- 1Department of Molecular Neurology, University Hospital Erlangen, Friedrich-Alexander Universität (FAU) Erlangen-Nürnberg, Erlangen, Germany
- 2Department of Applied Econometrics and International Political Economy, Goethe University Frankfurt, Frankfurt, Germany
- 3Department of Cellular and Molecular Medicine, University of California, San Diego, La Jolla, CA, United States
One striking observation in Parkinson’s disease (PD) is the remarkable gender difference in incidence and prevalence of the disease. Data on gender differences with regard to disease onset, motor and non-motor symptoms, and dopaminergic medication are limited. Furthermore, whether estrogen status affects disease onset and progression of PD is controversially discussed. In this retrospective single center study, we extracted clinical data of 226 ambulatory PD patients and compared age of disease onset, disease stage, motor impairment, non-motor symptoms, and dopaminergic medication between genders. We applied a matched-pairs design to adjust for age and disease duration. To determine the effect of estrogen-related reproductive factors including number of children, age at menarche, and menopause on the age of onset, we applied a standardized questionnaire and performed a regression analysis. The male to female ratio in the present PD cohort was 1.9:1 (147 men vs. 79 women). Male patients showed increased motor impairment than female patients. The levodopa equivalent daily dose was increased by 18.9% in male patients compared to female patients. Matched-pairs analysis confirmed the increased dose of dopaminergic medication in male patients. No differences were observed in age of onset, type of medication, and non-motor symptoms between both groups. Female reproductive factors including number of children, age at menarche, and age at menopause were positively associated with a delay of disease onset up to 30 months. The disease-modifying role of estrogen-related outcome measures warrants further clinical and experimental studies targeting gender differences, specifically hormone-dependent pathways in PD.
Introduction
Parkinson’s disease (PD) is a chronic, progressive neurodegenerative disease, and the most common movement disorder (1). The incidence and prevalence of PD in males substantially exceeds those of females (2, 3). Large meta-analyses of incidence studies determined 1.5–2 times higher risk for men to develop PD (2, 4, 5) and roughly 2-year earlier disease onset in men compared to women (4, 6).
The clinical phenotype of PD, which is defined by the presence of bradykinesia and at least one additional motor symptom including rigidity and resting tremor (7), may also differ between both genders. Tremor is more prevalent as initial symptom in women (6), whereas the progression rate of motor impairment and daily l-DOPA doses are increased by 15% in men (8, 9). Besides prototypical motor symptoms, a broad spectrum of non-motor symptoms such as obstipation, hyposmia, rapid eye movement sleep behavior disorder (RBD), depression, cognitive impairment, restless legs syndrome (RLS), and urinary dysfunction are highly prevalent in PD affecting quality of life and disease severity (10, 11). Hyposmia and RBD are more prevalent in men (12–15), whereas depression and anxiety are more prevalent in women (14, 16). In a Chinese PD cohort, female de novo PD patients showed more severe depressive symptoms than men (17).
Despite recent advances in deciphering the molecular pathogenesis and genetic predispositions in PD, the causal relationships underlying the observed gender differences are not well understood till date. In vivo studies of acute PD models (18–20) suggest a neuroprotective effect of the sex hormone estrogen, which may explain the differences in prevalence. This experimental evidence is further supported by epidemiological findings, such as (i) the age of PD onset correlates with the age of menopause, length of fertile life, and parity (6, 21, 22) and (ii) application of estrogen supplementation in postmenopausal women has been linked to a lower risk of developing PD (23, 24). Furthermore, estrogen supplementation improved motor function in patients with PD (25, 26). Genome-wide association studies also revealed common genetic variants in the estrogen-related gene PR domain 2 to an increased susceptibility for PD (27, 28).
The aim of this study is to explore gender differences in a German single center movement disorder outpatient clinic with regard to demographic characteristics, type and dosage of dopaminergic medication, and motor and non-motor symptoms in PD. Hence, we used our movement disorder center database to address (1) gender differences in dopaminergic medication and (2) the role of estrogen-related reproductive factors including number of children and reproductive life span on the age of disease onset.
Materials and Methods
Study Population
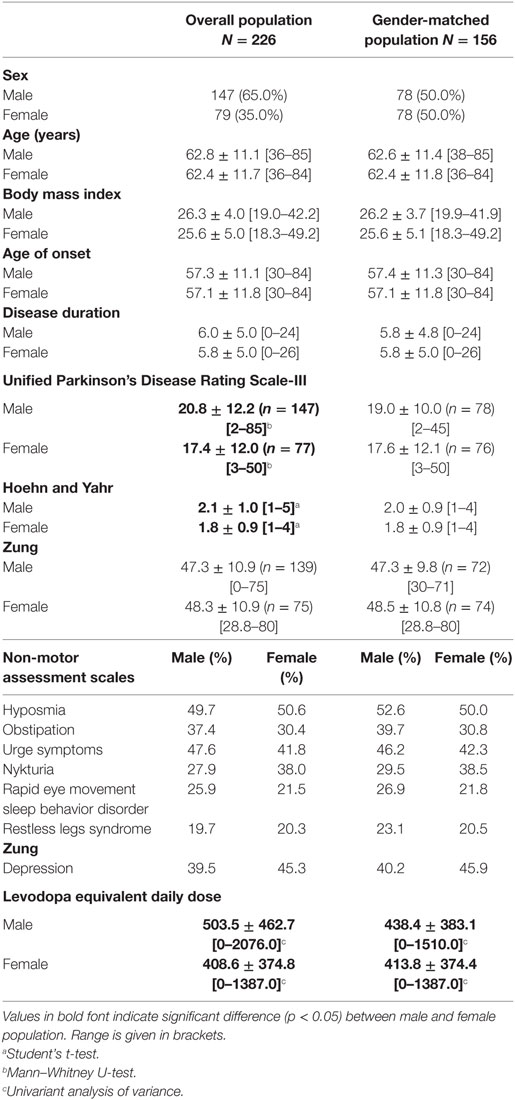
The study was approved by the Institutional Review Board for Ethics, FAU Erlangen-Nürnberg (152_15 Bc). We consecutively extracted the clinical data of 226 PD patients from our movement disorder center database between April 2010 and March 2015. Only patients diagnosed with PD according to the consensus criteria of the German Society of Neurology [analog to the National Institute of Neurological Disorders and Stroke diagnostic criteria for PD (29)] were included. Our database includes general demographic information such as age at presentation, sex, weight, height, body mass index (BMI) and a general dataset including diagnoses, medication, and family history. Disease staging was based on the Hoehn and Yahr (H&Y) Disability Scale (30). Motor impairment of PD subjects was assessed using the Unified Parkinson’s Disease Rating Scale (UPDRS) motor score part III rating (31). Type and dose of dopaminergic medication was compared using the levodopa equivalent daily dose (LEDD) calculated as previously described by Tomlinson et al. (32). We defined the age of disease onset as the year in which first signs of motor impairment were reported either by the patient or relatives. Non-motor symptoms were recorded using non-motor assessment scales (NMS) for PD (33), and patients were tested for the presence of depressive symptoms based on the Zung self-rating depression scale (34). We applied a matched-pairs design to assess the effect of gender on LEDD. Thus, each female patient was matched per age and disease duration to a male patient. This strategy enabled us to match 78 out of 79 female patients to male counterparts.
Estrogen Status Questionnaire
To assess the estrogen status of our female PD population, we used a brief self-rating, standardized instrument based on previously published reports (6, 21, 35). Aim of the questionnaire was to determine (i) the number of children, (ii) the age at menarche, (iii) age at menopause (defined as the age 12 months after last menstrual cycle), and (iv) hormonal replacement therapy (HRT). The following questions were included in the questionnaire: (1) How many children do you have? (2) At what age did you have your first menstruation? (3) At what age did you have your last menstruation? (4) Have you received any HRT including estrogens?
The questionnaire was mailed to 79 female patients with a 96% return quote (76/79). For the analysis on whether the age at menopause and fertile life span influenced the age of disease onset, we focused on the period of endogenous estrogen exposure prior to the onset of PD. Therefore, women reporting menopause after PD onset (n = 15) or who had undergone hysterectomy (n = 7) were excluded.
Statistical Analysis
Demographic characteristics, the age of onset and disease duration, and clinical rating scales were given as mean ± SD. Potential differences in these features between men and women were analyzed using a two-sample t-test. Normal distribution was ascertained by Kolmogorov–Smirnov test for all variables except UPDRS-III. For analysis of UPDRS-III, Mann–Whitney U-test was used accordingly. Non-motor symptoms were indicated as proportions and compared by a Chi-square statistics. LEDD was examined through univariate analyses of variances, with gender, H&Y disease staging, and BMI as independent variables. Type of dopaminergic medication was analyzed by means of different categories (l-DOPA only; l-DOPA + others; dopamine agonist only; dopamine agonist + others; l-DOPA + dopamine agonist; and others) and tested with Chi-square statistics.
Moreover, female reproductive data were given as mean ± SD and linear regression models were applied to demonstrate a relationship between hormonal and clinical parameters in PD. Consequently, the age of disease onset was regressed on age at menarche, age at menopause, number of children, and duration of fertile life. The correlation coefficient, standard error (SE), and beta were determined. The Pearson coefficient was calculated to quantify the correlation between the duration of fertile life and the age of PD onset. The application of hormone replacement therapy was not included as the sample size was too small (n = 11). All data were analyzed using IBM’s SPSS software (version 21.0). A value of p < 0.05 was set to be statistically significant.
Results
To detect differences in the clinical phenotype of PD between women and men, we retrospectively analyzed the data of 226 patients of the Erlangen movement disorder database. As presented in Table 1, male patients outnumbered females in a ratio of 1.9:1 (147 vs. 79 patients, respectively) at baseline. Age was similar between both groups. Age of onset and disease duration did not significantly differ between both groups. Compared to females, male PD patients were more severely affected, as indicated by higher scores in H&Y (2.1 ± 1.0 vs. 1.8 ± 0.9, two-sample t-test, p < 0.05) and UPDRS-III scale (20.8 ± 12.2 vs. 17.4 ± 12.0, Mann–Whitney U-test, p < 0.05).
As non-motor symptoms have a profound impact on the quality of life in patients with PD, we screened for differences in hyposmia, obstipation, urge symptoms, depression, RBD, and RLS using self-rating questionnaires, but there were no significant differences between males and females. According to the NMS questionnaire (33), hyposmia was the most prevalent syndrome reported by ~50% of the PD patients and REM-sleep behavior disorder was reported by ~25%. Based on the Zung self-rating depression scale (34), 39.5% of male and 45.3% of the female PD patients showed signs of depression. Whereas 24.4% of male patients and 28.0% of female patients have mild depressive symptoms, 15.0% of male patients and 17.3% of female patients were identified with moderate-to-severe depression.
Next, we asked if the dose of dopaminergic medication differed between males and females in our cohort. We observed a significantly increased LEDD in men compared with women (503.5 ± 462.7 vs. 408.6 ± 374.8 mg; univariate analysis of variance, F = 133.79, p < 0.001, adjusted R2 = 0.540).
Regarding the type of dopaminergic medication, the majority of patients were taking dopamine agonists in combination with other drugs including l-DOPA, amantadine, monoamine oxidase type B inhibitors, or anticholinergics (men 27.2%, women 22.8%). Only 6.1% male patients and 10.1% female patients were treated with an l-DOPA monotherapy, whereas 14.1 and 16.5% received a combination of l-DOPA and a dopamine agonist, respectively (Table 2). We detected no gender difference in the type of dopaminergic medication.
Since the difference in the daily dose of dopaminergic medication may be due to a higher age and a longer disease duration in males in the present cohort, we matched the population based on gender, age, and disease duration to rule out their confounding effects, resulting in 78 matched gender PD patient pairs. We did not detect any significant gender differences in the mean motor score (UPDRS-III: 19.0 ± 10.0 for men vs. 17.6 ± 12.1 for women, Mann–Whitney U-test, p = 0.411) and the disease stage (H&Y: 2.0 ± 0.9 in men vs. 1.8 ± 0.9 in women, two-sample t-test, p = 0.374; Table 1). Nevertheless, LEDD values were still significantly increased in men (438.4 ± 383.1 mg men vs. 413.8 ± 374.4 mg women, univariate analysis of variance, p < 0.001). When integrating further factors into the LEDD model (univariate analysis of variance, p < 0.001, adjusted R2 = 0.712), H&Y had a very profound effect (univariate analysis of variance, F = 20.8, p < 0.001, ηp2 = 0.308). Despite taking H&Y staging as one of the most relevant confounding factors into account, gender remained to be a significant and crucial factor for LEDD (univariate analysis of variance, F = 4.6, p < 0.05, ηp2 = 0.032). The BMI did not have a significant influence on LEDD (univariate analysis of variance, p = 0.457). Applying the matched-pairs design, we did not observe significant differences in the type of dopaminergic medication between both genders (Table S1 in Supplementary Material).
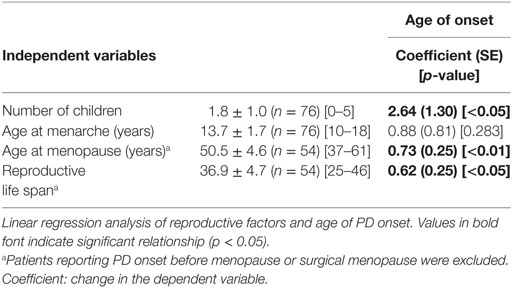
We then asked, whether estrogen status in female patients influenced the age of PD onset. To answer this question, we determined the number of children, the age of menarche, and the age of menopause of our female study population based on the applied questionnaire and conducted a linear regression analysis (Table 3). The age of disease onset correlated significantly with the number of children (linear regression analysis, beta = 0.229, p < 0.05, adjusted R2 = 0.053), i.e., birth of one child increased the age of disease onset by 2.6 years (Figure 1).
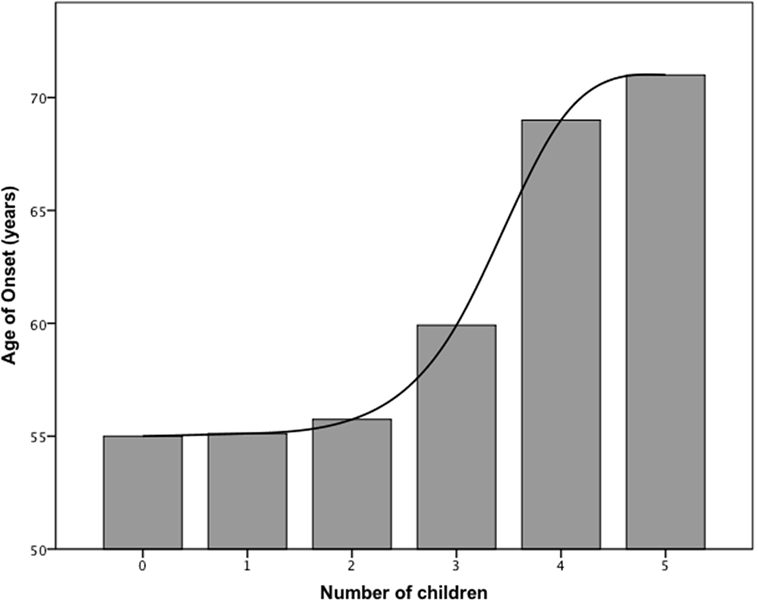
Figure 1. Bar graph showing the relationship between age of disease onset and number of children in females.
The mean age at menarche and menopause was 13.7 and 50.5 years, respectively, with a mean duration of fertile life of 36.8 years (Table 3). The age at menopause (linear regression analysis, beta = 0.370, p < 0.01, adjusted R2 = 0.121) and duration of fertile life (linear regression analysis, beta = 0.343, p < 0.05, adjusted R2 = 0.117) were associated with the delayed disease onset. Specifically, 1 year of prolonged fertility increased age of PD onset by 0.7 years (Figure 2). We did not observe effects of the abovementioned reproductive factors on LEDD or UPDRS.
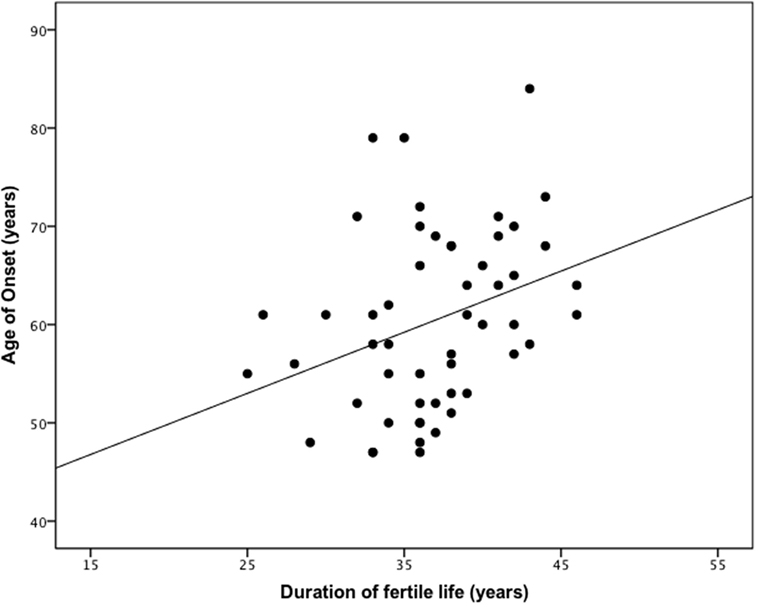
Figure 2. The duration of reproductive life influences the age of onset in female patients with Parkinson’s disease (PD). One year of prolonged fertility increases age of PD onset by 0.7 years. Pearson coefficient = 0.343 (p < 0.05).
Discussion
The present cross-sectional, retrospective single center study supports the general epidemiological observation of apparent gender differences in PD (2, 4–6). Interestingly, estrogen status in females had a major impact on the age of PD onset. We show that LEDD is lower in women when compared to men. However, we did not observe gender differences in the type of dopaminergic medication and clinical presentation of non-motor symptoms.
We included 226 PD patients from our movement disorder center database, resulting in a male to female ratio of 1.9:1. The gender ratio in the present cohort with a male predominance in PD is in line with previously published studies. An incidence study by Van den Eeden et al. revealed a 91% higher rate for men to develop PD in a Northern California cohort (2). In a meta-analysis of seven incidence studies (with the population size ranging from 182,934 to 3,869,162), Wooten et al. determined a weighted mean male to female ratio of 1.5 (5). Furthermore, Twelves et al. reviewed and identified a significantly greater ratio of males to females (1.5:2.0) in five of the nine incidence studies (4).
We observed significantly increased motor impairment and disease severity as measured by UPDRS-III and H&Y in male PD patients. However, differences regarding motor impairment and disease stage were not evident after the populations were matched to gender, age, and disease duration. The LEDD was overall lower in female patients, after controlling further for disease severity and BMI. Nyholm et al. (9) also described that men with PD required higher mean daily doses of l-DOPA, specifically by 17.4% (9). This finding was based on a nationwide study from Sweden conducted in 2007, in which 33,534 l-DOPA prescriptions from a drug register were analyzed and daily l-DOPA doses estimated for each patient, regardless of confirmed PD diagnosis, disease duration or body weight. Lyons et al. (8) reviewed 630 patients from a PD registry and controlled them for age and disease duration (8) and reported an increase in the mean daily l-DOPA dosages by 15.2% in men. This gender difference was even more prominent in advanced disease stages with disease duration greater than 5 years. Umeh et al. (36) detected no sex differences in type and dose of dopaminergic medications used in early PD (36). In this large multicenter study from the United States and Canada, 1,741 patients were registered within 5 years of PD diagnosis and adjusted for disease duration, motor impairment, and body weight. The study also employed LEDD instead of solely l-DOPA as a more appropriate overall measure for dopaminergic treatment. Of note, we did not observe that the BMI significantly interacts with the dosage of dopaminergic medication, confirming a previously published study (36).
We detected no gender differences in the presentation of non-motor symptoms. Approximately 40% of the male patients and 45% of the female patients in the present study fulfilled the criteria for depression, indicating a slight but not significant difference. Although two studies identified the female gender as a risk factor for depression in PD (16, 37), Tandberg et al. (38) described no difference between male and female PD patients (38). The mean frequency of depression in PD patients varies considerably, ranging from 22 to 46% (38–40), depending on the population and the method of diagnosis. While 50% of the patients reported olfactory impairment in the self-assessment of the NMS, hyposmia is generally described to be more common among PD patients (15). There is evidence that one-third of the patients with olfactory loss are not aware of the condition (41), and thus the NMS questionnaire is prone to underestimate the frequency of hyposmia in PD (42).
The role of estrogen and its relevance to PD is not well understood. Our findings indicate that endogenous estrogen affects age of disease onset. Number of children, age of menopause, and reproductive life span were associated with a delayed age of disease onset. Thus, increased circulating estrogen levels throughout a woman’s life delay the onset of PD supporting previous cross-sectional studies (6, 21, 22). Haaxma et al. (6) prospectively studied a cohort from the Netherlands and observed an increase of PD onset by 2.7 years per child and by 0.5 years per additional year of fertility (6). A case–control study further associated a shorter reproductive life span with an increased risk of developing PD (35). It should be noted that prospective cohort studies (43, 44) and case–control studies (45, 46) showed no significant relation between reproductive factors and PD risk. Our data support the observation that endogenous estrogen may be a protective factor for women against developing PD. The impact of exogenous estrogen on PD and its role as a disease-modifying hormone is similarly controversial. Postmenopausal hormonal therapy is reported to either improve motor function (25, 26) or to be without an effect on motor symptoms (47, 48). Moreover, estrogen level was associated with a decreased (23), increased (46), and equal risk to develop PD (43, 49).
The inconsistency among clinical studies may be due to the limited understanding of precise mechanisms by which estrogen affects the dopaminergic system in the brain. Estradiol was shown to be essential in maintaining the integrity of nigostratial pathways in rodents and in an acute rodent MPTP model of PD (20, 50). Estradiol has been demonstrated to exert antioxidant and neurotrophic properties, to modulate neuronal plasticity, and to decrease degeneration of dopaminergic neurons (51).
We acknowledge certain limitations in this study. To study the effect of reproductive factors on the age of PD onset, we performed a retrospective cohort study. Thus, we cannot rule out recall bias, e.g., regarding the disease onset or reproductive events such as age of menarche and menopause. However, it has been shown that recalling important reproductive events is very reliable and consistent (52). Furthermore, patient data from only one center with a Caucasian ethnicity were analyzed. For future studies, special attention should be paid on the effect of ethnicity on dopaminergic medication and gender differences.
Taken together, our study shows a strong association between reproductive factors and age of PD onset. A more detailed and in depth understanding of gender differences and the role of estrogen in PD may contribute to etiological conclusions and reveal novel treatment approaches.
Ethics Statement
The study was approved by the Institutional Review Board for Ethics, FAU Erlangen-Nürnberg (152_15 Bc).
Author Contributions
JW and JS designed the work; DF, GJ, JK, JW, and JS acquired data; OB and DF performed statistical analysis; DF, JW, and JS interpreted the data; DF and JS wrote the initial draft, and all authors revised the work critically for important intellectual content; all authors approved the final version of the manuscript and all authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The present work was performed in fulfillment of the requirements for obtaining the degree “Dr. med.” This work was supported by the Interdisciplinary Center for Clinical Research (IZKF) and by the DFG (JCMS, SCHL2102/1-1). We acknowledge support by Deutsche Forschungsgemeinschaft and Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU) within the funding program Open Access Publishing. We thank Pascal Schön and Georg Zweyer for their valuable help in updating, revising, and maintaining the movement disorder center database.
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fneur.2017.00397/full#supplementary-material.
References
1. Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov Disord (2014) 29(13):1583–90. doi:10.1002/mds.25945
2. Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, et al. Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol (2003) 157(11):1015–22. doi:10.1093/aje/kwg068
3. Mayeux R, Denaro J, Hemenegildo N, Marder K, Tang MX, Cote LJ, et al. A population-based investigation of Parkinson’s disease with and without dementia. Relationship to age and gender. Arch Neurol (1992) 49(5):492–7. doi:10.1001/archneur.1992.00530290076015
4. Twelves D, Perkins KS, Counsell C. Systematic review of incidence studies of Parkinson’s disease. Mov Disord (2003) 18(1):19–31. doi:10.1002/mds.10305
5. Wooten GF, Currie LJ, Bovbjerg VE, Lee JK, Patrie J. Are men at greater risk for Parkinson’s disease than women? J Neurol Neurosurg Psychiatry (2004) 75(4):637–9. doi:10.1136/jnnp.2003.020982
6. Haaxma CA, Bloem BR, Borm GF, Oyen WJ, Leenders KL, Eshuis S, et al. Gender differences in Parkinson’s disease. J Neurol Neurosurg Psychiatry (2007) 78(8):819–24. doi:10.1136/jnnp.2006.103788
7. Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord (2015) 30(12):1591–601. doi:10.1002/mds.26424
8. Lyons KE, Hubble JP, Troster AI, Pahwa R, Koller WC. Gender differences in Parkinson’s disease. Clin Neuropharmacol (1998) 21(2):118–21.
9. Nyholm D, Karlsson E, Lundberg M, Askmark H. Large differences in levodopa dose requirement in Parkinson’s disease: men use higher doses than women. Eur J Neurol (2010) 17(2):260–6. doi:10.1111/j.1468-1331.2009.02866.x
10. O’Sullivan SS, Williams DR, Gallagher DA, Massey LA, Silveira-Moriyama L, Lees AJ. Nonmotor symptoms as presenting complaints in Parkinson’s disease: a clinicopathological study. Mov Disord (2008) 23(1):101–6. doi:10.1002/mds.21813
11. Chaudhuri KR, Healy DG, Schapira AH; National Institute for Clinical Excellence. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol (2006) 5(3):235–45. doi:10.1016/S1474-4422(06)70373-8
12. Ozekmekci S, Apaydin H, Kilic E. Clinical features of 35 patients with Parkinson’s disease displaying REM behavior disorder. Clin Neurol Neurosurg (2005) 107(4):306–9. doi:10.1016/j.clineuro.2004.09.021
13. Yoritaka A, Ohizumi H, Tanaka S, Hattori N. Parkinson’s disease with and without REM sleep behaviour disorder: are there any clinical differences? Eur Neurol (2009) 61(3):164–70. doi:10.1159/000189269
14. Liu R, Umbach DM, Peddada SD, Xu Z, Troster AI, Huang X, et al. Potential sex differences in nonmotor symptoms in early drug-naive Parkinson disease. Neurology (2015) 84(21):2107–15. doi:10.1212/WNL.0000000000001609
15. Szewczyk-Krolikowski K, Tomlinson P, Nithi K, Wade-Martins R, Talbot K, Ben-Shlomo Y, et al. The influence of age and gender on motor and non-motor features of early Parkinson’s disease: initial findings from the Oxford Parkinson Disease Center (OPDC) discovery cohort. Parkinsonism Relat Disord (2014) 20(1):99–105. doi:10.1016/j.parkreldis.2013.09.025
16. Fernandez HH, Lapane KL, Ott BR, Friedman JH. Gender differences in the frequency and treatment of behavior problems in Parkinson’s disease. SAGE study group. Systematic assessment and geriatric drug use via epidemiology. Mov Disord (2000) 15(3):490–6. doi:10.1002/1531-8257(200005)15:3<490::AID-MDS1011>3.0.CO;2-E
17. Song Y, Gu Z, An J, Chan P; Chinese Parkinson Study Group. Gender differences on motor and non-motor symptoms of de novo patients with early Parkinson’s disease. Neurol Sci (2014) 35(12):1991–6. doi:10.1007/s10072-014-1879-1
18. Datla KP, Murray HE, Pillai AV, Gillies GE, Dexter DT. Differences in dopaminergic neuroprotective effects of estrogen during estrous cycle. Neuroreport (2003) 14(1):47–50. doi:10.1097/01.wnr.0000050300.92401.45
19. Liu B, Dluzen DE. Oestrogen and nigrostriatal dopaminergic neurodegeneration: animal models and clinical reports of Parkinson’s disease. Clin Exp Pharmacol Physiol (2007) 34(7):555–65. doi:10.1111/j.1440-1681.2007.04616.x
20. Rodriguez-Perez AI, Dominguez-Meijide A, Lanciego JL, Guerra MJ, Labandeira-Garcia JL. Inhibition of Rho kinase mediates the neuroprotective effects of estrogen in the MPTP model of Parkinson’s disease. Neurobiol Dis (2013) 58:209–19. doi:10.1016/j.nbd.2013.06.004
21. Cereda E, Barichella M, Cassani E, Caccialanza R, Pezzoli G. Reproductive factors and clinical features of Parkinson’s disease. Parkinsonism Relat Disord (2013) 19(12):1094–9. doi:10.1016/j.parkreldis.2013.07.020
22. Ragonese P, D’Amelio M, Callari G, Salemi G, Morgante L, Savettieri G. Age at menopause predicts age at onset of Parkinson’s disease. Mov Disord (2006) 21(12):2211–4. doi:10.1002/mds.21127
23. Currie LJ, Harrison MB, Trugman JM, Bennett JP, Wooten G. Postmenopausal estrogen use affects risk for Parkinson disease. Arch Neurol (2004) 61(6):886–8. doi:10.1001/archneur.61.6.886
24. Gatto NM, Deapen D, Stoyanoff S, Pinder R, Narayan S, Bordelon Y, et al. Lifetime exposure to estrogens and Parkinson’s disease in California teachers. Parkinsonism Relat Disord (2014) 20(11):1149–56. doi:10.1016/j.parkreldis.2014.08.003
25. Saunders-Pullman R, Gordon-Elliott J, Parides M, Fahn S, Saunders HR, Bressman S. The effect of estrogen replacement on early Parkinson’s disease. Neurology (1999) 52(7):1417–21. doi:10.1212/WNL.52.7.1417
26. Tsang KL, Ho SL, Lo SK. Estrogen improves motor disability in parkinsonian postmenopausal women with motor fluctuations. Neurology (2000) 54(12):2292–8. doi:10.1212/WNL.54.12.2292
27. Chung SJ, Armasu SM, Biernacka JM, Lesnick TG, Rider DN, Cunningham JM, et al. Variants in estrogen-related genes and risk of Parkinson’s disease. Mov Disord (2011) 26(7):1234–42. doi:10.1002/mds.23604
28. Maraganore DM, de Andrade M, Lesnick TG, Strain KJ, Farrer MJ, Rocca WA, et al. High-resolution whole-genome association study of Parkinson disease. Am J Hum Genet (2005) 77(5):685–93. doi:10.1086/496902
29. Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol (1999) 56(1):33–9. doi:10.1001/archneur.56.1.33
30. Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, et al. Movement disorder society task force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord (2004) 19(9):1020–8. doi:10.1002/mds.20213
31. Goetz CG, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stebbins GT, et al. Movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): process, format, and clinimetric testing plan. Mov Disord (2007) 22(1):41–7. doi:10.1002/mds.21198
32. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord (2010) 25(15):2649–53. doi:10.1002/mds.23429
33. Storch A, Odin P, Trender-Gerhard I, Fuchs G, Reifschneider G, Ray Chaudhuri K, et al. [Non-motor symptoms questionnaire and scale for Parkinson’s disease. Cross-cultural adaptation into the German language]. Nervenarzt (2010) 81(8):980–5. doi:10.1007/s00115-010-3010-z
34. Zung WW. A self-rating depression scale. Arch Gen Psychiatry (1965) 12:63–70. doi:10.1001/archpsyc.1965.01720310065008
35. Ragonese P, D’Amelio M, Salemi G, Aridon P, Gammino M, Epifanio A, et al. Risk of Parkinson disease in women: effect of reproductive characteristics. Neurology (2004) 62(11):2010–4. doi:10.1212/WNL.62.11.2010
36. Umeh CC, Pérez A, Augustine EF, Dhall R, Dewey RB Jr, Mari Z, et al. No sex differences in use of dopaminergic medication in early Parkinson disease in the US and Canada – baseline findings of a multicenter trial. PLoS One (2014) 9(12):e112287. doi:10.1371/journal.pone.0112287
37. Kuopio AM, Marttila RJ, Helenius H, Toivonen M, Rinne UK. The quality of life in Parkinson’s disease. Mov Disord (2000) 15(2):216–23. doi:10.1002/1531-8257(200003)15:2<216::AID-MDS1003>3.0.CO;2-#
38. Tandberg E, Larsen JP, Aarsland D, Cummings JL. The occurrence of depression in Parkinson’s disease. A community-based study. Arch Neurol (1996) 53(2):175–9. doi:10.1001/archneur.1996.00550020087019
39. Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP, et al. The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord (2009) 24(11):1641–9. doi:10.1002/mds.22643
40. Cummings JL. Depression and Parkinson’s disease: a review. Am J Psychiatry (1992) 149(4):443–54. doi:10.1176/ajp.149.4.443
41. Hawkes C. Olfaction in neurodegenerative disorder. Mov Disord (2003) 18(4):364–72. doi:10.1002/mds.10379
42. Romenets SR, Wolfson C, Galatas C, Pelletier A, Altman R, Wadup L, et al. Validation of the non-motor symptoms questionnaire (NMS-Quest). Parkinsonism Relat Disord (2012) 18(1):54–8. doi:10.1016/j.parkreldis.2011.08.013
43. Rugbjerg K, Christensen J, Tjønneland A, Olsen JH. Exposure to estrogen and women’s risk for Parkinson’s disease: a prospective cohort study in Denmark. Parkinsonism Relat Disord (2013) 19(4):457–60. doi:10.1016/j.parkreldis.2013.01.008
44. Simon KC, Chen H, Gao X, Schwarzschild MA, Ascherio A. Reproductive factors, exogenous estrogen use, and risk of Parkinson’s disease. Mov Disord (2009) 24(9):1359–65. doi:10.1002/mds.22619
45. Nicoletti A, Nicoletti G, Arabia G, Annesi G, De Mari M, Lamberti P, et al. Reproductive factors and Parkinson’s disease: a multicenter case-control study. Mov Disord (2011) 26(14):2563–6. doi:10.1002/mds.23951
46. Popat RA, Van Den Eeden SK, Tanner CM, McGuire V, Bernstein AL, Bloch DA, et al. Effect of reproductive factors and postmenopausal hormone use on the risk of Parkinson disease. Neurology (2005) 65(3):383–90. doi:10.1212/01.wnl.0000171344.87802.94
47. Blanchet PJ, Fang J, Hyland K, Arnold LA, Mouradian MM, Chase TN. Short-term effects of high-dose 17beta-estradiol in postmenopausal PD patients: a crossover study. Neurology (1999) 53(1):91–5. doi:10.1212/WNL.53.1.91
48. Strijks E, Kremer JA, Horstink MW. Effects of female sex steroids on Parkinson’s disease in postmenopausal women. Clin Neuropharmacol (1999) 22(2):93–7. doi:10.1097/00002826-199903000-00005
49. Ascherio A, Chen H, Schwarzschild MA, Zhang SM, Colditz GA, Speizer FE. Caffeine, postmenopausal estrogen, and risk of Parkinson’s disease. Neurology (2003) 60(5):790–5. doi:10.1212/01.WNL.0000046523.05125.87
50. Leranth C, Roth RH, Elsworth JD, Naftolin F, Horvath TL, Redmond DE Jr. Estrogen is essential for maintaining nigrostriatal dopamine neurons in primates: implications for Parkinson’s disease and memory. J Neurosci (2000) 20(23):8604–9.
51. Saunders-Pullman R. Estrogens and Parkinson disease: neuroprotective, symptomatic, neither, or both? Endocrine (2003) 21(1):81–7. doi:10.1385/endo:21:1:81
Keywords: Parkinson’s disease, gender, estrogen status, disease onset, dopamine
Citation: Frentzel D, Judanin G, Borozdina O, Klucken J, Winkler J and Schlachetzki JCM (2017) Increase of Reproductive Life Span Delays Age of Onset of Parkinson’s Disease. Front. Neurol. 8:397. doi: 10.3389/fneur.2017.00397
Received: 10 April 2017; Accepted: 25 July 2017;
Published: 21 August 2017
Edited by:
Oscar Arias-Carrión, Hospital General Dr. Manuel Gea González, MexicoReviewed by:
Silmar Teixeira, Federal University of Piauí, BrazilAngelo Quartarone, University of Messina, Italy
Copyright: © 2017 Frentzel, Judanin, Borozdina, Klucken, Winkler and Schlachetzki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jürgen Winkler, anVlcmdlbi53aW5rbGVyQHVrLWVybGFuZ2VuLmRl;
Johannes C. M. Schlachetzki, am9oYW5uZXMuc2NobGFjaGV0emtpQHVrLWVybGFuZ2VuLmRl, anNjaGxhY2hldHpraUB1Y3NkLmVkdQ==
 Dominik Frentzel
Dominik Frentzel Grigorij Judanin
Grigorij Judanin Olga Borozdina
Olga Borozdina Jochen Klucken
Jochen Klucken Jürgen Winkler
Jürgen Winkler Johannes C. M. Schlachetzki
Johannes C. M. Schlachetzki