- 1Department of Interventional Neuroadiology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 2Department of Neurology, Tong Ren Hospital Shanghai Jiaotong University School of Medicine, Shanghai, China
Background and purpose: Tirofiban and oral antiplatelet drugs can be used to inhibit reocclusion and restore microvascular reperfusion during endovascular treatment (EVT). This study compared recanalization rates, symptomatic intracranial hemorrhage (SICH), 90 day mortality, and functional outcomes between periprocedural tirofiban and antiplatelet therapy in patients with acute intracranial atherosclerosis-related vertebrobasilar artery occlusion.
Methods: A total of 105 consecutive patients with acute intracranial atherosclerosis-related vertebrobasilar artery occlusion who underwent EVT + tirofiban + oral antiplatelet or EVT + oral antiplatelet therapy at the Beijing Tiantan Hospital between January 2012 and July 2018 were included. Baseline characteristics, procedural parameters, and functional outcomes were assessed.
Results: Among the 105 patients, 74 underwent EVT + tirofiban + oral antiplatelet therapy, while 31 underwent EVT + oral antiplatelet drug therapy. EVT + tirofiban + oral antiplatelet therapy resulted in higher recanalization rates compared to EVT + oral antiplatelet drug therapy (93.24% vs. 77.42%; p = 0.038), whereas the risk for SICH, 90 day mortality, and functional independence outcomes did not differ between the groups. Logistic regression analysis revealed that EVT + tirofiban + oral antiplatelet therapy had an increased probability of higher recanalization rates (OR 0.18 [95% confidence interval (CI) 1.24–24.39]; p = 0.025). There were no differences in SICH (OR 0.00 [95% CI 0.00–Inf]; p = 0.998), 90 day mortality (OR 1.19 [95% CI 0.17–4.05]; p = 0.826), or functional independence (modified Rankin score 0 to ≤ 2) (OR 1.43 [95% CI 0.23–2.17]; p = 0.538) between the groups.
Conclusions: Ninety day functional outcomes of EVT + tirofiban + oral antiplatelet therapy were not superior to those of EVT + oral antiplatelet drug therapy; however, the recanalization rate was higher and the risks for SICH and 90 day mortality were lower.
Introduction
Many randomized trials have demonstrated the improved efficacy of endovascular treatment (EVT) compared with standard medical care in patients with acute ischemic stroke (AIS) caused by arterial occlusion in the anterior circulation (1–5). For patients with acute ischemia due to basilar artery occlusion, the rates of death or dependency are 76–78% (6), even when these patients are treated with intravenous or intra-arterial thrombolysis agents. Different case scenarios have shown that stent-retriever thrombectomy is a safe and feasible treatment technique in patients with acute basilar artery occlusion (BAO) (7–10). However, for acute intracranial atherosclerosis-related occlusion (ICASO), which is more common in Asia (11–14), salvage therapies (15), such as balloon angioplasty and stent angioplasty, are necessary to increase the rate of successful and complete reperfusion. The associated risks, such as perforator occlusion (16), endothelial damage (17, 18), microvascular dysfunction (19), and reocclusion of recanalized vessels (20–22), may cause intracranial hemorrhage or even worse outcomes.
Many studies have investigated methods to prevent the negative events caused by antithrombotic EVT, either in the anterior or the posterior circulation. A post hoc analysis from the Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke from the Netherlands reported that patients on a previous oral antiplatelet drug regimen were twice as likely to experience a favorable functional outcome (23). Injection of low-dose tirofiban combined with EVT can enhance recanalization rates (24–26). However, there have been no studies confirming which method is safer and/or more effective. The aim of the present study, therefore, was to compare outcomes in patients who underwent EVT plus tirofiban (EVT + tirofiban) therapy vs. those who underwent EVT in conjunction with oral antiplatelet drug therapy.
Methods
Study Design
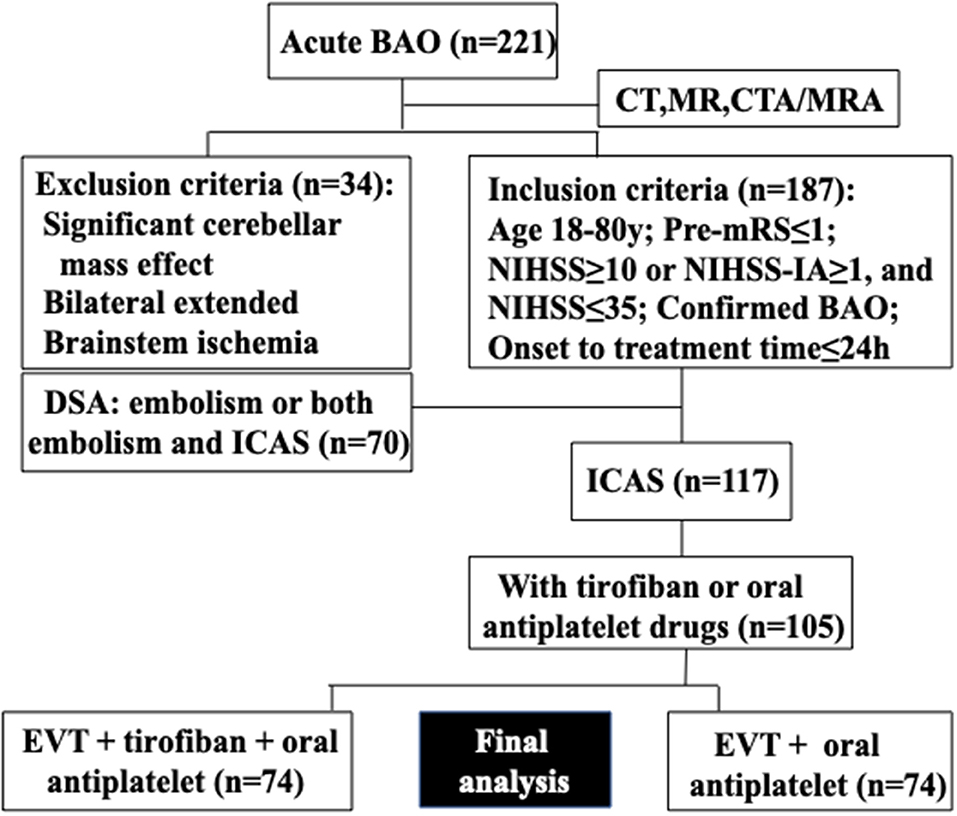
Consecutive patients presenting with ICAS-related acute BAO who underwent EVT + tirofiban + oral antiplatelet or EVT + oral antiplatelet drug therapy at Beijing Tiantan Hospital (Beijing, China) between January 2012 and July 2018 were included in this retrospective study. Intravenous thrombolysis with tissue plasminogen activator was allowed prior to EVT, consistent with current guidelines (27). The details of patient selection and grouping are presented in Figure 1 according to previous studies (28–30). Tirofiban was considered for application in the following situations/parameters: (1) rescue treatment with emergency stenting for residual artery stenosis or failed thrombectomy; (2) rescue treatment with emergency balloon angioplasty for residual artery stenosis or failed thrombectomy; (3) local new thrombosis or vascular dissection of the responsible vessel; and (4) severe atherosclerosis lesions with a high possibility of reocclusion after being recanalized. Informed consent was obtained from all participants or their relatives, and the protocol was approved by the Institutional Review Board of Beijing Tiantan Hospital.
Plain computed tomography (CT) of the brain was performed first to exclude intracranial bleeding. Magnetic resonance imaging (MRI) was used to exclude large brainstem infarctions. CT angiography (CTA) or magnetic resonance angiography (MRA) was used to confirm the presence of acute BAO. The clinical and radiological data of these patients were retrospectively collected and analyzed and included age; sex; stroke risk factors, including hypertension, diabetes mellitus, hypercholesterolemia, and current smoking status; initial stroke severity, as reflected by the National Institutes of Health Stroke Scale (NIHSS) score; initial imaging modality; previous intravenous thrombolysis; type of EVT device(s); onset to puncture time; procedure duration; onset to recanalization time; and collateral status.
EVT
The decision to perform EVT was made by a stroke neurologist and a neuroradiologist based on the CT, CTA, or MRA results of the patient. All EVT procedures were performed by a neurointerventionalist with sufficient experience in neurovascular intervention in mechanical thrombectomy for AIS (>50 cases). Cerebral angiography and EVT were performed under general anesthesia or conscious sedation after evaluation by a dedicated anesthesiology team. A stent retriever or aspiration was used first, with a possible switch to another strategy in the case of recanalization failure (mTICI grade = 0–2a) with the first approach. Underlying ICAS was determined when significant stenosis was seen on the initial diagnostic angiography or on follow-up angiography after a mechanical thrombectomy procedure (31). Causes of acute BAO were classified based on our previous research (32). ICAS was defined as significant fixed focal stenosis that could be resolved by means of angioplasty or stent insertion at the site of occlusion. Significant stenosis was defined as (1) fixed stenosis ≥70% or (2) fixed stenosis ≥50% in addition to either angiographical evidence of impaired perfusion or evidence of reocclusion after sufficient treatment with a stent retriever. The cause of acute BAO was classified as embolism based on the following: (1) after clot retrieval, there was no focal stenosis; (2) an embolus was removed with a stent retriever; or (3) MRA or CTA performed within 1 week after the procedure showed lack of stenosis in the responsible artery. In cases of recanalization failure (mTICI grade 0–2a after mechanical thrombectomy within 10 min), intracranial angioplasty or stenting was performed. All patients immediately received a loading dose of 300 mg of aspirin and 300 mg of clopidogrel orally. Patients with daily aspirin (100 mg) or/and clopidogrel (75 mg) medication before the intervention did not receive a loading dose. Alternatively, tirofiban therapy (Grand Pharmaceutical Co Ltd, China) was applied. Standard procedure was to administer 5 mg of tirofiban diluted in 100 ml of normal saline and infused with a 6- to 8-ml (0.3–0.4 mg) loading dose via a catheter at a rate of 1 ml/min. Intravenous tirofiban was continued at a rate of 0.15 μg/kg/min for the next 24 h. Intravenous tirofiban was continued at a rate of 0.15 μg/kg/min for the next 24 h. If there were no hemorrhagic complications seen on the head CT scan, aspirin and clopidogrel (one tablet each) were used with overlap for 4–6 h during the operation. If CT performed 24 h after the operation revealed no dynamic bleeding or hemorrhagic transformation, dual antiplatelet therapy was maintained for at least 3 months after the procedure, followed by lifelong aspirin or clopidogrel monotherapy thereafter.
Image Interpretation
Diffusion-weighted images were assessed using the pons-midbrain index (using the posterior communicating artery) and the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) (29, 33) grading system. All images were analyzed retrospectively by two neurologists blinded to the patient information and study protocol. If no consensus was reached, a third neurointerventionalist with 20 years of experience made the final decision.
Outcome Measures
For primary effectiveness, outcomes were assessed according to the BASICS definition (34). Functional independence and a favorable outcome were defined as mRS ≤ 2 and mRS ≤ 3, respectively. Other effectiveness outcome measures included the rates of successful recanalization [modified treatment for cerebral infarction (mTICI) = 2b−3] and complete recanalization (mTICI = 3) (35).
The safety outcome measures included 90 day mortality and rates of intracerebral hemorrhage and symptomatic intracerebral hemorrhage (SICH) within 24 h according to the criteria described in the European Cooperative Acute Stroke Study III (ECASS III) (36).
Statistical Analysis
Study data were collected on standard forms, evaluated for completeness, and double keyed into an EpiData statistics data document. Statistical analysis was performed using R (http://www.R-project.org, The R Foundation for Statistical Computing, Vienna, Austria) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA, USA). Continuous variables are expressed as medians and interquartile ranges (IQRs) and as absolute numbers and percentages, while categorical variables are expressed as the means and standard deviations (SDs). Shapiro–Wilk test, histogram, and QQ chart were used to confirm normal distribution of data. The chi-squared test was used to compare categorical variables, t-tests were used to compare continuous variables, and the Mann–Whitney U test was used to compare scores. Logistic regression was performed to estimate odds ratios (ORs) for 90 day mortality and primary effectiveness outcomes (mRS ≤ 2 and ≤ 3). Variables including the NIHSS score, sex, standard preoperative intravascular tissue plasminogen activator, general anesthesia, stent thrombectomy, and intraoperative heparinization were entered into a logistic regression model. All tests were two-tailed, and statistical significance was determined at a P level of 0.05.
Results
Of 105 patients, 71 [mean (± SD) age, 60.0 ± 8 years; 86.49% men] underwent EVT + tirofiban + oral antiplatelet drug therapy, whereas 34 (mean age, 60.0 ± 10 years; 83.87% men) underwent EVT + oral antiplatelet drug therapy.
A comparison of the baseline data and treatment procedures between the two groups is shown in Table 1. The NIHSS score was higher in patients who underwent EVT + tirofiban + oral antiplatelet drug therapy (25 [IQR 13–35] vs. 12 [IQR 10–23]; p = 0.005) than in those who underwent EVT + oral antiplatelet drug therapy (Table 1). More patients in the EVT + tirofiban + oral antiplatelet drug therapy group required stent thrombectomy (77.03% vs. 45.16%; p = 0.001) than patients in the EVT + oral antiplatelet drug therapy group, and fewer patients required intraoperative heparinization (40.54% vs. 70.97%; p = 0.004), which may have been related to differences in the severity of neurological deficits at the time of admission between the two groups.
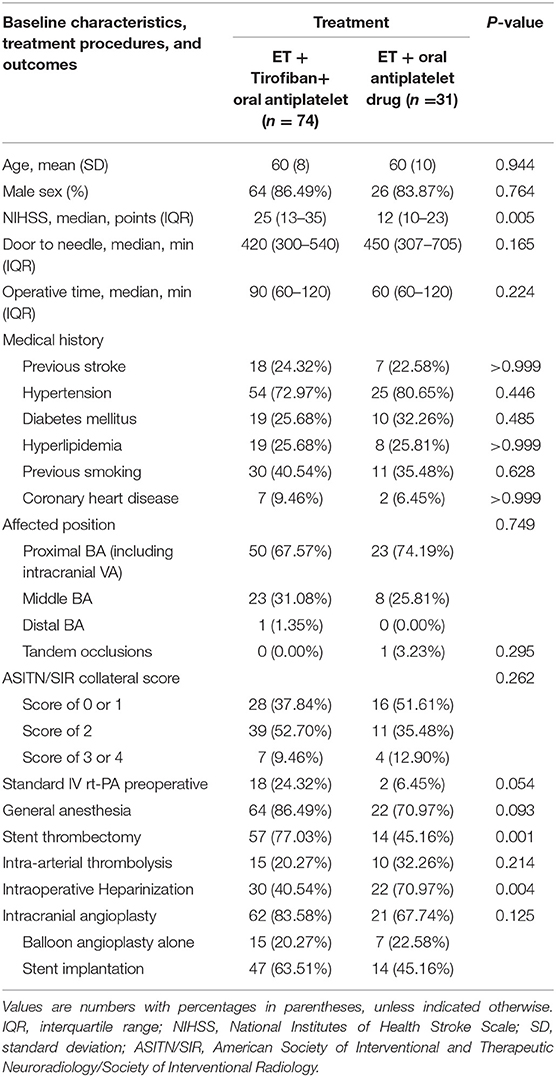
Table 1. Comparison of baseline data and treatment procedures between patients who underwent ET +tirofiban + oral antiplatelet and those who underwent ET + oral antiplatelet drug therapy.
A comparison of higher recanalization rates, 90 day mortality, SICH, and functional outcomes between the two groups is summarized in Table 2. In both groups, there was a higher rate of successful recanalization (mTICI 2b-3) among patients who underwent EVT + tirofiban + oral antiplatelet drug therapy (93.24% vs. 77.42%; p = 0.038). After comparing the two groups, the functional independence outcome rate (mRS 0 to ≤ 2) at 90 days (32.43% vs. 51.61%, p = 0.065), 7 day intracerebral hemorrhage (16.22% vs. 6.45%, p = 0.223), 90 day mortality (16.22% vs. 9.68%, p = 0.544), 7 day SICH (4.05% vs. 0.00%, p = 0.553), and 30 day responsible artery reocclusion (16.22% vs. 12.90%, p = 0.773) was not significantly different. The favorable outcome rate (mRS 0 to ≤ 3) in the EVT + tirofiban + oral antiplatelet drug therapy group was higher than that in the EVT + oral antiplatelet drug therapy group, owing to the differences in severe neurological deficits at admission.
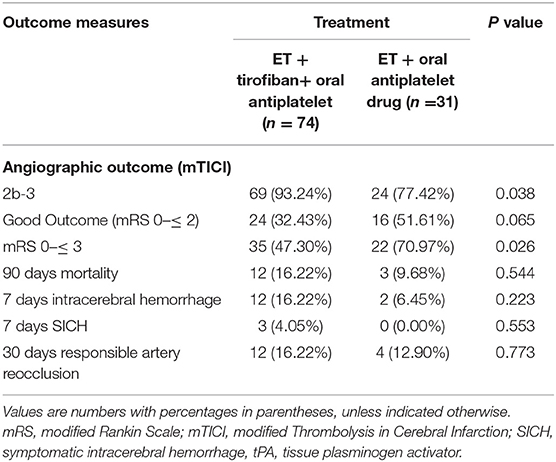
Table 2. Comparison of higher recanalization rates, mortality at 90 days, symptomatic intracerebral hemorrhage (SICH), and function outcomes between EVT + tirofiban + oral antiplatelet and EVT + oral antiplatelet drug therapy.
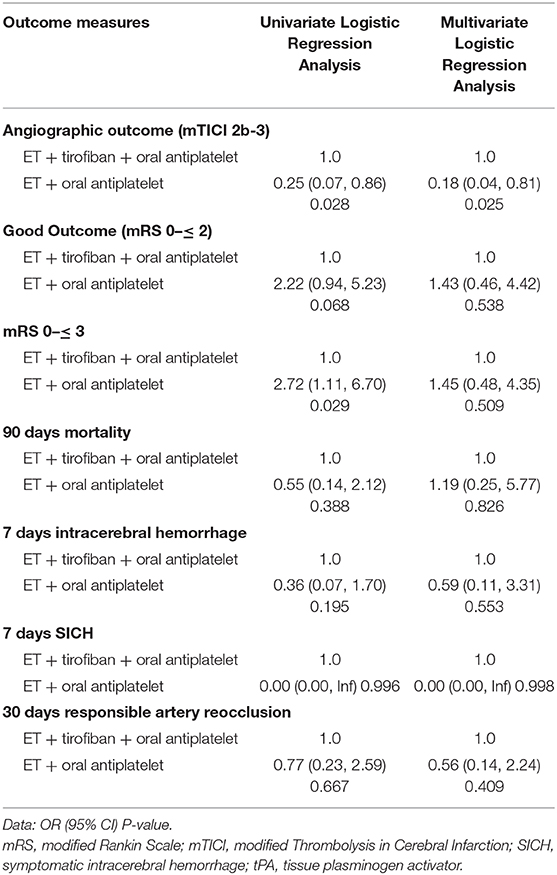
Adjusted logistic regression analysis revealed that treatment with EVT + tirofiban + oral antiplatelet drug therapy was associated with an increased rate of successful recanalization (OR 0.18 [95% confidence interval (CI) 1.24–24.39]; p = 0.025), whereas no differences were found in SICH (OR 0.00 [95% CI 0.00–Inf]; p = 0.998), 90 day mortality (OR 1.19 [95% CI 0.17–4.05]; p = 0.826), or functional independence outcomes (mRS 0 to ≤ 2) (OR 1.43 [95% CI 0.23–2.17]; p = 0.538) between the groups (Table 3).
Discussion
ICAS occlusion is more common in the posterior than in the anterior circulation, especially among the Asian population (22, 37). Therefore, rescue therapies such as emergency angioplasty or stenting are often required when treating these patients with EVT (15, 21), thereby inducing the removal of in situ thrombi (17), instability in the ICAS endothelium (38), and the “snowplow” effect (21). These steps result in perforator occlusion, microvascular dysfunction, and reocclusion of recanalized vessels. Many treatment strategies, including EVT + tirofiban + oral antiplatelet drug therapy (23) and EVT + oral antiplatelet drug therapy (24, 25), have been performed on patients with anterior and posterior circulation infarction in previous studies to prevent these drawbacks.
Due to the disparate characteristics of patients with posterior circulation cerebral infarction, intravascular tirofiban is more convenient than oral antiplatelet drug therapy. First, most patients with posterior circulation infarction exhibit consciousness disorders and difficulty swallowing. Oral antiplatelet drugs need to be administered through a gastric tube, the insertion of which is time-consuming. Second, it is important to differentiate an ICAS occlusion from an intracranial embolism before operating on patients without a clear history of intracranial artery stenosis. For these patients, intravascular tirofiban has the advantage of a rapid onset. Therefore, the present study compared the safety and outcomes in patients who underwent EVT+ tirofiban drug therapy vs. those who underwent EVT + oral antiplatelet drug therapy.
Our study found that acute intracranial atherosclerosis-related vertebrobasilar artery occlusion treated with EVT + tirofiban + oral antiplatelet drug therapy was associated with an increased rate of successful recanalization (OR 0.18 [95% (CI) 1.24–24.39]; p = 0.025), whereas no differences were found in SICH (OR 0.00 [95% CI 0.00–Inf]; p = 0.998) or 90 day mortality (OR 1.19 [95% CI 0.17–4.05]; p = 0.826). Functional independence outcomes at 90 days (mRS 0 to ≤ 2) were not significantly different (OR 1.43 [95% CI 0.23–2.17]; p = 0.538), which may be attributed to the severity of the neurological deficits between the groups at the time of admission and the small sample size. Since the investigation was an observational one based on a single-center prospective registry study, there are several limitations. First, the administration route, dose, and duration of tirofiban used in this study were pragmatic. Furthermore, inherent bias because of the retrospective and monocentric study design was inevitable. Compared to patients with EVT + oral antiplatelet drug therapy, those with EVT + tirofiban + oral antiplatelet drug therapy had a higher NIHSS score at baseline and a more frequent application of stent thrombectomy and intraoperative heparinization. However, after adjusting for potential confounders and conducting a multivariate analysis, we presume that this bias probably had little influence on our results. A multicenter study involving a larger sample size or a randomized controlled trial is needed to verify this result.
Conclusions
In summary, for patients who have trouble swallowing and those without a clear history of intracranial artery stenosis, intravascular tirofiban treatment can be performed to save time. Our results demonstrated that EVT + tirofiban + oral antiplatelet drug therapy was not superior to EVT + oral antiplatelet drug therapy in terms of the functional independence outcomes at 90 days. Therefore, a more effective perioperative administration route, time, and dosage await further study.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of Beijing Tiantan Hospital, Capital Medical University medical ethics committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Author Contributions
ZM designed the research and wrote the manuscript. XS and HZ designed the research. XS, HZ, and ZM performed the research. XT, FG, and GM analyzed the data.
Funding
This study was supported by the National Key Research and Development Program of China (Grant number 2016YFC1301501).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all relevant clinicians, statistician, and imaging technicians.
Abbreviations
EVT, endovascular treatment; SICH, symptomatic intracranial hemorrhage; AIS, acute ischemic stroke; ICASO, intracranial atherosclerosis-related occlusion; tPA, tissue plasminogen activator; CT, computed tomography; CTA, computed tomography angiography; MRI, magnetic resonance imaging; MRA, magnetic resonance angiography; NIHSS, National Institutes of Health Stroke Scale; mTICI, modified Thrombolysis in Cerebral Infarction; mRS, modified Rankin Scale; ORs, odds ratios.
References
1. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. (2015) 372:11–20. doi: 10.1056/NEJMoa1411587
2. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. (2015) 372:1009–18. doi: 10.1056/NEJMoa1414792
3. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. (2015) 372:1019–30. doi: 10.1056/NEJMoa1414905
4. Molina CA, Chamorro A, Rovira A, de Miquel A, Serena J, Roman LS, et al. REVASCAT: a randomized trial of revascularization with solitaire FR device vs. Best medical therapy in the treatment of acute stroke due to anterior circulation large vessel occlusion presenting within eight-hours of symptom onset. Int J Stroke. (2015) 10:619–26. doi: 10.1111/ijs.12157
5. Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-pa vs. T-pa alone in stroke. N Engl J Med. (2015) 372:2285–95. doi: 10.1056/NEJMoa1415061
6. Yeung JT, Matouk CC, Bulsara KR, Sheth KN. Endovascular revascularization for basilar artery occlusion. Interv Neurol. (2015) 3:31–40. doi: 10.1159/000368968
7. Gory B, Eldesouky I, Sivan-Hoffmann R, Rabilloud M, Ong E, Riva R, et al. Outcomes of stent retriever thrombectomy in basilar artery occlusion: an observational study and systematic review. J Neurol Neurosurg Psychiatry. (2016) 87:520–5. doi: 10.1136/jnnp-2014-310250
8. Mohlenbruch M, Stampfl S, Behrens L, Herweh C, Rohde S, Bendszus M, et al. Mechanical thrombectomy with stent retrievers in acute basilar artery occlusion. AJNR Am J Neuroradiol. (2014) 35:959–64. doi: 10.3174/ajnr.A3796
9. Baek JM, Yoon W, Kim SK, Jung MY, Park MS, Kim JT, et al. Acute basilar artery occlusion: outcome of mechanical thrombectomy with solitaire stent within 8 hours of stroke onset. Am J Neuroradiol. (2014) 35:989–93. doi: 10.3174/ajnr.A3813
10. Yoon W, Kim SK, Heo TW, Baek BH, Lee YY, Kang HK. Predictors of good outcome after stent-retriever thrombectomy in acute basilar artery occlusion. Stroke. (2015) 46:2972–5. doi: 10.1161/STROKEAHA.115.010840
11. Lee JS, Hong JM, Lee KS, Suh HI, Demchuk AM, Hwang YH, et al. Endovascular therapy of cerebral arterial occlusions: intracranial atherosclerosis versus embolism. J Stroke Cerebrovasc Dis. (2015) 24:2074–80. doi: 10.1016/j.jstrokecerebrovasdis.2015.05.003
12. De Silva DA, Woon FP, Lee MP, Chen CP, Chang HM, Wong MC. South Asian patients with ischemic stroke: intracranial large arteries are the predominant site of disease. Stroke. (2007) 38:2592–4. doi: 10.1161/STROKEAHA.107.484584
13. Lee SJ, Cho SJ, Moon HS, Shon YM, Lee KH, Kim DI, et al. Combined extracranial and intracranial atherosclerosis in Korean patients. Arch Neurol. (2003) 60:1561–4. doi: 10.1001/archneur.60.11.1561
14. Wang Y, Zhao X, Liu L, Soo YO, Pu Y, Pan Y, et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese intracranial atherosclerosis (cicas) study. Stroke. (2014) 45:663–9. doi: 10.1161/STROKEAHA.113.003508
15. Gao F, Lo WT, Sun X, Mo DP, Ma N, Miao ZR. Combined use of mechanical thrombectomy with angioplasty and stenting for acute basilar occlusions with underlying severe intracranial vertebrobasilar stenosis: preliminary experience from a single Chinese center. AJNR Am J Neuroradiol. (2015) 36:1947–52. doi: 10.3174/ajnr.A4364
16. Yoon W, Kim SK, Park MS, Kim BC, Kang HK. Endovascular treatment and the outcomes of atherosclerotic intracranial stenosis in patients with hyperacute stroke. Neurosurgery. (2015) 76:680–6. doi: 10.1227/NEU.0000000000000694
17. Gory B, Bresson D, Kessler I, Perrin ML, Guillaudeau A, Durand K, et al. Histopathologic evaluation of arterial wall response to 5 neurovascular mechanical thrombectomy devices in a swine model. AJNR Am J Neuroradiol. (2013) 34:2192–8. doi: 10.3174/ajnr.A3531
18. Arai D, Ishii A, Chihara H, Ikeda H, Miyamoto S. Histological examination of vascular damage caused by stent retriever thrombectomy devices. J Neurointerv Surg. (2016) 8:992–5. doi: 10.1136/neurintsurg-2015-011968
19. Dalkara T, Arsava EM. Can restoring incomplete microcirculatory reperfusion improve stroke outcome after thrombolysis? J Cereb Blood Flow Metab. (2012) 32:2091–9. doi: 10.1038/jcbfm.2012.139
20. Heo JH, Lee KY, Kim SH, Kim DI. Immediate reocclusion following a successful thrombolysis in acute stroke: a pilot study. Neurology. (2003) 60:1684–7. doi: 10.1212/01.WNL.0000063323.23493.98
21. Kang DH, Kim YW, Hwang YH, Park SP, Kim YS, Baik SK. Instant reocclusion following mechanical thrombectomy of in situ thromboocclusion and the role of low-dose intra-arterial tirofiban. Cerebrovasc Dis. (2014) 37:350–5. doi: 10.1159/000362435
22. Lee JS, Hong JM, Lee KS, Suh HI, Choi JW, Kim SY. Primary stent retrieval for acute intracranial large artery occlusion due to atherosclerotic disease. J Stroke. (2016) 18:96–101. doi: 10.5853/jos.2015.01347
23. Mulder MJ, Berkhemer OA, Fransen PS, van den Berg LA, Lingsma HF, den Hertog HM, et al. Does prior antiplatelet treatment improve functional outcome after intra-arterial treatment for acute ischemic stroke? Int J Stroke. (2017) 12:368–76. doi: 10.1177/1747493016677842
24. Goh DH, Jin SC, Jeong HW, Ha SY. Mechanical solitaire thrombectomy with low-dose booster tirofiban injection. Neurointervention. (2016) 11:114–9. doi: 10.5469/neuroint.2016.11.2.114
25. Seitz RJ, Hamzavi M, Junghans U, Ringleb PA, Schranz C, Siebler M. Thrombolysis with recombinant tissue plasminogen activator and tirofiban in stroke: preliminary observations. Stroke. (2003) 34:1932–5. doi: 10.1161/01.STR.0000080535.61188.A6
26. Seitz RJ, Siebler M. Platelet gpiib/iiia receptor antagonists in human ischemic brain disease. Curr Vasc Pharmacol. (2008) 6:29–36. doi: 10.2174/157016108783331303
27. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2018). 49:e46–110. doi: 10.1161/STR.0000000000000172
28. Sun X, Tong X, Gao F, Lao H, Miao Z. Endovascular treatment for acute basilar artery occlusion: a single center retrospective observational study. BMC Neurol. (2019) 19:315. doi: 10.1186/s12883-019-1551-8
29. van der Hoeven Erik JRJ, Schonewille Wouter J, Vos Jan, Algra Ale, Audebert Heinrich J, Berge Eivind, et al. The Basilar Artery International Cooperation Study (BASICS): study protocol for a randomised controlled trial. Trials. (2013) 14:200. doi: 10.1186/1745-6215-14-200
30. Writing Group for the BASILAR Group, Zi W, Qiu Z, Wu D, Li F, Liu H, et al. Assessment of endovascular treatment for acute basilar artery occlusion via a nationwide prospective registry. JAMA Neurol. (2020) 72:1295–303. doi: 10.1001/jamaneurol.2020.0156
31. Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. Am J Neuroradiol. (2000) 21:643–6. doi: 10.1016/S0925-4927(99)00050-5
32. Zhang H, Sun X, Huang Q, Wang XM, Yue YH, Ju MF, et al. Intracranial atherosclerotic disease-related acute middle cerebral artery occlusion can be predicted by diffusion-weighted imaging. Front Neurosci. (2019) 13:903. doi: 10.3389/fnins.2019.00903
33. Goyal N, Tsivgoulis G, Nickele C, Doss VT, Hoit D, Alexandrov AV, et al. Posterior circulation CT angiography collaterals predict outcome of endovascular acute ischemic stroke therapy for basilar artery occlusion. J Neurointerv Surg. (2016) 8:783–6. doi: 10.1136/neurintsurg-2015-011883
34. Singer OC, Berkefeld J, Nolte CH, Bohner G, Haring HP, Trenkler J, et al. Mechanical recanalization in basilar artery occlusion: the endostroke study. Ann Neurol. (2015) 77:415–24. doi: 10.1002/ana.24336
35. Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. (2013) 44:2650–63. doi: 10.1161/STROKEAHA.113.001972
36. Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. (2008) 359:1317–29. doi: 10.1056/NEJMoa0804656
37. Lee JS, Hong JM, Kim JS. Diagnostic and therapeutic strategies for acute intracranial atherosclerosis-related occlusions. J Stroke. (2017) 19:143–51. doi: 10.5853/jos.2017.00626
Keywords: endovascular treatment, tirofiban, antiplatelet, intracranial atherosclerosis-related vertebrobasilar artery occlusion, vertebrobasilar artery occlusion
Citation: Sun X, Zhang H, Tong X, Gao F, Ma G and Miao Z (2020) Effects of Periprocedural Tirofiban vs. Oral Antiplatelet Drug Therapy on Posterior Circulation Infarction in Patients With Acute Intracranial Atherosclerosis-Related Vertebrobasilar Artery Occlusion. Front. Neurol. 11:254. doi: 10.3389/fneur.2020.00254
Received: 19 July 2019; Accepted: 17 March 2020;
Published: 15 April 2020.
Edited by:
Jean-Claude Baron, University of Cambridge, United KingdomReviewed by:
Beom Joon Kim, Seoul National University Bundang Hospital, South KoreaJohannes Boltze, University of Warwick, United Kingdom
Copyright © 2020 Sun, Zhang, Tong, Gao, Ma and Miao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongrong Miao, bWlhb3pob25ncm9uZzEyM0AxMjYuY29t
†These authors have contributed equally to this work
 Xuan Sun1†
Xuan Sun1† Huijun Zhang
Huijun Zhang Xu Tong
Xu Tong Zhongrong Miao
Zhongrong Miao