- 1Department of Neurology and Clinical Neurophysiology, Canisius Wilhelmina Hospital, Nijmegen, Netherlands
- 2Department of Neurology and Clinical Neurophysiology, OLVG West, Amsterdam, Netherlands
- 3Department of Neurology, Franciscus Vlietland Hospital, Schiedam, Netherlands
Background: For the preoperatively often required confirmation of clinically defined carpal tunnel syndrome (CTS), sensory as well as motor nerve conduction studies can be applied. The aim of this study was to test the sensitivity of specific motor nerve conduction tests in comparison with, as well as in addition to, sensory nerve conduction tests.
Methods: In 162 patients with clinically defined CTS, sensory and motor nerve conduction tests were performed prospectively. Sensitivity and specificity of all tests were computed. Also, Receiver Operating Characteristic (ROC) analyses were conducted.
Results: Sensitivity for all sensory tests was at least 79.4% (DIG1). All tests had a specificity of at least 95.7%. The motor conduction test with the highest sensitivity was the TLI-APB (81.3%); its specificity was 97.9%.
Conclusion: In the electrophysiological confirmation of CTS, sensory nerve conduction tests and terminal latency index have a high sensitivity. If, however, sensory nerve action potentials cannot be recorded, all motor nerve conduction tests have a high sensitivity.
Introduction
Carpal tunnel syndrome (CTS) is the most common entrapment neuropathy (1, 2). Usually, the diagnosis can be reliably made on the basis of clinical signs and symptoms. In the Netherlands it is common that (neuro) surgeons require the clinical diagnosis to be confirmed electrodiagnostically prior to surgical treatment, which makes reliable electrodiagnostic tests an important issue (3).
It has previously been established that sensory nerve conduction studies are the most sensitive electrodiagnostic tests to confirm the diagnosis of CTS. Motor nerve conduction studies are important in the documentation of motor fiber involvement in CTS. In more severe cases, sensory nerve action potentials (SNAP) may not be recordable (4). In this case, motor nerve conduction studies are the only electrophysiological means to confirm the clinically defined diagnosis of CTS (5). However, median motor nerve conduction studies are supposed to be less sensitive than sensory nerve conduction studies (5). Several motor nerve conduction studies are available to confirm the diagnosis of CTS. The most commonly used test is the median distal motor latency (DML), obtained by recording over the abductor pollicis brevis (APB) muscle with stimulation at the wrist (1). Other motor nerve conduction studies are (1) the absolute value of the DML of the compound muscle action potential (CMAP) of the second lumbrical muscle (2) comparison of the motor latency of the CMAP of the lumbrical muscle with that of the interosseous muscle after stimulation of the median and ulnar nerve (6) and (3) the terminal latency index of the thenar CMAP (7). It has not been fully determined which motor nerve conduction study is the most reliable in the electrodiagnostic confirmation of CTS in patients whose sensory nerve action potentials cannot be recorded.
Therefore, in the present study we prospectively tested the sensitivity of both sensory as well as motor nerve conduction tests in a group of patients with clinically defined CTS. We particularly focused on the group of CTS patients whose sensory nerve action potentials could not be recorded; we tried to evaluate which motor nerve conduction test was the best alternative in these specific cases in terms of sensitivity.
Materials and Methods
Subjects
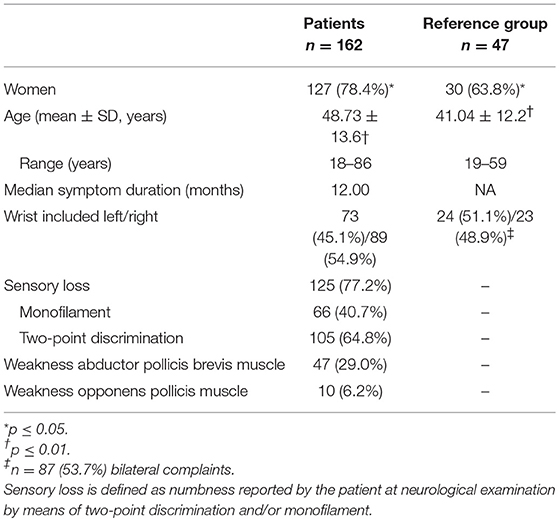
One hundred and sixty two patients with clinically defined CTS were included between 2006 and 2009. Data were collected prospectively. Carpal tunnel syndrome was considered to be clinically present in case of pain and/or paresthesias in the sensory distribution of the median nerve.
Two or more of the following criteria also needed to be present: (1) nocturnal paresthesias; (2) reproduction or aggravation of paresthesias or pain by provocative tests (Tinel or Phalen signs); aggravation of paresthesias by activities such as driving, riding a bike, holding a book or telephone; or (3) relief of symptoms by shaking the hand. These clinical criteria have previously been used in other studies (4, 8, 9). CTS with mild atrophy in combination with an MRC score ≥4 of the abductor pollicis brevis muscle and the opponens pollicis muscle was allowed.
In case of clinical signs of polyneuropathy or known hereditary neuropathy with a liability to pressure palsy, a history of trauma or any previous surgery to the symptomatic wrist, pregnancy, severe atrophy of the abductor pollicis brevis muscle, a history of rheumatoid arthritis or arthrosis of the wrist, known diabetes, thyroid disease, or alcoholism, patients were excluded from this study. Only the most symptomatic hand was included.
All candidates gave their written informed consent, and the local medical ethics committee approved the study (CWZ 1062006).
Control Subjects
Reference values were derived from 47 healthy, asymptomatic volunteers, who were recruited from the hospital staff. All were tested in the same laboratory according to the same electrodiagnostic test protocol.
Clinical Examination
All subjects underwent neurological examination, including inspection of the thenar, motor function tests of the hand muscles, the abductor pollicis brevis, and opponens pollicis muscle in particular, in accordance with Medical Research Council (10). Sensory tests, including a monofilament (10 g) and two-point discrimination were also performed. These data are not the subject of the present study.
Electrodiagnostic Evaluation
All patients and healthy volunteers underwent standardized motor and sensory nerve conduction studies (NCS) in accordance with our laboratory's standard procedure as recommended by the American Association of Neuromuscular and Electrodiagnostic Medicine (AANEM) guidelines (1). Additionally, the residual motor latency was calculated (7). NCS were performed using a Viking Monograph IV (Nicolet Biomedical Inc. Madison, WI, USA). Skin temperature of the hands was maintained at a minimum of 31.0°C by means of hot packs (11) and it was measured before and after each test. One examiner, who was not informed of the preceding history and physical examination results, performed all tests.
Sensory Nerve Conduction Studies
Ring electrodes were applied for recording SNAPs. In all subjects, the proximal electrode was placed at the first interphalangeal joint and the distal electrode at a distance of, preferably, 3 cm. The optimal stimulation site was determined carefully. Signal averaging was applied on all SNAPs in order to obtain a sharp potential take-off from baseline or to ensure the SNAP was not recordable.
Conduction distances were measured with a precision of 1 mm, using a tape measure.
Three different antidromic sensory nerve conduction studies were performed: 2 comparison tests, 1 short segment study.
- DIG1: sensory median-radial comparison test: the median and radial nerves were stimulated separately at the wrist, and the SNAPs were recorded from the thumb. Onset latency differences were computed. SNAP amplitudes were measured (peak-to-peak).
- DIG4: sensory median-ulnar comparison test: the median and ulnar nerves were stimulated separately at the wrist, and SNAPs were recorded from the ring finger. Onset latency differences were computed. SNAP amplitudes were measured (peak-to-peak).
- PALM3: sensory short segment forearm-wrist vs. wrist-to-palm segment (4). SNAPs were recorded from the third finger after stimulation of the median nerve at the palm, wrist, and elbow, respectively. Differences in sensory nerve conduction velocities between the wrist-to-palm segments and forearm-to-wrist segments (forearm) were calculated using onset latencies.
Motor Nerve Conduction Studies
Compound muscle action potentials (CMAP) were recorded by means of surface electrodes. The recording position was chosen in a way that enabled recording a maximal CMAP, if possible, with a sharp initial negative deflection.
2 motor NCS were performed:
- DML-APB: distal motor latency (DML) to the abductor pollicis brevis muscle. The median nerve was stimulated at the wrist and at the elbow. CMAPs were recorded from the thenar eminence at a distance of 6 cm from the stimulation site. The reference electrode was positioned over the metacarpal-phalangeal joint of the thumb.
- 2L-INT: lumbrical-interosseous comparison study. The median and ulnar nerves were both stimulated at the wrist, with the same conduction distance. However, the value of the conduction distance varied per patient as the optimal stimulation site of the stimulus cathode was variable in order to be able to search for the optimal stimulation site (between 6 and 7 cm). CMAPs were recorded from the second lumbrical (2L) and second interosseous muscle (INT), respectively, with the active recording electrode in the palm, between the second and third metacarpals. The reference electrode was placed at the distal phalanx of the index finger. Distal motor latencies of the lumbrical (DML-LUMB) and interosseous (DML-INT) muscles were recorded, and differences between the two latencies were computed. The optimal recording site was defined as the location at which the CMAP was maximal with a sharp initial negative deflection.
For both DML-APB as well as 2L-INT terminal latency indexes (TLI) and residual motor latency (RML) were calculated by means of the following equations:
- TLI-APB = terminal distance (mm)/[motor conduction velocity forearm(m/s) * DML-APB (ms)]
- TLI-LUMB = terminal distance (mm)/[motor conduction velocity forearm(m/s) * DML-LUMB (ms)]
- RML-APB = DML-APB (ms) – [terminal distance (mm)/motor nerve conduction velocity forearm (m/s)]
- RML-LUMB = DML-LUMB (ms) – [terminal distance (mm)/motor nerve conduction velocity forearm (m/s)]
Statistical Analysis
Data concerning clinical variables and nerve conduction studies were processed using Microsoft Office Excel and Access 2010; and all statistical analyses were performed using IBM SPSS Statistics 21.0.
In the reference group, mean differences, standard deviations, as well as upper and lower limits of normal (ULN and LLN, respectively) were calculated for all nerve conduction studies. ULN and LLN were defined as the mean, plus or minus twice the standard deviation, respectively.
The number of patients with an abnormal test result was determined using the ULN (or LLN in case of TLI). The sensitivity of each test was calculated as the number of patients that both met the criteria of clinical CTS and had an abnormal electrodiagnostic test result, divided by the number of patients meeting the criteria of clinical CTS times 100%. Specificity was calculated as the number of controls having normal test results, divided by the number of controls times 100%. Receiver Operating Characteristic (ROC) analyses were conducted. The Area Under the Curve (AUC) was computed for all nerve conduction studies.
Comparison between patients and the reference group was performed with a t-test for continuous variables or a χ2-test for categorical variables, as appropriate.
P < 0.05 values were considered as statistically significant test results.
Results
Clinical Features
One hundred and sixty two patients with clinical symptoms of CTS were included in this study, 35 men and 127 women. The mean age in this group was 48.7 (SD 13.6). The median duration of symptoms was 12 months. The mean age and gender distribution were significantly different between patients and controls (P < 0.01 and P < 0.05, respectively) (Table 1).
Electrophysiology
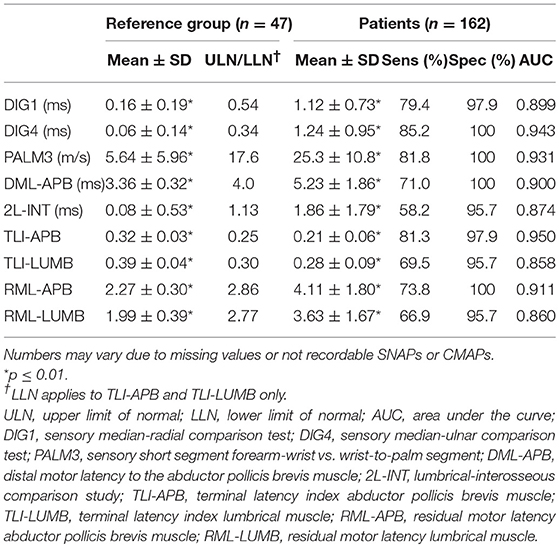
Details on electrophysiological features in both the patients and the reference group are presented in Table 2; ULN and LLN of performed tests are presented in Table 2.
The DML to the APB was 3.36 ± 0.32 and 5.23 ± 1.86 ms (mean ± SD) in the reference group and patients, respectively. 2L-INT was 0.08 ± 0.53 ms in the reference group vs. 1.86 ± 1.79 ms in patients. DML-APB was abnormal in 115 patients (70.6%); in 5 of them the CMAP was not recordable. 2L-INT was abnormal in 92 of 158 (58.2%), and in 4 of these the lumbrical CMAP was not recordable. These differences were statistically significant (P < 0.01).
TLI-APB was abnormal in 130 of 160 patients (81.3%), TLI-LUMB in 107 of 154 (69.5%). RML-APB was abnormal in 118 of 160 patients (73.8%), RML-LUMB in 103 of 154 (66.9%) (Table 2, P < 0.01). The number of tests varies between APB and LUMB because of missing values.
Sensitivity for all sensory tests was at least 79.4% (DIG1); for motor conduction tests sensitivity was considerably lower, except for the TLI-APB, which was 81.3%. All conduction tests had high specificity values (range 95.7–100%) with AUC values ranging from 0.858 to 0.950. Of the motor nerve conduction tests, it was the TLI-APB that had the highest sensitivity (81.3%), a high specificity (97.9%), and a high AUC value (0.950) (Figure 1).
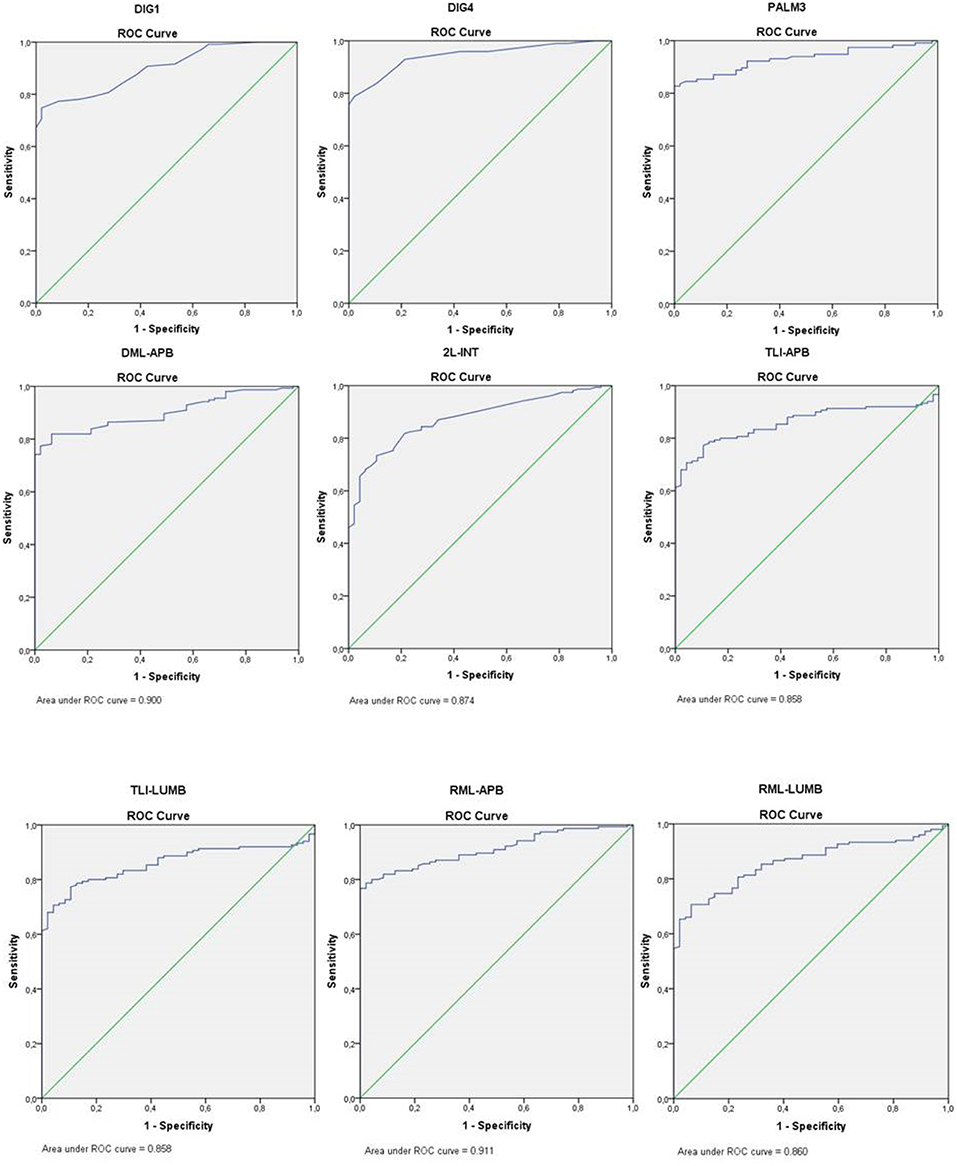
Figure 1. ROC curve nerve conduction studies. DIG1, sensory median-radial comparison test; DIG4, sensory median-ulnar comparison test; PALM3, sensory short segment forearm-wrist vs. wrist-to-palm segment; DML-APB, distal motor latency to the abductor pollicis brevis muscle; 2L-INT, lumbrical-interosseous comparison study; TLI-APB, terminal latency index abductor pollicis brevis muscle; TLI-LUMB, terminal latency index lumbrical muscle; RML-APB, residual motor latency abductor pollicis brevis muscle; RML-LUMB, residual motor latency lumbrical muscle.
In CTS patients with no recordable SNAPs [meaning that no median nerve SNAP could be recorded from DIG1, DIG4, and PALM3 (n = 27) with stimulation at the wrist], the percentage of abnormal motor nerve conduction tests was 100. This was significantly more than in patients with recordable SNAPs (P < 0.01).
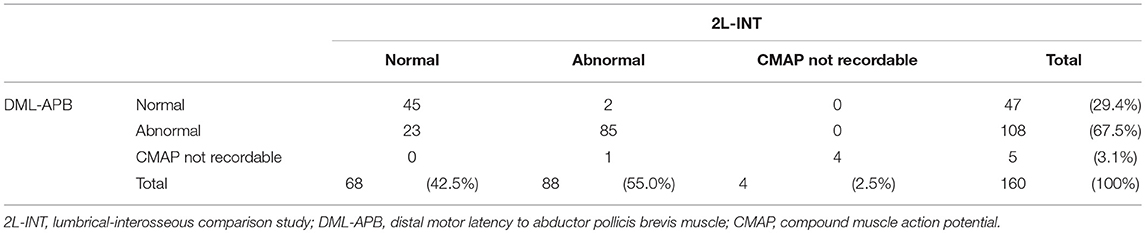
Numbers of normal and abnormal tests according to 2L-INT and DML-APB are presented in Table 3. Out of 47 CTS patients with normal DML-APB test results, only 2 patients had an abnormal 2L-INT test result. In contrast, 23 of 68 patients with normal 2L-INT test results had an abnormal DML-APB test result (Table 3).
Discussion
Our results confirm that out of all electrodiagnostic tests that are available to confirm the clinical diagnosis of CTS, sensory conduction studies are the most sensitive (1, 5). In severe CTS, however, sensory nerve action potentials often cannot be recorded. Motor nerve conduction studies have to be performed to show the presence of conduction slowing in the median nerve across the wrist, in order to differentiate between CTS and for example a proximal lesion of the median nerve. Several types of motor conduction studies can then be applied to show the presence of conduction slowing in the median nerve across the wrist. Most often, the distal motor latency of the median nerve is used, defined as the onset latency of the APB CMAP, which is obtained by stimulation of the median nerve at the crease of the wrist. A cut-off value of 4.0 ms without taking conduction distance into account, has a reported sensitivity of 6 to 65% (1).
In theory, a better alternative is the lumbrical-interosseous comparison test as described in the methods. The comparative aspect of this test is advantageous, as the motor conduction of the ulnar nerve is its reference. One may expect that nerve conduction velocities in distal segments of median and ulnar nerves do not differ much (12). In our data, however, the sensitivity of this test in the whole group of patients is even less than that of the classic DML-APB test. Moreover, 23 of 68 patients with a normal lumbrical-interosseous comparison test result showed an abnormal DML-APB. In the subgroup of patients with more severe CTS, whose SNAPs are not recordable, we found that all specific motor nerve conduction tests show a high sensitivity of up to 100%. In only one patient the CMAP to the APB was not recordable while the lumbrical-interosseous comparison test showed abnormal results, which proved a distal median neuropathy. The sensitivity which was found in this study is considerably lower compared to reported sensitivities in the literature (12, 13). This difference can be explained by the different cut-off values. Preston et al. (6) used a cut-off of 0.4 ms, and Chang et al. (13) used 0.6 ms. According to our reference population the cut-off value is 1.13 ms. We do not have a satisfying explanation for this difference.
The great advantage of the lumbrical-interosseous comparison test over the other motor nerve conduction studies is, that a DML of ulnar muscles can be used as a reference for the thenar DML. However, since in only one patient the lumbrical-interosseous comparison test had additional value to the DML-APB, we could not confirm the hypothesis that motor fibers to the lumbrical muscle at the level of the carpal tunnel are less vulnerable because of their anatomical/topographical position in the median nerve, which has been suggested by others (6, 12–17). Moreover, the association between 2L-INT and DML-APB is high (r = 0.87, P < 0.001), making the additional value of the 2L-INT to the traditionally performed DML only marginal. Values in the same order of magnitude were found in the subgroup of CTS patients whose SNAP could not be recorded.
As TLI gives a DML correction to nerve conduction velocity in the proximal segment of the median nerve as well as in the terminal distance, it is not very surprising that we found that the TLI of APB showed a high sensitivity, almost similar to sensory tests. This goes for the whole patient group as well as the subgroup of patients whose SNAPs are not recordable. This is in accordance with previous reports (7, 17–19), yet normative values may vary because of methodological differences and the electrophysiological techniques used. One may argue that, considering the tortuous course of the median nerve in the carpal tunnel (20) it is virtually impossible to measure the distal conduction distance precisely. As a consequence, the TLI value may be biased to lower values as it is to be expected that measured distances are underestimated. However, this is not an issue, since the same argument can be used for the TLI value acquired in healthy subjects, if reference values are collected in the same way as in the patients, which is the case in our study.
The conclusions of our study apply to patients with no or mild thenar atrophy. We excluded patients with severe thenar atrophy, which is probably the group of patients with the most severe CTS. Numerically, this is presumably not a very significant group of patients; according to the study of Yates et al. (16), only 5% of the total carpal tunnel syndrome population tested in the laboratory over a 2 and a half year period had severe thenar wasting. It could therefore be worthwhile to investigate the group of very severe CTS patients with the aforementioned tests.
Since we used the clinical diagnosis as the standard, and all patients in this study had clinical CTS, specificity could not be calculated. Knowledge of specificities of motor nerve conduction studies would have been of additional value. Also, the reference group was not completely matched for age and sex with the patient group and this may have influenced the results. However, when comparative tests within the same subject are used, differences in age or sex are probably less relevant.
In conclusion, it appears that in CTS patients with recordable SNAPs, motor nerve conduction tests are less sensitive to confirm the clinical diagnosis of CTS with the exception of the TLI of the APB. In CTS patients whose SNAPs are not recordable, all discussed motor tests have a high sensitivity. The TLI in particular, appears to be a robust electrodiagnostic test in CTS.
In cases when median nerve SNAPs cannot be recorded, but a CMAP to the APB can, the 2L-INT has no additional value. However, in our study the chance of recording a lumbrical CMAP in these specific cases is rather low.
Data Availability
The datasets for this manuscript are not publicly aware because several other papers related to the database are pending.
Author Contributions
KK: data processing and writing the manuscript. FC, WV, and JM: study design, data collection, and review the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Jablecki CK, Andary MT, Floeter MK, Miller RG, Quartly CA, Vennix MJ, et al. Practice parameter: electrodiagnostic studies in carpal tunnel syndrome: report of the American Association of Electrodiagnostic Medicine, American Academy of Neurology, and the American Academy of Physical Medicine and Rehabilitation. Neurology. (2002) 58:1589–92. doi: 10.1212/WNL.58.11.1589
2. Latinovic R, Gulliford MC, Hughes RA. Incidence of common compressive neuropathies in primary care. J Neurol Neurosurg Psychiatry. (2006) 77:263–5. doi: 10.1136/jnnp.2005.066696
3. Claes F, Verhagen WI, Meulstee J. Current practice in the use of nerve conduction studies in carpal tunnel syndrome by surgeons in the Netherlands. J Hand Surg Eur. (2007) 32E:663–7. doi: 10.1016/J.JHSE.2007.09.007
4. Kasius KM, Claes F, Verhagen WI, Meulstee J. The segmental palmar test in diagnosing carpal tunnel syndrome reassessed. Clin Neurophysiol. (2012) 123:2291–5. doi: 10.1016/j.clinph.2012.04.003
5. Werner RA, Andary M. Electrodiagnostic evaluation of carpal tunnel syndrome. Muscle Nerve. (2011) 44:597–607. doi: 10.1002/mus.22208
6. Preston DC, Logigian EL. Lumbrical and interossei recording in carpal tunnel syndrome. Muscle Nerve. (1992) 15:1253–7. doi: 10.1002/mus.880151106
7. Uzar E, Tamam Y, Acar A, Yucel Y, Palanci Y, Cansever S, et al. Sensitivity and specificity of terminal latency index and residual latency in the diagnosis of carpal tunnel syndrome. Eur Rev Med Pharmacol Sci. (2011) 15:1078–84.
8. Jablecki CK, Andary MT, So YT, Wilkins DE, Williams F. Literature review of the usefulness of nerve conduction studies and electromyography for the evaluation of patients with carpal tunnel syndrome. Muscle Nerve. (1993) 16:1392–414. doi: 10.1002/mus.880161220
9. Witt JC, Hentz JG, Stevens JC. Carpal tunnel syndrome with normal nerve conduction studies. Muscle Nerve. (2004) 29:515–22. doi: 10.1002/mus.20019
10. Medical Research Council. Aids to the Examination of the Peripheral Nervous System. Memorandum no. 45, Her Majesty's Stationary Office (1981).
11. Kasius KM, Riphagen JH, Verhagen WI, Meulstee J. An easily applicable alternative method for warming cold limbs in nerve conduction studies. Neurophysiol Clin. (2014) 44:219–26. doi: 10.1016/j.neucli.2014.03.001
12. Chang MH, Lee YC, Hsieh PF. The real role of forearm mixed nerve conduction velocity in the assessment of proximal forearm conduction slowing in carpal tunnel syndrome. J Clin Neurophysiol. (2008) 25:373–7. doi: 10.1097/WNP.0b013e31818e7930
13. Chang MH, Wei SJ, Chiang HL. Comparison of motor conduction techniques in the diagnosis of carpal tunnel syndrome. Neurology. (2002) 58:1603–7. doi: 10.1212/WNL.58.11.1603
14. Logigian EL, Busis NA, Berger AR, Bruyninckx F, Khalil N, Shahani BT, et al. Lumbrical sparing in carpal tunnel syndrome: anatomic, physiologic, and diagnostic implications. Neurology. (1987) 37:1499–505. doi: 10.1212/WNL.37.9.1499
15. Tan U. Velocities of motor and sensory nerve conduction are the same for right and left arms in right- and left-handed normal subjects. Percept Mot Ski. (1985) 60:625–6. doi: 10.2466/pms.1985.60.2.625
16. Yates SK, Yaworski R, Brown WF. Relative preservation of lumbrical versus thenar motor fibres in neurogenic disorders. J Neurol Neurosurg Psychiatry. (1981) 44:768–74. doi: 10.1136/jnnp.44.9.768
17. Kuntzer T. Carpal tunnel syndrome in 100 patients: sensitivity, specificity of multi-neurophysiological procedures and estimation of axonal loss of motor, sensory and sympathetic median nerve fibers. J Neurol Sci. (1994) 127:221–9. doi: 10.1016/0022-510X(94)90076-0
18. Simovic D, Weinberg DH. The median nerve terminal latency index in carpal tunnel syndrome: a clinical case selection study. Muscle Nerve. (1999) 22:573–7. doi: 10.1002/(SICI)1097-4598(199905)22:5<573::AID-MUS4>3.0.CO;2-A
19. Simovic D, Weinberg DH. Terminal latency index in the carpal tunnel syndrome. Muscle Nerve. (1997) 20:1178–80. doi: 10.1002/(SICI)1097-4598(199709)20:9<1178::AID-MUS14>3.0.CO;2-P
Keywords: carpal tunnel syndrome, nerve conduction studies, diagnostics, sensory nerve action potentials, motor nerve conduction tests
Citation: Kasius KM, Claes F, Verhagen WIM and Meulstee J (2019) Motor Nerve Conduction Tests in Carpal Tunnel Syndrome. Front. Neurol. 10:149. doi: 10.3389/fneur.2019.00149
Received: 20 June 2018; Accepted: 05 February 2019;
Published: 14 March 2019.
Edited by:
Ghazala Hayat, Saint Louis University, United StatesReviewed by:
Giuseppe Piscosquito, Fondazione Salvatore Maugeri, Telese (IRCCS), ItalyRaghav Govindarajan, University of Missouri, United States
Copyright © 2019 Kasius, Claes, Verhagen and Meulstee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kristel M. Kasius, ay5rYXNpdXNAb2x2Zy5ubA==
 Kristel M. Kasius
Kristel M. Kasius Franka Claes
Franka Claes Wim I. M. Verhagen
Wim I. M. Verhagen Jan Meulstee1
Jan Meulstee1