- Department of Radiation Oncology, Medical College of Wisconsin, Milwaukee, WI, United States
The incidence of brain metastases is projected to rise because survival rates of lung cancer, breast cancer, and melanoma continue to improve (1). The brain is being identified as a sanctuary site for harboring metastases despite excellent control of extracranial disease. This is thought to occur because the drug therapies that control extracranial disease have limited central nervous system (CNS) penetration. The development of brain metastases is a devastating diagnosis affecting both quality of life (QOL) and survival. Symptoms after diagnosis can include headache, nausea, vomiting, seizure, neurocognitive decline, and focal neurologic deficit. Some of these symptoms can be irreversible even after successful treatment of intracranial disease. Treatment of brain metastases often necessitates surgery and radiation. There have been some reports of systemic therapies offering an intracranial response however long-term data is lacking. These treatments for CNS metastases can also lead to neurocognitive sequelae impacting quality of life. Therefore, preventing disease from spreading to the brain is a topic that has generated much interest in oncology. Prophylactic cranial Irradiation (PCI) has been used in leukemia, small cell lung cancer (SCLC), and non-small cell lung cancer (NSCLC). While showing effectiveness in preventing intracranial disease development, its carries with it side effects of neurocognitive decline that can affect QOL. There are Clinical trials exploring novel delivery of PCI and concurrent neuroprotective drug therapy to try to mitigate these neurocognitive sequelae. These will be important trials to complete, as PCI has shown promise in controlling disease and prolonging survival in select patient populations. There are also drug therapies that have shown efficacy in preventing CNS metastases development. This review will explore the current therapies available to prevent CNS metastases.
Standard Brain Mets Treatment
Standard therapies for brain metastases often include surgery, whole brain radiotherapy (WBRT), stereotactic radiosurgery (SRS), or a combination of these treatment modalities. The decision for utilizing these therapies are often dependent upon the number of lesions, their location, and the severity of patients' symptoms.
The routine use of WBRT has been challenged with recent publications showing improved cognitive outcomes and equivalent survival in patients treated with SRS compared to SRS and WBRT in patients with limited brain metastases (2). In addition, SRS is also being favored over WBRT following resection of metastases as recent data has also shown good local control and equivalent survival with less neurocognitive decline in patients where WBRT following surgery was omitted (3, 4). Regardless of the reduction in neurocognitive sequelae when WBRT is withheld, it's important to recognize that patients still experienced neurocognitive decline even when focused radiotherapy was administered. This is a fact that is frequently omitted in the discussion of the results of these trials. The mere presence of metastatic disease can lend itself to neurocognitive symptoms. These may not be outwardly apparent to the patient or clinician but in trials where pre-treatment cognitive assessments were performed, pre-WBRT neurocognitive symptoms were uncovered with testing (5, 6). This underscores the need for prevention of brain metastases as opposed treatment after the development of intracranial disease.
PCI in the Management of Acute Lymphoblastic Leukemia (All)
PCI was initially introduced in order to address metastatic disease to the CNS in childhood leukemia. The CNS was known to be a sanctuary site for leukemic cells and CNS relapses were common and carried with it a poor prognosis (7–9). Early studies had shown that patients with high risk features (young age at diagnosis, T cell phenotype, WBC count >50,000–100,000, extra-medullary disease, presence of Philadelphia chromosome, and poor response to induction chemotherapy) had poor survival even after they had achieved remission, and this was attributed to CNS relapses (7–9). In high risk populations, PCI has been shown to decrease the rate of CNS recurrences from 42 to 100% down to 6% (10). These impressive results have been seen in both the pediatric and adult populations (11).
The unfortunate result of delivering radiation therapy to the brain in this disease process is the long-term repercussions of CNS radiation toxicity. Some of the side effects that children developed as a result of these therapies were neurocognitive decline, mood disturbances, short stature, abnormal skull growth, endocrinopathies, and secondary malignancies. As a result of these side effects the radiotherapy dose has been aggressively decreased from 24 to 12 Gy in the hopes of avoiding some or all of these long-term toxicities (12–14).
Currently, leukemia CNS prophylaxis without PCI has been the preferred approach. As an example, the Berlin-Franfurt-Munster (NHL-BFM 95) trial showed that in Stage III–IV lymphoblastic leukemia who received high dose systemic methotrexate, including intrathecal (IT) methotrexate, had very low rates of CNS relapse comparable to historic control who had received PCI (15). Additionally, the Children's Leukemia Group showed that even in patients with CNS involvement at diagnosis had high rates of cure and low rates of CNS relapse with appropriate systemic and IT therapies (16).
Current management of ALL, even with high risk features, excludes PCI. However, the early use of this treatment modality was the initial pioneering effort that led to cures of childhood ALL and paved the way for this treatment modality to be utilized in other malignancies where metastases can be harbored in the CNS and shielded from effective systemic chemotherapies.
PCI in Small Cell Lung Cancer
Small cell lung cancer (SCLC) is an additional malignancy where CNS failure rates are approximated to be 50–60% at 2 years following diagnosis (17). CNS failure in SCLC carries with it a poor prognosis (18). As a result of these high rates of CNS failure, consideration of delivering PCI to improve local CNS control was considered.
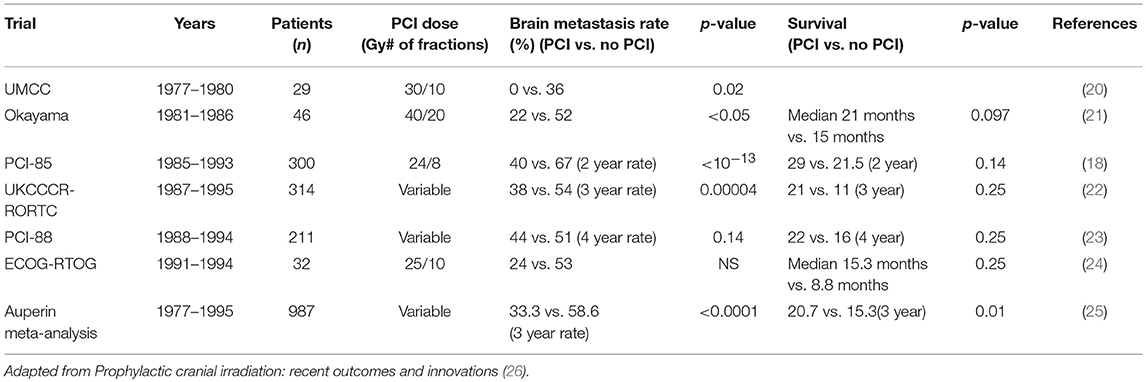
Initial early trials did not show a clear benefit to the delivery of PCI in SCLC (19). These early trials did not separate patients into limited disease (LD) or extensive disease (ED) or perform appropriate re-staging for response to chemotherapy prior to the delivery of PCI. The failure to show improvements in survival was likely due to the competing risk of death from systemic disease progression or the presence of CNS disease prior to the delivery of “prophylactic” CNS radiation. What became evident was that patients who had a complete response to systemic chemotherapy in LD SCLC and were re-staged prior to the delivery of PCI benefitted from PCI with both local control and survival (Table 1). The Aurperin meta-analysis demonstrated that the use of PCI at varying dose and fractionation schedules who had a complete response to systemic chemotherapies had a 50% reduction in the development of brain metastases and an improvement in overall survival (20.7% PCI vs. 15% observation) (25). A more recent analysis of 12 trials by Meert et al. showed similar results. PCI decreased brain metastases and improved survival in patients achieving a complete response (CR) after chemotherapy with hazard ratio [HR] of 0.48 (95% CI 0.39–0.60) for incidence of brain metastases, and HR of 0.82 (95% CI 0.71–0.96) for survival. However, when patients with less than a CR to chemotherapy were included in this analysis, the benefit of PCI on survival became non-significant (HR 0.94, 0.87–1.02) (27).
Recommendations for PCI in patients with ED-SCLC is less clear. Auperin's meta-analysis included a small number of patients with ED-SCLC and in those patients who achieved a complete response (CR) to systemic chemotherapy there was better survival and lower rates of brain metastases when PCI was administered (25).
In addition to this data, the European Organization for Research and Treatment of Cancer (EORTC) performed a Phase III trial investigating the role of PCI in patients with ED SCLC who had partial response (PR) or CR to chemotherapy (28). The risk of brain metastases at 1-year was significantly reduced in the PCI group (14.6% PCI vs. 40.4% No PCI), and the 1-year survival rate was also superior (27.1% PCI and 13.3% No PCI). A criticism of this study was its lack of re-staging brain MRI in asymptomatic patients which may have led to inclusion of patients who may have harbored brain metastases.
More evidence in support of PCI in ED-SCLC came from a North Central Cancer Treatment Group analysis examining patients with LD and ED-SCLC with stable disease following chemotherapy and thoracic radiotherapy. Three hundred eighteen patients were enrolled, and this showed improvement in survival at 1 and 3 years with limited toxicity using traditional radiation dose fractionation (29).
There are other studies that question the routine use of PCI in ED-SCLC. The Japanese closed their phase III trial early due to the lack of survival seen in patients who received PCI (25 Gy in 10 fractions). Median survival was shown to be 10.1 months in those receiving PCI compared to 15.1 months without PCI (p = 0.091). However, there was a significant reduction in the development of brain metastases (32% PCI vs. 58% No PCI) which matches the 50% reduction in brain metastases development seen in patients with LD-SCLC where PCI is administered (30).
There is a clear role for PCI in LD-SCLC who demonstrate a CR to systemic chemotherapy with improvements in both local control and survival. The routine use of PCI in ED-SCLC is less clear. However, it seems very reasonable to consider administering this therapy in patients with ED-SCLC who show response to initial systemic chemotherapies and who have not developed brain metastases upon restaging of the CNS prior to PCI delivery.
Roll of PCI in Non-Small Cell Lung Cancer (NSCLC)
Brain metastases occur with frequency in patients diagnosed with NSCLC and are also one of the first sites of relapse. Patient with early stage (I–II) disease are less likely to be diagnosed with brain metastases compared to those with more advanced disease (Stage III) (31–37).
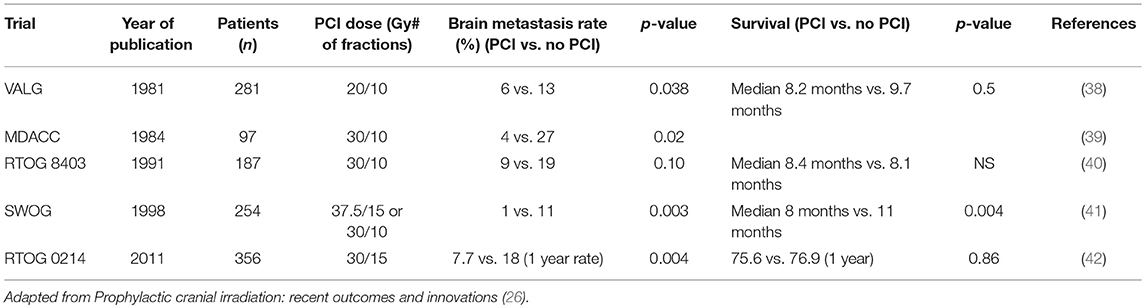
The role of PCI in NSCLC is not as well established as it is in those with SCLC. However, there are some older studies that demonstrated PCI reduced development of CNS metastases and prolonged the time to develop intracranial disease. Cox et al. had shown that PCI decreased the incidence of CNS metastases from 13% to 6% (p = 0.038) (38). Umsawasdi et al. showed a decrease in CNS metastases from 27% (No PCI) to 4% (PCI) (p = 0.002) with an increase in CNS metastases free survival (39).
However, the biggest criticism of PCI in NSCLC is that, while this treatment modality demonstrates reductions in the development of brain metastases, there is not a corresponding improvement in overall survival. As an example, the RTOG tried to demonstrate a benefit of PCI in Stage II and III NSCLC. With 187 patients enrolled, there were non-significant reductions in the development of brain metastases but also a non-significant reduction in survival in the PCI arm (40). There was however one trial that showed a significant benefit in brain metastases reduction and survival (41).
Based upon these mixed results, the RTOG tried to definitively answer the question of the benefit of PCI in NSCLC with RTOG 0214. This was a Phase III trial with Stage IIIA and IIIB NSCLC. Three hundred fifty-six patients were accrued to this study. After definitive treatment, patients were randomized to PCI, 30 Gy in 15 fractions or observation. This study closed early due to poor accrual. Unfortunately, it failed to show a difference in overall survival between the two arms, however, there was a statistically significant reduction in the development of brain metastases (18.0% No PCI vs. 7.7% PCI, p = 0.004) (42).
Based upon these trials, the routine use of PCI in NSCLC is not routinely recommended. (Table 2).
Side Effects and QOL
Cranial radiation can cause significant neurologic toxicity that can negatively impact QOL. This argument is used for forgoing PCI especially in settings where a survival benefit is not realized. However, when PCI is omitted, the competing risk of neurologic sequelae caused by the emergence of CNS metastases must also be considered (28).
Earlier studies reporting on the neurocognitive impact of PCI were small, retrospective, and did not establish a pre-treatment baseline (43). The absence of a pre-treatment baseline is critical because there are many factors that can lead to neurocognitive decline in patients other than the presence of metastatic disease or radiotherapy. Age, smoking, paraneoplastic syndromes, and depression are just a few factors that can lead to neurocognitive symptoms in the absence of radiotherapy. This is why it is absolutely necessary to perform neurocognitive assessments on patients at baseline to truly measure the impact that radiotherapy can have on posttreatment neurocognition.
Modern series assessing the efficacy of PCI have included more robust and reliable assessments of cognitive function assessed both before and after the administration of radiotherapy like mini mental status exam (MMSE), Hopkins Verbal Learning Test (HVLT) and Controlled Oral Word Association (COWA). Cognitive evaluation of RTOG 0212 showed a correlation between higher-dose PCI and increased, chronic neurological toxicity, but this was not associated with an impact on HVLT score (44).
Pooled analysis of RTOG 0212 and RTOG 0214 reported that patients treated with PCI had a greater risk of self-reported neurocognitive decline at 6 months (odds ratio [OR] 3.60, 95% CI 2.34–6.37; p < 0.0001) and 12 months (OR 3.44, 1.84–6.44; p < 0.0001) in addition to a decline in HVLT recall score at 6 and 12 months compared with the observation group (6, 44, 45).
QOL was also assessed in RTOG 0214 and showed that while global cognitive function and QOL was preserved between PCI and no PCI cohorts, there was decline in memory as measured by the HVLT in the group that received radiotherapy (6). Therefore, robust cognitive assessments may show neurocognitive decline in those receiving PCI, however, this does not always translate into patient's QOL being impacted.
There are currently efforts underway to try to deliver PCI in a way to try to mitigate cognitive effects. NRG Oncology CC003 “Randomized Phase II/III Trial of Prophylactic Cranial Irradiation with or without Hippocampal Avoidance for Small Cell Lung Cancer” is currently accruing patients in the hopes of enhancing the therapeutic ratio of PCI1; improve intracranial control while limiting neurocognitive toxicity. It has been hypothesized that radiation-induced injury to proliferating neuronal progenitor cells in the sub granular zone of the hippocampi may be responsible for the radiation induced NCF decline, thus, avoiding the hippocampal region of the brain may reduce cognitive side effects (46–48). The addition of neurocognitive protective agents is also being considered to further reduce the cognitive side effects of cranial irradiation (49).
Systemic Targeted or Immunotherapies Therapies for Brain Metastases Prevention
An interesting approach to the treatment of brain metastases to try to mitigate the deleterious effect of radiotherapy to the brain has been to consider targeted or immunotherapies upfront to treat intracranial disease. The Chinese Thoracic Oncology Group conducted a randomized trial looking at patients with NSCLC with epidermal growth factor receptor (EGFR) mutations, who were naive to treatment with EGFR-tyrosine kinase inhibitors (TKI) or radiotherapy and had at least three metastatic brain lesions to either icotinib or WBRT (30 Gy in ten fractions of 3 Gy) plus concurrent or sequential chemotherapy for 4–6 cycles. In patients with EGFR-mutant NSCLC and multiple brain metastases, icotinib had significantly longer intracranial PFS than WBI plus chemotherapy. Therefore, icotinib might be a better first-line therapeutic option for this patient population (50).
In another recently published trial, 303 patients with untreated, advanced ALK-positive NSCLC were treated with alectinib (600 mg twice daily) or crizotinib (250 mg twice daily). The primary end point was PFS. Secondary end points were time to CNS progression, objective CNS response rate, and overall survival. A CNS response was appreciated in 17 of 21 patients in the alectinib group (CNS response rate, 81%; 95% CI, 58 to 95) and in 11 of 22 patients in the crizotinib group (CNS response rate, 50%; 95% CI, 28 to 72). Eight patients (38%) in the alectinib group had a CNS complete response (CR), compared to 1 patient (5%) in the crizotinib group. The median duration of intracranial response was 17.3 months in the alectinib group (95% CI, 14.8 to not estimable) and 5.5 months in the crizotinib group (95% CI, 2.1 to 17.3), respectively. A CNS response occurred in 38 of 64 patients in the alectinib group (CNS response rate, 59%; 95% CI, 46 to 71) and in 15 of 58 patients in the crizotinib group (CNS response rate, 26%; 95% CI, 15 to 39) in patients who had measurable disease. Twenty-nine patients (45%) in the alectinib group had a CNS CR, as compared with 5 patients (9%) in the crizotinib group. This was an important trial as it showed that in patients who harbor an ALK-mutation, targeted therapies can be effective in treating and preventing CNS progression (51).
Similar studies have also been performed in patients with metastatic melanoma. In a recently published trial, patients with asymptomatic melanoma brain metastases with no prior local CNS therapy were randomly assigned to cohort A (nivolumab plus ipilimumab, n = 36) or cohort B (nivolumab, n = 27). With a median follow up of 17 months (IQR 8–25), intracranial responses were achieved by 16 (46%; 95% CI 29–63) of 35 patients in cohort A and five (20%; 7–41) of 25 in cohort B. Intracranial CR occurred in six (17%) patients in cohort A and three (12%) in cohort B. The effectiveness of these therapies came at the cost of treatment-related adverse events which occurred in 34 (97%) of 35 patients in cohort A and 17 (68%) of 25 in cohort B. Grade 3 or 4 treatment-related adverse events occurred in 19 (54%) patients in cohort A and four (16%) in cohort B indicating that the combination therapy was more toxic (52).
Another EGFR-TKI Lapatinib has also shown effectiveness in the treatment of metastatic HER2 positive breast cancer to the brain based upon 2, Phase II clinical trials (53, 54). Addition studies have also shown that Lapatinib in combination with chemotherapy can decrease the rate of CNS relapse of Her2 positive disease from 6% down the 1–2%. Currently, the Radiation Therapy Oncology Group (RTOG) 1119 is evaluating the complete response rate in the brain at 12 weeks post WBRT based upon MRI with the addition of Lapatinib and WBRT compared to WBRT alone in women with Her2 positive disease that has metastasized to the brain1. Another agent that has shown activity in the treatment of HER2 positive metastatic breast cancer to the brain is neratinib. There are trials currently accruing to determine if neratinib combined with other systemic chemotherapies will show activity against CNS metastases (55).
An interesting concept based upon these promising results is whether systemic targeted or immunotherapies could be used in the prevention of disease as opposed to treatment of metastases that have already developed. Trial concepts are currently being generated at the cooperative group level to address this question.
Conclusion
The prevention of metastases spreading to the CNS would have a significant benefit in preventing debilitating side effects. PCI has shown promise in preventing CNS metastases in ALL, LD and ED-SCLC, and NSCLC. However, a survival benefit has only been firmly established in ALL and SCLC. Some argue that in the absence of a survival benefit PCI should be omitted because of the neurologic and QOL sequelae that can occur in some patients. However, consideration needs to be given to the competing decline in cognition and QOL that can arise because of the development of CNS metastases. Novel radiation delivery techniques and targeted and immunotherapies may provide some hope of preventing CNS metastases without the negative impact on cognition and QOL.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
References
1. Posner JB, Chernik NL. Intracranial metastases from systemic cancer. Adv Neurol. (1978) 19:579–92.
2. Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA (2016) 316:401–9. doi: 10.1001/jama.2016.9839
3. Brown PD, Ballman KV, Cerhan JH, Anderson SK, Carrero XW, Whitton AC, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. (2017) 18:1049–60. doi: 10.1016/S1470-2045(17)30441-2
4. Soliman H, Ruschin M, Angelov L, Brown PD, Chiang VLS, Kirkpatrick JP, et al. Consensus contouring guidelines for postoperative completely resected cavity stereotactic radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. (2018) 100:436–42. doi: 10.1016/j.ijrobp.2017.09.047
5. Li J, Bentzen SM, Renschler M, Mehta MP. Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Clin Oncol. (2007) 25:1260–6. doi: 10.1200/JCO.2006.09.2536
6. Sun A, Bae K, Gore EM, Movsas B, Wong SJ, Meyers CA, et al. Phase III trial of prophylactic cranial irradiation compared with observation in patients with locally advanced non-small-cell lung cancer: neurocognitive and quality-of-life analysis. J Clin Oncol. (2011) 29:279–86. doi: 10.1200/JCO.2010.29.6053
7. Aur RJ, Hustu HO, Verzosa MS, Wood A, Simone JV. Comparison of two methods of preventing central nervous system leukemia. Blood (1973) 42:349–57.
8. Aur RJ, Simone JV, Husto HO, Verzosa MS, Pinkel D. Cessation of therapy during complete remission of childhood acute lymphocytic leukemia. N Engl J Med. (1974) 291:1230–4. doi: 10.1056/NEJM197412052912306
9. Pinkel D. (1987). Curing children of leukemia. Cancer 59:1683–91. doi: 10.1002/1097-0142(19870515)59:10<1683::AID-CNCR2820591002>3.0.CO;2-G
10. Hustu HO, Aur RJ. Extramedullary leukaemia. Clin Haematol. (1978) 7:313–37. doi: 10.1016/S0308-2261(78)80008-3
11. Hoelzer D, Gökbuget N, Digel W, Faak T, Kneba M, Reutzel R, et al. Outcome of adult patients with T-lymphoblastic lymphoma treated according to protocols for acute lymphoblastic leukemia. Blood (2002) 99:4379–85. doi: 10.1182/blood-2002-01-0110
12. Bongers ME, Francken AB, Rouwé C Kamps WA, Postma A. Reduction of adult height in childhood acute lymphoblastic leukemia survivors after prophylactic cranial irradiation. Pediatr Blood Cancer (2005) 45:139–43. doi: 10.1002/pbc.20334
13. Glover DA, Byrne J, Mills JL, Robison LL, Nicholson HS, Meadows A, et al. Impact of CNS treatment on mood in adult survivors of childhood leukemia: a report from the Children's Cancer Group. J Clin Oncol. (2003) 21:4395–401. doi: 10.1200/JCO.2003.04.089
14. Langer T, Martus P, Ottensmeier H, Hertzberg H, Beck JD, Meier W. CNS late-effects after ALL therapy in childhood. Part III: neuropsychological performance in long-term survivors of childhood ALL: impairments of concentration, attention, and memory. Med Pediatr Oncol. (2002) 38:320–8. doi: 10.1002/mpo.10055
15. Burkhardt B, Woessmann W, Zimmermann M, Kontny U, Vormoor J, Doerffel W, et al. Impact of cranial radiotherapy on central nervous system prophylaxis in children and adolescents with central nervous system-negative stage III or IV lymphoblastic lymphoma. J Clin Oncol. (2006) 24:491–9. doi: 10.1200/JCO.2005.02.2707
16. Uyttebroeck A, Suciu S, Laureys G, Robert A, Pacquement H, Ferster A, et al. Treatment of childhood T-cell lymphoblastic lymphoma according to the strategy for acute lymphoblastic leukaemia, without radiotherapy: long term results of the EORTC CLG 58881 trial. Eur J Cancer (2008) 44:840–6. doi: 10.1016/j.ejca.2008.02.011
17. Komaki R, Cox JD, Whitson W. Risk of brain metastasis from small cell carcinoma of the lung related to length of survival and prophylactic irradiation. Cancer Treat Rep. (1981) 65:811–4.
18. Arriagada R, Le Chevalier T, Borie F, Rivière A, Chomy P, Monnet I, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. J Natl Cancer Inst. (1995) 87:183–90. doi: 10.1093/jnci/87.3.183
19. Kristjansen PE, Kristensen CA. The role of prophylactic cranial irradiation in the management of small cell lung cancer. Cancer Treat Rev. (1993) 19:3–16. doi: 10.1016/0305-7372(93)90023-K
20. Aroney RS, Aisner J, Wesley MN, Whitacre MY, Van Echo DA, Slawson RG, et al. Value of prophylactic cranial irradiation given at complete remission in small cell lung carcinoma. Cancer Treat Rep. (1983) 67:675–82.
21. Ohonoshi T, Ueoka H, Kawahara S, Kiura K, Kamei H, Hiraki Y, et al. Comparative study of prophylactic cranial irradiation in patients with small cell lung cancer achieving a complete response: a long-term follow-up result. Lung Cancer (1993) 10:47–54. doi: 10.1016/0169-5002(93)90308-K
22. Gregor A, Cull A, Stephens RJ, Kirkpatrick JA, Yarnold JR, Girling DJ, et al. Prophylactic cranial irradiation is indicated following complete response to induction therapy in small cell lung cancer: results of a multicentre randomised trial. United Kingdom Coordinating Committee for Cancer Research (UKCCCR) and the European Organization for Research and Treatment of Cancer (EORTC). Eur J Cancer (1997) 33:1752–8. doi: 10.1016/S0959-8049(97)00135-4
23. Laplanche A, Monnet I, Santos-Miranda JA, Bardet E, Le Péchoux C, Tarayre M, et al. Controlled clinical trial of prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Lung Cancer (1998) 21:193–201. doi: 10.1016/S0169-5002(98)00056-7
24. Wagner HKK, Turrisi A. A randomized Phase III study of prophylactic cranial irradiation vs observationin patient with small cell lung cancerachieving a complete response: final report of an incomplete trial by the ECOG and RTOG. Proc Am Soc Clin Oncol. (1996) 15:376.
25. Aupérin A, Arriagada R, Pignon JP, Le Péchoux C, Gregor A, Stephens RJ, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. prophylactic cranial irradiation overview collaborative group N Engl J Med. (1999) 341:476–84. doi: 10.1056/NEJM199908123410703
26. Snider JW, Gondi V, Brown PD, Tome W, Mehta MP. Prophylactic cranial irradiation: recent outcomes and innovations. CNS Oncol. (2014) 3:219–30. doi: 10.2217/cns.14.22
27. Meert AP, Paesmans M, Berghmans T, Martin B, Mascaux C, Vallot F, et al. Prophylactic cranial irradiation in small cell lung cancer: a systematic review of the literature with meta-analysis. BMC Cancer (2001) 1:5. doi: 10.1186/1471-2407-1-5
28. Slotman B, Faivre-Finn C, Kramer G, Rankin E, Snee M, Hatton M, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. (2007) 357:664–72. doi: 10.1056/NEJMoa071780
29. Schild SE, Foster NR, Meyers JP, Ross HJ, Stella PJ, Garces YI, et al. Prophylactic cranial irradiation in small-cell lung cancer: findings from a North Central Cancer Treatment Group Pooled Analysis. Ann Oncol. (2012) 23:2919–24. doi: 10.1093/annonc/mds123
30. Seto T, Takahashi T, Yamanaka T. Prophylactic cranial irradiation has a detrimental effect on the overall survival of patients with extensive disease small cell lung cancer:results of a Japanese randomized Phase III trial. Proc Am Soc Clin Oncol. (2014) 32:663–71. doi: 10.1016/S1470-2045(17)30230-9
31. Albain KS, Rusch VW, Crowley JJ, Rice TW, Turrisi AT, Weick JK, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. (1995) 13:1880–92. doi: 10.1200/JCO.1995.13.8.1880
32. Andre F, Grunenwald D, Pujol JL, Girard P, Dujon A, Brouchet L, et al. Patterns of relapse of N2 nonsmall-cell lung carcinoma patients treated with preoperative chemotherapy: should prophylactic cranial irradiation be reconsidered. Cancer (2001) 91:2394–400. doi: 10.1002/1097-0142(20010615)91:12<2394::AID-CNCR1273>3.0.CO;2-6
33. Carolan H, Sun AY, Bezjak A, Yi QL, Payne D, Kane G, et al. Does the incidence and outcome of brain metastases in locally advanced non-small cell lung cancer justify prophylactic cranial irradiation or early detection. Lung Cancer (2005) 49:109–15. doi: 10.1016/j.lungcan.2004.12.004
34. Gaspar LE, Chansky K, Albain KS, Vallieres E, Rusch V, Crowley JJ, et al. Time from treatment to subsequent diagnosis of brain metastases in stage III non-small-cell lung cancer: a retrospective review by the Southwest Oncology Group. J Clin Oncol. (2005) 23:2955–61. doi: 10.1200/JCO.2005.08.026
35. Komaki R, Scott CB, Byhardt R, Emami B, Asbell SO, Russell AH, et al. Failure patterns by prognostic group determined by recursive partitioning analysis (RPA) of 1547 patients on four radiation therapy oncology group (RTOG) studies in inoperable nonsmall-cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys. (1998) 42:263–7. doi: 10.1016/S0360-3016(98)00213-2
36. Robnett TJ, Machtay M, Stevenson JP, Algazy KM, Hahn SM. Factors affecting the risk of brain metastases after definitive chemoradiation for locally advanced non-small-cell lung carcinoma. J Clin Oncol. (2001) 19:1344–9. doi: 10.1200/JCO.2001.19.5.1344
37. Strauss GM, Herndon JE, Sherman DD, Mathisen DJ, Carey RW, Choi NC, et al. Neoadjuvant chemotherapy and radiotherapy followed by surgery in stage IIIA non-small-cell carcinoma of the lung: report of a Cancer and Leukemia Group B phase II study. J Clin Oncol. (1992) 10:1237–44. doi: 10.1200/JCO.1992.10.8.1237
38. Cox JD, Stanley K, Petrovich Z, Paig C, Yesner R. Cranial irradiation in cancer of the lung of all cell types. JAMA (1981) 245:469–72. doi: 10.1001/jama.1981.03310300023013
39. Umsawasdi T, Valdivieso M, Chen TT, Barkley HT, Booser DJ, Chiuten DF, et al. Role of elective brain irradiation during combined chemoradiotherapy for limited disease non-small cell lung cancer. J Neurooncol. (1984) 2:253–9. doi: 10.1007/BF00253278
40. Russell AH, Pajak TE, Selim HM, Paradelo JC, Murray K, Bansal P, et al. Prophylactic cranial irradiation for lung cancer patients at high risk for development of cerebral metastasis: results of a prospective randomized trial conducted by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. (1991) 21:637–43. doi: 10.1016/0360-3016(91)90681-S
41. Miller TP, Canwell JJ, Mira J. A randomized trial of chemotherapy and radiotherapy for stage III non-small cell lung cancer. Cancer Ther. (1998) 1:229–36.
42. Gore EM, Bae K, Wong SJ, Sun A, Bonner JA, Schild SE, et al. Phase III comparison of prophylactic cranial irradiation versus observation in patients with locally advanced non-small-cell lung cancer: primary analysis of radiation therapy oncology group study RTOG 0214. J Clin Oncol. (2011) 29:272–8. doi: 10.1200/JCO.2010.29.1609
43. Le Péchoux C, Arriagada R. Prophylactic cranial irradiation in small cell lung cancer. Hematol Oncol Clin North Am. (2004) 18:355–72. doi: 10.1016/j.hoc.2003.12.004
44. Wolfson AH, Bae K, Komaki R, Meyers C, Movsas B, Le Pechoux C, et al. Primary analysis of a phase II randomized trial Radiation Therapy Oncology Group (RTOG) 0212: impact of different total doses and schedules of prophylactic cranial irradiation on chronic neurotoxicity and quality of life for patients with limited-disease small-cell lung cancer. Int J Radiat Oncol Biol Phys. (2011) 81:77–84. doi: 10.1016/j.ijrobp.2010.05.013
45. Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. (2003) 63:4021–7.
46. Ghia A, Tomé WA, Thomas S, Cannon G, Khuntia D, Kuo JS, et al. Distribution of brain metastases in relation to the hippocampus: implications for neurocognitive functional preservation. Int J Radiat Oncol Biol Phys. (2007) 68:971–7. doi: 10.1016/j.ijrobp.2007.02.016
47. Gondi V, Pugh SL, Tome WA, Caine C, Corn B, Kanner A, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. (2014) 32:3810–6. doi: 10.1200/JCO.2014.57.2909
48. Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. (2004) 162:39–47. doi: 10.1667/RR3206
49. Brown PD, Pugh S, Laack NN, Wefel JS, Khuntia D, Meyers C, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. (2013) 15:1429–37. doi: 10.1093/neuonc/not114
50. Yang JJ, Zhou C, Huang Y, Feng J, Lu S, Song Y, et al. Icotinib versus whole-brain irradiation in patients with EGFR-mutant non-small-cell lung cancer and multiple brain metastases (BRAIN): a multicentre, phase 3, open-label, parallel, randomised controlled trial. Lancet Respir Med. (2017) 5:707–16. doi: 10.1016/S2213-2600(17)30262-X
51. Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med. (2017) 377:829–38. doi: 10.1056/NEJMoa1704795
52. Long GV, Atkinson V, Lo S, Sandhu S, Guminski AD, Brown MP, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. (2018) 19:672–81. doi: 10.1016/S1470-2045(18)30139-6
53. Lin NU, Carey LA, Liu MC, Younger J, Come SE, Ewend M, et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. (2008) 26:1993–9. doi: 10.1200/JCO.2007.12.3588
54. Lin NU, Diéras V, Paul D, Lossignol D, Christodoulou C, Stemmler HJ, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. (2009) 15:1452–9. doi: 10.1158/1078-0432.CCR-08-1080
55. Freedman RA, Gelman RS, Wefel JS, Melisko ME, Hess KR, Connolly RM, et al. Translational Breast Cancer Research Consortium (TBCRC) 022: a phase II trial of neratinib for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J Clin Oncol. (2016) 34:945–52. doi: 10.1200/JCO.2015.63.0343
Keywords: CNS metastases, prevention, SRS, WBRT, immunotherapy, targeted therapy (TT)
Citation: Bovi JA (2018) Prevention of Brain Metastases. Front. Neurol. 9:758. doi: 10.3389/fneur.2018.00758
Received: 29 June 2018; Accepted: 21 August 2018;
Published: 28 September 2018.
Edited by:
Sunit Das, St. Michael's Hospital, CanadaReviewed by:
Riccardo Soffietti, Università degli Studi di Torino, ItalyArjun Sahgal, University of Toronto, Canada
Kaisorn Chaichana, Mayo Clinic, United States
Copyright © 2018 Bovi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joseph A. Bovi, amJvdmlAbWN3LmVkdQ==
 Joseph A. Bovi
Joseph A. Bovi