- 1 Department of Neurosurgery, Rush University Medical Center, Chicago, IL, USA
- 2 Department of Neurology, Rush University Medical Center, Chicago, IL, USA
Background and Purpose: A limited number of studies consisting predominantly of ruptured aneurysms have looked at differences in anatomical distribution of aneurysms between male and females. Unlike all other causes of stroke, subarachnoid hemorrhages (SAH) occur more often in women and are thought to be a result of both hormonal influences and variation in wall shear stress. This paper retrospectively looks at a cohort of largely unruptured intracranial aneurysms to determine if there exists a gender discrepancy in the anatomic distribution of cerebral aneurysms. Methods: A retrospective review of consecutive patients with ruptured and unruptured intradural saccular cerebral aneurysms treated endovascularly was performed. Results: Six hundred eighty-two aneurysms were treated. Seventy-two percentage of the patients were women and 27% of patients presented with SAH. Among women, most aneurysms were located along the ICA (54%) while men the ACA (29%, compared to 15% in women), a discrepancy evident in both unruptured and ruptured groups. Females tended to present later in life (59 vs. 55 years), with multiple aneurysms (11 vs. 6% in men), and with SAH (28 vs. 23% in men) – the majority of these ruptured aneurysms were located at the ICA (42%), while men at the ACA (47%). Additionally, the majority (68%) of ruptured ICA aneurysms were PCOM. Conclusion: Understanding the natural history of aneurysms is imperative in treating incidentally found aneurysms. Significant differences exist between the genders in relation to aneurysm location, the most pronounced at the ICA and ACA. Previously described hormonal and hemodynamic theories behind cerebral aneurysm pathogenesis seem like plausible reasons to explain these differences.
Introduction
Despite the increasing number of embolizations performed on unruptured, largely incidentally discovered cerebral aneurysms, the public health impact of subarachnoid hemorrhage (SAH) has remained largely unchanged over the last 20 years (Longstreth et al., 1985; Juvela et al., 1993a). Surgically treating incidental intracranial aneurysms has been a topic of debate, given the uncertainty of the natural history of the disease and the inherent risks of the procedure (Juvela et al., 1993a; Wiebers et al., 2003). This is a commonly encountered dilemma as asymptomatic unruptured aneurysms are detected in 1–6% of healthy individuals based on autopsy and imaging studies (Inagawa and Hirano, 1990a; Vernooij et al., 2007). A possible clue in better understanding the natural history of cerebral aneurysms may lie in the unique epidemiologic feature that unlike all other causes of stroke, SAH occur more often in women (2:1; Nishioka et al., 1984; Longstreth et al., 1985; Kassell et al., 1990; Kongable et al., 1996; Teunissen et al., 1996; Ellamushi et al., 2001; Molyneux et al., 2002; Wiebers et al., 2003; Eden et al., 2008; Aarhus et al., 2009). Studies evaluating de novo formation, growth, intraoperative rupture, and multiplicity of intracranial aneurysms all demonstrate an increased female occurrence and more often post-menopausal (Juvela et al., 1993a, 2001; Ellamushi et al., 2001; Kaminogo et al., 2003; Wiebers et al., 2003; Beck et al., 2006). The cause of this disparity is unknown, but likely is not the result of the well-known risk factors for SAH, including smoking, hypertension, atherosclerosis, and alcohol consumption, all of which are more common among men (Longstreth et al., 1985, 1994; Stober et al., 1985; Juvela et al., 1993b, 2001; Adamson et al., 1994; Mhurchu et al., 2001; Stirone et al., 2003; Jamous et al., 2005; Harrod et al., 2006).
Several pathophysiological mechanisms have been proposed to explain the female susceptibility to cerebral aneurysms and SAH. Because the greater female-to-male ratio of SAH is found to occur more so in the post-menopausal period, a mechanism involving compromised arterial integrity as a result of a drop in estrogen levels has been proposed (Juvela et al., 2001; Mhurchu et al., 2001; Stirone et al., 2003; Wiebers et al., 2003; Harrod et al., 2006). Additionally, vascular geometry and wall shear stress (WSS) may also be fundamentally different between the genders as demonstrated using computed fluid dynamics (CFD; Alnaes et al., 2007; Lindekleiv et al., 2010; Rahman et al., 2010). By elaborating on these differences, we may better define the pathophysiologic mechanisms underlying not only why aneurysms occur more commonly in women, but also possibly aneurysm pathophysiology in general.
A few studies evaluating exclusively ruptured cerebral aneurysms have demonstrated a variation between gender and aneurysm location (Inagawa and Hirano, 1990b; Kongable et al., 1996; Eden et al., 2008; Park et al., 2008; Aarhus et al., 2009). Therefore, we sought to describe the nature of a possible gender discrepancy in the anatomic distribution of a large cohort of mostly unruptured cerebral aneurysms around the circle of Willis.
Materials and Methods
We obtained institutional review board approval for our study protocol. We conducted a retrospective chart review over 9 years of intradural saccular cerebral aneurysms in patients treated via endovascular embolization by two physicians. Both ruptured and unruptured aneurysms were included. We collected information on patient’s age, gender, aneurysm location, and aneurysm rupture status, all determined retrospectively in databases specifically designed to collect data on aneurysm treatment. We also reviewed operative and imaging reports and determined aneurysm size by measuring the largest recorded diameter. We used the Koivisto charting system for categorizing aneurysm location which has been commonly used in related studies since 2000 (Table 1; Koivisto et al., 2000).

Table 1. Koivisto categories for grouping aneurysm locations Location of unruptured and ruptured aneurysms in males and females.
Statistical Analysis
All analyses were performed with Microsoft Excel 2007. A P-value of 0.05 was used as being statistically significant. Group comparisons were performed with Student’s t-test.
Results
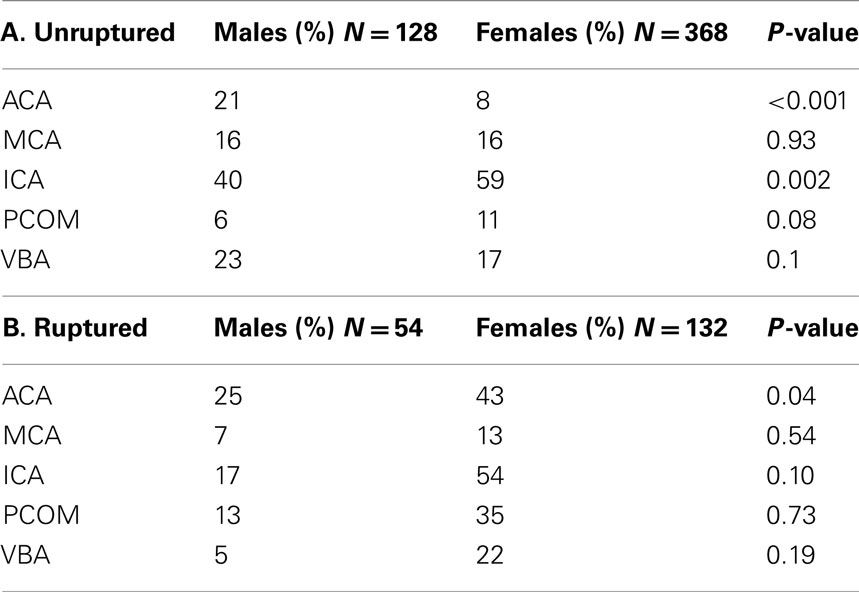
Between 2002 and 2011, 608 patients were included with a total of 682 treated aneurysms. Of the 682 aneurysms under review, women comprised 72% of the patients. The overall average age in our cohort was 57 years of age, with men presenting at an earlier age (55 years, compared to 59 in women, p = 0.05). Aneurysms ranged in size from 2 to 42 mm in largest diameter. Using the Koivisto categories for aneurysm location, our overall aneurysm location frequency and breakdown by gender is shown in Figure 1. Among women, most were along the ICA (54% in women, compared to 38% in men) while men had a disproportionate number of ACA territory aneurysms (29% in men, compared to 15% in women), both of which showed statistical significance (p = 0.001). No differences were seen in the frequency of aneurysms in the MCA and posterior circulation (VBA).
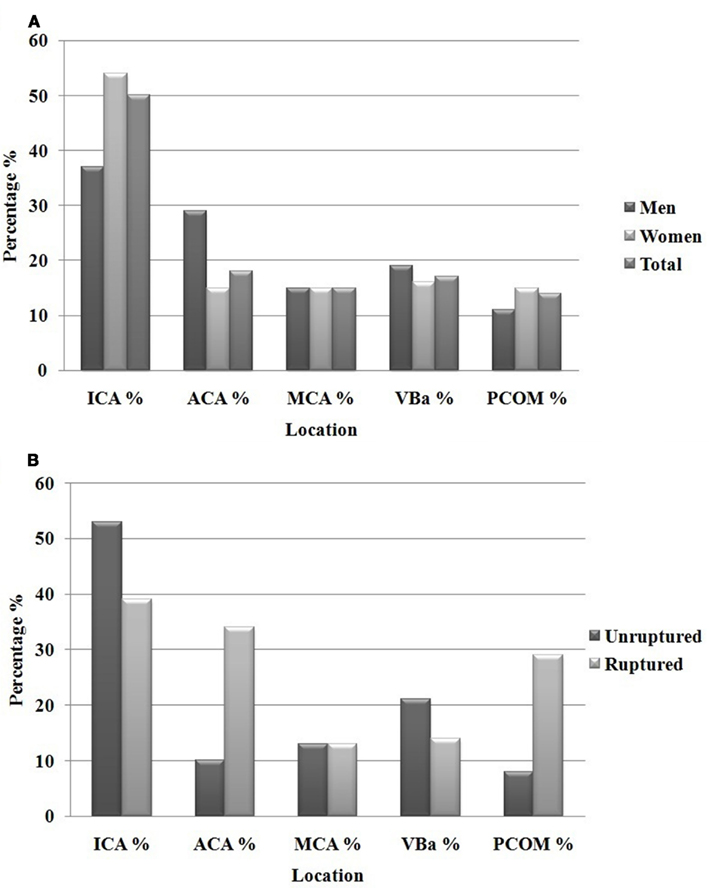
Figure 1. Total aneurysms men and women (682) (A) and location of unruptured compared to ruptured aneurysms (B).
Gender Difference among Unruptured Aneurysms
The average age of the patients treated for the 496 unruptured aneurysms was 57 (range 27–81), with men presenting significantly younger (55 vs. 59 years, p = 0.02). Combined, unruptured aneurysms were predominantly located at the ICA 54%, followed by VBA 19%, MCA 16%, and ACA 11% (Figure 1). For the ICA group, 49 of the 269 unruptured aneurysms were found at the PCOM.
The distribution of unruptured aneurysms were significantly different between women and men (Table 2) – 21% of the unruptured aneurysms found in males were located at the ACA, compared to only 6% in females (p < 0.001). Females, however, had 59% of their unruptured aneurysms found at the ICA, compared to 40% in males (p = 0.002). The size of the unruptured aneurysms was on average similar between the two genders (8 vs. 8.1 mm).
Gender difference among Ruptured Aneurysms
The average age of the 186 ruptured aneurysms was 56 (range 26–83), with men again presenting at a significantly younger age (53 vs. 59 years, p = 0.05). Collectively, ruptured aneurysms were predominantly located at the ICA 38%, followed by ACA 37%, VBA 14%, and MCA 11% (Table 2; Figure 1). For the ICA group, 48 of the 71 ruptured aneurysms were found at the PCOM (Table 3).
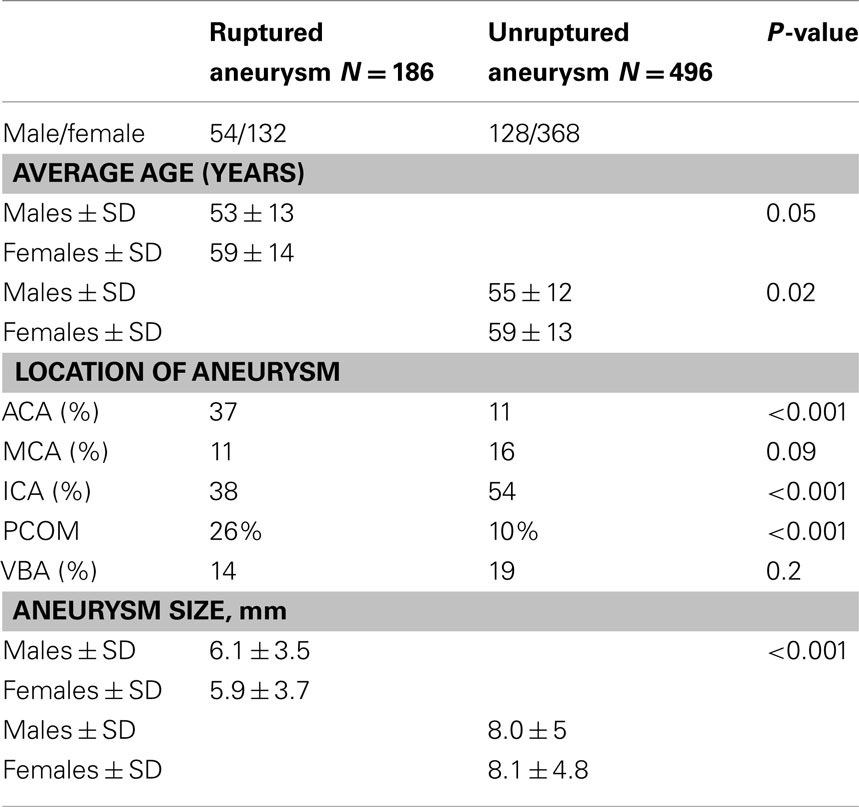
Table 3. Distribution of gender, age, size, and anatomical location of ruptured and unruptured aneurysms.
The location of the ruptured aneurysms also varied between women and men. The majority of ruptured female aneurysms arose at the ICA (42%), while in men the ACA (47%). When calculating the P-value for these differences, the discrepancy proved to be significant at the ACA. Overall, ACA aneurysms were much more frequent in the ruptured group (37 vs. 11%, p < 0.0001) and more frequent among males (47 vs. 32%, p = 0.02). The size of ruptured aneurysms was not significantly different between the two genders (5.9 vs. 6.1 mm). Additionally, a significant number of females were found to have multiple aneurysms compared to males (11 vs. 6%, p = 0.05).
Discussion
Our study showed a difference in gender with respect to aneurysm location, prevalence, and time of presentation. Unlike prior studies evaluating gender differences among ruptured aneurysms, our series consists mostly of unruptured aneurysms, providing additional clues not just to the pathophysiology of aneurysm rupture, but also of aneurysm formation.
Gender differences in the anatomical distribution of aneurysms have been studied, but only on a smaller scale and only among patients with ruptured aneurysms. Park et al. (2008) reviewed a series of 53 patients aged 20–39 with ruptured cerebral aneurysms and found the majority of aneurysms on the ICA in women and the majority on the ACA in men. In a larger, but still exclusively ruptured cohort, nearly 900 aneurysms were evaluated for gender differences in anatomical distribution. Again, the female-to-male discrepancy was most pronounced along the ICA (36% in women compared to 18% in men) and the ACA (46% in men compared to 27% found in women; Kongable et al., 1996). In our cohort of mostly unruptured aneurysms, the most pronounced gender discrepancy was also at the ICA and ACA, with women outnumbering men 54–37% in the former, and men outnumbering women 29–15% in the latter (Figure 1). This trend was also observed when exclusively comparing the unruptured and ruptured aneurysms (Figure 1). Therefore, our results were similar to those in the literature that have looked at gender discrepancies in ruptured aneurysm location. More importantly, our results show that these gender differences are evident in both ruptured and unruptured aneurysms as well.
Our study also demonstrated several additional gender specific features. Among our 682 treated intracranial aneurysms, the number of female aneurysms outnumbered males by nearly a factor of 3. This finding is consistent with autopsy studies as well as the 3:1 female-to-male ratio observed in the ISUIA study (Inagawa and Hirano, 1990a; Vernooij et al., 2007). A systematic review evaluating 23 studies including 56,304 patients found that aneurysms were not only more common among women, but that women were at a greater risk of rupture (relative risk of 2.1). Our findings suggested similar trends with 28% of our cohort of female patients presenting with SAH, while only 23% of men (Rinkel et al., 1998). In conjunction with findings in the literature, males with both unruptured and ruptured aneurysms presented at an earlier age (Kongable et al., 1996; Aarhus et al., 2009). In our analysis, males were diagnosed 6 years earlier in the ruptured cohort and 4 years earlier in the unruptured cohort. Additionally, the incidence of multiple aneurysms in the ruptured group was significantly higher among females (11 vs. 6% in men). This finding was also noted by Kongable et al. who showed multiple aneurysms in 32% of females presenting with SAH, while men with only 17% (Kongable et al., 1996; Aarhus et al., 2009).
The pathophysiological basis accounting for these dissimilarities remains unknown. Several mechanisms have been proposed to underlie cerebral aneurysm formation; two of the most prominent mechanisms are hemodynamic stress and compromised vascular remodeling secondary to a decline in estrogen.
Numerous studies suggest a role for hormones in aneurysm pathogenesis via its effect on vascular remodeling and have shown the benefits of hormone replacement therapy (HRT) on reducing aneurysm formation and rupture (Stober et al., 1985; Mhurchu et al., 2001; Stirone et al., 2003; Harrod et al., 2006; Lazzaro et al., 2012). The increase in female prevalence of cerebral aneurysms and SAH peaks between 50 and 59 years of age, in correlation with the fall in estrogen levels. Estrogen has been shown to promote normal physiologic vascular endothelial function and its effect on vascular structure and function has been described to occur via pleiotropic effects on vascular endothelial cells, collagen, and nitric oxide (Stirone et al., 2003). As such, changes in estrogen levels may have ramifications on vascular integrity. Longstreth et al. (1994) evaluated 103 women with SAH and found a higher frequency of cases in post-menopausal women and those who never took HRT. Likewise, Mhurchu et al. (2001) performed a prospective case control study looking at 286 patients and found that any use of HRT was associated with a significant 36% reduction in the odds of SAH. Additionally, Jamous et al. used animal experiments to demonstrate the protective role HRT plays in rats following oophorectomy. After 10 weeks of treatment with 17b-estradiol, only 7% of rats developed saccular aneurysms, as opposed to 53% of rats not given exogenous estrogen (Jamous et al., 2005). For these reasons, estrogen appears to play a protective role in the formation, development, and rupture of intracranial aneurysms in women by altering vascular integrity.
In addition to hormonal influences, the prevalence and distinct location of aneurysms in females can also be explained by vessel wall weakness and stress as a consequence of hemodynamic forces. It has been shown that hemodynamic factors may lead to aneurysm formation as a consequence of increased WSS leading to endothelial damage and vessel wall remodeling. In a study by Lindekleiv et al. (2010) the group looked at gender variation at the branch points of the MCA and ICA. They found significantly smaller vessel diameters among female subjects, which translated into greater blood flow velocity and higher WSS at the female MCA bifurcation (19%), and even greater at the ICA bifurcations (50%). Blood flow models using CFDs looking at the circle of Willis have demonstrated greater peak pressures and WSS in vessels with smaller radii and asymmetric branch angles (Alnaes et al., 2007). Using CFD, peak pressure at systole has been shown to be greatest at the VBA, ICA, ICA bifurcation, and ICA–PCOM junction. However, the bifurcation of the basilar artery is normally symmetric, generating low WSS. Therefore, it may be plausible to assume that because women have greater WSS as a result of smaller vessel diameters, they are more prone to generate aneurysms located at the ICA, ICA bifurcation, and ICA–PCOM junction. One hypothesis may be that the relative difference in vessel diameter is most pronounced between men and women at the supraclinoid ICA, and with likely more similar arterial blood pressures, the effective WSS is more conducive to aneurysm formation in women.
The clinical ramifications of smaller vessel caliber at the circle of Willis has also been demonstrated to increase the risk of rupture. Rahman et al. (2010) assessed if the “size ratio,” the ratio of the intracranial aneurysm diameter to the native vessel diameter, has any influence on risk of aneurysm rupture. The group looked at 16 ruptured and 24 unruptured aneurysms, and found the size ratio was significantly larger in ruptured aneurysms. Taken into consideration, these results can be interpreted to mean that unruptured aneurysms are more likely to rupture the larger they are in relation to the parent vessel artery diameter from which they arise. Given that females on average have smaller diameter intracranial vessels, females may be at greater risk for cerebral aneurysm rupture. Therefore, the disproportionate WSS at the ICA and the lack of endothelial protection by the decline in estrogen at locations of hemodynamic stress may explain why females are more prone to aneurysms at the ICA.
It is also important to note that ruptured aneurysms proved significantly smaller than incidentally treated aneurysms (6 vs. 8.1 mm, p = 0.02), without any significant difference between the genders. This finding is in accordance with those found in the ISAT trial and the numerous other studies in the literature – the ISAT trial showed that 52% of their ruptured aneurysms were <5 mm, and 92% <10 mm (Molyneux et al., 2002). Like the ISAT trial, our study contradicts those of the ISUIA trial, which concluded that anterior circulation aneurysms less than 7 mm have a 0% chance of rupturing at 5 years. Another study conducted by Juvela et al. (1993a) looked at 181 aneurysms, and showed that 67% of the ruptured aneurysms in their series were less than 6 mm, further validating our findings.
The primary limitation of this report is that our institution is an urban, tertiary center, and not all incidental aneurysms presenting to clinic were treated and therefore not included in this study. To account for this bias, it is important to mention that the aneurysms treated at our center were the aneurysms thought to pose a concerning natural history for risk of rupture, and therefore of more clinical consequence than those deemed to have a benign natural history risk. Additionally, when comparing our overall aneurysm distribution to other published studies that are more population based, the proportions are comparable. One of the larger clinical studies looking at the natural course of incidental aneurysms is the ISUIA study, which looked at 4060 patient with ruptured and unruptured aneurysms (Wiebers et al., 2003). When looking at all the patients included in the study, the majority of the aneurysms were located at the ICA (46%), with only 9% found at the PCOM, followed by MCA (28%), VBA (12%), and ACA (13%), findings comparable to ours (Table 4). Like our institution, Coon et al. (2011) looked at the tertiary center experience at John Hopkins with 400 intracerebral aneurysms and found a similar distribution except for a greater number of ACA aneurysms – 28%. Additionally, when comparing our ruptured to unruptured cohort with respect to aneurysm location, we find a similar trend to other published reports. When looking at our overall aneurysm location, there were more ACA and PCOM aneurysms in the ruptured group (Figure 1). In fact, they were 3 times more likely (37 vs. 11 and 26 vs. 10% respectively) to occur. This is consistent with a study by Kivisaari et al. (2004) which looked at more than 800 aneurysms and found the distribution of ruptured and unruptured ACA aneurysms to be 41 vs. 18%. Similarly, Aarhus et al. (2009) showed a greater tendency for ACA aneurysms to rupture (36 vs. 9.6%). ICA and VBA aneurysms, however, were more commonly seen in the unruptured group (53 vs. 39 and 21 vs. 14% respectively), while MCA aneurysms were found equally between the two groups. Therefore, despite the possible biases involved with our largely referred and treated cohort, the overall distribution of aneurysms along the circle of Willis among both genders is comparable to previous studies that were more population based.
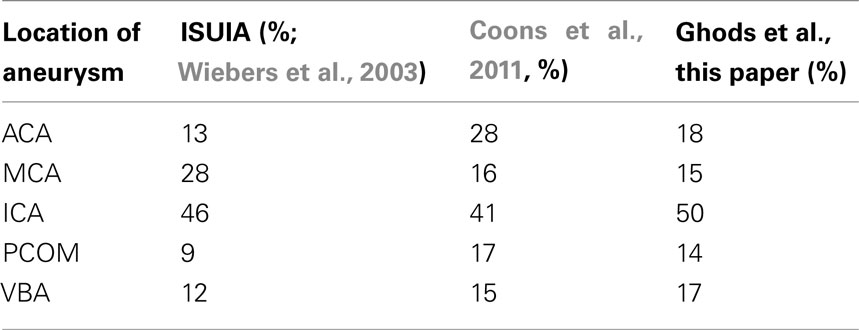
Table 4. Data of both ruptured and unruptured aneurysms from ISUIA and Coons compared to current series.
Conclusion
In summary, women with cerebral aneurysms more commonly harbor aneurysms located at the ICA, with the PCOM the most common site of rupture, and are more likely to present with SAH. Men, on the other hand, present with a significantly higher incidence of ACOM aneurysms, which are also the most common site of rupture among men. Previously described hormonal and hemodynamic theories behind cerebral aneurysm pathogenesis seem like plausible mechanisms to explain the differences we found.
Rather than continuing the trend of anatomically excluding many incidentally discovered cerebral aneurysms, to make a public health impact, patients who are truly at risk for aneurysm rupture need to be identified at the preclinical stage for possible treatment. Our findings demonstrate anatomic differences that give further support to the previously described pathophysiologic features of cerebral aneurysms. Our results on largely unruptured cerebral aneurysms may offer insight to future studies that can discern the critical steps in cerebral aneurysm pathogenesis.
Conflict of Interest Statement
The authors report no conflict of interest concerning the Section “Materials and methods” used in this study or the findings specified in this paper. This manuscript has not been previously published and is not under consideration in the same or substantially similar form in any other peer-reviewed media. To the best of our knowledge, no financial support was acquired. This has been approved by the IRB at the institution.
Acknowledgments
No other persons other than the authors have made contribution to this manuscript.
References
Aarhus, M., Helland, C. A., and Wester, K. (2009). Differences in anatomical distribution, gender, and sidedness between ruptured and unruptured intracranial aneurysms in a defined patient population. Acta Neurochir. (Wien) 151, 1569–1574.
Adamson, J., Humphries, S. E., Ostergaard, J. R., Voldby, B., Richards, P., and Powell, J. T. (1994). Are cerebral aneurysms atherosclerotic? Stroke 25, 963–966.
Alnaes, M. S., Isaksen, J., Mardal, K. A., Romner, B., Morgan, M. K., and Ingebrigtsen, T. (2007). Computation of hemodynamics in the circle of Willis. Stroke 38, 2500–2505.
Beck, J., Rohde, S., Berkefeld, J., Seifert, V., and Raabe, A. (2006). Size and location of ruptured and unruptured intracranial aneurysms measured by 3-dimensional rotational angiography. Surg. Neurol. 65, 18–25; discussion 25–27.
Coon, A. L., Paul, A. R., Colby, G. P., Lin, L. M., Pradilla, G., Huang, J., and Tamargo, R. J. (2011). Comparison of tertiary-center aneurysm location frequencies in 400 consecutive cases: decreasing incidence of posterior communicating artery region aneurysms. Surg. Neurol. Int. 2, 152.
Eden, S. V., Meurer, W. J., Sánchez, B. N., Lisabeth, L. D., Smith, M. A., Brown, D. L., and Morgenstern, L. B. (2008). Gender and ethnic differences in subarachnoid hemorrhage. Neurology 71, 731–735.
Ellamushi, H. E., Grieve, J. P., Jager, H. R., and Kitchen, N. D. (2001). Risk factors for the formation of multiple intracranial aneurysms. J. Neurosurg. 94, 728–732.
Harrod, C. G., Batjer, H. H., and Bendok, B. R. (2006). Deficiencies in estrogen-mediated regulation of cerebrovascular homeostasis may contribute to an increased risk of cerebral aneurysm pathogenesis and rupture in menopausal and postmenopausal women. Med. Hypotheses 66, 736–756.
Inagawa, T., and Hirano, A. (1990a). Autopsy study of unruptured incidental intracranial aneurysms. Surg. Neurol. 34, 361–365.
Inagawa, T., and Hirano, A. (1990b). Ruptured intracranial aneurysms: an autopsy study of 133 patients. Surg. Neurol. 33, 117–123.
Jamous, M. A., Nagahiro, S., Kitazato, K. T., Tamura, T., Kuwayama, K., and Satoh, K. (2005). Role of estrogen deficiency in the formation and progression of cerebral aneurysms. Part I: experimental study of the effects of hormone replacement therapy in rats. J. Neurosurg. 103, 1052–1057.
Juvela, S., Hillbom, M., Numminen, H., and Koskinen, P. (1993a). Cigarette smoking and alcohol consumption as risk factors for aneurysmal subarachnoid hemorrhage. Stroke 24, 639–646.
Juvela, S., Porras, M., and Heiskanen, O. (1993b). Natural history of unruptured intracranial aneurysms: a long-term follow-up study. J. Neurosurg. 79, 174–182.
Juvela, S., Poussa, K., and Porras, M. (2001). Factors affecting formation and growth of intracranial aneurysms: a long-term follow-up study. Stroke 32, 485–491.
Kaminogo, M., Yonekura, M., and Shibata, S. (2003). Incidence and outcome of multiple intracranial aneurysms in a defined population. Stroke 34, 16–21.
Kassell, N. F., Torner, J. C., Haley, E. C. Jr., Jane, J. A., Adams, H. P., and Kongable, G. L. (1990). The International Cooperative Study on the timing of aneurysm surgery. Part 1: overall management results. J. Neurosurg. 73, 18–36.
Kivisaari, R. P., Porras, M., Ohman, J., Siironen, J., Ishii, K., and Hernesniemi, J. (2004). Routine cerebral angiography after surgery for saccular aneurysms: is it worth it? Neurosurgery 55, 1015–1024.
Koivisto, T., Vanninen, R., Hurskainen, H., Saari, T., Hernesniemi, J., and Vapalahti, M. (2000). Outcomes of early endovascular versus surgical treatment of ruptured cerebral aneurysms. A prospective randomized study. Stroke 31, 2369–2377.
Kongable, G. L., Lanzino, G., Germanson, T. P., Truskowski, L. L., Alves, W. M., Torner, J. C., and Kassell, N. F. (1996). Gender-related differences in aneurysmal subarachnoid hemorrhage. J. Neurosurg. 84, 43–48.
Lazzaro, M. A., Ouyang, B., and Chen, M. (2012). The role of circle of Willis anomalies in cerebral aneurysm rupture. J. Neurointerv. Surg. 4, 22–26.
Lindekleiv, H. M., Valen-Sendstad, K., Morgan, M. K., Mardal, K.-A., Faulder, K., Magnus, J., Waterloo, K., Romner, B., and Ingebrigtsen, T. (2010). Sex differences in intracranial arterial bifurcations. Gen. Med. 7, 149–155.
Longstreth, W. T., Koepsell, T. D., Yerby, M. S., and van Belle, G. (1985). Risk factors for subarachnoid hemorrhage. Stroke 16, 377–385.
Longstreth, W. T., Nelson, L. M., Koepsell, T. D., and van Belle, G. (1994). Subarachnoid hemorrhage and hormonal factors in women. A population-based case-control study. Ann. Intern. Med. 121, 168–173.
Mhurchu, C. N., Anderson, C., Jamrozik, K., Hankey, G., Dunbabin, D., Australasian Cooperative Research on Subarachnoid Hemorrhage Study (ACROSS) Group. (2001). Hormonal factors and risk of aneurysmal subarachnoid hemorrhage: an international population-based, case-control study. Stroke 32, 606–612.
Molyneux, A., Kerr, R., Stratton, I., Sandercock, P., Clarke, M., Shrimpton, J., Holman, R., International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. (2002). International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 360, 1267–1274.
Nishioka, H., Torner, J. C., Graf, C. J., Kassell, N. F., Sahs, A. L., and Goettler, L. C. (1984). Cooperative study of intracranial aneurysms and subarachnoid hemorrhage: a long-term prognostic study., II. Ruptured intracranial aneurysms managed conservatively. Arch. Neurol. 41, 1142–1146.
Park, S. K., Kim, J. M., Kim, J. H., Cheong, J. H., Bak, K. H., and Kim, C. H. (2008). Aneurysmal subarachnoid hemorrhage in young adults: a gender comparison study. J. Clin. Neurosci. 15, 389–392.
Rahman, M., Smietana, J., Hauck, E., Hoh, B., Hopkins, N., Siddiqui, A., Levy, E. I., Meng, H., and Mocco, J. (2010). Size ratio correlates with intracranial aneurysm rupture status: a prospective study. Stroke 41, 916–920.
Rinkel, G. J., Djibuti, M., Algra, A., and van Gijn, J. (1998). Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke 29, 251–256.
Stirone, C., Duckles, S. P., and Krause, D. N. (2003). Multiple forms of estrogen receptor-alpha in cerebral blood vessels: regulation by estrogen. Am. J. Physiol. Endocrinol. Metab. 284, E184–E92.
Stober, T., Sen, S., Anstätt, T., Freier, G., and Schimrigk, K. (1985). Direct evidence of hypertension and the possible role of post-menopause oestrogen deficiency in the pathogenesis of berry aneurysms. J. Neurol. 232, 67–72.
Teunissen, L. L., Rinkel, G. J., Algra, A., and van Gijn, J. (1996). Risk factors for subarachnoid hemorrhage: a systematic review. Stroke 27, 544–549.
Vernooij, M. W., Arfan Ikram, M., Tanghe, H. L., Vincent Arnaud, J. P. E., Hofman, A., Krestin, G. P., Niessen, W. J., Breteler, M. M. B., and van der Lugt, A. (2007). Incidental findings on brain, MRI in the general population. N. Engl. J. Med. 357, 1821–1828.
Wiebers, D. O., Whisnant, J. P., Huston, J. III, Meissner, I., Brown, R. D. Jr., Piepgras, D. G., Forbes, G. S., Thielen, K., Nichols, D., O’Fallon, W. M., Peacock, J., Jaeger, L., Kassell, N. F., Kongable-Beckman, G. L., Torner, J. C., International Study of Unruptured Intracranial Aneurysms Investigators. (2003). Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 362, 103–110.
Keywords: subarachnoid hemorrhage, aneurysm, endovascular, wall shear stress, computed fluid dynamics
Citation: Ghods AJ, Lopes D and Chen M (2012) Gender differences in cerebral aneurysm location. Front. Neur. 3:78. doi: 10.3389/fneur.2012.00078
Received: 16 February 2012; Paper pending published: 27 March 2012;
Accepted: 23 April 2012; Published online: 21 May 2012.
Edited by:
Marc Lazzaro, Medical College of Wisconsin, USAReviewed by:
Angelos A. Konstas, Massachusetts General Hospital, USAEdgard Pereira, JFK Medical Center, USA
Copyright: © 2012 Ghods, Lopes and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Ali J. Ghods, Department of Neurosurgery, Rush University Medical Center, 1653 West Congress Parkway, Chicago, Il 60612-3244, USA. e-mail:YWxpamdob2RzQGhvdG1haWwuY29t

 Demetrius Lopes1
Demetrius Lopes1