- 1Princeton Neuroscience Institute, Princeton University, Princeton, NJ, United States
- 2Department of Molecular Biology, Princeton University, Princeton, NJ, United States
Sex differences are observed in several neurologic and psychiatric disorders. Many aberrant behavioral symptoms can be characterized clinically as either internalizing or externalizing, which tend to manifest disproportionately in females or males, respectively. Stress may precipitate or amplify these behavioral disturbances, which often start in childhood and adolescence but persist into adulthood. Increased understanding of sex differences in stress-induced behavioral changes and their underlying molecular mechanisms is integral to developing better therapeutics specifically tailored to males and females. Here, we highlight the potential of Drosophila melanogaster (D. melanogaster) as a model for the neurobiological study of sex differences in stress-altered behavior. We first review paradigms for stressing D. melanogaster, with an emphasis on social environmental stress. We then introduce behavioral tests that can be used to quantify stress-induced behaviors in flies and note sex differences that emerge in response to stress. Finally, we provide an overview of the known molecular and cellular mechanisms underlying stress-induced behavioral change, with a focus on sex differences and studies incorporating social isolation or crowding.
Introduction
Across human neuropsychiatric disorders, sex biases manifest through disease prevalence, severity, and development. Late-onset schizophrenia, anorexia, bulimia, post-traumatic stress disorder, anxiety, and depression are more prevalent in female patients, whereas attention deficit hyperactivity disorder, autism, dyslexia, stuttering, Tourette’s syndrome, and early-onset schizophrenia are more frequent in male patients (McCarthy, 2016). Over the life course, women are roughly twice as likely to develop depression and anxiety disorders compared to men (Kessler et al., 1994; Gater et al., 1998; Kuehner, 2017). Although it is important to consider the role of sex in the etiology of human mental illness and the biases that influence diagnosis of certain behavioral disorders, establishing relationships between biological sex and aberrations in behavior is the first step to elucidating those behaviors’ neurobiological underpinnings.
Sex differences in human disorders
One lens through which we can consider the contribution of biological sex to the development of maladaptive behavior is that of internalizing and externalizing psychopathology. Psychologists have long established internalizing and externalizing as broad categories of behaviors in humans, particularly in children and adolescents (Achenbach, 1966). Internalizing behavior is thought to be self-focused, while externalizing behavior relates to one’s interaction with the social environment. Internalizing behaviors include behaviors suggesting anxiety, depression, and emotional dysregulation. Externalizing behaviors encompass episodes of aggression, impulsivity, deviance, and hyperactivity (Nikstat and Riemann, 2020). One recent behavioral genetic study using data from over 3,000 twin families attributed roughly one-third of variance in internalizing and externalizing behavior to genetic influences, leaving the majority of variance to be accounted for by environmental factors, such as stress (Nikstat and Riemann, 2020). The authors suggest that many previous heritability estimates for internalizing and externalizing behaviors may be inflated, reflective of the “missing heritability” problem (Maher, 2008), whereby heritability estimates differ based on whether they were calculated using twin data or DNA-based approaches such as genome-wide association studies (GWAS).
Furthermore, there are prominent sex differences in the prevalence of internalizing versus externalizing disorders in human children and adolescents. Sexual selection theory posits that differences between boys and girls in biomarkers, molecular mechanisms, and development can produce differences in risk and resilience for various forms of psychopathology (Martel, 2013). Boys may be more predisposed to externalizing risk factors such as disinhibition and sensation-seeking due to higher levels of prenatal testosterone exposure, which result from the male fetus’ production of hormones in the testes beginning around week 8 of gestation (Wilson et al., 1981). This enhanced prenatal testosterone is also hypothesized to make males more susceptible to prenatal stressors (Martel and Roberts, 2014; Barrett and Swan, 2015) with downstream effects on dopaminergic signaling in the brain (Martel, 2013). Alternately, girls may be more at risk for developing internalizing disorders, with the risk markers being negative affect and rumination. These risk factors may arise during puberty from increases in estradiol and its interaction with cortisol and oxytocin, which may act on the serotonergic system to increase females’ stress sensitivity (Goel and Bale, 2010; Martel, 2013). Taken together, in human children and adolescents, psychologists posit that stress exposure in conjunction with differences in sex hormones may trigger different monoaminergic signaling processes in male versus female brains, which manifest as sex-specific stress phenotypes.
Comparative utility of model systems in the study of stress response
While mammalian models have proven useful for gaining mechanistic understanding of how stress differentially impacts male versus female brains and translationally relevant behaviors, Drosophila melanogaster (D. melanogaster) offers certain advantages. Beyond the decreased costs and ethical considerations of working with an invertebrate species, flies have a shorter lifespan than rodents, thus facilitating the study of stress and its effects at different developmental timepoints. In D. melanogaster, it is also simple to manipulate the sex determination hierarchy in certain cellular populations without manipulating the sex phenotype of the entire organism or its hormones. This strategy can be harnessed to perform sex-reversal studies, which shed light on how the genetic sex of a small neuronal population can regulate behaviors (Mundiyanapurath et al., 2009; Oyeyinka et al., 2022). The ease of sex determination hierarchy manipulation in combination with the recent mapping of the D. melanogaster connectome (Winding et al., 2023) positions D. melanogaster as a promising model system for identifying sex-specific neural circuits with fine-scale resolution.
While these qualities have made Caenorhabditis elegans (C. elegans) a good model system for studying the interrelationships among molecular mechanisms, neural circuit function, and behaviors that differ by sex (Salzberg et al., 2021), D. melanogaster enables us to study more complex behaviors with excellent availability of genetic tools and greater conservation of genes relevant to human disease (Sonnhammer and Durbin, 1997; Lloyd and Taylor, 2010). The simple body plan and rapid reproduction of C. elegans make it useful for studying cell-to-cell interactions or performing high-throughput genetic analyses (Riddle et al., 1997). However, with a more complex body plan (Alberts et al., 2002), higher genetic homology to humans, and superior availability of genetic tools (Irion and Nüsslein-Volhard, 2022), D. melanogaster may be a more useful invertebrate system for modeling human disease. Although flies lack a vertebrate nervous system, the complexities of mammalian hormonal signaling via sex hormones, and morphological and anatomical similarities to humans, fundamental molecular pathways have been found to be highly conserved across species in multiple neurological diseases (Rubin and Lewis, 2000). D. melanogaster has been used to discover new molecular pathways and interactions in transgenic fly models of neurodegenerative and metabolic brain diseases, epilepsy, tumors, and neurotrauma (Jeibmann and Paulus, 2009).
Overview
The goal of this review is to present D. melanogaster as a simple yet elegant model system through which we can probe the molecular mechanisms of sex-specific behavioral changes following stress experience. We begin by highlighting approaches that have been developed for stressing flies in the laboratory, with a focus on social isolation and crowding stress. We next summarize behavioral tests and key molecules that experimenters have studied in flies to assess phenotypes such as motivation, locomotion, aggression, activity, and sleep. Then, we consider sex differences in how these behaviors change in response to social or physical stressors. We conclude with an extensive discussion of the molecular mechanisms and neuromodulatory systems underlying D. melanogaster stress-induced behavioral changes, with an emphasis on the effects of social isolation and crowding and how they may differ between males and females.
Drosophila melanogaster as a model system for studying stress
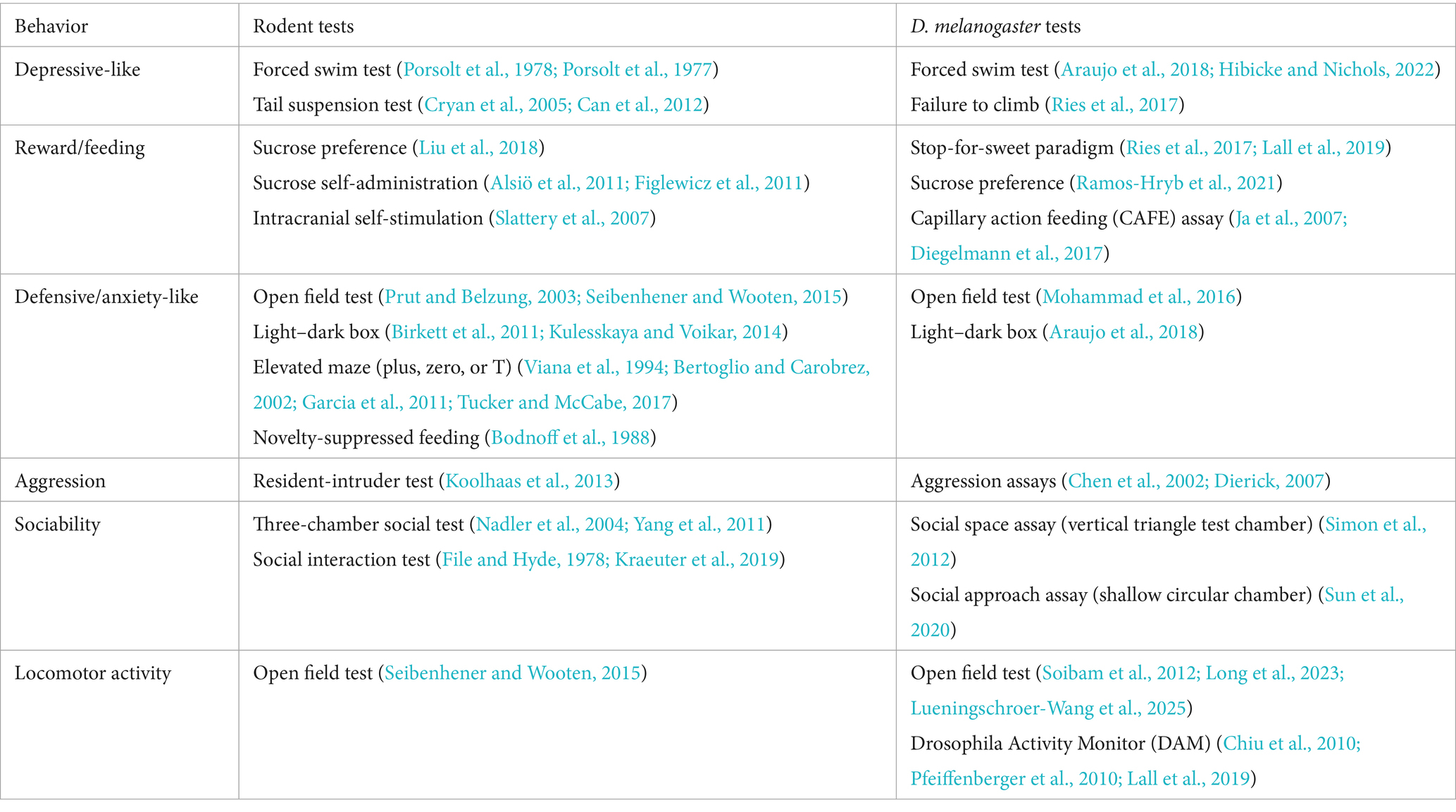
A vast literature exists on stress and its phenotypic and behavioral effects in male and female D. melanogaster (Table 1). Numerous paradigms have been developed to subject D. melanogaster to physical or psychosocial stress. Physical stressors include starvation (Neckameyer and Weinstein, 2005), oxidative stress-inducing chemical exposure (Neckameyer and Weinstein, 2005; Rzezniczak et al., 2011), temperature (Yang et al., 2013), mechanical stress (Neckameyer and Weinstein, 2005; Ries et al., 2017), sleep deprivation (Huber et al., 2004; Tanizawa and Takemoto, 2021), and electric shocks (Batsching et al., 2016). Social stressors consist of modifications to the fly’s social environment, including social isolation (Brown et al., 2017; Yadav et al., 2024) and overcrowding (Sørensen and Loeschcke, 2001; Lall et al., 2019; Chen and Sokolowski, 2022). Even the presence of dead conspecifics has been shown to impact fruit flies adversely through changes to the head metabolome and decreases in longevity (Chakraborty et al., 2019).
Some models of D. melanogaster stress use a chronic unpredictable mild stress approach, in which multiple stressors are used in combination (Ries et al., 2017; Araujo et al., 2018, 2020). For example, flies may be exposed to heat, cold, starvation, and sleep deprivation at unpredictable intervals over a period of 10 days (Araujo et al., 2018, 2020). Chronic unpredictable mild stress in male D. melanogaster has been shown to induce both aggression and depression-like behavior, measured through significantly greater numbers of aggressive encounters between male pairs and faster times to immobility in a forced swim test relative to controls (Araujo et al., 2018). The same study also found chronic unpredictable mild stress approach to decrease body weight, decrease preference for sucrose, reduce percentage of time spent in the illuminated compartment of a light–dark box (the preferred side of the apparatus for flies), and diminish whole head levels of serotonin and dopamine (Araujo et al., 2018). Although these tests imply depressive-like, anhedonia-like, and defensive behaviors in flies following chronic stress, it is important to consider ethological relevance in adapting animal behavior tests traditionally developed for rodents to flies. For instance, interpreting a reduction in time spent in the light portion of a light–dark box as a readout of depressive or defensive behavior is plausible in flies, given that flies demonstrate positive phototaxis and have been found to employ anti-predation strategies (Ferreira and Moita, 2020). However, while rodents generally avoid bright light as prey animals, young adult flies are attracted to light. These interspecies differences must be accounted for when modifying rodent behavioral tests for flies, and not all tests may be adaptable. For example, the forced swim test might not be easily translatable from rodents to flies. The interpretation of this test has not only been debated in the rodent literature (Molendijk and de Kloet, 2022), but rodents are largely considered natural swimmers, whereas flies are not. Thus, swimming might not be a behavior that makes sense to study in D. melanogaster in the laboratory, and an interpretation of the fly immobility response as behavioral despair is likely over-anthropomorphizing.
Social isolation
In studies of the effects of an individual stressor, social isolation has largely been used in D. melanogaster to examine the behavioral impact of environmental deprivation. Although fruit flies are not eusocial insects and thus are not commonly viewed as a model for sociality, they can demonstrate behaviors reflective of affective states (Anderson and Adolphs, 2014) and social learning (Sokolowski, 2010). D. melanogaster flies can form social networks (Schneider et al., 2012; Pasquaretta et al., 2016; Jezovit et al., 2021), perform collective behaviors (Wu et al., 2003; Durisko et al., 2014; Ramdya et al., 2017), and transmit mating preferences (Mery et al., 2009; Danchin et al., 2018). Laboratory assays have been developed for quantifying social space (Simon et al., 2012) and social attraction (Sun et al., 2020) within groups of flies, and freely-moving flies of both sexes demonstrate attraction to immobilized flies, regardless of sex, in a shallow circular chamber (Sun et al., 2020). In the absence of social interaction, flies show alterations in their lifespan (Leech et al., 2017; Lin et al., 2022; Vora et al., 2022), gene expression in the brain (Agrawal et al., 2020; Li et al., 2021), and behaviors (Wang et al., 2008; Li et al., 2021; Vora et al., 2022). Social interaction, relative to isolation, in male D. melanogaster has been found to modulate an array of behaviors, including aggression, feeding, and sleep (Ganguly-Fitzgerald et al., 2006; Chen and Sokolowski, 2022). These behavioral changes can persist for up to 72 h following social experience and correlate with increases in CREB-dependent neural activity and synaptic plasticity in the mushroom bodies (Gil-Martí et al., 2024). Another report suggests that social experience may drive social motivation in D. melanogaster through serotonergic signaling and activation of neurons in the γ lobe of the mushroom body (Sun et al., 2020). Hence, social interactions may be important for maintaining normal fly physiology, and disruption to sociality in the form of social isolation may induce stress and stress-associated physiological changes.
The social environment has been found to alter D. melanogaster sleep patterns such that socially isolated flies sleep less during the day compared to group-housed controls (Ganguly-Fitzgerald et al., 2006). Transcriptional changes in dopaminergic neurons specifically may be responsible for isolation-induced disruptions to sleep (Agrawal et al., 2019). A more recent study of social isolation in D. melanogaster replicated and extended these findings to a feeding context by establishing mechanistic connections between social deprivation, activity, and metabolism. This group found that chronic social isolation reduces sleep, enhances feeding behavior, and induces transcriptomic changes indicative of a starvation-like brain state in male flies (Li et al., 2021). Aggression is another behavior that increases in laboratory flies following social isolation (Hoffmann, 1987; Wang et al., 2008; Agrawal et al., 2020). This enhancement in aggression occurs in both sexes, with males fighting for food, territory, and mates (Hoffmann and Cacoyianni, 1989; Zwarts et al., 2012) and females fighting for food and egg-laying sites (Ueda and Kidokoro, 2002; Nilsen et al., 2004). Downregulation of the neuropeptide drosulfakinin (Dsk), a fly ortholog to mammalian cholecystokinin (CCK), has been identified as the potential neural substrate of chronic social isolation-induced aggression (Agrawal et al., 2020).
Finally, social experience and isolation have been shown to regulate male courtship maturation in D. melanogaster. Group-housing, compared to social isolation, increases mature males’ courtship behavior via enhanced activity of olfactory receptor neurons expressing the Or47b pheromone receptor (Sethi et al., 2019). Downstream signaling cascades convey information about reproductive maturity and population density, which can be integrated to modulate courtship. This specific process, whereby the fly flexibly tunes its reproductive behavior based on a combination of internal and external states, may be coordinated at the molecular level by the male fruitless (fruM) promoter (Zhao et al., 2020). FruM is a male-specific protein that acts as a master regulator of male courtship behavior. Group housing and signaling via juvenile hormone, a key hormone in the regulation of reproduction and metamorphosis, were found to increase fruM expression in Or47b neurons and active chromatin marks at the fruM promoter. However, social isolation or decreased juvenile hormone signaling decreased fruM expression and enhanced repressive marks around the fruM promoter.
Crowding stress
Crowding, the opposite of social isolation, is another potential source of social environmental stress. Much of the existing literature on overcrowding in flies has focused on the effects of larval crowding (Chen and Sokolowski, 2022). In adult flies, this manipulation has ranged from increasing the density of flies within a given physical space to full restraint, imitating chronic restraint stress in rodent models. One study, which employed full restraint, immobilized male flies between two soft plugs for 10 h a day for up to 3 days. These flies were then mated in order to examine the epigenetic, transcriptomic, and metabolic effects of paternal “psychological” stress on the offspring. It was found that paternal restraint stress induced epigenetic changes leading to energy metabolism deficiencies in F1 generation males (Seong et al., 2020). Three days of more moderate adult crowding has also been shown to decrease longevity in adult D. melanogaster, though it may be the case that exposure to high density living can build resilience (Joshi and Mueller, 1997).
A study investigating the effects of mechanical stress and adult crowding on behavior also found sex differences in sweet-seeking, activity, and exploration that were more pronounced following crowding than following mechanical perturbation (Lall et al., 2019). Specifically, males demonstrated increased sweet-seeking and activity following adult crowding, whereas females demonstrated reduced sweet-seeking and activity. Females also showed reduced exploratory behavior after chronic crowding. Further research is needed to gain a fuller understanding of the molecular and behavioral impacts of adult crowding in D. melanogaster. However, these preliminary findings suggest that crowding could be used in the future as a valid paradigm for uncovering the cellular and molecular mechanisms that generate stress-related behaviors.
Models for internalizing-like behaviors
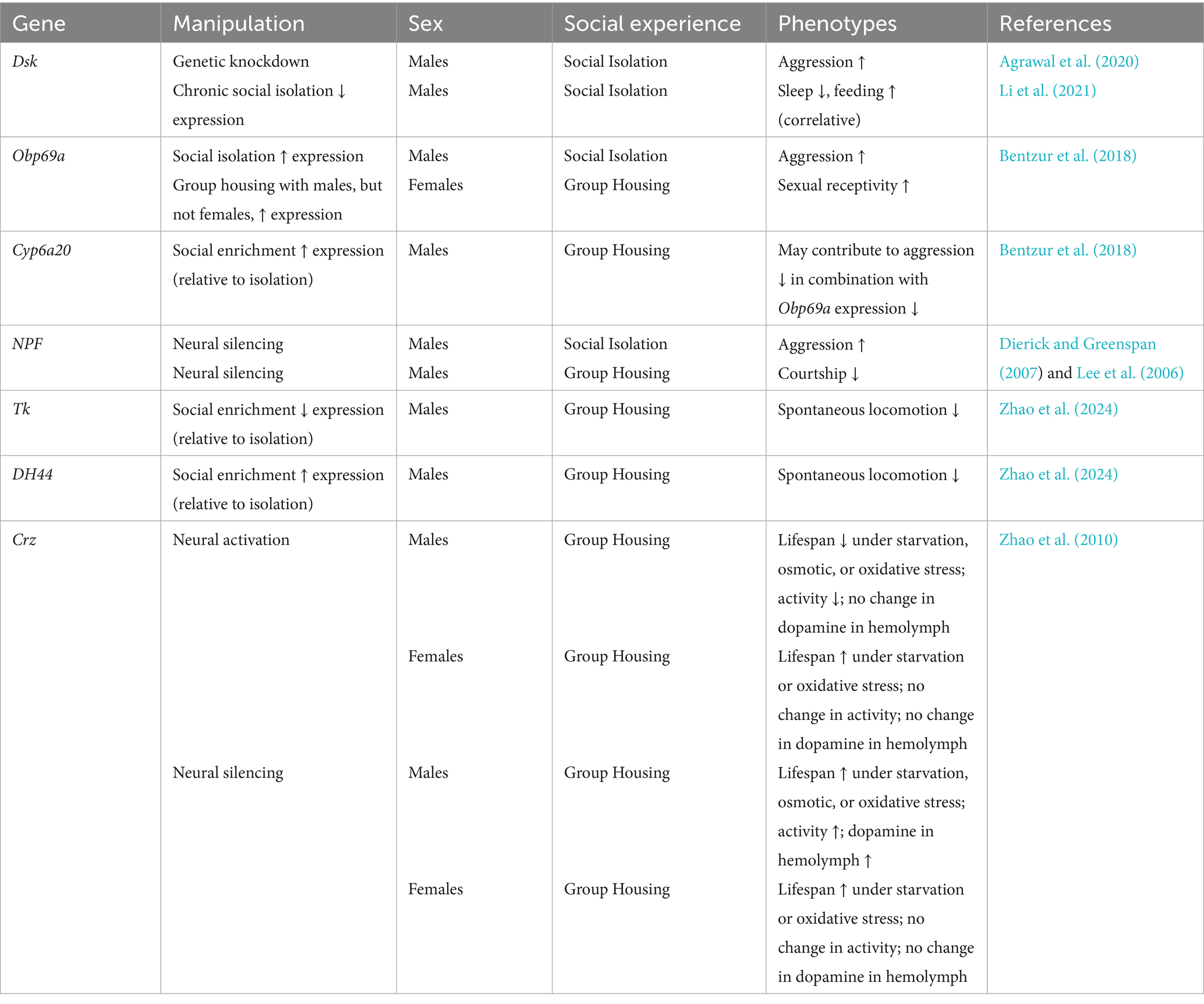
Human internalizing disorders are frequently marked by symptoms indicative of depression and anxiety. Although we do not believe flies to possess internalizing emotions like humans, they may experience persistent internal states, which generate behaviors with some resemblance to human internalizing symptoms. In humans, major depressive disorder has been characterized by depressed mood, or bias toward negative emotions; anhedonia, or impaired reward sensitivity; learning and memory dysfunction; appetite and weight changes; and disturbances in sleep and circadian rhythms, among other psychopathological endophenotypes (Hasler et al., 2004). Behavioral despair and anhedonia-like behavior are often interpreted in animal studies as indicative of a depression-like state, as human major depression may be marked by low motivation and persistence, social withdrawal, and a loss of interest or pleasure in enjoyable activities. While animal models cannot capture the full complexity of human depression and internalizing symptoms, they can be useful to the extent that they demonstrate face validity (common behaviors and symptoms), construct validity (common underlying biological mechanisms), and predictive validity (treatment response) (Willner, 1984). Table 2 presents a summary of rodent and fly behavioral tests across various domains of behavior, which reflect a degree of similarity between species.
Common rodent tests of depression-like behavior include the forced swim test and tail suspension test, in which a shorter time to immobility is interpreted as an expression of behavioral despair or entrapment (Lucki et al., 2001; Cryan et al., 2005). Rodent models can be used to study anhedonia-like behavior by measuring a decreased drive for food, sex, or other forms of pleasure through tests such as the sucrose preference test (Liu et al., 2018) or intracranial self-stimulation (Slattery et al., 2007). Similarly, behavioral tests, which will be detailed below, have been developed for flies to quantify stress-induced behavioral despair or anhedonia-like behavior.
Drosophila melanogaster tests of motivation
An important study of fly behavior consistent with learned helplessness found that following exposure to uncontrollable heat pulses, both male and female flies decreased their activity relative to controls and flies that could stop the onset of heat by moving (Yang et al., 2013). Of the flies that endured uncontrollable heat stress, females showed more pronounced reductions in walking and walking speed than males relative to other experimental groups of the same sex. In male flies, it has also been shown that chronic, uncontrollable vibration in combination with food and water deprivation is sufficient to produce what could be interpreted as learned helplessness and anhedonia-like phenotypes (Ries et al., 2017). These behaviors were characterized in male D. melanogaster by the failure to climb or the failure of hungry flies to feed on sweet-tasting glycerol, respectively. Upon feeding lithium chloride to male flies, simulating lithium as a pharmacological treatment for major depressive disorder, researchers observed an alleviation of these phenotypes, such that both stressed and control males demonstrated excessive climbing behavior. Serotonergic neurons were found to signal to the fly mushroom bodies to modulate the depression-like state (Ries et al., 2017).
In another study, short-term variable stress consisting of a random sequence of stressors such as social isolation, fasting, heat, and electric shock over a 24 h period was shown to decrease preference for sucrose in male and female flies. However, this phenotype was resistant to pharmacological treatment with fluoxetine and diazepam (Ramos-Hryb et al., 2021). These results are likely due to a combination of stress model design and differing pharmacodynamics between invertebrates and mammals. Because the short-term variable stress protocol encompasses four different types of stressors, it is not clear which of the stressors or which combination of stressors is the key driver of reduced sucrose preference, or how all of these stressors together are impacting the underlying neurobiology. Although the authors seemed to succeed in establishing a stress phenotype prior to treatment administration and behavioral testing, the combination of different stressors may be altering neural signaling in such a way that the primary changes are not serotonergic or GABAergic in nature. Alternatively, it may be that fluoxetine and diazepam differ in their binding affinity to target receptors in flies compared to vertebrate or mammalian models, or that the dose or time of administration was insufficient to ameliorate the stress-induced behavioral change.
Over time, the behavioral neuroscience field has shifted from using animals to model specific mental disorders to using them to understand neurobiological mechanisms underlying general behaviors. In mice and rats, learning how bottom-up neuroendocrine signaling combines with top-down circuit regulation from the medial prefrontal cortex may be key to understanding how animals switch between active and passive coping strategies in the presence of uncontrollable stress (Molendijk and de Kloet, 2022). Functionally analogous neural circuits or gene pathways in flies could be studied in the context of behavioral despair to evaluate whether common mechanisms produce internalizing-like behaviors across species.
Drosophila melanogaster tests of defensive behavior
Drosophila melanogaster has also been proposed as a possible model for probing the genetic basis of anxiety disorders. The elevated plus maze, open field, and light–dark box tests have been routinely used in rodents to measure approach or avoidance as a readout of defensive behavior (Griebel and Holmes, 2013). These tests are based on the hypothesis that a more “anxious” animal is thought to avoid light and exposure and prefer darkness and edges, tendencies which are adaptive in the wild for hiding from predators. The tendency of both flies and rodents to avoid the center of the open field arena is called thigmotaxis, centrophobism, or wall-following (WAFO).
In mammals, serotonin has long been the primary neurotransmitter associated with anxiety (Zangrossi et al., 2020), and diazepam is a benzodiazepine medication that is sometimes used in humans for the management of anxiety disorders. Diazepam exerts its anxiolytic effects via allosteric binding at gamma-aminobutyric acid (GABA)-A receptors, which induces neuronal hyperpolarization within the limbic system (Dhaliwal et al., 2023). D. melanogaster has orthologous genes which code for the serotonin receptor (1A, 1B, 2A, 2B, and 7), serotonin transporter, and GABA-A receptor. Researchers who measured avoidance behavior in male flies found that pharmacologic administration of diazepam, genetic overexpression of the serotonin class 1 receptor, or overexpression of the serotonin transporter were sufficient to decrease fly WAFO in the open field, similarly to mice (Mohammad et al., 2016). Knock-down of these genes increased mouse and fly WAFO. Some scientists characterize the D. melanogaster WAFO behavior as a preference for an arena’s boundaries rather than the centrophobism that mice demonstrate in the open field test (Soibam et al., 2012). However, these authors attributed the arena-boundary preference to the fly’s efficient search for escape routes, and they observed a preference for dark corners in males which may be indicative of shelter-seeking (Soibam et al., 2012). Regardless of whether the preference for spatial boundaries, attraction to the touch of the wall, or fear of the arena center is driving WAFO, these explanations are all rooted in predatory avoidance. A more recent study of WAFO further supports this phenotype as an indicator of emotion-like state in flies, by finding that negatively valenced stimuli (social isolation, starvation, sex or sleep deprivation, heat, mechanical stress, and electric foot shocks) generally increased WAFO, while positively valenced stimuli (social grouping, feeding, and relief from stress) decreased it (Lueningschroer-Wang et al., 2025). This group also found that both overexpression and downregulation of the serotonin transporter, as well as thermogenetic activation of NPF + neurons, decreased WAFO. Thermogenetic suppression of dopaminergic reward neurons increased WAFO. Finally, WAFO decreased significantly following the consumption of diazepam-containing food, consistent with previous results (Mohammad et al., 2016).
Hence, it is possible that flies could be used in the future as a neurogenetic model for emotion primitives and defensive behaviors, yet additional research is needed to validate this model. This could include adapting other tests of defensive behaviors in rodents, beyond the open field test, to flies. For instance, flies and mice could be tested on novelty-suppressed feeding (Bodnoff et al., 1988) or a food preference assay, to characterize similarities in other anxiety-like or anhedonia-like behaviors. Researchers could then apply the aforementioned manipulations of GABAergic and serotonergic signaling and measure changes across a battery of behaviors, to increase evidence supporting the use of flies as a neurogenetic model for human internalizing-like behaviors.
Models for externalizing-like behaviors
In addition to studying D. melanogaster as a potential model for stress-induced internalizing-like behaviors, flies have also been used to study behaviors relevant to externalizing disorders. Again, we do not believe that flies have externalizing emotions in the same way as humans, but changes in internal states may stimulate behaviors, such as aggression and hyperactivity, that are analogous to human externalizing symptoms. It should also be clarified that aggression and activity are not disorders, but these phenotypes may present as the outward manifestations of neuronal disorders. For example, hyperactivity is strongly linked to externalizing psychopathology in humans as a key symptom of attention-deficit hyperactivity disorder (ADHD). ADHD patients with pronounced hyperactivity are more likely to demonstrate hallmark features of externalizing disorders, including impulsivity and aggression (Ahmad and Hinshaw, 2017). Aggression and locomotor activity are two behaviors that can be quantified easily across species. In D. melanogaster, not all hyperactivity may be reflective of externalizing-like behavior. However, in some cases, there may be a common molecular substrate between fly hyperactivity and the hyperactivity observed in human ADHD, suggested by findings such as loss-of-function mutations in the dopamine D1 receptor ortholog DopR exacerbating environmentally-stimulated hyperactivity (Lebestky et al., 2009).
Drosophila melanogaster studies of aggression
As previously noted, social isolation has been found to enhance aggression in flies (Hoffmann, 1987; Hoffmann and Cacoyianni, 1989; Wang et al., 2008), similarly to food deprivation (Edmunds et al., 2021). A study examining genetic variance and gene–environment interactions in aggression in socially isolated and socially experienced individuals established that significant genetic variation exists in the aggressive response to social isolation (Rohde et al., 2017). The findings of this study support a polygenic basis of aggression in D. melanogaster, with several of these genes having orthologs previously implicated in mouse aggression and human neurological disorders. The D. melanogaster genes cac, dysf, Fas2, Fas3, ftz-f1, kirre, loco, and rst have orthologs in mice associated with aggression. Dysf and Fas2 also have human orthologs associated with bipolar disorder (Psychiatric GWAS Consortium Bipolar Disorder Working Group, 2011) and suicide attempts in depression and bipolar disorder (Mullins et al., 2014), respectively, in mixed-sex patient populations. Multiple candidate genes previously implicated in both D. melanogaster aggression and human disorders emerged, including Cad87A, CG42458, Dys, Gug, Ptp99A, Rbfox1, Ten-a, and Tl. The human orthologs of these genes have been associated with bipolar disorder, anxiety, depression, schizophrenia, and Alzheimer’s disease.
Although the study above (Rohde et al., 2017) has been critiqued for lack of replicability (Chowdhury et al., 2021) and the use of a behavior test that may not truly capture aggression (Kravitz and Fernandez, 2015), other studies have identified neuromodulatory systems and genes implicated in fly aggressive behavior. Increasing serotonin pharmacologically and genetically in serotonergic circuits increases aggression in D. melanogaster, as does silencing neuropeptide F (NPF) circuitry (Dierick and Greenspan, 2007). Serotonin and neuropeptide Y, the vertebrate ortholog of NPF, have been associated with aggression (Karl et al., 2004; Popova, 2006) among other behaviors (Lucki, 1998; Pedrazzini et al., 2003; Carvajal et al., 2006). These two neuromodulatory systems may be evolutionarily conserved across species and oppose each other to regulate aggression. Additionally, neuropeptide signaling from neurosecretory cells within the pars intercerebralis (PI) is a major molecular regulator of aggression in D. melanogaster (Davis et al., 2014). The PI functions as the Drosophila equivalent of the mammalian hypothalamus, and individual knock down of the genes tailless and Atrophin in the PI increases fly aggression (Davis et al., 2014). The physical interaction of the protein products encoded by these genes in the PI may modulate aggressive behavior by controlling neuropeptide release.
Drosophila melanogaster studies of locomotor activity
In addition to aggression, hyperactivity also follows social isolation in male and female flies (Ganguly-Fitzgerald et al., 2006; Li et al., 2021), which may be due to histone modifications altering the expression of activity-related transcription factors in dopaminergic neurons (Agrawal et al., 2019). In addition to isolation, short term variable stress (Ramos-Hryb et al., 2021) and starvation (Yu et al., 2016; Chi et al., 2021) have been shown to stimulate hyperactivity in male and female flies. Starvation stress may induce hyperactivity in flies to facilitate food location and acquisition. This behavior can be attributed to the binding of adipokinetic hormone, the insect analog of glucagon, to its receptor in adipokinetic hormone receptor+ octopaminergic neurons. Silencing these neurons, blocking octopaminergic transmission in these neurons, or binding D. melanogaster insulin-like peptides to the insulin-like receptor either eliminates or suppresses starvation-induced hyperactivity in females (Yu et al., 2016). It has also been found that a population of male-specific P1 neurons regulates sleep, feeding, and courtship in male Drosophila (Zhang et al., 2018). These cells could be important for understanding the neurochemical basis of how social isolation and starvation stress induce similar externalizing-like phenotypes (aggression and locomotor activity), as well as sex differences in the behavioral response to these stressors.
Behavioral sex differences in fly models of stress
As noted in the foregoing, behavioral sex differences in internalizing and externalizing disorders may also manifest in D. melanogaster models of stress. It has been previously suggested that D. melanogaster behavioral responses to stress may depend on sex and sexual maturity, and that stress-induced changes in one behavioral test may not predict performance in others (Neckameyer and Nieto, 2015). It should be noted that sex determination is more strongly regulated by genetics in D. melanogaster compared to mammals, which rely on both genetics and sex hormones for sexual differentiation. The ratio of X chromosomes to autosomes determines sex in each cell of the fruit fly embryo through a cascade of pre-mRNA alternative splicing events, without the influence of sex hormones (Gilbert, 2000; Heller, 2010). Stress may uncover latent sex differences in fly behaviors, which are generated as a result of sexual identity-based differences in certain populations of cells.
One study examining the effects of isolation versus social contact on aging found that lifelong same-sex pairing decreased lifespan more in males than in females (Leech et al., 2017). While same-sex pairing in females protected against functional senescence, measured by climbing ability, same-sex pairing produced climbing deficiencies in males. The detrimental effect of same-sex pairing on lifespan doubled in males in combination with wounding. These results suggest that male and female D. melanogaster respond differently to social contact and isolation, and that the adverse effects of social contact on longevity in male–male pairings may be due to the stress of increased competition for mates.
Another report that studied sex differences in D. melanogaster behavioral responses to chronic stress found that stressed females showed more behavioral changes than stressed males, and that the effects on behavior varied based on whether the stress could be categorized as abiotic (mechanical perturbation) or biotic (adult crowding) (Lall et al., 2019). In this study, adult crowding produced more sex differences in behavior than mechanical stress in tests of motivation and locomotion (Lall et al., 2019). Following chronic overcrowding, females showed greater anhedonia-like behavior, measured through decreased interest in glycerol-feeding, while males showed less. Females also had a decreased tendency to explore a novel environment, and females’ activity declined while males became hyperactive. Different results followed chronic vibration stress. After experiencing chronic vibration, both males and females demonstrated increased anhedonic-like behavior and activity. The only sex difference observed following vibration was decreased exploratory behavior in females that was not evident in males.
Hence, flies may respond differently to stress based on whether it is social or non-social in nature. The multiple behavioral sex differences observed following overcrowding demonstrate how a common stressor may influence internal state differentially in females versus males to produce divergent phenotypes. These divergent behavioral responses to social stress could potentially relate to female versus male reproductive demands and drives. The perception of increased competition for food and mates in a crowded environment could drive males to eat more glycerol and increase their activity, to locate these resources faster. In female Drosophila, glycerol is metabolized to support oogenesis (Heier and Kühnlein, 2018). If population density is too high, females may divert resources away from reproduction and consequently be less interested in glycerol-feeding or less motivated by food overall (leading to lower locomotion).
Molecular and neuromodulatory mechanisms in fly models of stress and stress-related behaviors
Well-characterized in mammalian systems, neurotransmitters, including the biogenic amines serotonin, dopamine, and octopamine, have been studied extensively as a mechanistic link between stress and behavioral changes in D. melanogaster. Stress can induce depression-like phenotypes in flies associated with the depletion of serotonin, dopamine, and octopamine, which can be rescued through several approaches with mixed success (Ries et al., 2017; Araujo et al., 2020; Ramos-Hryb et al., 2021).
Serotonin
Serotonin, or 5-hydroxytryptamine (5-HT), signaling pathways are evolutionarily conserved across a wide range of species (Turlejski, 1996; Hay-Schmidt, 2000; Buznikov et al., 2001), resulting in many similarities in serotonin signaling between flies and mammals in the areas of behavioral regulation, mechanism of action, and conservation of receptor families. Serotonin is known to modulate locomotion, sleep and circadian activity, feeding, aggression, anxiety and depressive-like behavior, and learning and memory in D. melanogaster (Bacqué-Cazenave et al., 2020). Serotonin also regulates many of these behaviors in mammals, including sleep–wake states (Monti, 2011), feeding (Vickers and Dourish, 2004; Voigt and Fink, 2015; D’Agostino et al., 2018), aggression (Miczek et al., 2001; Howell et al., 2007), anxiety-like behavior (Barnes and Sharp, 1999), depressive-like behavior (Artigas, 2013), and learning and memory (Buhot, 1997; Pittenger and Kandel, 2003). In both flies and mammals, serotonin exerts its effects through interactions with G protein-coupled receptors, which share considerable sequence similarity between species. D. melanogaster has the receptors 5-HT1A, 5-HT1B, 5HT-2, and 5HT-7 (Witz et al., 1990; Saudou et al., 1992; Colas et al., 1995), which are functional orthologs of the 5-HT1A, 5-HT2, and 5-HT7 receptors in mammals. However, despite these similarities, serotonin signaling differs between species due to mammals’ greater numbers of serotonergic neurons, diversity and number of receptor families, and complexity of neural circuits (Kasture et al., 2018). Although having a smaller and less complex brain can limit the extent to which D. melanogaster can model human disorders, the relative simplicity of the fly brain also allows for a reductionist study of the effects of neuromodulatory signaling on behavior.
Serotonin binding to its receptors influences the activity of cAMP-protein kinase A (PKA) (Ganguly et al., 2020), PLC-IP3 (Blenau et al., 2017; Tierney, 2018), GABAergic, and cholinergic signaling pathways (Alekseyenko et al., 2019) in D. melanogaster. Alterations in cAMP-PKA and PLC-IP3 signaling can induce downstream changes in gene expression, synaptic plasticity, and neuronal excitability. Serotonin also modulates the activity of GABAergic and cholinergic neurons, which, respectively, reduce and elevate aggressive behavior in males (Alekseyenko et al., 2019).
The literature on serotonergic signaling and aggression in D. melanogaster is substantial. Treatment with serotonin precursor 5-HTP or activation of 5-HT neurons enhances male aggression, while serotonergic neuronal silencing tends to decrease male aggression (Dierick and Greenspan, 2007; Alekseyenko et al., 2014; Hu et al., 2020). However, the effects of serotonin on aggression may be receptor subtype-specific. Although one report found that activation of the 5-HT1A receptor induces aggression in socially isolated male flies (Johnson et al., 2009), another group found that activating 5-HT1A neurons decreases male aggression (Alekseyenko et al., 2014). This discrepancy may be reconciled by more recent research, which has identified two distinct 5-HT1A receptor-expressing neuron types with opposing functions in regulating aggression (Alekseyenko et al., 2019). Activation of the first type, inhibitory GABAergic neurons, results in decreased aggression. Activation of the second type, excitatory cholinergic neurons, increases aggression. It is hypothesized that these two cell types converge in a common sensory integration area, the LC12 optic glomerulus, and that downstream signaling pathways from this brain region may refine the aggression response (Alekseyenko et al., 2019). The activation of specific receptor subtypes may also produce different forms of aggressive behaviors. For example, activation of 5-HT2 receptors has been associated with lunging and boxing, but manipulation of the 5-HT1A receptor alters wing threats and fencing (Johnson et al., 2009).
Dopamine
Dopaminergic signaling is another neuromodulatory system with functional parallels between flies and mammals. In both Drosophila and mammals, dopamine has been found to mediate locomotor activity, sexual behavior, response to drugs of abuse, memory, sleep, circadian rhythms, and aggression (Wilson et al., 1991; Mani et al., 1994; Yellman et al., 1997; Missale et al., 1998; Neckameyer, 1998; Andretic et al., 1999; Bainton et al., 2000; Schwaerzel et al., 2003; Willuhn et al., 2010; Riemensperger et al., 2011; Alekseyenko et al., 2013; Berry et al., 2015; Yamagata et al., 2015; Korshunov et al., 2017; Duszkiewicz et al., 2019; Hasegawa et al., 2022; Dai et al., 2025). In mammals, dopamine acts as a primary neurotransmitter in mesolimbic, mesocortical, nigrostriatal, and tuberoinfundibular pathways to modulate reward, motivation, motor control, and hormonal control (Björklund and Dunnett, 2007; Muthuraman et al., 2018; Tian et al., 2022). In D. melanogaster, clusters of dopaminergic neurons in the brain and ventral nerve cord project their axons to the mushroom bodies, central complex, and ventral lateral protocerebrum to regulate memory, locomotion, and aggression (White et al., 2010; Alekseyenko et al., 2013; Marquis and Wilson, 2022). Beyond the overlap in dopamine-regulated behaviors, the majority of genes involved in dopamine synthesis, secretion, and signaling are also conserved across species (Yamamoto and Seto, 2014). Several receptors and transporters involved in signal reception within dopaminergic pathways and dopamine reuptake, respectively, are conserved. Drosophila have four G protein-coupled dopamine receptors: DopR (Gotzes et al., 1994; Sugamori et al., 1995) and DopR2 (Feng et al., 1996; Han et al., 1996), which are orthologs of mammalian D1 receptors; D2R (Hearn et al., 2002), which is a D2-like receptor; and DopEcR (Srivastava et al., 2005), a non-canonical receptor.
However, there is some evolutionary divergence from mammals in genes involved in dopamine metabolism. Flies either (a) recycle dopamine through glial reuptake, whereby dopamine undergoes β-alanylation (Hovemann et al., 1998), and the resulting product is converted back to dopamine (True et al., 2005), or (b) metabolize dopamine via N-acetylation (Hintermann et al., 1996; Paxon et al., 2005). These processes differ from the mammalian metabolic mechanisms of oxidation and methylation (Meiser et al., 2013). Additionally, there are differences between flies and mammals regarding the organization of dopaminergic neurons and dopaminergic receptor subtypes. Dopaminergic neurons are largely concentrated in the substantia nigra and ventral tegmental area of the midbrain in mammals (Hegarty et al., 2013). In flies, they are found in specific clusters throughout the brain, such as clusters PAM, PPL, and PPM (Mao and Davis, 2009). The mammalian brain also has a greater number of dopaminergic receptor subtypes with greater diversity of splice variants and polymorphisms compared to the fly brain (Jackson and Westlind-Danielsson, 1994; Missale et al., 1998; Seeman et al., 2000; Hearn et al., 2002; Gurevich et al., 2016; Karam et al., 2019; Magistrelli et al., 2021), giving rise to a larger and more complex dopaminergic system.
There is substantial evidence that dopamine signaling in D. melanogaster is associated with behavioral and physiological indicators of stress. Dopaminergic transmission increases heart rate (Johnson et al., 1997), modulates arousal (Andretic et al., 2005), and increases with heat stress (Gruntenko et al., 2004). At baseline, adult female D. melanogaster also have higher levels of dopamine than adult males (Denno et al., 2015). Dopamine has been found to play a role in female sexual receptivity (Neckameyer, 1998) and to modulate the effects of drugs of abuse, including cocaine, nicotine, and ethanol (Neckameyer, 1998; Bainton et al., 2000). Stressors such as starvation, oxidative stress, and mechanical stress have been found to disrupt female sexual receptivity and ovarian development, potentially through alterations in tyrosine hydroxylase activity, which plays a critical role in dopamine biosynthesis (Neckameyer and Weinstein, 2005).
Social space among flies is also modulated by dopaminergic signaling, with dose-dependent effects differing by sex. This finding suggests a role for sexually dimorphic dopaminergic neurons in maintaining appropriate social spacing (Fernandez et al., 2017). Previously socially isolated male and female flies show reductions in physical distance to other flies, while prior mating increases social distance (Simon et al., 2012). Socially isolated male and female flies also decrease their sleep and have roughly one-third the level of whole brain dopamine as their socially enriched, longer-sleeping siblings (Ganguly-Fitzgerald et al., 2006). Both silencing dopaminergic circuitry and increasing endogenous dopamine prevent social deprivation from altering sleep, suggesting that dopaminergic transmission is crucial to this form of experience-dependent behavioral plasticity (Ganguly-Fitzgerald et al., 2006). An additional study found that social isolation-induced dopamine depletion, as well as dopamine recovery to normal levels following 3 days of group-housing, was specific to male flies (Yost et al., 2024). The previously mentioned study (Ganguly-Fitzgerald et al., 2006) found reductions in whole head dopamine levels in mixed-sex isolated versus socially enriched control flies. It is possible that this result was driven by male-specific dopamine depletion, given that this research group did not perform sex-specific comparisons, but further investigation is needed to explore whether these studies support or contradict one another.
Lastly, differential sensitivity of dopaminergic receptors or differences in receptor subtype expression may account for sex differences in behaviors, such as locomotion (Yellman et al., 1997). Dopaminergic neurons may also be differentially vulnerable to neurodegeneration on the basis of sex. Female D. melanogaster have elevated expression of vesicular glutamate transporter in dopaminergic neurons relative to males (Buck et al., 2021). This sex difference is conserved across flies, rodents, and humans and may confer protection to females against aging-related neurodegeneration and locomotor deficits (Buck et al., 2021).
Octopamine
Octopamine, the insect structural analog of mammalian norepinephrine, is expressed in roughly 70–100 neurons in the D. melanogaster nervous system and modulates a range of behaviors, from ovulation to learning (Monastirioti, 2003; Schwaerzel et al., 2003). Neural octopamine is required for D. melanogaster aggression (Zhou et al., 2008). Silencing of octopaminergic neurons and genetic knockdown or mutant approaches have generally yielded decreases in male and female aggressive behavior (Hoyer et al., 2008; Zhou et al., 2008; Watanabe et al., 2017; Sherer et al., 2020), while activation of octopaminergic neurons generates male aggression (Watanabe et al., 2017; Jia Y. et al., 2021). In one of these studies, the release of both octopamine and glutamate from neurons expressing both neurotransmitters was found to be necessary for male aggression (Sherer et al., 2020). Another research group induced a depression-like state in male flies, characterized by reduced climbing motivation, using a battery of chronic stressors, including vibration, overcrowding, and food deprivation. It was found that the depression-like state could be relieved through consumption of 5-HTP, fluoxetine, or sucrose, all of which increase serotonin in the brain (Hermanns et al., 2022). A sugar-induced restoration of climbing motivation was associated with octopaminergic signaling from the ventral unpaired medial neurons in the subesophageal ganglion to dopaminergic protocerebral anterior medial (PAM) neurons. The dopaminergic PAM neurons and serotonergic dorsal paired medial (DPM) neurons both synapse with cells in the mushroom bodies to modulate and relieve the stress-induced depression state. These findings reiterate the important ways in which monoaminergic signaling systems work together in behavioral regulation, and how disruption of these systems by stress dysregulates behavior.
In the context of stress, octopamine has also been identified as a substrate for starvation-induced foraging behavior, but not feeding itself, in virgin female flies (Yang et al., 2015). In males, overexpression of tyramine β hydroxylase (TβH), an enzyme involved in octopamine synthesis, increases aggression in group-housed flies but not socially isolated flies (Zhou et al., 2008). This result could be attributed to either a ceiling effect in the already high levels of aggression demonstrated by isolated flies, or a role for octopamine in the social regulation of aggression. Octopamine may also modulate male aggression by inducing the production and secretion of Dsk, a satiety-related neuropeptide that will be discussed in the subsequent section (Williams et al., 2014). Interestingly, male flies have been found to express higher levels of mRNA for the octopaminergic receptor Octα2R than females, which could be attributed to higher numbers of Octα2R + neurons in males or male-specific upregulation of Octα2R expression (Qi et al., 2017). It is possible that sex differences in gene expression related to octopaminergic signaling could contribute to the generation of sex-specific behaviors, such as male aggression.
Drosulfakinin
Neuropeptides are another subset of neuromodulators that have been found to play a role in behavior change driven by social stress. Cholecystokinin (CCK) is a mammalian gut hormone that also functions as a neurotransmitter. CCK + neurons regulate aggression, anxiety, and social defeat responses in rodents (Vasar et al., 1993; Panksepp et al., 2004; Becker et al., 2007; Li et al., 2007), and CCK-4 has been shown to induce panic attacks in humans (Bradwejn et al., 1990; Tõru et al., 2010). Dsk, the fly ortholog of CCK, has been implicated in regulating gut function, feeding and satiety, hyperactivity, aggression, locomotor behavior, and synaptic plasticity during the development of the neuromuscular junction (Nässel and Williams, 2014). The D. melanogaster genome encodes two distinct receptors to which Dsk binds: CCKLR1 (CCKLR-17D1) and CCKLR2 (CCKLR-17D3). It has been shown recently that the binding of Dsk to CCKLR-17D1 in insulin-producing cells modulates selectivity in mating choice in males (“male choosiness”), and that broad activation of Dsk + neurons rescues the adverse effect of female pheromone exposure on male lifespan (Fedina et al., 2023). Dsk signaling in male flies has also been implicated in early life social memory, which may have lasting effects on social behavioral plasticity (Jeong et al., 2024). Both Dsk and CCKLR-17D1 mutants show deficits in larval stress-induced escape behavior (Chen et al., 2012), and knockdown of these genes in the PI in adults increases aggression in socially isolated males (Agrawal et al., 2020). Consistent with the fact that social isolation increases aggression in D. melanogaster, another group has found that chronic social isolation decreases Dsk expression in males, which correlates with decreased sleep and increased feeding behavior (Li et al., 2021). Although there is abundant evidence that Dsk signaling may play a role in stress-related phenotypes and social behaviors in D. melanogaster, little is known about whether chronic stress induces sex differences at the transcriptomic level in this pathway, and if such differences could alter behaviors such as activity, feeding, or aggression.
Neuropeptide F
Neuropeptide F (NPF), the fly homolog of mammalian neuropeptide Y (NPY), has also been linked to essential behaviors which are impacted by stress. In mammals, NPY is involved in the regulation of diverse biological functions, including energy balance, circadian rhythms, feeding, reproduction, anxiety, learning and memory, and alcohol dependence (Lee et al., 2006). There is also some evidence that NPY pathways mediate physiological and behavioral stress responses differently between males and females (Painsipp et al., 2011; Forbes et al., 2012). In D. melanogaster, NPF regulates feeding, wakefulness, metabolism, and reproduction (Nässel and Wegener, 2011; Chung et al., 2017). A related class, short NPFs, which belong to a separate invertebrate neuropeptide family from NPFs but bind to NPY-like receptors, have been associated with feeding and growth, stress response, movement, olfaction, hormone release, and learning and memory (Nässel and Wegener, 2011). One study has found that inhibitory signaling from NPF + neurons to dopaminergic neurons confers resilience to chronic stress-induced learning deficits through the maintenance of normal autophagic flux (Chen et al., 2023). Silencing NPF + cells has also been shown to increase aggression in socially isolated males (Dierick and Greenspan, 2007) and decrease male courtship behavior in group-housed controls (Lee et al., 2006). NPF also demonstrates sex-specific expression patterns in D. melanogaster, which may influence sexual dimorphism and sex differences in behavior. Male flies have neurons which express male-specific NPF under regulation of the transformer (tra) sex determination pathway and may modulate male courtship (Lee et al., 2006). Male-specific NPF expression in neurons may also be regulated by circadian factors, suggesting a role of these cells in generating sex-specific behaviors which are also clock-controlled (Lee et al., 2006). There is currently limited work examining whether NPF signaling and sex-nonspecific behaviors diverge under stress between males and females, providing a needed avenue for future research.
Corazonin
Finally, corazonin (Crz) is a lesser studied but highly conserved neuropeptide in insects that may contribute to sexually dimorphic neural circuitry and behavioral responses to stress. D. melanogaster cells expressing Crz have receptors for two diuretic hormones, DH44 and DH31 (Johnson et al., 2005), which are related to the mammalian stress hormones corticotropin releasing factor and calcitonin gene-related peptide (Bale and Vale, 2004; Taylor and Samson, 2005). It has been suggested that Crz plays important functions in development and survival by regulating growth, feeding, mating behavior, and ethanol sensitivity (Khan et al., 2021). Although Crz is expressed in dorsomedial, dorsolateral, and ventral nerve cord neurons in the fly nervous system, there is a small population of four neurons in the abdominal ganglion which are present only in males and are involved in ejaculation and copulation duration (Lee et al., 2008). A study examining the role of Crz-expressing neurons in stress sensitivity and stress-related behavior identified sex differences in lifespan following starvation, osmotic, or oxidative stress. Activation of Crz + neurons decreased lifespan only in males, despite neural silencing improving lifespan in both sexes under conditions of starvation or oxidative stress (Zhao et al., 2010). This study also found male-specific effects of Crz + neuron activation and silencing on motor activity, with silencing resulting in male hyperactivity and activation resulting in male hypoactivity relative to wild type flies. Starvation and osmotic stress decreased transcript expression of Crz more in males compared to females, and males with silenced Crz + neurons showed elevations in dopamine in the hemolymph relative to parental genotypes. Activation and silencing of corazonin neurons did not alter dopamine levels compared to parental genotypes in females (Zhao et al., 2010). Finally, a separate sex-reversal study found that feminizing Crz + neurons in male flies was sufficient to reduce time to sedation by ethanol to female-like levels (Oyeyinka et al., 2022). Taken together, the results of these experiments suggest that corazonin neurons may receive sex-specific inputs or may directly or indirectly signal to sex-specific downstream targets to produce sex differences in stress phenotypes. To date, no studies have explored the effects of social isolation on corazonin neural signaling and behavior in D. melanogaster.
Molecular and cellular sex differences in social isolation stress
Overall, the studies outlined below offer many potential genetic targets for exploring male- versus female-specific cell types and circuits underlying stress-induced and social context-dependent sex differences in behaviors (Table 3). In the context of social isolation, a recent review (Yadav et al., 2024) summarized extensive research on the genes, neuromodulators, cell types, and behaviors that are differentially altered by single versus group housing conditions, with courtship, sleep, and aggression being the most widely studied behaviors. At a circuit level, both sexually monomorphic and dimorphic cell types underlie the Drosophila aggression response (Chiu et al., 2021). Common aggression-promoting (CAP) neurons mediate the approach (appetitive phase) of aggression in both sexes, but the attack (consummatory) phase relies on male-specific aggression-promoting (MAP) neurons in males and fpC1 neurons in females (Chiu et al., 2021). Social isolation may enhance aggressive behavior in both sexes by strengthening these monomorphic-to-dimorphic functional connections.
In addition to Dsk downregulation (Agrawal et al., 2020), social isolation-induced aggression has also been linked to upregulation of Obp69a and downregulation of Cyp6a20 (Bentzur et al., 2018). Interestingly, Obp69a, a gene encoding an odorant binding protein, and Cyp6a20, a gene encoding a cytochrome P450, are expressed in a sexually dimorphic manner. Obp69a is naturally overexpressed in males relative to females and increases only in males following single housing. In females, expression of Obp69a does not seem to be affected by single or group housing with same-sex conspecifics, but it increases when they are group-housed with males. Expression of Cyp6a20 does not vary based on social environment in females but is significantly reduced in males following single housing (Bentzur et al., 2018). The authors believe that the inverse regulation of Obp69a by social environment in males versus females may promote mating, by decreasing aggression and increasing aggregation in groups for males and enhancing sexual receptivity for females. It is plausible that pheromone-sensing pathways may be altered by social context differentially in males and females, due to male-specific initiation of courtship behavior. This is supported by studies cited earlier in this review, which found social experience to enhance Or47b neural activity and stimulate courtship in mature adult males (Sethi et al., 2019; Zhao et al., 2020).
NPF, which is altered by stress and associated with stress-induced behavior change, is regulated in both a sex-specific and sex-nonspecific manner (Lee et al., 2006). Male-specific NPF expression is regulated by fruitless (fru), a transcription factor which plays a critical role in the sex determination hierarchy in the fly central nervous system and is required for normal male courtship. NPF is also regulated by clock factors in a subset of male dorsolateral neurons, suggesting that this gene may play a role in sexually dimorphic courtship behavior which is also circadian (Lee et al., 2006). Since NPF + neuronal silencing increases aggression in socially isolated males and decreases courtship in group-housed males, it would be interesting to explore whether social isolation impacts NPF gene expression in a sex-specific way (e.g., decreases NPF expression in males), and, if so, whether NPF expression changes in this particular population of neurons. P2 neurons expressing NPF have also been identified as the cells through which chronic social isolation reduces sleep and increases feeding, but this study only tested male flies (Li et al., 2021).
In addition to NPF, tachykinin (Tk) is a neuropeptide which may play sex-specific roles in stress-related behaviors. Substance P, a member of the Tk family, has been linked to the initiation of aggressive behavior in mammals (Katsouni et al., 2009) and may perform a similar function in insects. A class of neurons in the male fly brain that express the male-specific form of fru and release D. melanogaster Tk have been found to generate male-specific aggression (Asahina et al., 2014). A recent report has also found that in male flies, social enrichment (relative to social isolation) upregulates Diuretic Hormone 44 (DH44) and downregulates Tk to decrease spontaneous locomotor activity (Zhao et al., 2024). These authors also uncovered a neural circuit in which male-specific P1 neurons stimulate movement through positive feedback with interneurons expressing both doublesex (dsx) and Tk and inhibit movement with negative feedback from interneurons co-expressing dsx and DH44 (Zhao et al., 2024). This neural circuit could be integral to understanding how changes to the social environment produce sexually dimorphic changes in innate behaviors. Taken together, the results of these studies allude to male-specific neural substrates in flies for two externalizing behaviors, aggression and hyperactivity.
Interestingly, the feminization of cholinergic neurons in male flies via expression of tra also increases aggression (Mundiyanapurath et al., 2009). Tra is an important gene in the sex determination hierarchy that is only functional in females. It has been found that a small number of cholinergic cells in female fly brains, pC1 dsx + neurons, are required for female hyperaggression (Palavicino-Maggio et al., 2019). Although females are typically less aggressive than males at baseline (Kravitz and Fernandez, 2015), they will become more aggressive, similarly to males, following social isolation (Palavicino-Maggio and Sengupta, 2022). Further research is needed to determine if social isolation induces female aggression by altering gene expression in such a way that makes pC1 dsx + or other cholinergic neurons more active.
Molecular and cellular sex differences in crowding stress
In D. melanogaster, there has been more research conducted on larval than adult crowding. Larval crowding has been associated with transcriptomic changes in adulthood, including the upregulation of genes relating to metabolism and DNA replication and downregulation of genes involved in immune signaling. Downregulation was also observed in gene classes relating to excretion and recycling pathways, namely taurine metabolism and lysosome and ABC transporters (Morimoto et al., 2023). Overall, high density living early in life may remodel metabolic pathways in flies to cope with a limited-resource environment and build tolerance to toxic compounds.
However, one study has suggested that the stress resistance built in adulthood by larval crowding may not be the same in both sexes. During adult heat stress, it was found that after multiple generations of larval crowding, males but not females improved their heat shock tolerance (Kapila et al., 2021). Crowding increased expression of the heat shock protein Hsp70 in larvae, but Hsp70 expression in adulthood did not account for the observed sex difference in heat shock tolerance, as there were no Hsp70 expression differences in the adult populations. Thus, the molecular basis of this observed sex difference in heat stress tolerance remains largely speculative. Another study of larval crowding in the fruit fly Bactrocera tryoni found that high density early living decreased emergence, body weight, energetic reserves, flight ability, and fertility and increased feeding in adulthood (Morimoto et al., 2019). Although flies of both sexes from the larval crowding treatment increased their total food consumption (relative to body weight), males ate more yeast, while females ate more sucrose compared to their respective uncrowded controls. Further research is needed to uncover the molecular mechanisms by which larval crowding induces sex differences in stress sensitivity and nutrient preference in adulthood.
In adult flies, one study (Chen et al., 2023) used adult crowding stress, in combination with mechanical shock, to examine the molecular and neuronal mechanisms underlying chronic stress-induced learning deficits. Learning and memory impairment is a common behavioral symptom of depression in humans and other mammals. Four days of crowding and mechanical shock treatment were sufficient to induce learning deficits (Jia J. et al., 2021), which correlated with disrupted autophagic degradation in neurons. RNA-seq differential expression analysis from fly heads collected after 96 h of stress treatment revealed changes in gene expression in the autophagy-lysosome pathway. This is an evolutionarily conserved stress pathway (Kroemer et al., 2010) that degrades abnormal intracellular macromolecules to maintain cellular homeostasis. This pathway has also been implicated in both neurodegenerative diseases (Nixon, 2013) and depression (Jia and Le, 2015; Gassen and Rein, 2019). Activation of NPF + cells during chronic stress protects against learning deficits by inhibiting dopaminergic neurons and thus maintaining autophagic flux (Chen et al., 2023). Future studies should examine whether these specific molecular changes in the autophagy-lysosome pathway can be linked to depressive-like behavior or other stress responses in flies.
Lastly, one paper studying adult restraint stress, which bears some similarity to overcrowding in that the flies were restricted in physical space in a group, found that restraint of fly fathers induced gene expression changes in the offspring (Seong et al., 2020). It was found that paternal restraint disrupted heterochromatin, upregulated the expression of genes involved in one-carbon metabolism, and downregulated the expression of genes involved in glycolysis and the tricarboxylic acid (TCA) cycle in F1 males. These authors identified a novel pathway by which paternal restraint stress reduces energy metabolism activity and produces epigenetic changes in male offspring to transmit stress information from somatic to germline cells. Overall, adult crowding or restraint stress seems to alter gene expression in D. melanogaster in a manner that is behaviorally maladaptive and detrimental to survival. However, the extent to which adult crowding or restraint stress produces behavioral sex differences in D. melanogaster remains fairly unknown, as well as the underlying molecular mechanisms if sexual dimorphism exists.
Conclusion
Despite the Drosophila nervous system being smaller and simpler than that of vertebrates, genetic homology and conserved neural circuits between flies and mammals could be key to understanding latent sex differences by which stress differentially alters emotion primitives and behaviors. This knowledge is important for informing targets in the treatment of human disorders. This review has provided an extensive, but not exhaustive, overview of the molecular and systems level mechanisms through which flies generate sexually dimorphic, stress-induced behavioral responses.
Although there is overlap in the symptoms of human internalizing disorders and similar, stress-induced behaviors in flies, ongoing research is working to define the neurobiological mechanisms underlying these behaviors in flies and whether they have translational relevance to vertebrates. It is possible that orthologous genes could be identified in mammals whose roles have not yet been defined in stress-related circuits and behaviors. Another avenue for future research is to determine whether similar or different molecular pathways contribute to common phenotypes (e.g., sweet-seeking, activity) induced by different forms of stress (e.g., social isolation versus overcrowding) in flies. Additionally, many stress studies in D. melanogaster fail to explore the long-term consequences of stress exposure. For how long do social isolation and other forms of chronic stress exacerbate internalizing-like and externalizing-like behaviors? Do different behaviors emerge over time? Do sex differences in stress-induced molecular mechanisms and behaviors persist or evolve over time? These unanswered questions could provide further insight as to whether stress responses in flies resemble those observed in vertebrate animal studies.
Overall, D. melanogaster has long served as a useful model system for identifying conserved molecular pathways underlying fundamental behaviors and diseases. The genetic toolkit combined with established methods for stressing flies and quantifying internalizing-like and externalizing-like behaviors make D. melanogaster an attractive organism for exploring the molecular basis of sex differences in stress-induced behavior change. It is possible that evolutionarily conserved molecular mechanisms could play a role in human psychiatric and behavioral disorders that are precipitated by stress and manifest differently in males and females. Combined with knowledge from rodent models, increased understanding of these relationships could inform novel therapeutic interventions for stress-related disorders and emphasize the need for equal representation of both sexes in preclinical research.
Author contributions
ET: Conceptualization, Writing – original draft, Writing – review & editing. DN: Supervision, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This publication was supported by the Princeton University Center for Health and Wellbeing (CHW). Portions of Elisabeth Tawa’s and Daniel Notterman’s effort were supported by R01HD076592 and R01HL149869.
Acknowledgments
We would like to thank Jodi Schottenfeld-Roames and Lisa Schneper (Princeton University, Department of Molecular Biology) for their insightful comments and feedback on this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Achenbach, T. M. (1966). The classification of children’s psychiatric symptoms: a factor-analytic study. Psychol. Monogr. 80, 1–37. doi: 10.1037/h0093906
Agrawal, P., Chung, P., Heberlein, U., and Kent, C. (2019). Enabling cell-type-specific behavioral epigenetics in Drosophila: a modified high-yield INTACT method reveals the impact of social environment on the epigenetic landscape in dopaminergic neurons. BMC Biol. 17:30. doi: 10.1186/s12915-019-0646-4
Agrawal, P., Kao, D., Chung, P., and Looger, L. L. (2020). The neuropeptide drosulfakinin regulates social isolation-induced aggression in Drosophila. J. Exp. Biol. 223:jeb207407. doi: 10.1242/jeb.207407
Ahmad, S. I., and Hinshaw, S. P. (2017). Attention-deficit/hyperactivity disorder, trait impulsivity, and externalizing behavior in a longitudinal sample. J. Abnorm. Child Psychol. 45, 1077–1089. doi: 10.1007/s10802-016-0226-9
Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K., and Walter, P. (2002). “Drosophila and the molecular genetics of pattern formation: genesis of the body plan” in Molecular biology of the cell. 4th ed (New York: Garland Science).
Alekseyenko, O. V., Chan, Y. B., Fernandez, M. P., Bülow, T., Pankratz, M. J., and Kravitz, E. A. (2014). Single serotonergic neurons that modulate aggression in Drosophila. Curr. Biol. 24, 2700–2707. doi: 10.1016/j.cub.2014.09.051
Alekseyenko, O. V., Chan, Y. B., Li, R., and Kravitz, E. A. (2013). Single dopaminergic neurons that modulate aggression in Drosophila. Proc. Natl. Acad. Sci. USA 110, 6151–6156. doi: 10.1073/pnas.1303446110
Alekseyenko, O. V., Chan, Y. B., Okaty, B. W., Chang, Y. J., Dymecki, S. M., and Kravitz, E. A. (2019). Serotonergic modulation of aggression in Drosophila involves GABAergic and cholinergic opposing pathways. Curr. Biol. 29, 2145–2156.e5. doi: 10.1016/j.cub.2019.05.070
Alsiö, J., Nordenankar, K., Arvidsson, E., Birgner, C., Mahmoudi, S., Halbout, B., et al. (2011). Enhanced sucrose and cocaine self-administration and Cue-induced drug seeking after loss of VGLUT2 in midbrain dopamine neurons in mice. J. Neurosci. 31, 12593–12603. doi: 10.1523/JNEUROSCI.2397-11.2011
Anderson, D. J., and Adolphs, R. (2014). A framework for studying emotions across species. Cell 157, 187–200. doi: 10.1016/j.cell.2014.03.003
Andretic, R., Chaney, S., and Hirsh, J. (1999). Requirement of circadian genes for cocaine sensitization in Drosophila’. Science 285:5430. doi: 10.1126/science.285.5430.1066
Andretic, R., van Swinderen, B., and Greenspan, R. J. (2005). Dopaminergic modulation of arousal in Drosophila. Curr. Biol. 15, 1165–1175. doi: 10.1016/j.cub.2005.05.025
Araujo, S. M., Bortolotto, V. C., Poetini, M. R., Dahleh, M. M. M., Couto, S. F., Pinheiro, F. C., et al. (2020). Γ-Oryzanol produces an antidepressant-like effect in a chronic unpredictable mild stress model of depression in Drosophila melanogaster. Stress 24, 282–293. doi: 10.1080/10253890.2020.1790519
Araujo, S. M., Poetini, M. R., Bortolotto, V. C., de Freitas Couto, S., Pinheiro, F. C., Meichtry, L. B., et al. (2018). Chronic unpredictable mild stress-induced depressive-like behavior and dysregulation of brain levels of biogenic amines in Drosophila melanogaster. Behav. Brain Res. 351, 104–113. doi: 10.1016/j.bbr.2018.05.016
Artigas, F. (2013). Serotonin receptors involved in antidepressant effects. Pharmacol. Ther. 137, 119–131. doi: 10.1016/j.pharmthera.2012.09.006
Asahina, K., Watanabe, K., Duistermars, B. J., Hoopfer, E., González, C. R., Eyjólfsdóttir, E. A., et al. (2014). Tachykinin-expressing neurons control male-specific aggressive arousal in Drosophila. Cell 156, 221–235. doi: 10.1016/j.cell.2013.11.045
Bacqué-Cazenave, J., Bharatiya, R., Barrière, G., Delbecque, J. P., Bouguiyoud, N., di Giovanni, G., et al. (2020). Serotonin in animal cognition and behavior. Int. J. Mol. Sci. 21:1649. doi: 10.3390/ijms21051649
Bainton, R. J., Tsai, L. T. Y., Singh, C. M., Moore, M. S., Neckameyer, W. S., and Heberlein, U. (2000). Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Curr. Biol. 10, 187–194. doi: 10.1016/s0960-9822(00)00336-5
Bale, T. L., and Vale, W. W. (2004). CRF and CRF receptors: role in stress responsivity and other behaviors. Annu. Rev. Pharmacol. Toxicol. 44, 525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410
Barnes, N. M., and Sharp, T. (1999). A review of central 5-HT receptors and their function. Neuropharmacology 38, 1083–1152. doi: 10.1016/s0028-3908(99)00010-6.
Barrett, E. S., and Swan, S. H. (2015). Stress and androgen activity during fetal development. Endocrinology 156, 3435–3441. doi: 10.1210/en.2015-1335
Batsching, S., Wolf, R., and Heisenberg, M. (2016). Inescapable stress changes walking behavior in flies -learned helplessness revisited. PLoS One 11:e0167066. doi: 10.1371/journal.pone.0167066
Becker, C., Zeau, B., Rivat, C., Blugeot, A., Hamon, M., and Benoliel, J. J. (2007). Repeated social defeat-induced depression-like behavioral and biological alterations in rats: involvement of cholecystokinin. Mol. Psychiatry 13, 1079–1092. doi: 10.1038/sj.mp.4002097
Bentzur, A., Shmueli, A., Omesi, L., Ryvkin, J., Knapp, J. M., Parnas, M., et al. (2018). Odorant binding protein 69a connects social interaction to modulation of social responsiveness in Drosophila. PLoS Genet. 14:e1007328. doi: 10.1371/journal.pgen.1007328
Berry, J. A., Cervantes-Sandoval, I., Chakraborty, M., and Davis, R. L. (2015). Sleep facilitates memory by blocking dopamine neuron-mediated forgetting. Cell 161, 1656–1667. doi: 10.1016/j.cell.2015.05.027
Bertoglio, L. J., and Carobrez, A. P. (2002). Anxiolytic effects of ethanol and phenobarbital are abolished in test-experienced rats submitted to the elevated plus maze. Pharmacol. Biochem. Behav. 73, 963–969. doi: 10.1016/s0091-3057(02)00958-9
Birkett, M. A., Shinday, N. M., Kessler, E. J., Meyer, J. S., Ritchie, S., and Rowlett, J. K. (2011). Acute anxiogenic-like effects of selective serotonin reuptake inhibitors are attenuated by the benzodiazepine diazepam in BALB/c mice. Pharmacol. Biochem. Behav. 98, 544–551. doi: 10.1016/j.pbb.2011.03.006
Björklund, A., and Dunnett, S. B. (2007). Dopamine neuron systems in the brain: an update. Trends Neurosci. 30, 194–202. doi: 10.1016/j.tins.2007.03.006
Blenau, W., Daniel, S., Balfanz, S., Thamm, M., and Baumann, A. (2017). Dm5-HT2B: pharmacological characterization of the fifth serotonin receptor subtype of Drosophila melanogaster. Front. Syst. Neurosci. 11, 1–11. doi: 10.3389/fnsys.2017.00028
Bodnoff, S. R., Suranyi-Cadotte, B., Aitken, D. H., Quirion, R., and Meaney, M. J. (1988). The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology 95, 298–302. doi: 10.1007/BF00181937
Bradwejn, J., Koszycki, D., and Meterissian, G. (1990). Cholecystokinin-Tetrapeptide induces panic attacks in patients with panic disorder*. Can. J. Psychiatry 35, 83–85. doi: 10.1177/070674379003500115
Brown, M. K., Strus, E., and Naidoo, N. (2017). Reduced sleep during social isolation leads to cellular stress and induction of the unfolded protein response. Sleep 40:zsx095. doi: 10.1093/sleep/zsx095
Buck, S. A., Steinkellner, T., Aslanoglou, D., Villeneuve, M., Bhatte, S. H., Childers, V. C., et al. (2021). Vesicular glutamate transporter modulates sex differences in dopamine neuron vulnerability to age-related neurodegeneration. Aging Cell 20:e13365. doi: 10.1111/acel.13365
Buhot, M. C. (1997). Serotonin receptors in cognitive behaviors. Curr. Opin. Neurobiol. 7, 243–254. doi: 10.1016/s0959-4388(97)80013-x
Buznikov, G. A., Lambert, H. W., and Lauder, J. M. (2001). Serotonin and serotonin-like substances as regulators of early embryogenesis and morphogenesis. Cell Tissue Res. 305, 177–186. doi: 10.1007/s004410100408
Can, A., Dao, D. T., Terrillion, C. E., Piantadosi, S. C., Bhat, S., and Gould, T. D. (2012). The tail suspension test. J. Visua. Exp. 59:e3769. doi: 10.3791/3769
Carvajal, C., Dumont, Y., and Quirion, R. (2006). Neuropeptide y: role in emotion and alcohol dependence. CNS Neurol. Disord. Drug Targets 5, 181–195. doi: 10.2174/187152706776359592
Chakraborty, T. S., Gendron, C. M., Lyu, Y., Munneke, A. S., DeMarco, M. N., Hoisington, Z. W., et al. (2019). Sensory perception of dead conspecifics induces aversive cues and modulates lifespan through serotonin in Drosophila. Nat. Commun. 10:2365. doi: 10.1038/s41467-019-10285-y
Chen, S., Lee, A. Y., Bowens, N. M., Huber, R., and Kravitz, E. A. (2002). Fighting fruit flies: a model system for the study of aggression. Proc. Natl. Acad. Sci. 99, 5664–5668. doi: 10.1073/pnas.082102599
Chen, X., Peterson, J., Nachman, R. J., and Ganetzky, B. (2012). Drosulfakinin activates CCKLR-17D1 and promotes larval locomotion and escape response in Drosophila. Fly 6, 290–297. doi: 10.4161/fly.21534
Chen, M., and Sokolowski, M. B. (2022). How social experience and environment impacts Behavioural plasticity in Drosophila. Fly 16, 68–84. doi: 10.1080/19336934.2021.1989248
Chen, T., Zhang, M., Ding, Z., Hu, J., Yang, J., He, L., et al. (2023). The Drosophila NPY-like system protects against chronic stress–induced learning deficit by preventing the disruption of autophagic flux. Proc. Natl. Acad. Sci. USA. 120:e2307632120. doi: 10.1073/pnas.2307632120
Chi, W., Liu, W., Fu, W., Xia, S., Heckscher, E. S., and Zhuang, X. (2021). RNA-binding protein syncrip regulates starvation-induced hyperactivity in adult Drosophila. PLoS Genet. 17:e1009396. doi: 10.1371/journal.pgen.1009396
Chiu, H., Hoopfer, E. D., Coughlan, M. L., Pavlou, H. J., Goodwin, S. F., and Anderson, D. J. (2021). A circuit logic for sexually shared and dimorphic aggressive behaviors in Drosophila. Cell 184, 507–520.e16. doi: 10.1016/j.cell.2020.11.048
Chiu, J. C., Low, K. H., Pike, D. H., Yildirim, E., and Edery, I. (2010). Assaying locomotor activity to study circadian rhythms and sleep parameters in Drosophila. J. Visual. Exp. 43:2157. doi: 10.3791/2157
Chowdhury, B., Wang, M., Gnerer, J. P., and Dierick, H. A. (2021). The divider assay is a high-throughput pipeline for aggression analysis in Drosophila. Commun. Biol. 4:85. doi: 10.1038/s42003-020-01617-6
Chung, B. Y., Ro, J., Hutter, S. A., Miller, K. M., Guduguntla, L. S., Kondo, S., et al. (2017). Drosophila neuropeptide F signaling independently regulates feeding and sleep-wake behavior. Cell Rep. 19, 2441–2450. doi: 10.1016/j.celrep.2017.05.085
Colas, J. F., Launay, J. M., Kellermann, O., Rosay, P., and Maroteaux, L. (1995). Drosophila 5-HT2 serotonin receptor: coexpression with fushi-tarazu during segmentation. Proc. Natl. Acad. Sci. USA 92, 5441–5445. doi: 10.1073/pnas.92.12.5441
Cryan, J. F., Mombereau, C., and Vassout, A. (2005). The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 29, 571–625. doi: 10.1016/j.neubiorev.2005.03.009
D’Agostino, G., Lyons, D., Cristiano, C., Lettieri, M., Olarte-Sanchez, C., Burke, L. K., et al. (2018). Nucleus of the solitary tract serotonin 5-HT2C receptors modulate food intake. Cell Metab. 28, 619–630.e5. doi: 10.1016/j.cmet.2018.07.017
Dai, B., Zheng, B., Dai, X., Cui, X., Yin, L., Cai, J., et al. (2025). Experience-dependent dopamine modulation of male aggression. Nature 639, 430–437. doi: 10.1038/s41586-024-08459-w
Danchin, E., Nöbel, S., Pocheville, A., Dagaeff, A. C., Demay, L., Alphand, M., et al. (2018). Cultural flies: conformist social learning in fruitflies predicts long-lasting mate-choice traditions. Science 362, 1025–1030. doi: 10.1126/science.aat1590
Davis, S. M., Thomas, A. L., Nomie, K. J., Huang, L., and Dierick, H. A. (2014). ‘Tailless and Atrophin control Drosophila aggression by regulating neuropeptide signalling in the pars intercerebralis’, nature. Communications 5:3177. doi: 10.1038/ncomms4177
Denno, M. E., Privman, E., and Venton, B. J. (2015). Analysis of neurotransmitter tissue content of Drosophila melanogaster in different life stages. ACS Chem. Neurosci. 6, 117–123. doi: 10.1021/cn500261e
Dhaliwal, J. S., Rosani, A., and Saadabadi, A. (2023). “Diazepam” in StatPearls (Treasure Island, FL: StatPearls Publishing).
Diegelmann, S., Jansen, A., Jois, S., Kastenholz, K., Velo Escarcena, L., Strudthoff, N., et al. (2017). The CApillary FEeder assay measures food intake in Drosophila melanogaster. J. Visual. Exp. 121:55024. doi: 10.3791/55024
Dierick, H. A. (2007). A method for quantifying aggression in male Drosophila melanogaster. Nat. Protoc. 2, 2712–2718. doi: 10.1038/nprot.2007.404
Dierick, H. A., and Greenspan, R. J. (2007). Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat. Genet. 39, 678–682. doi: 10.1038/ng2029
Durisko, Z., Kemp, R., Mubasher, R., and Dukas, R. (2014). Dynamics of social behavior in fruit Fly larvae. PLoS One 9:e95495. doi: 10.1371/journal.pone.0095495
Duszkiewicz, A. J., McNamara, C. G., Takeuchi, T., and Genzel, L. (2019). Novelty and dopaminergic modulation of memory persistence: a tale of two systems. Trends Neurosci. 42, 102–114. doi: 10.1016/j.tins.2018.10.002
Edmunds, D., Wigby, S., and Perry, J. C. (2021). “Hangry” Drosophila: food deprivation increases male aggression. Anim. Behav. 177, 183–190. doi: 10.1016/j.anbehav.2021.05.001
Fedina, T. Y., Cummins, E. T., Promislow, D. E. L., and Pletcher, S. D. (2023). The neuropeptide drosulfakinin enhances choosiness and protects males from the aging effects of social perception. Proc. Natl. Acad. Sci. USA. 120:e2308305120. doi: 10.1073/pnas.2308305120
Feng, G., Hannan, F., Reale, V., Hon, Y. Y., Kousky, C. T., Evans, P. D., et al. (1996). Cloning and functional characterization of a novel dopamine receptor from Drosophila melanogaster. J. Neurosci. 16, 3925–3933. doi: 10.1523/JNEUROSCI.16-12-03925.1996
Fernandez, R. W., Akinleye, A. A., Nurilov, M., Feliciano, O., Lollar, M., Aijuri, R. R., et al. (2017). Modulation of social space by dopamine in Drosophila melanogaster, but no effect on the avoidance of the Drosophila stress odorant. Biol. Lett. 13:20170369. doi: 10.1098/rsbl.2017.0369
Ferreira, C. H., and Moita, M. A. (2020). Behavioral and neuronal underpinnings of safety in numbers in fruit flies. Nat. Commun. 11:4182. doi: 10.1038/s41467-020-17856-4
Figlewicz, D. P., Bennett-Jay, J. L., Kittleson, S., Sipols, A. J., and Zavosh, A. (2011). ‘Sucrose self-administration and CNS activation in the rat’, American journal of physiology - regulatory, integrative and comparative. Physiology 300, R876–R884. doi: 10.1152/ajpregu.00655.2010
File, S. E., and Hyde, J. R. (1978). Can social interaction be used to measure anxiety? Br. J. Pharmacol. 62, 19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x
Forbes, S., Herzog, H., and Cox, H. M. (2012). A role for neuropeptide Y in the gender-specific gastrointestinal, corticosterone and feeding responses to stress. Br. J. Pharmacol. 166, 2307–2316. doi: 10.1111/j.1476-5381.2012.01939.x
Ganguly, A., Qi, C., Bajaj, J., and Lee, D. (2020). Serotonin receptor 5-HT7 in Drosophila mushroom body neurons mediates larval appetitive olfactory learning. Sci. Rep. 10:21267. doi: 10.1038/s41598-020-77910-5
Ganguly-Fitzgerald, I., Donlea, J., and Shaw, P. J. (2006). Waking experience affects sleep need in Drosophila. Science 313, 1775–1781. doi: 10.1126/science.1130408
Garcia, A. M., Cardenas, F. P., and Morato, S. (2011). The effects of pentylenetetrazol, chlordiazepoxide and caffeine in rats tested in the elevated plus-maze depend on the experimental illumination. Behav. Brain Res. 217, 171–177. doi: 10.1016/j.bbr.2010.09.032
Gassen, N. C., and Rein, T. (2019). Is there a role of autophagy in depression and antidepressant action? Front. Psychiatry 10:337. doi: 10.3389/fpsyt.2019.00337
Gater, R., Tansella, M., Korten, A., Tiemens, B. G., Mavreas, V. G., and Olatawura, M. O. (1998). Sex differences in the prevalence and detection of depressive and anxiety disorders in general health care settings: report from the World Health Organization collaborative study on psychological problems in general health care. Arch. Gen. Psychiatry 55, 405–413. doi: 10.1001/archpsyc.55.5.405
Gilbert, S. F. (2000). ‘Chromosomal sex determination in Drosophila’, in developmental biology. 6th Edn. Sunderland, MA: Sinauer Associates.
Gil-Martí, B., Isidro-Mézcua, J., Poza-Rodriguez, A., Asti Tello, G. S., Treves, G., Turiégano, E., et al. (2024). Socialization causes long-lasting behavioral changes. Sci. Rep. 14:22302. doi: 10.1038/s41598-024-73218-w
Goel, N., and Bale, T. L. (2010). Sex differences in the serotonergic influence on the hypothalamic-pituitary-adrenal stress Axis. Endocrinology 151, 1784–1794. doi: 10.1210/en.2009-1180
Gotzes, F., Balfanz, S., and Baumann, A. (1994). Primary structure and functional characterization of a Drosophila dopamine receptor with high homology to human D1/5 receptors. Receptors Channels 2, 131–141
Griebel, G., and Holmes, A. (2013). 50 years of hurdles and hope in anxiolytic drug discovery. Nat. Rev. Drug Discov. 12, 667–687. doi: 10.1038/nrd4075
Gruntenko, N., Chentsova, N. A., Bogomolova, E. V., Karpova, E. K., Glazko, G. V., Faddeeva, N. V., et al. (2004). The effect of mutations altering biogenic amine metabolism in Drosophila on viability and the response to environmental stresses. Arch. Insect Biochem. Physiol. 55, 55–67. doi: 10.1002/arch.10123
Gurevich, E. V., Gainetdinov, R. R., and Gurevich, V. V. (2016). G protein-coupled receptor kinases as regulators of dopamine receptor functions. Pharmacol. Res. 111, 1–16. doi: 10.1016/j.phrs.2016.05.010
Han, K. A., Millar, N. S., Grotewiel, M. S., and Davis, R. L. (1996). DAMB, a novel dopamine receptor expressed specifically in Drosophila mushroom bodies. Neuron 16, 1127–1135. doi: 10.1016/s0896-6273(00)80139-7
Hasegawa, E., Miyasaka, A., Sakurai, K., Cherasse, Y., Li, Y., and Sakurai, T. (2022). Rapid eye movement sleep is initiated by basolateral amygdala dopamine signaling in mice. Science 375, 994–1000. doi: 10.1126/science.abl6618
Hasler, G., Drevets, W. C., Manji, H. K., and Charney, D. S. (2004). Discovering Endophenotypes for major depression. Springer Nature 29, 1765–1781. doi: 10.1038/sj.npp.1300506
Hay-Schmidt, A. (2000). The evolution of the serotonergic nervous system. Proc. Biol. Sci. 267, 1071–1079. doi: 10.1098/rspb.2000.1111
Hearn, M. G., Ren, Y., McBride, E. W., Reveillaud, I., Beinborn, M., and Kopin, A. S. (2002). A Drosophila dopamine 2-like receptor: molecular characterization and identification of multiple alternatively spliced variants. Proc. Natl. Acad. Sci. USA 99, 14554–14559. doi: 10.1073/pnas.202498299
Hegarty, S. V., Sullivan, A. M., and O’Keeffe, G. W. (2013). Midbrain dopaminergic neurons: a review of the molecular circuitry that regulates their development. Dev. Biol. 379, 123–138. doi: 10.1016/j.ydbio.2013.04.014
Heier, C., and Kühnlein, R. P. (2018). Triacylglycerol metabolism in Drosophila melanogaster. Genetics 210, 1163–1184. doi: 10.1534/genetics.118.301583
Hermanns, T., Graf-Boxhorn, S., Poeck, B., and Strauss, R. (2022). Octopamine mediates sugar relief from a chronic-stress-induced depression-like state in Drosophila. Curr. Biol. 32, 4048–4056.e3. doi: 10.1016/j.cub.2022.07.016
Hibicke, M., and Nichols, C. D. (2022). Validation of the forced swim test in Drosophila, and its use to demonstrate psilocybin has long-lasting antidepressant-like effects in flies. Sci. Rep. 12:10019. doi: 10.1038/s41598-022-14165-2
Hintermann, E., Grieder, N. C., Amherd, R., Brodbeck, D., and Meyer, U. A. (1996). Cloning of an arylalkylamine N-acetyltransferase (aaNAT1) from Drosophila melanogaster expressed in the nervous system and the gut. Proc. Natl. Acad. Sci. USA 93, 12315–12320. doi: 10.1073/pnas.93.22.12315
Hoffmann, A. (1987). A laboratory study of male territoriality in the sibling species Drosophila melanogaster and D. simulans. Anim. Behav. 35, 807–818. doi: 10.1016/S0003-3472(87)80117-3
Hoffmann, A., and Cacoyianni, Z. (1989). Selection for territoriality in Drosophila melanogaster: correlated responses in mating success and other fitness components. Anim. Behav. 38, 23–34. doi: 10.1016/S0003-3472(89)80062-4
Hovemann, B. T., Ryseck, R. P., Walldorf, U., Störtkuhl, K. F., Dietzel, I. D., and Dessen, E. (1998). The Drosophila ebony gene is closely related to microbial peptide synthetases and shows specific cuticle and nervous system expression. Gene 221, 1–9. doi: 10.1016/s0378-1119(98)00440-5
Howell, S., Westergaard, G., Hoos, B., Chavanne, T. J., Shoaf, S. E., Cleveland, A., et al. (2007). Serotonergic influences on life-history outcomes in free-ranging male rhesus macaques. Am. J. Primatol. 69, 851–865. doi: 10.1002/ajp.20369
Hoyer, S. C., Eckart, A., Herrel, A., Zars, T., Fischer, S. A., Hardie, S. L., et al. (2008). Octopamine in male aggression of Drosophila. Curr. Biol. 18, 159–167. doi: 10.1016/j.cub.2007.12.052
Hu, S. W., Yang, Y. T., Sun, Y., Zhan, Y. P., and Zhu, Y. (2020). Serotonin signals overcome loser mentality in Drosophila. iScience 23:101651. doi: 10.1016/j.isci.2020.101651
Huber, R., Hill, S. L., Holladay, C., Biesiadecki, M., Tononi, G., and Cirelli, C. (2004). Sleep homeostasis in Drosophila melanogaster. Sleep 27, 628–639. doi: 10.1093/sleep/27.4.628
Irion, U., and Nüsslein-Volhard, C. (2022). Developmental genetics with model organisms. Proc. Natl. Acad. Sci. 119:e2122148119. doi: 10.1073/pnas.2122148119
Ja, W. W., Carvalho, G. B., Mak, E. M., de la Rosa, N. N., Fang, A. Y., Liong, J. C., et al. (2007). Prandiology of Drosophila and the CAFE assay. Proc. Natl. Acad. Sci. 104, 8253–8256. doi: 10.1073/pnas.0702726104
Jackson, D. M., and Westlind-Danielsson, A. (1994). Dopamine receptors: molecular biology, biochemistry and behavioural aspects. Pharmacol. Ther. 64, 291–370. doi: 10.1016/0163-7258(94)90041-8
Jeibmann, A., and Paulus, W. (2009). Drosophila melanogaster as a model organism of brain diseases. Int. J. Mol. Sci. 10, 407–440. doi: 10.3390/ijms10020407
Jeong, J., Kwon, K., Geisseova, T. K., Lee, J., Kwon, T., and Lim, C. (2024). Drosulfakinin signaling encodes early-life memory for adaptive social plasticity. eLife 13:e103973. doi: 10.7554/eLife.103973
Jezovit, J. A., Alwash, N., and Levine, J. D. (2021). Using flies to understand social networks. Front. Neural Circuits 15:755093. doi: 10.3389/fncir.2021.755093
Jia, J., He, L., Yang, J., Shuai, Y., Yang, J., Wu, Y., et al. (2021). A pair of dopamine neurons mediate chronic stress signals to induce learning deficit in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 118:e2023674118. doi: 10.1073/pnas.2023674118
Jia, Y., Jin, S., Hu, K., Geng, L., Han, C., Kang, R., et al. (2021). Gut microbiome modulates Drosophila aggression through octopamine signaling. Nat. Commun. 12:2698. doi: 10.1038/s41467-021-23041-y
Jia, J., and Le, W. (2015). Molecular network of neuronal autophagy in the pathophysiology and treatment of depression. Neurosci. Bull. 31, 427–434. doi: 10.1007/s12264-015-1548-2
Johnson, O., Becnel, J., and Nichols, C. D. (2009). Serotonin 5-HT2 and 5-HT1A-like receptors differentially modulate aggressive behaviors in Drosophila melanogaster. Neuroscience 158, 1292–1300. doi: 10.1016/j.neuroscience.2008.10.055
Johnson, E., Ringo, J., and Dowse, H. (1997). Modulation of Drosophila heartbeat by neurotransmitters. J. Comp. Physiol. B, Biochem. Syst. Environ. Physiol. 167, 89–97. doi: 10.1007/s003600050051
Johnson, E. C., Shafer, O. T., Trigg, J. S., Park, J., Schooley, D. A., Dow, J. A., et al. (2005). A novel diuretic hormone receptor in Drosophila: evidence for conservation of CGRP signaling. J. Exp. Biol. 208, 1239–1246. doi: 10.1242/jeb.01529
Joshi, A., and Mueller, L. D. (1997). Adult crowding effects on longevity in Drosophila melanogaster: increase in age-independent mortality. Curr. Sci. 72, 255–260.
Kapila, R., Kashyap, M., Gulati, A., Narasimhan, A., Poddar, S., Mukhopadhaya, A., et al. (2021). Evolution of sex-specific heat stress tolerance and larval Hsp70 expression in populations of Drosophila melanogaster adapted to larval crowding. J. Evol. Biol. 34, 1376–1385. doi: 10.1111/jeb.13897
Karam, C. S., Jones, S. K., and Javitch, J. A. (2019). Come Fly with me: an overview of dopamine receptors in Drosophila melanogaster. Basic Clin. Pharmacol. Toxicol. 126 Suppl 6, 56–65. doi: 10.1111/bcpt.13277
Karl, T., Lin, S., Schwarzer, C., Sainsbury, A., Couzens, M., Wittmann, W., et al. (2004). Y1 receptors regulate aggressive behavior by modulating serotonin pathways. Proc. Natl. Acad. Sci. USA 101, 12742–12747. doi: 10.1073/pnas.0404085101
Kasture, A. S., Hummel, T., Sucic, S., and Freissmuth, M. (2018). Big lessons from tiny flies: Drosophila melanogaster as a model to explore dysfunction of dopaminergic and serotonergic neurotransmitter systems. Int. J. Mol. Sci. 19:1788. doi: 10.3390/ijms19061788
Katsouni, E., Sakkas, P., Zarros, A., Skandali, N., and Liapi, C. (2009). The involvement of substance P in the induction of aggressive behavior. Peptides 30, 1586–1591. doi: 10.1016/j.peptides.2009.05.001
Kessler, R. C., McGonagle, K., Zhao, S., Nelson, C. B., Hughes, M., Eshleman, S., et al. (1994). Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch. Gen. Psychiatry 51, 8–19. doi: 10.1001/archpsyc.1994.03950010008002
Khan, Z., Tondravi, M., Oliver, R., and Vonhoff, F. J. (2021). ‘Drosophila Corazonin neurons as a hub for regulating growth, stress responses, ethanol-related behaviors, copulation persistence and sexually dimorphic reward pathways’, journal of. Dev. Biol. 9:26. doi: 10.3390/jdb9030026
Koolhaas, J. M., Coppens, C. M., de Boer, S. F., Buwalda, B., Meerlo, P., and Timmermans, P. J. A. (2013). The resident-intruder paradigm: a standardized test for aggression, violence and social stress. J. Visual. Exp. 77:e4367. doi: 10.3791/4367
Korshunov, K. S., Blakemore, L. J., and Trombley, P. Q. (2017). Dopamine: a modulator of circadian rhythms in the central nervous system. Front. Cell. Neurosci. 11:91. doi: 10.3389/fncel.2017.00091
Kraeuter, A.-K., Guest, P. C., and Sarnyai, Z. (2019). Free dyadic social interaction test in mice. Pre-Clin Model. doi: 10.1007/978-1-4939-8994-2_8
Kravitz, E. A., and Fernandez, M. P. (2015). Aggression in Drosophila. Behav. Neurosci. 129, 549–563. doi: 10.1037/bne0000089
Kroemer, G., Mariño, G., and Levine, B. (2010). Autophagy and the integrated stress response. Mol. Cell 40, 280–293. doi: 10.1016/j.molcel.2010.09.023
Kuehner, C. (2017). Why is depression more common among women than among men? Lancet Psychiatry 4, 146–158. doi: 10.1016/S2215-0366(16)30263-2
Kulesskaya, N., and Voikar, V. (2014). Assessment of mouse anxiety-like behavior in the light-dark box and open-field arena: role of equipment and procedure. Physiol. Behav. 133, 30–38. doi: 10.1016/j.physbeh.2014.05.006
Lall, S., Mudunuri, A., Santhosh, S., Malwade, A., Thadi, A., Kondakath, G., et al. (2019). Adult crowding induces sexual dimorphism in chronic stress-response in Drosophila melanogaster. BioRxiv, 702357. doi: 10.1101/702357
Lebestky, T., Chang, J. S. C., Dankert, H., Zelnik, L., Kim, Y. C., Han, K. A., et al. (2009). Two different forms of arousal in Drosophila are oppositely regulated by the dopamine D1 receptor Ortholog DopR via distinct neural circuits. Neuron 64, 522–536. doi: 10.1016/j.neuron.2009.09.031
Lee, G., Bahn, J. H., and Park, J. H. (2006). Sex- and clock-controlled expression of the neuropeptide F gene in Drosophila. Proc. Natl. Acad. Sci. 103, 12580–12585. doi: 10.1073/pnas.0601171103
Lee, G., Kim, K. M., Kikuno, K., Wang, Z., Choi, Y. J., and Park, J. H. (2008). Developmental regulation and functions of the expression of the neuropeptide corazonin in Drosophila melanogaster. Cell Tissue Res. 331, 659–673. doi: 10.1007/s00441-007-0549-5
Leech, T., Sait, S. M., and Bretman, A. (2017). Sex-specific effects of social isolation on ageing in Drosophila melanogaster. J. Insect Physiol. 102, 12–17. doi: 10.1016/j.jinsphys.2017.08.008
Li, Q., Deng, X., and Singh, P. (2007). ‘Significant increase in the aggressive behavior of transgenic mice overexpressing peripheral Progastrin peptides: associated changes in CCK2 and serotonin receptors in the CNS’, Neuropsychopharmacology: official publication of the American college of. Neuropsychopharmacology 32, 1813–1821. doi: 10.1038/sj.npp.1301304
Li, W., Wang, Z., Syed, S., Lyu, C., Lincoln, S., O’Neil, J., et al. (2021). Chronic social isolation signals starvation and reduces sleep in Drosophila. Nature 597, 239–244. doi: 10.1038/s41586-021-03837-0
Lin, Y.-C., Zhang, M., Wang, S. H., Chieh, C. W., Shen, P. Y., Chen, Y. L., et al. (2022). The deleterious effects of old social partners on Drosophila lifespan and stress resistance. Npj Aging 8, 1–10. doi: 10.1038/s41514-022-00081-2
Liu, M. Y., Yin, C. Y., Zhu, L. J., Zhu, X. H., Xu, C., Luo, C. X., et al. (2018). Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat. Protoc. 13, 1686–1698. doi: 10.1038/s41596-018-0011-z
Lloyd, T. E., and Taylor, J. P. (2010). Flightless flies: Drosophila models of neuromuscular disease. Ann. N. Y. Acad. Sci. 1184, e1–e20. doi: 10.1111/j.1749-6632.2010.05432.x
Long, X., du, H., Jiang, M., and Meng, H. (2023). A simple technique to assay locomotor activity in Drosophila. J. Visual. Exp. 192. doi: 10.3791/65092
Lucki, I. (1998). The spectrum of behaviors influenced by serotonin. Biol. Psychiatry 44, 151–162. doi: 10.1016/s0006-3223(98)00139-5
Lucki, I., Dalvi, A., and Mayorga, A. J. (2001). Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology 155, 315–322. doi: 10.1007/s002130100694
Lueningschroer-Wang, Y., Derksen, E., Steigmeier, M., and Wegener, C. (2025). Behavioural and neurogenetic evidence for emotion primitives in the fruit fly Drosophila: insights from the Open Field Test. eLife. 14:RP107110. doi: 10.7554/eLife.107110.1
Magistrelli, L., Ferrari, M., Furgiuele, A., Milner, A. V., Contaldi, E., Comi, C., et al. (2021). Polymorphisms of dopamine receptor genes and Parkinson’s disease: clinical relevance and future perspectives. Int. J. Mol. Sci. 22:3781. doi: 10.3390/ijms22073781
Maher, B. (2008). Personal genomes: the case of the missing heritability. Nature 456, 18–21. doi: 10.1038/456018a
Mani, S. K., Allen, J. M. C., Clark, J. H., Blaustein, J. D., and O’malley, B. W. (1994). Convergent pathways for steroid hormone- and neurotransmitter-induced rat sexual behavior. Science 265, 1246–1249. doi: 10.1126/science.7915049
Mao, Z., and Davis, R. L. (2009). Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Front. Neural Circuits 3:5. doi: 10.3389/neuro.04.005.2009
Marquis, M., and Wilson, R. I. (2022). Locomotor and olfactory responses in dopamine neurons of the Drosophila superior-lateral brain. Curr. Biol. 32, 5406–5414.e5. doi: 10.1016/j.cub.2022.11.008
Martel, M. M. (2013). Sexual selection and sex differences in the prevalence of childhood externalizing and adolescent internalizing disorders. Psychol. Bull. 139, 1221–1259. doi: 10.1037/a0032247
Martel, M. M., and Roberts, B. A. (2014). Prenatal testosterone increases sensitivity to prenatal stressors in males with disruptive behavior disorders. Neurotoxicol. Teratol. 44, 11–17. doi: 10.1016/j.ntt.2014.05.001
McCarthy, M. M. (2016). Multifaceted origins of sex differences in the brain. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 371:20150106. doi: 10.1098/rstb.2015.0106
Meiser, J., Weindl, D., and Hiller, K. (2013). Complexity of dopamine metabolism. Cell Commun. Signal. 11:34. doi: 10.1186/1478-811X-11-34
Mery, F., Varela, S. A. M., Danchin, É., Blanchet, S., Parejo, D., Coolen, I., et al. (2009). Public versus personal information for mate copying in an invertebrate. Curr. Biol. 19, 730–734. doi: 10.1016/j.cub.2009.02.064
Miczek, K. A., Maxson, S. C., Fish, E. W., and Faccidomo, S. (2001). Aggressive behavioral phenotypes in mice. Behav. Brain Res. 125, 167–181. doi: 10.1016/s0166-4328(01)00298-4
Missale, C., Nash, S. R., Robinson, S. W., Jaber, M., and Caron, M. G. (1998). Dopamine receptors: from structure to function. Physiol. Rev. 78, 189–225. doi: 10.1152/physrev.1998.78.1.189
Mohammad, F., Aryal, S., Ho, J., Stewart, J. C., Norman, N. A., Tan, T. L., et al. (2016). Ancient anxiety pathways influence Drosophila defense behaviors. Curr. Biol. 26, 981–986. doi: 10.1016/j.cub.2016.02.031
Molendijk, M. L., and de Kloet, E. R. (2022). Forced swim stressor: trends in usage and mechanistic consideration. Eur. J. Neurosci. 55, 2813–2831. doi: 10.1111/ejn.15139
Monastirioti, M. (2003). Distinct octopamine cell population residing in the CNS abdominal ganglion controls ovulation in Drosophila melanogaster. Dev. Biol. 264, 38–49. doi: 10.1016/j.ydbio.2003.07.019
Monti, J. M. (2011). Serotonin control of sleep-wake behavior. Sleep Med. Rev. 15, 269–281. doi: 10.1016/j.smrv.2010.11.003
Morimoto, J., Nguyen, B., Dinh, H., Than, A. T., Taylor, P. W., and Ponton, F. (2019). Crowded developmental environment promotes adult sex-specific nutrient consumption in a polyphagous fly. Front. Zool. 16, 1–11. doi: 10.1186/s12983-019-0302-4
Morimoto, J., Wenzel, M., Derous, D., Henry, Y., and Colinet, H. (2023). The transcriptomic signature of responses to larval crowding in Drosophila melanogaster. Insect Sci. 30, 539–554. doi: 10.1111/1744-7917.13113
Mullins, N., Perroud, N., Uher, R., Butler, A. W., Cohen-Woods, S., Rivera, M., et al. (2014). Genetic relationships between suicide attempts, suicidal ideation and major psychiatric disorders: a genome-wide association and polygenic scoring study. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 165B, 428–437. doi: 10.1002/ajmg.b.32247
Mundiyanapurath, S., Chan, Y. B., Leung, A. K. W., and Kravitz, E. A. (2009). Feminizing cholinergic neurons in a male Drosophila nervous system enhances aggression. Fly 3, 179–184. doi: 10.4161/fly.3.3.8989
Muthuraman, M., Koirala, N., Ciolac, D., Pintea, B., Glaser, M., Groppa, S., et al. (2018). Deep brain stimulation and L-DOPA therapy: concepts of action and clinical applications in Parkinson’s disease. Front. Neurol. 9:711. doi: 10.3389/fneur.2018.00711
Nadler, J. J., Moy, S. S., Dold, G., Trang, D., Simmons, N., Perez, A., et al. (2004). Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 3, 303–314. doi: 10.1111/j.1601-183X.2004.00071.x
Nässel, D. R., and Wegener, C. (2011). A comparative review of short and long neuropeptide F signaling in invertebrates: any similarities to vertebrate neuropeptide Y signaling? Peptides 32, 1335–1355. doi: 10.1016/j.peptides.2011.03.013
Nässel, D. R., and Williams, M. J. (2014). Cholecystokinin-like peptide (DSK) in Drosophila, not only for satiety signaling. Front. Endocrinol. 5:219. doi: 10.3389/fendo.2014.00219
Neckameyer, W. S. (1998). Dopamine modulates female sexual receptivity in Drosophila melanogaster. J. Neurogenet. 12, 101–114. doi: 10.3109/01677069809167259
Neckameyer, W. S., and Nieto, A. (2015). Response to stress in Drosophila is mediated by gender, age and stress paradigm. Stress 18, 254–266. doi: 10.3109/10253890.2015.1017465
Neckameyer, W. S., and Weinstein, J. S. (2005). Stress affects dopaminergic signaling pathways in Drosophila melanogaster. Stress 8, 117–131. doi: 10.1080/10253890500147381
Nikstat, A., and Riemann, R. (2020). On the etiology of internalizing and externalizing problem behavior: a twin-family study. PLoS One 15:e0230626. doi: 10.1371/journal.pone.0230626
Nilsen, S. P., Chan, Y. B., Huber, R., and Kravitz, E. A. (2004). Gender-selective patterns of aggressive behavior in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 101, 12342–12347. doi: 10.1073/pnas.0404693101
Nixon, R. A. (2013). The role of autophagy in neurodegenerative disease. Nat. Med. 19, 983–997. doi: 10.1038/nm.3232
Oyeyinka, A., Kansal, M., O’Sullivan, S. M., Gualtieri, C., Smith, Z. M., and Vonhoff, F. J. (2022). Corazonin neurons contribute to dimorphic ethanol sedation sensitivity in Drosophila melanogaster. Front. Neural Circuits 16:702901. doi: 10.3389/fncir.2022.702901
Painsipp, E., Herzog, H., Sperk, G., and Holzer, P. (2011). Sex-dependent control of murine emotional-affective behaviour in health and colitis by peptide YY and neuropeptide Y. Br. J. Pharmacol. 163, 1302–1314. doi: 10.1111/j.1476-5381.2011.01326.x
Palavicino-Maggio, C. B., Chan, Y. B., McKellar, C., and Kravitz, E. A. (2019). A small number of cholinergic neurons mediate hyperaggression in female Drosophila. Proc. Natl. Acad. Sci. USA 116, 17029–17038. doi: 10.1073/pnas.1907042116
Palavicino-Maggio, C. B., and Sengupta, S. (2022). The Neuromodulatory basis of aggression: lessons from the humble fruit Fly. Front. Behav. Neurosci. 16:836666. doi: 10.3389/fnbeh.2022.836666
Panksepp, J., Burgdorf, J., Beinfeld, M. C., Kroes, R. A., and Moskal, J. R. (2004). Regional brain cholecystokinin changes as a function of friendly and aggressive social interactions in rats. Brain Res. 1025, 75–84. doi: 10.1016/j.brainres.2004.07.076
Pasquaretta, C., Battesti, M., Klenschi, E., Bousquet, C. A., Sueur, C., and Mery, F. (2016). How social network structure affects decision-making in Drosophila melanogaster. Proc. Biol. Sci. 283:20152954. doi: 10.1098/rspb.2015.2954
Paxon, T. L., Powell, P. R., Lee, H. G., Han, K. A., and Ewing, A. G. (2005). Microcolumn separation of amine metabolites in the fruit fly. Anal. Chem. 77, 5349–5355. doi: 10.1021/ac050474m
Pedrazzini, T., Pralong, F., and Grouzmann, E. (2003). Neuropeptide Y: the universal soldier. Cell Mol. Life Sci. 60, 350–377. doi: 10.1007/s000180300029
Pfeiffenberger, C., Lear, B. C., Keegan, K. P., and Allada, R. (2010). Locomotor activity level monitoring using the Drosophila activity monitoring (DAM) system. Cold Spring Harb. Protoc. 2010:pdb.prot5518. doi: 10.1101/pdb.prot5518
Pittenger, C., and Kandel, E. R. (2003). In search of general mechanisms for long-lasting plasticity: Aplysia and the hippocampus. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 358, 757–763. doi: 10.1098/rstb.2002.1247
Popova, N. K. (2006). From genes to aggressive behavior: the role of serotonergic system. Bioessays 28, 495–503. doi: 10.1002/bies.20412
Porsolt, R. D., Anton, G., Blavet, N., and Jalfre, M. (1978). Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur. J. Pharmacol. 47, 379–391. doi: 10.1016/0014-2999(78)90118-8
Porsolt, R. D., Le Pichon, M., and Jalfre, M. (1977). Depression: a new animal model sensitive to antidepressant treatments. Nature 266, 730–732. doi: 10.1038/266730a0
Prut, L., and Belzung, C. (2003). The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur. J. Pharmacol. 463, 3–33. doi: 10.1016/s0014-2999(03)01272-x
Psychiatric GWAS Consortium Bipolar Disorder Working Group (2011). Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat. Genet. 43, 977–983. doi: 10.1038/ng.943
Qi, Y., Xu, G., Gu, G. X., Mao, F., Ye, G. Y., Liu, W., et al. (2017). A new Drosophila octopamine receptor responds to serotonin. Insect Biochem. Mol. Biol. 90, 61–70. doi: 10.1016/j.ibmb.2017.09.010
Ramdya, P., Schneider, J., and Levine, J. D. (2017). The neurogenetics of group behavior in Drosophila melanogaster. J. Exp. Biol. 220, 35–41. doi: 10.1242/jeb.141457
Ramos-Hryb, A. B., Ramirez, M. F., Lino-de-Oliveira, C., and Pagani, M. R. (2021). Stress-mediated hyperactivity and anhedonia resistant to diazepam and fluoxetine in drosophila. Stress 24, 96–106. doi: 10.1080/10253890.2020.1759547
Riddle, D. L., Blumenthal, T., Meyer, B. J., and Priess, J. R. (1997). The biological model ’, in C. elegans II. 2nd Edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
Riemensperger, T., Isabel, G., Coulom, H., Neuser, K., Seugnet, L., Kume, K., et al. (2011). Behavioral consequences of dopamine deficiency in the Drosophila central nervous system. Proc. Natl. Acad. Sci. USA 108, 834–839. doi: 10.1073/pnas.1010930108
Ries, A.-S., Hermanns, T., Poeck, B., and Strauss, R. (2017). Serotonin modulates a depression-like state in Drosophila responsive to lithium treatment. Nat. Commun. 8:15738. doi: 10.1038/ncomms15738
Rohde, P. D., Gaertner, B., Ward, K., Sørensen, P., and Mackay, T. F. C. (2017). Genomic analysis of genotype-by-social environment interaction for Drosophila melanogaster aggressive behavior. Genetics 206, 1969–1984. doi: 10.1534/genetics.117.200642
Rubin, G. M., and Lewis, E. B. (2000). A brief history of drosophila’s contributions to genome research. Science 287, 2216–2218. doi: 10.1126/science.287.5461.2216
Rzezniczak, T. Z., Douglas, L. A., Watterson, J. H., and Merritt, T. J. S. (2011). Paraquat administration in Drosophila for use in metabolic studies of oxidative stress. Anal. Biochem. 419, 345–347. doi: 10.1016/j.ab.2011.08.023
Salzberg, Y., Gat, A., and Oren-Suissa, M. (2021). One template, two outcomes: how does the sex-shared nervous system generate sex-specific behaviors? Curr. Top. Dev. Biol. 144, 245–268. doi: 10.1016/bs.ctdb.2020.08.003
Saudou, F., Boschert, U., Amlaiky, N., Plassat, J. L., and Hen, R. (1992). A family of Drosophila serotonin receptors with distinct intracellular signalling properties and expression patterns. EMBO J. 11, 7–17. doi: 10.1002/j.1460-2075.1992.tb05021.x
Schneider, J., Dickinson, M. H., and Levine, J. D. (2012). Social structures depend on innate determinants and chemosensory processing in Drosophila. Proc. Natl. Acad. Sci. USA 109, 17174–17179. doi: 10.1073/pnas.1121252109
Schwaerzel, M., Monastirioti, M., Scholz, H., Friggi-Grelin, F., Birman, S., and Heisenberg, M. (2003). Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J. Neurosci. 23, 10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003
Seeman, P., Nam, D., Ulpian, C., Liu, I. S. C., and Tallerico, T. (2000). New dopamine receptor, D2(longer), with unique TG splice site, in human brain. Brain Res. Mol. Brain Res. 76, 132–141. doi: 10.1016/s0169-328x(99)00343-5
Seibenhener, M. L., and Wooten, M. C. (2015). Use of the open field maze to measure locomotor and anxiety-like behavior in mice. J. Visual. Exp. 96:e52434. doi: 10.3791/52434
Seong, K.-H., Ly, N. H., Katou, Y., Yokota, N., Nakato, R., Murakami, S., et al. (2020). Paternal restraint stress affects offspring metabolism via ATF-2 dependent mechanisms in Drosophila melanogaster germ cells. Commun. Biol. 3:208. doi: 10.1038/s42003-020-0935-z
Sethi, S., Lin, H. H., Shepherd, A. K., Volkan, P. C., Su, C. Y., and Wang, J. W. (2019). Social context enhances hormonal modulation of pheromone detection in Drosophila. Curr. Biol. 29, 3887–3898.e4. doi: 10.1016/j.cub.2019.09.045
Sherer, L. M., Catudio Garrett, E., Morgan, H. R., Brewer, E. D., Sirrs, L. A., Shearin, H. K., et al. (2020). Octopamine neuron dependent aggression requires dVGLUT from dual-transmitting neurons. PLoS Genet. 16:e1008609. doi: 10.1371/journal.pgen.1008609
Simon, A. F., Chou, M. T., Salazar, E. D., Nicholson, T., Saini, N., Metchev, S., et al. (2012). A simple assay to study social behavior in Drosophila: measurement of social space within a group. Genes Brain Behav. 11, 243–252. doi: 10.1111/j.1601-183X.2011.00740.x
Slattery, D. A., Markou, A., and Cryan, J. F. (2007). Evaluation of reward processes in an animal model of depression. Psychopharmacology 190, 555–568. doi: 10.1007/s00213-006-0630-x
Soibam, B., Mann, M., Liu, L., Tran, J., Lobaina, M., Kang, Y. Y., et al. (2012). Open-field arena boundary is a primary object of exploration for Drosophila. Brain Behav. 2, 97–108. doi: 10.1002/brb3.36
Sokolowski, M. B. (2010). Social interactions in “simple” model systems. Neuron 65, 780–794. doi: 10.1016/j.neuron.2010.03.007
Sonnhammer, E. L., and Durbin, R. (1997). Analysis of protein domain families in Caenorhabditis elegans. Genomics 46, 200–216. doi: 10.1006/geno.1997.4989
Sørensen, J. G., and Loeschcke, V. (2001). Larval crowding in Drosophila melanogaster induces Hsp70 expression, and leads to increased adult longevity and adult thermal stress resistance. J. Insect Physiol. 47, 1301–1307. doi: 10.1016/s0022-1910(01)00119-6
Srivastava, D. P., Yu, E. J., Kennedy, K., Chatwin, H., Reale, V., Hamon, M., et al. (2005). Rapid, nongenomic responses to ecdysteroids and catecholamines mediated by a novel Drosophila G-protein-coupled receptor. J. Neurosci. 25, 6145–6155. doi: 10.1523/JNEUROSCI.1005-05.2005
Sugamori, K. S., Demchyshyn, L. L., McConkey, F., Forte, M. A., and Niznik, H. B. (1995). A primordial dopamine D1-like adenylyl cyclase-linked receptor from Drosophila melanogaster displaying poor affinity for benzazepines. FEBS Lett. 362, 131–138. doi: 10.1016/0014-5793(95)00224-w
Sun, Y., Qiu, R., Li, X., Cheng, Y., Gao, S., Kong, F., et al. (2020). Social attraction in Drosophila is regulated by the mushroom body and serotonergic system. Nat. Commun. 11:5350. doi: 10.1038/s41467-020-19102-3
Tanizawa, F., and Takemoto, H. (2021). Sleep contributes to preference for novel food odours in Drosophila melanogaster. Sci. Rep. 11:9395. doi: 10.1038/s41598-021-88967-1
Taylor, M. M., and Samson, W. K. (2005). Stress hormone secretion is altered by central administration of intermedin/adrenomedullin-2. Brain Res. 1045, 199–205. doi: 10.1016/j.brainres.2005.03.034
Tian, L., al-Nusaif, M., Chen, X., Li, S., and le, W. (2022). Roles of transcription factors in the development and reprogramming of the dopaminergic neurons. Int. J. Mol. Sci. 23:845. doi: 10.3390/ijms23020845
Tierney, A. J. (2018). Invertebrate serotonin receptors: a molecular perspective on classification and pharmacology. J. Exp. Biol. 221:jeb184838. doi: 10.1242/jeb.184838
Tõru, I., Aluoja, A., Võhma, Ü., Raag, M., Vasar, V., Maron, E., et al. (2010). Associations between personality traits and CCK-4-induced panic attacks in healthy volunteers. Psychiatry Res. 178, 342–347. doi: 10.1016/j.psychres.2010.04.003
True, J. R., Yeh, S. D., Hovemann, B. T., Kemme, T., Meinertzhagen, I. A., Edwards, T. N., et al. (2005). Drosophila tan encodes a novel hydrolase required in pigmentation and vision. PLoS Genet. 1:e63. doi: 10.1371/journal.pgen.0010063
Tucker, L. B., and McCabe, J. T. (2017). Behavior of male and female C57BL/6J mice is more consistent with repeated trials in the elevated zero maze than in the elevated plus maze. Front. Behav. Neurosci. 11:13. doi: 10.3389/fnbeh.2017.00013
Turlejski, K. (1996). Evolutionary ancient roles of serotonin: long-lasting regulation of activity and development. Acta Neurobiol. Exp. 56, 619–636. doi: 10.55782/ane-1996-1167
Ueda, A., and Kidokoro, Y. (2002). Aggressive behaviours of female Drosophila melanogaster are influenced by their social experience and food resources. Physiol. Entomol. 27, 21–28. doi: 10.1046/j.1365-3032.2002.00262.x
Vasar, E., Peuranen, E., Harro, J., Lang, A., Oreland, L., and Männistö, P. T. (1993). Social isolation of rats increases the density of cholecystokinin receptors in the frontal cortex and abolishes the anti-exploratory effect of caerulein. Naunyn Schmiedeberg's Arch. Pharmacol. 348, 96–101. doi: 10.1007/BF00168543
Viana, M. B., Tomaz, C., and Graeff, F. G. (1994). The elevated T-maze: a new animal model of anxiety and memory. Pharmacol. Biochem. Behav. 49, 549–554. doi: 10.1016/0091-3057(94)90067-1
Vickers, S. P., and Dourish, C. T. (2004). Serotonin receptor ligands and the treatment of obesity. Curr. Opin. Investig. Drugs 5, 377–388
Voigt, J. P., and Fink, H. (2015). Serotonin controlling feeding and satiety. Behav. Brain Res. 277, 14–31. doi: 10.1016/j.bbr.2014.08.065
Vora, A., Nguyen, A. D., Spicer, C., and Li, W. (2022). The impact of social isolation on health and behavior in Drosophila melanogaster and beyond. Brain Sci. Adv. 8, 183–196. doi: 10.26599/BSA.2022.9050016
Wang, L., Dankert, H., Perona, P., and Anderson, D. J. (2008). A common genetic target for environmental and heritable influences on aggressiveness in Drosophila. Proc. Natl. Acad. Sci. USA 105, 5657–5663. doi: 10.1073/pnas.0801327105
Watanabe, K., Chiu, H., Pfeiffer, B. D., Wong, A. M., Hoopfer, E. D., Rubin, G. M., et al. (2017). A circuit node that integrates convergent input from Neuromodulatory and social behavior-promoting neurons to control aggression in Drosophila. Neuron 95, 1112–1128.e7. doi: 10.1016/j.neuron.2017.08.017
White, K. E., Humphrey, D. M., and Hirth, F. (2010). The dopaminergic system in the aging brain of Drosophila. Front. Neurosci. 4:205. doi: 10.3389/fnins.2010.00205
Williams, M. J., Goergen, P., Rajendran, J., Klockars, A., Kasagiannis, A., Fredriksson, R., et al. (2014). Regulation of aggression by obesity-linked genes TfAP-2 and Twz through octopamine signaling in Drosophila. Genetics 196, 349–362. doi: 10.1534/genetics.113.158402
Willner, P. (1984). The validity of animal models of depression. Psychopharmacology 83, 1–16. doi: 10.1007/BF00427414
Willuhn, I., Wanat, M. J., Clark, J. J., and Phillips, P. E. M. (2010). Dopamine signaling in the nucleus Accumbens of animals self-administering drugs of abuse. Curr. Top. Behav. Neurosci. 3, 29–71. doi: 10.1007/7854_2009_27
Wilson, J. D., George, F. W., and Griffin, J. E. (1981). The hormonal control of sexual development. Science 211, 1278–1284. doi: 10.1126/science.7010602
Wilson, C. A., Thody, A. J., Hole, D. R., Grierson, J. P., and Celis, M. E. (1991). Interaction of estradiol, alpha-melanocyte-stimulating hormone, and dopamine in the regulation of sexual receptivity in the female rat. Neuroendocrinology 54, 14–22. doi: 10.1159/000125845
Winding, M., Pedigo, B. D., Barnes, C. L., Patsolic, H. G., Park, Y., Kazimiers, T., et al. (2023). The connectome of an insect brain. Science 379:eadd9330. doi: 10.1126/science.add9330
Witz, P., Amlaiky, N., Plassat, J. L., Maroteaux, L., Borrelli, E., and Hen, R. (1990). Cloning and characterization of a Drosophila serotonin receptor that activates adenylate cyclase. Proc. Natl. Acad. Sci. USA 87, 8940–8944. doi: 10.1073/pnas.87.22.8940
Wu, Q., Wen, T., Lee, G., Park, J. H., Cai, H. N., and Shen, P. (2003). Developmental control of foraging and social behavior by the Drosophila neuropeptide Y-like system. Neuron 39, 147–161. doi: 10.1016/s0896-6273(03)00396-9
Yadav, R. S. P., Ansari, F., Bera, N., Kent, C., and Agrawal, P. (2024). Lessons from lonely flies: molecular and neuronal mechanisms underlying social isolation. Neurosci. Biobehav. Rev. 156:105504. doi: 10.1016/j.neubiorev.2023.105504
Yamagata, N., Ichinose, T., Aso, Y., Plaçais, P. Y., Friedrich, A. B., Sima, R. J., et al. (2015). Distinct dopamine neurons mediate reward signals for short- and long-term memories. Proc. Natl. Acad. Sci. USA 112, 578–583. doi: 10.1073/pnas.1421930112
Yamamoto, S., and Seto, E. S. (2014). Dopamine dynamics and signaling in Drosophila: an overview of genes, drugs and behavioral paradigms. Exp. Anim. 63, 107–119. doi: 10.1538/expanim.63.107
Yang, Z., Bertolucci, F., Wolf, R., and Heisenberg, M. (2013). Flies cope with uncontrollable stress by learned helplessness. Curr. Biol. 23, 799–803. doi: 10.1016/j.cub.2013.03.054
Yang, M., Silverman, J. L., and Crawley, J. N. (2011). Automated three-chambered social approach task for mice. Curr. Protoc. Neurosci. Chapter 8:Unit 8.26. doi: 10.1002/0471142301.ns0826s56
Yang, Z., Yu, Y., Zhang, V., Tian, Y., Qi, W., and Wang, L. (2015). Octopamine mediates starvation-induced hyperactivity in adult Drosophila. Proc. Natl. Acad. Sci. 112, 5219–5224. doi: 10.1073/pnas.1417838112
Yellman, C., Tao, H., He, B., and Hirsh, J. (1997). Conserved and sexually dimorphic behavioral responses to biogenic amines in decapitated Drosophila. Proc. Natl. Acad. Sci. USA. 94, 4131–4136. doi: 10.1073/pnas.94.8.4131
Yost, R. T., Scott, A. M., Kurbaj, J. M., Walshe-Roussel, B., Dukas, R., and Simon, A. F. (2024). Recovery from social isolation requires dopamine in males, but not the autism-related gene nlg3 in either sex. R. Soc. Open Sci. 11:240604. doi: 10.1098/rsos.240604
Yu, Y., Huang, R., Ye, J., Zhang, V., Wu, C., Cheng, G., et al. (2016). Regulation of starvation-induced hyperactivity by insulin and glucagon signaling in adult Drosophila. eLife 5:e15693. doi: 10.7554/eLife.15693
Zangrossi Jr, H., Del Ben, C. M., Graeff, F. G., and Guimarães, F. S. (2020). Serotonin in panic and anxiety disorders. Handb. Behav. Neurosci. 31, 611–633. doi: 10.1016/B978-0-444-64125-0.00036-0
Zhang, W., Guo, C., Chen, D., Peng, Q., and Pan, Y. (2018). Hierarchical control of Drosophila sleep, courtship, and feeding behaviors by male-specific P1 neurons. Neurosci. Bull. 34, 1105–1110. doi: 10.1007/s12264-018-0281-z
Zhao, Y., Bretz, C. A., Hawksworth, S. A., Hirsh, J., and Johnson, E. C. (2010). Corazonin neurons function in sexually dimorphic circuitry that shape behavioral responses to stress in Drosophila. PLoS One 5:e9141. doi: 10.1371/journal.pone.0009141
Zhao, S., Deanhardt, B., Barlow, G. T., Schleske, P. G., Rossi, A. M., and Volkan, P. C. (2020). Chromatin-based reprogramming of a courtship regulator by concurrent pheromone perception and hormone signaling. Sci Adv. 6:eaba6913. doi: 10.1126/sciadv.aba6913
Zhao, H., Jiang, X., Ma, M., Xing, L., Ji, X., and Pan, Y. (2024). A neural pathway for social modulation of spontaneous locomotor activity (SoMo-SLA) in Drosophila. Proc. Natl. Acad. Sci. 121:e2314393121. doi: 10.1073/pnas.2314393121
Zhou, C., Rao, Y., and Rao, Y. (2008). A subset of octopaminergic neurons are important for Drosophila aggression. Nat. Neurosci. 11, 1059–1067. doi: 10.1038/nn.2164
Keywords: stress, Drosophila, sex differences, social isolation, cellular and molecular
Citation: Tawa E and Notterman D (2025) Drosophila melanogaster as a neurobehavioral model for sex differences in stress response. Front. Behav. Neurosci. 19:1581763. doi: 10.3389/fnbeh.2025.1581763
Edited by:
Nikolaos Kokras, National and Kapodistrian University of Athens, GreeceReviewed by:
Fernando Vonhoff, University of Maryland, Baltimore County, United StatesVikram Narayan, Embry–Riddle Aeronautical University, Prescott, United States
Copyright © 2025 Tawa and Notterman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel Notterman, ZGFuMUBwcmluY2V0b24uZWR1
 Elisabeth Tawa
Elisabeth Tawa Daniel Notterman
Daniel Notterman