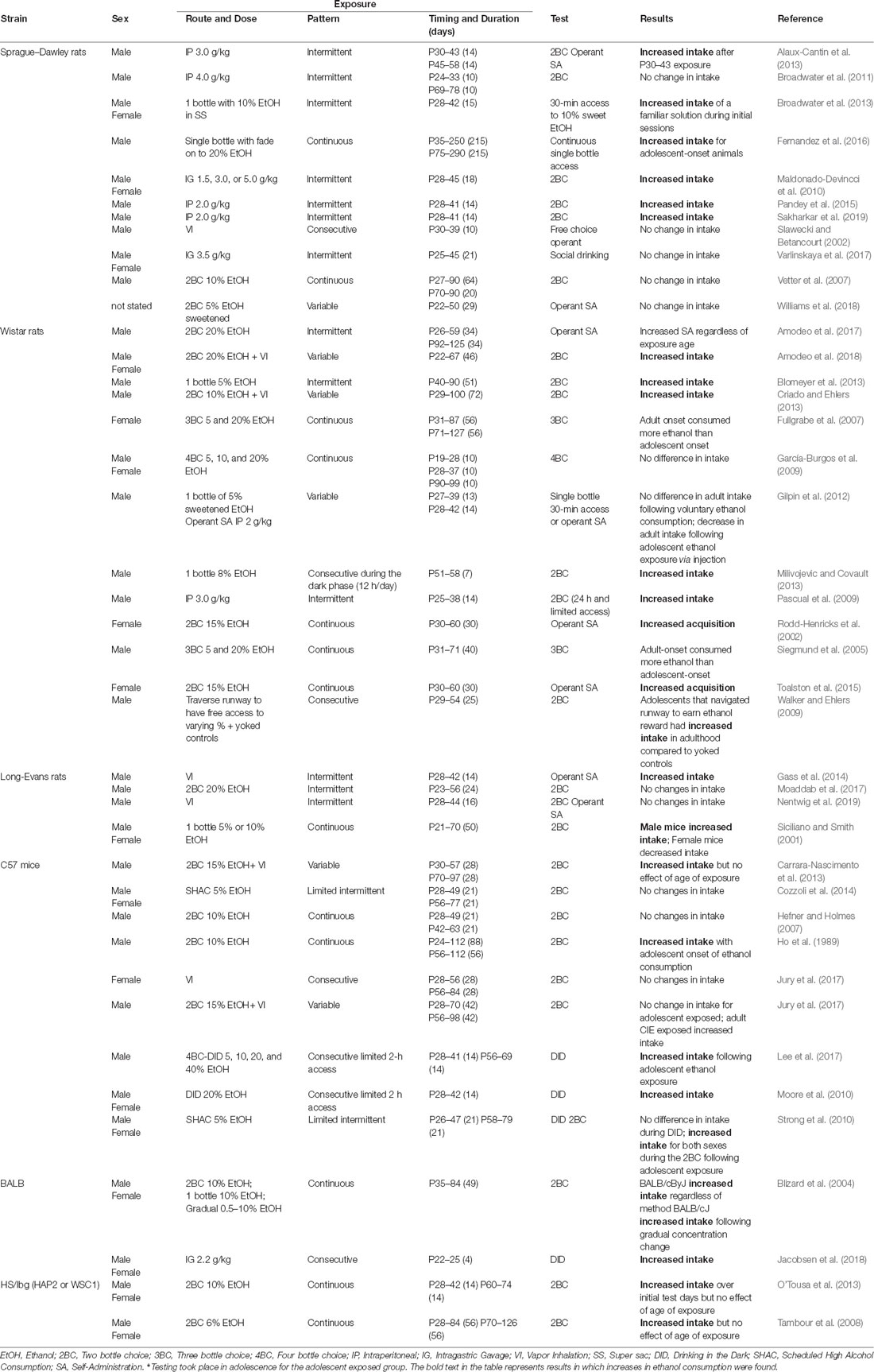
Different animal models of ethanol exposure have been used to determine whether adolescent experience with ethanol influences subsequent intake. Voluntary ethanol consumption in laboratory rodents is commonly assessed using a two-bottle choice (2BC) paradigm, in which animals are given free access to water and ethanol, with this paradigm also used for adolescent ethanol exposure. Intake levels in the 2BC paradigm generally do not produce blood ethanol concentrations (BECs) in the binge range (80 mg/dl and higher), but rather in the low-to-moderate range. The original 2BC paradigm has been modified to increase ethanol consumption, via models such as the drinking in the dark (DID) paradigm (reviewed in Thiele and Navarro, 2014) and scheduled high-alcohol consumption (SHAC) procedure (Finn et al., 2005). These modified voluntary consumption paradigms implement a restricted time of access to ethanol that allows for elevated ethanol intake resulting in higher BECs, similar to those achieved by forced exposure. Indeed, studies focused on ethanol exposures that produce BECs well into the binge range (100–250 mg/dl) use forced ethanol administration via intragastric gavage (IG), intraperitoneal injections (IP), and ethanol vapor inhalation (VI). Findings from studies evaluating the effects of adolescent ethanol exposure on later ethanol intake are presented in Table 1.
More than half of the studies outlined in Table 1 demonstrate that adolescent ethanol exposure results in persistent increases in ethanol intake in adulthood, findings across multiple models of exposure and intake assessment methods. For example, although Blomeyer et al. (2013) exposed male, Wistar rats via a 2BC paradigm during mid-adolescence, and Pandey et al. (2015) administered ethanol IP (2 g/kg) in male, Sprague–Dawley rats, both studies demonstrated higher intakes in ethanol-exposed animals relative to their age-matched controls. Similarly, Gass et al. (2014) reported increased ethanol intake in adulthood following adolescent VI exposure. Some studies have also shown increased intake in adulthood following a combination of voluntary (2BC) and forced (VI) ethanol exposures (Criado and Ehlers, 2013; Amodeo et al., 2018). However, 19 of the studies reviewed found either no effects of adolescent ethanol exposure, more pronounced effects of adult exposure on ethanol intake, or an equivalent response following adolescent and adult exposures (see Table 1).
Several factors potentially contribute to these inconsistent findings, with exposure duration and exposure timing representing two important variables that vary drastically between the different studies. Throughout the reviewed literature, exposure duration ranged from 4 days (Jacobsen et al., 2018) to as long as 8 weeks (Fullgrabe et al., 2007) during adolescence and in some cases well into adulthood (Fernandez et al., 2016). The results of the only study that directly addressed the issue of exposure timing within the adolescent period on ethanol intake later in life demonstrated that early adolescent males (postnatal days 30–43) are more vulnerable to ethanol-exposure-related increases in ethanol intake than their more mature (postnatal days 45–58) adolescent counterparts (Alaux-Cantin et al., 2013).
Only a third of the studies (5 out of 16) that used voluntary ethanol consumption, such as 2BC or similar models of adolescent exposure, demonstrated increased ethanol intake later in life. Very few studies reported BEC data during the voluntary consumption exposure phase, and those that did generally observed low BECs that ranged from 0 to 100 mg/dl with averages around 25–35 mg/dl (Gilpin et al., 2012; Broadwater et al., 2013; Amodeo et al., 2017; however see O’Tousa et al., 2013). However, findings of studies implementing voluntary consumption in which higher BECs (80–200 mg/dl) were achieved, commonly from DID and SHAC procedures, generally supported elevated intake levels in adulthood. Of the studies that report increased ethanol intake in adulthood, forced adolescent exposure appears to more effectively increase ethanol intake than voluntary exposure models. Indeed, more than half (11 out of 19) of the studies that employed forced exposure paradigms reported exposure-related increases in ethanol intake. Together, these findings suggest that to enhance ethanol intake later in life, adolescent exposure models, both voluntary and forced, should produce BECs well into the binge range.
Many studies that reported increases in ethanol intake following adolescent ethanol exposure did not include other age groups for comparison. Of the studies that included adolescents and adults, previous exposure to ethanol tended to increased ethanol intake later in life regardless of exposure timing (Hefner and Holmes, 2007; Tambour et al., 2008; Strong et al., 2010; Carrara-Nascimento et al., 2013; O’Tousa et al., 2013; Amodeo et al., 2017) or following adult exposure only (Fullgrabe et al., 2007; Jury et al., 2017), suggesting that exposure-related increases in ethanol consumption may not be specific to adolescent exposure. Therefore, it is still not clear whether adolescents are more vulnerable than adults to ethanol-exposure-related increases in ethanol intake.
The studies conducted to date indicate that many factors contribute to the effects of adolescent ethanol exposure on ethanol intake later in life, including exposure regiments (continuous vs. intermittent), exposure duration, exposure mode (voluntary vs. forced), exposure levels, BECs achieved, and strain. Given their respective effects and contributions, all these factors should be considered in future studies.
Ethanol Sensitivity Following Adolescent Ethanol Exposure
In general, adolescent laboratory rodents are less responsive than their adult counterparts to adverse effects of ethanol that may curb ethanol intake. These adverse effects of ethanol include social inhibition (Varlinskaya and Spear, 2002), sedation (Moy et al., 1998; Silveri and Spear, 1998; Draski et al., 2001), motor impairment (White et al., 2002; Ramirez and Spear, 2010), and aversion (Vetter-O’Hagen et al., 2009; Anderson et al., 2010; Schramm-Sapyta et al., 2014; Saalfield and Spear, 2015, 2019). In contrast, adolescent rats are uniquely responsive to social facilitation induced by low doses of ethanol (Varlinskaya and Spear, 2002, 2007, 2015; Trezza et al., 2009; Willey et al., 2009), with some evidence also suggesting higher responsiveness to the rewarding effects of ethanol during adolescence than in adulthood (Pautassi et al., 2008). Adolescent ethanol exposure produces alterations in responsiveness to ethanol that resemble these adolescent-typical ethanol sensitivities. This retention of adolescent-typical responding to ethanol has been termed as the “lock-in” effect of adolescent ethanol exposure (reviewed in Spear and Swartzwelder, 2014). The “locking in” of adolescent-typical responding to ethanol effects may play a substantial role in increased ethanol intake in adulthood following adolescent exposure.
Effects of chronic adolescent exposure to ethanol on ethanol-induced sedation indexed via the loss of the righting reflex (LORR) have been assessed in laboratory rodents. For instance, adult male rats exposed to ethanol during adolescence (P30–48, 1, 2, 3 or 4 g/kg ethanol, IP) and challenged with a hypnotic ethanol dose regained their righting reflex more rapidly than did their non-exposed counterparts (Matthews et al., 2008), with these alterations evident only following high exposure doses of ethanol (3 and 4 g/kg). These results were also replicated by the same group (Matthews et al., 2017) and others using mice (Jury et al., 2017). However, similar decreases in LORR duration were evident following adult exposure as well (Jury et al., 2017). These findings suggest that adolescent ethanol exposure results in relative insensitivity to ethanol-induced sedation, although the development of metabolic tolerance to ethanol cannot be ruled out. Indeed, the development of metabolic tolerance has been reported following adolescent ethanol exposure (Silvers et al., 2003). When adolescent and adult male rats were repeatedly exposed to an ethanol dose of 4 g/kg and challenged with the same dose 24 h after the last exposure, adult rats, but not their adolescent counterparts, demonstrated chronic tolerance to the sedative effects of ethanol that appeared to be metabolic, but not functional (Broadwater et al., 2011). Evidence of decreased sensitivity to ethanol-induced sedation associated with adolescent ethanol exposure came from the study of Quoilin et al. (2012): female Swiss mice exposed to ethanol during adolescence regained the righting reflex at higher BECs than controls.
Adult animals exposed to ethanol during adolescence become relatively insensitive to the aversive effects of ethanol assessed via ethanol-induced conditioned taste aversion (CTA). Diaz-Granados and Graham (2007) exposed adolescent male mice to ethanol vapor either continuously or intermittently and found attenuated CTA to ethanol later in life, with intermittent exposure producing greater attenuation and adult exposure not producing similar effects. Saalfield and Spear (2015), assessing the impact of ethanol exposure (4.0 g/kg, IG) during early (P25–45) and late (P45–65) adolescence on ethanol-induced CTA in male rats, found that both adolescent exposures resulted in decreased sensitivity to the aversive effects of ethanol. Alaux-Cantin et al. (2013) also found that male rats exposed to ethanol during early adolescence (3 g/kg, IP, P30–43) demonstrated attenuated ethanol-induced CTA in adulthood. The reductions in sensitivity to ethanol CTA following adolescent ethanol exposure appear to be sex-specific, with only male Long-Evans rats, but not females, demonstrating an attenuated CTA in adulthood following adolescent exposure (Sherrill et al., 2011).
Adolescent ethanol exposure (P25–45, IG, 4 g/kg) of Sprague–Dawley male rats resulted in precipitation of adolescent-typical responding to acute ethanol challenge with social facilitation (i.e., ethanol-induced increases in peer-directed social behavior) when these males were tested in adulthood (Varlinskaya et al., 2014). Enhanced sensitivity to ethanol reinforcement indexed via a significant leftward shift in the dose-response curve for ethanol self-administration into the posterior ventral tegmental area following adolescent ethanol exposure (4 g/kg IG, P28–48) was also evident in adult male and female Wistar rats, as well as in alcohol-preferring (P) male rats (Hauser et al., 2019). Carrara-Nascimento et al. (2014) showed that adult male Swiss mice exposed to ethanol during adolescence displayed a robust CPP to 2.0 g/kg ethanol, whereas adult exposure decreased sensitivity to the reinforcing properties of ethanol. Similarly, BALB/c adult mice demonstrated enhanced sensitivity to ethanol-induced CPP following only four exposures to ethanol given during the juvenile period on P22–25 (Jacobsen et al., 2018).
Taken together, the experimental findings demonstrate that exposure to ethanol during adolescence changes sensitivity to many ethanol effects later in life, decreasing sensitivity to adverse effects of ethanol and making adult laboratory rodents more sensitive to stimulatory and rewarding properties of ethanol. This pattern of sensitivity to the adverse and desired ethanol effects, reminiscent of that typically shown by adolescent rodents, may allow adult animals to ingest higher amounts of ethanol without experiencing negative consequences.
Adolescent Alcohol Exposure: Anxiety-Like Behavioral Alterations
Adolescents and young adults who engage in problematic drinking often drink for enhancement of positive emotional states or alleviation of negative affective states (Ham and Hope, 2003; Kuntsche et al., 2006). The association between negative reinforcement and alcohol use has been shown to become stronger in individuals with alcohol use disorder, with no changes evident in the association between positive reinforcement and alcohol consumption (Cho et al., 2019). Indeed, available research suggests relatively strong associations between adolescent alcohol use and increased prevalence of anxiety and depression disorders in adulthood (Rohde et al., 2001; Jeanblanc, 2015). In older adults, alcohol use disorder is frequently comorbid with depression and anxiety (Vorspan et al., 2015; Wiener et al., 2018). Therefore, the assessment of affective behavioral alterations in animal models of adolescent alcohol exposure seems utterly important.
Increases in anxiety-like behavior have been reported using different models of adolescent ethanol exposure. For instance, adult male Sprague–Dawley rats exposed to 2 g/kg ethanol (Kokare et al., 2017; Kyzar et al., 2017, 2019; Sakharkar et al., 2019) or 4 g/kg ethanol (Van Skike et al., 2015) given IP during adolescence, as well as male Long-Evans rats exposed IG to a 1.5 g/kg ethanol dose (Loxton and Canales, 2017), demonstrated elevated levels of anxiety-like behavior when tested on the elevated plus-maze (EPM). Our recent findings indicated that IG ethanol exposure of Sprague–Dawley males and females during early/mid-adolescence (P25–45) results in enhanced anxiety-like behavior on the EPM in adulthood regardless of sex (Varlinskaya et al., 2019). However, only males demonstrated enhanced anxiety-like behavior on the EPM following late-adolescent/emerging-adulthood exposure (P45–65), suggesting that the effects of ethanol exposure are sex- and exposure-timing dependent.
However, Torcaso et al. (2017), exposing male Wistar rats IG to ethanol (3 g/kg, P37–44) reported no behavioral changes on the EPM, whereas other researchers have demonstrated that adolescent ethanol exposure resulted in decreases of anxiety-like behavior on the EPM (Gilpin et al., 2012; Gass et al., 2014). For example, Long-Evans male rats were intermittently exposed to ethanol via VI during early-mid adolescence (P28–P42), and this exposure regimen resulted in decreased anxiety, as indexed by increased open arm behavior evident in adult rats (Gass et al., 2014). Similarly, adolescent (P28–P42) ethanol exposure of male Wistar rats via self-administration increased percent open arm time on the EPM when these males were tested in adulthood (Gilpin et al., 2012). These inconsistent results are likely associated with procedural differences such as rat strain, route of ethanol administration, phase of light/dark cycle during testing, and pre-test manipulations (see Hogg, 1996; Carobrez and Bertoglio, 2005). For example, the two studies that reported decreased anxiety-like behavior on the EPM tested animals in low-light conditions during the dark part of the light/dark cycle, conditions that may reduce anxiety-like behavior (Gilpin et al., 2012; Gass et al., 2014). It is possible that the observed increases in open arm entries and/or open arm time reflect disinhibition but not decreases in anxiety-like behavior, since the characteristics of the test situation determine whether anxiety or disinhibition are manifested in the EPM (Ennaceur, 2014).
Anxiety-like alterations associated with adolescent ethanol exposure were reported for other tests of anxiety as well. Experiments using the light/dark box have shown that adolescent ethanol exposure increases time spent in the dark portion of the apparatus and decreases entries into the light side in male Sprague–Dawley rats (Pandey et al., 2015; Vetreno et al., 2016; Sakharkar et al., 2019). Anxiety-like behavior in the open-field (Coleman et al., 2014; Yan et al., 2015) and marble-burying test (Lee et al., 2017) have also been enhanced following adolescent ethanol exposure of Sprague–Dawley males (Yan et al., 2015) and male C57BL/6J mice (Coleman et al., 2014; Lee et al., 2017). When tested in adulthood, male Sprague–Dawley rats exposed to ethanol during early-mid adolescence demonstrated social anxiety-like behavioral alterations indexed via decreases in social investigation and social preference (Varlinskaya et al., 2014), with no changes in social behavior evident following late adolescent ethanol exposure.
Increased anxiety-like behavior following adolescent ethanol exposure may also contribute to increases in ethanol intake due to the anxiolytic properties of ethanol. Although links between anxiety and alcohol consumption have been commonly reported in humans (Vorspan et al., 2015) and laboratory rodents (Pelloux et al., 2015), it remains to be investigated whether animals that demonstrate increases in anxiety following adolescent ethanol exposure drink more ethanol for its negatively reinforcing, anxiolytic effects.
Conclusions
Although adolescent alcohol exposure is associated with behavioral alterations and changes in ethanol sensitivity, it is still not clear whether these alterations contribute to increases in ethanol intake. Considering that very few studies assessing changes in ethanol intake following adolescent ethanol exposure included both sexes, the question of whether responding to ethanol exposure during adolescence differs in males and females remains unanswered. Furthermore, among the studies that assessed changes in ethanol intake, only a limited number included both adolescent and adult ethanol exposure conditions (see Table 1), producing mixed results and not allowing to conclude that enhanced ethanol intake in adulthood is specific to adolescent ethanol exposure. The impact of ethanol exposure timing within the adolescent period (i.e., during early vs. late adolescence) on ethanol intake and sensitivity is still not well understood, and this important issue should also be addressed in future studies (Spear, 2015). The relative insensitivity of adolescents to the acute effects of ethanol is related in part to age differences in compensatory responses, including acute tolerance, that serve to counteract ethanol-induced impairment. Therefore, it is important to investigate whether adolescent ethanol exposure decreases sensitivity to the adverse effects of acute ethanol by enhancing the development of acute tolerance. More studies are needed for a better understanding of the consequences of alcohol exposure during adolescence that might contribute to heavy drinking later in life and put individuals at risk for the development of alcohol use disorders.
Author Contributions
TT and EV contributed equally.
Funding
The work presented in this manuscript was funded by grants U01 AA019972 (Neurobiology of Adolescent Drinking in Adulthood Consortium—NADIA Project), P50 AA017823 (Developmental Exposure Alcohol Research Center), and T32 AA025606 (Development and Neuroadaptation in Alcohol and Addictions—DNAA Project).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alaux-Cantin, S., Warnault, V., Legastelois, R., Botia, B., Pierrefiche, O., Vilpoux, C., et al. (2013). Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology 67, 521–531. doi: 10.1016/j.neuropharm.2012.12.007
Amodeo, L. R., Kneiber, D., Wills, D. N., and Ehlers, C. L. (2017). Alcohol drinking during adolescence increases consumptive responses to alcohol in adulthood in Wistar rats. Alcohol 59, 43–51. doi: 10.1016/j.alcohol.2016.12.002
Amodeo, L. R., Wills, D. N., Sanchez-Alavez, M., Nguyen, W., Conti, B., and Ehlers, C. L. (2018). Intermittent voluntary ethanol consumption combined with ethanol vapor exposure during adolescence increases drinking and alters other behaviors in adulthood in female and male rats. Alcohol 73, 57–66. doi: 10.1016/j.alcohol.2018.04.003
Anderson, R. I., Varlinskaya, E. I., and Spear, L. P. (2010). Ethanol-induced conditioned taste aversion in male Sprague-Dawley rats: impact of age and stress. Alcohol. Clin. Exp. Res. 34, 2106–2115. doi: 10.1111/j.1530-0277.2010.01307.x
Blakemore, S. J. (2012). Development of the social brain in adolescence. J. R. Soc. Med. 105, 111–116. doi: 10.1258/jrsm.2011.110221
Blizard, D. A., Vandenbergh, D. J., Jefferson, A. L., Chatlos, C. D., Vogler, G. P., and McClearn, G. E. (2004). Effects of periadolescent ethanol exposure on alcohol preference in two BALB substrains. Alcohol 34, 177–185. doi: 10.1016/j.alcohol.2004.08.007
Blomeyer, D., Friemel, C. M., Buchmann, A. F., Banaschewski, T., Laucht, M., and Schneider, M. (2013). Impact of pubertal stage at first drink on adult drinking behavior. Alcohol. Clin. Exp. Res. 37, 1804–1811. doi: 10.1111/acer.12154
Broadwater, M., Varlinskaya, E. I., and Spear, L. P. (2011). Chronic intermittent ethanol exposure in early adolescent and adult male rats: effects on tolerance, social behavior, and ethanol intake. Alcohol. Clin. Exp. Res. 35, 1392–1403. doi: 10.1111/j.1530-0277.2011.01474.x
Broadwater, M., Varlinskaya, E. I., and Spear, L. P. (2013). Effects of voluntary access to sweetened ethanol during adolescence on intake in adulthood. Alcohol. Clin. Exp. Res. 37, 1048–1055. doi: 10.1111/acer.12049
Carobrez, A. P., and Bertoglio, L. J. (2005). Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci. Biobehav. Rev. 29, 1193–1205. doi: 10.1016/j.neubiorev.2005.04.017
Carrara-Nascimento, P. F., Lopez, M. F., Becker, H. C., Olive, M. F., and Camarini, R. (2013). Similar ethanol drinking in adolescent and adult C57BL/6J mice after chronic ethanol exposure and withdrawal. Alcohol. Clin. Exp. Res. 37, 961–968. doi: 10.1111/acer.12056
Carrara-Nascimento, P. F., Olive, M. F., and Camarini, R. (2014). Ethanol pre-exposure during adolescence or adulthood increases ethanol intake but ethanol-induced conditioned place preference is enhanced only when pre-exposure occurs in adolescence. Dev. Psychobiol. 56, 36–48. doi: 10.1002/dev.21089
Cho, S. B., Su, J., Kuo, S. I., Bucholz, K. K., Chan, G., Edenberg, H. J., et al. (2019). Positive and negative reinforcement are differentially associated with alcohol consumption as a function of alcohol dependence. Psychol. Addict. Behav. 33, 58–68. doi: 10.1037/adb0000436
Coleman, L. G. Jr., Liu, W., Oguz, I., Styner, M., and Crews, F. T. (2014). Adolescent binge ethanol treatment alters adult brain regional volumes, cortical extracellular matrix protein and behavioral flexibility. Pharmacol. Biochem. Behav. 116, 142–151. doi: 10.1016/j.pbb.2013.11.021
Cozzoli, D. K., Strong-Kaufman, M. N., Tanchuck, M. A., Hashimoto, J. G., Wiren, K. M., and Finn, D. A. (2014). The effect of mGluR5 antagonism during binge drinking on subsequent ethanol intake in C57BL/6J mice: sex- and age-induced differences. Alcohol. Clin. Exp. Res. 38, 730–738. doi: 10.1111/acer.12292
Crews, F. T., Robinson, D. L., Chandler, L. J., Ehlers, C. L., Mulholland, P. J., Pandey, S. C., et al. (2019). Mechanisms of persistent neurobiological changes following adolescent alcohol exposure: NADIA consortium findings. Alcohol. Clin. Exp. Res. 43, 1806–1822. doi: 10.1111/acer.14154
Crews, F. T., Vetreno, R. P., Broadwater, M. A., and Robinson, D. L. (2016). Adolescent alcohol exposure persistently impacts adult neurobiology and behavior. Pharmacol. Rev. 68, 1074–1109. doi: 10.1124/pr.115.012138
Criado, J. R., and Ehlers, C. L. (2013). Effects of adolescent onset voluntary drinking followed by ethanol vapor exposure on subsequent ethanol consumption during protracted withdrawal in adult Wistar rats. Pharmacol. Biochem. Behav. 103, 622–630. doi: 10.1016/j.pbb.2012.10.016
Dawson, D. A., Goldstein, R. B., Chou, S. P., Ruan, W. J., and Grant, B. F. (2008). Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcohol. Clin. Exp. Res. 32, 2149–2160. doi: 10.1111/j.1530-0277.2008.00806.x
DeWit, D. J., Adlaf, E. M., Offord, D. R., and Ogborne, A. C. (2000). Age at first alcohol use: a risk factor for the development of alcohol disorders. Am. J. Psychiatry 157, 745–750. doi: 10.1176/appi.ajp.157.5.745
Diaz-Granados, J. L., and Graham, D. L. (2007). The effects of continuous and intermittent ethanol exposure in adolesence on the aversive properties of ethanol during adulthood. Alcohol. Clin. Exp. Res. 31, 2020–2027. doi: 10.1111/j.1530-0277.2007.00534.x
Draski, L. J., Bice, P. J., and Deitrich, R. A. (2001). Developmental alterations of ethanol sensitivity in selectively bred high and low alcohol sensitive rats. Pharmacol. Biochem. Behav. 70, 387–396. doi: 10.1016/s0091-3057(01)00621-9
Ehlers, C. L., Slutske, W. S., Gilder, D. A., Lau, P., and Wilhelmsen, K. C. (2006). Age at first intoxication and alcohol use disorders in Southwest California Indians. Alcohol. Clin. Exp. Res. 30, 1856–1865. doi: 10.1111/j.1530-0277.2006.00222.x
Ennaceur, A. (2014). Tests of unconditioned anxiety—pitfalls and disappointments. Physiol. Behav. 135, 55–71. doi: 10.1016/j.physbeh.2014.05.032
Faden, V. B. (2006). Trends in initiation of alcohol use in the United States 1975 to 2003. Alcohol. Clin. Exp. Res. 30, 1011–1022. doi: 10.1111/j.1530-0277.2006.00115.x
Fernandez, G. M., Stewart, W. N., and Savage, L. M. (2016). Chronic drinking during adolescence predisposes the adult rat for continued heavy drinking: Neurotrophin and behavioral adaptation after long-term, continuous ethanol exposure. PLoS One 11:e0149987. doi: 10.1371/journal.pone.0149987
Finn, D. A., Belknap, J. K., Cronise, K., Yoneyama, N., Murillo, A., and Crabbe, J. C. (2005). A procedure to produce high alcohol intake in mice. Psychopharmacology 178, 471–480. doi: 10.1007/s00213-004-2039-8
Fullgrabe, M. W., Vengeliene, V., and Spanagel, R. (2007). Influence of age at drinking onset on the alcohol deprivation effect and stress-induced drinking in female rats. Pharmacol. Biochem. Behav. 86, 320–326. doi: 10.1016/j.pbb.2006.10.004
García-Burgos, D., González, F., Manrique, T., and Gallo, M. (2009). Patterns of ethanol intake in preadolescent, adolescent, and adult Wistar rats under acquisition, maintenance, and relapse-like conditions. Alcohol. Clin. Exp. Res. 33, 722–728. doi: 10.1111/j.1530-0277.2008.00889.x
Gass, J. T., Glen, W. B. Jr., McGonigal, J. T., Trantham-Davidson, H., Lopez, M. F., Randall, P. K., et al. (2014). Adolescent alcohol exposure reduces behavioral flexibility, promotes disinhibition, and increases resistance to extinction of ethanol self-administration in adulthood. Neuropsychopharmacology 39, 2570–2583. doi: 10.1038/npp.2014.109
Gilpin, N. W., Karanikas, C. A., and Richardson, H. N. (2012). Adolescent binge drinking leads to changes in alcohol drinking, anxiety, and amygdalar corticotropin releasing factor cells in adulthood in male rats. PLoS One 7:e31466. doi: 10.1371/journal.pone.0031466
Ham, L. S., and Hope, D. A. (2003). College students and problematic drinking: a review of the literature. Clin. Psychol. Rev. 23, 719–759. doi: 10.1016/s0272-7358(03)00071-0
Hauser, S. R., Knight, C. P., Truitt, W. A., Waeiss, R. A., Holt, I. S., Carvajal, G. B., et al. (2019). Adolescent intermittent ethanol increases the sensitivity to the reinforcing properties of ethanol and the expression of select cholinergic and dopaminergic genes within the posterior ventral tegmental area. Alcohol. Clin. Exp. Res. 43, 1937–1948. doi: 10.1111/acer.14150
Hefner, K., and Holmes, A. (2007). An investigation of the behavioral actions of ethanol across adolescence in mice. Psychopharmacology 191, 311–322. doi: 10.1007/s00213-006-0646-2
Ho, A., Chin, A. J., and Dole, V. P. (1989). Early experience and the consumption of alcohol by adult C57BL/6J mice. Alcohol 6, 511–515. doi: 10.1016/0741-8329(89)90060-8
Hogg, S. (1996). A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol. Biochem. Behav. 54, 21–30. doi: 10.1016/0091-3057(95)02126-4
Jacobsen, J. H. W., Buisman-Pijlman, F. T., Mustafa, S., Rice, K. C., and Hutchinson, M. R. (2018). Antagonising TLR4-TRIF signalling before or after a low-dose alcohol binge during adolescence prevents alcohol drinking but not seeking behaviour in adulthood. Neuropharmacology 128, 460–473. doi: 10.1016/j.neuropharm.2017.09.028
Jeanblanc, J. M. (2015). Comorbidity between psychiatric diseases and alcohol use disorders: impact of adolescent alcohol consumption. Curr. Addict. Rep. 2, 239–301. doi: 10.1007/s40429-015-0076-5
Jury, N. J., Pollack, G. A., Ward, M. J., Bezek, J. L., Ng, A. J., Pinard, C. R., et al. (2017). Chronic ethanol during adolescence impacts corticolimbic dendritic spines and behavior. Alcohol. Clin. Exp. Res. 41, 1298–1308. doi: 10.1111/acer.13422
Kokare, D. M., Kyzar, E. J., Zhang, H., Sakharkar, A. J., and Pandey, S. C. (2017). Adolescent alcohol exposure-induced changes in α-melanocyte stimulating hormone and neuropeptide Y pathways via histone acetylation in the brain during adulthood. Int. J. Neuropsychopharmacol. 20, 758–768. doi: 10.1093/ijnp/pyx041
Kuntsche, E., Knibbe, R., Gmel, G., and Engels, R. (2006). Who drinks and why? A review of socio-demographic, personality, and contextual issues behind the drinking motives in young people. Addict. Behav. 31, 1844–1857. doi: 10.1016/j.addbeh.2005.12.028
Kuntsche, E., Rossow, I., Engels, R., and Kuntsche, S. (2016). Is ‘age at first drink’ a useful concept in alcohol research and prevention? We doubt that. Addiction 111, 957–965. doi: 10.1111/add.12980
Kyzar, E. J., Zhang, H., and Pandey, S. C. (2019). Adolescent alcohol exposure epigenetically suppresses amygdala arc enhancer RNA expression to confer adult anxiety susceptibility. Biol. Psychiatry 85, 904–914. doi: 10.1016/j.biopsych.2018.12.021
Kyzar, E. J., Zhang, H., Sakharkar, A. J., and Pandey, S. C. (2017). Adolescent alcohol exposure alters lysine demethylase 1 (LSD1) expression and histone methylation in the amygdala during adulthood. Addict. Biol. 22, 1191–1204. doi: 10.1111/adb.12404
Lee, K. M., Coehlo, M. A., Solton, N. R., and Szumlinski, K. K. (2017). Negative affect and excessive alcohol intake incubate during protracted withdrawal from binge-drinking in adolescent, but not adult, mice. Front. Psychol. 8:1128. doi: 10.3389/fpsyg.2017.01128
Loxton, D., and Canales, J. J. (2017). Long-term cognitive, emotional and neurogenic alterations induced by alcohol and methamphetamine exposure in adolescent rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 74, 1–8. doi: 10.1016/j.pnpbp.2016.11.003
Maldonado-Devincci, A. M., Alipour, K. K., Michael, L. A., and Kirstein, C. L. (2010). Repeated binge ethanol administration during adolescence enhances voluntary sweetened ethanol intake in young adulthood in male and female rats. Pharmacol. Biochem. Behav. 96, 476–487. doi: 10.1016/j.pbb.2010.07.008
Masten, A. S., Faden, V. B., Zucker, R. A., and Spear, L. P. (2009). A developmental perspective on underage alcohol use. Alcohol Res. Health 32, 3–15.
Matthews, D. B., Novier, A., Diaz-Granados, J. L., Van Skike, C. E., Ornelas, L., and Mittleman, G. (2017). Impact of adolescent alcohol use across the lifespan: long-lasting tolerance to high-dose alcohol coupled with potentiated spatial memory impairments to moderate-dose alcohol. Alcohol 61, 33–42. doi: 10.1016/j.alcohol.2017.01.012
Matthews, D. B., Tinsley, K. L., Diaz-Granados, J. L., Tokunaga, S., and Silvers, J. M. (2008). Chronic intermittent exposure to ethanol during adolescence produces tolerance to the hypnotic effects of ethanol in male rats: a dose-dependent analysis. Alcohol 42, 617–621. doi: 10.1016/j.alcohol.2008.09.001
Milivojevic, V., and Covault, J. (2013). Alcohol exposure during late adolescence increases drinking in adult Wistar rats, an effect that is not reduced by finasteride. Alcohol Alcohol. 48, 28–38. doi: 10.1093/alcalc/ags105
Mills, K. L., Lalonde, F., Clasen, L. S., Giedd, J. N., and Blakemore, S. J. (2014). Developmental changes in the structure of the social brain in late childhood and adolescence. Soc. Cogn. Affect. Neurosci. 9, 123–131. doi: 10.1093/scan/nss113
Moaddab, M., Mangone, E., Ray, M. H., and McDannald, M. A. (2017). Adolescent alcohol drinking renders adult drinking BLA-dependent: BLA hyper-activity as contributor to comorbid alcohol use disorder and anxiety disorders. Brain Sci. 7:E151. doi: 10.3390/brainsci7110151
Moore, E. M., Mariani, J. N., Linsenbardt, D. N., Melon, L. C., and Boehm, S. L. II. (2010). Adolescent C57BL/6J (but not DBA/2J) mice consume greater amounts of limited-access ethanol compared to adults and display continued elevated ethanol intake into adulthood. Alcohol. Clin. Exp. Res. 34, 734–742. doi: 10.1111/j.1530-0277.2009.01143.x
Morean, M. E., Kong, G., Camenga, D. R., Cavallo, D. A., Connell, C., and Krishnan-Sarin, S. (2014). First drink to first drunk: age of onset and delay to intoxication are associated with adolescent alcohol use and binge drinking. Alcohol. Clin. Exp. Res. 38, 2615–2621. doi: 10.1111/acer.12526
Morean, M. E., L’Insalata, A., Butler, E. R., McKee, A., and Krishnan-Sarin, S. (2018). Age at drinking onset, age at first intoxication and delay to first intoxication: assessing the concurrent validity of measures of drinking initiation with alcohol use and related problems. Addict. Behav. 79, 195–200. doi: 10.1016/j.addbeh.2017.12.017
Moy, S. S., Duncan, G. E., Knapp, D. J., and Breese, G. R. (1998). Sensitivity to ethanol across development in rats: comparison to [3H]zolpidem binding. Alcohol. Clin. Exp. Res. 22, 1485–1492. doi: 10.1097/00000374-199810000-00018
Nentwig, T. B., Starr, E. M., Chandler, L. J., and Glover, E. J. (2019). Absence of compulsive drinking phenotype in adult male rats exposed to ethanol in a binge-like pattern during adolescence. Alcohol 79, 93–103. doi: 10.1016/j.alcohol.2019.01.006
Olsson, C. A., Romaniuk, H., Salinger, J., Staiger, P. K., Bonomo, Y., Hulbert, C., et al. (2016). Drinking patterns of adolescents who develop alcohol use disorders: results from the victorian adolescent health cohort study. BMJ Open 6:e010455. doi: 10.1136/bmjopen-2015-010455
O’Tousa, D. S., Matson, L. M., and Grahame, N. J. (2013). Effects of intoxicating free-choice alcohol consumption during adolescence on drinking and impulsivity during adulthood in selectively bred high-alcohol preferring mice. Alcohol. Clin. Exp. Res. 37, 141–149. doi: 10.1111/j.1530-0277.2012.01857.x
Pandey, S. C., Sakharkar, A. J., Tang, L., and Zhang, H. (2015). Potential role of adolescent alcohol exposure-induced amygdaloid histone modifications in anxiety and alcohol intake during adulthood. Neurobiol. Dis. 82, 607–619. doi: 10.1016/j.nbd.2015.03.019
Pascual, M., Boix, J., Felipo, V., and Guerri, C. (2009). Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J. Neurochem. 108, 920–931. doi: 10.1111/j.1471-4159.2008.05835.x
Patrick, M. E., Evans-Polce, R., and Terry-McElrath, Y. M. (2019). Faster escalation from first drink to first intoxication as a risk factor for binge and high-intensity drinking among adolescents. Addict. Behav. 92, 199–202. doi: 10.1016/j.addbeh.2019.01.003
Patrick, M. E., Schulenberg, J. E., Martz, M. E., Maggs, J. L., O’Malley, P. M., and Johnston, L. D. (2013). Extreme binge drinking among 12th-grade students in the United States: prevalence and predictors. JAMA Pediatr. 167, 1019–1025. doi: 10.1001/jamapediatrics.2013.2392
Pautassi, R. M., Myers, M., Spear, L. P., Molina, J. C., and Spear, N. E. (2008). Adolescent but not adult rats exhibit ethanol-mediated appetitive second-order conditioning. Alcohol. Clin. Exp. Res. 32, 2016–2027. doi: 10.1111/j.1530-0277.2008.00789.x
Pelloux, Y., Costentin, J., and Duterte-Boucher, D. (2015). Differential involvement of anxiety and novelty preference levels on oral ethanol consumption in rats. Psychopharmacology 232, 2711–2721. doi: 10.1007/s00213-015-3910-5
Quoilin, C., Didone, V., Tirelli, E., and Quertemont, E. (2012). Chronic ethanol exposure during adolescence alters the behavioral responsiveness to ethanol in adult mice. Behav. Brain Res. 229, 1–9. doi: 10.1016/j.bbr.2011.12.039
Ramirez, R. L., and Spear, L. P. (2010). Ontogeny of ethanol-induced motor impairment following acute ethanol: assessment via the negative geotaxis reflex in adolescent and adult rats. Pharmacol. Biochem. Behav. 95, 242–248. doi: 10.1016/j.pbb.2010.01.013
Rodd-Henricks, Z. A., Bell, R. L., Kuc, K. A., Murphy, J. M., McBride, W. J., Lumeng, L., et al. (2002). Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: I. Periadolescent exposure. Alcohol. Clin. Exp. Res. 26, 1632–1641. doi: 10.1111/j.1530-0277.2002.tb02465.x
Rohde, P., Lewinsohn, P. M., Kahler, C. W., Seeley, J. R., and Brown, R. A. (2001). Natural course of alcohol use disorders from adolescence to young adulthood. J. Am. Acad. Child Adolesc. Psychiatry 40, 83–90. doi: 10.1097/00004583-200101000-00020
Saalfield, J., and Spear, L. (2015). Consequences of repeated ethanol exposure during early or late adolescence on conditioned taste aversions in rats. Dev. Cogn. Neurosci. 16, 174–182. doi: 10.1016/j.dcn.2015.01.004
Saalfield, J., and Spear, L. (2019). Fos activation patterns related to acute ethanol and conditioned taste aversion in adolescent and adult rats. Alcohol 78, 57–68. doi: 10.1016/j.alcohol.2019.02.004
Sakharkar, A. J., Kyzar, E. J., Gavin, D. P., Zhang, H., Chen, Y., Krishnan, H. R., et al. (2019). Altered amygdala DNA methylation mechanisms after adolescent alcohol exposure contribute to adult anxiety and alcohol drinking. Neuropharmacology 157:107679. doi: 10.1016/j.neuropharm.2019.107679
Schramm-Sapyta, N. L., Francis, R., MacDonald, A., Keistler, C., O’Neill, L., and Kuhn, C. M. (2014). Effect of sex on ethanol consumption and conditioned taste aversion in adolescent and adult rats. Psychopharmacology 231, 1831–1839. doi: 10.1007/s00213-013-3319-y
Sherrill, L. K., Berthold, C., Koss, W. A., Juraska, J. M., and Gulley, J. M. (2011). Sex differences in the effects of ethanol pre-exposure during adolescence on ethanol-induced conditioned taste aversion in adult rats. Behav. Brain Res. 225, 104–109. doi: 10.1016/j.bbr.2011.07.003
Siciliano, D., and Smith, R. F. (2001). Periadolescent alcohol alters adult behavioral characteristics in the rat. Physiol. Behav. 74, 637–643. doi: 10.1016/s0031-9384(01)00623-0
Siegmund, S., Vengeliene, V., Singer, M. V., and Spanagel, R. (2005). Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcohol. Clin. Exp. Res. 29, 1139–1145. doi: 10.1097/01.alc.0000171928.40418.46
Silveri, M. M., Dager, A. D., Cohen-Gilbert, J. E., and Sneider, J. T. (2016). Neurobiological signatures associated with alcohol and drug use in the human adolescent brain. Neurosci. Biobehav. Rev. 70, 244–259. doi: 10.1016/j.neubiorev.2016.06.042
Silveri, M. M., and Spear, L. P. (1998). Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol. Clin. Exp. Res. 22, 670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x
Silvers, J. M., Tokunaga, S., Mittleman, G., and Matthews, D. B. (2003). Chronic intermittent injections of high-dose ethanol during adolescence produce metabolic, hypnotic, and cognitive tolerance in rats. Alcohol. Clin. Exp. Res. 27, 1606–1612. doi: 10.1097/01.alc.0000090141.66526.22
Slawecki, C. J., and Betancourt, M. (2002). Effects of adolescent ethanol exposure on ethanol consumption in adult rats. Alcohol 26, 23–30. doi: 10.1016/s0741-8329(01)00192-6
Spear, L. P. (2015). Adolescent alcohol exposure: are there separable vulnerable periods within adolescence? Physiol. Behav. 148, 122–130. doi: 10.1016/j.physbeh.2015.01.027
Spear, L. P. (2018). Effects of adolescent alcohol consumption on the brain and behaviour. Nat. Rev. Neurosci. 19, 197–214. doi: 10.1038/nrn.2018.10
Spear, L. P., and Swartzwelder, H. S. (2014). Adolescent alcohol exposure and persistence of adolescent-typical phenotypes into adulthood: a mini-review. Neurosci. Biobehav. Rev. 45, 1–8. doi: 10.1016/j.neubiorev.2014.04.012
Strong, M. N., Yoneyama, N., Fretwell, A. M., Snelling, C., Tanchuck, M. A., and Finn, D. A. (2010). “Binge” drinking experience in adolescent mice shows sex differences and elevated ethanol intake in adulthood. Horm. Behav. 58, 82–90. doi: 10.1016/j.yhbeh.2009.10.008
Tambour, S., Brown, L. L., and Crabbe, J. C. (2008). Gender and age at drinking onset affect voluntary alcohol consumption but neither the alcohol deprivation effect nor the response to stress in mice. Alcohol. Clin. Exp. Res. 32, 2100–2106. doi: 10.1111/j.1530-0277.2008.00798.x
Thiele, T. E., and Navarro, M. (2014). “Drinking in the dark” (DID) procedures: a model of binge-like ethanol drinking in non-dependent mice. Alcohol 48, 235–241. doi: 10.1016/j.alcohol.2013.08.005
Toalston, J. E., Deehan, G. A. Jr., Hauser, S. R., Engleman, E. A., Bell, R. L., Murphy, J. M., et al. (2015). The reinforcing properties of ethanol are quantitatively enhanced in adulthood by peri-adolescent ethanol, but not saccharin, consumption in female alcohol-preferring (P) rats. Alcohol 49, 513–518. doi: 10.1016/j.alcohol.2015.04.007
Torcaso, A., Asimes, A., Meagher, M., and Pak, T. R. (2017). Adolescent binge alcohol exposure increases risk assessment behaviors in male Wistar rats after exposure to an acute psychological stressor in adulthood. Psychoneuroendocrinology 76, 154–161. doi: 10.1016/j.psyneuen.2016.11.032
Trezza, V., Baarendse, P. J., and Vanderschuren, L. J. (2009). Prosocial effects of nicotine and ethanol in adolescent rats through partially dissociable neurobehavioral mechanisms. Neuropsychopharmacology 34, 2560–2573. doi: 10.1038/npp.2009.85
Van Skike, C. E., Diaz-Granados, J. L., and Matthews, D. B. (2015). Chronic intermittent ethanol exposure produces persistent anxiety in adolescent and adult rats. Alcohol. Clin. Exp. Res. 39, 262–271. doi: 10.1111/acer.12617
Varlinskaya, E. I., Hosová, D., Towner, T., Werner, D. F., and Spear, L. P. (2019). Effects of chronic intermittent ethanol exposure during early and late adolescence on anxiety-like behaviors and behavioral flexibility in adulthood. Behav. Brain Res. 378:112292. doi: 10.1016/j.bbr.2019.112292
Varlinskaya, E. I., Kim, E. U., and Spear, L. P. (2017). Chronic intermittent ethanol exposure during adolescence: effects on stress-induced social alterations and social drinking in adulthood. Brain Res. 1654, 145–156. doi: 10.1016/j.brainres.2016.03.050
Varlinskaya, E. I., and Spear, L. P. (2002). Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol. Clin. Exp. Res. 26, 1502–1511. doi: 10.1111/j.1530-0277.2002.tb02449.x
Varlinskaya, E. I., and Spear, L. P. (2007). Chronic tolerance to the social consequences of ethanol in adolescent and adult Sprague-Dawley rats. Neurotoxicol. Teratol. 29, 23–30. doi: 10.1016/j.ntt.2006.08.009
Varlinskaya, E. I., and Spear, L. P. (2015). Social consequences of ethanol: impact of age, stress, and prior history of ethanol exposure. Physiol. Behav. 148, 145–150. doi: 10.1016/j.physbeh.2014.11.062
Varlinskaya, E. I., Truxell, E., and Spear, L. P. (2014). Chronic intermittent ethanol exposure during adolescence: effects on social behavior and ethanol sensitivity in adulthood. Alcohol 48, 433–444. doi: 10.1016/j.alcohol.2014.01.012
Vetreno, R. P., Yaxley, R., Paniagua, B., and Crews, F. T. (2016). Diffusion tensor imaging reveals adolescent binge ethanol-induced brain structural integrity alterations in adult rats that correlate with behavioral dysfunction. Addict. Biol. 21, 939–953. doi: 10.1111/adb.12232
Vetter, C. S., Doremus-Fitzwater, T. L., and Spear, L. P. (2007). Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol. Clin. Exp. Res. 31, 1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x
Vetter-O’Hagen, C., Varlinskaya, E., and Spear, L. (2009). Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol Alcohol. 44, 547–554. doi: 10.1093/alcalc/agp048
Vorspan, F., Mehtelli, W., Dupuy, G., Bloch, V., and Lepine, J. P. (2015). Anxiety and substance use disorders: co-occurrence and clinical issues. Curr. Psychiatry Rep. 17:4. doi: 10.1007/s11920-014-0544-y
Walker, B. M., and Ehlers, C. L. (2009). Age-related differences in the blood alcohol levels of Wistar rats. Pharmacol. Biochem. Behav. 91, 560–565. doi: 10.1016/j.pbb.2008.09.017
White, A. M., Truesdale, M. C., Bae, J. G., Ahmad, S., Wilson, W. A., Best, P. J., et al. (2002). Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol. Biochem. Behav. 73, 673–677. doi: 10.1016/s0091-3057(02)00860-2
Wiener, C. D., Moreira, F. P., Zago, A., Souza, L. M., Branco, J. C., Oliveira, J. F., et al. (2018). Mood disorder, anxiety, and suicide risk among subjects with alcohol abuse and/or dependence: a population-based study. Braz. J. Psychiatry 40, 1–5. doi: 10.1590/1516-4446-2016-2170
Willey, A. R., Varlinskaya, E. I., and Spear, L. P. (2009). Social interactions and 50 kHz ultrasonic vocalizations in adolescent and adult rats. Behav. Brain Res. 202, 122–129. doi: 10.1016/j.bbr.2009.03.025
Williams, K. L., Nickel, M. M., and Bielak, J. T. (2018). Oral binge-like ethanol pre-exposure during juvenile/adolescent period attenuates ethanol-induced conditioned place aversion in rats. Alcohol Alcohol. 53, 518–525. doi: 10.1093/alcalc/agy040
Keywords: adolescence, alcohol, adolescent ethanol exposure, anxiety, ethanol intake, ethanol sensitivity
Citation: Towner TT and Varlinskaya EI (2020) Adolescent Ethanol Exposure: Anxiety-Like Behavioral Alterations, Ethanol Intake, and Sensitivity. Front. Behav. Neurosci. 14:45. doi: 10.3389/fnbeh.2020.00045
Received: 27 November 2019; Accepted: 16 March 2020;
Published: 31 March 2020.
Edited by:
Ricardo Marcos Pautassi, Universidad Nacional de Córdoba, ArgentinaReviewed by:
Waldo Cerpa, Pontifical Catholic University of Chile, ChileMariana Rae, University of São Paulo, Brazil
Copyright © 2020 Towner and Varlinskaya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Trevor T. Towner, dHRvd25lcjFAYmluZ2hhbXRvbi5lZHU=
 Trevor T. Towner
Trevor T. Towner Elena I. Varlinskaya
Elena I. Varlinskaya