- 1Department of Biobehavioral Health, Pennsylvania State University, University Park, PA, United States
- 2Department of Biology, Pennsylvania State University, University Park, PA, United States
- 3Neuroscience Curriculum, Pennsylvania State University, University Park, PA, United States
Forced abstinence (FA) from alcohol has been shown to produce a variety of anxiety- and depression-like symptoms in animal models. Somatostatin (SST) neurons, a subtype of GABAergic neurons found throughout the brain, are a novel neural target with potential treatment implications in affective disorders, yet their role in alcohol use disorders (AUD) remains to be explored. Here, we examined the neuroadaptations of SST neurons during forced abstinence from voluntary alcohol consumption. Following 6 weeks of two-bottle choice alcohol consumption and protracted forced abstinence, male and female C57BL/6J mice exhibited a heightened, but sex-specific, depressive-like behavioral profile in the sucrose preference test (SPT) and forced swim test (FST), without changes in anxiety-like behaviors in the elevated plus maze (EPM) and open field test (OFT). FST-induced cFos expressions in the prefrontal cortex (PFC) and ventral bed nucleus of the stria terminalis (vBNST) were altered in FA-exposed female mice only, suggesting a sex-specific effect of forced abstinence on the neural response to acute stress. SST immunoreactivity in these regions was unaffected by forced abstinence, while differences were seen in SST/cFos co-expression in the vBNST. No differences in cFos or SST immunoreactivity were seen in the lateral central nucleus of the amygdala (CEA) and the basolateral amygdala (BLA). Additionally, SST neurons in female mice displayed opposing alterations in the PFC and vBNST, with heightened intrinsic excitability in the PFC and diminished intrinsic excitability in the vBNST. These findings provide an overall framework of forced abstinence-induced neuroadaptations in these key brain regions involved in emotional regulation and processing.
Introduction
Alcohol use disorder (AUD) represents one of the most prevalent and costly neuropsychiatric disorders globally and domestically, costing the United States economy an estimated $249 billion due to losses in workplace productivity, and health care and criminal justice expenses (Sacks et al., 2015). Withdrawal from alcohol (both acute and protracted withdrawal) produces a host of negative emotional conditions, such as depression and anxiety (Hershon, 1977; Becker, 2014; Smith et al., 2019). These emotional states can increase the risk for relapse and further hamper an individual’s ability to abstain from alcohol. Importantly, the literature suggests that risk of relapse may be different across the sexes, with women more likely to relapse following negative affect-related situations, and men more likely to relapse when in the presence of other alcohol consumers (White et al., 2015; Peltier et al., 2019). Complementary research suggests that alleviating depressive symptoms following abstinence from alcohol may improve treatment outcomes for women (Annis et al., 1998; Holzhauer and Gamble, 2017). Taken together, these data point to a complex relationship between AUD, depression, and treatment outcome, likely moderated by sex.
Previous research has shown that forced abstinence (FA) from alcohol produces depressive-like behavior and a variety of neurobiological changes in rodent models. For instance, Vranjkovic et al. (2018) demonstrated that 24-h immediately following 6 weeks of alcohol drinking, female C57BL/6J mice showed decreased time spent in the open arm of the elevated plus maze (EPM), and increased latency to feed in the novelty suppression of feeding test (NSFT), two commonly used models of anxiety-like behavior. In addition, this model produced increased immobility in the forced swim test (FST) during protracted withdrawal (21 days of abstinence), though it should be noted males were not investigated in these studies (Holleran et al., 2016; Vranjkovic et al., 2018). Similarly, Valdez and Harshberger (2012) demonstrated that male Wistar rats exposed to chronic alcohol show increased immobility in the FST, and that this effect is further enhanced during protracted withdrawal.
Alcohol use disorder and major depressive disorder (MDD) are highly comorbid disorders with overlapping etiology. Novel fast acting antidepressants are able to reduce both binge drinking (Crowley et al., 2019a) and depressive-like behavior following alcohol exposure (Holleran et al., 2016; Vranjkovic et al., 2018) further highlighting the potential overlapping neural circuitry involved in AUD and MDD. Importantly, both the clinical and preclinical depression literature has continuously pointed to a novel subpopulation of gamma aminobutyric acidergic (GABAergic) neurons as a protective, resiliency-conferring population. Somatostatin (SST) neurons are a group of GABAergic neurons that express and release the neuropeptide SST in the cortex, hippocampus, thalamus and amygdala (Fuchs et al., 2017; Abbas et al., 2018; Ahrens et al., 2018). Recent reports have implicated these neurons in a host of neuropsychiatric disorders in addition to MDD, such as bipolar disorder, anxiety disorder, and schizophrenia (Fee et al., 2017). Global genetic upregulation of SST neuronal function reduces depression- and anxiety- like behaviors in male and female mice (Fuchs et al., 2017), and deficits in SST expression are seen in the amygdala of postmortem samples of MDD patients (Douillard-Guilloux et al., 2017). Ketamine reverses the effects of chronic stress on GABAergic transmission in PFC pyramidal neurons by restoring the excitation/inhibition balance in SST neurons (Ghosal et al., 2020). Despite the clear correlation between SST neuronal markers and their function in MDD, both in the human and preclinical animal literature, thus far SST neurons have been poorly investigated in the context of AUD and depression-like phenotypes seen during withdrawal from alcohol.
The current study had two aims: first, to replicate previous behavioral models of forced abstinence from alcohol with both male and female mice. The second aim was to bridge the resiliency-like effect of SST neuronal function seen in the animal depression literature with the alcohol literature, in order to establish whether SST neurons within the prefrontal cortex (PFC), bed nucleus of the stria terminalis (BNST), basolateral amygdala (BLA) and lateral central amygdala (CEA), brain regions known for their role in MDD (Fee et al., 2017) and chronic alcohol exposure (Pleil et al., 2015a) are altered following forced abstinence from alcohol. These findings may shed new insights into the overlapping neural etiology of these highly comorbid disorders, particularly the SST neurons, and provide new directions to the discovery of more effective treatments.
Materials and Methods
Animals
Male and female C57BL/6J (stock #000664) were purchased from the Jackson Laboratory. Hemizygous female SST-IRES-Cre::Ai9 were generated from homozygous SST-IRES-Cre (stock #013044, Jackson Laboratory) and homozygous Ai9 (stock #007909, Jackson Laboratory) parents. All mice were at least 8-weeks old at the beginning of alcohol drinking. Mice were individually housed with ad lib access to food and water, and were maintained on a 12 h reverse light/dark cycle (lights off at 7am) in temperature- and humidity-controlled vivarium for at least 1 week prior to alcohol exposure. Mouse weights were monitored weekly. All procedures were approved by the Pennsylvania State University’s Institutional Animal Care and Use Committee.
Two-Bottle Choice (2BC) Alcohol Drinking
A schematic of the experimental timeline is displayed in Figure 1A. After 1 week of acclimatization to single housing, mice were randomly assigned to either drinking or non-drinking groups. Alcohol-drinking mice received continuous access to one sipper bottle of tap water and another of unsweetened, diluted alcohol. Alcohol concentrations increased from 3% in day 1 to 3, to 7% in day 3 to 9, to 10% in day 9 to 42. Bottles were weighed and refilled every 48-h. The positions of the bottles were randomized across mice, and within individual mice were switched weekly to avoid position bias. Following alcohol drinking, mice underwent forced abstinence where they had access to water only. Non-drinking control mice received continuous access to two water bottles only throughout the experiment. Escalation of alcohol drinking and forced abstinence were modeled off of Holleran et al. (2016).
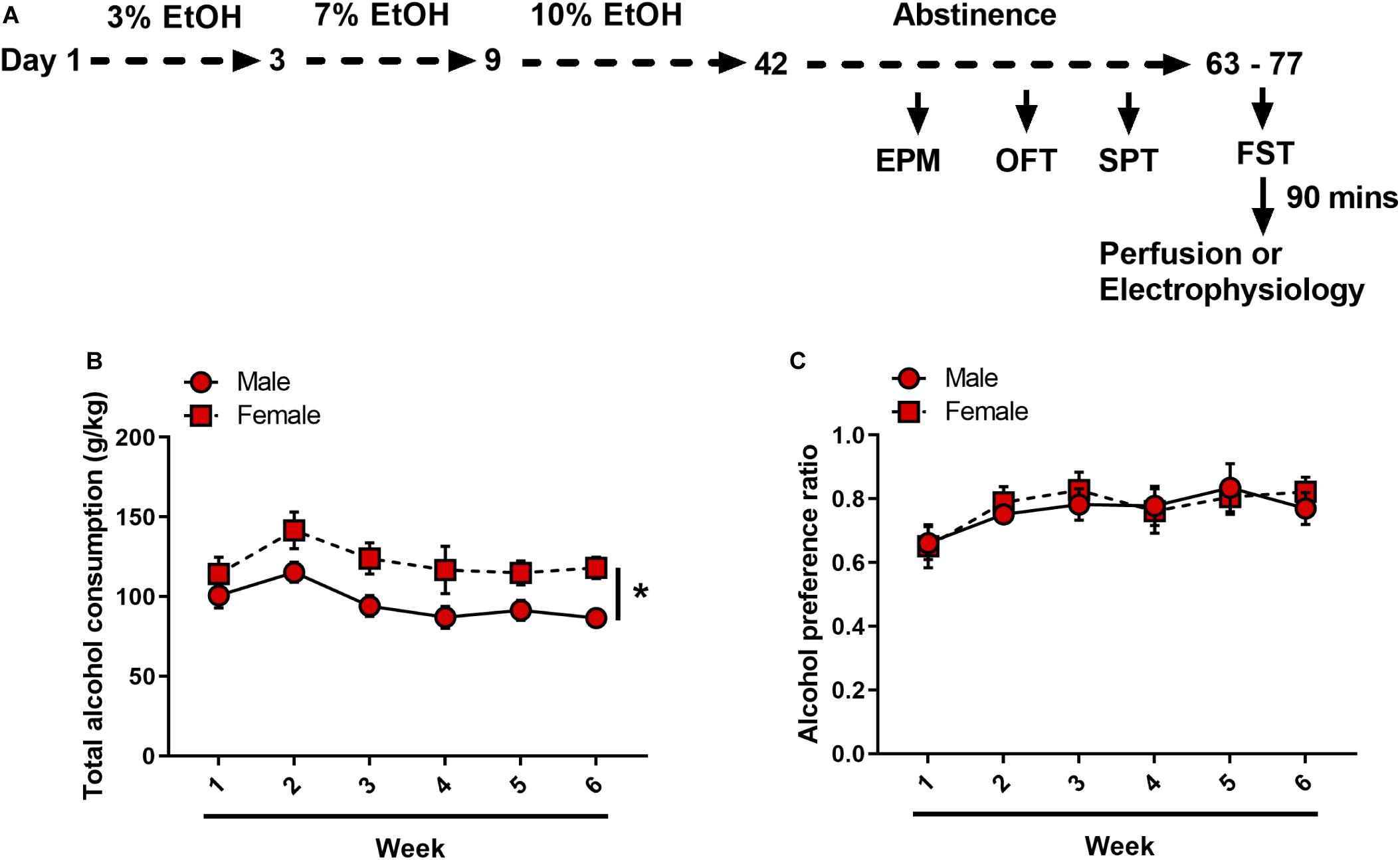
Figure 1. Forced abstinence from alcohol in alcohol ramp two-bottle choice paradigm. (A) Experimental timeline. Following 6 weeks of an alcohol ramp procedure, mice underwent a behavior battery (each test 1 week apart). Mice that were used in electrophysiology experiments did not undergo EPM, OPF or SPT. (B) Female mice consistently drank more than male mice. (C) Both sexes equally preferred alcohol over water. *p < 0.05.
Behavioral Testing
Mice were tested for anxiety- and depression- like behaviors during abstinence, starting with elevated plus maze (EPM), open field test (OFT), sucrose preference test (SPT) and lastly forced swim test (FST). Prior to behavior, mice were brought to the testing room and allowed to rest for at least 30 min. All tests were done 3-h into the dark cycle, under red light (6 lux). Female SST-Ai9 mice that were used in electrophysiology experiments did not undergo EPM, OFT, and SPT, and were tested in the FST under normal light.
Elevated Plus Maze
One week after the onset of abstinence, mice underwent EPM where they were placed in the center square of the maze (35 × 5 × 40 cm), facing a closed arm (20 cm arm wall height, transparent Plexiglass and gray floor). Mice were allowed to explore the maze for 5 min. Sessions were recorded with EthoVision XT video tracking system (Noldus, Leesburg, VA, United States). Total time spent in open arms and number of entries to open arms were automatically analyzed by EthoVision XT.
Open Field Test
Mice were placed in a corner of a black Plexiglass arena (50 × 50 × 20 cm) and allowed to explore for 20 min. Sessions were recorded with EthoVision XT, and total time spent in center zone and number of entries to center zones were automatically analyzed by EthoVison XT. Center zone was defined as a 12.5 × 12.5 cm area at the center of the arena.
Sucrose Preference Test
Mice received access to one bottle of tap water and another of 1% (w/v) sucrose solution for 12-h, starting 3-h into the dark cycle. Bottles were weighed before and after testing. Sucrose preference ratio was calculated as the volume of sucrose solution consumed divided by total volume of fluid consumed.
Forced Swim Test
Mice were placed in a transparent 5-liter glass beaker filled with approximately 3-liters of water (20 ± 1°C) for 6 min. Total time spent immobile during the last 4 min was recorded and manually scored by a researcher blind to experimental conditions.
Fluorescence Immunohistochemistry
Ninety minutes after FST, mice were deeply anesthetized with Avertin (250 mg/kg) and perfused transcardially with ice-cold phosphate buffered saline (PBS, pH 7.4) and 4% paraformaldehyde (PFA, pH 7.4). Brains were removed, post-fixed in PFA overnight and placed in 30% (w/v) sucrose solution until sunk. 50-μm free floating sections were sliced with a Compresstome vibrating microtome VF-300-0Z (Precisionary Instruments LLC, Greenville, NC, United States) in a 1:3 series and stored in 30% sucrose/30% ethylene glycol cryoprotectant at −20°C until processed.
Prior to immunostaining, slices underwent antigen retrieval in 10 mM sodium citrate buffer (pH 6.0) at 80°C for 30 min. Slices were washed three times in PBS for 10 min each, and permeabilized in 0.5% Triton X-100 in PBS for 60 min. Nonspecific binding was blocked with 5% normal goat serum (NGS) in 0.1% Triton X-100 in PBS for 60 min. Slices were then incubated in a primary antibody cocktail, including rabbit anti-cFos (1:4000, Cell Signaling Technology, Danvers, MA, United States) and rat anti-somatostatin (1:500, Millipore, Burlington, MA, United States) in 2.5% NGS in 0.1% Triton X-100 in PBS for 48-h at 4°C. Slices were rinsed three times with PBS for 10 min each, and incubated in a fluorophore-tagged secondary antibody cocktail, including goat anti-rabbit Alexa Fluor 488 (1:500, Cell Signaling Technology, Danvers, MA, United States) and goat anti-rat Cy3 (1:500, Millipore, Burlington, MA, United States) for 4-h at room temperature. Slices were rinsed again three times with PBS, with the last step including DAPI (1:10,000), mounted on glass slides, air-dried and coverslipped with Immunomount (Thermo Fisher Scientific, Waltham, MA, United States). Images were obtained with an Olympus BX63 upright microscope (Center Valley, PA, United States) under matched exposure settings. Four to eight images from both hemispheres were taken per region.
Total cFos counts, SST immunoreactivity (IR), and cFos+/SST+ counts were quantified by researchers blind to experimental conditions using ImageJ (National Institutes of Health, Bethesda, MD, United States). For total cFos counts, region of interest (ROI) was delineated, and cFos+ nuclei were automatically quantified under matched criteria for size, circularity and intensity. Each ROI’s total Fos count was divided by the ROI’s area to give a cFos total density value (Smith et al., 2019). cFos+/SST+ nuclei count was manually quantified, and divided by the ROI’s area to give a cFos+/SST+ density value. SST IR was quantified as mean fluorescence intensity of the ROI (Pleil et al., 2015b). At least three sections per region (PFC, dBNST, vBNST, lateral CeA, BLA) were quantified and averaged to obtain one value per mouse.
Electrophysiology
Whole-cell current clamp recordings were conducted similarly to those previously published (Crowley et al., 2016, 2019b). Regions for electrophysiology were determined by cFos and SST results. Based on these results and behavioral results, only female mice were explored. The regions of interest (PFC and vBNST) were identified according to the Allen Mouse Brain Atlas. Following alcohol exposure and abstinence, mice underwent FST. Ninety minutes following FST, female SST-IRES-Cre::Ai9 mice were deeply anesthetized via inhaled isoflurane and rapidly decapitated. Brains were rapidly removed and processed according to the NMDG protective recovery method (Ting et al., 2018). Briefly, brains were immediately placed in ice-cold, oxygenated N-methyl-D-glucamine (NMDG)-HEPES aCSF containing the following, in mM: 92 NMDG, 2.5 KCl, 1.25 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 glucose, 2 thiourea, 5 Na-ascorbate, 3 Na-pyruvate, 0.5 CaCl2⋅2H2O, and 10 MgSO4⋅7H2O (pH to 7.3–7.4). 300 μM coronal slices containing the PFC and the vBNST were prepared on a Compresstome vibrating microtome VF-300-0Z (Precisionary Instruments, Greenville, NC, United States), and transferred to heated (31°C) NMDG-HEPES aCSF for a maximum of 10 min. Slices were then transferred to heated (31°C), oxygenated normal aCSF (in mM: 124 NaCl, 4.4 KCl, 2 CaCl2, 1.2 MgSO4, 1 NaH2PO4, 10.0 glucose, and 26.0 NaHCO3, pH 7.4, mOsm 300–310), where they were allowed to rest for at least 1-h before use. Finally, slices were moved to a submerged recording chamber (Warner Instruments, Hamden, CT, United States) where they were continuously perfused with heated recording aCSF at a rate of 2 ml/min. Recording electrodes (3–6 MΩ) were pulled from thin-walled borosilicate glass capillaries with a Narishige P-100 Puller (Amityville, NY, United States).
SST-expressing neurons were identified in SST-IRES-Cre::Ai9 mice via presence of tdTomato fluorescence under a 40× immersed objective with 565 nm LED excitation. Measurements of intrinsic excitability included resting membrane potential (RMP), rheobase (the minimum amount of current needed to elicit an action potential during a current ramp protocol), action potential threshold (the membrane potential at which the first action potential fired), and the number of action potentials fired during a voltage-current plot protocol (V-I plot) with increasing steps of depolarizing currents (0–200 pA, 10 pA per step). Hyperpolarizing currents (not shown) were included as a control. Experiments were performed at both RMP and at the standard holding potential of −70 mV. Electrodes were filled with a potassium gluconate-based (KGluc) intracellular recording solution (in mM: 135 K-Gluc, 5 NaCl, 2 MgCl2, 10 HEPES, 0.6 EGTA, 4 Na2ATP, and 0.4 Na2GTP, 287–290 mOsm, pH 7.35).
Signals were digitized at 10 kHz and filtered at 3 kHz using a Multiclamp 700B amplifier, and analyzed using Clampfit 10.7 software (Molecular Devices, Sunnyvale, CA, United States). For all measures, recordings were performed in a maximum of two neurons per subregion, per mouse, and n values reported reflect the total number of neurons.
Exclusion Criteria
One male mouse did not consume alcohol (average of 14.75 g/kg per week vs. group average of 108.12 g/kg per week). Since this was the first behavioral assay, this mouse was excluded from all analyses. For immunofluorescence quantification, problematic images (e.g. tears, out of focus, debris) were excluded prior to de-blinding. For electrophysiology recordings, cells that did not fire during the current ramp or V-I plot protocol were excluded. Statistical outliers, identified by Grubb’s test, were excluded from the data set.
Statistical Analysis
Data was analyzed in Graphpad Prism 7.0 (San Diego, CA, United States). All datasets were checked for normality (D’Agostino-Pearson’s test) and homogeneity (Barlett’s test). If found violated, nonparametric Kruskall–Wallis test was performed and Dunn’s multiple comparison was used as a posthoc test. Ordinary two-way ANOVA was used for all behavior and fluorescence immunohistochemistry data, followed by Tukey’s multiple comparison posthoc test. One sample t-test was used to examine alcohol preference over water (theoretical mean 0.5). Student’s t-test was used to analyze RMP, rheobase and action potential thresholds, while mixed-model two-way ANOVA was used to analyze SST V-I protocol. Statistical significance threshold was set at α = 0.05. Data presented show means and standard error of the mean (SEM).
Results
Male and female C57BL/6J mice underwent either an alcohol ramp two-bottle choice procedure and forced abstinence (N = 11 males and 10 females) or control condition (N = 12 males and 10 females) (Figure 1A). On average, over the 6 weeks of alcohol exposure, male mice consumed significantly less alcohol per body weight as compared to female mice [Ftime(5, 95) = 6.314, p < 0.001, Fsex(1, 19) = 7.060, p = 0.015, Fsex × time(5, 95) = 0.685, p = 0.635] (Figure 1B). Alcohol preference over water did not differ between male and female mice [Ftime(5, 95) = 5.839, p < 0.001, Fsex(1, 19) = 0.041, p = 0.84, Fsex × time(5, 95) = 0.535, p = 0.749], and both sexes displayed a strong preference for alcohol over water [male: one-sample t(10) = 5.391, p < 0.001, female: one-sample t(9) = 7.102, p < 0.001] (Figure 1C).
Depressive-Like Behavioral Phenotypes Following Forced Abstinence From Alcohol
Following the first week of abstinence, mice underwent multiple assays (one per week) to assess anxiety- and depression- like behavioral states. In the EPM, open arm duration was comparable between sexes and alcohol conditions [Fsex(1, 39) = 0.379, p = 0.531, FFA(1, 39) = 0.991, p = 0.325, Fsex × FA(1, 39) = 0.4, p = 0.530; Figure 2A]. Females had higher frequency of entries into the open arms than males, [Fsex(1, 39) = 5.275, p = 0.027] with no effects of FA conditions [FFA(1, 39) = 0.485, p = 0.489, Fsex × FA(1, 39) = 0.044, p = 0.833; Figure 2B]. In the OFT, no differences were seen in either open field center duration [Fsex(1, 39) = 0.223, p = 0.639, FFA(1, 39) = 0.878, p = 0.354, Fsex × FA(1, 39) = 0.066, p = 0.797; Figure 2C] or total distance traveled [Fsex(1, 39) = 2.883, p = 0.097, FFA(1, 39) = 2.502, p = 0.121, Fsex × FA(1, 39) = 1.575, p = 0.217; Figure 2D].
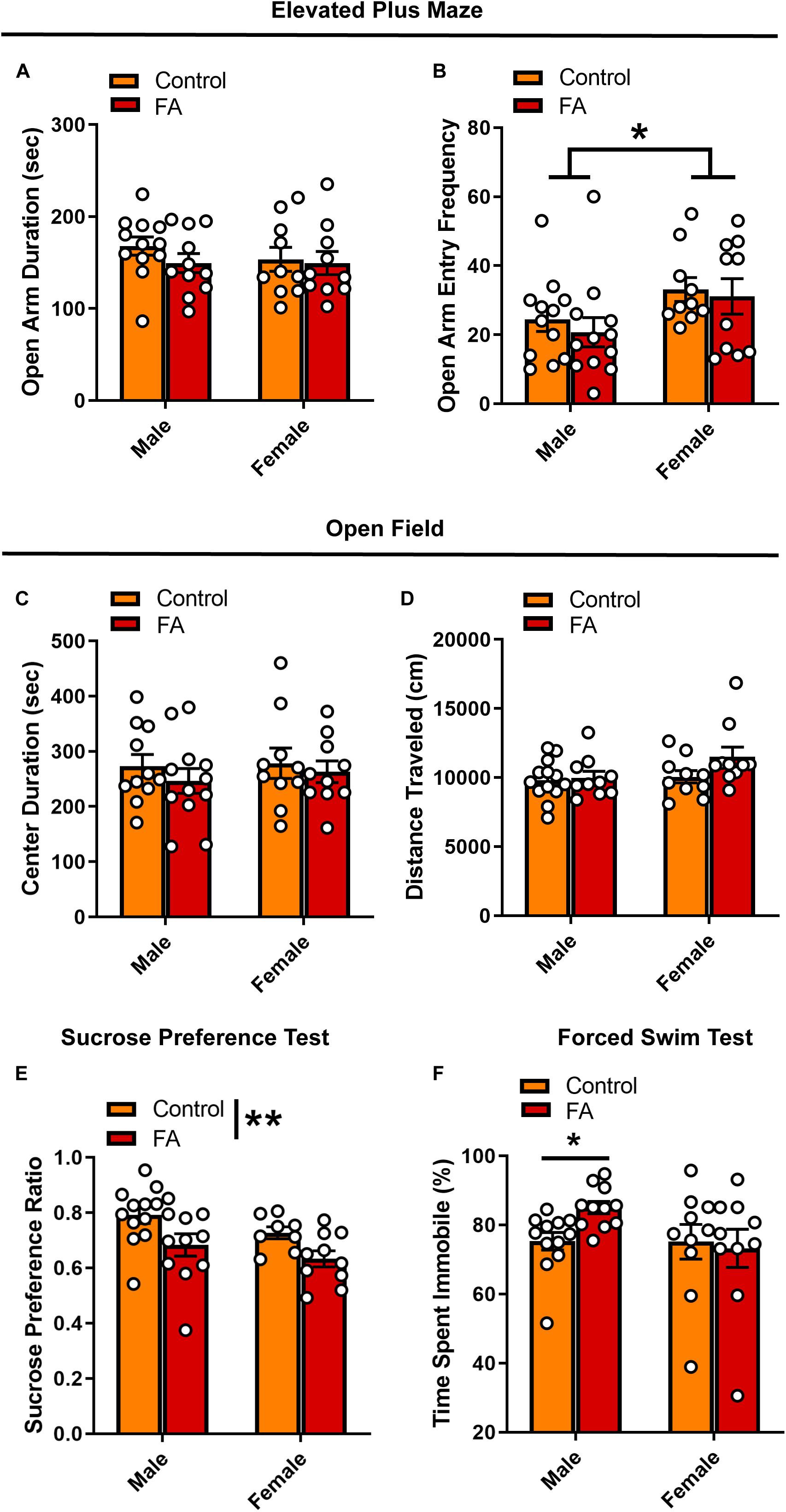
Figure 2. Forced abstinence from alcohol induces a depressive-like behavioral phenotype. (A) No significant difference in time spent in the open arm of the EPM was observed across sexes and FA conditions. (B) Female mice made more entries to the open arms than male mice, and this effect was not modulated by alcohol conditions. (C) No significant difference in time spent in the center zone of the OPT and (D) total distance traveled was observed across sexes and FA condition. (E) FA-exposed male and female mice displayed lower sucrose preference in the SPT than their control counterparts. (F) FA-exposed male mice spent more time immobile in the FST than control male mice. This effect was not observed in female mice. *p < 0.05, **p < 0.01.
When probing depression-like behavior using the SPT and FST, key differences in both FA exposure and sex emerged. There was a significant main effect of FA history on sucrose preference [FFA(1, 37) = 9.388, p = 0.004], but no effect of sex [Fsex(1, 37) = 2.983, p = 0.092] and no sex by FA interaction [Fsex × FA(1, 37) = 0.049, p = 0.825] (Figure 2E). Both male and female mice that underwent forced abstinence from FA showed lower sucrose preference, classically interpreted as an anhedonia phenotype. Interestingly, sex differences emerged in the FST. Though male mice exposed to FA showed a significant increase in immobility time, female mice did not (Kruskall–Wallis χ = 7.783, p = 0.05, male control vs. male EtOH: p = 0.02, female control vs. female EtOH: p > 0.99, Dunn’s posthoc test; Figure 2F). In sum, these data suggest that following forced abstinence from alcohol, male and female mice display a depressive-like behavioral profile without changes in anxiety-like behaviors.
Forced Swim Stress-Induced Neuronal Activation in the PFC Is Modulated by Sex and Forced Abstinence From Alcohol
Next, we probe the neural substrates that may underlie the changes in depressive-like behaviors in cortical and amygdalar areas, including the PFC, BNST, BLA and lateral CeA. In the PFC (Figure 3A for representative images), FST-induced neuronal activation, identified by expression of the immediately early gene marker cFos, showed significant main effect of sex [Fsex(1, 33) = 22.44, p < 0.001] and significant interaction between sex and FA conditions [Fsex × FA(1, 33) = 5.910, p = 0.020], without a main effect of FA [FFA(1, 33) = 1.198, p = 0.281; Figure 3B]. Tukey’s posthoc test revealed that FA-exposed female mice showed significantly lower number of cFos nuclei in the PFC than control female mice (p < 0.05), control male mice (p < 0.001) and FA-exposed male mice (p < 0.001). Control female mice also had less cFos nuclei than FA-exposed male mice (p < 0.05). SST neuron-specific cFos expression (cFos+/SST+ nuclei) was not altered by sex and FA conditions [Fsex(1, 36) = 2.166, p = 0.149, FFA(1, 36) = 2.140, p = 0.152, Fsex × FA(1, 36) = 0.202, p = 0.656; Figure 3C]. There were also no changes in SST immunoreactivity [Fsex(1, 35) = 1.889, p = 0.178, FFA(1, 35) = 0.082, p = 0.775, Fsex × FA(1, 35) = 0.026, p = 0.872; Table 1].
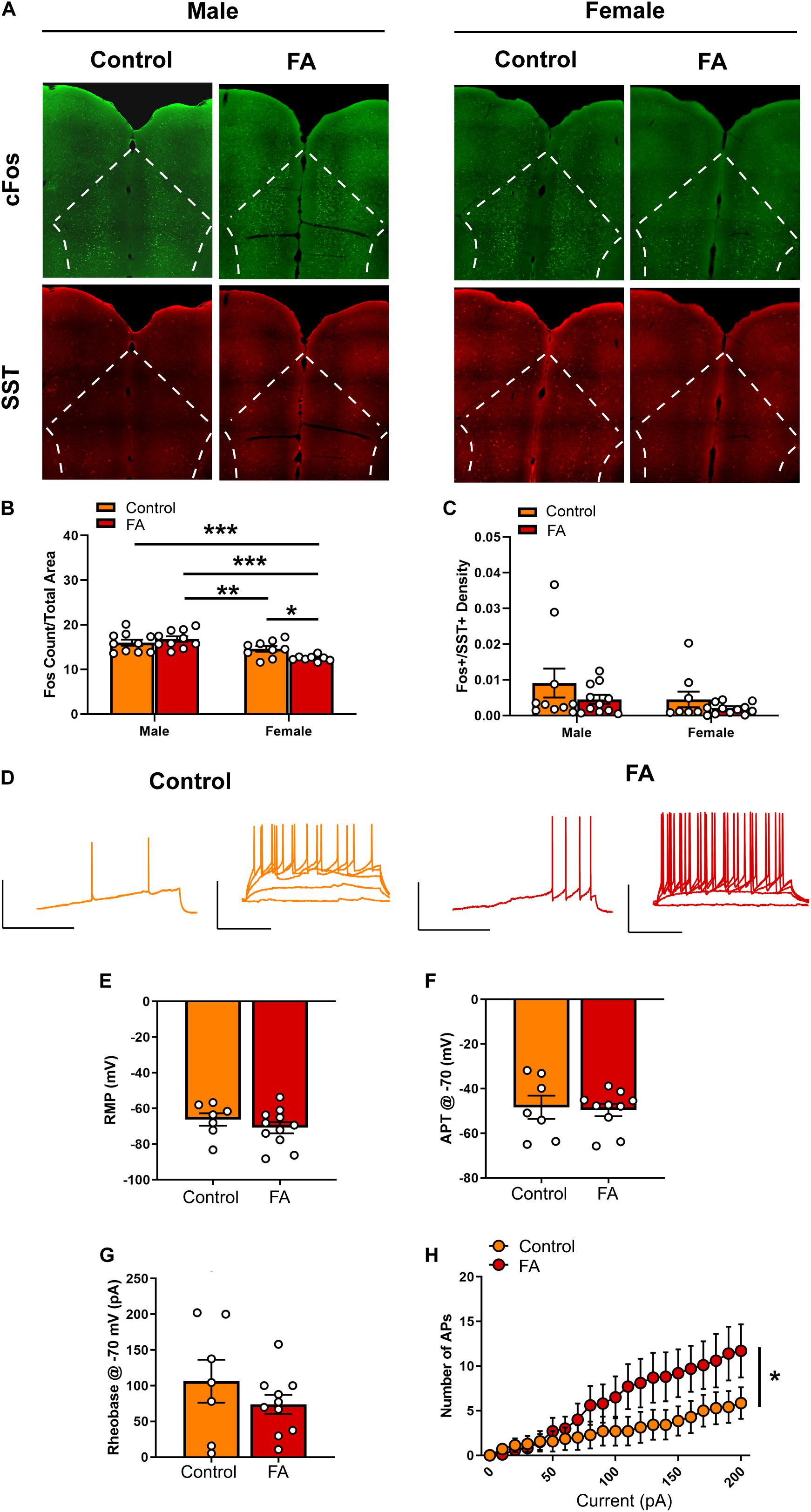
Figure 3. Forced abstinence from alcohol decreases forced swim stress-induced cFos expression and augments intrinsic excitability of SST neurons in the PFC of female mice. (A) Representative images of cFos (green) and SST (red) immunofluorescence with the PFC delineated for quantification. (B) Male mice displayed more cFos nuclei than female mice following forced swim, and FA-exposed female mice had less cFos nuclei than control female mice. (C) SST neuron-specific cFos expression was similar in both sexes and FA conditions. (D) Representative traces of current-injected firing from SST neurons in the PL during a ramp protocol (left) and V-I plot protocol (right) at –70 mV. Scale bars 50 mV × 200 ms. (E) RMP, (F) action potential threshold, and (G) rheobase were unaltered in female PL SST neurons. (H) FA-exposed female SST neurons fired significantly more action potentials in the V-I plot protocol than control female SST neurons. *p < 0.05, **p < 0.01, and ***p < 0.001.
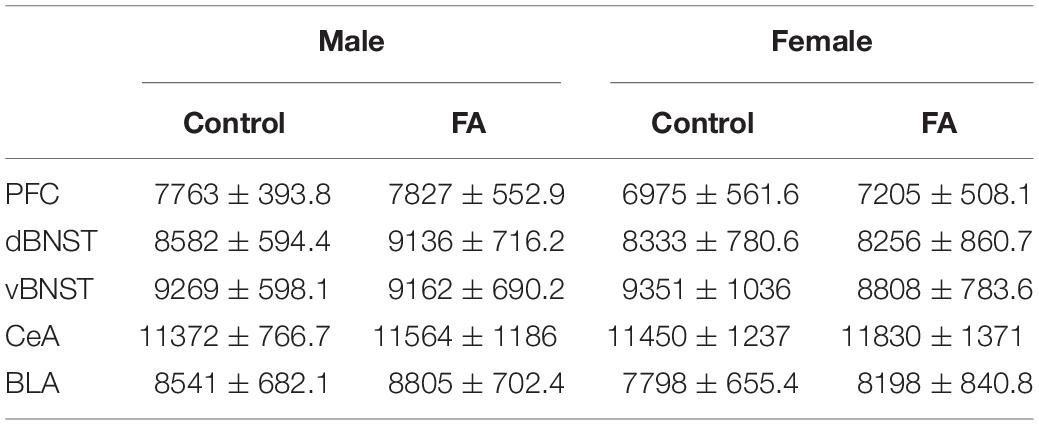
Table 1. Somatostatin Immunoreactivity was not modulated by sex or forced abstinence from alcohol drinking.
Given that only female mice displayed a change in FST-induced cFos expression in the PFC following forced abstinence from alcohol, we next performed whole-cell patch clamp electrophysiology in SST neurons in the PFC of female SST-Ai9 reporter (Figure 3D for representative recording traces). Current clamp experiments revealed that SST neurons had similar RMP [t(16) = 0.955, p = 0.353, Figure 3E]. When cells were held at −70 mV, action potential threshold [t(16) = 0.223, p = 0.825, Figure 3F] and rheobase [t(16) = 1.096, p = 0.290, Figure 3G] were unaltered between FA-exposed mice (n = 10 cells, N = 4 mice) and control mice (n = 7 cells, N = 3 mice). V-I plot at the holding potential of −70 mV indicated a significant main effect of current amplitude [Fcurrent(20, 300) = 15.257, p < 0.001[and a significant FA by current amplitude interaction [FFA × current(20, 300) = 2.970, p < 0.001), with no main effect of FA [FFA(1, 15) = 1.539, p = 0.234; Figure 3H]. SST neurons in the PFC of FA-exposed female mice fired significantly more action potentials in response to increasing steps of depolarizing currents than those in control female mice.
Recordings at RMP did not reveal any significant difference in rheobase [t(16) = 0.225, p = 0.824], action potential threshold [t(16) = 0.267, p = 0.792] and V-I plot [Fcurrent(20, 300) = 42.523, p < 0.001, FFA(1, 15) = 0.118, p = 0.735, FFA × current(20, 300) = 0.421, p = 0.987; data not shown] between FA-exposed mice and control mice.
Together, these data suggest that forced abstinence from alcohol dampened forced swim stress-induced neuronal activation in the female PFC, as indicated by reductions in cFos, likely via an increase in excitability of the GABAergic SST neurons.
Forced Swim Stress-Induced Neuronal Activation in the vBNST Is Modulated by Sex and Forced Abstinence From Alcohol
The dorsal BNST (Figure 4A for representative images) showed a significant main sex effect [Fsex(1, 37) = 13.88, p < 0.001], but no main effect of FA [FFA(1, 37) = 1.646, p = 0.207] or sex by FA interaction [Fsex × FA(1, 37) = 0.354, p = 0.149] in FST-induced cFos expression (Figure 4B). SST neuron-specific cFos expression (Fos+/SST+ nuclei) in the dorsal BNST was unaltered by sex and FA conditions [Fsex(1, 38) = 0.178, p = 0.675, FFA(1, 38) = 0.134, p = 0.715, Fsex × FA(1, 38) = 0.0, p = 0.988; Figure 4C]. SST immunoreactivity was intact across sexes and FA conditions [Fsex(1, 37) = 0.106, p = 0.446, FFA(1, 37) = 0.106, p = 0.746, Fsex × FA(1, 37) = 0.184, p = 0.669; Table 1].
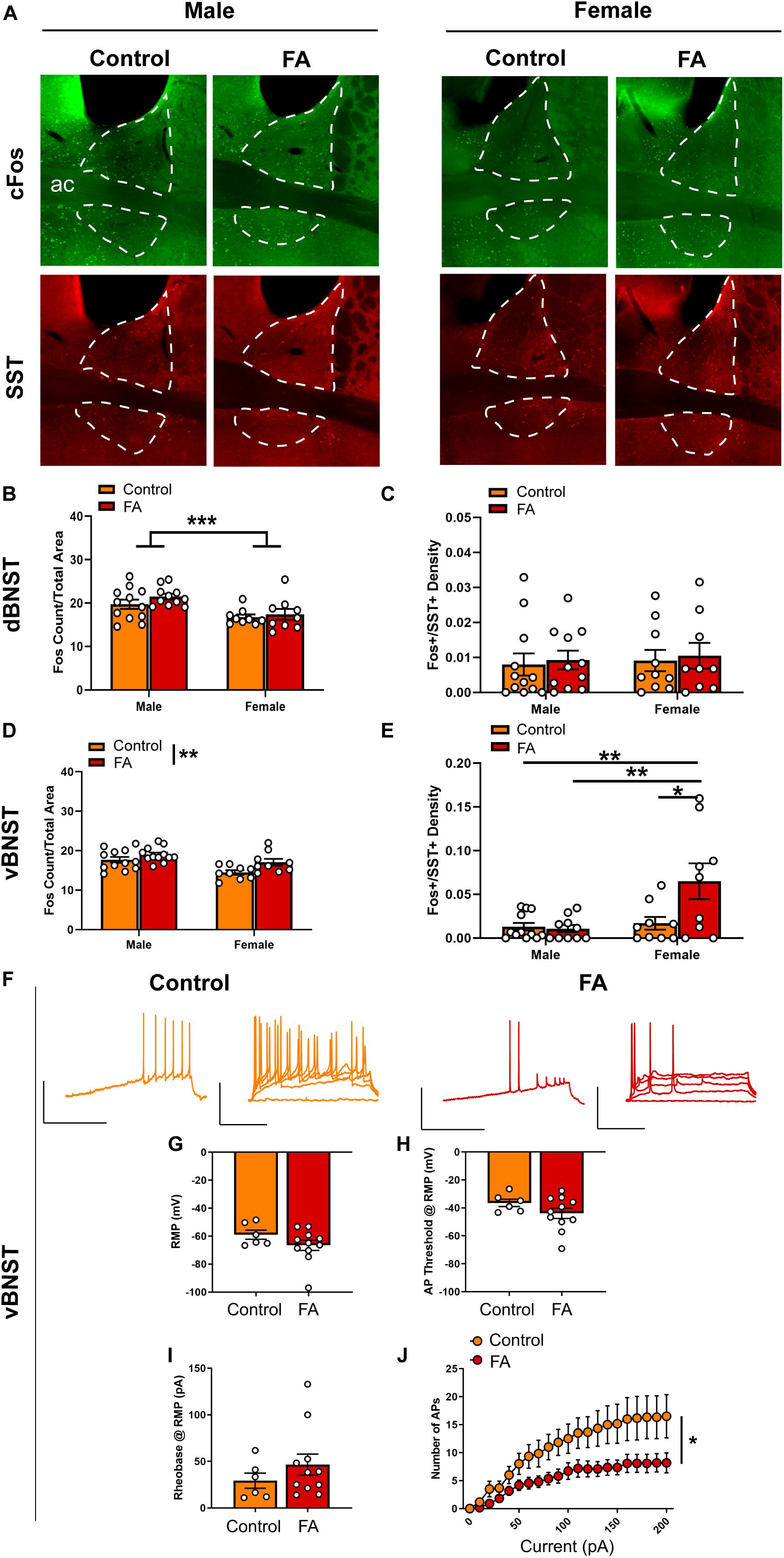
Figure 4. Forced abstinence from alcohol augments forced swim stress-induced neuronal activation and decreases intrinsic excitability of SST neurons in the vBNST. (A) Representative images of cFos (green) and SST (red) immunofluorescence in the BNST with the dorsal and ventral subregions delineated for quantification. (B) FA-exposed male mice had a higher number of cFos nuclei in the dorsal BNST than control female mice and FA-exposed female mice. (C) SST neuron-specific cFos expression in the dorsal BNST was comparable across sexes and FA conditions. (D) Control mice had a lower number of cFos nuclei in the vBNST than FA-exposed mice across sexes. (E) FA-exposed female mice had a higher number of SST neuron-specific cFos nuclei than control female mice, control male mice and FA-exposed male mice. (F) Representative traces of current-injected firing from SST neurons in the vBNST during a ramp protocol (left) and V-I plot protocol (right) at RMP. (G) RMP, (H) action potential threshold, and (I) rheobase were unaltered in female vBNST SST neurons. (J) FA-exposed female SST neurons fired significantly less action potentials in the V-I plot protocol than control female SST neurons. *p < 0.05, **p < 0.01, and ***p < 0.001.
The vBNST (Figure 4A for representative images) showed a significant main sex effect [Fsex(1, 38) = 13.59, p < 0.001], a significant main FA effect [FFA(1, 38) = 7.739, p = 0.008], without a sex by FA interaction [Fsex × FA(1, 38) = 0.815, p = 0.372] in FST-induced cFos expression (Figure 4D). Additionally, cFos+/SST+ nuclei in the vBNST revealed a significant main sex effect [Fsex(1, 36) = 7.905, p = 0.008], a significant main effect of FA [FFA(1, 36) = 4.944, p = 0.032] and a significant sex by FA interaction [Fsex × FA(1, 36) = 5.918, p = 0.020; Figure 4E]. Tukey’s posthoc test indicated that FA-exposed female mice showed higher number of Fos+/SST+ nuclei than control female mice (p < 0.05), control male mice (p < 0.01) and FA-exposed male mice (p < 0.01). SST immunoreactivity was similar across sexes and FA conditions [Fsex(1, 37) = 0.030, p = 0.862, FFA(1, 37) = 0.174, p = 0.678, Fsex × FA(1, 37) = 0.078, p = 0.780; Table 1].
Current clamp recordings from SST neurons in female vBNST (Figure 4F for representative recording traces) revealed that the RMP [t(15) = 1.371, p = 0.190; Figure 4G], action potential threshold at RMP [t(15) = 1.387, p = 0.185; Figure 4H], and rheobase at RMP [t(15) = 1.034, p = 0.317; Figure 4I] were unaltered in FA-exposed mice (n = 11 cells, N = 4 mice), compared to control mice (n = 6 cells, N = 3 mice). These neurons also fired significantly less action potentials in response to increasing steps of depolarizing currents at RMP, as revealed by a significant main effect of current amplitude [Fcurrent(20, 300) = 26.770, p < 0.001], a significant main effect of FA [FFA(1, 15) = 6.688, p = 0.021] and a significant current by FA interaction [Fcurrent × FA(20, 300) = 2.737, p < 0.001; Figure 4J]. Similar effects were seen when cells were held at −70 mV, including rheobase [t(15) = 0.851, p = 0.408], action potential threshold [t(15) = 0.508, p = 0.618], and V-I plot [Fcurrent(20, 300) = 23.391, p < 0.001, FFA(1, 15) = 4.329, p = 0.05, Fcurrent × FA(20, 300) = 3.426, p < 0.001; data not shown].
In sum, these data suggest forced abstinence from alcohol induced robust neuroadaptations in the vBNST of female mice, with an increase in overall vBNST neuronal activation, as indicated by cFos, following forced swim stress and a decrease in excitability of the SST subpopulation of vBNST GABAergic neurons.
Forced Swim Stress-Induced Neuronal Activation in the BLA and Lateral CeA Is Not Modulated by Sex or Forced Abstinence From Alcohol
Contrary to the PFC and the vBNST, both the BLA and lateral CeA did not display any appreciable alteration in forced swim stress-induced cFos expression across sexes and FA conditions. In the BLA (Figure 5A for representative images), there was a significant main sex effect [Fsex(1, 39) = 5.715, p = 0.021], but not main FA effect [FFA(1, 39) = 0.041, p = 0.839] or a sex by FA interaction [Fsex × FA(1, 39) = 0.2850, p = 0.596; Figure 5B]. SST immunoreactivity was similar across sexes and FA conditions in the BLA [FFA(1, 39) = 0.870, p = 0.356, Fsex(1, 39) = 0.211, p = 0.648, Fsex × FA(1, 39) = 0.008, p = 0.925, Table 1].
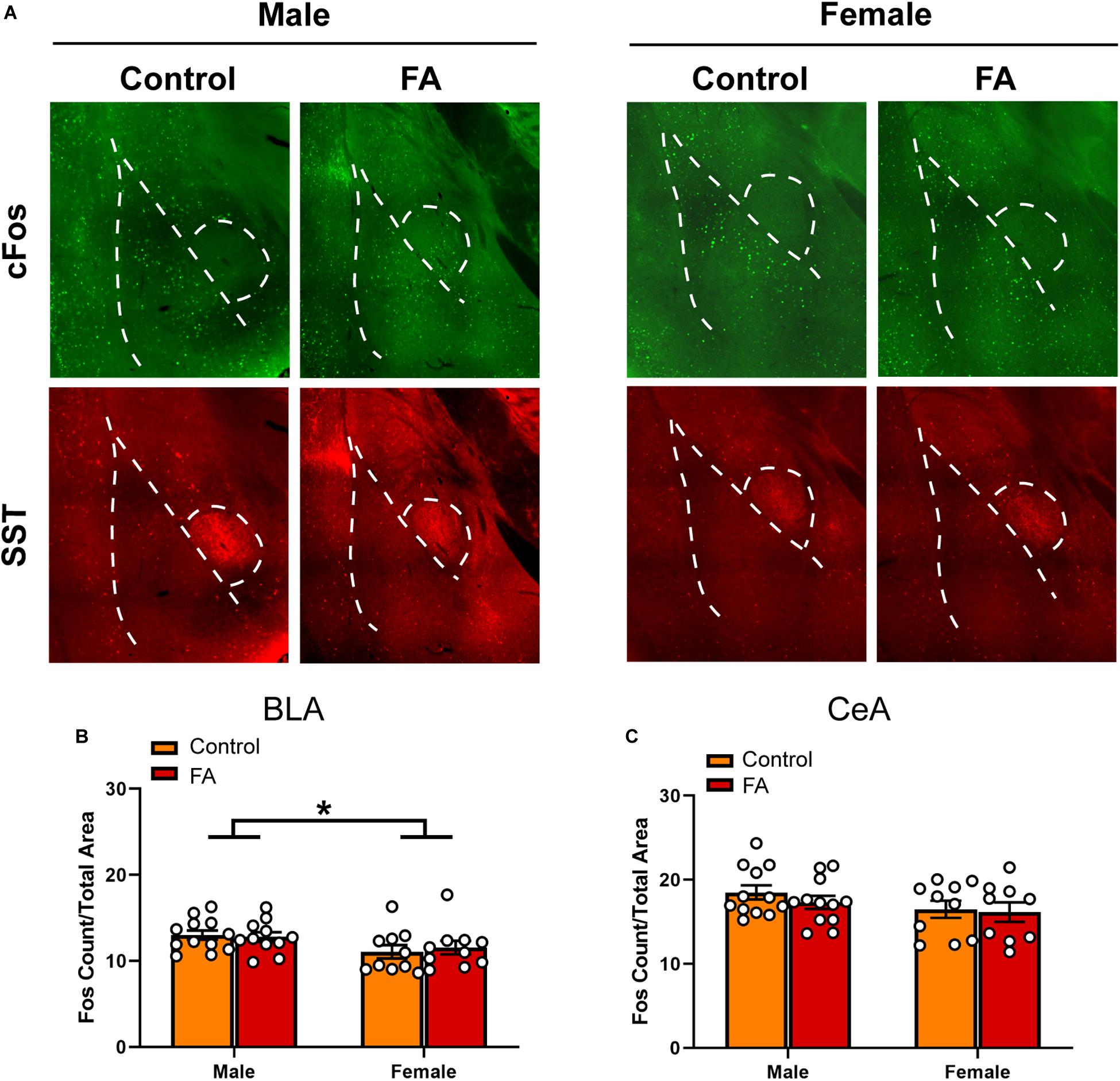
Figure 5. Forced abstinence from alcohol did not influence neuronal activation following forced swim stress in the BLA and CeA. (A) Representative images of cFos (green) and SST (red) immunofluorescence in the amygdala with the BLA and CeA delineated for quantification. (B) Male mice have a higher number of cfos nuclei in the BLA than female mice, and there was no significant difference in the number of cFos nuclei between FA conditions. (C) There was no significant difference in the number of cFos nuclei in the CeA between sexes and FA conditions.
In the CeA (Figure 5A), there was no main sex effect [Fsex(1, 39) = 2.836, p = 0.100], no main FA effect [FFA(1, 39) = 0.650, p = 0.424], and no sex by FA interaction [Fsex × FA(1, 39) = 0.2147, p = 0.645; Figure 5C] in FST-induced cFos expression. SST immunoreactivity in the CeA was unaltered by FA or sex [FFA(1, 39) = 0.063, p = 0.802, Fsex(1, 39) = 0.022, p = 0.881, Fsex × FA(1, 39) = 0.006, p = 0.934, Table 1]. Because of this, electrophysiology was not conducted in the BLA and lateral CeA.
Discussion
The current study aimed to replicate previously published findings of abstinence-induced depression in both male and female rodents, and to understand the role SST neurons throughout the brain may play in this phenotype. Both male and female C57BL/6J mice showed an increased depressive-like behavioral profile following forced abstinence from alcohol, without aberrations in anxiety-like behaviors. Though both sexes showed a decrease in sucrose preference (a behavioral phenotype classically interpreted as anhedonia), only male mice showed an increased time spent immobile in the FST. Additionally, we observed plasticity in cortical and amygdalar areas of FA-exposed female mice in response to forced swim stress, including alterations in neuronal activation and intrinsic excitability of the GABAergic SST neurons in the PFC and vBNST. These findings highlight the efficacy of this alcohol exposure procedure to model protracted, forced abstinence-induced affective disturbances in both sexes, as well as shed insight into the circuit and cell type-specific adaptations.
Our results corroborate previous preclinical animal studies on sex differences in voluntary alcohol drinking (Almeida et al., 1998; Priddy et al., 2017; Crowley et al., 2019a; Peltier et al., 2019) in which female mice consistently consumed more alcohol than male mice across 6 weeks of access, despite both sexes showing similar alcohol preference over water (Figures 1B,C). In humans, while women historically tend to consume less alcohol than men (Rehm et al., 2010), this gap is rapidly closing with substantial increases in the prevalence of alcohol use and binge drinking among women and not men (White et al., 2015; Grucza et al., 2018). Women are also more susceptible to development of alcohol-associated neuropsychiatric disorders, including MDD, anxiety, post-traumatic stress disorder (PTSD) and stress-mediated relapse in alcohol use (Grant et al., 2017; Peltier et al., 2019). A lack of observable differences in anxiety-like behaviors in the EPM and OFT between control and FA-exposed mice in our study could indicate a transition from the heightened anxious state associated with acute withdrawal and early abstinence (Lee et al., 2015; Pleil et al., 2015a) to a more depressive-like state during protracted abstinence (Driessen, 2001; Stevenson et al., 2009; Heilig et al., 2010).
Previous studies using the same two-bottle choice paradigm demonstrated that female mice develop a heightened depressive-like behavioral phenotype during protracted abstinence from alcohol, as indicated by longer time spent immobile in the FST and longer latency to feed in the novelty-suppressed feeding test (NSFT), that can be alleviated by physical activity (Pang et al., 2013) or administration of the novel fast-acting antidepressant ketamine (Holleran et al., 2016; Vranjkovic et al., 2018). Interestingly, here we observed a more robust depressive-like behavioral phenotype in the FST in FA-exposed male mice, and not FA-exposed female mice. One hypothesis for these differences is that females transition to the abstinence-induced depressive-like state at a more rapid rate than males, such that heightened depressive-like behaviors in females may be observable at an earlier timepoint during abstinence. Alcohol-dependent men and women progress through courses of mood states at different rates during alcohol withdrawal (Bokström et al., 1991). Future experiments should assess the same depressive-like behavioral assays across multiple timepoints during protracted withdrawal. Another possibility is that male and female mice cope with acute stress and manifest depressive-like behaviors differently. Multiple studies in the literature (Kokras et al., 2015; Colom-Lapetina et al., 2017, 2019; Molendijk and de Kloet, 2019) have cautioned against over-interpretation of immobility duration in the FST, which classically has been interpreted as ‘behavioral despair’ or diminished motivation to escape a stressful environment (Porsolt et al., 1977). Recent work from Colom-Lapetina et al. (2017, 2019) suggests that female rats employ more active strategies to cope with the FST, including climbing and headshaking, while males employ the more classic strategy of immobility. Similar sex differences in coping strategy have also been found in conditioned fear response (Gruene et al., 2015a). Furthermore, we observed a sex difference in the frequency of open arm entries in the EPM (Figure 2B), where more entries into the open arm is classically interpreted as lower level of anxiety. Overall, our results suggest that the behavioral adaptations in response to acute swim stress following forced abstinence may be more nuanced in female mice, and future studies should employ a more fine-tuned behavioral analysis to fully elucidate the affective perturbations associated with alcohol exposure and abstinence.
The corticolimbic circuit comprises of highly interconnected regions, including the medial prefrontal cortex (mPFC) and the amygdala and its subregions, that are critically involved in the regulation of emotional behaviors, stress response and reward seeking (Koob, 2009; Koob and Volkow, 2016). The mPFC, including the prelimbic (PL) and infralimbic (IL) subregions, receives glutamatergic inputs from the BLA that can promote anxiety-like behaviors (Felix-Ortiz et al., 2016) and are modulated by chronic stress (Lowery-Gionta et al., 2018; Marcus et al., 2019). Acute withdrawal from alcohol exposure has been found to disrupt synaptic transmission and intrinsic excitability of pyramidal neurons of the mPFC at different stages of alcohol dependence; particularly, the dependence-inducing chronic intermittent ethanol (CIE) model enhances excitatory drive and intrinsic excitability in pyramidal neurons (Pleil et al., 2015b; Cannady et al., 2018), whereas the drinking-in-the-dark (DID) model of pre-dependence binge-like drinking impaired excitatory transmission (Crowley et al., 2019b). Recent evidence further demonstrates an interaction between sex and alcohol exposure on the PFC neurophysiology. Chronic gavage administration of high-dose ethanol enhances excitability of Martinotti neurons (presumably SST neurons) in male PL but decreases excitability of female Martinotti neurons via a reduction in hyperpolarization-induced cation channel (HCN) current (Hughes et al., 2020). Interestingly, this model of alcohol exposure disrupts inhibitory transmission in layer 5/6 pyramidal neurons in both sexes (Hughes et al., 2019), likely via an uncharacterized microcircuit mechanism. Excitatory transmission in PL layer 2/3 pyramidal neurons is decreased following four cycles of DID via distinct cellular mechanisms between the sexes (Crowley et al., 2019a), in which DID targeted cell-surface expression of NMDA and AMPA receptors alterations occur in female PL only. Sex also modulates the effects of stress on the cortical circuitry. Male rats with high level of freezing in fear conditioning have higher spine density in IL neurons that project to the BLA than male rats with low level of freezing (Gruene et al., 2015b), but the effect is not observable in females. Reciprocal projections from the BLA to the PL and IL are more severely affected by early life adversity in females than in males (Honeycutt et al., 2020). Chronic unpredictable stress induces sex-specific alterations in the transcriptomic profiles of SST neurons in the PFC (Girgenti et al., 2019). Here our FST-induced cFos expression data revealed that forced abstinence from alcohol dampened neuronal activation in the female PFC (Figure 3B). Hypoactivation of the mPFC in response to stress and subsequent dysregulation of amygdala activity have indeed been observed in humans with MDD and alcoholism (Johnstone et al., 2007; Covington et al., 2010; Seo et al., 2013), as well as rodents in chronic social defeat (Covington et al., 2010) and chronic unpredictable stress (Lam et al., 2018). Withdrawal from CIE exposure similarly reduces cFos expression in the PL and IL of male mice (Smith et al., 2019). Our model, which combines both stress (potential hypoactivation of PFC) and alcohol (potential hyperactivation) may result in more nuanced alterations in this region.
The downregulation of neuronal activation in the PFC may have resulted from augmented inhibitory tone from the GABAergic SST neurons, as we identified an increase in intrinsic excitability of these neurons (Figures 3H, 6). In the cortex, SST neurons are often postulated to be the gatekeeper of thalamo- and cortico-cortical excitatory inputs to pyramidal neurons, via their soma- and dendrite-targeting synapses (Urban-Ciecko and Barth, 2016). These neurons recently gained substantial interest for their role in a host of neuropsychiatric disorders, including MDD, bipolar disorder, and schizophrenia (Fee et al., 2017; Pantazopoulos et al., 2017; Abbas et al., 2018). Global disinhibition of SST neurons via deletion of GABAA receptors expressing the Υ2 subunit produces an antidepressant-like behavioral and neural profile (Fuchs et al., 2017). Acute, chemogenetically induced inhibition of SST neurons in the mPFC is anxiogenic (Soumier and Sibille, 2014), pointing to an overall resiliency-conferring role of SST neurons in the cortex. In our study, it is unclear whether the hyperexcitability of SST neurons following forced abstinence from alcohol directly contributes to the depressive-like states, or is in fact a compensating mechanism to counteract the alcohol-induced adaptations. The latter interpretation, that SST hyperexcitability may be in response to alcohol-induced adaptations, is further supported by the opposing results seen in the vBNST. Future studies should further examine whether SST neurons in the PFC has a causal role in alcohol-related pathological behavioral adaptations.
The extended amygdala, including the BNST and the CeA, is critically involved in regulation of alcohol consumption and withdrawal-associated negative affect (Koob, 2009; Koob and Volkow, 2016; Vranjkovic et al., 2017; Torruella-Suárez et al., 2020). As in the mPFC, withdrawal from both the CIE and DID models modifies synaptic transmission and excitability in the extended amygdala, with heavy emphasis on hyperexcitability in the vBNST and enhanced inhibition of the CeA (Roberto et al., 2010; Lee et al., 2015; Pleil et al., 2015a; Smith et al., 2019). In accordance with these results, our FST-induced cFos expression data demonstrated elevated neuronal activation in the vBNST of FA-exposed female mice (Figure 4D). SST neurons in the vBNST were also highly activated in response to FST in FA-exposed female, whereas this activation was very minimal in all other groups (Figure 4E). Contrastingly, FA dampens intrinsic excitability in SST neurons of female mice, with fewer numbers of action potentials in response to depolarizing step currents (Figure 4J), which could reflect a compensating mechanism to counteract the heightened engagement of vBNST SST neurons following FST. These data suggest that forced abstinence from alcohol may remodel the engagement of the stress-responsive networks in the vBNST, including SST neurons (Figure 6).
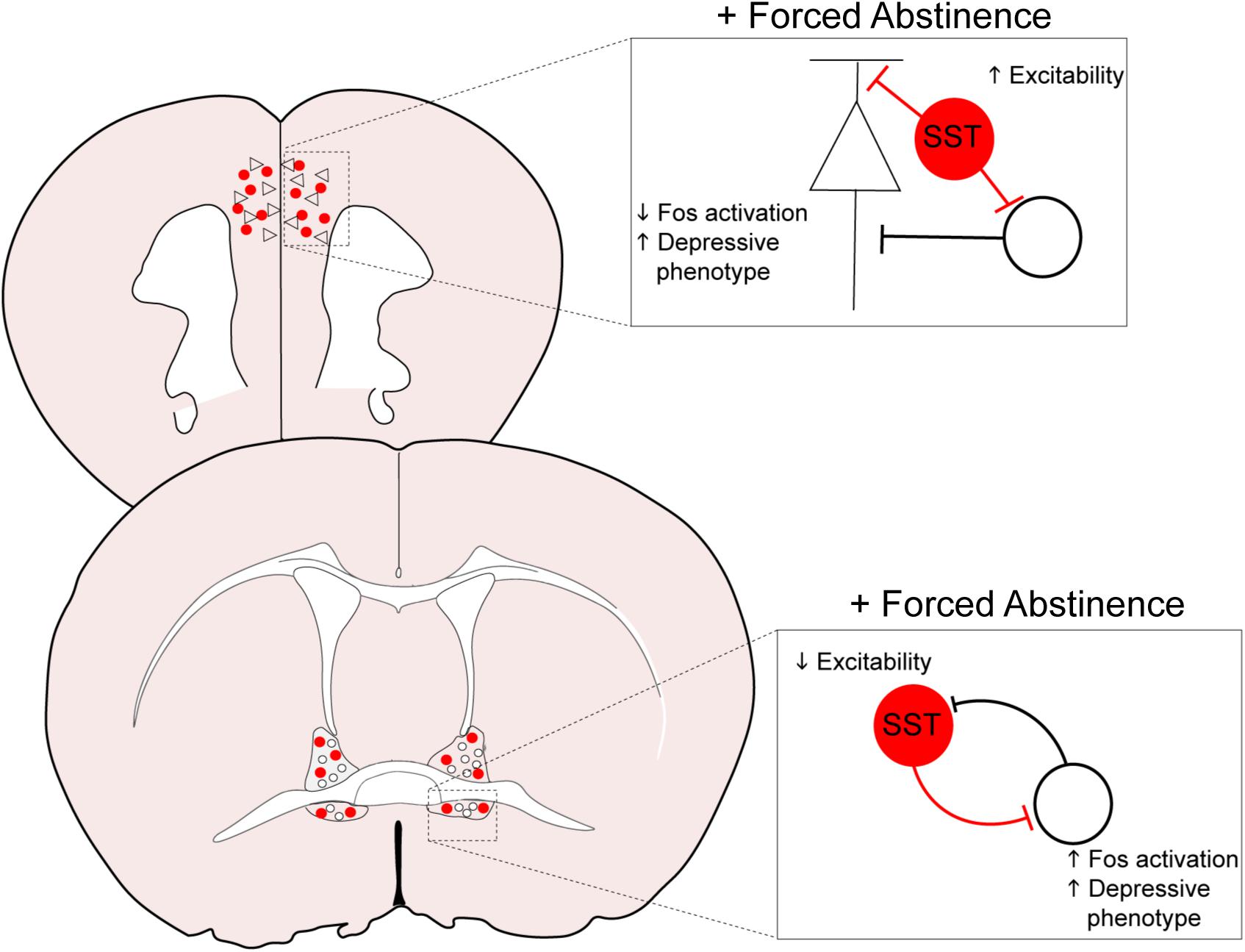
Figure 6. Overall model of neuroadaptations in cortical and amygdalar areas following forced abstinence from alcohol. In the PFC, forced abstinence-induced hyperexcitability of the GABAergic SST neurons may enhance inhibitory tone onto pyramidal neurons and other neighboring populations to dampen neuronal activation in response to forced swim stress. SST neurons in the vBNST otherwise showed diminished intrinsic excitability and consequently disinhibited the local network to enhance neuronal activation in response to forced swim stress.
The circuit organization and functionality of these neuronal populations in the extended amygdala are not fully characterized. There is evidence suggesting that SST neurons in the lateral CeA directly synapse onto SST neurons in the oval nucleus of the BNST (Ahrens et al., 2018). Enhancing excitatory drive on the CeA SST neurons paradoxically disinhibits SST neurons in the oval nucleus in a kappa opioid receptor dependent manner to promote anxiety (Ahrens et al., 2018). As we did not observe any changes in anxiety-like behaviors, or any SST neuron-specific plasticity in the lateral CeA and the dorsal BNST, it is possible that the neuroadaptations in these anxiety-promoting populations may be transient and occur during acute withdrawal, subsiding before transition to a depressive-like state. The otherwise robust plasticity in the vBNST SST neurons may implicate a functional distinction between the dorsal and ventral subregions of the BNST. In addition to a dense population of corticotropin-releasing factor (CRF) neurons that are anxiogenic and alcohol drinking-promoting (Pleil et al., 2015b), the vBNST includes an anxiolytic population of GABAergic neurons that project to the ventral tegmental area and the lateral hypothalamus (Marcinkiewcz et al., 2016; Pati et al., 2019). Withdrawal from CIE exposure augments excitability of CRF neurons and increases inhibition onto the anxiolytic midbrain-projecting neurons (Pati et al., 2019). Forced abstinence-induced decrease in intrinsic excitability of SST neurons in the vBNST may result in disinhibition of the CRF neurons to exacerbate negative affect and heighten the risk of stress-induced relapse. Nevertheless, there is no current evidence of direct synaptic input from SST neurons to CRF neurons or midbrain-projecting neurons in the vBNST. Further complicating the matter is the fact that the BNST organization and functionality in females have not been examined to the same degree as in males. In adult men, somatostatin and vasoactive intestinal peptide (VIP) immunostaining reveal larger BNST volume than in adult women (Chung et al., 2002). In rats, female BNST SST neurons do not express estrogen receptors and androgen receptors that are prevalent in male SST neurons (Herbison and Theodosis, 1993; Fernández-Guasti et al., 2000), suggesting that the FA-induced behavioral and neuronal adaptations in females might not be modulated by gonadal hormones. Studies examining the sex-specific properties of the BNST in regulation of emotions and reward seeking, and sex differences in AUD-related neuroadaptations in the BNST, are lacking. Future studies should examine the input and output pathways of these SST populations, as well as their functional role in regulation of alcohol drinking and emotional behaviors with an eye toward sex-specific properties.
Notably, we observed a consistent sex effect in total cFos expression the PFC and the dorsal and ventral subregions of the BNST, in which male mice showed markedly higher level of FST-induced cFos expression (Figures 3B, 4B,D). Given the fact that the majority of the previously cited studies on alcohol-induced neuroplasticity were performed in males, it is interesting to see that FA-exposed male mice seem to be relatively resilient against protracted forced abstinence-induced alterations. One possibility is that, since male mice consumed less alcohol than female mice in the two-bottle choice paradigm when normalized to body weight, the effect of forced abstinence from alcohol in male mice may be too subtle to be detected by the methods employed here. Additionally, as discussed with the sex differences in coping strategies in response to forced swim stress, passive coping in male mice may engage a different circuit and cell-type specificity that could be overlooked. These observations further highlight the importance of sex as a biological variate in neurobiological studies in order to have a better understanding of the pathophysiology underlying neuropsychiatric disorders.
We did not observe any changes in SST protein expression in any cortical or amygdalar regions. We have previously reported that SST neurons in the PL cortex release SST peptide tonically and phasically in a frequency-dependent manner (Dao et al., 2019), hence altered excitability of these neurons in the PFC and the BNST may affect their capacity for neuropeptide release. The SST peptide acts on a family of G protein-coupled receptors, that are mainly inhibitory through Gi/o-dependent signaling (Barnett, 2003). However, SST receptor signaling in these regions has not been characterized. Future studies may merit from examination of the modulatory action of SST peptide, in healthy states and alcohol abuse-associated conditions.
Conclusion
The current study identified a host of sex-dependent behavioral adaptations and neuroplasticity in corticolimbic regions that may underlie forced abstinence-induced affective perturbations. SST neurons in the PFC and vBNST emerge as a strong neural candidate that undergo robust plasticity following alcohol exposure and protracted abstinence. These results shed new insight into the neurobiological bases of highly comorbid neuropsychiatric diseases, including MDD and AUDs, and may aid in the development of new and effective treatments.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by the Pennsylvania State University Institutional Animal Care and Use Committee.
Author Contributions
ND and NC conceived and designed the experiments, analyzed and interpreted the experiments, and wrote the manuscript with feedback from all authors. ND, MS, SM, JM, VS, and DB conducted the experiments.
Funding
The experiments were funded by the Brain and Behavior Research Foundation (NARSAD Young Investigator Award; NC), The National Institute on Alcohol Abuse and Alcoholism (R21AA028008; NC), and the Penn State’s Social Science Research Institute (Level 2 Award; NC).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Dr. Janine Kwapis (Department of Biology, Pennsylvania State University) for assistance in imaging.
References
Abbas, A. I., Sundiang, M. J. M., Henoch, B., Morton, M. P., Bolkan, S. S., Park, A. J., et al. (2018). Somatostatin interneurons facilitate hippocampal-prefrontal synchrony and prefrontal spatial encoding. Neuron 100, 926–939. doi: 10.1016/j.neuron.2018.09.029
Ahrens, S., Wu, M. V., Furlan, A., Hwang, G. R., Paik, R., Li, H., et al. (2018). A central extended amygdala circuit that modulates anxiety. J. Neurosci. 38, 5567–5583. doi: 10.1523/JNEUROSCI.0705-18.2018
Almeida, O. F. X., Shoaib, M., Deicke, J., Fischer, D., Darwish, M. H., and Patchev, V. K. (1998). Gender differences in ethanol preference and ingestion in rats: the role of the gonadal steroid environment. J. Clin. Invest. 101, 2677–2685. doi: 10.1172/JCI1198
Annis, H. M., Sklar, S. M., and Moser, A. E. (1998). Gender in relation to relapse crisis situations, coping, and outcome among treated alcoholics. Addict. Behav. 23, 127–131. doi: 10.1016/S0306-4603(97)00024-5
Barnett, P. (2003). Somatostatin and somatostatin receptor physiology. Endocrine 20, 255–264. doi: 10.1385/endo:20:3:255
Becker, H. C. (2014). “Alcohol dependence, withdrawal, and relapse,” in Neurobiology of Alcohol Dependence, eds A. B. C. Noronha, C. Cui, R. A. Harris, and J. C. Crabbe (Cham: Springer), 377–410. doi: 10.1016/B978-0-12-405941-2.00019-5
Bokström, K., Balldin, J., and Långström, G. (1991). Individual mood profiles in alcohol withdrawal. Alcohol. Clin. Exp. Res. 15, 508–513. doi: 10.1111/j.1530-0277.1991.tb00552.x
Cannady, R., Rinker, J. A., Nimitvilai, S., Woodward, J. J., and Mulholland, P. J. (2018). Chronic alcohol, intrinsic excitability, and potassium channels: neuroadaptations and drinking behavior. Handb. Exper. Pharmacol. 248, 311–343. doi: 10.1007/164_2017_90
Chung, W. C. J., De Vries, G. J., and Swaab, D. F. (2002). Sexual differentiation of the bed nucleus of the stria terminalis in humans may extend into adulthood. J. Neurosci. 22, 1027–1033. doi: 10.1523/jneurosci.22-03-01027.2002
Colom-Lapetina, J., Begley, S. L., Johnson, M. E., Bean, K. J., Kuwamoto, W. N., and Shansky, R. M. (2017). Strain-dependent sex differences in a long-term forced swim paradigm. Behav. Neurosci. 131, 428–436. doi: 10.1037/bne0000215
Colom-Lapetina, J., Li, A. J., Pelegrina-Perez, T. C., and Shansky, R. M. (2019). Behavioral diversity across classic rodent models is sex-dependent. Front. Behav. Neurosci. 13:45. doi: 10.3389/fnbeh.2019.00045
Covington, H. E., Lobo, M. K., Maze, I., Vialou, V., Hyman, J. M., Zaman, S., et al. (2010). Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J. Neurosci. 30, 16082–16090. doi: 10.1523/JNEUROSCI.1731-10.2010
Crowley, N. A., Bloodgood, D. W., Hardaway, J. A., Kendra, A. M., McCall, J. G., Al-Hasani, R., et al. (2016). Dynorphin controls the gain of an amygdalar anxiety circuit. Cell Rep. 14, 2774–2783. doi: 10.1016/j.celrep.2016.02.069
Crowley, N. A., Dao, N. C., Magee, S. N., Bourcier, A. J., and Lowery-Gionta, E. G. (2019a). Animal models of alcohol use disorder and the brain: from casual drinking to dependence. Transl. Issues Psychol. Sci. 5, 222–242. doi: 10.1037/tps0000198
Crowley, N. A., Magee, S. N., Feng, M., Jefferson, S. J., Morris, C. J., Dao, N. C., et al. (2019b). Ketamine normalizes binge drinking-induced defects in glutamatergic synaptic transmission and ethanol drinking behavior in female but not male mice. Neuropharmacology 149, 35–44. doi: 10.1016/j.neuropharm.2019.02.003
Dao, N. C., Brockway, D. F., and Crowley, N. A. (2019). In vitro optogenetic characterization of neuropeptide release from prefrontal cortical somatostatin neurons. Neuroscience 419, 1–4. doi: 10.1016/j.neuroscience.2019.08.014
Douillard-Guilloux, G., Lewis, D., Seney, M. L., and Sibille, E. (2017). Decrease in somatostatin-positive cell density in the amygdala of females with major depression. Depress. Anxiety 34, 68–78. doi: 10.1002/da.22549
Driessen, M. (2001). The course of anxiety, depression and drinking behaviours after completed detoxification in alcoholics with and without comorbid anxiety and depressive disorders. Alcohol Alcohol. 36, 249–255. doi: 10.1093/alcalc/36.3.249
Fee, C., Banasr, M., and Sibille, E. (2017). Somatostatin-positive gamma-aminobutyric acid interneuron deficits in depression: cortical microcircuit and therapeutic perspectives. Biol. Psychiatry 82, 549–559. doi: 10.1016/j.biopsych.2017.05.024
Felix-Ortiz, A. C., Burgos-Robles, A., Bhagat, N. D., Leppla, C. A., and Tye, K. M. (2016). Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience 321, 197–209. doi: 10.1016/j.neuroscience.2015.07.041
Fernández-Guasti, A., Kruijver, F. P. M., Fodor, M., and Swaab, D. F. (2000). Sex differences in the distribution of androgen receptors in the human hypothalamus. J. Comp. Neurol. 7, 545–553.
Fuchs, T., Jefferson, S. J., Hooper, A., Yee, P. H., Maguire, J., and Luscher, B. (2017). Disinhibition of somatostatin-positive GABAergic interneurons results in an anxiolytic and antidepressant-like brain state. Mol. Psychiatry 22, 920–930. doi: 10.1038/mp.2016.188
Ghosal, S., Duman, C. H., Liu, R. J., Wu, M., Terwilliger, R., Girgenti, M. J., et al. (2020). Ketamine rapidly reverses stress-induced impairments in GABAergic transmission in the prefrontal cortex in male rodents. Neurobiol. Dis. 130:104669. doi: 10.1016/j.nbd.2019.104669
Girgenti, M. J., Wohleb, E. S., Mehta, S., Ghosal, S., Fogaca, M. V., and Duman, R. S. (2019). Prefrontal cortex interneurons display dynamic sex-specific stress-induced transcriptomes. Transl. Psychiatry 9:292. doi: 10.1038/s41398-019-0642-z
Grant, B. F., Chou, S. P., Saha, T. D., Pickering, R. P., Kerridge, B. T., Ruan, W. J., et al. (2017). Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the national epidemiologic survey on alcohol and related conditions. JAMA Psychiatry 74, 911–923. doi: 10.1001/jamapsychiatry.2017.2161
Grucza, R. A., Sher, K. J., Kerr, W. C., Krauss, M. J., Lui, C. K., McDowell, Y. E., et al. (2018). Trends in adult alcohol use and binge drinking in the early 21st-century united states: a meta-analysis of 6 national survey series. Alcohol. Clin. Exp. Res. 42, 1939–1950. doi: 10.1111/acer.13859
Gruene, T. M., Flick, K., Stefano, A., Shea, S. D., and Shansky, R. M. (2015a). Sexually divergent expression of active and passive conditioned fear responses in rats. eLife 4:e11352. doi: 10.7554/elife.11352
Gruene, T. M., Roberts, E., Thomas, V., Ronzio, A., and Shansky, R. M. (2015b). Sex-specific neuroanatomical correlates of fear expression in prefrontal-amygdala circuits. Biol. Psychiatry 78, 186–193. doi: 10.1016/j.biopsych.2014.11.014
Heilig, M., Egli, M., Crabbe, J. C., and Becker, H. C. (2010). Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict. Biol. 15, 169–184. doi: 10.1111/j.1369-1600.2009.00194.x
Herbison, A. E., and Theodosis, D. T. (1993). Absence of estrogen receptor immunoreactivity in somatostatin (SRIF) neurons of the periventricular nucleus but sexually dimorphic colocalization of estrogen receptor and SRIF immunoreactivities in neurons of the bed nucleus of the stria terminalis. Endocrinology 132, 1707–1714. doi: 10.1210/endo.132.4.7681764
Hershon, H. I. (1977). Alcohol withdrawal symptoms and drinking behavior. J. Stud. Alcohol 38, 953–971. doi: 10.15288/jsa.1977.38.953
Holleran, K. M., Wilson, H. H., Fetterly, T. L., Bluett, R. J., Centanni, S. W., Gilfarb, R. A., et al. (2016). Ketamine and MAG lipase inhibitor-dependent reversal of evolving depressive-like behavior during forced abstinence from alcohol drinking. Neuropsychopharmacology 41, 2062–2071. doi: 10.1038/npp.2016.3
Holzhauer, C. G., and Gamble, S. A. (2017). Depressive symptoms mediate the relationship between changes in emotion regulation during treatment and abstinence among women with alcohol use disorders. Psychol. Addict. Behav. 31, 284–294. doi: 10.1037/adb0000274
Honeycutt, J. A., Demaestri, C., Peterzell, S., Silveri, M. M., Cai, X., Kulkarni, P., et al. (2020). Altered corticolimbic connectivity reveals sex-specific adolescent outcomes in a rat model of early life adversity. eLife 9:e52651. doi: 10.7554/eLife.52651
Hughes, B. A., Bohnsack, J. P., O’Buckley, T. K., Herman, M. A., and Morrow, A. L. (2019). Chronic ethanol exposure and withdrawal impair synaptic GABAA receptor-mediated neurotransmission in deep-layer prefrontal cortex. Alcohol. Clin. Exp. Res. 43, 822–832. doi: 10.1111/acer.14015
Hughes, B. A., Crofton, E. J., O’Buckley, T. K., Herman, M. A., and Morrow, A. L. (2020). Chronic ethanol exposure alters prelimbic prefrontal cortical Fast-spiking and martinotti interneuron function with differential sex specificity in rat brain. Neuropharmacology 162:107805. doi: 10.1016/j.neuropharm.2019.107805
Johnstone, T., Van Reekum, C. M., Urry, H. L., Kalin, N. H., and Davidson, R. J. (2007). Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J. Neurosci. 27, 8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007
Kokras, N., Antoniou, K., Mikail, H. G., Kafetzopoulos, V., Papadopoulou-Daifoti, Z., and Dalla, C. (2015). Forced swim test: what about females? Neuropharmacology 99, 408–421. doi: 10.1016/j.neuropharm.2015.03.016
Koob, G. F. (2009). Brain stress systems in the amygdala and addiction. Brain Res. 1293, 61–75. doi: 10.1016/j.brainres.2009.03.038
Koob, G. F., and Volkow, N. D. (2016). Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3, 760–773. doi: 10.1016/S2215-0366(16)00104-8
Lam, V. Y. Y., Raineki, C., Takeuchi, L. E., Ellis, L., Woodward, T. S., and Weinberg, J. (2018). Chronic stress alters behavior in the forced swim test and underlying neural activity in animals exposed to alcohol prenatally: sex- and time-dependent effects. Front. Behav. Neurosci. 12:42. doi: 10.3389/fnbeh.2018.00042
Lee, K. M., Coehlo, M., McGregor, H. A., Waltermire, R. S., and Szumlinski, K. K. (2015). Binge alcohol drinking elicits persistent negative affect in mice. Behav. Brain Res. 291, 385–398. doi: 10.1016/j.bbr.2015.05.055
Lowery-Gionta, E. G., Crowley, N. A., Bukalo, O., Silverstein, S., Holmes, A., and Kash, T. L. (2018). Chronic stress dysregulates amygdalar output to the prefrontal cortex. Neuropharmacology 139, 68–75. doi: 10.1016/j.neuropharm.2018.06.032
Marcinkiewcz, C. A., Mazzone, C. M., D’Agostino, G., Halladay, L. R., Hardaway, J. A., Diberto, J. F., et al. (2016). Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature 537, 97–101. doi: 10.1038/nature19318
Marcus, D. J., Bedse, G., Gaulden, A., Ryan, J. D., Clauss, J., Delpire, E., et al. (2019). Endocannabinoid signaling collapse mediates stress-induced amygdalo-cortical strengthening. Neuron 105, 1062–1076. doi: 10.1016/j.neuron.2019.12.024
Molendijk, M. L., and de Kloet, E. R. (2019). Coping with the forced swim stressor: current state-of-the-art. Behav. Brain Res. 364, 1–10. doi: 10.1016/j.bbr.2019.02.005
Pang, T. Y., Renoir, T., Du, X., Lawrence, A. J., and Hannan, A. J. (2013). Depression-related behaviours displayed by female C57BL/6J mice during abstinence from chronic ethanol consumption are rescued by wheel-running. Eur. J. Neurosci. 37, 1803–1810. doi: 10.1111/ejn.12195
Pantazopoulos, H., Wiseman, J. T., Markota, M., Ehrenfeld, L., and Berretta, S. (2017). Decreased numbers of somatostatin-expressing neurons in the amygdala of subjects with bipolar disorder or schizophrenia: relationship to circadian rhythms. Biol. Psychiatry 81, 536–547. doi: 10.1016/j.biopsych.2016.04.006
Pati, D., Marcinkiewcz, C. A., DiBerto, J. F., Cogan, E. S., McElligott, Z. A., and Kash, T. L. (2019). Chronic intermittent ethanol exposure dysregulates a GABAergic microcircuit in the bed nucleus of the stria terminalis. Neuropharmacology 168:107759. doi: 10.1016/j.neuropharm.2019.107759
Peltier, M. R., Verplaetse, T. L., Mineur, Y. S., Petrakis, I. L., Cosgrove, K. P., Picciotto, M. R., et al. (2019). Sex differences in stress-related alcohol use. Neurobiol. Stress 10:100149. doi: 10.1016/j.ynstr.2019.100149
Pleil, K. E., Lowery-Gionta, E. G., Crowley, N. A., Li, C., Marcinkiewcz, C. A., Rose, J. H., et al. (2015a). Effects of chronic ethanol exposure on neuronal function in the prefrontal cortex and extended amygdala. Neuropharmacology 99, 735–749. doi: 10.1016/j.neuropharm.2015.06.017
Pleil, K. E., Rinker, J. A., Lowery-Gionta, E. G., Mazzone, C. M., McCall, N. M., Kendra, A. M., et al. (2015b). NPY signaling inhibits extended amygdala CRF neurons to suppress binge alcohol drinking. Nat. Neurosci. 18, 545–552. doi: 10.1038/nn.3972
Porsolt, R. D., Bertin, A., and Jalfre, M. (1977). Behavioral despair in mice: a primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 229, 327–336.
Priddy, B. M., Carmack, S. A., Thomas, L. C., Vendruscolo, J. C. M., Koob, G. F., and Vendruscolo, L. F. (2017). Sex, strain, and estrous cycle influences on alcohol drinking in rats. Pharmacol. Biochem. Behav. 152, 61–67. doi: 10.1016/j.pbb.2016.08.001
Rehm, J., Taylor, B., Mohapatra, S., Irving, H., Baliunas, D., Patra, J., et al. (2010). Alcohol as a risk factor for liver cirrhosis: a systematic review and meta-analysis. Drug Alcohol Rev. 29, 437–445. doi: 10.1111/j.1465-3362.2009.00153.x
Roberto, M., Cruz, M. T., Gilpin, N. W., Sabino, V., Schweitzer, P., Bajo, M., et al. (2010). Corticotropin releasing factor-induced amygdala gamma-aminobutyric acid release plays a key role in alcohol dependence. Biol. Psychiatry 67, 831–839. doi: 10.1016/j.biopsych.2009.11.007
Sacks, J. J., Gonzales, K. R., Bouchery, E. E., Tomedi, L. E., and Brewer, R. D. (2015). 2010 National and state costs of excessive alcohol consumption. Am. J. Prev. Med. 49, 73–79. doi: 10.1016/j.amepre.2015.05.031
Seo, D., Lacadie, C. M., Tuit, K., Hong, K. I., Todd Constable, R., and Sinha, R. (2013). Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA Psychiatry 70, 727–739. doi: 10.1001/jamapsychiatry.2013.762
Smith, R. J., Anderson, R. I., Haun, H. L., Mulholland, P. J., Griffin, W. C., Lopez, M. F., et al. (2019). Dynamic c-Fos changes in mouse brain during acute and protracted withdrawal from chronic intermittent ethanol exposure and relapse drinking. Addict. Biol. 9:e12804. doi: 10.1111/adb.12804
Soumier, A., and Sibille, E. (2014). Opposing effects of acute versus chronic blockade of frontal cortex somatostatin-positive inhibitory neurons on behavioral emotionality in mice. Neuropsychopharmacology 39, 2252–2262. doi: 10.1038/npp.2014.76
Stevenson, J. R., Schroeder, J. P., Nixon, K., Besheer, J., Crews, F. T., and Hodge, C. W. (2009). Abstinence following alcohol drinking produces depression-like behavior and reduced hippocampal neurogenesis in mice. Neuropsychopharmacology 34, 1209–1222. doi: 10.1038/npp.2008.90
Ting, J. T., Lee, B. R., Chong, P., Soler-Llavina, G., Cobbs, C., Koch, C., et al. (2018). Preparation of acute brain slices using an optimized n-methyl-D-glucamine protective recovery method. J. Vis. Exp. 132:53825. doi: 10.3791/53825
Torruella-Suárez, M. L., Vandenberg, J. R., Cogan, E. S., Tipton, G. J., Teklezghi, A., Dange, K., et al. (2020). Manipulations of central amygdala neurotensin neurons alter the consumption of ethanol and sweet fluids in mice. J. Neurosci. 40, 632–647. doi: 10.1523/JNEUROSCI.1466-19.2019
Urban-Ciecko, J., and Barth, A. L. (2016). Somatostatin-expressing neurons in cortical networks. Nat. Rev. Neurosci. 17, 401–409. doi: 10.1038/nrn.2016.53
Valdez, G. R., and Harshberger, E. (2012). Kappa opioid regulation of anxiety-like behavior during acute ethanol withdrawal. Pharmacol. Biochem. Behav. 102, 44–47. doi: 10.1016/j.pbb.2012.03.019
Vranjkovic, O., Pina, M., Kash, T. L., and Winder, D. G. (2017). The bed nucleus of the stria terminalis in drug-associated behavior and affect: a circuit-based perspective. Neuropharmacology 122, 100–106. doi: 10.1016/j.neuropharm.2017.03.028
Vranjkovic, O., Winkler, G., and Winder, D. G. (2018). Ketamine administration during a critical period after forced ethanol abstinence inhibits the development of time-dependent affective disturbances. Neuropsychopharmacology 43, 1915–1923. doi: 10.1038/s41386-018-0102-0
Keywords: forced abstinence, alcohol, sex, stress, somatostatin, prefrontal cortex, extended amygdala
Citation: Dao NC, Suresh Nair M, Magee SN, Moyer JB, Sendao V, Brockway DF and Crowley NA (2020) Forced Abstinence From Alcohol Induces Sex-Specific Depression-Like Behavioral and Neural Adaptations in Somatostatin Neurons in Cortical and Amygdalar Regions. Front. Behav. Neurosci. 14:86. doi: 10.3389/fnbeh.2020.00086
Received: 24 March 2020; Accepted: 07 May 2020;
Published: 27 May 2020.
Edited by:
Jessica R. Barson, Drexel University College of Medicine, United StatesReviewed by:
Sachin Patel, Vanderbilt University Medical Center, United StatesCharlis Raineki, The University of British Columbia, Canada
Katherine Mercedes Holleran, Wake Forest Baptist Medical Center, United States
Copyright © 2020 Dao, Suresh Nair, Magee, Moyer, Sendao, Brockway and Crowley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicole A. Crowley, bnpjMjdAcHN1LmVkdQ==
†Present address: Sarah N. Magee, Department of Pharmacology, The University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
 Nigel C. Dao
Nigel C. Dao Malini Suresh Nair
Malini Suresh Nair Sarah N. Magee2†
Sarah N. Magee2† Nicole A. Crowley
Nicole A. Crowley