- 1Neuroradiology Department, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milan, Italy
- 2Neurology Department, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milan, Italy
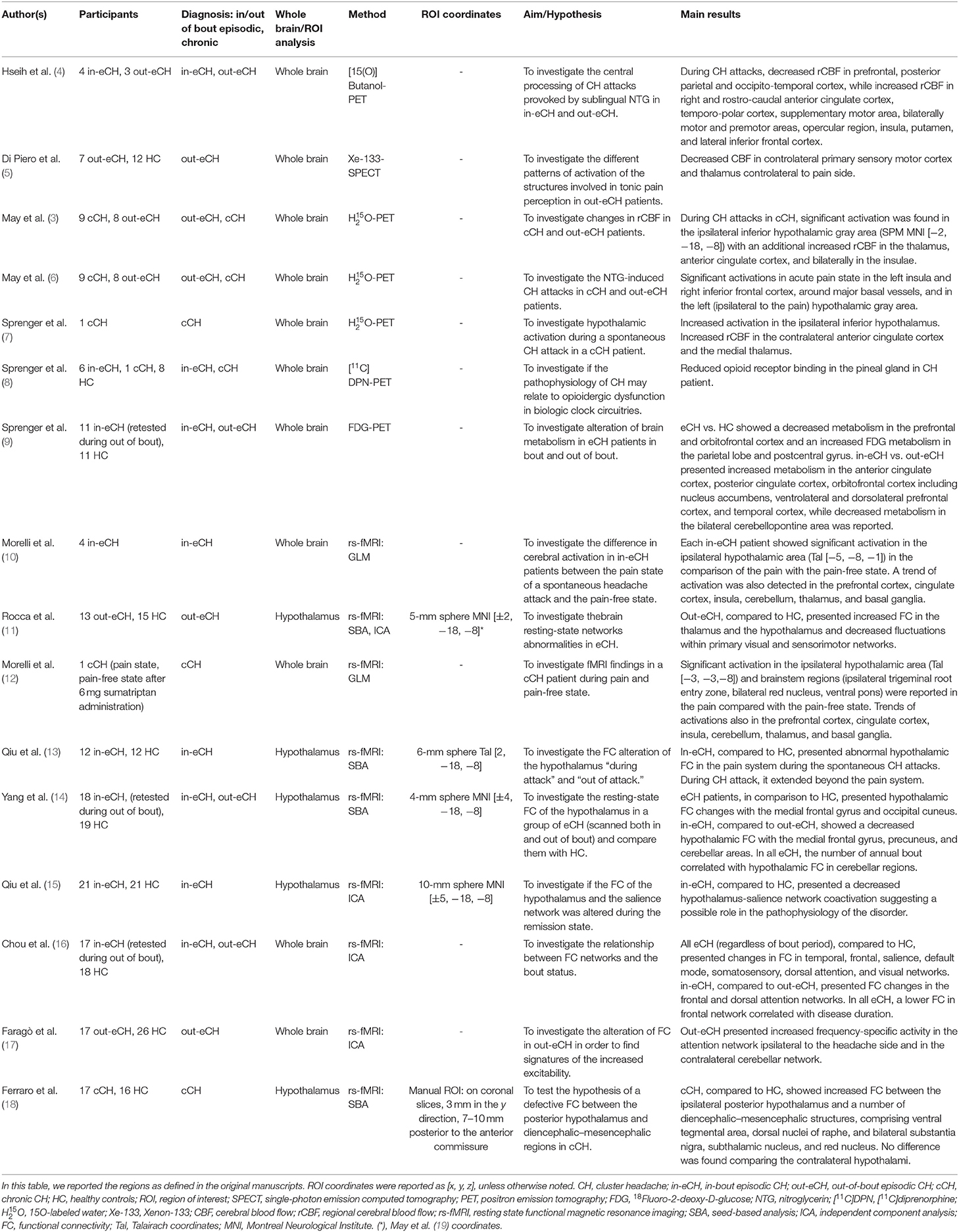
Cluster headache is an excruciating pain syndrome characterized by unilateral head pain attacks, lasting between 15 and 180 min, accompanied by marked ipsilateral cranial autonomic symptoms, such as lacrimation and conjunctival injection. Despite important insights provided by neuroimaging studies and deep brain stimulation findings, the pathophysiology of cluster headache and its pathways of chronicization are still elusive. In this mini-review, we will provide an overview of the functional and structural neuroimaging studies in episodic and chronic cluster headache conditions conducted to clarify the underlying pathophysiology.
Introduction
The distinctive clinical characteristic of cluster headache (CH), in particular the recurrence of excruciating unilateral attacks accompanied by marked ipsilateral cranial autonomic symptoms in periods separated by the spontaneous remission (1), suggested specific brain networks involved in seasonal adaptation (2) to have a role in the pathophysiology of this disorder. Neuroimaging can track these functional and anatomical changes (3), irrespective if they are the cause of the disease or represent a brain adaptation/maladaptation to the painful condition.
Brain networks involved in different phases of CH, namely, the in-bout (out of attacks and during attacks) and out-of-bout phases, will be presented. We will describe and discuss resting-state functional magnetic resonance imaging (rs-fMRI), positron emission tomography (PET) and single-photon emission computed tomography (SPECT), structural MRI, and diffusion tensor imaging (DTI) studies. Due to the recognized importance of the hypothalamus in CH pathophysiology, first, we will present studies investigating the hypothalamic/midbrain tegmentum. Then, we will describe MRI/PE/SPECT studies focusing on other cerebral areas and DTI investigations.
Search and Selection of Studies
We searched electronic databases PubMed and Google Scholar for articles published in English between January 1996 and December 2019 (see Tables 1, 2). The search terms were: (“cluster headache”) AND (“functional magnetic resonance imaging” OR “fMRI” OR “functional connectivity”); (“cluster headache”) AND (“positron emission tomography” OR “PET” OR “single photon emission computed tomography” OR “SPECT” OR “cerebral blood flow”); (“cluster headache”) AND (“gray matter” OR “voxel based morphometry” OR “VBM” OR “cortical thickness”); (“cluster headache”) AND (“white matter” OR “diffusion tensor imaging” OR “DTI” OR “tractography”). We did not consider reviews and conference abstracts.
Neuroimaging Studies Investigating the Hypothalamus/Midbrain Tegmentum
PET and rs-fMRI Studies
The circadian and circannual rhythmicity of attacks and neuroendocrinological findings pointed to hypothalamic involvement in the pathophysiology of the CH (2). In 1998, May et al., using PET, confirmed this hypothesis showing increased blood flow in the ipsilateral-to-the-pain posterior–inferior hypothalamus during nitroglycerin-induced attacks (3). This abnormal activity in the hypothalamus was considered to trigger the CH attacks because it was not observed in patients in out-of-bout phase who did not experience the attack under nitroglycerin (3). This seminal observation was confirmed by a subsequent PET study (6) and by a voxel-based morphometry study (19) showing hypothalamic volume abnormalities in CH patients (see the Structural MRI studies section). These results have opened the doors to hypothalamic deep brain stimulation (DBS) to successfully treat intractable chronic CH patients (36). Subsequently, the hypothesis of the hypothalamic involvement was supported by a single-case PET study (7) showing a metabolic activity during spontaneous attacks and by a ligand PET study showing opioidergic changes in the region identified by May et al. (3) in episodic CH patients during the in-bout phase (9).
More recently, the area reported as posterior–inferior hypothalamus by (3, 7, 19) has been suggested to localize in the midbrain tegmentum, possibly the ventral tegmental area (37, 38).
Despite this dispute, an fMRI study reported an evident activity in the ipsilateral-to-the pain hypothalamus during spontaneous attacks in a series of episodic CH patients (10). Importantly, this activity was not constrained by a region of interest approach centered on the results of the work of May et al. (3) but emerged using a whole-brain approach, reinforcing the hypothesis that the hypothalamus might play a role in CH. A paroxysmal activity during CH attacks in the red nuclei was also reported (12).
The observation that hypothalamic DBS in intractable chronic CH patients presents clinical effects after weeks of stimulation (39) and that it is not effective in terminating ongoing CH attacks (40) led us to hypothesize that the hypothalamus is a crucial region of a complex functional network that might disinhibit the hypothalamic–trigeminal pathway. In this new framework, the area reported by May et al. (3), hereafter “midbrain tegmentum” (37, 38), and the hypothalamus as such were investigated in a series of studies in CH patients using rs-fMRI to detect possible abnormalities in the functional connectivity (FC) of these regions.
The first study using rs-fMRI (11) showed that episodic CH patients have increased FC between the ipsilateral-to-the pain midbrain tegmentum and several regions known to be involved in pain processing, such as the anterior cingulate cortex, the bilateral secondary somatosensory cortex, the thalamus, and insula. Interestingly, abnormal FC was also observed between the midbrain tegmentum and striate and extra-striate visual regions, indicating the involvement of extra-pain-processing areas.
More recently, Qiu et al. (15) showed that episodic CH patients presented decreased bilateral midbrain tegmentum-salience network co-activation during the cluster period (in-bout phase). Previous works have shown that the dorsal anterior cingulate cortex and the fronto-insular cortex represent salient stimuli, such as hunger (41) and pain (42), and respond to emotional pain, such as during social rejection; in the seminal work of (43) these regions were appreciated with rs-fMRI as a robust functional network, namely, the salience network, also comprising subcortical structures such as thalamus, hypothalamus, and ventral tegmental area/substantia nigra. Seeley et al. (43) proposed that the relevant homeostatic stimuli, as sensory information, are integrated with visceral and autonomic functions, supporting a capital role of this network in pain processing. Based on this hypothesis (15), suggested that the bilateral midbrain tegmentum might play a role in the dysregulation of the salience network, in particular suggesting a defective pain control capable of generating CH attacks (15). The authors did not study CH patients out-of-bout phase: this does not allow one to make any inferences about the stability of this dysfunctional connectivity that might be dynamic (only during the in-bout phase) or a trait of CH patients. Notably, the same group showed that, in episodic CH patients investigated during attacks, the ipsilateral-to-the pain midbrain tegmentum presented abnormal dysfunctional connectivity with several cortical and subcortical areas (13). These areas comprised not only pain-processing regions but also extra-pain-processing areas. Notably, some of the identified areas (posterior cingulate cortex, inferior parietal lobule, ventral medial prefrontal cortex, and parahippocampal gyrus) belong to the default mode network.
Yang et al. (14) investigated for the first time the FC of the hypothalamus. They showed that, in accordance with its modulatory role, the hypothalamus is dynamically tuned, as it appears during the in-bout and the out-of-bout phases. During the in-bout phase, the ipsilateral-to-the pain hypothalamus presented, when compared to the out-of-bout phase, a decreased FC with the precuneus, a key region of the default mode network. This observation, with the results from the work of (13) during CH attacks, seems to confirm that dynamic alterations of the FC exist between the midbrain tegmentum/hypothalamus and regions belonging to the default mode network. Remarkably, the parasympathetic system is hypothesized to map onto the regions of the default mode network (44). Therefore, this dynamic dysregulation, present during the in-bout phase (13, 14), might indicate a parasympathetic dysfunction, possibly linked to the autonomic phenomena of CH. Further, Yang et al. (14) suggested that hypothalamic FC abnormalities in CH brain go beyond the pain matrix: when comparing patients in-bout vs. out-of-bout phase, the ipsilateral-to-the pain hypothalamus presented a decreased FC with the medial frontal gyrus and the cerebellar areas. These results seem to support dynamic alterations of hypothalamic FC in the disease. Moreover, the work of Yang et al. (14) showed that the annual bout frequency correlated significantly with the degree of FC between the hypothalamus and the cerebellar areas, suggesting that this might be an effect of the CH pathophysiology (14).
It is important to note that dysfunctional connectivity between the posterior hypothalamic regions and the midbrain areas was observed in chronic CH patients out of attacks (18). The authors showed an increased FC between the ipsilateral posterior hypothalamus and several diencephalic–mesencephalic structures as the ventral tegmental area, the dorsal nuclei of raphe, and the bilateral substantia nigra, the subthalamic nucleus, and the red nucleus. These results suggest a deranged FC of the hypothalamic–midbrain pathway in CH mainly involving structures that are part of (i.e., ventral tegmental area, substantia nigra) or modulate (dorsal nuclei of raphe, subthalamic nucleus) the midbrain dopaminergic systems. The latter may have a role in the chronicization of CH. Future studies should address the question if this abnormality is specific to chronic CH or it is already presented, with a lesser extent, in episodic CH.
As a whole, the above results show that the paroxysmal functional hyperactivity in the hypothalamus/midbrain tegmentum during induced and spontaneous CH attacks (3, 7, 12) is a dynamic process that appears to involve, in particular, FC changes between the midbrain tegmentum and regions belonging to the default mode network (13). This might suggest a paroxysmal activity of the parasympathethic system.
During in-bout and out-of-bout phases, the hypothalamus/midbrain tegmentum presents FC changes with different regions of the salience network (15) and the default mode network (14), suggesting, respectively, defective pain control and parasympathethic dysfunction. Moreover, it is essential to note that abnormal FC between the midbrain tegmentum and pain and extra-pain-processing regions is also observed in out-of-bout phase (11). This might indicate the presence of stable deranged connectivity between the hypothalamus/midbrain tegmentum and those areas. However, the observation of Yang et al. seems to indicate that further FC abnormalities between the hypothalamus and extra-pain-processing areas might superimpose on the already present FC alterations during the out-of-bout phase (14).
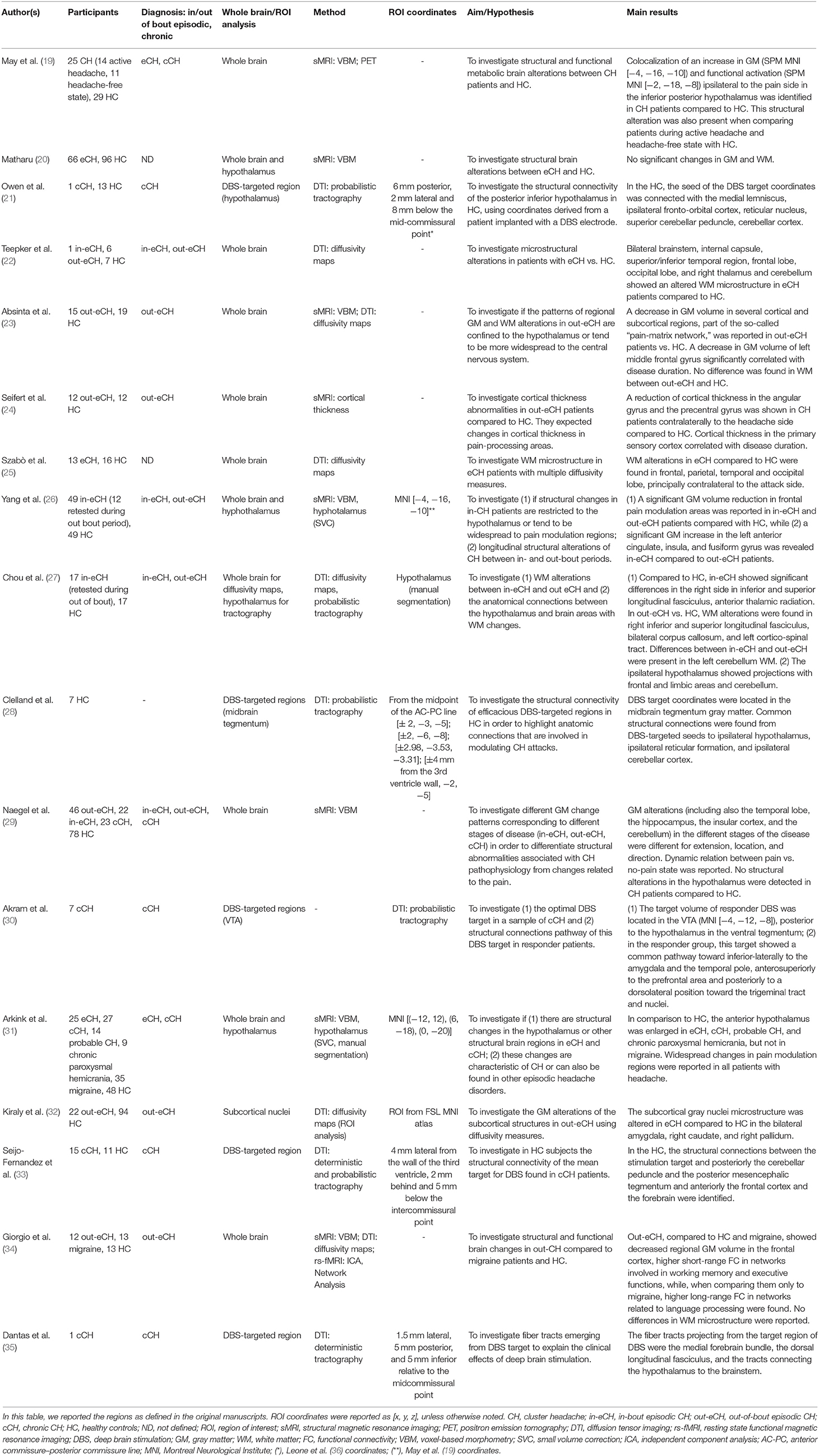
Structural MRI Studies
The possible involvement of the region defined as inferior–posterior hypothalamus in the work of May et al. (3) during CH attacks was supported by a VBM study of the same group showing morphological alterations of this region in a relatively large cohort of episodic (in-bout and out-of-bout phase) or chronic CH patients (19).
Matharu in his PhD thesis (20) investigated a large sample of patients and analyzed the morphological data with updated software using also small volume correction in the region identified in the work of (3). Importantly, Matharu did not find volumetric abnormalities in this region and concluded that the previous VBM results (19) were false positive due to methodological limitations, possibly due to the susceptibility of the employed technique (VBM) to several confounders (45, 46).
In agreement, the VBM results from (3) were not replicated in subsequent morphological studies (23, 26, 47).
Despite these inconsistencies, a recent investigation showed an increased volume of the bilateral anterior hypothalamus of individuals with episodic (out-of-bout) and chronic CH but not in individuals with migraine (31). This study directly pointed to alterations of the suprachiasmatic nucleus, the site of the endogenous biological clock, and the paraventricular nucleus, both part of the anterior hypothalamus. Their abnormalities could explain the typical circadian rhythms of the recurrent attacks of CH, as well as some autonomic phenomena of the disease. These results confirm hypothalamic morphological alteration in episodic (in both in-bout and out-of-bout) and in chronic CH patients. It is important to note that possible dynamic morphological changes of the hypothalamus might have been underestimated due to the difficulties in investigating this relatively small structure with MRI.
Neuroimaging Studies Investigating Pain-Processing Areas
PET/SPECT and rs-fMRI Studies
The excruciating nature of pain in CH led us to hypothesize a possible deficient top-down modulation of antinociceptive circuits (9). In line with this hypothesis, several works have shown functional alterations of the pain-processing areas. The first PET study on CH dated back to 1996: Hsieh et al. (4) found increased regional blood flow in the main cortical regions involved in pain processing (anterior cingulate cortex, insula cortex, and operculum) during induced CH attacks.
Decreased cerebral blood flow activity in controlateral primary sensory-motor cortex and thalamus was also described during the cold pressor test (5).
Metabolic alterations of several brain areas, comprising regions involved in pain processing, were also shown in episodic CH: during the in-bout phase, compared to the out-of-bout phase, increased metabolism was observed in the anterior and posterior cingulate cortex, prefrontal cortex, insula, thalamus, and temporal cortex, while a decreased metabolism in the cerebellopontine areas (9).
Alterations of the pain-processing pathways were also reported in rs-fMRI studies. Rocca et al. (11) reported reduced FC in the sensorimotor network in a group of episodic CH patients out-of-bout. Affected brain areas comprised the primary and secondary somatosensory area, the supplementary motor area, and the anterior cingulate cortex (11). These regions play a role in sensory discrimination and affective-cognitive processes evoked by painful conditions. Notably, the anterior cingulate cortex is part of the salience network (43, 48): alteration of the FC in this region reinforces the hypothesis of a strong involvement of this circuit in CH pathophysiology (15). These abnormalities were observed in out-of-bout phase, indicating stable brain alterations. Importantly, disease duration was negatively correlated with the strength of FC in the primary sensory-motor cortex, indicating that prolonged and severe painful condition may have induced those alterations.
FC alterations in the sensorimotor network were confirmed in another study in episodic CH, which reported abnormalities also in regions of the salience network (16). This study found no differences between the in-bout and out-of-bout phase, suggesting that the functional alterations in the sensorimotor and salience network could be a trait marker of CH.
As a whole, a dynamic dysregulation or adaptation in networks involved in pain processing and modulation of the parasympathetic activity, as suggested by functional abnormalities in regions of the default mode network (i.e., anterior cingulate cortex), seems to characterize CH patients. These functional alterations may represent a derangement of descending pain processing and autonomic pathways.
Structural MRI Studies
In patients with episodic CH during out-of-bout phase, volumetric alterations of the regions involved in pain processing such as thalamus, caudate nucleus, posterior cingulate cortex, prefrontal cortex, sensorimotor cortex, parietal cortex, insula, and middle temporal cortex have been reported (23, 34).
Notably, the chronic CH condition, compared to episodic CH, seems to be characterized by decreased gray matter in different regions of the pain matrix, i.e., anterior insula, cingulate cortex, secondary somatosensory cortex, hippocampus, left temporal lobe, and an increased gray matter in primary somatosensory cortex and supplementary motor cortex (47).
Neuroimaging Studies Investigating other Cortical and Subcortical Areas
PET and rs-fMRI Studies
In a large group of episodic CH patients, functional alterations in the default mode network have been observed in both in-bout and out-of-bout phases of CH (16). This suggests that the default mode network is dysfunctional in episodic CH patients, possibly as a trait marker of CH.
Rocca et al. (11) and Chou et al. (16) consistently reported alterations in the FC of the visual network in CH patients: these abnormalities might be linked to photophobia and retro-orbital pain, frequently observed in CH (49). These abnormalities were observed in in-bout (16) and out-of-bout phase (11, 16) and were negatively correlated to disease duration, suggesting that they might be the consequence of a prolonged and severe pain condition.
In agreement with widespread alterations, dysfunctional connectivity within the attention network (in the ipsilateral superior frontal gyrus and medial frontal cortex) and the cerebellar network was observed (17) in episodic CH patients in the out-of-bout phase. Abnormal FC in temporal and visual networks irrespective of the illness phase was also present.
Structural MRI Studies
In VBM studies, volumetric gray matter alterations of the visual cortex (cuneus and occipital fusiform gyrus) have been observed (23, 34).
Volumetric alterations were observed by Naegel et al. in the temporal lobe, the hippocampus, the insular cortex, and the cerebellum in CH; (47) the location and direction of gray matter alterations varied according to the state of disease as well as to pain state (pain vs. no-pain). These dynamic changes may provide an explanation of the non-homogeneous results in previous VBM studies in pain.
DTI
DTI is an advanced MRI technique measuring water molecule diffusion: it allows one to study the integrity and architecture of the tissues through several parameters, such as fractional anisotropy or mean, axial, and radial diffusivity (50). These quantitative indices are sensible to microstructural brain tissue properties such as axon diameter, density and orientations, axon myelination, and membrane permeability (51). Studying the principal diffusion direction, it is also possible to reconstruct through tractography continuous white matter pathways of significant clusters of parallel axons (51).
In the last decade, DTI has been applied in a CH population using both quantitative indices, employing the abovementioned parameters, or tractography.
The studies using the quantitative approach showed different results in episodic CH patients. While some groups did not find any significant differences in diffusion parameters (23, 34) in the out-of-bout phase, others reported patterns of stable alterations (i.e., in both in-bout and out-of-bout phase) in the white matter, mainly localized in frontal and limbic lobes (27). Importantly, these regions were shown to present anatomical connections with the hypothalamus when using probabilistic tractography (27).
Gray matter microstructure abnormalities were also reported in several subcortical structures, in particular in the right amygdala, caudate, and globus pallidum (32).
Interestingly, an increased axial diffusivity in the left cerebellar white matter when comparing patients in the in-bout and out-of-bout phase was also reported (27).
Other quantitative studies, focused on relatively small cohorts of episodic CH patients, highlighted very widespread diffusion microstructural abnormalities in different white matter regions, encompassing mainly temporal, frontal, occipital, and cerebellar regions (22, 25).
Tractography studies were mainly focused on the anatomical connections of successful DBS targets in chronic CH patients in the effort to reveal the anatomical network responsible for the amelioration of the disorder. These studies confirmed the relevance, in CH pathophysiology, of the target-brainstem projections. Notably, different DBS targets were considered: inferior–posterior hypothalamus (21), midbrain tegmentum (28, 33), and ventral tegmental area (35, 52). In chronic CH patients, the structural connectivity of these regions also showed extended and relevant connections to pain-related areas, supporting the hypothesis of large pain matrix modulating CH attacks.
In particular, important anatomical connections were highlighted between the hypothalamus and the midbrain tegmentum, including the medial lemniscus, the dorsal longitudinal and mamillo-tegmental fasciculi, the fronto-orbital cortex, the reticular shape, and the cerebellar cortex (21, 28, 33, 35).
In the case of DBS target stimulation in the VTA, further connections with the temporal cortex and brainstem areas in the proximity of the parabrachial nuclei, nucleus of the solitary tract, periaqueductal gray, and ending in the region of the trigeminal nucleus and tract and the superior salivatory nucleus were described (52). Despite these very interesting results, the small sample combined with heterogeneous inclusion criteria, the very different MRI sequences settings, and the different diffusion indices selection complicate the direct comparison between these works and may cause the observed discrepancy in particular in the results of quantitative diffusion analysis.
Conclusion
Notwithstanding the notable number of neuroimaging studies in CH, we are still far from fully understanding the brain mechanisms of this disorder. However, some, although tentative and not conclusive considerations, can be done. First of all, CH patients seem to present widespread FC and anatomical abnormalities across multiple networks and multiple cortical and subcortical areas, not only confined in regions involved in pain processing. This suggests that the CH brain is functionally and morphologically reorganized in a maladaptive or adaptive way. In particular, functional and anatomical abnormalities of cortical and subcortical areas involved in pain processing are consistently reported. In this perspective, the salience network seems to play a prominent role in CH pathophysiology: the here reviewed studies suggest that regions of this network presents a relatively stable functional alteration during the in-bout and the out-of-bout conditions (16). One can speculate that dysfunctional connectivity in the salience network might be the neural “tract” of the disease. Notably, alteration of the salience network suggests that CH patients present a dysfunctional ability to elaborate salient stimuli. In this regard, abnormalities of this network were observed in other chronic pain conditions, such as diabetic neuropathy (53), headache, (54, 55), and irritable bowel syndrome (56). This might indicate that the abnormalities in the salience network might predispose the chronification of CH. Importantly, disruption of the salience network has been reported in several neuropsychiatric conditions such as autism (57), schizophrenia (58), and addiction (59). Therefore, the observed alterations seem not to be specific to CH. Future studies should assess the role of this network in CH pathophysiology. Moreover, regions belonging to the default mode network seem to be abnormal in CH, irrespective of the phase (16). The parasympathetic system maps onto the default mode network (44), and this raises the possibility that default mode network alterations are consequences of the CH attacks. The default mode network plays a role in integrating sensory-visceromotor processing, self-referential activity, and recalling of previous experience (60). This might indicate that CH patients suffer a disturbance in the social–emotional spheres.
Second, supporting the above results, studies investigating the hypothalamic/midbrain tegmentum FC suggest a modulatory role of these structures within the salience network and the regions of the default mode network (15, 16).
Third, the abnormal FC between the hypothalamus and the midbrain dopaminergic system (in particular the ventral tegmental area) in chronic CH patients (18) suggests that the possible pathways of chronicization pass through the mesocorticolimbic system also in CH. It is now accepted that the midbrain dopaminergic system is also stimulated by aversive stimuli such as pain (61), and the nucleus accumbens, a key component of the mesocorticolimbic system receiving direct projections from the ventral tegmental area, seems to be involved in the chronicization of pain in humans (62–64). This possibility in CH is also fostered by the recent proposal of the ventral tegmental area as the main target of DBS (65) and by the observation that long-term DBS can revert chronic to episodic CH (66). It is important to note, however, that inhibition and facilitation of pain mechanisms were also suggested to be at the basis of the chronicization of the disease, as indicated by gray matter reorganization accordingly with the different pain states, possibly supported by highly dynamic changes in nociceptive and anti-nociceptive networks (29). Also, DBS in CH patients induces blood flow changes in the anterior cingulate, insula, and frontal lobe involved in pain chronicization (67).
Future studies should assess the validity of the above hypotheses clarifying the role of the hypothalamus/midbrain tegmentum in CH and chronic CH pathophysiology (68). Moreover, it would be of great interest to determine if the observed abnormalities in functional and anatomical networks are specific to CH or represent an unspecific response to pain.
Author Contributions
SF, AN, GD, CP, LC, LG, and AP went through on literature and collected the articles. SF, AN, and ML wrote the manuscript. ML supervised all the steps of this minireview. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Italian Ministry of Health (research grant RF-2016-02364909).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Headache Classification Comittee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia Int J headache. (2018). 38:1–211. doi: 10.1177/0333102417738202
2. Leone M, Bussone G. A review of hormonal findings in cluster headache. Evidence for hypothalamic involvement. Cephalalgia. (1993) 13:309–17.
3. May A, Bahra A, Büchel C, Frackowiak RS, Goadsby PJ. Hypothalamic activation in cluster headache attacks. Lancet. (1998) 352:275–8. doi: 10.1016/S0140-6736(98)02470-2
4. Hsieh JC, Hannerz J, Ingvar M. Right-lateralised central processing for pain of nictoglycerin-inudced clustre headache. Pain. (1996) 67:59–68.
5. Di Piero V, Fiacco F, Tombari D, Pantano P. Tonic pain: a SPET study in normal subjects and cluster headache patients. Pain. (1997) 70:185–91. doi: 10.1016/S0304-3959(96)03318-0
6. May A, Bahra A, Büchel C, Frackowiak RS, Goadsby PJ. PET and MRA findings in cluster headache and MRA in experimental pain. Neurology. (2000) 55:1328–35. doi: 10.1212/wnl.55.9.1328
7. Sprenger T, Boecker H, Tolle TR, Bussone G, May A, Leone M. Specific hypothalamic activation during a spontaneous cluster headache attack. Neurology. (2004) 62:516–7. doi: 10.1212/wnl.62.3.516
8. Sprenger T, Willoch F, Miederer M, Schindler F, Valet M, Berthele A, et al. Opioidergic changes in the pineal gland and hypothalamus in cluster headache: a ligand PET study. Neurology. (2006) 66:1108–10. doi: 10.1212/01.wnl.0000204225.15947.f8
9. Sprenger T, Ruether K V, Boecker H, et al. Altered metabolism in frontal brain circuits in cluster headache. Cephalalgia Int J Headache. (2007) 27:1033–42. doi: 10.1111/j.1468-2982.2007.01386.x
10. Morelli N, Rota E, Colombi D, Spallazzi M, Bodini FC, Marchesi G, et al. Functional magnetic resonance imaging in episodic cluster headache. J Headache Pain. (2009) 10:11–4. doi: 10.1007/s10194-008-0085-z
11. Rocca MA, Valsasina P, Absinta M, Colombo B, Barcella V, Falini A, et al. Central nervous system dysregulation extends beyond the pain-matrix network in cluster headache. Cephalalgia. (2010) 30:1383–91. doi: 10.1177/0333102410365164
12. Morelli N, Rota E, Gori S, Guidetti D, Michieletti E, De Simone R, et al. Brainstem activation in cluster headache: an adaptive behavioural response? Cephalalgia. (2013) 33:416–20. doi: 10.1177/0333102412474505
13. Qiu E, Wang Y, Ma L, Tian L, Liu R, Dong Z, et al. Abnormal brain functional connectivity of the hypothalamus in cluster headaches. Hou B, ed. PLoS ONE. (2013) 8:e57896. doi: 10.1371/journal.pone.0057896
14. Yang FC, Chou KH, Fuh JL, Lee PL, Lirng JF, Lin YY, et al. Altered hypothalamic functional connectivity in cluster headache: a longitudinal resting-state functional MRI study. J Neurol Neurosurg Psychiatry. (2015) 86:437–45. doi: 10.1136/jnnp-2014-308122
15. Qiu E, Tian L, Wang Y, Ma L, Yu S. Abnormal coactivation of the hypothalamus and salience network in patients with cluster headache. Neurology. (2015) 84:1402–8. doi: 10.1212/WNL.0000000000001442
16. Chou KH, Yang FC, Fuh JL, Kuo CY, Wang YH, Lirng JF, et al. Bout-associated intrinsic functional network changes in cluster headache: a longitudinal resting-state functional MRI study. Cephalalgia. (2017) 37:1152–63. doi: 10.1177/0333102416668657
17. Faragó P, Szabó N, Tóth E, Tuka B, Király A, Csete G, et al. Ipsilateral alteration of resting state activity suggests that cortical dysfunction contributes to the pathogenesis of cluster headache. Brain Topogr. (2017) 30:281–9. doi: 10.1007/s10548-016-0535-x
18. Ferraro S, Nigri A, Bruzzone MG, Brivio L, Proietti Cecchini A, Verri M, et al. Defective functional connectivity between posterior hypothalamus and regions of the diencephalic-mesencephalic junction in chronic cluster headache. Cephalalgia. (2018) 38:1910–8. doi: 10.1177/0333102418761048
19. May A, Ashburner J, Büchel C, McGonigle DJ, Friston KJ, Frackowiak RS, et al. Correlation between structural and functional changes in brain in an idiopathic headache syndrome. Nat Med. (1999) 5:836–8. doi: 10.1038/10561
20. Matharu MS. Functional and structural neuroimaging in primary headache syndromes. (PhD Thesis). London: Institute of Neurology, University of London (2006).
21. Owen SL, Green AL, Davies P, Stein JF, Aziz TZ, Behrens T, et al. Connectivity of an effective hypothalamic surgical target for cluster headache. J Clin Neurosci. (2007) 14:955–60. doi: 10.1016/j.jocn.2006.07.012
22. Teepker M, Menzler K, Belke M, Heverhagen JT, Voelker M, Mylius V, et al. Diffusion tensor imaging in episodic cluster headache. Headache. (2011) 52:274–82. doi: 10.1111/j.1526-4610.2011.02000.x
23. Absinta M, Rocca MA, Colombo B, Falini A, Comi G, Filippi M. Selective decreased grey matter volume of the pain-matrix network in cluster headache. Cephalalgia. (2012) 32:109–15. doi: 10.1177/0333102411431334
24. Seifert CL, Magon S, Staehle K, Zimmer C, Foerschler A, Radue EW, et al. A case-control study on cortical thickness in episodic cluster headache. Headache. (2012) 52:1362–8. doi: 10.1111/j.1526-4610.2012.02217.x
25. Szabó N, Kincses ZT, Párdutz Á, Tóth E, Szok D, Csete G, et al. White matter disintegration in cluster headache. J Headache Pain. (2013) 14:1–6. doi: 10.1186/1129-2377-14-64
26. Yang FC, Chou KH, Fuh JL, Huang CC, Lirng JF, Lin YY, et al. Altered gray matter volume in the frontal pain modulation network in patients with cluster headache. Pain. (2013) 154:801–7. doi: 10.1016/j.pain.2013.02.005
27. Chou KH, Yang FC, Fuh JL, Huang CC, Lirng JF, Lin YY, et al. Altered white matter microstructural connectivity in cluster headaches: a longitudinal diffusion tensor imaging study. Cephalalgia. (2014) 34:1040–52. doi: 10.1177/0333102414527649
28. Clelland CD, Zheng Z, Kim W, Bari A, Pouratian N. Common cerebral networks associated with distinct deep brain stimulation targets for cluster headache. Cephalalgia. (2014) 34:224–30. doi: 10.3851/IMP2701
29. Naegel S, Holle D, Desmarattes N, Theysohn N, Diener HC, Katsarava Z, et al. Cortical plasticity in episodic and chronic cluster headache. NeuroImage Clin. (2014) 6:415–23. doi: 10.1016/j.nicl.2014.10.003
30. Akram H, Miller S, Lagrata S, Hariz M, Ashburner J, Behrens T, et al. Optimal deep brain stimulation site and target connectivity for chronic cluster headache. Neurology. (2017) 89:2083–91. doi: 10.1212/WNL.0000000000005851
31. Arkink EB, Schmitz N, Schoonman GG, van Vliet JA, Haan J, van Buchem MA, et al. The anterior hypothalamus in cluster headache. Cephalalgia. (2016) 0:1–12. doi: 10.1177/0333102416660550
32. Király A, Szabó N, Párdutz Á, Tóth E, Tajti J, Csete G, et al. Macro- and microstructural alterations of the subcortical structures in episodic cluster headache. Cephalalgia. (2018) 38:662–73. doi: 10.1177/0333102417703762
33. Seijo-Fernandez F, Saiz A, Santamarta E, Nader L, Alvarez-Vega MA, Lozano B, et al. Long-term results of deep brain stimulation of the mamillotegmental fasciculus in chronic cluster headache. Stereotact Funct Neurosurg. (2018) 96:215–22. doi: 10.1159/000489937
34. Giorgio A, Lupi C, Zhang J, De Cesaris F, Alessandri M, Mortilla M, et al. Changes in grey matter volume and functional connectivity in cluster headache versus migraine. Brain Imaging Behav. (2019) 14:496–504. doi: 10.1007/s11682-019-00046-2
35. Dantas SAF, Alho EJL, da Silva JJ, Mendes Neto NN, Fonoff ET, Hamani C. Deep brain stimulation modulates hypothalamic-brainstem fibers in cluster headache: case report. J Neurosurgey. (2019) 11:1–4. doi: 10.3171/2018.11.JNS181412
36. Leone M, Franzini A, Bussone G. Stereotactic stimulation of posterior hypothalamic gray matter in a patient with intractable cluster headache. N Engl J Med. (2001) 345:1428–9. doi: 10.1056/NEJM200111083451915
37. Sánchez del Rio M, Alvarez Linera J. Functional neuroimaging of headaches. Lancet Neurol. (2004) 3:645–51. doi: 10.1016/S1474-4422(04)00904-4
38. Matharu MS, Zrinzo L. Deep brain stimulation in cluster headache: hypothalamus or midbrain tegmentum? Curr Pain Headache Rep. (2010) 14:151–9. doi: 10.1007/s11916-010-0099-5
39. Leone M, Franzini A, Broggi G, Bussone G. Hypothalamic stimulation for intractable cluster headache : Long-term experience. Neurology. (2006) 67:4–7. doi: 10.1212/01.wnl.0000223319.56699.8a
40. Leone M, Franzini A, Broggi G, Mea E, Cecchini AP, Bussone G. Acute hypothalamic stimulation and ongoing cluster. Neurology. (2006) 67:1844–5. doi: 10.1212/01.wnl.0000247273.93084.49
41. Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. (2002) 3:655–66. doi: 10.1038/nrn894
42. Peyron R, Laurent B, García-Larrea L. Functional imaging of brain responses to pain. a review and meta-analysis 2000. Neurophysiol Clin Neurophysiol. (2000) 30:263–88. doi: 10.1016/S0987-7053(00)00227-6
43. Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. (2007) 27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007
44. Beissner F, Meissner K, Bär KJ, Napadow V. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J Neurosci. (2013) 33:10503–11. doi: 10.1523/JNEUROSCI.1103-13.2013
45. Henley SM, Ridgway GR, Scahill RI, Klöppel S, Tabrizi SJ, Fox NC, et al. Pitfalls in the use of voxel-based morphometry as a biomarker:examples from huntington. AJNR Am J Neuroradiol. (2010) 31:711–9. doi: 10.3174/ajnr.A1939
46. Ashburner J. Computational anatomy with the SPM software. Magn Reson Imaging. (2009) 27:1163–74. doi: 10.1016/j.mri.2009.01.006
47. Naegel S, Holle D, Obermann M. Structural imaging in cluster headache. Curr Pain Headache Rep. (2014) 18:415. doi: 10.1007/s11916-014-0415-6
48. Borsook D, Edwards R, Elman I, Becerra L, Levine J. Pain and Analgesia the value of the salience circuit. Prog Neurobiol. (2013) 104:93–105. doi: 10.1016/j.pneurobio.2013.02.003.Pain
49. Bahra A, May A, Goadsby PJ. Cluster headache: a prospective clinical study with diagnostic implications. Neurology. (2002) 58:354–61. doi: 10.1212/wnl.58.3.354
50. Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. (1996) 201:637–48. doi: 10.1148/radiology.201.3.8939209
51. Jones DK, Knösche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. Neuroimage. (2013) 73:239–54. doi: 10.1016/j.neuroimage.2012.06.081
52. Akram H, Miller S, Lagrata S, Hyam J, Jahanshahi M, Hariz M, et al. Ventral tegmental area deep brain stimulation for refractory chronic cluster headache. Neurology. (2016) 86:1676–82. doi: 10.1212/WNL.0000000000002632
53. Cauda F, D' Agata F, Sacco K, Duca S, Cocito D, et al. Altered resting state attentional networks in diabetic neuropathic pain. J Neurol Neurosurg Psychiatry. (2010) 81:806–11. doi: 10.1136/jnnp.2009.188631
54. Eck J, Richter M, Straube T, Miltner WH, Weiss T. Affective brain regions are activated during the processing of pain-related words in migraine patients. Pain. (2011) 152:1104–13. doi: 10.1016/j.pain.2011.01.026
55. Maleki N, Becerra L, Borsook D. Migraine: maladaptive brain responses to stress. Headache J Head Face Pain. (2012) 52:102–6. doi: 10.1111/j.1526-4610.2012.02241.x
56. Elsenbruch S, Rosenberger C, Bingel U, Forsting M, Schedlowski M, Gizewski ER. Patients with irritable bowel syndrome have altered emotional modulation of neural responses to visceral stimuli. Gastroenterology. (2010) 139:1310–9. doi: 10.1053/j.gastro.2010.06.054
57. Uddin LQ. The self in autism: an emerging view from neuroimaging. Neurocase. (2011) 17:201–8. doi: 10.1080/13554794.2010.509320
58. Palaniyappan L, Simmonite M, White TP, Liddle EB, Liddle PF. Article neural primacy of the salience processing system in schizophrenia. Neuron. (2013) 79:814–28. doi: 10.1016/j.neuron.2013.06.027
59. Geng X, Hu Y, Gu H, Salmeron BJ, Adinoff B, Stein EA, et al. Salience and default mode network dysregulation in chronic cocaine users predict treatment outcome. Brain. (2017) 140:1513–24. doi: 10.1093/brain/awx036
60. Raichle ME. The brain's default mode network. Annu Rev Neurosci. (2015) 38:433–47. doi: 10.1146/annurev-neuro-071013-014030
61. Taylor AM, Becker S, Schweinhardt P, Cahill C. Mesolimbic dopamine signaling in acute and chronic pain. Pain. (2016) 157:1. doi: 10.1097/j.pain.0000000000000494
62. Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, et al. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci. (2013) 15:1117–9. doi: 10.1038/nn.3153
63. Chang PC, Pollema-Mays SL, Centeno MV, Procissi D, Contini M, Baria AT, et al. Role of nucleus accumbens in neuropathic pain: linked multi-scale evidence in the rat transitioning to neuropathic pain. Pain. (2014) 155:1128–39. doi: 10.1016/j.pain.2014.02.019
64. Makary MM, Polosecki P, Cecchi GA, DeAraujo IE, Barron DS, Constable TR, et al. Loss of nucleus accumbens low-frequency fluctuations is a signature of chronic pain. Proc Natl Acad Sci USA. (2020) 117:10015–23. doi: 10.1073/pnas.1918682117
65. Miller S, Akram H, Lagrata S, Hariz M, Zrinzo L, Matharu M. Ventral tegmental area deep brain stimulation in refractory short-lasting unilateral neuralgiform headache attacks. Brain. (2016) 139:2631–40. doi: 10.1093/aww233
66. Leone M, Franzini A, Proietti Cecchini A, Bussone G. Success, failure, and putative mechanisms in hypothalamic stimulation for drug-resistant chronic cluster headache. Pain. (2013) 154:89–94. doi: 10.1016/j.pain.2012.09.011
67. May A, Leone M, Boecker H, Sprenger T, Juergens T, Bussone G, et al. Hypothalamic deep brain stimulation in positron emission tomography. J Neurosci. (2006) 26:3589–93. doi: 10.1523/jneurosci.4609-05.2006
Keywords: cluster headache, resting-state fMRI, structural MRI, PET, DTI, hypothalamus
Citation: Ferraro S, Nigri A, Demichelis G, Pinardi C, Chiapparini L, Giani L, Proietti Cecchini A and Leone M (2020) Understanding Cluster Headache Using Magnetic Resonance Imaging. Front. Neurol. 11:535. doi: 10.3389/fneur.2020.00535
Received: 21 November 2019; Accepted: 14 May 2020;
Published: 30 June 2020.
Edited by:
Roberta Messina, Vita-Salute San Raffaele University, ItalyReviewed by:
Lars Neeb, Charité—Universitätsmedizin Berlin, GermanyAynur Özge, Mersin University, Turkey
Copyright © 2020 Ferraro, Nigri, Demichelis, Pinardi, Chiapparini, Giani, Proietti Cecchini and Leone. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna Nigri, YW5uYS5uaWdyaUBpc3RpdHV0by1iZXN0YS5pdA==
 Stefania Ferraro
Stefania Ferraro Anna Nigri
Anna Nigri Greta Demichelis
Greta Demichelis Chiara Pinardi1
Chiara Pinardi1 Luca Giani
Luca Giani Alberto Proietti Cecchini
Alberto Proietti Cecchini