- 1Clinic of Psychiatry, Social Psychiatry and Psychotherapy, Hannover Medical School, Hanover, Germany
- 2Department of Bioethics, Medical University of Warsaw, Warsaw, Poland
- 3Department of Neurology, Medical University of Warsaw, Warsaw, Poland
- 4Department of Neurology, Hannover Medical School, Hanover, Germany
- 5Department of Psychiatry, Institute of Psychiatry, University of São Paulo, São Paulo, Brazil
Gilles de la Tourette syndrome (GTS) is a neuropsychiatric disorder characterized by motor and vocal tics. There are several hypotheses as to the cause of the disease. One of which suggests that the immune system is involved in the pathophysiology of GTS. Here, we present a 40-year-old female patient with a typical history and clinical presentation of GTS with tics and psychiatric comorbidities, in whom positive oligoclonal bands (OCBs) type 2 in cerebral spinal fluid (CSF) were detected in an earlier study. At that time point, all other investigations were unremarkable (including neurological examination and cMRI), but 2 years later, she presented with further neurological symptoms including tetraparesis mostly affecting the left limbs, distal hypesthesia and paresthesia, and dyspnea. Since further examinations (including EMG, MRI, CSF, virological, and bacteriological tests, as well as autoimmune-encephalitis antibodies) revealed normal results, based on clinical presentation, the diagnosis of Guillain-Barré-like immune-mediated neuropathy was made and treatment with intravenous immunoglobulins (IVIg) (30 g/day for 5 days) was initiated resulting in complete remission of immune-mediated neuropathy. Based on the “immune hypothesis” of GTS, we were interested in whether in this patient positive CSF OCBs might serve as a biomarker for treatment response of tics and additional GTS-related psychiatric symptoms to IVIg, and therefore assessed tics, premonitory urges, and psychiatric comorbidities before and several times after the IVIg treatment. However, even though immune-mediated neuropathy resolved after treatment, tics, premonitory urges, and comorbidities remained unchanged. Thus, this case study suggests that treatment with IVIg is not effective in the treatment of GTS—even in a patient with intrathecal antibody synthesis as expressed by CSF isolated OCBs.
Case Report
Gilles de la Tourette syndrome (GTS) is a childhood-onset tic disorder often accompanied by different comorbidities such as attention deficit/hyperactivity disorder (ADHD), obsessive-compulsive disorder (OCD), depression, and anxiety. Multiple neurotransmitter systems are thought to be involved in the pathophysiology of GTS including the dopaminergic, GABAergic, serotonergic, glutamatergic, histaminergic, and endocannabinoid systems. In addition, there is certainly a complex interplay of both genetic and environmental factors (1).
With regard to non-genetic influential factors, numerous findings support the hypothesis of the involvement of the immune system in the pathology of GTS. Many studies have investigated the relationship between the occurrence of tics and group A Streptococcus (GAS) infections also known as pediatric autoimmune neuropsychiatric disorders associated with streptococcus infections (PANDAS) (2–4). To shed further light into the proposed association between various environmental factors including GAS infections and different immunological factors and chronic tic disorders including GTS, the large European multicenter study EMTICS was initiated (5). The main conclusion of the EMTICS study is that there are no indications for a causal relation between GAS exposure and the onset or exacerbation of tics and related psychiatric comorbidities such as obsessive-compulsive symptoms (for consulting the final report, see https://cordis.europa.eu/project/rcn/102102/reporting/en?rcn=59137). However, the data provided further evidence for the involvement of immunological factors in the pathogenesis of chronic tic disorders.
Thus, the EMTICS findings are in accordance with several other studies that have consistently shown either an overall activation or distinct abnormalities in the immune system in GTS such as increased levels of cytokines (6, 7) and immunoglobulins (8–10), activation of different types of immune cells (11), and gene expression related to the immune system (12). In line with these data, positive oligoclonal bands (OCB) in cerebral spinal fluid (CSF) have been found in 20–30% of adult patients with GTS indicating an intrathecal antibody synthesis (13, 14). However, until now, no corresponding antibodies could be identified (14). Furthermore, microglia activation was demonstrated both in a postmortem study indicated by increased numbers of CD45+ microglial cells in the striatum (15) and in an imaging study using positron emission tomography (PET) and (11)C-[R]-PK11195 (PK) showing increased binding potential values in the caudate nuclei in children with GTS (16).
Further support for the “immune hypothesis” of GTS comes from studies conducted in animal models and cell cultures based on active immunization with bacterial and viral agents, direct injection of cytokines or a patient's serum antineuronal antibodies, as well as transgenic techniques used to create animal models resulting in the causation of stereotyped behaviors similar to tics [for review, see (17)]. Examples of this causal relationship are as follows: (i) that stereotypic behaviors have been reported in a mouse strain model with altered immune function (BTBR T+tf/J) (18); (ii) in mice treated with interleukin (IL)-6, increased locomotor behavior has been demonstrated (19); (iii) also other immune molecules such as TGF-β1 (20) and soluble IL-2 receptors (sIL-2R) α or β (21) have been found to increase stereotypic behaviors or repetitive movements; and (iv) exposure of male Fischer 344 rats to unfractionated sera or IgG extracted to antibodies from patients with GTS led to motor stereotypies and episodic vocalizations similar to tics (22).
Based on these findings that suggest a dysfunctional immune system in GTS, an immune-based treatment using intravenous immunoglobulins (IVIg) has been proposed as an alternative to current pharmacotherapy with antipsychotics. Until now, available results are limited and highly inconsistent: data from an uncontrolled study (23) in a small number of patients—mostly children with otherwise treatment-resistant GTS—suggest that treatment with IVIg may improve tics, but in contrast, a double-blind placebo-controlled study in 30 unselected patients (aged 16–63 years) failed to demonstrate efficacy (24). However, in another double-blinded study (in 29 children) comparing IVIg not only to placebo but also to plasma exchange, both active interventions were comparably efficacious in the treatment of tics and OCD compared to placebo (25).
Here we present the unique case of a female patient with GTS, who not only exhibited intrathecal antibody synthesis documented by positive OCBs, but in addition received IVIg for the treatment of an unrelated Guillain-Barré-like immune-mediated neuropathy. Based on the hypothesis that positive OCBs might serve as a biomarker not only for an involvement of the immune system in GTS but also for successful treatment of tics and comorbidities, we were interested in whether her GTS-related symptoms might improve after the IVIg treatment.
Case Description
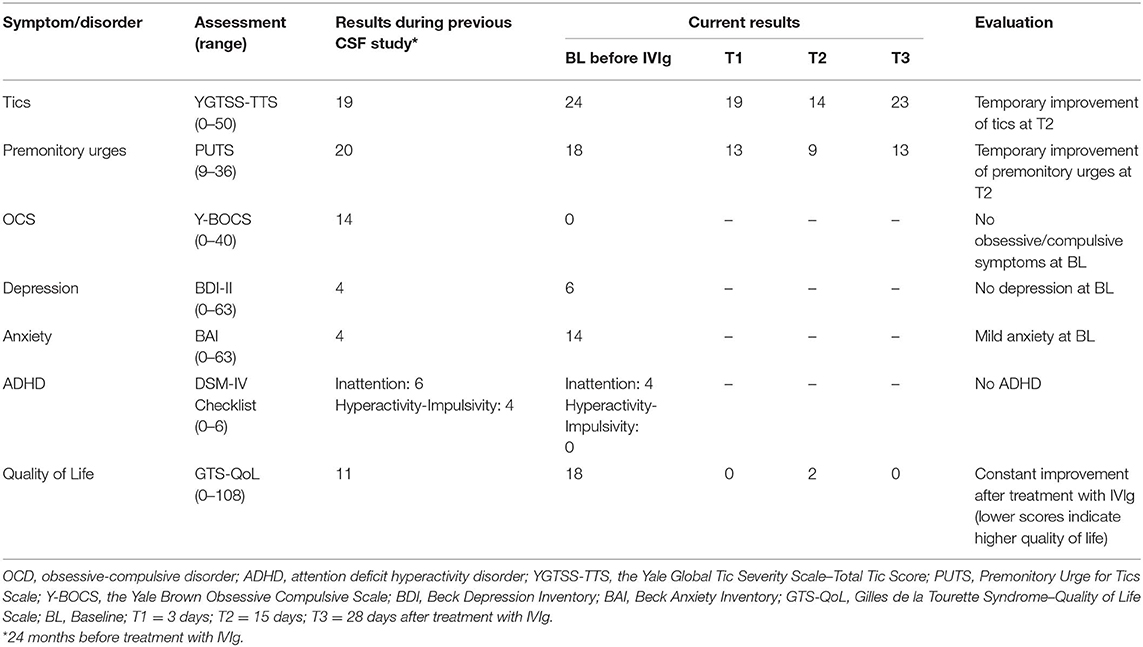
For the first time, the patient presented in our Tourette outpatient clinic at the age of 40. She was diagnosed with GTS several years before at the age of 5. At the first consultation, she was suffering from multiple motor tics such as head and shoulder shaking, jerks of the whole body, blocking tics that impaired walking, and muscle cramp-like tics, as well as different vocal tics including teeth grinding, deep exhaling, screaming of syllables such as “ha,” and grunting. Further clinical exploration revealed the comorbid diagnoses of OCD, self-injurious behavior, sleeping problems, as well as a history of a depressive episode, alcohol abuse, and benzodiazepine abuse. Treatments with habit reversal training and antipsychotics did not result in a significant improvement of tics or caused intolerable side effects. At age 44, the patient agreed to participate in a study at our clinic that aimed to investigate different CSF parameters in patients with GTS (14). At that time point, she suffered from severe motor tics that provoked pain resulting in a further increase in tics, but further neurological examination and cMRI results were unremarkable and there was no history of any further neurological symptoms or diseases. Her CSF analysis revealed positive OCBs (type 2), while all other immunological investigations were normal.
Two years later, at age 46, she presented in our clinic with a tetraparesis predominantly affecting the left limbs, distal hypesthesia and paresthesia, and dyspnea. Medical history revealed that 3 months before, similar symptoms had occurred for the first time. After admission to an external clinic, lumbar puncture was performed and showed CSF pleocytosis (but no OCBs). Because of this finding, antibiotic treatment was initiated, which resulted in a complete remission of neuropathy-associated symptoms. During hospitalization in our clinic (3 months after the first episode), further examinations revealed normal results for EMG, MRI, CSF, and virological and bacteriological tests, as well as negative results for autoimmune-encephalitis antibodies. Based on the clinical presentation, the diagnosis of Guillain-Barré-like immune-mediated neuropathy was made and treatment with IVIg (30g/day for 5 days) was initiated, which resulted in a complete remission of neuropathy. Another relapse occurred 3 months later, but the patient again responded well to treatment with IVIg (30 g/day for 5 days). At that time, she received no medication for tics or comorbidities.
Because OCBs were found to be positive before the first clinical manifestation of the immune-mediated neuropathy, we were interested in whether her GTS-related symptoms including tics and psychiatric comorbidities might also respond to the IVIg treatment. Therefore, we assessed tics, premonitory urges, and psychiatric symptoms including OCD, ADHD, depression, and anxiety disorder before and several times after treatment with IVIg (results of all clinical assessments are presented in Table 1). However, while the patient recovered from immune-mediated neuropathy, tics and premonitory urges improved only slightly and temporarily 2 weeks after initiation of the IVIg treatment and returned to baseline level thereafter, while psychiatric symptoms remained unchanged.
Discussion
Anecdotal reports suggest that IVIg might be effective in the treatment of tics in patients with GTS. Since available data are contradictory, we hypothesized that positive CSF OCBs might serve as a biomarker for successful treatment of tics, premonitory urges, and psychiatric comorbidities using IVIg. We present the unique case of a patient with GTS, in whom positive OCBs had been detected in the context of an earlier study that aimed to investigate immunological factors in GTS (14), and who later developed a Guillain-Barré-like immune-mediated neuropathy. However, while the immune-mediated neuropathy resolved after the IVIg treatment, tics, premonitory urges, and comorbidities remained unchanged. In our opinion, slight and temporary improvement of tics 2 weeks after treatment should be interpreted in the context of the recovery from immune-mediated neuropathy with consecutive reduction of stress and improved mood and anxiety, and subsequent improvement of general well-being and quality of life, but not as a direct treatment effect on tics. Although theoretically conceivable, from our data, it is not suggested that, in this patient, muscle weakness due to immune-mediated neuropathy has influenced the severity of motor tics.
In summary, this case report suggests that treatment with IVIg is not effective in the treatment of GTS—even in patients with intrathecal antibody synthesis as expressed by CSF-isolated OCBs.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
Written informed consent was obtained from the patient for the publication of this Case Report.
Author Contributions
KM-V and K-WS conceived and designed the study. KM-V, K-WS, and CF acquired data. CF set up the electronic database. KM-V, K-WS, PM, and NS analyzed and interpreted the data, performed visualization, and reviewed and edited the manuscript. NS wrote the original draft of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to kindly thank Adam Lombroso from The Child Study Center, Yale School of Medicine, for help in revision of this case.
References
1. Hoekstra PJ, Dietrich A, Edwards MJ, Elamin I, Martino D. Environmental factors in Tourette syndrome. Neurosci Biobehav Rev. (2013) 37:1040–9. doi: 10.1016/j.neubiorev.2012.10.010
2. Swedo SE, Snider LA, Garvey MA. Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infections (PANDAS). In: Shoenfeld Y, Rose N, editors. Infection and Autoimmunity. 2nd ed. London: Elsevier (2004). p. 333–43.
3. Swedo SE, Leonard HL, Garvey M, Mittleman B, Allen AJ, Perlmutter S, et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatr. (1998) 155:264–71. doi: 10.1176/ajp.155.2.264
4. Bombaci M, Grifantini R, Mora M, Reguzzi V, Petracca R, Meoni E, et al. Protein array profiling of tic patient sera reveals a broad range and enhanced immune response against Group A Streptococcus antigens. PLoS ONE. (2009) 4:e6332. doi: 10.1371/journal.pone.0006332
5. Schrag A, Martino D, Apter A, Ball J, Bartolini E, Benaroya-Milshtein N, et al. European Multicentre Tics in Children Studies (EMTICS): protocol for two cohort studies to assess risk factors for tic onset and exacerbation in children and adolescents. Eur Child Adolesc Psychiatr. (2019) 28:91–109. doi: 10.1007/s00787-018-1190-4
6. Bos-Veneman NGP, Bijzet J, Limburg PC, Minderaa RB, Kallenberg CG, Hoekstra PJ. Cytokines and soluble adhesion molecules in children and adolescents with a tic disorder. Prog Neuro-Psychopharmacol Biol Psychiatr. (2010) 34:1390–5. doi: 10.1016/j.pnpbp.2010.06.028
7. Yeon S-M, Lee JH, Kang D, Bae H, Lee KY, Jin S, et al. A cytokine study of pediatric Tourette's disorder without obsessive compulsive disorder. Psychiatr Res. (2017) 247:90–6. doi: 10.1016/j.psychres.2016.11.005
8. Söhs KW, Skripuletz T, Pul R, Alvermann S, Schwenkenbecher P, Stangel M, et al. Gilles de la tourette syndrome is not linked to contactin-associated protein receptor 2 antibodies. Mol Brain. (2015) 8:62. doi: 10.1186/s13041-015-0154-6
9. Morris-Berry CM, Pollard M, Gao S, Thompson C, Singer HS. Anti-streptococcal, tubulin, and dopamine receptor 2 antibodies in children with PANDAS and Tourette syndrome: single-point and longitudinal assessments. J Neuroimmunol. (2013) 264:106–13. doi: 10.1016/j.jneuroim.2013.09.010
10. Cheng YH, Zheng Y, He F, Yang JH, Li WB, Wang ML, et al. Detection of autoantibodies and increased concentrations of interleukins in plasma from patients with Tourette's syndrome. J Mol Neurosci. (2012) 48:219–24. doi: 10.1007/s12031-012-9811-8
11. Möller JC, Tackenberg B, Heinzel-Gutenbrunner M, Burmester R, Oertel WH, Bandmann O, et al. Immunophenotyping in Tourette syndrome - a pilot study. Eur J Neurol. (2008) 15:749–53. doi: 10.1111/j.1468-1331.2008.02159.x
12. Lei J, Xu H, Liang H, Su L, Zhang J, Huang X, et al. Gene expression changes in peripheral blood from Chinese han patients with Tourette syndrome. Am J Med Genet B Neuropsychiatr Genet. (2012) 159:977–80. doi: 10.1002/ajmg.b.32103
13. Wenzel C, Wurster U, Müller-Vahl KR. Oligoclonal bands in cerebrospinal fluid in patients with Tourette's syndrome. Mov Disord. (2011) 26:343–6. doi: 10.1002/mds.23403
14. Baumgaertel C, Skripuletz T, Kronenberg J, Stangel M, Schwenkenbecher P, Sinke C, et al. Immunity in Gilles de la Tourette-syndrome: results from a cerebrospinal fluid study. Front Neurol. (2019) 10:732. doi: 10.3389/fneur.2019.00732
15. Lennington JB, Coppola G, Kataoka-Sasaki Y, Fernandez TV, Palejev D, Li Y, et al. Transcriptome analysis of the human striatum in Tourette syndrome. Biol Psychiatr. (2016) 79:372–82. doi: 10.1016/j.biopsych.2014.07.018
16. Kumar A, Williams MT, Chugani HT. Evaluation of basal ganglia and thalamic inflammation in children with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection and Tourette syndrome: a Positron Emission Tomographic (PET) study using 11C-[R]-PK11195. J Child Neurol. (2015) 30:749–56. doi: 10.1177/0883073814543303
17. Hornig M, Lipkin WI. Immune-mediated animal models of Tourette syndrome. Neurosci Biobehav Rev. (2013) 37:1120–38. doi: 10.1016/j.neubiorev.2013.01.007
18. Pearson BL, Pobbe RLH, Defensor EB, Oasay L, Bolivar VJ, Blanchard DC, et al. Motor and cognitive stereotypies in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. (2011) 10:228–35. doi: 10.1111/j.1601-183X.2010.00659.x
19. Zalcman S, Murray L, Dyck DG, Greenberg AH, Nance DM. Interleukin-2 and−6 induce behavioral-activating effects in mice. Brain Res. (1998) 944:157–64. doi: 10.1016/S0006-8993(98)00904-4
20. Depino AM, Lucchina L, Pitossi F. Early and adult hippocampal TGF-β1 overexpression have opposite effects on behavior. Brain Behav Immun. (2011) 25:1582–91. doi: 10.1016/j.bbi.2011.05.007
21. Zalcman SS, Patel A, Mohla R, Zhu Y, Siegel A. Soluble cytokine receptors (sIL-2Rα, sIL-2Rβ) induce subunit-specific behavioral responses and accumulate in the cerebral cortex and basal forebrain. PLoS ONE. (2012) 7:e36316. doi: 10.1371/journal.pone.0036316
22. Hallett JJ, Harling-Berg CJ, Knopf PM, Stopa EG, Kiessling LS. Anti-striatal antibodies in Tourette syndrome cause neuronal dysfunction. J Neuroimmunol. (2000) 111:195–202. doi: 10.1016/S0165-5728(00)00320-9
23. Zykov VP, Shcherbina AY, Novikova EB, Shvabrina T V. Neuroimmune aspects of the pathogenesis of Tourette's syndrome and experience in the use of immunoglobulins in children. Neurosci Behav Physiol. (2009) 39:635–8. doi: 10.1007/s11055-009-9184-9
24. Hoekstra PJ, Minderaa RB, Kallenberg CGM. Lack of effect of intravenous immunoglobulins on tics: a double-blind placebo-controlled study. J Clin Psychiatr. (2004) 65:537–42. doi: 10.4088/JCP.v65n0413
Keywords: Gilles de la Tourette syndrome, intrathecal antibody synthesis, intravenous immunoglobulin treatment, cerebral spinal fluid, oligoclonal bands
Citation: Szejko N, Fremer C, Sühs K-W, Macul Ferreira de Barros P and Müller-Vahl KR (2020) Intravenous Immunoglobulin Treatment Did Not Improve Tics in a Patient With Gilles de la Tourette Syndrome and Intrathecal Antibody Synthesis. Front. Neurol. 11:110. doi: 10.3389/fneur.2020.00110
Received: 20 November 2019; Accepted: 31 January 2020;
Published: 28 February 2020.
Edited by:
Sharon Glynn Lynch, University of Kansas Medical Center, United StatesReviewed by:
A. Cavanna, Birmingham and Solihull Mental Health NHS Foundation Trust, United KingdomAndreas Hartmann, Hôpitaux Universitaires Pitié Salpêtrière, France
Copyright © 2020 Szejko, Fremer, Sühs, Macul Ferreira de Barros and Müller-Vahl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Natalia Szejko, bmF0YWxpYS5zemVqa29Ad3VtLmVkdS5wbA==
 Natalia Szejko
Natalia Szejko Carolin Fremer1
Carolin Fremer1 Kurt-Wolfram Sühs
Kurt-Wolfram Sühs Pedro Macul Ferreira de Barros
Pedro Macul Ferreira de Barros Kirsten R. Müller-Vahl
Kirsten R. Müller-Vahl