- 1Department of Radiology, Zhengzhou University People's Hospital and Henan Provincial People's Hospital, Zhengzhou, China
- 2Department of Radiology, Union Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 3School of Life Science and Technology, Xidian University, Xi'an, China
- 4Institute of Automation, Chinese Academy of Sciences, Beijing, China
- 5Huaxi MR Research Center, Department of Radiology, West China Hospital of Sichuan University, Chengdu, China
Background: There is accumulating evidence showing that patients with autism spectrum disorder (ASD) have obvious changes in resting-state functional brain activity. So far, there have been no meta-analyses of the resting-state brain activity alterations in patients with ASD. We attempted to explore the resting-state functional activity changes in patients with ASD, possibly providing a new perspective for investigating the pathophysiology of patients with ASD.
Methods: We screened relevant studies published before August 2017 in PubMed, Ovid, Web of Science, China National Knowledge Infrastructure (CNKI), and the Wan-fang database. Fifteen resting-state functional neural activity datasets (including 382 patients and 348 healthy controls) were included. Through the use of the effect-size signed differential mapping (ES-SDM) method, we carried out a meta-analysis of resting-state functional activity studies of patients with ASD.
Results: Compared with healthy controls, patients with ASD showed hyperactivity in the right supplementary motor area, middle frontal gyrus, inferior frontal gyrus, the left precentral gyrus, and the bilateral cerebellum hemispheric lobule (VIII/IX), and hypoactivity in the right middle temporal gyrus, superior temporal gyrus, and the left precuneus, posterior cingulate cortex, median cingulate cortex, and bilateral cerebellum (crus I).
Conclusion: This meta-analysis indicates that patients with ASD have significant and robust resting-state brain activity alterations in the language comprehension network, inferior-posterior cerebellum, default mode network (DMN), and cerebellar crus I. These brain regions may serve as specific regions of interest for further studies of ASD, which will allow us to further clarify the neurobiological mechanisms in patients with ASD.
Introduction
The main clinical symptoms of autism spectrum disorder (ASD) include impairments in social communication and interaction, as well as patterns of stereotypic and repetitive behaviors (1). Although the neuropathology of ASD is still not clear, there is a broad consensus that abnormal activity in related brain regions plays a very important role in the neurodevelopmental disorder of autism.
The resting state refers to the state during which a subject is not performing an explicit task. In addition, resting-state brain activity, also known as the default mode network, is observed through changes in blood flow in the brain that creates what is referred to as the blood oxygen level-dependent (BOLD) signal, which can be measured using functional magnetic resonance imaging (fMRI). Because brain activity is intrinsic, which is present even in the absence of an externally prompted task, any brain region will have spontaneous fluctuations in the BOLD signal (2–4). Resting-state neuroimaging, which monitors intrinsic brain activity, has been proven to be a popular and reliable research tool that can provide important insights into the pathophysiology of ASD (5). So far, multiple neuroimaging modalities, such as fMRI, single-photon emission computed tomography (SPECT), and positron emission tomography (PET), have been used to investigate brain activity alterations in patients with ASD. In the previous resting-state studies, participants were asked to close their eyes and not perform any explicit tasks during acquisition of the data. In general, fMRI depends on BOLD signal fluctuations to show patterns of brain activity. Roughly two analytical methods have been used to quantify fMRI resting-state brain activity, including regional homogeneity (ReHo) and the amplitude of low-frequency fluctuations (ALFF). In addition, ASL-fMRI/PET/SPECT monitors brain activity alterations by means of cerebral blood flow (CBF) measurements. The three different techniques, ReHo, ALFF, and CBF, reflect local spontaneous neuronal activity in a similar manner.
Many of the previous studies showed resting-state neuronal activity abnormalities in the cerebral cortex and limbic regions, mainly including the thalamus, inferior frontal gyrus, right superior temporal gyrus, medial frontal gyrus, middle/posterior cingulate cortex, and cerebellum, in patients with ASD (6, 7). However, because of the interstudy differences in the clinical symptoms of the participants, the sample sizes, and the technical methods used for the data acquisition and analysis, the detailed results were different. Along with the increasing number of published studies of resting-state neuronal activity in patients with ASD, we attempted to carry out a meta-analysis to identify common abnormalities.
As a new meta-analytic technique, ES-SDM is a method based on the use of voxel coordinates to identify resting-state brain activity abnormalities in the whole brain rather than within discrete brain regions that have been widely applied to neuroimaging studies of several diseases, such as depression (8, 9) and migraine(10). However, there have been no meta-analyses of resting-state neuronal activity studies in patients with ASD using ES-SDM. Therefore, we tried to conduct a meta-analysis of resting-state brain activity studies to provide a path to understand the pathological underpinnings of patients with ASD.
Materials and Methods
Study Selection
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (11). We performed systematic and comprehensive searches in PubMed, Ovid, Web of Science, China National Knowledge Infrastructure (CNKI), and the Wan-fang database. Correlative studies were published from January 1985 to August 2017. The search keywords were as follows: (1) “autism” < OR > “Asperger” < OR > “ASD”; (2) “rest” < OR > “resting state”; and (3) ReHo < or > CBF < or > ALFF < or > ASL < or > PET < or > SPECT < or > amplitude of low-frequency fluctuations < or > regional homogeneity < or > cerebral blood flow < or > positron emission tomography < or > single-photon emission computed tomography < or > arterial spin labeling < or > neuroimaging.
We selected the included studies according to the following inclusion criteria: (1) autistic disorder or Asperger's disorder was diagnosed on the basis of the Autism Diagnostic Observation Schedule (ADOS), the Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV), or the Autism Diagnostic Interview-Revised (ADI-R) diagnostic criteria; (2) comparisons of patients with ASD with healthy controls over the whole brain; (3) original studies published in English or Chinese; (4) the whole-brain results shown in three-dimensional coordinates (x, y, and z) in the standard stereotactic space (Talairach or MNI); and (5) use of the ReHo or CBF or ALFF method at the whole-brain level. Studies were excluded according to the following exclusion criteria: (1) any other meta-analytical studies or literature reviews; (2) sufficient data for the meta-analysis were unavailable from the original study or after contacting the authors; (3) studies using the seed voxel-based analysis method or using region-of-interest (ROI)-based methods.
Quality Assessment
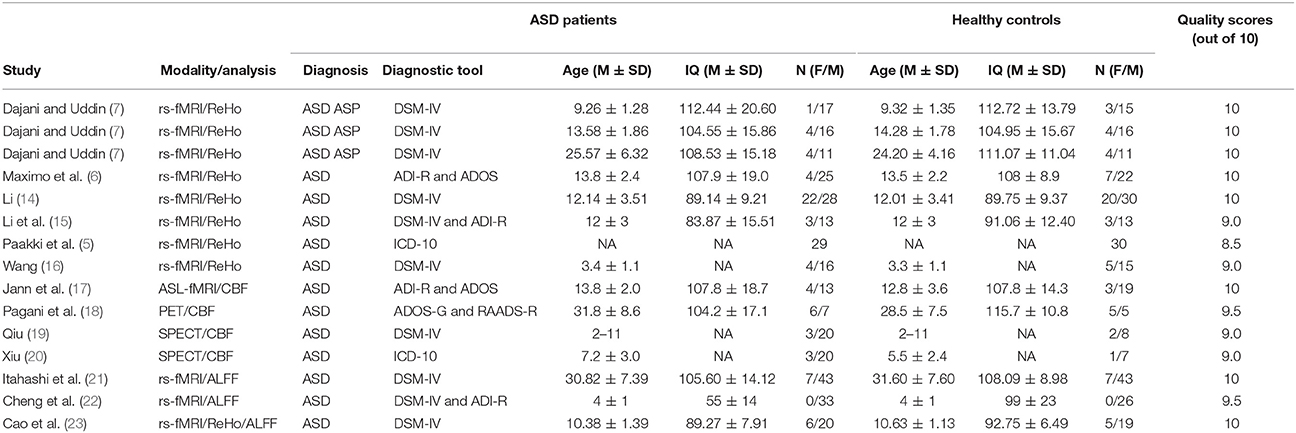
The quality of the included studies was assessed by a 10-point checklist that focused not only on methods for image acquisition and analysis but also on the demographic and clinical characteristics of the study participants. The checklist was based on previous meta-analytic studies (12, 13). The assessment details mainly included the diagnostic criteria applied, the sample size, the demographic and clinical characteristics, the imaging technique, the quality of the reported results, and the analytical technique (see Appendix, Table S1). Although the checklist may need to be improved, it still provided some objective indicators for the rigor of each study. The checklist requires that no fewer than two authors independently reviewed each paper and completed a full-scale rating. If the 2 ratings were inconsistent, after discussing the scores, a unified quality score was obtained. The final quality scores of the studies are shown in Table 1.
Voxelwise Meta-Analysis
Meta-analytical group differences in resting-state functional brain activity between patients and controls were assessed using ES-SDM, a voxel-based meta-analytic approach (http://www.sdmproject.com/software). The SDM methods have been described in detail elsewhere (24–26) and are only described briefly in this study. First, we extracted the peak coordinates and effect sizes (e.g., t-values or z-scores) of all functional brain activity differences from each included study, which were statistically significant at the whole-brain level. Importantly, to avoid potential bias toward liberally thresholded regions, we ensured that each included study used the same statistical threshold. When only z-scores were reported in a study, an online converter (www.sdmproject.com/utilities/?show=Statistics) was used to convert the z-scores to t-values. Second, we used the peak coordinates and effect sizes of the group differences to recreate a standard Talairach map of the differences in ReHo, CBF, or ALFF for each study by means of a Gaussian kernel that assigned higher effect sizes to the voxels closer to the peaks. In the assignment, a relatively full-width at half-maximum (FWHM) was set at 20 mm to control for false-positive results (26). Unlike previous meta-analytic methods, such as the activation likelihood estimation (27) and multilevel kernel density analysis (28), both positive and negative coordinates were reconstructed in the same map, which prevented particular voxels from erroneously appearing to be positive and negative at the same time (24). Third, the mean map was obtained via voxelwise calculation of the random-effects mean of the study maps, weighted by the squared root of the sample size of each study, so that studies with large sample sizes contributed more. Finally, using standard randomization tests, we obtained statistical significance and then created null distributions from which the p values were obtained directly. The default ES-SDM kernel size and thresholds were used (full-width at half-maximum = 20 mm, voxel p = 0.005, peak height Z = 1, and cluster extent = 50 voxels) (26).
Sensitivity Analysis and Heterogeneity Analysis
We conducted a systematic voxel-based jackknife sensitivity analysis to test the robustness of the results from different studies at the whole-brain level. After discarding only one study each time, the rest of the studies were included to repeat the jackknife sensitivity analysis. Hence, the analysis was repeated 15 times. When a previously significant brain region was replicated in all or most of the included studies, the results were considered highly replicable and conclusive.
Meta-Regression of Confounding Biases
All the potentially confounding effects that may have influenced the analytic results, such as the mean age, gender ratios, and IQs of the patients and controls, were evaluated. We performed a simple linear regression of these factors to determine whether these factors influenced the meta-analytic resting-state brain activity results.
Results
Studies Included in the Meta-Analyses
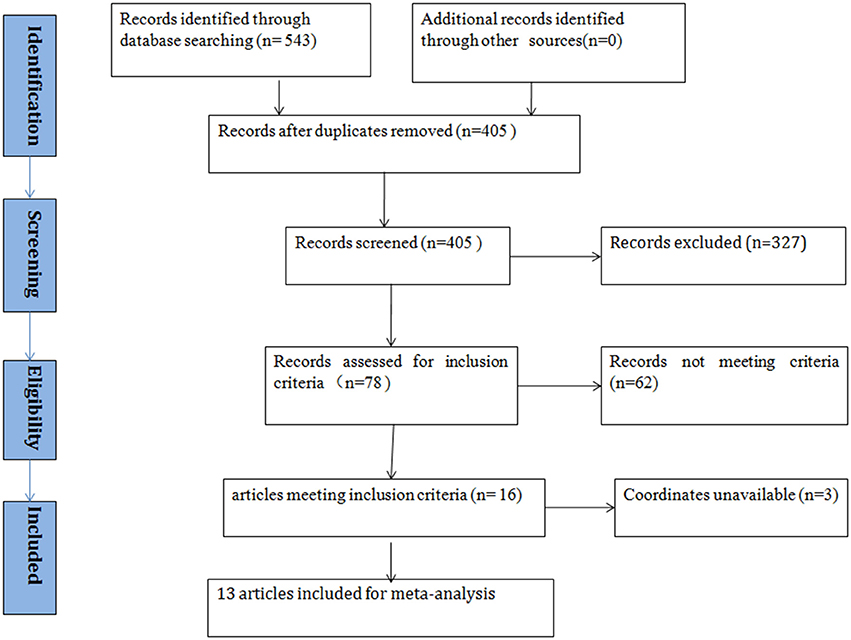
According to the search strategy, we found a total of 405 studies. Ultimately, 13 studies met the inclusion criteria and were included in the ASD vs. healthy controls (HCs) comparison analysis. There were no additional eligible studies found in the reference lists. Participants were further stratified into Child, Adolescent, and Adult groups in one study, which was therefore treated as three unique studies. Thus, a total of 15 datasets were used to conduct the meta-analysis. Seven of the selected studies were written in Chinese and were translated into English for assessment, and the others were written in English. A flow diagram of the screening process is shown in Figure 1. In addition, some basic information about the subjects is presented in Table 1. In each study, there were no significant differences in age or sex between the patients with ASD and the healthy controls. However, the mean IQ of the healthy control group was marginally significantly higher than that of the ASD group.
Meta-Analysis of Studies of ASD vs. HCs
In the pooled, whole-brain meta-analysis, compared with the HCs, resting-state activity was increased mainly in the right inferior frontal gyrus (IFG), middle frontal gyrus (MFG), supplementary motor area (SMA), left precentral gyrus, and bilateral cerebellum (hemispheric lobule IX, VIII) in patients with ASD. Compared with the healthy controls, the resting-state hypoactivity in the patients with ASD was mainly found in the right middle temporal gyrus (MTG), superior temporal gyrus (STG), left precuneus, posterior cingulate cortex (PCC), median cingulate cortex (MCC), and bilateral cerebellum (crus I) (Table 2, Figure 2).
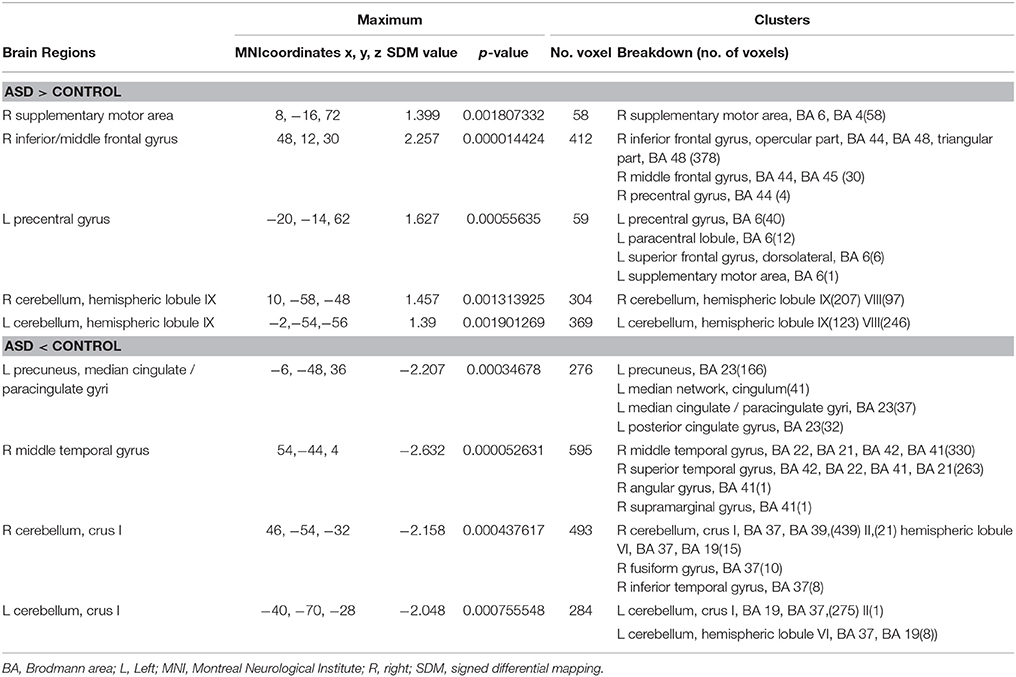
Table 2. Brain regions showing greater and less activity in patients with ASD and healthy controls in the pooled meta-analysis (voxel-wise p < 0.005 and full-width at half-maximum 20 mm).
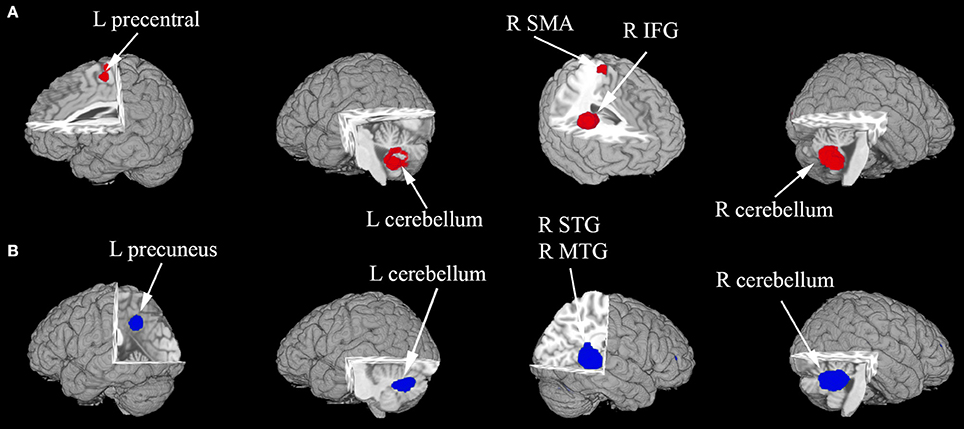
Figure 2. (A,B) The areas of decreased (blue) and increased (red) brain activity in patients with ASD compared with controls in the pooled meta-analysis. Altered resting-state brain activity in patients with ASD is displayed on a three-dimensionally rendered brain, with part of the left or right hemisphere removed. IFG, inferior frontal gyrus; L, left; MTG, middle temporal gyrus; R, right; SMA, supplementary motor area; STG, superior temporal gyrus.
Reliability Analysis
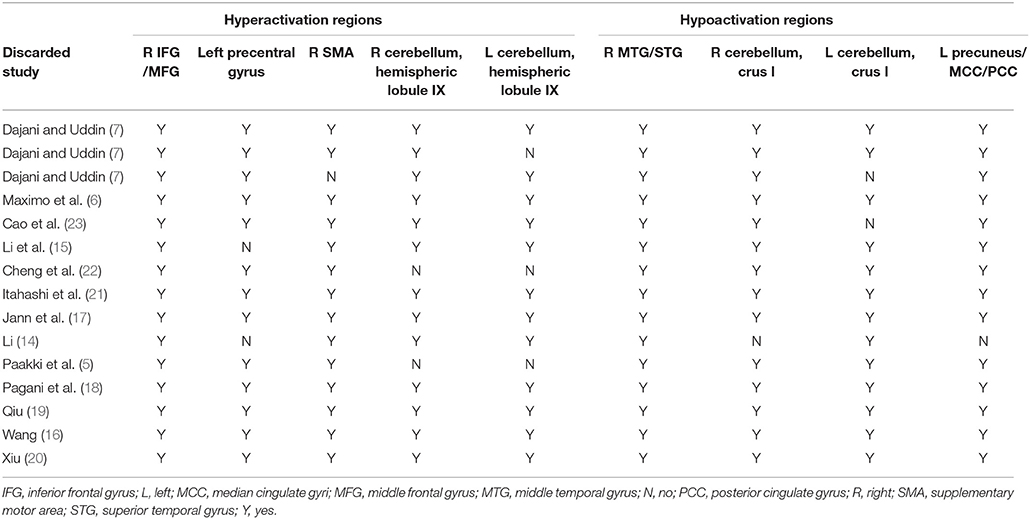
The whole-brain jackknife sensitivity analysis of the ASD vs. HCs (Table 3) showed that the resting-state hyperactivity in the right IFG was highly replicable, as this finding was replicated across all 15 combinations of the datasets. The resting-state hyperactivity in the right SMA was also significant in all but one of the datasets. The resting-state hyperactivity in the left precentral gyrus and right cerebellum (hemispheric lobules IX and VIII) was also significant in all but two of the datasets. The resting-state hyperactivity in the left cerebellum (hemispheric lobules IX and VIII) was also significant in all but three of the datasets. The resting-state hypoactivity in the right MTG was highly replicated across all 15 combinations of the datasets. The resting-state hypoactivity in the right cerebellum (crus I), left precuneus, PCC, and MCC was significant in all but one of the datasets. The resting-state hypoactivity in the left cerebellum (crus I) was also significant in all but two of the datasets (Table 3).
Meta-Regression
The mean age, sex ratio, and IQ were used as factors of influence to conduct a regression analysis. In the meta-regression analysis of the studies of ASD vs. HC, no effects of mean age, sex ratio, or IQ were detected.
Discussion
As far as we know, this whole-brain, voxelwise meta-analysis, which evaluated resting-state brain activity anomalies in patients with ASD compared with healthy controls, is original. Our results revealed that the most robust resting-state brain activity was increased in the language comprehension network (29, 30) and in the inferior-posterior cerebellum (31), which are composed of the frontal cortex and cerebellar hemispheric lobules (VIII/IX), respectively. Meanwhile, the most significant regions of hypoactivation were found in the default mode network (DMN) and cerebellar crus I. The findings were powerful and reliable according to the jackknife sensitivity analysis. There were no detectable effects of the mean age, sex ratio, or IQ on resting-state brain activity in patients with ASD.
In this meta-analysis, increased resting-state brain activity was mainly observed in the right SMA, MFG, IFG, left precentral gyrus, and cerebellar hemispheric lobules (VIII/IX). Because of the prominent role of the cerebellum in motor functioning, its linguistic functions have been overlooked for a very long time (32). Currently, it is thought that the cerebellum is actively involved in language, cognition, and affective modulation (33). Hodge et al.'s study supported that the posterior lateral cerebellum was highly linked to the frontal cortex, including Broca's area, which together form the fronto-cortico cerebellar language circuits, and the abnormal development of language circuits was related to cognitive function and language impairments (31). An increasing amount of neuroimaging research has focused on the language comprehension networks, which play a significant role in underlying language comprehension, such as semantic understanding and integration of information (34), understanding vocabulary in context (35), and flexible application of pragmatics and grammar (36). In healthy controls, the language comprehension network is left-cerebral hemisphere dominant (37, 38). However, individuals with ASD tend to use right-cerebral hemisphere regions (39, 40). Recently, more studies have used resting-state fMRI (rs-fMRI) to assess abnormal connectivity in the language comprehension neural networks in individuals with ASD (41, 42). Previous research has shown that the compensatory neural networks for language comprehension include the supplementary motor regions of the right hemisphere and left precentral gyrus (30). In line with the findings of anatomic abnormalities, previous studies on individuals with ASD have shown that partial cerebral regions in the language comprehension network overlapped with the regions of increased gray matter. For example, Ecker et al. (43) found that the gray matter (GM) volumes of the dorsolateral prefrontal regions were significantly increased in individuals with ASD. This observation of structural abnormalities is compatible with the findings of functional alterations in resting-state brain activity.
In our study, decreased resting-state brain activity was observed mainly in the left precuneus, PCC, MCC and right MTG, right STG, and bilateral cerebellum (crus I). These brain regions overlap with the components of the DMN (44), which greatly contribute to autism spectrum traits (45, 46). As one of the major resting-state brain networks, the DMN plays an important role in performing emotional and social processes, self-referential thought, and theory of mind; therefore, the DMN has become a focus of research (47). Raichle et al. found that the DMN was active during the resting state (44); on the contrary, performing cognitive tasks induces decreased activation (deactivation) in the DMN. In addition, Kay Jann et al. found decreased resting brain functional connectivity in the precuneus/posterior cingulate cortex areas of the DMN in children with ASD (17). It is possible that all of these functional abnormalities are due to structural abnormalities. Yang et al. (48) found reduced GM volume in the temporal gyrus/cerebellum crus I in patients with ASD. He et al. (49) performed one study combining ReHo and structural MRI that showed that patients with Alzheimer's disease (AD) had decreased ReHo in the PCC/precuneus and atrophy in the PCC/precuneus region. Perhaps, decreased resting-state brain activity in ASD is accompanied by reduced GM volume in the DMN. These changes in GM likely play significant roles in establishing interregional information processing in the brain (50). In addition, an increasing amount of research has shown that cerebellar abnormalities are associated with autism and contribute to different higher order cortical networks, including frontoparietal networks, as well as the DMN. Allin et al. (51) indicated that the cerebellar crus I has functional connectivity with the DMN. Furthermore, Halko et al.'s study (52) demonstrated that, by mediating the activity of default regions, crus I/II participates in the DMN. In line with this anatomical evidence (53), the Stereotyped Behaviors and Restricted Interests scores in the population with ASD were found to be correlated with GM differences in the cerebellar crus I/II. There may be a close association between altered resting-state brain activity in the above-mentioned brain regions and neuropathological mechanisms that lead to the clinical symptoms in ASD.
Limitations
The present meta-analytical study had some limitations. First, the study needed larger samples to increase the power of the analysis. Second, the accuracy of our voxelwise meta-analysis may have been limited because the accuracy was not derived from an original study rooted in raw statistical image but instead from published studies. Third, it is worth noting that different ages would cause different results. During the natural progression of autism, there are developmental changes in anatomy, and in cognitive function, and these changes may affect resting-state brain activity. Fourth, our study included both patients with autism and patients with Asperger's syndrome; however, different subtypes of the classical autistic spectrum may have different etiologies, which may have influenced the results of our findings. However, there were an insufficient number of studies to carry out these subgroup analyses. Finally, common artifacts, such as head motion and breathing effects, may have influenced the acquisition of neuroimaging data.
Conclusion
Despite the limitations mentioned above, our voxelwise meta-analysis found decreased resting-state brain activity in the left precuneus, PCC and MCC, and in the right MTG, right STG, and bilateral cerebellar crus I, as well as increased resting-state brain activity in the right SMA, MFG, and IFG, the left precentral gyrus, and the bilateral cerebellum hemispheric lobule (VIII/IX) in patients with ASD by using the ES-SDM method. However, the meta-regression analysis suggested that the mean age, sex ratio, and IQ had no effects on resting-state brain activity, and further studies with larger samples and more accurate assessments of the ASD stages are necessary. There may be a close association between altered resting-state brain activity and impaired cognitive and affective function in these brain regions. These resting-state brain activity alterations, combined with demographic characteristics, may help us understand the neuropathological changes in the population with ASD.
Author Contributions
MW contributed to the conception of the study. WW, JL, TL, LM, XM, and SS contributed significantly to the analysis and manuscript preparation. WW and JL performed the data analyses and wrote the manuscript. JT and QG contributed to the interpretation and discussion of the results of the analyses.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported by the National Natural Science Foundation (No. 31470047, 81271565) and the Henan Province Scientific and Technological Innovation Talents Project.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2018.00556/full#supplementary-material
References
1. American Psychiatric Association. Desk Reference to the Diagnostic Criteria from DSM-5. Washington, DC, American Psychiatric Publishing (2013).
2. Rosazza C, Minati L. Resting-state brain networks: literature review and clinical applications. Neurol Sci. (2011) 32:773–85. doi: 10.1007/s10072-011-0636-y
3. Biswal BB. Resting state fMRI: a personal history. Neuroimage (2012) 62:938–44. doi: 10.1016/j.neuroimage.2012.01.090
4. Buckner RL, Krienen FM, Thomas Yeo BT. Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci. (2013) 16:832–7. doi: 10.1038/nn.3423
5. Paakki J-J, Rahko J, Long X, Moilanen I, Tervonen O, Nikkinen J, et al. Alterations in regional homogeneity of resting-state brain activity in autism spectrum disorders. Brain Res. 1321:169–79. doi: 10.1016/j.brainres.2009.12.081
6. Maximo JO, Keown CL, Nair A, Müller R-A. (2013). Approaches to local connectivity in autism using resting state functional connectivity MRI. Front Hum Neurosci. (2010) 7:605. doi: 10.3389/fnhum.2013.00605
7. Dajani DR, Uddin LQ. Local brain connectivity across development in autism spectrum disorder: a cross-sectional investigation. Autism Res. (2016) 9:43–54. doi: 10.1002/aur.1494
8. Du M, Liu J, Chen Z, Huang X, Li J, Kuang W, et al. Brain grey matter volume alterations in late-life depression. J Psychiatry Neurosci. (2014) 39:397–406. doi: 10.1503/jpn.130275
9. Zhao YJ, Du MY, Huang XQ, Lui S, Chen ZQ, Liu J, et al. Brain grey matter abnormalities in medication-free patients with major depressive disorder: a meta-analysis. Psychol Med. (2014) 44:2927–37. doi: 10.1017/S0033291714000518
10. Dai Z, Zhong J, Xiao P, Zhu Y, Chen F, Pan P, et al. Gray matter correlates of migraine and gender effect: a meta-analysis of voxel-based morphometry studies. Neuroscience (2015) 299:88–96. doi: 10.1016/j.neuroscience.2015.04.066
11. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
12. Brambilla P, Hardan A, di Nemi SU, Perez J, Soares JC, Barale F. Brain anatomy and development in autism: review of structural MRI studies. Brain Res Bull. (2003) 61:557–69. doi: 10.1016/j.brainresbull.2003.06.001
13. Shepherd AM, Matheson SL, Laurens KR, Carr VJ, Green MJ. Systematic meta-analysis of insula volume in schizophrenia. Biol Psychiatry (2012) 72:775–84. doi: 10.1016/j.biopsych.2012.04.020
14. Li JF. The application value of magnetic resonance imaging in autism. Mater Child World. (2017) 8:5–7.
15. Li X, Liu J, Yang W, et al. A resting-state functional magnetic resonance imaging study of brain activity in children with autism. Chin J Psychiatry (2013) 46:137–41.
16. Wang LL. A Study on Regional Homogeneity of Resting-State Brain Activity in Preschool Children with Autism Spectrum Disorder. Master thesis. Chong Qing: Chongqing Medical University (2016).
17. Jann K, Hernandez LM, Beck-Pancer D, McCarron R, Smith RX, Dapretto M, et al. Altered resting perfusion and functional connectivity of default mode network in youth with autism spectrum disorder. Brain Behav. (2015) 5:e00358. doi: 10.1002/brb3.358
18. Pagani M, Manouilenko I, Stone-Elander S, Odh R, Salmaso D, Hatherly R, et al. Brief report: alterations in cerebral blood flow as assessed by PET/CT in adults with autism spectrum disorder with normal IQ. J Autism Dev Disord. (2012) 42:313–8. doi: 10.1007/s10803-011-1240-y
19. Qiu XY. Research on Brain Function of Childhood Autism. Master thesis. Guang Dong: Sun Yat-sen University (2009).
20. Xiu LJ. Regional Cerebral Blood Flow in Children with Autism Spectrum Disorder a SPECT Study. Master thesis. Guang Dong: Sun Yat-sen University (2010).
21. Itahashi T, Yamada T, Watanabe H, Nakamura M, Ohta H, Kanai C, et al. Alterations of local spontaneous brain activity and connectivity in adults with high-functioning autism spectrum disorder. Mol Autism (2015) 6:30. doi: 10.1186/s13229-015-0026-z
22. Cheng H, Gong GL, Peng Y, et al. Abnormalities of spontaneous neuronal activity low-frequency fluctuation of typical autistic children: a functional magnetic resonance imaging study. Chin Mental Health J. (2016) 30:441–7.
23. Cao XH, Zhang AX, Liu ZF, et al. A resting-state fMRI study in adolescents with autism spectrum disorder. Chin J Clin. (2016) 10:325–30.
24. Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry (2009) 195:393–402. doi: 10.1192/bjp.bp.108.055046
25. Radua J, Via E, Catani M, Mataix-Cols D. Voxel-based meta-analysis of regional white-matter volume differences in autism spectrum disorder versus healthy controls. Psychol Med. (2011) 41:1539–50. doi: 10.1017/S0033291710002187
26. Radua J, Mataix-Cols D, Phillips ML, El-Hage W, Kronhaus DM, Cardoner N, et al. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur Psychiatry (2012) 27:605–11. doi: 10.1016/j.eurpsy.2011.04.001
27. Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, et al. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. (2005) 25:155–64. doi: 10.1002/hbm.20136
28. Wager TD, Lindquist MA, Nichols TE, Kober H, Van Snellenberg JX. Evaluating the consistency and specificity of neuroimaging data using meta-analysis. Neuroimage (2009) 45(1 Suppl.):S210–21. doi: 10.1016/j.neuroimage.2008.10.061
29. Murdaugh DL, Maximo JO, Kana RK. Changes in intrinsic connectivity of the brain's reading network following intervention in children with autism. Hum Brain Mapp. (2015) 36:2965–79. doi: 10.1002/hbm.22821
30. Eigsti IM, Stevens MC, Schultz RT, Barton M, Kelley E, Naigles L, et al. Language comprehension and brain function in individuals with an optimal outcome from autism. Neuroimage Clin. 10:182–91. doi: 10.1016/j.nicl.2015.11.014
31. Hodge SM, Makris N, Kennedy DN, Caviness VS Jr, Howard J, McGrath L, et al. Cerebellum, language, and cognition in autism and specific language impairment. J Autism Dev Disord. (2010) 40:300–16. doi: 10.1007/s10803-009-0872-7
32. Beaton A, Marien P. Language, cognition and the cerebellum: grappling with an enigma. Cortex (2010) 46:811–20. doi: 10.1016/j.cortex.2010.02.005
33. Murdoch BE. The cerebellum and language: historical perspective and review. Cortex (2010) 46:858–68. doi: 10.1016/j.cortex.2009.07.018
34. Groen WB, Tesink C, Petersson KM, van Berkum J, van der Gaag RJ, Hagoort P, et al. Semantic, factual, and social language comprehension in adolescents with autism: an FMRI study. Cereb Cortex (2010) 20:1937–45. doi: 10.1093/cercor/bhp264
35. Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain (2004) 127(Pt 8): 1811–21. doi: 10.1093/brain/awh199
36. Groen WB, Zwiers MP, van der Gaag RJ, Buitelaar JK. The phenotype and neural correlates of language in autism: an integrative review. Neurosci Biobehav Rev. (2008) 32:1416–25. doi: 10.1016/j.neubiorev.2008.05.008
37. Turken AU, Dronkers NF. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Front Syst Neurosci. (2011) 5:1. doi: 10.3389/fnsys.2011.00001
38. Tomasi D, Volkow ND. Resting functional connectivity of language networks: characterization and reproducibility. Mol Psychiatry (2012) 17:841–54. doi: 10.1038/mp.2011.177
39. Mason RA, Williams DL, Kana RK, Minshew N, Just MA. Theory of Mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia (2008) 46:269–80. doi: 10.1016/j.neuropsychologia.2007.07.018
40. Kana RK, Wadsworth HM. The archeologist's career ended in ruins: hemispheric differences in pun comprehension in autism. Neuroimage (2012) 62:77–86. doi: 10.1016/j.neuroimage.2012.04.034
41. Koyama MS, Di Martino A, Zuo XN, Kelly C, Mennes M, Jutagir DR, et al. Resting-state functional connectivity indexes reading competence in children and adults. J Neurosci. (2011) 31:8617–24. doi: 10.1523/JNEUROSCI.4865-10.2011
42. Verly M, Verhoeven J, Zink I, Mantini D, Peeters R, Deprez S, et al. Altered functional connectivity of the language network in ASD: role of classical language areas and cerebellum. Neuroimage Clin. (2014) 4:374–82. doi: 10.1016/j.nicl.2014.01.008
43. Ecker C, Suckling J, Deoni SC, Lombardo MV, Bullmore ET, Baron-Cohen S, et al. Brain anatomy and its relationship to behavior in adults with autism spectrum disorder: a multicenter magnetic resonance imaging study. Arch Gen Psychiatry (2012) 69:195–209. doi: 10.1001/archgenpsychiatry.2011.1251
44. Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL, et al. A default mode of brain function. Proc Natl Acad Sci USA. (2001) 98:676–82. doi: 10.1073/pnas.98.2.676
45. Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain's default network. Neuron (2010) 65:550–62. doi: 10.1016/j.neuron.2010.02.005
46. Rudie JD, Hernandez LM, Brown JA, Beck-Pancer D, Colich NL, Gorrindo P, et al. Autism-associated promoter variant in MET impacts functional and structural brain networks. Neuron (2012) 75:904–15. doi: 10.1016/j.neuron.2012.07.010
47. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. (2008) 1124:1–38. doi: 10.1196/annals.1440.011
48. Yang X, Si T, Gong Q, Qiu L, Jia Z, Zhou M, et al. Brain gray matter alterations and associated demographic profiles in adults with autism spectrum disorder: a meta-analysis of voxel-based morphometry studies. Aust N Z J Psychiatry (2016) 50:741–53. doi: 10.1177/0004867415623858
49. He Y, Wang L, Zang Y, Tian L, Zhang X, Li K, et al. Regional coherence changes in the early stages of Alzheimer's disease: a combined structural and resting-state functional MRI study. Neuroimage (2007) 35:488–500. doi: 10.1016/j.neuroimage.2006.11.042
50. Hagmann P, Sporns O, Madan N, Cammoun L, Pienaar R, Wedeen VJ, et al. White matter maturation reshapes structural connectivity in the late developing human brain. Proc Natl Acad Sci USA. (2010) 107:19067–72. doi: 10.1073/pnas.1009073107
51. Allin MP. Novel insights from quantitative imaging of the developing cerebellum. Semin Fetal Neonatal Med. (2016) 21:333-8. doi: 10.1016/j.siny.2016.06.003
52. Halko MA, Farzan F, Eldaief MC, Schmahmann JD, Pascual-Leone A. Intermittent theta-burst stimulation of the lateral cerebellum increases functional connectivity of the default network. J Neurosci. (2014) 34:12049–56. doi: 10.1523/JNEUROSCI.1776-14.2014
Keywords: autism spectrum disorder, meta-analysis, regional homogeneity, functional neuroimaging, resting state
Citation: Wang W, Liu J, Shi S, Liu T, Ma L, Ma X, Tian J, Gong Q and Wang M (2018) Altered Resting-State Functional Activity in Patients With Autism Spectrum Disorder: A Quantitative Meta-Analysis. Front. Neurol. 9:556. doi: 10.3389/fneur.2018.00556
Received: 10 May 2017; Accepted: 20 June 2018;
Published: 24 July 2018.
Edited by:
Maria Assunta Rocca, San Raffaele Hospital (IRCCS), ItalyReviewed by:
Martin Gorges, Universität Ulm, GermanyDomenico De Berardis, Azienda Usl Teramo, Italy
Copyright © 2018 Wang, Liu, Shi, Liu, Ma, Ma, Tian, Gong and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meiyun Wang, bXl3YW5nQGhhLmVkdS5jbg==
†These authors have contributed equally to this work.
 Wenhui Wang
Wenhui Wang Jia Liu
Jia Liu Shaojie Shi
Shaojie Shi Taiyuan Liu
Taiyuan Liu Lun Ma
Lun Ma Xiaoyue Ma
Xiaoyue Ma Jie Tian3,4
Jie Tian3,4 Qiyong Gong
Qiyong Gong