- 1Department of Pharmacy, Evidence-Based Pharmacy Center, West China Second Hospital, Sichuan University, Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Chengdu, China
- 2Department of Health Policy and Management, West China School of Public Health, and West China Fourth Hospital, Sichuan University, Chengdu, China
- 3Department of Pediatric Clinic, West China Second Hospital, Sichuan University, Chengdu, China
- 4Department of Children's Genetic Endocrinology and Metabolism, West China Second Hospital, Sichuan University, Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Chengdu, China
Background: Real-world evidence includes data from retrospective/prospective observational studies and observational registries, and provides insights beyond those addressed by randomized controlled trials. This study aimed to evaluate the effectiveness and safety of a clonidine adhesive patch (CAP) for children with tic disorder (TD) in a real-world setting (RWS).
Methods: This was an open-label, non-interventional, post-marketing, observational study in a RWS. Children diagnosed with TDs were enrolled from a pediatric neurology clinic in China, and the change in tic symptom severity following 6 weeks pharmacologic treatments was investigated using Yale Global Tic Severity Scale (YGTSS) during visits at weeks 0, 4, 8, and 12.
Results: Of 150 patients, 76% (114/150) were male (age range, 3.03–14.24 years; mean, 8.11 ± 2.48 years). Patients were divided into three groups: tiapride (n = 94), CAP (n = 14), and CAP + tiapride (n = 42). The mean YGTSS improved 11.02, 15.14, 11.13 points from baseline to posttreatment for tiapride, CAP, and CAP + tiapride, respectively, but variance analysis showed there was no significant difference in YGTSS related to different pharmacologic intervention during subsequent visits at weeks 4, 8, and 12. Repeated measure analysis showed there was no significant difference between different medication types for reducing the YGTSS score (F = 0.553, P = 0.576). No serious adverse events (AEs) occurred, and there was no significant difference in the prevalence of AEs between the three groups.
Conclusion: The CAP is effective and safe for TD management in a RWS, because of the limitation of sample size and the period of follow up, observational studies with longer-term outcomes, and larger sample size are needed.
Introduction
Tic disorders (TDs) have been conceptualized as hyperkinetic-movement disorders and chronic neuropsychiatric disorder with childhood onset. TDs are characterized by multiple motor and one or more phonic tics; males are three-times more likely to suffer from TDs than females (1, 2). There are three types of TDs: transient tic disorder (TTD), chronic tic disorder (CTD), and Tourette syndrome (TS). One meta-analysis showed the worldwide prevalence of TTD to be 2.99%, followed by CTD (1.61%), and TS (0.77%) (3). In China, the combined prevalence of TDs has been reported to be 6.1%. One meta-analysis stated the prevalence of TTD, CTD, and TS in China to be 1.7, 1.2, and 0.3%, respectively (4). Despite the core symptom being tics, co-occurring psychiatric disorders are common in children suffering from TDs, such as attention deficit and hyperactivity disorder (ADHD), obsessive-compulsive disorder (OCD), and anxiety disorder (5–7).
Pharmacologic treatment is the main therapy for the management of tics and comorbidities (8, 9). Several randomized controlled trials (RCTs) have shown that clonidine can reduce some of the tics and other behavioral symptoms associated with TDs, and that clonidine is safe to use (10–12). In 2016, a systematic review by Wang and colleagues encompassing six RCTs (1,145 participants) evaluated the effectiveness of a clonidine adhesive patch (CAP) for TD treatment (13). They showed that the CAP may be as effective as haloperidol or tiapride for TDs, and that the prevalence of adverse events (AEs) of the CAP was low. Nevertheless, additional studies are needed urgently to reevaluate the effectiveness and safety of the CAP.
RCTs are undertaken according to regulatory and scientific standards, so extrapolation of research results is limited. Hence, RCTs may not necessarily reflect what happens in real-world settings (14). Real-world evidence helps to improve decision-making in healthcare settings. Hence, this study aimed to evaluate the effectiveness and safety of CAP for children with tic disorder (TD) in a real-world setting (RWS).
Materials and Methods
Study Design
This was an open-label, non-interventional, post-marketing, observational study to investigate the change in severity of tic symptoms in TD patients following different pharmacologic treatments in a real-world practice. The study was conducted from January to May 2019. The non-interventional, post-marketing nature of our study meant that registration in a clinical trial registry was not mandatory. Nevertheless, the study protocol was approved by the Office of Research Ethics Committees of West China Second Hospital (Chengdu, China).
Inclusion Criteria
The inclusion criteria were: (i) a clinically confirmed diagnosis of TD according to the Diagnostic and Statistical Manual of Mental Disorders-IV-Text Revision; (ii) age <18 years; (iii) provision of written informed consent.
Exclusion Criteria
The exclusion criteria were: (i) delays or problems with mental development (Wechsler intelligence-quotient score <70 points); (ii) cerebral palsy, neurodevelopmental delay, history of inherited metabolism, or poor development of motor language; (iii) non-provision of written informed consent. Voluntary written informed consent was provided by all caregivers or children aged >8 years.
Treatment
Assignment of patients to pharmacologic therapy was decided according to standard practice and medical indications assessed by the treating physician. Eligible patients were prescribed a drug dose by their treating physician depending on its effectiveness and toxicity and patient weight. This dosing regimen conformed to standard medical practices and the terms of local marketing authorization and reimbursement guidelines.
For CAPs, patients of weight 20–40 kg were given 1.0 mg/film; 40–60 kg were given 1.5 mg/film; >60 kg were given 2.0 mg/film. For tiapride, each child was started on 50 mg/day and the dose increased gradually to a maximum of 400 mg/day. The stable dose of medication was maintained for 6 weeks.
Assessments
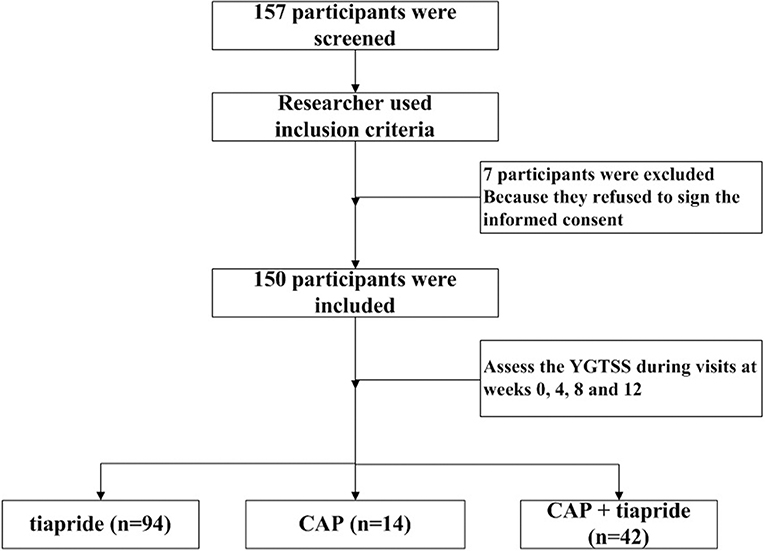
Baseline data (age, sex, disorder duration, type of TD) were obtained by the treating physician, followed by assessments during subsequent visits at weeks 0, 4, 8, and 12. The primary outcome was measured by the Yale Global Tic Severity Scale (YGTSS). This consists of a separate rating of severity for motor and vocal tics along five discriminant dimensions (number, frequency, intensity, complexity, and interference) on a scale of 0–5 for each. Summation of these scores (i.e., 0–50) results in a total tic score (TTS). The tic impairment score (TIS), from zero to a maximum of 50 points, is based on the impact of the tic disorder on self-esteem, family life, social acceptance, and school performance. The TIS is added to the TTS to obtain the total YGTSS (YGTSS-T) score. The secondary outcome was AEs, which were assessed via an interview of self-report by participants or their caregivers during the follow-up. The study flow of screening the participants was shown in Figure 1.
Statistical Analyses
The intent-to-treat population (which comprised all patients who received the study drug and who had a baseline visit and at least one on-treatment post-baseline visit) was used to assess all effectiveness and safety variables. For the comparability of baseline variables with continuous variables, they are shown as the mean ± standard deviation. If data followed a normal distribution, one-way ANOVA was used. If data did not follow a normal distribution, they were analyzed by the Wilcoxon rank sum test. Categorical variables are shown as frequencies and percentages, and the chi-square test or Fisher's exact test were used as appropriate.
Repeated measure analysis of variance was used to test the difference of treatment effects for different drugs. First, we used the Mauchly sphericity test to ascertain if the measured data satisfied the test hypothesis. If not, the Greenhouse–Geisser correction was carried out. P ≤ 0.05 was considered significant. We used SPSS v22 (IBM, Armonk, NY, USA) for statistical analyses.
Results
Characteristics of Patients (Table 1)
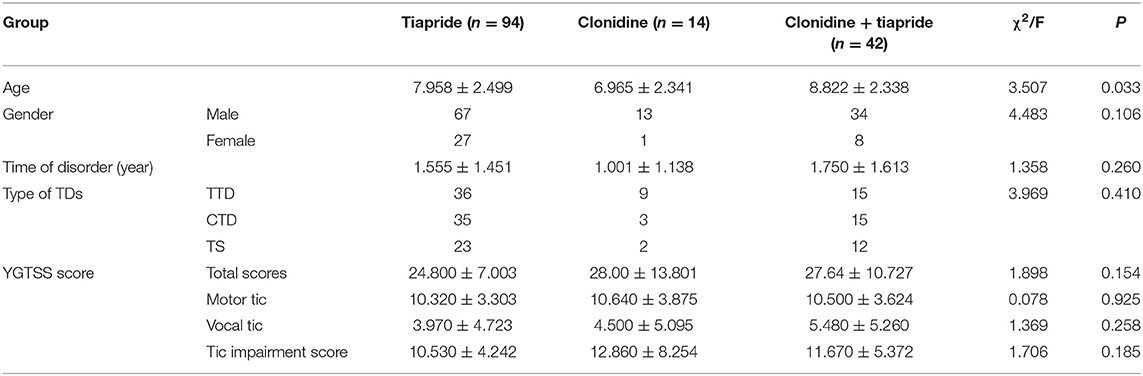
Children diagnosed with TDs were enrolled from a pediatric neurology clinic at West China Second Hospital. Initially, 157 patients were recruited. Seven patients discontinued treatment because they refused to sign the informed consent, so 150 patients completed the study. Among these 150 children, 76% (114/150) were male, and ranged in age from 3.03 years to 14.24 (mean, 8.11 ± 2.48) years. These patients were divided into three intervention groups: tiapride (n = 94), CAP (n = 14), and CAP + tiapride (n = 42).
There was no significant difference between the three groups in terms of sex, disorder duration, type of TD, or baseline YGTSS score, but there was a significant difference in age, so we included age as a covariate in the statistical model for analyses.
Effectiveness of Different Pharmacologic Interventions
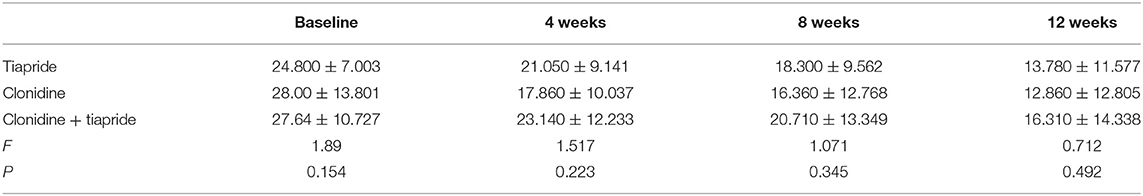
The YGTSS score was reduced at different times after pharmacologic intervention. For tiapride, the mean YGTSS improved 11.02 points from baseline to posttreatment (24.8 vs. 13.78). For CAP, the mean YGTSS improved 15.14 points from baseline to posttreatment (28.0 vs. 12.86). For CAP + tiapride, the mean YGTSS improved 11.13 points from baseline to posttreatment (27.64 vs. 16.31), but variance analysis showed there was no significant difference in YGTSS related to different pharmacologic intervention during subsequent visits at weeks 4, 8, and 12 (Table 2).
Repeated measure analysis also showed a significant difference for the YGTSS score for different medications at different follow-up times (F = 18.949, P = 0.000), but we found no interaction between the medication time and medication type (F = 1.043, P = 0.389) or between the medication time and age (F = 2.384, P = 0.069). A test of within-participant effects showed no significant difference between different medication types for reducing the YGTSS score (F = 0.553, P = 0.576).
Safety
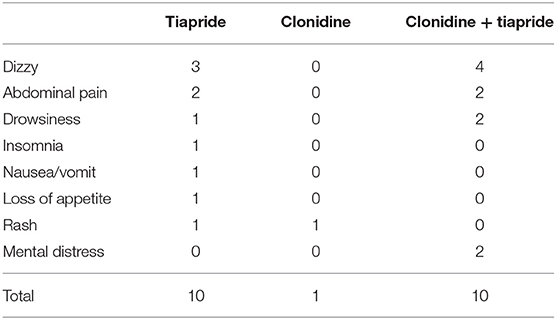
We found that 10.6% (10/94) of patients reported AEs in the tiapride group (Table 3); the most common AEs were dizziness and abdominal pain. Also, 7.1% (1/14) of patients reported AEs in the CAP group; the most common AEs were rash. In addition, 23.1% (10/42) of patients reported AEs in the CAP + tiapride group; the most common AEs were dizziness, abdominal pain, and drowsiness. The chi-square test showed no significant difference in the prevalence of AEs between the three groups.
Discussion
We used an observational study design to investigate the effectiveness and safety of different pharmacologic treatments in a real-world practice. We compared three pharmacologic interventions (tiapride, CAP, tiapride+ CAP) in 150 patients with TDs. We observed no significant difference between the medication groups in terms of reducing the YGTSS score after 8 weeks of treatment. In general, tiapride and the CAP were well-tolerated, and elicited few AEs.
Clonidine use is strongly recommended in Canadian guidelines, and it is regarded as first-line treatment in Chinese clinical guidelines, for TDs (15, 16). However, European guidelines recommend clonidine as second-line treatment for TDs (antipsychotic agents are first-line treatment) (17). Clonidine is seldom chosen to treat TDs in Japan (but clonidine is the only α2 adrenergic receptor agonist used to treat hypertension in Japan) (18). North American guidelines recommend α2 adrenergic agonists for the treatment of tics if the benefits of treatment outweigh the risks (with evidence level B), but physicians should monitor common side effects, such as sedation, heart rate, and blood pressure (level-A evidence) (19). Hence, the safety of the medication is an important concern for long-term treatment of TDs. We did not find serious AEs in TD treatment in a real-world practice during a short follow-up period, but common side effects must be monitored.
No clinical guidelines have mentioned CAP use, and there may be two reasons for this. First, formulations applied as an adhesive patch are not used widely. Nevertheless, a CAP releases clonidine at a relatively invariable rate for 1 week without trough or peak changes in plasma concentrations, so it is a very convenient treatment. Second, adhesive patches are relatively expensive, but the convenience of treatment may improve the quality of life of patients with TDs. Hence, cost–benefit analyses should be carried out. Therefore, future research should be considered from these two perspectives.
Our findings are similar to the study conducted by Joo and Kim (20), they assessed the effectiveness and safety of clonidine extended release (ER) treatment in Korean youth with ADHD and/or TS and included 29 children treated with clonidine ER, the result showed significant decreases in the CGI-S scores for both ADHD and tic symptoms were noted over 12 weeks, and life-threatening adverse effects were not observed.
Our study had three main limitations. First, included patients were from a single center, so a selection bias was evident. Nevertheless, the West China Second Hospital is the largest hospital in western China, so the research results carry a certain representativeness. Second, the observational, non-interventional design of our study may have introduced some bias toward overestimation of the treatment effect. Nevertheless, our study provides important insights into the real-world management of TDs. Finally, an observational study may underestimate AEs because adverse events were only assessed via an interview of self-report by participants or their caregivers, sometimes they may ignore reporting adverse reactions. Further studies are needed to overcome the shortcomings mentioned above.
Conclusions
The CAP is effective and safe for TD management in a RWS, because of the limitation of sample size and the period of follow up, observational studies with longer-term outcomes and larger sample size are needed.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
The studies involving human participants were reviewed and approved by the Office of Research Ethics Committees of West China Second Hospital. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author Contributions
CY designed the review, collected data, carried out analysis and interpretation of the data, and wrote the review. BK and DY designed the review, collected data, checked the data, and wrote the review. LZhao and LZhan designed the review, commented on drafts for previous version.
Funding
This study was funded by Sichuan Health and Wellness Committee: Evidence-based construction of clinical drug route for children with tic disorder (18PJ528). The sponsor had no role in the study design, writing of the manuscript, or decision to submit this or future manuscripts for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We also thank Rachel James, Ph.D. from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
References
1. Sanger TD, Chen D, Fehlings DL, Hallett M, Lang AE, Mink JW, et al. Definition and classification of hyperkinetic movements in childhood. Mov Disord. (2010) 25:1538–49. doi: 10.1002/mds.23088
2. Robertson MM. The gilles de la tourette syndrome: the current status. Arch Dis Child. (2012) 97:166–75. doi: 10.1136/archdischild-2011-300585
3. Knight T, Steeves T, Day L, Lowerison M, Jette N, Pringsheim T. Prevalence of tic disorders: a systematic review and meta-analysis. Pediatr Neurol. (2012) 47:77–90. doi: 10.1016/j.pediatrneurol.2012.05.002
4. Yang C, Zhang L, Zhu P, Zhu C, Guo Q. The prevalence of tic disorders for children in China:a systematic review and meta-analysis. Medicine. (2016) 95:e4354. doi: 10.1097/MD.0000000000004354
5. Yang C, Cheng X, Zhang Q, Yu D, Li J, Zhang L. Interventions for tic disorders: an updated overview of systematic reviews and meta analyses. Psychiatry Res. (2020) 287:112905. doi: 10.1016/j.psychres.2020.112905
6. Robertson MM. A personal 35 year perspective on gilles de la tourette syndrome: assessment, investigations, and management. Lancet Psychiatry. (2015) 2:88–104. doi: 10.1016/S2215-0366(14)00133-3
7. Freeman RD, Fast DK, Burd L, Kerbeshian J, Robertson MM, Sandor P. An international perspective on Tourette syndrome: selected findings from 3,500 individuals in 22 countries. Dev Med Child Neurol. (2000) 42:436–47. doi: 10.1017/S0012162200000839
8. Ganos C, Martino D, Pringsheim T. Tics in the pediatric population: pragmatic management. Mov Disord Clin Pract. (2017) 4:160–72. doi: 10.1002/mdc3.12428
9. Hollis C, Pennant M, Cuenca J, Glazebrook C, Kendall T, Whittington C, et al. Clinical effectiveness and patient perspectives of different treatment strategies for tics in children and adolescents with Tourette syndrome: a systematic review and qualitative analysis. Health Technol Assess. (2016) 20:1–450. doi: 10.3310/hta20040
10. Goetz CG, Tanner CM, Wilson RS, Carroll VS, Como PG, Shannon KM. Clonidine and Gilles de la Tourette's syndrome: double-blind study using objective rating methods. Ann Neurol. (1987) 21:307–10. doi: 10.1002/ana.410210313
11. Leckman JF, Hardin MT, Riddle MA, Stevenson J, Ort SI, Cohen DJ. Clonidine treatment of Gilles de la Tourette's syndrome. Arch Gen Psychiatry. (1991) 48:324–8. doi: 10.1001/archpsyc.1991.01810280040006
12. Du YS, Li HF, Vance A, Zhong YQ, Jiao FY, Wang HM, et al. Randomized double-blind multicentre placebo-controlled clinical trial of the clonidine adhesive patch for the treatment of tic disorders. Aust N Z J Psychiatry. (2008) 42:807–13. doi: 10.1080/00048670802277222
13. Wang S, Wei YZ, Yang J, Zhou Y, Zheng Y. Clonidine adhesive patch for the treatment of tic disorders: a systematic review meta-analysis. Eur J Paediatr Neurol. (2017) 21:614–20. doi: 10.1016/j.ejpn.2017.03.003
14. Schwartz D, Lellouch J. Explanatory and pragmatic attitudes in therapeutic trials. J Clin Epidemiol. (2009) 62:499–505. doi: 10.1016/j.jclinepi.2009.01.012
15. Pringsheim T, Doja A, Gorman D, McKinlay D, Day L, Billinghurst L, et al. Canadian guidelines for the evidence-based treatment of tic disorders: pharmacotherapy. Can J Psychiatry. (2012) 57:133–43. doi: 10.1177/070674371205700302
16. The Branch of Neurology of Chinese Medical Association. The guideline of the diagnosis and treatment for tic disorders in children. J Clin J Appl Clin Pediatr. (2017) 32:1137–40. doi: 10.3760/cma.j.issn.2095-428X
17. Roessner V, Plessen KJ, Rothenberger A, Ludolph AG, Rizzo R, Skov L, et al. European clinical guidelines for Tourette syndrome and other tic disorders. part II: pharmacological treatment. Eur Child Adolesc Psychiatry. (2011) 20:173–96. doi: 10.1007/s00787-011-0163-7
18. Hamamoto Y, Fujio M, Nonaka M, Matsuda N, Kono T, Kano Y. Expert consensus on pharmacotherapy for tic disorders in Japan. Brain Dev. (2019) 41:501–6. doi: 10.1016/j.braindev.2019.02.003
19. Pringsheim T, Okun MS, Müller-Vahl K, Martino D, Jankovic J, Cavanna AE, et al. Practice guideline recommendations summary: treatment of tics in people with Tourette syndrome and chronic tic disorders. Neurology. (2019) 92:896–906. doi: 10.1212/WNL.0000000000007466
Keywords: effectiveness, safety, clonidine adhesive patch, tic disorders, real-world study
Citation: Yang C, Kang B, Yu D, Zhao L and Zhang L (2020) Effectiveness and Safety of a Clonidine Adhesive Patch for Children With Tic Disorders: Study in a Real-World Practice. Front. Neurol. 11:361. doi: 10.3389/fneur.2020.00361
Received: 07 November 2019; Accepted: 14 April 2020;
Published: 08 May 2020.
Edited by:
Changlian Zhu, Third Affiliated Hospital of Zhengzhou University, ChinaReviewed by:
Giovanni Messina, University of Foggia, ItalyEmily Ricketts, University of California, Los Angeles, United States
Copyright © 2020 Yang, Kang, Yu, Zhao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Zhao, emhhb2xpQHNjdS5lZHUuY24=; Lingli Zhang, emhsaW5nbGlAc2luYS5jb20=
†These authors have contributed equally to this work
 Chunsong Yang
Chunsong Yang BingYao Kang3†
BingYao Kang3†