- 1Department of Neurology, Peking Union Medical College Hospital, Chinese Academy of Medical Science, Beijing, China
- 2Neuroscience Center, Chinese Academy of Medical Sciences, Beijing, China
- 3Department of Radiology, Peking Union Medical College Hospital, Chinese Academy of Medical Science, Beijing, China
Background: Few studies have investigated structural imaging findings of cerebral venous thrombosis (CVT) in China. The structural imaging [head computed tomography (CT) and routine brain magnetic resonance imaging (MRI)] can assess any parenchymal lesion secondary to the venous thrombosis and reveal direct signs of intraluminal thrombus. In recent years, many patients can be diagnosed with CVT more rapidly and directly by structural imaging. The aim of the present study is to determine the performance of structural imaging in the diagnosis and outcome of CVT in a large cohort single center of Chinese patients.
Methods: We evaluated consecutive patients admitted to our hospital with CVT receiving structural imaging from 1991 to 2015. A neuroradiologist, blinded to clinical data, independently reviewed the structural imaging, including head CT and routine MRI for parenchymal lesions and signs of dural venous sinus thrombosis, as well as the MRV/DSA findings. The Clinical and laboratory data were reviewed and recorded for further analysis.
Results: 117 patients were included in this study, 68 (58.1%) were females. Parenchymal lesions were identified in 56.4% (66/117) of the patients on structural imaging, including focal edema in 30.8%, hemorrhage in 19.7%, and brain swelling in 4.3% of the patients. Patients with parenchymal lesions presented with more often seizures (P < 0.001) and less often headache (P = 0.049). Intraluminal thrombus within the sinuses or veins on structural imaging was found in 28.2% (33/117) of the patients. Patients with both intraluminal thrombus and parenchymal lesions on structural imaging had more acute onset (P = 0.01) and present more consciousness disturbance (P = 0.007).
Conclusion: Intracranial lesions on structural imaging are frequently found in patients with CVT. Patients with parenchymal lesions on structural imaging, especially with intraluminal thrombus simultaneously, tend to have a severe clinical picture and might lead to a devastating or fatal outcome. Structural imaging may help on early diagnosis and predict the poor outcome of CVT.
Introduction
Cerebral venous thrombosis (CVT) is an uncommon but potentially serious and life-threatening cause of stroke, accounting for 0.5–1% of all strokes (1). Since the underlying risk factors and clinical manifestations of CVT are highly diverse which make early diagnosis much difficult, neuroimaging plays a key role in the diagnosis and further treatment (2).
The structural imaging [head computed tomography (CT) and routine brain magnetic resonance imaging (MRI)] can assess any parenchymal lesion secondary to the venous thrombosis and reveal direct signs of intraluminal thrombus (3). As some special MRI sequences, such as susceptibility-weighted images, are used in routine MRI protocols to detect the presence of intraluminal thrombus, many patients can be diagnosed with CVT more rapidly and directly by structural imaging in recent years (4, 5). On the other hand, although the parenchymal lesions are not specific, they could draw attention and prompt a search for direct visualization of a thrombus on MRI or ordering a venography. Therefore, understanding the characteristics of structural imaging for the evaluation of CVT is especially important when CVT is not clinically suspected or magnetic resonance venography (MRV) has not been ordered. In addition, systematic studies investigating structural imaging characteristics in CVT are scarce from China. The aim of the present study is to determine the structural imaging performance on the diagnosis and outcome of CVT in a large number of Chinese patients from a single center.
Materials and Methods
Patients
This was a single-center retrospective hospital-based study. We reviewed our stroke database of all consecutive patients diagnosed as CVT admitted to Peking Union Medical College Hospital between July 1991 and December 2015. All of the patients enrolled in this study had at least one of the following neuroradiological studies to confirm the diagnosis of CVT: CT scan, MRI, MRV, or cerebral angiography, following established diagnostic criteria (3). Of 176 consecutive patients with a diagnosis of CVT,39 without available head CT or brain MRI or with low-quality images were excluded. Given some patients before 2000 might be misdiagnosed due to the limited imaging information, all clinical and neuroimaging records were reviewed by our cerebrovascular group and reconfirmed the diagnosis of CVT. Based on the group review, 20 patients with an equivocal diagnosis were further excluded, leaving 117 patients for analysis in the present study (Figure 1). The ethics committee of Peking Union Medical College Hospital approved the protocol of the study.
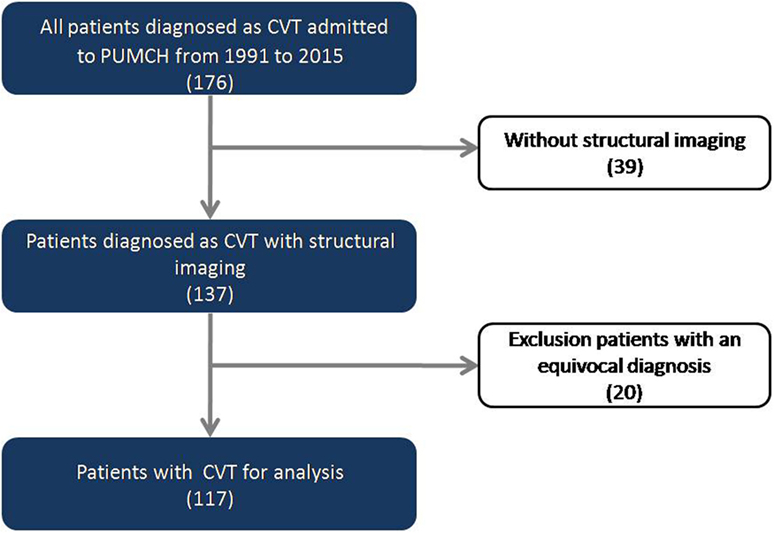
Figure 1. Flowchart of the screening process for identifying patients with cerebral venous thrombosis (CVT).
Baseline Data
We reviewed medical records extensively and recorded demographic data, clinical presentations, risk factors such as genetic and acquired prothrombotic states, trauma, and infections or inflammatory conditions, pregnancy and puerperium, laboratory testing, treatments, and outcome on discharge.
Imaging Protocol
The MRI protocol used in the study routinely includes T1-weighted, T2- weighted, diffusion-weighted images (DWI), non-echo-planar fluid-attenuated inversion recovery (FLAIR), and MRV or cerebral angiography. T2*-weighted gradient echo (GRE) was conducted in some of the patients after 2000. The admission imaging was used for analysis. The initial imaging performed after symptom onset was used in the study if the patients were not admitted to our hospital primarily. Of 117 patients enrolled in the present study, 89 performed MRV and 52 performed cerebral angiographies.
In this study, the parenchymal lesions secondary to CVT on structural imaging were categorized as three subtypes: brain swelling, focal edema, and hemorrhage [parenchymal hemorrhage or subarachnoid hemorrhage (SAH)] (6). Brain swelling was defined as there were no abnormalities in attenuation or signal intensity on structural images, but sulcal effacement, diminished cistern visibility, and a reduction in ventricular size may occur. Focal edema is visible on CT or MR images by focal abnormalities in attenuation or signal intensity without visible hemorrhage, which could be subclassified as either vasogenic edema (with increased ADC values presumably related to venous congestion) or cytotoxic edema (with decreased ADC values related to cellular energy disruption) by DWI techniques. The definition of intraluminal thrombus was the cord sign and the dense sign on plain CT or the empty delta sign on enhance CT scan or abnormal high signal on T1, loss of flow void on T2, loss of flow void or hyperintense signal on FLAIR in the corresponding venous sinus and veins, accompanied by filling defect. In each case, evaluation of all sequences of interest was performed at the same time to reflect usual clinical practice.
A radiologist blinding to clinical information rated all images, including head CT, MRI, MRV, and digital subtraction angiography. The supervisor will further review and confirm the lesions, which were originally doubted.
Statistical Analysis
The continuous demographic data were analyzed using mean ± SD and the discrete data using number and percentage. Differences in proportions were tested with chi-square test or Fisher’s exact test when required. Data analysis was processed on SPSS Statistics Bass 20.0 (SPSS Inc., Chicago, IL, USA). Student’s t-test was used for continuous variables. All P values were two-tailed and criteria for significance were P < 0.05.
Results
Baseline Clinical Data
The mean age of patients diagnosed with CVT was 34 ± 14.3 years. There was a female predominance (58.1%, 68/117). The onset of CVT in the study was almost acute or subacute (within 2 weeks) in 89.7% (105/117) of the cases. The most common manifestation was headache (87.2%, 102/117), followed by seizures (31.6%, 37/117), focal neurological deficits (29.9%, 35/117), visual impairment (26.5%, 31/117), and consciousness disturbance (15.4%, 18/117). At least one underlying risk factor was identified in 71.8% (84/117) of patients. By location, the common affected sinuses were transverse sinus (65.0%, 76/117), sigmoid sinus (55.6%, 65/117), and superior sagittal sinus (54.7%, 64/117). Only 4.3% (5/117) of patients had isolate thrombosis within the cortical veins.
In hospitalization, most of the patients (82.1%, 96/117) received anticoagulation with intravenous heparin or subcutaneous low-molecular weight heparin (LMWH) in therapeutic dosages. Data of clinical outcome at discharge were available for 86.3% (101/117) of the patients. A total recovery (41.2%) or clinical improvements (50.5%) were found in majority of the cases (92.1%, 93/101). In-hospital mortality was 4.0% (4/101) in our cohort.
Parenchymal Lesions on Structural Imaging
Parenchymal lesions were identified on structural imaging in 56.4% of the patients (66/117). Focal edema was the most common structural imaging findings, which was visible on CT and MRI in 30.8% (36/117) of the patients. Hemorrhage was detected in 19.7% (23/117) of cases, including parenchymal hemorrhage in 15.4% (18/117) and SAH in 4.3% (5/117) of the cases. Brain swelling was visualized on CT and MRI in only 4.3% (5/117) of cases. In two patients, multiple dilated subcortical and medullar veins were observed on T2*-GRE (Figure 2).
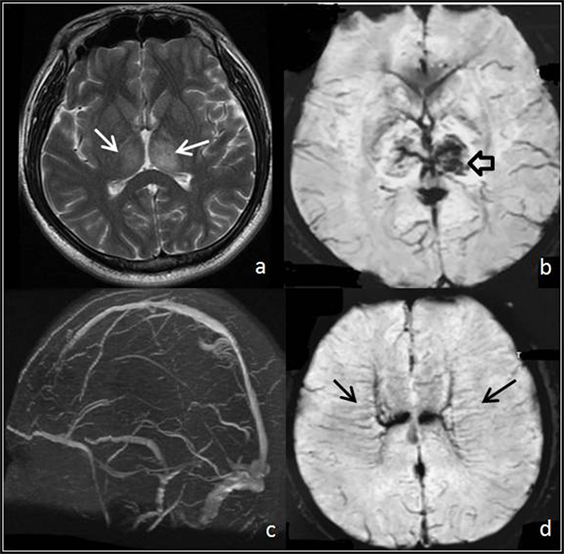
Figure 2. Thrombosis of deep venous system. (A,B) T2 and T2* images show focal edema (white arrows) and hemorrhage (hollow arrow) within both thalami. (C) MR venogram demonstrates a portion of the deep venous system with no signal, a finding consistent with occlusion. (D) T2* images show multiple linear abnormal signals within the deep white matter (black arrows), which demonstrate medullar veins dilated compensatory.
Compared to the patients without parenchymal lesions, those with parenchymal lesions presented with more often seizures (P < 0.001) and less often headache (P = 0.049). More patients with parenchymal lesions were anticoagulated than those without parenchymal lesions (P = 0.019). There was no significant difference in demographic data, onset of the CVT, occluded sinus and veins, underlining risk factors, and outcome at discharge between two groups. The comparison of baseline data, clinical presentation, risk factors, and occluded sinus or vein between patients with and without parenchymal lesions is presented in Table 1.
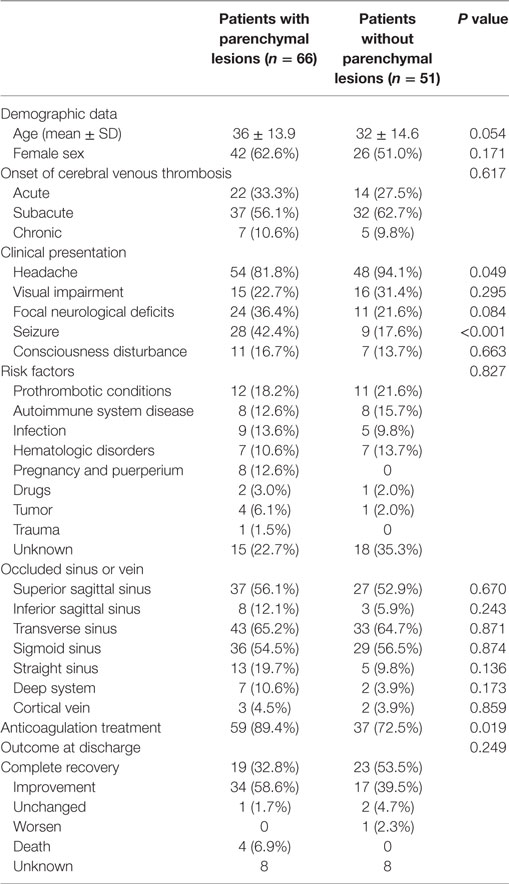
Table 1. The comparison of baseline data, clinical presentation, risk factors and radiological findings of patients with and without parenchymal lesions.
Intraluminal Thrombus in Sinuses or Veins on Structural Imaging
Intraluminal thrombus within the sinuses or veins was found on structural imaging in 28.2% (33/117) of the patients (Figure 3). Of these, intraluminal thrombus was detected by head CT in 6.0% (7/117), by brain MRI in 20.5% (24/117), and by both CT and MRI in 1.7% (2/117) based on the definition.
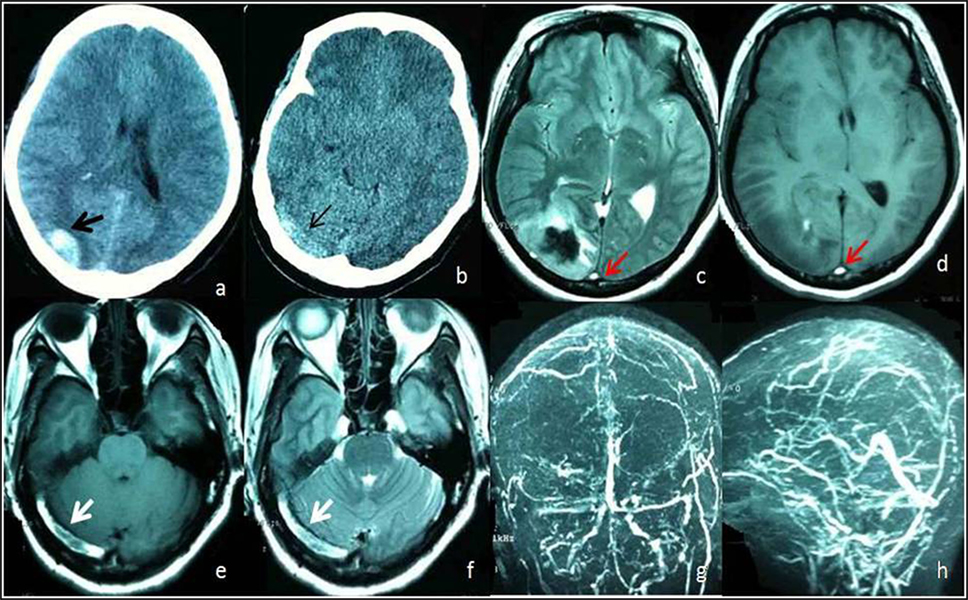
Figure 3. A 48-year female admitted to Emergency Room with headache and vomiting. (A) Head computed tomography (CT) shows hemorrhage on right occipital lobe (black arrow). (B) Unenhanced CT image shows areas of abnormal hyperattenuation consistent with thrombus in right transverse sinuses (thin arrow). Axial T1-weighted (D,E) and axial T2-weighted (C,F) MR images acquired 10 days after onset show an area of abnormal increased signal intensity in the superior sagittal sinus (red arrow) and right transverse sinus (white arrow) consistent with subacute thrombi of the superior sagittal sinus and right transverse sinus. Magnetic resonance venography (G,H) shows occlusion of the superior sagittal sinus and filling defects within the right transverse sinus (hollow arrow) due to thrombi.
In the present study, 13 patients have both parenchymal lesions and intraluminal thrombus on structural imaging. Compared to the patients (n = 31) with neither parenchymal lesions nor intraluminal thrombus, they had significantly more acute onset (P = 0.01) and presented more consciousness disturbance (P = 0.007). In-hospital mortality was higher (15.3% vs 0%).
Discussion
The present study is the first report of the structural imaging findings in a large Chinese CVT cohort. In the present series, the female predominance, age of onset, frequency of onset, and varied clinical manifestations are those classically reported in previous large series of CVT (7). Our finding on mortality at discharge was quite similar to the result of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT), in which mortality at discharge was only 4.3% (7).
Parenchymal Lesions on Structural Imaging
Parenchymal lesions were observed in 56.4% of a total of 117 patients on structural imaging, in line with the previous report (8). However, brain swelling was detected in only 4.3% of the patients in our cohort. Previous studies have reported that brain swelling may occur in as many as 42% of patients with CVT (9). A single center design and the ethnic difference might partly explain the relative low percentage of brain swelling observed in our cohort. An alternative explanation is that potential different parameters among patients would affect the diagnosis even all the imaging were re-evaluated. Finally, the duration between symptoms onset and performance of imaging might affect the visibility of brain swelling. Patients presented with only brain swelling but without parenchymal signal intensity changes tend to have intrasinus pressures in the intermediate range (20–25 mmHg); however, intrasinus pressures could also be markedly elevated (10). Such patients typically have more prominent clinical symptoms than expected based on the imaging findings. Thus, we suggest clinicians and radiologists to promptly identify the brain swelling in the patients with CVT.
In addition, we found that seizures occurred more frequently in patients with parenchymal lesions. Our finding was in line with that reported from the ISCVT, showing that 245 (39%) of 624 patients presented with seizures (11). The findings demonstrated that patients with parenchymal lesions have a higher risk of seizures and supported the prescription of antiepileptic agents in CVT patients with parenchymal lesions who present with the first attack of seizure. However, for those patients without seizure attack but with parenchymal lesions, whether the anticonvulsants should be used or not still remains controversial (11, 12). Further large-sample randomized studies are warrant to investigate the safety and effect of the prophylactic use of antiepileptic drugs on CVT patients with parenchymal lesions but without seizure attack.
Intraluminal Thrombus in Sinuses or Veins on Structural Imaging
In our cohort, only 28.2% (33/117) of the patients were detected with the direct signs of intraluminal thrombus in sinuses or veins on structural imaging. A few recent studies demonstrated that the combination of routine MR sequences had an overall sensitivity of 79%–83% on detecting intraluminal thrombus (5, 13). The lower prevalence of intraluminal thrombus on structural imaging in our study might be related to the variable interval between the onset of thrombus formation and the time of imaging. As we know, the magnetic resonance signal intensity of venous thrombus varies according to the time of imaging from the onset of thrombus formation. In addition, recognition of these direct signs might be challenged due to less knowledge of the different stages of thrombus evolution and pitfalls. Another reason might be that only a small part of patients in our center performed GRE sequences. As GRE sequences are increasingly used to detect the presence of blood breakdown products, their usefulness in depicting intraluminal thrombosis in CVT has been appreciated (14, 15).
In our study, patients with both intraluminal thrombus and parenchymal lesions on structural imaging had significantly more frequent seizures with consciousness disturbance and high percentage of in-hospital mortality, which suggest that these patients have a severe clinical picture and might lead to a devastating or fatal outcome. Thus, the clinicians should be highly alert the early signs of intraluminal thrombus on structural imaging and give the patients early diagnosis and prompt treatment in order to prevent the unfavorable outcome.
Our study had some limitations. First, the retrospective nature of the study resulted in a non-uniform method of neuroimaging studies. Second, given the study was carried out with patients from a single referral center, selected bias might have contributed to the inclusion of more acute-onset and severe cases in the study than would otherwise have been the case. Finally, patients were just followed until hospital discharge, and thus, there were inadequate data to compare the long-term clinical outcome.
In conclusion, intracranial lesions on structural imaging are frequently found in patients with CVT in our cohort. Seizures occurred more frequently in patients with parenchymal lesions. Patients with parenchymal lesions and intraluminal thrombus simultaneously on structural imaging tend to have a severe clinical picture and might lead to a devastating or fatal outcome. Structural imaging may be of help on early diagnosis and prediction of the outcome of CVT.
Author Contributions
L-xZ and JN contributed to the writing of the article. MY and Y-cZ contributed to the collection of data. M-lL contributed to the rating of images. L-yC and BP contributed to the revising of the article.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This study was supported by Capital Medical Development Research funding of China (No. 2016-1-40117)
References
1. Stam J. Thrombosis of the cerebral veins and sinuses. N Engl J Med (2005) 352:1791–8. doi:10.1056/NEJMra042354
2. Bousser MG, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol (2007) 6:162–70. doi:10.1016/S1474-4422(07)70029-7
3. Bousser MG, Russell R. Cerebral Venous Thrombosis: Major Problem in Neurology. Philadelphia, PA: WB Saunders (1997).
4. Idbaih A, Boukobza M, Crassard I, Porcher R, Bousser MG, Chabriat H. MRI of clot in cerebral venous thrombosis: high diagnostic value of susceptibility-weighted images. Stroke (2006) 37(4):991–5. doi:10.1161/01.STR.0000206282.85610.ae
5. Patel D, Machnowska M, Symons S, Yeung R, Fox AJ, Aviv RI, et al. Diagnostic performance of routine brain MRI sequences for dural venous sinus thrombosis. AJNR Am J Neuroradiol (2016) 37(11):2026–32. doi:10.3174/ajnr.A4843
6. Saposnik G, Barinagarrementeria F, Brown RD Jr, Bushnell CD, Cucchiara B, Cushman M, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke (2011) 42:1158–92. doi:10.1161/STR.0b013e31820a8364
7. Ferro JM, Canhao P, Stam J, Bousser MG, Barinagarrementeria F; ISCVT Investigators. Prognosis of cerebral vein and dural sinus thrombosis: results of the International study on cerebral vein and dural sinus thrombosis (ISCVT). Stroke (2004) 35:664–70. doi:10.1161/01.STR.0000117571.76197.26
8. Leach JL, Fortuna RB, Jones BV, Gaskill-Shipley MF. Imaging of cerebral venous thrombosis: current techniques, spectrum of findings, and diagnostic pitfalls. Radiographics (2006) 26:S19–41. doi:10.1148/rg.26si055174
9. Yuh WT, Simonson TM, Wang AM, Koci TM, Tali ET, Fisher DJ, et al. Venous sinus occlusive disease: MR findings. AJNR Am J Neuroradiol (1994) 15:309–16.
10. Tsai FY, Wang AM, Matovich VB, Lavin M, Berberian B, Simonson TM, et al. MR staging of acute dural sinus thrombosis: correlation with venous pressure measurements and implications for treatment and prognosis. AJNR Am J Neuroradiol (1995) 16:1021–9.
11. Ferro JM, Canhão P, Bousser MG, Stam J, Barinagarrementeria F; ISCVT Investigators. Early seizures in cerebral vein and dural sinus thrombosis: risk factors and role of antiepileptics. Stroke (2008) 39(4):1152–558. doi:10.1161/STROKEAHA.107.487363
12. Messe SR, Sansing LH, Cucchiara BL, Herman ST, Lyden PD, Kasner SE, et al. Prophylactic antiepileptic drug use is associated with poor outcome following ICH. Neurocrit Care (2009) 11:38–44. doi:10.1007/s12028-009-9207-y
13. Sari S, Verim S, Hamcan S, Battal B, Akgun V, Akgun H, et al. MRI diagnosis of dural sinus – cortical venous thrombosis: immediate post-contrast 3D GRE T1-weighted imaging versus unenhanced MR venography and conventional MR sequences. Clin Neurol Neurosurg (2015) 134:44–54. doi:10.1016/j.clineuro.2015.04.013
14. Selim M, Fink J, Linfante I, Kumar S, Schlaug G, Caplan LR. Diagnosis of cerebral venous thrombosis with echo-planar T2*-weighted magnetic resonance imaging. Arch Neurol (2002) 59:1021–6. doi:10.1001/archneur.59.6.1021
Keywords: cerebral venous thrombosis, imaging, parenchymal lesion, intraluminal thrombosis, magnetic resonance imaging
Citation: Zhou L-x, Yao M, Cui L-y, Li M-l, Zhu Y-c, Ni J and Peng B (2017) The Structural Imaging Characteristics and Its Clinical Relevance in Patients with Cerebral Venous Thrombosis—A Retrospective Analysis from One Single Center in China. Front. Neurol. 8:648. doi: 10.3389/fneur.2017.00648
Received: 18 May 2017; Accepted: 17 November 2017;
Published: 30 November 2017
Edited by:
Antonio Arauz, Instituto Nacional de Neurología y Neurocirugía, MexicoReviewed by:
Jose L. Ruiz Sandoval, Hospital Civil de Guadalajara, MexicoCarlos Cantu-Brito, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico
Copyright: © 2017 Zhou, Yao, Cui, Li, Zhu, Ni and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Ni, cHVtY2huaWp1bkAxNjMuY29t;
Bin Peng, cGVuZ2JpbjNAaG90bWFpbC5jb20=
 Li-xin Zhou
Li-xin Zhou Ming Yao1
Ming Yao1 Li-ying Cui
Li-ying Cui Yi-cheng Zhu
Yi-cheng Zhu Jun Ni
Jun Ni