- 1School of Public Health, Dalian Medical University, Dalian, China
- 2Department of Epidemiology and Health Statistics, School of Public Health, Ningxia Medical University, Ningxia, China
- 3Laboratory of Neurobiology, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC, United States
- 4Department of Cardiology, Institute of Cardiovascular Diseases, First Affiliated Hospital of Dalian Medical University, Dalian, China
Background: Hypertension has been associated with cognitive dysfunction in the general population and patients with Alzheimer’s disease (AD). However, there are contradictory data regarding the potential association between hypertension and diagnosis of Parkinson’s disease (PD), the second most common neurodegenerative disorder after AD. The purpose of this meta-analysis is to synthesize data from cohort studies to explore the potential association between preexisting hypertension and subsequent PD diagnosis.
Methods: The PubMed and Embase databases were searched to identify all relevant studies. Two independent investigators performed the data extraction. Eligible cohort studies providing risk and precision estimates related to hypertension and PD were selected. Pooled risk ratios (RRs) with 95% confidence interval (CI) were calculated by using a random-effects model or a fixed-effects model. Sensitivity analyses after excluding one study at a time were performed to assess the stability of the results. Publication bias was assessed with Begg’s test and Egger’s test.
Results: Seven cohort studies were identified, including 3,170 persons who were confirmed to have developed PD and 339,517 participants who did not have PD during follow-up. The onset of hypertension before PD diagnosis was significantly associated with an increased risk of motor stage PD (RR = 1.799, 95% CI [1.066–3.037]). This relationship was further confirmed by secondary analyses based on estimates adjusted for potential vascular confounders (RR = 1.319, 95% CI [1.073–1.622]). After excluding one study at a time, the sensitivity analyses still showed that hypertension history was significantly associated with an increased risk of motor stage PD (RR with 95% CI ranging from 1.11 [1.075–1.35] to 1.42 [1.65–1.83]). No publication bias was observed in this meta-analysis.
Conclusion: The findings of this meta-analysis suggest that hypertension may be a risk factor for motor stage PD, which may provide novel insights into the etiology and pathogenesis of this neurodegenerative disorder. However, large-scale well-designed studies that consider various confounders are still needed to further verify and clarify the association between hypertension and PD diagnosis.
Introduction
The incidence of Parkinson’s disease (PD), which is the second most common neurodegenerative disorder after Alzheimer’s disease (AD), rises rapidly with age and affects more than 1.7% of the population over 65 years of age (1). As populations age globally, the number of individuals with PD worldwide will increase more than double by 2030 (2). Traditionally, PD was considered a motor disorder characterized by the progressive loss of dopaminergic neurons in the substantia nigra pars compacta and the presence of Lewy bodies mainly composed of α-synuclein in remaining neurons (3). PD is now recognized as having a more complex phenotype and widespread α-synuclein pathology that starts in the myenteric plexus and/or the olfactory system and progresses upwardly in a prion-like fashion (4, 5). In addition to classic motor impairments (6), many non-motor symptoms, such as constipation, fatigue, sleep disorder, depression, apathy, and anxiety, are also identified in patients with PD (7, 8), and these symptoms occur even before the onset of motor deficits and greatly decrease the quality of life of patients (9). Current treatments for PD patients provide symptom relief of motor impairments but fail to improve the non-motor symptoms and the progressive neurodegenerative process (6, 10).
The etiology of PD is largely unknown, which greatly hampers the development of novel preventive or therapeutic strategies for combating this neurodegenerative disorder. PD is a multifactorial brain disorder, in which both genetic and environmental factors play important roles. The inherited forms of PD account for only 10–15% of all cases, and the majority of PD cases are likely due to different combinations of environmental exposures and genetic susceptibility (11). However, at present, the environmental risk factors for developing PD are incompletely known. Considering that typical PD neuropathology may be encountered in clinically healthy individuals (12), we assumed that certain environmental factors might be risk factors for the severity of the motor manifestation of PD.
Abnormalities in blood pressure (BP) due to autonomic dysfunction can occur even in the early stages of PD, often preceding the onset of the classic motor symptoms of PD (13). In addition to orthostatic and postprandial hypotension, PD patients also experience nocturnal and supine hypertension, which suggests that BP regulation is impaired in these patients (13, 14). According to Tsukamoto’s report, nocturnal hypertension exists in up to 64.9% of patients with PD (14). Accumulating evidence suggests that hypertension is associated with cognitive dysfunction in the general population and patients with AD (15–22). Since supine hypertension may be a sign of pre-motor PD (23), we hypothesized that preexisting hypertension might result in faster progression of nigral dopaminergic neurodegeneration and related motor symptoms. However, epidemiological studies on the association between hypertension and motor stage PD/PD diagnosis have generated inconclusive results to date, with some studies suggesting an association (24–27) and others not (28–30). To address the inconsistency of the relationship between hypertension and motor stage PD/PD diagnosis, a meta-analysis was conducted. Our study may enhance our understanding for the etiology of motor stage PD and therefore improve clinical practice in term of prevention for patients suffering from this neurodegenerative disorder.
Materials and Methods
Literature Search Strategy
Our meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement (31) and was written according to the Meta-analysis of Observational Studies in Epidemiology guidelines (32).
The PubMed and Embase databases were searched to identify all relevant literature. The following search strategy was used: (“Parkinson’s disease” OR “Parkinson disease”) AND (“hypertension” OR “high blood pressure” OR “high blood pressures”) in Title/abstract. The subjects of studies and the languages of articles were limited to humans and English, respectively. If more than one article was found that used the same data, we included the study with the largest sample size only. We undertook a database search from April 15 to May 1, 2017.
Inclusion Criteria
Studies meeting the following criteria were included in this meta-analysis: (1) evaluated the association between hypertension and PD risk; (2) had a cohort design; (3) had clearly stated diagnostic criteria for PD and hypertension; (4) the study population had no history of PD; and (5) the study reported at least a risk [relative risk (RR) or hazard ratio (HR)] with 95% confidence interval (CI) or provided sufficient data to calculate them.
Exclusion Criteria
Studies were excluded if they met the following criteria: (1) cohort studies that lack the systematic case identification or have bias in patient ascertainment (for PD); (2) did not provide RR/HR and RR/HR could not be calculated from the results; (3) studies without original data such as comments, letters, or reviews; and (4) case–control study design. The case–control study design was not included mainly due to the high burden of orthostatic hypertension observed in PD patients (33), which might mask the actual association between hypertension and PD diagnosis. The other reason was the selection bias among the case–control studies. A previous study showed that the use of antihypertensive agents, especially calcium channel blockers, is associated with a reduced incidence of PD in hypertensive patients (34). People with hypertension are more likely to seek health-care service than normotensive people, which may reduce the prevalence of PD in hypertensive populations.
Data Extraction
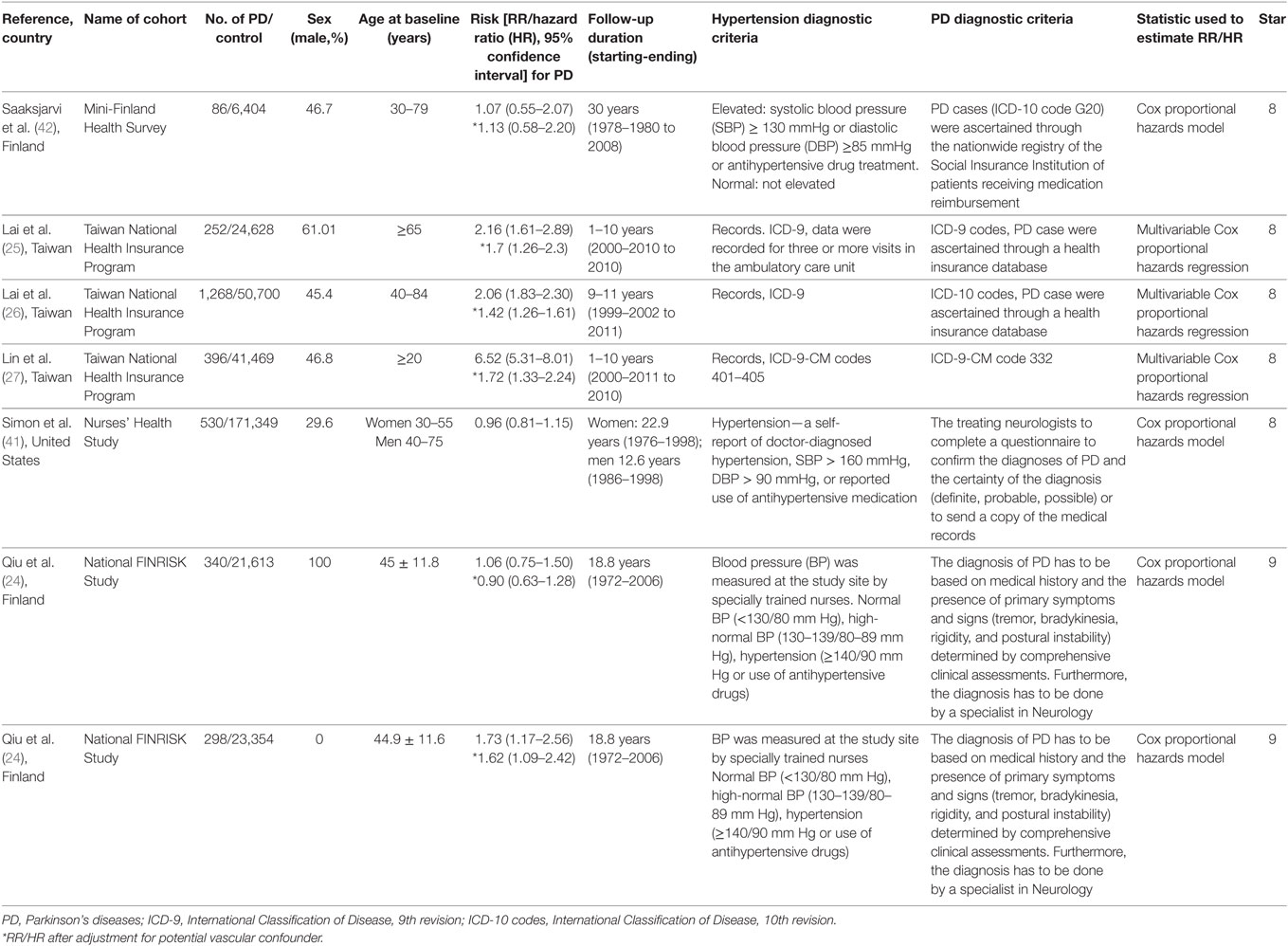
Two authors (Liyan Hou and Liping Jiang) independently assessed the studies according to the inclusion/exclusion criteria and any discrepancies were resolved by discussion with senior authors (Qingshan Wang and Huihua Li). The extracted information included the first author, publication year, geographical location, study design, number of participants, sex ratio, follow-up duration, PD and hypertension diagnostic criteria, risk estimates for PD and analytical methods. The Newcastle Ottawa scale was used to access the methodological quality of the studies (35) by two authors independently. The maximum score of 9 stars reflects the highest quality, 7–8 stars reflects medium quality, and ≤6 stars reflects low quality.
Data Analyses
Meta-analysis of the relationship between hypertension and risk of subsequent motor PD was conducted to calculate the pooled RR and corresponding 95% CI. The heterogeneity across studies was assessed using the Cochran’s Q and I2 statistics (36), and a value of I2 > 50% was considered to indicate significant heterogeneity (37, 38). In the presence of heterogeneity, pooled RR and corresponding 95% CI were calculated by using a random-effects model (39). Otherwise, the fixed-effects model was used. The significance of pooled RR was determined by a Z-test. Meta-regression analysis was used to assess the potentially important covariates that might exert a substantial impact on between-study heterogeneity (40). Sensitivity analyses after excluding one study at a time were performed to assess the stability of the results (40). Publication bias was assessed using Begg’s test and Egger’s test (37). All analyses were performed using the STATA 12 software. Statistical significance was considered to be P < 0.05.
Results
Literature Search and Characteristics of Selected Studies
The initial search identified a total of 1,126 records in which 510 studies were duplicates. Scanning the titles and abstracts resulted in the exclusion of 582 studies. After detailed assessment of the remaining 34 full-text studies, 28 studies were excluded since they did not provide RR (or HR) with 95% CI (5 studies), lacked a non-PD group (7 studies), lacked a hypertension-free group (1 study), or were case–control designs (15 studies). The excluded studies with the corresponding reasons for exclusion were presented in Figure 1. Since the data in one study were separated by sex, we analyzed the data separately and considered them to be separate studies (24). Therefore, a total of seven studies (24–27, 41, 42) were included in this meta-analysis. Based on NOS, all the studies were assigned a medium-high score (Table 1).
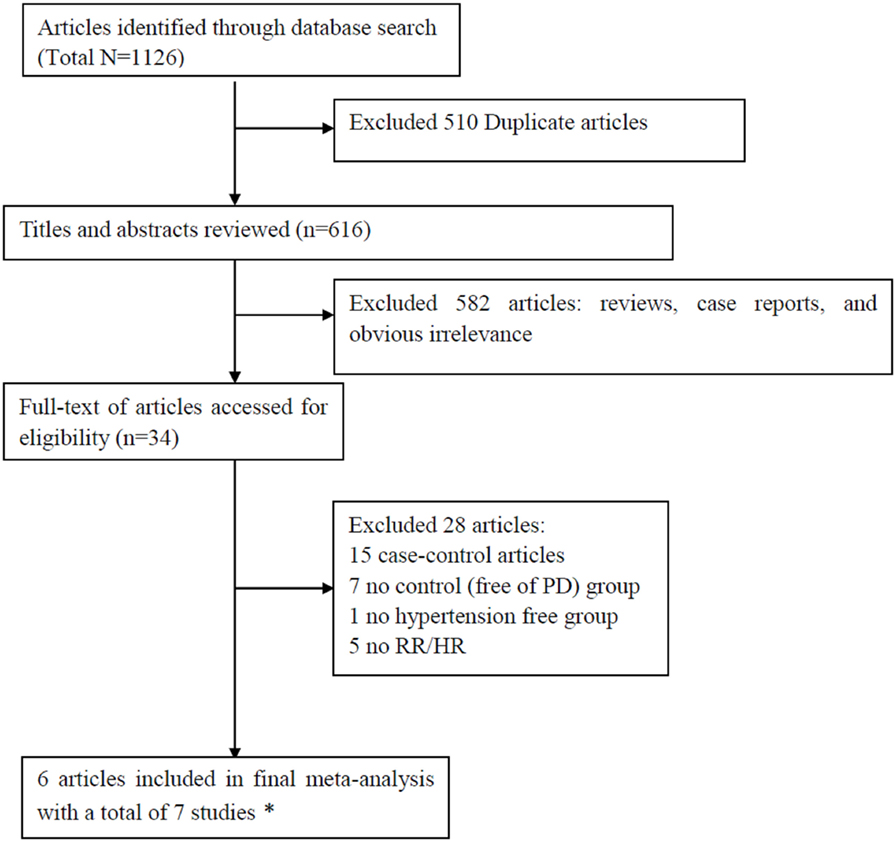
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-analyses flowchart of the literature search.
Hypertension and Risk of Subsequent Motor PD
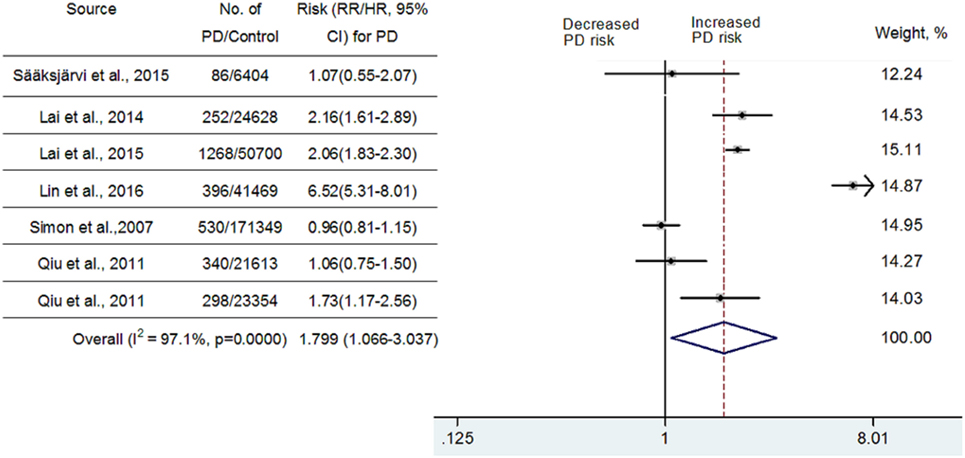
Seven cohort studies published between 2007 and 2015 included 3,170 persons who met the criteria for PD diagnosis and 339,517 participants who had not met the criteria for PD diagnosis during follow-up. Among them, four studies reported that hypertension is associated with an increased risk of PD (24–27), whereas the other three studies indicated that there was no significant association (24, 41, 42).
Because significant heterogeneity (heterogeneity P = 0.0000, I2 = 97.1%,) was observed, a random-effects model was chosen to calculate pooled RR and corresponding 95% CI. Pooled analysis based on adjusted estimates of seven cohort studies showed that hypertension history before the onset of PD was significantly associated with an increased risk of PD (RR = 1.799, 95% CI [1.066–3.037], Figure 2). Although three cohort studies that were included (25–27) came from Taiwan National Health Insurance, we verified with the author that these three studies were performed separately and contained a very limited number of overlapping individuals. Similarly, we also verified with the authors that two studies performed in Finland (24, 42) contain a very limited number of overlapped individuals. Since diabetes, hyperlipidemia, cerebrovascular disease, and coronary artery disease may contribute to vascular parkinsonism (43), a secondary analysis was carried out after adjustment for potential vascular confounders. As seen in Figure 3, the association remained significant (RR = 1.319, 95% CI [1.073–1.622]; P = 0.0000, I2 = 76.5%). In one study, researchers did not investigate whether vascular disorders could influence the combined estimates or not. The exclusion of this study still showed a significant association between hypertension and PD (RR = 1.429, 95% CI [1.199–1.704]; P = 0.0686, I2 = 52.8%, Figure 3). We conducted a subgroup analysis by ethnicity as well (Asian vs Caucasian). The results showed that hypertension was a risk factor for PD in an Asian population (RR = 1.523, 95% CI [1.338–1.735]) but not in a Caucasian population (RR = 1.083, 95% CI [0.84–1.396]).
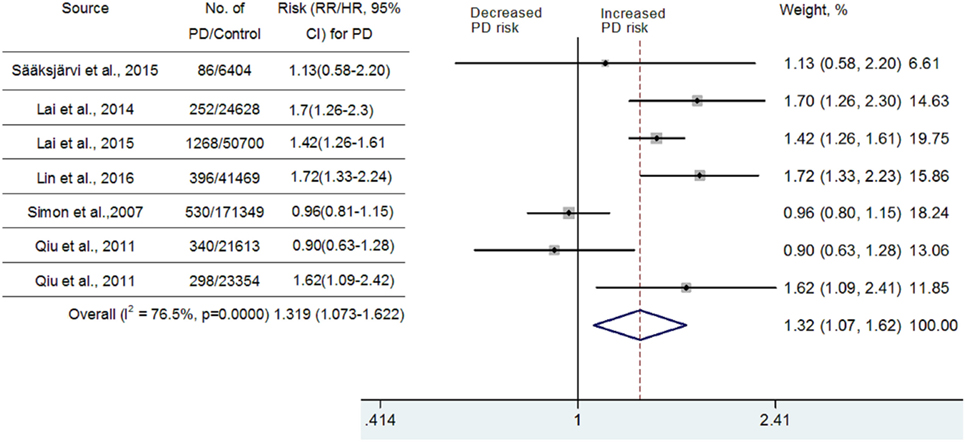
Figure 3. Meta-analysis of the association between hypertension and Parkinson’s disease (PD) risk after adjustment for the potential confounding vascular factors.
Substantial heterogeneity was noted in this meta-analysis. However, as seen in Figure 2, almost all of the seven included cohort studies showed a similar effect, although three of them showed no significant association, which suggested that the partial heterogeneity might come from the variations in the magnitude of the estimated risk instead of the direction. Seven studies that adjusted risk factors for confounders were mostly adjusted for different confounders. The results indicated that adjustment for different confounders was the main source of heterogeneity. In addition, the impact of heterogeneity (I2) was reduced 20.6% after adjustment for the possibility of confounding vascular factors. After exclusion of one study that did not investigate the effects of vascular disorders on the combined estimates, the impact of heterogeneity was further reduced 23.7%. Since the I2 (52.8%) was still slightly higher than 50%, meta-regression analysis was further used to explore the other sources of heterogeneity. However, all the variables, including publication year, number of PD diagnoses during follow-up, follow-up duration, and sex ratio, could not explain the source of heterogeneity.
Sensitivity Analyses and Publication Bias Evaluation
The sensitivity analyses performed by excluding one study at a time revealed that hypertension history before the diagnosis of PD was significantly associated with increased risk of PD. The values of RRs with 95% CIs ranged from 1.11 [1.075–1.35] to 1.42 [1.65–1.83].
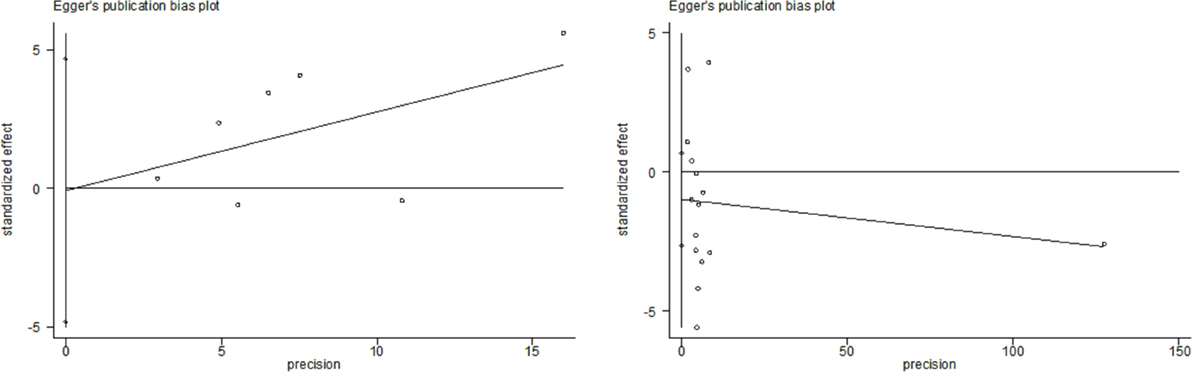
All studies included in this meta-analysis were considered high quality (Table 1). Publication bias was not detected in these studies (P = 0.548 and 0.790 in Begg’s test and Egger’s test, respectively) (Figure 4).
Discussion
To the best of our knowledge, this meta-analysis is the first that aimed to quantitatively investigate the association between hypertension and motor stage PD/PD diagnosis. Here, we found that preexisting hypertension may be a risk factor for PD diagnosis. Our conclusion should be more credible than that of individual studies since our study involved a total of 3,170 persons who met the criteria for PD diagnosis and 339,517 participants who had not met the criteria for PD diagnosis. Our conclusion was drawn based on the evidence derived from cohort studies. A major advantage of the cohort study is that the study participants completed the hypertension history survey before onset of PD. By contrast, the case–control study mainly depends on recall of the subjects (risk factors) and a real causal relationship between factors is difficult to infer. Therefore, the prospective cohort study design is usually preferred in etiologic epidemiology, although it is more expensive and time-consuming than case–control study design.
The subgroup analyses by ethnicity in this meta-analysis showed that hypertension was significantly associated with increased risk of PD diagnosis in the Asian population, but not in the Caucasian population. It is well documented that the prevalence of both PD and hypertension varies by ethnicity. Zhang and Roman (44) found that the prevalence of PD is lower in China, Japan, and Africa and higher among western industrialized nations, especially United States and Europe. Analysis of different populations living in Northern California also showed that the prevalence of PD is lower in Asians than Caucasians (45). In agreement with PD, a similar low prevalence of hypertension is also observed in Asians compared with Caucasians (46). The genetic differences along with differences in environmental factors exposed between Asians and Caucasians may have, at least partially, accounted for the different frequency of hypertension and PD between ethnicities (47, 48). However, the cause for the ethnic difference on association between hypertension and motor stage PD remains unclear. We suspected that some unknown confounding factors included in this meta-analysis might mask the relationship between hypertension and PD diagnosis in Caucasians. In addition, a possibly insufficient number of studies available for pooling might also be responsible for the ethnic difference on association between hypertension and PD diagnosis. Further clinical and large cohort studies are warranted in the future to confirm this ethnic difference.
Currently, the mechanisms for linking hypertension and motor stage PD have yet to be investigated. Since the pathogenesis of the selective dopaminergic neurodegeneration in the SN is obscure (49), the biological mechanisms linking hypertension to PD can only be speculated on. First, patients with hypertension display decreased resting cerebral blood flow, which may reduce the delivery of oxygen to ischemia-sensitive brain regions such as the SN, which results in dopaminergic neurodegeneration and subsequent motor deficits. Second, chronic high BP causes hypertensive neurovascular unit dysfunction in multiple brain regions, including the basal ganglia, which may result in dopaminergic neurodegeneration in the SN and then reduction of dopamine transmitters in the striatum (24, 50). Third, inflammation is a common mechanism that leads to both hypertension and PD. The inflammatory response that exists in hypertensive patients may contribute to neuroinflammation and related damage of dopaminergic neurons in the SN, leading to motor deficits.
Hypertension contributes to cognitive decline in both the general population and patients with AD. Previous studies suggested that increased BP is associated with increased brain atrophy, gray matter atrophy, and white matter injury (51, 52). Recently, a community-based cohort study showed that midlife systolic hypertension and persistence of systolic hypertension into late life are associated with an elevated risk of incident dementia (53). Similarly, Moonga et al. reported that hypertension is also associated with worse cognitive function in patients with AD (22). Similar to these reports, hypertension was recognized as a risk factor for motor stage PD in our study. The results of this meta-analysis underline once more the importance of early diagnosis and treatment of hypertension, which may be especially critical for those with preclinical or prodromal PD. Considering the results of the meta-analysis, monitoring supine hypertension should be considered, at least in those at risk for PD or fulfilling the 2015 Movement Disorders Society criteria for prodromal PD.
Limitations and Future Perspectives
Several potential limitations should be addressed for this meta-analysis. First, there were a limited number of eligible studies to obtain raw data regarding hypertension duration, levels of BP, and gender of PD patients for comparison. Our meta-analysis therefore could not provide detailed results on these issues. Second, substantial heterogeneity was observed in the meta-analysis. Although subgroup analysis revealed ethnicity as a potential reason for the heterogeneity, the interpretation of this finding should be cautious due to the small number of studies available for pooling. Meta-regression analysis for geographic locations, publication year, and number of PD individuals failed to reveal the source of heterogeneity, which suggests that other unknown confounders might be involved. Among the subjects, the use of antihypertensive drugs might be one of the important confounders. Nationwide Cohort Studies performed in Finland (24) and Taiwan (34) showed that the usage of antihypertensive drugs is associated with a reduced incidence of PD in hypertensive patients. Due to the limited studies included in this meta-analysis that evaluate the effects of antihypertensive drugs, the confounder of usage of antihypertensive drugs cannot be adjusted. Third, although the diagnostic criteria for PD among studies were consistent, the criteria for hypertension were not. The Nurses’ Health Study performed by Simon et al. (41) used a significantly different definition for hypertension (i.e., more than 160 mmHg instead of equal to or more than 130 mmHg in other studies). Fourth, we included studies published only in English. Eligible articles with other languages were not included in this meta-analysis. Finally, the cohort studies included in this meta-analysis were only performed in Asia, Finland, and United States and our findings might not be extended to populations of other ethnicities.
Further well-designed studies with consideration of different levels of hypertension are still required to verify and clarify the association between hypertension and motor stage PD/PD diagnosis. In addition, the possible relation between ethnicity, PD diagnosis, and hypertension may warrant further investigation due to the different conclusions derived from the subgroup analysis by ethnicity. Furthermore, experimental studies are also needed to explore the biological mechanisms linking hypertensive status to PD.
Conclusion
The results of the meta-analysis confirm and expand the already available evidence suggesting that hypertension may be a risk factor for motor stage PD/PD diagnosis, especially in Asian populations. This may provide novel insights into the etiology of this neurodegenerative disorder. However, more population-based prospective studies which consider various confounders (e.g., age, sex, hypertension duration, the use of antihypertensive drugs, other cardiovascular risk factors, caffeine, and alcohol intake) are still needed to clarify the association between hypertension and PD diagnosis. The results of the meta-analysis underline once more the importance of early diagnosis and correct treatment of hypertension, which may be especially challenging in those with preclinical and prodromal PD.
Author Contributions
LH and QW conceived the study and wrote the paper; QL and LJ performed the literature search and data extraction; HQ and CG analyzed and interpreted data; HL and J-SH revised the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was supported by “QiZhen” Talent Project of Dalian Medical University (No. 201122), Liaoning BaiQianWan Talents Program (No. [2017]90).
References
1. Di Giovanni G, Di Matteo V, Esposito E. Birth, life and death of dopaminergic neurons in the substantia nigra. J Neural Transm Suppl (2009) (73):1, preceeding table of contents.
2. Achey M, Aldred JL, Aljehani N, Bloem BR, Biglan KM, Chan P, et al. The past, present, and future of telemedicine for Parkinson’s disease. Mov Disord (2014) 29(7):871–83. doi:10.1002/mds.25903
3. Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson’s disease. Annu Rev Neurosci (1999) 22:123–44. doi:10.1146/annurev.neuro.22.1.123
4. Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging (2003) 24(2):197–211. doi:10.1016/S0197-4580(02)00065-9
5. Goedert M, Masuda-Suzukake M, Falcon B. Like prions: the propagation of aggregated tau and alpha-synuclein in neurodegeneration. Brain (2017) 140(2):266–78. doi:10.1093/brain/aww230
6. Dunnett SB, Bjorklund A. Prospects for new restorative and neuroprotective treatments in Parkinson’s disease. Nature (1999) 399(6738 Suppl):A32–9. doi:10.1038/399a032
7. Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol (2009) 8(5):464–74. doi:10.1016/S1474-4422(09)70068-7
8. Khoo TK, Yarnall AJ, Duncan GW, Coleman S, O’Brien JT, Brooks DJ, et al. The spectrum of nonmotor symptoms in early Parkinson disease. Neurology (2013) 80(3):276–81. doi:10.1212/WNL.0b013e31827deb74
9. Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP, et al. The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord (2009) 24(11):1641–9. doi:10.1002/mds.22643
10. Schapira AH. Neuroprotection in Parkinson’s disease. Parkinsonism Relat Disord (2009) 15(Suppl 4):S41–3. doi:10.1016/s1353-8020(09)70834-x
11. Fleming SM. Mechanisms of gene-environment interactions in Parkinson’s disease. Curr Environ Health Rep (2017) 4(2):192–9. doi:10.1007/s40572-017-0143-2
12. Fengler S, Liepelt-Scarfone I, Brockmann K, Schaffer E, Berg D, Kalbe E. Cognitive changes in prodromal Parkinson’s disease: a review. Mov Disord (2017) 32(12):1655–66. doi:10.1002/mds.27135
13. Asahina M, Vichayanrat E, Low DA, Iodice V, Mathias CJ. Autonomic dysfunction in parkinsonian disorders: assessment and pathophysiology. J Neurol Neurosurg Psychiatry (2013) 84(6):674–80. doi:10.1136/jnnp-2012-303135
14. Tsukamoto T, Kitano Y, Kuno S. Blood pressure fluctuation and hypertension in patients with Parkinson’s disease. Brain Behav (2013) 3(6):710–4. doi:10.1002/brb3.179
15. Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol (2006) 5(9):735–41. doi:10.1016/s1474-4422(06)70537-3
16. Anstey KJ, Lipnicki DM, Low LF. Cholesterol as a risk factor for dementia and cognitive decline: a systematic review of prospective studies with meta-analysis. Am J Geriatr Psychiatry (2008) 16(5):343–54. doi:10.1097/JGP.0b013e31816b72d4
17. Irie F, Fitzpatrick AL, Lopez OL, Kuller LH, Peila R, Newman AB, et al. Enhanced risk for Alzheimer disease in persons with type 2 diabetes and APOE epsilon4: the cardiovascular health study cognition study. Arch Neurol (2008) 65(1):89–93. doi:10.1001/archneurol.2007.29
18. Ho AJ, Raji CA, Becker JT, Lopez OL, Kuller LH, Hua X, et al. Obesity is linked with lower brain volume in 700 AD and MCI patients. Neurobiol Aging (2010) 31(8):1326–39. doi:10.1016/j.neurobiolaging.2010.04.006
19. Qiu C, Xu W, Fratiglioni L. Vascular and psychosocial factors in Alzheimer’s disease: epidemiological evidence toward intervention. J Alzheimers Dis (2010) 20(3):689–97. doi:10.3233/jad-2010-091663
20. Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol (2011) 10(9):819–28. doi:10.1016/S1474-4422(11)70072-2
21. Polidori MC, Pientka L, Mecocci P. A review of the major vascular risk factors related to Alzheimer’s disease. J Alzheimers Dis (2012) 32(3):521–30. doi:10.3233/JAD-2012-120871
22. Moonga I, Niccolini F, Wilson H, Pagano G, Politis M. Hypertension is associated with worse cognitive function and hippocampal hypometabolism in Alzheimer’s disease. Eur J Neurol (2017) 24(9):1173–82. doi:10.1111/ene.13374
23. Sharabi Y, Goldstein DS. Mechanisms of orthostatic hypotension and supine hypertension in Parkinson disease. J Neurol Sci (2011) 310(1–2):123–8. doi:10.1016/j.jns.2011.06.047
24. Qiu C, Hu G, Kivipelto M, Laatikainen T, Antikainen R, Fratiglioni L, et al. Association of blood pressure and hypertension with the risk of Parkinson disease: the National FINRISK Study. Hypertension (2011) 57(6):1094–100. doi:10.1161/HYPERTENSIONAHA.111.171249
25. Lai SW, Liao KF, Lin CL, Lin CC, Sung FC. Hearing loss may be a non-motor feature of Parkinson’s disease in older people in Taiwan. Eur J Neurol (2014) 21(5):752–7. doi:10.1111/ene.12378
26. Lai SW, Lin CL, Liao KF, Chang-Ou KC. Increased risk of Parkinson’s disease in cataract patients: a population-based cohort study. Parkinsonism Relat Disord (2015) 21(1):68–71. doi:10.1016/j.parkreldis.2014.11.005
27. Lin JC, Lin CS, Hsu CW, Lin CL, Kao CH. Association between Parkinson’s disease and inflammatory bowel disease: a nationwide Taiwanese retrospective cohort study. Inflamm Bowel Dis (2016) 22(5):1049–55. doi:10.1097/MIB.0000000000000735
28. McCann SJ, LeCouteur DG, Green AC, Brayne C, Johnson AG, Chan D, et al. The epidemiology of Parkinson’s disease in an Australian population. Neuroepidemiology (1998) 17(6):310–7. doi:10.1159/000026185
29. Miyake Y, Tanaka K, Fukushima W, Sasaki S, Kiyohara C, Tsuboi Y, et al. Case-control study of risk of Parkinson’s disease in relation to hypertension, hypercholesterolemia, and diabetes in Japan. J Neurol Sci (2010) 293(1–2):82–6. doi:10.1016/j.jns.2010.03.002
30. Cereda E, Cassani E, Barichella M, Spadafranca A, Caccialanza R, Bertoli S, et al. Low cardiometabolic risk in Parkinson’s disease is independent of nutritional status, body composition and fat distribution. Clin Nutr (2012) 31(5):699–704. doi:10.1016/j.clnu.2012.02.004
31. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol (2009) 62(10):e1–34. doi:10.1016/j.jclinepi.2009.06.006
32. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA (2000) 283(15):2008–12. doi:10.1001/jama.283.15.2008
33. Mazza A, Ravenni R, Antonini A, Casiglia E, Rubello D, Pauletto P. Arterial hypertension, a tricky side of Parkinson’s disease: physiopathology and therapeutic features. Neurol Sci (2013) 34(5):621–7. doi:10.1007/s10072-012-1251-2
34. Lee YC, Lin CH, Wu RM, Lin JW, Chang CH, Lai MS. Antihypertensive agents and risk of Parkinson’s disease: a nationwide cohort study. PLoS One (2014) 9(6):e98961. doi:10.1371/journal.pone.0098961
35. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi:10.1007/s10654-010-9491-z
36. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (2003) 327(7414):557–60. doi:10.1136/bmj.327.7414.557
37. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (1997) 315(7109):629–34. doi:10.1136/bmj.315.7109.629
38. Song F, Sheldon TA, Sutton AJ, Abrams KR, Jones DR. Methods for exploring heterogeneity in meta-analysis. Eval Health Prof (2001) 24(2):126–51. doi:10.1177/016327870102400203
39. DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials (2015) 45(Pt A):139–45. doi:10.1016/j.cct.2015.09.002
40. Higgins JP, Thompson SG. Controlling the risk of spurious findings from meta-regression. Stat Med (2004) 23(11):1663–82. doi:10.1002/sim.1752
41. Simon KC, Chen H, Schwarzschild M, Ascherio A. Hypertension, hypercholesterolemia, diabetes, and risk of Parkinson disease. Neurology (2007) 69(17):1688–95. doi:10.1212/01.wnl.0000271883.45010.8a
42. Saaksjarvi K, Knekt P, Mannisto S, Lyytinen J, Heliovaara M. Prospective study on the components of metabolic syndrome and the incidence of Parkinson’s disease. Parkinsonism Relat Disord (2015) 21(10):1148–55. doi:10.1016/j.parkreldis.2015.07.017
43. Korczyn AD. Vascular parkinsonism – characteristics, pathogenesis and treatment. Nat Rev Neurol (2015) 11(6):319–26. doi:10.1038/nrneurol.2015.61
44. Zhang ZX, Roman GC. Worldwide occurrence of Parkinson’s disease: an updated review. Neuroepidemiology (1993) 12(4):195–208. doi:10.1159/000110318
45. Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, et al. Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol (2003) 157(11):1015–22. doi:10.1093/aje/kwg068
46. Rodriguez F, Ferdinand KC. Hypertension in minority populations: new guidelines and emerging concepts. Adv Chronic Kidney Dis (2015) 22(2):145–53. doi:10.1053/j.ackd.2014.08.004
47. Chan DK, Cordato D, Bui T, Mellick G, Woo J. Comparison of environmental and genetic factors for Parkinson’s disease between Chinese and Caucasians. Neuroepidemiology (2004) 23(1–2):13–22. doi:10.1159/000073970
48. Minor DS, Wofford MR, Jones DW. Racial and ethnic differences in hypertension. Curr Atheroscler Rep (2008) 10(2):121–7. doi:10.1007/s11883-008-0018-y
49. Saeedi Saravi SS, Khoshbin K, Dehpour AR. Current insights into pathogenesis of Parkinson’s disease: approach to mevalonate pathway and protective role of statins. Biomed Pharmacother (2017) 90:724–30. doi:10.1016/j.biopha.2017.04.038
50. Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol (2009) 8(2):165–74. doi:10.1016/S1474-4422(09)70013-4
51. DeCarli C, Murphy DG, Tranh M, Grady CL, Haxby JV, Gillette JA, et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology (1995) 45(11):2077–84. doi:10.1212/WNL.45.11.2077
52. Swan GE, DeCarli C, Miller BL, Reed T, Wolf PA, Jack LM, et al. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology (1998) 51(4):986–93. doi:10.1212/WNL.51.4.986
Keywords: hypertension, Parkinson’s disease, cohort studies, meta-analysis, Parkinson’s disease diagnosis
Citation: Hou L, Li Q, Jiang L, Qiu H, Geng C, Hong J-S, Li H and Wang Q (2018) Hypertension and Diagnosis of Parkinson’s Disease: A Meta-Analysis of Cohort Studies. Front. Neurol. 9:162. doi: 10.3389/fneur.2018.00162
Received: 10 October 2017; Accepted: 05 March 2018;
Published: 19 March 2018
Edited by:
Maurizio A. Leone, Casa Sollievo della Sofferenza (IRCCS), ItalyReviewed by:
Radu Tanasescu, University of Nottingham, United KingdomKeryn Louise Taylor, University of Melbourne, Australia
Copyright: © 2018 Hou, Li, Jiang, Qiu, Geng, Hong, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huihua Li, aGhsaTE5MzVAYWxpeXVuLmNvbQ==;
Qingshan Wang, d2FuZ3E0QDEyNi5jb20=
 Liyan Hou1
Liyan Hou1 Qingshan Wang
Qingshan Wang