- 1Klinik und Hochschulambulanz für Neurologie, Charité–Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, Berlin Institute of Health, Berlin, Germany
- 2Berlin Institute of Health (BIH), Berlin, Germany
- 3Center for Stroke Research Berlin (CSB), Charité – Universitätsmedizin Berlin, Berlin, Germany
- 4German Centre for Cardiovascular Research (DZHK), Partner Site, Berlin, Germany
- 5German Center for Neurodegenerative Diseases (DZNE), Partner Site, Berlin, Germany
Background: Randomized controlled trials indicate that patent foramen ovale (PFO) closure reduces risk of stroke recurrence in patients with cryptogenic stroke and PFO. However, the optimal time point for PFO closure is unknown and depends on the risk of stroke recurrence.
Objective: We aimed to investigate risk of early new ischemic lesions on cerebral magnetic resonance imaging (MRI) in cryptogenic stroke patients with and without PFO.
Methods: Cryptogenic stroke patients underwent serial MRI examinations within 1 week after symptom onset to detect early new ischemic lesions. Diffusion-weighted imaging (DWI) lesions were delineated, co-registered, and analyzed visually for new hyperintensities by raters blinded to clinical details. A PFO was classified as stroke-related in patients with PFO and a Risk of Paradoxical Embolism (RoPE) score >5 points.
Results: Out of 80 cryptogenic stroke patients, risk of early recurrent DWI lesions was not significantly different in cryptogenic stroke patients with and without PFO. Similar results were observed in patients ≤ 60 years of age. Patients with a stroke-related PFO even had a significantly lower risk of early recurrent ischemic lesions compared to all other patients with cryptogenic stroke (unadjusted odds ratio 0.23 [95% confidence interval 0.06–0.87], P = 0.030).
Conclusion: Our data argue against a high risk of early stroke recurrence in patients with cryptogenic stroke and PFO.
Introduction
In the general population, prevalence of patent foramen ovale (PFO) is ~25% (1). Prevalence of PFO in patients with cryptogenic stroke is significantly higher than in the general population (2–5). In these patients, PFO is considered a possible etiology of stroke. The suggested pathophysiologic mechanisms include paradoxical embolism and local intraseptal thrombosis (5–9).
Randomized controlled trials indicate that patent foramen ovale (PFO) closure combined with antiplatelet therapy compared to antiplatelet therapy alone significantly reduces risk of stroke recurrence in young patients with cryptogenic stroke and PFO (10–12). Evidence in favor of PFO closure raises the question whether PFO closure is an urgent matter (13). However, the optimal time point for PFO closure is unknown and depends on the risk of stroke recurrence (14). New diffusion-weighted imaging (DWI) lesions after acute ischemic stroke are a sensitive marker for new ischemic events and are detected more frequently than clinical stroke recurrence alone (15–20). Here we analyzed, whether presence of PFO in cryptogenic stroke patients is associated with occurrence of early recurrent DWI lesions within 1 week after stroke.
Materials and Methods
Study Design and Patients
We performed a post-hoc analysis of data drawn from an observational study conducted by the Center for Stroke Research Berlin (CSB) at the Charité—Universitätsmedizin Berlin, Campus Benjamin Franklin (Berlin, Germany; clinicaltrials.gov: NCT00715533). The study included acute ischemic stroke patients that were able to undergo MRI within 24 h after symptom onset (16–18, 21). We included patients recruited between March, 2008 and December, 2010 with a complete set of three MRI examinations within the first week after symptom onset and an undetermined etiology of stroke according to Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria (22). Diagnostic work-up in all patients included MRI, MR-angiography, carotid ultrasonography and cardiac rhythm monitoring for at least 24 h. Patients with multiple potential causes of stroke (22) and patients who underwent endovascular revascularization procedures were excluded. We excluded patients who underwent endovascular revascularization procedures, because endovascular procedures may cause new DWI lesions on MRI (23, 24). Patients who received thrombolysis were not excluded. All patients included in this study received standard stroke unit care following the guidelines of the European Stroke Organization (ESO) and the German Stroke Society (DSG; https://www.dsg-info.de/stroke-units/stroke-units-uebersicht.html). None of the patients included in this study underwent PFO closure during the study period. The study was approved by the local Ethics Committee (EA4/026/08). All patients gave written informed consent.
MRI
Details have been reported previously (18, 21). In short, we conducted three cerebral MRI examinations on a 3-Tesla MRI scanner (Tim Trio, Siemens Medical, Erlangen, Germany): on admission, on the following day, and 4 to 7 days after symptom onset. DWI images were pseudonymized and afterwards reviewed in random order. Raters were blinded to clinical information. Hyperintensities on initial DWIs were delineated manually and then co-registered. Co-registered DWIs were analyzed visually for new hyperintensities through slice-by-slice comparison of the first and second, as well as the second and third DWI. New hyperintensities had to be clearly separate from the index lesion. All new diffusion hyperintensities regardless of apparent diffusion coefficient value were considered (17, 18, 21) Number of new DWI lesions was counted.
Clinical Data
Sociodemographic and laboratory data were collected from the medical records. All patients were assessed for stroke severity directly before the first MRI examination by physicians certified to assess the National Institutes of Health Stroke Scale (NIHSS) (25). PFO was diagnosed by transesophageal echocardiography (TEE) or transcranial Doppler. Both techniques have similar sensitivity and specificity (5, 26, 27). An associated atrial septum aneurysm (ASA) was diagnosed in patients with septum primum excursion >10 mm on TEE (11). The Risk of Paradoxical Embolism (RoPE) score was used to differentiate between patients with a high probability of a stroke-related PFO (high attributable risk) vs. an incidental PFO.(26, 28) The RoPE score is externally validated (28, 29) and varies from 0 to 9 points with higher scores indicating a higher attributable risk. In stroke patients with PFO and a RoPE score >5 points, the PFO has an attributable risk for stroke of 62% or more (28). Therefore in this study, patients with an undetermined etiology of stroke (22), PFO and a RoPE score >5 points were assumed to have a stroke-related PFO.
Statistics
For comparisons of nominal and categorical variables, we used unadjusted, univariate logistic regression to obtain odds ratios (OR) and corresponding 95% confidence intervals (CI). Additionally, we used chi-square test to compare (1) patients with and without data available regarding PFO and (2) patients with and without tested PFO parameters (any PFO, stroke-related PFO, PFO in patients ≤ 60 years of age) regarding appearance of new DWI lesions. For comparisons of continuous variables, we used the Mann–Whitney U-test. Statistical significance was determined at an alpha level of 0.05 (16–18, 21).
Results
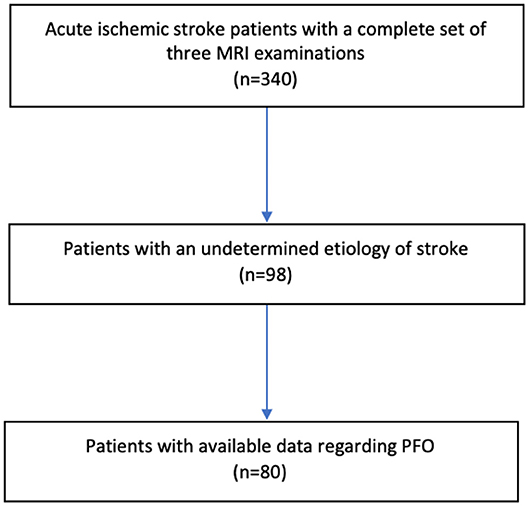
Out of 340 acute ischemic stroke patients examined, 98 patients (29%) had a cryptogenic stroke defined as stroke of undetermined etiology. Of these, 80 patients had data available regarding PFO and constitute the study population (Figure 1, Table 1). Cryptogenic stroke patients with and without data available regarding PFO did not differ regarding sex and NIHSS > 3 points. Patients without data available regarding PFO were more often > 60 years of age (88.9 vs. 57.5%, p = 0.013).
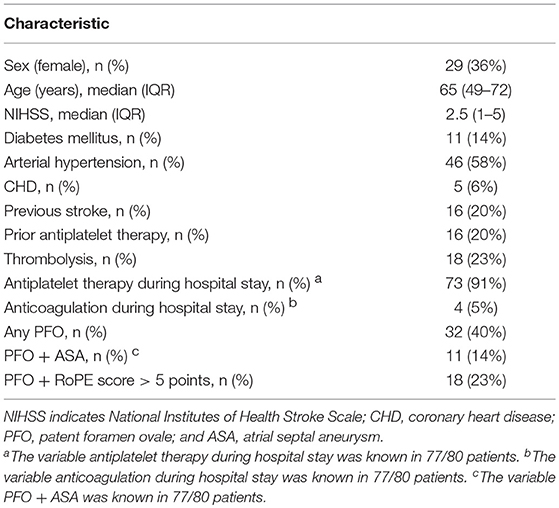
Thirty-two out of 80 patients (40%) had any PFO and 18/80 patients (23%) with PFO had a RoPE score >5 points (stroke-related PFO). Early recurrent DWI lesions were detected in 11 of 32 patients (34%) with any PFO and in 3 of 18 patients (17%) with a stroke-related PFO.
Neither any PFO (unadjusted OR 0.67 [95%CI 0.27–1.70], p = 0.403; 34% [with PFO] vs. 44% [without PFO], p = 0.402) nor stroke-related PFO were significantly positively associated with early recurrent DWI lesions. On the contrary, stroke-related PFO was negatively associated with early recurrent DWI lesions (unadjusted OR 0.23 [95%CI 0.06–0.87], p = 0.030; 17% [with stroke-related PFO] vs. 47% [without PFO or with incidental PFO], p = 0.022). Factors associated with early recurrent DWI lesions were diabetes mellitus, arterial hypertension, and older age (Table 2).
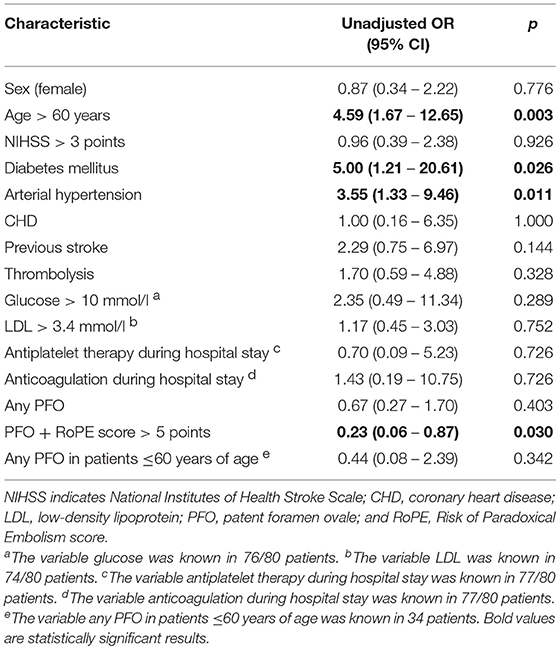
Table 2. Sociodemographic and clinical characteristics of the study population by the presence vs. absence of early recurrent DWI lesions.
Out of the 80 patients with cryptogenic stroke, 34 patients were ≤ 60 years of age (29% women; median age [years]: 48 [interquartile range {IQR}, 41–52]; median NIHSS: 2 [IQR, 1–4]). Of these, 20 patients (59%) had a PFO (18/20 patients had a stroke-related PFO). Early recurrent DWI lesions were detected in 3 of 20 patients (15%) with PFO. PFO was not positively associated with early recurrent DWI lesions [unadjusted OR 0.44 [95%CI 0.08–2.39], p = 0.342; 15% [with PFO] vs. 29% [without PFO], p = 0.335].
Results did not change when PFO and an associated atrial septal aneurysm (ASA) was taken into account (PFO+ASA: unadjusted OR 0.51 [95%CI 0.12–2.09], p = 0.349; PFO+ASA in patients ≤ 60 years of age: unadjusted OR 0.54 [95%CI 0.05 – 5.50], p = 0.605).
The number of early recurrent DWI lesions was significantly lower in cryptogenic stroke patients with any PFO (32/80) compared to patients without PFO (median: 2 [IQR, 1-2] vs. 5 [IQR, 2–8]; p = 0.014).
Discussion
In this population of patients with cryptogenic stroke undergoing serial MRI examinations, PFO was not associated with early recurrent ischemic lesions.
Risk of stroke recurrence may vary over time. For example, in stroke patients with symptomatic carotid stenosis, endarterectomy should be done within the first 2 weeks after the initial event because the survival curve for recurrence is front-loaded (30, 31).
In general stroke cohorts, early recurrent ischemic lesions are reported to appear in 24–34% of patients while higher rates are reported, for example, in acute stroke patients with large artery atherosclerosis (20). In this study, frequency of early recurrent ischemic lesions was lower both in patients with a stroke-related PFO (17%) and in young stroke patients ≤ 60 years of age with PFO (15%). In all tested conditions (any PFO, stroke-related PFO, PFO in patients ≤ 60 years of age) presence of PFO was not positively associated with early recurrent ischemic lesions. In the CLOSE trial (Patent Foramen Ovale Closure or Anticoagulants vs. Antiplatelet Therapy to Prevent Stroke Recurrence) (11) only stroke patients with PFO and additional echocardiographic features like ASA were included. Therefore, we included PFO and an associated ASA as an additional parameter but lack of association regarding early recurrent ischemic lesions remained. Patients with a stroke-related PFO even had a significantly lower risk of early recurrent ischemic lesions compared to all other patients with cryptogenic stroke (patients without PFO and patients with an incidental PFO)–this result complements a previous study that reported a low long-term risk of a clinically diagnosed stroke recurrence in patients with a stroke-related PFO (28). In addition, number of early recurrent ischemic lesions in patients with any PFO was significantly lower compared to patients without PFO. In contrast to PFO, well-known risk factors for both clinically overt and silent stroke recurrence like diabetes mellitus, arterial hypertension, and older age (32, 33) were associated with an increased risk of early recurrent ischemic lesions in this cohort.
Limitations of this study have to be considered. First, this is a single-center post-hoc analysis. Second, the number of patients was small. We cannot exclude a type-2 error with respect to non-significant findings. Still, the point estimates argue against a clinically relevant, increased risk of early recurrent ischemic lesions in patients with PFO. Patients with a stroke-related PFO had a significantly lower risk of early recurrent ischemic lesions. In addition, the number of early recurrent ischemic lesions in patients with any PFO was significantly lower.
In conclusion, our data argue against a high risk of early stroke recurrence in patients with cryptogenic stroke and PFO, especially in patients eligible for PFO closure (≤ 60 years of age, patients with PFO and an associated ASA). Therefore, our findings suggest that PFO closure does not necessarily have to be performed early after the initial stroke. Rather a comprehensive clinical assessment to exclude other potential causes of stroke should be prioritized.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator for analysis at the Department of Neurology, Charité–Universitätsmedizin Berlin, Berlin. Data sharing will be restricted to non-commercial and academic purposes only. The corresponding author will keep a copy of the final data set for at least 10 years.
Author Contributions
TBB: conception/design of the work, acquisition of data, data analysis and interpretation, drafting the article, and final approval; TU: acquisition of data, data interpretation, critical review, and final approval; JFS: data analysis and interpretation, critical review, and final approval; HE: data analysis and interpretation, critical review, and final approval; JBF: acquisition of data, data interpretation, critical review, and final approval; HJA: data interpretation, critical review, and final approval; ME: data interpretation, critical review, and final approval; CHN: conception/design of the work, data analysis and interpretation, critical review, and final approval.
Funding
TBB is participant in the BIH Charité Clinician Scientist Program funded by the Charité—Universitätsmedizin Berlin and the Berlin Institute of Health. JBF has received consulting, lecture, and advisory board fees from BioClinica, Cerevast, Artemida, and Brainomix as well as a grant from the German Federal Ministry of Education and Research (01EO0801 and 01EO01301) As PI he receives funding from the European Union Seventh Framework Program [FP7/2007–2013] under grant agreement no. 278276 (WAKE-UP). HJA has received funding from the DFG, BMBF, Innovationsfonds, Future-Funds Berlin with cofounding of the European Union, Pfizer and Stiftung Deutsche Schlaganfall-Hilfe. He has received speaker or consultancy honoraria from Boehringer Ingelheim, Siemens healthcare, Pfizer, BMS, Bayer, Sanofi, Dayichi Sankyo, Ever Pharma, Lundbeck Pharma, and NovoNordisk. ME reports grant support from the DFG (NeuroCure, SFB TR 43, KFO 247), BMBF (Center for Stroke Research Berlin), DZHK and DZNE (for PRAISE Study), EU (WakeUp, Counterstroke), Corona Foundation (Vascular Senescence); Fondation Leducq, and Bayer (Mondafis, Berlin Afib registry), and fees paid to the Charite for lectures and/or consulting from Bayer, BI, BMS/Pfizer, Daiichi Sankyo, Amgen, Sanofi, Covidien, GSK, Ever, Novartis, all outside the submitted work. CHN has received consulting and lecture fess from Boehringer Ingelheim, W. L. Gore and Associates, Bristol-Myers Squibb, Pfizer, Sanofi. We acknowledge support from the German Research Foundation (DFG) and the Open Access Publication Fund of Charité—Universitätsmedizin Berlin.
Conflict of Interest Statement
TBB has received travel support from W. L. Gore and Associates. JFS has received a speaker's honorarium from W. L. Gore and Associates. CHN has received speaker's honoraria from W. L. Gore and Associates.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Meissner I, Khandheria BK, Heit JA, Petty GW, Sheps SG, Schwartz GL, et al. Patent foramen ovale: innocent or guilty? J Am Coll Cardiol. (2006) 47:440–5. doi: 10.1016/j.jacc.2005.10.044
2. Lamy C, Giannesini C, Zuber M, Arquizan C, Meder JF, Trystram D, et al. Clinical and imaging findings in cryptogenic stroke patients with and without patent foramen ovale the PFO-ASA study. Stroke (2002) 33:706–11. doi: 10.1161/hs0302.104543
3. Overell JR, Bone I, Lees KR. Interatrial septal abnormalities and stroke: a meta-analysis of case-control studies. Neurology (2000) 55:1172–1179. doi: 10.1212/WNL.55.8.1172
4. Handke M, Harloff A, Olschewski M, Hetzel A, Geibel A. Patent foramen ovale and cryptogenic stroke in older patients. N Engl J Med. (2007) 357:2262–8. doi: 10.1056/NEJMoa071422
5. Bang OY, Lee MJ, Ryoo S, Kim SJ, Kim JW. Patent foramen ovale and stroke–current status. J Stroke (2015) 17:229–37. doi: 10.5853/jos.2015.17.3.229
6. Koullias GJ. Massive paradoxical embolism: caught in the act. Circulation (2004) 109:3056–7. doi: 10.1161/01.CIR.0000132371.91318.6D
7. Meier B, Kalesan B, Mattle HP, Khattab AA, Hildick-Smith D, Dudek D, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. (2013) 368:1083–91. doi: 10.1056/NEJMoa1211716
8. Kitsios GD, Lasker A, Singh J, Thaler DE. Recurrent stroke on imaging and presumed paradoxical embolism: a cross-sectional analysis. Neurology (2012) 78:993–7. doi: 10.1212/WNL.0b013e31824d58bc
9. Berthet K, Lavergne T, Cohen A, Guize L, Bousser MG, Le Heuzey JY, et al. Significant association of atrial vulnerability with atrial septal abnormalities in young patients with ischemic stroke of unknown cause. Stroke (2000) 31:398–403. doi: 10.1161/01.STR.31.2.398
10. Søndergaard L, Kasner SE, Rhodes JF, Andersen G, Iversen HK, Nielsen-Kudsk JE, et al. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. (2017) 377:1033–42. doi: 10.1056/NEJMoa1707404
11. Mas J-L, Derumeaux G, Guillon B, Massardier E, Hosseini H, Mechtouff L, et al. Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med. (2017) 377:1011–21. doi: 10.1056/NEJMoa1705915
12. Saver JL, Carroll JD, Thaler DE, Smalling RW, MacDonald LA, Marks DS, et al. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. (2017) 377:1022–32. doi: 10.1056/NEJMoa1610057
13. Braemswig TB, Scheitz JF, Nolte CH. Trials of patent foramen ovale closure. N Engl J Med. (2017) 377:2599. doi: 10.1056/NEJMc1714320
14. Piccolo R, Franzone A, Siontis GCM, Stortecky S, Pilgrim T, Meier B, et al. Patent foramen ovale closure vs. medical therapy for recurrent stroke prevention: evolution of treatment effect during follow-up. Int J Cardiol. (2018) 255:29–31. doi: 10.1016/j.ijcard.2018.01.001
15. Kang D-W, Latour LL, Chalela JA, Dambrosia J, Warach S. Early ischemic lesion recurrence within a week after acute ischemic stroke. Ann Neurol. (2003) 54:66–74. doi: 10.1002/ana.10592
16. Nolte CH, Albach FN, Heuschmann PU, Brunecker P, Villringer K, Endres M, et al. Silent New DWI Lesions within the first week after stroke. Cerebrovasc Dis. (2012) 33:248–54. doi: 10.1159/000334665
17. Usnich T, Albach FN, Brunecker P, Fiebach JB, Nolte CH. Incidence of new diffusion-weighted imaging lesions outside the area of initial hypoperfusion within 1 week after acute ischemic stroke. Stroke (2012) 43:2654–8. doi: 10.1161/STROKEAHA.112.655993
18. Braemswig TB, Usnich T, Albach FN, Brunecker P, Grittner U, Scheitz JF, et al. Early new diffusion-weighted imaging lesions appear more often in stroke patients with a multiple territory lesion pattern. Stroke (2013) 44:2200–4. doi: 10.1161/STROKEAHA.111.000810
19. Erdur H, Scheitz JF, Ebinger M, Rocco A, Grittner U, Meisel A, et al. In-hospital stroke recurrence and stroke after transient ischemic attack: frequency and risk factors. Stroke (2015) 46:1031–7. doi: 10.1161/STROKEAHA.114.006886
20. Lee E-J, Kang D-W, Warach S. Silent new brain lesions: innocent bystander or guilty party? J Stroke (2016) 18:38–49. doi: 10.5853/jos.2015.01410
21. Braemswig TB, Nolte CH, Fiebach JB, Usnich T. Early new ischemic lesions located outside the initially affected vascular territory appear more often in stroke patients with elevated glycated hemoglobin (HbA1c). Front Neurol. (2017) 8:606. doi: 10.3389/fneur.2017.00606
22. Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke (1993) 24:35–41.
23. Bendszus M, Stoll G. Silent cerebral ischaemia: hidden fingerprints of invasive medical procedures. Lancet Neurol. (2006) 5:364–72. doi: 10.1016/S1474-4422(06)70412-4
24. Bang OY, Kim GM, Chung CS, Kim SJ, Kim KH, Jeon P, et al. Differential pathophysiological mechanisms of stroke evolution between new lesions and lesion growth: perfusion-weighted imaging study. Cerebrovasc Dis. (2010) 29:328–35. doi: 10.1159/000278928
25. Berger K, Weltermann B, Kolominsky-Rabas P, Meves S, Heuschmann P, Böhner J, et al. Untersuchung zur Reliabilität von Schlaganfallskalen. Fortschritte Neurol · Psychiatr. (1999) 67:81–6. doi: 10.1055/s-2007-993985
26. Thaler DE, Ruthazer R, Weimar C, Mas J-L, Serena J, Di Angelantonio E, et al. Recurrent stroke predictors differ in medically treated patients with pathogenic vs other PFOs. Neurology (2014) 83:221–6. doi: 10.1212/WNL.0000000000000589
27. Droste DW, Reisener M, Kemény V, Dittrich R, Schulte-Altedorneburg G, Stypmann J, et al. Contrast transcranial Doppler ultrasound in the detection of right-to-left shunts. Reproducibility, comparison of 2 agents, and distribution of microemboli. Stroke (1999) 30:1014–8.
28. Kent DM, Ruthazer R, Weimar C, Mas J-L, Serena J, Homma S, et al. An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurology (2013) 81:619–25. doi: 10.1212/WNL.0b013e3182a08d59
29. Prefasi D, Martínez-Sánchez P, Fuentes B, Díez-Tejedor E. The utility of the RoPE score in cryptogenic stroke patients ≤ 50 years in predicting a stroke-related patent foramen ovale. Int J Stroke (2016) 11:NP7–8. doi: 10.1177/1747493015607505
30. Rothwell P, Eliasziw M, Gutnikov S, Warlow C, Barnett H. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet (2004) 363:915–24. doi: 10.1016/S0140-6736(04)15785-1
31. Rothwell PM, Mehta Z, Howard SC, Gutnikov SA, Warlow CP. From subgroups to individuals: general principles and the example of carotid endarterectomy. Lancet (2005) 365:256–65. doi: 10.1016/S0140-6736(05)17746-0
32. Vermeer SE, Longstreth WT, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol. (2007) 6:611–9. doi: 10.1016/S1474-4422(07)70170-9
Keywords: patent foramen ovale, stroke, MRI–magnetic resonance imaging, diffusion-weighted (DW) imaging, new ischemic lesions
Citation: Braemswig TB, Usnich T, Scheitz JF, Erdur H, Fiebach JB, Audebert HJ, Endres M and Nolte CH (2018) Early Recurrent Ischemic Lesions in Patients With Cryptogenic Stroke and Patent Foramen Ovale: An Observational Study. Front. Neurol. 9:996. doi: 10.3389/fneur.2018.00996
Received: 09 September 2018; Accepted: 05 November 2018;
Published: 22 November 2018.
Edited by:
Maurizio Acampa, Azienda Ospedaliera Universitaria Senese, ItalyReviewed by:
Nishant K. Mishra, Icahn School of Medicine at Mount Sinai, United StatesSvetlana Lorenzano, La Sapienza University of Rome, Italy
Cumara B. O'Carroll, Mayo Clinic Arizona, United States
Copyright © 2018 Braemswig, Usnich, Scheitz, Erdur, Fiebach, Audebert, Endres and Nolte. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tim Bastian Braemswig, dGltLWJhc3RpYW4uYnJhZW1zd2lnQGNoYXJpdGUuZGU=
 Tim Bastian Braemswig
Tim Bastian Braemswig Tatiana Usnich
Tatiana Usnich Jan F. Scheitz
Jan F. Scheitz Hebun Erdur
Hebun Erdur Heinrich J. Audebert
Heinrich J. Audebert Matthias Endres
Matthias Endres Christian H. Nolte
Christian H. Nolte