- 1St George's University Hospitals NHS Foundation Trust, London, United Kingdom
- 2Genetics and Genomics Clinical Academic Group, Molecular and Clinical Sciences Research Institute, St George's, University of London, London, United Kingdom
- 3Alder Hey Children's NHS Foundation Trust, Liverpool, United Kingdom
- 4University Hospitals Birmingham NHS Foundation Trust, Birmingham, United Kingdom
- 5King's College Hospital NHS Foundation Trust, London, United Kingdom
- 6York Teaching Hospitals NHS Foundation Trust, York, United Kingdom
- 7Wirral University Teaching Hospitals NHS Foundation Trust, Merseyside, United Kingdom
- 8Tuberous Sclerosis Association, London, United Kingdom
- 9Leeds Teaching Hospitals NHS Trust, Leeds, United Kingdom
- 10Department of Renal Medicine, University College London, London, United Kingdom
- 11Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, United Kingdom
- 12Centre for Genomic Medicine, St. Mary's Hospital, Central Manchester University Hospitals NHS Foundation Trust, Manchester, United Kingdom
- 13Centre for Genomic Medicine, Manchester Academic Health Science Centre, University of Manchester, Manchester, United Kingdom
- 14Division of Respiratory Medicine, Faculty of Medicine & Health Sciences, University of Nottingham, Nottingham, United Kingdom
- 15National Centre for Lymphangioleiomyomatosis, Nottingham, United Kingdom
- 16NHS Greater Glasgow and Clyde, Glasgow, United Kingdom
- 17Birmingham Children's Hospital NHS Foundation Trust, Birmingham, United Kingdom
- 18Tuberous Sclerosis Clinic, Belfast Health and Social Care Trust, Belfast, United Kingdom
- 19Great Ormond Street Hospital for Children NHS Foundation Trust, London, United Kingdom
- 20Evelina London Children's Hospital, St. Thomas' Hospital, London, United Kingdom
- 21School of Life Course Sciences, King's College London, London, United Kingdom
- 22Institute of Neuroscience, Newcastle University, Newcastle upon Tyne, United Kingdom
- 23Kidney Genetics Group, Academic Nephrology Unit, University of Sheffield Medical School, Sheffield, United Kingdom
- 24Sheffield Kidney Institute, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, United Kingdom
- 25Institute of Medical Genetics, Cardiff University School of Medicine, Cardiff, United Kingdom
- 26Nobles Hospital, Douglas, United Kingdom
- 27Brighton and Sussex University Hospitals NHS Trust, Brighton, United Kingdom
Introduction
Tuberous sclerosis complex (TSC) is an autosomal dominant multisystem genetic disorder characterized by benign tumors in multiple organs, including the skin, brain, kidneys, and lungs and occasional malignant tumors. Hamartomas in the brain, retina, and sometimes other organs also occur (1–3). The estimated prevalence is 1:600–1:10,000 live births in the general population (4–6). Patients present at different ages with different manifestations, and varying degrees of organ involvement (Figure 1). CNS manifestations of TSC mainly present in childhood, affect around 85% of patients (8), frequently resulting in epilepsy refractory to treatment, intellectual impairment, autistic spectrum disorder, attention deficit hyperactivity disorder, and behavioral problems (1–3). Renal angiomyolipomas (AMLs) occur in ~80% of patients (9); kidney disease is the leading cause of death in adults with TSC (10). TSC is complex and highly varied (Figure 1) necessitating careful coordination of care, which is lacking for most patients in the UK. Some TSC manifestations are rarer; e.g., subependymal giant cell astrocytoma (SEGA) occurs in around 20–24% of patients (11, 12) (Figure 2).
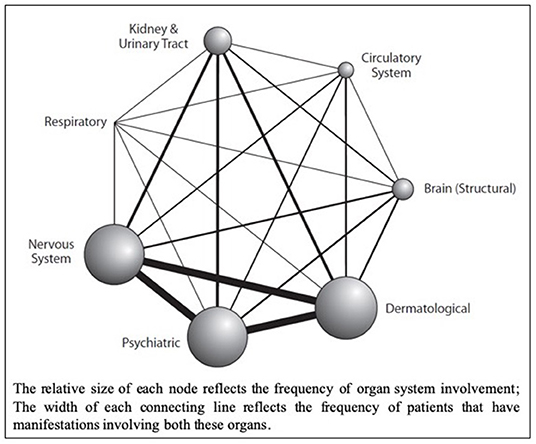
Figure 1. Network Diagram showing primary organ systems affected for each patient from a retrospective cohort analysis of UK TSC patient data (n = 324); sourced from the Clinical Practice Research Datalink (CPRD), linked to secondary care data from Hospital Episode Statistics (HES) database, and the Office for National Statistics (ONS) mortality register (Reproduced with permission from Eur J Paediatr Neurol) (7).
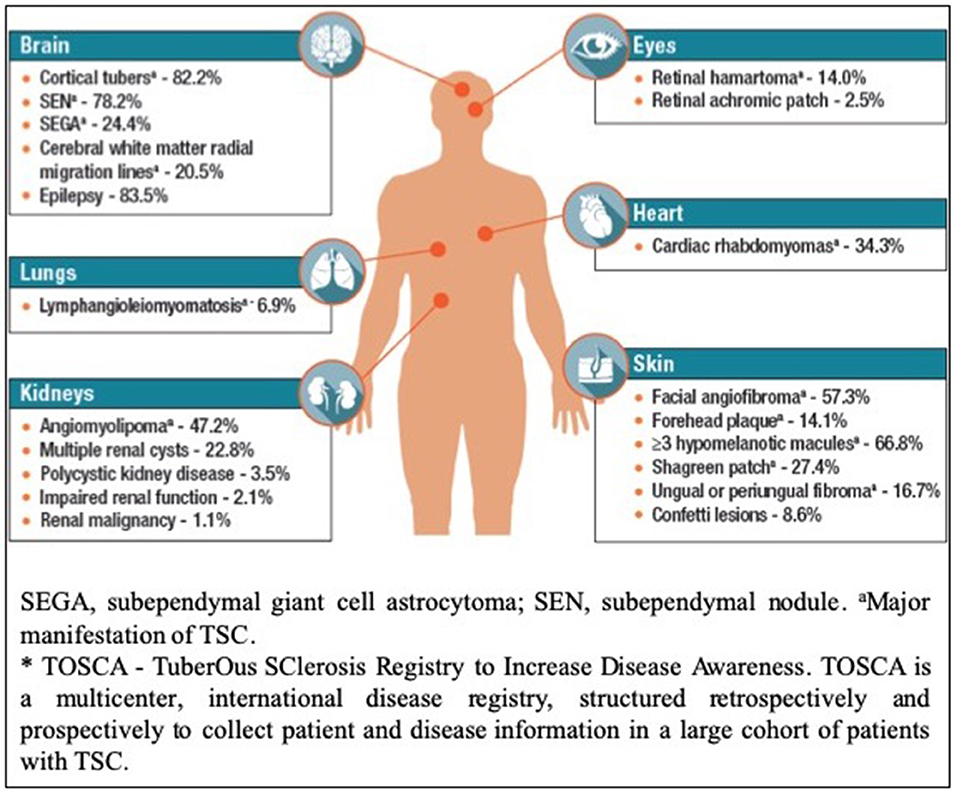
Figure 2. Disease Manifestations of TSC Reported at Baseline in TOSCA* Participants (n = 2,093) (Reproduced with permission of TOSCA consortium, presented at The International TSC research Conference Tokyo 2018).
The major unsolved problem in TSC is refractory epilepsy and TSC-associated neuropsychiatric disorders (TAND); of which preliminary evidence suggests refractory epilepsy is a major cause (13–15).
TSC, like other complex rare diseases, is a major burden to patients, families and healthcare systems. Optimizing care will alleviate some of this while waiting for medical research to deliver a cure.
Classically, a clinical diagnosis of TSC is made by identifying major and minor features (Table 1) (1, 16). With wider availability of genetic testing, identification of pathogenic mutations in TSC1 or TSC2 is now sufficient to establish a diagnosis, regardless of the presence of clinical features (1, 16), and is particularly useful in confirming a suspected diagnosis, as many clinical TSC manifestations are infrequent in young patients (1, 16).

Table 1. Major and minor clinical manifestations of tuberous sclerosis complex (1).
The approval of the mTORC1 inhibitor—everolimus—for the treatment of AMLs, SEGA, and refractory epilepsy represents a significant advance in the potential management of the disease (17–19). Whilst not licensed in Europe, the Federal Drugs Agency (FDA) have also approved sirolimus for use in pulmonary lymphangioleiomyomatosis (LAM) (18). Refractory seizures adversely affect early development (20). Furthermore, appropriate early treatment of infantile spasms with vigabatrin has been shown to reduce the long-term impact of the neurological and neuropsychiatric aspects of TSC on patients (13, 14).
A retrospective UK cohort study linking Clinical Practice Research Datalink (CPRD) to Hospital Episode Statistics (HES) data identified 334 patients with TSC revealed a much lower frequency of complications than would be expected from previous research; the disparity possibly reflecting under-recognition, and hence suggestive of inadequate medical care (7).
It is clear from these findings, and the observation that many new patients referred to TSC clinics have never had holistic systematic monitoring, that many patients receive inadequate care. In the UK, about 1000 TSC families are known to the UK Tuberous Sclerosis Association, known as the TSA (Patient organization), and a similar number (usually the same families) attend UK specialist TSC clinics. Therefore, in most other cases, the quality of care delivered is unknown.
Given the range of organ systems affected by TSC, its treatment requires coordination across a number of medical specialties over a patient's lifetime (Table 2). Currently in the UK, 16 centers host specialist TSC clinics—but most UK TSC patients are not currently managed within them. These specialist clinics have often been founded by enthusiastic clinicians but are frequently inadequately funded.
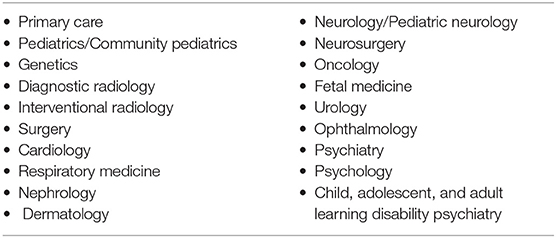
Table 2. A wide range of healthcare services are involved in the diagnosis, management, and treatment of the various manifestations of TSC. These include:
The transition from pediatric to adult services can be particularly challenging in the absence of a systematic service. In Wales, a specialist TSC clinic that has been established through a partnership, between a pharmaceutical company and the NHS, awaits the development of a fully sustainable commissioning model. In Northern Ireland, a TSC clinic has been running since 1995, and directly reviews the majority of TSC patients in the region.
In the UK, specialized service specifications are in place for adults and children with genetic disorders such as cystic fibrosis and inherited metabolic disorders. These are funded by NHS England, the Department of Health, Social Services and Public Safety in Northern Ireland, and Welsh Health Specialized Services Committee in Wales. However, no similar service or service specification is yet available for TSC patients.
We propose a comprehensive, holistic model of care—to manage patients that present with a range of manifestations, requiring specialist management from a wide range of specialties (Figures 1, 2).
Review of International Guidelines on TSC Surveillance and Management
The 2012 consensus statement on TSC surveillance and management, together with UK guidelines published this year make a number of recommendations for patient screening (1, 16, 21), with additional recommendations specific to AML, SEGA, LAM, and TSC-related epilepsy reported in disease-specific guidelines (22–24). A summary of the UK clinical guidelines, targeted at patients and general physicians has been made available by the UK TSA (25).
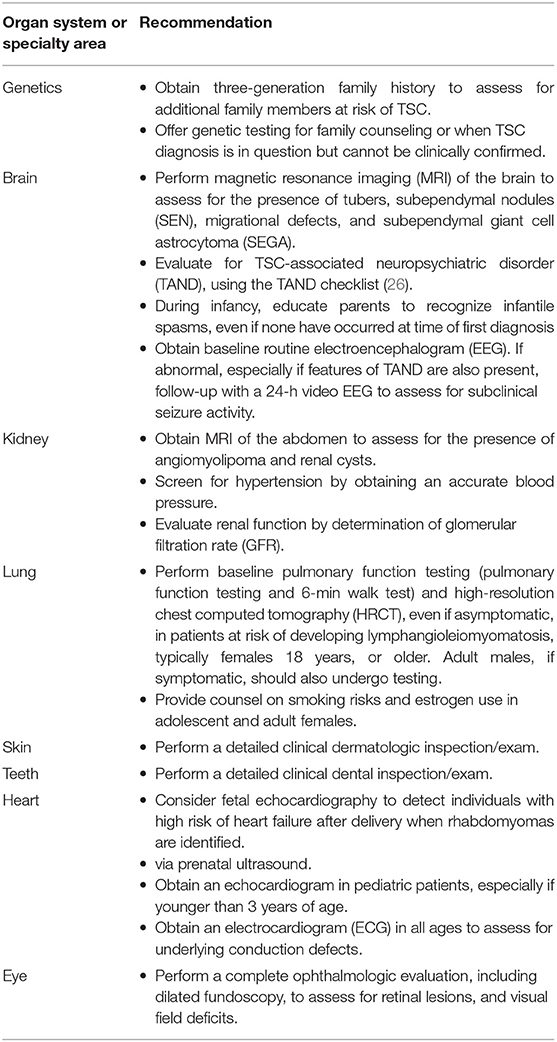
Additional baseline investigations to assess the extent of disease and organ involvement (Table 3), play an important role in guiding later treatment decisions.
The treatment and long-term surveillance needs (Table 4) (16) should be determined, based on the extent of disease at baseline, and tailored to the patient.

Table 4. Surveillance and management recommendations for patients already diagnosed with definite or possible TSC (16, 21).
Recommendations for the Delivery of Services for TSC Patients
A “hub and spoke” model of care is proposed, with a central network of TSC-centers, co-ordinated by specialists, and supported by a regional network of clinicians, that offer access to a comprehensive set of TSC-related specialist services. Hospital specialists should work collaboratively with patients, their families and their community doctors (General Practitioners or general physician) to provide support and advice and a pathway for dealing with problems that need specialist care. Since holistic care of TSC patients requires input from many different specialties, treatment of TSC patients should be discussed within the regional network by a multidisciplinary team (MDT), with the aim of ensuring that each TSC patient and their family have a tailored care plan to manage current disease manifestations, and surveillance for future TSC manifestations.
To achieve this, Specialist TSC services should ensure:
• Diagnosis: Patients with TSC are identified by clinical evaluation and/or genetic testing.
• Surveillance: Provision of multi-disciplinary evaluation–through alignment with regional genetic services (for genetic counseling to patients and their families), and with other clinical specialties to ensure access to appropriate care for all patients.
• Treatment: The appropriate access and use of TSC therapies.
• Safe transition from pediatric to adult care.
• Information and Support: Collaboration with patients/family and other organizations to provide access to TSC-specific information.
• Research: Facilitate patients and their families to become involved in relevant research projects.
Regional TSC clinics should be responsible for the diagnosis of patients with TSC, and the provision of routine care and support for patients and their families. Regional clinics should be supported by a dedicated TSC specialist coordinator, who has responsibility for coordinating the service, ensuring timely surveillance, and coordinating care between different specialist services, developing individualized plans for patient follow-up, and ensuring continuity of care for TSC patients transitioning to adulthood. Alongside this, linking regional clinics with TSC patient support groups (e.g., in the UK, the Tuberous Sclerosis Association (TSA), is vital to ensure that patients and their families receive comprehensive support). Regional clinics are in an ideal position to gather clinical and prevalence data to monitor needs locally and facilitate future research.
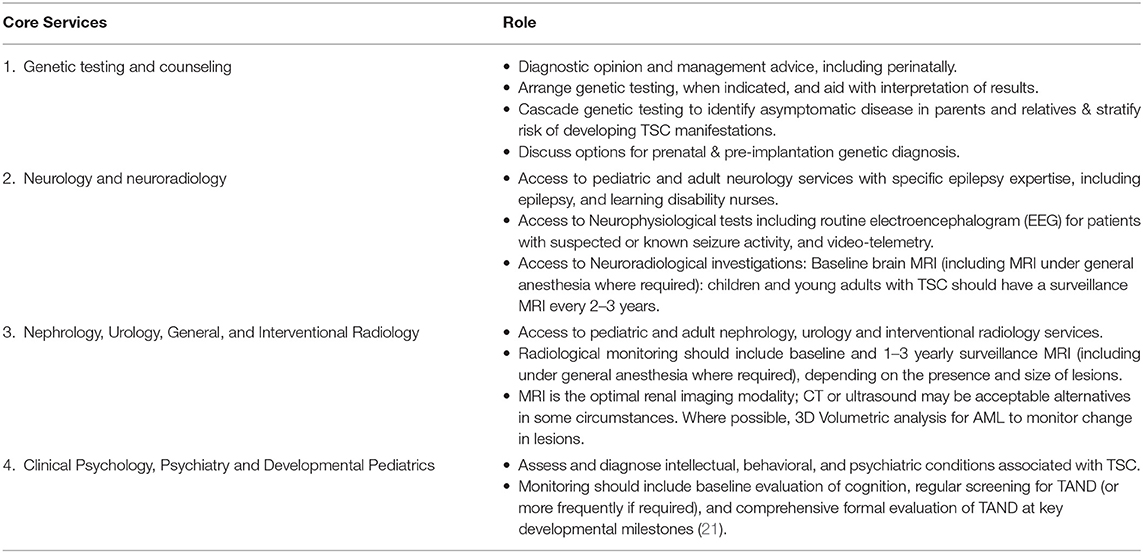
To allow regional TSC clinics to fulfill this pluripotent role, they need to offer or have access to a range of core services, including:
• Genetic Testing and Genetic Counseling.
• Neurology and Neuroimaging.
• Nephrology, Urology, General and Interventional Radiology services.
• Clinical Psychology, Psychiatry, and Developmental Pediatrics.
• Collaboration with patient/family organizations.
• Collaboration with patient's community physician (General Practitioner).
The roles of each of these core services is summarized in Table 5. Where regional centers are unable to provide a core service, there should be a clear pathway through which that service can be accessed. Furthermore, regional centers should also have access to the necessary facilities to cater for the specific needs of TSC patients. For example, TSC-related intellectual impairment and autistic spectrum disorder may necessitate that surveillance brain and renal imaging be performed under general anesthetic. This requires co-ordination of such procedures in an appropriate day-unit, or via a formal inpatient admission, with the support of specialized pediatric and adult anesthetists.
In addition to the “core” services, in order to provide comprehensive treatment to TSC patients, regional TSC centers would also need to have access to additional specialist support services, including Dermatology, Respiratory, Cardiology, Neuropsychiatry, and Obstetrics/Gynecology. The role of each of these additional services in relation to TSC patients is summarized in Table 6.
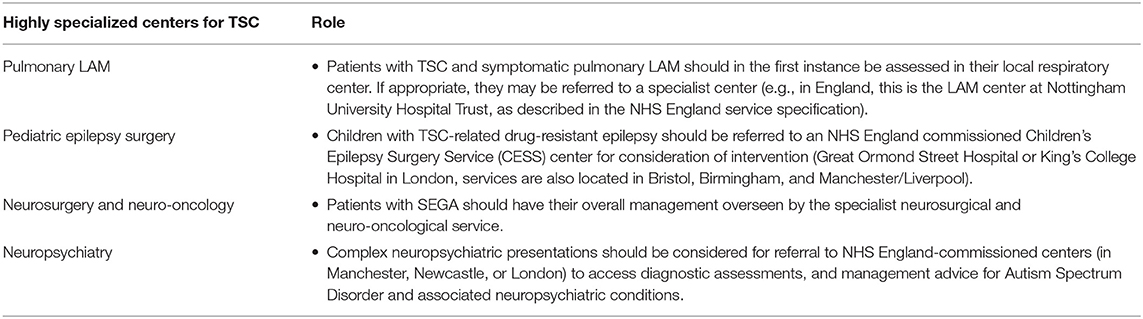
TSC Clinics need access to highly specialized services, of which there are four in the UK, including for Pulmonary LAM, Pediatric epilepsy surgery, Neurosurgery & Neuro-oncology and Neuropsychiatric services (summarized in Table 7).
In addition to ensuring access to appropriate services, there are key responsibilities for the regional centers in ensuring holistic care for TSC patients and their families.
Regional services need to ensure provision of the supportive care needed by patients and their families, including referral for individualized education plans for patients, genetic counseling for family members, and ongoing support for both the patient and their family from a patient association.
There is a need to monitor patient movement through the service to ensure that all patients are offered appropriate, regular surveillance and timely follow-up. Patients should be offered the most up-to-date, evidence-based surveillance, and those patients with multiple complications of TSC should attend joint clinics, or have the monitoring of different manifestations performed in a single session (e.g., combined surveillance/monitoring of SEGA and renal AML through a coordinated MRI scan of both brain & renal tract—particularly where a general anesthetic is required to achieve the imaging), in order to minimize individual patients' time in hospital. Such efforts would not only help to reduce the costs of patient monitoring but help to improve patients' and careers' experience of care, and their quality of life.
TSC regional centers should ensure that the service is aligned with National guidelines such as those published by NICE or the Renal Association on how to manage transitional care for patients moving from pediatric to adult services, with bespoke plans drawn up for individual patients where necessary.
TSC centers and networks should collaborate with the current available networks of local/community services (e.g., Community pediatricians and mental health services) to optimize care and minimize cost.
Pediatric and adult TSC centers, if not co-located, need to collaborate proactively to ensure safe transition of care from children's' to adult services. This is a time when patients are often lost to follow up.
Finally, there is a need to audit the services offered to and used by patients with TSC, so as to ensure that patients are treated appropriately. A very helpful way to ensure that clinic services develop into exactly what is needed by patients and families is to audit services using PREMS (Patient reported experience measures) (29) and PROMS (Patient reported outcome measures) (30).
Regular review of services will help to identify any potential opportunities for improved efficiency, as well as ensure that patients are consistently screened and treated according to best practice. With this aim, a national database should be established to facilitate the coordination of care between centers, auditing of services, planning of resource allocation, and TSC-related research.
Discussion
The rarity and heterogeneity of TSC presentations offers a number of challenges to the implementation of best practice care; treatment and follow-up is consequently frequently fragmented, disjointed, and suboptimal.
There is a need to improve TSC management to ensure patients have early access to appropriate treatment and preventive measures—both to minimize long-term effects of TSC where possible, and to support a frequently vulnerable patient group and their families. In particular, there are three elements that are both essential for the success of a TSC clinic, yet frequently missing. These include dedicated neuropsychiatric input, access to CT/MRI imaging under general anesthetic, and perhaps most importantly, a dedicated specialist TSC co-ordinator. A mechanism to deliver optimal care is essential if patients are to gain the best outcomes; including monitoring and intervention for SEGA, renal AMLs, LAM, and TAND, and early improvement in refractory epilepsy.
Hepatic lesions are common in TSC but very rarely cause any clinical problems (31). They do not need to be regularly monitored.
The primary physician of a patient is usually their general pediatrician or, in adults, their general practitioner or general physician. They may delegate responsibility for holistic management of TSC care to a hospital specialist but remain responsible for other aspects of their patient's care, so that collaboration and good communication is essential.
Finally, it should be acknowledged that optimal management of TSC is a field of active research and new recommendations will continue to be made. For example, It is now recognized that regular surveillance EEGs in infants can identify infants who are about to begin having seizures (32, 33). Emerging evidence from the Epistop trial and historic case series suggest starting therapy promptly or before the onset of clinical seizures, may markedly improve outcomes (13, 14). Similarly, genetic testing cannot yet be used to accurately predict an individual's prognosis, only the average risk in a group, but this is likely to change in the near future (9, 34).
We advocate that specialist expertise be provided by centralized TSC “hubs,” with routine patient management coordinated centrally and undertaken in regional TSC networks to facilitate optimal resource use and improve the comprehensive care of TSC patients. The TSC hub-and-spoke model will form a coordinated care network, that will also provide a structure to facilitate the education of health care professionals and affected families, and to facilitate TSC research. This model for TSC care may also serve as a blueprint for improving the quality of care for patients with other rare diseases in evolving, ever more efficient, healthcare services.
Author Contributions
NA and FE participated in the development of the service specification and the drafting of the manuscript. JK participated in the development of the service specification, the drafting, and reviewing of the manuscript. PC advised on adult neurological aspects of TSC in particular relating to epilepsy. PM helped to draft the manuscript and has read and approved the final manuscript. RA, ZB, RB, PB, AC, MT, EF, DG, AH, EJ, SJ, SRJ, LK, GL, FO'C, JC, AO, JS, and CS read and approved the final manuscript.
Funding
Editorial assistance with the preparation of this manuscript was provided by Cathy Jarrold and Mark Robinson of Open Access Consulting and Bernard Kerr of Succinct Medical Communications, and was funded by a grant from Novartis UK. Publication costs were funded by the UK Tuberous Sclerosis Association (TSA). The authors maintained full control over the content of the manuscript and the decision to publish.
Conflict of Interest
NA has received honoraria for lectures given and travel subsidies to attend specialist TSC meetings from Novartis UK. FO'C has received grants from the UK Tuberous Sclerosis Association (TSA), the UK NIHR, and Wellcome Trust to support research on tuberous sclerosis. He has received Honoraria from Novartis for lectures and conference attendance. PB was a Trustee of the TSA. He has received grants from the TSA and the NIHR and Action Medical Research to support research on tuberous sclerosis. He has received Honoraria from Novartis for lectures and conference attendance. AC has been involved in a virtual webex venture to discuss patients with TSC amongst clinicians across Cheshire and Merseyside, supported by Novartis. FE has received a payment from Novartis for participating in a study day on TSC. She is also a Trustee of the TSA. EF Leeds TSC clinic is funded by Novartis through the MHRA joint working initiative. EF has received two honoraria from Novartis for speaking at TSC conferences and is a member of the TSA's Research committee. LK has been a paid member of a Novartis advisory board and is the Rare Disease Lead at Birmingham Children's Hospital who currently receive funding from the Roald Dahl's Marvelous Children's Charity for Rare Disease Nursing Post, and from Novartis to fund Psychology, and Transition Support for TSC clinics. GL was Director of the adult Birmingham Center for Rare Disease. He has received an unrestricted educational grant for acting as an expert speaker at a regional education forum on TSC. Novartis are funding a clinical nurse specialist post to help support a multi-specialty TSC clinic in Queen Elizabeth Hospital Birmingham. JS has received grant funding and lecture fees from Novartis and grant funding from the TSA. JK has received grant funding and lecture fees from Novartis, and is Medical Advisor to, and a Trustee of the TSA. RB has received travel and subsidence from Novartis. DG has received fees for consulting from Novartis, who partly support the TSC service at the Royal Free Hospital. AH has received an honorarium from Novartis for conference attendance. SRJ has received lecture fees from Novartis and is a member of the TSA's research committee. PM has received a one-off honorarium from Novartis for speaking at a TSC conference but has no other competing interests. JC has received an honorarium from Novartis for lecture fees. JC also works at Evelina London Children's Hospital, Guys and St Thomas's NHS Trust, Novartis are currently supporting salary costs for a clinical nurse specialist post within the TSC specialist service.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to the TSC community, especially patients and their families for focused discussion and informal feedback that has informed the approach in this paper and for their encouragement.
References
1. Northrup H, Krueger DA, Roberds S, Smith K, Sampson J, Korf B, et al. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 international tuberous sclerosis complex consensus conference. Pediatr Neurol. (2013) 49:243–54. doi: 10.1016/j.pediatrneurol.2013.08.001
2. Curatolo P, Bombardieri R, Jozwiak S. Seminar tuberous sclerosis. Lancet. (2008) 372:657–68. doi: 10.1016/S0140-6736(08)61279-9
3. Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. (2006) 355:1345–56. doi: 10.1056/NEJMra055323
4. Sampson JR, Scahill SJ, Stephenson JB, Mann L, Connor JM. Genetic aspects of tuberous sclerosis in the west of Scotland. J Med Genet. (1989) 26:28–31. doi: 10.1136/jmg.26.1.28
5. O'Callaghan FJ, Shiell AW, Osborne JP, Martyn CN. Prevalence of tuberous sclerosis estimated by capture-recapture analysis. Lancet. (1998) 351:1490. doi: 10.1016/S0140-6736(05)78872-3
6. Kingswood JC, Crawford P, Johnson SR, Sampson JR, Shepherd C, Demuth D, et al. The economic burden of tuberous sclerosis complex in the UK: a retrospective cohort study in the Clinical Practice Research Datalink. J Med Econ. (2016) 19:1087–98. doi: 10.1080/13696998.2016.1199432
7. Kingswood C, Bolton P, Crawford P, Harland C, Johnson SR, Sampson JR, et al. The clinical profile of tuberous sclerosis complex (TSC) in the United Kingdom: a retrospective cohort study in the Clinical Practice Research Datalink (CPRD). Eur J Paediatr Neurol. (2016) 20:296–308. doi: 10.1016/j.ejpn.2015.11.011
8. Curatolo P, Cusmai R, Cortesi F, Chiron C, Jambaque I, Dulac O. Neuropsychiatric aspects of tuberous sclerosis. Ann N Y Acad Sci. (1991) 615:8–16. doi: 10.1111/j.1749-6632.1991.tb37743.x
9. Kingswood JC, Belousova E, Benedik MP, Carter T, Cottin V, Curatolo P, et al. Renal angiomyolipoma in patients with tuberous sclerosis complex: findings from the tuberous sclerosis registry to increase disease Awareness. Nephrol Dial Transplant. (2019) 34:502–8. doi: 10.1093/ndt/gfy063
10. Shepherd CW, Gomez MR, Lie JT, Crowson CS. Causes of death in patients with tuberous sclerosis. Mayo Clin Proc. (1991) 66:792–6. doi: 10.1016/S0025-6196(12)61196-3
11. Adriaensen MEAPM, Schaefer-Prokop CM, Stijnen T, Duyndam DAC, Zonnenberg BA, Prokop M. Prevalence of subependymal giant cell tumors in patients with tuberous sclerosis and a review of the literature. Eur J Neurol. (2009) 16:691–6. doi: 10.1111/j.1468-1331.2009.02567.x
12. Jansen AC, Belousova E, Benedik MP, Carter T, Cottin V, Curatolo P, et al. Newly diagnosed and growing subependymal giant cell astrocytoma in adults with tuberous sclerosis complex: results from the international TOSCA study. Front Neurol. (2019) 10:821. doi: 10.3389/fneur.2019.00821
13. Jóźwiak S, Kotulska K, Domańska-Pakieła D, Łojszczyk B, Syczewska M, Chmielewski D, et al. Antiepileptic treatment before the onset of seizures reduces epilepsy severity and risk of mental retardation in infants with tuberous sclerosis complex. Eur J Paediatr Neurol. (2011) 15:424–31. doi: 10.1016/j.ejpn.2011.03.010
14. Bombardieri R, Pinci M, Moavero R, Cerminara C, Curatolo P. Early control of seizures improves long-term outcome in children with tuberous sclerosis complex. Eur J Paediatr Neurol. (2010) 14:146–9. doi: 10.1016/j.ejpn.2009.03.003
15. Curatolo P, Moavero R, de Vries PJ. Neurological and neuropsychiatric aspects of tuberous sclerosis complex. Lancet Neurol. (2015) 14:733–45. doi: 10.1016/S1474-4422(15)00069-1
16. Krueger DA, Northrup H, Northrup H, Krueger DA, Roberds S, Smith K, et al. Tuberous sclerosis complex surveillance and management: recommendations of the 2012 international tuberous sclerosis complex consensus conference. Pediatr Neurol. (2013) 49:255–65. doi: 10.1016/j.pediatrneurol.2013.08.002
17. Franz DN, Belousova E, Sparagana S, Bebin EM, Frost M, Kuperman R, et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. (2013) 381:125–32. doi: 10.1016/S0140-6736(12)61134-9
18. Bissler JJ, Kingswood JC, Radzikowska E, Zonnenberg BA, Frost M, Belousova E, et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. (2013) 381:817–24. doi: 10.1016/S0140-6736(12)61767-X
19. French JA, Lawson JA, Yapici Z, Ikeda H, Polster T, Nabbout R, et al. Adjunctive everolimus therapy for treatment-resistant focal-onset seizures associated with tuberous sclerosis (EXIST-3): a phase 3, randomised, double-blind, placebo-controlled study. Lancet. (2016) 388:P2153–63. doi: 10.1016/S0140-6736(16)31419-2
20. Capal JK, Bernardino-Cuesta B, Horn PS, Murray D, Byars AW, Bing NM, et al. Influence of seizures on early development in tuberous sclerosis complex. Epilepsy Behav. (2017) 70(Pt A):245–52. doi: 10.1016/j.yebeh.2017.02.007
21. Amin S, Kingswood JC, Bolton PF, Elmslie F, Gale DP, Harland C, et al. The UK guidelines for management and surveillance of Tuberous Sclerosis Complex. QJM An Int J Med. (2019) 112:171–82. doi: 10.1093/qjmed/hcy215
22. Roth J, Roach ES, Bartels U, Jóźwiak S, Koenig MK, Weiner HL, et al. Subependymal giant cell astrocytoma: diagnosis, screening, and treatment. Recommendations from the international tuberous sclerosis complex consensus conference 2012. Pediatr Neurol. (2013) 49:439–44. doi: 10.1016/j.pediatrneurol.2013.08.017
23. Curatolo P, Jóźwiak S, Nabbout R. Management of epilepsy associated with tuberous sclerosis complex (TSC): clinical recommendations. Eur J Paediatr Neurol. (2012) 16:582–6. doi: 10.1016/j.ejpn.2012.05.004
24. Johnson SR, Cordier JF, Lazor R, Cottin V, Costabel U, Harari S, et al. European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur Respir J. (2010) 35:14–26. doi: 10.1183/09031936.00076209
25. UK Guidelines for Managing Tuberous Sclerosis Complex: A Summary for Clinicians in the NHS (2019). Available online at: http://www.tuberous-sclerosis.org/new-tsa-news/guideline-summary (accessed October 14, 2019).
26. De Vries PJ, Whittemore VH, Leclezio L, Byars AW, Dunn D, Ess KC, et al. Tuberous sclerosis associated neuropsychiatric disorders (TAND) and the TAND checklist. Pediatr Neurol. (2015) 52:25–35. doi: 10.1016/j.pediatrneurol.2014.10.004
27. Koenig MK, Bell CS, Hebert AA, Roberson J, Samuels JA, Slopis JM, et al. Efficacy and safety of topical rapamycin in patients with facial angiofibromas secondary to tuberous sclerosis complex the TREATMENT randomized clinical trial. JAMA Dermatol. (2018) 154:773–80. doi: 10.1001/jamadermatol.2018.0464
28. Jóźwiak S, Sadowski K, Kotulska K, Schwartz RA. Topical use of mammalian target of rapamycin (mTOR) inhibitors in tuberous sclerosis complex—a comprehensive review of the literature. Pediatric Neurol. (2016). 61:21–7. doi: 10.1016/j.pediatrneurol.2016.04.003
29. Breckenridge K, Bekker HL, Gibbons E, van der Veer SN, Abbott D, Briançon S, et al. How to routinely collect data on patient-reported outcome and experience measures in renal registries in Europe: an expert consensus meeting. Nephrol Dial Transplant. (2015) 30:1605–14. doi: 10.1093/ndt/gfv209
30. Black N. Patient reported outcome measures could help transform healthcare. BMJ. (2013) 346:f167. doi: 10.1136/bmj.f167
31. Jóźwiak S, Sadowski K, Borkowska J, Domańska-Pakieła D, Chmielewski D, Jurkiewicz E, et al. Liver angiomyolipomas in tuberous sclerosis complex—their incidence and course. Pediatr Neurol. (2018) 78:20–26. doi: 10.1016/j.pediatrneurol.2017.09.012
32. Whitney R, Jan S, Zak M, McCoy B. The utility of surveillance electroencephalography to guide early antiepileptic drug therapy in infants with tuberous sclerosis complex. Pediatr Neurol. (2017) 72:76–80. doi: 10.1016/j.pediatrneurol.2017.04.009
33. Wu JY, Peters JM, Goyal M, Krueger D, Sahin M, Northrup H, et al. Clinical electroencephalographic biomarker for impending epilepsy in asymptomatic tuberous sclerosis complex infants. Pediatr Neurol. (2016) 54:29–34. doi: 10.1016/j.pediatrneurol.2015.09.013
Keywords: tuberous sclerosis complex, service specification, commissioning, surveillance, guidelines, clinics, rare disease, United Kingdom
Citation: Annear NMP, Appleton RE, Bassi Z, Bhatt R, Bolton PF, Crawford P, Crowe A, Tossi M, Elmslie F, Finlay E, Gale DP, Henderson A, Jones EA, Johnson SR, Joss S, Kerecuk L, Lipkin G, Morrison PJ, O'Callaghan FJ, Cadwgan J, Ong ACM, Sampson JR, Shepherd C and Kingswood JC (2019) Tuberous Sclerosis Complex (TSC): Expert Recommendations for Provision of Coordinated Care. Front. Neurol. 10:1116. doi: 10.3389/fneur.2019.01116
Received: 15 June 2019; Accepted: 07 October 2019;
Published: 06 November 2019.
Edited by:
Vincenzo Belcastro, Ospedale Sant Anna, ItalyReviewed by:
Romina Moavero, Bambino Gesù Children Hospital (IRCCS), ItalyAglaia Vignoli, University of Milan, Italy
Copyright © 2019 Annear, Appleton, Bassi, Bhatt, Bolton, Crawford, Crowe, Tossi, Elmslie, Finlay, Gale, Henderson, Jones, Johnson, Joss, Kerecuk, Lipkin, Morrison, O'Callaghan, Cadwgan, Ong, Sampson, Shepherd and Kingswood. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicholas M. P. Annear, bmlja2FubmVhckBkb2N0b3JzLm9yZy51aw==
 Nicholas M. P. Annear
Nicholas M. P. Annear Richard E. Appleton3
Richard E. Appleton3 Zahabiyah Bassi
Zahabiyah Bassi Maureen Tossi
Maureen Tossi Frances Elmslie
Frances Elmslie Finbar J. O'Callaghan
Finbar J. O'Callaghan Jill Cadwgan
Jill Cadwgan Albert C. M. Ong
Albert C. M. Ong J. Chris Kingswood
J. Chris Kingswood