- 1Department of Neurology, Stroke Center, University Hospital Basel and University of Basel, Basel, Switzerland
- 2Pharmaceutical Care Research Group, Department of Pharmaceutical Sciences, University of Basel, Basel, Switzerland
- 3Neurorehabilitation Unit, University Center for Medicine of Ageing and Rehabilitation, University of Basel and Felix-Platter Hospital, Basel, Switzerland
- 4Clinical Trial Unit, University Hospital Basel, Basel, Switzerland
Background: Non-adherence to direct oral anticoagulants (DOACs) remains a matter of concern, especially for patients with a recent stroke. However, data on electronically monitored adherence and adherence-improving interventions are scarce.
Aims: We aim to use electronic monitoring in DOAC-treated stroke patients to (i) evaluate the effect of an educational, reminder-based adherence-improving intervention, (ii) investigate predictors of non-adherence, (iii) identify reliable self-report measures of adherence, and (iv) explore the association of non-adherence with clinical outcomes.
Methods: Single-center, randomized, crossover, open-label study. Adherence to DOACs of polymedicated patients self-administering their medication will be monitored electronically throughout the 12-month-long study following hospitalization for ischemic stroke. After a 6-month observational phase, patients will receive pharmaceutical counseling with feedback on their intake history and be given a multi-compartment pillbox for the subsequent 6-month interventional phase. The pillbox will provide intake reminders either during the first or the last three interventional-phase months. Patients will be randomly allocated to reminders-first or reminders-last.
Study outcomes: Primary: non-optimal timing adherence; Secondary: non-optimal taking adherence; timing adherence; taking adherence; self-reported adherence; clinical outcomes including ischemic and hemorrhagic events; patient-reported device usability and satisfaction.
Sample size estimates: A sample of 130 patients provides 90% power to show a 20% improvement of the primary adherence outcome with intake reminders.
Discussion: MAAESTRO will investigate various aspects of non-adherence and evaluate the effect of an adherence-improving intervention in DOAC-treated patients with a recent stroke using electronic monitoring.
Clinical Trial Registration: ClinicalTrials.gov identifier: NCT03344146, Swiss National Clinical Trials Portal SNCTP000002410
Background and aims
Non-adherence to direct oral anticoagulants (DOACs) remains a matter of concern due to their short half-lives and lack of coagulation monitoring (1). So far, research on adherence to DOACs has been sparse and inconsistent, especially among patients treated for recurrent stroke prevention. Studies using self-reporting (2, 3) and prescription claims data (1) yielded discrepant results on the extent of non-adherence and failed to identify concrete predictors. Although electronic monitoring is considered the most accurate method in adherence research (4), data remain scarce (5).
Non-adherence to DOACs is associated with increased risk for stroke, bleeding, and death (1, 2). The need for effective adherence-improving strategies is increasingly recognized (5, 6). Patients with a recent stroke are at high risk for both recurrence and non-adherence due to neurological and cognitive deficits (7). Although they would especially benefit from adherence-improving interventions, to our knowledge just one study used electronic devices to enhance adherence in DOAC-treated stroke patients (8). So far, no studies investigated multi-compartment compliance aids (MCCAs) with reminder function, i.e., compartmentalized pillboxes with discrete sections for each dosing occasion that also provide audiovisual intake reminders.
We aim to use electronic monitoring and reminder-delivering MCCAs in DOAC-treated patients with a recent stroke to evaluate the effect of an educational, reminder-based adherence-improving intervention, investigate predictors of non-adherence, identify reliable self-report measures of adherence, and explore the association of non-adherence with clinical outcomes. Our main hypothesis is that intake reminders improve adherence.
Methods
Design
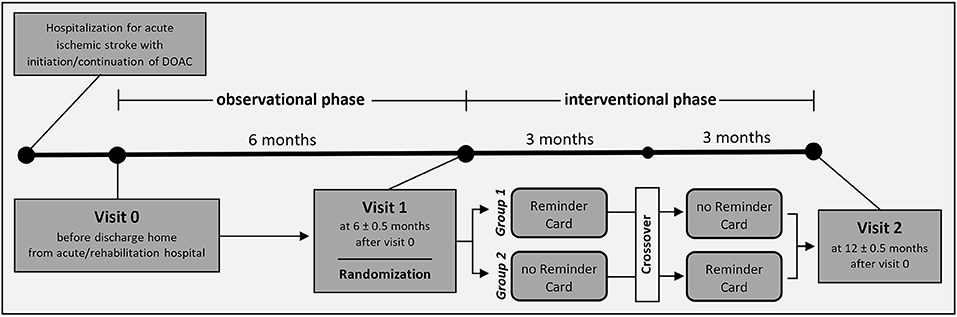
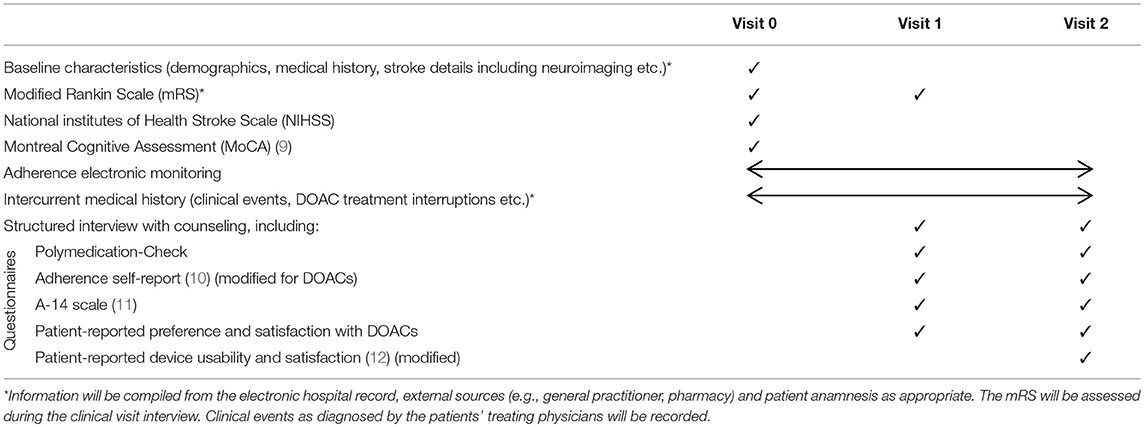
This is a single-center, randomized, crossover, open-label study. Figure 1 shows the study flowchart and Table 1 the assessments schedule.
Patient Population
Inclusion criteria:
• Age ≥ 18 years
• Hospitalization for ischemic stroke
• DOAC (dabigatran, rivaroxaban, apixaban, edoxaban) treatment for recurrent stroke prevention (e.g., in non-valvular atrial fibrillation or other indications)
• Polypharmacy (≥ 3 medications, including DOACs)
• Medication self-administration
Exclusion criteria:
• Medication administration by a third person (pillbox prefilling by a third person, physical disability and cognitive deficits do not exclude patients provided they self-administer their medication)
• Unable or unwilling to participate
Randomization
Patients will be openly randomized to one of two groups in a 1:1 ratio at visit 1 in a crossover design: During the 6-month interventional phase, Group 1 will receive intake reminders for the first 3 months and Group 2 for the last 3 months. Randomization will be performed by a central computer through the web-based electronic case report form and include a variance minimization algorithm to ensure balancing for sex, age, NIHSS, and prior pillbox use.
Intervention
Key methodological instrument for MAAESTRO is the novel Time4Med medication system (Adherence Innovations, Hong Kong, China) which includes (i) the Smart Card, a small electronic device featuring a button that records intake date and time when pressed, (ii) the Reminder Card, a small electronic device that provides audiovisual intake reminders, and (iii) a MCCA with discrete medication storage compartments for each time of the day and day of the week, upon which the Reminder Card can be attached. Patients will record their intakes using Smart Cards throughout the 12-month-long study. After the 6-month observational phase, patients will receive pharmaceutical counseling during a medication review, the Polymedication-Check (13), and feedback on their intake history (Visit 1). Based on these, the optimal intake timepoints will be identified for each patient. Patients will receive MCCAs for use throughout the 6-month interventional phase along with attachable Reminder Cards, which will be programmed to provide reminders at the optimal timepoints for either the first or the last 3 months.
Study Outcomes
The primary outcome is non-optimal timing adherence to DOACs, defined as ≥ 1 dose omitted or taken outside of 25% of the prescribed dosing schedule (± 6 h for once-daily and ± 3 h for twice-daily DOACs). It will be calculated for the observational phase and for the two interventional-phase periods separately. Physician-initiated temporary treatment interruptions will not be considered non-adherence.
Secondary outcomes are:
• non-optimal taking adherence (i.e., ≥ 1 dose omitted)
• timing adherence (i.e., proportion of doses taken within 25% of the prescribed dosing schedule)
• taking adherence (i.e., proportion of prescribed doses taken)
• self-reported adherence (based on the “Adherence self-report” and “A-14 scale” questionnaires, Table 1)
• clinical events including recurrent ischemic stroke, other ischemic events, bleeding and death
• patient-reported device usability and satisfaction
Sample Size Estimates
A sample of 130 participants is required to show a significant difference in non-optimal timing adherence with reminders, based on simulations using a mixed-effects logistic model with a power of 0.9 and a two-sided 0.05 alpha level and assuming (i) 20% adherence-enhancing effect of reminders, (ii) 45% rate of non-optimal timing adherence without reminders and (iii) 12% drop-out rate. These assumptions were informed by our previous experience with this patient population (3).
Statistical Analyses
The primary analysis will evaluate the reminders' effect on the primary outcome with a mixed-effects logistic model with Reminder Card (yes/no) as fixed and patient as random effect. Ancillary analyses will include the addition to the model of the following covariates:
(i) the interventional-phase period (first/second), in order to assess for period effects (adherence differences between the two periods independent of the reminders' effect). We designed the study with a crossover design, assuming that adherence will be generally stable over the relatively short study duration and thus no period effects are expected.
(ii) the interaction term “Reminder Card * period”, in order to assess for modification of the reminders' effect by the interventional-phase period (e.g., through a “carry-over effect”). We expect any interaction to be negligible.
(iii) the type of DOAC (once-/twice-daily), in order to assess for association between adherence and type of DOAC.
(iv) the interaction term “Reminder Card * type of DOAC”, in order to assess for modification of the reminders' effect by the type of DOAC.
Secondary analyses include the comparison of adherence outcomes between (i) the two interventional-phase periods and (ii) observational and interventional phase, as in the primary analysis. Non-adherence predictors (e.g., clinical and neuroimaging variables, mRS, MoCA test scores) will be assessed using logistic mixed lasso models. Descriptive statistics will be used for the comparison of self-reported with electronically recorded adherence, the association between adherence and clinical events and to present the rest of the data.
Compliance With Ethical Standards
MAAESTRO has been approved by the Ethics Committee northwest/central Switzerland (EKNZ2017-01552). All study procedures are in accordance with the provisions of ICH GCP and the 1964 Declaration of Helsinki and its later amendments. Written informed consent is obtained from each patient before enrolment. MAAESTRO is registered on ClinicalTrials.gov (NCT03344146) and on the Swiss National Clinical Trials Portal (SNCTP000002410).
Safety and Data Monitoring Body
No safety issues are expected. Any serious study-related events, along with the events captured as secondary study outcomes will be reported to the ethics committee. There will be no independent steering or safety committee. An external study monitor will routinely perform on-site visits to ensure per-protocol study conduct.
Study Organization and Funding
MAAESTRO is the joint initiative of the Stroke Center, University Hospital Basel and the Pharmaceutical Care Research Group, University of Basel. This study is funded by the University of Basel and a grant from the Swiss Academy of Medical Sciences and the Bangerter-Foundation (YTCR 31/17).
Discussion
MAAESTRO is designed with pragmatic eligibility criteria to ensure participants are as representative as possible of stroke survivors self-administering their medication. Our study uses MCCAs and includes polymedicated patients, for whom pillbox use is meaningful, as they comprise the vast majority of stroke patients in clinical practice (3).
To obtain objective adherence data in a real-life setting, we use electronic monitoring during a long observational phase without further interventions that might modify medication-taking behavior (4). This will allow us to search for non-adherence predictors, with emphasis on cognitive and neuroimaging characteristics, and for reliable self-report measures of adherence that best discriminate adherers from non-adherers. These will provide healthcare professionals with simple, inexpensive ways to identify patients at risk for non-adherence.
For the adherence-improving intervention we hypothesize that reminders will have the largest effect (14). We therefore designed and powered MAAESTRO to provide randomized evidence for their use. We expect that counseling and MCCAs will have an additional adherence-improving effect, which we will assess in before-after comparisons. Furthermore, we will capture patient-reported device usability and satisfaction to complement the intervention's evaluation.
MAAESTRO is not powered to determine the impact of non-adherence on clinical outcomes. However, the detailed electronic intake records will allow close examination of possible deviations preceding clinical outcomes on an individual basis, which may provide insights into the mechanisms of stroke and bleeding occurrence in DOAC-treated patients.
In summary, MAAESTRO will provide comprehensive data on electronically monitored adherence and most importantly, randomized evidence for an adherence-improving intervention in the yet unexplored population of DOAC-treated patients with a recent stroke. Patient enrolment began in January 2018.
Author Contributions
AP and VA contributed equally to study design, writing, and reviewing the manuscript. IA and PL contributed equally to study conception and design and manuscript review. KH and SE contributed to study design and manuscript review. SS contributed to statistical aspects of the protocol.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Borne RT, O'Donnell C, Turakhia MP, Varosy PD, Jackevicius CA, Marzec LN, et al. Adherence and outcomes to direct oral anticoagulants among patients with atrial fibrillation: findings from the veterans health administration. BMC Cardiovasc Disord.(2017) 17:236. doi: 10.1186/s12872-017-0671-6
2. Emren SV, Senoz O, Bilgin M, Beton O, Aslan A, Taskin U, et al. Drug adherence in patients with nonvalvular atrial fibrillation taking non-vitamin k antagonist oral anticoagulants in Turkey. Clin Appl Thromb Hemost. (2018) 24:525–31. doi: 10.1177/1076029617693940.
3. Polymeris AA, Traenka C, Hert L, Seiffge DJ, Peters N, De Marchis GM, et al. Frequency and determinants of adherence to oral anticoagulants in stroke patients with atrial fibrillation in clinical practice. Eur Neurol. (2016) 76:187–93. doi: 10.1159/000450750
4. Sutton S, Kinmonth AL, Hardeman W, Hughes D, Boase S, Prevost AT, et al. Does electronic monitoring influence adherence to medication? Randomized controlled trial of measurement reactivity. Ann Behav Med. (2014) 48:293–9. doi: 10.1007/s12160-014-9595-x
5. Desteghe L, Vijgen J, Koopman P, Dilling-Boer D, Schurmans J, Dendale P, et al. Telemonitoring-based feedback improves adherence to non-vitamin K antagonist oral anticoagulants intake in patients with atrial fibrillation. Eur Heart J. (2018) 39:1394–403. doi: 10.1093/eurheartj/ehx762
6. Boehme P, Wienand P, Herrmann M, Truebel H, Mondritzki T. New digital adherence devices could prevent millions of strokes from atrial fibrillation by the end of the next century. Med Hypotheses (2017) 108:46–50. doi: 10.1016/j.mehy.2017.07.034
7. Al AlShaikh S, Quinn T, Dunn W, Walters M, Dawson J. Predictive factors of non-adherence to secondary preventative medication after stroke or transient ischaemic attack: a systematic review and meta-analyses. Eur Stroke J (2016) 1:65–75. doi: 10.1177/2396987316647187
8. Labovitz DL, Shafner L, Reyes Gil M, Virmani D, Hanina A. Using artificial intelligence to reduce the risk of nonadherence in patients on anticoagulation therapy. Stroke (2017) 48:1416–9. doi: 10.1161/STROKEAHA.116.016281
9. Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
10. Noens L, van Lierde MA, De Bock R, Verhoef G, Zachee P, Berneman Z, et al. Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: the ADAGIO study. Blood (2009) 113:5401–11. doi: 10.1182/blood-2008-12-196543
11. Jank S, Bertsche T, Schellberg D, Herzog W, Haefeli WE. The A14-scale: development and evaluation of a questionnaire for assessment of adherence and individual barriers. Pharm World Sci. (2009) 31:426–31. doi: 10.1007/s11096-009-9296-x
12. Allemann SS, Hersberger HK, Arnet I. Patient views on an electronic dispensing device for prepackaged polypharmacy: a qualitative assessment in an ambulatory setting. Integ Pharm Res Pract. (2015) 4:167-−74. doi: 10.2147/IPRP.S90923
13. Messerli M, Blozik E, Vriends N, Hersberger KE. Impact of a community pharmacist-led medication review on medicines use in patients on polypharmacy–a prospective randomised controlled trial. BMC Health Serv Res. (2016) 16:145. doi: 10.1186/s12913-016-1384-8
Keywords: ischemic stroke, direct oral anticoagulants, adherence, electronic monitoring, adherence-improving intervention, polypharmacy, multi-compartment compliance aid
Citation: Polymeris AA, Albert V, Hersberger KE, Engelter ST, Schaedelin S, Arnet I and Lyrer PA (2018) Protocol for MAAESTRO: Electronic Monitoring and Improvement of Adherence to Direct Oral Anticoagulant Treatment—A Randomized Crossover Study of an Educational and Reminder-Based Intervention in Ischemic STROke Patients Under Polypharmacy. Front. Neurol. 9:1134. doi: 10.3389/fneur.2018.01134
Received: 01 November 2018; Accepted: 10 December 2018;
Published: 21 December 2018.
Edited by:
Andreas Charidimou, Harvard Medical School, United StatesReviewed by:
Aristeidis H. Katsanos, University of Ioannina, GreeceTerence J. Quinn, University of Glasgow, United Kingdom
Copyright © 2018 Polymeris, Albert, Hersberger, Engelter, Schaedelin, Arnet and Lyrer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexandros A. Polymeris, QWxleGFuZHJvcy5Qb2x5bWVyaXNAdXNiLmNo
† These authors share first authorship
‡ These authors share senior authorship
 Alexandros A. Polymeris
Alexandros A. Polymeris Valerie Albert2†
Valerie Albert2† Kurt E. Hersberger
Kurt E. Hersberger Isabelle Arnet
Isabelle Arnet