- 1Social Neuropsychiatry Department, Bekhterev National Medical Research Center for Psychiatry and Neurology, Saint-Petersburg, Russia
- 2Department of Natural Sciences, Technology and Environmental Studies, Södertörn University, Stockholm, Sweden
- 3Geriatric Psychiatry Department, Bekhterev National Medical Research Center for Psychiatry and Neurology, Saint-Petersburg, Russia
- 4Psychiatry and Addictions Department, Pavlov First Saint-Petersburg State Medical University, Saint-Petersburg, Russia
Currently, there is little published data on the effects of antidepressants on normal gut microbiota and the consequences of such effects on treatment outcomes.
The aim of the study: was to evaluate the growth kinetics of normal human gut microorganisms with antidepressants most common in routine clinical practice.
Materials and methods: Research objects were species of microorganisms representing normal gut microbiota: Staphylococcus aureus ATCC 25923, Escherichia coli ATCC 25922, Candida albicans ATCC 24433, Bifidobacterium 791, Enterococcus faecalis ATCC 29212, Lactobacillus rhamnosus ATCC 53103. All microorganisms were cultivated in Schaedler broth (HiMedia) under aerobic/anaerobic conditions. The active substances of all studied antidepressants (fluvoxamine, fluoxetine, escitalopram, duloxetine, venlafaxine, mirtazapine) were extracted from ground preparations by dimethyl sulfoxide and centrifuged. Each solution of antidepressants was added to a Schaedler broth containing a certain microorganism’s strain and diluted to final concentrations—200 μg/ml, 500 μg/ml, and 700 μg/ml. For a quantitative assessment of the effect, the specific growth rates (μ, h-1) of microorganisms were calculated as the slope of the initial part of the growth curve in coordinates (lnA, t). To evaluate the antidepressant effects on representatives of the normal microbiota in vitro, the following parameters were chosen: specific growth rate and IC50.
Results: All antidepressants had an inhibitory effect on the growth of all studied microorganisms. Fluvoxamine and venlafaxine had the least effect on the growth activity of all studied microorganisms. Fluoxetine showed a pronounced effect on growth activity against E. coli, E. feacalis, S. aureus, and the least effect against C. albicans. Escitalopram had a greater effect on the growth rate of E. coli, E. feacalis, B. bifidum, L. rhamnosus, and C. albicans, which puts it among the leaders in terms of its effect on the growth activity of the microorganisms we studied. Mirtazapine, according to the results of our experiment, showed the greatest activity against L. rhamnosus and C. albicans.
Conclusions: Our results confirm the effects of antidepressants on the growth activity of the normal gut microbiota individual strains. Further study of the antimicrobial activity of antidepressants may become one of the new directions for optimizing the personalized therapy of patients with depression.
1. Introduction
The data from the preclinical studies showed that the microbiota and the whole microbiome both could play a significant role in neurodegenerative and neuropsychiatric disorders (e.g., depressive disorder, bipolar disorder, schizophrenia, autism, Parkinson’s disease; Kelly et al., 2016; Codagnone et al., 2019). The association between the microbiota and the central nervous system is based on the concept of a bidirectional microbiota-gut-brain axis (Osadchiy et al., 2019). However, it is still not clear whether the changes in the microbiome are causally related to mental disorders or their direct consequences.
The available experimental data showed that the gut microbiota is able to improve depressive symptoms, and the use of pro- and prebiotics can lead to a decrease in cortisol levels and attenuate depressive-like and anxiety behavior in mice (Messaoudi et al., 2011). Moreover, there is data on the microbiota effects on the levels of neurotransmitters involved in the mechanisms of antidepressants action (Stasi et al., 2019).
There are also publications confirming the antimicrobial effects of antidepressants (Munoz-Bellido et al., 2000; Ayaz et al., 2015; Karine de Sousa et al., 2018). Thus, the selective serotonin reuptake inhibitors (SSRIs) showed synergistic activity when combined with some antibiotics against several bacteria, shown by a decrease in MICs, that converts strains previously resistant to the category of sensitivity, and modify physiological aspects related to pathogenicity (Munoz-Bellido et al., 2000). Sertraline previously showed strong intrinsic antibacterial and antifungal activities and has augmented the antibacterial activities of antibiotics (Ayaz et al., 2015). The association of fluoxetine with gentamicin and erythromycin P. aeruginosa and E. coli presented synergistic effects, demonstrating that this drug can selectively modulate the activity of antibiotics of clinical use (Karine de Sousa et al., 2018). Although the effects of antidepressants on microorganisms have been studied for a long period of time, the available research is primarily focused on pathogenic strains and the synergistic activity of antidepressants and antibiotics (Munoz-Bellido et al., 2000; Ayaz et al., 2015; Karine de Sousa et al., 2018). The antibacterial effect of SSRIs (sertraline, fluoxetine, and paroxetine) has been described against gram-positive bacteria such as Staphylococcus aureus and Enterococcus (Kaatz et al., 2003; Sun et al., 2019). However, there is little published data on the effect of antidepressants on normal gut microbiota and the consequences of their antibacterial effect on treatment outcomes.
The study of the antidepressants’ effects on the gut microbiota will allow both to determine microorganism’s potential role in the tolerance and side effects of psychopharmacological drugs and to assess the feasibility of using microbiota-modeling tactics to optimize the therapeutic strategies for patients with depression.
The aim of the study was to evaluate the growth kinetics of normal human gut microorganisms with antidepressants most common in routine clinical practice.
2. Materials and methods
2.1. Strains and growth conditions
Experiments with microorganisms were performed on the basis of the Microbiology Department of the Khanty-Mansiysk State Medical Academy. We used standard methods of microorganism cultivation on solid and liquid nutrient media. Research objects were species of facultative anaerobe or aerotolerant anaerobe microorganisms representing minor normal microbiota. Firmicutes: Staphylococcus aureus ATCC 25923 (S. aureus), Bifidobacterium bifidum 791 (B. bifidum), Enterococcus faecalis ATCC 29212 (E. faecalis), Lactobacillus rhamnosus ATCC 53103 (L. rhamnosus); Gracilicutes: Escherichia coli ATCC 25922 (E. coli) and Fungi: Candida albicans ATCC 24433 (C. albicans). All microorganisms were cultivated in Schaedler broth (HiMedia) at 37°C under aerobic/anaerobic conditions with shaking for the amount of time required.
2.2. Drugs
To study the effects of antidepressants on microorganisms, we selected antidepressants from different pharmacological groups that are most often used in clinical practice for major depression treatment. SSRIs (fluvoxamine, fluoxetine, escitalopram), selective serotonin, and noradrenaline reuptake inhibitors (SNRIs—duloxetine, venlafaxine) and noradrenergic and specific serotonergic antidepressants (NaSSA—mirtazapine) were used in the study.
All antidepressant preparations were ground under aseptic conditions. The active substances from ground preparations were been extracting by dimethyl sulfoxide (DMSO) for 12 h at 25°C. To separate the active substance from the excipients, the solutions were centrifuged at 5,000 rpm for 30 min and the supernatant (active substance) was separated from the sediment (excipients). In order to control that active substance was tuned into DMSO we use the classical gravimetric method (Ballinger and Gershon, 2011). We weighted a substance before and after incubation with DMSO on an analytical balance (Sartorius) having a maximum weighing capacity between 60 g and 520 g after heating the sample (30–45°C) to constant mass.
2.3. The growth kinetics of microorganisms with antidepressants
Each solution of antidepressants in DMSO was added to a Schaedler broth containing a certain microorganism’s strain and diluted to final concentrations—200 μg/ml, 500 μg/ml, and 700 μg/ml. A mixture of microorganisms and preparations was dispensed into a 96-well plate. Microorganism cultures in Schaedler broth with DMSO without antidepressants were used as controls. Further, microorganisms were cultivated in a photometer (Multiscan FC) at 37°C, wavelength λ = 540 nm, and optical density of suspension (A, optic unit) measurements were performed every hour (20 min) during the cultivation period.
For a quantitative assessment of the effect of the preparations, the specific growth rates (μ, h-1) of microorganisms were calculated as the slope of the initial part of the growth curve in coordinates (lnA, t).
To evaluate the antidepressant effects on representatives of the normal microbiota in vitro, the following parameters were chosen: specific growth rate and IC50. The IC50 is the concentration of an antidepressant at which the microbial growth rate was reduced to 50% of its original value.
2.4. Statistical analysis
All experiments were performed thrice (n = 3) with similar results. The data were analyzed and graphically visualized using Rstudio and Windows (GraphPad Software, United States1). The inhibition concentration (IC50) of the specific growth rate was determined using GraphPad Prism version 6.00 for Windows (GraphPad Software, United States1). The data points that were used to calculate such parameters were those that produced linear regression with correlation coefficients (r2) higher than 0.94.
3. Results
The addition of antidepressants to the medium led to a decrease in the growth activity of all studied microorganisms.
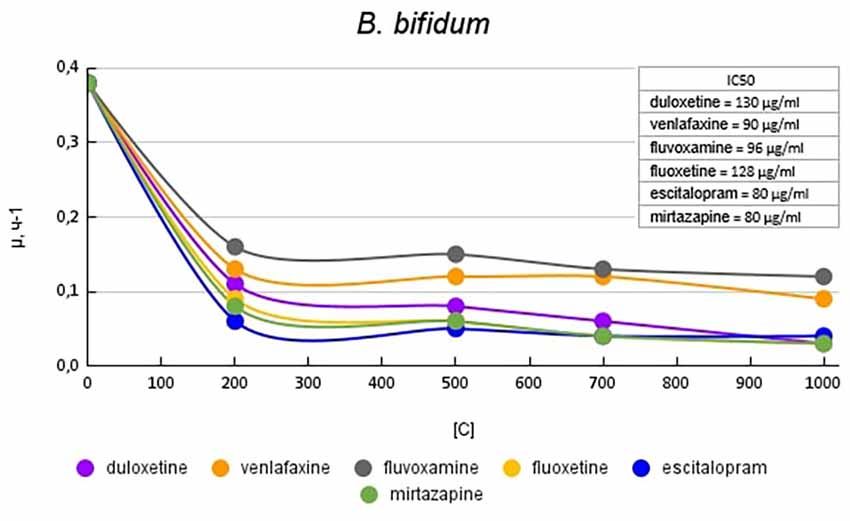
Figure 1 plots the growth rate of B. bifidum in the presence of antidepressants. The antidepressants escitalopram and mirtazapine had the greatest effect on growth activity. At a concentration of 200 μg/ml escitalopram and mirtazapine reduced the growth activity by 6.3 (84%) and 4.2 (76%) times compared with the control, respectively. Venlafaxine and fluvoxamine at a concentration of 200 μg/ml had the least effect on growth activity. The growth rate was 2.9 (65%) and 2.3 (56%) times compared with the control, respectively. When duloxetine and fluoxetine were added to the medium, a dose-dependent effect was observed. Duloxetine at a concentration of 200 μg/ml reduced the growth rate of B. bifidum by 3.4 (70%) times compared with the control. With a subsequent increase in the concentration of duloxetine, a consistent decrease in the specific growth rate of 4.7 (79%); 6.3 (84%), and 12.6 times compared to control was reported. Fluoxetine at a concentration of 200 μg/ml reduced the growth rate of B. bifidum by 4.2 (76%) times. With a subsequent increase in the concentration of fluoxetine, a decrease in the specific growth rate was observed by 6.3 (84%), 9.5 (89%), and 12.6 (92%) times compared with the control.
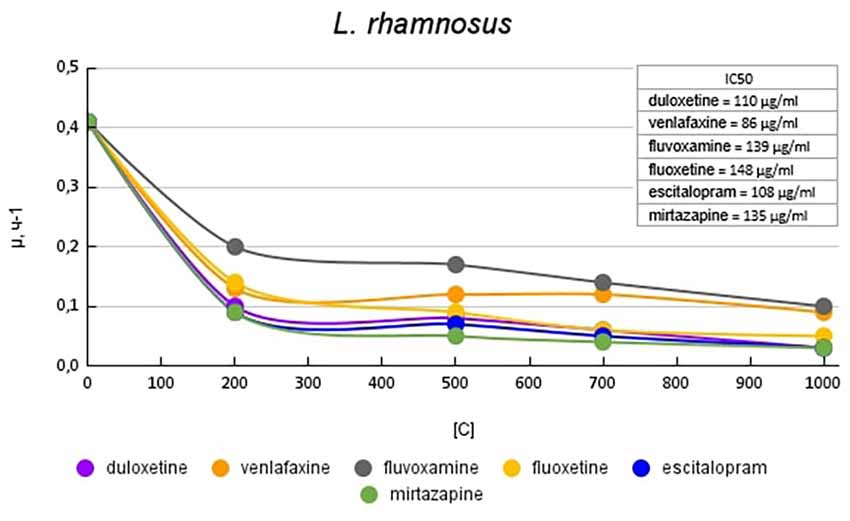
Figure 2 shows graphs of the specific growth rate of L. rhamnosus in the presence of antidepressants. The antidepressants escitalopram, mirtazapine, and duloxetine had the greatest effect on growth activity. At a concentration of 200 μg/ml, escitalopram, and mirtazapine reduced the growth activity by 4.5 (77%) times, and duloxetine at the same concentration by 4.1 (76%) times compared with the control. With a subsequent increase in the concentration of antidepressants in the medium, the growth rate changed less significantly. Fluvoxamine had the least effect on the growth activity of L. rhamnosus. At a concentration of 200 mg/ml, it reduced the growth rate by 2 (50%) times compared with the control.
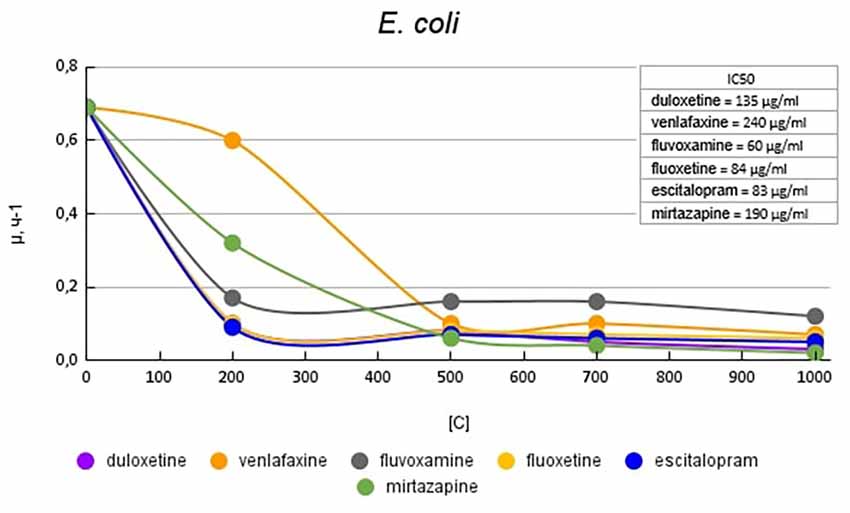
Figure 3 shows graphs of the specific growth rate of E. coli in the presence of antidepressants. The antidepressants duloxetine, fluoxetine, and escitalopram, had the greatest effect on growth activity. Duloxetine and fluoxetine at a concentration of 200 μg/ml reduced the growth rate by 6.9 (85%) times, and escitalopram at the same concentration reduced the growth rate by 7.6 (87%) times compared with the control. Venlafaxine and mirtazapine at a concentration of 200 μg/ml reduced the growth rate of E. coli to a lesser extent, by 1.2 (17%) and 2.2 (54%) times compared with the control, respectively. However, with a subsequent increase in the concentration of antidepressants, it led to a decrease in growth rate comparable to the above-described antidepressants. Fluvoxamine at a concentration of 200 mg/ml reduced the growth rate by 4.1 (76%) times compared with the control.
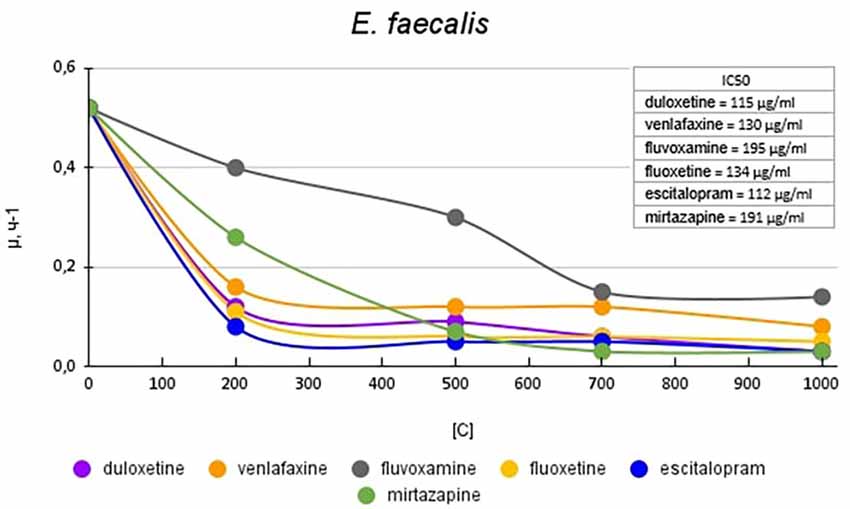
Figure 4 shows graphs of the specific growth rate of E. faecalis in the presence of antidepressants. The antidepressants duloxetine, fluoxetine, and escitalopram, had the greatest effect on growth activity. Duloxetine and fluoxetine at a concentration of 200 μg/ml reduced the growth rate by 4.1 (76%) and 4.7 (79%) times, and escitalopram at the same concentration reduced the growth rate by 6.5 (85%) times compared with the control. Mirtazapine at a concentration of 200 mg/ml reduced the growth rate of E. faecalis to a lesser extent, by 2 (50%) times compared to the control. However, with a subsequent increase in the concentration of antidepressants, it led to a decrease in growth rate comparable to the above-described antidepressants. Venlafaxine at a concentration of 200 mg/ml reduced the growth rate of E. faecalis by 3.3 (70%) times compared with the control. Fluvoxamine had the least effect on growth activity. At a concentration of 200 μg/ml, it reduced the growth rate by 1.3 (23%) times compared with the control.
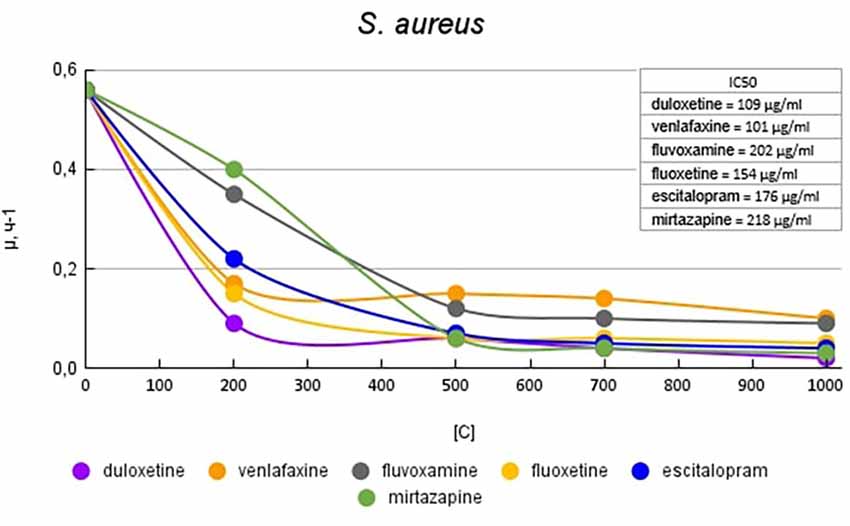
Figure 5 shows graphs of the specific growth rate of S. aureus in the presence of antidepressants. Duloxetine had the greatest effect on growth activity; at a concentration of 200 μg/ml, it reduced the growth rate by 6.2 (84%) times compared with the control. Escitalopram and fluoxetine at a concentration of 200 mg/ml reduced the growth rate by 2.5 (60%) and 3.7 (73%) times compared with the control, respectively. However, a subsequent increase in the concentration of antidepressants led to a decrease in growth rate comparable to duloxetine. Mirtazapine at a concentration of 200 mg/ml reduced the growth rate by 1.4 (28%) times compared with the control. However, a subsequent increase in the concentration of antidepressants led to a decrease in growth rate comparable to duloxetine. Venlafaxine at a concentration of 200 mg/ml reduced the growth rate by 2.5 (60%) and 3.2 (69%) times compared with the control. Fluvoxamine at a concentration of 200 mg/ml reduced the growth rate by 1.6 (37%) times compared with the control. A subsequent increase in the concentration of antidepressants led to a decrease in growth rate comparable to that of venlafaxine.
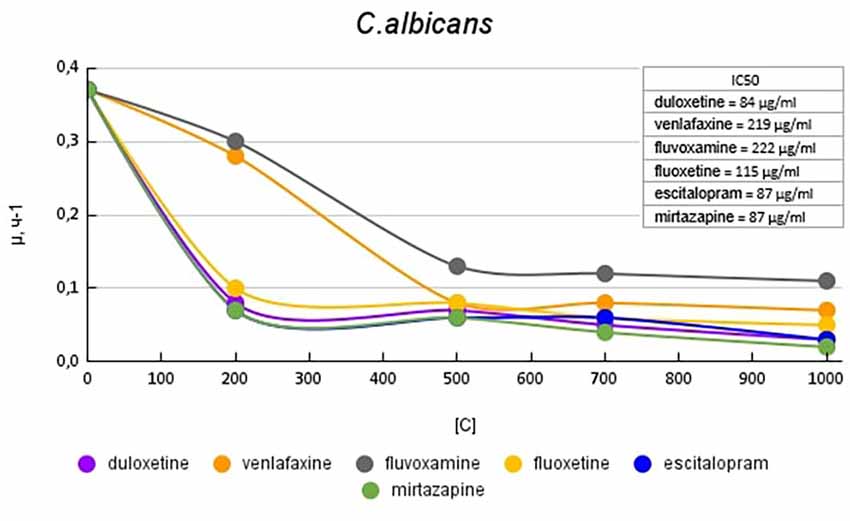
Figure 6 shows plots of the specific growth rate of C. albicans in the presence of antidepressants. Duloxetine, mirtazapine, escitalopram, and fluoxetine had the greatest effect on growth activity. Mirtazapine and escitalopram at a concentration of 200 μg/ml reduced the growth rate by 5.3 (81%) times compared with the control. Fluoxetine and duloxetine at a concentration of 200 μg/ml reduced the growth rate by 3.7 (73%) and 4.6 (78%) times compared with the control. With a subsequent increase in the concentration of the antidepressant in the medium, the growth rate changed less significantly. Venlafaxine at a concentration of 200 μg/ml reduced the growth rate by 1.3 (23%) times compared with the control. However, with a subsequent increase in the concentration of antidepressants, it led to a decrease in growth rate comparable to the abovementioned antidepressants. Fluvoxamine had the least effect on growth activity. At a concentration of 200 μg/ml, it reduced the growth rate by 1.2 (17%) times compared with the control.
4. Discussion
The current study results confirm and expand the existing data on the antidepressant’s’ potential antimicrobial effects. In our experiment, we evaluated the in vitro effect of the most commonly prescribed antidepressants on commensal bacterial strains of the normal human gut microbiota. The results clearly showed that all antidepressants had an inhibitory effect on the growth of all studied microorganisms. All evaluated microorganisms play important role in the functioning of the host organism. Members of the B. bifidum species constitute one of the dominant taxa amongst the bifidobacterial communities and have been shown to display significant physiological and genetic aspects in encompassing adhesion to epithelia as well as the metabolism of host-derived glycans (Turroni et al., 2019). S. aureus is able to colonize multiple niches within humans, however the clinical implications of intestinal colonization by it are still relatively ill-defined (Sannasiddappa et al., 2011). The healthy gut microbiota including certain strains of E. coli, E. faecalis, and L. rhamnosus promotes intestinal homeostasis and can exert anti-inflammatory or even anti-cancer effects (Alhinai et al., 2019). The evolutionary advantage of C. albicans colonization may be the education of the host immune system and competition with other microorganisms with an impact on the balance of the intestinal microbiota (Kumamoto et al., 2020).
There are studies that show the association between a decrease in the taxonomic diversity of normal intestinal microflora representatives with depression (Simpson et al., 2021), which may be the reason for the decrease in the therapeutic effect with prolonged use of the drugs. On the other hand, experimental data suggest that the effect on the microbiota may be one of the mechanisms of antidepressant activity (O’Mahony et al., 2015). The direct effect of the intestinal microbiota on the serotonergic system is by limiting the availability of tryptophan for the host organism due to its need for the growth of bacterial strains and, subsequently, the synthesis of indole (Lee and Lee, 2010; Li and Young, 2013). Thus, it is possible that in certain cases, the antimicrobial activity of antibiotics can alter the balance of tryptophan, which will affect the level of serotonin and other neurotransmitters and, accordingly, the potential therapeutic effect.
Fluvoxamine and venlafaxine had the least effect on the growth activity of all studied microorganisms. These results are inconsistent with a previous experiment where venlafaxine at a maximum dosage of 600 μg/ml had no effect on the growth activity of both E. coli and L. rhamnosus (Ayaz et al., 2015; Ait Chait et al., 2020).
The effect of fluvoxamine on the growth activity of microorganisms was comparable to that of venlafaxine. Previous studies evaluating the antimicrobial activity of fluvoxamine and other SSRIs have shown that fluvoxamine has greater antibacterial activity against E. coli than S. aureus (McGovern et al., 2019). The results of these studies reported that the minimum inhibitory concentrations of fluvoxamine for E. coli and S. aureus were 32 and 4,000 mg/L, respectively. In general, this correlates with the results of our study, where fluvoxamine reduced the growth activity of E. coli and S. aureus by 4.1 and 1.2 times, respectively, at minimal concentrations.
Previous studies with venlafaxine have shown no in vitro antibacterial effect of venlafaxine against L. rhamnosus and E. coli (Cussotto et al., 2019; Ait Chait et al., 2020).
According to the results of our experiment, fluoxetine showed a pronounced effect on growth activity against E. coli, E. feacalis, S. aureus, and the least effect against C. albicans. In another study, fluoxetine showed the greatest activity against gram-positive bacteria, including S. aureus. In relation to gram-negative, it also showed a pronounced inhibitory, but to a lesser extent (Kalayci et al., 2015). Our results showed that fluoxetine had the greatest effect on the growth activity to E. coli, reducing the growth rate by 6.9 times at a concentration of 200 μg/ml compared with the control. The antifungal activity of fluoxetine shown by our study is confirmed by other researchers (Lass-Flörl et al., 2001; Costa Silva et al., 2017).
Escitalopram in our experiment had a greater effect on the growth rate of E. coli, E. feacalis, B. bifidum, L. rhamnosus, and C. albicans, which puts it among the leaders in terms of its effect on the growth activity of the microorganisms we studied. These results do not confirm the earlier studies, where escitalopram showed one of the lowest antibacterial activities. In a study by Hendricks et al. (2007), the value of the minimum inhibitory concentration of escitalopram for E. feacalis was >256 mg/L. And in a study by Kalayci et al. (2015), escitalopram showed no effect on the growth of E. feacalis, E. coli, C. albicans, and S. aureus. However, a study by Irish scientists confirms the antibacterial activity of escitalopram against E. coli (Cussotto et al., 2019).
Mirtazapine, according to the results of our experiment, showed the greatest activity against L. rhamnosus and C. albicans. With respect to S. aureus, mirtazapine had a pronounced effect on growth activity only at high concentrations. Studies of mirtazapine and duloxetine are not numerous; therefore, it is not possible to provide a comparative analysis of our results. The only study of mirtazapine found showed no antimicrobial effect against all microorganisms studied by us (Kalayci et al., 2015).
Our results showed the variability of the antibacterial activity of the tested antidepressants in relation to different types of microorganisms. These differences in microbial suppression may contribute to changes in gut microbial diversity, causing a shift in microbial communities towards dysbiosis and eubiosis. Presumably the basis of antibacterial action of antidepressants, in particular SSRIs, are their ability to inhibit the active excretion of the drug from the microorganism cell (efflux effect; Kaatz et al., 2003). The complete understanding of such mechanisms still remains obscure. However, antidepressants may influence microbial diversity through strong selection for mutant bacteria with increased AcrAB-TolC activity, an efflux pump that removes antibiotics from cells. Moreover, a new group of genes that contribute to cross-resistance between antidepressants and antibiotics, act by regulating efflux activity, underscoring overlapping mechanisms, was identified (Ou et al., 2022).
5. Limitations
The main limitation of our study was its in vitro methodology. In order to apply current results to clinical practice, it is necessary to understand whether antidepressants reach the gastrointestinal tract in sufficient concentration to exert antimicrobial effects. Since the pharmacokinetics of drugs are affected by many factors, such as dosage, solubility, absorption, distribution, etc. it is difficult to estimate the actual concentrations of orally administered drugs in the gastrointestinal tract. Moreover, the time of exposure and the cumulative effect of antidepressants in the colon may be a determining factor for increased antimicrobial activity, since antidepressants are taken daily for a prolonged period of time.
6. Conclusions
Our results confirm the effects of antidepressants on the growth activity of individual strains of representatives of the normal gut microbiota. The results were depending on the studied drug and the dosage evaluated in the experiment. Therefore, there is evidence to believe that antidepressants may affect the gut microbiota, which may possibly influence the therapeutic process. But this assumption can be confirmed or refuted only by conducting further clinical studies, including the analysis of the taxonomic diversity of the microbiome of patients with affective disorders. Further study of the antimicrobial activity of antidepressants on the intestinal microbiota may become one of the new directions for optimizing the personalized therapy of patients with depression, considering individual microbiome profiles.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Author contributions
GM, NN, GR, and EK: study concept and supervision, manuscript revision and editing. LL and VL: methodology, experiment organization and conduction, statistical analysis. LL: text original draft. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Ait Chait, Y., Mottawea, W., Tompkins, T. A., and Hammami, R. (2020). Unravelling the antimicrobial action of antidepressants on gut commensal microbes. Sci. Rep. 10:17878. doi: 10.1038/s41598-020-74934-9
Alhinai, E. A., Walton, G. E., and Commane, D. M. (2019). The role of the gut microbiota in colorectal cancer causation. Int. J. Mol. Sci. 20:5295. doi: 10.3390/ijms20215295
Ayaz, M., Subhan, F., Ahmed, J., Khan, A. U., Ullah, F., Ullah, I., et al. (2015). Sertraline enhances the activity of antimicrobial agents against pathogens of clinical relevance. J. Biol. Res. (Thessalon) 22:4. doi: 10.1186/s40709-015-0028-1
Ballinger, J. T., and Gershon, J. S. (2011). “Gravimetric analysis,” in Chemical Technicians’ Ready Reference Handbook, 5th Edn. (New York: McGraw-Hill Companies, Inc.), 281–312. Available online at: https://www.accessengineeringlibrary.com/content/book/9780071745925/chapter/chapter15.
Codagnone, M. G., Spichak, S., O’Mahony, S. M., O’Leary, O. F., Clarke, G., Stanton, C., et al. (2019). Programming bugs: microbiota and the developmental origins of brain health and disease. Biol. Psychiatry 85, 150–163. doi: 10.1016/j.biopsych.2018.06.014
Costa Silva, R. A., da Silva, C. R., de Andrade Neto, J. B., da Silva, A. R., Campos, R. S., Sampaio, L. S., et al. (2017). in vitro anti-Candida activity of selective serotonin reuptake inhibitors against fluconazole-resistant strains and their activity against biofilm-forming isolates. Microb. Pathog. 107, 341–348. doi: 10.1016/j.micpath.2017.04.008
Cussotto, S., Strain, C. R., Fouhy, F., Strain, R. G., Peterson, V. L., Clarke, G., et al. (2019). Differential effects of psychotropic drugs on microbiome composition and gastrointestinal function. Psychopharmacology 236, 1671–1685. doi: 10.1007/s00213-018-5006-5
Hendricks, O., Kristiansen, J. E., Nielsen, H. W., Jakobsen, D., Jacobsen, K., and Posselt, J. (2007). Antimicrobial Effects of Selected Non-antibiotics on Sensitivity and Invasion of Gram-positive Bacteria. PhD Thesis. Odense: University of Southern Denmark.
Kaatz, G. W., Moudgal, V. V., Seo, S. M., Hansen, J. B., and Kristiansen, J. E. (2003). Phenylpiperidine selective serotonin reuptake inhibitors interfere with multidrug efflux pump activity in Staphylococcus aureus. Int. J. Antimicrob. Agents 22, 254–261. doi: 10.1016/s0924-8579(03)00220-6
Kalayci, S., Demirci, S., and Sahin, F. (2015). Antimicrobial properties of various psychotropic drugs against broad range microorganisms. Curr. Psychopharmacol. 3, 195–202. doi: 10.2174/2211556004666150520230121
Karine de Sousa, A., Rocha, J. E., Gonçalves de Souza, T., Sampaio de Freitas, T., Ribeiro-Filho, J., and Melo Coutinho, H. D. (2018). New roles of fluoxetine in pharmacology: antibacterial effect and modulation of antibiotic activity. Microb. Pathog. 123, 368–371. doi: 10.1016/j.micpath.2018.07.040
Kelly, J. R., Borre, Y., O’ Brien, C., Patterson, E., El Aidy, S., Deane, J., et al. (2016). Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 82, 109–118. doi: 10.1016/j.jpsychires.2016.07.019
Kumamoto, C. A., Gresnigt, M. S., and Hube, B. (2020). The gut, the bad and the harmless: Candida albicans as a commensal and opportunistic pathogen in the intestine. Curr. Opin. Microbiol. 56, 7–15. doi: 10.1016/j.mib.2020.05.006
Lass-Flörl, C., Dierich, M. P., Fuchs, D., Semenitz, E., Jenewein, I., and Ledochowski, M. (2001). Antifungal properties of selective serotonin reuptake inhibitors against Aspergillus species in vitro. J. Antimicrob. Chemother. 48, 775–779. doi: 10.1093/jac/48.6.775
Lee, J. H., and Lee, J. (2010). Indole as an intercellular signal in microbial communities. FEMS Microbiol. Rev. 34, 426–444. doi: 10.1111/j.1574-6976.2009.00204.x
Li, G., and Young, K. D. (2013). Indole production by the tryptophanase TnaA in Escherichia coli is determined by the amount of exogenous tryptophan. Microbiology 159, 402–410. doi: 10.1099/mic.0.064139-0
McGovern, A. S., Hamlin, A. S., and Winter, G. (2019). A review of the antimicrobial side of antidepressants and its putative implications on the gut microbiome. Aust. N. Z. J. Psychiatry 53, 1151–1166. doi: 10.1177/0004867419877954
Messaoudi, M., Lalonde, R., Violle, N., Javelot, H., Desor, D., Nejdi, A., et al. (2011). Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 105, 755–764. doi: 10.1017/S0007114510004319
Munoz-Bellido, J. L., Munoz-Criado, S., and Garcìa-Rodrìguez, J. A. (2000). Antimicrobial activity of psychotropic drugs: selective serotonin reuptake inhibitors. Int. J. Antimicrob. Agents 14, 177–180. doi: 10.1016/s0924-8579(99)00154-5
O’Mahony, S. M., Clarke, G., Borre, Y. E., Dinan, T. G., and Cryan, J. F. (2015). Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 277, 32–48. doi: 10.1016/j.bbr.2014.07.027
Osadchiy, V., Martin, C. R., and Mayer, E. A. (2019). The gut-brain axis and the microbiome: mechanisms and clinical implications. Clin. Gastroenterol. Hepatol. 17, 322–332. doi: 10.1016/j.cgh.2018.10.002
Ou, J., Elizalde, P., Guo, H. B., Qin, H., Tobe, B. T. D., and Choy, J. S. (2022). TCA and SSRI antidepressants exert selection pressure for efflux-dependent antibiotic resistance mechanisms in Escherichia coli. mBio 13:e0219122. doi: 10.1128/mbio.02191-22
Sannasiddappa, T. H., Costabile, A., Gibson, G. R., and Clarke, S. R. (2011). The influence of Staphylococcus aureus on gut microbial ecology in an in vitro continuous culture human colonic model system. PloS One 6:e23227. doi: 10.1371/journal.pone.0023227
Simpson, C. A., Diaz-Arteche, C., Eliby, D., Schwartz, O. S., Simmons, J. G., and Cowan, C. S. M. (2021). The gut microbiota in anxiety and depression - A systematic review. Clin. Psychol. Rev. 83:101943. doi: 10.1016/j.cpr.2020.101943
Stasi, C., Sadalla, S., and Milani, S. (2019). The relationship between the serotonin metabolism, gut-microbiota and the gut-brain axis. Curr. Drug Metab. 20, 646–655. doi: 10.2174/1389200220666190725115503
Sun, L., Zhang, H., Cao, Y., Wang, C., Zhao, C., Wang, H., et al. (2019). Fluoxetine ameliorates dysbiosis in a depression model induced by chronic unpredicted mild stress in mice. Int. J. Med. Sci. 16, 1260–1270. doi: 10.7150/ijms.37322
Keywords: antidepressants, depression, antibacterial, microbiota-gut-brain axis, growth activity
Citation: Rukavishnikov G, Leonova L, Kasyanov E, Leonov V, Neznanov N and Mazo G (2023) Antimicrobial activity of antidepressants on normal gut microbiota: Results of the in vitro study. Front. Behav. Neurosci. 17:1132127. doi: 10.3389/fnbeh.2023.1132127
Received: 26 December 2022; Accepted: 02 March 2023;
Published: 22 March 2023
Edited by:
Suzan Gonçalves Rosa, Federal University of Pampa, BrazilReviewed by:
Paloma Birmann, Federal University of Pelotas, BrazilNatalia Jardim, Lancaster University, United Kingdom
Vanessa Zborowski, Université Paris-Saclay, France
Copyright © 2023 Rukavishnikov, Leonova, Kasyanov, Leonov, Neznanov and Mazo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Grigory Rukavishnikov, Z3JpZ29yeV92X3JAbWFpbC5ydQ==
 Grigory Rukavishnikov
Grigory Rukavishnikov Lubov Leonova1
Lubov Leonova1 Evgeny Kasyanov
Evgeny Kasyanov Galina Mazo
Galina Mazo