- 1 Vanderbilt Stroke Center, Vanderbilt University Medical Center, Nashville, TN, USA
- 2 Stroke Institute, University of Pittsburgh Medical Center, Pittsburgh, PA, USA
- 3 University of Miami School of Medicine, Miamia, FL, USA
- 4 University of Louisville School of Medicine, Louisville, KY, USA
A commentary on
Stenting versus endarterectomy for treatment of carotid-artery stenosis.
by Thomas G. Brott et al. (2010). CREST Trial. N. Engl. J. Med. May 26. (10.1056/NEJMoa0912321)
Carotid revascularization with carotid endarterectomy (CEA) has been shown to be superior to medical therapy for stroke prevention in symptomatic and asymptomatic patients with moderate to severe stenosis who meet well defined medical and surgical selection criteria. The benefit of CEA is significantly higher in symptomatic compared to asymptomatic patients. Carotid artery stenting (CAS) has emerged as an alternative in patients who are considered high surgical-risk due to co-existent medical co-morbidities or anatomical high-risk features. Since its development in the early 1990’s, the technique of endovascular carotid revascularization has been undergoing a continuous maturation process mainly due to a change from the initial use of balloon expandable stents to self-expanding stents, the introduction of and continuously improving array of emboli prevention devices (EPD’s) and last but not least increasing operator experience. This culminated in the randomized SAPPHIRE trial of protected CAS [i.e., CAS performed with EPD] vs. CEA in high surgical-risk patients, that showed that CAS was non-inferior to CEA with lower peri-procedural complication rates as well as lower rates of restenosis (Yadav et al., 2004). Furthermore, increased experience with this technique has led to the realization that just like with CEA, there are patients (e.g., older age, excessive vascular tortuosity or calcification) who are high-risk for CAS. (Chaturvedi et al., 2010).
The question of whether CAS is an alternative to CEA in patients without high surgical-risk, is addressed by the results of three randomized, European studies comparing CEA to CAS in patients without high surgical-risk medical or anatomical features (Mas et al., 2006; Ringleb et al., 2006; Ederle et al., 2010) (EVA-3S, SPACE, ICSS). More recently the North American CREST study results were presented at the International Stroke Conference in 2010 and subsequently published. (Brott et al., 2010). The results of these trials were discrepant with two (EVA-3S and ICSS; Mas et al., 2006; Ederle et al., 2010) showing worse outcomes with CAS, one failing to prove non-inferiority (SPACE) (Ederle et al., 2010) and one showing equivalence (CREST) (Brott et al., 2010). Why were the results so different? To better understand these discrepancies, it is important to understand the differences in trial methodology.
The first and most important methodologic difference was operator experience across the four studies. In EVA 3S, operators had to have performed at least five carotid stent procedures or be supervised by a physician who was qualified (Mas et al., 2006). Following publication of the EVA3s manuscript it was revealed that only 16% of patients were treated by operators with more than 50 CAS cases of experience and 39% of patients were treated by physicians in training (Clark, 2010). In SPACE, the operator had to have performed 25 percutaneous angioplasty or stent procedures, without a specific requirement for carotid procedures. Operators who had insufficient CAS experience (10 cases) could enroll patients if they had assistance of a tutor (Ringleb et al., 2006). In ICSS, the requirement was 50 stent procedures, of which a minimum of 10 were required to be carotid artery procedures (Ederle et al., 2010). As with EVA3s and SPACE inexperienced operators could have the assistance of a tutor. The trend across all of these studies is that many operators may have had some experience with peripheral stent placement but this experience was not necessarily acquired in the carotid arteries. As aortic arch tortuosity is emerging as one of the critical factors determining procedural risk with CAS, lack of proof of experience with carotid catheterization as a prerequisite for participation in the trial seen across all the European studies is arguably the most important factor responsible for the overall high rates of stroke reached in these studies. By contrast prospective CAS registries in North America, which preceded CREST, required a higher level of experience with brachiocephalic catheterization and carotid interventions and have reported rates of stroke that are significantly less that those reported in the European studies. Table 1 summarizes the differences in selection criteria for stenting across the recent randomized trials. Site selection may also have been an issue; in ICSS, two centers were found to have an extraordinarily high rate of complications and were removed from the study after 5 of 11 patients experienced disabling stroke or death. The inclusion of inexperienced physicians and allowing them to perform the procedure in the presence of a tutor as part of a randomized trial casts doubt on the validity of these trials.
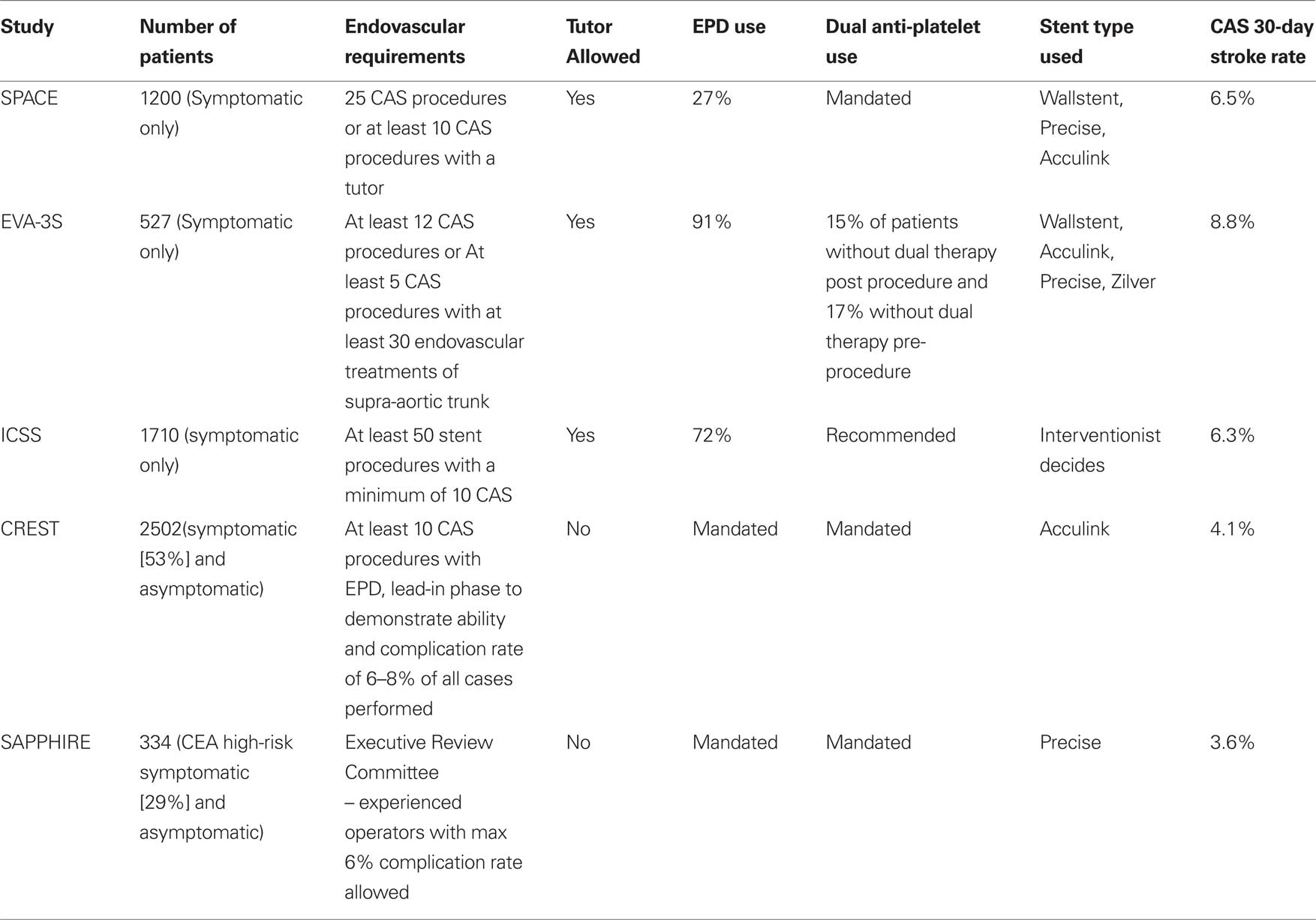
Table 1. Summary of randomized controlled studies comparing carotid artery stenting to carotid endarterecomy.
By contrast the vetting process for the CREST study was more rigorous and required a minimum experience of 10–30 carotid stent procedures with 0.14ʹ wire systems, experience with EPD, and a documented 30-day stroke and death rate of “6–8%” (Hopkins et al., 2010). In addition after admittance into the study there was a required lead-in phase of up to 20 patients designed to ensure operators had adequate experience and acceptable complication rates prior to randomizing patients. The standards of rigorous vetting for proceduralists performing carotid revascularization were set by NASCET and ACAS, the first trials to show benefit of CEA compared to medical therapy (North American Symptomatic Carotid Endarterectomy Trial, 1991; Endarterectomy for asymptomatic carotid artery stenosis, 1995) in which only experienced surgeons chosen according to strict criteria were allowed to participate. As opposed to the stenting arm operators, carotid surgeons in the European randomized trials of CAS vs. CEA were more experienced compared to their interventionalist counterparts: no inexperienced surgeon was allowed to perform the procedure whether or not a tutor was present.
The second major protocol difference was the use of peri-procedural dual anti-platelet medications. In the ICSS and EVA-3S studies, the use of dual anti-platelet medications was “recommended”. In EVA-3S, 17% of patients were not on dual anti-platelet medications prior to the procedure and nearly 15% did not have these medications post procedure (Mas et al., 2006). Data regarding peri-procedural anti-platelet medications were surprisingly not reported in ICSS (Ederle et al., 2010).
The issue of anti-platelet therapy with carotid stenting is of great importance because following stent implantation especially within the first week there is activation of platelets and increases of ADP-induced platelet aggregation (Szapary et al., 2009). Vast experience from the coronary literature confirmed by carotid stenting studies has shown that the use of dual anti-platelet therapy is essential in the prevention of peri-procedural ischemic events and acute stent thrombosis (Bhatt et al., 2001). In the CREST study, the use of dual anti-platelet therapy was required as part of the protocol. An issue not addressed in any of the hitherto conducted studies and which requires further study is that of biochemical resistance to anti-platelet agents. The recently described association of the cytochrome P450 2C19 genotype to reduced effectiveness of clopidogrel (Shuldiner et al., 2009) and associated cardiovascular death raises concerns that may be of great relevance for carotid stenting; adverse thromboembolic events following carotid stenting may be reduced in the future by tailoring peri-procedural antithrombotic agents to patient specific response to the drug.
The third consideration was the lack of exclusion criteria for stenting. By contrast high surgical-risk criteria precluding randomization were present for the CEA arms in all the above randomized trials. The EVA-3S trial did not include angiographic exclusion criteria for stenting, which combined with low operator experience, could account for the significant rate of perioperative stroke and death seen in the CAS arm, a rate not reported since the very first series of carotid stenting reported in the late 1990’s (Naylor et al., 1998). What has become clear is that akin to CEA patient selection for CAS is key to minimizing peri-procedural risks. Consistent with this experience gained from CEA the CREST trial had rigorous angiographic exclusion criteria such as severe tortuosity and calcification, intraluminal thrombi and large, bulky, plaques (Roubin et al., 2006), which further explains these discrepant results.
Fourthly, ICSS, EVA 3S and SPACE allowed the use of different stent and EPD types. By allowing operators to select the stent and EPD type, there may have been unfamiliarity with the devices particularly when there is a lack of consistent use. In contrast, the CREST study utilized one single stent and EPD system the Acculink™ stent and Accunet™ EPD (Abbott Vascular, Santa Clara, CA, USA) for each patient including for those patients treated within the lead-in phase. This allowed the operator to become familiar with the particularities of one single device.
Lastly, a potentially critical consideration was the inconsistency of EPD use across all of the European studies. EPD’s were used in 27%, 72% and 91% of patients in SPACE, ICSS and EVA 3S respectively (Mas et al., 2006; Ringleb et al., 2006; Ederle et al., 2010). Although there has been controversy with regards to the benefit of EPD in preventing ischemic stroke associated with CAS the lack of a consistent protocol represents a significant shortcoming. The CREST study protocol required the use of an EPD for all patients enrolled. To what extent this requirement contributes to the lower peri-procedural risk of stroke of 4.1% observed in CREST vs. 7.5–8.8% in the European studies is not entirely clear. Although no randomized trials have compared protected CAS vs. unprotected CAS, several large series, including a registry comprising >12,000 patients comparing the two approaches, have shown an approximately 50% reduction in perioperative stroke risk with their use (Wholey et al., 2003). The ICAROS trial also showed a significant reduction of events with the use of EPD in patients with echolucent plaques (Biasi et al., 2004). Although MRI based studies have not shown a difference in the number of DWI lesions when comparing protected to unprotected CAS, the studies were not powered to detect if a clinical difference may exist. Moreover, the use of an EPD may be a surrogate for operator experience. Despite the controversy surrounding EPD’s, a lack of a consistent protocol with regards to EPD’s likely accounts for some of the peri-procedural stroke rates noted amongst the trials.
The inclusion of asymptomatic and symptomatic carotid stenosis patients in the CREST and SAPPHIRE trials as opposed to only symptomatic carotid stenosis in the other trials is another important difference that raises the question regarding its contribution to the inconsistent results amongst the trials. Registries for CAS have shown differences in major adverse clinical event rates when comparing symptomatic lesions to asymptomatic stenosis. The CREST trial and SAPPHIRE trials included 47% and 71% of patients respectively with asymptomatic stenosis. This may also account for the differences in the overall 30-day event rates, but does not account for the final results of the North American studies compared to their European counterparts.
In conclusion, the CREST study showed non-inferiority of protected CAS to CEA. The ICSS, EVA-3S and SPACE studies failed to reach the same conclusion due to higher stroke rates in the CAS group. For several reasons outlined above but particularly because of insufficiently rigorous vetting standards used for carotid stent operators, the latter studies showed that inexperienced operators without a defined protocol will achieve inferior results to CEA performed by experienced operators. Available data from CREST a trial with vigorous standards for operator experience allow us to reach the conclusion that in addition to aggressive medical therapy there are two equivalent treatment options for symptomatic or asymptomatic carotid stenoses: CEA and protected CAS. Therefore, the task ahead and one that should be pursued in daily practice by endovascular specialists and carotid surgeons as part of multidisciplinary teams is to determine which procedure is best suited for each individual patient according to patient specific anatomical and medical considerations.
References
Bhatt, D. L., Kapadia, S. R., Bajzer, C. T., Chew, D. P., Ziada, K. M., Mukherjee, D., Roffi, M., Topol, E. J., and Yadav, J. S. (2001). Dual antiplatelet therapy with clopidogrel and aspirin after carotid artery stenting. J. Invasive. Cardiol. 13, 767–771.
Biasi, G. M., Froio, A., Diethrich, E. B., Deleo, G., Galimberti, S., Mingazzini, P., Nicolaides, A. N., Griffin, M., Raithel, D., Reid, D. B., and Valsecchi, M. G. (2004). Carotid plaque echolucency increases the risk of stroke in carotid stenting: the Imaging in Carotid Angioplasty and Risk of Stroke (ICAROS) study. Circulation 110, 756–762.
Brott, T. G., Hobson, R. W. II, Howard, G., Roubin, G. S., Clark, W. M., Brooks, W., Mackey, A., Hill, M. D., Leimgruber, P. P., Sheffet, A. J., Howard, V. J., Moore, W. S., Voeks, J. H., Hopkins, L. N., Cutlip, D. E., Cohen, D. J., Popma, J. J., Ferguson, R. D., Cohen, S. N., Blackshear, J. L., Silver, F. L., Mohr, J. P., Lal, B. K., and Meschia, J. F.; the CREST Investigators. (2010). Stenting versus endarterectomy for treatment of carotid-artery stenosis. N. Engl. J. Med. [Epub ahead of print].
Chaturvedi, S., Matsumura, J. S., Gray, W., Xu, C., Verta, P., and CAPTURE 2 Investigators and Executive Committee. (2010). Carotid artery stenting in octogenarians: periprocedural stroke risk predcitro analysis from the multicenter Carotid ACCULINK/ACCUNET Post approval trial to uncover rare events (CAPTURE 2) clinical trial. Stroke 41, 757–764.
Clark, W. M.; for the CREST Investigators. (2010). Carotid Revascularization endarterectomy vs. stenting trial. Presented at the International Stroke Conference, San Antonio, TX.
Endarterectomy for asymptomatic carotid artery stenosis. (1995). Executive committee for the asymptomatic carotid atherosclerosis study. JAMA 273, 1421–1428.
Hopkins, L. N., Roubin, G. S., Chakhtoura, E. Y., Gray, W. A., Ferguson, R. D., Katzen, B. T., Rosenfield, K., Goldstein, J., Cutlip, D. E., Morrish, W., Lal, B. K., Sheffet, A. J., Tom, M., Hughes, S., Voeks, J., Kathir, K., Meschia, J. F., Hobson, R. W., and Brott, T. G. (2010). The Carotid revascularization endarterectomy versus stenting trial: credentialing of interventionalists and final results of lead-in phase. J. Stroke Cerebrovasc. Dis. 19, 153–162.
International Carotid Stenting Study Investigators, Ederle, J., Dobson, J., Featherstone, R. L., Bonati, L. H., van der Worp, H. B., de Borst, G. J., Lo, T. H., Gaines, P., Dorman, P. J., Macdonald, S., Lyrer, P. A., Hendriks, J. M., McCollum, C., Nederkoorn, P. J., and Brown, M. M. (2010). Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. Lancet 375, 985–997.
Mas, J. L., Chatellier, G., Beyssen, B., Branchereau, A., Moulin, T., Becquemin, J. P., Larrue, V., Lievre, M., Leys, D., Bonneville, J. F., Watelet, J., Pruvo, J. P., Albucher, J. F., Viguier, A., Piquet, P., Garnier, P., Viader, F., Touze, E., Giroud, M., Hosseini, H., Pillet, J. C., Favrole, P., Neau, J. P., and Ducrocq, X. (2006). Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N. Engl. J. Med. 355, 1660–1671.
Naylor, A. R., Bolia, A., Abbott, R. J., Pye, I. F., Smith, J., Lennard, N., Lloyd, A. J., London, N. J., and Bell, P. R. (1998). Randomized study of carotid angioplasty and stenting versus carotid endarterectomy: a stopped trial. J. Vasc. Surg. 28, 326–334.
North American Symptomatic Carotid Endarterectomy Trial. (1991). Methods, patient characteristics, and progress. Stroke 22, 711–720.
Ringleb, P. A., Allenberg, J., Bruckmann, H., Eckstein, H. H., Fraedrich, G., Hartmann, M., Hennerici, M., Jansen, O., Klein, G., Kunze, A., Marx, P., Niederkorn, K., Schmiedt, W., Solymosi, L., Stingele, R., Zeumer, H., and Hacke, W. (2006). 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet 368, 1239–1247.
Roubin, G. S., Iyer, S., Halkin, A., Vitek, J., and Brennan, C. (2006). Realizing the potential of carotid artery stenting: proposed paradigms for patient selection and procedural technique. Circulation 113, 2021–2030.
Shuldiner, A. R., O’Connell, J. R., Bliden, K. P., Gandhi, A., Ryan, K., Horenstein, R. B., Damcott, C. M., Pakyz, R., Tantry, U. S., Gibson, Q., Pollin, T. I., Post, W., Parsa, A., Mitchell, B. D., Faraday, N., Herzog, W., and Gurbel, P. A. (2009). Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA 302, 849–857.
Szapary, L., Bagoly, E., Kover, F., Feher, G., Pozsgai, E., Koltai, K., Hanto, K., Komoly, S., Doczi, T., and Toth, K. (2009). The effect of carotid stenting on rheological parameters, free radical production and platelet aggregation. Clin. Hemorheol. Microcirc. 43, 209–217.
Wholey, M. H., Al-Mubarek, N., and Wholey, M. H. (2003). Updated review of the global carotid artery stent registry. Catheter. Cardiovasc. Interv. 60, 259–266.
Yadav, J. S., Wholey, M. H., Kuntz, R. E., Fayad, P., Katzen, B. T., Mishkel, G. J., Bajwa, T. K., Whitlow, P., Strickman, N. E., Jaff, M. R., Popma, J. J., Snead, D. B., Cutlip, D. E., Firth, B. G., and Ouriel, K. (2004). Protected carotid-artery stenting versus endarterectomy in high-risk patients. N. Engl. J. Med. 351, 1493–1501.
Citation: Gupta R, Jovin TG, Yavagal D and Abou-Chebl A (2010) Carotid endarterectomy vs. carotid stenting: fairly comparable or unfairly compared? Front. Neur. 1:14. doi: 10.3389/fneur.2010.00014
Received: 10 June 2010;
Accepted: 10 June 2010;
Published online: 16 July 2010.
Copyright: © 2010 Gupta, Jovin, Yavagal and Abou-Chebl. This is an open-access article subject to an exclusive license agreement between the authors and the Frontiers Research Foundation, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
*Correspondence: a0abou03@louisville.edu
