- 1Department of Medical and Surgical Science, School of Medicine, University of Catanzaro, Catanzaro, Italy
- 2Department of Pharmacy Health and Nutritional Sciences, University of Calabria, Rende, Italy
- 3Department of Biological Sciences, Centre for Neuroscience, Xi'an Jiaotong-Liverpool University, Suzhou, China
- 4Department of Law, Ecenomy and Sociology, University of Catanzaro, Catanzaro, Italy
- 5Section of Child and Adolescent Neuropsychiatry, Department of Human Neuroscience, “Sapienza” University, Rome, Italy
- 6Clinical Pharmacology and Pharmacovigilance Operative Unit, Department of Health Science, University of Catanzaro, Mater Domini Hospital Catanzaro, Catanzaro, Italy
Background: Palmitoyl ethanol amide (PEA) is an endogenously produced substance showing anti-nociceptive effect through both receptor and non-receptor mediated effects at the level of different cellular and tissue sites. This study showed the results of a single blind study that was conducted to evaluate both the safety and the efficacy of ultramicronized PEA (umPEA; 1,200 mg/day) for up 90 days in patients suffering of Migraine with Aura (MA) treated with NSAIDs.
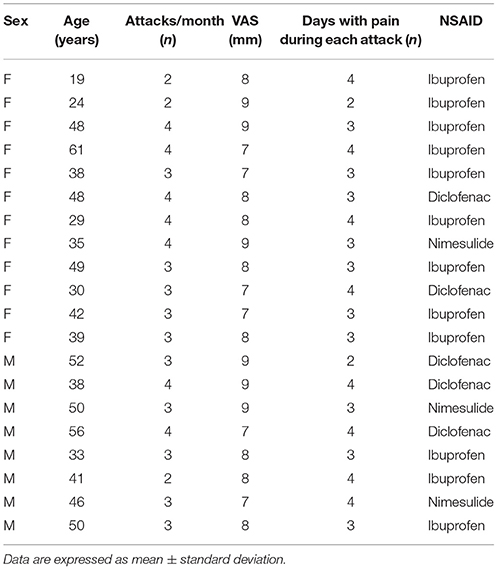
Methods: A total of 20 patients, 8 male (33–56-years, average 41.4 ± 7.8) and 12 female (19–61-years, average 38.5 ± 11.9) with MA were admitted to our observation and diagnosed according to ICHD-3 criteria, they received umPEA (1,200 mg/day) in combination with NSAIDs for up to 90 days. They were revaluated at 30, 60, and 90 days after treatment.
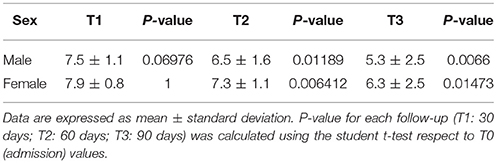
Results: umPEA administration induced a statistically significant and time dependent pain relief. In particular, these effects were evident at 60 days (male P = 0.01189; female P = <0.01) and they lasted until the end of the study (male P = 0.0066; female P = 0.01473).
Conclusion: Although further studies are needed, our findings indicate that in patients suffering of MA treatment with umPEA had good efficacy and safety which candidate this compound as a therapeutic tool in pain migraine management.
Introduction
Migraine is a common disabling primary headache disorder. It is the sixth highest cause of years lost due to disability worldwide (1, 2) with high prevalence in young adults (3, 4). Migraine can be classified in two major types: Migraine without aura characterized by headache with specific features and associated symptoms and Migraine with aura (MA) characterized by the transient focal neurological symptoms that usually precede or sometimes accompany the headache (5).
In MA, the word “aura” denotes recurrent attacks of reversible neurologic symptoms (e.g., visual, sensory, speech, motor, or other central nervous symptoms) usually lasting few minutes. Often the symptoms are unilateral, occurring on only one side of the body or of the visual field; the aura is generally followed by a headache (5).
Traditionally migraine treatment (with or without aura) includes both prophylactic therapy, aimed at reducing the frequency and severity of attacks, and acute therapy for halting the progression of attacks. Unfortunately prophylactic therapy rarely eliminates migraine (6), even though it is effective in improving responsiveness to acute therapy, thus ameliorating the level of disability. Triptans, non-steroidal anti-inflammatory drugs (NSAIDs), and antiemetics represent the mainstay of acute therapy (7, 8); however, in MA pain is less responsive to triptans (9) and NSAIDs use is hampered by the development of several adverse drug reactions (ADRs) (10, 11). Other drugs such as antidepressants (duloxetine and amitriptyline) and anticonvulsants (e.g., pregabalin and gabapentin) are also able to induce pain relief through the modulation of synaptic neurotransmitter levels leading to an improvement of quality of life (12). However, like NSAIDs their use is limited by the development of heavy side effects. Palmitoyl ethanol amide (PEA) is an endogenous fatty acid amide widely distributed in different tissues, including nervous tissues; it is synthesized on demand. PEA is emerging as a novel therapeutic approach in pain and inflammatory conditions (13). PEA has been reported to be effective in animal models of chronic pain and inflammation as well as in several clinical trials on various pain states (14–17). However, to date no studies have been performed to evaluate the role of PEA in the management of MA.
The aim of this study was to evaluate the efficacy and the safety of chronic administration of ultramicronized PEA (um-PEA) in patients with MA treated with NSAIDs.
Methods
Study
We performed a prospective single-blind study from 2014 to 2015 in patients admitted to the Neurosurgery Division of “Mater Domini” University Hospital in Catanzaro. The study protocol was approved by the Local Ethics Committee (Catanzaro Centro protocol number 235/2017), the enrolled patients signed the written informed consent, and the work was conducted in compliance with the Institutional Review Board/Human Subjects Research Committee requirements. In order to exclude any risk for the patients, both patients and physicians that evaluated the patients knew the protocol and the group of treatment, while physicians that evaluated the data were blinded to both protocol and treatments.
Inclusion Criteria
Patients of both sexes >18 year-old and with 12 months history of MA and with ≥2 attacks/month in the least 12 months, diagnosed according to ICHD-3 criteria, and upon treatment with NSAIDs (ibuprofen or diclofenac or nimesulide) were eligible for the study.
Exclusion Criteria
Hypersensitivity to study drugs, progressive serious clinical conditions (cancer, chronic hepatitis, human immunodeficiency virus), neuropsychiatric diseases (e.g., psychosis and depression, for the risk of low compliance), renal diseases (serum creatinine concentration more than 1.2 times the upper limit of the normal range according to the central laboratory reference values) and liver dysfunction (serum alanine or aspartate transaminase concentration more than 1.5 times the upper limit of normal range according to the central laboratory reference values). Patients with disorders capable of inducing the development of aura (i.e., patent foramen ovale, ischaemic stroke, restless legs syndrome, Parkinson's disease, and psychiatric disorders), patients with other diagnosis of headache (e.g., tension-type headache) and patients who did not sign the informed consent were also not considered eligible for the study.
Sample
The study sample includes 20 patients with MA, 8 male (33–56-years, average 45.8 ± 7.8) and 12 female (19–61-years, average 38.5 ± 11.9). All enrolled patients received a daily treatment with umPEA (1,200 mg/day) for 90 days and used a NSAID (ibuprofen, 600 mg as requested and up to 1,200 mg; diclofenac sodium, 50 mg as requested and up to 100 mg/day, and nimesulide, dosage 100 mg as requested and up to 200 mg/day) in presence of acute headache pain.
Moreover, 20 patients with MA 10 male (35–59-years, average 42.4 ± 8.5) and 10 female (19–60-years, average 37.3 ± 10.6) were also enrolled in this study as positive control-group receiving a treatment with NSAIDs alone (ibuprofen, 600 mg as requested and up to 1,200 mg; diclofenac sodium, 50 mg as requested and up to 100 mg/day, and nimesulide, dosage 100 mg as requested and up to 200 mg/day) in presence of acute headache pain.
In both groups, the follow-ups were performed at 30 (T1), 60 (T2), and 90 (T3) days after the starting from the time of enrollment. Moreover, patients enrolled in these groups did not receive any prophylaxis treatment for MA.
Assessment of Efficacy
In agreement with our previous study (18), a visual analogical scale (VAS) was used to measure pain intensity before and after the pharmacological treatment. A total VAS summary score was calculated for each individual, adjusted, and reported on a 0–100 scale. Lower scores were associated with less pain and better function.
Assessment of Safety
Safety was assessed by monitoring drug-drug interactions and the incidence of adverse drug reactions (ADRs) which were assessed for both severity and causality, in agreement with our previous studies (19–22).
Efficacy End-Points
The primary efficacy end-point was defined as a statistically significant difference (P < 0.05) in the improvement of pain after um-PEA treatment measured during the three follow-up visits (T1–T3) compared to admission (T0). Another primary efficacy end-point was the improvement of disability (evaluated as the reduction of days with headache) after um-PEA treatment measured during the follow-up visits (T1–T3) compared to admission (T0). The secondary efficacy end-point was assessed measuring the reduction of NSAIDs consumption (ibuprofen, diclofenac sodium, or nimesuilde) in enrolled patients.
Safety End-Points
The primary safety end-point was defined as a statistically significant difference (P < 0.05) in the development of any adverse drug reaction. The secondary safety end-point was the development of drug-drug interactions during the study.
Experimental Protocol
For ruling out secondary headache, patients underwent a neurological examination, clinical biochemistry panel and radiological evaluation (X-ray, computed tomography and magnetic resonance imaging). Additionally a questionnaire was administered in order to confirm the clinical diagnosis of MA, according to ICHD-3 criteria (5), then a VAS was also administered.
All patients enrolled in umPEA-group received a daily treatment with umPEA (1,200 mg/day) for 90 days (end of the study); during the study, an add-on treatment with NSAIDs (ibuprofen, diclofenac sodium, or nimesuilde) was used for pain relief during acute migraine attack (about 2 days for each attack). Both umPEA and NSAIDs were bought by patients over the counter from the open market.
Patients enrolled in control-group did not received umPEA using ibuprofen or diclofenac sodium or nimesulide alone as symptomatic treatment.
The follow-up visits were performed, in all groups, at 1 (T1), 2 (T2), and 3 (T3, end of the study) months after the first administration of umPEA.
Statistical Analysis
A 17% difference in the VAS score was considered as minimal clinical improvement threshold. In order to assess the clinically relevant difference between each group, almost 20 subjects were enrolled in each group (power >80%, alpha 0.05, two-tailed). All data are expressed as mean ± standard deviation (SD). Data were checked for normality using the Kolmogorove–Smirnov test, while the Student's t-test was used as post-hoc test. The differences between multiple means was assessed using one-way ANOVA and the Kruskal–Wallis test. A multivariate analysis for age (continuous), sex (categorical), VAS score (continuous), and ADRs (continuous) was also performed. The threshold of statistical significance was set at P < 0.05. Statistical analysis was performed using SPSS software version 21 (SPSS Inc., Chicago, USA) was used.
Results
Patients
In all enrolled patients (umPEA-group and control-group), the laboratory parameters were in the normal range highlighting no systemic diseases. Neurological examinations and radiological findings confirmed the diagnosis of primary migraine excluding secondary causes, while the questionnaire confirmed the presence of aura.
All enrolled patients suffered from visual aura (with phosphenes and teicopsies) and severe pain (VAS 7–10; umPEA-group mean 8.0 ± 0.8: control-group mean: 8.05 ± 0.8, P = 0.288) (Tables 1, 2).
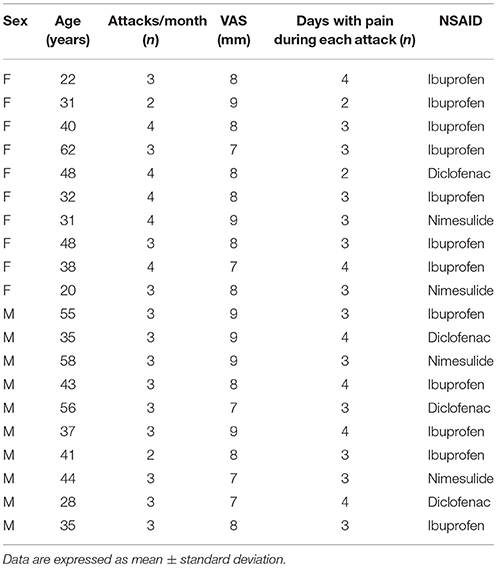
Table 2. Demographic characteristics of enrolled patients in Control-group at the time of admission.
As shown in Tables 1, 2, at the time of enrollment (T0), in both groups, the patients showed about 3 attacks/month (umPEA-group mean: 3.2 ± 0.7; control-group male 3.15 ± 0.6, P = 0.333) and each pain attack lasted 2–4 days (umPEA-group mean 3.3 ± 0.7; control-group mean 3.2 ± 0.6, P = 0.27).
All enrolled patients (umPEA-group and control-group), did not receive any prophylactic treatment for MA, but during the acute headache attack used NSAIDs: ibuprofen (umPEA-group: 12 patients; control group: 12 patients), diclofenac sodium (umPEA-group: 5 patients; control group: 4 patients), and nimesulide (umPEA-group: 3 patients; control group: 4 patients) with no difference for sex or age (see Tables 1, 2).
As shown in Table 3, at T1 follow-up, umPEA treatment did not significantly affect pain headache (P = 0.0675), in both sexes, whereas a dramatic improvement in pain symptom was observed at T2 patients, and this effect was maintained at the last follow up (T3), irrespective to gender.
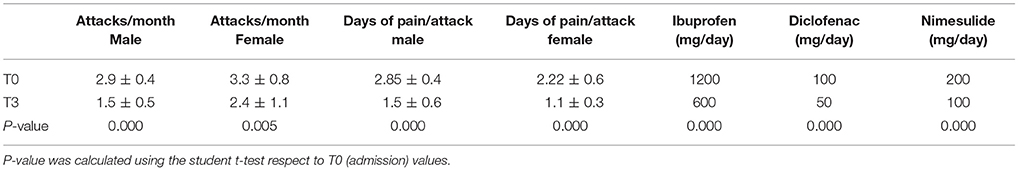
Moreover, at T3 both the days of pain and the number of attacks/months were significantly reduced (primary end-point) without difference for gender or age (T0: 3.1 ± 0.6; T3: 2.0 ± 1; P = 0.000, Table 3); a decrease in NSAIDs dosage was also observed (primary end-point; Table 4).
In contrast, in control group, the treatment with NSAIDs alone, even if induced a significant decrease in the intensity of pain during each attack (P < 0.05), they failed to modify the pain intensity during the recurrence of attacks (T0: 3.15 ± 0.6; T3: T0: 3.1 ± 0.6, P = 0.164) or their number/month (T0: 2.85 ± 0.4; T3: 2.75 ± 0.4, P = 0.08; Table 5).
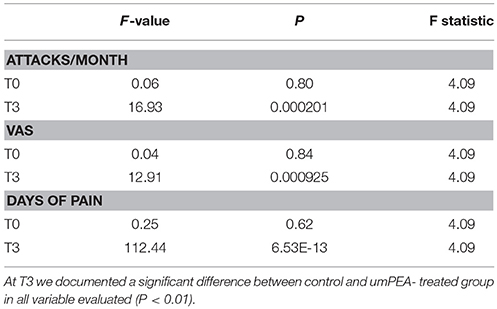
Table 5. ANOVA test analysis between case and control groups at admission (T0) and at the end of the study (T3).
In both groups clinical examination and laboratory assays excluded the occurrence of major clinical event (see section Methods), therefore we excluded the incidence of side effects related to drug administration. All enrolled patients concluded the study and no patients were missing to the follow-up.
Discussion
Migraine is recognized as a neurogenic disorder associated with secondary changes in brain perfusion (5). However, while the neuroinflammation affecting cranial blood vessels and dura sustains pain migraine in the early stages, the presence of allodynia, hyperalgesia, and expansion of nociceptive fields during migraine attacks is evocative of neuropathic pain, thus implying the involvement of further mechanisms, such as peripheral and central sensitizations (23, 24). As with other neuropathic conditions the therapeutic management of migraine is still a clinical challenge and several drugs have been proposed (7, 25), since their use may be related with the development of side effects (10, 25) or chronic migraine (26, 27).
In a previous case series, Hesselink (28) documented that the administration of PEA 1,200 mg/day in patients with neuropathic pain was able to induce a significant pain decrease. In agreement, we documented that the administration of topiramate and PEA was able to decrease pain symptoms in patients with nummular headache (15). Herein, we demonstrate for the first time that umPEA administration to patients with MA (1,200 mg/day for up 90 days) treated with common NSAIDs induced a significant pain relief (evaluated considering the VAS score and the number of attacks/month), irrespective to age or gender. These effects were evident at 60 days after the beginning of umPEA-treatment and lasted throughout the study. These results are in agreement with previous reports showing the anti-nociceptive action of umPEA in both preclinical models of neuropathic pain and with clinical trials performed in a variety of pain states (14, 23). The efficacy of PEA in reducing pain is related to its capability to interfere with the inflammatory mechanism within the nociceptive axis, allowing for a reduction of both peripheral and central sensitization. PEA activity encompasses both neuronal and non-neuronal cells (29, 30); the latter concerns the down-regulation of mast-cell hyperactivity mediated by this compound (31–33). Indeed, this cell population is often found in proximity to sensory nerve endings and through the release of inflammatory mediators and cytokines, stored in intracellular granules, they can enhance the nociceptive signal. Remarkably, mast-cells also colonize the spinal dura, the thalamus and the dura mater (31–33).
Moreover, in our study we also documented that patients treated with umPEA reduced the NSAIDs consumption, while this was not recorded in control-group.
In this frame, PEA might represent a useful therapeutic approach for migraine, as meningeal nociceptors can be activated locally through a neuro-immune interplay with resident mast cells populating the dura mater (34).
Previous studies reported that treatment with PEA does not cause adverse events or drug interactions and it doesn't induce pharmacological tolerance (16, 35–37). Our data demonstrate that um-PEA chronically administered for 90 days and occasionally added on to NSAIDs significantly reduces the score of pain intensity, the number of attacks/month, and the days of pain during each attack irrespective to age and gender, suggesting a synergic effect of these compounds.
This synergic effect is possibly related to the different mechanisms of action of the drugs used. In fact, PEA has dose-dependent anti-inflammatory and analgesic effects related to the modulation of mast-cell and microglia and is able to reduce pain, to preserve peripheral nerve morphology, to reduce endoneural edema, the recruitment and activation of mast cells, and the production of pro-inflammatory mediators (38–40). On the other hand NSAIDs have a dose-dependent anti-inflammatory and analgesic effect related to the inhibition of prostaglandins; however their use must be monitored, particularly in elderly patients, for potential gastrointestinal and hepatic risks, cardiovascular and renal side effects, and drug-drug interactions (41–43).
In our study, we did not record the occurrence of any major ADRs related to NSAIDs administration and this could be related to the short time of treatment (occasional use for 2 days) and also to the low dosage used. In particular, the treatment with um-PEA allowed a decrease in NSAIDs dosage in all patients suggesting that this combination may be useful to reduce the toxicity in patients underwent to polytherapy. The data also confirm the safety of treatment with um-PEA. Indeed, no adverse drug reactions or interactions were recorded during the study highlighting an optimal um-PEA pharmacological profile and the adherence with the umPEA regimen was good with a rate of 100%.
Finally, even without having performed a pharmacoeconomic analysis, these data suggest that this combination may help to reduce the cost of migraine, including drugs, hospitalization, and toxicity.
However, this study had some major limitations represented by the method used (single blind), the limited sample size and the brief duration of follow-up, so we defined this study as a pilot study and other clinical trials in a large population must be performed to confirm these data.
Although PEA is not reported in guidelines of migraine treatment, in our study the chronic administration of um-PEA to patients with MA in combination with NSAIDs, induced a significant pain relief allowing the reduction of the NSAID dosage. Remarkably, the compound was also able to reduce the number of migraine attacks. No major adverse drug reactions or interactions were recorded during the study. These data, compatibly with the design of the trial, are suggestive of an optimal pharmacological profile forum-PEA.
Author Contributions
DC, GD, and SS recorded clinical data. EC, MC, MW, TG, and NF analyzed clinical data. DC, LG, and VG conceived the study, had full access to all of the data, and wrote the manuscript. All authors have read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer RM and handling Editor declared their shared affiliation.
Acknowledgments
The authors thank Dr. Martin Laurenzi for his thorough English language editing that greatly improved the manuscript.
References
1. GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet (2016) 388:1545–602. doi: 10.1016/S0140-6736(16)31678-6
2. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet (2017) 390:1211–59. doi: 10.1016/S0140-6736(17)32154-2
3. Steiner TJ, Stovner LJ, Vos T. GBD 2015: migraine is the third cause of disability in under 50s. J Headache Pain (2016) 17:104. doi: 10.1186/s10194-016-0699-5
4. Gallelli L, Iannacchero R, De Caro E, Peltrone F, Colosimo M, De Sarro G. A questionnaire-based study on prevalence and treatment of headache in young children. J Headache Pain (2005) 6:277–80. doi: 10.1007/s10194-005-0206-x
5. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition. Cephalalgia (2018) 38:1–211. doi: 10.1177/0333102417738202
6. Termine C, Ferri M, Balottin U. Acute treatment of migraine in children and adolescents. Funct Neurol. (2008) 23:63–9.
7. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the american headache society evidence assessment of migraine pharmacotherapies. Headache (2015) 55:3–20. doi: 10.1111/head.12499
8. Worthington I, Pringsheim T, Gawel MJ, Gladstone J, Cooper P, Dilli E, et al. Canadian headache society guideline: acute drug therapy for migraine headache. Can J Neurol. (2013) 40(5 Suppl. 3):S1–80.
9. Hansen JM, Goadsby PJ, Charles A. Reduced efficacy of sumatriptan in migraine with aura vs without aura. Neurology (2015) 84:1880–5. doi: 10.1212/WNL.0000000000001535
10. Gallelli L, Colosimo M, Pirritano D, Ferraro M, De Fazio S, Marigliano NM, et al. Retrospective evaluation of adverse drug reactions induced by nonsteroidal anti-inflammatory drugs. Clin Drug Invest. (2007) 27:115–22. doi: 10.2165/00044011-200727020-00004
11. Gallelli L, Galasso O, Urzino A, Sacca S, Falcone D, Palleria C, et al. Characteristics and clinical implications of the pharmacokinetic profile of ibuprofen in patients with knee osteoarthritis. Clin Drug Invest. (2012) 32:827–33. doi: 10.1007/s40261-012-0011-7
12. Kremer M, Salvat E, Muller A, Yalcin I, Barrot M. Antidepressants and gabapentinoids in neuropathic pain: mechanistic insights. Neuroscience (2016) 338:183–206. doi: 10.1016/j.neuroscience.2016.06.057
13. Paterniti I, Impellizzeri D, Crupi R, Morabito R, Campolo M, Esposito E, et al. Molecular evidence for the involvement of PPAR-delta and PPAR-gamma in anti-inflammatory and neuroprotective activities of palmitoylethanolamide after spinal cord trauma. J Neuroinflamm. (2013) 10:20. doi: 10.1186/1742-2094-10-20
14. Hesselink JMK. New targets in pain, non-neuronal cells, and the role of palmitoylethanolamide. Open Pain J. (2012) 5:2–23. doi: 10.2174/1876386301205010012
15. Chirchiglia D, Della Torre A, Signorelli F, Volpentesta G, Guzzi G, Stroscio CA, et al. Administration of palmitoylethanolamide in combination with topiramate in the preventive treatment of nummular headache. Int Med Case Rep J. (2016) 9:193–5. doi: 10.2147/IMCRJ.S106323
16. Chirchiglia D, Paventi S, Seminara P, Cione E, Gallelli L. N-palmitoyl ethanol amide pharmacological treatment in patients with nonsurgical lumbar radiculopathy. J Clin Pharmacol. (2018) 58:733–9. doi: 10.1002/jcph.1070
17. Gabrielsson L, Mattsson S, Fowler CJ. Palmitoylethanolamide for the treatment of pain: pharmacokinetics, safety and efficacy. Br J Clin Pharmacol. (2016) 82:932–42. doi: 10.1111/bcp.13020
18. Gallelli L, Avenoso T, Falcone D, Palleria C, Peltrone F, Esposito M, et al. Effects of acetaminophen and ibuprofen in children with migraine receiving preventive treatment with magnesium. Headache (2014) 54:313–24. doi: 10.1111/head.12162
19. Gallelli L, Staltari O, Palleria C, De Sarro G, Ferraro M. Hepatotoxicity induced by methimazole in a previously healthy patient. Curr Drug Saf. (2009) 4:204–6. doi: 10.2174/157488609789006912
20. Palleria C, Di Paolo A, Giofre C, Caglioti C, Leuzzi G, Siniscalchi A, et al. Pharmacokinetic drug-drug interaction and their implication in clinical management. J Res Med Sci. (2013) 18:601–10.
21. Gallelli L, Gallelli G, Codamo G, Argentieri A, Michniewicz A, Siniscalchi A, et al. Recognizing severe adverse drug reactions: two case reports after switching therapies to the same generic company. Curr Drug Saf. (2016) 11:104–8. doi: 10.2174/1574886311207040309
22. Gallelli L, Colosimo M, Tolotta GA, Falcone D, Luberto L, Curto LS, et al. Prospective randomized double-blind trial of racecadotril compared with loperamide in elderly people with gastroenteritis living in nursing homes. Eur J Clin Pharmacol. (2010) 66:137–44. doi: 10.1007/s00228-009-0751-3
23. Rice AS, Farquhar-Smith WP, Nagy I. Endocannabinoids and pain: spinal and peripheral analgesia in inflammation and neuropathy. Prostaglandins Leukot Essent Fatty Acids (2002) 66:243–56. doi: 10.1054/plef.2001.0362
24. Biondi DM. Is migraine a neuropathic pain syndrome?. Curr Pain Headache Rep. (2006) 10:167–78. doi: 10.1007/s11916-006-0042-y
25. Mayans L, Walling A. Acute migraine headache: treatment strategies. Am Fam Physician (2018) 97:243–51. doi: 10.1111/head.12201
26. Lipton RB, Serrano D, Nicholson RA, Buse DC, Runken MC, Reed ML. Impact of NSAID and triptan use on developing chronic migraine: results from the american migraine prevalence and prevention (AMPP) study. Headache (2013) 53:1548–63.
27. Raggi A, Schiavolin S, Leonardi M, Giovannetti AM, Bussone G, Curone M, et al. Chronic migraine with medication overuse: association between disability and quality of life measures, and impact of disease on patients' lives. J Neurol Sci. (2015) 348:60–6. doi: 10.1016/j.jns.2014.11.004
28. Hesselink JM. Chronic idiopathic axonal neuropathy and pain, treated with the endogenous lipid mediator palmitoylethanolamide: a case collection. Int Med Case Rep J. (2013) 6:49–53. doi: 10.2147/IMCRJ.S51572
29. Loria F, Petrosino S, Mestre L, Spagnolo A, Correa F, Hernangomez M, et al. Study of the regulation of the endocannabinoid system in a virus model of multiple sclerosis reveals a therapeutic effect of palmitoylethanolamide. Eur J Neurosci. (2008) 28:633–41. doi: 10.1111/j.1460-9568.2008.06377.x
30. Keppel Hesselink JM. Glia as a new target for neuropathic pain, clinical proof of concept for palmitoylethanolamide, a glia modulator. Anesth Pain Intensive Care (2011) 15:143–45. Available online at: http://www.apicareonline.com/editorial-view-glia-as-a-new-target-for-neuropathic-pain-clinical-proof-of-concept-for-palmitoylethanolamide-a-glia-modulator/
31. Esposito E, Paterniti I, Mazzon E, Genovese T, Di Paola R, Galuppo M, et al. Effects of palmitoylethanolamide on release of mast cell peptidases and neurotrophic factors after spinal cord injury. Brain Behav Immun. (2011) 25:1099–112. doi: 10.1016/j.bbi.2011.02.006
32. Cerrato S, Brazis P, della Valle MF, Miolo A, Puigdemont A. Effects of palmitoylethanolamide on immunologically induced histamine, PGD2 and TNFalpha release from canine skin mast cells. Vet Immunol Immunopathol. (2010) 133:9–15. doi: 10.1016/j.vetimm.2009.06.011
33. De Filippis D, Luongo L, Cipriano M, Palazzo E, Cinelli MP, de Novellis V, et al. Palmitoylethanolamide reduces granuloma-induced hyperalgesia by modulation of mast cell activation in rats. Mol Pain (2011) 7:3. doi: 10.1186/1744-8069-7-3
34. Levy D, Burstein R, Kainz V, Jakubowski M, Strassman AM. Mast cell degranulation activates a pain pathway underlying migraine headache. Pain (2007) 130:166–76. doi: 10.1016/j.pain.2007.03.012
35. Skaper SD, Facci L, Fusco M, Della Valle MF, Zusso M, Costa B, et al. Palmitoylethanolamide, a naturally occurring disease-modifying agent in neuropathic pain. Inflammopharmacology (2014) 22:79–94. doi: 10.1007/s10787-013-0191-7
36. Varrassi G, Fusco M, Coaccioli S, Paladini A. Chronic pain and neurodegenerative processes in elderly people. Pain Pract. (2015) 15:1–3. doi: 10.1111/papr.12254
37. Paladini A, Fusco M, Cenacchi T, Schievano C, Piroli A, Varrassi G. Palmitoylethanolamide, a special food for medical purposes, in the treatment of chronic pain: a pooled data meta-analysis. Pain Physician (2016) 19:11–24.
38. Luongo L, Guida F, Boccella S, Bellini G, Gatta L, Rossi F, et al. Palmitoylethanolamide reduces formalin-induced neuropathic-like behaviour through spinal glial/microglial phenotypical changes in mice. CNS Neurol Disord Drug Targets (2013) 12:45–54. doi: 10.2174/1871527311312010009
39. Costa B, Comelli F, Bettoni I, Colleoni M, Giagnoni G. The endogenous fatty acid amide, palmitoylethanolamide, has anti-allodynic and anti-hyperalgesic effects in a murine model of neuropathic pain: involvement of CB(1), TRPV1 and PPARgamma receptors and neurotrophic factors. Pain (2008) 139:541–50. doi: 10.1016/j.pain.2008.06.003
40. Bettoni I, Comelli F, Colombo A, Bonfanti P, Costa B. Non-neuronal cell modulation relieves neuropathic pain: efficacy of the endogenous lipid palmitoylethanolamide. CNS Neurol Disord Drug Targets (2013) 12:34–44. doi: 10.2174/1871527311312010008
41. Gallelli L, Galasso O, Falcone D, Southworth S, Greco M, Ventura V, et al. The effects of nonsteroidal anti-inflammatory drugs on clinical outcomes, synovial fluid cytokine concentration and signal transduction pathways in knee osteoarthritis. A randomized open label trial. Osteoarthritis Cartilage (2013) 21:1400–8. doi: 10.1016/j.joca.2013.06.026
42. Barkin RL, Beckerman M, Blum SL, Clark FM, Koh EK, Wu DS. Should nonsteroidal anti-inflammatory drugs (NSAIDs) be prescribed to the older adult?. Drugs Aging (2010) 27:775–89. doi: 10.2165/11539430-000000000-00000
Keywords: Migraine with aura, ultramicronized palmitoyl ethanol amide, pain, clinical trial, efficacy, safety
Citation: Chirchiglia D, Cione E, Caroleo MC, Wang M, Di Mizio G, Faedda N, Giacolini T, Siviglia S, Guidetti V and Gallelli L (2018) Effects of Add-On Ultramicronized N-Palmitol Ethanol Amide in Patients Suffering of Migraine With Aura: A Pilot Study. Front. Neurol. 9:674. doi: 10.3389/fneur.2018.00674
Received: 09 May 2018; Accepted: 26 July 2018;
Published: 17 August 2018.
Edited by:
Massimiliano Valeriani, Bambino Gesù Ospedale Pediatrico (IRCCS), ItalyReviewed by:
Romina Moavero, Bambino Gesù Ospedale Pediatrico (IRCCS), ItalyIshaq Abu-Arafeh, Royal Hospital for Sick Children, United Kingdom
Copyright © 2018 Chirchiglia, Cione, Caroleo, Wang, Di Mizio, Faedda, Giacolini, Siviglia, Guidetti and Gallelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vincenzo Guidetti, dmluY2Vuem8uZ3VpZGV0dGlAdW5pcm9tYTEuaXQ=
 Domenico Chirchiglia
Domenico Chirchiglia Erika Cione
Erika Cione Maria C. Caroleo2
Maria C. Caroleo2 Minyan Wang
Minyan Wang Giulio Di Mizio
Giulio Di Mizio Noemi Faedda
Noemi Faedda Teodosio Giacolini
Teodosio Giacolini Vincenzo Guidetti
Vincenzo Guidetti Luca Gallelli
Luca Gallelli