- 1Pediatric and Adolescence Unit, Department of Internal Medicine, University of Pavia, Pavia, Italy
- 2Pediatric Unit, Department of the Mother and Child Health, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy
- 3Laboratory of Dietetics and Clinical Nutrition, Department of Public Health, Experimental and Forensic Medicine, University of Pavia, Pavia, Italy
- 4Unit of Internal Medicine and Endocrinology, Clinical Nutrition and Dietetics Service, ICS Maugeri IRCCS, Pavia, Italy
- 5Biometry & Clinical Epidemiology, Scientific Direction, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy
- 6Pediatric Surgery Department, Children's Hospital “G. Di Cristina”, ARNAS Civico-di Cristina-Benfratelli, Palermo, Italy
Background: Insulin resistance (IR) plays a key role in the pathogenesis of type 2 diabetes (T2D). In neurologically impaired (NI) children unfavorable cardio-metabolic risk profile with high prevalence of IR has been reported. We evaluated the prevalence of T2D in NI children and adolescents, in order to define if a dedicated glucose monitoring may be recommended in these subjects.
Methods: We retrospectively evaluated 63 patients (11.4 ± 4.0 years) with severe disabilities. Auxological parameters were recorded. Metabolic blood assays included fasting blood glucose (FBG), fasting insulin, triglycerides (TG). IR was detected with the homeostasis model assessment for insulin resistance (HOMA-IR > 97.5th percentile for age and sex) and triglyceride-glucose index (TyG index > 7.88). Elevated FBG was defined with values >100 mg/dl. T2D was defined according to American Diabetes Association criteria.
Results: Impaired insulin sensitivity, pathological TyG index and elevated FBG were observed, respectively, in 41.3, 63.5, and 11.1% patients. T2D was diagnosed in 3.2% asymptomatic patients. The prevalence of diabetes was higher in pre-pubertal compared to pubertal subjects (p = 0.03).
Conclusions: T2D in NI children and adolescents without obesity could represent a new emerging entity. IR and/or surrogate markers of IR index may be useful for the primary screening of this at-risk disabled population so as to prevent diabetes.
Introduction
Youth type 2 diabetes (T2D) is increasing, linked with obesity and declining physical activity in high-risk population (1). In the United States, the prevalence of T2D in 2009 among adolescents aged 10 through 19 years was 0.46 per 1,000 or 0.046%, with highest prevalence in American Indians, followed by black, Hispanic, and Asian Pacific Islander youths, with lowest prevalence in white ones (1–3). Studies in Europe indicate that T2D remains rare in largely white populations (4–8).
Insulin resistance (IR) plays a key role in the pathogenesis of T2D (1). Previously, we described that neurologically impaired (NI) children showed an unfavorable cardio-metabolic risk profile with a high prevalence of IR which was not related to BMI (9–11).
In this brief report, we evaluated the prevalence of T2D in NI children and adolescents without obesity, in order to describe if a dedicated glucose profile monitoring may be recommended in this “fragile” population.
Patients and Methods
Patients
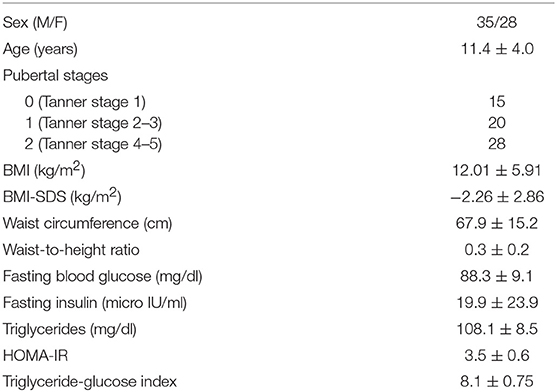
We retrospectively evaluated 63 caucasian patients (35 males/28 females, mean age 11.4 ± 4.0 years, range 1.9–19.1 yr) with severe disabilities (cerebral palsy 34.9%, epileptic encephalopathy 28.6%, severe psychomotor developmental delay in dysmorphic syndromes 36.5%). All patients presented with neuromotor damage that could alter their ability to self-feed and were bedridden.
All subjects had been previously scheduled for surgical treatment (gastrostomy/jejunostomy tube positioning, anti-reflux surgical treatment) and/or management of nutritional support devices.
Enteral feeding was adopted in 58/63 participants (bolus 82.7%, continuous pump feeding 17.3%) and oral feeding in 5/63 subjects (8%). Anticonvulsive drugs (at least two of the following: phenobarbital, valproic acid, phentoyn, lamotrigine, topiramate, carbamazepine, and clonazepam) were reported in 56/63 (88.8%) of the whole sample; other therapies were recorded in 5 patients (in 3 anti-hypertensive drugs, in 1 growth hormone therapy, in 1 L-thyroxine).
In all subjects physical activity was conducted for <60 min/week.
The study was performed according to the Declaration of Helsinki and with the approval of the Institutional Review Board. Parents and/or legal guardian, after receiving information about the study, gave their written consent.
Methods
Clinical and Anthropometric Parameters
Physical examination of the subjects included anthropometric parameters as well as pubertal stage evaluation according to Marshall and Tanner (12, 13) (pre-pubertal characteristics corresponding to Tanner stage 1).
Weight, height, waist circumference (WC) were measured as previously reported (11) and consequently BMI and waist to height ratio (WHtR) were calculated.
Metabolic Parameters
Metabolic blood assays included fasting blood glucose (FBG), insulin, triglycerides (TG). Insulin resistance was calculated with the homeostasis model assessment for insulin resistance (HOMA-IR) (14). Triglyceride-glucose index (TyG index) was also evaluated using the formula (ln[fasting triglycerides (mg/dl) × fasting plasma glucose (mg/dl)/2]) (15), as a surrogate marker of IR and predictor of diabetes.
Elevated FBG was defined with values exceeding 100 mg/dl and impaired insulin sensitivity (ISI) with a HOMA-IR exceeding the 97.5th percentile for age and sex (16).
According to Vieira Ribeiro, TyG index was considered pathological with a cut-off exceeding 7.88.
T2D was defined as FBG ≥ 126 mg/dL and/or 2 h plasma glucose (PG) ≥ 200 mg/dL and/or random PG ≥ 200 mg/dL (in patients with continuous pump feeding only random PG ≥ 200 mg/dL was considered) plus symptoms of diabetes (given the feeding difficulties, of these children, only polyurea was considered) and HbA1c ≥ 6.5% (17).
In diabetic patients the presence of islet autoimmunity [glutamic acid decarboxylase (GAD), islet antigen type 2 (IA2), anti-insulin (IAA), and zinc transporter 8 (ZnT8) autoantibodies] were excluded.
The waist to height ratio (WHtR) was then calculated with the standard formula and a cut-off of 0.5 was used to differentiate low from high WHtR (18).
Treatment following diabetes diagnosis was recorded.
Statistical Analysis
The Shapiro-Wilk test was used to assess the normal distribution of quantitative variables. When quantitative variables were normally distributed, the results were expressed as the mean value and standard deviation (SD), otherwise median and interquartile range (IQR; 25th−75th percentile) were reported. Qualitative variables were summarized as counts and percentages. Associations between sex or pubertal stage and continuous variables were tried through t-test or non parametric Mann-Whitney test for the former and oneway analysis of variance followed by Scheffe corrected 2 × 2 post hoc test for the latter; chi square test was used for qualitative variables. A p < 0.05 was considered statistically significant. Multivariate (including sex and pubertal stage as independent variables) linear or logistic regression models were fitted.
Results
In Table 1, the clinical features and metabolic parameters of the patients are reported.
The average HOMA-IR was 3.5 ± 0.6, with higher levels in pubertal compared to pre-pubertal patients (p = 0.03) and without significant difference between sex (p = 0.3). Insulin resistance was detected in 26/63 (41.3%) subjects, with a similar prevalence in males and females (p = 0.7) and in pre-pubertal and pubertal subjects (p = 0.1).
The mean values of TyG index observed in the sample was 8.1 ± 0.75, which is higher in pubertal compared to pre-pubertal patients (p = 0.03) and did not differ between genders (p = 0.6). Pathological TyG index was noted in 40/63 patients (63.5%), without significant difference according to sex (p = 0.3) and pubertal status (p = 0.6).
No significant correlation between HOMA-IR and TyG was found (r = 0.17 p = 0.20).
Pathological FBG was detected in 7/63 children (11.1%), without difference between sex (p = 0.3 and pubertal stage (p = 0.09).
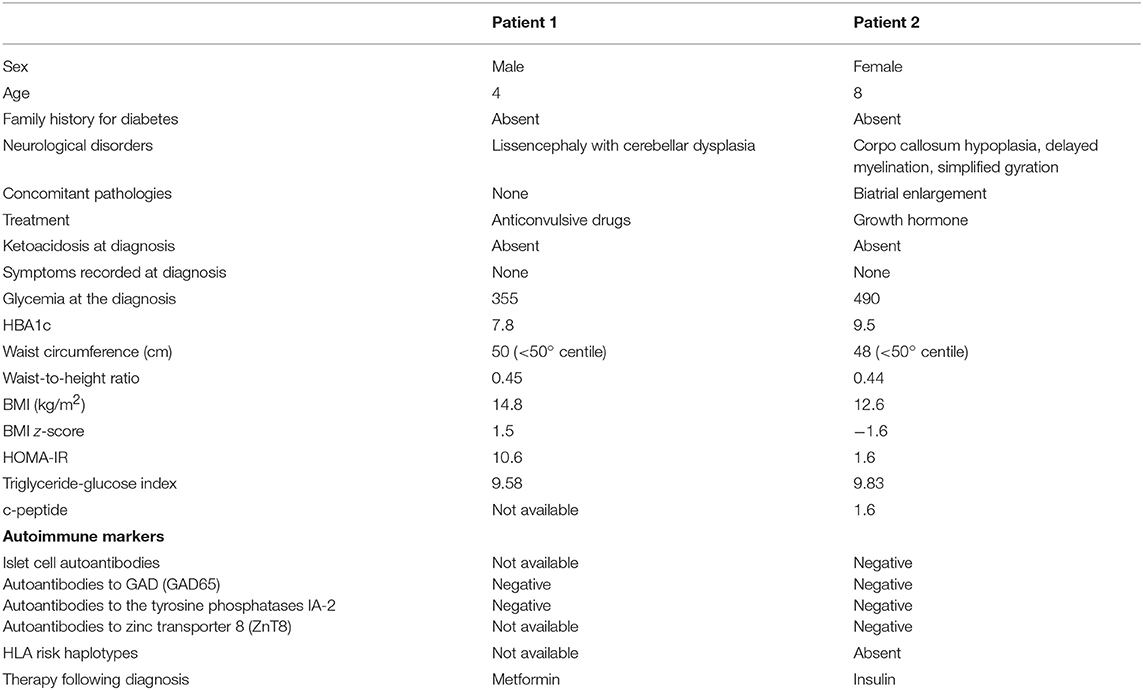
T2D was diagnosed in 2/63 patients (3.2%; 1 male and female with dysmorphic syndromes in which inborn errors of metabolism and mitochondrial disease were excluded) at the age of 4 and 8 years, respectively. Both patients were asymptomatic and diabetes was incidentally detected during a routine checkup. Clinical and biochemical data and treatment following diagnosis was reported in Table 2. In both patients, IR or surrogate markers of IR were detected.
The prevalence of diabetes was higher in pre-pubertal compared to pubertal subjects (p = 0.03), similarly in males and females (p = 0.8).
No association between metabolic alterations and type of nutritional support (p = 0.1) or drug exposure was noted (p = 0.9).
Discussion
Individuals with neurological impairment are at increased risk for frailty and chronic disease due to factors experienced throughout lifespan, such as excessive sedentary behaviors and malnutrition (19). Unfavorable cardio-metabolic profile and high allostatic load have been previously reported in early childhood (9–11). However, little is known about diabetes in disabled population.
This is the first study to report a certain prevalence of diabetes mellitus (3.2%) in the NI pediatric population. We demonstrated that ISI and pathological surrogate markers of insulin resistance index are common in children and adolescents with NI and showed that a high prevalence of diabetes is also relevant in pre-pubertal subjects.
Glucose homeostasis is maintained by a delicate coupling of insulin secretion, from pancreatic β-cells, with insulin sensitivity (skeletal muscle, adipose tissue, and hepatic) (1). When insulin sensitivity declines, insulin secretion must increase to maintain glucose tolerance and finally when β-cells are no longer able to secrete sufficient insulin to overcome insulin resistance, IGT ensues progressing to T2D (1).
Overweight and obesity are major contributors to the development of T2D, which represents the 1% of diabetes subtype in the italian pediatric population (20). There are few data on T2D prevalence in children without obesity and in this population diabetes is described as a major complication of post-transplant immunosuppressive therapy for kidney (21), liver (22), or hematological diseases (23) with a broad range (1.8–13%) depending of the treatment process (21–23).
We showed that the development of T2D and IR may also be observed in NI patients without obesity, not on immunosuppressive drugs, with no difference between sex. As previously reported, IR in these disabled patients was not correlated to BMI nor to energy intake (11). Negative regulation of insulin signaling could be viewed as a physiologic “adaptive mechanism for human survival” that is activated whenever the organism needs to switch from an anabolic to a catabolic or “insulin resistance” state, such as undernutrition, and to mobilize energy to support vital metabolic processes (9–11, 24–26). The restricted physical activity (27) may also play a crucial role on insulin resistance; it causes a rapid loss of lean mass, which is associated with a decline in basal metabolic rate and increased whole body and regional adiposity leading to insulin signaling interference (10). Additionally, chronic stress also tends to alter the anabolic/catabolic hormonal balance and may be involved in increased cortisol levels and insulin resistance (28). Finally, long-term therapy, such as anticonvulsive drugs and hormonal therapy may be associated with several metabolic abnormalities and their effects on insulin resistance and diabetes should be considered (9–11).
Glucose and lipid metabolism are linked to each other in many ways (29). Hypertriglyceridemia is the characteristic dyslipidemia in subjects with IR (29). Although no definitive explanation is still available for the correlation between hypertriglyceridemia and IR, it has been reported that elevated TG levels interfere with glucose metabolism in muscles, a finding consistent with the hypothesis that TG elevation in serum and tissue is related to decreased insulin sensitivity (29–31). Markers of insulin action based on lipids may help identify subjects with IR (32–36). The TyG index which is a surrogate marker of IR, is known to be associated with metabolic parameters and CVD and it had some prognostic value to predict T2D also in normoglycemic patients and normal-weight patients (32–34, 36). In our report a higher percentage of patients without obesity, with significant difference between sexes, showed pathological TyG index, in accordance with the presence of IR. Considering the unfavorable cardio-metabolic risk profile of this disabled population (9–11), this marker may be useful for predicting T2D in clinical practice and may provide a feasible alternative to the expensive and invasive gold standard test for IR.
We observed a lack of correlation between HOMA-IR and TyG index; this result could be due to the fact that the HOMA-IR does not change at the same time as the TyG index. Youth T2D is associated with insulin resistance, together with progressive deterioration in cell function and relative insulin deficiency in the absence of diabetes-related immune markers (1). Therefore HOMA-IR may not be altered when insulin reserves are running out. Further longitudinal studies are mandatory to clarify the natural history of ISI and β-cell Function in neurologically impaired youth.
We must acknowledge that the study has some limitations. The sample size was too small to define a prevalence of diabetes mellitus, moreover due to the difficulties in enrolling and studying neuro-cognitively disabled children, this metabolic aspect is interesting to consider for disabled young patients monitoring. The gold standard method for the determination of insulin sensitivity is the hyperinsulinemic euglycemic glucose clamp, moreover it is impractical in these young disabled people so we used HOMA-IR and TyG index because. Finally, several factors, such as inadequate nutritional status, low physical activity level, polypharmacotherapy, may synergically affect metabolic profile, however the effects of any single event was not measured. Additionally, a genetic component of diabetes is not excluded.
In conclusion, T2D in NI children and adolescents could represent a new emerging entity in subjects without obesity. Insulin resistance and/or surrogate marker of insulin resistance index may be useful for the early screening of these at-risk disabled populations in order to prevent diabetes.
Data Availability
All datasets generated for this study are included in the manuscript.
Author Contributions
VC and HC designed experiments, wrote, and supervised the manuscript. VG and DB carried out experiments. AD performed statistical analysis. GP provided patient samples, wrote, and supervised the manuscript. All authors have read and approved the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Hannon TS, Arslanian SA. The changing face of diabetes in youth: lessons learned from studies of type 2 diabetes. Ann N Y Acad Sci. (2015) 1353:113–37. doi: 10.1111/nyas.12939
2. Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, et al. Search for diabetes in youth study: incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med. (2017) 376:1419–29. doi: 10.1056/NEJMoa1610187
3. Dabelea D, Mayer-Davis EJ, Saydah S, Imperatore G, Linder B, Divers J, et al. Search for diabetes in youth study: prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. (2014) 311:1778–86. doi: 10.1001/jama.2014.3201
4. The NS, Richardson AS, Gordon-Larsen P. Timing and duration of obesity in relation to diabetes: findings from an ethnically diverse, nationally representative sample. Diabetes Care. (2013) 36:865–72. doi: 10.2337/dc12-0536
5. Dorenbos E, Drummen M, Rijks J, Adam T, Stouthart P, Alfredo Martínez J, et al. Preview (prevention of diabetes through lifestyle intervention and population studies in Europe and around the world) study in children aged 10 to 17 years: design, methods and baseline results. Diabetes Obes Metab. (2018) 20:1096–101. doi: 10.1111/dom.13216
6. Koskinen J, Magnussen CG, Sinaiko A, Woo J, Urbina E, Jacobs DR Jr, et al. Childhood age and associations between childhood metabolic syndrome and adult risk for metabolic syndrome, type 2 diabetes mellitus and carotid intima media thickness: the International Childhood Cardiovascular Cohort Consortium. J Am Heart Assoc. (2017) 6:e005632. doi: 10.1161/JAHA.117.005632
7. O'Dea MI, O'Connell SM, O'Grady MJ. Prevalence and characteristics of paediatric type 2 diabetes in the Republic of Ireland. Diabet Med. (2017) 34:1603–07. doi: 10.1111/dme.13425
8. Zimmermann E, Bjerregaard LG, Gamborg M, Vaag AA, Sørensen TIA, Baker JL. Childhood body mass index and development of type 2 diabetes throughout adult life-A large-scale Danish cohort study. Obesity. (2017) 25:965–71. doi: 10.1002/oby.21820
9. Calcaterra V, Cena H, Casali PM, Iacobellis G, Albertini R, De Amici M, et al. Epicardial fat thickness in non-obese neurologically impaired children: association with unfavorable cardiometabolic risk profile. Ann Nutr Metab. (2018) 72:96–103. doi: 10.1159/000484326
10. Calcaterra V, Cena H, de Silvestri A, Albertini R, De Amici M, Valenza M, et al. Stress measured by allostatic load in neurologically impaired children: the importance of nutritional status. Horm Res Paediatr. (2017) 88:224–30. doi: 10.1159/000477906
11. Pelizzo G, Calcaterra V, Carlini V, Fusillo M, Manuelli M, Klersy C, et al. Nutritional status and metabolic profile in neurologically impaired pediatric surgical patients. J Pediatr Endocrinol Metab. (2017) 30:289–300. doi: 10.1515/jpem-2016-0369
12. Marshall WA, Tanner JM. Variations in patterns of pubertal changes in boys. Arch Dis Child. (1996) 45:13–23. doi: 10.1136/adc.45.239.13
13. Marshall WA, Tanner JM. Variations in patterns of pubertal changes in girls. Arch Dis Child. (1996) 44:291–303. doi: 10.1136/adc.44.235.291
14. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Naylor BA, Treacher DF, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. (1985) 28:412–19. doi: 10.1007/BF00280883
15. Vieira-Ribeiro SA, Fonseca PCA, Andreoli CS, Ribeiro AQ, Hermsdorff HHM, Pereira PF, et al. The TyG index cutoff point and its association with body adiposity and lifestyle in children. J Pediatr. (2018) 95:217–23. doi: 10.1016/j.jped.2017.12.012
16. d'Annunzio G, Vanelli M, Pistorio A, Minuto N, Bergamino L, Iafusco D, et al. Insulin resistance and secretion indexes in healthy Italian children and adolescents: a multicentre study. Acta Biomed. (2009) 80:21–8.
17. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. (2010) 33(Suppl. 1):S62–9. doi: 10.2337/dc10-S062
18. Maffeis C, Banzato C, Talamini G, Obesity Study Group of the Italian Society of Pediatric Endocrinology and Diabetology. Waist-to-height ratio, a useful index to identify high metabolic risk in overweight children. J Pediatr. (2008) 152:207–13. doi: 10.1016/j.jpeds.2007.09.021
19. Whitney DG, Hurvitz EA, Ryan JM, Devlin MJ, Caird MS, French ZP, et al. Noncommunicable disease and multimorbidity in young adults with cerebral palsy. Clin Epidemiol. (2018) 10:511–19. doi: 10.2147/CLEP.S159405
20. Delvecchio M, Mozzillo E, Salzano G, Iafusco D, Frontino G, Patera PI, et al. Monogenic diabetes accounts for 6.3% of cases referred to 15 Italian pediatric diabetes centers during 2007 to 2012. J Clin Endocrinol Metab. (2017) 102:1826–34. doi: 10.1210/jc.2016-2490
21. Prokai A, Fekete A, Kis E, Reusz GS, Sallay P, Korner A, et al. Post-transplant diabetes mellitus in children following renal transplantation. Pediatr Transplant. (2008) 12:643–9. doi: 10.1111/j.1399-3046.2007.00862.x
22. Hathout E, Alonso E, Anand R, Martz K, Imseis E, Johnston J, et al. Post-transplant diabetes mellitus in pediatric liver transplantation. Pediatr Transplant. (2009) 13:599–605. doi: 10.1111/j.1399-3046.2007.00603.x
23. Hirabayashi K, Nakazawa Y, Matsuura H, Hara Y, Kurata T, Hirabayashi K, et al. Risk factors for diabetes mellitus and impaired glucose tolerance following allogeneic hematopoietic stem cell transplantation in pediatric patients with hematological malignancies. Int J Hematol. (2014) 99:477–86. doi: 10.1007/s12185-014-1536-8
24. Bjorntorp P. Visceral fat accumulation: the missing link between psychosocial factors and cardiovascular disease? J Intern Med. (1991) 230:195–201. doi: 10.1111/j.1365-2796.1991.tb00431.x
25. Holloszy JO. Exercise-induced increase in muscle insulin sensitivity. J Appl Physiol. (2005) 99:338–43. doi: 10.1152/japplphysiol.00123.2005
26. Benson AC, Torode ME, Fiatarone Singh MA. Effects of resistance training on metabolic fitness in children and adolescents: a systematic review. Obes Rev. (2008) 9:43–66. doi: 10.1111/j.1467-789X.2007.00388.x
27. Venkatasamy VV, Pericherla S, Manthuruthil S, Mishra S, Hanno R. Effect of physical activity on insulin resistance, inflammation and oxidative stress in diabetes mellitus. J Clin Diagn Res. (2013) 7:1764–6. doi: 10.7860/JCDR/2013/6518.3306
28. Eek MN, Tranberg R, Zügner R, Alkema K, Beckung E. Muscle strength training to improve gait function in children with cerebral palsy. Dev Med Child Neurol. (2008) 50:759–64. doi: 10.1111/j.1469-8749.2008.03045.x
29. Parhofer KG. Interaction between glucose and lipid metabolism: more than diabetic dyslipidemia. Diabetes Metab J. (2015) 39:353–62. doi: 10.4093/dmj.2015.39.5.353
30. Furler SM, Poynten AM, Kriketos AD, Lowy AJ, Ellis BA, Maclean EL, et al. Independent influences of central fat and skeletal muscle lipids on insulin sensitivity. Obes Res. (2001) 9:535–43. doi: 10.1038/oby.2001.70
31. Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C, et al. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes. (1997) 46:983–8. doi: 10.2337/diab.46.6.983
32. Unger G, Benozzi SF, Perruzza F, Pennacchiotti GL. Triglycerides and glucose index: a useful indicator of insulin resistance. Endocrinol Nutr. (2014) 61:533–40. doi: 10.1016/j.endonu.2014.06.009
33. Mohd Nor NS, Lee S, Bacha F, Tfayli H, Arslanian S. Triglyceride glucose index as a surrogate measure of insulin sensitivity in obese adolescents with normoglycemia, prediabetes, and type 2 diabetes mellitus: comparison with the hyperinsulinemic-euglycemic clamp. Pediatr Diabetes. (2016) 17:458–65. doi: 10.1111/pedi.12303
34. Lee EY, Yang HK, Lee J, Kang B, Yang Y, Lee SH, et al. Triglyceride glucose index, a marker of insulin resistance, is associated with coronary artery stenosis in asymptomatic subjects with type 2 diabetes. Lipids Health Dis. (2016) 15:155. doi: 10.1186/s12944-016-0324-2
35. Low S, Khoo KCJ, Irwan B, Sum CF, Subramaniam T, Lim SC, et al. The role of triglyceride glucose index in development of Type 2 diabetes mellitus. Diabetes Res Clin Pract. (2018) 143:43–9. doi: 10.1016/j.diabres.2018.06.006
36. Navarro-González D, Sánchez-Íñigo L, Pastrana-Delgado J, Fernández-Montero A, Martinez JA. Triglyceride-glucose index (TyG index) in comparison with fasting plasma glucose improved diabetes prediction in patients with normal fasting glucose: the vascular-metabolic CUN cohort. Prev Med. (2016) 86:99–105. doi: 10.1016/j.ypmed.2016.01.022
Keywords: type 2 diabetes, children, adolescents, neurologically impaired, disability
Citation: Calcaterra V, Cena H, De Silvestri A, Girgenti V, Bommarito D and Pelizzo G (2019) Diabetes Type 2 in Neurologically Impaired Children and Adolescents Without Obesity: A New Emerging Entity? Front. Neurol. 10:947. doi: 10.3389/fneur.2019.00947
Received: 17 May 2019; Accepted: 16 August 2019;
Published: 29 August 2019.
Edited by:
Alberto Verrotti, University of L'Aquila, ItalyReviewed by:
Chiara Fiorillo, University of Genoa, ItalyMaurizio Elia, Oasi Maria SS. Association ONLUS (IRCCS), Italy
Copyright © 2019 Calcaterra, Cena, De Silvestri, Girgenti, Bommarito and Pelizzo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valeria Calcaterra, di5jYWxjYXRlcnJhQHNtYXR0ZW8ucHYuaXQ=
†These authors have contributed equally to this work
 Valeria Calcaterra
Valeria Calcaterra Hellas Cena3,4†
Hellas Cena3,4† Annalisa De Silvestri
Annalisa De Silvestri