- 1Department of Neurology, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China
- 2Department of Pathology, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China
Background: The associations between the clinical characteristics and diffusion-weighted imaging (DWI) positivity in patients with a clinical diagnosis of transient ischemic attack (TIA) are still poorly understood. The purpose of our study was to determine the incidence of TIA related acute infarction by DWI, and to determine the underlying predictors of DWI positivity in TIA patients.
Methods: Between Jan 2017 and Dec 2018, we retrospectively enrolled 430 patients with a clinical diagnosis of TIA who underwent DWI. Patients were divided into those with acute ischemic lesions (DWI positive group) and those without (DWI negative group). The clinical characteristics, laboratory data, and imaging parameters were compared between the two groups.
Results: A total of 430 time-based TIA patients (mean age, 61.4 ± 13.0) were enrolled in this study. About 126 (29.3%) of TIA patients had a DWI positive lesion in our series. Comparing TIA patients with positive DWI to those with negative DWI, acute lesions were more likely to be more male, have higher hyperlipidemia and a smoking history, more speech abnormalities and increased motor weakness; and higher systolic and diastolic blood pressure, homocysteine, fasting blood glucose, and the scores of ABCD2, ABCD3, ABCD3-I, and Dawson. Several independent predictors of DWI positivity were identified with logistic regression analysis: motor weakness (odds ratio 4.861, P = 0.021), speech abnormalities (odds ratio 4.029, P = 0.024), and ABCD3-I (odds ratio 13.141, P = 0.001). ABCD3-I showed the greatest area under the ROC curve, with a sensitivity of 85.7% and specificity of 72.4%.
Conclusion: In patients with a clinical diagnosis of TIA, 29.3% demonstrated acute DWI lesions on brain magnetic resonance imaging (MRI). They were associated with motor weakness, speech abnormalities and higher ABCD3-I score at admission.
Introduction
Stroke is the second leading cause of death worldwide, and the first cause of death in China. About 20% of stroke is preceded by an episode of transient ischemic attack (TIA). Patients with TIA have the highest risk of early stroke especially in the first 48 h (1, 2) and subsequent adverse events (3). The time-based definition of TIA was classically defined as the presence of focal neurological symptoms ascribable to a vascular etiology lasting <24 h. The tissue-based definition of TIA was proposed to classify patients only if the symptoms fit the clinical syndrome and if no acute ischemic lesion was identified by magnetic resonance imaging (MRI) (4). Thus, diffusion-weighted imaging (DWI) has been a mandatory tool of the diagnosis and treatment of patients with TIA (5). However, it has been reported that only 11.1% of patients with TIA could be detected by DWI (6). Several studies suggested DWI positivity in TIA was associated with clinical characteristics such as age (7), longer symptom duration (8), motor deficit, aphasia, and NIHSS score (6), and large-vessel occlusion of magnetic resonance angiography (MRA) (9). However, these associations between the clinical characteristics and DWI positivity are still incompletely understood.
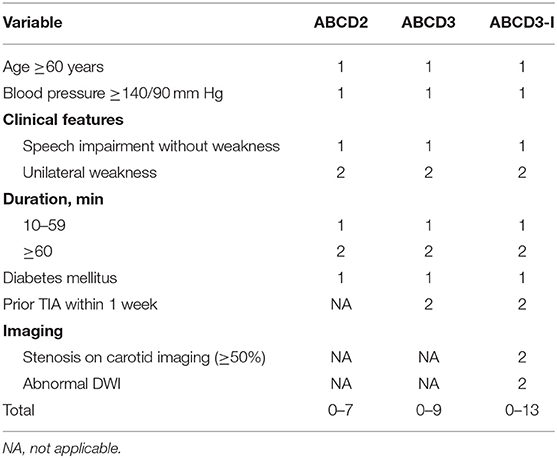
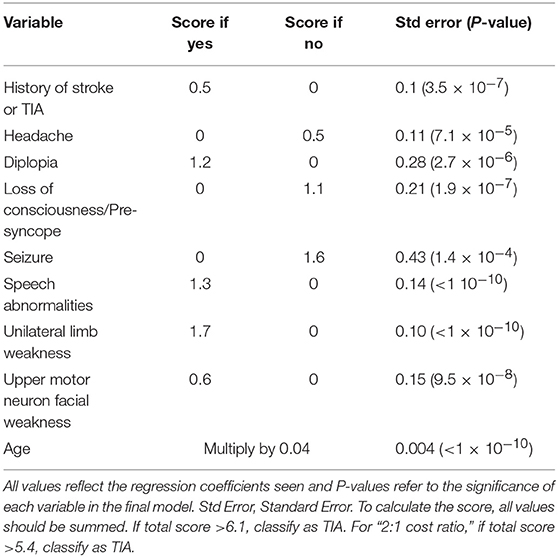
In resource-limited settings, clinicians need to rely on patient history, neurological examination, and patient-specific risk factors to stratify. Thus, it is worthwhile to develop some reliable risk scores to prevent stroke in an early stage after the onset of TIA. The ABCD2 score was first developed to provide a structured way to stratify the early risk of stroke after TIA (10, 11). ABCD2-I score (12), ABCD3 (13), and ABCD3-I score (14, 15) were then also widely used to stratify the risk factors of stroke. The ABCD score system was widely used, however, up to a third of mimics were also found to have ABCD2 scores ≥ 4 (16). Furthermore, the management of ABCD3-I needs the immediate stroke specialist assessment, and urgent brain MRI and vascular imaging, which will be much more difficult with higher cost-effectiveness. The Dawson score (17), while not widely used, incorporates more specific clinical characteristics of patient presentation and is designed for sensitivity, thus we sought to investigate its utility in our population.
Thus, the aims of the present study are to determine the incidence of TIA related acute lesions by DWI and to investigate its utility of ABCD score system and Dawson score in our population, and finally to determine underlying predictors of DWI positivity in TIA patients.
Materials and Methods
Patients
Our study was a retrospective, observational study in the Department of Neurology, Beijing Chaoyang Hospital, Capital Medical University. We retrospectively enrolled consecutive 430 patients between Jan 2017 and Dec 2018 and they all underwent brain MRI. The mean time from symptom onset to MRI was 72 h. TIA was classically defined as a focal cerebral ischemic event with neurological symptoms lasting <24 h regardless of brain lesions detected by brain imaging, including computed tomography (CT), and MRI (4). The detection of acute ischemic lesions was based on DWI. Based on DWI, patients were divided into those with acute ischemic lesions (DWI positive group) and those without (DWI negative group), and their demographics, clinical data, and imaging variables were analyzed. Ischemic stroke subtypes were classified only limited to DWI positive group according to the criteria in the Trial of Org 10172 in Acute Stroke Treatment (TOAST) (18).
Clinical Variables
We retrospectively obtained the following clinical data: age, gender; vascular risk factors including hypertension, diabetes mellitus, hyperlipidemia, stroke, coronary heart disease, atrial fibrillation, and current smoking. The score s of ABCD2, ABCD3, ABCD3-I, and Dawson were calculated (Tables 1, 2). The laboratory blood tests were obtained from medical records, including the counts of red blood cell, white blood cell, platelet; hemoglobin, fibrinogen, D-dimer, fasting blood glucose (FBG), hemoglobin A1c, glycated albumin, uric acid, homocysteine, total cholesterol, low-density lipoprotein, high-density lipoprotein, and C-reactive protein. The presenting symptoms of TIA included unilateral motor weakness, sensory disturbance, speech abnormalities (dysarthria/aphasia), dizziness, loss of consciousness, hemianopia, and amnesia. Duration of TIA was classified into 3 categories: <10, 10–59 min, or more than 1 h.
Imaging Data
The sequences of MRI included T1-weighted images, T2-weighted images, fluid-attenuated inversion recovery images, DWI, and the accompanying MRA with 3-dimensional time-of-flight images were obtained. The presence of acute ischemic lesions was defined by areas of high signal intensity on DWI. All neuroimaging data and clinical reports were reviewed blindly by neurologists and neuroradiologists, without prior knowledge of baseline clinical data. For analysis of MRA, intracranial as well as extracranial stenoses >50% were considered to be significant and if the stenosis was in a territory appropriate to the patient's symptom, it was defined as the “MRA abnormality.”
Treatment
We retrospectively examined the content of antithrombotic treatments administered after admission, including anticoagulant treatment, antiplatelet treatment (aspirin or clopidogrel), and administered either as a single or dual treatment.
Statistical Analysis
The data were described using mean and standard deviation, or median and interquartile range values for continuous variables, and absolute numbers and percentages for nominal and categorical variables, and we compared the groups using the non-parametric Mann-Whitney U-test. We performed a chi-square test to determine the correlation between categorical variables and a t-test between continuous variables. The multivariate logistic regression analysis was performed to find predictors for the presence of DWI lesions. The receiver operating characteristic curve (ROC) analysis was performed to discriminate DWI-positive from DWI-negative patients using the value of area under the curve (AUC). We used the Statistical Package for Social Sciences (SPSS) version 16.0 (SPSS Inc., Chicago, IL, USA) for data analysis. A P value <0.05 was considered statistically significant.
Results
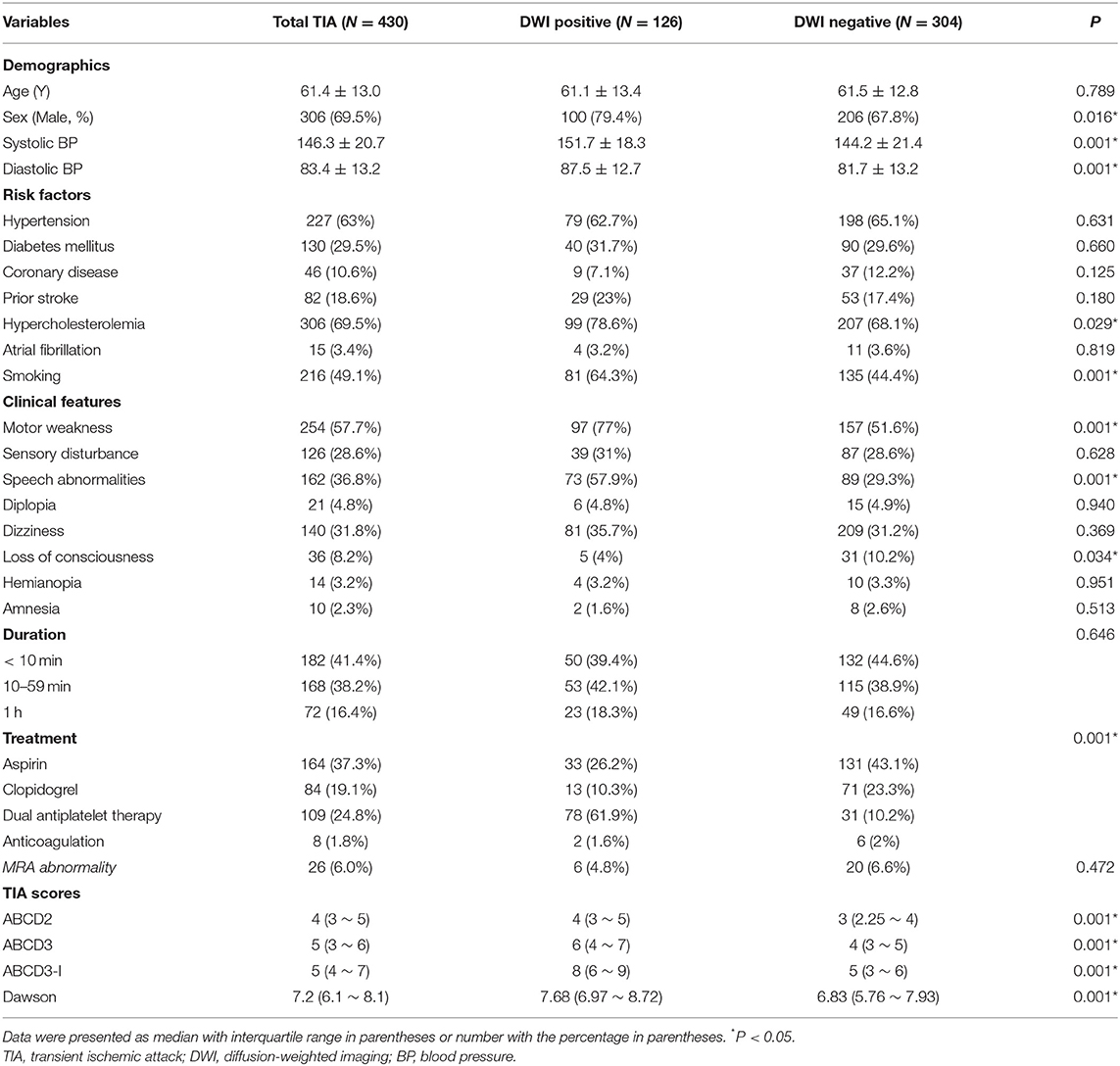
Among a total of 430 patients with time-defined TIA, the mean age was 61.4 ± 13.0 years, and 69.5% were male. One hundred twenty-six (29.3%) patients had high-intensity lesions on DWI. We classified the patients with positive DWI as stroke etiology according to the TOAST: 48 (38.1%) of large-artery atherosclerosis, 14 (11.1%) of cardioembolism, 40 (31.7%) of small artery occlusion, 4 (3.2%) of other determined etiology, 20 (15.9%) of undetermined etiology.
Table 3 showed the demographics, baseline clinical characteristics, vascular risk factors, TIA symptoms, treatment of all TIA patients (N = 430), DWI positive group (N = 126), and DWI negative group (N = 304). There were significant differences in sex, systolic and diastolic blood pressure, hypercholesterolemia and smoking history, motor weakness, speech abnormalities, acute antithrombotic treatment, and the scores of ABCD2, ABCD3, ABCD3-I, and Dawson between the two groups (P < 0.05). The percentage of patients who were medicated with dual antiplatelet therapy was significantly higher in the DWI positive group than DWI negative group (61.9 vs. 10.2%, P < 0.001). There were no significant differences in the other baseline clinical characteristics and MRA abnormality between the two groups.
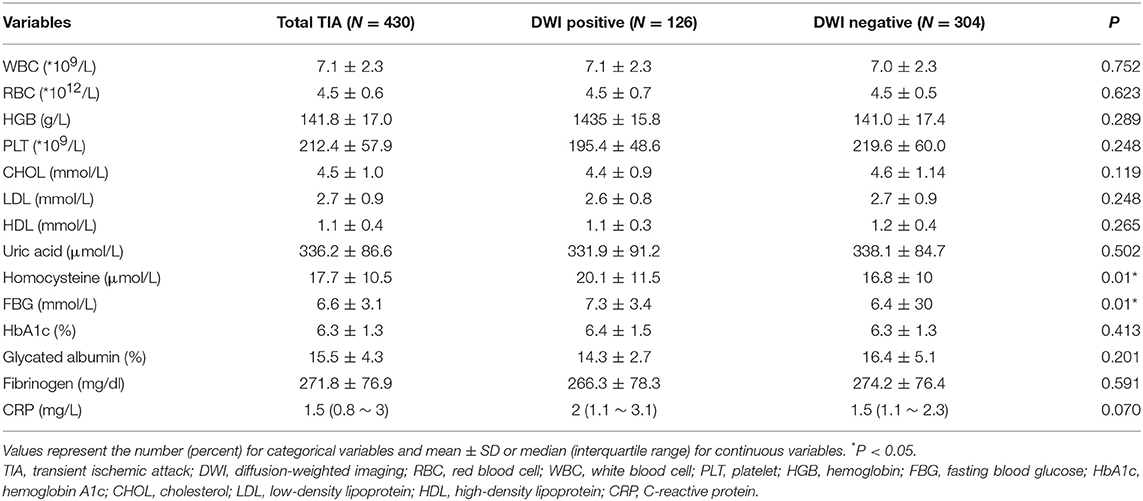
The laboratory findings were presented in Table 4. The levels of homocysteine and FBG were significantly higher in DWI-positive group than those in DWI-negative group. There were no differences in the other laboratory data between the two groups.
Logistic regression analysis was shown in Table 5, and we found motor weakness (odds ratio 4.861, 95% CI 1.264–18.344, P = 0.021), speech abnormalities (odds ratio 4.029, 95% CI 1.203–13.496, P = 0.024) and ABCD3I (odds ratio 13.141, 95% CI 7.392–23.360, P = 0.001) were independently associated with positive DWI lesions.
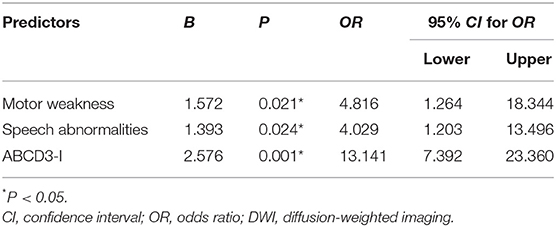
Table 5. Associated factors with acute infarction detected by DWI using logistic regression analysis.
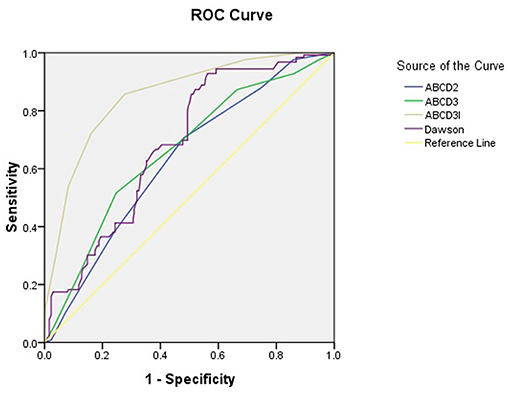
The area under the ROC curve (95% CI) of the scores of ABCD2, ABCD3, ABCD3-I, and Dawson for the prediction of acute ischemic events were 0.629 (95% CI, 0.573–0.684), 0.660 (95% CI, 0.604–0.716), 0.854 (95% CI, 0.815–0.892), 0.684 (95% CI, 0.633–0.736), respectively. Figure 1 showed the ROC for selected predictors of DWI positive TIA patients. ABCD3-I showed the greatest area under the ROC curve, with the sensitivity of 85.7% and specificity of 72.4%, respectively.
Discussion
In our present study, we found 29.3% of TIA patients had a positive DWI lesion on brain MRI in patients with clinically diagnosed TIA. They were independently associated with motor weakness, speech abnormalities, and higher ABCD3-I score at admission. ABCD3-I showed the greatest area under the ROC curve (0.854), and Dawson was 0.684.
DWI has been a mandatory tool in the diagnosis of a TIA or acute ischemic stroke (19). DWI is more sensitive than CT for detecting acute ischemia, and up to one-third of patients diagnosed with TIA were found to have an acute infarct on DWI (20). The frequency of positive DWI findings varied from 9 to 67% among different cohorts of TIA (12, 21, 22). A meta-analysis of the 9,078 patients in 47 studies showed that 34.3% of patients had a DWI lesion (23). The incidence of DWI positivity in our series was similar to previous reports (9, 23, 24). It has been reported that one of the important factors that affect DWI positivity rate was the duration from stroke onset to MRI examination (25). In our present study, the mean time from symptom onset to MRI was 72 h. It is probable that if patients receiving MRI within the first 24–48 h were more likely to have acute DWI lesions than patients receiving MRI after 72 h.
In resource-limited settings, the clinical evaluation could be quite important to stratify different risk factors of TIA patients. In patients with TIA, symptom duration of more than 1 h, motor deficits, and aphasia were independently correlated with detecting an abnormality with DWI (21). Subcortical acute lesions were also reported in patients with TIA, that were associated with recurrent episodes, dysarthria, and motor weakness (26). The data from Germany also indicated that the evidence of acute infarction by DWI in TIA patients was detected in 11.1 % of patients and associated with motor weakness, aphasia, and NIHSS score of ≥10 at admission (6). Our results were also consistent with these previous findings (6, 21, 24, 26, 27), especially positive DWI in TIA patients performed with the motor weakness and speech abnormalities (27). We also found that ABCD3-I showed the greatest area under the ROC curve. It was reported that the area under the ROC curve of ABCD2 score and ABCD3-I score was 0.50 and 0.58 in South Korea population (28). Our study was also in line with this study (15, 29). As for the Dawson score, we found that the AUC for the Dawson score was 0.684 in our study, which was also quite similar to the population from the United Kingdom (30). However, before applying the ABCD system or Dawson score as risk stratification, the physician should always keep in mind to never neglect the individual examination of TIA patient (31).
Our study had several limitations. First, this study was designed as a retrospective study from a single center. A study with a larger number of patients from multiple centers in China is urgently needed to confirm our results. Second, it has been reported that patients with acute DWI lesions more often performed a cardioembolic etiology or large artery atherosclerosis cause (32). As for the etiology of the TIA, however, we only classified the TIA patients with positive DWI group according to the TOAST, which were not performed in all the TIA patients. Third, we did not assess the risk factors of the recurrence of TIA or stroke, lack of subgroup analysis such as low, medium, and high risk according to ABCD2/ABCD3/ABCD3-I scores. Fourth, another limitation was the lack of data regarding prior treatment and follow-up in our study. However, the strengths of the study were listed as follows. First, as far as we know, the present study is the largest ever reported MRI including DWI investigation of TIA that identifies clinical predictors for evidence of acute infarction in patients suffering from TIA in China. Secondly, we found the levels of homocysteine and FBG were significantly higher in the DWI-positive group compared to DWI-negative group, which was a novelty in this study. Third, we further validated its utility of Dawson score in a Chinese population.
Conclusion
In summary, our findings demonstrated that an acute infarction was detected in 29.3% of clinical diagnosed TIA patients, and it was independently associated with motor weakness, speech abnormalities, and higher ABCD3-I score at admission. A larger number of patients from multiple centers in China are urgently needed to confirm our findings, especially higher ABCD3-I score as predictors of positive DWI.
Data Availability
The datasets analyzed in this manuscript are not publicly available. Requests to access the datasets should be directed to d2VubGlodTMzNjZAMTI2LmNvbQ==.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Beijing Chaoyang Hospital, Capital Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
JY and WH conceived and designed the experiments. JY analyzed the data and drafted the manuscript. ZJ, YS, SY, YL, LY, and WQ collected data. All authors have read and approved the final manuscript to be published.
Funding
This work was supported by the National Natural Science Foundation of China (81301016) and the Beijing Municipal Administration of Hospitals Incubating Program (PX2019009).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank all the study participants. We thank Yicheng Xu, Weixue Wang, Shang Wang, Zihan Yan, Yu Zhang for their great help for the data collection.
References
1. Giles MF, Rothwell PM. Risk of stroke early after transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol. (2007) 6:1063–72. doi: 10.1016/S1474-4422(07)70274-0
2. Coutts SB, Modi J, Patel SK, Demchuk AM, Goyal M, Hill MD. CT/CT angiography and MRI findings predict recurrent stroke after transient ischemic attack and minor stroke: results of the1. prospective CATCH study. Stroke. (2012) 43:1013–17. doi: 10.1161/STROKEAHA.111.637421
3. Hao Q, Tampi M, O'Donnell M, Foroutan F, Siemieniuk RA, Guyatt G. Clopidogrel plus aspirin versus aspirin alone for acute minor ischaemic stroke or high risk transient ischaemic attack: systematic review and meta-analysis. BMJ. (2018) 363:k5108. doi: 10.1136/bmj.k5108
4. Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. (2009) 40:2276–93. doi: 10.1161/STROKEAHA.108.192218
5. Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2014) 45:2160–236. doi: 10.1161/STR.0000000000000024
6. Al-Khaled M, Matthis C, Munte TF, Eggers J, Qug SSS. The incidence and clinical predictors of acute infarction in patients with transient ischemic attack using MRI including DWI. Neuroradiology. (2013) 55:157–63. doi: 10.1007/s00234-012-1091-z
7. Schulz UG, Briley D, Meagher T, Molyneux A, Rothwell PM. Diffusion-weighted MRI in 300 patients presenting late with subacute transient ischemic attack or minor stroke. Stroke. (2004) 35:2459–65. doi: 10.1161/01.STR.0000143455.55877.b9
8. Kidwell CS, Alger JR, Di Salle F, Starkman S, Villablanca P, Bentson J, et al. Diffusion MRI in patients with transient ischemic attacks. Stroke. (1999) 30:1174–80. doi: 10.1161/01.STR.30.6.1174
9. Coutts SB, Simon JE, Eliasziw M, Sohn CH, Hill MD, Barber PA, et al. Triaging transient ischemic attack and minor stroke patients using acute magnetic resonance imaging. Ann Neurol. (2005) 57:848–54. doi: 10.1002/ana.20497
10. Johnston SC, Rothwell PM, Nguyen-Huynh MN, Giles MF, Elkins JS, Bernstein AL, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. (2007) 369:283–92. doi: 10.1016/S0140-6736(07)60150-0
11. Giles MF, Rothwell PM. Systematic review and pooled analysis of published and unpublished validations of the ABCD and ABCD2 transient ischemic attack risk scores. Stroke. (2010) 41:667–73. doi: 10.1161/STROKEAHA.109.571174
12. Giles MF, Albers GW, Amarenco P, Arsava MM, Asimos A, Ay H, et al. Addition of brain infarction to the ABCD2 Score (ABCD2I): a collaborative analysis of unpublished data on 4574 patients. Stroke. (2010) 41:1907–13. doi: 10.1161/STROKEAHA.110.578971
13. Johansson E, Bjellerup J, Wester P. Prediction of recurrent stroke with ABCD2 and ABCD3 scores in patients with symptomatic 50–99% carotid stenosis. BMC Neurol. (2014) 14:223. doi: 10.1186/s12883-014-0223-y
14. Dai Q, Sun W, Xiong Y, Hankey GJ, Xiao L, Zhu W, et al. From clinical to tissue-based dual TIA: validation and refinement of ABCD3-I score. Neurology. (2015) 84:1426–32. doi: 10.1212/WNL.0000000000001444
15. Kiyohara T, Kamouchi M, Kumai Y, Ninomiya T, Hata J, Yoshimura S, et al. ABCD3 and ABCD3-I scores are superior to ABCD2 score in the prediction of short- and long-term risks of stroke after transient ischemic attack. Stroke. (2014) 45:418–25. doi: 10.1161/STROKEAHA.113.003077
16. Wardlaw JM, Brazzelli M, Chappell FM, Miranda H, Shuler K, Sandercock PA, et al. ABCD2 score and secondary stroke prevention: meta-analysis and effect per 1,000 patients triaged. Neurology. (2015) 85:373–80. doi: 10.1212/WNL.0000000000001780
17. Dawson J, Lamb KE, Quinn TJ, Lees KR, Horvers M, Verrijth MJ, et al. A recognition tool for transient ischaemic attack. QJM. (2009) 102:43–9. doi: 10.1093/qjmed/hcn139
18. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
19. Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. (2011) 42:227–76. doi: 10.1161/STROKEAHA.111.614933
20. Wardlaw J, Brazzelli M, Miranda H, Chappell F, McNamee P, Scotland G, et al. An assessment of the cost-effectiveness of magnetic resonance, including diffusion-weighted imaging, in patients with transient ischaemic attack and minor stroke: a systematic review, meta-analysis and economic evaluation. Health Technol Assess. (2014) 18:1–368, v–vi. doi: 10.3310/hta18270
21. Crisostomo RA, Garcia MM, Tong DC. Detection of diffusion-weighted MRI abnormalities in patients with transient ischemic attack: correlation with clinical characteristics. Stroke. (2003) 34:932–37. doi: 10.1161/01.STR.0000061496.00669.5E
22. Redgrave JN, Coutts SB, Schulz UG, Briley D, Rothwell PM. Systematic review of associations between the presence of acute ischemic lesions on diffusion-weighted imaging and clinical predictors of early stroke risk after transient ischemic attack. Stroke. (2007) 38:1482–8. doi: 10.1161/STROKEAHA.106.477380
23. Brazzelli M, Chappell FM, Miranda H, Shuler K, Dennis M, Sandercock PA, et al. Diffusion-weighted imaging and diagnosis of transient ischemic attack. Ann Neurol. (2014) 75:67–76. doi: 10.1002/ana.24026
24. Al-Khaled M, Eggers J. MRI findings and stroke risk in TIA patients with different symptom durations. Neurology. (2013) 80:1920–6. doi: 10.1212/WNL.0b013e318293e15f
25. Pavlovic AM, Barras CD, Hand PJ, Tress BM, Desmond PM, Davis SM. Brain imaging in transient ischemic attack–redefining TIA. J Clin Neurosci. (2010) 17:1105–10. doi: 10.1016/j.jocn.2010.01.011
26. Purroy F, Begue R, Gil MI, Quilez A, Sanahuja J, Brieva L, et al. Patterns of diffusion-weighted magnetic resonance imaging associated with etiology improve the accuracy of prognosis after transient ischaemic attack. Eur J Neurol. (2011) 18:121–8. doi: 10.1111/j.1468-1331.2010.03080.x
27. Anticoli S, Pezzella FR, Pozzessere C, Gallelli L, Bravi MC, Caso V, et al. Transient ischemic attack fast-track and long-term stroke risk: role of diffusion-weighted magnetic resonance imaging. J Stroke Cerebrovasc Dis. (2015) 24:2110–6. doi: 10.1016/j.jstrokecerebrovasdis.2015.05.016
28. Nah HW, Kwon SU, Kang DW, Lee DH, Kim JS. Diagnostic and prognostic value of multimodal MRI in transient ischemic attack. Int J Stroke. (2014) 9:895–901. doi: 10.1111/ijs.12212
29. Purroy F, Jimenez-Caballero PE, Mauri-Capdevila G, Torres MJ, Gorospe A, Ramirez Moreno JM, et al. Predictive value of brain and vascular imaging including intracranial vessels in transient ischaemic attack patients: external validation of the ABCD3-I score. Eur J Neurol. (2013) 20:1088–93. doi: 10.1111/ene.12141
30. Dutta D. Diagnosis of TIA (DOT) score–design and validation of a new clinical diagnostic tool for transient ischaemic attack. BMC Neurol. (2016) 16:20. doi: 10.1186/s12883-016-0535-1
31. Wolf ME, Held VE, Hennerici MG. Risk scores for transient ischemic attack. Front Neurol Neurosci. (2014) 33:41–68. doi: 10.1159/000351891
Keywords: transient ischemic attack, ischemic stroke, diffusion-weighted imaging, ABCD3-I score, dawson score
Citation: Yuan J, Jia Z, Song Y, Yang S, Li Y, Yang L, Qin W and Hu W (2019) Incidence and Predictors of Acute Ischemic Lesions on Brain Magnetic Resonance Imaging in Patients With a Clinical Diagnosis of Transient Ischemic Attack in China. Front. Neurol. 10:764. doi: 10.3389/fneur.2019.00764
Received: 15 May 2019; Accepted: 01 July 2019;
Published: 16 July 2019.
Edited by:
Nishant K. Mishra, Icahn School of Medicine at Mount Sinai, United StatesReviewed by:
Hakan Ay, Eskişehir Osmangazi University, TurkeyYan Ji, University of Kansas Medical Center, United States
Copyright © 2019 Yuan, Jia, Song, Yang, Li, Yang, Qin and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenli Hu, d2VubGlodTMzNjZAMTI2LmNvbQ==
 Junliang Yuan1
Junliang Yuan1 Zejin Jia
Zejin Jia Yangguang Song
Yangguang Song Shuna Yang
Shuna Yang Yue Li
Yue Li Lei Yang
Lei Yang Wei Qin
Wei Qin Wenli Hu
Wenli Hu