- 1Nazarbayev University School of Medicine (NUSOM), Nur-Sultan, Kazakhstan
- 2National Research Oncology Center, Nur-Sultan, Kazakhstan
- 3Astana Medical University, Nur-Sultan, Kazakhstan
- 4Division of Respirology, Department of Medicine, University Health Network, Toronto, ON, Canada
- 5Interdepartmental Division of Critical Care Medicine, University of Toronto, Toronto, ON, Canada
- 6Toronto General Hospital Research Institute, Toronto, ON, Canada
Background: Risk factors for medium to long-term mortality after stroke are well-established but predictors of in-hospital stroke mortality are less clearly characterized. Kazakhstan has the highest age-standardized mortality rate from ischemic stroke in the world.
Methods: We performed a retrospective analysis of patients with stroke who were admitted over a 3.5-years period to the neurocritical care unit of a tertiary care hospital in Nur-Sultan, Kazakhstan.
Results: In total, 148 critically ill patients were included in the analysis (84 ischemic stroke, 64 hemorrhagic stroke). The mean age was 63 years, 45% were male and the mean Glasgow Coma Score (±SD) at baseline was 10.3 (±3.4). The in-hospital mortality rate was similar in patients with ischemic (36%) and hemorrhagic (39%) stroke (HR 0.88, 95%CI 0.48–1.60). Median survival was 38 days (range: 1–89 days) in patients with ischemic stroke and 39 days (range: 1–63 days) in patients with hemorrhagic stroke. Univariable analysis found that patients who had a lower Glasgow Coma Scale, were in coma and who had cerebral edema were more likely to die in-hospital (P = 0.04, 0.02, <0.01, respectively).
Conclusions: Our analysis showed that mortality risk in critically ill patients with hemorrhagic stroke was closer to mortality risk in patients with ischemic stroke than has been reported in other analyses. Hypertension, chronic heart failure, ischemic heart disease and atrial fibrillation were the most frequent comorbidities in patients who developed severe (life-threatening) stroke. Coma and cerebral edema on admission appear to be associated with poor outcome. This is the first publication of in-hospital stroke mortality from a Central Asian population and could form the basis for future research including development of risk scores and identifying modifiable risk factors.
Background
Stroke remains the second most common cause of death and the predominant cause of disability worldwide despite recent advances in its management (1, 2). Stroke-related complications most frequently occur during the 1st week after stroke and are associated with higher mortality risk during hospital stay and increased length of hospital stay (3–5). Admissions to intensive care units for stroke are increasing and this trend can be expected to continue in aging populations (6). Intensive care units are extremely important for management of critically ill stroke patients and admission criteria usually include single or multiple organ dysfunction/failure, low Glasgow Coma Scale score, necessity for advanced monitoring and postoperative observation.
The prognosis is generally considered to be poor for stroke patients admitted to critical care units. Short term in-hospital mortality rates as high as 50% (7–9). Currently available therapeutic options are limited and even if patients survive the acute phase and are discharged from intensive care, persistent paralysis and cognitive dysfunction are common and require long-term rehabilitation. Therefore, the identification of risk factors for poor outcomes in critically ill stroke patients could improve patient management and allow for more precise prognosis estimation. Factors that have previously been identified as predictors for in-hospital mortality in other studies include age, localization of ischemia, National Institute of Health Stroke Scale (NIHSS) score, Acute Physiology and Chronic Health Evaluation score (APACHEII), Glasgow Coma Scale (GCS) score, and the Simplified Acute Physiology Score (SAPS) (9). The main goal of neurocritical care is directed toward prevention, early recognition and management of secondary brain damage that includes cerebral edema, intracranial hypertension, impaired cerebral circulation, hypotension, hypertension, hypoxia, hypercapnia, and hyperthermia. Therefore, the identification of modifiable risk factors in critically ill stroke patients could improve patient outcomes.
Until recently, very little has been known about the prevalence of modifiable and non-modifiable risk factors for cerebrovascular disease in Central Asia. Kazakhstan has the highest age-standardized mortality rate from ischemic stroke in the world (149–174 deaths per 100,000 person years) (10). Recent cross-sectional surveys have indicated that the prevalence of dyslipidemia, diabetes and hypertension is high in this region (11–13). Therefore, in this study, we aimed to identify prognostic factors for in-hospital mortality in critically ill Central Asian patients with acute ischemic or hemorrhagic stroke.
Methods
We performed a retrospective analysis of routinely collected data from patients diagnosed with ischemic or hemorrhagic stroke at the University Medical Center in Nur-Sultan, Kazakhstan. We included all adults (age >18 years) who were admitted to the neurocritical care unit from October 2009 to March 2013.
All patients presented to our hospital with stroke underwent a standardized workup by a neurologist and stroke diagnosis was confirmed by computed tomography or magnetic resonance tomography. The management of acute stroke was conducted in accordance with our local stroke management protocol based on the American Heart Association/American Stroke Council recommendations (14, 15).
In our center, criteria used to determine admission to the neurocritical care unit included impaired consciousness (GCS ≤ 12); need for mechanical ventilation (apnoea, ataxic or cluster breathing), respiratory failure (tachypnoea, dyspnoea at rest, bradypnoea, hypopnoea, apnoea, ataxic, or cluster breathing, use of accessory muscles), inability to protect airway, clinically significant brain edema, evidence of raised intracranial pressure, monitoring required (level of consciousness, respiratory function, intracranial pressure, and continuous electroencephalography) and post-neurosurgical intervention.
This study was approved by two local Ethics Committees (Nazarbayev University and the University Medical Center). Patient consent was not requested because the study was retrospective and data were extracted excluding personal identifiers.
Statistical Analysis
Descriptive analyses on normally distributed continuous variables were performed by calculating means and standard deviations while medians and interquartile ranges for non-normally distributed numeric variables. Frequencies and percentages were summarized for categorical variables. Bivariable analysis by stroke type was done using the chi-squared test or Fisher exact test for categorical variables, and independent t-test with equal variances for continuous variables. To examine the survival probabilities by stroke type and by presence of coma signs on admission, Kaplan Meier estimators with log rank test were used. Since one of the main predictors—history of hypertension—was highly associated with the outcome variable (quasi-separation) this caused numerical issues when calculating the hazard ratio in Cox proportional hazards regression analysis. Therefore, we used survival analysis with Firth's penalized estimation to calculate crude and adjusted hazard ratio estimates (16). To analyze statistical differences in repeated measurements of the Glasgow Coma Scale and arterial blood pressure over time between categories and the interaction between time and categories, we used two-way ANOVA with repeated measurements. Statistical analysis was performed using STATA (version 15.1, StataCorp USA) and R (version 3.5.3) statistical software.
Results
Characteristics of Patients at Admission
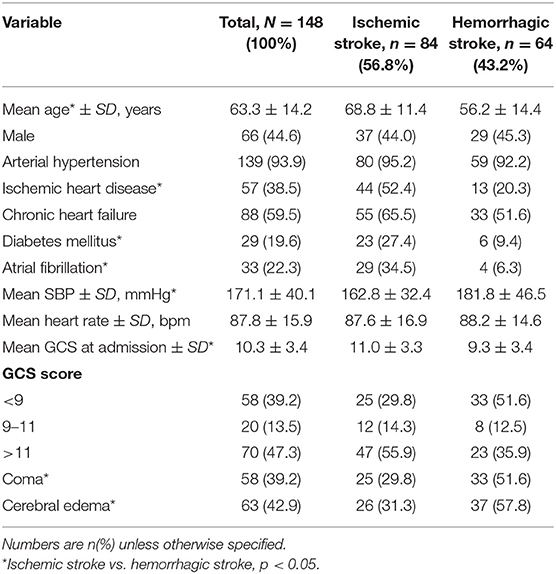
One hundred and forty eight patients were admitted to the neurocritical care unit at our center for stroke between October 2009 and March 2013. The most common co-morbidities were hypertension, ischemic heart disease, chronic heart failure and atrial fibrilation (Table 1). Most of the patients were admitted directly to neurocritical care upon presentation to the emergency department of our center (127/148 [85.8%]) or during the 1st day after presentation to our center (11/148 [7.4%]). The remaining 10 (6.8%) patients were transferred to neurocritical care between day 2 and 14 after admission to the hospital. Eighty four of 148 patients (56.8%) were diagnosed with ischemic stroke and 64/148 (43.2%) were diagnosed with hemorrhagic stroke (Table 1). Of the patients with ischemic stroke, 56/84 (66.7%) had middle cerebral artery occlusion and 27/84 (32.1%) had vertebrobasilar artery occlusion. Mean Glasgow Coma Scale scores were higher at admission in patients with ischemic stroke, compared to patients with hemorrhagic stroke (Table 1).
Out of 148 patients, 58 (39.1%) were in coma at time of admission to neurocritical care and had a Glasgow Coma Scale <9 points. A smaller proportion of the patients with ischemic stroke were in coma (30%) compared to patients with hemorrhagic stroke (52%). Patients with ischemic stroke were, on average, older than patients with hemorrhagic stroke. History of ischemic heart disease, diabetes mellitus and atrial fibrillation was more common in patients who had an ischemic stroke, relative to patients who had a hemorrhagic stroke (Table 2). Eighteen (12.1%) patients had chronic renal failure. History of arterial hypertension was common (94%) with mean systolic blood pressure (±SD) at admission of 171(±40) mmHg and mean diastolic blood pressure at admission of 95(±15) mmHg; however, only 105 (71%) patients were taking antihypertensive medications at admission.
Patient Outcomes
The median duration (interquartile range) of hospital stay was 12 days (6–22.5 days) and the median neurocritical care unit stay was 7 days (range: 1–68 days). Neurosurgical procedures were performed in 27 (18%) patients. One patient with ischemic stroke underwent decompression craniotomy and 26 (41%) patients with hemorrhagic stroke underwent craniotomy with hematoma removal.
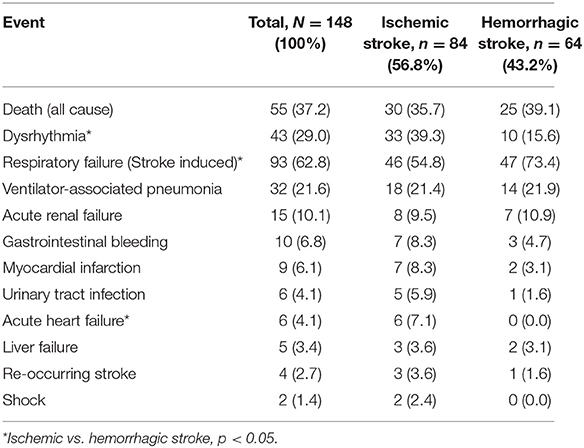
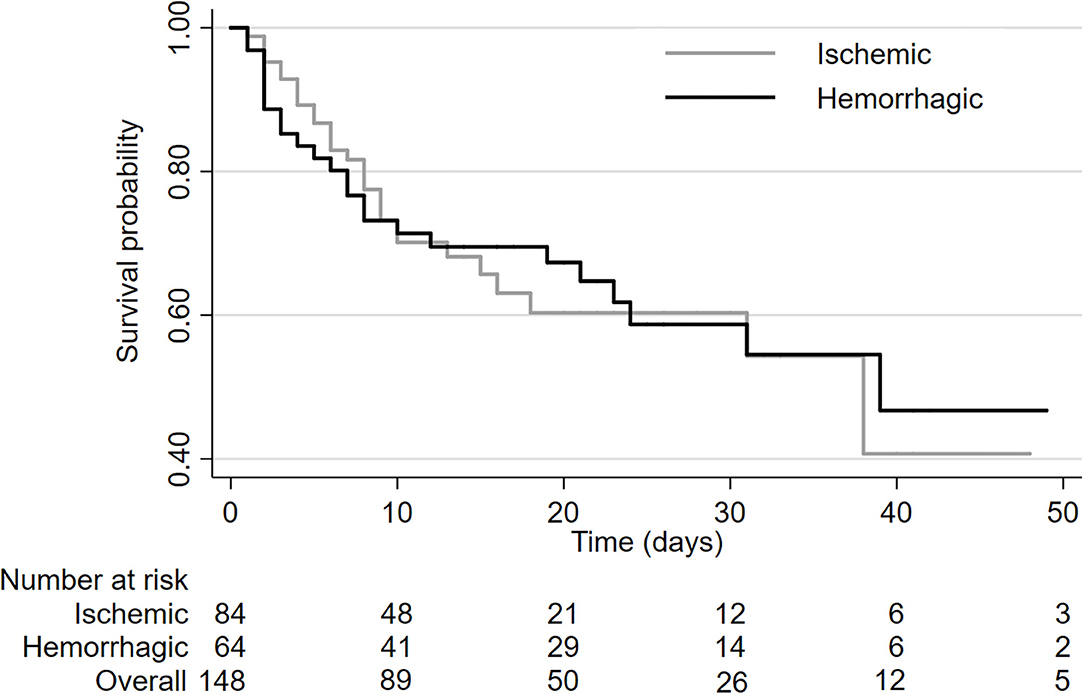
Of the patients with stroke who were admitted to neurocritical care, 55/148 patients (37%) died in hospital. The in-hospital mortality rate was similar in patients with ischemic (36%) and hemorrhagic (39%) stroke (HR 0.88, 95%CI 0.48–1.60, log-rank P = 0.99; Figure 1). Median survival was 38 days (range: 1–89 days) in patients with ischemic stroke and 39 days (range: 1–63 days) in patients with hemorrhagic stroke.
Respiratory complications were the most common extra-cerebral complications and 93 (63%) patients developed stroke-related respiratory failure (coma, loss of airway protective reflexes, reduced minute ventilation, pneumonia, and acute lung injury) requiring intubation with mechanical ventilation (Table 2). Respiratory failure was less common in patients with ischemic stroke (55%) than in patients with hemorrhagic stroke (73%). Hemorrhagic transformation of stroke occurred in three patients.
Predictors of In-Hospital Mortality
Univariable analysis revealed several characteristics that were correlated with mortality rate (Table 3). All patients in our analysis were admitted to neurocritical intensive care. At admission, patients who had a lower Glasgow Coma Scale, were in coma and who had cerebral edema were more likely to die in-hospital (P = 0.05, 0.02, < 0.01, respectively). Statistically significant variables from the univariable analysis and clinically important variables were used in the multivariable analysis (age, sex, hypertension, ischemic heart disease, chronic heart failure, systolic blood pressure, heart rate, presence of cerebral edema, and stroke type). In multivariable analysis, male sex (HR 0.48, 95%CI: 0.22–0.98) and hemorrhagic stroke (vs. ischemic stroke, HR 0.44 95%CI:0.23–1.22) trended toward a lower risk of in-hospital mortality. Cerebral edema was associated with a higher risk of mortality (HR 2.45 95%CI: 1.04–6.13).
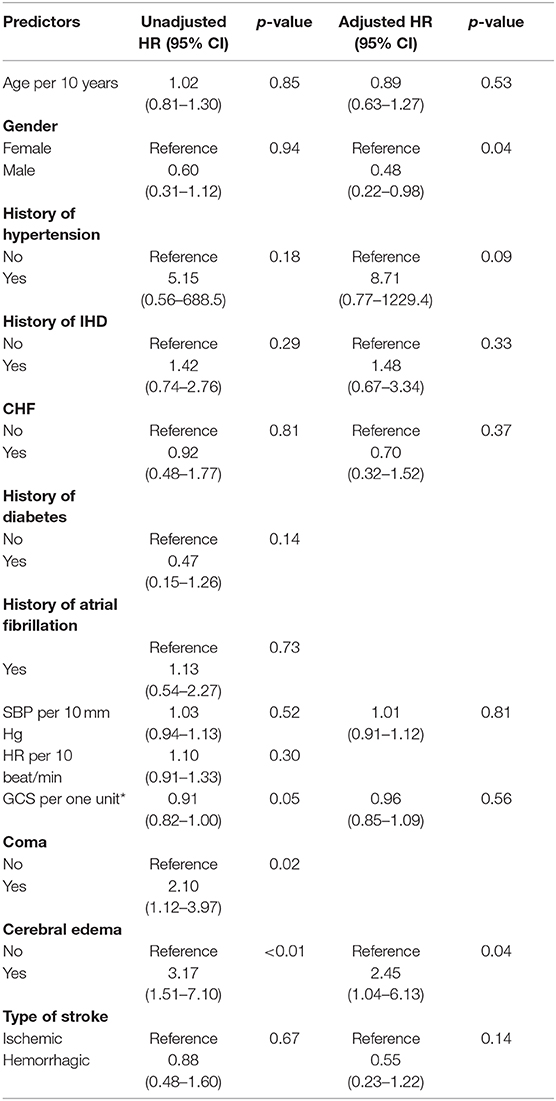
Table 3. In-hospital stroke mortality predictors using simple and multivariable Cox regression analysis.
Discussion
The lifetime risk of ischemic or hemorrhagic stroke in adults is 25% and the risk of ischemic stroke is 18% from the age of 25 years onward (17). Estimates for the incidence of stroke range from 90 to 180 per 100,000 population in North America and Western Europe to 241–360 per 100,000 population in East Asia, Eastern Europe and Central Asia (18). Recent epidemiologic data suggest that the highest incidence of stroke is in East Asia, followed by Eastern Europe and Central Asia (18). Data from the Global Burden of Ischemic and Hemorrhagic Stroke (GBIHS) study indicate that while Kazakhstan has a low prevalence of ischemic stroke (0–170 cases per 100,000 person years), it has the highest age-standardized mortality rate from ischemic stroke in the world (149–174 deaths per 100,000 person years) (10). Age standardized prevalence of hemorrhagic stroke is 39–78 cases per 100,000 person-years. Mortality due to hemorrhagic stroke is 64–95 deaths per 100,000 person-years, a rate which is higher than developed countries and higher than most developing countries.
In our study, mortality was high because we included only severely ill patients. The unadjusted rates of mortality were similar for patients with ischemic and hemmorhagic stroke, but in the multivariable analysis male sex was protective. In comparison to ischemic stroke, hemmorhagic stroke was associated with reduced risk of death and this is consistent with the GBIHS study.
Overall rate of in-hospital mortality was similar in patients with ischemic stroke compared to patients with hemorrhagic stroke despite the fact that mean age was higher amongst patients with ischemic stroke (mean age 68.8 years) compared to patients with hemorrhagic stroke (mean age 56.2 years). A majority of the deaths occurred within the first 10 days after admission and deaths tended to occur earlier in patients with ischemic stroke, though this did not reach statistical significance in the multivariable analysis (HR 0.55, 95%CI 0.23–1.22; p = 0.14; Figure 1). Hemorrhagic stroke was associated with a slightly longer length of hospital stay. While it would be expected that patients with hemorrhagic stroke would have a higher in-hospital mortality risk, we did not observe this in our analysis. This could be a spurious finding because of the sample size was not large. It could also be a related to the older age of our patients and a high prevalence of comorbidities (atrial fibrillation, ischemic heart disease, hypertension, and diabetes) in ischemic stroke patients.
The univariable analysis identified coma and cerebral edema as the most strongly correlated risk factors for subsequent in-hospital mortality (Table 2). None of the modifiable risk factors showed a statistically significant impact on mortality in the multivariable model, but history of hypertension had a large hazard ratio with high statistical uncertainty [HR(95%CI) 8.71(0.77–1,229)]. The prevalence of hypertension in our study is higher than a recent study performed in the same city which showed a prevalence of at 70% in adults aged 50–75 years (11). Recent studies reported that hypertension is associated with unfavorable outcome in patients with acute ischemic stroke (19). Another study showed that hypertension was correlated with death and disability in patients with hemorrhagic stroke but not with ischemic stroke in Inner Mongolia (20).
Gastrointestinal Complications
All patients were given an acid-suppressive therapy (proton pump inhibitor or H2-blocker), however, 10 patients (7%) still developed gastrointestinal bleeding. Gastrointestinal bleeding was more common in patients with ischemic stroke (N = 7) and this difference was likely due to the use of antiplatelet medications in this group. Nine cases of GI bleeding were successfully treated either medically or endoscopically and there was only one severe case which required surgery. All patients who developed bleeding were in coma and were mechanically ventilated. GI bleeding is a well-documented complication of acute phase of stroke with a reported incidence of 0.1–8.0% (21, 22).
Mucosal injury is extremely common in ICU patients and was reported to be 75–100% in those who were admitted in shock (23). Sympathetic drive and vasoconstriction in neurosurgical patients is a probable causative mechanism of the high incidence of gastric ulcers (24) and although a high proportion of critically ill patients develop mucosal injury, not all of these progress to gastrointestinal bleeding (23). Another potential mechanism leading to stress ulcer is reduced mucosal circulation and mucosal hypoxia (25). The impact of acid suppressing medication in stroke patients deserves further study to identify patients who could benefit most. Several meta-analysis have been published to date but the results are inconclusive. Acid suppression strategies have not demonstrated a mortality benefit (26, 27).
Cardiovascular Complications
Central nervous system pathology has been clearly associated with myocardial damage and dysfunction (28). The mechanism of myocardial injury in acute ischemic stroke is not well-understood but elevated serum catecholamine level has been hypothesized to be a causative factor (29). Conversely, the autoregulation of cerebral blood flow in ischemic stroke is reduced and depends on cardiac function, therefore this can result in vicious cycle of neurocardic dysfunction (30–32).
We found that 8.3% of patients with ischemic stroke and 3.1% of patients with hemorrhagic stroke developed myocardial infarction. Patients with ischemic stroke were more likely to develop myocardial infarction likley related to advanced ischemic heart disease. Previous studies have shown that myocardial infarction is a probable complication in ischemic stroke patients and that this may be related to release of endogenous catecholamines (33).
A strength of this study is the wide range of risk factors that were assessed in a population not previously studied and idenfied several predictors of mortality in this Central Asian population. The main limitation of this study is that it was a retrospective analysis of a small sample. We did not have data on the time from stroke onset to presentation at our center. Thrombolytic therapy with recombinant tissue plasminogen activator was uncommonly used in Kazakhstan during the patient enrollment period (2009–2013) because of access and the likelihood of presentation to hospital more than 4.5 h after stroke onset, therefore, none of the patients in our study received this intervention.
Conclusion
This is the first analysis of its type in Central Asian patients with stroke, providing both a descriptive element and estimates of the relative contributions to mortality of several risk factors. Our analysis showed that mortality risk in patients with hemorrhagic stroke was closer to mortality risk in patients with ischemic stroke than presented in other analyses. Whether this was an artifact of the sample reflects a regional difference needs to be clarified. Hypertension, chronic heart failure, ischemic heart disease and atrial fibrillation were the most frequent comorbidities in patients who developed severe (life-threatening) stroke. Coma and cerebral edema on admission appear to be associated with poor outcome. Future research directions could include pooled analyses from multiple centers in the region to identify risk factors for in-hospital stroke mortality with greater precision.
Data Availability Statement
The dataset will be available upon request. Requests to access these datasets should be directed to ZG1pdHJpeS52aWRlcm1hbkBudS5lZHUua3o=.
Ethics Statement
This study was reviewed and approved by the Nazarbayev University IREC. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author's Note
This study was performed at the University Medical Center.
Author Contributions
DV: conception, design of the study, interpretation of data, drafting the manuscript, manuscript revision, and administrative support. AI: interpretation, statistical analysis, data management, and manuscript revision. TT: administrative support. EG: manuscript revision. PF: obtaining funding, design of the study, interpretation of data, drafting the manuscript, and manuscript revision. All authors contributed to the article and approved the submitted version.
Funding
Internal funding from Nazarbayev University.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Langhorne P, Dey P, Woodman M, Kalra L, Wood-Dauphinee S, Patel N, et al. Is stroke unit care portable? A systematic review of the clinical trials. Age Ageing. (2005) 34:324–30. doi: 10.1093/ageing/afi038
2. World Health Organization. The Top 10 Causes of Death. World Health Organization (2020). Available online at: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed October 27, 2020).
3. Ingeman A, Andersen G, Hundborg HH, Svendsen ML, Johnsen SP. In-hospital medical complications, length of stay, and mortality among stroke unit patients. Stroke. (2011) 42:3214–8. doi: 10.1161/STROKEAHA.110.610881
4. Hong KS, Saver JL, Kang DW, Bae HJ, Yu KH, Koo J, et al. Years of optimum health lost due to complications after acute ischemic stroke: disability-adjusted life-years analysis. Stroke. (2010) 41:1758–65. doi: 10.1161/STROKEAHA.109.576066
5. Indredavik B, Rohweder G, Naalsund E, Lydersen S. Medical complications in a comprehensive stroke unit and an early supported discharge service. Stroke. (2008) 39:414–20. doi: 10.1161/STROKEAHA.107.489294
6. Kirkman MA, Citerio G, Smith M. The intensive care management of acute ischemic stroke: an overview. Intensive Care Med. (2014) 40:640–53. doi: 10.1007/s00134-014-3266-z
7. Bushnell CD, Phillips-Bute BG, Laskowitz DT, Lynch JR, Chilukuri V, Borel CO. Survival and outcome after endotracheal intubation for acute stroke. Neurology. (1999) 52:1374–81. doi: 10.1212/WNL.52.7.1374
8. Ho WM, Lin JR, Wang HH, Liou CW, Chang KC, Lee JD, et al. Prediction of in-hospital stroke mortality in critical care unit. SpringerPlus. (2016) 5:1051–8. doi: 10.1186/s40064-016-2687-2
9. Alonso A, Ebert AD, Kern R, Rapp S, Hennerici MG, Fatar M. Outcome predictors of acute stroke patients in need of intensive care treatment. Cerebrovasc Dis. (2015) 40:10–7. doi: 10.1159/000430871
10. Feigin VL, Krishnamurthi RV, Parmar P, Norrving B, Mensah GA, Bennett DA, et al. Update on the global burden of ischemic and hemorrhagic stroke in 1990–2013: the GBD 2013 study. Neuroepidemiology. (2015) 45:161–76. doi: 10.1159/000441085
11. Supiyev A, Kossumov A, Utepova L, Nurgozhin T, Zhumadilov Z, Bobak M. Prevalence, awareness, treatment and control of arterial hypertension in Astana, Kazakhstan. A cross-sectional study. Public Health. (2015) 129:20. doi: 10.1016/j.puhe.2015.02.020
12. Supiyev A, Kossumov A, Kassenova A, Nurgozhin T, Zhumadilov Z, Peasey A, et al. Diabetes prevalence, awareness and treatment and their correlates in older persons in urban and rural population in the Astana Region, Kazakhstan. Diabetes Res Clin Practice. (2016) 112:11. doi: 10.1016/j.diabres.2015.11.011
13. Supiyev A, Nurgozhin T, Zhumadilov Z, Peasey A, Hubacek JA, Bobak M. Prevalence, Awareness, Treatment and Control of Dyslipidemia in Older Persons in Urban and Rural Population in the Astana Region, Kazakhstan. BMC Public Health. (2017) 17:5. doi: 10.1186/s12889-017-4629-5
14. Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, Krieger D, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke. (2007) 38:2001–23. doi: 10.3410/f.1087891.540830
15. Adams HP, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Circulation. (2007) 115:e478–534. doi: 10.1161/CIRCULATIONAHA.107.181486
16. Heinze G, Schemper M. A solution to the problem of monotone likelihood in Cox regression. Biometrics. (2001) 57:114–9. doi: 10.1111/j.0006-341X.2001.00114.x
17. Collaborators GBDLRoS, Feigin VL, Nguyen G, Cercy K, Johnson CO, Alam T, et al. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med. (2018) 379:2429–37. doi: 10.1056/NEJMoa1804492
18. Collaborators GBDN. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. (2019) 18:459–80. doi: 10.1016/S1474-4422(18)30499-X
19. Nayak AR, Shekhawat SD, Lande NH, Kawle AP, Kabra DP, Chandak NH, et al. Incidence and clinical outcome of patients with hypertensive acute ischemic stroke: an update from tertiary care center of Central India. Basic Clin Neurosci. (2016) 7:351–60. doi: 10.15412/J.BCN.03070408
20. Zhang Y, Reilly KH, Tong W, Xu T, Chen J, Bazzano LA, et al. Blood pressure and clinical outcome among patients with acute stroke in Inner Mongolia, China. J Hypertens. (2008) 26:1446–52. doi: 10.1097/HJH.0b013e328300a24a
21. Wijdicks EF, Fulgham JR, Batts KP. Gastrointestinal bleeding in stroke. Stroke. (1994) 25:2146–8. doi: 10.1161/01.STR.25.11.2146
22. Donnell MJ, Kapral MK, Fang J, Saposnik G, Eikelboom JW, Oczkowski W, et al. Gastrointestinal bleeding after acute ischemic stroke. Neurology. (2008) 71:650–5. doi: 10.1212/01.wnl.0000319689.48946.25
23. Bardou M, Quenot JP, Barkun A. Stress-related mucosal disease in the critically ill patient. Nat Rev Gastroenterol Hepatol. (2015) 12:98–107. doi: 10.1038/nrgastro.2014.235
24. Choi YH, Lee JH, Shin JJ, Cho YS. A revised risk analysis of stress ulcers in burn patients receiving ulcer prophylaxis. Clin Exp Emerg Med. (2015) 2:250–5. doi: 10.15441/ceem.15.076
25. Yandrapu H, Sarosiek J. Protective factors of the gastric and duodenal mucosa: an overview. Curr Gastroenterol Rep. (2015) 17:24. doi: 10.1007/s11894-015-0452-2
26. Krag M, Perner A, Wetterslev J, Wise MP, Hylander Moller M. Stress ulcer prophylaxis versus placebo or no prophylaxis in critically ill patients. A systematic review of randomised clinical trials with meta-analysis and trial sequential analysis. Intensive Care Med. (2014) 40:11–22. doi: 10.1007/s00134-013-3125-3
27. Ro Y, Eun CS, Kim HS, Kim JY, Byun YJ, Yoo KS, et al. Risk of Clostridium difficile infection with the use of a proton pump inhibitor for stress ulcer prophylaxis in critically ill patients. Gut Liver. (2016) 10:581–6. doi: 10.5009/gnl15324
28. Oppenheimer SM, Hachinski VC. The cardiac consequences of stroke. Neurol Clin. (1992) 10:167–76. doi: 10.1016/S0733-8619(18)30239-1
29. Wira C, Rivers E, Martinez-Capolino C, Silver B, Iyer G, Sherwin R, et al. Cardiac complications in acute ischemic stroke. Western J Emergency Med. (2011) 12:414–20. doi: 10.5811/westjem.2011.2.1785
30. Keller TS, McGillicuddy JE, LaBond VA, Kindt GW. Volume expansion in focal cerebral ischemia: the effect of cardiac output on local cerebral blood flow. Clin Neurosurg. (1982) 29:40–50. doi: 10.1093/neurosurgery/29.CN_suppl_1.40
31. Keller TS, McGillicuddy JE, LaBond VA, Kindt GW. Modification of focal cerebral ischemia by cardiac output augmentation. J Surg Res. (1985) 39:420–32. doi: 10.1016/0022-4804(85)90096-4
32. Tranmer BI, Keller TS, Kindt GW, Archer D. Loss of cerebral regulation during cardiac output variations in focal cerebral ischemia. J Neurosurg. (1992) 77:253–9. doi: 10.3171/jns.1992.77.2.0253
Keywords: cerebrovascular disease, intensive care unit, critical care, mortality, risk factors, hemorrhagic stroke, ischemic stroke
Citation: Viderman D, Issanov A, Temirov T, Goligher E and la Fleur P (2020) Outcome Predictors of Stroke Mortality in the Neurocritical Care Unit. Front. Neurol. 11:579733. doi: 10.3389/fneur.2020.579733
Received: 30 July 2020; Accepted: 18 November 2020;
Published: 15 December 2020.
Edited by:
Vasileios-Arsenios Lioutas, Beth Israel Deaconess Medical Center and Harvard Medical School, United StatesReviewed by:
Christos Krogias, Ruhr University Bochum, GermanyMaurice Giroud, Centre Hospitalier Regional Universitaire De Dijon, France
Copyright © 2020 Viderman, Issanov, Temirov, Goligher and la Fleur. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dmitriy Viderman, ZG1pdHJpeS52aWRlcm1hbkBudS5lZHUua3o=
 Dmitriy Viderman
Dmitriy Viderman Alpamys Issanov
Alpamys Issanov Talgat Temirov2,3
Talgat Temirov2,3 Philip la Fleur
Philip la Fleur