- 1Department of Neurology, Faculty of Medicine, Cairo University, Cairo, Egypt
- 2Department of Neuroradiology, Ospedale Niguarda Ca' Granda, Milan, Italy
- 3Department of Neurology, Faculty of Medicine, Al-Azhar University, Cairo, Egypt
- 4Department of Neurology, Faculty of Medicine, Alexandria University, Alexandria, Egypt
Intracranial atherosclerotic disease (ICAD) is considered a major cause of recurrent cerebrovascular events. ICAD continues to be a disease without an effective method of reducing the risk of recurrent stroke and death, even with aggressive, highly monitored medical treatment. We reviewed data from three randomized controlled studies that published data comparing intracranial stenting vs. medical treatment for symptomatic severe-ICAD. Ethnic, demographic, clinical, and procedural differences were observed among the data from these trials that might influence their results. Future research should aim at establishing refined selection criteria that can identify high-risk ICAD patients who may benefit from intracranial stenting.
Introduction
Intracranial atherosclerotic disease (ICAD) is considered a major cause of recurrent stroke and transient ischemic attacks (TIAs) (1). The optimal treatment for ICAD is a crucial issue in Stroke Medicine. Data from recent trials demonstrated that aggressive medical treatment and lifestyle modifications are better than endovascular treatment for stroke prevention in high-risk patients with ICAD (2, 3). However, the annual stroke risk in patients with intracranial atherosclerosis is still high even with aggressive, highly monitored medical management. Questions remain as to what Stroke physicians should do, if patients suffer ischaemic events in spite of optimal medical treatment? Is endovascular therapy a viable treatment option for a certain subgroup of ICAD patients who are not responding to optimal medical therapy?
Methods
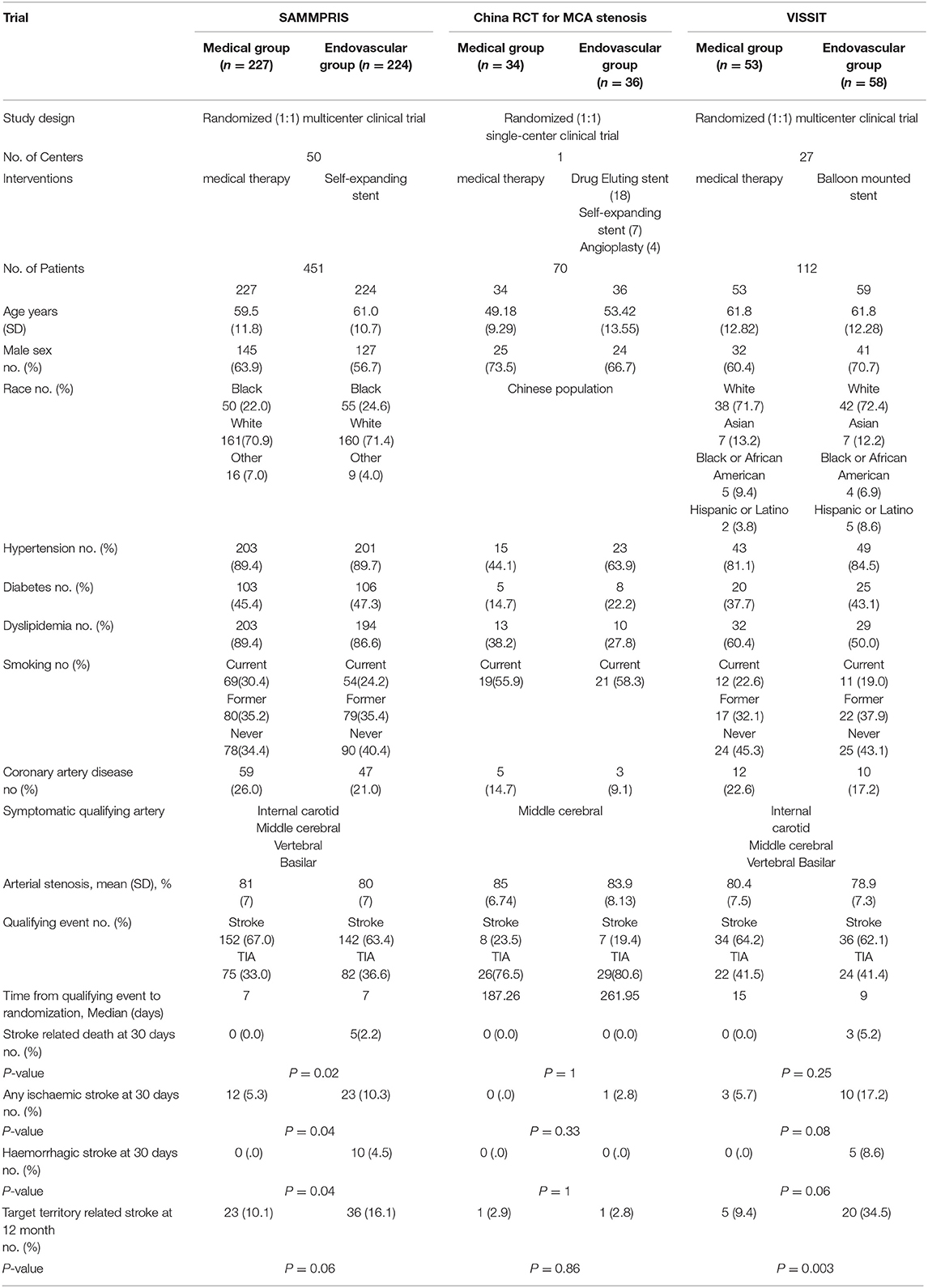
We searched PubMed and Cochrane Library for Randomized Controlled Trials (RCTs) comparing efficacy and safety of medical treatment vs. intracranial stenting as treatment options for intracranial arterial stenosis. The key words included “intracranial atherosclerosis,” “stroke,” “medical treatment,” “intracranial stenting,” and “randomized controlled trial.” Three main trials were found and included for data extraction. We reviewed the data from the three trials including: patient and procedural characteristics, and outcome parameters (Table 1).
Trials Overview
The SAMMPRIS (2) and VISSIT (3) trials were initiated to compare aggressive medical treatment vs. intracranial stenting in patients with symptomatic, severe ICAD. Both trials concluded that medical therapy was better than intracranial stenting. In contrary, a single-center randomized controlled trial in China, that randomized patients with stroke or TIA attributable to significant Middle Cerebral Artery (MCA) stenosis, to either endovascular or aggressive medical treatment concluded that endovascular treatment could be safe and efficient treatment modality for carefully selected patients with MCA stenosis (4). Sample sizes were estimated in each trial according to power analysis. The intent of the intervention was to prevent stroke or death within 30 days after enrollment or any stroke, death in the territory of the symptomatic intracranial artery beyond 30 days through 12 months. Enrollment was discontinued in SAMMPRIS and VISSIT trials after negative results and a higher rate of stroke and deaths in the stenting group (2, 3). The technical success rate was 92.9% in the endovascular arm of SAMMPRIS, and 54% in the endovascular arm of VISSIT, and 100% in the endovascular arm of China RCT. The rate of adverse events was significantly higher among stent group compared to optimal medical therapy group in SAMMPRIS and VISSIT trials. In China RCT, there was similar adverse events rate in the optimal medical therapy and stent groups.
Differences in Patient Selection and Procedural Characteristics
Post-stenting adverse events in the endovascular arm of China RCT were considerably lower compared to the endovascular arm of both SAMMPRIS and VISSIT trials (2–4). The mechanisms of post-stenting adverse events in ICAD patients are usually uncertain (5). Presumed mechanisms include perforator occlusion, distal embolization, wire perforation, cerebral hyperperfusion, or in-stent thrombosis. Some post-procedural adverse events might be related to patient's non-adherence to antiplatelet therapy, risk factor control, or even follow-up visits (6).
Many reasons may contribute to better outcome and less adverse events after intracranial stenting in China RCT. First, the operators were highly experienced in a single center having operated more than 500 ICAD interventions in the 5 years before enrollment. This might guarantee technical success and safe outcome of procedures and maintain the continuity of operators' experience which is critical in reducing periprocedural complications and adverse events (7, 8). In SAMMPRIS, of the 224 patients enrolled, 208 underwent stent placement in 50 participating sites in the United States over 29 months with an average of <2 ICAD interventions at each site per year. This may indicate that SAMMPRIS operators may not have had enough experience with ICAD interventions. Secondly, Chinese neurointerventionists were free in choosing between different endovascular modalities with options of a balloon-expandable stent or self-expanding stent placement based on lesion morphology, vessels tortuosity and the operators' own judgment. Generally, using balloon-mounted stents or primary balloon angioplasty usually do not require over wire catheter exchange with low risk of wire perforation and subsequent haemorrhagic complications (9). Thirdly, the mean age in the Chinese stenting group was 53 years, younger than the VISSIT and SAMMPRIS trials, suggesting easier vascular access for stenting and less procedural complications (10, 11). Additionally, the study was conducted only on Chinese patients excluding diverse ethnic groups with varied stroke pathologies and responses to treatment (12).
In the China RCT, the study population showed less prevalence of stroke risk factors, than the SAMMPRIS and VISSIT trials, e.g., HTN, DM, dyslipidaemia, and coronary artery disease which are associated with increased risk of procedural complications (13). SAMMPRIS trial received criticism for its patient selection and failure to differentiate the most likely stroke mechanism (perforator-occlusion, hypoperfusion, and embolic stroke). SAMMPRIS did not mention inclusion of ICAD patients with hypoperfusion in the territory supplied by the target vessel. Those patients might be more prone to benefit from endovascular revascularization (14). China RCT included only proximal MCA stenosis. Basilar artery stenosis, which may carry a higher periprocedural risk for endovascular treatment was included in the SAMMPRIS and VISSIT trials (8, 13, 15). Finally, most of qualifying events in China RCT were TIAs and time from qualifying events to randomization was longer than the time interval in SAMMPRIS and VISSIT. Recent stroke may be a major risk factor for periprocedural adverse events, which could result from plaque instability (8, 16, 17). The long interval between qualifying event and procedure may allow for stabilization of the atherosclerotic plaque via medical treatment, thus reducing the thromboembolic events in the periprocedural period (8).
Current Status and Future Directions
Current Stroke guidelines do not recommend endovascular treatment as an initial modality (18). Even for ICAD patients with recurrent stroke or TIAs despite aggressive medical treatment, the usefulness of endovascular treatment is considered unknown and investigational. However, the recently published results of Wingspan stEnt system post-mArket surVEillance (WEAVE) trial showed extremely low periprocedural stroke and death rate (2.6%) with Wingspan stent use (19). The trial data suggest that proper patient selection and operator experience may maximize benefit from endovascular treatment with the Wingspan Stent System. Additionally, China Angioplasty and Stenting for Symptomatic Intracranial Severe Stenosis (CASSISS) trial is an ongoing, multicentre RCT with refined inclusion criteria aiming at determining whether intracranial stenting was superior to aggressive medical treatment for preventing recurrent cerebrovascular events in patients with severe symptomatic ICAD (20). The participating centers in CASSISS trial had to prove an annual volume of more than 30 ICAD patients treated with intracranial stenting sustained over the past 3 years. In CASSISS, patients with perforator ischaemia alone without distal hypoperfusion or artery-to-artery embolism would not be included in the study. Also, pre-procedural perfusion imaging would be used to evaluate for cerebral hypoperfusion. Additionally, time from qualifying event to procedure should not be <3 weeks. Another multicentre RCT is currently underway to compare intracranial stenting with aggressive medical therapy in patients with severe symptomatic ICAD and cerebral hypoperfusion (21). Cerebral hypoperfusion would be assessed initially with transcranial Doppler measuring cerebral blood flow velocity and would be confirmed using Magnetic Resonance perfusion imaging. The final results may clarify the potential role of intracranial stenting in management of ICAD.
Conclusion
Endovascular treatment of ICAD remains a feasible viable option, but its efficacy in preventing recurrent cerebrovascular events still needs validation. Careful patient selection and technical advancement of the devices utilized may improve the long-term clinical outcomes of intracranial stenting. Future large randomized controlled trials with refined selection criteria are warranted.
Author Contributions
AA, FA-A, and GP drafted the article. All authors were involved in data acquisition, critical analysis, organization, engaged in the manuscript review, manuscript editing, and final approval of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Gorelick P, Wong K, Bae H, Pandey D. Large artery intracranial occlusive disease. Stroke. (2008) 39:2396–9. doi: 10.1161/STROKEAHA.107.505776
2. Chimowitz M, Lynn M, Derdeyn C, Turan T, Fiorella D, Lane B, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. (2011) 365:993–1003. doi: 10.1056/NEJMoa1105335
3. Zaidat O, Fitzsimmons B, Woodward B, Wang Z, Killer-Oberpfalzer M, Wakhloo A, et al. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA. (2015) 313:1240–8. doi: 10.1001/jama.2015.1693
4. Miao Z, Jiang L, Wu H, Bao Y, Jiao L, Li S, et al. Randomized controlled trial of symptomatic middle cerebral artery stenosis: endovascular versus medical therapy in a Chinese population. Stroke. (2012) 43:3284–90. doi: 10.1161/STROKEAHA.112.662270
5. Derdeyn C, Fiorella D, Lynn M, Rumboldt Z, Cloft H, Gibson D, et al. Mechanisms of stroke after intracranial angioplasty and stenting in the SAMMPRIS trial. Neurosurgery. (2013) 72:777–95. doi: 10.1227/NEU.0b013e318286fdc8
6. Roger V, Go A, Lloyd-Jones D, Adams R, Berry J, Brown T, et al. Heart disease and stroke statistics−2011 update. Circulation. (2011) 123:e18–209. doi: 10.1161/CIR.0b013e3182009701
7. Yu S, Leung T, Lee K, Wong L. Learning curve of Wingspan stenting for intracranial atherosclerosis: single-center experience of 95 consecutive patients. J Neurointerv Surg. (2013) 6:212–8. doi: 10.1136/neurintsurg-2012-010593
8. Nahab F, Lynn MJ, Kasner SE, Alexander MJ, Klucznik R, Zaidat OO, et al. Risk factors associated with major cerebrovascular complications after intracranial stenting. Neurology. (2009) 72:2014–9. doi: 10.1212/01.wnl.0b013e3181a1863c
9. Connors J, Wojak J, Hoppe B. The technique of endovascular intracranial revascularization. Front Neurol. (2014) 5:246. doi: 10.3389/fneur.2014.00246
10. Lin S, Trocciola S, Rhee J, Dayal R, Chaer R, Morrissey N, et al. Analysis of anatomic factors and age in patients undergoing carotid angioplasty and stenting. Ann Vasc Surg. (2005) 19:798–804. doi: 10.1007/s10016-005-8045-4
11. Snelling B, Sur S, Shah S, Chen S, Menaker S, McCarthy D, et al. Unfavorable vascular anatomy is associated with increased revascularization time and worse outcome in anterior circulation thrombectomy. World Neurosurg. (2018) 120:e976–83. doi: 10.1016/j.wneu.2018.08.207
12. Kim J, Bonovich D. Research on intracranial atherosclerosis from the east and west: why are the results different? J Stroke. (2014) 16:105–13. doi: 10.5853/jos.2014.16.3.105
13. Cheng L, Jiao L, Gao P, Song G, Chen S, Wang X, et al. Risk factors associated with in-hospital serious adverse events after stenting of severe symptomatic intracranial stenosis. Clin Neurol Neurosurg. (2016) 147:59–63. doi: 10.1016/j.clineuro.2016.05.019
14. Alexander M. Intracranial stenting for intracranial atherosclerotic disease: still much to learn. J Neurointerv Surg. (2012) 4:85–6. doi: 10.1136/neurintsurg-2012-010269
15. Groschel K, Schnaudigel S, Pilgram SM, Wasser K, Kastrup A. A systematic review on outcome after stenting for intracranial atherosclerosis. Stroke. (2009) 40:e340–7. doi: 10.1161/STROKEAHA.108.532713
16. Kurre W, Berkefeld J, Brassel F, Bruning R, Eckert B, Kamek S, et al. In-hospital complication rates after stent treatment of 388 symptomatic intracranial stenoses: results from the INTRASTENT multicentric registry. Stroke. (2010) 41:494–8. doi: 10.1161/STROKEAHA.109.568063
17. Qureshi A, Tariq N, Hassan A, Vazquez G, Hussein H, Suri M, et al. Predictors and timing of neurological complications following intracranial angioplasty and/or stent placement. Neurosurgery. (2011) 68:53–61. doi: 10.1227/NEU.0b013e3181fc5f0a
18. Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2014) 45:2160–236. doi: 10.1161/STR.0000000000000024
19. Alexander M, Zauner A, Chaloupka J, Baxter B, Callison R, Gupta R, et al. WEAVE trial. Stroke. (2019) 50:889–94. doi: 10.1161/STROKEAHA.118.023996
20. Gao P, Zhao Z, Wang D, Wu J, Cai Y, Li T, et al. China angioplasty and stenting for symptomatic intracranial severe stenosis (CASSISS): a new, prospective, multicenter, randomized controlled trial in China. Interv Neuroradiol. (2015) 21:196–204. doi: 10.1177/1591019915581778
Keywords: intracranial atherosclerosis, medical therapy, endovascular treatment, stroke, intracranial stenting, angioplasty
Citation: Abualhasan A, Abd-Allah F, Pero G, Sobh K, Mansour O, El-Serafy O and Boccardi E (2019) Intracranial Stenting: Is It Still an Option for Treatment of Patients With Intracranial Atherosclerosis? Front. Neurol. 10:1248. doi: 10.3389/fneur.2019.01248
Received: 03 July 2019; Accepted: 08 November 2019;
Published: 22 November 2019.
Edited by:
Sunil Sheth, McGovern Medical School, University of Texas Health Science Center at Houston, United StatesReviewed by:
Hamidreza Saber, School of Medicine, Wayne State University, United StatesVictor Lopez, University of Texas Health Science Center at Houston, United States
Copyright © 2019 Abualhasan, Abd-Allah, Pero, Sobh, Mansour, El-Serafy and Boccardi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Foad Abd-Allah, Zm9hZC5hYmRhbGxhaEBrYXNyYWxhaW55LmVkdS5lZw==; Zm9hZG5ldXJvQGhvdG1haWwuY29t
 Ahmed Abualhasan
Ahmed Abualhasan Foad Abd-Allah
Foad Abd-Allah Guglielmo Pero
Guglielmo Pero Khaled Sobh
Khaled Sobh Ossama Mansour
Ossama Mansour Omar El-Serafy1
Omar El-Serafy1